- 1Neonatal Intensive Care Unit (NICU), Women’s Wellness and Research Center (WWRC), Hamad Medical Corporation (HMC), Doha, Qatar
- 2Medical Research Center, Hamad Medical Corporation (HMC), Doha, Qatar
- 3Research Department, Women’s Wellness and Research Center (WWRC), Hamad Medical Corporation (HMC), Doha, Qatar
- 4Qatar University, Doha, Qatar
- 5Obstetrics and Gynecology Department, Women’s Wellness and Research Center (WWRC), Hamad Medical Corporation (HMC), Doha, Qatar
Background: Pregnant women and newborns are at-risk groups for coronavirus disease 2019 (COVID-19). There is a paucity of evidence to prove the degree of perinatal passive immunity transfer from COVID-19-vaccinated or COVID-19-infected mothers to their newborns.
Methods: We prospectively investigated the vaccination and infection status of 70 women included in the study, as well as the serological characteristics of 72 newborns, to investigate the in utero transmission of maternal antibodies against COVID-19 to newborn infants between 2021 and 2022.
Results: A total of 70 pregnant mothers were included in the study after providing signed informed consent. After delivery, cord blood samples were collected from all 72 newborns included in the study. The COVID-19-vaccinated group had significantly higher (p < 0.001) values of both antibodies (NTAb*3.31 and S-RBD*1.15) in the cord blood across both the COVID-19-positive and COVID-19-negative groups. The antibody titres were the lowest in mothers who were not vaccinated and the highest in those who received three vaccination doses (p < 0.001). Multivariate linear regression analysis was performed using dependent variables NTAb*3.31 and S-RBD*1.15 antibodies and independent predictor variables nationality, infant’s gender, COVID-19 vaccination status, and COVID-19 test status; the multivariate linear regression analysis results indicated that vaccination against COVID-19 remained a potential significant (p < 0.0001) predictor for both NTAb*3.31 and S-RBD*1.15 antibodies after adjusting other potential predictor variables.
Conclusions: In our study, we found significantly higher titres of NTAb*3.31 and S-RBD*1.15 antibodies in newborns’ cord blood whose mothers had previous COVID-19 infection or received COVID-19 vaccination; however, these titres were higher in the case of vaccination than previous infection. The more doses of vaccine received, the higher the antibody levels in newborns’ cord blood. This indicates transplacental immunity transmission from mothers to their newborns after previous COVID-19 vaccination or infection.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is highly infectious. SARS-CoV-2 was first described by Huang et al. (1), and it continues to pose a global public health concern (2).
Pregnant women and newborns are at-risk groups for COVID-19 (3, 4). The COVID-19 virus was detected in various tissues of the body as well as body fluids, including the umbilical cord, amniotic fluid, and placental tissue and placental circulation. Maternal antibodies have dual effects. They supply passive protection and prime the immune system of the child for subsequent exposure to an antigen. In humans, maternal antibodies wane over 6–12 months. The kinetics of maternal antibody decline are correlated with the amount of maternal antibody present in the neonate after birth. Higher titres persist for a longer time. Furthermore, the number of antibodies could be pathogen- and dose-dependent (5).
During an infection, the immune response is activated to produce antibodies by plasma cells (activated B cells) specific to spike proteins (or other viral structural proteins). Antibodies specific to S1 could neutralise and block the attachment and fusion of SARS-CoV-2 to the host cell before its replication within the lungs and other tissues, including the gastrointestinal tract (6).
Tests for IgG and IgM antibodies for SARS-CoV-2 became available in February 2020. On March 4, 2020, the seventh edition of The New Coronavirus Pneumonia Prevention and Control Protocol for SARS-CoV-2 was released by the National Health Commission of the People’s Republic of China and added serological diagnostic criteria (7, 8).
A recent study found that in a woman positive for SARS-CoV-2, RNA was initially detected in nasopharyngeal samples but not in serum or breast milk samples. The presence of SARS-CoV-2 RNA does not mean vertical transmission to the newborn, as this transmission remains very unlikely (9–11). However, limited studies have been conducted to prove perinatal passive immunity transfer from COVID-19-infected or COVID-19-vaccinated mothers to their newborns. There has been no evidence of mother-to-newborn vertical transmission of SARS-CoV-2 (3, 12), and little is known about susceptibility and immunological characteristics.
In this study, we aimed to prospectively investigate the immunological characteristics of newborns delivered to mothers with previous COVID-19 infection or vaccination for the possibility of acquiring passive transplacental transmission immunity.
Methods
This prospective observational study was conducted in the Neonatal Intensive Care Unit (NICU) at the Women’s Wellness and Research Center (WWRC), Hamad Medical Corporation (HMC), Doha, Qatar. The WWRC is Qatar’s main hospital for tertiary care obstetrics and neonatal–perinatal services. The NICU in the WWRC is equipped with 128 tertiary-level beds. The WWRC has >18,000 deliveries per year. The NICU has >3,000 admissions per year.
This prospective study was conducted over 2 years in 2021 and 2022 after obtaining institutional review board (IRB) approval (Protocol Number: MRC-01-20-1132) in full conformance with the principles of the Declaration of Helsinki and Good Clinical Practice (GCP) within the laws and regulations of the Ministry of Public Health in Qatar.
We prospectively investigated the vaccination and infection status of the 70 women included in the study, as well as the serological characteristics of the 72 newborns, to investigate the in utero transmission of maternal antibodies against COVID-19 to newborn infants.
Informed consent was obtained from pregnant women by a research team member before the participation of the women and their newborns. The consent was obtained while the pregnant mothers were in the hospital before delivery, and enough time was given according to their preference and acceptance to participate in this study. The confidentiality and privacy of all participants were protected, and patient identifiers were not disclosed.
Knowing the maternal COVID-19 infection and vaccination status provided us with data and directed us to further investigate newborn serological profiling. We believed that these data would help in proving or discouraging the notion of passive immunity transmission from pregnant mothers to their newborns after COVID-19 infection or vaccination. Nasopharyngeal aspirate samples were collected from the mothers upon hospital admission for delivery. A cord blood sample from each newborn was evaluated. A 4-mL volume of cord blood was collected after delivery in an ethylenediaminetetraacetic acid (EDTA) tube by the maternity nurse in the labour room/operating theatre of the WWRC. The cord blood samples were tested for neutralising antibodies (NTAbs) and spike-receptor binding domain (S-RBD) antibodies. All collected samples were kept in a refrigerator (for a maximum period of 12 hours) until collected by the research team and sent to the laboratory.
Laboratory methods
The cord blood was tested by rapid lateral flow immunochromatography assay (LFA; Lionex, Braunschweig, Germany). The level of antibody was measured by serial dilution of the specimen, and then the titration score was measured. All samples positive by ELISA and lateral flow assay were tested for the presence of neutralising antibodies (NTAbs) using the surrogate virus neutralisation assay (GenScript, New Jersey, USA) as described by the manufacturer. The ELISA and the nAb assay were performed in the Qatar University Laboratory. The level of neutralising antibody was measured by calculating the present inhibition score. A sample with more than 20% inhibition score was considered positive for NTAbs. All mothers were tested using the same assays to confirm vertical transmission from mother to infant.
Data collected included gender, nationality, maternal year of birth, date of delivery, collection date of the cord blood, maternal vaccination status against COVID-19, type of vaccine, number of vaccine doses, timing and date of vaccination, date of COVID-19 infection, symptoms of COVID-19 infection, and COVID-19 antibody test results, titres, and interpretation for both the mother and neonate. A comparison and a correlation were performed between the level of maternal antibodies and severity of infection, date of infection, timing during pregnancy, age of the pregnant woman, age of the infant, and stability of antibodies after birth (peak and the waning time). Data were coded and collected in an Excel sheet specifically designed for the study purpose. No patients’ identifiers were disclosed.
Inclusion criteria
1. We included all pregnant mothers who previously had a COVID-19 infection, were vaccinated, or were not infected or vaccinated in 2021 and 2022.
2. We included all newborns with gestational age >34 weeks delivered to the previously mentioned mothers in 2021 and 2022.
Exclusion criteria
1. We excluded mothers receiving immunosuppressive medications and those who received Intravenous Immunoglobulins (IVIG) before delivery.
