- 1Department of Neurology Medicine, The Second Hospital of Shandong University, Cheeloo College of Medicine of Shandong University, Shandong University, Jinan, China
- 2The Second Hospital of Shandong University, The Second Clinical College of Shandong University, Shandong University, Jinan, China
- 3State Key Laboratory of Neurology and Oncology Drug Development, Jiangsu Simcere Diagnostics Co., Ltd., Nanjing, China
We summarized the clinical manifestations, auxiliary examinations, treatment, and prognostic characteristics of a patient with neurexin-3a IgG-mediated autoimmune encephalitis. On March 2, 2024, a 43-year-old male patient was admitted to the Second Hospital of Shandong University and had prodromal symptoms of infection before the onset of encephalitis. The main manifestations were episodic loss of consciousness, eyes turned upward to the right, clenched teeth, bleeding from tongue bite, and limb twitching. Imaging results showed that the left frontal lobe was characterized by a patchy, slightly longer T1 and T2 signal foci, with a slightly higher signal in the pressurized water image. The CSF virus test was normal; both the serum and CSF were positive for neurexin-3a antibodies using CBA, which were confirmed by TBA. The patient’s symptoms improved after glucocorticosteroid therapy. Neurexin-3a IgG-mediated autoimmune encephalitis is a new type of autoimmune encephalitis, and suspicion of associated disease requires further testing for neurexin-3a IgG for a definitive diagnosis.
1 Introduction
Autoimmune encephalitis (AE) is an inflammatory disease of the brain parenchyma with clear triggers of infection and tumor. It is mediated by autoimmune mechanisms and pathologically characterized by inflammatory lesions in the gray matter and brain neurons, with some involvement of white matter. The clinical manifestations caused by AE are complex and varied. Patients present with an acute or subacute onset of disease, and AE is mainly characterized by near-memory deficits, psychiatric-behavioral abnormalities, seizures, and altered consciousness (1). Antibodies to neuronal intracellular antigens typically associate with paraneoplastic neurological syndromes and poor prognosis, whereas antibodies to synaptic/neuronal cell surface antigens characterize many AE subtypes that associate with tumors less frequently, and that are often immunotherapy-responsive (2). With the development of antibody detection technology and the discovery of neurological antibodies, knowledge of AE has become more extensive, and the differences in the clinical features and immunotherapy of various antibody-mediated AE have been reported. In 2016, Gresa-Arribas et al. identified a novel antibody against neurexin-3a in five patients with AE (3); however, reports on this antibody-mediated AE are rare. Herein, we described a case of neurexin-3a antibody-mediated AE. This report contributes to a better understanding of this type of disease, paving the way for improved diagnosis and treatment.
2 Case presentation
2.1 Clinical information
On March 2, 2024, a 43-year-old man was admitted to our hospital due to “episodic loss of consciousness for more than a month.” The patient had a sore throat, runny nose, cough, small amount of sputum, no fever, and above-mentioned symptoms improved with cold medication. Three days later, he lost consciousness, fell to the ground, and had both eyes fixed to the upper right, teeth clenching, and limb convulsions, which lasted approximately 30 s and then got better, with no vomiting or incontinence. Eight days prior to admission to the local hospital, the patient had a recurrence of the above-mentioned seizures with tongue biting, which lasted approximately 1 minute before improving. He was brought to the local hospital, and a thyroid ultrasound was performed, showing diffuse lesions in the thyroid parenchyma and the possibility of Hashimoto’s thyroiditis. Chest computed tomography(CT) results suggested inflammation in the upper lobe of the right lung, but those of the epigastric region, pelvis, and cranial brain, as well as magnetic resonance imaging (MRI), showed no obvious abnormalities. The patient underwent tongue suture placement and administered sodium valproate 0.2g tid, acyclovir 500 mg q8h, and piperacillin 4.5g q8h orally. Because the patient continued to have several seizures lasting a few seconds since being discharged from the local hospital, he was admitted to the emergency department of the same hospital with “cause of encephalitis to be investigated.” Since disease onset, the patient was on a general diet and had poor sleep quality, no abnormalities in urination and defecation, and no recent significant changes in weight. Physical examination on emergency admission showed a body temperature of 36.8°C, pulse of 79 beats/min, respiration of 17 beats/min, and blood pressure of 120/77 mmHg. The patient’s consciousness was clear, and he was slightly unresponsive. He also had a decline in gross near-memory, computation, and orientation, with a total score of 15 on the Brief Mental Status Examination Scale (MMSE), suggesting moderate cognitive deficits. His Montreal Cognitive Assessment Scale (MOCA) score was 11, suggesting cognitive dysfunction. The rest of the neurological examination showed no abnormality.
