- 1Department of Pediatrics, Gifu University Graduate School of Medicine, Gifu, Japan
- 2Department of Early Diagnosis and Preventive Medicine for Rare Intractable Pediatric Diseases, Graduate School of Medicine, Gifu University, Gifu, Japan
- 3Department of Pediatrics, Yokohama City University Graduate School of Medicine, Yokohama, Japan
- 4Department of Pediatrics, Shinshu University School of Medicine, Matsumoto, Japan
- 5Department of Medicine (Neurology and Rheumatology), Shinshu University School of Medicine, Matsumoto, Japan
- 6Department of Child Health, Institute of Medicine, University of Tsukuba, Tsukuba, Japan
- 7Department of Allergy and Rheumatology, Chiba Children’s Hospital, Chiba, Japan
- 8Department of General Medical Science, Graduate School of Medicine, Chiba University, Chiba, Japan
- 9Department of Pediatrics, Kitasato University School of Medicine, Sagamihara, Japan
- 10Department of Pediatrics, Kyoto University Graduate School of Medicine, Kyoto, Japan
- 11Department of Rheumatology, Fukushima Medical University School of Medicine, Fukushima, Japan
- 12Department of Pediatrics, Seirei Hamamatsu General Hospital, Hamamatsu, Japan
- 13Division of Clinical Nephrology and Rheumatology, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan
- 14Department of Pediatrics, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan
- 15Division of Immunology, National Center for Child Health and Development, Tokyo, Japan
- 16Department of Gastrointestinal and Pediatric Surgery, Mie University Graduate School of Medicine, Tsu, Mie, Japan
- 17Department of Rheumatism, Infectious Disease, Miyagi Children’s Hospital, Sendai, Japan
- 18Department of Gastroenterology and Hepatology, Miyagi Children’s Hospital, Sendai, Japan
- 19Department of Pediatrics, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki, Japan
- 20Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- 21Department of Pediatrics, Gunma University Graduate School of Medicine, Maebashi, Japan
- 22Department of Pediatrics and Child Health, Kurume University School of Medicine, Kurume, Japan
- 23Department of Pediatrics, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan
- 24Department of Rheumatology and Allergology, Japanese Red Cross Medical Center, Tokyo, Japan
- 25Department of Pediatrics, Tokyo Bay Urayasu-Ichikawa Medical Center, Urayasu, Japan
- 26Department of Pediatrics, Kawaguchi Municipal Medical Center, Saitama, Japan
- 27Department of Pediatrics and Developmental Biology, Institute of Science Tokyo, Tokyo, Japan
- 28Department of Child Health and Development, Institute of Science Tokyo, Tokyo, Japan
- 29Department of Clinical Immunology, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan
- 30Department of Pediatrics, School of Medicine, Iwate Medical University, Yahaba, Japan
- 31Department of Pediatrics, School of Medicine, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Kanazawa, Japan
- 32Japan Environment and Children’s Study (JECS) Kyoto Regional Center, Kyoto University Graduate School of Medicine, Kyoto, Japan
- 33Clinical Genetics Center, Gifu University Hospital, Gifu, Japan
- 34Center for One Medicine Innovative Translational Research, Gifu University, Gifu, Japan
- 35Department of Laboratory of Intractable and Rare Diseases, Graduate School of Medicine, Gifu University, Gifu, Japan
Background: The severity of A20 haploinsufficiency (HA20) varies, with no established clinical guidelines for treatment. This study aimed to elucidate the clinical characteristics of, and the efficacy of treatments attempted in, patients with HA20 in Japan.
Methods: Clinical information on HA20 patients from medical records was retrospectively collected through the attending physicians.
Results: Seventy-two HA20 patients were identified in Japan. And, 54 patients from 37 unrelated families were analyzed in detail. HA20 patients exhibited common features, including recurrent fever, gastrointestinal and musculoskeletal symptoms, and autoimmune disease; various organ disorders (e.g. neurological, liver, and pulmonary diseases) were less common complications. Molecular target drugs (MTDs) were administered in 44.4% of patients, among which anti-tumor necrosis factor (TNF)-α agents showed efficacy in 59.5% of patients. Eleven patients did not experience control of inflammation with initial MTDs, most commonly because of relapse due to secondary failure of MTDs. Anti-drug antibodies were related to the secondary failure of adalimumab in one patient and infusion reactions to infliximab in two patients. In such refractory cases, other treatments (e.g. switching the first MTD to an alternative agent or adding a Janus kinase inhibitor or immunomodulators, or allogeneic hematopoietic cell transplantation [HCT]) were attempted.
Conclusions: Our survey revealed that anti-TNF-α agents showed high efficacy. However, secondary failure of MTDs was a significant refractory-related factor in HA20 patients in Japan. Although anti-interferon therapies, thalidomide, and HCT might be potential treatment options, the results of this study suggest that further research is necessary to establish suitable treatments for HA20, especially for those with refractory disease.
1 Introduction
A20 haploinsufficiency (HA20) is a hereditary autoinflammatory disease caused by heterozygous loss-of-function variants in the TNFAIP3 gene (1). The A20 protein is involved in ubiquitin modification, exhibiting suppression of two pathways: nuclear factor κ light-chain enhancer of activated B cells (NF-κB) signaling and interferon (IFN) regulatory factor signaling related to type I IFN production (2). HA20 attenuates the inhibitory effects of these signaling pathways, leading to overproduction of proinflammatory cytokines and subsequently inducing a state of systemic inflammation. Consequently, patients with HA20 present with autoinflammatory Behcet’s disease (BD)-like symptoms and may also develop autoimmune diseases (3).
The severity of HA20 varies, with no established clinical guidelines for treatment. For severe cases, administration of molecular target drugs (MTDs), such as anti-tumor necrosis factor (TNF)-α agents, anti-interleukin (IL)-1 agents, and Janus kinase (JAK) inhibitors, is reportedly effective (1, 3, 4). By contrast, MTDs were found to be ineffective or insufficiently effective in patients with refractory disease (5). Therefore, it is necessary to elucidate the clinical characteristics of, and suitable treatment methods for, HA20 patients. Herein, we conducted an epidemiological survey of the accumulated data and treatment strategies used in 54 patients with HA20 in Japan. Additionally, we focused on HA20 patients with refractory disease and analyzed their characteristics and treatments.
2 Methods
2.1 Patients and clinical information
Records of diagnosed or suspected cases of HA20 were extracted from the registry of the Primary Immunodeficiency Database in Japan (PIDJ) (6), comprising patients who were treated at hospitals collaborating with the PIDJ project or were referred for consultation to the Japanese Society for Immunodeficiency and Autoinflammatory Disease. The diagnosis of HA20 was confirmed via functional analysis of the TNFAIP3 variant. Questionnaires were sent to the attending physicians for each case, and the following clinical information was retrospectively obtained from medical records: demographics, clinical symptoms, laboratory data, initial diagnosis, treatment, and treatment effects. Treatment effects were evaluated by the attending physician as “effective” (defined as a condition in which symptoms improved to the extent that additional treatment was not required), “improvement”, or “ineffective”. In this study, “refractory patients” were defined as those who required a change of the initial MTD to alternative agents. Details on the other methods (e.g. genetic analysis, in vitro functional evaluation of TNFAIP3 variants, minigene splicing assay, evaluation of type I IFN scores, and assays of anti-drug antibodies) are described in the Supplementary Material.
