- 1Department of Respiratory Medicine, The Second Hospital of Jilin University, Changchun, Jilin, China
- 2Department of Surgery, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
This report details the case of a 64-year-old male with well-controlled type 2 diabetes mellitus and hypertension. The patient presented with a 20-day history of progressive dyspnea, cough, and intermittent fever, which worsened despite antibiotic treatment. The initial assessment revealed leukocytosis, neutrophilia, and abnormal chest computed tomography (CT) findings, which led to a provisional diagnosis of pulmonary infection. However, empirical antibacterial therapy was ineffective. Further investigations revealed a right perinephric abscess and empyema caused by an oral anaerobic bacterial infection. Although the sputum cultures were negative, targeted next-generation sequencing (tNGS) identified multiple oral anaerobes. The patient was treated with metronidazole and drainage. After 33 days, the symptoms and laboratory abnormalities gradually resolved. Follow-up over one year demonstrated complete resolution of symptoms, normalization of inflammatory markers and no recurrence of the infection. This case highlights the importance of considering occult anaerobic infections in refractory febrile patients with diabetes, while also raising awareness of the rare complication of perinephric abscess and highlighting the value of tNGS in pathogen identification.
1 Introduction
Perinephric abscess (PA), characterized by the invasion of pathogens within the perinephric space, is a relatively infrequent yet severe medical condition (1). Recent evidence has refined our understanding of its etiology, clinical manifestations, diagnosis, and management (2). Hematogenous spread from a distant site, ascending urinary tract infections, and direct extension from adjacent organs are the major causes of PA. Moreover, there is an emerging recognition of the role of underlying immunocompromised states, such as those associated with advanced malignancies or chronic immunosuppressive therapy (3, 4). Despite controversy, research has further elaborated on the impact of comorbidities such as diabetes mellitus on the development of PA (5). Patients with diabetes often have impaired immune function and hyperglycemia-induced tissue damage, creating a favorable environment for bacterial growth and abscess formation (6). Escherichia coli is the most commonly isolated pathogen, followed by Staphylococcus aureus, as demonstrated in multiple contemporary microbiological surveys (2, 7). Clinically, patients with PA present with non-specific symptoms and typically experience fever, flank pain, and malaise (2). Nevertheless, these symptoms can be easily overlooked or misinterpreted, especially in patients with preexisting comorbidities (7). Currently, diagnosis relies heavily on advanced imaging modalities. High-resolution computed tomography (CT) remains the gold standard for detecting the presence, location, and extent of the abscess (2, 8). Ultrasonography has auxiliary value in diagnosing PA, but the diagnostic utility of magnetic resonance imaging (MRI) is limited (2). PA management includes antibiotic administration with concurrent percutaneous drainage, if necessary (2, 9). Surgical intervention may be used when PA is not successfully controlled with antibiotic treatment or percutaneous catheter drainage (2). However, the choice between percutaneous drainage and surgical intervention still depends on various factors such as size, location, and complexity of the abscess, as well as the patient’s overall condition. Furthermore, the use of adjunctive therapies, such as hyperbaric oxygen therapy, has been investigated to enhance the efficacy of standard treatment by facilitating an improvement in the bactericidal properties of neutrophils, increasing the penetration of antibiotics into damaged tissues, and reducing the systemic inflammatory response, thus aiding in the repair of damaged tissues (10). When considering patients with PA who also have concurrent pulmonary infections, the clinical scenario becomes more complex. A previous study reported pleural empyema in a patient with a PA and diaphragmatic defect (11). The patient was successfully treated with systemic antibiotics and drainage of both pleural and retroperitoneal collections (11). The authors concluded that the spread of intra-abdominal sepsis through diaphragmatic defects to the pleural cavity represented a potential source of empyema (11). Thus, understanding the coexistence of pulmonary infection and PA is of the utmost importance, as it impacts the diagnostic approach, treatment strategy, and overall prognosis. Herein, we report a case of a patient presenting with concurrent pulmonary infection and PA to contribute to the growing body of literature on this complex and relatively rare clinical entity.
2 Case presentation
A 64-year-old male patient presented with a 20-day history of progressive dyspnea, cough, and intermittent fever, which worsened despite a 10-day course of cephalosporins and penicillin from a local medical institution. Initial laboratory tests revealed leukocytosis (white blood cell [WBC] count: 20.94 × 109/L) with neutrophilia (87.6%). Chest CT revealed bilateral pleural effusion, compressive atelectasis in the right lower lung, and diffuse chronic inflammation (Figure 1). A provisional diagnosis of pulmonary infection was made, however, empiric antibacterial therapy failed to alleviate the symptoms. The patient was subsequently transferred to the respiratory department for further management. At admission, he reported persistent high-grade fever (peak: 39.5°C), chills, cough, and exertional dyspnea relieved by rest. No history of aspiration, chest pain, gastrointestinal symptoms, or weight loss was noted, and the patient denied any history of smoking or alcohol consumption. His medical history included well-controlled type 2 diabetes mellitus, hypertension, and mild obstructive sleep apnea syndrome (OSA). Vital signs included a temperature of 38.5°C, blood pressure of 151/79 mmHg, heart rate of 88/min, respiratory rate of 23/min, and oxygen saturation of 93% on low-flow oxygen. Physical examination revealed diminished breath sounds and moist rales in both lower lung fields. Repeat blood tests showed marked leukocytosis (WBC: 23.10 × 109/L), with neutrophilia (90.4%) and lymphopenia (4.2%). Urinalysis results were unremarkable.
