- 1Department of Critical Care Medicine, Tianjin Medical University General Hospital, Tianjin, China
- 2Department of General Surgery, Tianjin Medical University General Hospital, Tianjin, China
- 3Advertising Center, Tianjin Daily, Tianjin, China
- 4Department of Neurosurgery, Tianjin Medical University General Hospital Airport Hospital, Tianjin, China
Background: Despite early goal-directed therapy, sepsis mortality remains high. Statins exhibit pleiotropic effects, including anti-inflammatory and antimicrobial properties, which may be beneficial during sepsis.
Objective: To determine whether statins could improve the clinical outcomes in patients with sepsis.
Methods: We conducted a retrospective cohort study using data from the Medical Information Mart in Intensive Care-IV (MIMIC-IV) database. Adult patients with sepsis were included in the analysis. The exposure factor of this study was statin use during the Intensive Care Unit (ICU) stay. The primary outcome was 28-day all-cause mortality. The secondary outcomes were ICU and in-hospital mortality, length of ICU stay and hospital stay, duration of mechanical ventilation (MV) and continuous renal replacement therapy (CRRT). Both propensity score matching (PSM) and stepwise regression analyses were employed to adjust for potential confounders.
Results: The unmatched cohort comprised 20230 eligible patients, with 8972 patients in the statin group and 11258 in the no statin group. Propensity score matching generated balanced cohorts with 6070 patients in each group. Post-PSM analysis revealed significantly lower 28-day all-cause mortality in the statin group (14.3% [870/6070]) compared to the no statin group (23.4% [1421/6070]). Statin use was associated with decreased 28-day all-cause mortality (hazard ratio [HR], 0.56; 95% confidence interval [CI], 0.52-0.61; p < 0.001). In subgroup analysis, this beneficial effect was consistent across the different baseline characteristics of patients. Additionally, statin use was associated with decreased ICU mortality (odds ratio [OR], 0.43; 95% CI, 0.37-0.49; p < 0.001) and reduced in-hospital mortality (OR, 0.50; 95% CI, 0.45-0.57; p < 0.001). Sensitivity analysis using the unmatched cohort also showed a significant difference in 28-day all-cause mortality between the statin group and the no statin group (HR, 0.56; 95% CI, 0.52-0.61; p < 0.001).
Conclusion: Statins were associated with decreased mortality in critically ill patients with sepsis. Further high-quality prospective studies are still needed to verify our findings.
Background
Sepsis was defined as a life-threatening organ dysfunction caused by a deregulated inflammatory response to infection in the Third International Consensus Definitions released in 2016 (1). Treatment strategies for sepsis include early clinical recognition, adequate fluid resuscitation, prompt infectious source control, appropriate antibiotic therapy, and vasoactive medications as needed (2). Despite the implementation of early goal-directed therapy, the mortality rate of sepsis patients remains alarmingly high, with nearly 28% nationally (3). Sepsis is a major cause of hospitalization and mortality in the US (4, 5). To date, there is a lack of innovative adjunctive therapies to improve survival in sepsis patients (6). The complex pathophysiology of sepsis involves dysregulation of the inflammatory response, leading to an imbalance of pro- and anti-inflammatory mediators, enhanced leukocyte adhesion, inappropriate vasodilation, and impaired endothelial barrier function (7, 8). Thus, therapies aimed at modulating inflammation may hold great promise for improving clinical outcomes in sepsis (9).
Three-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, commonly known as statins, have become one of the most widely prescribed anti-hypercholesterolemic agents (10), and play an important role in lowering morbidity and mortality associated with cardio-cerebrovascular diseases (11, 12). Besides their lipid-lowering benefits in coronary artery disease, statins exhibit lipid-independent pleiotropic effects on pro-inflammatory/anti-inflammatory cytokines, inducible nitric oxide synthase, leukocyte adhesion and rolling (13). These properties have sparked great interest in using statins as an adjunctive therapy for a variety of inflammatory disorders, including autoimmune diseases, multiple sclerosis, chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome (ARDS) and sepsis (14). In addition, studies have shown that statins have antibacterial effects, providing an additional benefit for patients with sepsis (15). Several previous studies, including real-world observational studies and meta-analyses, have demonstrated an association between statin use and improved clinical outcomes in patients with sepsis or other life-threatening inflammatory conditions (16–18). Conversely, conflicting results from other studies have shown that statin use does not always result in better health outcomes (19, 20). Therefore, it is notable that the evidence on the association between statin use and the risk of mortality and other clinical outcomes from sepsis remains inconclusive (19), partly due to their pilot design and relatively small sample sizes. While randomized controlled trials (RCTs) are considered the gold standard for generating evidence, they are difficult or impractical to conduct for their high cost, resource-intensive, time-consuming, and sometimes ethical limitations (21). Therefore, employing an advanced analytical method to mitigate the impact of measurable confounders and biases inherent in observational studies is highly recommended. The closest approximation to such a scenario is to stratify sepsis patients base on statin use during their intensive care unit (ICU) stay and to match cases to controls by propensity score on key clinical characteristics. Thus, we conducted a retrospective propensity score matched cohort study using MIMIC-IV, a large real-world database, to investigate the effect of statins on clinical outcomes in patients with sepsis.
Materials and methods
Data sources
We conducted a retrospective propensity score matched cohort study using the Medical Information Mart for Intensive Care-IV (MIMIC-IV), a large, freely available, de-identified, comprehensive database that includes patients admitted to the BIDMC ICUs from 2008 to 2019 (22). The database contains non-identifiable bedside health data, including demographics, vital signs, laboratory data, prescriptions, fluid balance, caregivers notes, procedural and diagnostic codes (22). A member of our team (LCF) passed the Examination of Protection of Human Research Participants and was granted access to the database (record ID: 33047414). This study was conducted and reported following the STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) statement (23).
Study population
All consecutive patients were considered for inclusion. The inclusion criteria were as follows: (1) Diagnosed with sepsis (Sepsis-3)(1) upon hospital admission. (2) Aged 18 years or older. (3) For patients with multiple sepsis episodes and ICU stay records, only the first sepsis episode was evaluated. Patients with an ICU stay of less than 24 hours were excluded from the study.
Medication exposure and clinical outcomes
Medication prescriptions were identified from the prescription drug file based on both generic and brand names. The medication exposure was defined simply as any statin use or no statin use during ICU stay, regardless of the statin type. The primary outcome was 28-day all-cause mortality. The secondary outcomes were ICU mortality, in-hospital mortality, length of ICU stay, length of hospital stay, duration of mechanical ventilation (MV) and continuous renal replacement therapy (CRRT). To assess the impact of statin use on clinical outcomes, eligible patients were allocated into either the statin group or the no statin group based on whether they received statins or not during their ICU stay.
Data extraction and selection
All data were extracted from the MIMIC-IV database using Structured Query Language (SQL). The SQL script codes for data extraction were available on GitHub (https://github.com/MIT-LCP/mimic-iv). The following data were collected: demographics, including age, gender, race and body mass index (BMI); vital signs, including temperature, heart rate, respiratory rate (RR) and mean blood pressure (MBP); comorbidities, including cerebrovascular disease, congestive heart failure, chronic pulmonary disease, renal disease, severe liver disease, cancer and diabetes; severity scores, including acute physiology score III (APS III), charlson comorbidity index (CCI), glasgow coma scale (GCS), logistic organ dysfunction system (LODS), oxford acute severity of illness score (OASIS) and sequential organ failure assessment (SOFA); laboratory tests, including serum vitamin D, hemoglobin, white blood cells (WBC), platelets, creatinine, blood urea nitrogen (BUN), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, glucose, potential of hydrogen (pH), partial pressure of oxygen (pO2), partial pressure of carbon dioxide (pCO2), partial pressure of arterial oxygen to fraction of inspired oxygen ratio (PaO2/FiO2 ratio), base excess, lactate, sodium, potassium, calcium, chloride, anion gap and international normalized ratio (INR); clinical measures, including first-day vasopressor, antibiotic lag, duration of MV and duration of CRRT. Comorbidities were assessed upon admission. Initial vital signs and clinical indices acquired within 24 hours of ICU admission were used as baseline characteristics. Variables with missing values of more than 50% were excluded from the analysis, while those with less than 50% were included. The missing rate for each variable is presented in the Supplementary Material: Supplementary Table S1, Supplementary Figure S1. Missing values of the included variables were imputed using the missForest method to decrease bias and avoid participant exclusion (24).