2. We excluded newborns suspected of having an immunodeficiency disease and preterms with gestational age <34 weeks.
Statistical analysis
Descriptive statistics were used to summarise and determine the participants’ characteristics and data distribution. The normally distributed data were presented as mean with standard deviation (SD), whereas median and inter-quartile range (IQR) were used for skewed or non-normal data distribution. Categorical data were summarised using frequencies and respective percentages. Various parameters measured on categorical scales and their association with categorised NTAb and S-RBD antibody levels in cord blood (non-reactive and reactive) and the number of vaccination doses were evaluated using the chi-square (χ2) test, Fisher’s exact, or Yates’s corrected chi-square tests, as appropriate. Quantitative data outcomes (NTAb and S-RBD antibody levels in cord blood) between the two independent groups (reactive and non-reactive) were compared using a non-parametric Mann–Whitney U test.
Furthermore, linear regression analysis was performed using dependent variables NTAb*3.31 and S-RBD*1.15 antibody levels in cord blood and independent predictor variables nationality, infant’s gender, vaccination status against COVID-19, and COVID-19 test status; the results were reported using respective values of regression coefficients along with 95% confidence interval (CI). Receiver operating characteristic (ROC) curves were calculated to derive the best suitable cutoff values for both antibodies NTAb*3.31 and S-RBD*1.15 against vaccination status; sensitivity, specificity, and positive and negative likelihood ratio values were computed to evaluate and assess predictive accuracy. Because sensitivity and specificity were considered equally important, the best cutoff points were determined using Youden’s index, which maximises sensitivity and specificity. ROC curves provide a comprehensive and visually attractive way to summarise the accuracy of predictions. The ROC curve shows the tradeoff between sensitivity and specificity. It is a widely adopted method to detect the performance of a developed test, which classifies subjects into two categories: those who received and those who did not receive the COVID-19 vaccination. A box plot was constructed to depict the distribution of both antibodies (NTAb*3.31 and S-RBD*1.15) in the cord blood across the status of COVID-19 and vaccination groups. A two-sided p-value <0.05 was considered statistically significant. All statistical analyses were conducted using statistical software packages SPSS version 29.0 (IBM Corp., Armonk, NY, USA) and Epi Info (Centers for Disease Control and Prevention, Atlanta, GA, USA).
Results
The mothers were classified based on their COVID-19 infection and vaccination status, and newborns were investigated for possible passive immunity transmission through the placenta.
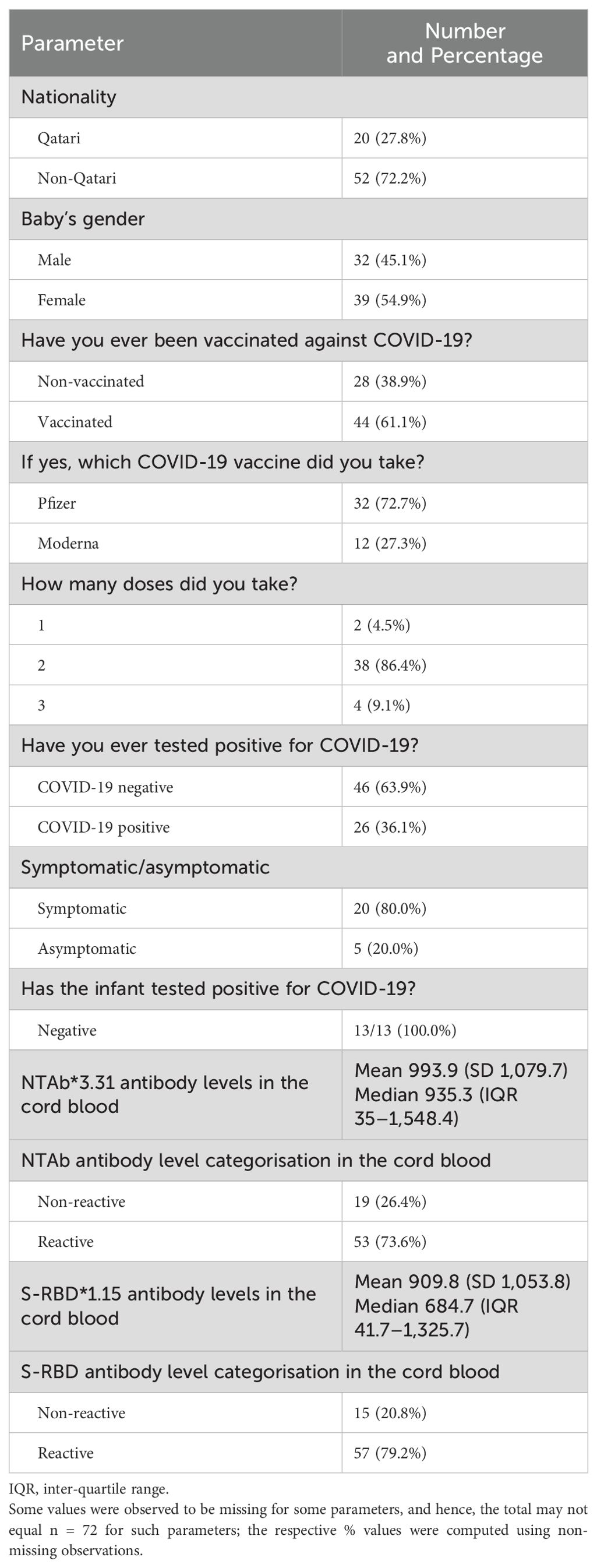
A total of 70 pregnant mothers were included in the study after providing signed informed consent. After delivery, cord blood samples were collected from all 72 newborns included in the study. The samples were sent to the laboratory and investigated for specific COVID-19 antibodies. Table 1 shows the descriptive statistics of the study population.
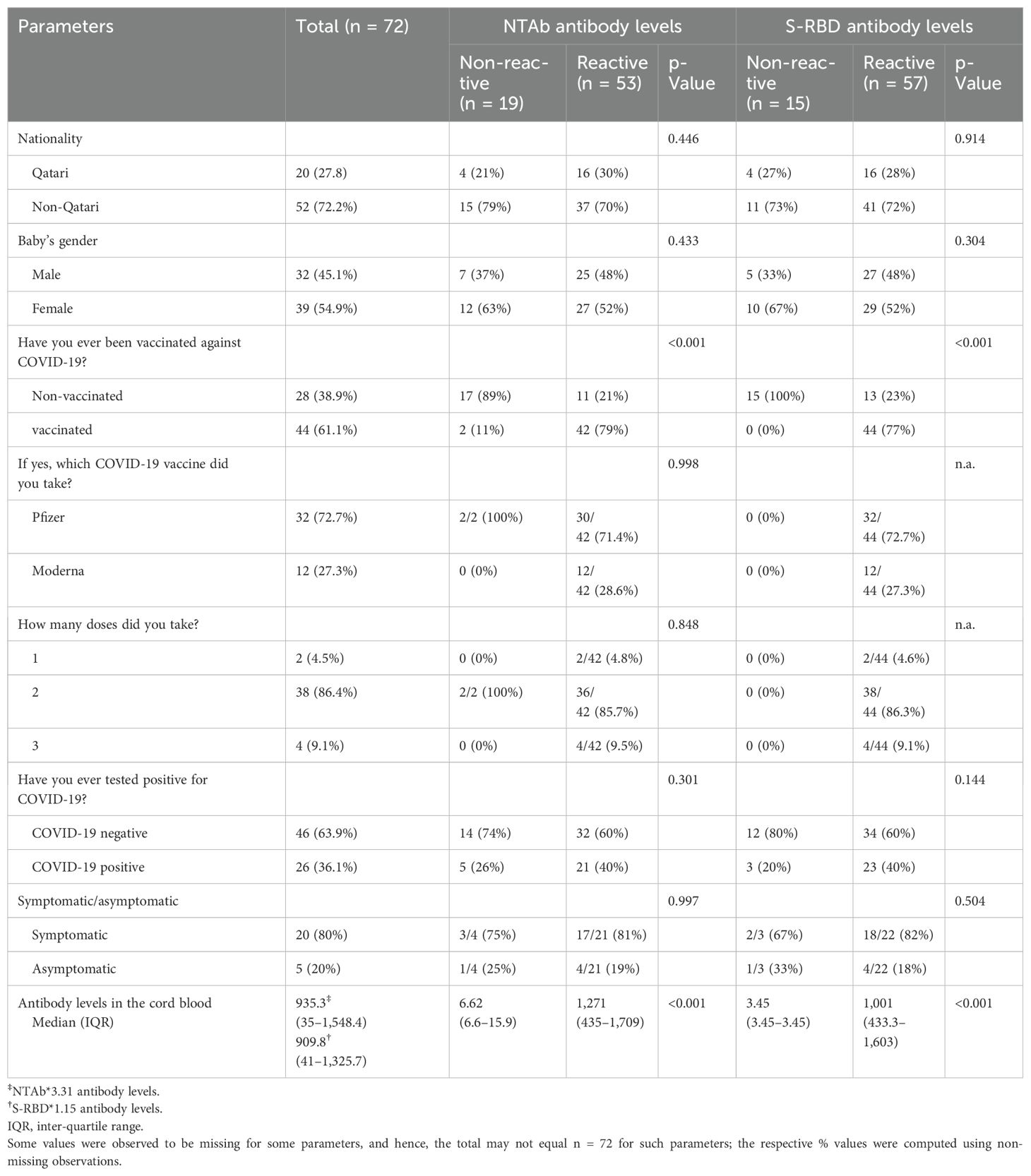
The box plot in Figure 1 shows that the COVID-19-vaccinated group had significantly higher (p < 0.001) values of both antibodies (NTAb*3.31 and S-RBD*1.15) in the cord blood across both the COVID-19-positive and COVID-19-negative groups. Table 2 shows NTAb*3.31 and SRBD* 1.15 antibody levels in the cord blood of the study population.