2.2 Complementary examination
During electroencephalography(EEG) monitoring, each lead showed paroxysmal medium-to-high amplitude 2–3 Hz δ waves, with a few 4–7 Hz theta waves. MRI showed small patchy foci of slightly longer T1 and T2 signals in the left frontal lobe, with a slightly higher signal in the pressurized water image. Diffusion-weighted imaging(DWI) was unremarkable (Figure 1). Abnormal low-signal foci were not detected in T2 star-weighted angiography(SWAN), and any abnormality in the blood flow in the lesion area was not detected in arterial spin labeling(ASL). A complete blood count showed a leukocyte count of 2.34 × 109/L, monocyte proportion of 14.5%, neutrophil count of 1.51 × 109/L, lymphocyte count of 0.47 × 109/L, and calcitonin of 0.058 ng/ml.
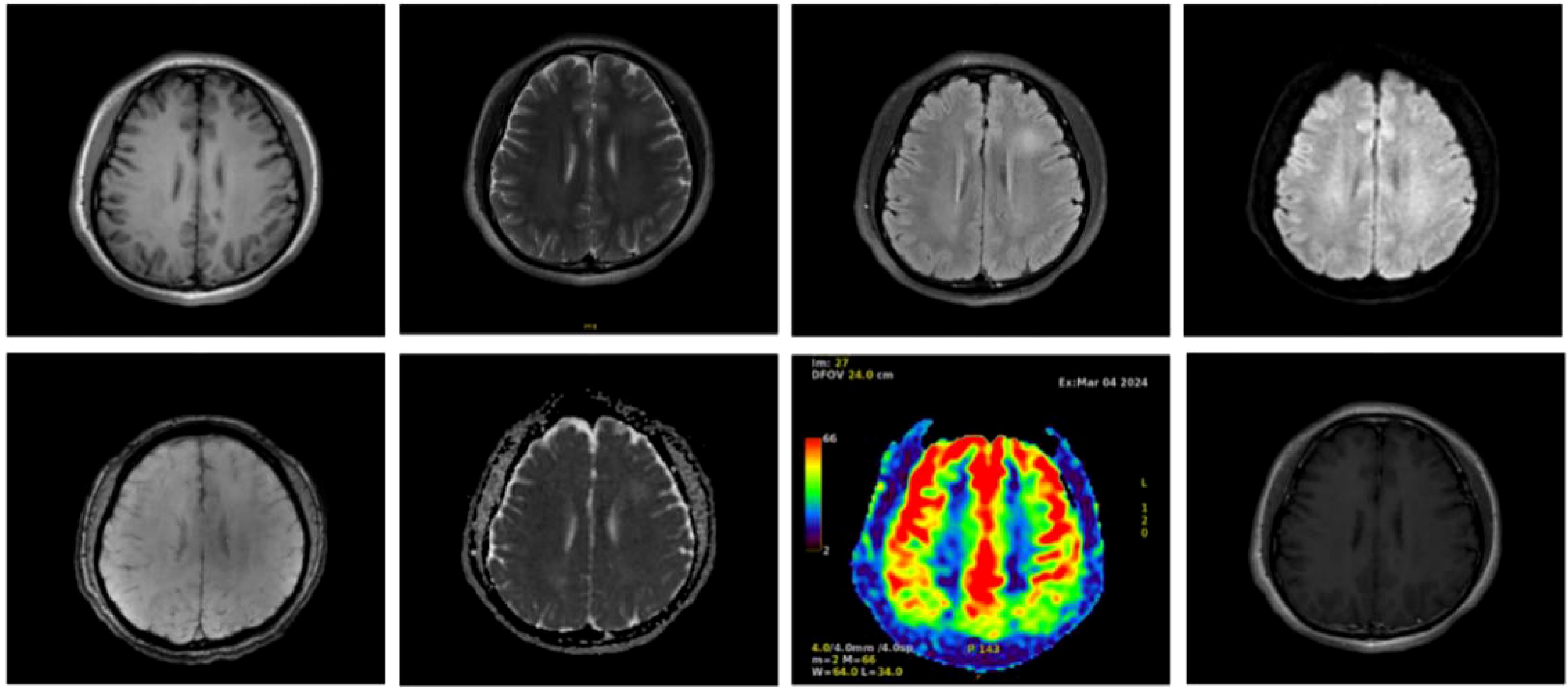
Figure 1. Cranial magnetic resonance imaging of the patient with autoimmune encephalitis showing a frontal lobe lesion, no abnormal low-signal foci in SWAN, and no significant abnormalities in blood flow in the lesion area in ASL.