Statistical analysis
No sample size calculation was performed as this was a retrospective exploratory study (25), and the sample size was determined by the number of patients available in the MIMIC-IV database (over 364,627 patients). Continuous variables were presented as mean (standard deviation [SD]) or median (interquartile range [IQR]) and were analyzed using either the Student’s t-test or the Mann-Whitney U-test depending on their distribution (26). Categorical variables were expressed as numbers (percentages) and compared using either the chi-square test or Fisher’s exact test (27). For the primary outcome, the Cox proportional hazards model was employed to estimate the hazard ratio (HR) and 95% confidence interval (CI). The Kaplan-Meier method and the log-rank test were used to calculate and compare the cumulative incidence of 28-day all-cause mortality. For dichotomous secondary outcomes, the logistic regression model was applied to compute the odds ratio (OR) and 95% CI. The Hodgese-Lehmann method was utilized to determine the median difference (MD) and 95% CI for continuous secondary outcomes. Multicollinearity between variables was assessed using the variance inflation factor (VIF), with VIF values of less than 5 indicating no multicollinearity (Supplementary Material: Supplementary Table S2, Supplementary Figure S2; Supplementary Table S3, Supplementary Figure S3). A two-tailed p < 0.05 was considered statistically significant for all analyses. All statistical analyses were performed using R software (version 4.2.3; R Foundation for Statistical Computing, Vienna, Austria).
Propensity score matching
To address potential confounding factors and selection bias inherent in observational studies, we performed PSM following the methodological guidelines proposed by Lonjon and colleagues (28). According to a consensus statement (29), the following variables were included in the propensity score model for matching: age, gender, congestive heart failure, cerebrovascular disease, diabetes, malignant cancer, severe liver disease, APS III, CCI, heart rate, first care unit, ALT, total bilirubin, base excess, calcium, anion gap and INR. The propensity score, which represents the predicted probability of receiving statins, was calculated using baseline covariates in a logistic regression model. Patients were matched using the 1:1 nearest neighbor method without replacement and with a caliper width of 0.05. After PSM, a matched cohort of patients with comparable baseline characteristics was assembled. The covariate balance between groups was evaluated using standardized mean differences (SMD) before and after matching, with a SMD < 0.1 indicating negligible differences (30). Furthermore, Stepwise Cox regression analyses were employed to build adjusted models while adequately considering possible confounders in the matched cohort. Variables with a p-value < 0.1 in the univariate analysis were selected for further stepwise multivariate analysis. The independent variables included in the final model were age, gender, race, BMI, APS-III, CCI, LODS, OASIS, SOFA, GCS, respiratory rate, temperature, hemoglobin, WBC, creatinine, ALT, total bilirubin, pH, pCO2, lactate, calcium, potassium, anion gap, INR, antibiotic lag, first-day vasopressor and statin use (Supplementary Material: Supplementary Table S4).
Subgroup analyses
To evaluate the impact of different variables on 28-day all-cause mortality in patients with sepsis, we conducted subgroup analyses in the matched cohort based on the following variables: age (>60 versus <=60 years), gender (female versus male), race (white, black, unknown, other), BMI (obesity, overweight, normal, underweight) and CCI (<6 versus >=6).
Sensitivity analysis
To validate the robustness of the findings in the matched cohort, sensitivity analyses were conducted in the unmatched cohort. Stepwise Cox regression analyses were employed to identify independent prognostic factors and adjust for potential confounders. Variables with a p-value < 0.1 in the univariable analysis were entered into the multivariable analysis by stepwise selection. The independent variables incorporated into the final model were: age, gender, race, BMI, APS-III, CCI, LODS, OASIS, SOFA, GCS, respiratory rate, temperature, hemoglobin, WBC, creatinine, ALT, total bilirubin, pH, lactate, Sodium, potassium, chloride, anion gap, INR, antibiotic lag, first-day vasopressor and statin use (Supplementary Material: Supplementary Table S5).
Result
Patient selection
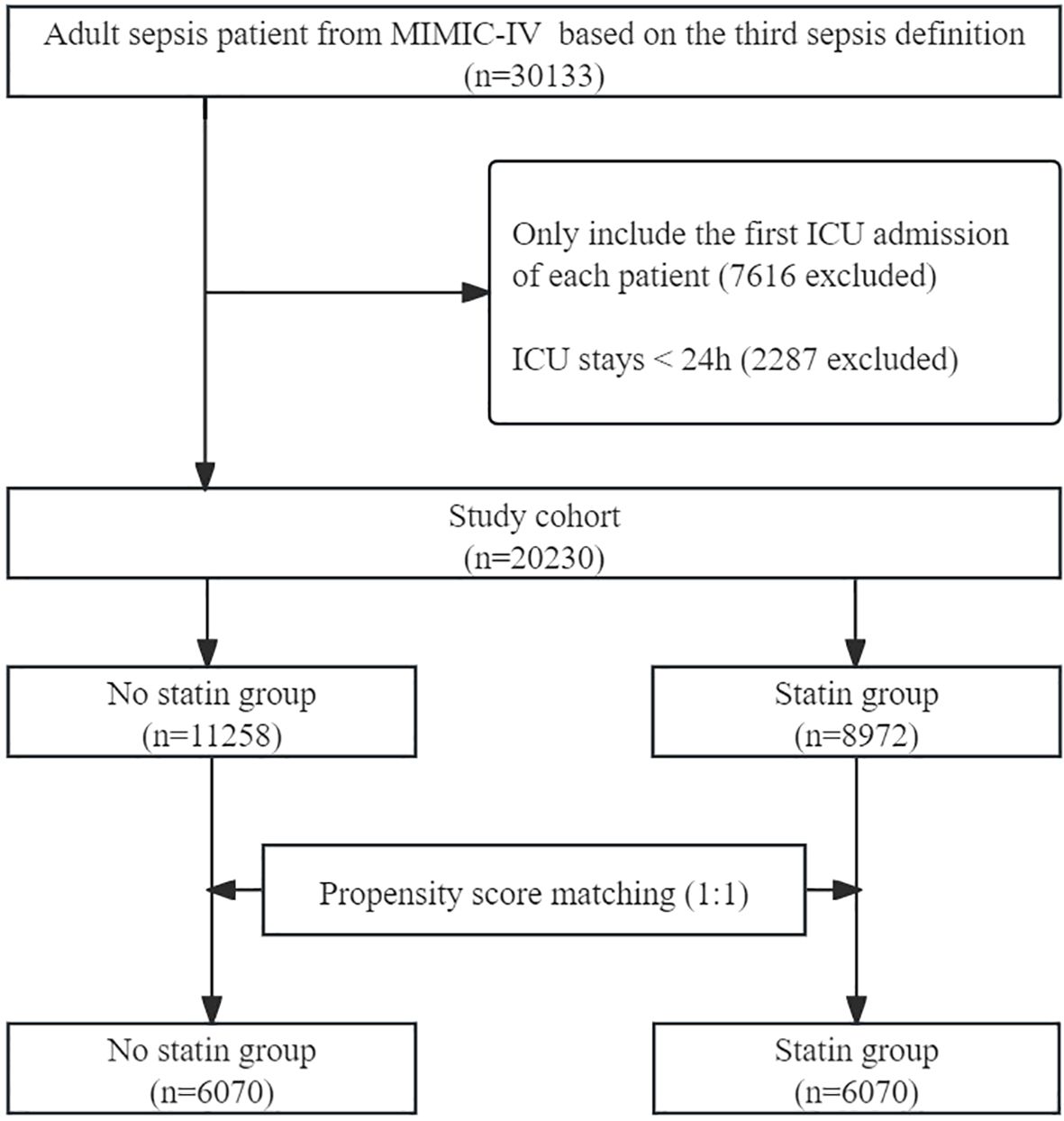
A total of 30133 adult patients with sepsis were identified from the database. After excluding ineligible records, 20230 patients were included in the unmatched cohort, with 8972 (44.34%) in statin group and 11258 (55.66%) in no statin group. After PSM, 12140 patients were included in the matched cohort, with 6070 in the statin group and 6070 in the no statin group. The process of patient selection is illustrated in Figure 1.
