- 1Department of Respiratory Medicine, Shanghai Children’s Medical Center, Shanghai Jiaotong University School of Medicine, Shanghai, China
- 2International Medical Department, Shanghai Children’s Medical Center, Shanghai Jiaotong University School of Medicine, Shanghai, China
- 3Department of Respiratory Medicine, Sanya Women and Children’s Hospital Affiliated to Hainan Medical College, Hainan Branch of Shanghai Children’s Medical Center, Sanya, Hainan, China
- 4Department of Respiratory Medicine, Linyi Maternal and Child Healthcare Hospital, Linyi, Shandong, China
- 5Shanghai Children’s Medical Center Pediatric Medical Complex (Pudong), Shanghai, China
- 6Pediatric Artificial Intelligence (AI) Clinical Application and Research Center, Shanghai Children’s Medical Center, Shanghai, China
Objective: Lung consolidation (LC) in pediatric pneumonia could lead to complicated clinical outcomes, yet the underlying immunological mechanisms are not fully understood. This study aimed to investigate the roles of local and systemic cytokines in the development of pulmonary complications and disease progression in children with pneumonia-associated LC.
Design: Conducted at the Shanghai Children’s Medical Center, this study included 169 children admitted between June 2022 and October 2023.
Methods: We analyzed levels of fifteen cytokines in bronchoalveolar lavage fluid (BALF) and blood. Classification and regression tree (CART) analysis identified specific cytokines associated with pulmonary complications and hypoxemia.
Results: In children with LC, most local cytokines were found at higher levels than systemic cytokines, with no apparent correlation between the two. Notably, an elevated level of IL-8 (≥ 6615 pg/ml) in BALF was associated with an increased risk of hypoxemia. Additionally, elevated levels of IL-4 and INF-γ in BALF were closely associated with the development of multi-segmental LC. Furthermore, elevated levels of IL-2R in BALF were significantly associated with the occurrence of atelectasis, in contrast to their levels in peripheral blood.
Conclusion: IL-4, INF-γ, IL-2R, and IL-8 levels in BALF are closely associated with pulmonary complications and disease progression in children with LC. Exploring targeted immunomodulatory therapies in these children may mitigate lung injury caused by excessive local inflammatory responses.
Introduction
Pneumonia remains one of the most prevalent childhood illnesses worldwide, making it a substantial contributor to pediatric hospitalization rates (1). It accounts for a significant portion of child mortality, particularly among those under the age of 5, with a heightened impact observed in developing regions (2). Lung consolidation (LC) refers to the pathological process in which the alveolar air content is replaced by fluid, inflammatory exudates, or cells, leading to a solidification-like appearance on radiological imaging. In children, LC can result in more severe clinical manifestations and complications such as prolonged hospital stays, increased risk of hypoxemia, and atelectasis. However, the mechanisms driving the varied outcomes associated with LC remain poorly understood (3).
During the course of pneumonia, previous studies have found that infections by different pathogens may lead to similar inflammatory responses (4, 5). Cytokines, as pivotal signaling molecules, assume a central role in the intricate orchestration of the immune response and the regulation of inflammatory processes (6). Therefore, cytokine profiles hold promise for unraveling the immune response mechanisms associated with pneumonia (7). The levels of cytokines in bronchoalveolar lavage fluid (BALF) is closely associated with pulmonary inflammation, elevated levels of interleukin(IL)-6 and tumor necrosis factor-alpha (TNF-α) have been documented as being closely associated with the severity of pneumonia (5). Therefore, analysis of cytokine profiles may reveal the underlying mechanisms responsible for the diverse clinical outcomes in children with pneumonia-associated LC, and provide therapeutic targets for lung injury caused by excessive inflammatory responses.
Considering the factors above, this study assessed the BALF and peripheral blood cytokine levels in children with pneumonia-associated LC. The aim of this study is to explore the differences in cytokine profiles between BALF and serum, and to elucidate the immune response mechanisms that influence outcomes of the pneumonia-associated LC.
Methods
Data source
This was a single-center, observational study conducted at the Shanghai Children’s Medical Center between June 2022 and October 2023. Data were collected from pediatric patients admitted to the Department of Respiratory Medicine. Ethical approval for the study was obtained from the Ethics Committee of the Shanghai Children’s Medical Center, and all procedures were conducted in accordance with the guidelines outlined in the Declaration of Helsinki. Patient information was managed with strict confidentiality and anonymity.
Study population
Inclusion criteria for this study included: 1) children hospitalized due to pneumonia, 2) aged between 1 month and 18 years, 3) chest computed tomography (CT) scans showing lung consolidation affecting two or more segments, and 4) informed consent obtained from the guardians and from children aged over 8 years for participation in the study. Conversely, the exclusion criteria encompassed any of the following conditions: 1) patients whose guardians declined bronchoscopy examination and treatment, 2) patients with hematologic malignancies, immunodeficiency, tumors, congenital cardiopulmonary diseases, muscular dystrophy, or cystic fibrosis, 3) long term use of immunosuppressants, 4) situations where the guardian declined participation in the study, 5) cases with significant omissions of important information. A total of 257 children were initially enrolled. After screening by the exclusion criteria, 88 children were excluded, resulting in a final sample size of 169. The detailed information of screening process was shown in Supplementary Figure S1.
Sample collection and cytokine testing
Samples (BALF and blood) were collected within 48 hours of admission to capture the acute immune response. We collected 2 milliliters (mL) of peripheral blood from children, placing the samples in EDTA-coated anticoagulant tubes. Plasma samples were obtained by centrifuging the blood at 3000g for 10 minutes and immediately stored at -80°C until further use. Five mL of BALF was collected during bronchoscopy at the site of the lesion selected based on pulmonary imaging. Final BALF samples were obtained by centrifuging the primary BALF at 4000g for 5 minutes and stored immediately at -80°C until further use.