Rheumatological parameters showed a positive ANA test of nuclear antibodies with titer 1:3200, with titer 1:3200 nuclear granulomatous and titer 1:320 cytoplasmic granulomatous karyotypes. In addition, SSA/Ro52 and Sm antibodies were positive. No significant abnormalities in thyroid function, RF, ASO, antineutrophil antibodies, lupus anticoagulant, infectious disease series, liver and renal function glycolipid biochemistry, folic acid and vitamins, tumor markers, and T-SPOT were detected. The cerebrospinal fluid (CSF) was colorless and clear and had 15/mm3 leukocyte count and 85% lymphocyte proportion, the intracranial pressure was 160 mmH2O, the cytology was lymphocytic, and the tryptophan test was negative. CSF biochemistry suggested normal protein, sugar, and chloride levels.
The results of ink stain, Gram stain, Alisinolan stain, cryptococcal pod antigen stain, and antacid stain were negative. The CSF was also negative for viral antibodies. Neural antibodies associated with AE and paraneoplastic syndrome, including neurexin-3a, NMDAR, LGI1, CASPR2, GABABR, AMPAR1, AMPAR2, DPPX, GAD65, mGluR5, GlyR, D2R, IgLON5, Hu, Yo, and Ri, in the serum and CSF were tested using cell-based assays (CBA) with immunofluorescence double staining. The screening titration was started from 1:10 and 1:1 for the serum and CSF samples, respectively, followed by a 10-fold dilution for the positive sample. The antibodies in the serum and CSF were further confirmed by tissue-based assay (TBA) using rat brain and kidney tissues (1:100). All tests were performed in Jiangsu Simcere Diagnostic Laboratory (Nanjing, China). CBA results suggested that both the blood and CSF were 1:10 positive for neurexin-3a antibody (Figure 2); meanwhile, TBA results indicated that the hippocampus, cerebellum, and cerebral cortex showed neuronal membrane antibody-positive fluorescence (Figure 3).
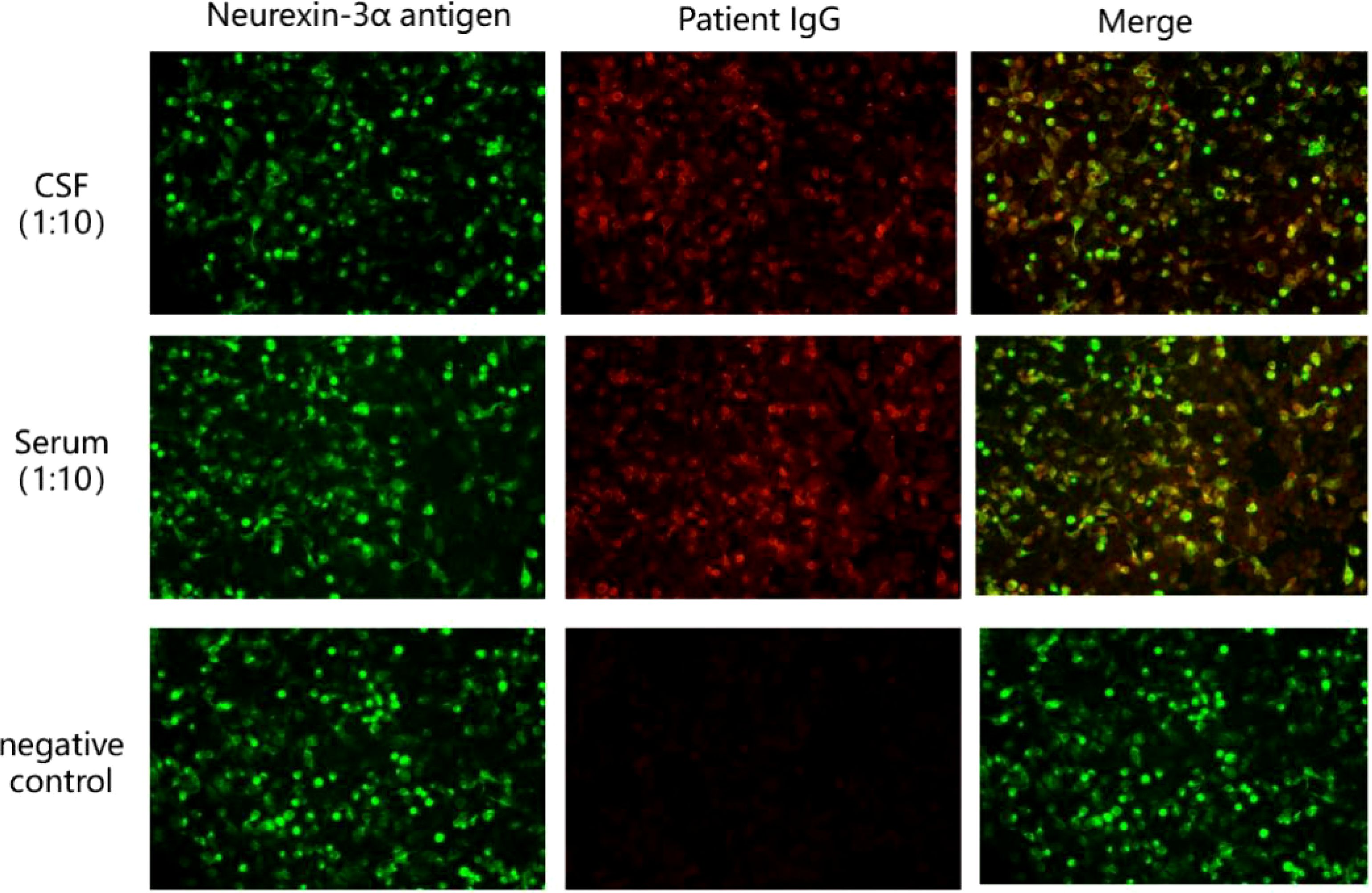
Figure 2. Presence of neurexin-3a antibody was confirmed by cell-based assays in the patient’s serum and CSF. Neurexin-3a-transfected HEK293T cells are shown in green, while Neurexin-3a IgG are in red.
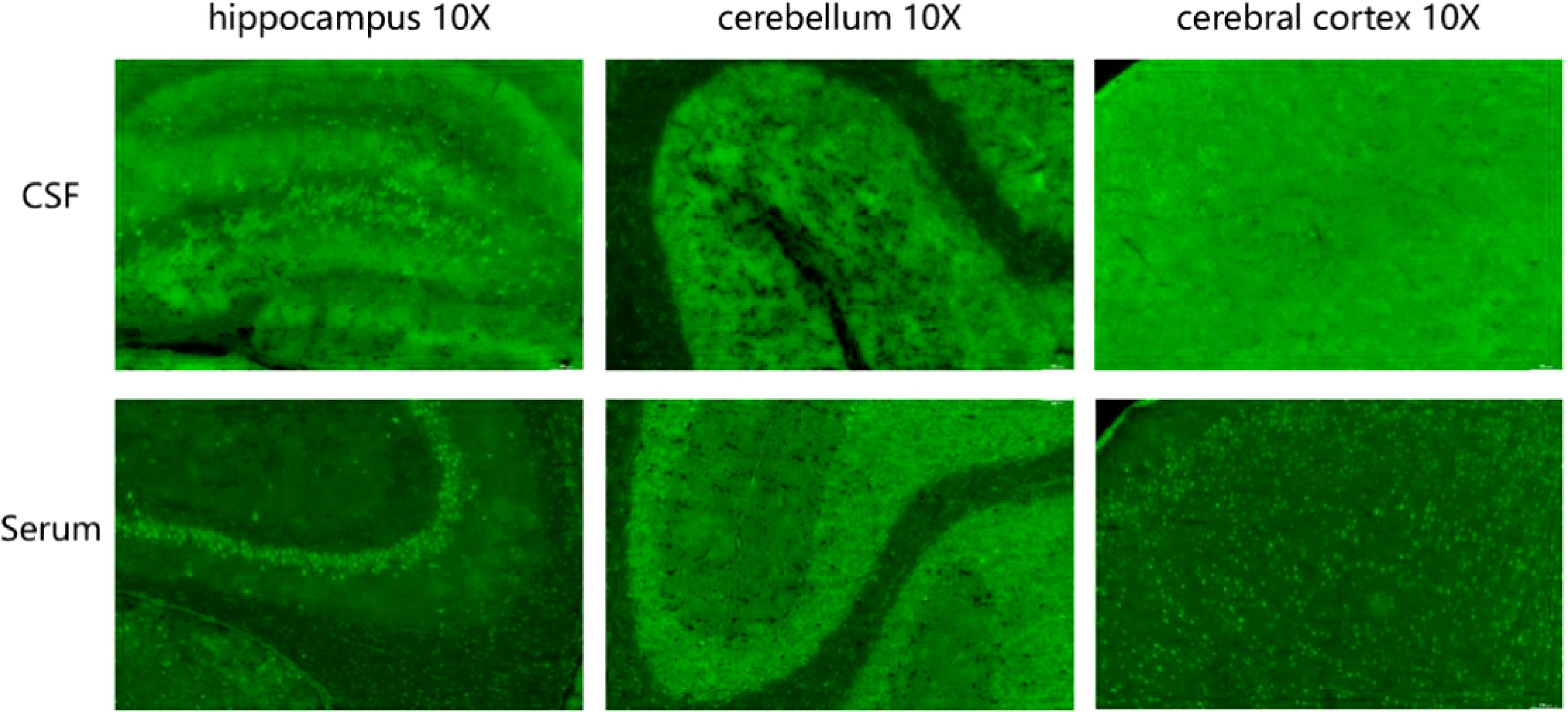
Figure 3. Tissue-based assay (TBA) showing the presence of immunoreactivity of cerebrospinal fluid and serum antibodies in the rat hippocampus, cerebellum, and cerebral cortex.