2.2 Ethics approval
The ethics committees of Kyoto University and Gifu University approved this national study (protocol numbers R2259 and 29–322 and 2023-005, respectively), which was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from individual adult patients or the legal guardian/next of kin of minor patients. Where it was difficult to contact individual adult patients or the legal guardian/next of kin of minor patients directly, or in patients who consented to the secondary use of their clinical information (e.g. during genetic testing), informed consent was obtained through an opt-out process.
3 Results
3.1 Patients
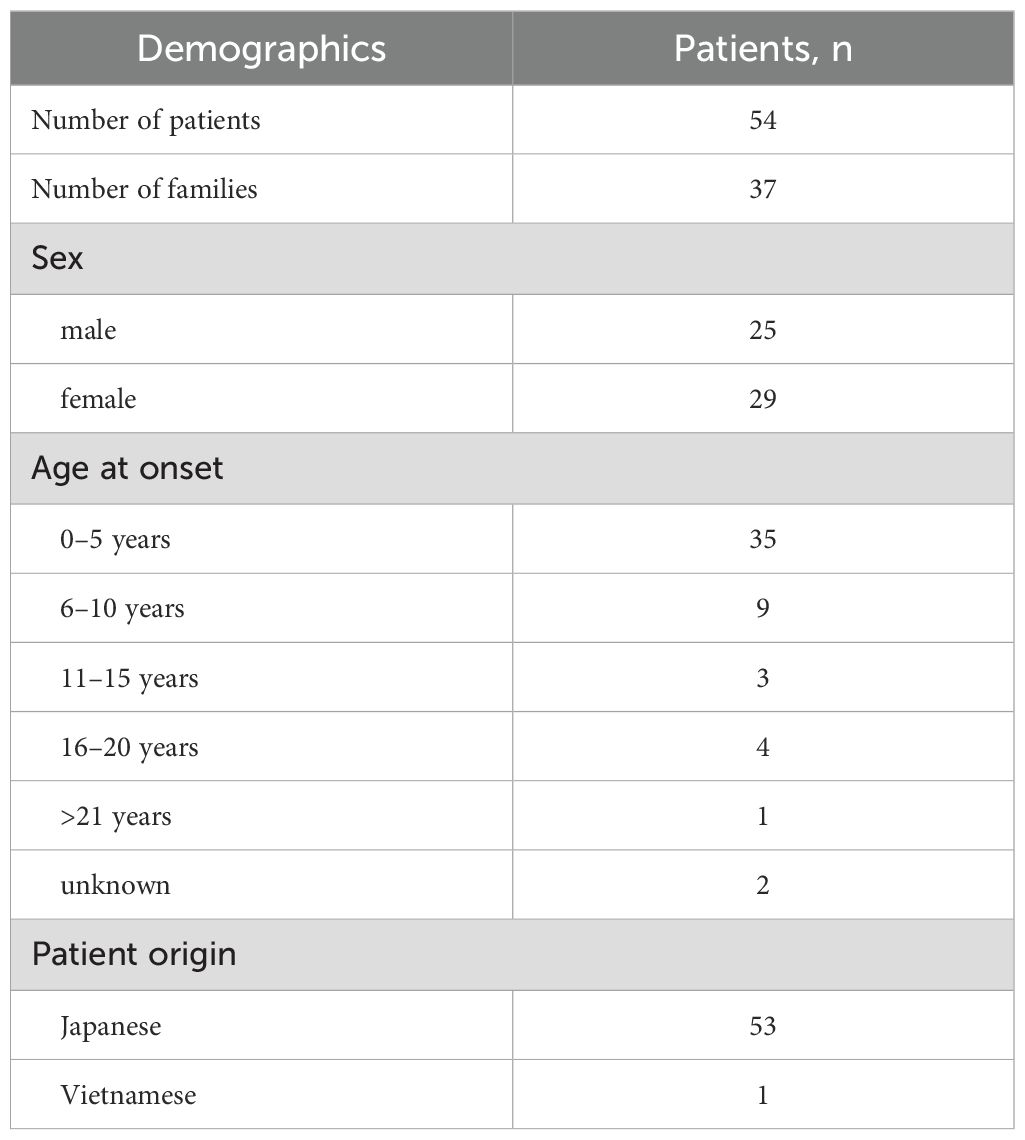
We obtained questionnaire responses from 54 patients (37 families) with HA20 who consented to our study (Table 1). The pedigrees of these 37 families are shown in Supplementary Figure 1. Records of 37 families with HA20 were examined to estimate the existence of a total of 72 patients with pathogenic TNFAIP3 variants in Japan. Of these, 70 patients had or were theorized to have germline heterozygous variants while two had somatic mosaicism. The excluded cases and low frequency somatic TNFAIP3 mosaicism are described in the Supplementary Material.
Fifty-three of the patients were Japanese and one was Vietnamese (patient 51). Twenty-five of 54 patients were male and 29 were female, with no sex-specific difference observed. The median age of onset was 2.8 years (range: 0–27 years), with approximately 60% of patients showing onset before age 5 years (Table 1, Supplementary Table 1).
3.2 TNFAIP3 variants and their pathogenicity
All 54 patients analyzed had heterozygous variants in TNFAIP3, comprising a total of 35 distinct pathogenic variants. The domain structure of A20 and the sites of TNFAIP3 variation in our survey are shown in Figure 1. Additionally, genetic analysis of family members revealed two cases of low frequency somatic mosaicism of TNFAIP3 in peripheral blood, with 10.06% in the mother of patient 3 and 16.7% in the father of patient 18 (3). The results of functional analyses of newly identified TNFAIP3 variants are shown in Figure 2 and Supplementary Figure 2.
