- 1Division of Abdominal Tumor Multimodality Treatment, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Radiation Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
There is currently no established standard treatment for patients with metastatic hepatocellular carcinoma after resistance to tyrosine kinase inhibitors. Given that radiotherapy can reprogram the tumor microenvironment and convert “cold” tumors into “hot” tumors, combining radiotherapy with immunotherapy shows significant potential. In this case, we present a male patient with HBV-related metastatic hepatocellular carcinoma (HCC) accompanied by portal vein tumor thrombosis. The patient achieved abscopal effect with a progression-free survival of 10 months and an overall survival of 21 months with the combination of anti-PD-1 therapy and stereotactic body radiotherapy (SBRT) as third-line treatment. This combination therapy demonstrates relative efficacy and favorability in treating HBV-related advanced HCC.
Introduction
Hepatocellular carcinoma (HCC) is the sixth leading cause of cancer morbidity and the third major cause of cancer-related death globally, with a death toll of 830,180 in 2020. While a fortunate few are deemed suitable for surgical intervention upon early diagnosis, the majority of cases are detected at advanced stages (1). Targeted therapy has emerged as a standard treatment for advanced HCC with extrahepatic metastases. However, the efficacy of current options remains limited, with sorafenib demonstrating a median time to progression of only 2.8 months (2), and median overall survival (OS) times for sorafenib and lenvatinib standing at 12.3 and 13.6 months, respectively (3). The suboptimal outcomes associated with these therapies are often attributed to the development of drug resistance (4).
Immunotherapy has been recognized as a viable choice for patients with advanced HCC who have failed sorafenib and lenvatinib. However, the response rate of immune checkpoint inhibitors (ICIs) in advanced HCC remains low, at less than 20% (5). Objective response rate of regorafenib combined with sintilimab reached 30% as a second-line treatment, which was superior to regorafenib monotherapy (6). Radiation therapy, particularly stereotactic body radiotherapy (SBRT), not only exerts direct cytotoxic effects on cancer cells but also stimulates a potent antitumor immune response by modulating the tumor microenvironment (7). Encouraging results have been reported by scholars regarding disease control with the combination therapy of radiotherapy (RT) and immunotherapy (8–10). Nevertheless, there is limited research on the abscopal effect of integrating RT and immunotherapy in HCC (11). Here, we present a case of advanced HCC with distal lymph node metastasis that experienced abscopal effect with a prolonged period of disease control and improved survival following treatment with SBRT and nivolumab as third-line therapy.
Case description
In June 2017, a 45-year-old male initially presented with a mass on the right side of neck. Subsequently, he underwent a biopsy of the enlarged lymph node, and the pathological examination confirmed metastatic carcinoma at a local hospital. On June 19, 2017, the patient was referred to our clinic for additional assessment. An abdominal computed tomography (CT) scan revealed an infiltrative mass in the upper right anterior and left inner regions of the liver, accompanied by multiple metastatic lymph nodes in the hepatogastric, hepatoduodenal, and aortocaval areas. The tumor marker alpha-fetoprotein (AFP) was 948.5 ng/ml (normal range ≤ 8 ng/ml), and the protein induced by the absence of vitamin K or antagonist-II (PIVKA-II) level was 387 mAU/ml (normal range ≤ 32.5 mAU/ml). Additionally, he had chronic hepatitis B virus (HBV) infection with a DNA level of 8.06E+04 IU/ml. he was clinically diagnosed with advanced hepatocellular carcinoma (stage C according to the Barcelona Clinic Liver Cancer criteria), with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 and a Child-Pugh class of A.