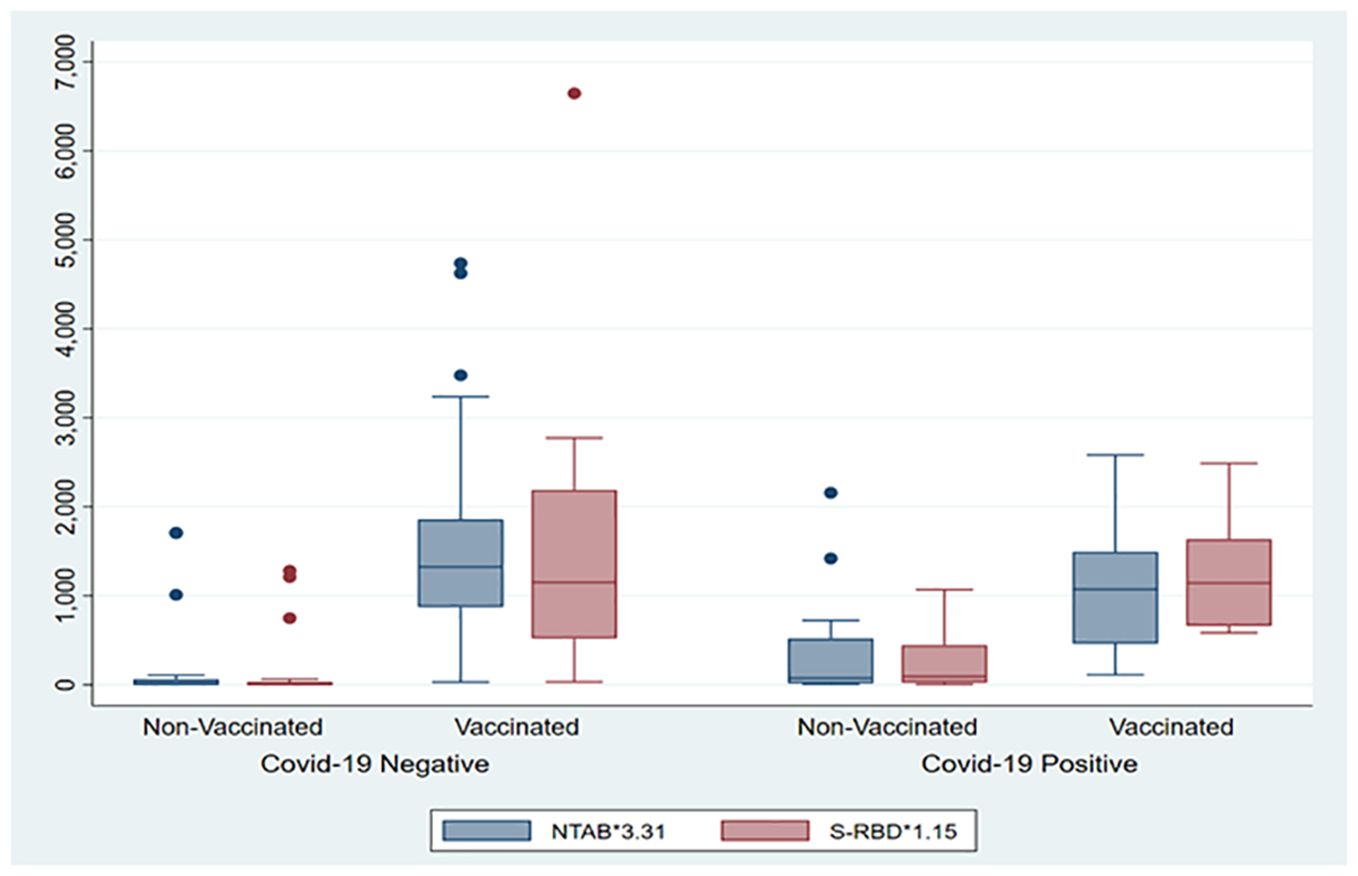
Figure 1. The box plot depicts the distribution of both antibodies (NTAb*3.31 and S-RBD*1.15) in the cord blood across the status of COVID-19 and vaccination groups.
ROC curves were calculated to derive the best suitable cutoff values for both antibodies NTAb*3.31 and S-RBD*1.15 and to assess predictive accuracy. ROC curves depicted in Figures 2 and 3 demonstrate an optimum cutoff value for both antibodies NTAb*3.31 and S-RBD*1.15 in the COVID-19-vaccinated group with their respective Area Under the Curve (AUC) values of 0.85 (95% CI 0.76, 0.95) and 0.89 (95% CI 0.82, 0.97), and high level of various diagnostic indices such as sensitivity, specificity, accuracy, and positive and negative likelihood ratio values indicates that the derived cutoff values for both antibodies exhibit high predictive accuracy.
