Abstract
Background:
Influx and establishment of key endometrial immune factors in the mid-luteal phase of the menstrual cycle is paramount for successful embryo implantation. Endometrial immune dysregulation is associated with repeated embryo implantation failure and miscarriage. In in vitro fertilisation cycles, approximately 30% of embryos diagnosed as chromosomally normal will still fail to produce a viable live birth, yet factors such as the endometrium are rarely clinically explored.
Methods:
In this retrospective analysis, clinical outcomes were compared between patients undergoing their first euploid transfer in a conventional substituted cycle (n=612), patients undergoing a euploid transfer in a similar cycle after previous euploid failure (n=149) and the study group of patients with previous euploid transfer failure who received a modified endometrial preparatory regimen following endometrial immune profiling targeting uterine natural killer cell recruitment, maturity and activity as well as their key regulatory counterparts (n=37).
Results:
Significant differences were found between first euploid attempt outcomes and patients with previous failures who didn’t use endometrial testing (implantation rate 63% vs 51, P=0.02; clinical pregnancy rates 55% vs 40%, P=0.002; live birth rates 50% vs 38%, P=0.02). Patients with previous failures who underwent endometrial immune profiling and a subsequent personalised plan exhibited a trend towards improved clinical outcomes than those with previous failures and no testing (implantation rate 65% vs 51%; clinical pregnancy rate 57% vs 40%; live birth rate 54% vs 38%, respectively) although statistical significance was not demonstrated. Clinical outcomes were comparable between the endometrial immune profiling group and those undergoing a first euploid attempt (implantation rate 65% vs 63%; clinical pregnancy rate 57% vs 55%; live birth rate 54% vs 50%, respectively).
Conclusions:
Patients who had a failed attempt when using a euploid embryo had lower chances of pregnancy when repeating their treatment, unless they received a personalised endometrial preparation regimen derived from the results of endometrial immune profiling. These preliminary findings indicate the potential value of guiding management based on immune endometrial testing.
Introduction
The central dogma for establishing a pregnancy is the arrival of a viable competent embryo into the uterus when the endometrium is receptive. This biological event is essential for implantation to commence; aberrances in either embryo or endometrium may ultimately lead to implantation failure and embryo rejection.
Treatment with in vitro fertilisation (IVF) aims to overcome reproductive challenges for patients. A major factor affecting patients’ ability to conceive from IVF is oocyte-derived aneuploidies which increase in incidence with age, reaching 66% by 40-42 years (1). A clinical technique used to ameliorate the clinical impact of this is Pre-Implantation Genetic Testing for Aneuploidy (PGT-A), which aims to determine chromosomal copy number from biopsied trophectodermal cells of a day 5 blastocyst. Recent non-selection studies have shown that embryos known to be chromosomally abnormal, referred to as aneuploid, rarely result in viable births. In a review of five non-selection studies, embryos diagnosed as aneuploid were reported to have a 98% positive predictive value of embryo lethality (2). Most non-viable embryos will usually fail to implant, some may succeed but result in miscarriage (3, 4). Evidence that encompasses both the embryo and the endometrium’s role in implantation suggest that an aneuploid or poorly viable embryo can secrete distinct molecules that are detected by the endometrial stroma, preventing further maternal investment in a non-viable pregnancy. (5, 6). This suggests there may be a dual function in modulating acceptance of the embryo into the endometrium.
Despite the use of techniques to eliminate aneuploidies, patients still fail to conceive. With 30% of euploid transfers in IVF failing to implant, other factors such as the endometrium could be attributable (7). However clinically, the complexity of the endometrium and its function in mediating embryo implantation and sustained pregnancy is poorly recognised. Routine endometrial investigations rarely surpass ultrasound scans and measuring endometrial thickness, with some routine measurements of serum progesterone levels during luteal phase support. Proper investigation of the endometrium is thus relatively disregarded and any treatments therefore tend to be empirical.
The concept of endometrial receptivity is based on the distinct transformation of the endometrium in its secretory phase. Aside from development into a glandular structure, an influx of immune cells and immunomodulatory factors establish a balance of both pro-inflammatory and pro-modulatory effects. These milieus play a key role in ensuring the endometrium is in a state that is receptive to an implanting blastocyst. Within the very complex local uterine immune milieu, involving multiple cell types (uNK, regulatory T-cells, dendritic cells) and cytokines (Th1, Th2, Th17), the Th1/Th2 balance has a key role. Pro-immunomodulatory Th2 cells are considered to provide protection to the foetus, while regulating pro-inflammatory Th1 cytokines (8, 9). While Th1 cells and their cytokines in excess are thought to be embryonically cytotoxic, a tightly-controlled concentration is required to promote trophoblastic invasion into the endometrium during implantation (10, 11).
Unique in their distinctive CD56bright, CD16dim antigen expression, uterine Natural Killer (uNK) cells exert key functions at the time of implantation in the endometrium and are crucial for governing endometrial receptivity in the mid-luteal immune milieu (12–14). Unlike circulating peripheral NK cells, uNKs are within an immune niche regulated by elevating mid-luteal progesterone concentrations, and by specific local cytokines within the endometrium (15). Namely, local secretions of IL-15 recruit uNK cell populations, while IL-18 promotes uNK cell maturation and activity. Thus uNK cells are required to establish a balanced pseudo-inflammatory environment in the decidua (16).
Early hypotheses suggested that changes in uNK cell counts, recruitment and maturity may be potential causes for implantation failure or miscarriage. Indeed, in patients experiencing recurrent embryo implantation failures (RIF) (17, 18) and recurrent miscarriages (RM) (19), significant differences in the proportion of uNK cells and their associated markers have been noted (18, 20). uNK cell immunomodulation depends on two key cytokines, IL-18 and IL-15 (21). More recently, modulators of these cytokines were unveiled, namely transmembrane protein TWEAK and its respective ligand Fn-14. These act to immunomodulate uNKs by inhibiting Th1-driven differentiation of uNK cells into cytotoxic cells and neutralise high IL-18 concentrations (22).