The patient underwent comprehensive therapy as first-line treatment, consisting of local transarterial chemoembolization to the liver lesion and oral sorafenib. Additionally, entecavir 0.5mg was orally prescribed for daily use. After two months, the AFP level dropped to 622.8 ng/ml, and a CT scan showed a stable disease (SD) according to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1). However, enlargement of the abdominal lymph nodes were detected by CT scan, and the AFP level was 926.8 ng/ml after four months, which was assessed as progressive disease (PD). Coincidentally, a phase III trial (REFLECT) comparing lenvatinib with sorafenib in first-line therapy demonstrated non-inferiority objective response rates and progression-free survival (PFS) (12). The RESORCE study, published in 2017, has demonstrated that regofenib can provide survival benefit in patients following progression of sorafenib. However, this drug was not chosen because it was not available in China at the time. Consequently, the patient switched to oral lenvatinib in October 2017. Unfortunately, subsequent CT scans after two months revealed progressive sagittal portal vein thrombosis (PVTT) and enlargement in the hepatogastric lymph nodes. Meanwhile, the serum AFP level increased to more than 1210 ng/ml. The patient was assessed as PD again. A summary of the treatment history is presented in Figure 1A.
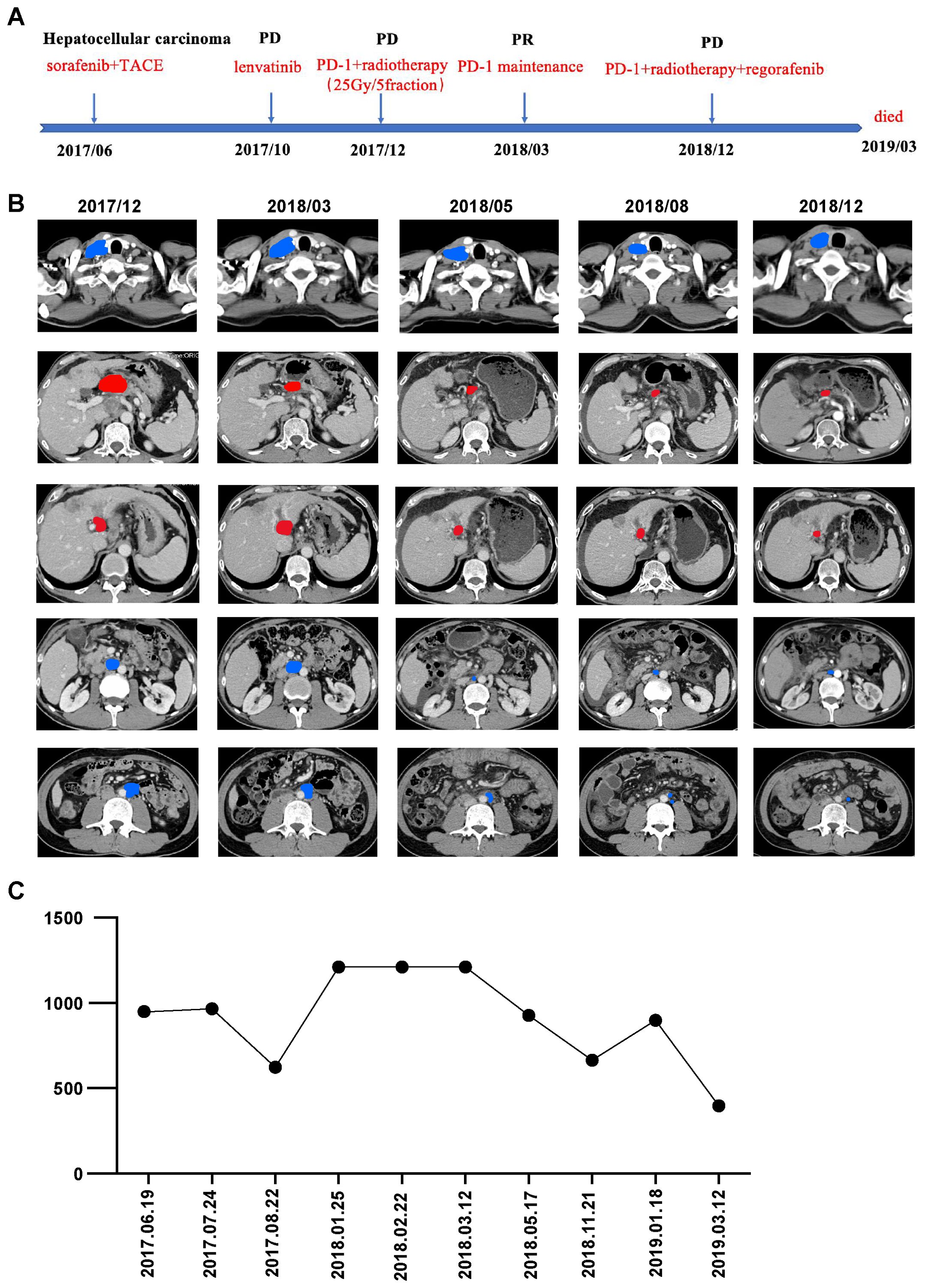
Figure 1. (A) Timeline of the whole treatment process for the patient. PD, progressive disease; PR, partial response; SBRT, stereotactic body radiotherapy; PVTT, progressive sagittal portal vein thrombosis. (B) CT scans before (2017-12) and after (2018-03,2018-05,2018-08,2018-12) the combination therapy of immunotherapy and SBRT. From top to bottom are right supraclavicular lymph node, hilar lymph node, right para-aortic lymph node, left para-aortic lymph node and PVTT lesions gradually shrunk significantly. Red, irradiated tumor sites; Blue, non-irradiated tumor sites. (C) Dynamics of alpha-fetoprotein(AFP)(IU/mL) levels during the entire disease course.
With informed consent, immunotherapy in combination with SBRT to PVTT and abdominal lymph nodes near the hilum of the liver was determined as third-line therapy. The patient began receiving nivolumab (240mg), an anti-PD-1 antibody, at 2-week intervals in December 2017. We administered 5Gy×5 fractions of radiotherapy, delivered every other day using volumetric modulated arc therapy, following two cycles of nivolumab. Three months after