- 1Department of Thoracic Surgery, the Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 2Department of CT&MRI, the Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 3Department of Thoracic Surgery, the Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 4Laboratory of Pathology, Hebei Cancer Institute, the Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
Background: While neoadjuvant chemoimmunotherapy shows promise for locally advanced esophageal squamous cell carcinoma (ESCC), optimal regimen selection remains challenging. This study compares perioperative outcomes between camrelizumab- and tislelizumab-based neoadjuvant chemoimmunotherapy in ESCC.
Methods: We conducted a retrospective analysis of 209 clinical stage II-IVA ESCC patients treated at Hebei Medical University Fourth Hospital (October 2020-December 2023) who underwent neoadjuvant chemoimmunotherapy (camrelizumab, n=119; tislelizumab, n=90) followed by esophagectomy.
Results: Comparable pathological responses were observed between groups: pathological complete response (31.1% vs 30.3%, P=1.00), major pathological response (44.4% vs 42.9%, P=0.89), and pathological downstaging (67.8% vs 73.9%, P=0.36). Perioperative complication rates, including hematologic toxicities, immune-related adverse events, and surgical complications, were similar (all P>0.05). The tislelizumab group demonstrated significantly lower unplanned ICU transfer rates (P=0.04), while operative parameters (duration, blood loss, R0 resection) showed no differences.
Conclusion: Tislelizumab-based chemoimmunotherapy demonstrates comparable efficacy and safety to camrelizumab-based regimens, potentially representing a viable neoadjuvant option for locally advanced ESCC.
Introduction
Esophageal cancer ranks seventh in incidence and sixth as an overall cause of mortality worldwide (1). The incidence of esophageal cancer exhibits significant regional variations. In China, esophageal squamous cell carcinoma (ESCC) accounts for over 90% of all cases of esophageal cancer (2). Surgery remains the standard treatment for locally advanced ESCC. However, it has been reported that approximately 33% of patients with ESCC who undergo surgery alone experience local recurrence and distant metastasis (3, 4). Since the publication of the results of the CROSS, NEOCRTEC5010, and JCOG9907 trials, neoadjuvant therapy has emerged as the first-line treatment option for locally advanced ESCC (5–7). In East Asia, thoracic surgeons tend to favor neoadjuvant chemotherapy over neoadjuvant chemoradiotherapy (8, 9).
The remarkable therapeutic efficacy of immunotherapy (including programmed cell death-ligand 1 [PD-L1] and programmed cell death protein-1 [PD-1] inhibitors) in advanced esophageal cancer has prompted numerous researchers to investigate its potential application as neoadjuvant therapy (10). A recent multicenter retrospective study demonstrated that patients with locally advanced ESCC who received neoadjuvant chemoimmunotherapy achieved superior 2-year overall survival (OS) and disease-free survival (DFS) outcomes compared to those treated with neoadjuvant chemoradiotherapy alone (11). The open-label, randomized Phase III ESCORT-NEO/NCCES01 trial showed that neoadjuvant chemotherapy plus camrelizumab is a safe treatment option for locally advanced ESCC and can enhance the pathological complete response (pCR) rate (12). Tislelizumab, a PD-1 inhibitor, has demonstrated promising efficacy and safety in the treatment of advanced ESCC, as evidenced by the RATIONALE-302 study (13). The TD-NICE study also found that tislelizumab combined with chemotherapy as neoadjuvant therapy improved the major pathological response (MPR), pCR, and R0 resection rates in resectable ESCC while maintaining an acceptable level of tolerability (14). In clinical practice, both of these immunotherapeutic agents are used in combination with neoadjuvant chemotherapy for locally advanced ESCC. However, direct comparisons of the therapeutic efficacy of these two agents during the perioperative period are currently limited.
In this study, we compared the perioperative efficacy and safety of neoadjuvant chemotherapy plus camrelizumab with that of neoadjuvant chemotherapy plus tislelizumab in patients with locally advanced ESCC.
Methods
Inclusion and exclusion criteria
This study had a single-center, retrospective real-world design and included patients with resectable locally advanced ESCC who received neoadjuvant chemotherapy plus camrelizumab or tislelizumab followed by esophagectomy between October 2020 and December 2023 at the Fourth Hospital of Hebei Medical University. The primary objective of this study was to evaluate and compare the therapeutic efficacy and safety outcomes between the two neoadjuvant treatment strategies in the perioperative setting. Data for these patients were collected from the electronic medical records. The inclusion criteria were as follows: pathological diagnosis of ESCC; resectable clinical stage II–IVA ESCC according to the American Joint Committee on Cancer 8th edition TNM staging system; received a complete cycle of neoadjuvant chemotherapy combined with camrelizumab or tislelizumab; and resection performed. Patients with cervical esophageal cancer, T4b disease, other tumors, or incomplete medical records were excluded.This study was conducted per the Declaration of Helsinki (as revised in 2013).
