- 1Xiamen Xianyue Hospital, Xianyue Hospital Affiliated to Xiamen Medical College, Fujian Psychiatric Center, Fujian Clinical Research Center for Mental Disorders, Xiamen, Fujian, China
- 2School of Medicine, Huaqiao University, Quanzhou, Fujian, China
- 3Medical Research Center, Xiamen Chang Gung Hospital, Xiamen, Fujian, China
- 4Xiamen Chang Gung Allergology Consortium, Xiamen Chang Gung Hospital, Xiamen, Fujian, China
- 5Department of Clinical Laboratory, The Third Hospital of Xiamen, Xiamen, Fujian, China
Background: Multiple lines of evidence indicate a connection between the pathogenesis of coronavirus disease 2019 (COVID-19) and psychiatric diseases (PDs). To improve the treatment and management of individuals with psychosis and COVID-19, we evaluated biomarkers of PD patients, including those with schizophrenia (SCZ), bipolar disorder (BD), and major depression (MDD), along with the biomarkers of COVID-19.
Methods: In this study, 104 inpatients with concurrent PD and COVID-19 (PD+), the same 104 PD patients after they had recovered from COVID-19 (PD-), and 97 healthy controls (HCs) were evaluated. We analyzed the peripheral blood hematological parameters, serum biochemical parameters, and cytokine levels of the participants and compared the results among the three groups.
Results: The monocyte count; neutrophil-to-lymphocyte ratio (NLR); monocyte-to-lymphocyte ratio (MLR); systemic immune-inflammation index (SII); and C-reactive protein (CRP), serum CK isoenzyme MB (CK-MB), glucose (GLU), and interleukin (IL)-6 levels were significantly greater (P < 0.05), whereas the magnesium (Mg) level was lower (P < 0.05) in both the PD+ and PD- groups than in the HC group. Moreover, the above indicators were significantly different between the PD+ and PD- groups (P < 0.05).
Conclusion: Neutrophil count, monocyte count, NLR, MLR, SII, CRP, CK-MB, GLU and IL-6 levels were positively correlated with COVID-19 and PD. The Mg level was negatively correlated with COVID-19 and PD. Our findings suggest that Mg supplementation might be considered a potential treatment approach for PD patients with COVID-19. Despite these insights, the underlying pathophysiological mechanisms remain unclear, highlighting the vital need for further research to validate and build upon these findings.
1 Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a tremendous impact on the global economy and human health (1). As of July 28, 2024, the number of people infected with SARS-CoV-2 had reached 775,830,200 (2), and among them, the number of deaths was as high as 7,056,108 (3). Several studies have shown that patients with psychiatric diseases (PDs) are more prone to contracting COVID-19 (4, 5). Additionally, COVID-19, an illness capable of triggering systemic inflammation and a cytokine storm, is also associated with an increase in the number of diagnoses of PDs, including schizophrenia (SCZ), bipolar disorder (BD), and major depressive disorder (MDD) (6–8). These findings indicate that there may be a potential link between the pathogenesis of COVID-19 and PDs.
The involvement of inflammatory processes in both PDs and COVID-19, leading to changes in a series of indicators, such as cytokines and laboratory factors, has been consistently reported in numerous studies (8–12). For example, several hematological parameters, such as the neutrophil count (Neu#), monocyte count (Mon#), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), and systemic immune-inflammation index (SII), are positively associated with COVID-19 (13–15), along with the levels of several biochemical parameters and cytokines, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), C-reactive protein (CRP), and interleukin 6 (IL-6) (16, 17). When inflammatory stimuli or infections occur, cytokine levels increase, causing systemic symptoms, especially during acute inflammation (9). An increase in the levels of inflammatory markers has also been observed in individuals with severe PDs such as SCZ, BD, and MDD (18, 19).
The NLR, MLR, PLR, and SII are commonly used as markers of inflammation in various diseases, such as infections and mental disorders, and these markers provide valuable prognostic insights (11, 20–25). For example, the NLR is positively correlated with patients’ self-perceived illness severity (26). Recently, an increase in the NLR was reported in patients with a first episode of psychosis (FEP), and this increase in the NLR could increase the expression of proinflammatory factors related to the stress response in these patients (27). Moreover, these ratios might be useful tools for identifying patients with BD at risk for metabolic syndrome (24, 28). On the basis of this rationale, the NLR seems to be a promising neuroinflammatory biomarker. Although these ratios lack specificity, they can assist in making treatment decisions, monitoring treatment efficacy, and predicting disease progression and complications (20, 24, 25, 29, 30).
In the study carried out by Raony et al., changes in cytokine levels were considered to be a link between immune system changes during COVID-19 and PDs, as these changes may play a role in disrupting neurotransmitter metabolism, leading to behavioral changes (31). Wang et al. reported that the levels of several interleukins, specifically IL-6, in the cerebrospinal fluid of patients with SCZ were significantly greater than those in a control group (32). Additionally, Severance et al. suggested that exposure to coronaviruses might be a concurrent risk factor for individuals with severe mental disorders (33). Furthermore, psychiatric patients with COVID-19 (PD+) require longer hospital stays and have higher rates of complications and mortality, indicating poorer outcomes (5). Therefore, the identification of indicators that are correlated with both COVID-19 and mental illness is crucial.
To date, few studies have described the hematological, serum biochemical, and cytokine parameters of patients with psychosis during and after COVID-19. Since hematological and serum biochemical analyses are the most commonly performed tests in the clinic, to determine whether other parameters from general laboratory tests have prognostic value in PD+ patients and to provide more evidence for clinical diagnosis and treatment, we investigated peripheral blood hematological and serum biochemical parameters, as well as cytokine levels, in PD+ patients.
2 Methods
2.1 Participants
One hundred four participants with PDs (83 with SCZ, 19 with BD, and 2 with MDD) and 97 healthy controls (HCs) were included in this study. PD patients who were inpatients at Xiamen Xianyue Hospital from December 1, 2020, to January 31, 2024, were recruited, and the details of the psychotropic medications they used are presented in Supplementary Table 1. SCZ, BD, and MDD were diagnosed according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders-IV Text Revision (DSM-IV-TR). The diagnoses were confirmed by two trained psychiatrists using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I).