2.3 Treatment and follow-up
The patient was treated with glucocorticosteroids (1000 mg) and antiepileptic treatment. No further seizures occurred during hospitalization, and cognitive impairment was significantly improved at discharge. After discharge, the patient was given oral prednisone 60 mg per day and sodium valproate 500 mg bid. During follow-up 1 month later, no seizures were reported, and cognitive impairment improved. His MMSE score improved to 20 and his MOCA score was 15.
3 Discussion
Since the first report of N-methyl-D-aspartate receptor (NMDAR) encephalitis by Dalmau et al. in 2007 (4), other antibodies such as γ-aminobutyric acid type B receptors (GABABR) and Leucine-rich glioma inactivated 1 (LGI1) that can cause AE have been reported. Synaptic proteins are among the most diverse families of proteins in the mammalian nervous system, with more than 1000 isoforms (5). They are highly expressed in the presynaptic membrane and play important roles in synaptic cell adhesion and neurotransmitter secretion (6). The pathogenesis of neurexin-3a antibody-associated encephalitis is hypothesized as follows: neurexin-3a antibodies suppress neuronal neurexin-3a expression and decrease the total number of synapses, thus affecting synapse development (3). In addition, neurexin-3a antibodies can disrupt the balance between inhibitory and excitatory synapses in different regions of the CNS regulated by neurexin-3a (7).
The clinical features of neurexin-3a antibody-mediated AE are preceded by infection-like prodromal symptoms, which may be accompanied by headache and gastrointestinal symptoms and followed by seizures, memory loss, confusion, loss of consciousness, central hypoventilation, behavioral abnormalities, and speech disorders (8). Of the 15 patients (including the case patient) with neurexin-3a antibody-mediated AE reported thus far, 8 were women, and 7 were men. Among them, 10 had symptoms of antecedent infection (e.g., fever, headache, high temperature, nausea, or diarrhea); 13, decreased level of consciousness and seizures; 4, central hypoventilation; and 2, severe hallucinations and hallucinations (Table 1). In this case report, the patient had prodromal symptoms of infection, including sore throat, runny nose, cough, coughing up a small amount of sputum, and no fever. These were followed by episodic loss of consciousness, which appeared as eyes fixed to the upper right, teeth clenching, bleeding from tongue bite, and limb convulsions, lasting for 1 minute. A history of antecedent infections can contribute to the disruption of the blood–brain barrier due to infectious inflammation, and antibodies can enter the CNS through the damaged blood–brain barrier, leading to the development of immune-inflammation in the CNS. Because neurexin-3a antibody-mediated encephalitis can be preceded by symptoms of prodromal infection, some scholars believe that neurexin-3a antibody-mediated encephalitis is post-infectious encephalitis (18).