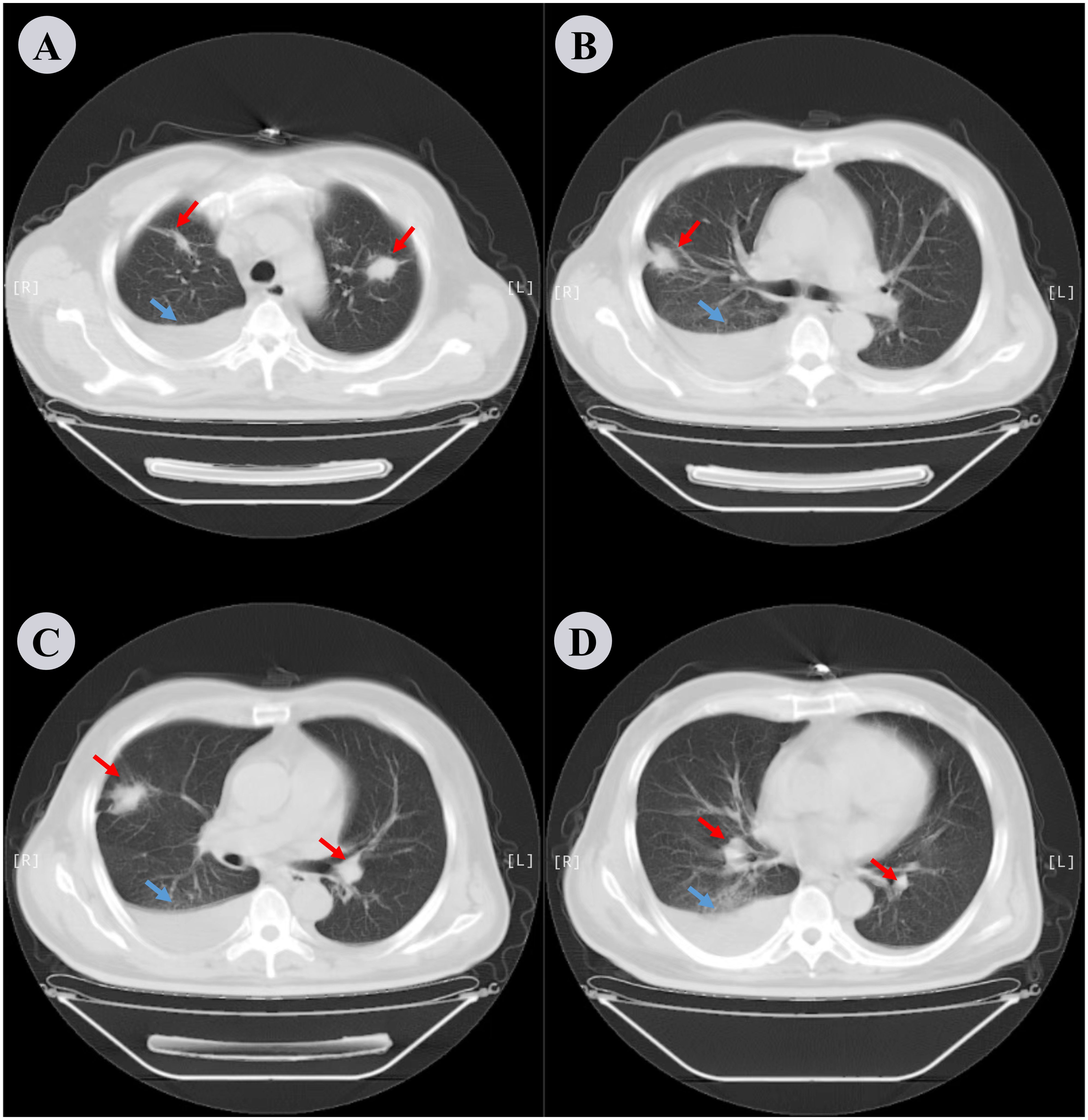
Figure 1. Chest CT scan performed the day before hospitalization. (A−D) depict different slices of the lung CT scans in the lung window. Bilateral pleural effusion is noted along with compressive atelectasis in the lower right lung and scattered chronic inflammation in both lungs. Red arrows indicate inflammation in the lungs and blue arrows indicate pleural effusion.
The patient was diagnosed with a pulmonary infection, type 2 diabetes mellitus, and grade 2 hypertension. He received insulin therapy, oxygen support, intravenous hydration, and meropenem as empirical anti-infective treatment, (1 g IV q8h). Despite treatment, fever persisted on day 3. Sputum cultures remained negative, and serum-tNGS detected only human herpesvirus. Elevated B-type natriuretic peptide (BNP: 481 pg/mL) and hypoalbuminemia (22.3 g/L) were noted. The differential diagnoses included bacteremia and occult extrapulmonary infection, prompting an abdominal CT examination. This revealed a heterogeneous mass-like lesion with internal septations adjacent to the right renal upper pole (Figures 2A–C). Repeat chest CT showed unchanged pulmonary infiltrates, but increased right pleural effusion (Figures 3A, D). Ultrasound-guided drainage of the right perirenal lesion yielded 200 mL of pink purulent fluid, confirming a PA. Pus cultures were negative; however, tNGS identified multiple oral anaerobic bacteria (Table 1). Two days later, the patient reported chest tightness. A bedside chest X-ray in the supine position demonstrated bilateral pleural effusion, which was more pronounced on the right side (Supplementary Figure S1). Concurrent bilateral thoracentesis under ultrasound guidance drained yellow fluid. The left-sided pleural effusion was close to leaking, while the right-sided effusion met the Light criteria for exudate (Table 2). Consistently, tNGS of the right-sided pleural fluid detected oral anaerobes (Table 1). Tinidazole (0.5 g IV q12h) was initiated for anaerobic coverage. Clinical improvement ensued with the resolution of fever, cough, and dyspnea. Repeat abdominal and chest CT on day 13 showed reduced PA (Figures 2D–F) and pleural effusion, although pulmonary inflammation persisted with right pleural septations (Figures 3B, E). Ultrasound-guided urokinase intrapleural injection was used to treat the right pleural septations, and the gradual resolution of symptoms and laboratory abnormalities was observed over 33 days. Follow-up chest CT revealed significant inflammatory absorption (Figures 3C, F).The timeline of the patient’s diagnostic workup and clinical care throughout the case is detailed in Supplementary Figure S2, Supplementary Figure S3.