Two patient groups were included in this study: a) patients with concurrent PD and COVID-19 (PD+): This group was composed of patients hospitalized in psychiatric units. The study population comprised individuals with laboratory-confirmed SARS-CoV-2 infection, with all cases definitively diagnosed through real-time reverse transcription PCR (RT–PCR) testing of nasopharyngeal swab samples . b) Patients in the PD group and post-COVID-19 group (PD-): This study cohort comprised the PD+ patients in the post-COVID-19 recovery phase (≥30 days since symptom resolution), with confirmed SARS-CoV-2 clearance via consecutive negative RT–PCR tests and the absence of concurrent viral/bacterial infections, as validated through comprehensive microbiological screening. Blood samples from PD+ patients were collected before antiviral therapy was initiated. HCs were recruited from Xiamen Changgeng Hospital. HCs had neither current nor lifetime Axis I disorders, nor did any of their first-degree relatives have a history of such disorders. The following participants were excluded: those with concomitant major medical disorders, neurological diseases, or head injuries accompanied by loss of consciousness and/or those with substance/alcohol abuse or dependence and SARS-CoV-2 or other bacterial or viral infections.
This study was approved by the Medical Research Ethics Committee of Xiamen Xianyue Hospital in accordance with the guidelines of the Declaration of Helsinki. All participants provided written informed consent after receiving a detailed description of the study.
2.2 Primary outcomes
In this study, all the laboratory data, including complete blood count (XN-1000, Sysmex, Japan) and serum biochemistry data (AU5800, Beckman Coulter, USA), were retrieved from the laboratory information system (LIS). The concentrations of human serum cytokines were measured with a flow cytometer (RaiseCyte2L6C, Raisecare, Qingdao, China). PD patients were diagnosed with COVID-19 with a SARS-CoV-2 nucleic acid detection kit (fluorescent PCR method, Shanghai BioGerm Medical Biotechnology Co., Ltd.) and a nasopharyngeal swab.
2.3 Statistical analysis
SPSS statistics software (version 19.0) and GraphPad Prism software (version 6; 07 Graph Pad Software, Inc.) were used for statistical data analysis. Quantitative data with a normal distribution are presented as the means ± standard deviations, whereas nonnormally distributed variables are expressed as the medians (interquartile ranges, IQRs). Parametric analysis employed one-way ANOVA for group comparisons of normally distributed variables followed by Bonferroni-adjusted pairwise comparisons, whereas nonparametric analyses utilized the Mann–Whitney U test for two-group comparisons. The significance level was set at P < 0.05.
3 Results
3.1 Demographic and clinical characteristics
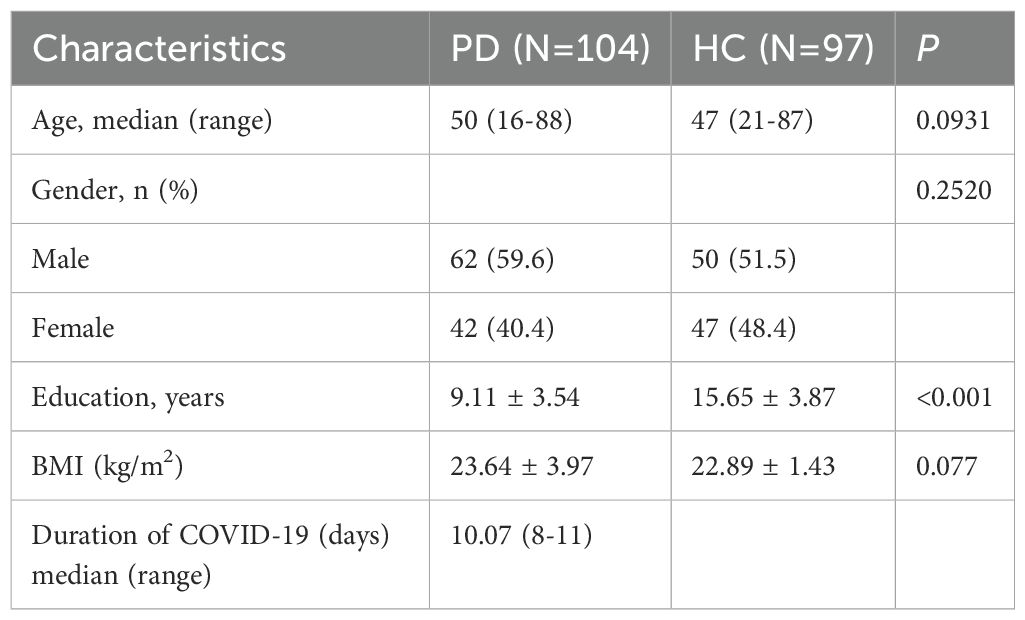
Table 1 presents the participant demographics and clinical characteristics. All PD patients were receiving antipsychotic treatment, and the psychotropic drugs (Supplementary Table 1) used remained unchanged during and after COVID-19. All participants ranged in age from 14 to 88 years. There was no significant difference in the median age between the PD and HC groups (P > 0.05). Among the PD patients, 62 were male (59.6%), and 42 were female (40.4%). Additionally, there was no significant difference in sex distribution or body mass index (BMI) between the groups (P > 0.05). Because the PD patients were inpatients in a long-term care psychiatric hospital, they had not received higher education; therefore, the number of years of education of PD patients significantly differed from that of the HCs (P < 0.05). The average duration of COVID-19 was 10.07 days.
3.2 Hematological parameters of the PD+, PD-, and HC groups
Table 2 shows the hematological parameters of the three groups. Compared with those in the HCs, the WBC#, Neu#, Mon#, platelet count (PLT#), NLR, MLR, PLR, SII and CRP levels in PD patients were significantly greater (P < 0.05). Moreover, the WBC#, Neu#, Mon#, NLR, MLR, PLR, SII and CRP levels were significantly greater (P < 0.05), whereas the lymphocyte count (Lym#), eosinophil count (Eos#) and basophil count (Bas#) were significantly lower (P < 0.05) in the PD+ group than in the other two groups. However, the Lym#, Eos#, Bas#, and PLT# values in the PD+ group were lower than those in the PD- group, whereas the Mon#, NLR, MLR, SII and CRP levels were greater in PD+ patients than in PD- patients (P < 0.05).
