- 1Innovative Research Center for Basic Medicine on Autoimmune Diseases, Ministry of Education, Hangzhou, Zhejiang, China
- 2Key Laboratory of Chinese Medicine Rheumatology of Zhejiang Province, College of Basic Medical Science, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 3Department of Party and Government Comprehensive Office, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 4School of Pharmaceutical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
Background: Atopic dermatitis (AD) is a common recurrent chronic inflammatory skin disease, and there is increasing evidence of a possible association between AD and autoimmune diseases.
Objectives: This study aimed to summarize existing epidemiological studies on the association between AD and autoimmune diseases and to perform a meta-analysis of combinable results.
Methods: We conducted a thorough search for cohort studies, case-control studies and cross-sectional studies across the PubMed, Cochrane Library, and Embase databases, from their inception to May 24, 2024, using medical subject headings and relevant keywords. All data were meticulously analyzed using Stata statistical software version 17.0. The protocol was registered on PROSPERO (CRD42024547282).
Result: A total of 26 cohort studies, comprising 1,629,723 patients with atopic dermatitis and 15,106,889 control subjects, were included in this meta-analysis. These studies were published between 2014 and 2024 and included 19 cohort studies, 2 case-control studies, and 5 cross-sectional studies. The current study demonstrated a significant association of atopic dermatitis with autoimmune diseases[HR 1.49, 95% CI (1.31-1.70); P<0.001], including celiac disease, systemic lupus erythematosus, Sjogren’s syndrome, ankylosing spondylitis, alopecia areata, rheumatoid arthritis, vitiligo, thyroid dysfunction, ulcerative colitis.
Conclusion: The results of our study indicate a clear association between atopic dermatitis and autoimmune diseases, both in adults and children. Additionally, women were more likely to have autoimmune disease complications than men. However, due to the limited number of participants in our study, further research is needed to thoroughly investigate the relationship.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024547282.
Introduction
Atopic dermatitis (AD) is a recurrent, chronic inflammatory skin disease that affects approximately 20% of children and 7 to 10% of adults in high-income countries. It is also prevalent in developing world, posing a significant public health concern due to its presence and increasing prevalence across most countries (1, 2). The defining features of AD include generalized dry skin, recurrent eczematous lesions, and pruritus (3). These symptoms can significantly impact daily activities, potentially leading to sleep disorders, thereby reducing an individual’s quality of life (4). The severity of the disease correlates with the frequency of recurrences and healthcare utilization, imposing a substantial financial burden on patients (5).
Evidence suggests that AD may possess an autoimmune component, with disease progression resembling that of known autoimmune disorders characterized by alternating relapse and remission phases. Furthermore, significant associations have been identified between AD and multiple autoimmune diseases (6). Autoimmune diseases represent a group of chronic, systemic disorders characterized by aberrant immune responses, excessive inflammation, and widespread deposition of immune complexes in tissues and organs. Epidemiological studies indicate that these conditions affect approximately 5–8% of the global population, highlighting their significant public health burden (7). A Mendelian randomization analysis has demonstrated that atopic dermatitis (AD) significantly increases the risk of rheumatoid arthritis (RA), type 1 diabetes (T1D), and autoimmune alopecia (AA), supporting a substantial causal relationship between these conditions. Although the precise pathogenic mechanisms linking AD to RA, T1D, and AA remain unclear, emerging evidence suggests that immune dysregulation and shared genetic susceptibility may underlie this association (8).
Several large-scale population-based studies have recently reported associations between AD and multiple autoimmune diseases. A meta-analysis on a similar topic was published in 2021 (9), however, the subgroup analyses did not stratify by age, sex, and AD severity. Simultaneously, numerous well-executed cohort studies have recently been published, presenting new evidence regarding the association between AD and autoimmune diseases. Given the importance of this subject, the limitations of prior reviews, and the availability of new data, we conducted a systematic review and meta-analysis to evaluate the association between AD and autoimmune diseases in adults compared to children.
Materials and methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (10).
Data sources
A thorough search encompassing the PubMed, Cochrane Library and Embase databases was conducted for cohort studies from the database’s inception to 26 May 2024, without any restrictions applied. Subject terms (Embase: Emtree; PubMed: MeSH) and keywords were utilized to identify relevant studies. The search terms comprised terms associated with atopic dermatitis, chronic atopy, autoimmune diseases, immune system disorders, autoantibodies, autoimmunity and specific autoimmune diseases. The detailed search strategy for the three databases is outlined in Supplementary Tables 1-3.
Eligibility criteria
The studies included in this analysis fulfilled the following eligibility criteria: (1) Population: This study includes individuals of all ages, both children and adults. The diagnosis of ADwas based on ICD-10-CM or ICD-9-CM codes.(2) Exposure: Diagnosis of AD. (3) Comparator: Individuals without AD (general population or healthy controls). (4) Outcomes: Incidence of autoimmune diseases in AD patients compared to the non-AD population. (5)Study Design: Eligible studies include cohort studies, case-control studies, and cross-sectional studies. (6) Exclusion criteria: conference abstracts; duplicate publications; incomplete data or no results of interest.
Study selection
Two reviewers, HL and WMZ, were responsible for independently screening and selecting eligible records based on the established eligibility criteria. The initial screening involved excluding duplicate records and irrelevant articles based on the titles and abstracts. In the second stage, the full-text articles were downloaded and reviewed to determine which studies could be included in the meta-analysis. Any discrepancies between the two reviewers during the study selection process were resolved through group discussion.
Data extraction
A data extraction form was designed in Microsoft Excel by three authors, WHL, CM, WTY and CWY. The principal elements extracted were the first author, publication date, country, age, and diagnosis of atopic dermatitis. The extracted data were subjected to a rigorous cross-checking process, with any discrepancies resolved through discussion.
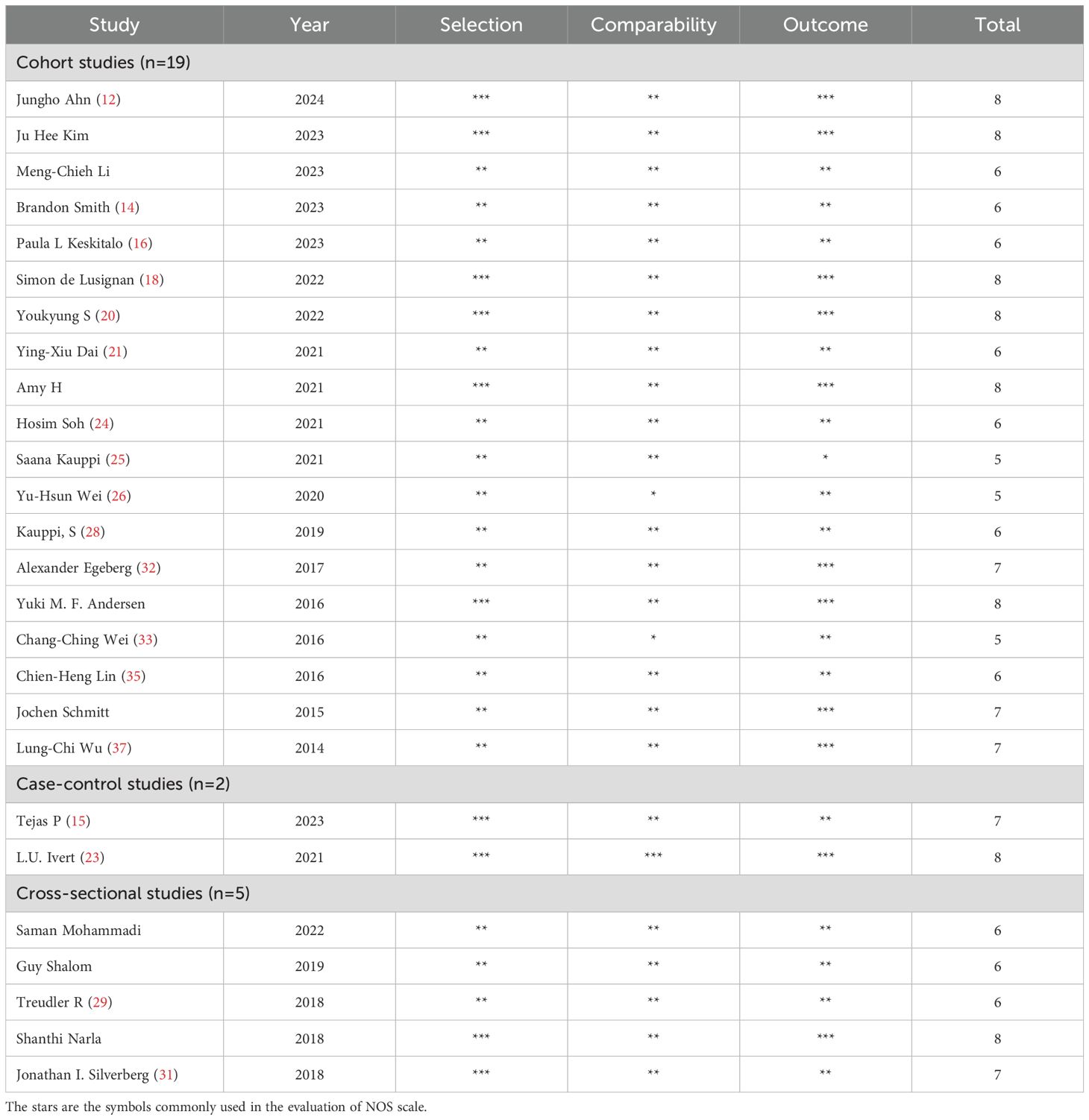
Quality assessment and risk of bias
Assessment: The quality of the included cohort studies, case-control studies and cross-sectional studies were evaluated using the Newcastle-Ottawa Scale (NOS) (11). The NOS employs a star-based system, with a maximum score of 9 stars. The stars are awarded based on the following criteria: Selection (4 stars): Represents the quality of participant selection and measurement of exposure; Comparability (2 stars): Reflects the comparability of the study design and statistical analyses; Outcome (3 stars): Evaluates the adequacy of the outcome indicator and the length of follow-up. The number of stars assigned to each study corresponds to the quality of the study. Studies with 0–3 stars were considered to be of low quality, those with 4–6 stars were deemed to be of moderate quality, and high-quality studies were those with 7–9 stars. The results of the quality assessment are provided in Table 1 (9). Additionally, PRISMA tables (Figure 1) were constructed to report the meta-analysis in a standardized format.