We used a multiplex bead-based detection kit from Nanjing Atom Life Technology Co., LTD (https://mp.weixin.qq.com/s/4kmGjAWv0TN1gavD7RMp1g), which simultaneously measures 15 cytokines in a single assay. The kit was employed to assess the levels of 15 cytokines, including IL-1β, IL-2, soluble interleukin-2 receptor (IL-2R), IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17, IL-18, Interferon alpha (IFN-α), IFN-γ, TNF-α, and tumor necrosis factor-beta (TNF-β). The reagent kit comprises 15 fluorescent coding microspheres, each respectively coupled with one of these 15 different monoclonal antibodies, mixed in a specific ratio. During the incubation process, the capture antibody microspheres capture the fifteen cytokines in the sample. Subsequently, a ‘capture antibody microsphere antigen biotin-labeled detection antibody SA-PE’ detection complex is formed after adding biotin-labeled paired detection antibodies and streptavidin phycoerythrin (SA-PE). The immunoassay was conducted using the Luminex MAGPIX detection system (MAGPIX, Luminex, USA). Standard curves and cytokine concentrations were determined using the Atansys software (Nanjing Atom Life Technology Co., LTD). Standardized methods for cytokine dosage in serum and BALF were referenced from previous studies (8). For cytokine values below the lower limit of detection (LLOD), the values were divided by the square root of 2 [LLOD/sqrt(2)].
Data extraction
The collected data encompassed baseline information (sex, age, weight, height), clinical symptoms, chest imaging results, infectious pathogens, and diagnosis. This information was extracted from our PLRTI database (9). For the detection of infectious pathogens, different methods were employed based on the type of pathogen: 1) For viruses and Mycoplasma pneumoniae, qualitative polymerase chain reaction (PCR) was performed to identify the presence of these pathogens. Quantitative analysis was not conducted. 2) For bacteria, detection was performed using bacterial culture. The results were expressed as colony-forming units per milliliter (CFU/mL), which reflects the number of viable bacteria in the sample.
Definitions
The diagnosis of pneumonia adhered to the 2019 edition of diagnostic and treatment standards for pneumonia in children in China (10). The definition of hypoxemia is as follows: 1) transcutaneous oxygen saturation ≤92% or arterial oxygen tension less than 60mmHg without supplemental oxygen; 2) increased respiratory rate; 3) presence of clinical signs such as nasal flaring and tracheal tug.
LC refers to the pathological process in which the alveolar air content is replaced by fluid, inflammatory exudates, or cells, leading to a solidification-like appearance on radiological imaging. Based on clinical observations that consolidation involving three or more segments often indicates more severe disease, we established the following operational definitions: consolidation confined to two segments was categorized as limited segmental lung consolidation (LSLC), while cases involving three or more segments were classified as multi-segmental lung consolidation (MSLC).
Statistical analysis
Continuous variables were compared by using t-tests if variables were normally distributed and while were presented as X ± standard deviation (SD), while Wilcoxon test if variables were not normally distributed and were presented as medians with the 25th and 75th percentiles (P25, P75). Data normality was assessed using the Shapiro-Wilk test. Since most cytokines were not normally distributed, their levels were presented as medians with medians with the 25th and 75th percentiles (P25, P75). Categorical variables were analyzed by the χ2 test or the Fisher’s exact test and expressed as numbers (n) and percentages (%). Since most cytokines were not normally distributed, comparisons of cytokine levels were performed using non-parametric tests. Specifically, the Wilcoxon rank-sum test was used to compare cytokine levels between different patients, while the Wilcoxon signed-rank test was applied to compare cytokine levels in serum and BALF from the same patients. For the heatmap visualization of cytokines, Spearman’s rank correlation coefficients (rs) were calculated to assess monotonic relationships between cytokine levels in serum and BALF. The rs values range from -1 to 1, where absolute values closer to 1 indicate stronger correlations and values near 0 suggest weaker associations. Corresponding p-values were computed for each pairwise comparison to determine statistical significance (p < 0.05).
To determine which cytokines were most predictive of pulmonary consolidation, we applied classification and regression tree (CART) analysis using the ‘rpart’ package in R software. CART, also known as recursive partitioning, constructs a decision tree by repeatedly splitting the data into increasingly homogeneous subsets based on the dependent variable (11). At each step, the algorithm selects the cytokine and its optimal cutoff value that yields the best separation between outcome groups. The resulting tree structure includes decision nodes that show the predicted class, the total number of observations in that node, and the class distribution (i.e., number of observations from each outcome group). The optimal tree was obtained by selecting the complexity parameter value that minimized the cross-validated error in the model’s complexity table, followed by pruning the initial tree using the prune() function. The final tree was visualized using the ‘rpart.plot’ package. P<0.05 was considered to indicate statistical significance. All statistical analyses were performed using R 4.1 software.