the nivolumab treatment, a follow-up CT scan revealed significant shrinkage of the PVTT and hilar lymph nodes within the irradiation field, along with a continuous decline in AFP levels (Figures 1B, C). Surprisingly, there was also notable shrinkage of metastases in the aortocaval areas and right supraclavicular lymph nodes outside of the irradiation field, resulting in a partial remission (PR).
As the regimen showed effectiveness, systemic therapy with nivolumab was continued for one year. The patient tolerated the combination treatment well, experiencing only slight adverse effects such as grade 1 anemia and grade 1 nausea, which were resolved with symptomatic medication. A follow-up CT scan showed metastasis in the anterior mediastinal lymph nodes and lymph nodes behind the vena cava. Subsequently, the patient received systemic therapy with nivolumab and regorafenib, in addition to palliative conventional fractionated radiotherapy for newly enlarged lymph nodes. However, due to complications arising from decompensated cirrhosis, the patient discontinued treatment and unfortunately passed away in March 2019. The OS period reached 21 months, and the timeline of significant clinical events is depicted in Figure 1.
Discussion
In the CheckMate 040 trial, 20% of patients who was treated with nivolumab as second-line treatment experienced an objective response (5). In the phase 2 trial, KEYNOTE-224 study, pembrolizumab obtained response rate of 17% (13). While ICIs have significantly improved efficacy for advanced hepatocellular carcinoma, the issue of drug resistance remains profoundly problematic (14). Response rates of only 15% for single-agent immunotherapy and 30% for two-agent immunotherapy fall far short of clinical needs (15).RT induces immunogenic cell death, remodel the immune microenvironment, overcome immune depletion, and sensitize tumors to immunotherapy (16–19). Specifically, RT stimulates the release of tumor antigens, promotes danger-associated molecular patterns (DAMPs), generates reactive oxygen species (ROS), and increases the expression of major histocompatibility complex I and FAS, leading to anti-tumor activity (20–22). However, radiation therapy also upregulates programmed cell death-Ligand 1, leading to radio resistance, which can be overcome by combining it with ICIs (23). Preclinical studies have shown that combining RT with immunotherapy results in a significant increase in intertumoral T-cell infiltration, tumor regression, and survival compared to RT or immunotherapy alone (24).
