- 1Department of Emergency Medicine, Research Center of Cardiovascular Disease, The People’s Hospital of Guangxi Zhuang Autonomous Region, Guangxi Academy of Medical Sciences, Nanning, China
- 2Department of Cardiology, Research Center of Cardiovascular Disease, The People’s Hospital of Guangxi Zhuang Autonomous Region, Guangxi Academy of Medical Sciences, Nanning, China
Background: The Naples Prognostic Score (NPS) is innovatively constructed to comprehensively evaluate the inflammatory and nutritional status according to several basic blood examinations. This study aimed to investigate the correlation between NPS and long-term prognosis in patients with coronary artery disease (CAD).
Methods: The analysis data of this retrospective cohort study were collected from electronic health records in the People’s Hospital of Guangxi Zhuang Autonomous Region. All adult patients who underwent coronary angiology (CAG) and were diagnosed as having CAD at the People’s Hospital of Guangxi Zhuang Autonomous Region from March 2013 to December 2023 were enrolled. The primary endpoint was all-cause death during follow-up.
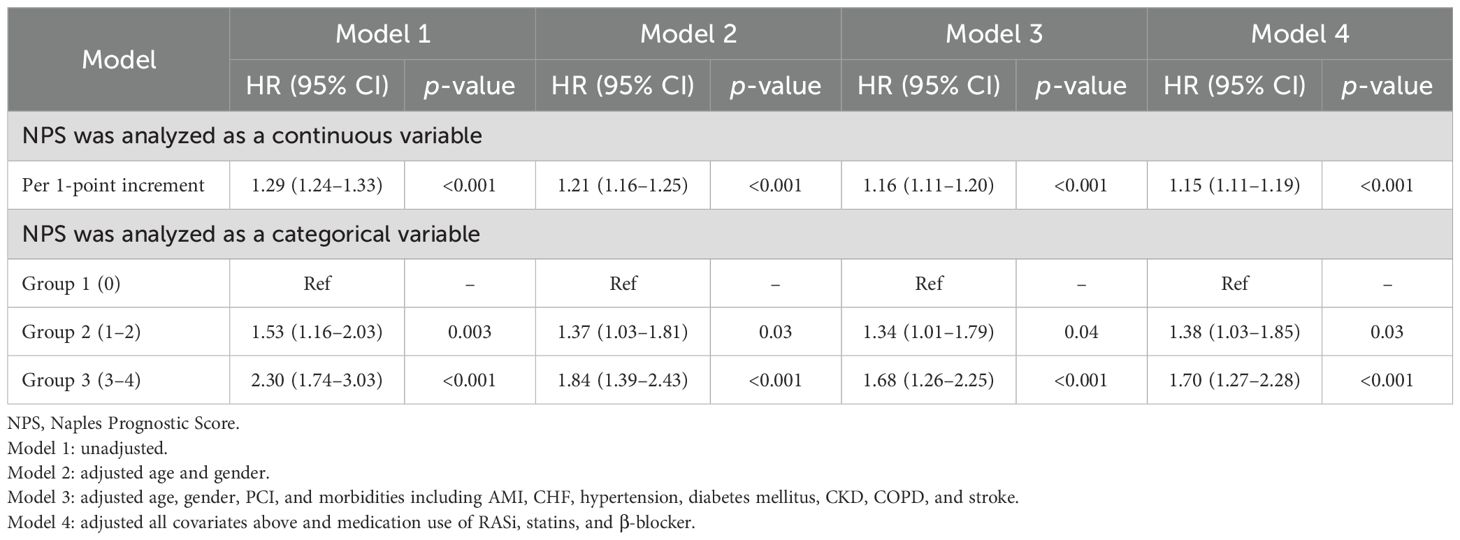
Results: The 28,799 patients were divided into three groups according to the NPS value, with 803 (2.79%) in group 0, 12,130 (42.12%) in group 1, and 15,866 (55.09%) in group 2. Over the median follow-up period of 6.12 years, 3,630 patients (12.60%) died. Long-term all-cause mortality was significantly higher in group 2 and group 1 compared with group 0 (log-rank p < 0.001). Cox regression analysis showed that both continuous NPS and categorical NPS groups were significantly associated with the risk of all-cause mortality in patients with CAD [per 1-point decrement: full adjusted HR = 1.15; 95% CI, 1.11–1.19; compared with group 0 (NPS of 0), group 1 (NPS of 1 or 2), full adjusted HR = 1.38, 95% CI: 1.03–1.85, and group 2 (NPS of 3 or 4), full adjusted HR = 1.70, 95% CI: 1.27–2.28]. Restricted cubic spline analyses showed a linear relationship between NPS and risk of long-term all-cause death.
Conclusions: The present study demonstrates that the NPS was independently associated with long-term all-cause mortality among patients with CAD.
Introduction
The high prevalence and mortality rate caused by coronary artery disease (CAD) pose a serious public health challenge worldwide (1, 2). The identification of modifiable risk factors is crucial for implementing interventions on these variables to lower the risk of poor long-term prognosis. Previous studies reported that malnutrition and high inflammation status played important roles in the poor prognosis of patients with CAD (3–5). The immune and nutritional status was increasingly determined as a substantial prognostic risk factor in patients with CAD, independent of traditional CAD risk factors (6), whereas most validated predictors were just solitary inflammatory or nutrition-related markers, which caused the evaluations to become incomprehensive.
The Naples Prognostic Score (NPS) is innovatively constructed to comprehensively evaluate the inflammatory and nutritional status according to several basic blood examinations. It has been demonstrated that NPS is correlated to prognosis in various diseases (7–9). However, no studies verified the association between NPS and long-term outcomes in patients with CAD.
Accordingly, this research intended to explore the prognostic significance of baseline NPS at admission, aiming to offer a simple and reliable approach to identify high-risk individuals among the CAD population.
Methods
Study design and data collection
A total of 39,653 individuals who underwent coronary angiography (CAG) for proven CAD at the People’s Hospital of Guangxi Zhuang Autonomous Region during March 2013 and December 2023 were enrolled. This research adopted a retrospective cohort design. A retrospective cohort design was used in this study. The following clinical characteristics were collected from electronic health records (EHRs): demographic characteristics, laboratory examination, and medication at discharge. The final database was cross-validated across multiple sources (e.g., laboratory systems and billing records) to minimize inconsistencies. Follow-up information was obtained and preserved by trained researchers. The study was approved by the Ethics Committee of Guangxi Zhuang Autonomous Region People’s Hospital and conducted following the Declaration of Helsinki (IRB No. KY-QT-202103).