In light of these findings, development of a clinical diagnostic test that profiles the mid-luteal phase endometrial immune milieu, specifically utilising uNK cell count, maturation and key associated immunomodulatory molecules (17, 23), found imbalances in these markers in 80% of RIF and RM patients (19, 24). Moreover, subsequent treatment cycles aiming to correct the dysregulation reported promising results, with increased LBRs (19, 24).
Within this study, we aim to explore whether patients who had at least one embryo implantation failure or miscarriage with a euploid blastocyst clinically benefit from endometrial immune profiling and a subsequent personalised treatment cycle, in terms of achieving both embryo implantation and a live birth, in comparison to those that did not undergo endometrial immune profiling prior to a euploid blastocyst transfer and had a conventional treatment cycle.
Materials and methods
Patients/participants
This was a retrospective cohort study of 609 patients who underwent PGT between 2019-2024 at a single UK-based centre. Inclusion criteria were patients who created blastocysts through IVF or ICSI and underwent PGT between January 2019- January 2024 via Next Generation Sequencing (NGS) and generated at least one euploid blastocyst. Further inclusion criteria were those who transferred a vitrified-warmed euploid blastocyst that resulted in implantation failure or miscarriage and subsequently underwent further euploid transfers, with or without endometrial immune profiling. Exclusion criteria were patients who underwent immune profiling but did not follow the treatment suggested or transferred outside of the test validity, array CGH-tested PGT embryos and non-euploid embryos (mosaics or no result). Any patients with pre-existing autoimmune diseases or taking immunosuppressants or steroids were further excluded.
Embryo biopsy & chromosomal analysis via PGT-A
On day 5, 6 or 7 of embryo culture, 5-10 cells of the blastocyst trophectoderm were biopsied using the laser technique, placed in sterile tubes and sent for NGS (25), as described previously (CooperGenomics, UK). Only patients that transferred blastocysts diagnosed as euploid were included in this study. Mosaic and no result embryos were excluded from analysis.
All blastocysts were morphologically assessed using a validated modified Gardner system for up to day 3 embryos, and the ACE/NEQAS grading system for day 5 blastocysts (26, 27). All embryos were cultured in EmbryoScopes (EmbryoScope time-lapse system - Vitrolife) (Vitrolife, UK) (28). Euploid blastocysts were prioritised for transfer, but where patients had more than one euploid blastocyst, these were secondarily prioritised for transfer by morphology and time-lapse morphokinetics (27).
Endometrial assessment
Criteria for referral for endometrial immune profiling was as follows. Patients who had at least one previous failed euploid transfer were eligible for referral for testing. Exclusion criteria were patients with poor morphological blastocyst quality or history of a thin endometrial lining.
All endometrial biopsies were performed in a mock substituted cycle, with oestradiol 8-10mg daily (Progynova, Bayer plc, UK). Luteal support was given as micronised vaginal progesterone 400mg three times daily (Cyclogest, L.D. Collins & Co Ltd., UK). Endometrial biopsy was performed with or without sedation following five days of progesterone with a pipelle. Endometrial tissue was stored in RNA later for transport.
All biopsies were sent for immunological profiling, as described previously (Matrice Lab Innove, Paris, France) (18, 20). To summarise, immune profiling analysed variables such as uNK cell count, recruitment and activation by testing for key markers such as IL-18/TWEAK mRNA, IL-15/Fn-14 and CD56+ counts using RT-qPCR.
Patients could receive one of four results:
-
“Balanced”- characterised by IL-18/TWEAK and IL-15/Fn-14 mRNA ratios and CD56+counts within the same range as defined by a fertile cohort.
-
“Overactive”- characterised by high IL-18/TWEAK and/or IL-15/Fn-14 mRNA ratios and/or a high CD56+count.
-
“Underactive”- characterised by IL-15/Fn-14 mRNA ratios and/or a low CD56+count and/or a very low local IL-18/TWEAK mRNA ratio.
-
“Mixed”- characterised by a high ratio of IL-18/TWEAK mRNA in tandem with low IL-15/Fn-14 mRNA ratio (20).
Endometrial preparation for frozen embryo transfer
All patients referred for endometrial assessment were prescribed a substituted frozen embryo transfer (FET) cycle based on their immune profiling results, as described previously (20). The protocols for each immune profile are as follows:
-
Balanced: Underwent a standard substituted cycle, commencing oestradiol on cycle day 2-3 until endometrial thickness measured 8mm, followed by 5 days of progesterone pessary administration 400mg 3x daily.
-
Overactive: Corticoids were prescribed at the dose of 20mg daily, with the aim to decrease Th1 cytokines and uNK cytotoxicity, reduce IL-15 overexpression and modulate Th1/Th2 cytokine ratios. Oral oestradiol was prescribed to downregulate IL-18 in cases displaying overexpression. High doses of progesterone were administered by dual route (vaginal and intramuscular/subcutaneous) to exert an immunosuppressive function.
-
Underactive: An endometrial scratch was performed during the mid-luteal phase of the preceding cycle. Supplementation with hCG was recommended on day 4, 6 and 8 after starting progesterone in cases with low CD56+ mobilisation or uNK cell immaturity.
-
Mixed: An endometrial scratch was performed and hCG was administered on days 4, 6 and 8 following commencement of progesterone. Patients were also prescribed corticoids at a dose of 10mg daily and oral oestradiol (20).
Only single euploid embryo transfers were performed following immune profiling and personalised plan.