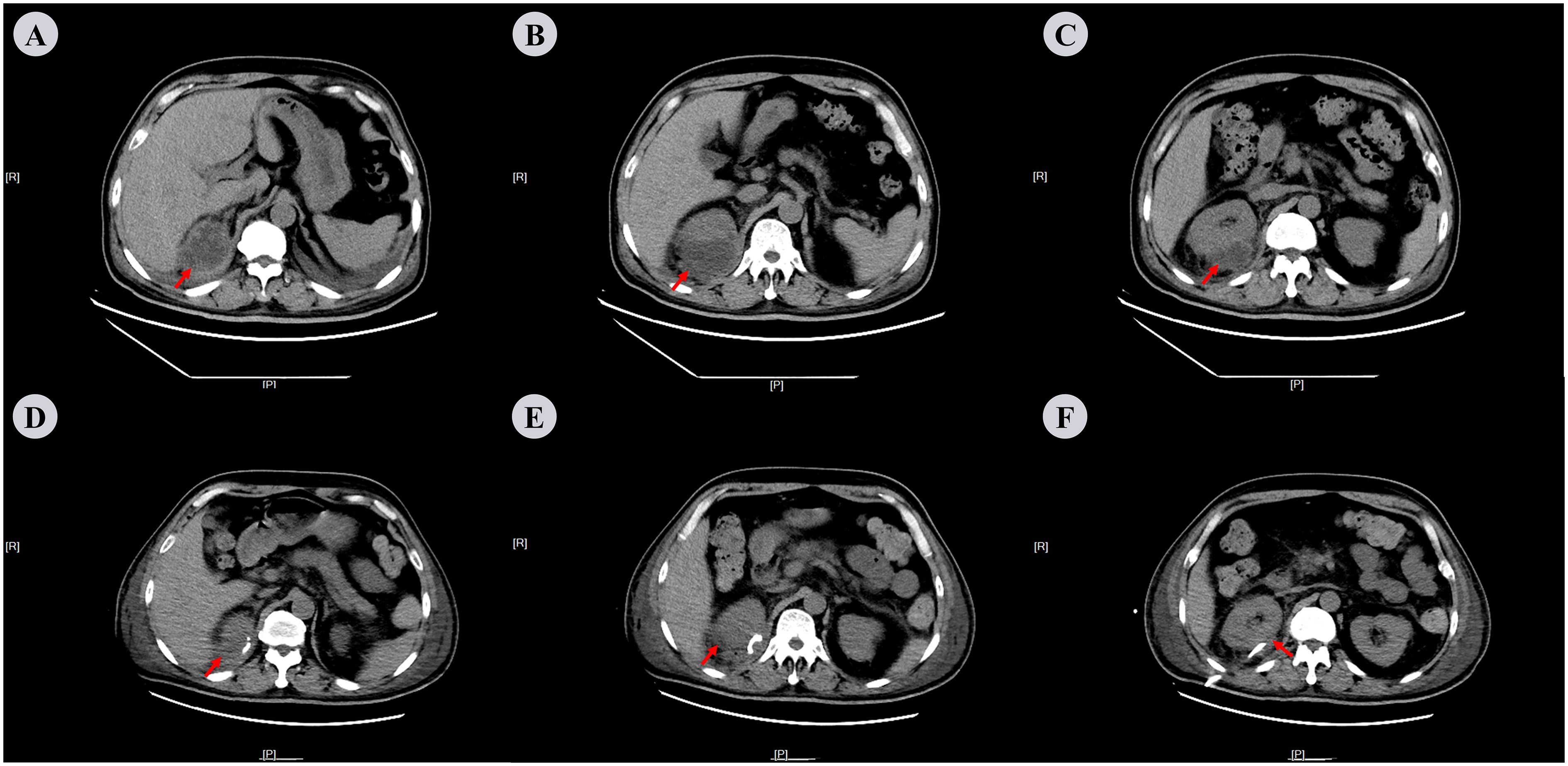
Figure 2. Abdominal CT scan on the 3rd and 33rd days after admission. (A−C) On the 3rd day: Mass-like heterogeneous density shadow with irregular margins, adjacent to the upper pole of the right kidney. The lesion shows internal septations and measures approximately 62 mm × 49 mm × 61 mm in dimensions with the CT attenuation value varying from -21 Hu to -12 Hu. (D−F) On the 13th day: Following the right renal drainage procedure, a small patchy low-density shadow was visible adjacent to the right kidney, with measures around 39 mm × 14 mm × 3 mm in size and a CT attenuation value of roughly 15Hu. The red arrows indicate perinephric abscess.