Staging and treatment
The patients underwent baseline staging using contrast-enhanced thoracoabdominal computed tomography, endoscopic ultrasound, and cervical lymph node ultrasound. Positron emission tomography–computed tomography was used for tumor staging when necessary. Clinical and pathological staging was performed using the American Joint Committee on Cancer 8th edition TNM staging system. Before surgical resection, all patients received 1–4 cycles of immunotherapy (camrelizumab 200 mg or tislelizumab 200 mg) and chemotherapy (platinum-based agent and albumin-bound paclitaxel/docetaxel) every 3–4 weeks. The specific chemotherapy regimens were as follows: cisplatin (75 mg/m² D1) or carboplatin (AUC=5 D1) combined with albumin bound paclitaxel (260 mg/m² D1); cisplatin (75 mg/m² D1) or carboplatin (AUC=5 D1) combined with docetaxel (75 mg/m² D1). Surgical procedures included McKeown and Ivor Lewis esophagectomy. Two-field lymph node dissection was routinely performed, with three-field lymph node dissection applied only when cervical lymph node metastasis was considered. All procedures were performed by high-volume surgeons with >5 years of specialization in this field. The criteria for unplanned postoperative ICU transfer included severe pneumonia, respiratory failure, significant arrhythmias, septic shock, and other life-threatening complications.
Outcome measures
A pCR was defined as the absence of evidence of residual tumor cells at the primary site in the surgical specimen and in the resected lymph nodes. An MPR was defined as having less than 10% viable residual tumor cells in the resection specimen. The tumor regression grade (TRG) was determined as follows: TRG 0, no visible viable cancer cells; TRG 1, single cells or rare small groups of cancer cells; TRG 2, residual cancer cells present, indicating significant tumor regression but extending beyond single cells or rare small groups; and TRG 3, a large number of residual cancer cells without obvious tumor regression. Operation time was calculated as the time between incision and wound closure.
Statistical analysis
As this was a retrospective study, our sample size was limited to all available patients who satisfied the inclusion criteria. Categorical variables are expressed as the count and percentage and were compared between groups using the chi-squared test or Fisher’s exact test. Continuous variables are shown as the median and range and were compared between groups using the Wilcoxon rank sum test. All statistical analyses were performed using STATA version 15.0 software (StataCorp LLC, College Station, TX, USA). A two-sided P-value of <0.05 was considered statistically significant. A post-hoc power calculation (two-sided α=0.05, power=80%, β=0.20) indicated adequate statistical power to identify a clinically meaningful 15 percentage-point absolute difference in pCR rates.
Results
Clinicopathological features
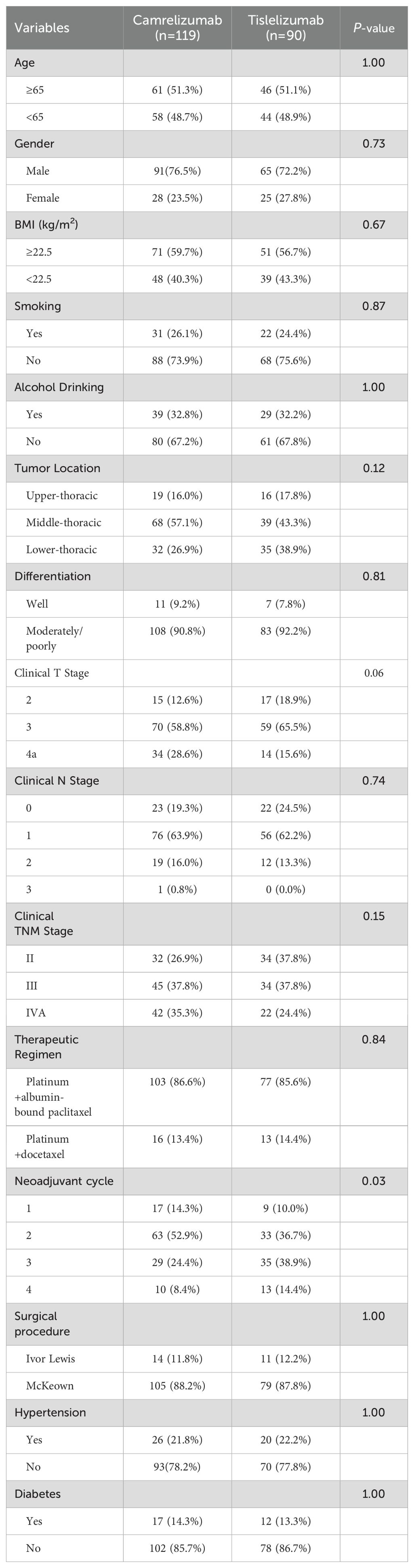
In total, 209 patients who were treated for locally advanced resectable ESCC during the study period were eligible for enrolment in the study. There were 119 patients in the camrelizumab group and 90 in the tislelizumab group. Their clinicopathological characteristics are shown in Table 1. There was no significant between-group difference in any of the baseline characteristics, including age, sex, body mass index, smoking status, alcohol consumption, tumor location, tumor differentiation, clinical T stage, clinical N stage, clinical TNM stage, therapeutic regimen, surgical procedure performed, hypertension, or diabetes. However, more neoadjuvant treatment cycles were administered in the tislelizumab group than in the camrelizumab group.