Patients who did not undergo endometrial assessment, comprising the control group, underwent a standard substituted FET cycle, commencing oestradiol 8mg daily (Progynova, Bayer plc, UK) on cycle day 2-3 until endometrial thickness measured 8mm or above via transvaginal ultrasonography, with all euploid blastocysts transferred following 5 days of progesterone administration (Cyclogest, L.D. Collins & Co Ltd., UK).
Outcome definitions
Primary outcome measure was implantation rate (IR), defined as a positive serum βhCG test. Evolution of pregnancy was confirmed with viable intrauterine scans/foetal hearts at 7 and 12-weeks gestation. Secondary outcome measures were clinical pregnancy rate, defined as confirmation of foetal heart at 7 weeks via ultrasound scans, and live birth rate (LBR). Another outcome calculated was miscarriage rate (MR), defined as the total number of pregnancy losses between a positive βhCG test and 12 weeks gestation. Outcome measures are calculated as per embryo transferred.
Statistical analyses
Univariate analyses comprised of non-parametric and parametric t-tests for continuous variable comparisons, with data reported as median [interquartile range (IQR)] for non-normally distributed variables and mean ± SD for normal distributions. Distribution normality was tested via histograms and Shapiro-Wilk tests. For comparison of multiple non-normally distributed continuous variables, Kruskal-Wallis tests were used for univariate analyses. For multiple normally distributed continuous variables, ANOVAs were used. For categorical variables, Fisher’s Exact tests were applied to compare proportional rates in small sample sizes. Post-hoc comparison tests were conducted for results returning with significance. P<0.05 denoted statistical significance. All analyses were conducted on R. 4.3.1.
Ethical approval
Since this was a retrospective service evaluation using anonymised clinical data further ethical approval by an IRB was not considered to be required by the University of Kent Central Research Ethics Advisory Group.
Results
A total of 609 patients underwent 640 PGT cycles in which at least one euploid blastocyst was transferred between 2019-2024. This first euploid transfer (n=609) comprises the first comparative group for this study (First attempt group). Following a first euploid blastocyst transfer attempt, 301 patients’ outcome resulted in implantation failure or miscarriage (49%). All patients who had a miscarriage were tested for the usual causes of miscarriage (thrombophilia, dysthyroidism, diabetes, uterine cavity assessment). Of these, 173 patients (57%) underwent at least one subsequent euploid embryo transfer after failure. Following an adopted clinical policy, patients were offered to undergo immune profiling of the endometrium after undergoing at least one failed euploid transfer. Overall, 50 patients were referred for immune profiling after one or more euploid transfer failures, of which 37 completed the treatment (Previous euploid failure/s [PEF] + endometrial immune profiling [EIP] group). In total, 149 patients underwent a second euploid transfer without undergoing immune profiling, comprising a second comparison group (PEF + no EIP group). Some patients underwent multiple euploid embryo transfer failures prior to being referred for immune profiling. There is therefore patient cross-over present between groups, where some patients underwent a second euploid transfer attempt without immune profiling, and then underwent immune profiling for a subsequent attempt.
The mean oocyte age in this cohort (n=640) was 36.7 ± 4.2 with a mean patient age of 37.8 ± 4.1. Overall, 95% (608/640) of cycles used own oocytes and 5% (32/640) with donor oocytes. The cohort had a median AMH of 17.9 (1.1, 34.8) and median BMI of 24 (18.5, 30). Characteristics stratified by group can be found in Table 1. Overall, there was a statistically significant difference in the oocyte age across all groups (P<0.001), with post-hoc analyses revealing significant differences between both the first attempt and PEF + no EIP group (36.8 ± 4.2 vs 35.8 ± 4.1, P=0.03) and between first attempt and the PEF + EIP group (36.8 ± 4.2 vs 37.8 ± 4.1, P=0.03). All other characteristics did not differ between groups (Table 1).
Table 1
| Patient/cycle characteristics | First attempt, euploid transfer (n=609) | Previous euploid failure + no immune profiling (n=149) | Previous euploid failure + immune profiling (n=37) | P-value |
|---|---|---|---|---|
| Oocyte age, mean ± SD | 36.8 ± 4.2 | 35.8 ± 4.1 | 37.8 ± 4.1 | P<0.001 |
| Patient age, mean ± SD | 37.8 ± 4.1 | 37.5 ± 4.5 | 38.6 ± 3.7 | P=0.3 |
| BMI (kg/m2), median [IQR] | 24 [19, 30] | 26.5 [19, 31] | 22.8 ± [19, 28] | P=1 |
| AMH (pmol/L), median [IQR] | 17.4 [0.6, 34.2] | 18.1 [3.4, 32.8] | 17.9 [3.8, 32] | P=1 |
| Own oocytes, n (%) | 95% (583/612) | 91% (138/149) | 90% (35/37) | P=0.4 |
| Donor oocytes, n (%) | 5% (29/612) | 9% (11/149) | 10% (2/37) | – |
Patient and PGT cycle characteristics between 2019-2024.
-Statistical test not applicable.
The median number of oocytes collected per fresh IVF collection cycle was 13 [IQR 3, 23]. In total, 9620 oocytes were collected, of which 62% were fertilised via ICSI and 38% via IVF, with fertilisation rates equating to 64%. Eighty-one percent used partner sperm and 19% with donor sperm. Blastulation rates reached 64%. Out of all created blastocysts, 96% were biopsied for PGT-A.
Clinical outcomes
A total of 805 euploid blastocysts were transferred in 795 FET cycles. A directive single blastocyst transfer policy is in place at this centre, however three patients underwent a double embryo transfer in their first euploid attempt, and seven in their second euploid transfer attempt. On average, patients underwent a mean of 1.3 ± 0.5 euploid blastocyst transfers within the study period. Of these, 99% were single euploid transfers.