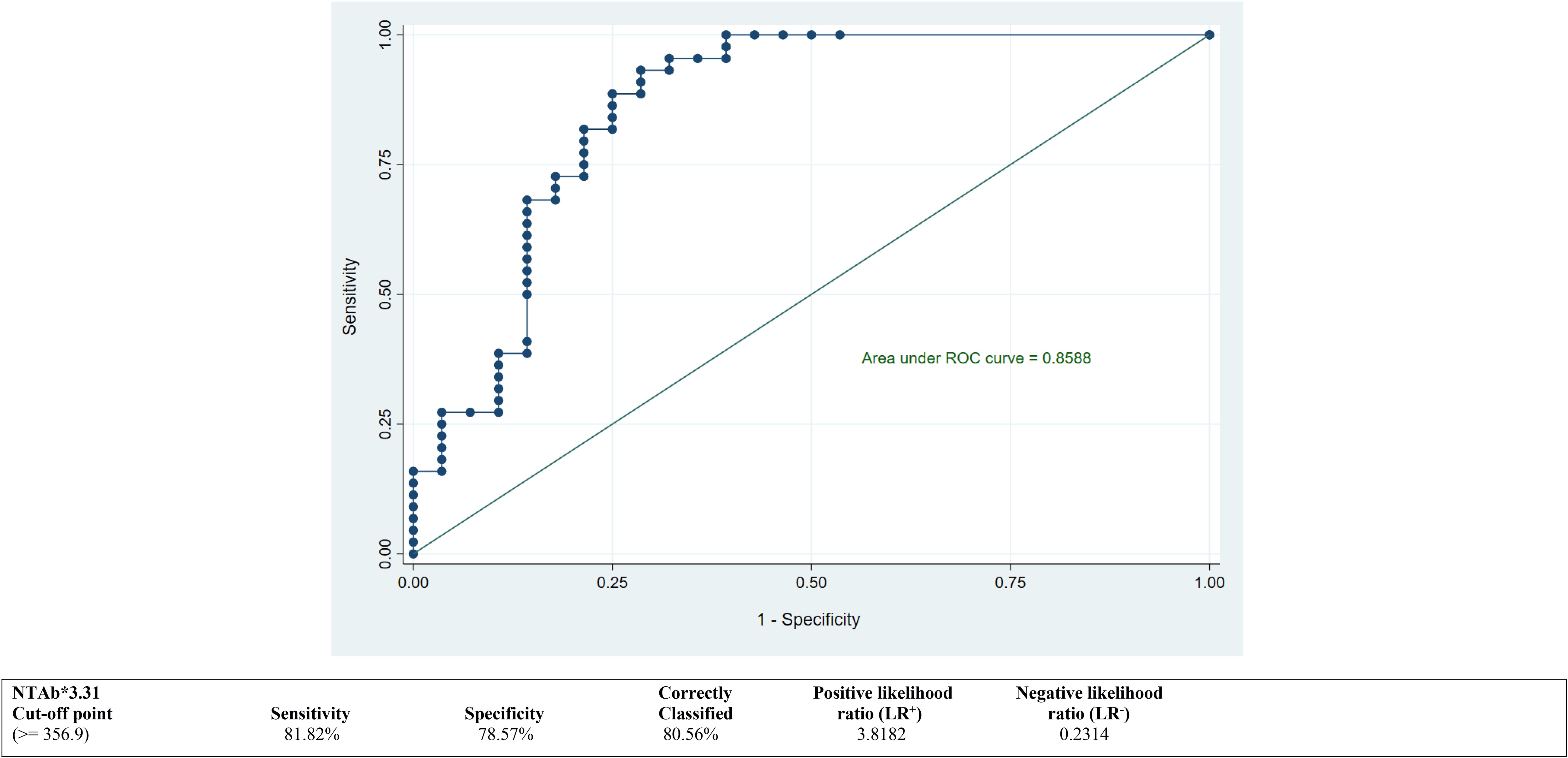
Figure 2. ROC curve to determine an optimum cutoff value for NTAb*3.31 antibodies in the COVID-19-vaccinated group. ROC, receiver operating characteristic.
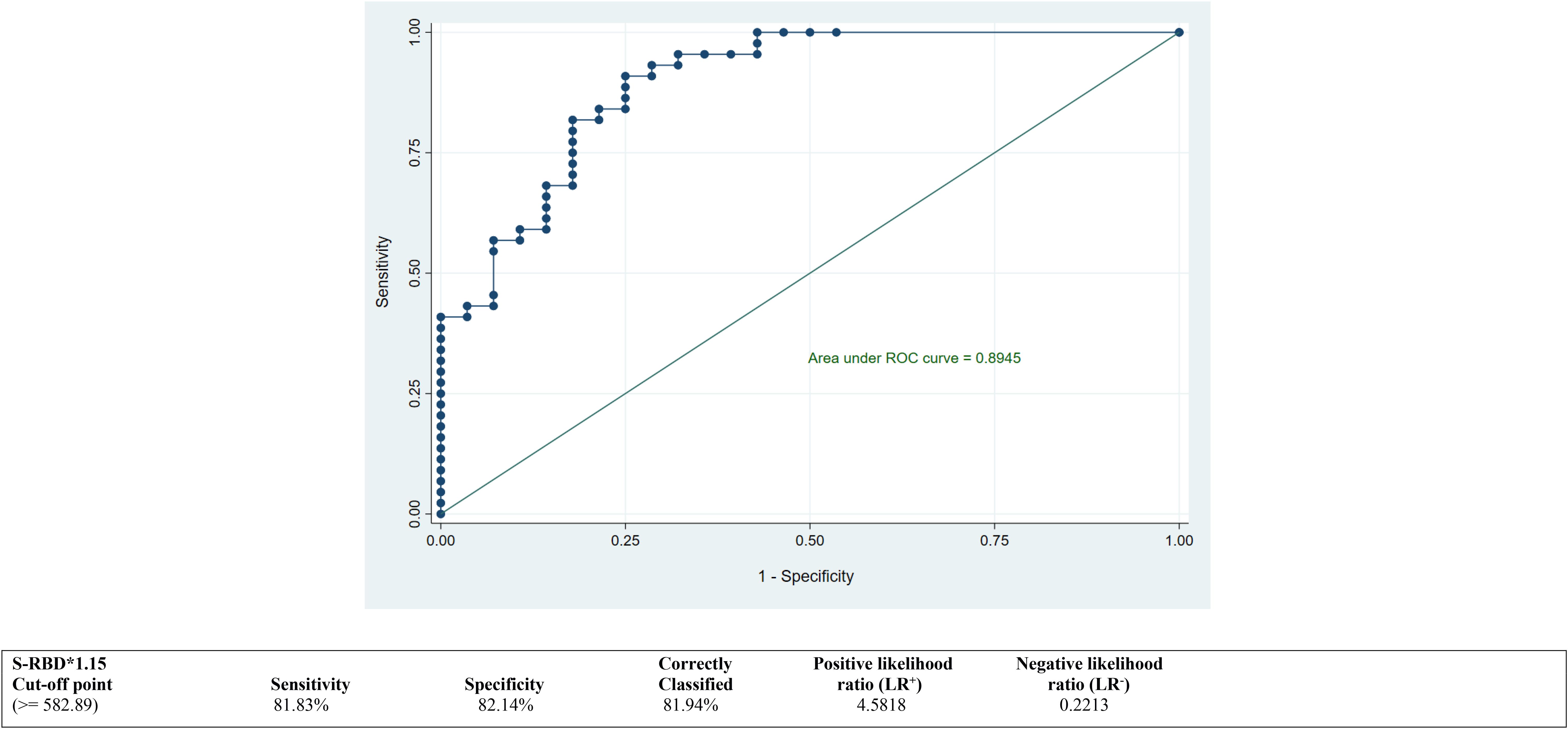
Figure 3. ROC curve to determine an optimum cutoff value for S-RBD*1.15 antibodies in the COVID-19-vaccinated group. ROC, receiver operating characteristic.