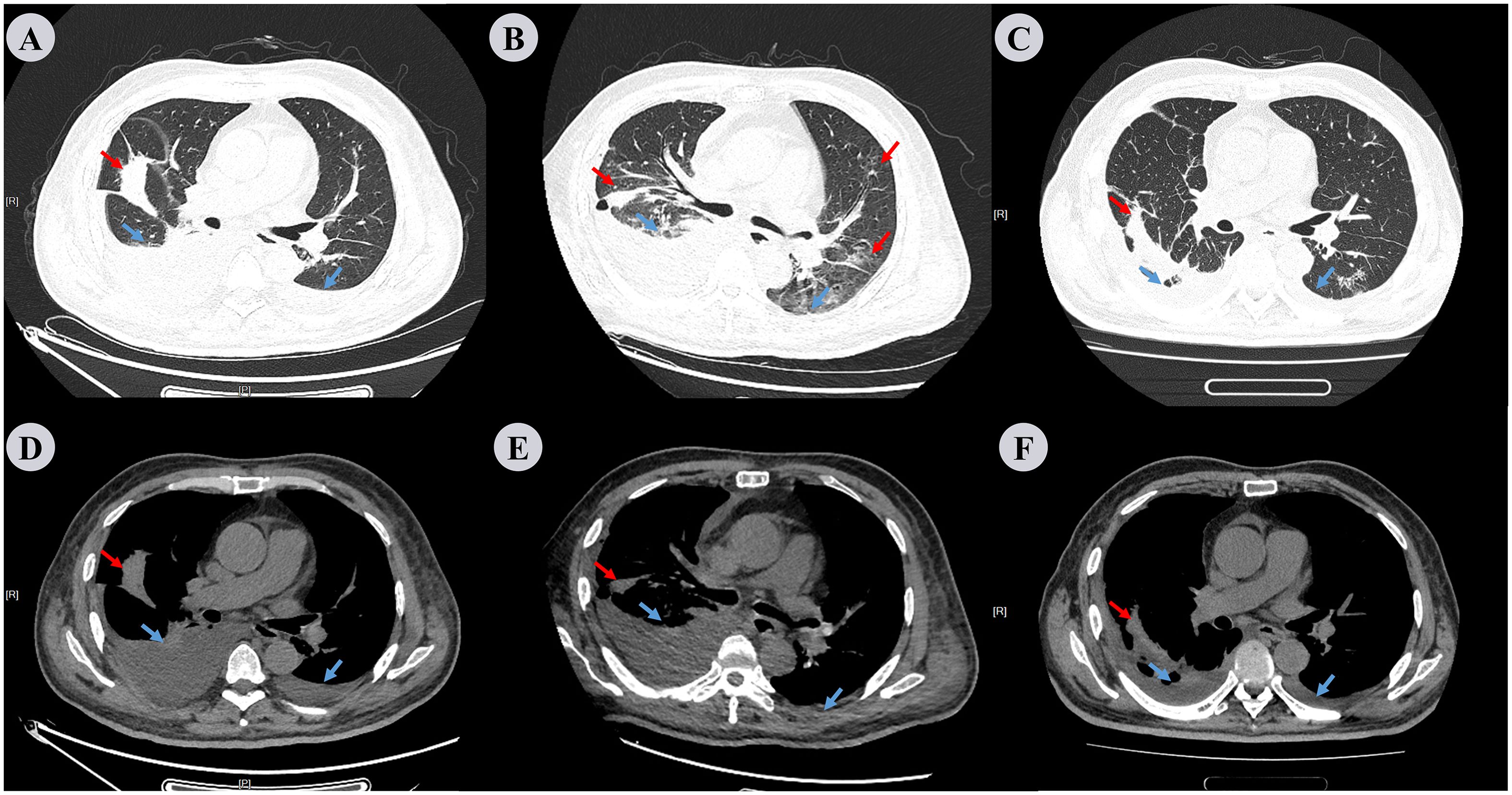
Figure 3. Chest CT scan performed on the 3rd, 13th, and 33rd days. (A−C) represent the lung window, while (D−F) represent the mediastinal window. Repeat chest CT on the 3rd day (A, D), compared to the pre-admission scan, revealed that the lesions in both lungs had no change; however, there was an increase in the amount of pleural effusion, especially on the right side. Repeat chest CT on 13th day (B, E) reveals increased inflammatory lesions in both lungs and decreased pleural effusion. Repeat chest CT on the 33rd day (C, F) shows that the inflammation in both lungs had decreased significantly compared to the previous images, and the right pleural effusion also decreased markedly. Red arrows denote inflammation in the lungs and blue arrows indicate pleural effusion.