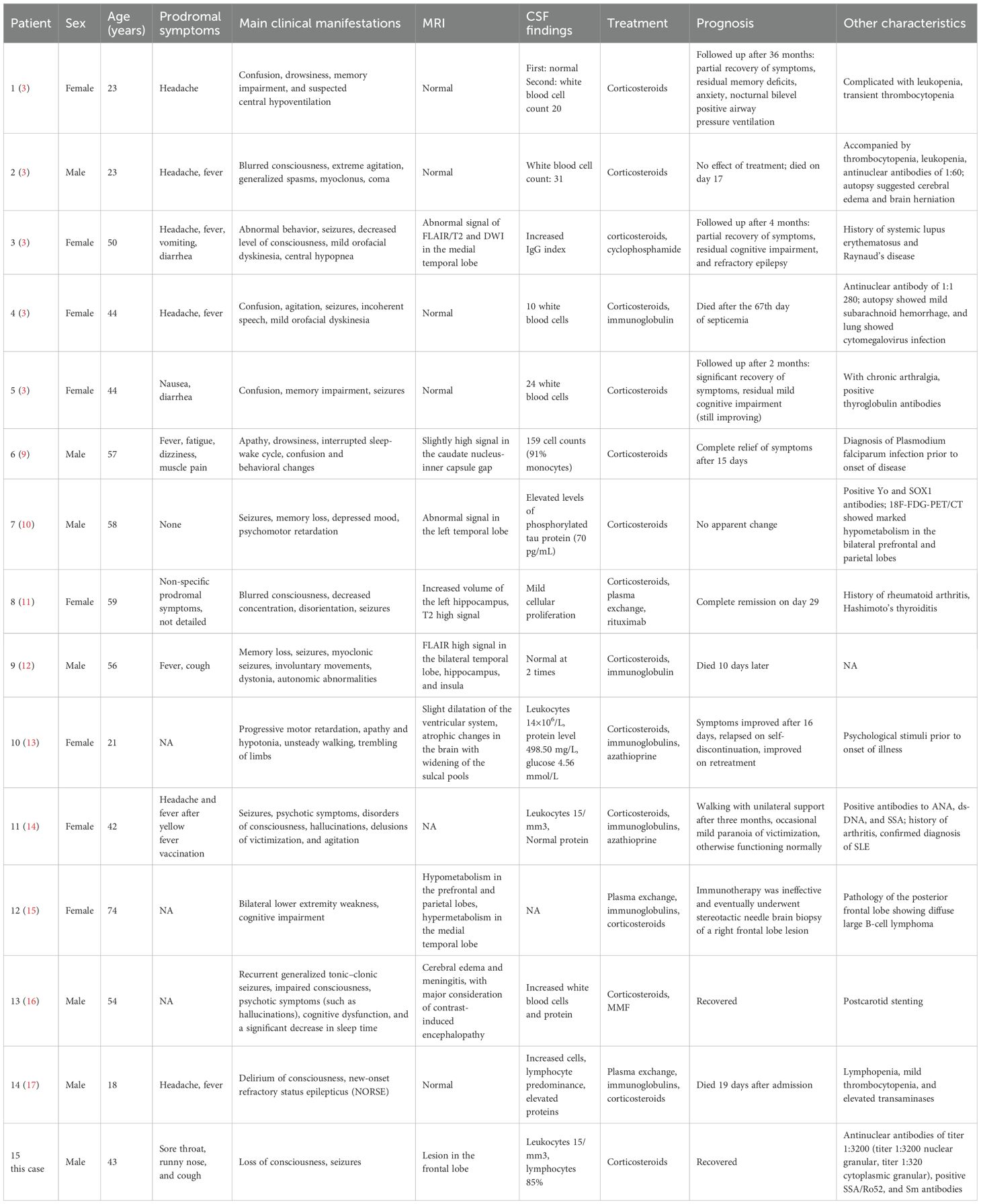
Table 1. Main clinical data of reported patients with neurexin-3a antibody-mediated encephalitis (n = 15).
The cranial MRI findings of patients with neurexin-3a antibody-mediated encephalitis may be normal or abnormal. Among the 15 patients with neurexin-3a antibody-mediated encephalitis reported thus far, 4 had normal cranial MRI manifestations, 9 had abnormal signals in different brain regions, and 2 lacked detailed information (Table 1). Abnormal MRI signals were found in the brain of some cases, such as in the temporal lobe, hippocampus, medial temporal lobe or caudate-capsule-lenticulate regions. In our patient, the MRI scan showed a patchy, slightly longer T1 and slightly longer T2 signal foci in the left frontal lobe, with a slightly higher signal in the pressurized water image. All patients with neurexin-3a antibody-mediated encephalitis had abnormal CSF findings, including 11 with abnormal cell counts, 1 with elevated levels of phosphorylated tau protein, and 3 with elevated levels of protein (Table 1). The CSF of the case patient had elevated leukocytes, and the percentage of lymphocytes was 85%. Then, various abnormal rheumatologic markers were assessed (Table 1). Autoimmune antibodies were present in some cases such as antinuclear antibodies, anti-dsDNA antibodies, anti-SSA/Ro52 antibodies, anti-Sm antibodies, et al. Herein, the patient had positive autoimmune antibodies but had no clinical manifestations such as recurrent fever, arthralgias, skin rashes, photoallergy, and mucosal ulcers. Moreover, cardiac ultrasound and chest CT scans did not reveal pericardial or pleural cavity effusion; thus, systemic lupus erythematosus could not be diagnosed. Nevertheless, close follow-up was required.