Results
Study population and the relationship between local and systemic cytokines
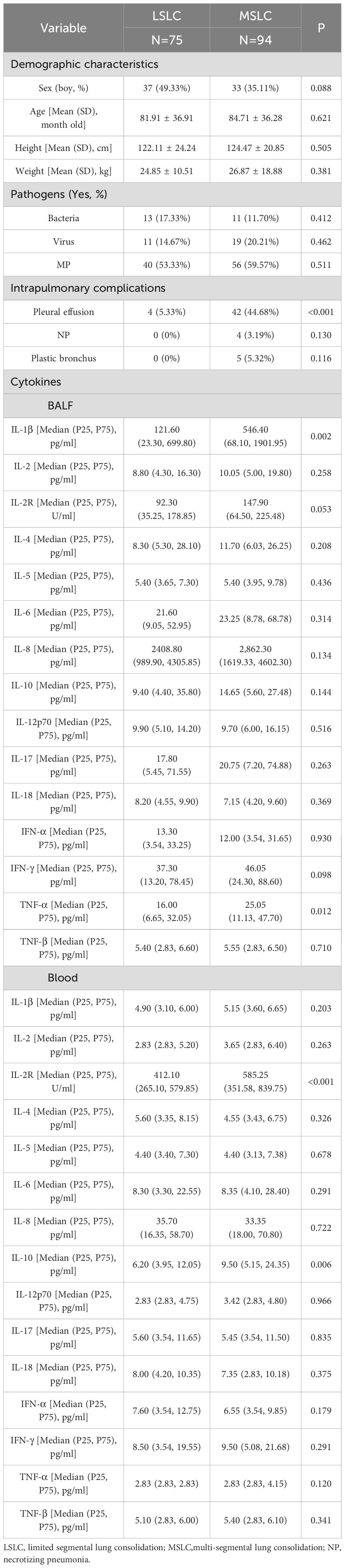
The mean age of the children included in our study was 83.47 ± 36.48 months old. Supplementary Table S1 presents the baseline clinical characteristics of the study population. The concentrations of local and systemic cytokines were assessed. Significantly elevated levels of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17, IFN-α, IFN-γ, TNF-α, and TNF-β were observed in BALF compared to peripheral blood (all p<0.05). In contrast, the concentration of IL-2R was significantly lower in BALF than in peripheral blood (p < 0.001). No significant difference was noted for IL-18 between the two sample types (p > 0.05) (Figure 1). The heatmap revealed varying degrees of association between local and systemic cytokines in children with pneumonia (Figure 2). Several cytokines showed moderate positive correlations, including IFN-α (rs = 0.574), IL-18 (rs = 0.525), and IL-2 (rs = 0.574) (all p<0.001). In contrast, several cytokines exhibited little to no significant correlation, such as IL-10 (rs = 0.118, p = 0.126), IL-4 (rs = 0.045, p = 0.565), and IL-5 (rs = 0.056, p = 0.471).
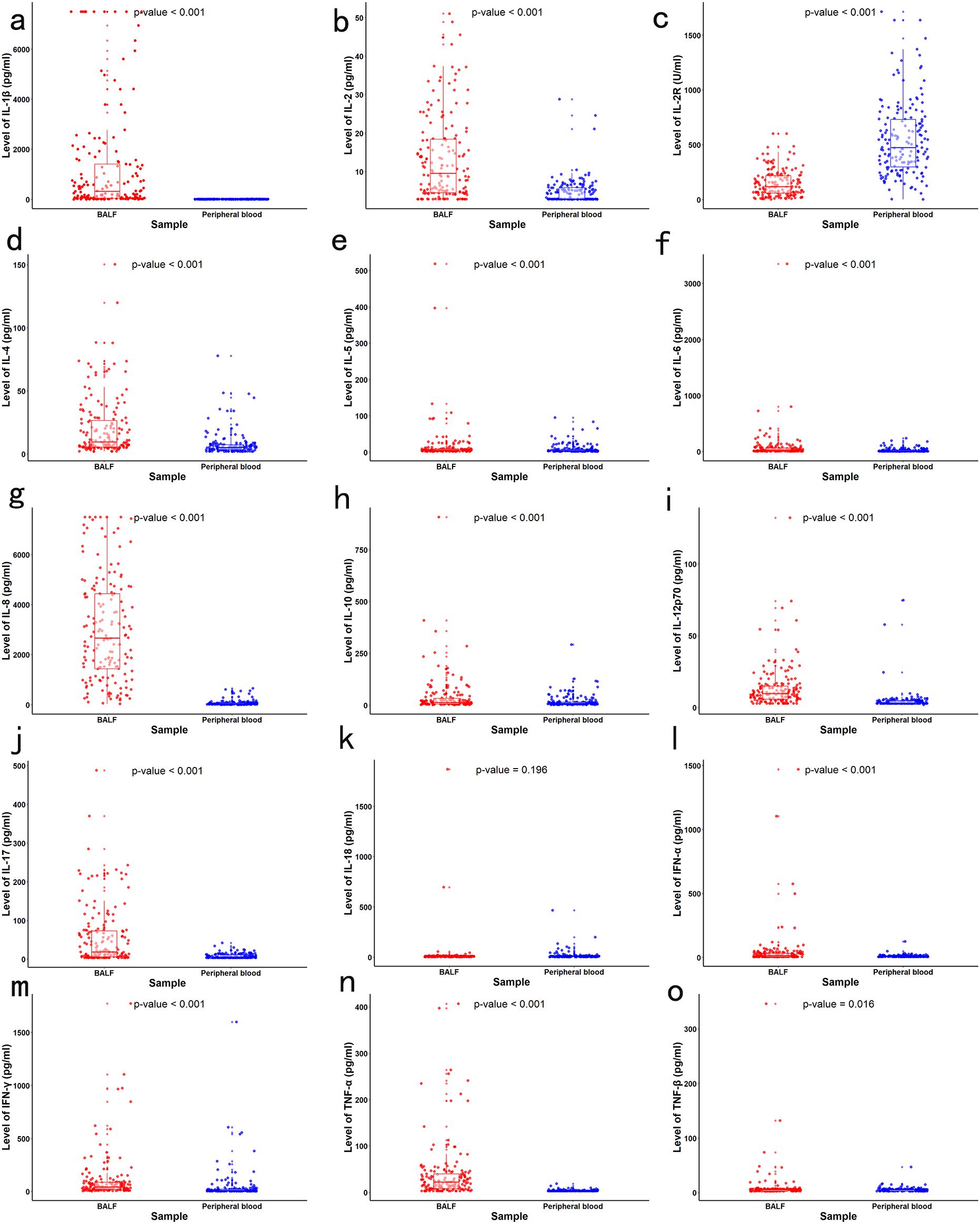
Figure 1. Concentrations of local (BALF) and systemic (peripheral blood) cytokines in children with LC. Paired comparisons between BALF and blood samples were conducted using the Wilcoxon signed-rank test, (a) IL-1β, (b) IL-2, (c) IL-2R, (d) IL-4, (e) IL-5, (f) IL-6, (g) IL-8, (h) IL-10, (i) IL-12p70, (j) IL-17, (k) IL-18, (l) IFN-α, (m) IFN-γ, (n) TNF-α, (o) TNF-β.