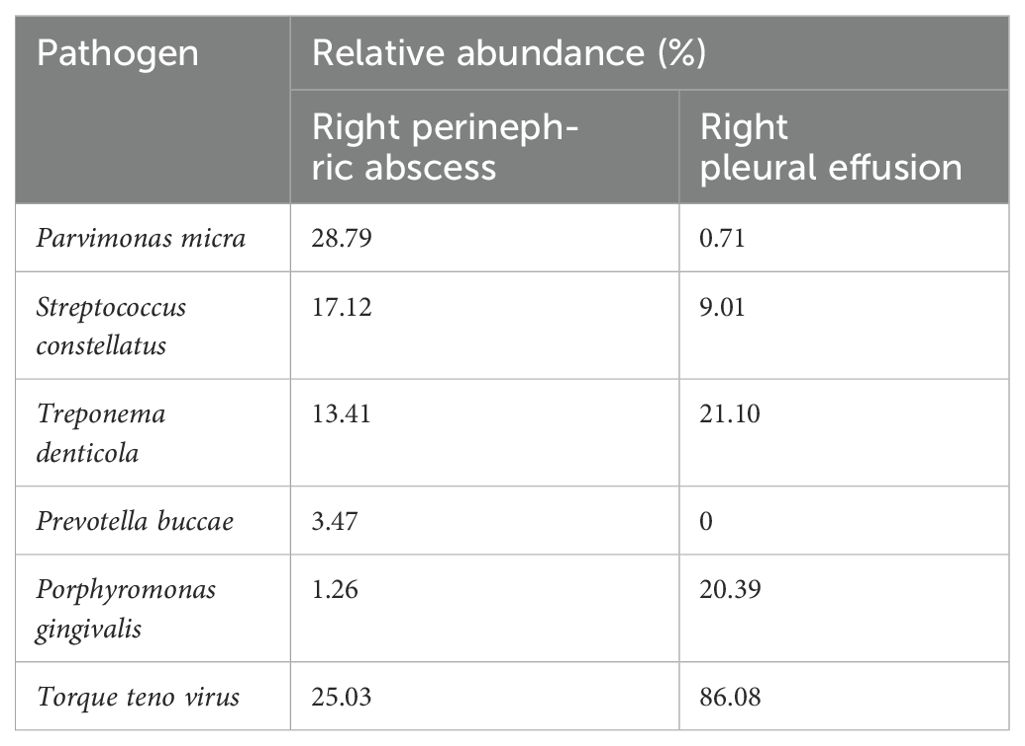
Table 1. Species distribution of pathogens detected by right perinephric abscess and right pleural effusion tNGS.
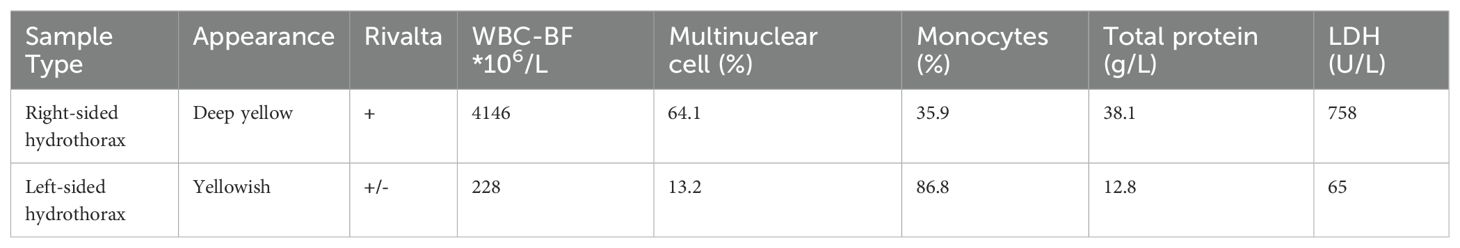
Table 2. Examination results of bilateral pleural effusion, including both routine and biochemical analyses.
The patient underwent regular clinical and radiographic follow-ups for over one year after discharge. At the one-month follow-up, dyspnea, cough, and fever had completely resolved, with no recurrence of chills. Repeat laboratory tests revealed normalization of inflammatory markers (WBC: 6 × 109/L, neutrophils: 51.6%). One-year follow-up chest CT revealed complete resolution of the bilateral pleural effusion and pulmonary infiltrates, with no residual abscess or septations (Figures 4A, B). Abdominal CT confirmed the absence of recurrent perirenal collections (Figures 4C, D). Functional recovery was notable as the patient resumed daily activities without exertional limitations. No antibiotic-related adverse effects (e.g., gastrointestinal disturbances or neurotoxicity) or procedural complications (e.g., catheter-site infections or pneumothorax) were observed during or after treatment. Tinidazole was discontinued after a 4-week course, with no signs of relapse at subsequent evaluations. This case underscored the importance of investigating occult anaerobic infections in patients with diabetes and refractory fever, particularly when thoracic and abdominal imaging studies reveal discordant findings. The integration of tNGS facilitated pathogen identification despite negative conventional cultures, thereby guiding targeted antimicrobial therapy.