Six-hundred and nine patients transferred 612 euploid blastocysts in their first euploid transfer attempt. Out of 609, 301 patients’ first euploid transfer ended in implantation failure (37%) or miscarriage (13%). One patient terminated their pregnancy (0.2%). One hundred and forty-nine patients underwent a second euploid blastocyst transfer with standard substituted cycle, transferring a total of 156 blastocysts. Fifty patients were referred for endometrial immune profiling after at least one failed euploid transfer. Patients referred for endometrial immune profiling (n=50) underwent between 1-4 failed euploid transfers and between a combined total of 1-9 failed untested and euploid transfers at this centre prior to referral. Of the final cohort that completed endometrial immune profiling (n=37), 43% of patients were diagnosed with an overactive profile, 27% a balanced profile, 16% an underactive profile and 14% a mixed profile. A personalised protocol was prescribed for FET according to the profile, as outlined in the methodology (Table 2). All patients who completed endometrial immune profiling and subsequent personalised FET protocol underwent single euploid blastocyst transfer.
Table 2
| % | Medication | |
|---|---|---|
| Overactive | 43% (16/37) | • Oestradiol tablets 8-10mg daily • Prednisolone 20mg daily • Progesterone pessaries 400mg 3x daily + intramuscular progesterone |
| Balanced | 27% (10/37) | • Oral oestradiol 8-10mg daily • Progesterone pessaries 400mg 3x daily |
| Underactive | 16% (6/37) | • Endometrial scratching • Oestradiol tablets 8-10mg daily • Progesterone pessaries 400mg 3x daily • Low-dose hCG during luteal support |
| Mixed | 14% (5/37) | • Oestradiol tablets 8-10mg daily • Prednisolone 10mg daily • Progesterone pessaries 400mg 3x daily + intramuscular progesterone |
Endometrial immune profiling and subsequent treatment for patients referred between 2019-2024.
Across all clinical outcomes, the PEF + EIP group achieved the highest rates (Table 3). This group achieved an implantation rate of 65%, a CPR of 57% with a final LBR of 54%. These rates were comparable with the first attempt group (65% vs 63%, P=1, 57% vs 55%, P= 0.9, 54% vs 50%, P= 0.7, respectively). This was the case despite the first attempt group having a significantly younger average oocyte age, greater number of embryos transferred per transfer and a greater proportion of embryos transferred morphologically graded as “BB” and above than both the PEF +EIP and no EIP groups (Table 3). In contrast, the PEF + no EIP group had a 14% lower implantation rate (51%) and a 16% lower LBR (38%) than the EIP group (Figure 1), despite having the youngest oocyte age across all groups and the greatest number of double embryo transfers, as well as no apparent differences in the proportion of “good quality” embryos transferred than the EIP group (Table 3). The IRs, CPRs and LBRs between the first attempt and PEF + no EIP groups significantly differed (63% vs 51%, P=0.02, 55% vs 40%, P=0.003, 50% vs 38%, P=0.02, respectively). Most likely due to small cohort size providing limited statistical power, no significant differences were found between the PEF + EIP group and the PEF + no EIP group, despite the largest differences in rates seen between these groups (IR; 65% vs 51%, P=0.2, LBR; 54% vs 38%, P=0.1, respectively). Miscarriage rates were comparable between groups, with a 2% lower miscarriage rate seen in the PEF + EIP group (PEF + EIP 11%; PEF + no EIP 13%; First attempt 13%).
Table 3
| Clinical outcomes (per embryo transferred) | First euploid attempt (n=612) | Previous euploid failure + no immune profiling (n=156) | Previous euploid failure/s + immune profiling (n=37) | P-value* |
|---|---|---|---|---|
| Previous euploid failures [mean ± SD] | 0 ± 0 | 1 ± 0 | 1.5 ± 0.8 | P<0.001 |
| Embryos transferred [mean ± SD] | 1.0 ± 0.07 | 1.04 ± 0.2 | 1.0 ± 0 | P<0.001 |
| Embryos transferred graded BB and above, % (n) | 81% (498/612) | 73% (114/156) | 73% (27/37) | P=0.04 |
| Implantation Rate, % (n) | 63% (387/612) | 51% (80/156) | 65% (24/37) | P=0.02 |
| Clinical Pregnancy Rate, % (n) | 55% (336/612) | 40% (62/156) | 57% (21/37) | P=0.002 |
| Live birth Rate, % (n) | 50% (306/612) | 38% (59/156) | 54% (20/37) | P=0.02 |
Clinical outcomes of euploid FETs between 2019-2024, per embryo transferred.
*P-value displayed is the unadjusted P-value across all three groups from a Kruskal Wallis test. Further pairwise post-hoc comparisons were also performed.