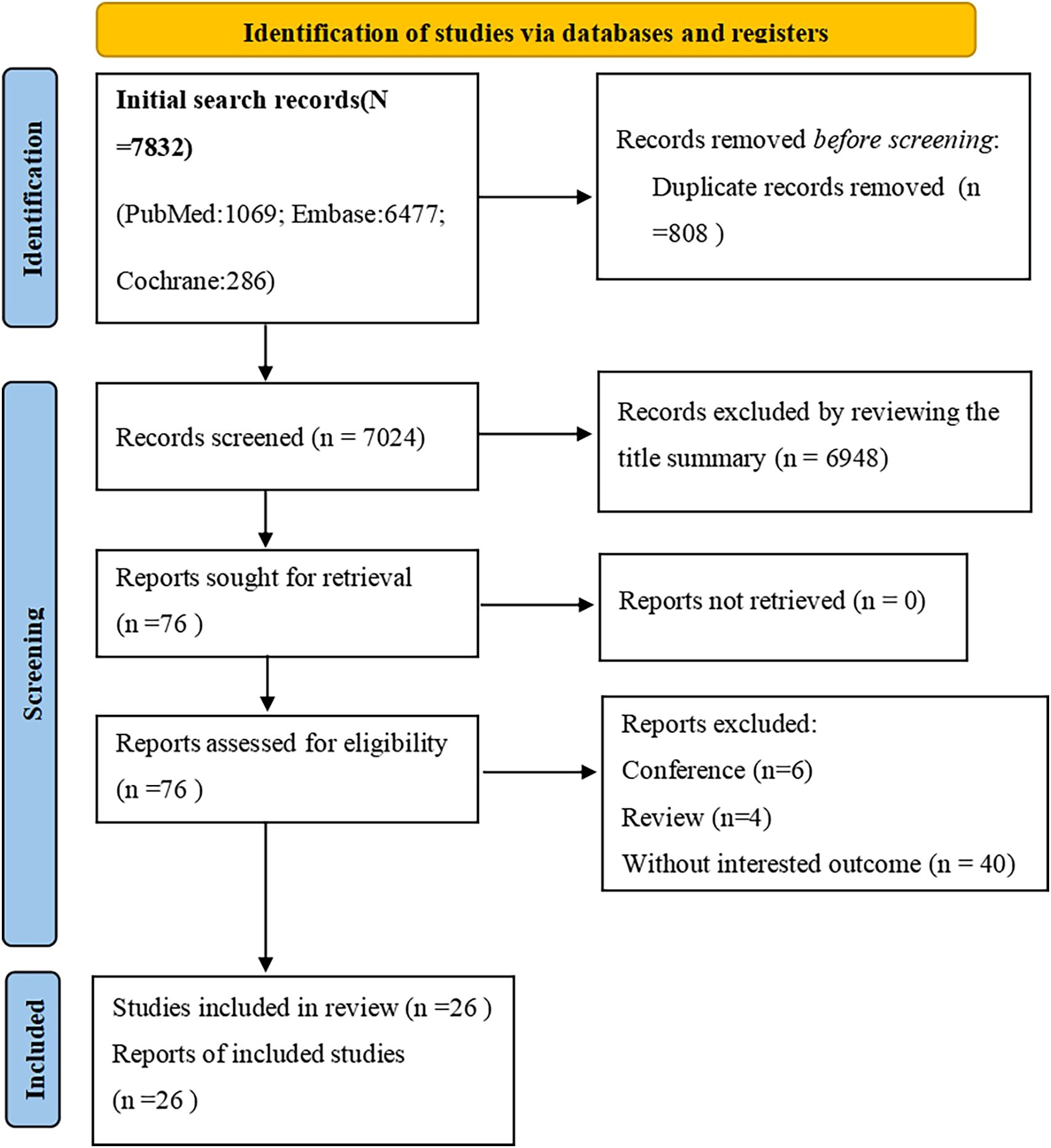
Figure 1. Studies screening process. PRISMA flow diagram of the screening and selection process according to PRISMA 2020 guidelines.
A subgroup analysis was conducted according to the following criteria: specific autoimmune disease, age, sex, and study design. In the subgroup analysis of age, the risk of onset of autoimmune diseases in adults and children is initially examined. Subsequently, the risk of the onset of specific autoimmune diseases in adults and children is analyzed.
Statistical analysis
All statistical analyses were conducted using Stata Statistical Software, version 17.0. The adjusted Hazard Ratio (HR) and its corresponding 95% confidence interval (CI) were employed to evaluate the correlation between atopic dermatitis and the risk of autoimmune disease. To assess heterogeneity, the I² statistic was employed. In consideration of the degree of heterogeneity identified, a random effects model was selected for analysis in instances where I² exceeded 50%, while a fixed effects model was employed when I² was below this threshold.
A sensitivity analysis was conducted to ensure the robustness of the overall results. The potential for publication bias was evaluated through a visual examination of the funnel plot and a statistical assessment using Egger regression.
Results
Literature search
The systematic search for studies published prior to May 26, 2024 yielded a total of 7,832 results. After the initial screening, 808 duplicate records were excluded. Additionally, 6872 articles were removed based on the screening of titles and abstracts, as they were deemed unrelated to the topic. The remaining 76 studies underwent a full-text review. Finally, 26 cohort studies were identified that reported on the association between atopic dermatitis and the risk of autoimmune disease flare-ups. The detailed study selection process is illustrated in Figure 1 (12–37).
Study characteristics
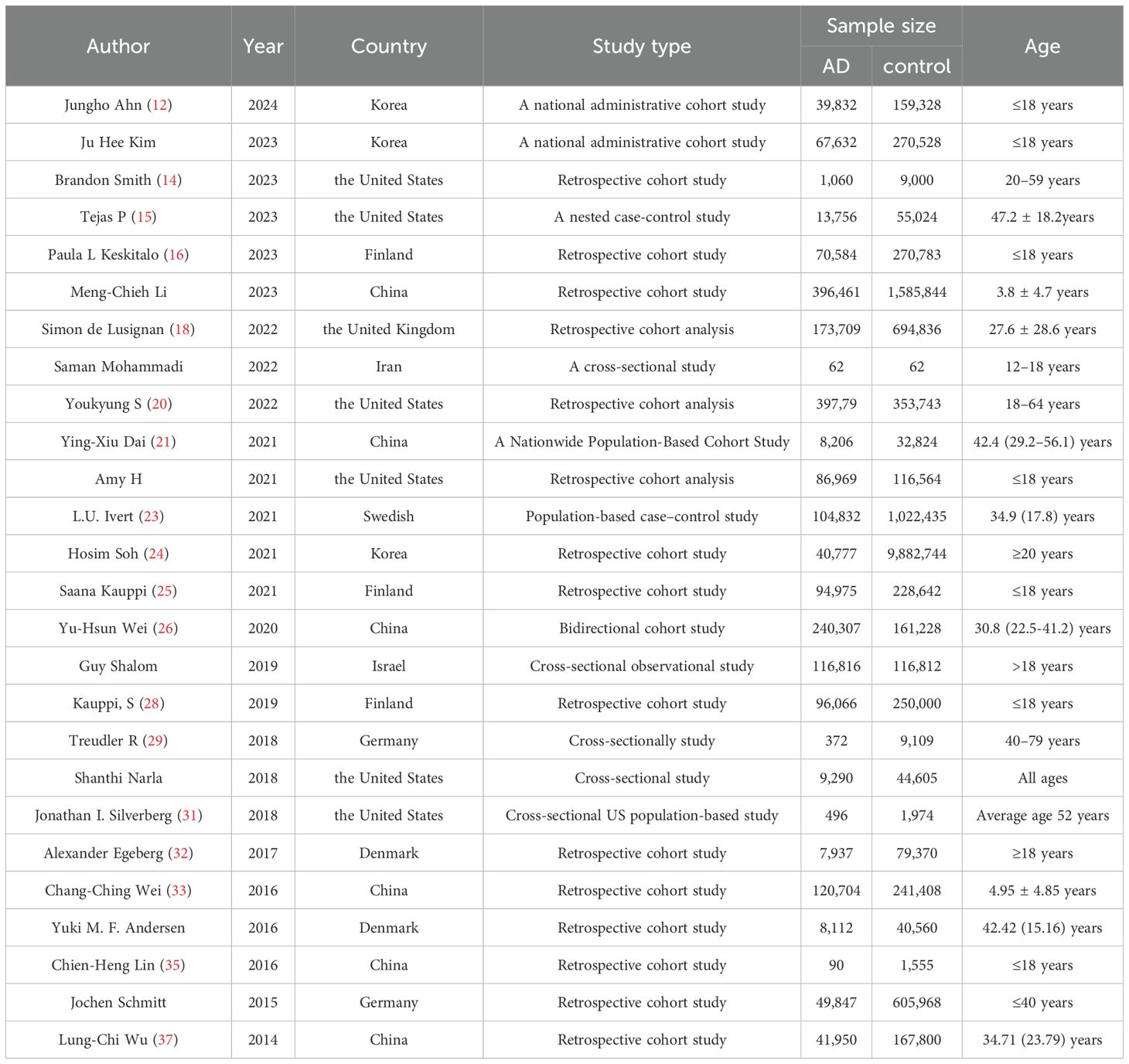
This meta-analysis included a total of 26 studies published between 2014 and 2024, comprising 19 cohort studies, 2 case-control studies, and 5 cross-sectional studies. The age distribution of the included populations was as follows: 9 studies with populations younger than 18 years of age, 8 studies with populations older than 18 years of age, 8 studies included participants across all age groups. In terms of the geographic distribution, the studies were conducted in the following countries: 6 studies each from China and the United States, 3 studies each from Korea and Finland, 2 studies each from Germany and Denmark, 1 study each from the United Kingdom, Israel, Switzerland, and Iran. All the included studies provided adjusted estimates, although the specific confounding factors adjusted for (e.g., age, sex, marital status, education level) varied slightly across the studies. The main characteristics of the included studies are shown in Table 1. Due to space limitations, additional details will be provided in Supplementary Table 4.