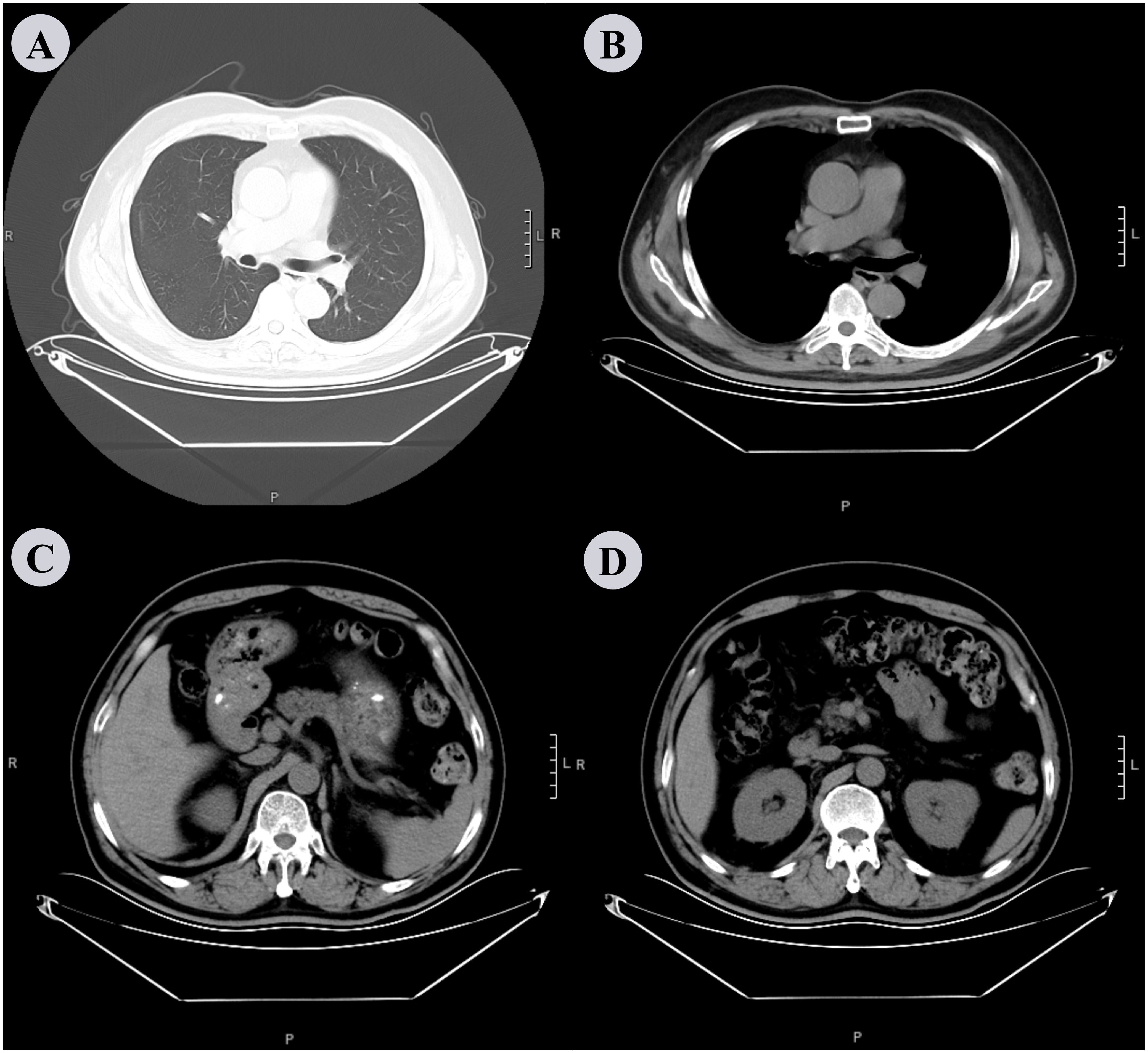
Figure 4. Chest and abdominal CT scan performed after 1 year. (A, B) The chest CT scan shows complete resolution of bilateral pleural effusion and pulmonary infiltrates, with no residual abscess or septations. (C, D) shows the absence of recurrent perirenal collections, although there is slight thickening of the perirenal fascia.
3 Discussion
Previous studies have indicated that most cases of PA result from the direct spread of pyelonephritis, with gram-negative bacilli, especially E. coli, being the most common pathogen (51.4%) (2). PA caused by S. aureus is usually secondary to hematogenous seeding (2). Additionally, certain anaerobes can also cause PA, and routine bacterial cultures are typically negative (12). Anaerobic bacteria have been isolated from PA associated with previous abdominal surgeries and renal transplants (12). In the present case, the detection of multiple oral anaerobic bacteria in PA using tNGS suggested that anaerobic infections should be considered, especially when conventional cultures are negative. Notably, the patient did not undergo abdominal surgery or renal transplantation. Regarding the origin of the pulmonary infection with pleural effusion, two main possibilities exist: aspiration pneumonia and respiratory tract colonization. Aspiration pneumonia often occurs when oral or gastric contents are inhaled into the lower respiratory tract. Although the patient denied a history of aspiration, he did have a history of mild obstructive sleep apnea (OSA). Therefore, the presence of oral anaerobes in both the PA and pleural effusion could not rule out the possibility of occult aspiration. Some studies have shown that, in patients with poor oral hygiene, the load of oral anaerobes increases, and even minor aspiration events, such as microaspiration during sleep, can lead to the entry of these bacteria into the lungs, causing infection (13, 14). Conversely, respiratory tract colonization by oral anaerobes may also be a factor, as oral anaerobes can colonize the respiratory tract in patients with a compromised immune system or airway clearance function (15, 16). The repeated negative sputum cultures in this case made it difficult to determine whether the presence of oral anaerobes in the pleural effusion was due to aspiration pneumonia or colonization. It is possible that the sampling method for sputum cultures was not optimal for detecting these fastidious anaerobes, or that the organisms were present in low numbers in the sputum at the time of sampling.