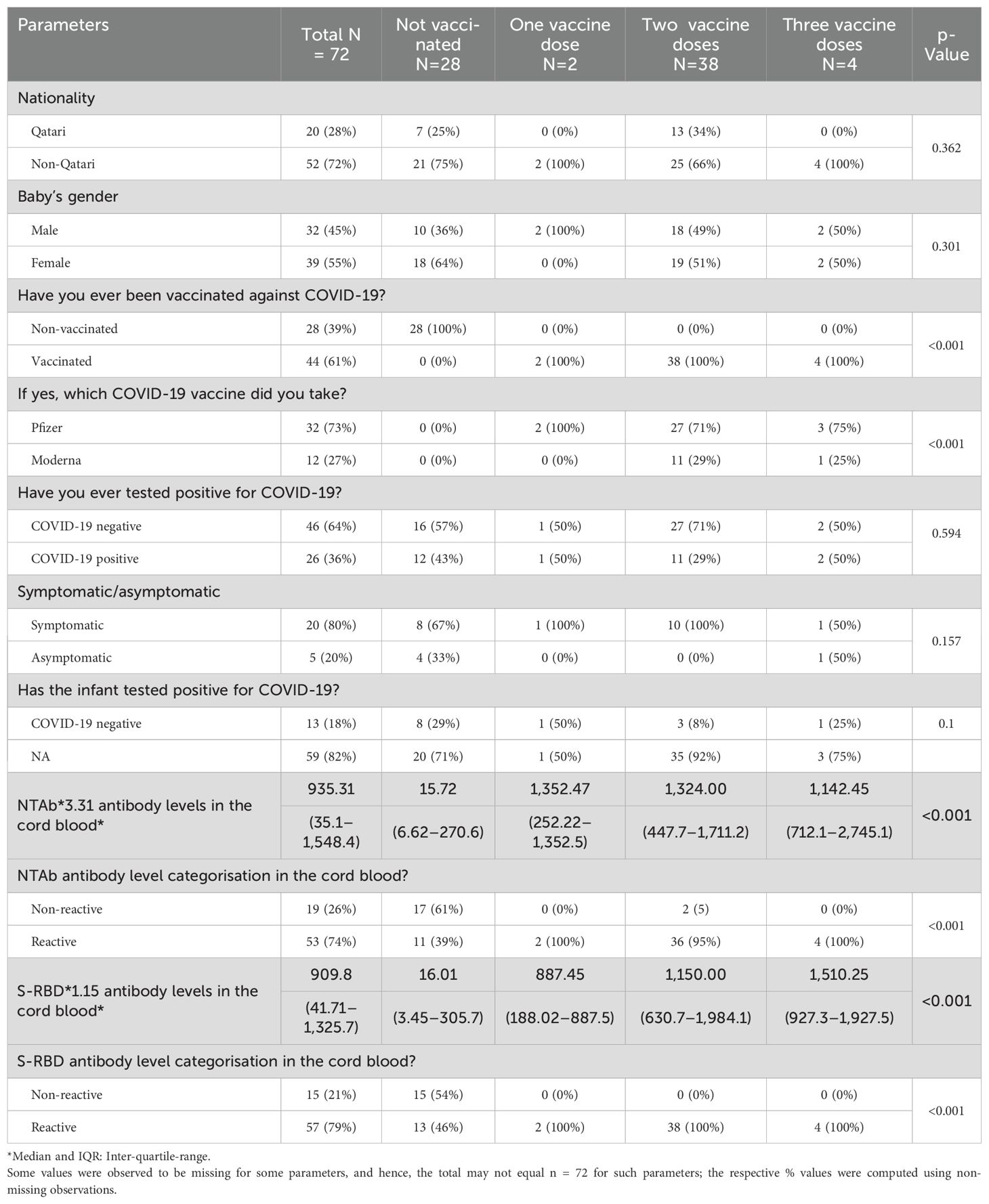
We found that the mother’s number of vaccination doses is significantlyt and directly proportional to the antibody levels in the cord blood. The antibody titres were the lowest in those who were not vaccinated and the highest in mothers who received three vaccination doses (p < 0.001). The number of vaccination doses in relation to other parameters is shown in Table 3.
Mothers who received more vaccination doses against COVID-19 had higher values of both antibodies (NTAb*3.31 and S-RBD*1.15) in the serum of their offspring, as shown in the box plots in Figures 4 and 5.
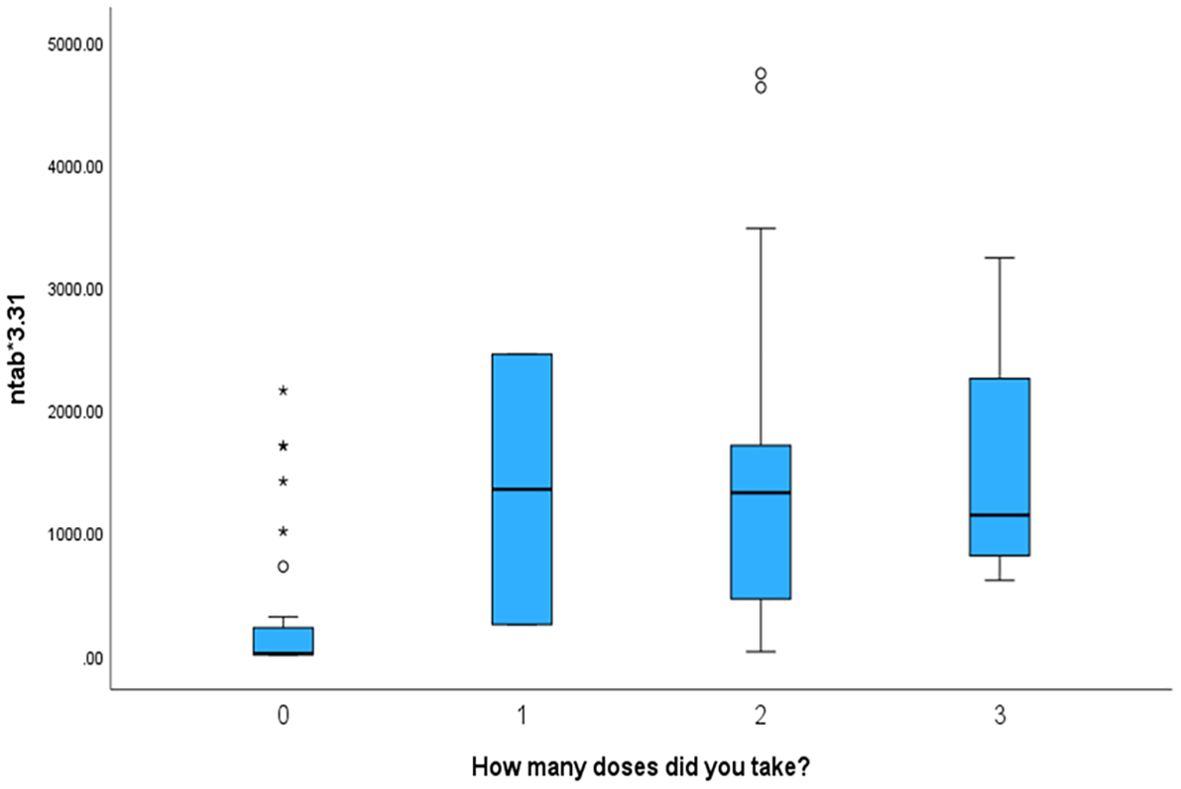
Figure 4. The box plot depicts that mothers who received more vaccination doses against COVID-19 had higher values of NTAb*3.31 antibodies in the serum of their offspring.
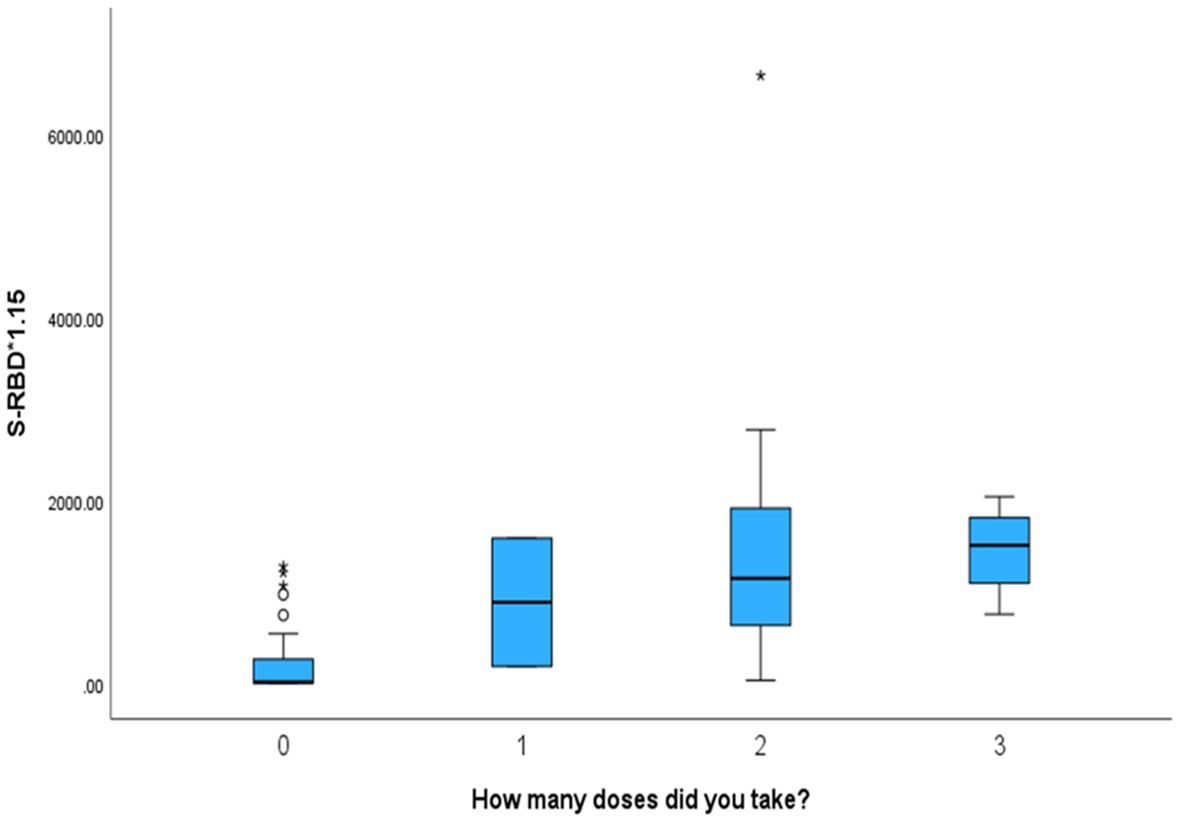
Figure 5. The box plot depicts that mothers who received more vaccination doses against COVID-19 had higher values of S-RBD*1.15 antibodies in the serum of their offspring.
