- 1Department of Cancer Center, The First Hospital of Jilin University, Changchun, Jilin, China
- 2Department of Radiology, The First Hospital of Jilin University, Changchun, Jilin, China
- 3Department of Thoracic Surgery, The First Hospital of Jilin University, Changchun, Jilin, China
Pulmonary pleomorphic carcinoma (PPC) is a rare and poorly differentiated subtype of non-small cell lung cancer (NSCLC), primarily originating from non-epithelial tissue. Currently, there is no consensus on optimal treatment strategies for patients with PPC presenting diverse clinical features. This report describes an elderly male with stage I pulmonary pleomorphic carcinoma. Five months after surgery, the disease progressed rapidly, resulting in vertebral metastasis. Following a second surgery, the patient was treated with a programmed cell death protein-1 (PD-1) inhibitor combined with cytotoxic therapy. This regimen led to significant tumor shrinkage, achieving a partial response (PR). These findings suggest that combining immune checkpoint inhibitors (ICIs) with chemotherapy may improve outcomes for patients with advanced PPC.
1 Introduction
PPC is an aggressive subtype of NSCLC classified under pulmonary sarcomatoid cancers, accounting for less than 1% of all lung cancer cases (1). This cancer demonstrates resistance to traditional chemotherapy and radiotherapy, is associated with a poor prognosis, and exhibits rapid progression in advanced stages. According to previous studies, the 5-year survival rate for PPC patients is approximately 23%, with a median overall survival of 9 months (2). Compared to other NSCLC subtypes, PPC shows higher PD-L1 expression and greater immune cell infiltration (3). Currently, no standard treatment protocol exists for PPC. Surgical resection is considered the most effective approach for early-stage PPC and plays a key role in reducing recurrence and metastasis. However, postoperative recurrence and survival rates are influenced by multiple factors; for example, PPC with adenocarcinoma components has been associated with higher recurrence rates (4). This report highlights the case of a patient with early-stage PPC containing an adenocarcinoma component, who experienced rapid postoperative progression. Remarkably, significant clinical improvement was achieved following the administration of the PD-1 inhibitor sintilimab combined with cytotoxic drugs and therapies targeting vascular and bone metastasis. The clinical and pathological features of this patient were analyzed to provide a novel treatment reference for managing advanced PPC.
2 Case presentation
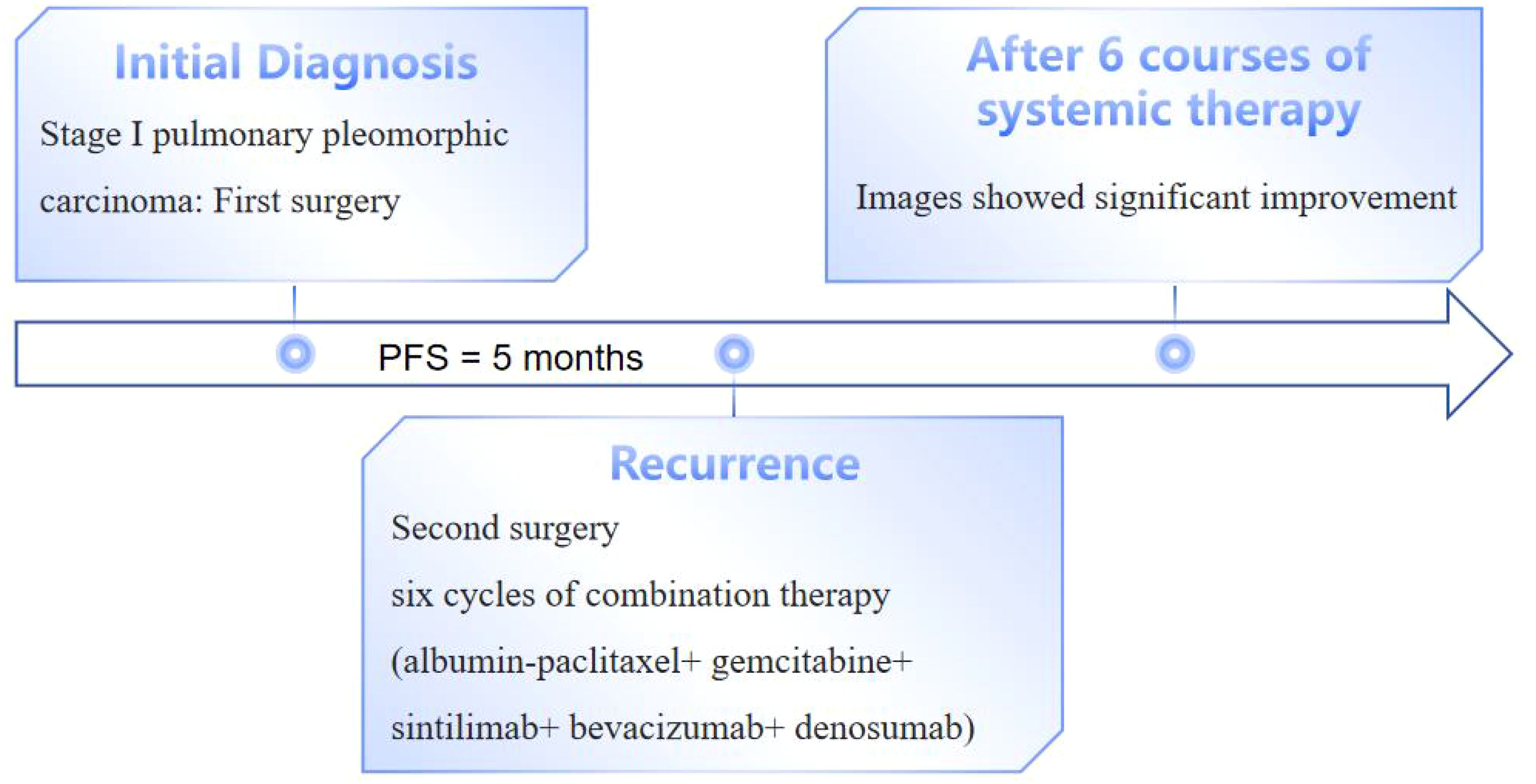
The patient, a 67-year-old male, presented to the Department of Thoracic Surgery in November 2023 for evaluation following a physical examination that detected a space-occupying lesion in the upper lobe of the left lung. The lesion was a subsolid nodule located adjacent to the pleura of the apical segment of the upper lobe of the left lung, measuring approximately 15 x 10 mm and containing a solid nodule approximately 7 mm in diameter (Figure 1). He had a smoking history of approximately 20 pack-years but no abnormalities in tumor markers including CEA, CYFRA21-1, SCCA, NSE. The patient had been healthy, with no specific family history of malignancy. His performance status was European Cooperative Oncology Group (ECOG) 0. The patient underwent a comprehensive examination, including computed tomography(CT) scans of the head, chest, and abdomen, an ultrasound of the superficial lymph nodes, and a whole-body bone scan. Based on clinical findings, the diagnosis was stage IA occupational lesion of the left lung. The patient underwent intrinsic upper lobe resection of the left lung. Intraoperative frozen-section histopathology revealed microinvasive adenocarcinoma and negative margins, so total lobectomy was not performed. Postoperative pathology identified pleomorphic carcinoma, measuring 1.3 cm. The tumor consisted of approximately 50% spindle cell carcinoma and 50% hyperdifferentiated adenocarcinoma. Immunohistochemistry results included Ki-67 (+40%), Napsin A (partly +), TTF-1 (+), CK7 (+), CK5/6 (-), P40 (-), and CKpan (+). The final diagnosis was stage IA (pT1N0M0) pleomorphic carcinoma of the left lung. The comprehensive genetic sequencing revealed no abnormal gene mutations in the patient. During the first postoperative follow-up in April 2024 (progression-free survival [PFS] = 5 months), the patient presented with a cough and hemoptysis. Lung computed tomography (CT) revealed a mass at the surgical site (Figure 2B) and metastases in the thoracic third vertebral body (Figure 3), leading to a diagnosis of stage IV recurrent pleomorphic carcinoma of the left lung with bone metastases. The patient’s head magnetic resonance imaging (MRI) and abdominal CT scan examination showed no metastases to other sites.