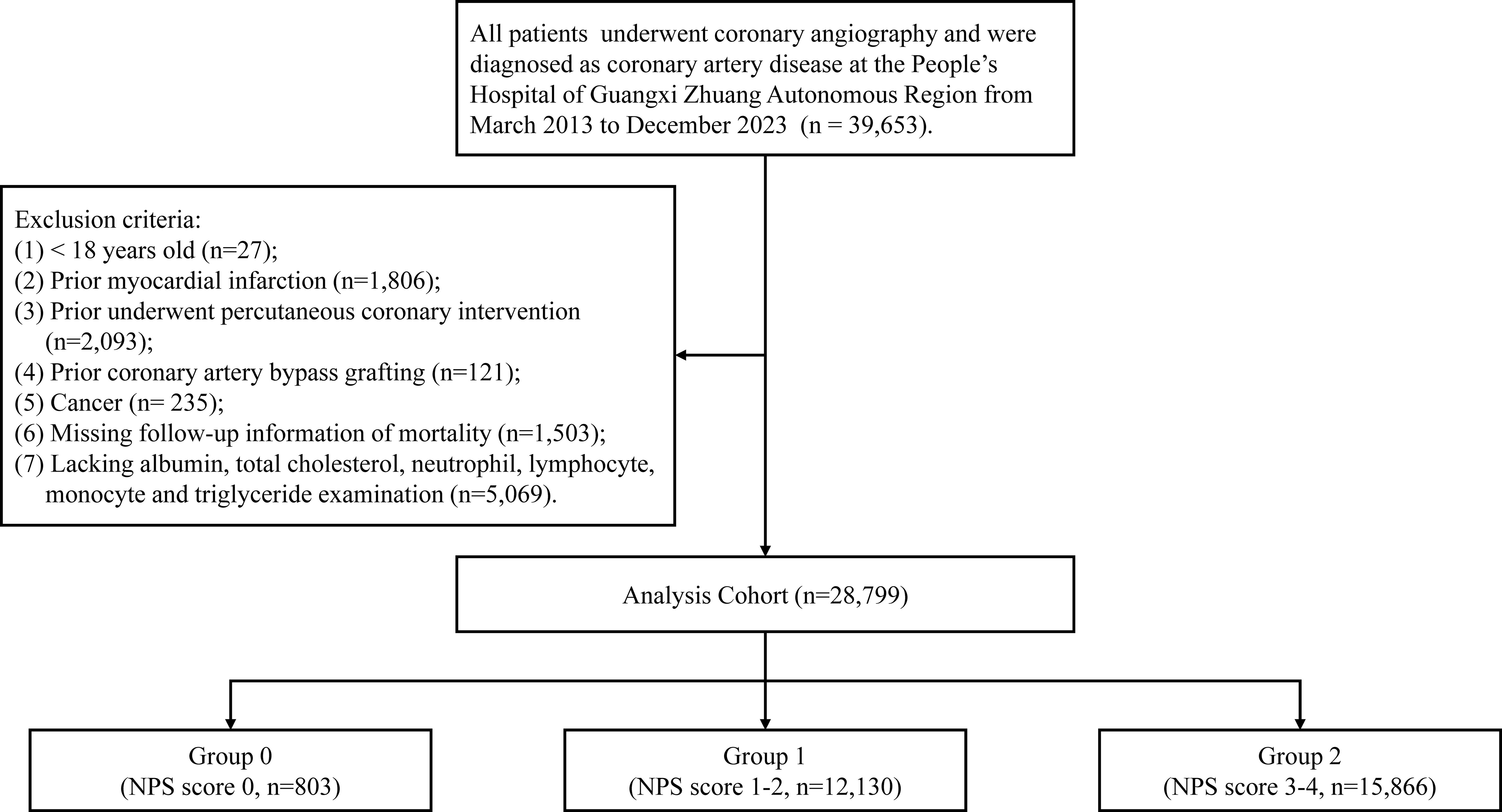
Patients fulfilling the following criteria were excluded: (1) individuals aged less than 18 years; (2) individuals with a history of myocardial infarction; (3) individuals who had undergone percutaneous coronary intervention (PCI) previously; (4) individuals who had undergone coronary artery bypass grafting previously; (5) individuals diagnosed with cancer; (6) individuals lacking follow-up information; and (7) individuals lacking albumin, total cholesterol, neutrophil, lymphocyte, monocyte, or triglyceride examination results. Figure 1 shows the flowchart of this study.
Clinical definitions
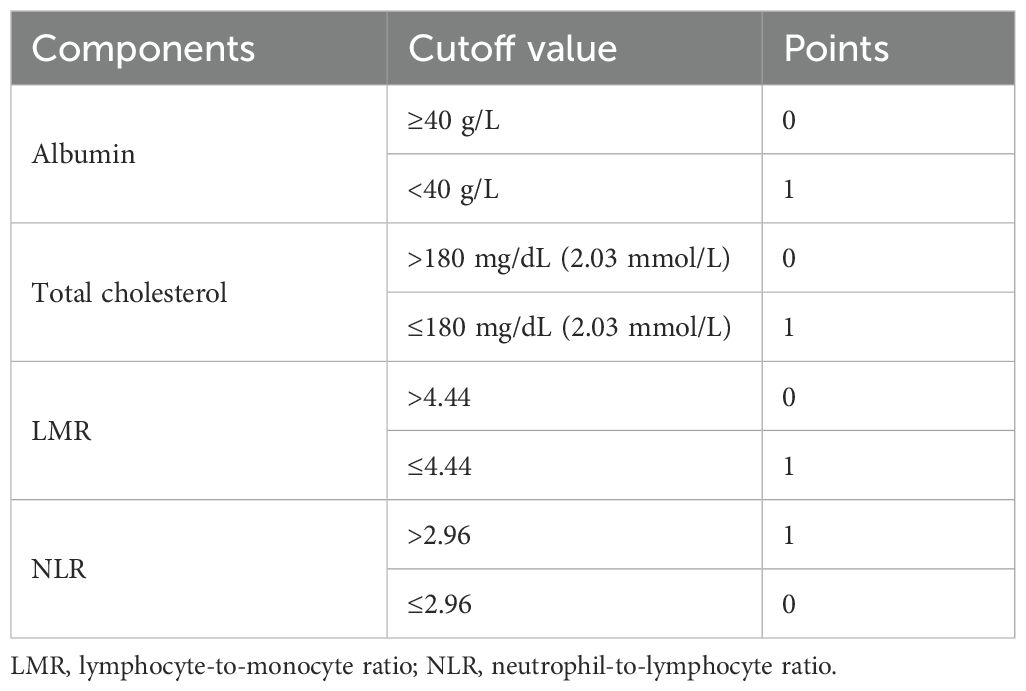
The primary outcome of this study was the all-cause mortality. The diagnosis of CAD was derived from angiographic confirmation (>50% stenosis in one vessel at the lowest), which was derived from structured EHR data (e.g., procedural reports and cardiologist notes). To assess NPS value, the following four variables were applied: serum albumin concentrations, total cholesterol concentrations, neutrophil-to-lymphocyte ratio (NLR) levels, and lymphocyte-to-monocyte ratio (LMR) levels (6). According to previous reports, serum albumin ≥40 g/L, total cholesterol > 180 mg/dL (2.03 mmol/L), LMR > 4.44, or NLR ≤ 2.96 was scored as 0, while serum albumin <40 g/L, total cholesterol ≤ 180– mg/dL (2.03 mmol/L), LMR ≤ 4.44, or NLR > 2.96 was scored as 1. NPS is the sum of the scores of each of the four factors. To convert total cholesterol from mmol/L to mg/dL, multiply by 88.6. Details were reported in Table 1. All procedures for PCI as well as CAG followed the clinical standard guidelines (10–12). A variety of concomitant diseases were taken into consideration, including acute myocardial infarction (AMI), congestive heart failure (CHF), hypertension, diabetes mellitus, chronic kidney disease (CKD), anemia, chronic obstructive pulmonary disease (COPD), and stroke. CHF was defined as New York Heart Association (NYHA) class >2 or Killip class >1 (13). CKD was determined as estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2 (14). The level of eGFR was calculated based on the Modification of Diet in Renal Disease (MDRD) formula (15). Other comorbidities were derived from ICD codes plus clinician diagnosis.