Cohort characteristics
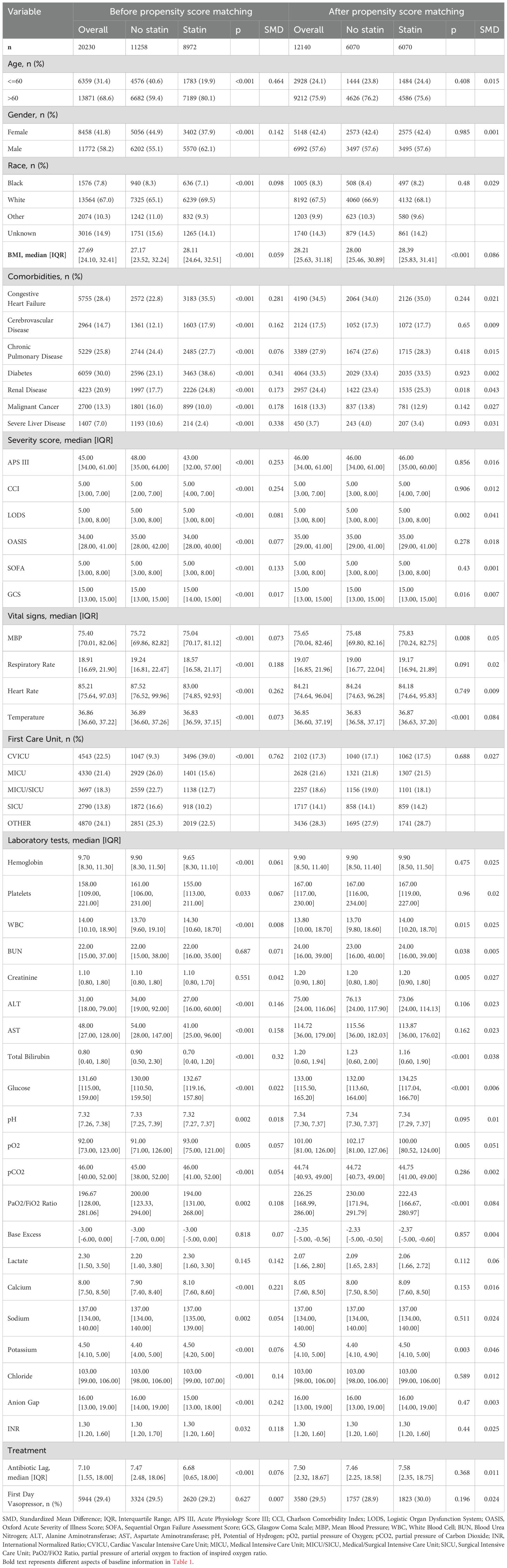
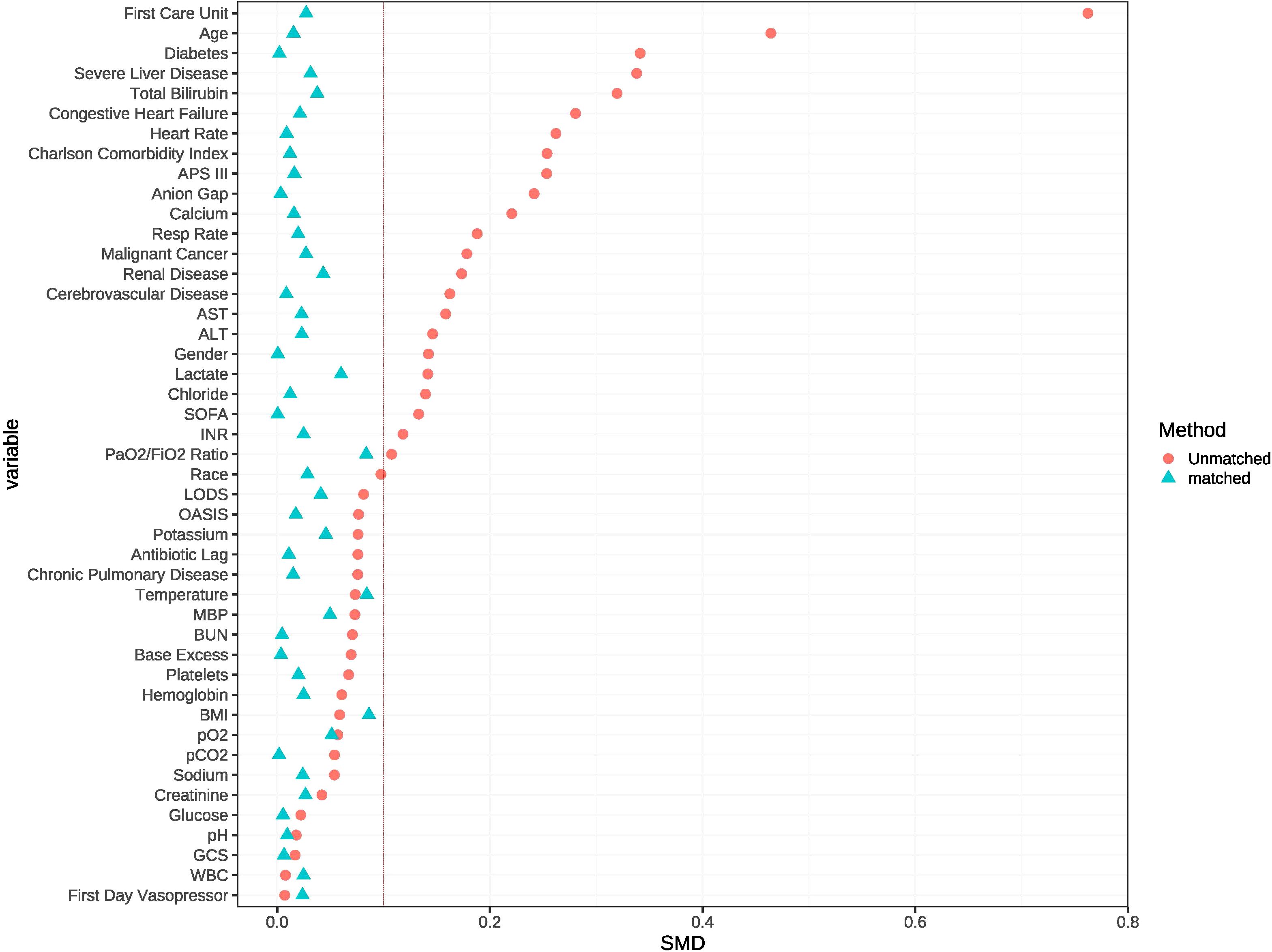
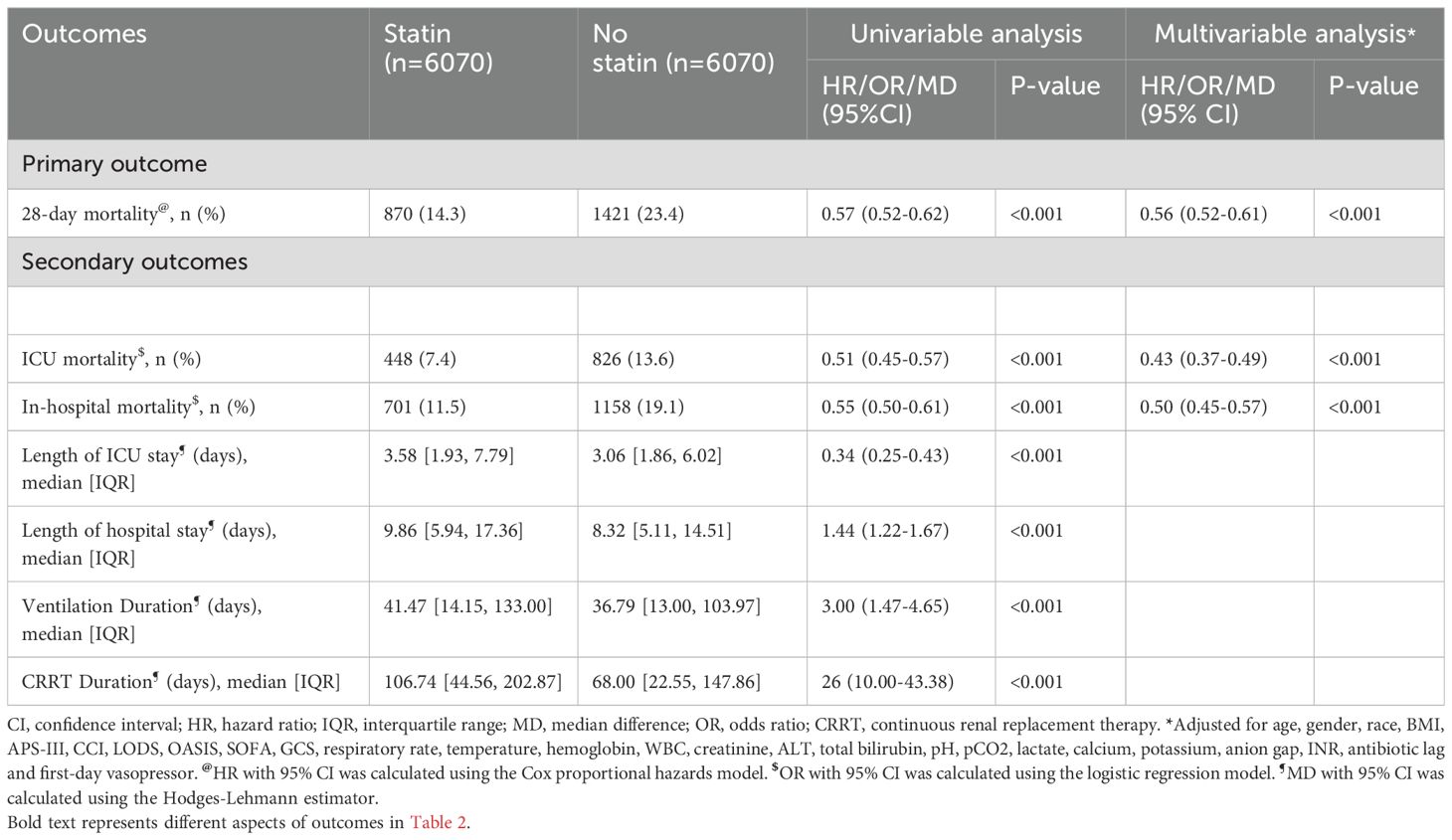
In the unmatched cohort, patients who received statin tended to be older and more likely to be male, exhibited lower APS-III and OASIS, as well as a shorter antibiotic lag. The baseline characteristics of both the unmatched and matched cohorts are shown in Table 1. After PSM, all variables were well-balanced in the matched cohort (SMD < 0.10) (Figure 2). The distribution of propensity scores of the two groups before and after matching are depicted in Figure 3.
Figure 3. The distributional balance of propensity scores before and after propensity score matching in the two groups.
Statin regimen
In the unmatched cohort, approximately 44.34% (8972/20230) patients received statins, while in the matched cohort, approximately 50% (6070/12140) patients received statins. Various forms of statins were used during ICU stay, including atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, simvastatin and other statins. Clinical indications for the initiation and discontinuation of statins were not available in the database.
Primary outcome
28-day all-cause mortality
In the matched cohort, the 28-day all-cause mortality rate was 14.3% (870/6070) in the statin group and 23.4% (1421/6070) in the no statin group (p < 0.001). Figure 4a displays the Kaplan-Meier curve for 28-day all-cause mortality stratified by statin use in the matched cohort. Cox regression analysis indicated that statin use was associated with decreased 28-day all-cause mortality in both univariable analysis (HR, 0.57; 95% CI, 0.52-0.62; p < 0.001) and multivariable analysis (HR, 0.56; 95% CI, 0.52-0.61; p < 0.001) in the matched cohort.
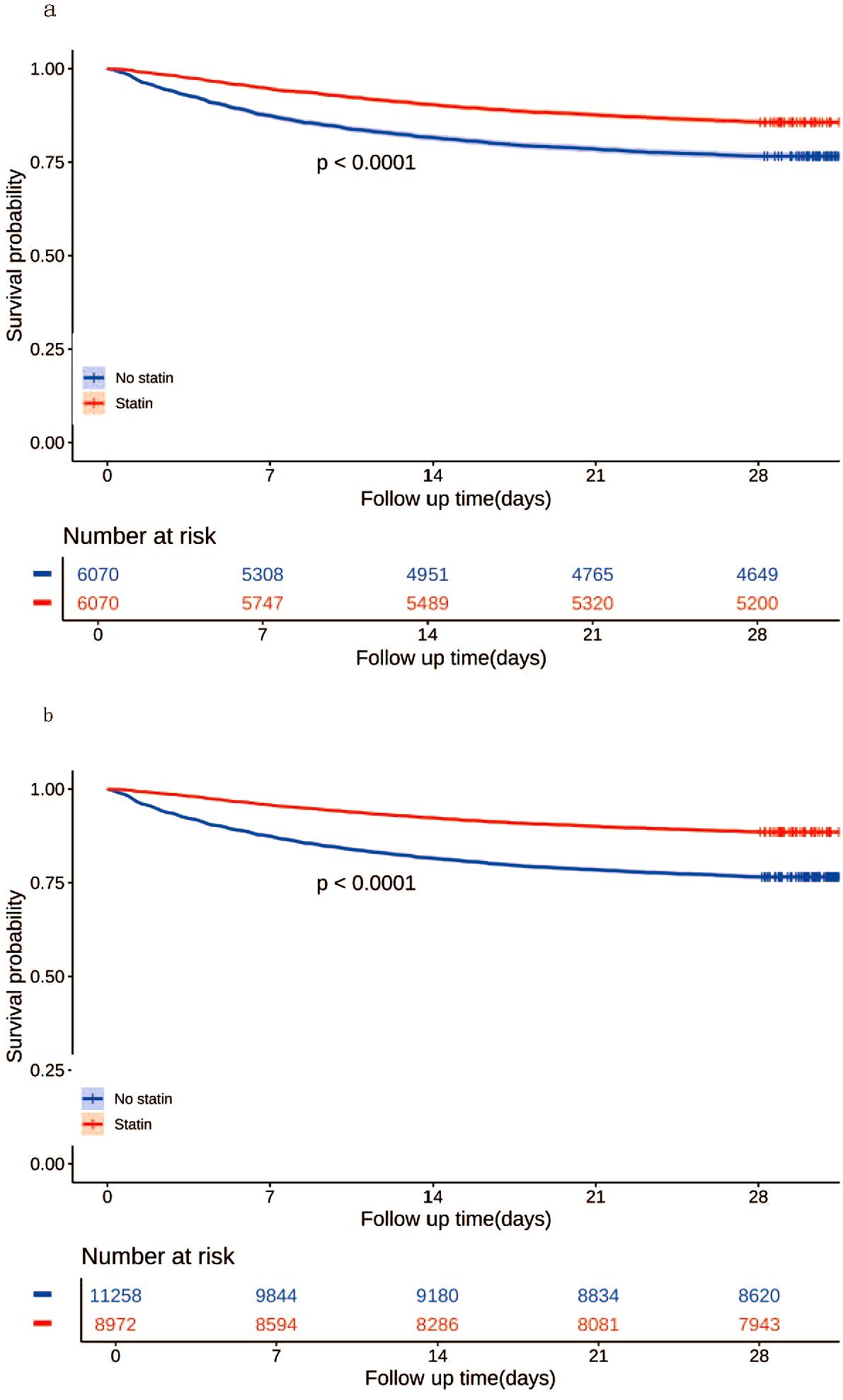
Figure 4. Kaplan-Meier curves for 28-day all-cause mortality according to statin use in the matched cohort (a) and the unmatched cohort (b).