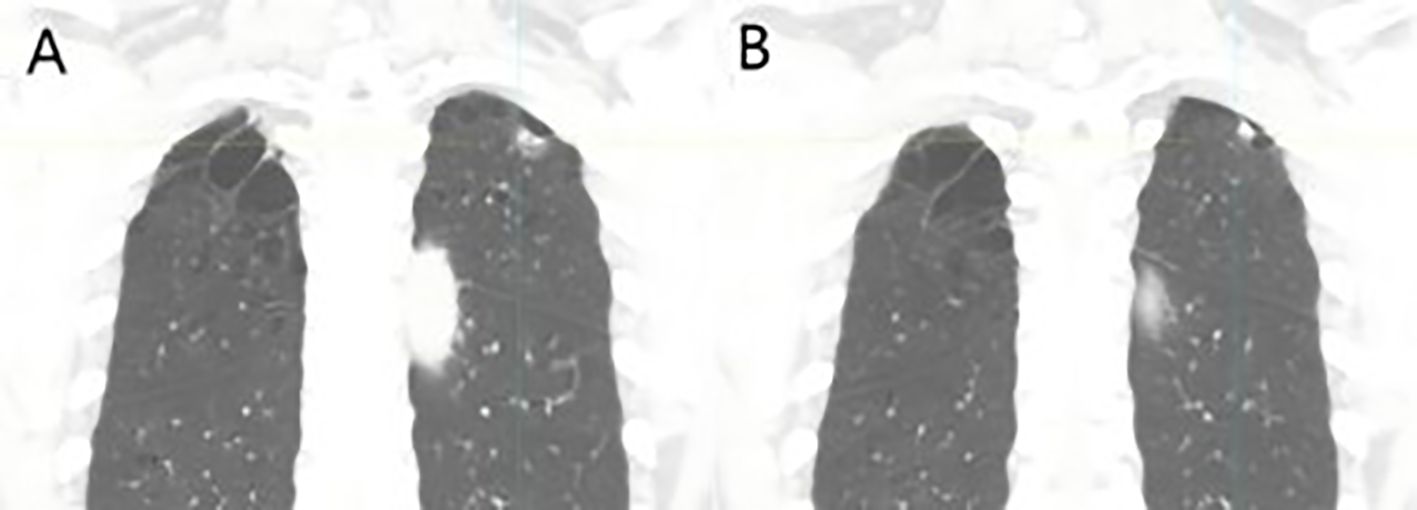
Figure 1. Coronal reconstructed images at initial diagnosis: (A) A subsolid nodule located on the parietal pleura in the apical segment of the left upper lobe, measuring approximately 15 × 10 mm, and (B) a solid component within the nodule, approximately 7 mm in diameter.
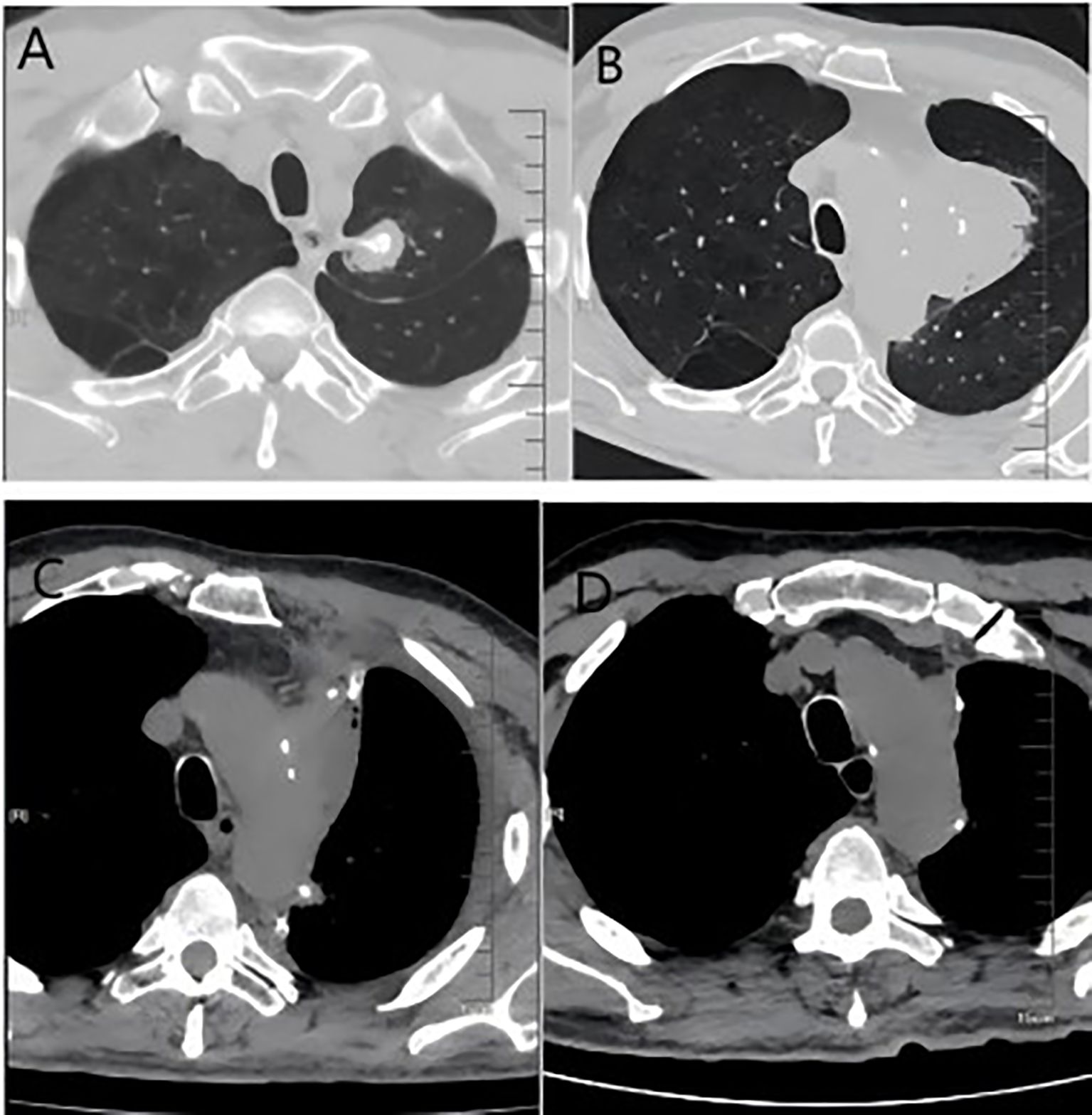
Figure 2. (A) Imaging from the first follow-up after the initial surgery showing