Statistical analysis
Based on the final score, study population was categorized into 3 groups (group 0: NPS value of 0; group 1: NPS value of 1 or 2; group 2: NPS value of 3 or 4) (6). This research compared continuous variables using the Wilcoxon rank sum test or Student’s t test and expressed them as mean with median with interquartile range (IQR) or standard deviation (SD). For categorical variables, the chi-square test was applied and number and percentage (%) were reported. The Kaplan–Meier curves were applied to estimate the effect of different groups on long-term all-cause mortality. To determine and quantify the magnitude of risk, univariate and multivariate COX regression were conducted. The study carefully selected covariates to create four models: (1) univariate; (2) adjusted age and gender; (3) adjusted age, gender, PCI and morbidities; and (4) adjusted all covariates above as well as medication use of renin–angiotensin system inhibitor (RASi), statins, and β-blocker. The same covariates were used in restricted cubic spline (RCS) analyses to investigate potential nonlinear correlations. R software (version 3.6.3) was used for all statistical analyses. A p-value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
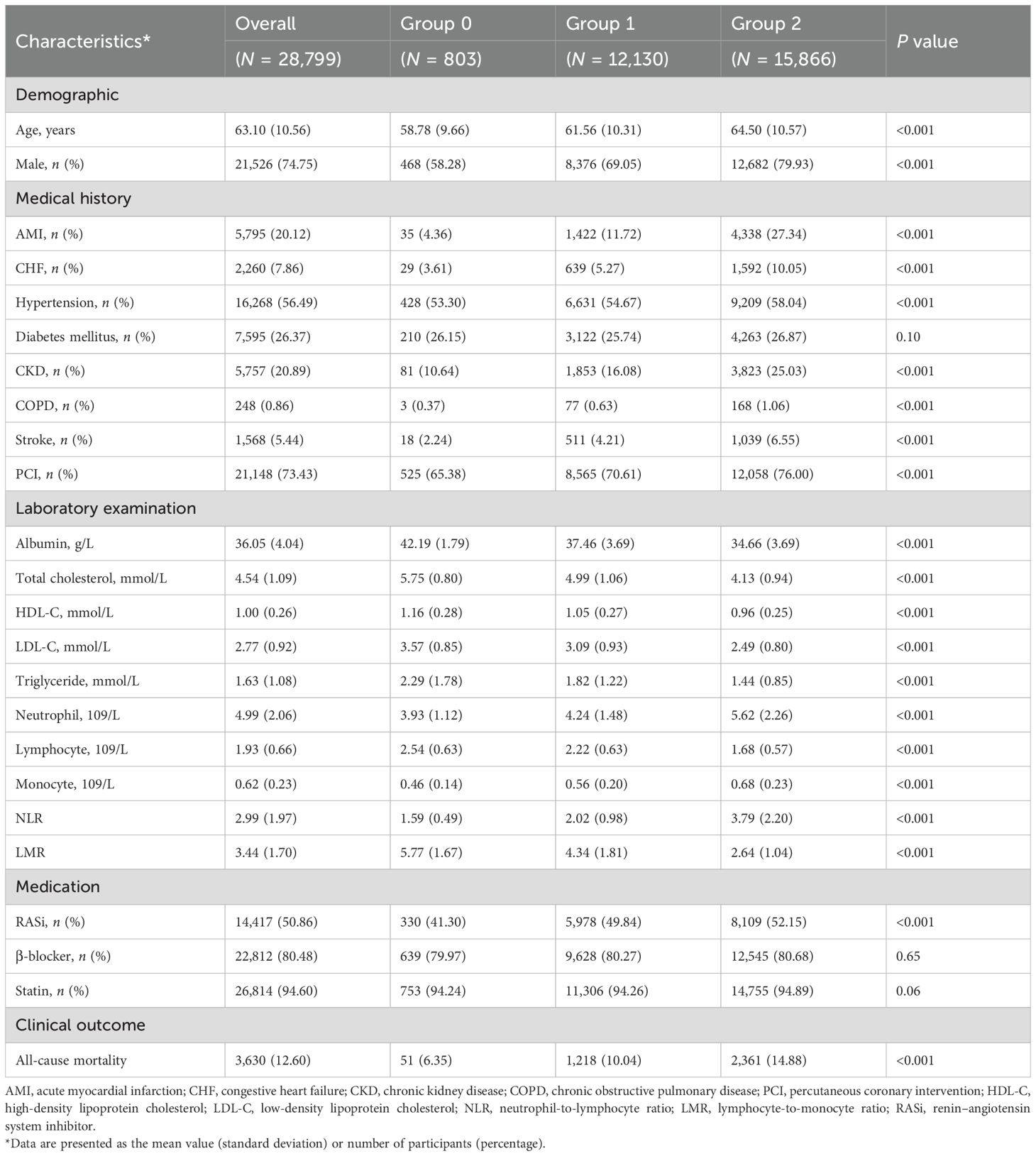
Study participants were 28,799 patients (mean age 63.10 ± 10.56 years, 74.75% men) suffering from CAD (Table 2). Of these patients, 803 (2.79%) were classified as group 0, 12,130 (42.12%) were classified as group 1, and 15,866 (55.09%) were classified as group 2. There were 21,148 (73.43%) patients who underwent PCI, 5,795 (20.12%) had AMI, 2,260 (7.86%) had CHF, 16,268 (56.49%) had hypertension, 7,595 (26.37%) had diabetes mellitus, 5,757 (20.89%) had CKD, 248 (0.86%) had COPD, and 1,568 (5.44%) had stroke.