Figure 1
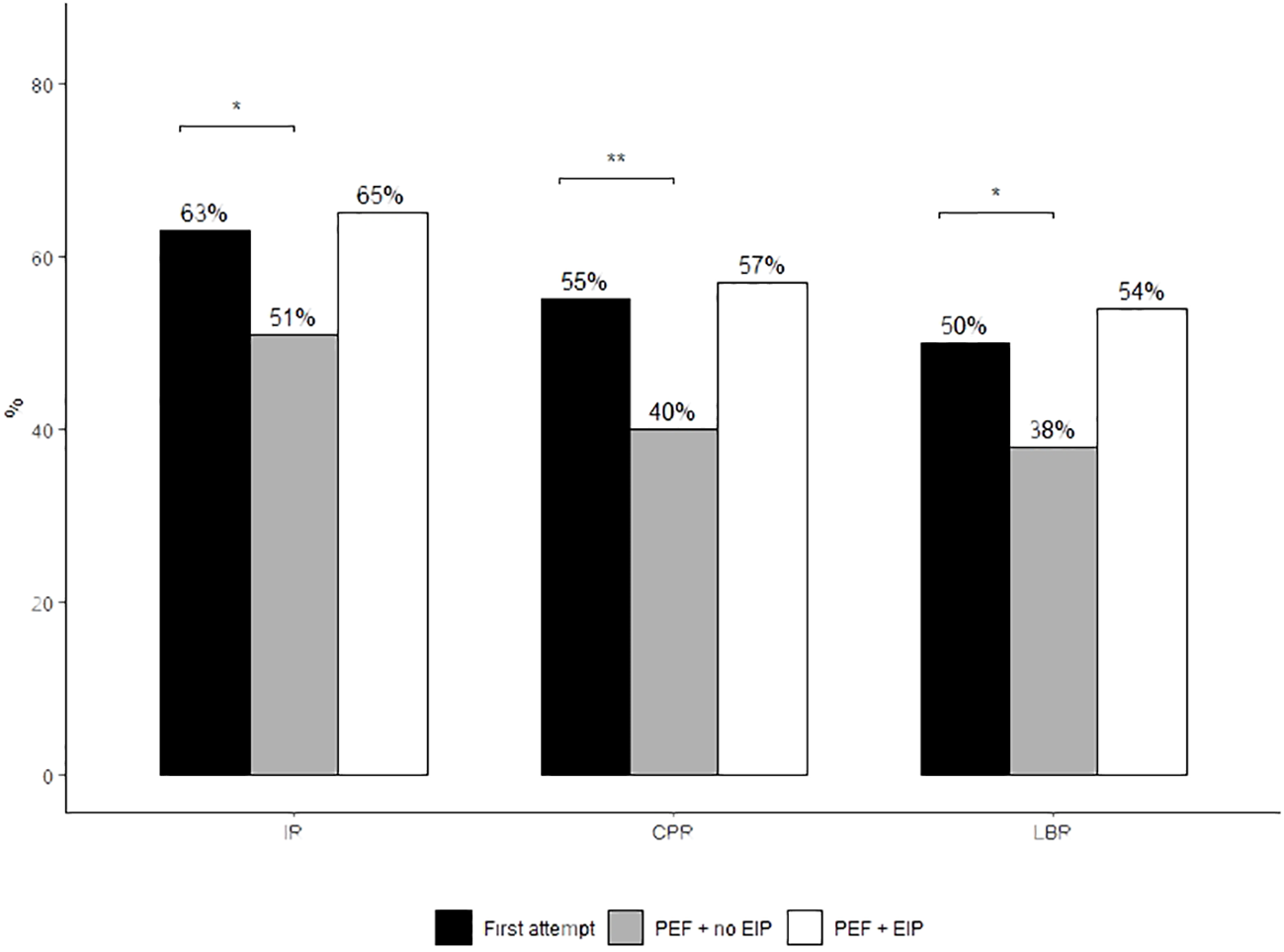
Implantation, clinical pregnancy and live birth rates of euploid FETs between study treatment groups. Proportions were compared using Fisher’s Exact test. Post-hoc comparisons were conducted using Tukey’s post-hoc test. *P<0.05 denotes statistical significance. **P<0.01. Only results with statistical significance are displayed on the graph. Absence of significance bars denotes no significant difference between groups.
When exploring immune profiling results within each clinical outcome (live birth, implantation failure or miscarriage), there were no strong evident correlations noted between said two variables (Table 4).
Table 4
| IP group (n=37) | Live birth (n=20) | Implantation failure (n=13) | Miscarriage (n=4) |
|---|---|---|---|
| Overactive, % (n) | 45% (9/20) | 46% (6/13) | 25% (1/4) |
| Balanced, % (n) | 30% (6/20) | 14% (2/13) | 50% (2/4) |
| Underactive, % (n) | 20% (4/20) | 8% (1/13) | 25% (1/4) |
| Mixed, % (n) | 5% (1/20) | 31% (4/13) | 0% (0/4) |
Immune profiling results by clinical outcome.
Further, stratifying immune profiling results by patients with at least one previous euploid miscarriage (n=11) versus patients who only experienced previous euploid implantation failure/s (n=26) found a trend in the latter group of a slightly greater number of “overactive” profiles (36% vs 46%, respectively), and a greater number of “balanced” profiles in the previous miscarriage group (36% vs 23%, respectively). In terms of clinical outcomes, the previous miscarriage group achieved a 73% LBR following immune profiling and subsequent personalised protocol, whereas the IF group achieved a 46% LBR. Due to small sample sizes, statistics could not be performed to test for significance of these comparisons.
There were slightly less patients with a balanced profile who experienced implantation failure after immune profiling. Conversely, there were slightly more mixed profiles present in patients who experienced implantation failure after immune profiling. However, due to small numbers, statistical analysis could not be completed to draw robust conclusions from this data (Table 4).
Discussion
In the present study, patients who underwent EIP after PEF achieved higher embryo implantation and LBRs than patients with PEF + no EIP. In comparison to the first euploid transfer attempt, the second conventional euploid transfer yielded significantly lower clinical outcomes. Outcomes between first euploid transfer attempts and the PEF + EIP group were comparable. Overall, this suggests EIP and personalised treatment plan may “restore” clinical outcomes back to rates seen in the first euploid attempt.
Transferring euploid-diagnosed embryos helps patients of advanced maternal age to conceive from the first attempt in IVF by eliminating aneuploidies (29–31). After the first euploid attempt, rates in the subsequent attempts decrease, for reasons that are unknown (32). One potential cause for failure is dysregulation in the endometrium, an observed symptom in those suffering from RIF or RM, particularly with aberrances in the recruitment, activation and regulation of key immunomodulatory markers that maintain the balance between embryo invasion and the mounted maternal immune response (10, 22). These aberrances are correlated with decreased implantation and LBRs (16, 17, 33). In this study, the PEF + EIP group achieved LBRs comparable to the first euploid transfer attempt. Contrastingly, the PEF + no EIP group had significantly lower clinical outcomes than the first attempt. This was the case despite the PEF + EIP group having a significantly older oocyte age, significantly more PEFs and only undergoing single embryo transfers.