post-surgical scarring; (B) imaging at recurrence, demonstrating a new soft tissue mass in the operated area; (C) Images after the second surgery; (D) Images after 6 courses of systemic therapy.
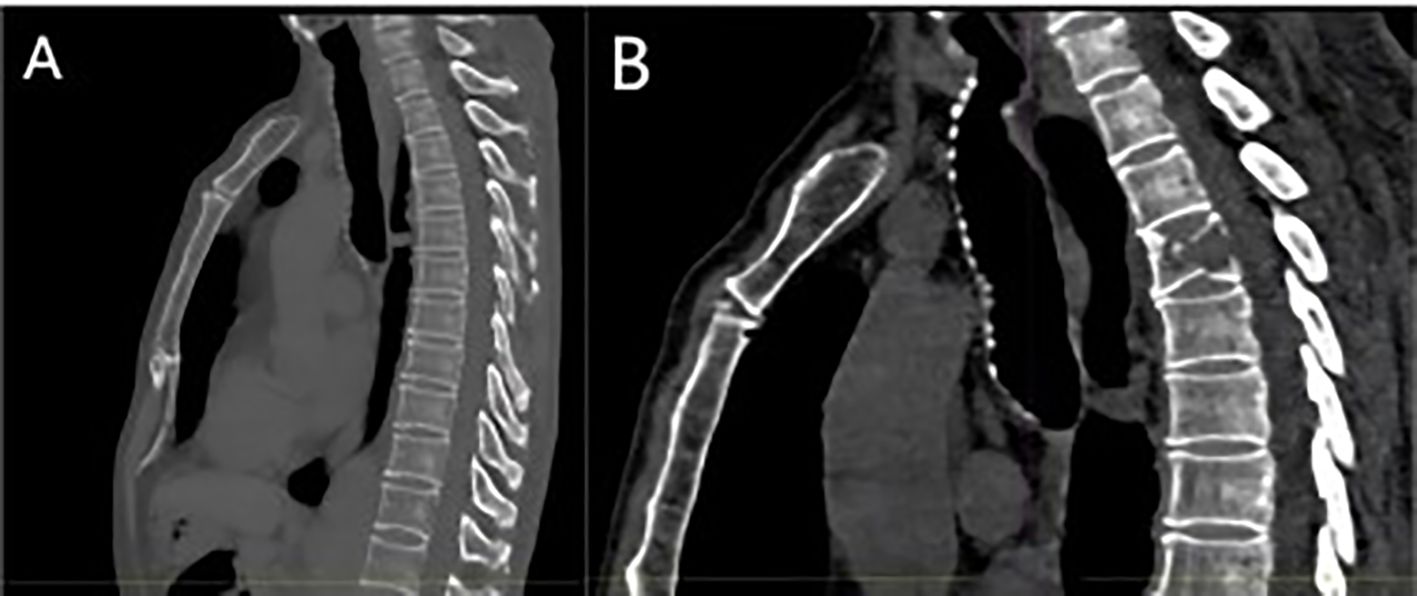
Figure 3. (A) Baseline bone window examination showing no evidence of destructive bone changes; (B) imaging at recurrence demonstrating hypodense bone destruction and a fracture of the third lumbar vertebra.