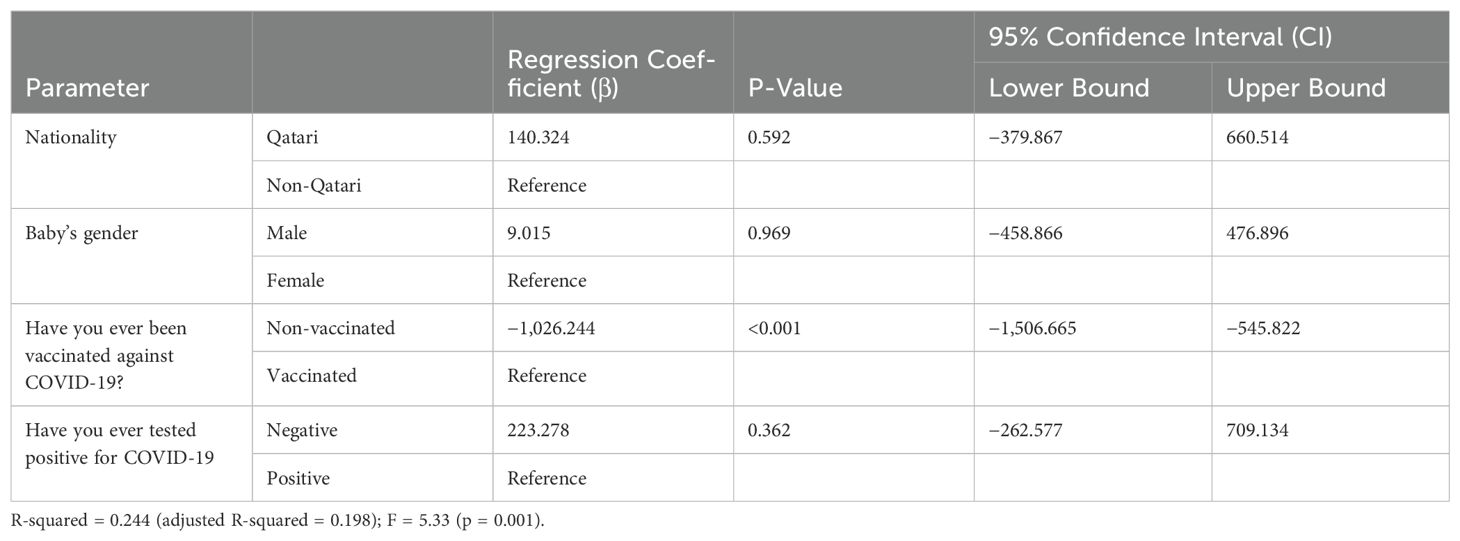
Multivariate linear regression analysis was performed using dependent variables NTAb*3.31 and S-RBD*1.15 antibodies and independent predictor variables nationality, infant’s gender, vaccination status against COVID-19, and COVID-19 test status; the multivariate linear regression analysis results indicated that vaccination against COVID-19 remained a potential significant (p < 0.0001) predictor for both NTAb*3.31 and S-RBD*1.15 antibodies after adjusting other predictor variables outlined in Tables 4 and 5.
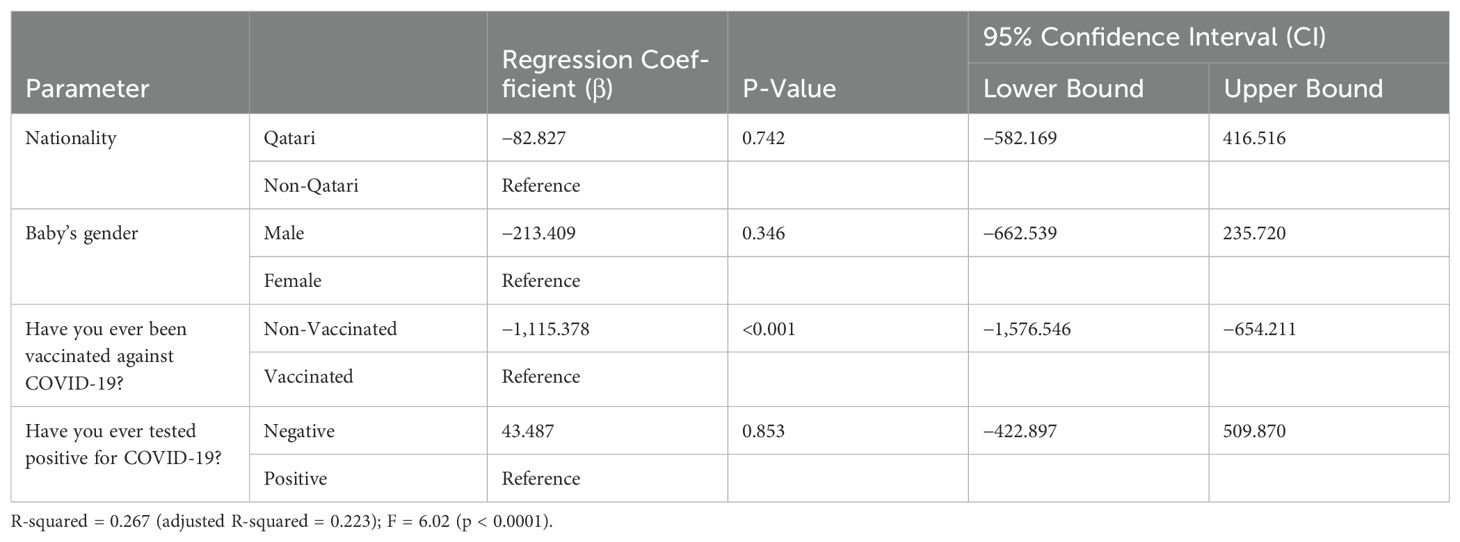
Table 5. Multivariate linear regression analysis using the dependent variable S-RBD*1.15 antibody levels.
Discussion
COVID-19, caused by SARS-CoV-2, is highly infectious (13). SARS-CoV-2 was first described by Huang et al. (1). Pregnant women and newborns are at-risk groups for COVID-19 (3, 4). Tests for IgG and IgM antibodies for SARS-CoV-2 became available in February 2020. On March 4, 2020, the seventh edition of The New Coronavirus Pneumonia Prevention and Control Protocol for SARS-CoV-2 was released by the National Health Commission of the People’s Republic of China and added serological diagnostic criteria (8).
NTAb*3.31 antibody is a species-specific, monoclonal antibody that targets SARS-CoV-2 and plays a significant role in the prevention of COVID-19 infection as well as virus elimination by inhibiting the interaction of the virus with the host cell receptors, as well as by interacting with phagocytes, natural killer cells, and complement. It is associated with early viral control, highlighting its relevance as a reliable immunological correlate of protection (CoP) against infection. S-RBD*1.15 is a recombinant monoclonal antibody that prevents viral interaction between RBD and angiotensin-converting enzyme 2 (ACE2) receptor, located on the surface of host cells (14). Studies have shown that the mean NTAb*3.31 and S-RBD*1.15 antibody titres of previously infected and vaccinated COVID-19 patients are higher than those of uninfected and vaccinated individuals. Among those infected, patients with moderate-to-severe symptoms had higher mean NTAb*3.31 and S-RBD*1.15 antibody titres than those with mild symptoms. It was also noted that vaccinated individuals had significantly higher NTAb*3.31 and S-RBD*1.15 antibody titres than COVID-19 patients. Further studies are required to investigate the usefulness of NTAb*3.31 and S-RBD*1.15 antibody titres in potential future reinfections with new viral variants (15, 16).