Outcomes of neoadjuvant treatment
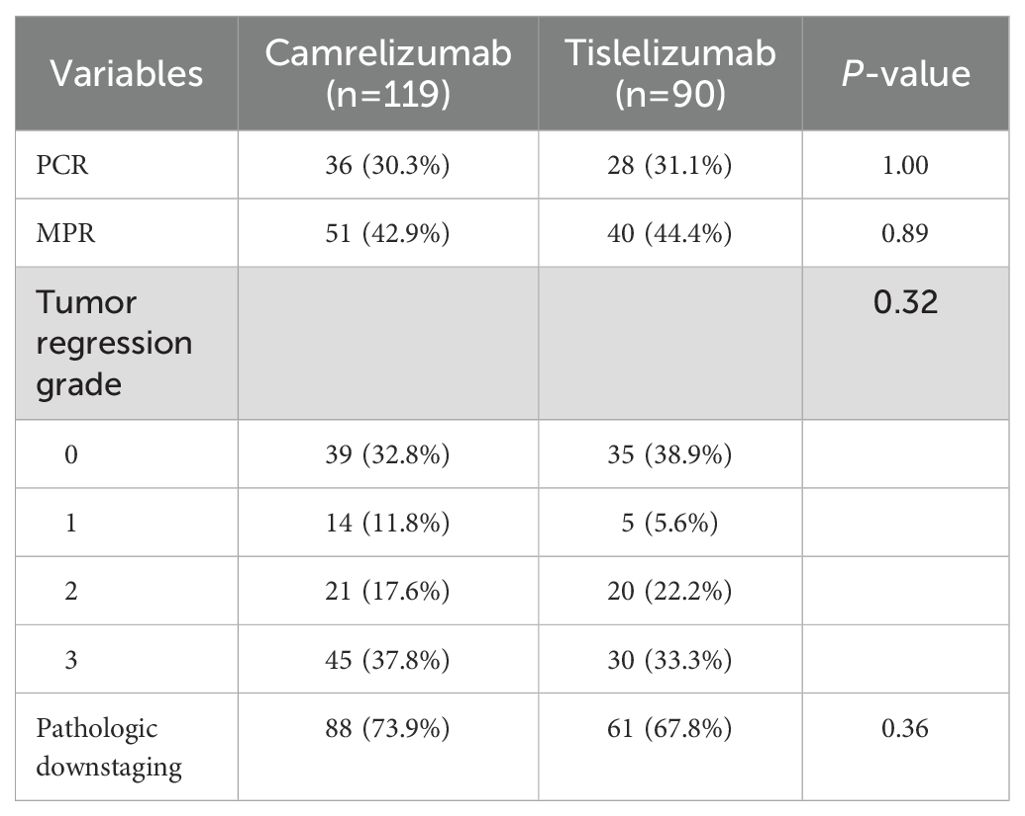
The postoperative pathological results for the camrelizumab group indicated achievement of a pCR in 36 patients (30.3%), an MPR in 51 (42.9%), TRG 0 in 39 (32.8%), and pathological downstaging in 88 (73.9%) (Table 2). Similarly, in the tislelizumab group, 28 patients (31.1%) achieved a pCR, 40 (44.4%) achieved an MPR, 35 (38.9%) achieved TRG 0, and 61 (67.8%) achieved pathological downstaging. There was no statistically significant difference in the pathological response between the two groups.
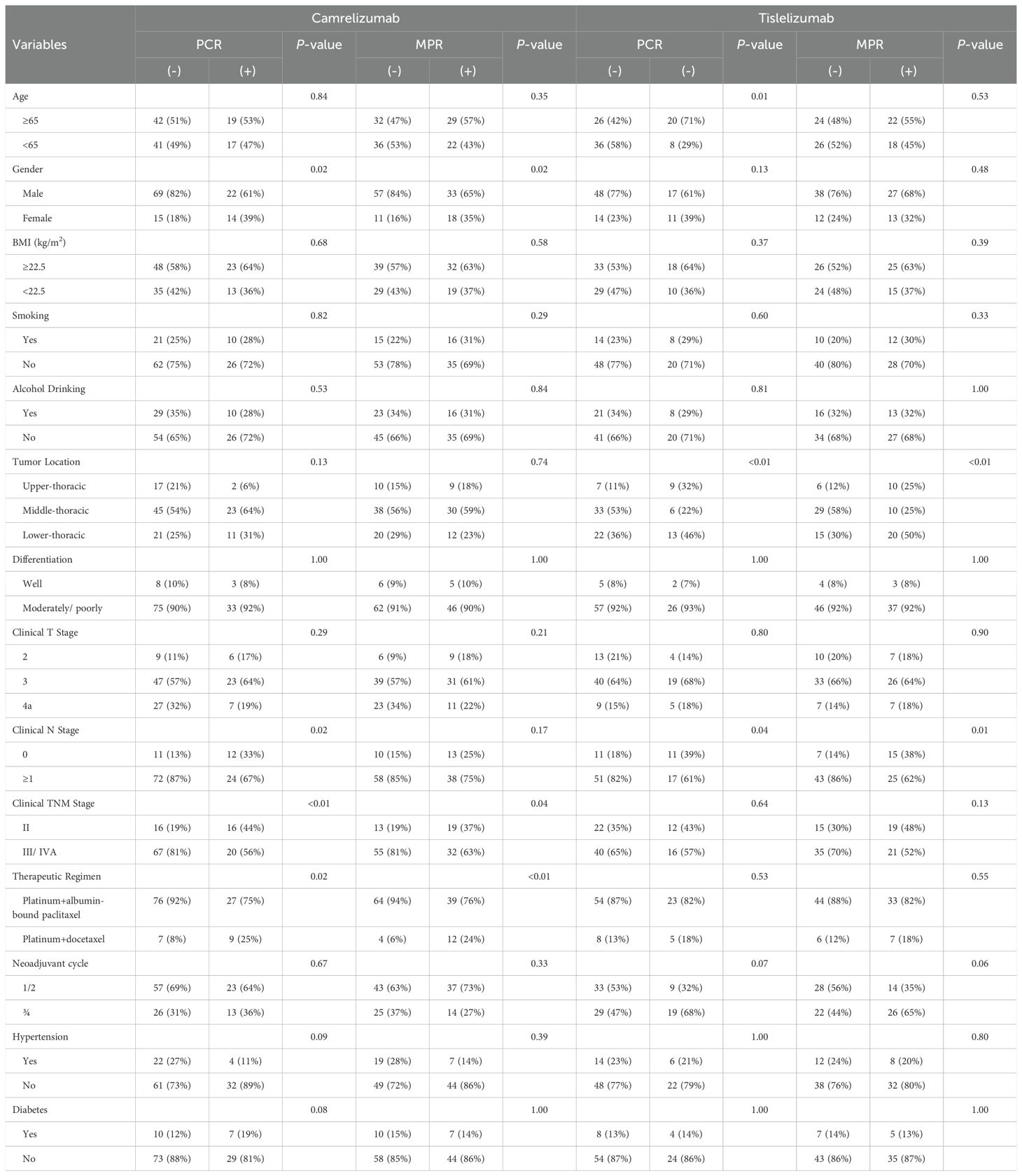
Subgroup analyses revealed that in the camrelizumab group, significantly higher pCR rates were observed among female patients, those with clinical N0 stage, clinical stage II disease, and docetaxel-treated individuals. Similarly, MPR rates were significantly elevated in female patients, clinical stage II cases, and docetaxel-treated subgroups within the camrelizumab cohort. In contrast, the tislelizumab group demonstrated distinct predictive patterns, with pCR rates showing significant associations with patient age, tumor location, and clinical N stage. Furthermore, MPR rates in the tislelizumab cohort were significantly correlated with tumor location and clinical N stage. (Table 3).
Safety and complications
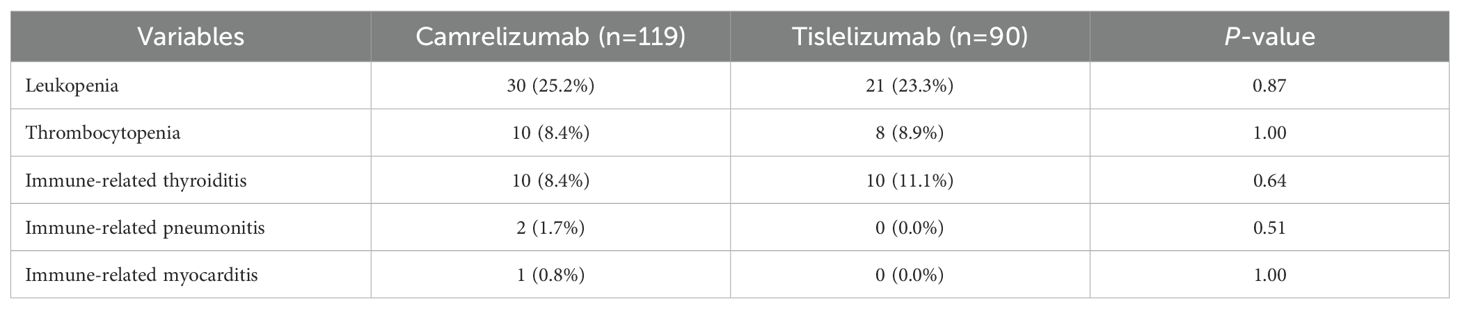
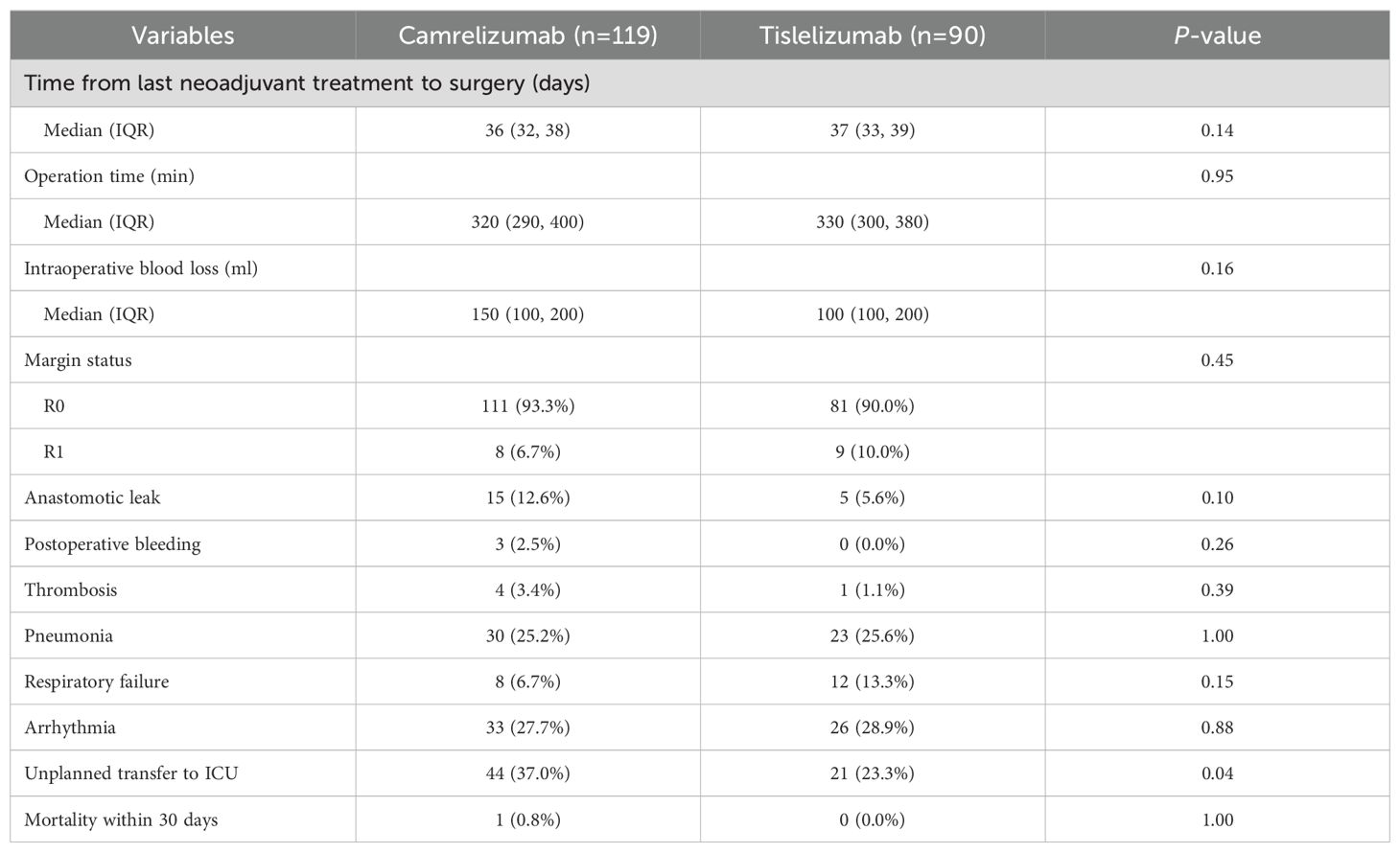