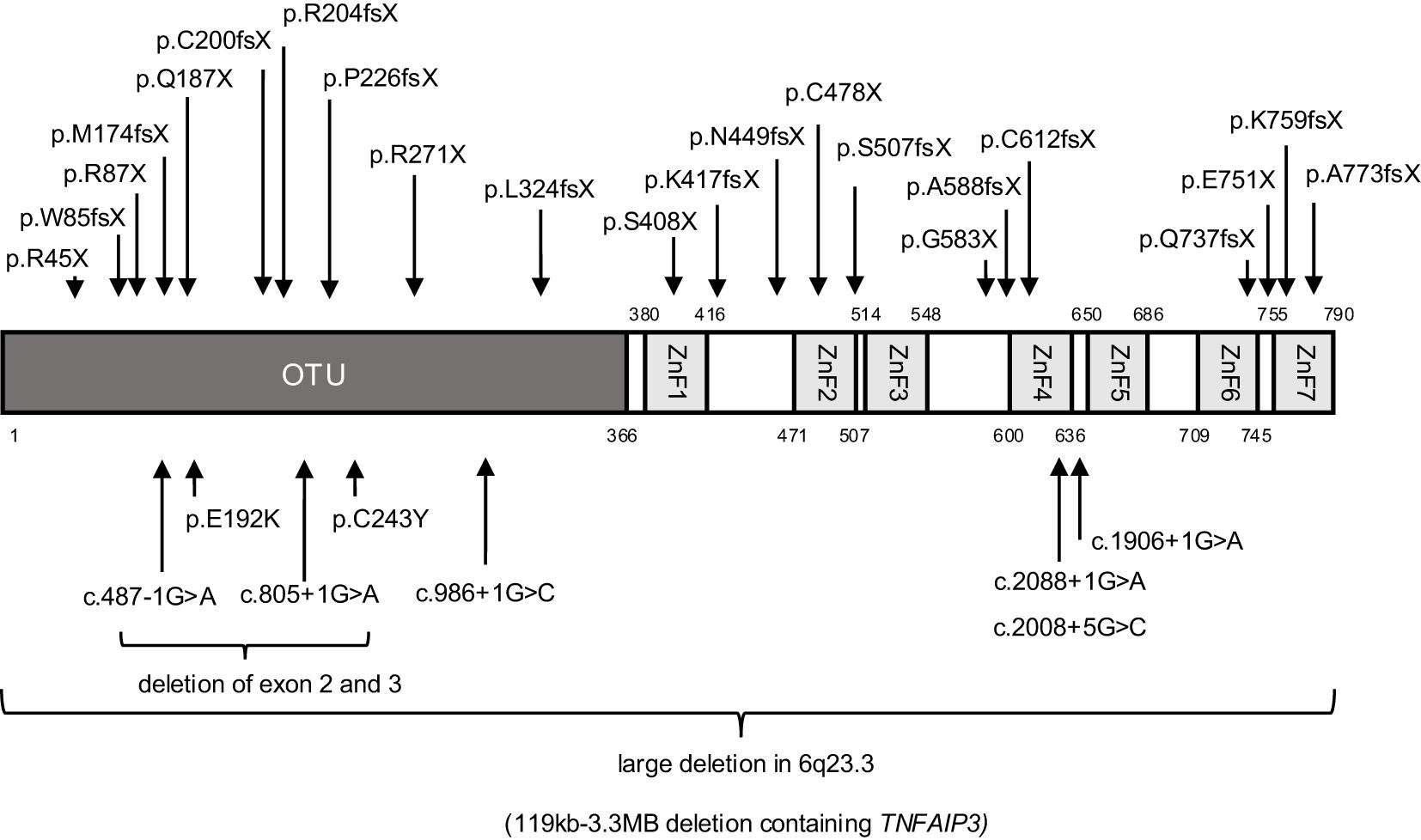
Figure 1. Domain structure of TNFAIP3 (A20) and sites of variation identified in 54 patients with A20 haploinsufficiency in Japan. OTU, operational taxonomic unit; ZnF, zinc finger domain.
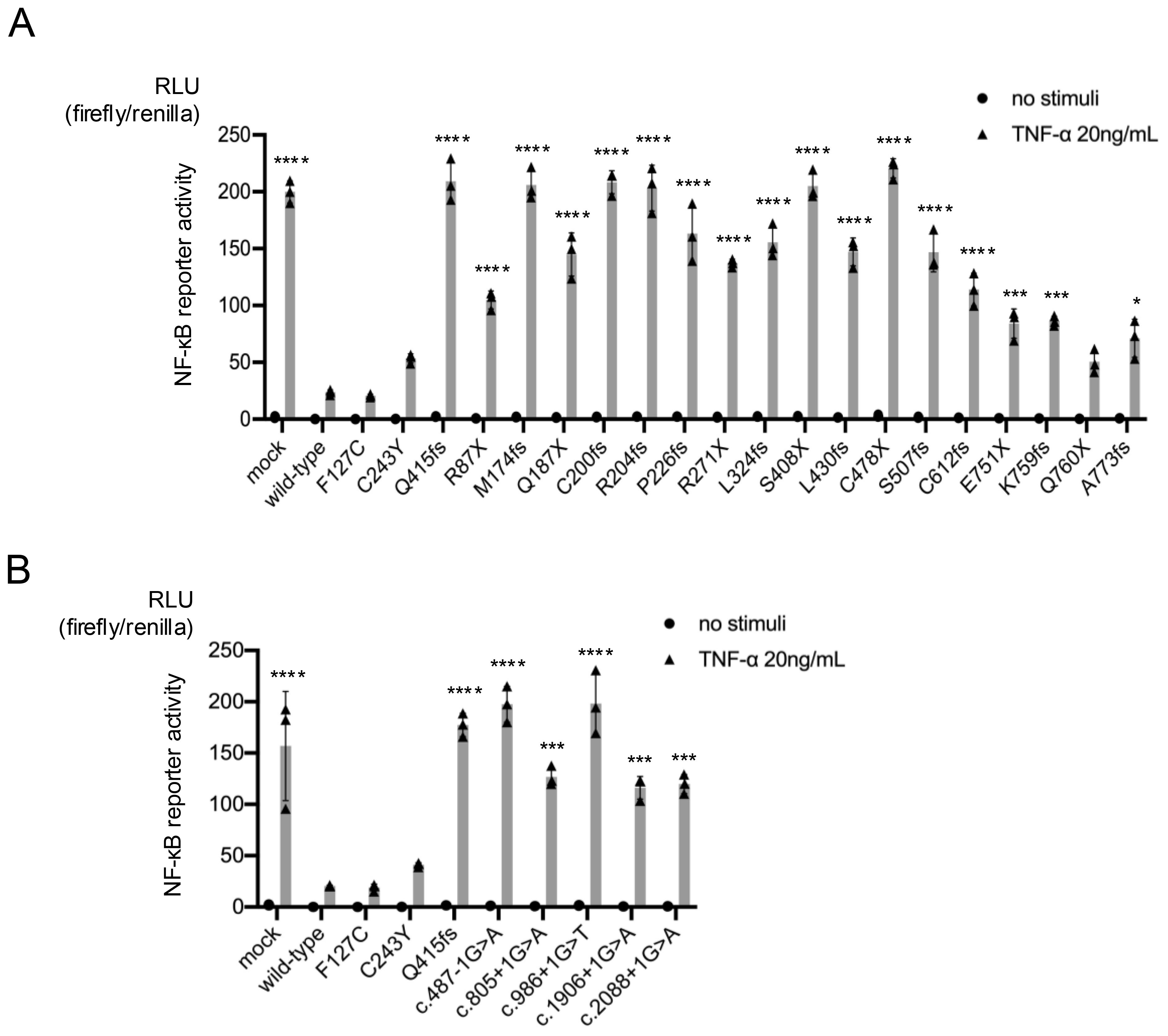
Figure 2. Reporter gene activity of truncating TNFAIP3 variants. The inhibitory effects of the wild-type gene and each variant on nuclear factor (NF)-κB reporter gene activity were compared. The pathogenicity of the truncating variants was examined using an NF-κB-driven luciferase reporter gene activity assay in A20-deficient HEK293 cells stimulated with tumor necrosis factor (TNF)-α (20 ng/mL). The wild-type gene had an inhibitory effect on NF-κB reporter gene activity, while the pathological variants had a disrupted inhibitory effect. Activities of frameshift and nonsense variants (A) and splice variants (B) of TNFAIP3 (A20). *P < 0.05, ***P < 0.001, ****P < 0.0001, as determined by one-way analysis of variance with Tukey’s multiple comparison test. RLU, relative light unit.