Several studies have documented aberrant immune milieus in non-tested transfers, reporting correlations with aberrances in RIF (20, 34) and RM (19). However, when embryo ploidy is not controlled for, it can be difficult to discern whether failed embryo transfer is solely due to the endometrium. One study confirmed the presence of dysregulated cytotoxic immune cell pathways in women with failed euploid transfer (35). Another study analysed immune ratios and profiles in patients with previous euploid transfers, but with no subsequent treatment and transfers (36). Unlike these studies, the present study reports the usage of an endometrial immune diagnostic tool and subsequent treatment and with final treatment outcomes. In the present study, solely euploid embryo transfers were included to isolate the role of the endometrium in embryo implantation as best as possible. To the author’s knowledge, this is the first study investigating endometrial immune profiling with confirmation of embryo ploidy for previous failures and subsequent treatment.
Possibly reflecting a lack of statistical power, no significant differences were seen between clinical outcomes of the PEF + no EIP group and the PEF + EIP group, despite the largest differences in rates seen between these groups. While the present study’s intervention cohort is too small to draw robust conclusions, these findings show potential trends that future studies may endeavour to explore on a larger scale.
Recent studies and extrapolated data (37, 38) have developed a narrative that RIF is a very rare phenomenon that can essentially be avoided by serial transfer of euploid embryos. These datasets predict that after three euploid embryo transfers, 93% of patients can achieve a LB and after five euploid embryo transfers, a predicted cumulative LBR of 98%. Moreover, they report implantation rates being sustained through serial euploid transfers. The further implication of these studies is therefore that the endometrium is not a significant determining factor for successful euploid transfer.
However, this extrapolated data is calculated under optimistic assumptions, such as that no patient discontinues treatment and that patients have sufficient euploid embryos to complete five transfers. The real-life challenge is that very few patients receive 3-5 euploid-diagnosed embryos, as well as the financial, emotional and physical burdens that repeated cycles could inflict. Thus, in scenarios where an endometrial factor is suspected, the available solution is rarely eradication of the endometrial factor through multiple euploid transfers, but rather to correct any identifiable endometrial factor and achieve a LB within a confined attempt limit.
Indeed, in our clinical setting, only 22% of cycles from patients over 38 years old had at least one embryo diagnosed as euploid, averaging 0.9 euploid embryos per cycle. Cumulative live birth rates after three euploid transfers for this age group is 66%, and after five reaches only 68% [unpublished data]. This is comparable with a recent report comparing cumulative LBRs in IVF cycles with euploid embryos (36). To conclude, why 20-30% of euploids fail to implant, and why euploids in subsequent transfers achieve lower clinical outcomes, remains unanswered. And while this remains so, factors such as the endometrium cannot be entirely ruled out as a possible reason for failure when embryo factors appear controlled-for.
The primary limitation of this study is the small cohort size that meant there was lack of statistical power to detect a significant difference between the EIP group and other groups. The second limitation is its retrospective nature, which means there are known and unknown confounders present throughout the data, such as variation in patient & cycle variables and differences in sample n for each study arm. Although previous studies have confirmed immune profile correction following treatment (19, 24, 39, 40), there were no repeat biopsies performed after personalised treatment in this study.
However, the strengths of this study include the standardisation of all treatments through a single centre with consistent policies applying to PGT technique and the platform used throughout the period reported. It is also one of the first studies investigating endometrial immune profiling within euploid transfers to control for embryo factor with treatment. Thirdly, in context of the existing literature, this study provides data that challenges current narratives regarding the role of the endometrium in RIF, and the widespread practice of empirical interventions provided without any attempt at an ‘endometrial diagnosis’.
Conclusions
Overall, these findings suggest that profiling of the endometrial immune milieu, with adjusted treatment cycles in those with dysregulated profiles, may improve chances of conception in subsequent euploid attempts for specific patient subsets, and may restore rates back to those seen in the first euploid transfer attempt. While only able to report initial trends, these findings hold promise that endometrial immune assessment could improve the likelihood of achieving a successful pregnancy for some patients who are struggling to conceive with a euploid embryo. Future work is needed to expand cohort size and reinforce the trends reported here with robust statistical conclusions.
Statements
Data availability statement
Data can be made available on reasonable request to the corresponding author. Requests to access these datasets should be directed to mona.rahmati@londonwomensclinic.com.