The adverse reactions associated with neoadjuvant therapy are summarized in Table 4. The incidence rates of leukopenia, thrombocytopenia, immune-related thyroiditis, immune-related pneumonitis, and immune-related myocarditis was comparable between the two groups (all P>0.05). There was no statistically significant between-group difference in operation time, intraoperative blood loss, or the R0 resection rate (Table 5), or in the incidence rates of postoperative complications, including anastomotic leak, postoperative bleeding, thrombosis, pneumonia, respiratory failure, arrhythmia, and mortality within 30 days. However, the rate of unplanned postoperative transfer to the intensive care unit (ICU) was lower in the tislelizumab group than in the camrelizumab group. Sensitivity analyses excluding elderly patients (>75 years) and prolonged surgeries (>10 hours) confirmed the robustness of our primary finding, with tislelizumab group maintaining significantly reduced ICU transfer rates (Supplementary Tables 1, 2). Multivariate logistic regression analysis identified clinical TNM stage, PD-1 inhibitor type, postoperative pneumonia, and respiratory failure as risk factors for unplanned postoperative ICU transfer (Supplementary Table 3).
Discussion
At this time, standard neoadjuvant therapy for locally advanced ESCC continues to consist of chemotherapy or chemoradiotherapy. However, the advent of immunotherapy, immune checkpoint inhibitors in particular, marks a significant advance in the treatment of advanced esophageal cancer. Multiple studies indicate that neoadjuvant chemotherapy combined with immunotherapy offers