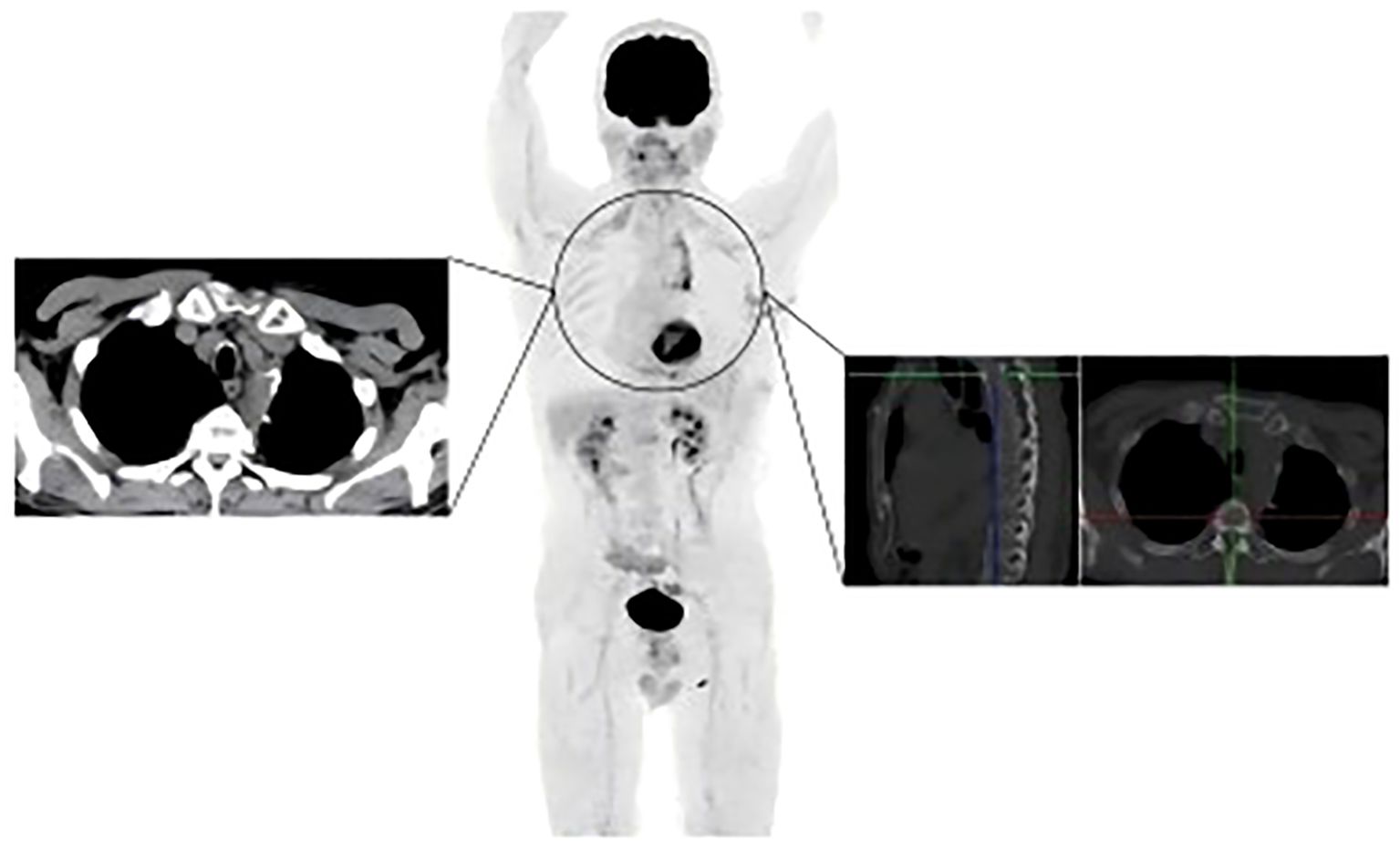
In May 2024, the patient underwent resection of the lingual segment of the left upper lobe (Figure 2C). Pathology confirmed pleomorphic carcinoma, with tumor composition of 50% spindle cell carcinoma, 30% giant cell carcinoma, and 20% adenocarcinoma. The tumor measured 2.5 cm × 2 cm × 1 cm. Immunohistochemistry results included Ki-67 (+60%), CKpan (+), CK7 (partly +), Napsin A (partly +), TTF-1 (partly +), P40 (-), P63 (-), vimentin (+), SMA (partly +), desmin (-), SMARCA4 (+), INI-1 (+), and PD-L1 (SP263; tumor proportion score [TPS]: 80%). Postoperatively, the patient received six cycles of combination therapy, including albumin-paclitaxel, gemcitabine, sintilimab, bevacizumab, and denosumab. The treatment consistently achieved a partial response (PR) based on efficacy evaluations (Figure 2D). In December 2024, PET-CT scanning showed that the patient had achieved PR in the lesion, suggesting that the treatment regimen was effective (Figure 4). The clinical course of the patient is illustrated in Figure 5. As of February 2025, the patient is continuing with 6 cycles of treatment using sintilimab and bevacizumab, achieving a progression-free survival of up to 9 months.
3 Discussion and literature review
Studies have indicated that PPC is more prevalent in older adults, with a median age range of 60–70 years. It is more common in men, with a male-to-female ratio of approximately 1.40:1, and is strongly associated with smoking. Across racial groups, about 80% of patients with PPC are Caucasian (2). Clinical symptoms of PPC can include chest pain, a persistent cough, hemoptysis, dyspnea, fever, and weight loss (5), though rare manifestations such as glandular cystitis and secretion of βHCG have also been reported (6). In this case, the patient, a 67-year-old male with a history of smoking, presented with a space-occupying lesion in the upper lobe of the left lung detected during a physical examination. The patient reported occasional dry cough and a weight loss of 4 kg. No other significant clinical symptoms or abnormal test results were observed, likely due to the tumor’s initial location and microscopic nature.
On chest computed tomography (CT), PPC lesions are typically peripheral, located in the upper lobes, with smooth margins and large sizes. They often show internal necrosis, unevenly thickened margins on enhanced scans, and “ice-floating” changes. Peritumoral ground-glass opacities are frequently observed, along with pleural invasion, hilar involvement, and mediastinal lymph node metastases (7). Studies suggest that lesions in the lobes have a better prognosis than those in the main bronchus, and patients with right lung lesions may have better overall survival (OS) than those with left lung lesions (2). At initial diagnosis, this patient’s CT revealed a lesion in the upper lobe of the left lung measuring approximately 1.6 cm × 1.1 cm. The lesion displayed ground-glass opacities with a solid nodule component pulling on the adjacent pleura. The imaging findings differed from those typically seen in larger lesions, likely due to the tumor being detected at an earlier stage.
Pathologically, pleomorphic carcinoma, a subtype of sarcomatoid carcinoma, is defined by the presence of at least 10% spindle cell or giant cell components, or by tumors entirely composed of spindle cells or neoplastic giant cells (8, 9). The mixed subtype of PPC has a better prognosis than the pure subtype (10). In this case, the initial tumor consisted of approximately 50% spindle cell carcinoma and 50% hyperdifferentiated adenocarcinoma. After recurrence, the tumor pathology showed 50% spindle cell carcinoma, 30% giant cell carcinoma, and 20% adenocarcinoma, indicating increased tumor heterogeneity. The higher proportions of spindle cell and giant cell components raise questions about their potential impact on prognosis, warranting further investigation. And in this patient (Figure 6), immunohistochemistry revealed positive expression of CK7, Napsin A, TTF-1, and vimentin, consistent with the immunohistochemical profiles of most pleomorphic carcinomas (7).