Quality assessment
The mean score for all included cohort studies was 7.69 based on the NOS criteria. In excess of 88% of studies achieved a score of 6 or above, with over 50% scoring 7 or above. The included scores are presented in Table 2.
Atopic dermatitis and risk of autoimmune disease
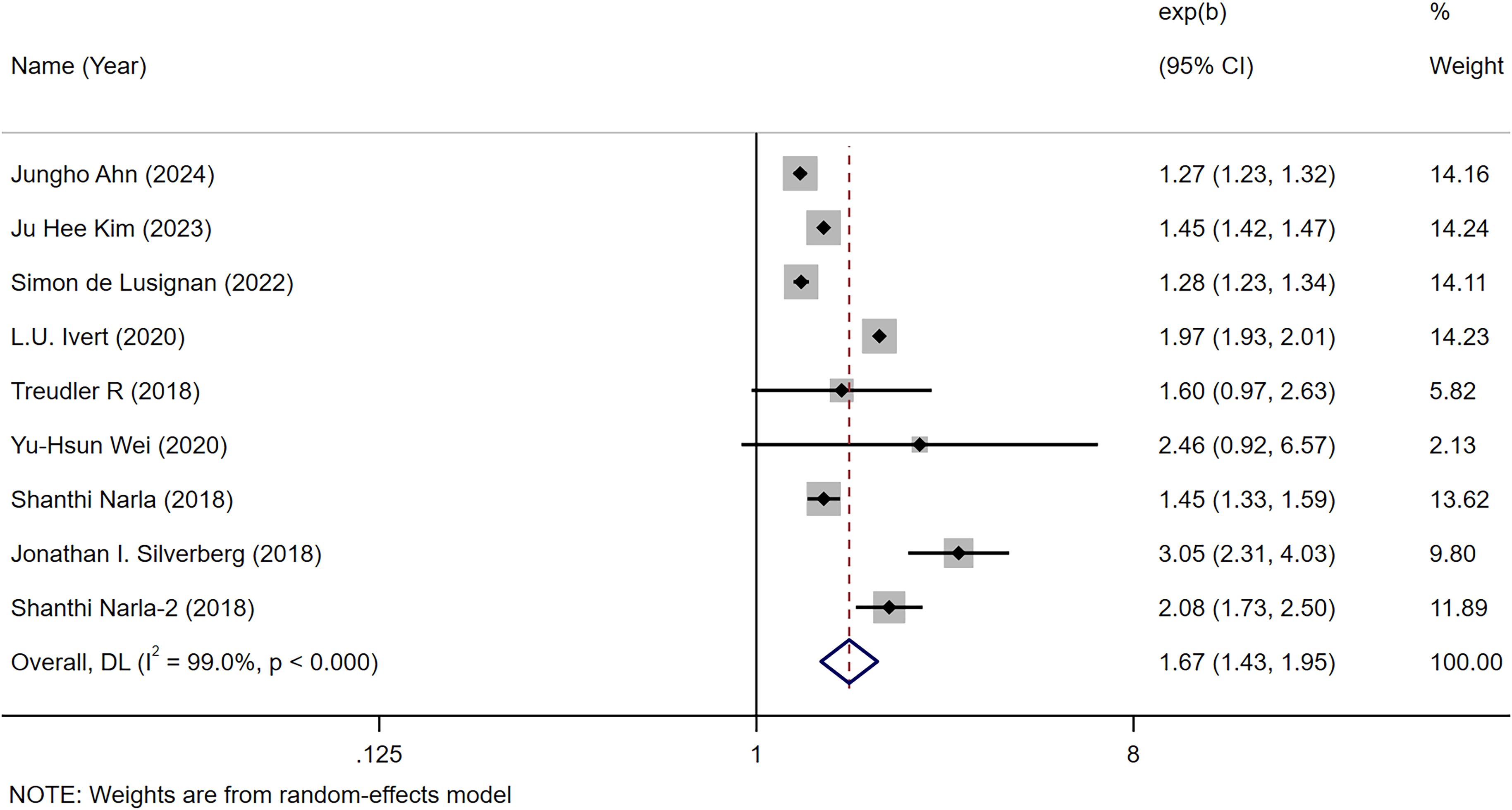
We hereby clarify that among the 26 studies included in the meta-analysis, only 9 studies explicitly reported HRs quantifying the association between AD and autoimmune diseases (collective assessment rather than disease-specific evaluation). The remaining studies primarily examined associations between AD and specific autoimmune diseases (e.g., Crohn’s disease, alopecia areata, etc.). Consequently, we conducted in-depth secondary analyses focusing specifically on these 9 studies. The combined analyses demonstrated a significant correlation between atopic dermatitis and an increased risk of autoimmune disease [OR = 1.67, 95% CI (1.43-1.95), I² = 99.0%, P<0.001] (12–37) (Figure 2). One of the articles included the risk of both AD and autoimmune disease in children and adults. This was done because the association between AD and autoimmune disease was analyzed across all ages.
Atopic dermatitis and risk of specific autoimmune disease
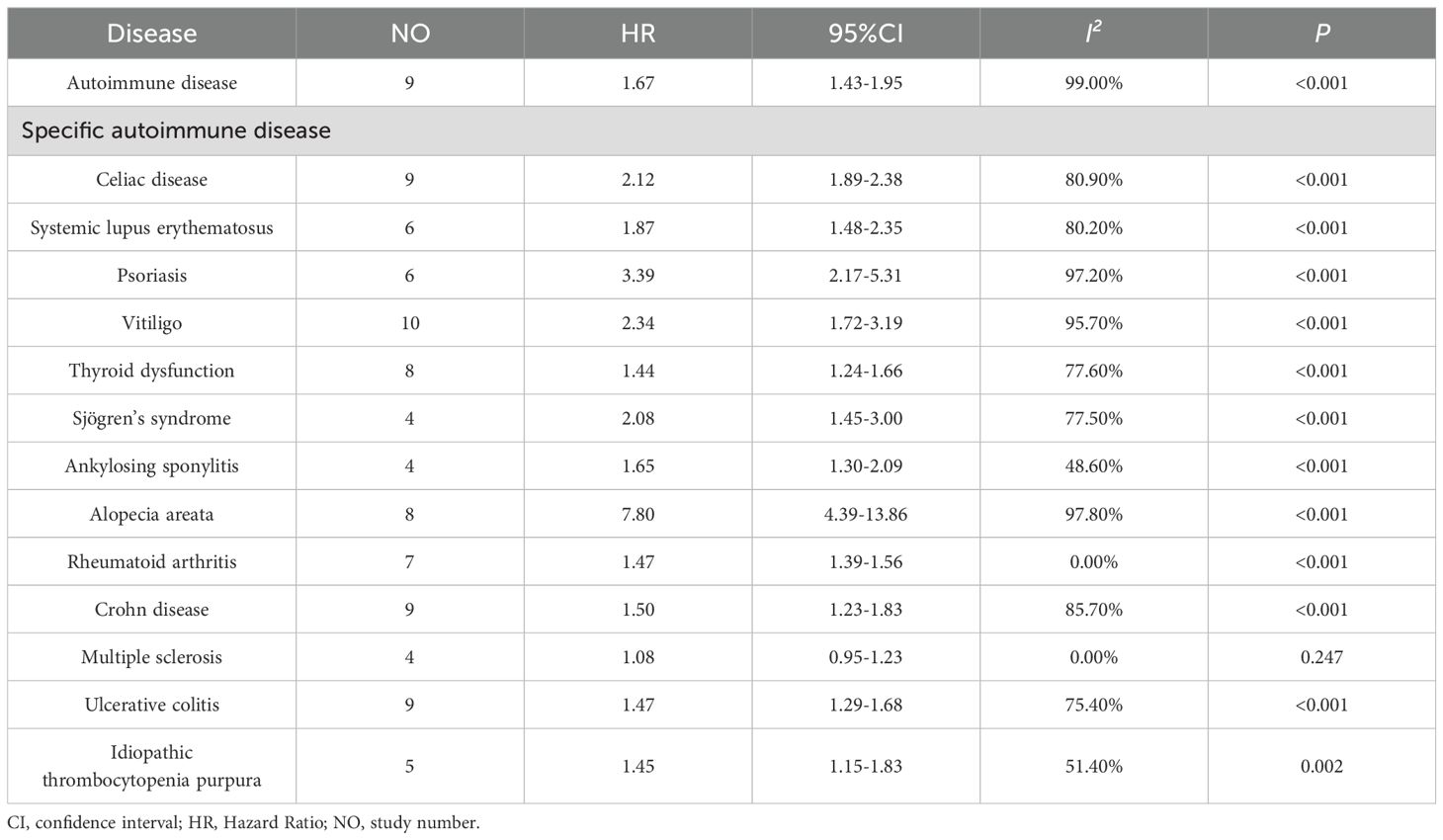
The meta-analysis identified a total of 9 studies that investigated the association between atopic dermatitis and the following autoimmune conditions: coeliac disease (9 studies) (20, 22, 23, 25, 27, 28, 30, 34), Crohn’s disease (9 studies) (12, 18, 23, 24, 30, 32, 34, 36),ulcerative colitis (9 studies) (12, 18, 23, 24, 30, 32, 34, 36), alopecia areata (8 studies) (18, 20, 22, 23, 26, 30, 34), thyroid dysfunction (8 studies) (12, 13, 18, 23, 26, 29–31), rheumatoid arthritis (7 studies) (23, 29, 30, 34, 36, 37), psoriasis (6 studies) (12, 13, 21–23, 26), systemic lupus erythematosus (6 studies) (12, 20, 23, 30, 34), idiopathic thrombocytopenic purpura (5 studies) (12, 13, 30, 33, 34), Sjogren’s syndrome (4 studies) (12, 20, 30, 34) and ankylosing spondylitis (4 studies) (12, 23, 30, 34).