a new treatment opportunity for locally advanced ESCC (15–18). Most surgeons believe that esophagectomy is no more difficult after neoadjuvant chemoimmunotherapy than after neoadjuvant chemoradiotherapy. Yu et al. demonstrated that neoadjuvant chemoimmunotherapy in patients with ESCC was associated with R0 resection and pCR rates that were comparable with those of neoadjuvant chemoradiotherapy and that the prognosis appeared to be better after neoadjuvant chemoimmunotherapy (19). Wang et al. also found neoadjuvant chemoimmunotherapy to be a safe and feasible strategy for locally advanced ESCC (20). Among the various immunotherapeutic agents currently used for ESCC, only camrelizumab has been shown in a Phase III clinical study to be more effective than chemotherapy alone when used in combination with chemotherapy in the neoadjuvant setting (12). Therefore, selecting the optimal neoadjuvant immunotherapy regimen for locally advanced ESCC is a challenge that still needs to be addressed.
In this study, we compared the perioperative efficacy and safety of neoadjuvant chemotherapy combined with camrelizumab with that of neoadjuvant chemotherapy combined with tislelizumab for locally advanced ESCC. Although the tislelizumab group had received more treatment cycles at baseline, both groups had comparable pCR, MPR, and tumor downstaging rates. The two groups also performed similarly well in terms of safety. However, the rate of unplanned postoperative transfer to the ICU was lower in the tislelizumab group.