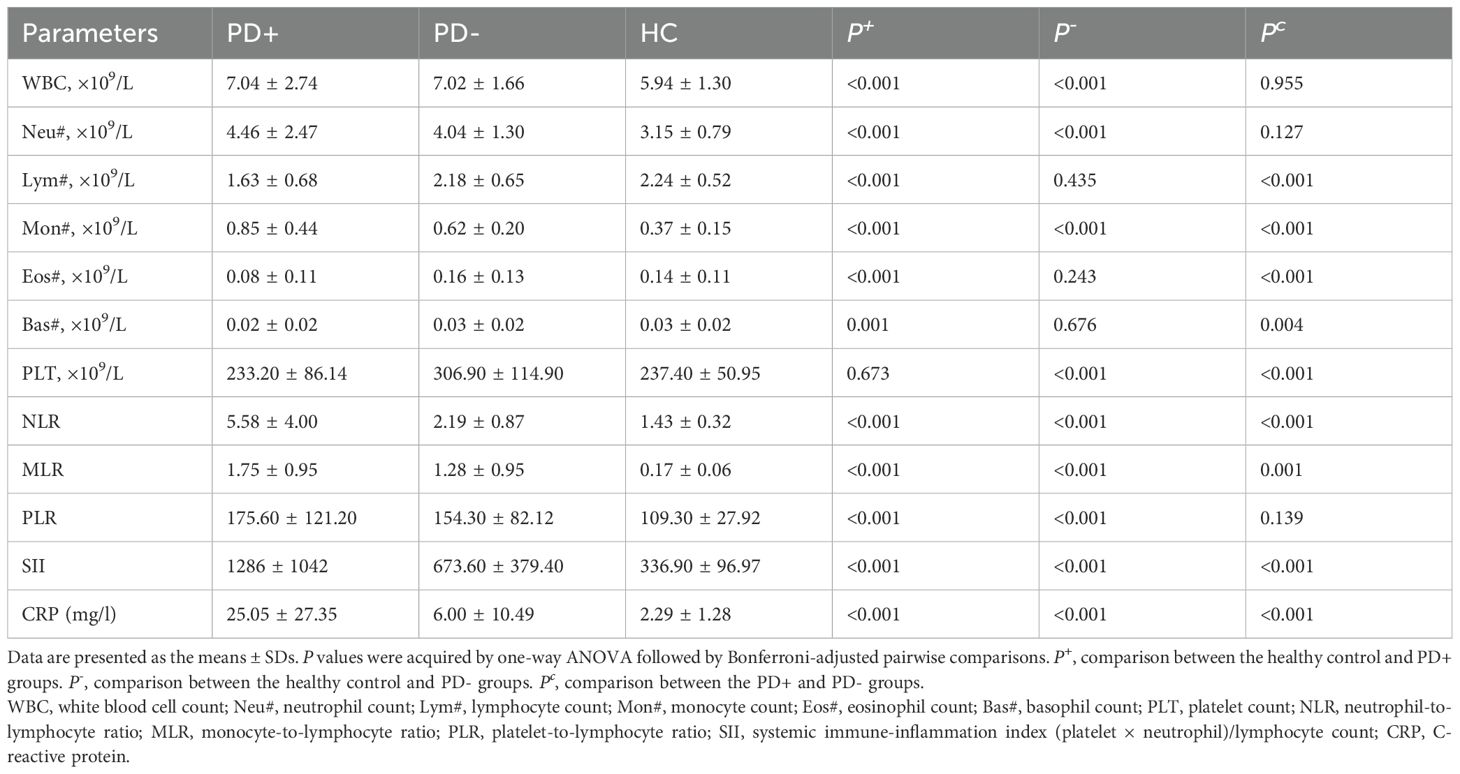
Table 2. Hematology findings and inflammatory markers in venous samples taken from the PD+, PD- and HC groups.
3.3 Serum biochemical parameters of the PD+, PD-, and HC groups
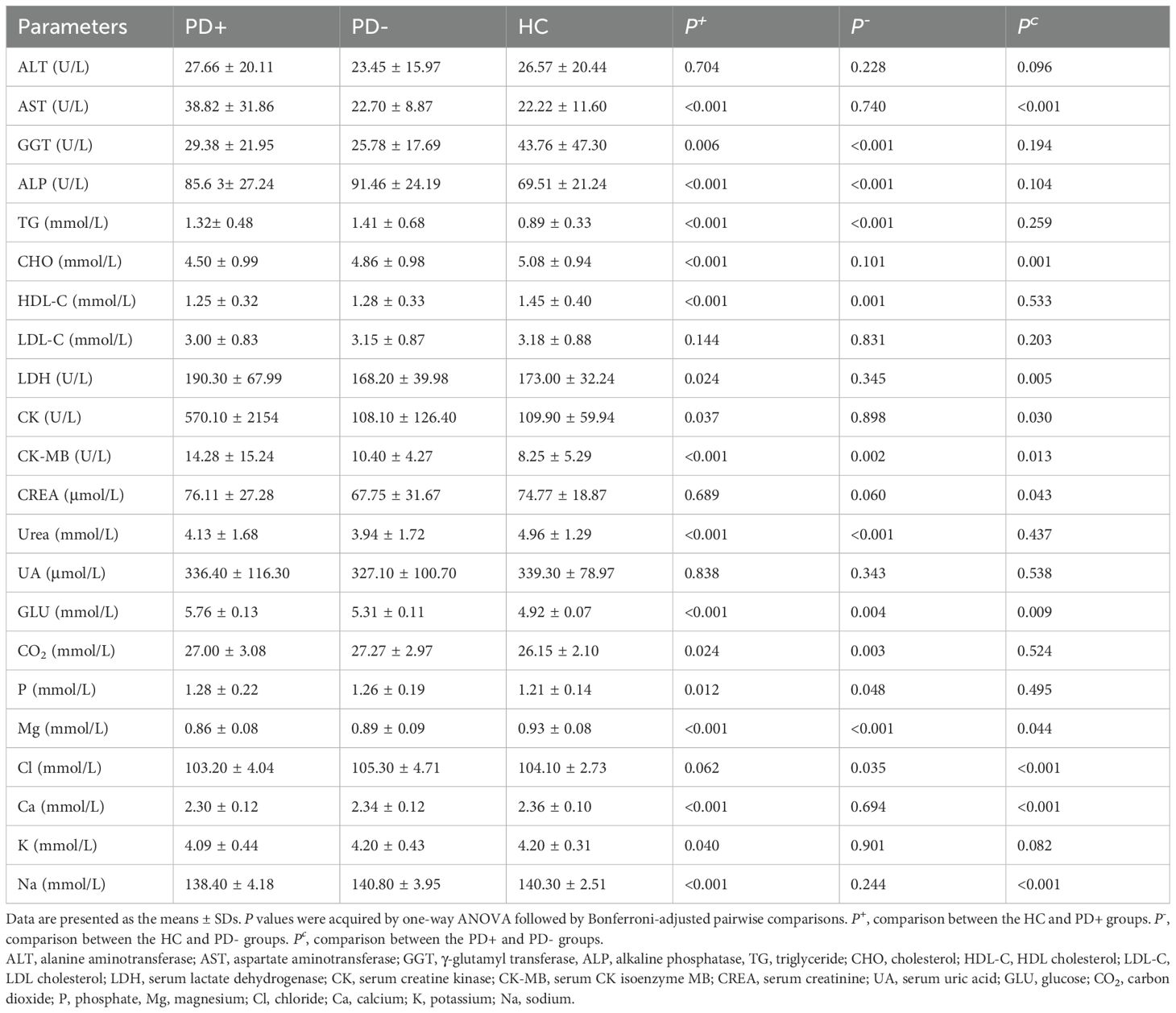
Table 3 shows the serum biochemical parameters of the three groups. The samples for biochemical testing were all collected in the morning while the participants were in a fasted state. Compared with those in the HC group, alkaline phosphatase (ALP), triglyceride (TG), serum creatine kinase (CK) isoenzyme MB (CK-MB), glucose (GLU), carbon dioxide (CO2), phosphate (P), and chloride (Cl) levels were greater in the PD+ group (P < 0.05); moreover, γ-glutamyl transferase (GGT), HDL-cholesterol (HDL-C), urea and magnesium (Mg) levels were lower in the PD+ group than in the HC group (P < 0.05). Compared with those in the HC group, the AST, ALP, TG, LDH, serum CK, CK-MB, GLU, CO2, and P levels in the PD+ group were greater (P < 0.05), but the GGT, cholesterol (CHO), HDL-C, urea, Mg, calcium (Ca), potassium (K) and sodium (Na) levels in the PD+ group were lower (P < 0.05). We found that the indicators ALT, LDL-cholesterol (LDL-C), serum creatinine (CREA), serum uric acid (UA), and Cl in the PD+ group and the indicators ALT, AST, CHO, LDL-C, LDH, CK, CREA, UA, Ca, K, and Na in the PD- group were mostly within the reference range. Furthermore, when the parameters of PD patients during and after COVID-19 were compared, the levels of AST, LDH, CK, CK-MB, CREA, and GLU in PD+ patients were significantly greater (P < 0.05), whereas the levels of CHO, Mg, Cl, Ca, and Na were lower (P < 0.05) than those in PD- patients.
3.4 Cytokine levels in the PD+, PD-, and HC groups
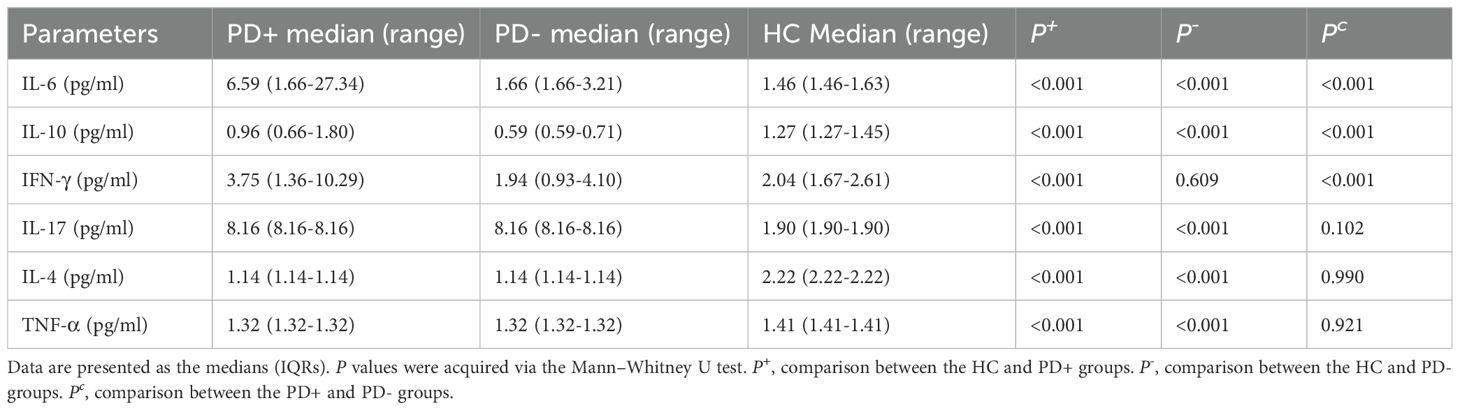