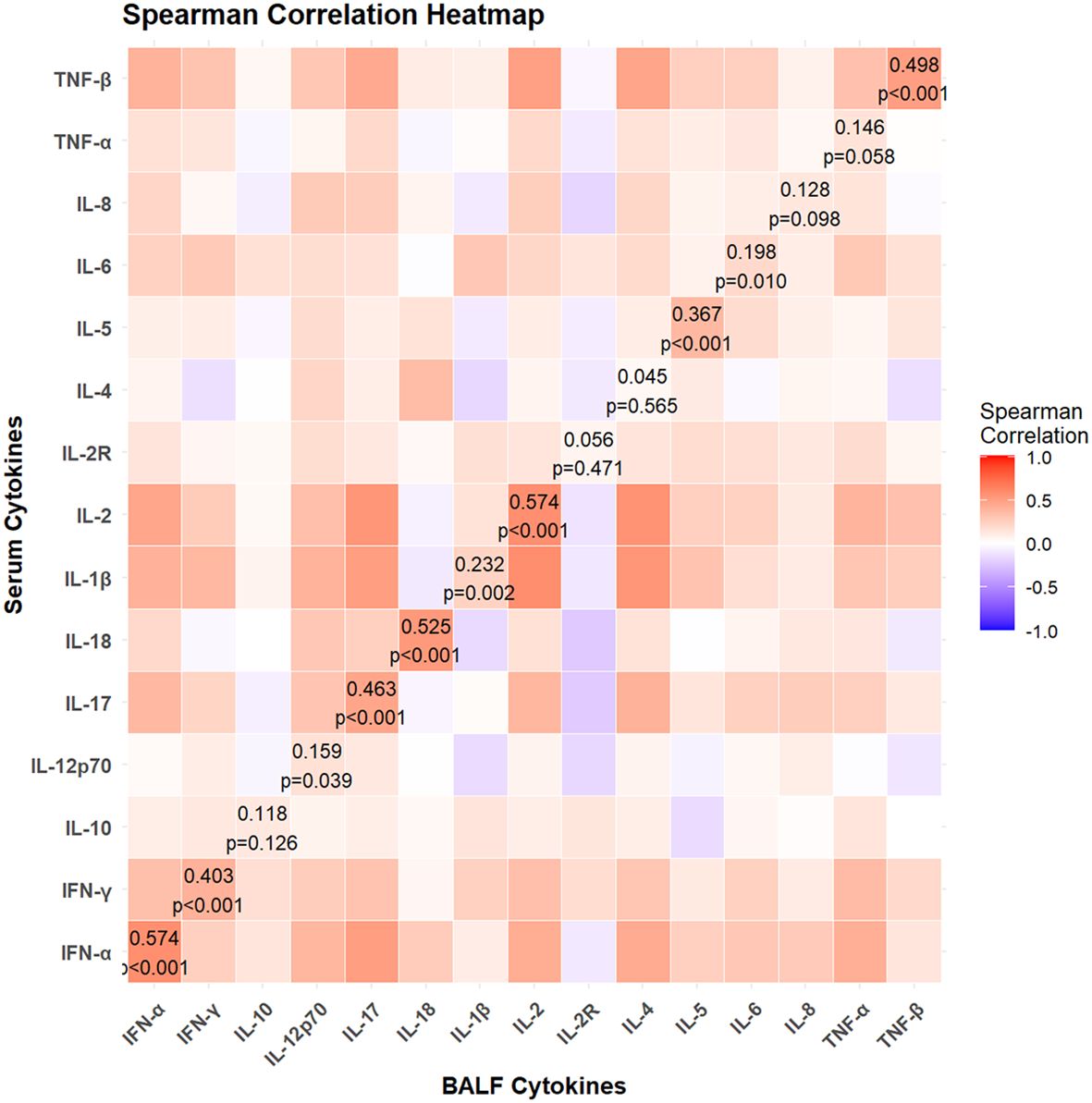
Figure 2. Spearman correlation heatmap between local and systemic cytokines in children with lung consolidation. The color intensity indicates the strength and direction of correlation (red for positive, blue for negative). Statistically significant correlations (p < 0.05) are labeled with corresponding correlation coefficients (rs) and p-values.
Alterations in cytokine profiles in children with hypoxemia
Among these children, a total of 26 children had hypoxemia. No statistically significant differences were noted in the demographic characteristics between hypoxemia group and non-hypoxemia group (Table 1). Compared to children without hypoxemia, those with hypoxemia exhibited significantly elevated levels of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17, IFN-α, IFN-γ, TNF-α, and TNF-β in BALF, as well as slightly higher IL-2 levels in blood (all p<0.05). No significant differences were observed in other cytokines in either BALF or blood.
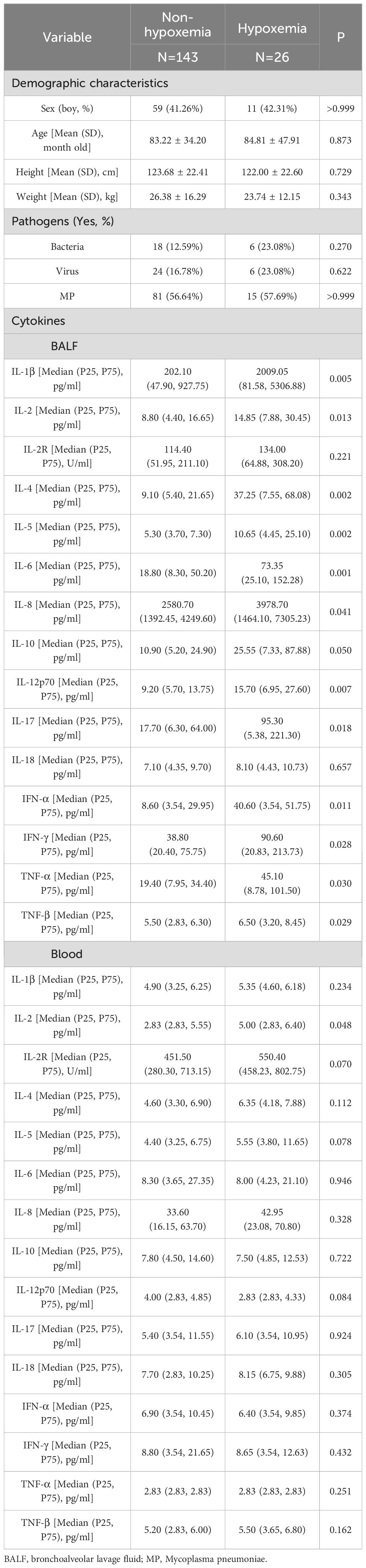
Table 1. The demographic characteristics and cytokines between hypoxemia group and non-hypoxemia group.