Subgroup analyses
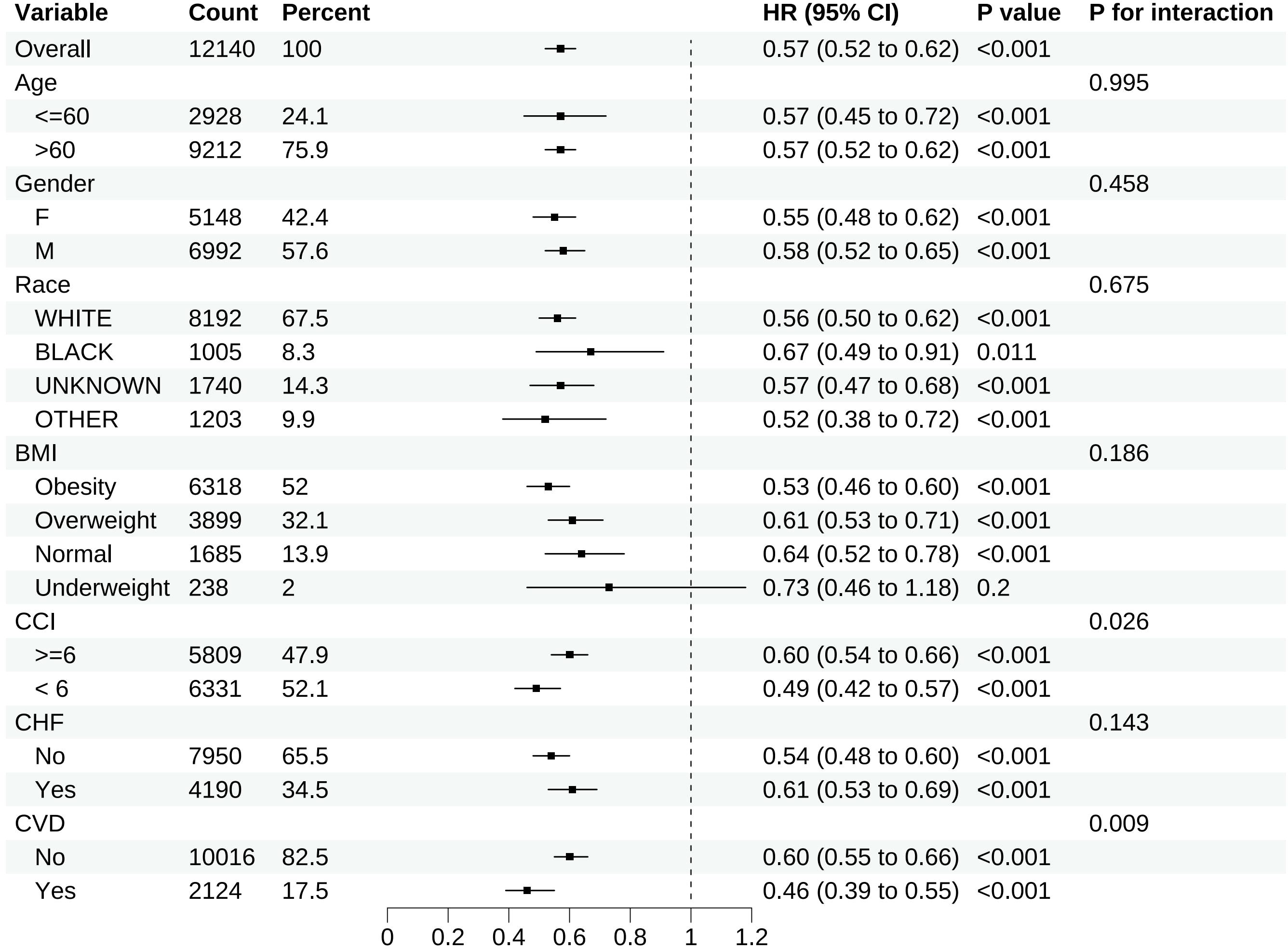
Except for individuals categorized as underweight subgroup based on BMI, the upper limits of the 95% CIs for all other subgroups were < 1.00, indicating a reduction in 28-day all-cause mortality following in-hospital statin use regardless of baseline characteristics. Nonetheless, due to the limited sample size (n = 238) of the underweight subgroup based on BMI, this finding may be due to chance and should be interpreted with caution. The results of subgroup analyses for 28-day all-cause mortality in the matched cohort are demonstrated in Figure 5.
Sensitivity analyses
In the unmatched cohort, the 28-day all-cause mortality rate was 11.5% (1029/8972) in the statin group and 23.4% (2638/11258) in the no statin group (p < 0.001). Figure 4b displays the Kaplan-Meier curve for 28-day all-cause mortality stratified by statin use in the unmatched cohort. Cox regression analysis indicated that statin use was associated with decreased 28-day all-cause mortality in both univariable analysis (HR, 0.57; 95% CI, 0.52-0.62; p < 0.001) and multivariable analysis (HR, 0.56; 95% CI, 0.52-0.61; p < 0.001) in the unmatched cohort.
Secondary outcomes
ICU mortality and in-hospital mortality
The ICU mortality rate was 7.4% (448/6070) in the statin group and 13.6% (826/6070) in the no statin group (p < 0.001). Logistic regression analysis showed that statin use was associated with decreased ICU mortality rate in both univariable analysis (OR, 0.51; 95% CI, 0.45-0.57; p < 0.001) and multivariable analysis (OR, 0.43; 95% CI, 0.37-0.49; p < 0.001). The in-hospital mortality rate was 11.5% (701/6070) in the statin group and 19.1% (1158/6070) in the no statin group (p < 0.001). Logistic regression analysis showed that statin use was associated with decreased in-hospital mortality rate in both univariable analysis (OR, 0.55; 95% CI, 0.50-0.61; p < 0.001) and multivariable analysis (OR, 0.50; 95% CI, 0.45-0.57; p < 0.001) (Table 2).
Duration of MV and CRRT
The median duration of MV was 41.47 hours (IQR, 14.15-133.00) in the statin group, while in the no-statin group, it was 36.79 hours (IQR, 13.00-103.97). Similarly, the median duration of CRRT was 106.74 hours (IQR, 44.56-202.87) in the statin group, while in the no-statin group, it was 68.00 hours (IQR, 22.55-147.86). Statin use was associated with prolonged duration of MV (MD, 3.00 hours; 95% CI, 1.47-4.65; p < 0.001) and CRRT (MD, 26.00 hours; 95% CI, 10.00-43.38; p < 0.001), but not with shortened duration of MV and CRRT (Table 2).
Length of ICU stay and hospital stay
The median length of ICU stay was 3.58 days (IQR, 1.93-7.79) in the statin group and 3.06 days (IQR, 1.86-6.02) in the no statin group. Similarly, the median length of hospital stay was 9.86 days (IQR, 5.94-17.36) in the statin group and 8.32 days (IQR, 5.11-14.51) in the no statin group. Statin use was associated with prolonged length of ICU stay (MD, 0.34 days; 95% CI, 0.25-0.43; p < 0.001) and hospital stay (MD, 1.44 days; 95% CI, 1.22-1.67; p < 0.001), but not with shortened length of ICU stay and hospital stay (Table 2).