Table 4 shows the cytokine levels of the three groups. In the PD+ and PD- groups, the levels of IL-6, IL-17 and TNF-α were elevated compared with those in the HC group (p < 0.05), whereas the level of IL-4 was decreased (p < 0.05). IL-10 and interferon-gamma (IFN-γ) levels were elevated only in the PD+ group compared with those in the PD- and HC groups (p < 0.05). Additionally, Wilcoxon tests revealed significant increases in the levels of IL-6 and IFN-γ (p < 0.05) in the PD+ group compared with those in the PD- group; however, the levels of IL-17, IL-4, and TNF-α were not significantly different between the PD+ group and the PD- group (p > 0.05).
4 Discussion
This study is one of the first in which the hematological and biochemical markers and cytokine levels of hospitalized psychiatric patients with COVID-19 were evaluated. First, we detected elevated Mon# and inflammatory marker levels (NLR, MLR, SII and CRP) in these patients and PD- patients compared with HCs. Our findings are consistent with those of previous studies regarding the presence of elevated inflammatory markers in relation to COVID-19 and mental disorders (13, 22, 25, 28). Higher levels of inflammatory mediators are linked to the pathogenesis and progression of PDs, suggesting that abnormal NLRs, MLRs, and SIIs are not specific to any particular disorder but might indicate an underlying pathophysiological process leading to brain dysfunction (30, 34). These results support the theory that neuroinflammation plays a significant role in the development of PDs. Studies have suggested that patients with PDs who are experiencing acute illness may exhibit a heightened systemic response to SARS-CoV-2 infection (7, 31). However, the debate over whether inflammation triggers severe psychiatric conditions remains unresolved, and the precise mechanisms underlying such associations are yet to be fully understood. Research, including Mazza et al.’s study, revealed that the SII measured during hospitalization is positively correlated with subsequent depression and anxiety scores, indicating a potential link between systemic inflammation and the development of psychiatric symptoms (29). Wang G et al. reported an association between the risk of COVID-19 exacerbation and the levels of the inflammatory marker CRP (35), and we also demonstrated that CRP levels tended to be higher in PD+ patients.