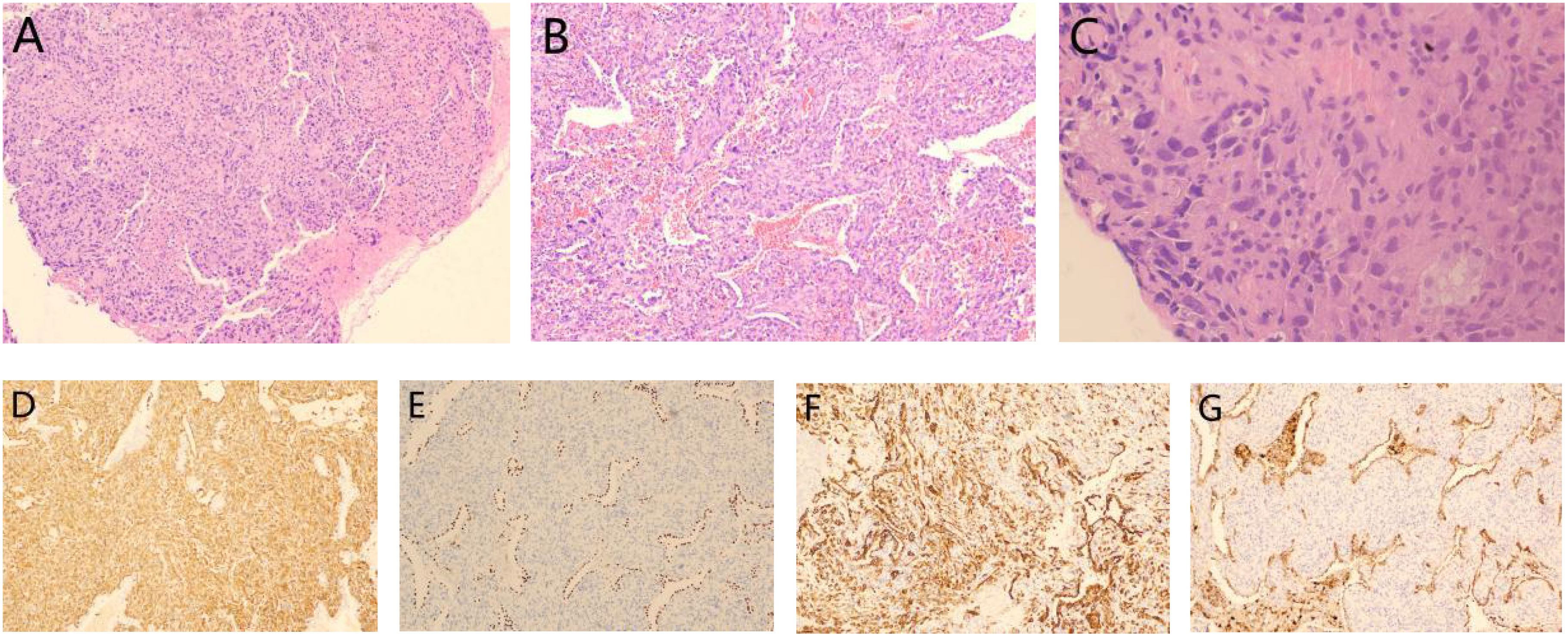
Figure 6. Pathological results from the second surgery: (A) Mixed presence of giant cells and spindle cells: HE staining(x40); (B) Adenocarcinoma components: HE staining(x100) and (C) Giant cells in tumor tissue: HE staining(x400). Immunohistochemistry results showed (D) vimentin (+), (E) TTF-1 (+), (F) CK7 (+) and (G) Napsin A (+).
Previous studies have shown that adjuvant chemotherapy reduces the risk of death by 46% in patients with distant metastases but does not significantly improve survival in patients with early-stage or locally advanced disease (2). In this case, the patient was initially diagnosed with stage IA and did not receive adjuvant therapy after surgery. However, the disease recurred within 5 months, suggesting that adjuvant therapy may be necessary even for stage IA patients to reduce the risk of recurrence. Following the second surgery, the patient was treated with a combination regimen of albumin-paclitaxel, gemcitabine, sintilimab, and bevacizumab, resulting in significant efficacy and a PR. Here we list some previously reported clinical cases of pleomorphic carcinoma of the lung (Table 1).
Regarding the choice of cytotoxic drugs, previous reports indicate that cytotoxic chemotherapy in cases of postoperative recurrence or unresectable PPC often leads to disease progression, with a median overall survival of approximately 5 months (11). However, a few patients with advanced PPC and distant metastases who were treated with gemcitabine or paclitaxel combined with platinum experienced recurrence-free survival exceeding 6 years (12–14). Based on this, a paclitaxel plus gemcitabine regimen was selected for this patient. For immunotherapy, PD-L1 expression is often positive in pleomorphic lung cancer, particularly in the sarcomatoid component (15, 16), and is associated with longer survival following immune checkpoint inhibitor (ICI) therapy (17). Given the patient’s high PD-L1 expression (tumor proportion score [TPS]: 80%), sintilimab, a PD-1 inhibitor, was administered.
PD-L1 expression in tumor cells and tumor-infiltrating lymphocytes (TILs) assessed by immunohistochemistry (IHC) is considered the most reliable predictor of response to immunotherapy in different tumors (18). There are two commonly used PD-L1 scoring methods for lung cancer: TPS (tumor proportion score)/TC (tumor cell score) and IC (immune cell score) (19). The Food and Drug Administration (FDA) approves the use of ICIs such as Pembrolizumab, Atezolizumab, and Durvalumab, provided that the PD-L1 assessment relies on a specific clone and scoring system (20). In contrast, the European Medicines Agency (EMA) permits treatment with ICIs without specific platforms or antibodies for immunohistochemistry testing.