Patients in groups 2 and 1 were older on average compared to group 0. Moreover, the prevalence of comorbid conditions such as AMI, CHF, hypertension, CKD, COPD, and stroke was also higher in group 2 and group 1 than in group 0. For laboratory examination, the concentrations of albumin, total cholesterol, HDL-C, LDL-C, triglyceride, lymphocyte, and LMR were lower in groups 2 and 1. Meanwhile, neutrophil, monocyte, and NLR were higher in groups 2 and 1.
Primary outcomes
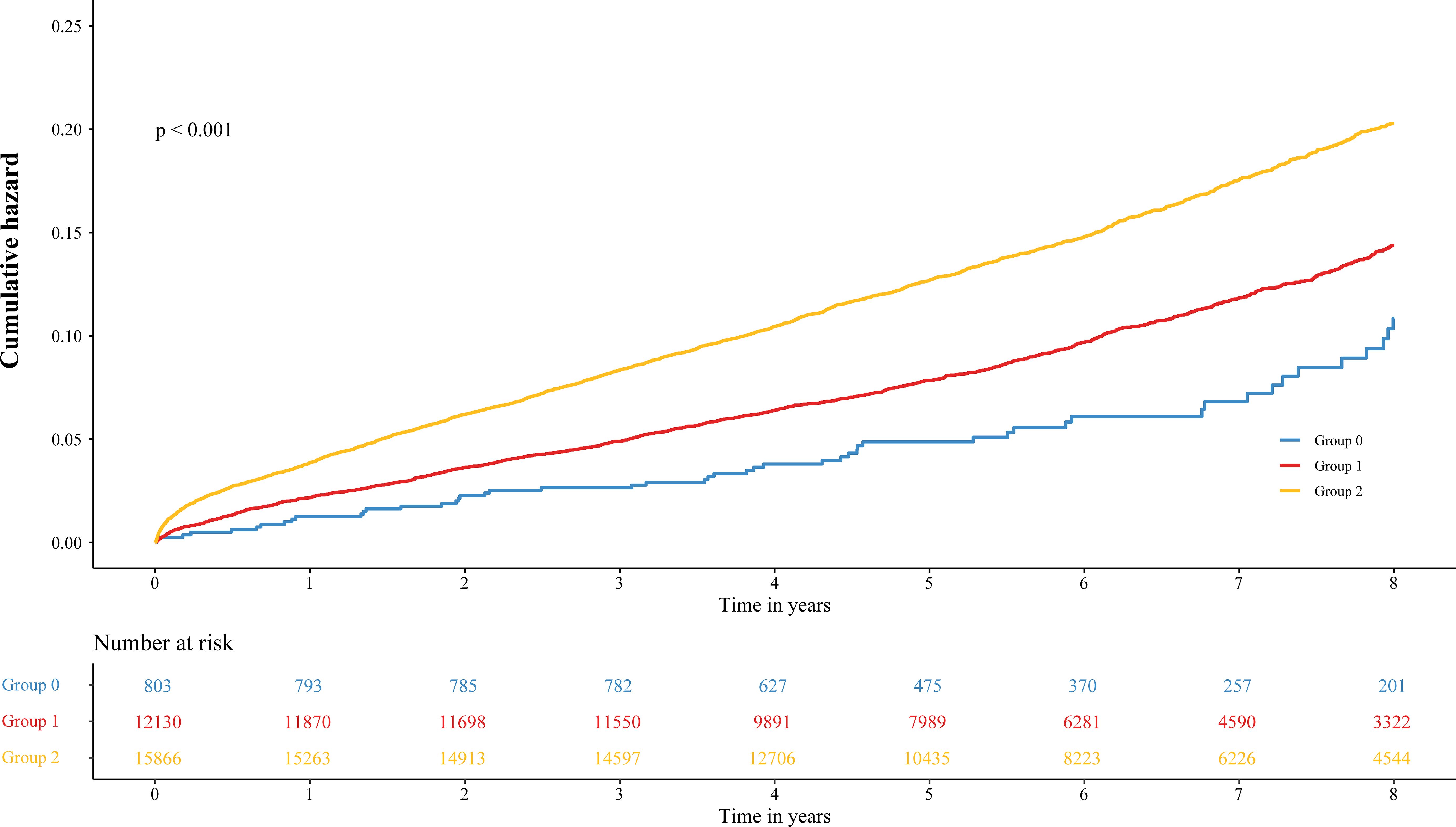
During the median follow-up period of 6.12 years, 3,630 patients (12.60%) died. Of these, 51 (6.35%), 1,218 (10.04%), and 2,361 (14.88%) were in groups 0, 1, and 2, respectively. On the basis of Kaplan–Meier cumulative hazard curves shown in Figure 2, a significantly higher cumulative incidence was determined from group 2 (log-rank p < 0.001) versus group 0 and group 1. In Table 3, results of Cox regression are shown. When NPS value was analyzed as a continuous variable, per 1-point increasement was related to 15% increased risk (adjusted HR, 1.15; 95% CI, 1.11–1.19). When NPS value was analyzed as a categorical variable, patients in group 2 (NPS of 3 or 4) and group 1 (NPS of 1 or 2) have a 70% and 38% increased risk compared to group 0 (NPS of 0) patients with low levels.
In order to examine the existing potential nonlinear correlation, RCS analysis was conducted with the same covariates form four Cox models (Figure 3). The result illustrated positive linear correlation between NPS and risk of outcome (all p for nonlinear > 0.05, Figure 3).