The confirmatory test for AE is positive anti-neuronal cell antibodies in the serum and/or CSF. The recommended methodology for detecting neuronal surface-antigen antibodies and some neurosynaptic intracellular antigen antibodies (e.g., GAD antibodies) is CBA. Other methodologies include protein blotting and TBA. Simultaneous testing for CSF and serum specimens is recommended (19). Because serum antineuronal antibody testing alone may lead to false-positive or false-negative results, CSF testing can improve the accuracy of the results (3). Of the 15 patients with neurexin-3a antibody-associated encephalitis reported to date, 12 were positive for both serum and CSF, 2 were seropositive only, and 1 lacked detailed information. The case patient’s blood and CSF samples were positive for neurexin-3a antibodies by CBA. TBA confirmed that both specimens were immunoreactive, exhibiting neuronal membrane antibody-positive fluorescence. Hence, the final diagnosis of neurexin-3a antibody-mediated encephalitis was confirmed.
Immunotherapy is the most effective treatment for AE according to the current guidelines; however, there is no consensus on the specific treatment for neurexin-3a antibody-mediated encephalitis. The current first-line agents used to treat AE are corticosteroids, plasma exchange, or intravenous immunoglobulin. When first-line interventions fail, second-line options include rituximab (CD20 receptor monoclonal antibody), cyclophosphamide (DNA alkylating agent), and other compounds, such as mycophenolate mofetil and azathioprine (20). Of the 15 patients with neurexin-3a antibody-mediated encephalitis reported thus far, 9 showed improvement in clinical manifestations, 2 showed no improvement, and 4 died, after treatment with steroid glucocorticosteroids, immunosuppression, or plasma exchange. The prognosis was poor in some cases, and even death occured. The potential reasons may be related to the severity of the patients’ clinical symptoms and poor efficacy of immunotherapy. According to literature reports, the clinical symptoms of some cases were similar to severe NMDAR encephalitis. Early intervention of first-line immunotherapy is very important.
In summary, neurexin-3a antibody-mediated encephalitis is very rare. It’s a new type of AE characterized by non-specific symptoms or mood changes in the early stage, followed by the rapid appearance of epilepsy, memory loss, confusion, loss of consciousness, central hypoventilation, behavioral abnormality, or speech disorders. Autoimmune antibodies were present in some cases and abnormal MRI signals were found in the brain of some patients. Some patients responded to immunotherapy, but the prognosis was poor in some cases, and even death occured. Therefore, when the patient has the above manifestations, clinicians should consider this disease and improve the relevant auxiliary examinations to clarify the diagnosis and guide the treatment, hence minimizing disability and saving the patient’s life.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Second Hospital of Shandong University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PW: Funding acquisition, Writing – review & editing. LS: Writing – review & editing. YW: Writing – original draft. LC: Data curation, Writing – original draft. HC: Writing – original draft. PS: Methodology, Writing – original draft. YX: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Natural Science Foundation of Shandong (No. ZR2023MH015) and the project of Shandong Medical Association (No.YXH2022ZX03230).
Conflict of interest
Author PS was employed by the company Jiangsu Simcere Diagnostics Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dalmau J and Graus F. Antibody-mediated encephalitis. N Engl J Med. (2018) 378:840–51. doi: 10.1056/NEJMra1708712
2. Costa D, Sardoeira A, Carneiro P, Neves E, Santos E, da Silva AM, et al. Autoimmune encephalitis: suspicion in clinical practice and mimics. J Neuroimmunol. (2022) 365:577824. doi: 10.1016/j.jneuroim.2022.577824
3. Gresa-Arribas N, Planagumà J, Petit-Pedrol M, Kawachi I, Katada S, Glaser CA, et al. Human neurexin-3α antibodies associate with encephalitis and alter synapse development. Neurology. (2016) 86:2235–42. doi: 10.1212/WNL.0000000000002775
4. Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. (2007) 61:25–36. doi: 10.1002/ana.21050
5. Ullrich B, Ushkaryov YA, and Südhof TC. Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron. (1995) 14:497–507. doi: 10.1016/0896-6273(95)90306-2
6. Vaags AK, Lionel AC, Sato D, Goodenberger M, Stein QP, Curran S, et al. Rare deletions at the neurexin 3 locus in autism spectrum disorder. Am J Hum Genet. (2012) 90:133–41. doi: 10.1016/j.ajhg.2011.11.025
7. Kattenstroth G, Tantalaki E, Südhof TC, Gottmann K, and Missler M. Postsynaptic N-methyl-D-aspartate receptor function requires alpha-neurexins. Proc Natl Acad Sci U S A. (2004) 101:2607–12. doi: 10.1073/pnas.0308626100
8. Waters PJ and Irani SR. Neurexin-3α: A new antibody target in autoimmune encephalitis. Neurology. (2016) 86:2222–3. doi: 10.1212/WNL.0000000000002781
9. Costa A, Silva-Pinto A, Alves J, Neves N, Martínez-Hernández E, Abreu P, et al. Postmalaria neurologic syndrome associated with neurexin-3α antibodies. Neurol Neuroimmunol Neuroinflamm. (2017) 4:e392. doi: 10.1212/NXI.0000000000000392
10. Hansen N, Lange C, Maass F, Hassoun L, Bouter C, Stöcker W, et al. Mild amnestic cognitive impairment and depressive symptoms in autoimmune encephalitis associated with serum anti-neurexin-3α Autoantibodies. Brain Sci. (2021) 11:673. doi: 10.3390/brainsci11060673
11. Loehrer PA, Bien CI, Dusoi AE, Timmermann L, and Simon OJ. Neurexin-3α-associated autoimmune encephalitis: a case report of full recovery after rituximab therapy. Eur J Neurol. (2020) 27:e91–3. doi: 10.1111/ene.14481
12. Zhang ZH, Huang RM, Lin H, Li JJ, Fu LH, Zhang XL, et al. Neurexin-3α IgG mediated autoimmune encephalitis: acase report13. Chin J Neurol. (2021) 54:258–62. doi: 10.3760/cma.j.cn113694-20201010-00770
13. Zheng HQ, Wu LX, Tian S, Liu P, Liu MX, and Wu W. Anti-neurexin-3α antibody-associated encephalitis with parkinsonism as the main manifestation: acase report. Chin J Neurol. (2022) 55:497–500. doi: 10.3760/cma.j.cn113694-20211219-00899
14. Guedes BF, Ribeiro AF, Pinto LF, Vidal JE, de Oliveira FG, Sztajnbok J, et al. Potential autoimmune encephalitis following yellow fever vaccination: A report of three cases. J Neuroimmunol. (2021) 355:577548. doi: 10.1016/j.jneuroim.2021.577548
15. Zhang C, Hao Y, Shao H, Xin M, Bai S, Guan Y, et al. Neurexin-3α-associated autoimmune encephalitis with intracranial diffuse large B lymphoma diagnosed on FDG and TSPO PET/MRI. Eur J Nucl Med Mol Imaging. (2023) 50:1270–2. doi: 10.1007/s00259-022-06054-7
16. Zhu L, Shang Q, Zhao CW, Dai S, and Wu Q. Case report: Anti-neurexin-3α-associated autoimmune encephalitis secondary to contrast-induced encephalopathy. Front Neurol. (2023) 14:1060110. doi: 10.3389/fneur.2023.1060110
17. Koh SJ, Ang CH, Tham, HLC, and Chua HC. Refractory status epilepticus secondary to Neurexin-3a encephalitis: A case report. Neurol Asia. (2018) 23:273–7.
18. Costa A, Silva-Pinto A, Alves J, Neves N, Martínez-Hernández E, Abreu P, et al. Postmalaria neurologic syndrome associated with neurexin-3α antibodies. Neurol Neuroimmunol Neuroinflamm. (2017) 4:e392. doi: 10.1212/NXI.0000000000000392
19. Masciocchi S, Businaro P, Scaranzin S, Morandi C, Franciotta D, and Gastaldi M. General features, pathogenesis, and laboratory diagnostics of autoimmune encephalitis. Crit Rev Clin Lab Sci. (2024) 61:45–69. doi: 10.1080/10408363.2023.2247482
Keywords: autoimmune encephalitis, neurexin-3a IgG-mediated autoimmune encephalitis, cell-based assay, tissue-based assay, immunotherapy
Citation: Wang P, Sun L, Wei Y, Cheng L, Cheng H, Shi P and Xu Y (2025) Neurexin-3a IgG-mediated autoimmune encephalitis: a case report and literature review. Front. Immunol. 16:1542578. doi: 10.3389/fimmu.2025.1542578
Received: 07 January 2025; Accepted: 29 April 2025;
Published: 21 May 2025.
Edited by:
Shanchao Zhang, Shandong Provincial Qianfoshan Hospital, ChinaCopyright © 2025 Wang, Sun, Wei, Cheng, Cheng, Shi and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingying Xu, eHV5aW5neWluZ0BlbWFpbC5zZHUuZWR1LmNu
 Pin Wang
Pin Wang Lin Sun1
Lin Sun1 Yingying Xu
Yingying Xu