Second, in our study, among the biochemical markers analyzed, only CK-MB and GLU levels were increased in both PD+ patients and PD- patients compared with HCs. An increase in GLU stimulates the proliferation of reactive astrocytes and promotes inflammation (36). Moreover, hyperglycemia can have profound effects on the central nervous system, thereby contributing to the pathogenesis of neuroinflammatory and neurodegenerative disorders (37, 38). In previous studies, CK-MB and GLU were found to be weak biochemical indicators of a good outcome of COVID-19 (39–42), and, consistent with our study, CK-MB and GLU levels were found to be higher in the PD+ group than in the HC group. This finding indicates the potential for viral myocarditis, cardiac injury resulting from progression to multiorgan failure (MOF), and secondary cardiac injury caused by organ-targeted pathologies (43). Among individuals with MOFs, cardiovascular diseases contribute to approximately 20% or more of life-years lost in patients with mental disorders (21). Emerging evidence indicates that common pathophysiological mechanisms exist between PDs and cardiovascular diseases, including biological, genetic, behavioral, and neurohormonal factors (44, 45). Severe inflammation and damage to multiple organs lead to lipid metabolism dysfunction and electrolyte imbalance (5, 28, 46–48). Dyslipidemia has frequently been reported in inpatients with severe mental disorders and COVID-19 (49, 50). TG, HDL-C, and LDL-C levels remained unchanged after COVID-19 in our study, which contrasts with the findings of other studies (51).
To our knowledge, we are the first to find that Mg levels are lower in both patients with COVID-19 and patients with mental disorders. Consistent with our findings, it has been reported that Mg levels decrease throughout the course of several mental disorders and COVID-19 (48, 52–54). Mg is an indispensable cation that is involved in many functions within the central nervous system, including signal transmission and intracellular signal transduction (48). Several studies have demonstrated the utility of Mg supplementation in neurological diseases and PDs (55, 56). Mg might be associated with the modulation of glutamatergic signals, which play a crucial role in neuroprotection, and Mg functions as an antagonist of NMDA receptors (48). On the basis of the available evidence, Mg supplementation might be beneficial in treating PD+ patients (52). A prompt diagnosis of electrolyte imbalance may minimize COVID-19-related morbidity and mortality.
As studies have shown that acute systemic infection, inflammatory states, and inflammation flares are accompanied by cytokine-mediated changes in plasma lipid and GLU levels (57, 58), we evaluated the cytokine levels of the three groups of participants. Additionally, according to the hypothesis of Raony et al., changes in cytokine levels can be regarded as a connection between immune system modifications and mental health disorders during COVID-19 (31). In the context of our study, significantly greater increases in IL-6 levels were observed in the PD+ and PD- groups than in the HC group. In line with previous studies, SARS-CoV-2-infected glial cells increased their secretion of IL-6 (49, 59). When the peripheral immune system is persistently activated, such as in chronic infections, autoimmune disorders, or PDs, the release of proinflammatory cytokines such as IL-6 might affect brain tissue related to neurological disorders, driving neuroinflammation and activating the neuroendocrine axis, thereby triggering depressive behaviors and cognitive impairments (32, 58). IL-6 may induce insulin resistance and increase the production of CRP in the liver by suppressing the expression of the insulin-sensitive glucose transporter in peripheral tissues (57). In line with this possibility, increased Mon# and protein levels associated with the immune response to bacterial infections have been identified in the blood of acutely ill unmedicated patients with mental disorders (32, 58). Several studies have demonstrated that high levels of IL-10 lead to poor outcomes in COVID-19 patients (59, 60), which is in line with the findings of our study. However, the role of IL-10 in psychosis is controversial. Some studies have shown no change in IL-10 in patients with SCZ or depression (58), whereas others have shown that IL-10 is elevated in patients with mental disorders (23, 58, 61). However, in our study, the level of IL-10 was decreased in PD patients, and this finding needs verification in subsequent multicenter studies with larger samples. Our study revealed that the level of IFN-γ was increased in PD patients only while they had COVID-19, suggesting that IFN-γ may be involved in the immune response after COVID-19 but not in mental illness. In addition, regardless of whether PD patients had COVID-19, the levels of TNF-α and IL-17 were greater in PD patients than in HCs, whereas the level of IL-4 was lower in PD patients than in HCs. These findings suggest that TNF-α, IL-17, and IL-4 might be related only to mental illness. However, TNF-α levels are increased in most COVID-19 patients and are correlated with the severity of the disease (59). This difference may be due to psychiatric drug therapy, which can reduce the levels of TNF-α.
The relationship between IL-17 and IL-4 in mental illness is controversial. Research has revealed that IL-17 levels are increased in individuals at ultrahigh risk of psychosis (62), whereas some studies have reported decreased IL-17 levels in BD patients (23). Consistent with the findings of Lorkiewicz et al., IL-4 levels were lower in MDD patients than in HCs (63). In addition, other studies have shown that the levels of IL-4 and IL-17 do not significantly change in patients with SCZ, BD, or MDD (58). These indicators, obtained through comprehensive collection and methodical screening, are valuable for differential diagnosis. This systematic screening method can identify significant indicators. The screened indicators suggest that PD+ patients have more severe inflammatory reactions, organ impairments, and disturbances in electrolytes and metabolism than PD- and non-COVID-19 patients do. Several mechanisms account for the neural effects of the inflammatory response. One hypothesis is that inflammatory response activation leads to increased production of proinflammatory cytokines and free radicals, potentially causing neuronal degeneration, white matter abnormalities, and decreased neurogenesis associated with the pathophysiology of SCZ (64). Cytokines can also directly modify the activity of enzymes involved in tryptophan catabolism (65). Although there are connections between immunological responses and the development of mental disorders, further exploration is needed to determine the underlying effects of immune mechanisms on brain neurochemical processes.
The limitations of our study are as follows: first, owing to the sudden onset of the COVID-19 outbreak and the fact that our hospital is a specialized psychiatric hospital, we were unable to collect data from non-PD patients with COVID-19. Second, although peripheral blood analysis provides valuable information, it is crucial to recognize the possible disparities between peripheral and central nervous system immune responses. In addition, our test results could have been affected by the fact that our samples were obtained from hospitalized PD patients on long-term antipsychotic drug therapy. Therefore, data from FEP or drug-naive patients could be collected to determine whether the findings were solely due to the use of antipsychotic drugs. Moreover, a larger sample is needed to confirm the results reported here.