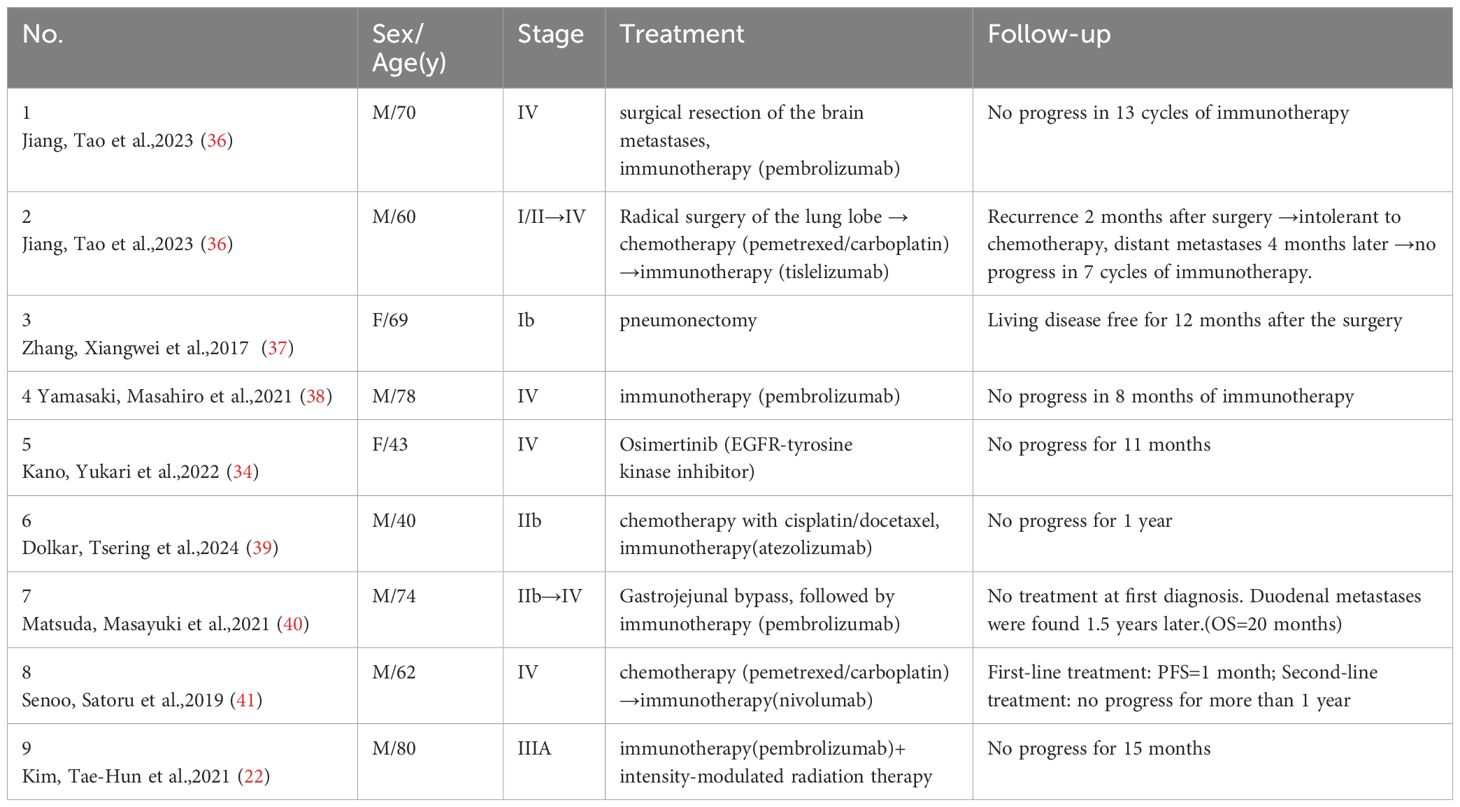
Previous clinical reports suggest that patients with pleomorphic NSCLC may benefit from ICI treatment, but the efficacy can vary. For example, among patients treated with pembrolizumab alone or in combination with chemotherapy or radiation, some achieved progression-free survival of up to 15 months, while others showed no response (21–23). In three patients with high PD-L1-expressing PPC treated with pembrolizumab, two experienced prolonged progression-free survival, while one developed brain and bone metastases during therapy (24). These findings suggest that immunotherapy is effective for most patients with PD-L1-expressing PPC, although tumor heterogeneity may lead to variability in outcomes.
Additional predictive factors beyond PD-L1 expression may also influence the effectiveness of ICIs. Regarding anti-angiogenic therapy, evidence suggests that anti-angiogenic agents enhance ICI efficacy by improving the tumor microenvironment and boosting the immune response. Clinical trials have demonstrated the benefits of combining ICIs with anti-angiogenic agents in advanced NSCLC (25–27). Studies have shown that patients with PPC exhibit high levels of vascular endothelial growth factor (VEGF) (28). Oda T et al. reported that two patients with PPC were treated with carboplatin, paclitaxel, and bevacizumab, resulting in partial responses (29). Based on these findings, bevacizumab was added to this patient’s regimen, resulting in a synergistic anti-tumor effect and potentially reducing the risk of drug resistance. For bone metastases, the patient received targeted therapy with denosumab. As the first fully human monoclonal antibody, denosumab inhibits the activity of the Receptor activator of the nuclear factor-κB ligand (RANKL) and has been used to prevent bone-related adverse events (SREs) in a variety of solid tumors. Importantly, it showed synergistic anti-tumor efficacy without increased toxicity when combined with ICIs in patients with advanced non-small cell lung cancer with bone metastases (30).The patient tolerated the treatment well, with only mild bone marrow suppression and gastrointestinal side effects observed. The Common Terminology Criteria for Adverse Events (CTCAE) was created by the National Cancer Institute to classify toxicity. This patient experienced grade I leukopenia and grade I nausea, which resolved with supportive therapy.
Although the patient underwent a comprehensive genetic evaluation, and no genetic mutations were found. The epithelial and sarcomatoid components of specimens from patients with PPC were found to share genomic alterations (31). KRAS mutations are the most common in PPC, and current targeted therapies for KRAS G12C mutations show promise for NSCLC patients, further studies are still needed to investigate the therapeutic role of ras-targeted inhibitors in patients with PPC (31, 32). MET exon 14 skipping mutation (METΔex14) and epidermal growth factor receptor(EGFR) mutations are the second most common types of mutations in PPC. Capmatinib and tepotinib are approved for NSCLC patients with MET exon 14 skipping, and these drugs are expected to be used in treating PPC patients with MET exon 14 skipping (33). A patient with advanced PPC carrying an EGFR mutation with exon 19 deletion was reported to have progression-free survival up to 11 months after treatment with ositinib (34). In addition, Lin L et al. reported a case of an advanced PPC patient with a mesenchymal lymphoma kinase (ALK) rearrangement treated with crizotinib and achieved partial remission for 7 months (35). Therefore, it is particularly important to monitor genetic alterations in patients with PPC and targeted therapies against activating driver mutations may be effective in patients.