SBRT offers the advantage of precisely targeting the lesion while minimizing damage to surrounding healthy tissue, making it more immunogenic compared to conventional fractionated radiotherapy (25). For patients with advanced HCC, particularly those with portal vein tumor thrombosis and inferior vena cava tumor thrombosis, SBRT has demonstrated benefits (26, 27). In a reported case, five patients with unresectable hepatocellular carcinoma who received SBRT and anti-PD-1 antibody showed two complete responses (CR) and three PR (28). However, there is a lack of prospective trial data on the combination of SBRT and immunotherapy. In a phase II clinical trial, patients receiving combination therapy as second-line treatment exhibited a median PFS of 5.8 months and an OS of 14.2 months (29). Additionally, in a propensity score matching analysis comparing SBRT combined with immunotherapy (SBRT-IO) with transarterial chemoembolization (TACE) in patients with locally advanced HCC, the 12-month PFS and 12-month OS were significantly better in the SBRT-IO arm (93.3% vs. 16.7% and 93.8% vs. 31.3%, respectively; P < 0.001) (30).
The presence of underlying HBV infection may influence the selection and effectiveness of treatment strategies. Patients testing positive for HBV exhibited a limited response to sorafenib (31). A study conducted by Pfister D et al. revealed that ICIs therapy had a lower response rate in non-viral HCC compared to viral HCC (32). This finding was corroborated by the results of the IMbrave150 trial, which demonstrated a notable improvement in the prognosis of patients with viral HCC following a combination treatment of atezolizumab and bevacizumab (33). Based on these findings, ICIs therapy may be more suitable for individuals with HBV-associated HCC, while targeted therapy may be more effective for those with non-HBV-related HCC. In the phase 3 KEYNOTE-240 trial, median OS and PFS in patients with advanced HCC, previously treated with sorafenib, was greater for pembrolizumab than for the placebo(OS 13.9 months versus 10.6 months,P=0.0238; PFS 3.0 months versus 2.8 months,P=0.0186) (34). Another phase 3 KEYNOTE394 trial mainly involved Asian patients, with a significantly higher proportion of hepatitis patients than KEYNOTE-240 trial. Median OS were 14.6 months for pembrolizumab and 13 months for placebo, respectively(P=0.018). And median PFS were 2.6 months for pembrolizumab and 2.3 months for placebo (P=0.0032), respectively (35). Therefore, we hypothesize that HBV-associated advanced HCC may possess immunogenic characteristics, potentially enhancing its responsiveness to emerging immunotherapeutic strategies. Attention should be given to the management of HBV-related cirrhosis. In this study, disease progression was observed in patients who discontinued treatment during the later stages due to decompensated cirrhosis. These findings highlight the significance of long-term treatment tolerability and the potential relationship between treatment-related toxicity and hepatic dysfunction, underscoring the necessity for careful assessment and continuous monitoring of liver function and hepatic status in future clinical management.
It has been widely recognized that RT can trigger a systemic immune response, with the abscopal effect being one of the most notable examples. This effect occurs when radiation applied to one site leads to the regression of tumors at distant, non-irradiated sites (36). RT-induced reprogramming of the tumor microenvironment serves as a “game changer”, transforming “cold” tumors, with limited immune cells infiltration, into “hot” tumors, which are characterized by lymphocytic infiltration (37, 38). This transformation creates a favorable condition for the response to immune checkpoint inhibitors (39, 40). Regarding the rationale, the predominant role of combination RT and immunotherapy has been well established (41). We considered this case as an abscopal effect of RT; however, the possibility that it was an immunotherapy effect cannot be ruled out.