5 Conclusion
The findings of our research indicated that both COVID-19 and psychosis can cause a cytokine storm, leading to the secretion of large amounts of IL-6. IL-6 can increase the levels of inflammatory biomarkers such as the NLR, MLR, SII, and CRP. Additionally, elevated IL-6 increases biochemical indicators such as CK-MB and GLU and decreases Mg, suggesting that both COVID-19 and psychosis affect multiple organ systems and may be related to cardiac injury, glycometabolic disorders, and electrolyte imbalance. In addition, our findings suggest that Mg supplementation might be considered a potential treatment approach for PD patients with COVID-19. A transdisciplinary approach is essential to ensure effective treatment with a focus on personalized medicine. Therefore, we suggest that both inflammatory and biochemical parameters, such as Neu#, Mon#, IL-6, NLR, MLR, SII, CRP, CK-MB, GLU, and Mg, should be monitored in PD+ patients throughout their hospitalization to improve outcomes. More research is needed to thoroughly comprehend the implications of these immune alterations throughout a longitudinal process to lay the foundation for potential progress in clinical practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Xiamen Xianyue Hospital Ethical Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
HD: Investigation, Writing – original draft. C-JC: Investigation, Writing – review & editing. ZL: Investigation, Methodology, Writing – review & editing. FL: Data curation, Resources, Writing – review & editing. QZ: Data curation, Methodology, Writing – review & editing. YB: Data curation, Methodology, Writing – review & editing. PY: Conceptualization, Funding acquisition, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Guiding Projects of Combinations of Engineering with Medicine of the Xiamen Science and Technology Program (Grant number 3502Z20244ZD2064) and the China Scholarship Council (Grant number 201809350002).
Acknowledgments
The authors are grateful to all the researchers for their kind collaboration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1536117/full#supplementary-material
References
1. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New Engl J Med. (2020) 382:1199–207. doi: 10.1056/NEJMoa2001316
2. World Health Organization. WHO COVID-19 dashboard. Available online at: https://data.who.int/dashboards/covid19/cases?n=c (Accessed July 28, 2024).
3. World Health Organization. WHO COVID-19 dashboard. Available online at: https://data.who.int/dashboards/covid19/deaths?n=c (Accessed July 28, 2024).
4. Barlati S, Nibbio G, Vita A. Schizophrenia during the COVID-19 pandemic. Curr Opin Psychiatry. (2021) 34:203–10. doi: 10.1097/YCO.0000000000000702
5. X. R, Wang Q, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. (2021) 20(1):124–30. doi: 10.1002/wps.20806
6. Octavius GS, Rahman A, Russell M, Zheng W, Eckrich D, Ahmed I. SARS-CoV-2 infection is associated with an increase in new diagnoses of schizophrenia spectrum and psychotic disorder: A study using the US national COVID cohort collaborative (N3C). PloS One. (2024) 19(5):e0295891. doi: 10.1371/journal.pone.0295891
7. S. J, Bednarova A, Sopkova D, Jarcuska P. Evaluation of neuropsychiatric complications in hospitalized COVID-19 patients. Psychiatr Danub. (2022) 34:752–7. doi: 10.24869/psyd.2022.752
8. S. S, Jansen van Vuren E, Brink CB, Möller M, Viljoen FP, Harvey BH. The neuropsychiatric manifestations of COVID-19: Interactions with psychiatric illness and pharmacological treatment. BioMed Pharmacother. (2021) 135:111200. doi: 10.1016/j.biopha.2020.111200
9. Tuna Ö, Ermis C, Enez Darcin A, Dagistan E, Salman S. Comparison of inflammation markers and severity of illness among patients with COVID-19, acute psychiatric disorders and comorbidity. Eur J Psychiatry. (2023) 37:125–32. doi: 10.1016/j.ejpsy.2022.01.008
10. W. R, Yin Y. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. (2018) 23:130–7. doi: 10.1111/resp.2018.23.issue-2
11. Dunleavy C, Elsworthy RJ, Upthegrove R, Wood SJ, Aldred S. Inflammation in first-episode psychosis: The contribution of inflammatory biomarkers to the emergence of negative symptoms, a systematic review and meta-analysis. Acta Psychiatrica Scand. (2022) 146:6–20. doi: 10.1111/acps.v146.1
12. Hughes HK, Yang H, Lesh TA, Carter CS, Ashwood P. Evidence of innate immune dysfunction in first-episode psychosis patients with accompanying mood disorder. J Neuroinflamm. (2022) 19(1):287. doi: 10.1186/s12974-022-02648-y
13. L. J, Yang AP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. (2020) 84:106504. doi: 10.1016/j.intimp.2020.106504
14. W.-B. B, Kosidło JW, Matowicka-Karna J, Dymicka-Piekarska V, Dorf J. Clinical significance and diagnostic utility of NLR, LMR, PLR and SII in the course of COVID-19: A literature review. J Inflammation Res. (2023) 16:539–62. doi: 10.2147/JIR.S395331
15. Wang C, Deng R, Gou L, Fu Z, Zhang X, Shao F, et al. Preliminary study to identify severe from moderate cases of COVID-19 using combined hematology parameters. Ann Trans Med. (2020) 8:593–3. doi: 10.21037/atm-20-3391
16. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med (CCLM). (2020) 58:1021–8. doi: 10.1515/cclm-2020-0369
17. Y. T, Zhou F, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
18. Y. N, Bulut NS, Çarkaxhiu Bulut G. The severity of inflammation in major neuropsychiatric disorders: comparison of neutrophil-lymphocyte and platelet-lymphocyte ratios between schizophrenia, bipolar mania, bipolar depression, major depressive disorder, and obsessive compulsive disorder. Nord J Psychiatry. (2021) 75:624–32. doi: 10.1080/08039488.2021.1919201
19. S. P, Bhikram T. Neutrophil-lymphocyte ratios as inflammatory biomarkers in psychiatric patients. Brain Behav Immun. (2022) 105:237–46. doi: 10.1016/j.bbi.2022.07.006