Patients who achieve a pCR after neoadjuvant therapy generally have a favorable long-term prognosis despite the risk of recurrence (21, 22). Therefore, the pCR and MPR are currently regarded as reliable surrogate endpoints for assessing the effectiveness of neoadjuvant therapy. In this study, the pCR rate for camrelizumab was 30.3% and that for tislelizumab was 31.1%. Compared with the 9% pCR rate for standard neoadjuvant chemotherapy, our outcomes were better and numerically in line with the 32% seen after neoadjuvant chemoradiotherapy for locally advanced ESCC in a meta-analysis (23). Subgroup analyses demonstrated differential predictive factors for pCR and MPR in the camrelizumab and tislelizumab cohorts. While certain variables reached significance in univariate analysis, none remained independently predictive in multivariate regression models. In ESCC, PD-L1 expression levels—an important biomarker of immune checkpoint inhibitor efficacy—are integral to risk stratification and treatment personalization. Both the ESCORT-NEO/NCCES01 and TD-NICE datasets demonstrated that elevated PD-L1 expression was significantly associated with improved pCR rates (12, 14). As a retrospective study, our analysis lacked PD-L1 expression data, which may explain the limited predictive value for pCR and MPR observed in subgroup analyses. At present, there are no clinical tools that can reliably predict the pCR in patients with ESCC (24). Several studies have found that CT radiomics can predict pCR status after neoadjuvant therapy for ESCC (25–27). Feng et al. also reported that the pretreatment integrative inflammatory and nutritional score is an independent predictor of pCR in patients with ESCC treated using chemoimmunotherapy (28). Prospective multicenter studies incorporating novel biomarkers and advanced machine learning algorithms are warranted to establish a robust predictive model for pCR following neoadjuvant therapy in ESCC patients. R0 resection status is important when assessing the success of surgery for ESCC because it correlates with patient outcomes and is a measure of effective treatment (29). In our study, there was no notable difference in the R0 resection rate between the two groups. The R0 resection rate was high in both the camrelizumab and tislelizumab groups (93.3% vs 90.0%). Perioperative morbidity and mortality are the primary concerns in surgery following neoadjuvant therapy. A recent retrospective analysis of 133 patients with ESCC demonstrated favorable perioperative safety profiles for neoadjuvant chemoimmunotherapy. While subgroup analyses indicated comparable safety outcomes across different immunotherapeutic agents, the limited sample size in each treatment subgroup warrants cautious interpretation of these findings (30). Zhang et al. reported that camrelizumab plus neoadjuvant chemotherapy yielded superior surgical outcomes versus chemoradiotherapy in ESCC, with shorter operations, less bleeding, and reduced ICU stays (4). The safety of tislelizumab during the perioperative period has been confirmed in other large-scale clinical studies (31, 32). In a retrospective study of gastric cancer, tislelizumab did not increase the incidence of postoperative complications (33). We did not detect a statistically significant difference in the incidence of neoadjuvant therapy-related adverse events between our two groups. In terms of postoperative complications, the proportion of patients who underwent unplanned transfer to the ICU after surgery was lower in the tislelizumab group. While ESCORT-NEO reported comparable perioperative morbidity between camrelizumab and chemotherapy arms, our observed ICU transfer reduction with tislelizumab may reflect differential immune-related toxicity profiles. Several immunologically plausible explanations merit consideration. First, as a humanized IgG4 anti-PD-1 antibody with engineered Fc receptor binding, tislelizumab may cause less cytokine-mediated toxicity compared to other checkpoint inhibitors. Second, its preserved T-regulatory cell function might maintain immunological homeostasis during surgical stress. Third, the observed effect could reflect reduced subclinical cardiotoxicity or pulmonary inflammation (34, 35). The observed discrepancy may be also attributable to several factors, including patient selection bias, heterogeneity in ICU admission criteria across clinical scenarios, and variations in postoperative management protocols within our institution. Prospective trials incorporating serial immune monitoring are needed to test these hypotheses.
This study had some limitations. First, it had a single-center retrospective real-world design with a small sample size, which meant that selection bias was unavoidable. Our study remains underpowered to detect rare outcomes such as myocarditis and mortality due to their low incidence rates. Future multi-center prospective head-to-head comparative studies would provide more robust evidence to address this issue. Second, this study was limited to comparing perioperative outcomes, with no data available on OS or DFS. Future studies with long-term follow-up are warranted to evaluate potential differences in survival outcomes. Third, there were no data on PD-L1 expression, so it was not possible to assess the relationship between PD-L1 expression and the response to neoadjuvant therapy in detail. The precise molecular mechanisms responsible for the differential therapeutic effects remain to be elucidated.
In conclusion, this real-world analysis has demonstrated that neoadjuvant chemotherapy plus tislelizumab is safe and feasible in patients with locally advanced ESCC, and has satisfactory pCR and MPR rates. However, long-term survival requires further investigation. Chemoimmunotherapy that includes tislelizumab may become a neoadjuvant treatment option for locally advanced ESCC in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethics Committee of the Fourth Hospital of Hebei Medical University (2021KY138). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
QZ: Conceptualization, Writing – original draft. YY: Conceptualization, Writing – original draft. TX: Formal analysis, Software, Writing – original draft. NY: Investigation, Methodology, Writing – original draft. FL: Data curation, Investigation, Writing – review & editing. JL: Formal analysis, Software, Writing – review & editing. MH: Supervision, Writing – review & editing. ZY: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Hebei Natural Science Foundation (no. H2025206311), Medical Science Research Project of Hebei (no.20220132), Innovative Research Team Support Program of the Fourth Hospital of Hebei Medical University (no. 2023C15).