3.3 Clinical characteristics
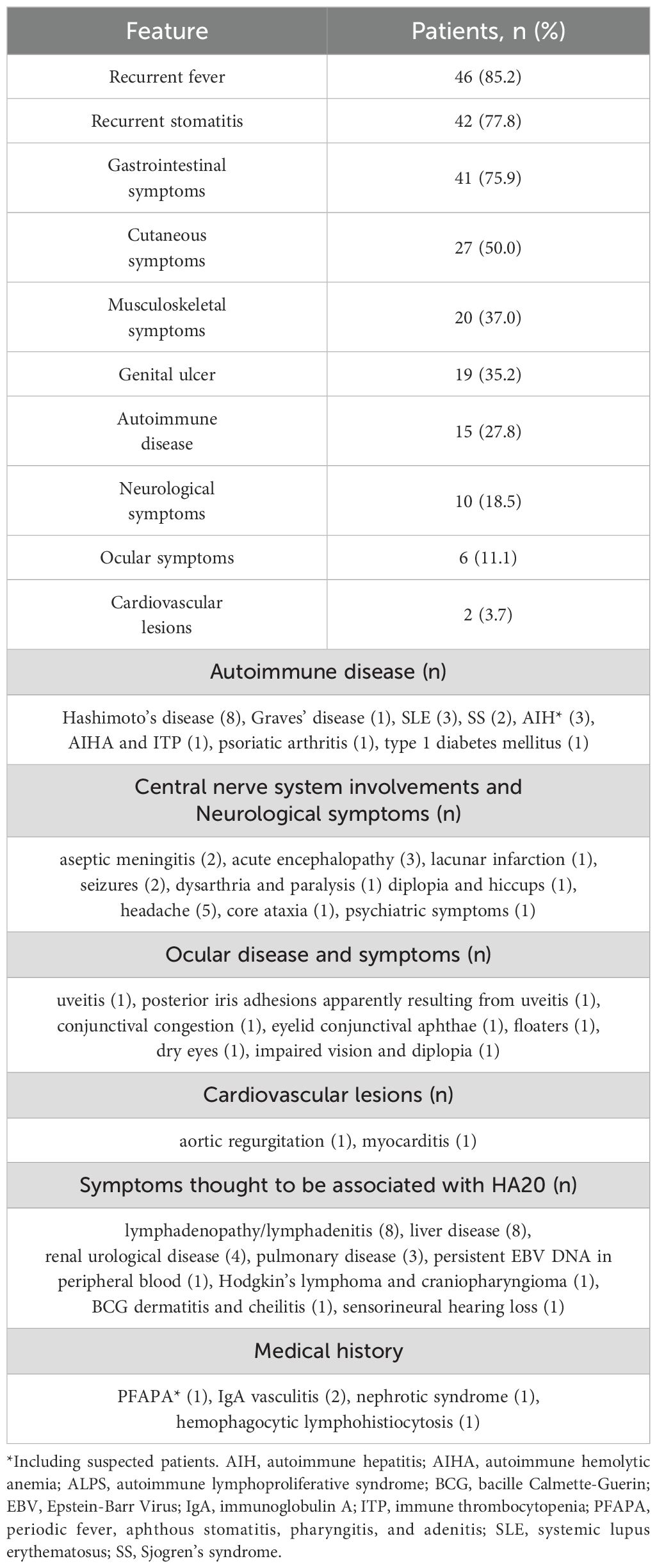
The 54 patients investigated all were symptomatic. Additionally, family analysis revealed that three cases with heterozygous variants were asymptomatic (penetrance: 95.7%). The two patients with somatic mosaicism of TNFAIP3 variants were asymptomatic. Table 2 and Supplementary Table 2 show the clinical characteristics of the 54 HA20 patients that were surveyed. Recurrent fever, recurrent stomatitis, and gastrointestinal symptoms were common features, followed by cutaneous symptoms, musculoskeletal symptoms, genital ulcers and autoimmune diseases. Central nervous system (CNS) involvement was observed in 5 patients. Patients 10 and 33 presented with aseptic meningitis, with patient 10 showing elevated IL-6 in the cerebrospinal fluid. Patient 38 developed acute encephalopathy with seizures and impaired consciousness. Patient 44 developed dysarthria and severe seizures, with magnetic resonance imaging (MRI) of the head showing lacunar infarction with leukoencephalopathy. Patient 47 had diplopia and hiccups with cerebral white matter lesions on head MRI with elevated IL-6 in the cerebrospinal fluid. Other symptoms thought to be associated with HA20 included eight patients with lymphadenopathy/lymphadenitis, eight with liver disease (including three patients with autoimmune hepatitis [AIH] or suspected AIH), one with Hodgkin’s lymphoma and craniopharyngioma (patient 8). Pulmonary disease was observed in three patients, in whom chest computed tomography examinations revealed unidentified pneumonia with nodular and granular shadow (patient 10), granular shadows with consolidation and bronchiectasis (patient 47), and multiple small nodules (patient 54), respectively.
Interestingly, patients carrying the same pathogenic variant exhibited different phenotypes and varying degrees of severity. Regarding phenotypes, in family 27, which carried the p.A773fs variant, the younger brother (patient 43) experienced recurrent episodes of fever and lymphadenopathy from 3 months of age, whereas the elder brother (patient 44) developed recurrent fevers and severe neurological symptoms in adulthood. Regarding severity, in three families (families 1, 3 and 7), patients 1, 4 and 16 presented with refractory disease, whereas other members with pathogenic variants exhibited only mild symptoms. Additionally, the severity differed between patient 3 (c.2088 + 5G>C) and patient 49 (c.2099 + 1G>A), despite both exhibiting exon 8 skipping (p.H636fsX).
3.4 Laboratory data
Inflammation markers were elevated in many patients during flares, with an average white blood cell count of 11,886/μl (range 600-23,380) and an average C-reactive protein (CRP) level of 7.89 mg/dl (range 0.31-19.89). Additionally, during non-flare periods, the average CRP level was 0.44 mg/dl (range 0-6.42), with nine patients testing positive. Liver enzymes were elevated in eight patients, and patient 53 had aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels of 1,659 and 1,446 U/L, respectively.
Human leukocyte antigen (HLA) data were examined in 31 patients, with HLA-A26 confirmed in five patients, B27 in one patient, B51 in six patients, and B52 in four patients. Patient 3, who required hematopoietic cell transplantation (HCT), was both HLA-B51-positive and HLA-B27-positive. Nineteen patients were evaluated for the type I IFN score, of whom 16 patients had an elevated IFN score and three patients carrying missense variants p.E192K or p.C243Y exhibited IFN levels within the normal range (Supplementary Table 2, Supplementary Figure 3). Anti-drug antibodies were measured in 11 patients (Supplementary Table 3). Of these, anti-adalimumab (ADA) antibodies were positive in one patient with secondary failure of ADA and in two patients still using ADA. Anti-infliximab (IFX) antibodies were positive in two patients. Four out of five patients with positive anti-drug antibodies were treated with concomitant immunosuppressants.