The combined analysis revealed that AD was most strongly associated with an elevated risk of alopecia areata [HR = 7.80, 95% CI (4.39-13.86), I² = 97.80%, P<0.001]. The results demonstrated that AD was significantly associated with an increased risk of psoriasis [HR = 3.39, 95% CI (2.17-5.31), I² = 97.20%, P<0.001], celiac disease [HR = 2.12, 95% CI (1.88-2.38), I² = 80.9%, P<0.001], vitiligo [HR = 2.34, 95% CI (1.72-3.19), I² = 95.70%, P<0.001]. Furthermore, AD was also found to be significantly associated with an increased risk of systemic lupus erythematosus [HR=1.87,95%CI(1.48-2.35,I² = 80.2%, P<0.001], Sjogren’s syndrome [HR = 2.08, 95% CI (1.45-3.00), I² = 77.50%, P<0.001], ankylosing spondylitis [HR = 1.65, 95% CI (1.30-2.09), I² = 48.60%, P<0.001], rheumatoid arthritis (HR = 1.47, 95% CI (1.39-1.56), I² = 0.00%, P<0.001], ankylosing spondylitis [HR = 1.65, 95% CI (1.30-2.10), I² = 48.60%, P<0.001], Crohn’s disease [HR= 1.50, 95% CI (1.23-1.83), I² = 85.70%, P<0.001], ulcerative colitis [HR=1.47,95%CI(1.29-1.68), I² =75.40%, P<0.001], idiopathic thrombocytopenia purpura [HR=1.45, 95% CI (1.15-1.83), I² = 51.40%, P = 0.002], thyroid dysfunction [HR=1.44, 95% CI (1.24-1.66), I² = 77.60%, P<0.001]. AD was hardly associated with an increased risk of multiple sclerosis [HR = 1.08, 95% CI (0.95-1.23, I² = 0.00%, P = 0.247 >0.05] (Table 3). A detailed forest map are shown in Supplementary Figures 1-14.
Subgroup analysis
It is noteworthy that among the 26 selected pieces of literature, only nine explicitly proposed the HR value between AD and autoimmune diseases. Of these nine, three explicitly discussed the risk of children and autoimmune diseases, two discussed the risk of adults and autoimmune diseases, and the remaining four covered all age groups it was not possible to discern whether the age group was related.
There is a statistically significant correlation between individuals with AD aged ≤18 years and autoimmune disease[HR=1.49, 95% CI 1.31-1.70; I²=96.5%, P<0.001]. In the context of AD and specific autoimmune diseases, adolescents with AD exhibit an extreme susceptibility to the complication of psoriasis [HR=4.12, 95% CI (2.38-7.13); I²=98.3%, P<0.001], followed by coeliac disease [HR=2.08, 95% CI(1.91-2.26); I²=0.00%, P<0.001]. Furthermore, there is an increased susceptibility to juvenile arthritis [HR=1.36, 95% CI (1.20-1.54); I²=58.60%, P<0.001], vitiligo[HR=1.97,95% CI (1.47-2.64); I²=93.30%, P<0.001], idiopathic thrombocytopenia purpura [HR=1.39, 95% CI (1.10-1.76);I²=53.90%, P=0.006], and thyroid dysfunction [HR=1.60, 95% CI (1.08-2.36); I²=74.50%, P=0.018]. A detailed forest map are shown in Supplementary Figures 14, 15.
In contrast, only two studies demonstrated a statistically significant correlation between atopic dermatitis and autoimmune disease in the AD population aged over 18 years [HR=1.75, 95% CI (1.12-2.72); I²=0.00%, P=0.014]. In the case of adult AD patients, there is an increased susceptibility to celiac disease [HR=2.60, 95% CI (1.61-4.20); I²=94.80%, P<0.001], Crohn’s disease [HR=1.88, 95% CI (1.52-2.31); I²=0.00%, P<0.001], IBD [HR=1.72, 95% CI (1.43-2.06); I²=26.30%, P<0.001], ulcerative colitis [HR=1.70, 95% CI (1.48-1.96); I²=0.00%, P<0.001], thyroid dysfunction [HR=1.43, 95% CI (1.28-1.70); I²=77.80%, P<0.001]. A detailed forest map are shown in Supplementary Figures 14, 16.
Of the nine articles of atopic dermatitis and the risk of autoimmune disease, four addressed the risk of autoimmune disease with AD in women, and two addressed the risk of autoimmune disease in men with AD. Female patients with AD were more likely to have autoimmune diseases [HR=1.49, 95%CI(1.15-1.91); I²=98.8%, P=0.002]. A detailed forest map are shown in Supplementary Figure 17.
In subgroup analyses by study design, meta-analyses of Cross-sectional studies demonstrated a significant association between AD and an increased risk of autoimmune disease [HR=1.96, 95% CI (1.39-2.77); I²=90.8%, P<0.001], whereas meta-analyses of cohort studies showed a similar association between AD and the risk of autoimmune disease [HR=1.34, 95% (CI 1.22-1.48); I²=95.0%, P<0.001] (Table 4). A detailed forest maps are shown in Supplementary Figure 18.
Publication bias
A visual inspection of the funnel plot did not reveal any evidence of significant publication bias in the assessment of the risk of comorbid autoimmune diseases in patients with atopic dermatitis. To formally evaluate publication bias, the Egger test was conducted. The results of this test confirmed the absence of statistically significant publication bias (P = 0.928). The funnel plot is presented in Figure 3.
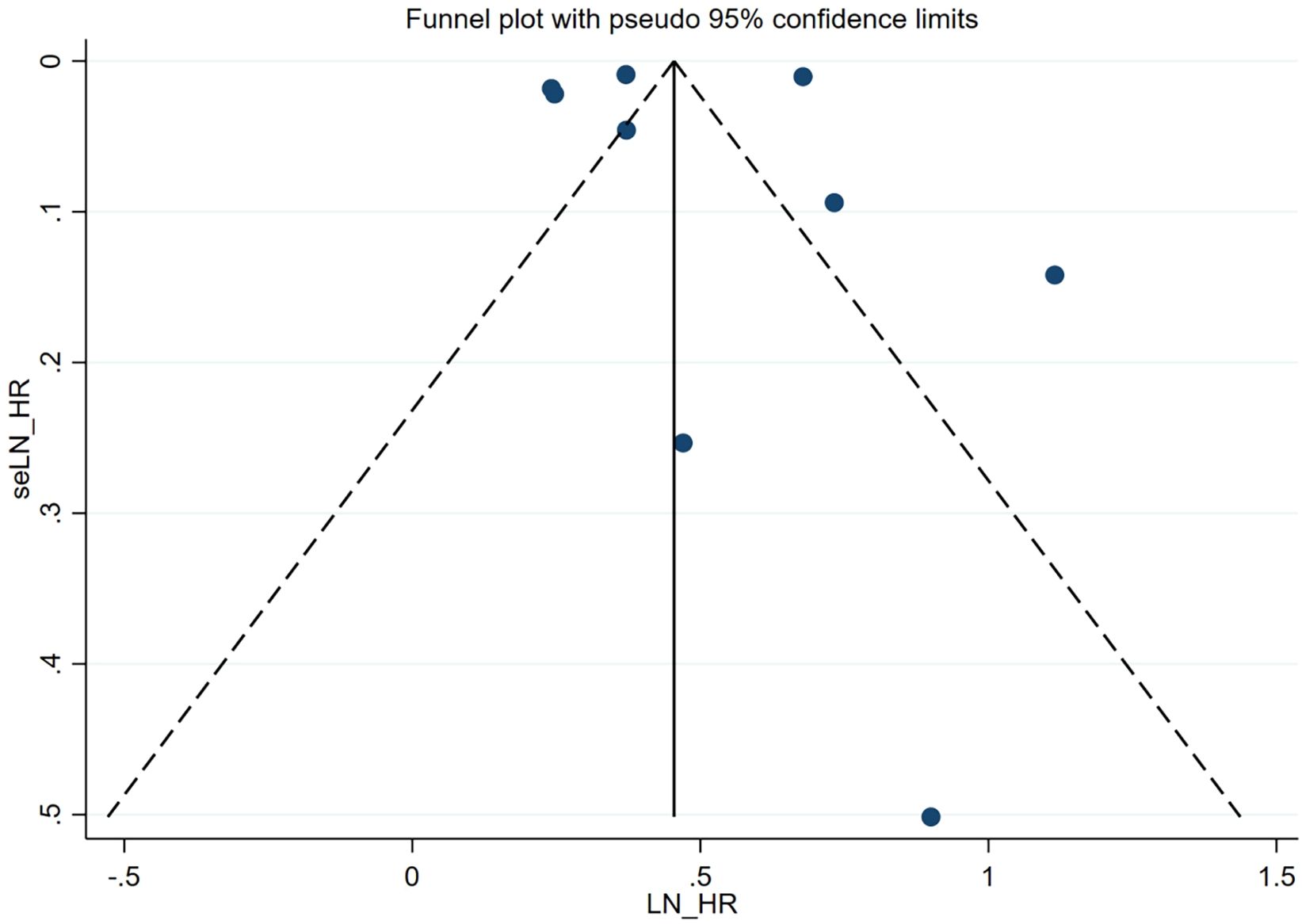
Figure 3. Funnel plot. Publication bias of the risk of atopic dermatitis associated with autoimmune diseases.
Discussion
Main findings
The meta-analysis included a total of 26 studies providing a comprehensive assessment of the association between AD and the risk of developing autoimmune diseases. The results demonstrated a significant association between AD and an increased risk of autoimmune conditions across all age groups, including conditions such as coeliac disease, systemic lupus erythematosus, alopecia areata, rheumatoid arthritis, Crohn’s disease, ulcerative colitis and idiopathic thrombocytopenia purpura. Further subgroup analyses, including age, gender, and studies design (cohort vs case-control), revealed that the significant correlation between AD and elevated autoimmune disease risk was consistent across these different factors.