Ethics statement
Ethical approval was not required for the studies involving humans because This was a retrospective analysis of a licenced procedure routinely performed at London Women’s Clinic. No procedure was performed for research purposes. There was no study-directed intervention and all data is reported as a service evaluation. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
JG: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft. BM: Project administration, Writing – review & editing. BA-H: Project administration, Writing – review & editing. EL: Project administration, Supervision, Writing – review & editing. RB: Project administration, Writing – review & editing. KA: Supervision, Writing – review & editing. NM: Conceptualization, Investigation, Project administration, Supervision, Writing – review & editing. MR: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to personally extend our gratitude to Ms Alessandra Ripanelli, Ms Rianna Taylor and Ms Paige Anderson who coordinated all the clinical follow-up of the patient cohort included in this study. We would also like to acknowledge all the clinicians at our centre who referred patients for endometrial immune profiling. We finally would like to acknowledge all our team who contributed to the achievements of these clinical tests and treatments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Morris J Brezina P Kearns W . The rate of aneuploidy and chance of having at least one euploid tested embryo per ivf cycle in 21,493 preimplantation genetic screening for aneuploidy (pgt-a) tested embryos as determined by a large genetic laboratory. Fertility Sterility. (2021) 116:e15. doi: 10.1016/j.fertnstert.2021.05.024
2
Capalbo A et al . On the reproductive capabilities of aneuploid human preimplantation embryos. Am J Hum Genet. (2022) 109:1572–81. doi: 10.1016/j.ajhg.2022.07.009
3
Scott RT Jr Ferry K Su J Tao X Scott K Treff NR et al . Comprehensive chromosome screening is highly predictive of the reproductive potential of human embryos: a prospective, blinded, nonselection study. Fertility Sterility. (2012) 97:870–5. doi: 10.1016/J.FERTNSTERT.2012.01.104
4
Barad DH et al . IVF outcomes of embryos with abnormal PGT-A biopsy previously refused transfer: a prospective cohort study. Hum Reprod. (2022) 37:1194–206. doi: 10.1093/HUMREP/DEAC063
5
Teklenburg G Salker M Molokhia M Lavery S Trew G Aojanepong T et al . Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PloS One. (2010) 5:e10258. doi: 10.1371/JOURNAL.PONE.0010258
6
Weimar CHE Kavelaars A Brosens JJ Gellersen B Vreeden-Elbertse JMT Heijnen CJ et al . Endometrial stromal cells of women with recurrent miscarriage fail to discriminate between high- and low-quality human embryos. PloS One. (2012) 7:e41424. doi: 10.1371/JOURNAL.PONE.0041424
7
Sato T Sugiura-Ogasawara M Ozawa F Yamamoto T Kato T Kurahashi H et al . Preimplantation genetic testing for aneuploidy: a comparison of live birth rates in patients with recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum Reprod. (2019) 34:2340–8. doi: 10.1093/HUMREP/DEZ229
8
Szekeres-Bartho J et al . Reactivity of lymphocytes to a progesterone receptor-specific monoclonal antibody. Cell Immunol. (1990) 125:273–83. doi: 10.1016/0008-8749(90)90083-4
9
Piccinni MP et al . Cytokines, hormones and cellular regulatory mechanisms favouring successful reproduction. Front Immunol. (2021) 12:717808. doi: 10.3389/FIMMU.2021.717808
10
Chaouat G Ledee-Bataille N Zourbas S Ostojic S Dubanchet S Martal J et al . Cytokines, implantation and early abortion: re-examining the Th1/Th2 paradigm leads to question the single pathway, single therapy concept. Am J Reprod Immunol (New York N.Y. : 1989). (2003) 50:177–86. doi: 10.1034/J.1600-0897.2003.00080.X
11
Chaouat G Lédée-Bataille N Dubanchet S Zourbas S Sandra O Martal J . Th1/th2 paradigm in pregnancy: paradigm lost? Int Arch Allergy Immunol. (2004) 134:93–119. doi: 10.1159/000074300
12
Moffett-King A Burrows T Loke YW . Human uterine natural killer cells. Nat Immunol. (1996) 15(1):41–52.
13
Ashkar AA Di Santo JP Croy BA . Interferon γ Contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. (2000) 192:259–70. doi: 10.1084/JEM.192.2.259
14
Von Woon E et al . Number and function of uterine natural killer cells in recurrent miscarriage and implantation failure: a systematic review and meta-analysis. Hum Reprod Update. (2022) 28:548–82. doi: 10.1093/HUMUPD/DMAC006
15
Szekeres-Bartho J . The role of progesterone in feto-maternal immunological cross talk. Med Principles Pract. (2018) 27:301. doi: 10.1159/000491576
16
Quenby S Nik H Innes B Lash G Turner M Drury J et al . Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum Reprod. (2009) 24:45–54. doi: 10.1093/HUMREP/DEN348
17
Lédée-Bataille N Dubanchet S Coulomb-L'hermine A Durand-Gasselin I Frydman R Chaouat G . A new role for natural killer cells, interleukin (IL)-12, and IL-18 in repeated implantation failure after in vitro fertilization. Fertility Sterility. (2004) 81:59–65. doi: 10.1016/j.fertnstert.2003.06.007
18
Lédée N Petitbarat M Chevrier L Vitoux D Vezmar K Rahmati M et al . The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am J Reprod Immunol. (2016) 75:388–401. doi: 10.1111/AJI.12483
19
Cheloufi M Kazhalawi A Pinton A Rahmati M Chevrier L Prat-Ellenberg L et al . The endometrial immune profiling may positively affect the management of recurrent pregnancy loss. Front Immunol. (2021) 12:656701. doi: 10.3389/fimmu.2021.656701
20
Lédée N Petitbarat M Prat-Ellenberg L Dray G Cassuto GN Chevrier L et al . The uterine immune profile: A method for individualizing the management of women who have failed to implant an embryo after IVF/ICSI. J Reprod Immunol. (2020) 142:103207. doi: 10.1016/J.JRI.2020.103207
21
Lédée-Bataille N Bonnet-Chea K Hosny G Dubanchet S Frydman R Chaouat G . Role of the endometrial tripod interleukin-18, -15, and -12 in inadequate uterine receptivity in patients with a history of repeated in vitro fertilization-embryo transfer failure. Fertility Sterility. (2005) 83:598–605. doi: 10.1016/j.fertnstert.2004.11.021
22
Petitbarat M Serazin V Dubanchet S Wayner R Mazancourt P Chaouat G et al . Tumour necrosis factor-like weak inducer of apoptosis (TWEAK)/fibroblast growth factor inducible-14 might regulate the effects of interleukin 18 and 15 in the human endometrium. Fertility sterility. (2010) 94:1141–3. doi: 10.1016/J.FERTNSTERT.2009.10.049
23
Chaouat G Zourbas S Ostojic S Lappree-Delage G Dubanchet S Lédée N et al . A brief review of recent data on some cytokine expressions at the materno-foetal interface which might challenge the classical Th1/Th2 dichotomy. J Reprod Immunol. (2002) 53:241–56. doi: 10.1016/s0165-0378(01)00119-x
24
Lédée N Petitbarat M Prat-Ellenberg L Dray G Cassuto GN Chevrier L et al . Endometrial immune profiling: A method to design personalized care in assisted reproductive medicine. Front Immunol. (2020) 11:1032. doi: 10.3389/FIMMU.2020.01032
25
Fiorentino F Bono S Biricik A Nuccitelli A Cotroneo E Cottone G et al . Application of next-generation sequencing technology for comprehensive aneuploidy screening of blastocysts in clinical preimplantation genetic screening cycles. Hum Reprod. (2014) 29:2802–13. doi: 10.1093/HUMREP/DEU277
26
A-NEQAS Grading System . (2017). Available online at: https://gamete-expert.com/news/article-32.html (Accessed May 20, 2024).