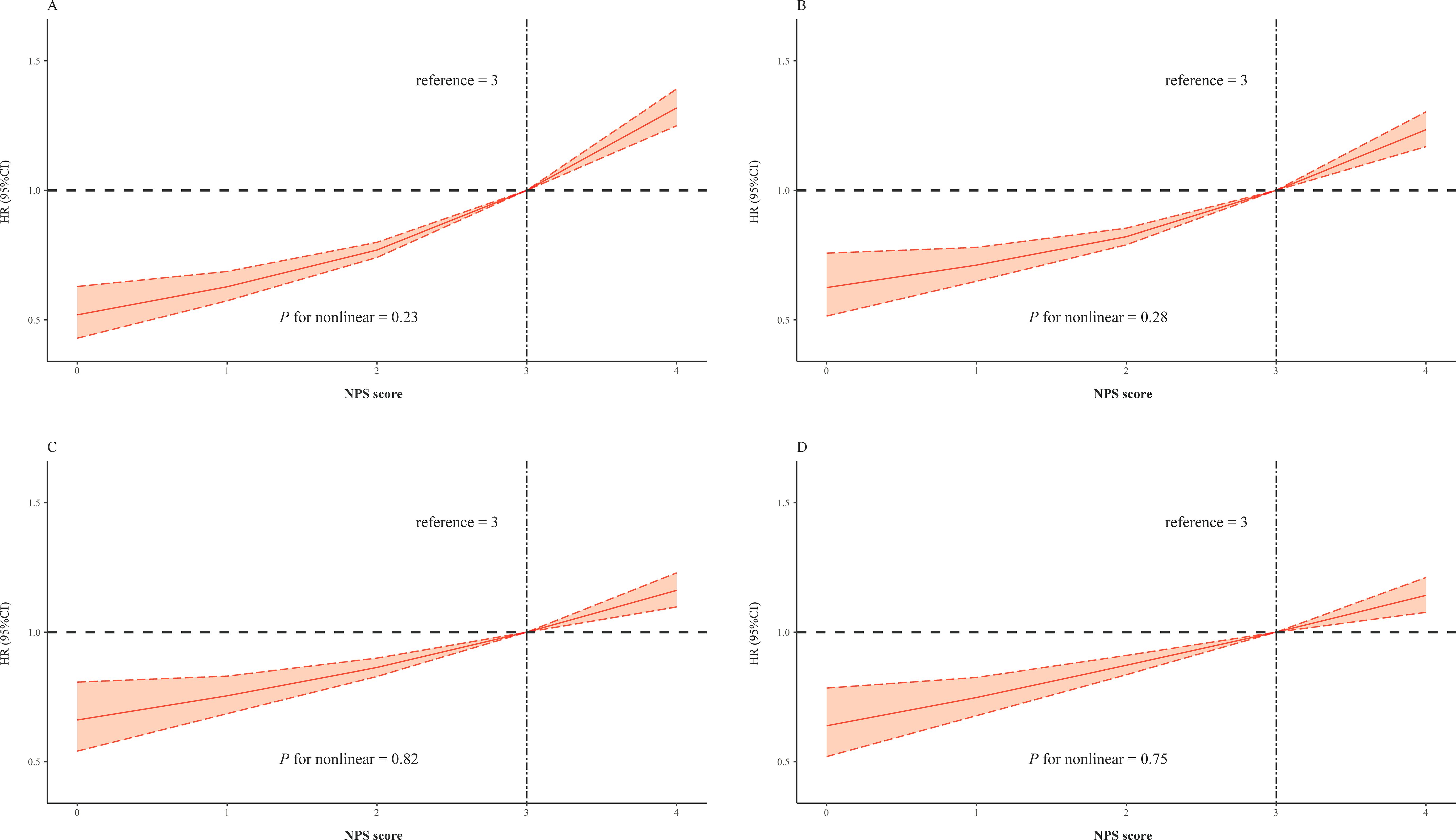
Figure 3. Restricted cubic splines of the NPS and hazard ratio for mortality. (A) The restrict spline curve of the univariate Cox model. (B) The restrict spline curve of multivariate Cox model 2, adjusted age and gender. (C) The restrict spline curve of multivariate Cox model 3, adjusted age, gender, PCI, and morbidities including AMI, CHF, hypertension, diabetes mellitus, CKD, COPD, and stroke. (D) The restrict spline curve of multivariate Cox model 4, adjusted all covariates above and medication use of RASi, statins, and β-blocker.
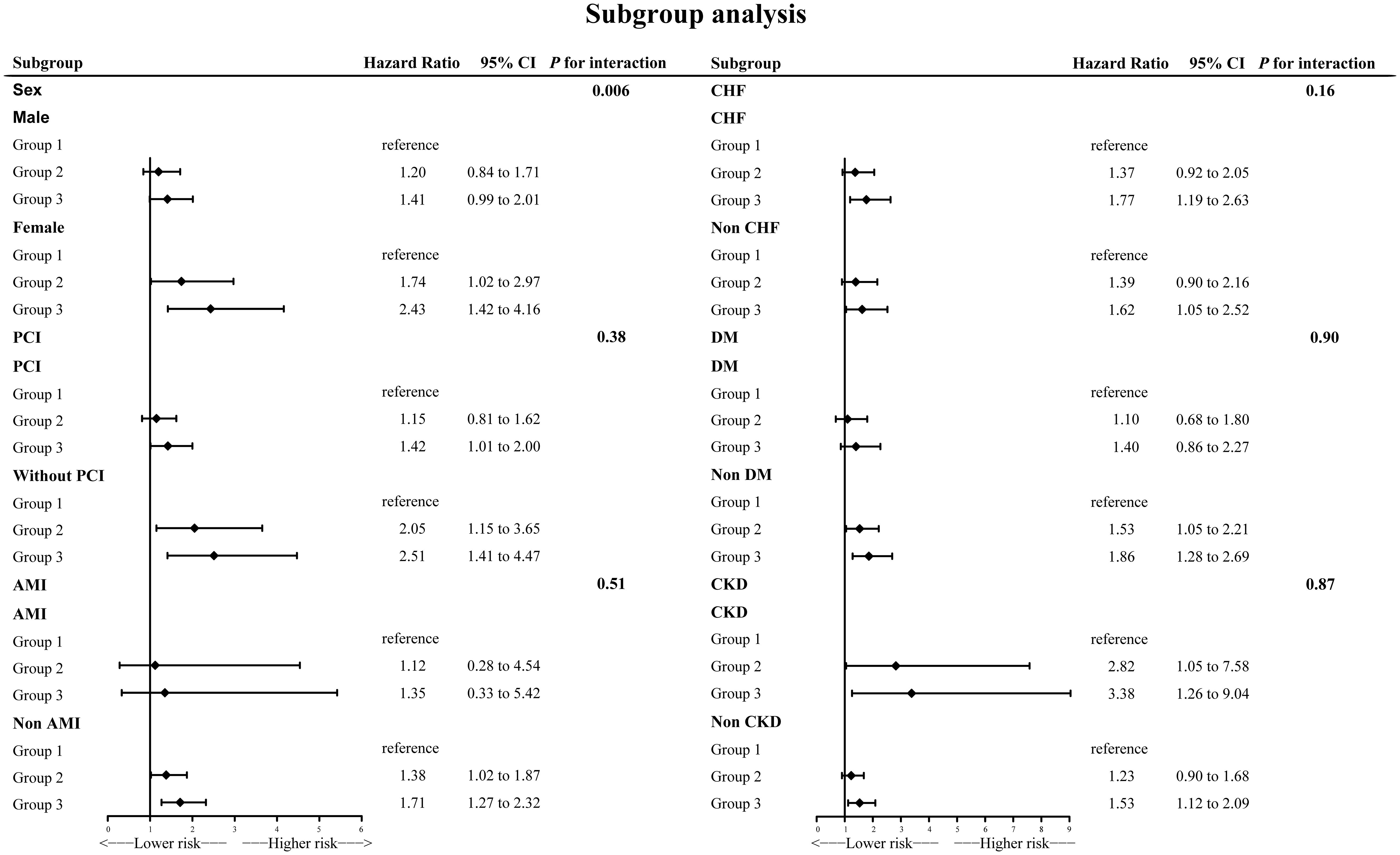
Subgroup analysis
Subgroup analyses generally agreed with main analysis results (Figure 4). There was a significant interaction effect for gender (p for interaction = 0.006). Among female individuals, the results were even more dramatic.