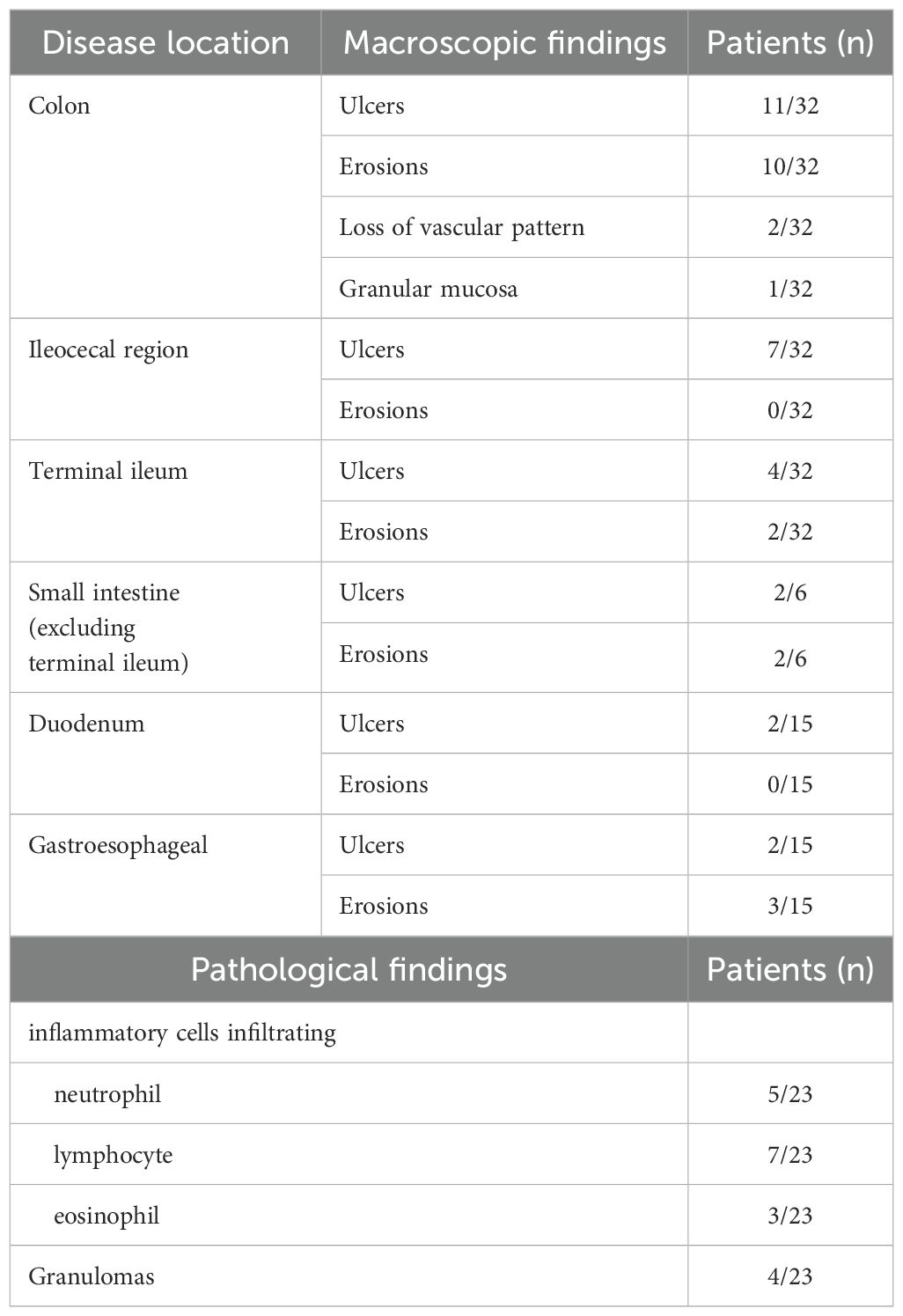
Gastrointestinal endoscopy was performed in 35 patients (including capsule or small bowel endoscopy in six patients), with confirmed lesions throughout the gastrointestinal tract (Table 3). Most of the lesions were skip lesions, although three patients had diffuse lesions and two of them showed loss of vascular patterning or granular mucosa, as observed in ulcerative colitis. Pathological findings showed that the infiltrating inflammatory cells varied from neutrophil-dominant cases to lymphocyte- and plasma cell-dominant cases, with granulation tissue observed in four patients.
Liver biopsy was performed in three patients with liver dysfunction. Pathology in two of these patients showed interface hepatitis and plasma cell infiltration (patients 6 and 18), consistent with AIH. Pathological findings in the third patient (patient 53) showed extensive hepatocyte loss (suspicious of atrophy of the liver parenchyma).
3.5 Treatments
The previous and current anti-inflammatory and surgical treatments and their effects in the surveyed patients are shown in Supplementary Table 1 and Figure 3A. While nine of the 54 patients (16.7%) were followed without treatment, the other 45 patients (83.3%) were administered anti-inflammatory treatment. Colchicine was used in 37 patients (68.5%), as combination therapy in some, with this treatment evaluated as “effective” or “improvement” by the attending physicians of 20 patients (54.1%). Systemic corticosteroids were administered in 38 patients (70.4%), either continuously or episodically, whenever symptoms appeared, with many showing efficacy (57.9%). Thalidomide was used in combination with an MTD and was effective in two patients (patients 16 and 29). Immunosuppressants were administered to 19 patients. These immunosuppressants are still being used in 12 patients, concomitantly with MTDs in nine patients, for whom immunosuppressant monotherapy did not completely suppress the HA20 symptoms.
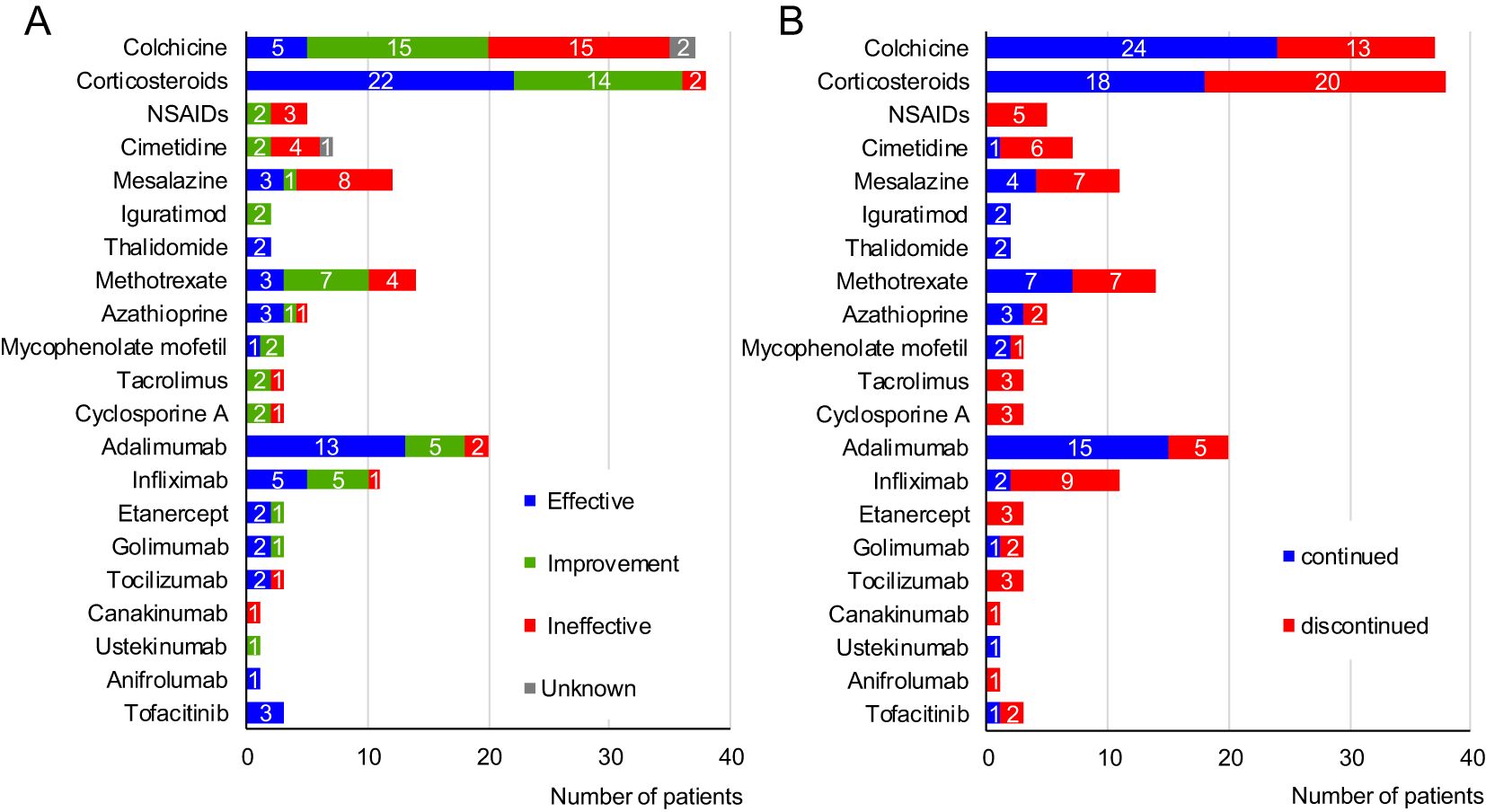
Figure 3. Treatments used for 54 patients with A20 haploinsufficiency in Japan. (A) Effects of each drug. (B) Continuity rate of each drug. NSAIDs, non-steroidal anti-inflammatory drugs.