Hematogenous spread is a common route by which bacteria reach the perinephric area (2). However, in this case, serum tNGS results were negative for oral anaerobes. It should be noted that the sensitivity of blood NGS for detecting low-level bacteremia is relatively low, with a high false-negative rate. Anaerobic bacteria may be present transiently or in small quantities in the bloodstream, which makes them difficult to detect (17). Direct extension is another possible route despite the absence of anatomical abnormalities in the diaphragm (11). The anatomical structure known as the lumbar rib triangle is located between the lumbar and rib regions of the diaphragm. This area lacks muscle fibers and is composed only of the supradiaphragmatic and infradiaphragmatic fasciae, making it a weak part of the diaphragm. Additionally, the lumbar rib triangle is closely related to the kidneys, especially the upper pole of the kidneys. In this patient, there was significant right-sided pleural effusion, which could cause physical pressure on the diaphragm, particularly in the area of the lumbar rib triangle, affecting its normal function. Additionally, inflammatory damage to the lungs and pleural cavity may have weakened the integrity of the diaphragm (18), providing a potential pathway for the spread of oral anaerobes from the thoracic cavity to the perinephric area. Although this route is less common, it cannot be completely excluded, especially considering the proximity of the right lung infection to the diaphragm and the fact that the perinephric abscess was also located at the upper pole of the kidney.
A comprehensive treatment approach was adopted. Meropenem was initially used as a broad-spectrum antibacterial treatment. Meropenem has a wide antibacterial spectrum and strong antibacterial activity, and can cover many common aerobic and anaerobic bacteria. However, the patient’s fever persisted, indicating that the initial treatment was ineffective. Considering the tNGS results, tinidazole was then added. Tinidazole is highly effective against anaerobic bacteria and can penetrate their cell membranes, interacting with their DNA, inhibiting DNA synthesis and leading to bacterial death (19). In addition to antibacterial therapy, drainage is also crucial. Ultrasound-guided percutaneous drainage of the perinephric abscess effectively removed the purulent fluid, reducing the bacterial load and the source of infection. Similarly, bilateral thoracentesis under ultrasound guidance drained the pleural effusion, relieving pressure on the lungs and providing samples for microbiological analyses. When right pleural septations occurred, the use of urokinase for intrapleural injection was an important adjunctive measure. Urokinase can dissolve fibrin clots and septations in the pleural cavity, thereby improving the drainage effect (11).
This case study provides several important insights. First, occult anaerobic infections should be suspected in patients with diabetes and refractory fever, especially when accompanied by abnormal chest and abdominal findings. Diabetes can compromise the immune system and increase the risk of infection by opportunistic pathogens (6). Second, the limitations of traditional culture methods in detecting anaerobic bacteria highlight the importance of advanced molecular techniques, such as tNGS, which can identify pathogens even when conventional cultures are negative, guiding targeted antimicrobial therapy (20, 21). Third, for patients with concurrent thoracic and abdominal manifestations, possible routes of bacterial spread, such as hematogenous and direct extension, should be carefully considered. Finally, long-term management should focus on optimizing glycemic control and providing nutritional support to improve overall immune function and reduce the risk of infection recurrence.
4 Conclusion
In conclusion, this case demonstrated the complexity of diagnosing and treating patients with diabetes and refractory fever. The identification of oral anaerobic bacteria as the causative agents through tNGS was crucial for effective treatment. The possible routes of dissemination of oral anaerobes to the perinephric area require further investigation. An integrated treatment approach, including appropriate antibiotic selection and drainage procedures, was key to the patient’s recovery. This case emphasizes the importance of considering anaerobic infections in similar patients and the value of advanced diagnostic techniques. Therefore, clinicians should be vigilant in such cases to improve the diagnosis and treatment of complex infections.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cncb.ac.cn/, PRJCA038549.
Ethics statement
The studies involving humans were approved by Ethics Committee of The Second Hospital of Jilin University, The Second Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
LG: Conceptualization, Writing – original draft. PG: Conceptualization, Funding acquisition, Writing – review & editing. LM: Supervision, Writing – review & editing. WR: Supervision, Writing – review & editing. XT: Conceptualization, Writing – original draft. YH: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the National Science Foundation of China (82070037).
Acknowledgments
We sincerely thank our patient and his family and acknowledge the support provided by Gene+ for their data assistance. The authors also express their gratitude for the invaluable help from the Radiology Department and the Anatomy Teaching and Research Office. We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1528542/full#supplementary-material
Supplementary Figure 1 | Chest X-ray examination. A blurring of the costophrenic angles shows the existence of substantial bilateral pleural effusion.
Supplementary Figure 2 | Timeline of the patient’s diagnostic workup. This figure illustrates the key chronological events in the patient’s diagnostic and therapeutic process. The timeline includes: onset of symptoms, initial presentation, diagnostic evaluations, date of diagnosis, initiation of Treatment, follow-up visits. Arrows indicate the sequence of events.
Supplementary Figure 3 | Nursing record. (A) Shows the patient’s temperature, heart rate, and blood oxygen level changes at key time points during the illness. (B) Shows the changes in pleural effusion drainage and perinephric abscess drainage at key time points during the illness.
Abbreviations
PA, perinephric abscess; CT, computed tomography; MRI, magnetic resonance imaging; tNGS, targeted next-generation sequencing; OSA, obstructive sleep apnea syndrome.