We prospectively investigated the immunological characteristics of newborns delivered to mothers with either previous COVID-19 infection or who received COVID-19 vaccination for the possibility of acquiring transplacental passive immunity in 2021 and 2022. Results from our study revealed significantly higher titres of NTAb*3.31 and S-RBD*1.15 antibodies in newborns’ cord blood whose mothers had previous COVID-19 infection or received COVID-19 vaccination; however, these titres were higher in the case of vaccination than previous infection. This indicates that the rate of transplacental immunity transmission from mothers to their newborns after previous COVID-19 vaccination is higher in those with previous infections. Boelig et al. had similar results, concluding that the rate of transplacental transmission of anti-COVID-19 antibodies is higher in pregnant women with previous COVID-19 vaccination compared to previous COVID-19 infection (17). Our results are similar to those of Lubrano et al., who concluded that vaccinating mothers against COVID-19 is protective for their foetuses/newborns against infection (18, 19).
Another key result from our study is that the more doses of vaccine received, the higher the antibody levels in newborns’ cord blood (p < 0.001). This can be explained by enhanced maternal binding during pregnancy as well as the in utero transmission of antibodies from the maternal serum to the foetus. Munoz et al. and Enengl et al. found similar results (20–22). Enengl et al. concluded that more than half of women will be positive for anti-SARS-CoV-2 IgG antibodies at 1–2 weeks after COVID-19 infection. They found that the higher the maternal antibody level, the higher the concentration in the serum of their newborns (22). Consequently, the in utero transmission of antibodies has a protective role in neonatal immunity against infection (10, 21–23). Mugo et al. found a positive correlation between maternal and cord blood anti-spike concentration, which suggests that interventions that increase maternal antibody concentrations, such as vaccination, may increase passive immunity and protection against severe COVID-19 in neonates (21). Oz-Alcalay et al. also concluded the same findings of a strong positive correlation between the maternal antibody levels and those in the neonatal serum. Of note, the authors also concluded that the levels of antibodies in the infants’ serum linearly decreased if the mother was vaccinated before 30 weeks of gestation. They recommended that future research studies investigate the effectiveness of booster vaccination doses during pregnancy on the infants’ antibody titres (23). Sturrock et al. found that both maternal infection and vaccination produced an antibody response in 100% of mothers and 92.9% and 93.8% of neonates, respectively. This antibody titre persisted at 6 weeks of age in 95% of neonates. Interestingly, they found that the anti-spike antibody response decreased by almost 25-fold from the first to third trimester of maternal vaccination (24). In continuation of their recommendation, our study and other researchers have shown that the more the maternal vaccination doses, the higher the infants’ antibody titres (17, 24–28).
SARS-CoV-2 vaccination is safe, and published studies have reported no major side effects, especially during the second and third trimesters of pregnancy and during breastfeeding. Conversely, available studies have revealed that infants received specific SARS-CoV-2 antibodies after maternal vaccination (29).
The study has some limitations. Firstly, it is a single-centre study. Secondly, the relatively small sample size limits the statistical power and generalisability of the findings. Additionally, as a prospective observational study, it can only establish associations, not causation. Also, the distribution of participants by vaccination and infection status was not fully detailed, which could introduce bias or complicate the interpretation of subgroup differences. However, the prospective study design, relevance, and the laboratory-based results enhance its strength, power, validity, and generalisability. Studies with large sample sizes are required to reinforce the results of our study and to investigate the mechanism by which the timing of vaccination affects the in utero transmission of antibodies (24).
Conclusions
In our study, we found significantly higher titres of NTAb*3.31 and S-RBD*1.15 antibodies in newborns’ cord blood whose mothers had previous COVID-19 infection or received COVID-19 vaccination; however, these titres were higher in the case of vaccination than previous infection. The more doses of vaccine received, the higher the antibody levels in newborns’ cord blood. This indicates transplacental immunity transmission from mothers to their newborns after previous COVID-19 vaccination or infection.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the corresponding authors, upon reasonable request.
Ethics statement
This study was approved by Institutional Review Board at Hamad Medical Corporation (Protocol Number: MRC-01-20-1132). The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
AM: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MB: Conceptualization, Data curation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PC: Data curation, Formal analysis, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HA: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. TO: Conceptualization, Formal analysis, Resources, Writing – review & editing. IA: Data curation, Software, Validation, Visualization, Writing – review & editing. ST: Data curation, Software, Validation, Visualization, Writing – review & editing. RP: Data curation, Software, Validation, Visualization, Writing – review & editing. AS: Data curation, Software, Validation, Visualization, Writing – review & editing. JA: Data curation, Software, Validation, Visualization, Writing – review & editing. MA: Data curation, Software, Validation, Visualization, Writing – review & editing. YA: Data curation, Software, Validation, Visualization, Writing – review & editing. MA-S: Data curation, Software, Validation, Visualization, Writing – review & editing. GN: Data curation, Investigation, Software, Validation, Visualization, Writing – review & editing. JJ: Investigation, Validation, Visualization, Writing – review & editing. CS: Investigation, Validation, Visualization, Writing – review & editing. SJ: Investigation, Validation, Visualization, Writing – review & editing. SG: Investigation, Validation, Visualization, Writing – review & editing. NA-D: Investigation, Validation, Visualization, Writing – review & editing. MA-Q: Conceptualization, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was funded and supported by the Medical Research Center (MRC), Hamad Medical Corporation, Doha, Qatar (Protocol number MRC-01-20-1132).
Acknowledgments
Special thanks to the entire NICU, obstetric, and infection control teams in the WWRC who provided high-quality family-integrated care to our newborns. We thank our colleagues from the Medical Research Center and Qatar National Library for sharing their pearls of wisdom with us during this research. The authors would like to thank and appreciate Palli Valapila Abdulrouf, Asma Tarannum, Salma Younes, Parveen Banu, Fathima Humaira Amanullah, John Paul Silang, Mary Grace Flores, and Maha Jouini for their valuable contributions to this study.
Conflict of interest
Authors AM, MB, PC, HA, TO, VA, IA, ST, RP, AS, JA, MA, YA, MA-S, JS, MF, MJ, JJ, CS, SG, NA-D, MA-Q are employed by Hamad Medical Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
WWRC, Women’s Wellness and Research Center; IRB, institutional review board; HMC, Hamad Medical Corporation; MRC, Medical Research Center; NICU, Neonatal Intensive Care Unit; CI, confidence interval; OR, odds ratio; RR, relative risk; SD, standard deviation; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; NTAb, neutralising antibody; S-RBD, spike-receptor binding domain antibody.