Several important questions remain, particularly concerning the optimal RT dose. The immune response induced by RT is dose-dependent, suggesting that the optimal dose should maximize tumor immunity while remaining tolerable for patients. Previous studies indicate that hypofractionated radiotherapy can enhance the coordination between RT and immunotherapy (42, 43). In our study, the dose of 5 Gy × 5 fractions appeared to result in effective disease control. However, there is still limited experience regarding the optimal SBRT dose and segmentation modalities. The study by Dewan et al. demonstrated that a dose of 24 Gy delivered in three fractions (8 Gy × 3 fractions) resulted in superior local tumor control and distant effects compared to two other regimens (20 Gy × 1 fraction or 6 Gy × 5 fractions) (44). Previous studies have indicated that the most common grade 3/4 treatment-related adverse events were elevated transaminases, colitis, or skin reactions, with no occurrences of grade 4 or 5 treatment-related toxicities (7, 28). The timing of ICIs in combination with RT remains a topic of debate. Some studies have provided evidence that administering ICIs and RT concurrently leads to the most significant improvement in OS (45). Moreover, a mathematical model suggests that the maximum response is observed when RT and ICIs are administered simultaneously, and the response diminishes notably with increased time intervals between RT and ICIs (46). Currently, there are several trials exploring the feasibility of SBRT and ICIs, two of which are specific to microvascular invasion (MVI) (NCT04167293, NCT04169399).
A limitation of this case report is the lack of analysis of immunological parameters, which could have strengthened the hypothesis that the observed effects are of immunological origin. Monitoring factors such as immune cells dynamics, as well as longitudinal changes in inflammatory cytokines and growth factors in the patient’s peripheral blood, would have provided valuable insights.
Conclusion
In this paper, we present a case of HBV-associated advanced HCC with lymph node metastases, which developed an abscopal effect after a combination of immunotherapy and radiotherapy. The factors responsible for inducing the abscopal effect remain unclear and warrant further exploration through additional clinical trials in the future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
PS: Data curation, Methodology, Writing – original draft, Writing – review & editing. FW: Data curation, Formal analysis, Methodology, Writing – original draft. XW: Conceptualization, Data curation, Funding acquisition, Project administration, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82073338), Sichuan Science and Technology Support Project (2021YFSY0039), the 1•3•5 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (2020HXFH002), the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21059).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/S0140-6736(22)01200-4
2. Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. (2009) 10:25–34. doi: 10.1016/S1470-2045(08)70285-7
3. Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
4. Chen J, Jin RN, Zhao J, Liu JH, Ying HN, Yan H, et al. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Letters. (2015) 367:1–11. doi: 10.1016/j.canlet.2015.06.019
5. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. (2017) 389:2492–502. doi: 10.1016/S0140-6736(17)31046-2
6. Huang JJ, Guo YJ, Huang WS, Hong XT, Quan Y, Lin LT, et al. Regorafenib combined with PD-1 blockade immunotherapy versus regorafenib as second-line treatment for advanced hepatocellular carcinoma: A multicenter retrospective study. J Hepatocellular Carcinoma. (2022) 9:157–70. doi: 10.2147/JHC.S353956
7. Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res. (2017) 23:1388–96. doi: 10.1158/1078-0432.CCR-16-1432