Discussion
This cohort study of participants with CAD examined the association between NPS value and risk of all-cause death. The result clearly demonstrated a linear positive correlation between NPS and the risk of long-term all-cause death, with each point increase in NPS increasing the risk by 15% in CAD population. Additionally, patients in the high NPS value group had a poorer prognosis compared to the group with an NPS value of 0. After adjustment of all confounders, the results were still robust. According to further subgroup analysis, most results were consistent.
The NPS is a newly detected scoring system that can comprehensively evaluate the individual’s immunological and nutritional status according to several basic blood examinations. These easily accessible and routinely tested indicators included albumin level, total cholesterol concentration, lymphocyte, neutrophil, and monocyte. NPS was initially constructed and verified as an independent prognostic risk factor in colorectal cancer (6). Subsequently, the NPS’s ability to predict prognosis was validated in various diseases, and increased NPS levels were found to be independently correlated to increased risk of poor prognosis (7–9). Erdogan et al.’s study, which included 1,887 consecutive patients with STEMI who were undergoing PCI, reported that the high-NPS group had a higher rate of death compared to the low-NPS group (19.1% vs 7.8%, p < 0.001) (16). After adjusting confounders, evaluated NPS was associated with poorer prognosis and high NPS (3–4) increased the risk 1.49-fold. In Saygi et al.’s study that recruited 3,828 patients with STEMI with emergency PCI, the result illustrated that the rate of in-hospital death was elevated in the high-NPS group in contrast to the medium- and low-NPS group (17). Multivariable logistic regression also showed that the high- and medium-NPS group significantly increased the risk of in-hospital death.
NPS encompasses not only immunoinflammatory markers like NLR and LMR, but also total cholesterol and serum albumin. These indicators mirrored an individual’s nutritional status and inflammation level. As a result, the body condition of the patient could be evaluated in a more comprehensive and efficient manner. According to the NPS scoring system, high NPS levels most likely presented underlying low albumin, low total cholesterol, low LMR, and high NLR, suggesting malnutrition and high inflammation status. The significance of inflammation in initiating, promoting, and destabilizing atherosclerotic plaques is paramount (18, 19). Systemic inflammation was commonly related to vascular wall inflammation (20). Several clinical studies demonstrated that taking anti-inflammatory drugs, such as therapeutic monoclonal antibodies against IL-1β (canakinumab) and colchicine is useful in modulating inflammation, reducing adverse events risk and improving the prognosis of chronic CAD (21, 22). A growing body of research has suggested that inflammation-related indexes, such as LMR and NLR, are related to CAD severity and prognosis (23, 24). NLR and LMR are straightforward and economically efficient biomarkers that mirror the complex equilibrium between innate and adaptive immune responses (25).
Notably, NLR has been demonstrated to be a reliable biomarker of inflammation in the vascular wall, and it is readily accessible (26). Through reactive oxygen species, cytokines, proteases, and neutrophil extracellular traps, neutrophils were known to adversely affect chronic inflammatory disorders in individuals with increased NLR (27). LMR is another inflammatory indicator calculated based on lymphocytes and monocytes. Decreased LMR was related to the poor prognosis. Inflammatory cell infiltration is an important mechanism of atherosclerosis (20, 28). Lymphocytes and monocytes played crucial roles in atherosclerosis’ early stage; meanwhile, neutrophils were involved in plaque destabilization and thrombosis (29).
Furthermore, nutrition, being a modifiable element, had a further impact on the prognosis of patients with CAD. Malnutrition, in particular, significantly affects prognosis. Several studies have identified malnutrition as the most prevalent cause of secondary immunologic disorders. Low serum total cholesterol levels and albumin concentrations were important objective indicators of malnutrition. Previous research has demonstrated that a low total cholesterol level serves as a biological indicator for concurrent cachexia, malnutrition, cancer, and other chronic diseases, which have confirmed detrimental effects for prognosis (30). Additionally, there is emerging evidence suggesting that cholesterol levels were clearly associated with regulation of immune cell function. Decreased cholesterol levels result in diminished activation of immune signaling and reduced antitumor activity (31). Serum albumin concentrations were linked to both nutritional status and the acute phase reaction, as well as chronic inflammatory diseases (32, 33). Existing studies indicated that decreased albumin concentrations might serve as an indicator of sustained arterial injury and advancement of thrombosis as well as atherosclerosis (34). As a result of decreased albumin concentrations, catabolic cytokines were produced, muscle breakdown occurred, and appetite was suppressed (33). Given the established roles of neutrophils, monocytes, lymphocytes, serum albumin, and total cholesterol in the poor prognosis of patients with CAD, NPS emerges as a promising tool. NPS calculated by combining these factors not only is less susceptible to various non-pathological factors than individual indicators, but also captures the patient’s inflammatory and nutritional status, which are crucial in the prognosis of patients with CAD. Our study validated the association between NPS and poor prognosis in patients with CAD using large sample data, and found a linear correlation between NPS level and the risk of poor prognosis. This may make NPS a good prognostic assessment tool for patients with CAD. It enables clinicians to identify high-risk patients at an early stage. However, precisely because NPS is a comprehensive assessment of nutritional status and inflammation levels, timely clinical interventions targeting high-risk patients need to be further explored.
According to the results of subgroup analysis, the association between NPS and long-term all-cause death was more significant in female patients (p for interaction = 0.006). The value of HR was also higher in female patients. There may be several possible explanations. Firstly, women passed through adverse metabolic disturbances and lipid profile deterioration more than men (35, 36). A previous study conducted in humans and mice confirmed that estrogen preserves endothelial function (37, 38). Thus, because of older age at onset and decreased estrogen levels, endothelial dysfunction is more severe in female patients with CAD than in male patients. The above factors together resulted in a worse nutritional status and higher levels of inflammation played more important roles in female patients’ prognosis. Secondly, women tend to present later CAD than men and suffered more from chronic comorbidities. Several present studies including Steg et al.’s CLARIFY study and Chen et al.’s study demonstrated this (39, 40). Furthermore, the clinical presentation was more atypical in women, and female patients received fewer interventions and drug therapies (41, 42). NPS may provide additional clues apart from the control of comorbidities such as hypertension, CHF, and CKD to improve prognosis in female patients with CAD.