MTDs were administered to 24 patients (44.4%), with biologics tending to be the first choice. Among these, the anti-TNF-α agents were the most common; overall, these agents showed efficacy, with a complete response rate of 59.5%. The median duration of effective anti-TNF-α agent was 1.63 years (range 0.17-9.83). Tocilizumab was administered to three patients and was initially effective in two but was discontinued because of relapse. Canakinumab (CAN) was administered to one patient but was ineffective. Ustekinumab improved the symptoms of one patient but did not lead to remission. Anifrolumab (ANI) was effective in one patient. Tofacitinib (TOF) was administered in patients with refractory disease (see below), showing partial effectiveness (5).
Seven patients received surgical treatment: enterectomy in three patients, tonsillectomy in three patients, and craniopharyngioma removal in one patient. Enterectomy was performed for perforation of the gastrointestinal tract (patient 29), in addition to endoscopic balloon dilation for stenotic sites (patient 16). Tonsillectomy was performed in patient 20 with an initial diagnosis of periodic fever, aphthous stomatitis, pharyngitis and adenitis (PFAPA), in patient 11, in whom PFAPA symptoms persisted despite ADA therapy, and in patient 50 with recurrent histiocytic necrotizing lymphadenitis. After tonsillectomy, PFAPA symptoms disappeared in the former two cases, whereas lymphadenitis remained in the latter case.
3.6 The reasons for discontinuation of MTDs
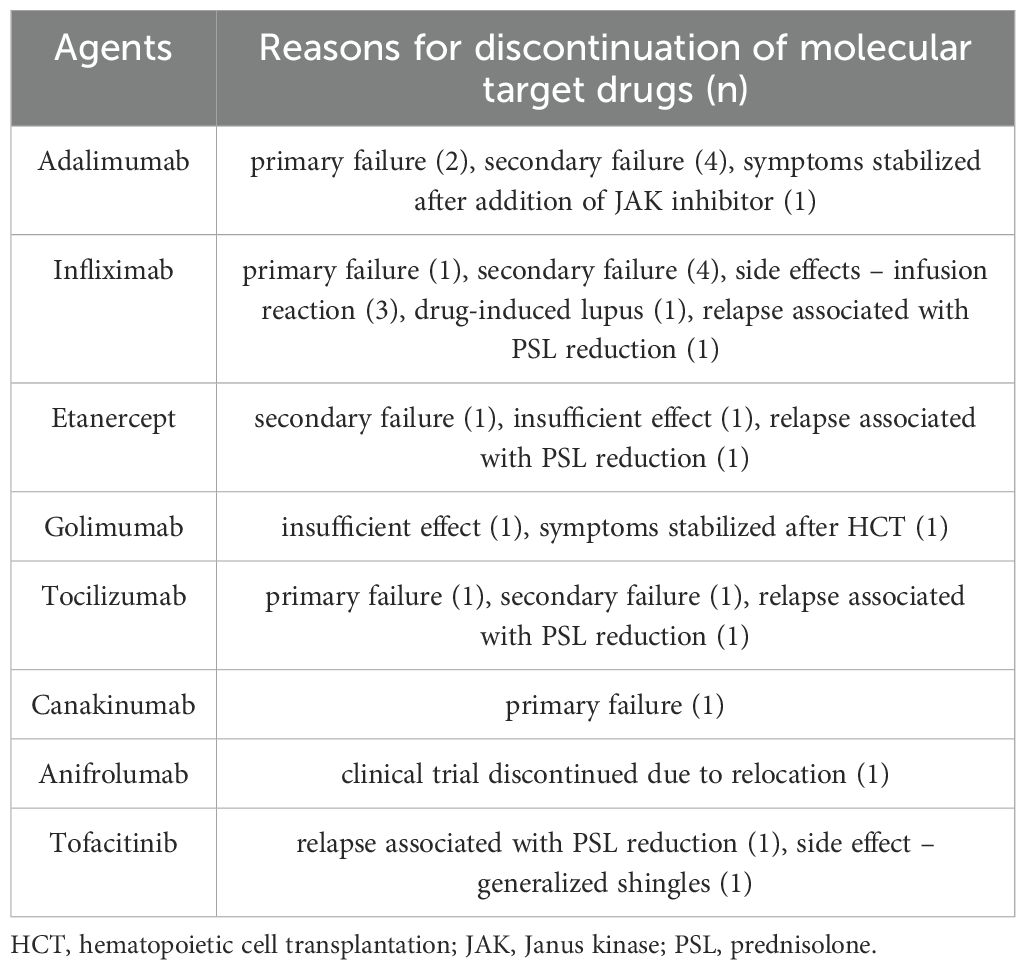
Several MTD treatments were discontinued due to primary or secondary treatment failure, relapse associated with prednisolone (PSL) tapering, or adverse effects of MTDs (Figure 3B, Table 4). The most common reason for discontinuation was secondary failure of MTDs, observed in 10 agents across seven patients. The rate of concomitant immunosuppressant use did not differ between MTDs with or without secondary failure (Supplementary Table 4). The period from initiation of treatment to confirmation of secondary failure varied, ranging from 2 months to several years. As indicated above, one patient was positive for anti-ADA antibodies at the time of secondary failure. Additionally, IFX was discontinued because of side effects in four patients; infusion reaction in three patients, and drug-induced lupus in one patient. Three patients who had infusion reactions initially responded well to IFX but experienced a decrease in the effectiveness of IFX during the course of treatment. Anti-IFX antibodies were detectable at the onset of infusion reaction in two patients.