We employed the CART algorithm for in-depth node classification of these children, focusing on the expression patterns of these significantly changed cytokines. The CART results indicated that elevated level of IL-8 (≥ 6615 pg/ml) in BALF was associated with an increased risk of hypoxemia (Figure 3a).
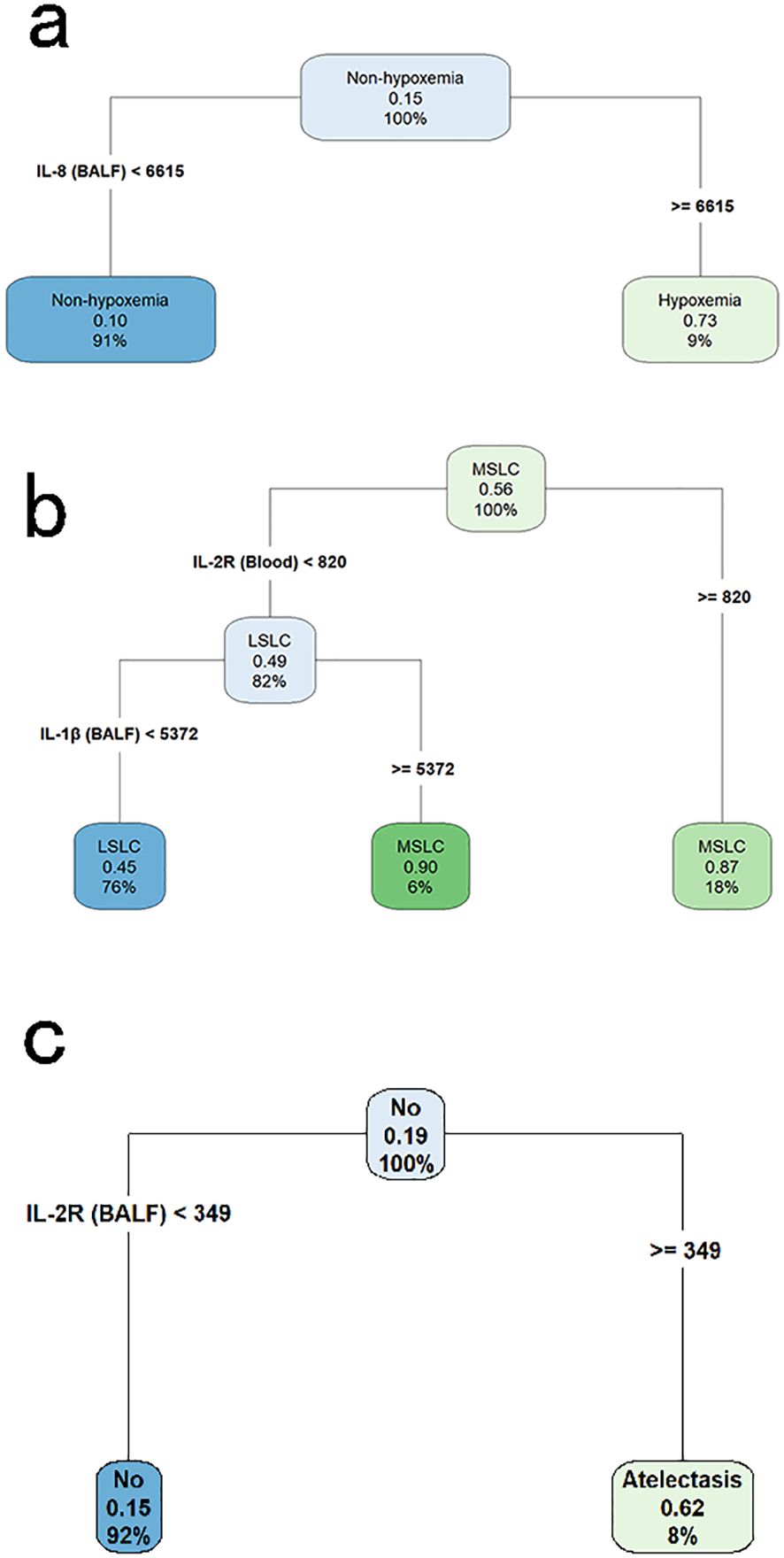
Figure 3. Classification and regression tree analyses for identifying key cytokine predictors of pulmonary complications in children with lung consolidation. (a) Decision tree for hypoxemia, with IL-8 in BALF identified as the primary discriminating cytokine. (b) Decision tree for multi-segmental lung consolidation, showing IL-2R in blood and IL-1β in BALF as key variables. (c) Decision tree for atelectasis, with IL-2R in BALF as the most informative predictor. Each node displays the predicted class, the probability of the outcome, and the percentage of samples represented.
Variations in cytokine profiles between LSLC and MSLC
Of the 169 children, 75 had LSLC and 94 had MSLC. Demographic characteristics were comparable between the LSLC and MSLC groups, with no significant differences in infectious pathogens (Table 2). Among these local and systemic cytokines, IL-1β, and TNF-α in BALF, as well as IL-2R in blood, were significantly elevated in the MSLC group compared with the LSLC group (all p< 0.05).
The CART results revealed a pivotal node at the top level of the tree, indicating that children with IL-2R levels ≥ 820 U/ml in blood were at a higher risk of MSLC. Among those with lower IL-2R levels, further stratification was based on IL-1β concentrations in BALF, where higher IL-1β levels (≥ 5372 pg/ml) were also associated with MSLC (Figure 3b).