It is necessary to admit that there existed several certain limitations. Firstly, although this research was derived from a single-center retrospective cohort, the enrolled patients originated from the largest cardiac intervention center in the Guangxi Zhuang Autonomous Region, which rendered the sample representative and ensured the study’s quality control. Secondly, the generalization of our results is restricted to the Chinese population without taking into account other races. Thirdly, this study only evaluated the baseline admission NPS levels and did not assess the effect of NPS changes during follow-up. This should be further investigated. Fourthly, there were limited data on the included patients, without information about body mass index (BMI), smoking status, and socioeconomic factors. This may partially affect the results. However, we adjusted for potential confounders whenever possible and constructed three multivariate Cox regression models to ensure the robustness of the results.
Conclusion
This research indicated the significance of inflammation and nutritional status in case of an unfavorable prognosis. Based on routine examination, NPS can comprehensively evaluate the prognosis of patients with CAD. There was a linear positive correlation between NPS value and prognosis.
Data availability statement
The datasets presented in this article are not readily available because it is not applicable at this stage. The datasets analyzed during the current study will be available from the corresponding author on reasonable request when the study is finished. Requests to access the datasets should be directed to LL aWN1bHZsaXdlbkAxNjMuY29t.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of Guangxi Zhuang Autonomous Region People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because all traceable personal identifiers were removed from the analytic dataset to protect patients’ privacy. For this reason, the requirement for informed consent was waived, which was approved by the Research Ethics Committee of Guangxi Zhuang Autonomous Region People’s Hospital (No. KY-QT-202103).
Author contributions
BW: Conceptualization, Writing – original draft. WC: Conceptualization, Writing – original draft. LS: Writing – original draft. MP: Writing – original draft. YZ: Writing – original draft. YW: Writing – original draft. YT: Writing – original draft. GQ: Writing – original draft. WD: Writing – original draft. SC: Project administration, Writing – original draft. XC: Project administration, Writing – original draft. ZZ: Writing – original draft. YS: Project administration, Writing – original draft. QJ: Writing – review & editing. LL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Guangxi Natural Science Foundation (2023GXNSFBA026088, 2023GXNSFBA026036, 2023GXNSFAA026059, 2024GXNSFAA010012 and 2025GXNSFAA069574), the Guangxi Science and Technology Program (GKAB25069064, GKAB24010174 and GKAB23026019), the Guangxi Medical and Health Appropriate Technology Research and Development Project (S2020076, S2020080, S2022015, S2022025 and S2023011), Guangxi Science and Technology Base and Special Talents' Project (GKAD17129026) and the Guangxi Self-funded Research project (Z20200743 and Z-A20220030).
Acknowledgments
We thank Home for Researchers editorial team (www.home-for-researchers.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 39210159:1736–88. doi: 10.1016/s0140-6736(18)32203-7
2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 7625:2982–3021. doi: 10.1016/j.jacc.2020.11.010
3. Wang B, Liu J, Chen S, Ying M, Chen G, Liu L, et al. Malnutrition affects cholesterol paradox in coronary artery disease: a 41,229 Chinese cohort study. Lipids Health Dis. (2021) 201:36. doi: 10.1186/s12944-021-01460-6
4. Raposeiras Roubín S, Abu Assi E, Cespón Fernandez M, Barreiro Pardal C, Lizancos Castro A, Parada JA, et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J Am Coll Cardiol. (2020) 767:828–40. doi: 10.1016/j.jacc.2020.06.058
5. Wang J, Chen L, Huang Z, Lu J, Yang Y, Zhao X, et al. A synergistic association between inflammation, malnutrition, and mortality in patients with diabetics. Front Nutr. (2022) 9:872512. doi: 10.3389/fnut.2022.872512
6. Galizia G, Lieto E, Auricchio A, Cardella F, Mabilia A, Podzemny V, et al. Naples prognostic score, based on nutritional and inflammatory status, is an independent predictor of long-term outcome in patients undergoing surgery for colorectal cancer. Dis Colon Rectum. (2017) 6012:1273–84. doi: 10.1097/dcr.0000000000000961
7. Jin J, Wang H, Peng F, Wang X, Wang M, Zhu F, et al. Prognostic significance of preoperative Naples prognostic score on short- and long-term outcomes after pancreatoduodenectomy for ampullary carcinoma. Hepatobil Surg Nutr. (2021) 106:825–38. doi: 10.21037/hbsn-20-741
8. Du CF, Gao ZY, Xu ZD, Fang ZK, Yu ZC, Shi ZJ, et al. Prognostic value of the Naples prognostic score in patients with intrahepatic cholangiocarcinoma after hepatectomy. BMC Cancer. (2024) 241:727. doi: 10.1186/s12885-024-12502-4
9. Zhu N, Lin S, Yu H, Liu F, and Huang W. Cao C Naples prognostic score as a novel prognostic prediction indicator in adult asthma patients: A population-based study. World Allergy Organ J. (2023) 1610:100825. doi: 10.1016/j.waojou.2023.100825
10. Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE Jr., et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2012) 607:645–81. doi: 10.1016/j.jacc.2012.06.004
11. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. (2016) 6710:1235–50. doi: 10.1016/j.jacc.2015.10.005
12. Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. (2023) 4438:3720–826. doi: 10.1093/eurheartj/ehad191
13. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. (2004) 447:1393–9. doi: 10.1016/j.jacc.2004.06.068
14. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. (2002) 392 Suppl 1:S1–266. Available online at: https://pubmed.ncbi.nlm.nih.gov/11904577/
15. Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. (2003) 411:47–55. doi: 10.1016/s0735-1097(02)02663-3
16. Erdogan A, Genc O, Ozkan E, Goksu MM, Ibisoglu E, Bilen MN, et al. Impact of naples prognostic score at admission on in-hospital and follow-up outcomes among patients with ST-segment elevation myocardial infarction. Angiology. (2023) 7410:970–80. doi: 10.1177/00033197231151559