8. Grassberger C, King G, Apisarnthanarax S. Combining immunotherapy and radiotherapy in hepatocellular carcinoma: the importance of irradiated tumor burden and the possible role of a low dose radiotherapy induction strategy. Trans Cancer Res. (2023) 12:701–4. doi: 10.21037/tcr-23-192
9. Mowery YM, Patel K, Chowdhary M, Rushing CN, Choudhury KR, Lowe JR, et al. Retrospective analysis of safety and efficacy of anti-PD-1 therapy and radiation therapy in advanced melanoma: A bi-institutional study. Radiother Oncol. (2019) 138:114–20. doi: 10.1016/j.radonc.2019.06.013
10. Postow MA, Knox SJ, Goldman DA, Elhanati Y, Mavinkurve V, Wong P, et al. A prospective, phase 1 trial of nivolumab, ipilimumab, and radiotherapy in patients with advanced melanoma. Clin Cancer Res. (2020) 26:3193–201. doi: 10.1158/1078-0432.CCR-19-3936
11. Yano R, Hirooka M, Morita M, Okazaki Y, Nakamura Y, Imai Y, et al. Hepatocellular carcinoma showing tumor shrinkage due to an abscopal effect. Internal Med. (2024) 63:241–6. doi: 10.2169/internalmedicine.1844-23
12. Vogel A, Qin S, Kudo M, Su Y, Hudgens S, Yamashita T, et al. Lenvatinib versus sorafenib for first-line treatment of unresectable hepatocellular carcinoma: patient-reported outcomes from a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. (2021) 6:649–58. doi: 10.1016/S2468-1253(21)00110-2
13. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. (2018) 19:940–52. doi: 10.1016/S1470-2045(18)30351-6
14. Parikh ND, Pillai A. Recent advances in hepatocellular carcinoma treatment. Clin Gastroenterol Hepatol. (2021) 19:2020–4. doi: 10.1016/j.cgh.2021.05.045
15. Pinter M, Jain RK, Duda DG. The current landscape of immune checkpoint blockade in hepatocellular carcinoma: A review. JAMA Oncol. (2021) 7:113–23. doi: 10.1001/jamaoncol.2020.3381
16. Boone BA, Lotze MT. Targeting damage-associated molecular pattern molecules (DAMPs) and DAMP receptors in melanoma. Methods Mol Biol (Clifton NJ). (2014) 1102:537–52.
17. Hönscheid P, Datta K, Muders MH. Autophagy: Detection, regulation and its role in cancer and therapy response. Int J Radiat Biol. (2014) 90:628–35. doi: 10.3109/09553002.2014.907932
18. Liu Y, Dong YP, Kong L, Shi F, Zhu H, Yu JM. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. (2018) 11. doi: 10.1186/s13045-018-0647-8
19. Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. (2018) 18:313–22. doi: 10.1038/nrc.2018.6
20. Chen LJ, Zhang RG, Lin ZY, Tan QY, Huang ZY, Liang BY. Radiation therapy in the era of immune treatment for hepatocellular carcinoma. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1100079
21. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. (2005) 174:7516–23. doi: 10.4049/jimmunol.174.12.7516
22. Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev. (2004) 23:311–22. doi: 10.1023/B:CANC.0000031769.14728.bc
23. Kreidieh M, Zeidan YH, Shamseddine A. The combination of stereotactic body radiation therapy and immunotherapy in primary liver tumors. J Oncol. (2019) 2019. doi: 10.1155/2019/4304817
24. Yu JL, Green MD, Li SS, Sun YL, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. (2021) 27:152. doi: 10.1038/s41591-020-1131-x
25. Brooks ED, Schoenhals JE, Tang C, Micevic G, Gomez DR, Chang JY, et al. Stereotactic ablative radiation therapy combined with immunotherapy for solid tumors. Cancer J. (2016) 22:257–66. doi: 10.1097/PPO.0000000000000210
26. Xi M, Zhang L, Zhao L, Li QQ, Guo SP, Feng ZZ, et al. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PloS One. (2013) 8. doi: 10.1371/journal.pone.0063864
27. Choi HS, Kang KM, Jeong BK, Jeong H, Lee YH, Ha IB, et al. Effectiveness of stereotactic body radiotherapy for portal vein tumor thrombosis in patients with hepatocellular carcinoma and underlying chronic liver disease. Asia-Pacific J Clin Oncol. (2021) 17:209–15. doi: 10.1111/ajco.13361
28. Chiang CL, Chan ACY, Chiu KWH, Kong FM. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: A potential synergistic treatment strategy. Front Oncol. (2019) 9. doi: 10.3389/fonc.2019.01157