Changes in cytokine profiles in children with atelectasis
Among children with LC, a total of 32 children had atelectasis. No statistically significant differences in demographic characteristics were observed between the atelectasis group and the non-atelectasis group (Table 3). Notably, levels of IL-1β, IL-2R, and IL-12p70 in BALF, as well as IL-2R and IL-10 in blood, were significantly higher in children with atelectasis compared to those without. These elevated cytokine levels were utilized in constructing the CART, where a pivotal node at the top level of the tree indicated a higher risk of atelectasis in children with IL-2R levels ≥349 U/ml in BALF (Figure 3c).
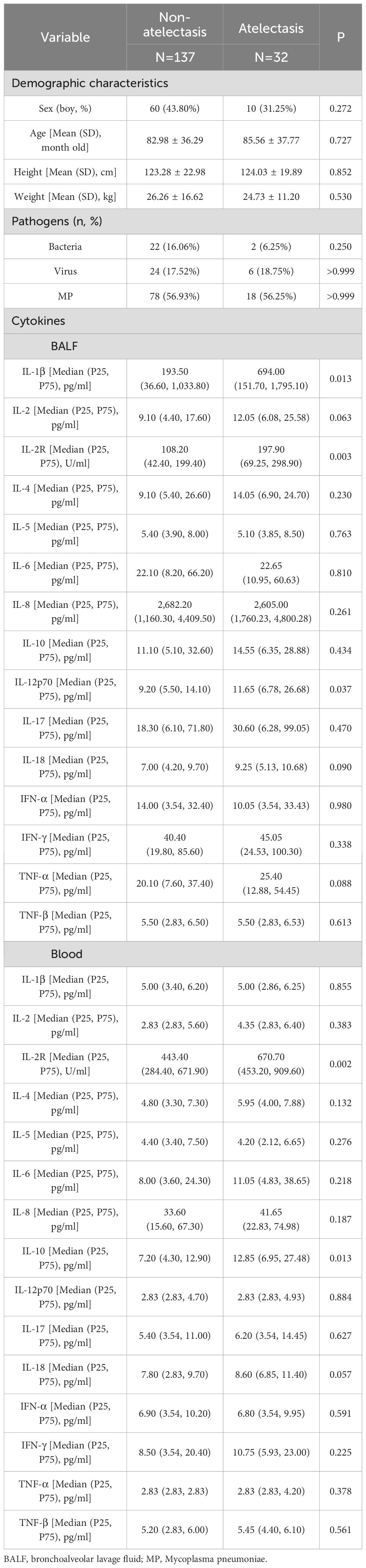
Table 3. The demographic characteristics and cytokines between atelectasis group and non-atelectasis group.
Discussion
In this study, we explored the intricate landscape of local and systemic cytokine expression in children with LC. Noteworthy findings include significant differences in cytokine profiles between BALF and blood, with elevated levels of key inflammatory markers like IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17, IFN-α, IFN-γ, TNF-α, and TNF-β in BALF indicating a localized inflammatory response in children with LC. IL-1β, IL-2R, and IL-8 are closely associated with pulmonary complications and disease progression in pediatric pneumonia with LC.
The alignment of local and systemic immune responses remains inconsistent in respiratory infections (12, 13). BALF offers a more direct reflection of immune activity at the infection site than peripheral blood, which may provide a diluted representation of the local response (14). Our correlation analysis confirmed that some cytokines exhibit parallel changes (e.g., IL-2, IFN-α, IL-18), whereas others differ substantially. This underscores the importance of assessing the local cytokine environment for a more precise understanding of pathological mechanisms, particularly in severe pediatric pneumonia. Currently, there is no consensus regarding the role cytokines play in the severity of pneumonia (15, 16). Luo’s research demonstrated that circulating levels of IL-4 and IFN-γ are not associated with the severity of COVID-19 symptoms (17). However, our study suggests that elevated levels of IL-4 and INF-γ in BALF are closely associated with the development of MSLC, which often represents more severe pneumonia in children. These disparate results suggest that systemic cytokine levels cannot be equated with those in BALF, highlighting the latter’s more direct involvement in the pathogenesis of pneumonia. Therefore, our study, through a comprehensive analysis of cytokines in peripheral blood and BALF, provides deeper insights into the potential mechanisms underlying the progression and severity of lung pathology in pneumonia.
In clinical practice, children with pneumonia accompanied by LC often do not exhibit hypoxemia (18). CART analysis revealed that heightened local levels of IL-8 in BALF may impair oxygen exchange in the pulmonary alveoli in LC. Previous studies have found that IL-8 is closely associated with the development of ARDS, which may explain some of our results (19, 20). Therefore, in children with LC and hypoxemia, targeting IL-8 for therapeutic modulation may be a potential focus of future research, with the aim of reducing mortality in pediatric pneumonia.
Interestingly, elevated IL-2R levels in BALF are significantly associated with the occurrence of atelectasis, rather than in peripheral blood. It is well known that elevated IL-2R levels in BALF are associated with increased T cell activation and immune system activation (21, 22). Notably, peripheral blood IL-2R was also significantly elevated in children with MSLC. This finding suggests that systemic IL-2R elevation might be used as a prognostic indicator or a marker of potential complications. Previous studies have primarily focused on systemic IL-2R levels as a marker of systemic inflammatory responses after infection, even suggesting a link to hemophagocytic syndromes (23, 24). In contrast, our results indicate that local IL-2R levels in BALF are more closely associated with pulmonary complications, while systemic IL-2R levels may reflect the overall burden of disease severity in MSLC. It remains unclear whether IL-2R serves as a biomarker indicating the intensity of the immune response or can be targeted therapeutically to reduce the occurrence of atelectasis or other complications. Therefore, further analysis is needed to explore the role of the IL-2/IL-2R pathway in different contexts of pneumonia.