17. Saygi M, Tanalp AC, Tezen O, Pay L, Dogan R, Uzman O, et al. The prognostic importance of the Naples prognostic score for in-hospital mortality in patients with ST-segment elevation myocardial infarction. Coron Artery Dis. (2024) 351:31–7. doi: 10.1097/mca.0000000000001285
18. Borghini A, Mercuri A, and Andreassi MG. Neutrophil-to-lymphocyte, platelet-to-lymphocyte ratios, and systemic immune-inflammation index as predictors of mortality in coronary artery disease. J Cardiovasc Transl Res. (2023) 162:473–5. doi: 10.1007/s12265-022-10312-2
19. Shi S, Gao J, Zhang Y, Zhan M, Tan Z, Wang P, et al. Inflammation and platelet hyperresponsiveness in coronary artery disease and the influence of Talin-1/αIIbβ3-mediated bidirectional signaling pathway. Front Pharmacol. (2025) 16:1535182. doi: 10.3389/fphar.2025.1535182
20. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. (2012) 329:2045–51. doi: 10.1161/atvbaha.108.179705
21. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 37712:1119–31. doi: 10.1056/NEJMoa1707914
22. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. (2020) 38319:1838–47. doi: 10.1056/NEJMoa2021372
23. Song S, Chen L, and Yu R. Zhu J Neutrophil-to-lymphocyte ratio as a predictor of all-cause and cardiovascular mortality in coronary heart disease and hypertensive patients: a retrospective cohort study. Front Endocrinol (Lausanne). (2024) 15:1442165. doi: 10.3389/fendo.2024.1442165
24. Si Y, Liu J, Shan W, Zhang Y, Han C, Wang R, et al. Association of lymphocyte-to-monocyte ratio with total coronary plaque burden in patients with coronary artery disease. Coron Artery Dis. (2020) 317:650–5. doi: 10.1097/mca.0000000000000857
25. Liu Z, Li Y, Wang Y, Zhang H, and Lian Y. Cheng X the neutrophil-to-lymphocyte and monocyte-to-lymphocyte ratios are independently associated with the severity of autoimmune encephalitis. Front Immunol. (2022) 13:911779. doi: 10.3389/fimmu.2022.911779
26. Afari ME. Bhat T Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. (2016) 145:573–7. doi: 10.1586/14779072.2016.1154788
27. Jorch SK. Kubes P An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. (2017) 233:279–87. doi: 10.1038/nm.4294
28. Libby P and Ridker PM. Hansson GK Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. (2009) 5423:2129–38. doi: 10.1016/j.jacc.2009.09.009
29. Yayan J. Emerging families of biomarkers for coronary artery disease: inflammatory mediators. Vasc Health Risk Manag. (2013) 9:435–56. doi: 10.2147/vhrm.S45704
30. Nakagomi A, Seino Y, Noma S, Kohashi K, Kosugi M, Kato K, et al. Relationships between the serum cholesterol levels, production of monocyte proinflammatory cytokines and long-term prognosis in patients with chronic heart failure. Intern Med. (2014) 5321:2415–24. doi: 10.2169/internalmedicine.53.2672
31. Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. (2016) 5317596:651–5. doi: 10.1038/nature17412
32. Chen SC, Yang YL, Wu CH, Huang SS, Chan WL, Lin SJ, et al. Association between preoperative nutritional status and clinical outcomes of patients with coronary artery disease undergoing percutaneous coronary intervention. Nutrients. (2020) 125:1295. doi: 10.3390/nu12051295
33. Merker M, Felder M, Gueissaz L, Bolliger R, Tribolet P, Kägi-Braun N, et al. Association of baseline inflammation with effectiveness of nutritional support among patients with disease-related malnutrition: A secondary analysis of a randomized clinical trial. JAMA netw Open. (2020) 33:e200663. doi: 10.1001/jamanetworkopen.2020.0663
34. Kuller LH, Eichner JE, Orchard TJ, Grandits GA, and McCallum L. Tracy RP The relation between serum albumin levels and risk of coronary heart disease in the Multiple Risk Factor Intervention Trial. Am J Epidemiol. (1991) 13411:1266–77. doi: 10.1093/oxfordjournals.aje.a116030
35. Du T, Fernandez C, Barshop R, Guo Y, Krousel-Wood M, Chen W, et al. Sex differences in cardiovascular risk profile from childhood to midlife between individuals who did and did not develop diabetes at follow-up: the bogalusa heart study. Diabetes Care. (2019) 424:635–43. doi: 10.2337/dc18-2029
36. Logue J, Walker JJ, Colhoun HM, Leese GP, Lindsay RS, McKnight JA, et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. (2011) 5412:3003–6. doi: 10.1007/s00125-011-2313-3
37. Sotnikova R, Bacova B, Vlkovicova J, Navarova J, and Tribulova N. Sex differences in endothelial function of aged hypertriglyceridemic rats - effect of atorvastatin treatment. Interdiscip Toxicol. (2012) 53:155–8. doi: 10.2478/v10102-012-0025-2
38. Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, et al. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation. (2001) 10310:1410–5. doi: 10.1161/01.cir.103.10.1410
39. Steg PG, Greenlaw N, Tardif JC, Tendera M, Ford I, Kääb S, et al. Women and men with stable coronary artery disease have similar clinical outcomes: insights from the international prospective CLARIFY registry. Eur Heart J. (2012) 3322:2831–40. doi: 10.1093/eurheartj/ehs289
40. Chen SQ, Liu J, Zhou Y, Huang ZD, Xie Y, Huang HZ, et al. Sex differences in characteristics, treatments, and in-hospital outcomes of patients undergoing coronary angiography or intervention. Front Cardiovasc Med. (2022) 9:878566. doi: 10.3389/fcvm.2022.878566
41. Lu J, Lu Y, Yang H, Bilige W, Li Y, Schulz W, et al. Characteristics of high cardiovascular risk in 1.7 million Chinese adults. Ann Intern Med. (2019) 1705:298–308. doi: 10.7326/m18-1932
Keywords: coronary artery disease, Naples Prognostic Score, long-term prognosis, mortality, inflammatory and nutritional status
Citation: Wang B, Chen W, Shi L, Pei M, Zhou Y, Wei Y, Tang Y, Qiu G, Duan W, Chen S, Chen X, Zhang Z, Shi Y, Ji Q and Lyu L (2025) Impact of the Naples Prognostic Score at admission on long-term prognosis among patients with coronary artery disease. Front. Immunol. 16:1529779. doi: 10.3389/fimmu.2025.1529779
Received: 17 November 2024; Accepted: 15 May 2025;
Published: 11 June 2025.
Edited by:
Di Wang, Jilin Agriculture University, ChinaReviewed by:
Jiale Zhang, China Science and Technology Development Center for Chinese Medicine, ChinaBartłomiej K. Sołtysik, Medical University of Lodz, Poland
Copyright © 2025 Wang, Chen, Shi, Pei, Zhou, Wei, Tang, Qiu, Duan, Chen, Chen, Zhang, Shi, Ji and Lyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liwen Lyu, aWN1bHZsaXdlbkAxNjMuY29t; Qingwei Ji, anF3MTI0QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Bo Wang
Bo Wang Wan Chen1†
Wan Chen1† Yao Zhou
Yao Zhou Yanlin Wei
Yanlin Wei Qingwei Ji
Qingwei Ji Liwen Lyu
Liwen Lyu