Discussion
In a large real-world clinical setting, we conducted a retrospective propensity score matched cohort study to evaluate the association between statin use and mortality among 20230 patients with sepsis. We found that statin users exhibited decreased 28-day all-cause mortality in both the matched and unmatched cohorts. Our subgroup analyses by BMI category revealed statistically significant protective effects of statins on sepsis in normal weight (HR, 0.64; 95% CI, 0.52-0.78; p < 0.001), overweight (HR, 0.61; 95% CI, 0.53-0.71; p < 0.001), and obese patients (HR, 0.53; 95% CI, 0.46-0.60; p < 0.001). While the point estimate for underweight patients showed a similar trend (HR, 0.73; 95% CI, 0.46-1.18; p = 0.2), this subgroup did not reach statistical significance, likely due to limited sample size (n=238, 2%) rather than a true biological difference. The result was consistent and stable in sensitivity analyses, indicating the robustness of our finding. Notably, statin therapy demonstrated associations with reduced ICU mortality and in-hospital mortality, prolonged ICU and hospital stay, and increased duration of MV and CRRT. These paradoxical findings likely reflect competing risk dynamics, wherein the mortality benefit permits extended survival of critically ill patients requiring prolonged intensive care and organ support (Table 2). This is supported by evidence from multiple studies demonstrating that statin use is associated with reduced mortality in critically ill patients, including those with sepsis (31). The prolonged duration of mechanical ventilation and CRRT should therefore be interpreted as a reflection of the complex interplay between disease severity, comorbidities, and the potential benefits of statin therapy, rather than as a negative outcome. In conclusion, our study suggests that statin use during the ICU stay may exert a protective effect in patients with sepsis.
Relation with previous evidence
While randomized controlled trials are widely regarded as the gold standard of evidence-based medicine, conducting prospective randomized controlled trials to assess the effect of statin use on sepsis prognosis is challenging due to the large number of patients required to achieve a sufficient cohort of patients who actually develop sepsis. We believe that the best alternative to a prospective randomized controlled trial is exactly what we have done: identify a cohort, follow them over time, even if not concurrently, and match cases to controls by propensity matching on important clinical characteristics.
To account for the selection bias and unmeasured confounders inherent in observational studies, we employed the PSM approach (32) to ensure that all patients were pseudo-randomized to the treatment and control groups as in a typical RCT. PSM enables the generation of an unbiased average treatment effect of statin on clinical outcomes among patients with sepsis admitted to the ICU. PSM allows simultaneous modeling of the propensity for unbiased group allocation and modeling of the outcomes using multivariate regression adjustment, thereby obtaining double robust and unbiased estimates of the average treatment effect of statins (32).
After adjusting for various biases inherent in observational studies using PSM, we observed a beneficial effect of statin use on the outcome of sepsis, which is contrary to the findings of several RCTs. Though the methodological differences between RCTs and observational studies are frequently cited as the primary source of such discrepancy, our study employed a pseudo-randomized quasi-experimental approach that successfully adjusted for selection biases and supported the results of most observational studies (16, 33, 34).
Our findings challenge the previous assertion made by Majumda that a healthy user effect explains why observational studies demonstrated the beneficial effects of statins on sepsis patients (35). Because the use of the PSM approach allows individuals to be assigned randomly to different groups, thus eliminating the possibility of a healthy user effect (20).
There are several possible reasons why most RCTs failed to detect a beneficial effect of statins in patients with sepsis. A comprehensive review of these RCTs revealed that sepsis diagnoses were often underreported, and many trials could not provide additional data upon request, increasing the risk that a non-representative sample of statin-treated patients was enrolled and assessed for sepsis outcomes (20). It is noteworthy that the PSM approach used in this study is not inherently superior to large-scale RCTs with complete data reporting, but it helps mitigate biases in observational studies and address noncompliance issues in RCTs.
Possible explanations for our findings
Subgroup analyses revealed consistent beneficial effects of statin therapy in sepsis patients irrespective of pre-existing cerebrovascular diseases and chronic heart failure. Notably, while the plaque-stabilizing properties of statins constitute the primary mechanism underlying their cardiovascular protective effects, this observed sepsis-associated mortality reduction in both subgroups suggest potential pleiotropic mechanisms independent of atherosclerotic plaque modulation.
The pleiotropic effects of statins have been well documented in the literature. However, despite this, the underlying mechanism by which statins confer benefit in sepsis remains unclear (36). Potential explanations for this beneficial effect include: First, statins may attenuate the severity of sepsis through their anti-inflammatory, immunomodulatory, antioxidative, and antithrombotic effects (37–40). In animal models of sepsis, statins have been demonstrated to inhibit the elevation of inflammatory mediators (41, 42), resulting in improved survival rates (43, 44). Previous clinical studies have shown that statins may exert potential antioxidant properties in models of sepsis, which could help mitigate tissue damage and organ dysfunction (45). Statins have been reported to inhibit the expression of toll-like receptors (TLR) 4 and 2 on monocytes in human endotoxemia models, leading to a decrease in inflammatory cytokine production (45). Statins may interfere with transcription factors such as nuclear factor kappaB (NF-kappaB) and activation protein-1 (AP-1), which could result in a reduction in the synthesis of proinflammatory cytokines, including interleukin-1 (IL-1) and IL-6 (46). Similarly, an association between statin treatment and reduced levels of tumor necrosis factor (TNF) and IL-6 has been observed in patients experiencing acute bacterial infections (47). Statins have been demonstrated to inhibit adhesion molecules in both neutrophils/monocytes and endothelial cells, resulting in a decreased migration of polynuclear neutrophils into tissues (48–50). Statins may assist in restoring the balance between endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS), which is disrupted in sepsis (51). By substantially boosting eNOS expression while downregulating iNOS, statins have the potential to prevent or reverse sepsis-related endothelial dysfunction (51). Furthermore, statins may play a crucial role in mitigating the negative effects of sepsis on the coagulation system by inhibiting the expression of tissue factor and plasminogen activator inhibitor-1, improving protein C function (52), lowering prothrombin fragment levels, and significantly upregulating the expression of thrombomodulin (53, 54). Second, Statin use was associated with a lower risk of bacterial infection. Statins may have direct antimicrobial properties (37, 55), as the enzymes in the mevalonate pathway, which are potentially modified by statin therapy, are also involved in the development of Gram-positive bacterial infections (55). It is noteworthy that statins may also exhibit antifungal properties due to the similarities between the ergosterol biosynthetic pathway in fungi and the cholesterol synthesis in humans, implying a direct effect on Candida species (56, 57). The immunomodulatory, antioxidative, anti-inflammatory, antithrombotic, and direct antimicrobial effects of statins may account for the beneficial effects against sepsis observed in our study.
Strength and limitation
The main strength of this study lies in the utilization of the PSM analytical approach, which allows for the generation of doubly robust unbiased estimates of the average treatment effects of statins in patients with sepsis. However, this study also has several limitations. First, the observational design inherently precludes definitive causal inferences, as unmeasured confounding factors may influence the observed associations despite our rigorous propensity score matching and multivariable adjustment approaches. Second, the study may be subject to potential residual confounders that are not recorded in the MIMIC-IV database. Although PSM is a robust method for addressing multiple baseline differences between groups, variables included in this study are confined to relevant variables available in the MIMIC-IV database, potentially introducing bias from unmeasured confounders. Third, the study did not identify the specific effects of individual statins on sepsis. In this study, the exposure was simply defined as either the use of any statin or no statin during the ICU stay. Previous studies have demonstrated that simvastatin, atorvastatin and rosuvastatin exhibit antibacterial properties, while other statins do not (15, 58). As a result, studies conducted without distinguishing the effects of different statins are prone to underestimate their effects, and future studies should be conducted to compare the clinical outcomes associated with individual statins. Fourth, the impact of prior statin use on clinical outcomes was not investigated. The study focused only on statin use during the ICU stay. However, pretreatment with simvastatin has been demonstrated to improve sepsis survival in mouse models by preserving cardiac function, lowering circulatory inflammatory cytokines, decreasing neutrophil migration to the lung, and enhancing T-cell function (42, 43, 59). Therefore, studies conducted without considering the impact of prior statin exposure may overestimate the beneficial effects of statins.
Conclusion
From a large, population-based cohort study, we found an association between statin use and reduced sepsis-related mortality. Given the wide use of statins for the prevention of cardiovascular disease, it is likely that their use in this population has also conferred benefits in combating infections and sepsis.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Publicly available datasets were used in this study. This data is available here: https://physionet.org/content/mimiciv/2.0/.
Ethics statement
This study followed the Helsinki Declaration, due to participant anonymity and data standardization in the MIMIC-IV database, no ethics committee approval was required.