This large-scale, comprehensive meta-analysis comprehensively characterizes the heightened risk of a broad spectrum of autoimmune conditions in patients with atopic dermatitis, underscoring the importance of this relationship for clinical practice and patient management.
Interpretation of findings
A previous review encompassed 14 studies that investigated the markedly elevated risk of selected autoimmune diseases in patients with AD (9). The findings indicated that AD can markedly increase the risk of autoimmune diseases, including systemic lupus erythematosus, vitiligo, coeliac disease, Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and others (9). We conducted separate analyses of autoimmune disease incidence in both pediatric (≤18 years) and adult AD patient populations. The results demonstrated significant associations between AD and autoimmune disease incidence in both age groups. Notably, substantial heterogeneity (I²=96.5%) was observed in the pediatric AD analysis. Given the limited number of included studies (n=3, comprising two South Korean studies and one US study), we performed additional country-stratified analyses. These analyses revealed a marked reduction in heterogeneity following country stratification (Supplementary Figure 19), strongly suggesting that geographic variations (potentially including differences in diagnostic criteria, environmental factors, or genetic backgrounds) represent a key contributor to the initial high heterogeneity. These findings provide novel insights into the AD-autoimmune disease relationship while highlighting the importance of considering geographic factors in future multicenter studies.
While the previous review incorporated a study on both adults and children (30), it did not elaborate on the potential differences in the autoimmune disease risk between these two populations.
This is an important consideration, as a substantial body of literature has documented the high prevalence of AD in pediatric patients, with rates reaching up to 20% in children in high-income countries (2, 38, 39). Children with AD often experience disrupted sleep, daytime fatigue, and academic underperformance (40, 41), which can result in depressive and psychological symptoms (42). Furthermore, psychological stress can influence the progression and outcome of the autoimmune disease (43).
Furthermore, the relative immaturity of the adolescent immune system (44, 45) — characterized by incomplete development of secondary lymphoid organs and immature immunoregulatory networks—must be carefully considered. Notably, given that 70-80% of immune cells reside in the gut, intricate interactions exist among the gut microbiota, intestinal epithelium, and local mucosal immune system (44). In pediatric AD patients (particularly preschoolers), the developmental stage of the immune system(e.g., gut) differs substantially from adolescents. This immunological dynamism may profoundly influence the patterns of autoimmune comorbidity, necessitating age-stratified investigations (45).
Of particular significance, genomic studies have identified numerous shared genetic susceptibility factors between AD and autoimmune diseases. These include: (1) Specific HLA haplotypes (e.g., HLA-DR4, HLA-DQ8) that demonstrate established associations with both AD and multiple autoimmune disorders (46, 47); and (2) Variations in key epithelial barrier gene networks (e.g., FLG, SPINK5) that not only compromise skin barrier integrity but may also participate in systemic autoimmune responses through epigenetic regulation (48). Importantly, the expression patterns of these genetic factors in children may exhibit unique characteristics due to immune system immaturity, offering novel insights into the pathogenesis of autoimmune comorbidities in pediatric AD patients.
Additionally, given that the immune system of adolescents is not yet fully developed (49, 50), the potential for autoimmune complications in children with AD requires careful consideration and further investigation.
The findings of our study indicate that both adults and children with AD are susceptible to complications associated with autoimmune diseases. Interestingly, the results suggest that adolescents may experience a greater range of autoimmune disease-related complications compared to other age groups.
There is a possibility that children may be more susceptible to atopic dermatitis as a result of a number of factors. As individuals age, a strong immune-activating effect has been observed in the skin lesions of pediatric AD patients in response to Th2, Th9 and Th17, with elevated levels of Th2 and Th17 markers in the blood (51, 52). The prevailing view of pediatric AD is that it is driven primarily by Th2 signaling.Th2 cells secrete cytokines such as IL-4 and IL-13, which not only mediate IgE class switching in B cells but also upregulate FcϵRI receptor expression on mast cells/basophils, thereby enhancing IgE-mediated immune responses (53, 54). Elevated IgE levels have been identified as a potential risk factor for the development of allergic diseases (55, 56). Following birth and throughout childhood, when the gut microbial system is not yet fully developed, the natural maturation of the immune system is inhibited, and Tregs fail to mature sufficiently to regulate certain balances, such as those between Th1 and Th2 (57). Furthermore, the human skin barrier is structurally and functionally immature at birth. This is evidenced by elevated skin surface pH, lower lipid levels, and lower resistance to chemicals and pathogens (58). It is evident that these factors have the potential to increase the incidence of AD in children. Furthermore, children are more susceptible to the influence of family members. For example, there is a clear association between the mother and the child’s AD disease. It has been documented that Maternal diet during pregnancy、timing of complementary food introduction、prenatal/early-life probiotic/prebiotic supplementation (59, 60),the status of maternal intestinal flora (61), maternal antibiotic use during pregnancy (62) and even maternal constipation (63) seems to be related to the pathophysiological development of atopic dermatitis in children. As will be discussed subsequently, it seems reasonable to posit that the fact that children are undergoing growth and that their immune systems are inherently unstable, coupled with the fact that they are also susceptible to genetic influences that are contributing to an increase in the prevalence of childhood conditions, is the reason behind this phenomenon.
In comparison to the immune system of a child, the adult immune system has matured and is capable of effectively regulating the body’s immune response. Similarly, adults are susceptible to increased morbidity as a result of prolonged stress, encompassing familial, occupational, and social stressors (64).The prevalence of AD in adults is associated with a significant financial burden on healthcare systems, including increased direct and indirect costs of care and lost productivity (65). It is evident that greater attention should be paid to the impact of AD on our lives, and to the disease itself.
Lastly, the study reveals that women with AD are at a higher risk of developing autoimmune diseases compared to men. This phenomenon may be related to the differential effects of sex hormones on Th2/Treg and Th1/Th17 cell activities (66–69). These comprehensive findings underscore the complex interplay between AD, the immune system, the gut microbiome, and various genetic, hormonal, and environmental factors in modulating the risk of autoimmune diseases, particularly in the adolescent population.
Implications and limitations
The principal strength of our meta-analysis was the inclusion of 26 pertinent observational studies, thereby ensuring a robust assessment of the association between atopic dermatitis and the risk of autoimmune disease. The large combined sample size enabled an effective assessment of this association. Our findings indicate that atopic dermatitis is a significant risk factor for autoimmune and other diseases. It is noteworthy that the analysis included both gender and article type, effectively managing confounding factors and improving the reliability of the conclusions.
It is important to consider the limitations of the meta-analysis. Firstly, the present study only considered articles published during the ten years 2014-2024. However, it would be possible to extend the period under review in future studies, thereby increasing the sample size for analysis. Secondly, although the fully adjusted estimated effect with 95% CI was extracted, adjusted confounders (e.g. medication use and socioeconomic status, etc.) were not consistent across the included studies. Thirdly, when discussing subgroup analyses of age, we undertook a strict differentiation between the discussion of samples of children and adults. As the inclusion of the majority of the literature was straightforward in discussing each age group together, the number of studies examining the onset of autoimmune disease in children versus adults with AD alone was limited. Further studies are required to analyze and discuss this issue in greater depth.
Fourthly, our analysis demonstrates a significant association between AD and autoimmune diseases (rather than specific autoimmune diseases), despite considerable heterogeneity (I²=96.5%). The limited number of eligible studies meeting our inclusion criteria (n=3) precluded further subgroup analyses to investigate potential sources of this heterogeneity. To address this limitation, we conducted supplementary analyses in Table 4 examining associations between ≤18-years AD patients and specific autoimmune diseases, including type 1 diabetes, juvenile idiopathic arthritis, and autoimmune thyroiditis. These analyses revealed statistically significant associations for all evaluated conditions (all P<0.05), except for type 1 diabetes which showed no significant correlation. While these findings hold potential clinical relevance, the interpretation requires caution due to the limited number of primary studies and substantial heterogeneity, underscoring the need for validation through larger-scale, well-designed prospective studies.
Finally, approximately 75% of studies included in our analysis employed retrospective designs, which indeed introduces limitations, particularly impacting the reliability of causal inferences for children and adolescents (≤18 years). While our meta-analysis demonstrated significant associations between AD (especially childhood-onset) and various autoimmune diseases, the predominantly retrospective nature of included studies suggests these findings should be interpreted as suggestive rather than conclusive evidence. The conclusion explicitly emphasizes the need for cautious interpretation of results for the ≤18 years population and strongly advocates for validation through future prospective cohort studies.
Future prospects
Based on current Mendelian randomization evidence and the meta-analysis findings from our study, there exists a clear epidemiological association and potential causal relationship between AD and autoimmune diseases. Building upon these discoveries, future research should focus on elucidating the precise pathogenic mechanisms linking these conditions and exploring clinical translation. Regarding mechanistic investigations, the ‘epithelial-immune axis’ hypothesis provides an important framework for understanding this association: persistent skin barrier dysfunction in AD patients may trigger systemic immune dysregulation through the release of epithelial-derived alarmins such IL-33, ultimately leading to aberrant activation of autoimmune responses. This hypothesis can be tested including but not limited to: (1) establishing an MC903-induced AD animal model followed by long-term (≥6 months) follow-up observations to determine whether spontaneous autoimmune manifestations develop; (2) employing organoid co-culture systems to simulate epithelial-immune cell interactions; and (3) utilizing humanized mouse models to investigate the role of alarmins such as IL-33 (54, 70–72).
At the clinical practice level, we recommend implementing risk-stratified management for AD patients across both adult and pediatric populations, with particular surveillance recommended for comorbid autoimmune conditions including alopecia areata, psoriasis, celiac disease, rheumatoid arthritis, and ulcerative colitis (see Table 5 for detailed stratification criteria). Particular attention should be given to patients with either a family history of autoimmune diseases or refractory dermatitis. Furthermore, conducting randomized controlled studies to compare the therapeutic efficacy between conventional immunosuppressants (e.g., methotrexate 10–15 mg/week) and novel biologics (e.g., anti-IL-4Rα monoclonal antibodies) in AD patients with comorbid autoimmune diseases will provide crucial evidence-based guidance for clinical decision-making.