20. Zhu X, Zhou J, Zhu Y, Yan F, Han X, Tan Y, et al. Neutrophil/lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in schizophrenia. Australas Psychiatry. (2021) 30:95–9. doi: 10.1177/10398562211022753
21. Westman J, Hällgren J, Wahlbeck K, Erlinge D, Alfredsson L, Ösby U. Cardiovascular mortality in bipolar disorder: a population-based cohort study in Sweden. BMJ Open. (2013) 3(4):e002373. doi: 10.1136/bmjopen-2012-002373
22. Daray FM, Grendas LN, Arena ÁR, Tifner V, Álvarez Casiani RI, Olaviaga A, et al. Decoding the inflammatory signature of the major depressive episode: insights from peripheral immunophenotyping in active and remitted condition, a case–control study. Trans Psychiatry. (2024) 14(1):254. doi: 10.1038/s41398-024-02902-2
23. Wu X, Chen Z, Liao Y, Yang Z, Liang X, Guan N, et al. Are serum levels of inflammatory markers associated with the severity of symptoms of bipolar disorder? Front Psychiatry. (2023) 13. doi: 10.3389/fpsyt.2022.1063479
24. Dadouli K, Janho MB, Hatziefthimiou A, Voulgaridi I, Piaha K, Anagnostopoulos L, et al. Neutrophil-to-lymphocyte, monocyte-to-lymphocyte, platelet-to-lymphocyte ratio and systemic immune-inflammatory index in different states of bipolar disorder. Brain Sci. (2022) 12(8):1034. doi: 10.3390/brainsci12081034
25. Yağcı İ, İnaltekin A. Evaluation of simple markers of inflammation and systemic immune inflammation index in schizophrenia, bipolar disorder patients and healthy controls. Turkish J Psychiatry. (2021) 34(1):11–5. doi: 10.5080/u26248
26. Hu Y, Chen Y, Zheng Y, You C, Tan J, Hu L, et al. Factors related to mental health of inpatients with COVID-19 in Wuhan, China. Brain Behav Immun. (2020) 89:587–93. doi: 10.1016/j.bbi.2020.07.016
27. L.-B. V, Bioque M, Salmerón S, García-Bueno B, MacDowell KS, Moreno C, et al. Association between neutrophil to lymphocyte ratio and inflammatory biomarkers in patients with a first episode of psychosis. J Psychiatr Res. (2024) 172:334–9. doi: 10.1016/j.jpsychires.2024.02.044
28. A. B, Sanchez-Autet M, Sierra P, Safont G, Garcia-Blanco A, de la Fuente L, et al. Association between neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and C-reactive protein levels and metabolic status in patients with a bipolar disorder. World J Biol Psychiatry. (2022) 23(6):464–74. doi: 10.1080/15622975.2021.2013089
29. Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun. (2020) 89:594–600. doi: 10.1016/j.bbi.2020.07.037
30. Paniagua G, González-Blanco L, Sáiz PA, Moya-Lacasa C, Gutiérrez L, Martínez-Botía P, et al. Platelet and white blood-cell-based ratios: Differential inflammatory markers of severe mental disorders? Spanish J Psychiatry Ment Health. (2023) 12:S2950-2853(23)00008-X. doi: 10.1016/j.sjpmh.2023.03.002
31. d.F. C, Raony Í, Pandolfo P, Giestal-de-Araujo E, Oliveira-Silva Bomfim P, Savino W. Neuroendocrine-immune interactions in COVID-19: potential impacts on mental health. Psycho-Front Immunol. (2020) 11:1170. doi: 10.3389/fimmu.2020.01170
32. B M, Wang AK. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull. (2018) 44:75–83. doi: 10.1093/schbul/sbx035
33. D. F, Severance EG, Viscidi RP, Bossis I, Stallings CR, Origoni AE, et al. Coronavirus immunoreactivity in individuals with a recent onset of psychotic symptoms. Schizophr Bull. (2011) 37:101–7. doi: 10.1093/schbul/sbp052
34. Yang C, Tian Y, Yang X, Liu L, Ling C, Xia L, et al. Hematological and inflammatory markers in Han Chinese patients with drug-free schizophrenia: relationship with symptom severity. Front Immunol. (2024) 15. doi: 10.3389/fimmu.2024.1337103
35. W. C, Wang G, Zhang Q, Wu F, Yu B, Lv J, et al. C-reactive protein level may predict the risk of COVID-19 aggravation. Open Forum Infect Dis. (2020) 7:153. doi: 10.1093/ofid/ofaa153
36. Lee K-S, Yoon S-H, Hwang I, Ma J-H, Yang E, Kim RH, et al. Hyperglycemia enhances brain susceptibility to lipopolysaccharide-induced neuroinflammation via astrocyte reprogramming. J Neuroinflamm. (2024) 21(1):137. doi: 10.1186/s12974-024-03136-1
37. Ouwens DM, van Duinkerken E, Schoonenboom SN, Herzfeld de Wiza D, Klein M, van Golen L, et al. Cerebrospinal fluid levels of Alzheimer’s disease biomarkers in middle-aged patients with type 1 diabetes. Diabetologia. (2014) 57:2208–14. doi: 10.1007/s00125-014-3333-6
38. Komici K, Femminella GD, Bencivenga L, Rengo G, Pagano G. Diabetes mellitus and parkinson’s disease: A systematic review and meta-analyses. J Parkinsons Dis. (2021) 11:1585–96. doi: 10.3233/JPD-212725
39. W. A, Martha JW, Pranata R. Prognostic value of elevated lactate dehydrogenase in patients with COVID-19: a systematic review and meta-analysis. Postgrad Med J. (2022) 98:422–7. doi: 10.1136/postgradmedj-2020-139542
40. Z. L, Gao J, Wu M, Ji J, Liu Z, Wang C, et al. Risk factors for mortality in critically ill patients with COVID-19: a multicenter retrospective case-control study. BMC Infect Dis. (2021) 21:602. doi: 10.1186/s12879-021-06300-7
41. A. M, Ghaith MM, Aldairi AF, Iqbal MS, Almaimani RA, AlQuthami K, et al. Potential predictors of poor prognosis among severe COVID-19 patients: A single-center study. Can J Infect Dis Med Microbiol. (2021) 2021:6656092. doi: 10.1155/2021/6656092
42. Ma X, Wang H, Huang J, Geng Y, Jiang S, Zhou Q, et al. A nomogramic model based on clinical and laboratory parameters at admission for predicting the survival of COVID-19 patients. BMC Infect Dis. (2020) 20(1):899. doi: 10.1186/s12879-020-05614-2
43. Qi J, He D, Yang D, Wang M, Ma W, Cui H, et al. Severity-associated markers and assessment model for predicting the severity of COVID-19: a retrospective study in Hangzhou, China. BMC Infect Dis. (2021) 21(1):774. doi: 10.1186/s12879-021-06509-6
44. Goldfarb M, De Hert M, Detraux J, Di Palo K, Munir H, Music S, et al. Severe mental illness and cardiovascular disease. J Am Coll Cardiol. (2022) 80:918–33. doi: 10.1016/j.jacc.2022.06.017