Author contributions
CL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KZ: Writing – review & editing. QR: Visualization, Writing – review & editing. LC: Data curation, Writing – review & editing. YZ: Data curation, Writing – review & editing. GW: Validation, Visualization, Writing – review & editing. KX: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
Author QR was employed by Tianjin Daily.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1537172/full#supplementary-material
Supplementary Figure 1 | Percentage of missing data of each variable.
Supplementary Figure 2 | Variance inflation factor of each variable in the matched cohort.
Supplementary Figure 3 | Variance inflation factor of each variable in the unmatched cohort.
Supplementary Table 1 | Percentage of missing data of each variable.
Supplementary Table 2 | Variance inflation factor of each variable in the matched cohort.
Supplementary Table 3 | Variance inflation factor of each variable in the unmatched cohort.
Supplementary Table 4 | Cox regression model for 28-day all-cause mortality using stepwise selection in the matched cohort.
Supplementary Table 5 | Cox regression model for 28-day all-cause mortality using stepwise selection in the unmatched cohort.
Abbreviations
MIMIC-IV, Medical Information Mart for Intensive Care IV; ICU, Intensive Care Unit; MV, Mechanical Ventilation; CRRT, Continuous Renal Replacement Therapy; PSM, Propensity Score Matching; HR, Hazard Ratio; CI, Confidence Interval; OR, Odds Ratio; US, The United State; HMG-CoA, Three-hydroxy-3-methylglutaryl coenzyme A; COPD, Chronic Obstructive Pulmonary Disease; ARDS, Acute Respiratory Distress Syndrome; RCTs, Randomized Controlled Trials; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; SQL, Structured Query Language; BMI, Body Mass Index; RR, Respiratory Rate; MBP, Mean Blood Pressure; WBC, White Blood Cell; BUN, Blood Urea Nitrogen; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; PH, Potential of Hydrogen; pO2, partial pressure of Oxygen; pCO2, partial pressure of Carbon Dioxide; INR, International Normalized Ratio; GCS, Glasgow Coma Scale; SD, Standard Deviation; IQR, Interquartile Range; VIF, Variance Inflation Factor; MD, Median Difference; SMD, Standardized Mean Difference; APS-III, Acute Physiology Score III; LODS, Logistic Organ Dysfunction System; OASIS, Oxford Acute Severity of Illness Score; CCI, Charlson Comorbidity Index; SOFA, Sequential Organ Failure Assessment Score; TLR, Toll-like receptor; NF-kappaB, Nuclear transcription factor-kappa B; AP-1, Activation protein-1; IL, Interleukin; TNF, Tumor Necrosis Factor; eNOS, endothelial Nitric Oxide Synthase; iNOS, inducible Nitric Oxide Synthase.
References
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Levy MM, Evans LE, and Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. (2018) 44:925–8. doi: 10.1007/s00134-018-5085-0
3. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, and Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. (2001) 29:1303–10. doi: 10.1097/00003246-200107000-00002
4. Abraham E and Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. (2007) 35:2408–16. doi: 10.1097/01.ccm.0000282072.56245.91
5. Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007). Chest. (2011) 140:1223–31. doi: 10.1378/chest.11-0352
6. Kopterides P and Falagas ME. Statins for sepsis: a critical and updated review. Clin Microbiol Infect. (2009) 15:325–34. doi: 10.1111/j.1469-0691.2009.02750.x
7. Gotts JE and Matthay MA. Sepsis: pathophysiology and clinical management. BMJ (Clinical Res ed). (2016) 353:i1585. doi: 10.1136/bmj.i1585
8. Angus DC and van der Poll T. Severe sepsis and septic shock. New Engl J Med. (2013) 369:840–51. doi: 10.1056/NEJMra1208623
9. Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. New Engl J Med. (2001) 344:699–709. doi: 10.1056/nejm200103083441001
10. Jackevicius CA, Chou MM, Ross JS, Shah ND, and Krumholz HM. Generic atorvastatin and health care costs. New Engl J Med. (2012) 366:201–4. doi: 10.1056/NEJMp1113112
11. Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. (2013) 2013:Cd004816. doi: 10.1002/14651858.CD004816.pub5
12. Taylor FC, Huffman M, and Ebrahim S. Statin therapy for primary prevention of cardiovascular disease. Jama. (2013) 310:2451–2. doi: 10.1001/jama.2013.281348
13. Mermis JD and Simpson SQ. HMG-coA reductase inhibitors for prevention and treatment of severe sepsis. Curr Infect Dis Reports. (2012) 14:484–92. doi: 10.1007/s11908-012-0277-1
14. Smeeth L, Douglas I, Hall AJ, Hubbard R, and Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. (2009) 67:99–109. doi: 10.1111/j.1365-2125.2008.03308.x
15. Masadeh M, Mhaidat N, Alzoubi K, Al-Azzam S, and Alnasser Z. Antibacterial activity of statins: a comparative study of atorvastatin, simvastatin, and rosuvastatin. Ann Clin Microbiol Antimicrobials. (2012) 11:13. doi: 10.1186/1476-0711-11-13
16. Lee MG, Lee CC, Lai CC, Hsu TC, Porta L, Lee M, et al. Preadmission statin use improves the outcome of less severe sepsis patients - a population-based propensity score matched cohort study. Br J Anaesthesia. (2017) 119:645–54. doi: 10.1093/bja/aex294
17. Falagas ME, Makris GC, Matthaiou DK, and Rafailidis PI. Statins for infection and sepsis: a systematic review of the clinical evidence. J Antimicrobial Chemother. (2008) 61:774–85. doi: 10.1093/jac/dkn019
18. Janda S, Young A, Fitzgerald JM, Etminan M, and Swiston J. The effect of statins on mortality from severe infections and sepsis: a systematic review and meta-analysis. J Crit Care. (2010) 25:656.e7–22. doi: 10.1016/j.jcrc.2010.02.013
19. Wan YD, Sun TW, Kan QC, Guan FX, and Zhang SG. Effect of statin therapy on mortality from infection and sepsis: a meta-analysis of randomized and observational studies. Crit Care (London England). (2014) 18:R71. doi: 10.1186/cc13828
20. van den Hoek HL, Bos WJ, de Boer A, and van de Garde EM. Statins and prevention of infections: systematic review and meta-analysis of data from large randomised placebo controlled trials. BMJ (Clinical Res ed). (2011) 343:d7281. doi: 10.1136/bmj.d7281
21. Schuemie MJ, Ryan PB, DuMouchel W, Suchard MA, and Madigan D. Interpreting observational studies: why empirical calibration is needed to correct p-values. Stat Med. (2014) 33:209–18. doi: 10.1002/sim.5925
22. Johnson A, Bulgarelli L, Pollard T, Celi LA, Mark R, and Horng S. MIMIC-IV-ED (version 2.2). PhysioNet. (2023).
23. Lachat C, Hawwash D, Ocké MC, Berg C, Forsum E, Hörnell A, et al. Strengthening the Reporting of Observational Studies in Epidemiology - nutritional epidemiology (STROBE-nut): An extension of the STROBE statement. Nutr Bulletin. (2016) 41:240–51. doi: 10.1111/nbu.12217
24. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ (Clinical Res ed). (2009) 338:b2393. doi: 10.1136/bmj.b2393
25. Cheung NK, Zhang J, Lu C, Parker M, Bahrami A, Tickoo SK, et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. Jama. (2012) 307:1062–71. doi: 10.1001/jama.2012.228
26. Zhou L, Zhao X, Heizhati M, Abulikemu S, Zhang D, Cheng Q, et al. Trends in lipids and lipoproteins among adults in Northwestern Xinjiang, China, from 1998 through 2015. Journal of epidemiology. (2019) 29(7):257–63. doi: 10.2188/jea.JE20180018
27. Wang X, Zhu A, Wang J, Ma F, Liu J, Fan Y, et al. Steroidal aromatase inhibitors have a more favorable effect on lipid profiles than nonsteroidal aromatase inhibitors in postmenopausal women with early breast cancer: a prospective cohort study. Ther Adv Med Oncol. (2020) 12:1758835920925991. doi: 10.1177/1758835920925991
28. Lonjon G, Porcher R, Ergina P, Fouet M, and Boutron I. Potential pitfalls of reporting and bias in observational studies with propensity score analysis assessing a surgical procedure: A methodological systematic review. Ann Surg. (2017) 265:901–9. doi: 10.1097/sla.0000000000001797
29. Cecconi M, Evans L, Levy M, and Rhodes A. Sepsis and septic shock. Lancet (London England). (2018) 392:75–87. doi: 10.1016/s0140-6736(18)30696-2
30. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28:3083–107. doi: 10.1002/sim.3697
31. Yao Y, Zhao X, Wang M, Zhou F, Li C, Le X, et al. Association between the use of statins and in-hospital mortality risk in patients with sepsis-induced coagulopathy during ICU stays: a study based on medical information mart for intensive care database. BMC Infect Disease. (2024) 24:738. doi: 10.1186/s12879-024-09636-y
32. Lamm M and Yung Y-F. Estimating causal effects from observational data with the CAUSALTRT procedure. In: Proceedings of the SAS Global Forum 2017 Conference. SAS Institute Inc, Cary, NC (2017). Available at: http://support.sas.com/resources/papers/proceedings17/SAS0374-2017.pdf.
33. Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, Albertson TE, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. New Engl J Med. (2014) 370:2191–200. doi: 10.1056/NEJMoa1401520
34. Kyu Oh T, Song IA, Lee JH, Lim C, Jeon YT, Bae HJ, et al. Preadmission statin use and 90-day mortality in the critically ill: A retrospective association study. Anesthesiol. (2019) 131:315–27. doi: 10.1097/aln.0000000000002811
35. Majumdar SR, McAlister FA, Eurich DT, Padwal RS, and Marrie TJ. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ (Clinical Res ed). (2006) 333:999. doi: 10.1136/bmj.38992.565972.7C
36. Oesterle A, Laufs U, and Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. (2017) 120:229–43. doi: 10.1161/circresaha.116.308537
37. Hennessy E, Adams C, Reen FJ, and O’Gara F. Is there potential for repurposing statins as novel antimicrobials? Antimicrobial Agents Chemother. (2016) 60:5111–21. doi: 10.1128/aac.00192-16
38. Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. New Engl J Med. (2009) 360:1851–61. doi: 10.1056/NEJMoa0900241
39. Mulder DJ, van Haelst PL, Wobbes MH, Gans RO, Zijlstra F, May JF, et al. The effect of aggressive versus conventional lipid-lowering therapy on markers of inflammatory and oxidative stress. Cardiovasc Drugs Ther. (2007) 21:91–7. doi: 10.1007/s10557-007-6010-x
40. McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, et al. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet (London England). (2004) 363:2015–21. doi: 10.1016/s0140-6736(04)16449-0
41. Ando H, Takamura T, Ota T, Nagai Y, and Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther. (2000) 294:1043–6. doi: 10.1016/S0022-3565(24)39169-4
42. Zhang S, Rahman M, Zhang S, Qi Z, and Thorlacius H. Simvastatin antagonizes CD40L secretion, CXC chemokine formation, and pulmonary infiltration of neutrophils in abdominal sepsis. J Leukoc Biol. (2011) 89:735–42. doi: 10.1189/jlb.0510279
43. Merx MW, Liehn EA, Janssens U, Lütticken R, Schrader J, Hanrath P, et al. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. (2004) 109:2560–5. doi: 10.1161/01.Cir.0000129774.09737.5b
44. Merx MW, Liehn EA, Graf J, van de Sandt A, Schaltenbrand M, Schrader J, et al. Statin treatment after onset of sepsis in a murine model improves survival. Circulation. (2005) 112:117–24. doi: 10.1161/circulationaha.104.502195
45. Durant R, Klouche K, Delbosc S, Morena M, Amigues L, Beraud JJ, et al. Superoxide anion overproduction in sepsis: effects of vitamin e and simvastatin. Shock (Augusta Ga). (2004) 22:34–9. doi: 10.1097/01.shk.0000129197.46212.7e
46. Frost FJ, Petersen H, Tollestrup K, and Skipper B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest. (2007) 131:1006–12. doi: 10.1378/chest.06-1997
47. Novack V, Eisinger M, Frenkel A, Terblanche M, Adhikari NK, Douvdevani A, et al. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: a randomized double-blind placebo controlled clinical trial. Intensive Care Med. (2009) 35:1255–60. doi: 10.1007/s00134-009-1429-0
48. Almog Y. Statins, inflammation, and sepsis: hypothesis. Chest. (2003) 124:740–3. doi: 10.1378/chest.124.2.740
49. Arnaud C and Mach F. Potential antiinflammatory and immunomodulatory effects of statins in rheumatologic therapy. Arthritis Rheum. (2006) 54:390–2. doi: 10.1002/art.21757
50. Braga Filho JAF, Abreu AG, Rios CEP, Trovão LO, Silva DLF, Cysne DN, et al. Prophylactic treatment with simvastatin modulates the immune response and increases animal survival following lethal sepsis infection. Front Immunol. (2018) 9:2137. doi: 10.3389/fimmu.2018.02137
51. McGown CC, Brown NJ, Hellewell PG, and Brookes ZL. ROCK induced inflammation of the microcirculation during endotoxemia mediated by nitric oxide synthase. Microvascular Res. (2011) 81:281–8. doi: 10.1016/j.mvr.2011.02.003
52. Terblanche M, Almog Y, Rosenson RS, Smith TS, and Hackam DG. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Disease. (2007) 7:358–68. doi: 10.1016/s1473-3099(07)70111-1
53. Niessner A, Steiner S, Speidl WS, Pleiner J, Seidinger D, Maurer G, et al. Simvastatin suppresses endotoxin-induced upregulation of toll-like receptors 4 and 2 in vivo. Atherosclerosis. (2006) 189:408–13. doi: 10.1016/j.atherosclerosis.2005.12.022
54. Shi J, Wang J, Zheng H, Ling W, Joseph J, Li D, et al. Statins increase thrombomodulin expression and function in human endothelial cells by a nitric oxide-dependent mechanism and counteract tumor necrosis factor alpha-induced thrombomodulin downregulation. Blood Coagulation Fibrinolysis. (2003) 14:575–85. doi: 10.1097/00001721-200309000-00010
55. Jerwood S and Cohen J. Unexpected antimicrobial effect of statins. J Antimicrobial Chemother. (2008) 61:362–4. doi: 10.1093/jac/dkm496
56. Sun HY and Singh N. Antimicrobial and immunomodulatory attributes of statins: relevance in solid-organ transplant recipients. Clin Infect Dis. (2009) 48:745–55. doi: 10.1086/597039
57. Parks LW and Casey WM. Physiological implications of sterol biosynthesis in yeast. Annu Rev Microbiol. (1995) 49:95–116. doi: 10.1146/annurev.mi.49.100195.000523
58. Thangamani S, Mohammad H, Abushahba MF, Hamed MI, Sobreira TJ, Hedrick VE, et al. Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent. Sci Reports. (2015) 5:16407. doi: 10.1038/srep16407
Keywords: critical illness, mortality, intensive care unit, statin, sepsis
Citation: Li C, Zhao K, Ren Q, Chen L, Zhang Y, Wang G and Xie K (2025) Statin use during intensive care unit stay is associated with improved clinical outcomes in critically ill patients with sepsis: a cohort study. Front. Immunol. 16:1537172. doi: 10.3389/fimmu.2025.1537172
Received: 30 November 2024; Accepted: 17 April 2025;
Published: 06 June 2025.
Edited by:
Sasha Shafikhani, Rush University Medical Center, United StatesCopyright © 2025 Li, Zhao, Ren, Chen, Zhang, Wang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caifeng Li, bGNmdGlhbnlpbmVpZmVubWlAMTYzLmNvbQ==; Keliang Xie, bXprMjAxMUAxMjYuY29t
 Caifeng Li
Caifeng Li Ke Zhao
Ke Zhao Qian Ren3
Qian Ren3 Keliang Xie
Keliang Xie