3.7 Treatment methods in refractory patients
The 11 patients (20.4%) with refractory disease required treatment modification to control the inflammatory conditions. The first strategy in all 11 patients was to switch the current MTD to an alternative one, which was successful in six patients. If the alternative biologic also failed to work, other agents were added: TOF in three patients, thalidomide in one patient, and an immunosuppressant in one patient. TOF was initially effective but was discontinued in 2/3 patients because of a side effect of generalized shingles and relapse associated with PSL reduction, respectively. Thalidomide was successfully introduced. One patient (patient 3) who underwent HCT because of resistance to various immunosuppressive therapies has remained in remission without anti-inflammatory therapy for 5 years post HCT.
4 Discussion
In this study, we described the clinical characteristics of, and the efficacy of treatments attempted in, HA20 patients in Japan. After familial Mediterranean fever and cryopyrin-associated periodic syndrome, HA20 is the third most frequent hereditary autoinflammatory disorder in Japan. Furthermore, we found that secondary failure of MTDs was a major factor contributing to refractory disease and limiting treatment options.
Regarding the characteristic findings of organ disorders, inflammatory bowel symptoms are recorded at high frequencies in HA20 patients. Endoscopically, in very-early-onset IBD, Crohn’s disease-like symptoms and colonic involvement are reportedly more common in patients with monogenic IBD than in those with non-monogenic IBD. Patients with TNFAIP3 variants tend to show involvement of both the upper and lower gastrointestinal tract, with about 80% of patients having colonic ulcers (7). Our study confirmed these findings and also identified lesions in the small intestine. Although it is not easy to perform endoscopy in younger children, this study demonstrates the importance of confirming intestinal involvement with endoscopy in patients with gastrointestinal symptoms.
Although HA20 was originally described as a disease that presents with BD-like symptoms, subsequent reports and our investigation found that HA20 patients presented with a wide variety of symptoms indicating disorders in important organs, including neurological, liver, and pulmonary diseases and malignant lymphomas at low frequencies. Infrequent organ disorders should be noted at diagnosis and follow-up and evaluated whenever new symptoms occur. CNS involvement of HA20 includes aseptic meningitis, encephalitis/encephalopathy, CNS vasculitis, cerebral vasculopathy, and intracerebral calcifications (4, 8, 9). In this survey, elevated levels of IL-6 in the cerebrospinal fluid, which were reported as a biomarker of neuro-BD (10), were observed in two HA20 patients presenting with aseptic meningitis or encephalopathy. JAK inhibitor treatment was reportedly effective for the intracranial mass lesions in which the presence of necrotizing granulomatous inflammation was confirmed by brain biopsy (8). In our survey, three HA20 patients with encephalopathy who were started on mycophenolate mofetil or anti-TNF-α agents after being treated with corticosteroids for CNS involvement have shown no relapse of neurological disease to date. Generally, antibody products administered through normal routes are not expected to reach the CNS; thus, it is not known whether biologics are effective in the prevention or treatment of acute phase CNS involvement. Spontaneous neuroinflammation was observed in the brains of A20 knockout and A20 heterozygous deficient mice (11). However, spontaneous cytokine production was not the predominant cause of neurological lesions in A20 heterozygous deficient mice, indicating that CNS lesions of HA20 patients may not be caused solely by inflammatory cytokines produced in the brain. Furthermore, in our patient, HCT improved symptoms in the skin, joints, lungs, and gastrointestinal lesions and normalized his IFN score (5), suggesting that peripheral immune cells or inflammatory cytokines produced by peripheral immune cells may influence inflammation in these organs. Studying the immune cells that directly affect neurological lesions may help to establish treatments for these lesions. A20 is a crucial hepatoprotective factor, and liver involvement (e.g. AIH) is an important form of organ damage in HA20 (12–16). The present study and previous reports suggested that acute onset or exacerbation of HA20-associated liver involvement/AIH may be ameliorated by appropriate treatment (14, 15). However, deleterious variants in TNFAIP3 have been reported to predispose patients to AIH with cirrhosis (17), with one report of death from progressive liver disease with cirrhosis (16), necessitating careful follow-up when liver dysfunction is observed. Reports of pulmonary disease associated with HA20 have included interstitial pneumonia, nodular lesions, alveolar hemorrhage, and bronchiectasis (9, 16, 18, 19). Many cases had no respiratory symptoms associated with lung lesions, while others exhibited poor respiratory status requiring administration of MTDs (16). Given the pathogenesis of interferonopathy and vasculitis, the possibility of pulmonary involvement should be considered in HA20. Three cases of malignant lymphoma have been reported in HA20 patients (including suspected cases) in Japan: two cases with HA20 (patient 8 and the father of patient 38 (20)) and a case with suspected HA20 (the grandmother of patient 15 (3)). It has been reported that HA20 patients exhibit the skewed immune repertoire typically found in B- and T-cell lymphomas and that the risk of lymphoma may be increased in HA20 patients (21). The median age of the patients at the time of this study was 16 years; considering the age of onset of each malignant lymphoma (21, 35, and 70 years, respectively), malignant lymphoma may be an important complication in adulthood that requires attention.
The phenotype and severity of HA20 in our survey differed, even among cases with the same variant of TNFAIP3. HLA alleles affecting T cells are reportedly a factor influencing the phenotypes of Behcet’s spectrum disorders (e.g. recurrent aphthous stomatitis, PFAPA, and BD), including severity and tissue involvement, with the strongest association between HLA and BD (22). Although, we were unable to identify any significant associations between HLA types and the severity of HA20 (Supplementary Table 5), further detailed study may uncover new HLA alleles involved in HA20 phenotypes. Additionally, Karri et al. (23). noted a difference in pathogenicity between missense variants and frameshift or truncating variants of TNFAIP3. In this survey, there were no refractory cases among the patients with missense variants, and the type I IFN scores were lower in patients with missense variants than in those with truncating variants (Supplementary Figure 3), suggesting that missense variants may lead to less severe disease.
Approximately 20% of the HA20 patients surveyed had refractory disease, mainly due to secondary failure of MTD treatment. One cause of such secondary failures is thought to be the production of anti-drug antibodies, as reported in inflammatory bowel disease (IBD) and rheumatic disease (24, 25). Indeed, anti-ADA antibodies were detected at the time of secondary failure in one patient in this survey. Intriguingly, the anti-ADA antibodies were detected very early (2 months after the start of drug administration) and disappeared after ADA discontinuation (Supplementary Table 3). However, anti-drug antibodies were not detected at the onset of secondary failure in many of the surveyed patients, suggesting that other factors were involved in the secondary failure. Factors that reportedly influence the blood levels of IFX include polymorphism in FCGR3A, which is involved in the removal and excretion of human IgG1 (26), and in FcRn, the neonatal Fc receptor responsible for prolonging the half-life of IgG (27). Alternatively, if the blood trough level of an MTD is high enough, as it was in patient 3, the influence of inflammatory cytokines from different pathways or the presence of MTD-resistant lesions may need to be considered. Because HA20 patients experience an early disease onset and many severe patients require long-term use of MTDs, the identification of biomarkers associated with secondary failure of MTDs in HA20 is key.