References
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
3. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. (2020) 395:809–15. doi: 10.1016/S0140-6736(20)30360-3
4. Wu Z and McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
5. Moza A, Duica F, Antoniadis P, Bernad ES, Lungeanu D, Craina M, et al. Outcome of newborns with confirmed or possible SARS-coV-2 vertical infection-A scoping review. Diagnostics (Basel). (2023) 13(2):245. doi: 10.3390/diagnostics13020245
6. Sungnak W, Huang N, Becavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. (2020) 26:681–7. doi: 10.1038/s41591-020-0868-6
7. Vendola N, Stampini V, Amadori R, Gerbino M, Curatolo A, and Surico D. Vertical transmission of antibodies in infants born from mothers with positive serology to COVID-19 pneumonia. Eur J Obstet Gynecol Reprod Biol. (2020) 253:331–2. doi: 10.1016/j.ejogrb.2020.08.023
8. Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. (2020) 323:1848–9. doi: 10.1001/jama.2020.4861
9. Yu Y, Li Y, Hu Y, Li B, and Xu J. Breastfed 13 month-old infant of a mother with COVID-19 pneumonia: a case report. Int Breastfeed J. (2020) 15:68. doi: 10.1186/s13006-020-00305-9
10. Auriti C, De Rose DU, Mondì V, Stolfi I, and Tzialla C. On behalf of the study group of neonatal infectious diseases. Neonatal SARS-coV-2 infection: practical tips. Pathogens. (2021) 10:611. doi: 10.3390/pathogens10050611
11. Auriti C, De Rose DU, Tzialla C, Caforio L, Ciccia M, Manzoni P, et al. Vertical transmission of SARS-coV-2 (COVID-19): are hypotheses more than evidences? Am J Perinatol. (2020) 37:S31–8. doi: 10.1055/s-0040-1714346
12. Yang P, Wang X, Liu P, Wei C, He B, Zheng J, et al. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol. (2020) 127:104356. doi: 10.1016/j.jcv.2020.104356
13. Jain S, Allen IE, Song D, and Piao X. Cytokine responses to SARS-COV2 infection in mother-infant dyads: a systematic review and meta-analysis. Front Pediatr. (2023) 11:1277697. doi: 10.3389/fped.2023.1277697
14. Wan Shuaib WMA, Badaruddin IA, Mansor M, Salleh SA, Hassan MR, Lindong S, et al. SARS-CoV-2 S-RBD IgG & Neutralizing antibodies among different categories of health care workers post third dose BNT162b2 mRNA COVID-19 vaccine. Hum Vaccin Immunother. (2023) 19:2266931. doi: 10.1080/21645515.2023.2266931
15. Tomassetti F, Nuccetelli M, Sarubbi S, Gisone F, Ciotti M, Spinazzola F, et al. Evaluation of S-RBD and high specificity ACE-2-binding antibodies on SARS-CoV-2 patients after six months from infection. Int Immunopharmacol. (2021) 99:108013. doi: 10.1016/j.intimp.2021.108013
16. Nowakowska A, Lee SM, Kim M, Chun J, Kim S, Kim BC, et al. Timing of maternal vaccination against COVID-19 for effective protection of neonates: cohort study. Front Immunol. (2024) 15:1359209. doi: 10.3389/fimmu.2024.1359209
17. Boelig RC, Chaudhury S, Gromowski GD, Mayer S, King J, Aghai ZH, et al. Reduced maternal immunity and vertical transfer of immunity against SARS-CoV-2 variants of concern with COVID-19 exposure or initial vaccination in pregnancy. Front Immunol. (2023) 14:1216410. doi: 10.3389/fimmu.2023.1216410
18. Lubrano C, Mancon A, Anelli GM, Gagliardi G, Corneo R, Bianchi M, et al. Immune response and transplacental antibody transfer in pregnant women after COVID-19 vaccination. J Pers Med. (2023) 13(4):689. doi: 10.3390/jpm13040689
19. Martinez-Quezada R, Valencia-Ledezma OE, Ramirez-Lozada T, Miguel-Rodriguez CE, Fernandez-Hernandez JC, and Acosta-Altamirano G. Influence of Maternal and Neonatal Factors on Transplacental Passive Immunity after Vaccination against COVID-19. Vaccines (Basel). (2024) 12(8):860. doi: 10.3390/vaccines12080860
20. Munoz FM, Posavad CM, Richardson BA, Badell ML, Bunge KE, Mulligan MJ, et al. COVID-19 booster vaccination during pregnancy enhances maternal binding and neutralizing antibody responses and transplacental antibody transfer to the newborn. Vaccine. (2023) 41:5296–303. doi: 10.1016/j.vaccine.2023.06.032
21. Mugo AG, Koech A, Cantrell L, Mukhanya M, Mwaniki I, Mutunga J, et al. Placental transfer of SARS-CoV-2 antibodies in mother-neonate pairs: a prospective nested cohort study. BMC Infect Dis. (2025) 25:875. doi: 10.1186/s12879-025-11225-6
22. Enengl S, Pecks U, Oppelt P, Stelzl P, Trautner PS, Shebl O, et al. Antibody response and maternofetal antibody transfer in SARS-coV-2-positive pregnant women: A multicenter observational study. Geburtshilfe Frauenheilkd. (2022) 82:501–9. doi: 10.1055/a-1768-0415
23. Oz-Alcalay L, Elron E, Davidovich R, Chodick G, Osovsky M, Chen R, et al. The association of neonatal SARS-CoV-2 anti-spike protein receptor-binding domain antibodies at delivery with infant SARS-CoV-2 infection under the age of 6 months: a prospective cohort study. Clin Microbiol Infect. (2023) 29:789–94. doi: 10.1016/j.cmi.2023.01.023
24. Sturrock S, Cavell B, Alexander F, Apostolakis K, Barro C, Daniel O, et al. Maternal and placental antibody responses in SARS-coV-2 vaccination and natural infection during pregnancy. Pediatr Infect Dis J. (2025) 44:S32–7. doi: 10.1097/INF.0000000000004704
25. Govindaraj S, Cheedarla N, Cheedarla S, Irby LS, Neish AS, Roback JD, et al. COVID-19 vaccine induced poor neutralization titers for SARS-CoV-2 omicron variants in maternal and cord blood. Front Immunol. (2023) 14:1211558. doi: 10.3389/fimmu.2023.1211558
26. Chiang CJ, Hsu WL, Su MT, Ko WC, Hsu KF, and Tsai PY. Impact of antenatal SARS-coV-2 exposure on SARS-coV-2 neutralization potency. Vaccines (Basel). (2024) 12(2):164. doi: 10.3390/vaccines12020164
27. Yang YJ, Murphy EA, Singh S, Sukhu AC, Wolfe I, Adurty S, et al. Association of Gestational Age at Coronavirus Disease 2019 (COVID-19) Vaccination, History of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, and a Vaccine Booster Dose With Maternal and Umbilical Cord Antibody Levels at Delivery. Obstet Gynecol. (2022) 139:373–80. doi: 10.1097/AOG.0000000000004693
28. Rottenstreich A, Zarbiv G, Oiknine-Djian E, Vorontsov O, Zigron R, Kleinstern G, et al. Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: a prospective cohort study. Clin Microbiol Infect. (2022) 28:419–25. doi: 10.1016/j.cmi.2021.10.003
Keywords: COVID-19, antibody titer, SARS-CoV-2 coronavirus, vaccination, transplacental immunity, newborn, neonatal intensive care unit, neonate
Citation: Masry A, Bayoumi MAA, Chandra P, Abukhadijah HJ, Olukade T, Abdelhady I, Thazhe S, Paramban R, Sudarsanan A, Abraham J, Abugubba M, Al-Matar Y, Al-Shanwar MS, Nasrallah GK, Joseph J, Sajor C, Joy S, George S, Al-Dewik N and Al-Qubaisi MA (2025) Impact of pregnant mothers’ previous COVID-19 infection and vaccination on newborns’ serological profiling. Front. Immunol. 16:1526264. doi: 10.3389/fimmu.2025.1526264
Received: 11 November 2024; Accepted: 17 July 2025;
Published: 19 September 2025.
Edited by:
Domenico Umberto De Rose, Bambino Gesù Children’s Hospital (IRCCS), ItalyReviewed by:
Stelvio Tonello, University of Eastern Piedmont, ItalyDelia Vanessa Lopez-Guerrero, Autonomous University of the State of Morelos, Mexico
Copyright © 2025 Masry, Bayoumi, Chandra, Abukhadijah, Olukade, Abdelhady, Thazhe, Paramban, Sudarsanan, Abraham, Abugubba, Al-Matar, Al-Shanwar, Nasrallah, Joseph, Sajor, Joy, George, Al-Dewik and Al-Qubaisi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alaa Masry, YV9lbG1hc3J5MTBAeWFob28uY29t; Mohammad A. A. Bayoumi, bW9oLmFiZGVsd2FoYWJAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share the last senior authorship
 Alaa Masry
Alaa Masry Mohammad A. A. Bayoumi
Mohammad A. A. Bayoumi Prem Chandra
Prem Chandra Hana J. Abukhadijah
Hana J. Abukhadijah Tawa Olukade3
Tawa Olukade3 Ratheesh Paramban
Ratheesh Paramban Gheyath Khaled Nasrallah
Gheyath Khaled Nasrallah Nader Al-Dewik
Nader Al-Dewik