4 Conclusion
In summary, pleomorphic carcinoma of the lung is a highly aggressive tumor with a high risk of rapid recurrence, even in stage IA1 disease treated with surgery alone. The role of adjuvant chemotherapy, radiotherapy, and immunotherapy requires further investigation. As a sarcomatoid carcinoma containing spindle cell and/or giant cell components, PPC responds well to gemcitabine combined with paclitaxel. For patients with high PD-L1 expression, the use of PD-1/PD-L1 inhibitors is essential. In this case, the combination of chemotherapy, immunotherapy, and anti-angiogenic therapy effectively controlled the disease, leading to significant tumor shrinkage. The patient continues to receive maintenance therapy with combined immunotherapy and anti-angiogenic therapy to prolong survival.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Conceptualization, Writing – original draft. WX: Investigation, Writing – original draft. HW: Formal Analysis, Writing – original draft. LS: Validation, Writing – original draft. CC: Formal Analysis, Writing – original draft. XS: Validation, Writing – original draft. XM: Validation, Writing – original draft. LY: Supervision, Writing – review & editing. ZL: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 world health organization classification of lung tumors. J Thorac Oncol. (2015) 10:1243–60. doi: 10.1097/Jto.0000000000000630
2. Chen ZZ, Liu JC, and Min LF. Clinicopathological characteristics, survival outcomes and prognostic factors in pleomorphic carcinoma: A seer population-based study. BMC Pulm Med. (2022) 22:116. doi: 10.1186/s12890-022-01915-1
3. Hsieh MS, Lin MW, and Lee YH. Lung adenocarcinoma with sarcomatoid transformation after tyrosine kinase inhibitor treatment and chemotherapy. Lung Cancer. (2019) 137:76–84. doi: 10.1016/j.lungcan.2019.08.029
4. Iijima Y, Nakajima Y, Kinoshita H, Nishimura Y, Iizuka T, Akiyama H, et al. Clinicopathological analysis of 17 surgically resected pulmonary pleomorphic carcinoma cases. Ann Thorac Cardiovas. (2021) 27:1–9. doi: 10.5761/atcs.oa.20-00018
5. Kaira K, Horie Y, Ayabe E, Murakami H, Takahashi T, Tsuya A, et al. Pulmonary pleomorphic carcinoma. J Thorac Oncol. (2010) 5:460–5. doi: 10.1097/JTO.0b013e3181ce3e3c
6. de Sousa MD, Barata M, Miranda AR, Sequeira P, Oliveira A, Xavier L, et al. Beta-Hcg secretion by a pulmonary pleomorphic carcinoma: A case report. Respir Med Case Rep. (2021) 34:101528. doi: 10.1016/j.rmcr.2021.101528
7. Liu Y, Ren G, Cai R, and Wang X. Ct and clinicopathology features of pulmonary pleomorphic carcinoma. Department Radiol. (2023) 38:285–9. doi: 10.13609/j.cnki.1000-0313.2023.03.008
8. Nishida A, Abiru H, Hayashi H, Uetani M, Matsumoto K, Tsuchiya T, et al. Clinicoradiological outcomes of 33 cases of surgically resected pulmonary pleomorphic carcinoma: correlation with prognostic indicators. Eur Radiol. (2016) 26:25–31. doi: 10.1007/s00330-015-3811-3
9. Mochizuki T, Ishii G, Nagai K, Yoshida J, Nishimura M, Mizuno T, et al. Pleomorphic carcinoma of the lung clinicopathologic characteristics of 70 cases. Am J Surg Pathol. (2008) 32:1727–35. doi: 10.1097/PAS.0b013e3181804302
10. Bao L and Shi L. Correlation of pd-L1 expression and the density of immune cell infiltration with prognosis in 46 cases of pulmonary pleomorphic carcinoma. Chin J Of Clin Oncol. (2024) 51:716–21. doi: 10.12354/j.issn.1000-8179.2024.20240533
11. Bae H-M, Min HS, Lee S-H, Kim D-W, Chung DH, Lee J-S, et al. Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Cancer (Amsterdam Netherlands). (2007) 58:112–5. doi: 10.1016/j.lungcan.2007.05.006
12. Kato D, Chihara Y, Shirase T, Takahashi T, Takahashi KI, and Sakai N. Successful treatment of two consecutive cases of pulmonary pleomorphic carcinoma with platinum chemotherapy. Oncol Lett. (2015) 10:3040–2. doi: 10.3892/ol.2015.3678
13. Yamanashi K, Marumo S, Miura K, and Kawashima M. Long-term survival in a case of pleomorphic carcinoma with a brain metastasis. Case Rep Oncol. (2014) 7:799–803. doi: 10.1159/000368186
14. Tamiya A, Asami K, Shimizu S, Matsumura A, Isa SI, Okishio K, et al. Long-term complete response in a patient with disseminated pulmonary pleomorphic carcinoma induced by cisplatin and gemcitabine. Internal Med. (2014) 53:2625–8. doi: 10.2169/internalmedicine.53.2637
15. Chang YL, Yang CY, Lin MW, Wu CT, and Yang PC. High co-expression of Pd-L1 and Hif-1α Correlates with tumour necrosis in pulmonary pleomorphic carcinoma. Eur J Cancer. (2016) 60:125–35. doi: 10.1016/j.ejca.2016.03.012
16. Kim S, Kim MY, Koh J, Go H, Lee DS, Jeon YK, et al. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: comparison of sarcomatous and carcinomatous areas. Eur J Cancer. (2015) 51:2698–707. doi: 10.1016/j.ejca.2015.08.013
17. Lee J, La Choi Y, Jung HA, Lee SH, Ahn JS, Ahn MJ, et al. Outstanding clinical efficacy of Pd-1/Pd-L1 inhibitors for pulmonary pleomorphic carcinoma. Eur J Cancer. (2020) 132:150–8. doi: 10.1016/j.ejca.2020.03.029
18. Nocini R, Vianini M, Girolami I, Calabrese L, Scarpa A, Martini M, et al. Pd-L1 in oral squamous cell carcinoma: A key biomarker from the laboratory to the bedside. Clin Exp Dental Res. (2022) 8:690–8. doi: 10.1002/cre2.590
19. Kerr KM, Tsao M-S, Nicholson AG, Yatabe Y, Wistuba II, and Hirsch FR. Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thorac Oncol. (2015) 10:985–9. doi: 10.1097/jto.0000000000000526