There were several strengths in this study. Firstly, the research specifically targeted the pediatric population, addressing a critical gap in the literature. Pediatric pneumonia has unique characteristics, and this study contributes valuable insights to this domain. Secondly, the study not only examines cytokine levels but also correlates them with clinical outcomes such as hypoxemia and complications like atelectasis. This connection between cytokines and real-world clinical consequences enhances the practical applicability of the research. Finally, by identifying specific cytokine profiles associated with disease severity and complications, the research lays the groundwork for personalized medicine approaches. Tailoring interventions based on individual immune responses, particularly in children with pneumonia who exhibit excessive immune and inflammatory reactions, may be a promising avenue for future research.
Limitations of this study should also be noted. Firstly, Firstly, it is noteworthy that factors such as previous or concurrent immunomodulant therapies (e.g., macrolides, corticosteroids, high-dose IVIG), environmental exposures (e.g., passive smoking, air pollution), prematurity, vaccination status, and history of respiratory conditions (e.g., asthma, RSV infections) could influence cytokine levels. These variables represent important potential confounders that may bias the observed cytokine profiles. Our current data did not allow for in-depth subgroup analyses to fully account for their effects, but future multicenter studies with larger cohorts and more comprehensive data collection may address these limitations. Secondly, although all samples were collected early during hospitalization, and the majority of patients received cephalosporins or penicillin-class antibiotics at admission, the potential influence of antibiotic use on serum cytokines cannot be entirely ruled out and warrants further investigation. Thirdly, the study focused on a specific panel of cytokines, and other potentially relevant cytokines or inflammatory markers may not have been included. A broader cytokine profile analysis could provide a more comprehensive view of the immune response. Another limitation of this study is the lack of long-term follow-up data on serum cytokine levels. While we observed significant differences in serum cytokines during the acute phase of pneumonia, it remains unclear whether these changes persist or normalize over time. Future studies with longitudinal designs could collect serum samples at 6 and 12 months post-recovery to assess whether the observed cytokine alterations are transient or indicative of long-term immune dysregulation.
In addition to the cytokine profiles identified in this study, future research should explore the potential correlations between cytokine patterns and environmental factors such as pollution exposure, as well as their interactions with respiratory and gut dysbiosis. Air pollution disrupts immune responses and exacerbates inflammation, potentially altering cytokines like IL-8 and TNF-α (25–27). Gut microbiota, via the “gut-lung axis,” regulates systemic immunity, and dysbiosis may increase infection susceptibility. Investigating these interactions could reveal novel therapeutic targets for mitigating lung injury in children.
Conclusion
In children with pneumonia-associated LC, local cytokine levels are significantly higher than systemic cytokine levels, and systemic cytokine levels cannot directly reflect the levels of local cytokines. IL-1β, IL-2R, and IL-8 are closely associated with pulmonary complications and disease progression in pediatric pneumonia with LC. Future research targeting these cytokines and their related pathways may play an important role in developing specific immunomodulatory therapies to reduce lung injury caused by excessive inflammatory responses in children with pneumonia.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of the Shanghai Children’s Medical Center and conducted according to the Declaration of Helsinki guidelines (SCMCIRB-YF2021003). Written informed consent was obtained from all participants or their representatives. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Validation, Writing – original draft. JC: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. SY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Writing – original draft. MT: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing – original draft. GY: Data curation, Investigation, Validation, Visualization, Writing – original draft. HSZ: Data curation, Investigation, Validation, Writing – original draft. WL: Data curation, Supervision, Validation, Visualization, Writing – original draft. HZ: Data curation, Validation, Visualization, Writing – original draft. JZ: Methodology, Supervision, Validation, Visualization, Writing – review & editing. LZ: Conceptualization, Investigation, Project administration, Resources, Supervision, Writing – review & editing. YY: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Translational Medicine Cross Research Fund of Shanghai Jiao Tong University (YG2021QN109); Shanghai Municipal Health Commission Health Industry Clinical Research Special Project (20224Y0180); Shanghai Qimingxing Project (Yangfan Special Project) (23YF1425100); National Natural Science Foundation of China (82300020); Special Livelihood Research Project of Pudong New Area Science and Technology Development Fund Public Institution (PKJ2023-Y50).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1546730/full#supplementary-material
References
1. Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Swartz S. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. (2017) 17:1133–61. doi: 10.1016/S1473-3099(17)30396-1
2. O’Brien KL, Baggett HC, Brooks WA, Feikin DR, Hammitt LL, Higdon MM. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. (2019) 394:757–79. doi: 10.1016/S0140-6736(19)30721-4
3. Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. (2020) 55:327–31. doi: 10.1097/RLI.0000000000000672