27
Gardner DK Schoolcraft WB . Culture and transfer of human blastocysts. Curr Opin Obstetrics Gynaecol. (1999) 11:307–11. doi: 10.1097/00001703-199906000-00013
28
EmbryoScope time-lapse system - Vitrolife . Available online at: https://www.vitrolife.com/why-vitrolife/the-patient-ivf-journey/embryoscope-time-lapse-system/ (Accessed May 20, 2024).
29
Ubaldi FM Cimadomo D Capalbo A Vaiarelli A Buffo L Trabucco E et al . Preimplantation genetic diagnosis for aneuploidy testing in women older than 44 years: a multicentre experience. Fertility sterility. (2017) 107:1173–80. doi: 10.1016/J.FERTNSTERT.2017.03.007
30
Munné S Kaplan B Frattarelli JL Child T Nakhuda G Shamma FN et al . Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicentre randomized clinical trial. Fertility sterility. (2019) 112:1071–1079.e7. doi: 10.1016/J.FERTNSTERT.2019.07.1346
31
Haviland MJ Murphy LA Modest AM Fox MP Wise LA Nillni YI et al . Comparison of pregnancy outcomes following preimplantation genetic testing for aneuploidy using a matched propensity score design. Hum Reprod (Oxford England). (2020) 35:2356–64. doi: 10.1093/HUMREP/DEAA161
32
Yan J Qin Y Zhao H Sun Y Gong F Li R et al . Live birth with or without preimplantation genetic testing for aneuploidy. New Engl J Med. (2021) 385:2047–58. doi: 10.1056/NEJMOA2103613
33
Quenby S Bates M Doig T Brewster J Lewis-Jones DI Johnson PM et al . Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Hum Reprod (Oxford England). (1999) 14:2386–91. doi: 10.1093/humrep/14.9.2386
34
Lédée N Prat-Ellenberg L Chevrier L Balet R Simon C Lenoble C et al . Uterine immune profiling for increasing live birth rate: A one-to-one matched cohort study. J Reprod Immunol. (2017) 119:23–30. doi: 10.1016/J.JRI.2016.11.007
35
Cheng Y Wang H Shang J Wang J Yin J Zhang J et al . Transcriptomic analysis of mid-secretory endometrium reveals essential immune factors associated with pregnancy after single euploid blastocyst transfer. Am J Reprod Immunol (New York N.Y. : 1989). (2023) 89(3):e13672. doi: 10.1111/AJI.13672
36
Ganeva R Parvanov D Vidolova N Ruseva M Handzhiyska M Arsov K et al . Endometrial immune cell ratios and implantation success in patients with recurrent implantation failure. J Reprod Immunol. (2023) 156:103816. doi: 10.1016/J.JRI.2023.103816
37
Pirtea P Ziegler De D Tao X Sun L Zhan Y Ayoubi JM et al . Rate of true recurrent implantation failure is low: results of three successive frozen euploid single embryo transfers. Fertility Sterility. (2021) 115:45–53. doi: 10.1016/J.FERTNSTERT.2020.07.002
38
Gill P Ata B Arnanz A Cimadomo D Vaiarelli A Fatemi HM et al . Does recurrent implantation failure exist? Prevalence and outcomes of five consecutive euploid blastocyst transfers in 123 987 patients. Hum Reprod. (2024) 39:974–80. doi: 10.1093/HUMREP/DEAE040
39
Lédée N Prat-Ellenberg L Petitbarat M Chevrier L Simon C El Irani E et al . Impact of prednisone in patients with repeated embryo implantation failures: Beneficial or deleterious? J Reprod Immunol. (2018) 127:11–5. doi: 10.1016/J.JRI.2018.03.003
40
Lédée N Petitbarat M Prat-Ellenberg L Dray G Vaucoret V Kazhalawi A et al . The next frontier in ART: harnessing the uterine immune profile for improved performance. Int J Mol Sci. (2023) 24:11322. doi: 10.3390/IJMS241411322
Summary
Keywords
endometrial immune profiling, embryo implantation, embryo implantation failure, euploid embryo, PGT-A
Citation
Garratt J, Mohammadi B, Al-Hashimi B, Linara-Demakakou E, Bhattacharya R, Ahuja KK, Macklon N and Rahmati M (2025) Endometrial immune assessment in patients with a history of previous euploid blastocyst failure. Front. Immunol. 16:1547159. doi: 10.3389/fimmu.2025.1547159
Received
17 December 2024
Accepted
20 March 2025
Published
07 April 2025
Volume
16 - 2025
Edited by
Roberta Bulla, University of Trieste, Italy
Reviewed by
Soledad Gori, Universidad de Buenos Aires, Argentina
Yasmin Mohseni, A2 Biotherapeutics, Inc., United States
Updates
Copyright
© 2025 Garratt, Mohammadi, Al-Hashimi, Linara-Demakakou, Bhattacharya, Ahuja, Macklon and Rahmati.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mona Rahmati, mona.rahmati@londonwomensclinic.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.