Acknowledgments
We thank Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1544739/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. He Y, Li D, Shan B, Liang D, Shi J, Chen W, et al. Incidence and mortality of esophagus cancer in China, 2008-2012. Chin J Cancer Res. (2019) 31:426–34. doi: 10.21147/j.issn.1000-9604.2019.03.04
3. Demarest CT and Chang AC. The landmark series: multimodal therapy for esophageal cancer. Ann Surg Oncol. (2021) 28:3375–82. doi: 10.1245/s10434-020-09565-5
4. Zhang B, Zhao H, Wu X, Gong L, Yang D, Li X, et al. Perioperative outcomes of neoadjuvant chemotherapy plus camrelizumab compared with chemotherapy alone and chemoradiotherapy for locally advanced esophageal squamous cell cancer. Front Immunol. (2023) 14:1066527. doi: 10.3389/fimmu.2023.1066527
5. Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. (2015) 16:1090–8. doi: 10.1016/S1470-2045(15)00040-6
6. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. (2018) 36:2796–803. doi: 10.1200/JCO.2018.79.1483
7. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. (2012) 19:68–74. doi: 10.1245/s10434-011-2049-9
8. Xu J, Yan C, Li Z, Cao Y, Duan H, and Ke S. Efficacy and safety of neoadjuvant chemoimmunotherapy in resectable esophageal squamous cell carcinoma: a meta-analysis. Ann Surg Oncol. (2023) 30:1597–613. doi: 10.1245/s10434-022-12752-1
9. Kitagawa Y, Ishihara R, Ishikawa H, Ito Y, Oyama T, Oyama T, et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus. (2023) 20:343–72. doi: 10.1007/s10388-023-00993-2
10. Wang R, Liu S, Chen B, and Xi M. Recent advances in combination of immunotherapy and chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Cancers (Basel). (2022) 14:5168. doi: 10.3390/cancers14205168
11. Guo X, Chen C, Zhao J, Wang C, Mei X, Shen J, et al. Neoadjuvant chemoradiotherapy vs chemoimmunotherapy for esophageal squamous cell carcinoma. JAMA Surg. (2025) 19:e250220. doi: 10.1001/jamasurg.2025.0220
12. Qin J, Xue L, Hao A, Guo X, Jiang T, Ni Y, et al. Neoadjuvant chemotherapy with or without camrelizumab in resectable esophageal squamous cell carcinoma: the randomized phase 3 ESCORT-NEO/NCCES01 trial. Nat Med. (2024) 30:2549–57. doi: 10.1038/s41591-024-03064-w
13. Shen L, Kato K, Kim SB, Ajani JA, Zhao K, He Z, et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): a randomized phase III study. J Clin Oncol. (2022) 40:3065–76. doi: 10.1200/JCO.21.01926
14. Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: a prospective, single-arm, phase II study (TD-NICE). Int J Surg. (2022) 103:106680. doi: 10.1016/j.ijsu.2022.106680
15. Waters JK and Reznik SI. Update on management of squamous cell esophageal cancer. Curr Oncol Rep. (2022) 24:375–85. doi: 10.1007/s11912-021-01153-4
16. Puhr HC, Prager GW, and Ilhan-Mutlu A. How we treat esophageal squamous cell carcinoma. ESMO Open. (2023) 8:100789. doi: 10.1016/j.esmoop.2023.100789
17. He W, Wang C, Li C, Nie X, Li H, Li J, et al. The efficacy and safety of neoadjuvant immunotherapy in resectable locally advanced esophageal squamous cell carcinoma: A systematic review and meta-analysis. Front Immunol. (2023) 14:1118902. doi: 10.3389/fimmu.2023.1118902
18. Shang X, Xie Y, Yu J, Zhang C, Zhao G, Liang F, et al. A prospective study of neoadjuvant pembrolizumab plus chemotherapy for resectable esophageal squamous cell carcinoma: The Keystone-001 trial. Cancer Cell. (2024) 42:1747–1763.e7. doi: 10.1016/j.ccell.2024.09.008
19. Yu YK, Meng FY, Wei XF, Chen XK, Li HM, Liu Q, et al. Neoadjuvant chemotherapy combined with immunotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. (2024) 168:417–428.e3. doi: 10.1016/j.jtcvs.2023.12.030
20. Wang Y, Ma K, Zhang H, Wu L, Liu L, Zhou Y, et al. Comparison of pathologic response and survival outcomes between neoadjuvant chemoradiotherapy (nCRT) and neoadjuvant immunochemotherapy (nICT) in patients with locally advanced esophageal squamous cell carcinoma: a propensity score-matched analysis. BMC Cancer. (2024) 24:1228. doi: 10.1186/s12885-024-12946-8
21. Wu YY, Dai L, Yang YB, Yan WP, Cheng H, Fan MY, et al. Long-term survival and recurrence patterns in locally advanced esophageal squamous cell carcinoma patients with pathologic complete response after neoadjuvant chemotherapy followed by surgery. Ann Surg Oncol. (2024) 31:5047–54. doi: 10.1245/s10434-023-14809-1
22. Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. (2014) 32:385–91. doi: 10.1200/JCO.2013.51.2186