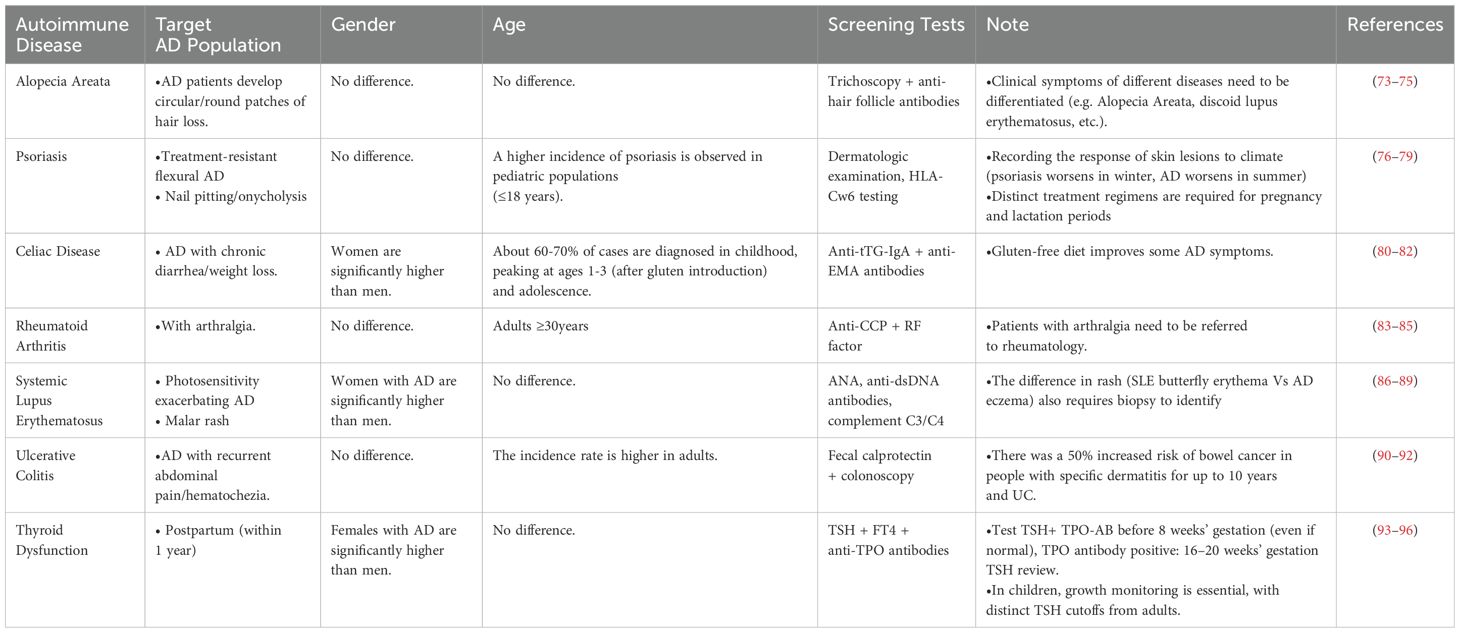
Table 5. Evidence-Based Screening Recommendations for Autoimmune Diseases in Atopic Dermatitis (AD) Patients.
Conclusion
This comprehensive meta-analysis assessed the risk of autoimmune diseases in patients with atopic dermatitis. The results demonstrated a significant association between AD and an increased risk of developing a broad range of autoimmune conditions, including alopecia areata, coeliac disease, Sjögren’s syndrome, ulcerative colitis, and others.
These findings underscore the importance for clinicians to have a high index of suspicion for potential autoimmune diseases when evaluating and managing patients diagnosed with atopic dermatitis.
The strong link between AD and elevated autoimmune disease risk highlighted by this meta-analysis has critical implications for clinical practice. Clinicians should consider screening for and closely monitoring AD patients for the development of associated autoimmune conditions to enable early intervention and improved patient outcomes.
These comprehensive meta-analysis results emphasize the need for a multidisciplinary, integrated approach to the management of atopic dermatitis, incorporating vigilance for comorbid autoimmune diseases. This will be essential for providing optimal, holistic care for individuals suffering from this complex, multisystem condition.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
HW: Data curation, Investigation, Writing – original draft. MC: Investigation, Writing – original draft. TW: Investigation, Writing – original draft. WC: Data curation, Writing – original draft. XL: Data curation, Writing – original draft. LH: Writing – review & editing. MW: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was funded by the Key R&D Program of Zhejiang (NO. 2023C03040), the Research Project of Zhejiang Chinese Medical University(2023RCZXZK18).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1539997/full#supplementary-material
Abbreviations
AD, Atopic dermatitis; PRISMA, Preferred reporting items for systematic reviews and meta-analyses; PROSPERO, International prospective register of systematic reviews; MeSH, Medical subject headings; HR, Hazard Ratio; CI, Confidence interval; NOS, Newcastle-Ottawa scale; ICD, International classification of diseases; IgE, Immunoglobulin E; US, the United States; UK, the United Kingdom; PAN, cells Paneth cells; IL-10, Interleukin-10.
References
1. Laughter MR, Maymone MBC, Mashayekhi S, Arents BWM, Karimkhani C, Langan SM, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990-2017. Br J Dermatol. (2021) 184:304–9. doi: 10.1111/bjd.v184.2
2. Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. (2015) 66 Suppl 1:8–16. doi: 10.1159/000370220
3. Bylund S, Kobyletzki LB, Svalstedt M, and Svensson Å. Prevalence and incidence of atopic dermatitis: A systematic review. Acta Derm Venereol. (2020) 100:adv00160. doi: 10.2340/00015555-3510
5. Sroka-Tomaszewska J and Trzeciak M. Molecular mechanisms of atopic dermatitis pathogenesis. Int J Mol Sci. (2021) 22(8):4130. doi: 10.3390/ijms22084130
6. Holmes J, Fairclough LC, and Todd I. Atopic dermatitis and autoimmunity: the occurrence of autoantibodies and their association with disease severity. Arch Dermatol Res. (2019) 311:141–62. doi: 10.1007/s00403-019-01890-4
7. Fugger L, Jensen LT, and Rossjohn J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell. (2020) 181:63–80. doi: 10.1016/j.cell.2020.03.007
8. Zhou W, Cai J, Li Z, and Lin Y. Association of atopic dermatitis with autoimmune diseases: A bidirectional and multivariable two-sample mendelian randomization study. Front Immunol. (2023) 14:1132719. doi: 10.3389/fimmu.2023.1132719
9. Lu Z, Zeng N, Cheng Y, Chen Y, Li Y, Lu Q, et al. Atopic dermatitis and risk of autoimmune diseases: a systematic review and meta-analysis. Allergy Asthma Clin Immunol. (2021) 17:96. doi: 10.1186/s13223-021-00597-4
10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, and Mulrow CD. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
11. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
12. Ahn J, Shin S, Lee GC, Han BE, Lee E, and Ha EK. Unraveling the link between atopic dermatitis and autoimmune diseases in children: Insights from a large-scale cohort study with 15-year follow-up and shared gene ontology analysis. Allergol Int. (2024) 73:243–54. doi: 10.1016/j.alit.2023.12.005
13. Kim JH, Lee E, Ha EK, Shin J, Lee GC, and Rha YH. Cascade of atopic dermatitis comorbidities in children after birth for 15 years. Allergy. (2024) 79:153–63. doi: 10.1111/all.15917
14. Smith B, Collier MR, Devjani S, Han G, and Wu JJ. Association between atopic dermatitis and thyroid disease among U.S. adults in the 2001–2006 National Health and Nutrition Examination Survey. J Am Acad Dermatol. (2023) 88:889–91. doi: 10.1016/j.jaad.2022.10.017
15. Joshi TP, Bancroft A, Garcia D, Kahla JA, McBee DB, Duvic M, et al. Association of atopic dermatitis with Graves’ disease and Hashimoto’s thyroiditis: A case-control study in the All of Us research program. J Am Acad Dermatol. (2023) 89:e175–6. doi: 10.1016/j.jaad.2023.04.073
16. Keskitalo PL, Jokelainen J, Tasanen K, Sinikumpu SP, and Huilaja L. Juvenile idiopathic arthritis in children and adolescents with atopic dermatitis: A Finnish nationwide registry study. J Am Acad Dermatol. (2023) 88:1187–9. doi: 10.1016/j.jaad.2022.12.025
17. Li MC, Wu CY, Chang YT, Lyu YS, and Wu CY. Risk of type 1 diabetes mellitus in patients with atopic dermatitis: A nationwide population-based cohort study. Dermatology. (2024) 240:254–61. doi: 10.1159/000535848
18. Lusignan S, Alexander H, Broderick C, Dennis J, McGovern A, Feeney C, et al. Atopic dermatitis and risk of autoimmune conditions: Population-based cohort study. J Allergy Clin Immunol. (2022) 150:709–13. doi: 10.1016/j.jaci.2022.03.030
19. Campos-Alberto E, Hirose T, Napatalung L, and Ohyama M. Prevalence, comorbidities, and treatment patterns of Japanese patients with alopecia areata: A descriptive study using Japan medical data center claims database. J Dermatol. (2023) 50:37–45. doi: 10.1111/1346-8138.16615
20. Roh YS, Huang AH, Sutaria N, Choi U, Wongvibulsin S, Choi J, et al. Real-world comorbidities of atopic dermatitis in the US adult ambulatory population. J Am Acad Dermatol. (2022) 86:835–45. doi: 10.1016/j.jaad.2021.11.014
21. Dai YX, Tai YH, Chang YT, and Chen TJ, and Chen MH. Bidirectional association between psoriasis and atopic dermatitis: A nationwide population-based cohort study. Dermatology. (2021) 237:521–7. doi: 10.1159/000514581
22. Huang AH, Roh YS, Sutaria N, Choi J, Williams KA, Canner JK, et al. Real-world comorbidities of atopic dermatitis in the pediatric ambulatory population in the United States. J Am Acad Dermatol. (2021) 85:893–900. doi: 10.1016/j.jaad.2021.03.016
23. Ivert LU, Wahlgren CF, Lindelöf B, Dal H, Bradley M, Johansson EK, et al. Association between atopic dermatitis and autoimmune diseases: a population-based case-control study. Br J Dermatol. (2021) 185:335–42. doi: 10.1111/bjd.v185.2
24. Soh H, Lee HJ, Han K, Park S, Hong SW, Moon JM, et al. Atopic diseases are associated with development of inflammatory bowel diseases in Korea: A nationwide population-based study. Clin Gastroenterol Hepatol. (2021) 19:2072–2081.e2076. doi: 10.1016/j.cgh.2020.07.049