4. McCullers JA. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. (2014) 12:252–62. doi: 10.1038/nrmicro3231
5. Deng F, Cao H, Liang X, Li Q, Yang Y, Zhao Z, et al. Analysis of cytokine levels, cytological findings, and MP-DNA level in bronchoalveolar lavage fluid of children with Mycoplasma pneumoniae pneumonia. Immun Inflammation Dis. (2023) 11:e849. doi: 10.1002/iid3.v11.5
6. Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood. (2011) 118:9–18. doi: 10.1182/blood-2010-08-265892
7. Berg J, Zscheppang K, Fatykhova D, Tönnies M, Bauer TT, Schneider P, et al. Tyk2 as a target for immune regulation in human viral/bacterial pneumonia. Eur Respir J. (2017) 50:1601953. doi: 10.1183/13993003.01953-2016
8. Lesueur J, Walachowski S, Barbey S, Cebron N, Lefebvre R, Launay F, et al. Standardized whole blood assay and bead-based cytokine profiling reveal commonalities and diversity of the response to bacteria and TLR ligands in cattle. Front Immunol. (2022) 13:871780. doi: 10.3389/fimmu.2022.871780
9. Lin J, Yuan S, Dong B, Zhang J, Zhang L, Wu J, et al. Establishment of a simple pediatric lower respiratory tract infections database based on the structured electronic medical records. Front Pediatr. (2022) 10:917994. doi: 10.3389/fped.2022.917994
10. Feng Q, Wang J, Wang X, Tian J, Zhang L, Dilmurat D, et al. Clinical epidemiological characteristics of hospitalized pediatric viral community-acquired pneumonia in China. J Infect. (2025) 90:106450. doi: 10.1016/j.jinf.2025.106450
11. Song YY, Lu Y. Decision tree methods: applications for classification and prediction. Shanghai Arch Psychiatry. (2015) 27:130–5. doi: 10.11919/j.issn.1002-0829.215044
12. Smith N, Goncalves P, Charbit B, Grzelak L, Beretta M, Planchais C, et al. Distinct systemic and mucosal immune responses during acute SARS-CoV-2 infection. Nat Immunol. (2021) 22:1428–39. doi: 10.1038/s41590-021-01028-7
13. Wimmers F, Burrell AR, Feng Y, Zheng H, Arunachalam PS, Hu M, et al. Multi-omics analysis of mucosal and systemic immunity to SARS-CoV-2 after birth. Cell. (2023) 186:4632–4651.e4623. doi: 10.1016/j.cell.2023.08.044
14. Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. (2020) 9:761–70. doi: 10.1080/22221751.2020.1747363
15. Dukhinova M, Kokinos E, Kuchur P, Komissarov A, Shtro A. Macrophage-derived cytokines in pneumonia: Linking cellular immunology and genetics. Cytokine Growth Factor Rev. (2021) 59:46–61. doi: 10.1016/j.cytogfr.2020.11.003
16. Rendon A, Rendon-Ramirez EJ, Rosas-Taraco AG. Relevant cytokines in the management of community-acquired pneumonia. Curr Infect Dis Rep. (2016) 18:10. doi: 10.1007/s11908-016-0516-y
17. Luo W, Zhang JW, Zhang W, Lin YL, Wang Q. Circulating levels of IL-2, IL-4, TNF-α, IFN-γ, and C-reactive protein are not associated with severity of COVID-19 symptoms. J Med Virol. (2021) 93:89–91. doi: 10.1002/jmv.26156
18. Meyer Sauteur PM. Childhood community-acquired pneumonia. Eur J Pediatr. (2023) 183:1129–36. doi: 10.1007/s00431-023-05366-6
19. Dahmer MK, Yang G, Zhang M, Quasney MW, Sapru A, Weeks HM, et al. Identification of phenotypes in paediatric patients with acute respiratory distress syndrome: a latent class analysis. Lancet Respir Med. (2022) 10:289–97. doi: 10.1016/S2213-2600(21)00382-9
20. Slim MA, van Amstel RBE, Bos LDJ, Cremer OL, Wiersinga WJ, van der Poll T, et al. Inflammatory subphenotypes previously identified in ARDS are associated with mortality at intensive care unit discharge: a secondary analysis of a prospective observational study. Crit Care. (2024) 28:151. doi: 10.1186/s13054-024-04929-9
21. Spolski R, Li P, Leonard WJ. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat Rev Immunol. (2018) 18:648–59. doi: 10.1038/s41577-018-0046-y
22. Permanyer M, Bošnjak B, Glage S, Friedrichsen M, Floess S, Huehn J, et al. Efficient IL-2R signaling differentially affects the stability, function, and composition of the regulatory T-cell pool. Cell Mol Immunol. (2021) 18:398–414. doi: 10.1038/s41423-020-00599-z
23. Knauft J, Schenk T, Ernst T, Schnetzke U, Hochhaus A, La Rosée P, et al. Lymphoma-associated hemophagocytic lymphohistiocytosis (LA-HLH): a scoping review unveils clinical and diagnostic patterns of a lymphoma subgroup with poor prognosis. Leukemia. (2024) 38:235–49. doi: 10.1038/s41375-024-02135-8
24. Lin M, Park S, Hayden A, Giustini D, Trinkaus M, Pudek M, et al. Clinical utility of soluble interleukin-2 receptor in hemophagocytic syndromes: a systematic scoping review. Ann Hematol. (2017) 96:1241–51. doi: 10.1007/s00277-017-2993-y
25. Dondi A, Manieri E, Betti L, Dormi A, Carbone C, Biagi C, et al. Exposure to outdoor air pollution and risk of hospitalization for bronchiolitis in an urban environment: A 9-year observational study. Pediatr Pulmonol. (2023) 58:2786–94. doi: 10.1002/ppul.v58.10
26. Roggiani S, Zama D, D’Amico F, Rocca A, Fabbrini M, Totaro C, et al. Gut, oral, and nasopharyngeal microbiota dynamics in the clinical course of hospitalized infants with respiratory syncytial virus bronchiolitis. Front Cell Infect Microbiol. (2023) 13:1193113. doi: 10.3389/fcimb.2023.1193113
Keywords: children, cytokine, pneumonia, lung consolidation, bronchoalveolar lavage fluid
Citation: Lin J, Chen J, Yuan S, Tang M, Yang G, Zhang H, Li W, Zhao H, Zhang J, Zhang L and Yin Y (2025) Local and systemic cytokine profiles in children with pneumonia-associated lung consolidation. Front. Immunol. 16:1546730. doi: 10.3389/fimmu.2025.1546730
Received: 17 December 2024; Accepted: 15 April 2025;
Published: 08 May 2025.
Edited by:
Laura Maggi, University of Florence, ItalyReviewed by:
Eugenia Granado, National Cancer Institute (INCA), BrazilMattia Moratti, University of Rome Tor Vergata, Italy
Copyright © 2025 Lin, Chen, Yuan, Tang, Yang, Zhang, Li, Zhao, Zhang, Zhang and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhang, emhhbmdsZWlAc2NtYy5jb20uY24=; Yong Yin, eW9uZ195aW4xOTcwQDE2My5jb20=
†These authors have contributed equally to this work
 Jilei Lin
Jilei Lin Jiande Chen1†
Jiande Chen1† Jing Zhang
Jing Zhang Yong Yin
Yong Yin