23. Gaber CE, Sarker J, Abdelaziz AI, Okpara E, Lee TA, Klempner SJ, et al. Pathologic complete response in patients with esophageal cancer receiving neoadjuvant chemotherapy or chemoradiation: A systematic review and meta-analysis. Cancer Med. (2024) 13:e7076. doi: 10.1002/cam4.7076
24. Wu Y, Chen J, Zhao L, Li Q, Zhu J, Yang H, et al. Prediction of pathologic response to neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma incorporating hematological biomarkers. Cancer Res Treat. (2021) 53:172–83. doi: 10.4143/crt.2020.594
25. Shi L, Li C, Bai Y, Cao Y, Zhao S, Chen X, et al. CT radiomics to predict pathologic complete response after neoadjuvant immunotherapy plus chemoradiotherapy in locally advanced esophageal squamous cell carcinoma. Eur Radiol. (2024) 35(3):1594–1604. doi: 10.1007/s00330-024-11141-4
26. Zhang M, Lu Y, Sun H, Hou C, Zhou Z, Liu X, et al. CT-based deep learning radiomics and hematological biomarkers in the assessment of pathological complete response to neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma: A two-center study. Transl Oncol. (2024) 39:101804. doi: 10.1016/j.tranon.2023.101804
27. Fan L, Yang Z, Chang M, Chen Z, and Wen Q. CT-based delta-radiomics nomogram to predict pathological complete response after neoadjuvant chemoradiotherapy in esophageal squamous cell carcinoma patients. J Transl Med. (2024) 22:579. doi: 10.1186/s12967-024-05392-4
28. Feng J, Wang L, Yang X, Chen Q, and Cheng X. Pathologic complete response prediction to neoadjuvant immunotherapy combined with chemotherapy in resectable locally advanced esophageal squamous cell carcinoma: real-world evidence from integrative inflammatory and nutritional scores. J Inflammation Res. (2022) 15:3783–96. doi: 10.2147/JIR.S367964
29. Whited WM, Trivedi JR, Bond ER, van Berkel VH, and Fox MP. Optimal therapy in locally advanced esophageal cancer: a national cancer database analysis. J Gastrointest Surg. (2018) 22:187–93. doi: 10.1007/s11605-017-3548-1
30. Guo Y, Xu X, Wang T, Liu Y, Gu D, Fang Y, et al. Efficacy, safety, and survival of neoadjuvant immunotherapy plus chemotherapy in locally advanced esophageal squamous cell carcinoma: A real-world retrospective study. Int Immunopharmacol. (2024) 10:138. doi: 10.1016/j.intimp.2024.112558
31. Zhao ZR, Liu SL, Zhou T, Chen G, Long H, Su XD, et al. Stereotactic body radiotherapy with sequential tislelizumab and chemotherapy as neoadjuvant therapy in patients with resectable non-small-cell lung cancer in China (SACTION01): a single-arm, single-centre, phase 2 trial. Lancet Respir Med. (2024) 12(12):988–996. doi: 10.1016/S2213-2600(24)00215-7
32. Yue D, Wang W, Liu H, Chen Q, Chen C, Liu L, et al. Perioperative tislelizumab plus neoadjuvant chemotherapy for patients with resectable non-small-cell lung cancer (RATIONALE-315): an interim analysis of a randomised clinical trial. Lancet Respir Med. (2024) 13(2):119–129. doi: 10.1016/S2213-2600(24)00269-8
33. Jiang Q, Liu W, Zeng X, Zhang C, Du Y, Zeng L, et al. Safety and efficacy of tislelizumab plus chemotherapy versus chemotherapy alone as neoadjuvant treatment for patients with locally advanced gastric cancer: real-world experience with a consecutive patient cohort. Front Immunol. (2023) 14:1122121. doi: 10.3389/fimmu.2023.1122121
34. Lee SH, Lee HT, Lim H, Kim Y, Park UB, and Heo YS. Crystal structure of PD-1 in complex with an antibody-drug tislelizumab used in tumor immune checkpoint therapy. Biochem Biophys Res Commun. (2020) 527:226–31. doi: 10.1016/j.bbrc.2020.04.121
Keywords: esophageal squamous cell carcinoma, neoadjuvant therapy, tislelizumab, camrelizumab, immunotherapy, esophagectomy, efficacy, safety
Citation: Zhao Q, Yuan Y, Xu T, Yan N, Li F, Lu J, He M and Yan Z (2025) Perioperative outcomes of neoadjuvant chemotherapy plus camrelizumab versus neoadjuvant chemotherapy plus tislelizumab for locally advanced esophageal squamous cell cancer: a real-world retrospective study. Front. Immunol. 16:1544739. doi: 10.3389/fimmu.2025.1544739
Received: 13 December 2024; Accepted: 29 July 2025;
Published: 21 August 2025.
Edited by:
Cheng Zhan, Fudan University, ChinaReviewed by:
Masaichi Ohira, Osaka City University, JapanBin Yang, University of Pittsburgh, United States
Copyright © 2025 Zhao, Yuan, Xu, Yan, Li, Lu, He and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoyang Yan, eHd5enkxOTkwQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Qi Zhao1†
Qi Zhao1† Ming He
Ming He Zhaoyang Yan
Zhaoyang Yan