25. Kauppi S, Jokelainen J, Timonen M, and Tasanen K, and Huilaja L. Atopic dermatitis is associated with dermatitis herpetiformis and celiac disease in children. J Invest Dermatol. (2021) 141:191–193.e192. doi: 10.1016/j.jid.2020.05.091
26. Wei YH, Tai YH, Dai YX, Chang YT, and Chen TJ, Chen MH, et al. Bidirectional association between alopecia areata and atopic dermatitis: A population-based cohort study in Taiwan. Clin Exp Allergy. (2020) 50:1406–14. doi: 10.1111/cea.v50.12
27. Shalom G, Kridin K, Raviv KO, Freud T, Comaneshter D, Friedland R, et al. Atopic dermatitis and celiac disease: A cross-sectional study of 116,816 patients. Am J Clin Dermatol. (2020) 21:133–8. doi: 10.1007/s40257-019-00474-2
28. Kauppi S, Jokelainen J, Timonen M, and Tasanen K, and Huilaja L Adult patients with atopic eczema have a high burden of psychiatric disease: A finnish nationwide registry study. Acta Derm Venereol. (2019) 99:647–51. doi: 10.2340/00015555-3165
29. Treudler R, Zeynalova S, Walther F, and Engel C, and Simon JC. Atopic dermatitis is associated with autoimmune but not with cardiovascular comorbidities in a random sample of the general population in Leipzig, Germany. J Eur Acad Dermatol Venereol. (2018) 32:e44–6. doi: 10.1111/jdv.2018.32.issue-2
30. Narla S and Silverberg JI. Association between atopic dermatitis and autoimmune disorders in US adults and children: A cross-sectional study. J Am Acad Dermatol. (2019) 80:382–9. doi: 10.1016/j.jaad.2018.09.025
31. Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Association of atopic dermatitis with allergic, autoimmune, and cardiovascular comorbidities in US adults. Ann Allergy Asthma Immunol. (2018) 121:604–612.e603. doi: 10.1016/j.anai.2018.07.042
32. Egeberg A, Andersen YM, Gislason GH, and Skov L, and Thyssen JP. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy. (2017) 72:783–91. doi: 10.1111/all.2017.72.issue-5
33. Wei CC, Lin CL, and Shen TC, and Tsai JD. Atopic dermatitis and association of risk for primary immune thrombocytopenia and autoimmune diseases among children: A nationwide population-based cohort study. Med (Baltimore). (2016) 95:e4226. doi: 10.1097/MD.0000000000004226
34. Andersen YM, Egeberg A, Gislason GH, Skov L, and Thyssen JP. Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. (2017) 76:274–280.e271. doi: 10.1016/j.jaad.2016.08.047
35. Lin CH, Lin CL, Shen TC, and Wei CC. Epidemiology and risk of juvenile idiopathic arthritis among children with allergic diseases: a nationwide population-based study. Pediatr Rheumatol Online J. (2016) 14:15. doi: 10.1186/s12969-016-0074-8
36. Schmitt J, Schwarz K, Baurecht H, Hotze M, Fölster-Holst R, Rodríguez E, et al. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J Allergy Clin Immunol. (2016) 137:130–6. doi: 10.1016/j.jaci.2015.06.029
37. Wu LC, Hwang CY, Chung PI, Hua TC, Chen YD, Chu SY, et al. Autoimmune disease comorbidities in patients with atopic dermatitis: a nationwide case-control study in Taiwan. Pediatr Allergy Immunol. (2014) 25:586–92. doi: 10.1111/pai.2014.25.issue-6
38. Anania C, Brindisi G, Martinelli I, Bonucci E, D'Orsi M, Ialongo S, et al. Probiotics function in preventing atopic dermatitis in children. Int J Mol Sci. (2022) 23(10):5409 doi: 10.3390/ijms23105409
39. Al-Naqeeb J, Danner S, Fagnan LJ, Ramsey K, Michaels L, Mitchell J, et al. The burden of childhood atopic dermatitis in the primary care setting: A report from the meta-LARC consortium. J Am Board Fam Med. (2019) 32:191–200. doi: 10.3122/jabfm.2019.02.180225
40. Tracy A, Bhatti S, and Eichenfield LF. Update on pediatric atopic dermatitis. Cutis. (2020) 106:143–6. doi: 10.12788/cutis
41. Drucker AM. Atopic dermatitis: Burden of illness, quality of life, and associated complications. Allergy Asthma Proc. (2017) 38:3–8. doi: 10.2500/aap.2017.38.4005
42. Kyung Y, Choi MH, Jeon YJ, Lee JS, Lee JH, Jo SH, et al. Association of atopic dermatitis with suicide risk among 788,411 adolescents: A Korean cross-sectional study. Ann Allergy Asthma Immunol. (2020) 125:55–64. doi: 10.1016/j.anai.2020.03.023
43. Ilchmann-Diounou H and Menard S. Psychological stress, intestinal barrier dysfunctions, and autoimmune disorders: an overview. Front Immunol. (2020) 11:1823. doi: 10.3389/fimmu.2020.01823
44. Wiertsema SP, Bergenhenegouwen van J, Garssen J, and Knippels LMJ. The interplay between the gut microbiome and the immune system in the context of infectious diseases throughout life and the role of nutrition in optimizing treatment strategies. Nutrients. (2021) 13(3):886. doi: 10.3390/nu13030886
45. Degboe Y, Vastert SJ, Prakken BJ, and McInnes IB. How does age determine the development of human immune-mediated arthritis? Nat Rev Rheumatol. (2022) 18:501–12. doi: 10.1038/s41584-022-00814-3
46. Margolis DJ, Duke JL, Mitra N, Berna RA, Hoffstad OJ, Wasserman JR, et al. A combination of HLA-DP α and β chain polymorphisms paired with a SNP in the DPB1 3’ UTR region, denoting expression levels, are associated with atopic dermatitis. Front Genet. (2023) 14:1004138. doi: 10.3389/fgene.2023.1004138
47. Zhu X and Wu W. The causal relationship between immune cells and atopic dermatitis: A bidirectional Mendelian randomization study. Skin Res Technol. (2024) 30:e13858. doi: 10.1111/srt.13858
48. Baloh CH and Mathias RA. Recent progress in the genetic and epigenetic underpinnings of atopy. J Allergy Clin Immunol. (2023) 151:60–9. doi: 10.1016/j.jaci.2022.10.027
49. Simon AK, Hollander GA, and McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. (2015) 282:20143085. doi: 10.1098/rspb.2014.3085
50. Pedersen CJ, Uddin MJ, Saha SK, and Darmstadt GL. Prevalence and psychosocial impact of atopic dermatitis in Bangladeshi children and families. PloS One. (2021) 16:e0249824. doi: 10.1371/journal.pone.0249824
51. Esaki H, Brunner PM, Renert-Yuval Y, Czarnowicki T, Huynh T, Tran G, et al. Early-onset pediatric atopic dermatitis is T(H)2 but also T(H)17 polarized in skin. J Allergy Clin Immunol. (2016) 138:1639–51. doi: 10.1016/j.jaci.2016.07.013
52. Brunner PM, Israel A, Zhang N, Leonard A, Wen HC, Huynh T, et al. Early-onset pediatric atopic dermatitis is characterized by T(H)2/T(H)17/T(H)22-centered inflammation and lipid alterations. J Allergy Clin Immunol. (2018) 141:2094–106. doi: 10.1016/j.jaci.2018.02.040
53. Ogulur I, Mitamura Y, Yazici D, Pat Y, Ardicli S, Li M, et al. Type 2 immunity in allergic diseases. Cell Mol Immunol. (2025) 22:211–42. doi: 10.1038/s41423-025-01261-2
54. Charles N and Blank U. IgE-mediated activation of mast cells and basophils in health and disease. Immunol Rev. (2025) 331:e70024. doi: 10.1111/imr.v331.1
55. Renert-Yuval Y, Thyssen JP, Bissonnette R, Bieber T, Kabashima K, Hijnen D, et al. Biomarkers in atopic dermatitis-a review on behalf of the International Eczema Council. J Allergy Clin Immunol. (2021) 147:1174–90.e1171. doi: 10.1016/j.jaci.2021.01.013
56. Kawamoto N, Fukao T, Kaneko H, Hirayama K, Sakurai S, Arai T, et al. Risk factors for infantile atopic dermatitis and recurrent wheezing. J Investig Allergol Clin Immunol. (2012) 22:116–25.