45. De Hert M, Detraux J, Vancampfort D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin Neurosci. (2018) 20:31–40. doi: 10.31887/DCNS.2018.20.1/mdehert
46. Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—A systematic review and meta-analysis. Schizophr Bull. (2013) 39:306–18. doi: 10.1093/schbul/sbr148
47. W.-M. C, Häfner S, Schulz M, Noelle R, Wiegand HF, Seemüller F, et al. Diagnostik metabolischer Risikofaktoren bei stationär-psychiatrischen Patienten, Diagnosis of Metabolic Risk Factors in Psychiatric Inpatients. Psychiatr Prax. (2016) 43:312–7. doi: 10.1055/s-0034-1387623
48. Botturi A, Ciappolino V, Delvecchio G, Boscutti A, Viscardi B, Brambilla P. The role and the effect of magnesium in mental disorders: A systematic review. Nutrients. (2020) 12(6):1661. doi: 10.3390/nu12061661
49. Mohammadi K, Sleeman MW, Boyapati A, Bigdelou P, Geba GP, Fazio S. Effect of IL-6R blockade on plasma lipids and clinical outcomes among hospitalized patients with COVID-19 infection. J Lipid Res. (2024) 65(6):100568. doi: 10.1016/j.jlr.2024.100568
50. Wei X, Zeng W, Su J, Wan H, Yu X, Cao X, et al. Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol. (2020) 14:297–304. doi: 10.1016/j.jacl.2020.04.008
51. Agouridis AP, Pagkali A, Zintzaras E, Rizos EC, Ntzani EE. High-density lipoprotein cholesterol: A marker of COVID-19 infection severity? Atheroscl Plus. (2021) 44:1–9. doi: 10.1016/j.athplu.2021.08.007
52. Cancarevic I, Nassar M, Foster A, Umar Z, Parikh A, Ahammed MR, et al. Electrolyte disturbances in patients hospitalized for COVID-19 infection: An observational study. Medicine. (2024) 103(20):e37749. doi: 10.1097/MD.0000000000037749
53. Ordak M, Matras J, Muszynska E, Nasierowski T, Bujalska-Zadrozny M. Magnesium in schizophrenia. Pharmacol Rep. (2017) 69:929–34. doi: 10.1016/j.pharep.2017.03.022
54. Al-Hakeim HK, Al-Jassas HK, Morris G, Maes M. Increased ACE2, sRAGE, and Immune Activation, but Lowered Calcium and Magnesium in COVID-19. Recent Adv Inflammation Allergy Drug Discovery. (2022) 16:32–43. doi: 10.2174/2772270816666220318103929
55. Camardese G, De Risio L, Pizi G, Mattioli B, Buccelletti F, Serrani R, et al. Plasma magnesium levels and treatment outcome in depressed patients. Nutr Neurosci. (2012) 15:78–84. doi: 10.1179/1476830512Y.0000000002
56. Shohag H, Ullah A, Qusar S, Rahman M, Hasnat A. Alterations of serum zinc, copper, manganese, iron, calcium, and magnesium concentrations and the complexity of interelement relations in patients with obsessive-compulsive disorder. Biol Trace Elem Res. (2012) 148:275–80. doi: 10.1007/s12011-012-9371-3
57. Li X, Qiu W, Li N, Da X, Ma Q, Hou Y, et al. Susceptibility to hyperglycemia in rats with stress-induced depressive-like behavior: involvement of IL-6 mediated glucose homeostasis signaling. Front Psychiatry. (2020) 11. doi: 10.3389/fpsyt.2020.00557
58. Schmitt Junior AA, Primo de Carvalho Alves L, Padilha BL, da Rocha NS. Serum cytokine variations among inpatients with major depression, bipolar disorder, and schizophrenia versus healthy controls: a prospective ‘true-to-life’ study. Ther Adv Psychopharmacol. (2023) 13:20451253221135463. doi: 10.1177/20451253221135463
59. Islam F, Habib S, Badruddza K, Rahman M, Islam MR, Sultana S, et al. The association of cytokines IL-2, IL-6, TNF-α, IFN-γ, and IL-10 with the disease severity of COVID-19: A study from Bangladesh. Cureus. (2024) 16(4):e57610. doi: 10.7759/cureus.57610
60. Al-Gebori AM, Mohsen AM, Al-Hindawi MS, Al-Hashimi NH. Enoxaparin effect on interleukin-10 levels in Iraqi patients with COVID-19: A case–control study. Front Bioscience-Scholar. (2024) 16. doi: 10.31083/j.fbs1602009
61. Chenniappan R, Nandeesha H, Kattimani S, Nanjaiah ND. Interleukin-17 and interleukin-10 association with disease progression in schizophrenia. Ann Neurosci. (2020) 27:24–8. doi: 10.1177/0972753120929565
62. Ouyang L, Li D, Li Z, Ma X, Yuan L, Fan L, et al. IL-17 and TNF-β: Predictive biomarkers for transition to psychosis in ultra-high risk individuals. Front Psychiatry. (2022) 13. doi: 10.3389/fpsyt.2022.1072380
63. Bravi B, Melloni EMT, Paolini M, Palladini M, Calesella F, Servidio L, et al. Choroid plexus volume is increased in mood disorders and associates with circulating inflammatory cytokines. Brain Behav Immun. (2024) 116:52–61. doi: 10.1016/j.bbi.2023.11.036
64. Williams JA, Burgess S, Suckling J, Lalousis PA, Batool F, Griffiths SL, et al. Inflammation and brain structure in schizophrenia and other neuropsychiatric disorders: A mendelian randomization study. JAMA Psychiatry. (2022) 79:498–507. doi: 10.1001/jamapsychiatry.2022.0407
Keywords: COVID-19, psychiatric diseases, neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, systemic immune-inflammation index, C-reactive protein, magnesium, interleukin 6
Citation: Dai H, Chang C-J, Li Z, Liu F, Zhang Q, Bai Y and You P (2025) Hematological and biochemical markers and cytokine levels in hospitalized psychiatric patients with COVID-19. Front. Immunol. 16:1536117. doi: 10.3389/fimmu.2025.1536117
Received: 28 November 2024; Accepted: 25 March 2025;
Published: 11 April 2025.
Edited by:
Lucas Murrins Marques, Santa Casa de Sao Paulo School of Medical Sciences, BrazilReviewed by:
Luiz Antonio Vesco Gaiotto, Santa Casa de Sao Paulo School of Medical Sciences, BrazilGermán Alberto Nolasco-Rosales, Universidad Juárez Autónoma de Tabasco, Mexico
Copyright © 2025 Dai, Chang, Li, Liu, Zhang, Bai and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pan You, cGFueW91MDAxQHlhaG9vLmNvbQ==
†These authors have contributed equally to this work
 Huirong Dai
Huirong Dai Chih-Jung Chang
Chih-Jung Chang Zishun Li
Zishun Li Farong Liu
Farong Liu Qiao Zhang
Qiao Zhang Yixuan Bai
Yixuan Bai Pan You
Pan You