References
1. Lai J, Safonova A, Pathak S, and O’Rourke P. Psoas abscess as a complication of a perinephric abscess. Am J Med. (2022) 135:e245–7. doi: 10.1016/j.amjmed.2022.02.044
2. Okafor CN and Onyeaso EE. Perinephric abscess. Treasure Island (FL: StatPearls Publishing LLC (2025).
3. Liu XQ, Wang CC, Liu YB, and Liu K. Renal and perinephric abscesses in West China Hospital: 10-year retrospective-descriptive study. World J Nephrol. (2016) 5:108–14. doi: 10.5527/wjn.v5.i1.108
4. Ngoo A, Eisemann J, Matsika A, and Winkle D. Ureaplasma urealyticum infection presenting as pyelonephritis and perinephric abscess in an immunocompromised patient. BMJ Case Rep. (2020) 13. doi: 10.1136/bcr-2020-234538
5. Lai SW, Lin HF, Lin CL, and Liao KF. Splenectomy and risk of renal and perinephric abscesses: A population-based cohort study in Taiwan. Medicine. (2016) 95:e4438. doi: 10.1097/md.0000000000004438
6. Holt RIG, Cockram CS, Ma RCW, and Luk AOY. Diabetes and infection: review of the epidemiology, mechanisms and principles of treatment. Diabetologia. (2024) 67:1168–80. doi: 10.1007/s00125-024-06102-x
7. Gardiner RA, Gwynne RA, and Roberts SA. Perinephric abscess. BJU Int. (2011) 107 Suppl 3:20–3. doi: 10.1111/j.1464-410X.2011.10050.x
8. Coelho RF, Schneider-Monteiro ED, Mesquita JL, Mazzucchi E, Marmo Lucon A, and Srougi M. Renal and perinephric abscesses: analysis of 65 consecutive cases. World J Surg. (2007) 31:431–6. doi: 10.1007/s00268-006-0162-x
9. El-Nahas AR, Faisal R, Mohsen T, Al-Marhoon MS, and Abol-Enein H. What is the best drainage method for a perinephric abscess? Int Braz J Urol: Off J Braz Soc Urol. (2010) 36:29–37. doi: 10.1590/s1677-55382010000100005
10. Vega J, Goecke H, Manriquez F, Escobar C, Escobar M, Videla C, et al. Hyperbaric oxygen therapy in a patient with autosomal dominant polycystic kidney disease with a perinephritic abscess. Clin Exp Nephrol. (2011) 15:141–6. doi: 10.1007/s10157-010-0348-2
11. Tan PSC, Badiei A, Fitzgerald DB, Kuok YJ, and Lee YCG. Pleural empyema in a patient with a perinephric abscess and diaphragmatic defect. Respirol Case Rep. (2019) 7:e00400. doi: 10.1002/rcr2.400
12. Brook I. The role of anaerobic bacteria in perinephric and renal abscesses in children. Pediatrics. (1994) 93:261–4. doi: 10.1542/peds.93.2.261
13. Janssens JP. Pneumonia in the elderly (geriatric) population. Curr Opin Pulmonary Med. (2005) 11:226–30. doi: 10.1097/01.mcp.0000158254.90483.1f
14. Hata R, Noguchi S, Kawanami T, Yamasaki K, Akata K, Ikegami H, et al. Poor oral hygiene is associated with the detection of obligate anaerobes in pneumonia. J Periodontol. (2020) 91:65–73. doi: 10.1002/jper.19-0043
15. Chen J, Li T, Ye C, Zhong J, Huang JD, Ke Y, et al. The lung microbiome: A new frontier for lung and brain disease. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24032170
16. Natalini JG, Singh S, and Segal LN. The dynamic lung microbiome in health and disease. Nat Rev Microbiol. (2023) 21:222–35. doi: 10.1038/s41579-022-00821-x
17. Poulsen SH, Søgaard KK, Fuursted K, and Nielsen HL. Evaluating the diagnostic accuracy and clinical utility of 16S and 18S rRNA gene targeted next-generation sequencing based on five years of clinical experience. Infect Dis (London England). (2023) 55:767–75. doi: 10.1080/23744235.2023.2241550
18. López-Viñas L, Vega-Villar J, Rocío-Martín E, García-García P, de la Rosa Santiago E, Galván-Román JM, et al. Diaphragm impairment in patients admitted for severe COVID-19. Eur J Trans Myol. (2022) 32. doi: 10.4081/ejtm.2022.10460
19. Reinhardt T, Lee KM, Niederegger L, Hess CR, and Sieber SA. Indolin-2-one nitroimidazole antibiotics exhibit an unexpected dual mode of action. ACS Chem Biol. (2022) 17:3077–85. doi: 10.1021/acschembio.2c00462
20. Jing C, Chen H, Liang Y, Zhong Y, Wang Q, Li L, et al. Clinical evaluation of an improved metagenomic next-generation sequencing test for the diagnosis of bloodstream infections. Clin Chem. (2021) 67:1133–43. doi: 10.1093/clinchem/hvab061
Keywords: pulmonary infection, perinephric abscess, targeted next-generation sequencing, oral anaerobes, case report
Citation: Guo L, Ma L, Ren W, Tang X, Hao Y and Gao P (2025) Concurrent pulmonary infection and perinephric abscess: a case report and literature review. Front. Immunol. 16:1528542. doi: 10.3389/fimmu.2025.1528542
Received: 15 November 2024; Accepted: 14 June 2025;
Published: 07 July 2025.
Edited by:
Beiwen Zheng, Zhejiang University, ChinaReviewed by:
Alexey V. Rakov, Central Research Institute of Epidemiology (CRIE), RussiaSammuel Shahzad, United States Department of Agriculture (USDA), United States
Copyright © 2025 Guo, Ma, Ren, Tang, Hao and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuqiu Hao, aGFvLnl1LnFpLnVAMTYzLmNvbQ==; Peng Gao, Z2FvcGVuZzEyMzRAamx1LmVkdS5jbg==
 Lixin Guo1
Lixin Guo1 Peng Gao
Peng Gao