57. Reddel S, Chierico Del F, Quagliariello A, Giancristoforo S, Vernocchi P, Russo A, et al. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci Rep. (2019) 9:4996. doi: 10.1038/s41598-019-41149-6
58. Schachner LA, Blume-Peytavi U, Andriessen A, Izakovic J, Maruani A, Micali G, et al. Algorithm to attenuate atopic dermatitis and for promoting a healthy skin barrier using skincare in newborns and infants. Ital J Dermatol Venerol. (2023) 158:224–35. doi: 10.23736/S2784-8671.23.07336-X
59. Su YC, Xie JS, Jan RH, and Hsieh CJ. Association between a maternal vegetarian diet during pregnancy and the occurrence of atopic dermatitis in children. Pediatr Allergy Immunol. (2023) 34:e14052. doi: 10.1111/pai.v34.12
60. Trikamjee T, Comberiati P, D'Auria E, Peroni D, and Zuccotti GV. Nutritional factors in the prevention of atopic dermatitis in children. Front Pediatr. (2020) 8:577413. doi: 10.3389/fped.2020.577413
61. Fan X, Zang T, Dai J, Wu N, Hope C, Bai J, et al. The associations of maternal and children’s gut microbiota with the development of atopic dermatitis for children aged 2 years. Front Immunol. (2022) 13:1038876. doi: 10.3389/fimmu.2022.1038876
62. Chang YC, Wu MC, Wu HJ, Liao PL, and Wei JC. Prenatal and early-life antibiotic exposure and the risk of atopic dermatitis in children: A nationwide population-based cohort study. Pediatr Allergy Immunol. (2023) 34:e13959. doi: 10.1111/pai.13959
63. Guo JY, Wu MC, Wang YH, and Wei JC. Association of maternal constipation and risk of atopic dermatitis in offspring. Int J Med Sci. (2024) 21:1790–8. doi: 10.7150/ijms.96326
64. Nie X, Kitaoka S, Tanaka K, Segi-Nishida E, Imoto Y, Ogawa A, et al. The innate immune receptors TLR2/4 mediate repeated social defeat stress-induced social avoidance through prefrontal microglial activation. Neuron. (2018) 99:464–79.e467. doi: 10.1016/j.neuron.2018.06.035
65. Sacotte R and Silverberg JI. Epidemiology of adult atopic dermatitis. Clin Dermatol. (2018) 36:595–605. doi: 10.1016/j.clindermatol.2018.05.007
66. Osinka K, Dumycz K, Kwiek B, and Feleszko W. Novel therapeutic approaches to atopic dermatitis. Arch Immunol Ther Exp (Warsz). (2018) 66:171–81. doi: 10.1007/s00005-017-0487-1
67. Furue M, Ulzii D, Vu YH, Tsuji G, Kido-Nakahara M, and Nakahara T. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. (2019) 16:97–107. doi: 10.22034/iji.2019.80253
68. Furue M, Chiba T, Tsuji G, Ulzii D, Kido-Nakahara M, Nakahara T, et al. Atopic dermatitis: immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol Int. (2017) 66:398–403. doi: 10.1016/j.alit.2016.12.002
69. Roved J, Westerdahl H, and Hasselquist D. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm Behav. (2017) 88:95–105. doi: 10.1016/j.yhbeh.2016.11.017
70. Hou D.D, Zhang W, Gao YL, Sun YZ, Wang HX, Qi RQ, et al. Anti-inflammatory effects of quercetin in a mouse model of MC903-induced atopic dermatitis. Int Immunopharmacol. (2019) 74:105676. doi: 10.1016/j.intimp.2019.105676
71. Guerrero-Aspizua S, Carretero M, Conti CJ, and Río Del M. The importance of immunity in the development of reliable animal models for psoriasis and atopic dermatitis. Immunol Cell Biol. (2020) 98:626–38. doi: 10.1111/imcb.12365
72. Bouffi C, Wikenheiser-Brokamp KA, Chaturvedi P, Sundaram N, Goddard GR, Wunderlich M, et al. In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice. Nat Biotechnol. (2023) 41:824–31. doi: 10.1038/s41587-022-01558-x
73. Pratt CH, King LE Jr., Messenger AG, Christiano AM, and Sundberg JP. Alopecia areata. Nat Rev Dis Primers. (2017) 3:17011. doi: 10.1038/nrdp.2017.11
74. Rossi A, Muscianese M, Piraccini BM, Starace M, Carlesimo M, Mandel VD, et al. Italian Guidelines in diagnosis and treatment of alopecia areata. G Ital Dermatol Venereol. (2019) 154:609–23. doi: 10.23736/S0392-0488.19.06458-7
75. Conic RZ, Tamashunas NL, Damiani G, Fabbrocini G, Cantelli M, Bergfeld WF, et al. Comorbidities in pediatric alopecia areata. J Eur Acad Dermatol Venereol. (2020) 34:2898–901. doi: 10.1111/jdv.v34.12
76. Patra P, Harrison T, and Khoury M. Challenges in diagnosis and treatment of pediatric psoriasis and atopic dermatitis. Arch Dermatol Res. (2025) 317:393. doi: 10.1007/s00403-025-03862-3
77. Cohen SN, Baron SE, and Archer CB. Guidance on the diagnosis and clinical management of psoriasis. Clin Exp Dermatol. (2012) 37 Suppl 1:13–8. doi: 10.1111/j.1365-2230.2012.04337.x
78. Kim JE, Lee J, Huh YJ, Kim K, Chaparala V, Krueger JG, et al. Genomic profiling of the overlap phenotype between psoriasis and atopic dermatitis. J Invest Dermatol. (2024) 144:43–52.e46. doi: 10.1016/j.jid.2023.06.194
79. Yang Z, Yao X, Wang M, Li H, and Li R. Updates in psoriasis diagnosis and treatment status in China: results from the National Psoriasis Center Registry. Chin Med J (Engl). (2023) 136:2874–6. doi: 10.1097/CM9.0000000000002563
80. Martín Mauro San I, Oliva López S, Vilar Garicano E, Niño Sánchez GM, Penadés BF, Lora Terrén A, et al. Effects of gluten on gut microbiota in patients with gastrointestinal disorders, migraine, and dermatitis. Nutrients. (2024) 16(8):1228. doi: 10.3390/nu16081228
81. Schuppan D and Zimmer KP. The diagnosis and treatment of celiac disease. Dtsch Arztebl Int. (2013) 110:835–46. doi: 10.3238/arztebl.2013.0835
82. Pallav K, Kabbani T, Tariq S, Vanga R, Kelly CP, and Leffler DA. Clinical utility of celiac disease-associated HLA testing. Dig Dis Sci. (2014) 59:2199–206. doi: 10.1007/s10620-014-3143-1
83. Schneider M and Krüger K. Rheumatoid arthritis–early diagnosis and disease management. Dtsch Arztebl Int. (2013) 110:477–84. doi: 10.3238/arztebl.2013.0477
84. Radu AF and Bungau SG. Management of rheumatoid arthritis: an overview. Cells. (2021) 10(11):2857. doi: 10.3390/cells10112857
85. Figueroa-Parra G, Castañeda-Martinez MM, Herrera-Sandate P, Castañeda-Martinez DD, Esquivel-Valerio JA, Vega-Morales D, et al. Clinical features of patients with hands arthralgia referred from primary care physicians to rheumatologists: A cohort study. Reumatol Clin (Engl Ed). (2024) 20:67–72. doi: 10.1016/j.reuma.2023.06.005
86. Kiriakidou M and Ching CL. Systemic lupus erythematosus. Ann Intern Med. (2020) 172:Itc81–itc96. doi: 10.7326/AITC202006020
87. Durcan L, O’Dwyer T, and Petri M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet. (2019) 393:2332–43. doi: 10.1016/S0140-6736(19)30237-5
88. Castro-Gutierrez A, Young K, and Bermas BL. Pregnancy and management in women with rheumatoid arthritis, systemic lupus erythematosus, and obstetric antiphospholipid syndrome. Med Clin North Am. (2021) 105:341–53. doi: 10.1016/j.mcna.2020.10.002
89. Boodhoo KD, Liu S, and Zuo X. Impact of sex disparities on the clinical manifestations in patients with systemic lupus erythematosus: A systematic review and meta-analysis. Med (Baltimore). (2016) 95:e4272. doi: 10.1097/MD.0000000000004272
90. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, and Colombel JF. Ulcerative colitis. Lancet. (2017) 389:1756–70. doi: 10.1016/S0140-6736(16)32126-2
91. Chou WY, Lai PY, Hu JM, Hsu CH, Chen YC, Tian YF, et al. Association between atopic dermatitis and colorectal cancer risk: A nationwide cohort study. Med (Baltimore). (2020) 99:e18530. doi: 10.1097/MD.0000000000018530
92. Shi X, Chen Q, and Wang F. The bidirectional association between inflammatory bowel disease and atopic dermatitis: A systematic review and meta-analysis. Dermatology. (2020) 236:546–53. doi: 10.1159/000505290
93. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. (2017) 27:315–89. doi: 10.1089/thy.2016.0457
94. D'Aurizio F, Kratzsch J, Gruson D, Petranović Ovčariček P, and Giovanella L. Free thyroxine measurement in clinical practice: how to optimize indications, analytical procedures, and interpretation criteria while waiting for global standardization. Crit Rev Clin Lab Sci. (2023) 60:101–40. doi: 10.1080/10408363.2022.2121960
95. LaFranchi SH. Thyroid function in preterm/low birth weight infants: impact on diagnosis and management of thyroid dysfunction. Front Endocrinol (Lausanne). (2021) 12:666207. doi: 10.3389/fendo.2021.666207
Keywords: atopic dermatitis, autoimmune diseases, meta-analysis, adults, children cohort studies (n=19)
Citation: Wang H, Chen M, Wang T, Cai W, Li X, Huang L and Wang M (2025) Atopic dermatitis and risk of autoimmune diseases: a systematic review and meta-analysis. Front. Immunol. 16:1539997. doi: 10.3389/fimmu.2025.1539997
Received: 05 December 2024; Accepted: 23 May 2025;
Published: 12 June 2025.
Edited by:
Veena Taneja, Mayo Clinic, United StatesReviewed by:
Enza D’Auria, Vittore Buzzi Children’s’ Hospital, ItalyBárbara Roque Ferreira, Centre Hospitalier de Mouscron, Belgium
Yi Xue, National Clinical Research Center for Child Health., China
Copyright © 2025 Wang, Chen, Wang, Cai, Li, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingzhu Wang, MTc4MjY4NjY3NDFAMTYzLmNvbQ==; Lin Huang, aHVhbmdsaW5AemNtdS5lZHUuY24=
†These authors have contributed equally to this work
 Hongli Wang
Hongli Wang Min Chen
Min Chen Tengyue Wang1,2†
Tengyue Wang1,2† Lin Huang
Lin Huang Mingzhu Wang
Mingzhu Wang