20. Marletta S, Fusco N, Munari E, Luchini C, Cimadamore A, Brunelli M, et al. Atlas of Pd-L1 for pathologists: indications, scores, diagnostic platforms and reporting systems. J Personalized Med. (2022) 12:1073. doi: 10.3390/jpm12071073
21. Hayashi K, Tokui K, Inomata M, Azechi K, Mizushima I, Takata N, et al. Case series of pleomorphic carcinoma of the lung treated with immune checkpoint inhibitors. In Vivo. (2021) 35:1687–92. doi: 10.21873/invivo.12428
22. Kim TH, Park SH, Hwang I, Lee JH, Kim JH, Kim HW, et al. Robust response of pulmonary pleomorphic carcinoma to pembrolizumab and sequential radiotherapy: A case report. Respirol Case Rep. (2021) 9:e0875. doi: 10.1002/rcr2.875
23. Luo YX, Wei JP, Zhang J, Zeng LT, Zeng ZM, and Liu AW. Two different patients with pulmonary pleomorphic carcinoma response to Pd-1 inhibitor plus anlotinib. Lung Cancer. (2021) 155:170–4. doi: 10.1016/j.lungcan.2021.03.019
24. Kanazu M, Uenami T, Yano Y, Nakatsubo S, Hosono Y, Ishijima M, et al. Case series of pleomorphic carcinomas of the lung treated with nivolumab. Thorac Cancer. (2017) 8:724–8. doi: 10.1111/1759-7714.12505
25. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous Nsclc. New Engl J Med. (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
26. Chu TQ, Zhong RB, Zhong H, Zhang B, Zhang W, Shi CL, et al. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced Nsclc. J Thorac Oncol. (2021) 16:643–52. doi: 10.1016/j.jtho.2020.11.026
27. Zhou CC, Wang YN, Zhao J, Chen GY, Liu ZH, Gu KS, et al. Efficacy and biomarker analysis of camrelizumab in combination with apatinib in patients with advanced nonsquamous Nsclc previously treated with chemotherapy. Clin Cancer Res. (2021) 27:1296–304. doi: 10.1158/1078-0432.Ccr-20-3136
28. Kaira K, Endo M, Abe M, Nakagawa K, Ohde Y, Okumura T, et al. Biologic correlates of 18f-Fdg uptake on pet in pulmonary pleomorphic carcinoma. Lung Cancer. (2011) 71:144–50. doi: 10.1016/j.lungcan.2010.05.021
29. Oda T, Sekine A, Kato T, Baba T, Okudela K, and Ogura T. Promising effect of chemotherapy with bevacizumab for patients with pulmonary pleomorphic carcinoma: two case reports and a literature review. Respir Invest. (2015) 53:296–9. doi: 10.1016/j.resinv.2015.05.004
30. Li H-S, Lei S-Y, Li J-L, Xing P-Y, Hao X-Z, Xu F, et al. Efficacy and safety of concomitant immunotherapy and denosumab in patients with advanced non-small cell lung cancer carrying bone metastases: A retrospective chart review. Front Immunol. (2022) 13:908436. doi: 10.3389/fimmu.2022.908436
31. Nagano M, Kohsaka S, Hayashi T, Ueno T, Kojima S, Shinozaki-Ushiku A, et al. Comprehensive molecular profiling of pulmonary pleomorphic carcinoma. NPJ Precis Oncol. (2021) 5:57. doi: 10.1038/s41698-021-00201-3
32. Román M, Baraibar I, López I, Nadal E, Rolfo C, Vicent S, et al. Kras oncogene in non-small cell lung cancer: clinical perspectives on the treatment of an old target. Mol Cancer. (2018) 17:33. doi: 10.1186/s12943-018-0789-x
33. Fujino T, Suda K, and Mitsudomi T. Lung cancer with met exon 14 skipping mutation: genetic feature, current treatments, and future challenges. Lung Cancer: Targets Ther. (2021) 12:35–50. doi: 10.2147/lctt.S269307
34. Kano Y, Kataoka N, Kunimatsu Y, Tsutsumi R, Sato I, Tanimura M, et al. Pulmonary pleomorphic carcinoma harboring Egfr mutation successfully treated with osimertinib: A case report. Medicina. (2022) 58:706. doi: 10.3390/medicina58060706
35. Lin L, Huang F, Chen F, He Y, Hu J, and Cao X. Anaplastic lymphoma kinase (Alk)-rearranged pulmonary pleomorphic carcinoma successfully treated with crizotinib. J Int Med Res. (2018) 46:3491–7. doi: 10.1177/0300060517748262
36. Jiang T, Wang H, Xue F, Wu X, Ni M, Wang Y, et al. Pulmonary pleomorphic carcinoma treated with pd-1 inhibitor: Two case reports. Thorac Cancer. (2023) 14(32):3240–4. doi: 10.1111/1759-7714.15120
37. Zhang X, Wang Y, Zhao L, Jing H, Sang S, and Du J. Pulmonary pleomorphic carcinoma. Medicine (2017) 96(29). doi: 10.1097/md.0000000000007465
38. Yamasaki M, Matsumoto Y, Nakamoto K, and Hattori N. Pulmonary pleomorphic carcinoma in an elderly patient treated with pembrolizumab shows a marked response. J Cancer Res Ther. (2021) 17(6):1580–2. doi: 10.4103/jcrt.JCRT_361_19
39. Dolkar T, Absher K, and Hao Z. Pulmonary pleomorphic carcinoma: Current understanding illustrated through a case. BMJ Case Rep (2024) 17(8). doi: 10.1136/bcr-2024-261076
40. Matsuda M, Kai Y, Harada S, Suzuki K, Hontsu S, and Muro S. Duodenal metastasis of pulmonary pleomorphic carcinoma: A case report. Case Rep Oncol (2021) 14(3):1511–5. doi: 10.1159/000519664
Keywords: pulmonary pleomorphic carcinoma, sintilimab, immune checkpoint inhibitors, immunotherapy, non-small cell lung cancer
Citation: Zhang J, Xiong W, Wang H, Sun L, Chen C, Sun X, Man X, Yang L and Li Z (2025) Treatment strategies for rapid recurrence and progression after surgery for stage I pulmonary pleomorphic carcinoma: a case report and literature review. Front. Immunol. 16:1547872. doi: 10.3389/fimmu.2025.1547872
Received: 18 December 2024; Accepted: 13 May 2025;
Published: 04 June 2025.
Edited by:
Mohamed Rahouma, NewYork-Presbyterian, United StatesReviewed by:
Armida D’Incecco, Hospital of Teramo, ItalyAlbino Eccher, University of Modena and Reggio Emilia, Italy
Copyright © 2025 Zhang, Xiong, Wang, Sun, Chen, Sun, Man, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Yang, eWFuZ2xlaUBqbHUuZWR1LmNu; Zhihong Li, bGl6aGlob25nQGpsdS5lZHUuY24=
 Jing Zhang
Jing Zhang Wenji Xiong2
Wenji Xiong2 Hong Wang
Hong Wang Chen Chen
Chen Chen Xiuyue Man
Xiuyue Man Lei Yang
Lei Yang