29. Li JX, Su TS, Gong WF, Zhong JH, Yan LY, Zhang J, et al. Combining stereotactic body radiotherapy with camrelizumab for unresectable hepatocellular carcinoma: a single-arm trial. Hepatol Int. (2022) 16:1179–87. doi: 10.1007/s12072-022-10396-7
30. Chiang C-L, Chiu KW-H, Lee FA-S, Kong F-MS, Chan AC-Y. Combined stereotactic body radiotherapy and immunotherapy versus transarterial chemoembolization in locally advanced hepatocellular carcinoma: A propensity score matching analysis. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.798832
31. Yang Y, Wen F, Li JL, Zhang PF, Yan WH, Hao P, et al. A high baseline HBV load and antiviral therapy affect the survival of patients with advanced HBV-related HCC treated with sorafenib. Liver Int. (2015) 35:2147–54. doi: 10.1111/liv.2015.35.issue-9
32. Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. (2021) 592:450–6. doi: 10.1038/s41586-021-03362-0
33. Cheng AL, Qin SK, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. (2022) 76:862–73. doi: 10.1016/j.jhep.2021.11.030
34. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol. (2020) 38:193. doi: 10.1200/JCO.19.01307
35. Qin SK, Chen ZD, Fang WJ, Ren ZG, Xu RC, Ryoo BY, et al. Pembrolizumab versus placebo as second-line therapy in patients from Asia with advanced hepatocellular carcinoma: A randomized, double-blind, phase III trial. J Clin Oncol. (2023) 41:1434. doi: 10.1200/JCO.22.00620
36. Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. (2015) 41:503–10. doi: 10.1016/j.ctrv.2015.03.011
37. Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. (2015) 15:409–25. doi: 10.1038/nrc3958
38. McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. (2020) 20:203–17. doi: 10.1038/s41568-020-0246-1
39. Demaria S, Coleman CN, Formenti SC. Radiotherapy: changing the game in immunotherapy. Trends Cancer. (2016) 2:286–94. doi: 10.1016/j.trecan.2016.05.002
40. Jarosz-Biej M, Smolarczyk R, Cichoń T, Kułach N. Tumor microenvironment as A “Game changer” in cancer radiotherapy. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20133212
41. Park JH, Kim HY, Lee A, Seo YK, Kim IH, Park ET, et al. Enlightening the immune mechanism of the abscopal effect in a murine HCC model and overcoming the late resistance with anti-PD-L1. Int J Radiat Oncol Biol Physics. (2021) 110:510–20. doi: 10.1016/j.ijrobp.2020.12.031
42. Marconi R, Strolin S, Bossi G, Strigari L. A meta-analysis of the abscopal effect in preclinical models: Is the biologically effective dose a relevant physical trigger? PloS One. (2017) 12:e0171559. doi: 10.1371/journal.pone.0171559
43. Zhang Z, Liu X, Chen D, Yu J. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduct Target Ther. (2022) 7:258. doi: 10.1038/s41392-022-01102-y
44. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but Not Single-Dose Radiotherapy Induces an Immune-Mediated Abscopal Effect when Combined with Anti-CTLA-4 Antibody. Clin Cancer Res. (2009) 15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265
45. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. (2014) 74:5458–68. doi: 10.1158/0008-5472.CAN-14-1258
Keywords: hepatocellular carcinoma, immunotherapy, stereotactic body radiotherapy, abscopal effect, HBV
Citation: Shu P, Wang F and Wang X (2025) Case Report: Abscopal effect in a patient with refractory metastatic hepatocellular carcinoma treated with stereotactic body radiotherapy and PD-1 inhibitor. Front. Immunol. 16:1536689. doi: 10.3389/fimmu.2025.1536689
Received: 29 November 2024; Accepted: 08 April 2025;
Published: 02 May 2025.
Edited by:
Masahiko Ito, Hamamatsu University School of Medicine, JapanReviewed by:
Inigo Martinez, UiT The Arctic University of Norway, NorwayYan Ma, Second Affiliated Hospital of Jilin University, China
Copyright © 2025 Shu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Wang, d2FuZ3hpbjIxM0BzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Pei Shu
Pei Shu Fang Wang
Fang Wang Xin Wang1,2*
Xin Wang1,2*