For treatment, approximately 40% of HA20 patients required MTDs. Anti-TNF-α agents were the recommended first-line MTDs and the most common choice, consistent with previous reports (23, 28, 29), showing efficacy in 59.5% of patients. However, because patients may develop infusion reactions, caution should be exercised in patients with temporarily decreased efficacy during IFX administration. Regarding anti-IL-1 agents, CAN was used in only one patient and was administered during an exacerbation phase, making it difficult to evaluate the efficacy of CAN in our study. ANI was used in one patient initially diagnosed with systemic lupus erythematosus (SLE); in addition to the SLE symptoms, recurrent fever and cutaneous symptoms thought to be associated with HA20 disappeared, suggesting that ANI may be effective in the inflammatory stage of HA20 rather than JAK inhibitors. In fact, ANI efficacy was also reported in a case of the other monogenic interferonopathy, recently (30). The observation that many of our HA20 patients had increased levels of type I IFN supports a rationale for ANI as a potential therapeutic option. Moreover, the prevalence of SLE in HA20 has been reported to be 8.4% (31). ANI may be considered for HA20 patients with SLE, in whom type I IFN plays a pathogenic role.
As a treatment strategy for refractory disease, when an MTD results in primary or secondary failure, or intolerance develops, switching to an alternative MTD is recommended. Switching between anti-TNF-α agents is useful in HA20 patients, as reported in IBD (32, 33). If a biologic produced a partial effect, the addition of an immunosuppressant, thalidomide, and a JAK inhibitor were also considered as treatment options. A20 is involved in various cytokine production pathways, and which cytokines have a major influence on the pathogenesis of HA20 may vary over time and by patient. Therefore, when treating refractory disease, the response to each therapeutic agent may need to be evaluated individually and different treatment options considered. JAK inhibitors are reportedly effective in HA20 patients (8, 34). In our survey, two of three patients who received TOF discontinued TOF because of flare-ups or side effects. Possible reasons for the failure of TOF in this case include the disease being too severe during TOF administration, and the TOF-mediated suppression of type I IFN being insufficient to reduce HA20 inflammation. Therefore, the timing of its use with regard to severity of disease may need to be considered. Additionally, JAK inhibitors carry a risk of viral infection (35) and should be considered with caution. Thalidomide, which promotes TNF-α mRNA degradation and suppresses TNF-α production by monocytes, is reportedly effective in patients with intestinal BD and pediatric IBD (36–38), and in children with HA20 (39, 40). Thus, thalidomide has therapeutic value for HA20 patients, with the notable side effect of peripheral neuropathy (37, 38). HCT for HA20 has been reported in four patients, including the patient in our survey, and was effective in two patients (4, 5, 41). However, given the presence of residual disease in the two cases of successful transplantation, further study on the timing of HCT is needed. HCT has also been attempted in other autoinflammatory diseases (42), but the intensity of pretreatment and the choice of transplant source remain to be investigated.
This retrospective study had several limitations. First, anti-inflammatory therapies were often initiated in combination, especially in severe cases, making it difficult to assess the efficacy of each drug separately. Second, the timing at which the IFN score was measured varied from patient to patient. The type I IFN signature reportedly correlates with disease activity (43, 44), thus, IFN scoring at consistent time points might be helpful to determine whether type I IFN levels can be used to assess the severity of HA20. Third, early use of anti-IL-1 agents and anti-IFN agents, including JAK inhibitors and ANI, has been difficult, especially in children, in Japan. Increasing the choice of available MTDs may allow early suppression of the inflammatory stage of HA20.
In conclusion, this large-scale survey of 54 patients with HA20 in Japan revealed the involvement of multiple organs and the high efficacy of anti-TNF-α agents. However, secondary failure of MTDs was an important factor leading to refractory disease. Although anti-IFN therapies, thalidomide, and HCT might be potential treatment options, the results of this study suggest that further research is necessary to elucidate the mechanism of secondary failures and establish effective treatments for HA20, especially in patients with refractory disease.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by Kyoto University and Gifu University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HO: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. MS: Conceptualization, Investigation, Writing – original draft. SaK: Investigation, Writing – review & editing. YM: Investigation, Writing – review & editing. KN: Investigation, Writing – review & editing. YM: Investigation, Writing – review & editing. DK: Investigation, Writing – review & editing. CK: Investigation, Writing – review & editing. HT: Investigation, Writing – review & editing. KM: Investigation, Writing – review & editing. YI: Investigation, Writing – review & editing. TE: Investigation, Writing – review & editing. TM: Investigation, Writing – review & editing. EH: Investigation, Writing – review & editing. SS: Investigation, Writing – review & editing. KM: Investigation, Writing – review & editing. TM: Investigation, Writing – review & editing. DK: Investigation, Writing – review & editing. EH: Investigation, Writing – review & editing. UK: Investigation, Writing – review & editing. TI: Investigation, Writing – review & editing. MO: Investigation, Writing – review & editing. KM: Investigation, Writing – review & editing. YK: Investigation, Writing – review & editing. HU: Investigation, Writing – review & editing. FK: Investigation, Writing – review & editing. DA: Investigation, Writing – review & editing. YF: Investigation, Writing – review & editing. MI: Investigation, Writing – review & editing. YO: Investigation, Writing – review & editing. TN: Investigation, Writing – review & editing. TI: Investigation, Writing – review & editing. RH: Investigation, Writing – review & editing. ST: Investigation, Writing – review & editing. ShoK: Investigation, Writing – review & editing. KN: Investigation, Writing – review & editing. HS: Investigation, Writing – review & editing. YH: Investigation, Writing – review & editing. FM: Investigation, Writing – review & editing. ShuK: Investigation, Writing – review & editing. AS: Investigation, Writing – review & editing. MS: Investigation, Writing – review & editing. HK: Investigation, Writing – review & editing. MH: Investigation, Writing – review & editing. NN: Investigation, Writing – review & editing. TY: Investigation, Writing – review & editing. YW: Investigation, Writing – review & editing. MU: Investigation, Writing – review & editing. TW: Investigation, Writing – review & editing. KIz: Investigation, Project administration, Writing – review & editing. TY: Investigation, Writing – review & editing. RN: Investigation, Writing – review & editing. KIm: Writing – review & editing, Investigation.
Collaborators
PIDJ (Primary Immunodeficiency Database in Japan) members in the JSIAD (Japanese Society for immunodeficiency and Autoinflammatory Diseases): Kohsuke Imai, Takaki Asano, Katsuhiro Arai, Hiroaki Ida, Norimitsu Inoue, Masahiro Ueki, Mariko Eguchi, Satoshi Okada, Nobuo Kanazawa, Naoki Kimura, Toshinao Kawai, Naotomo Kambe, Tomohiro Koga, Masanori Kono, Yoji Sasahara, Sanami Takada, Ichiro Takeuchi, Hiroshi Tsujimoto, Etsushi Toyofuku, Ryusuke Nambu, Junki Hiura, Yukiko Hidaka, Hiroko Fukushima, Takahiko Horiuchi, Yoshitaka Honda, Yusuke Matsuda, Tomoyuki Mizukami, Takako Miyamae, Hideki Muramatsu, Kunihiko Moriya, Takashi Yamazaki, Motoi Yamashita, and Manabu Wakamatsu.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the following grants: a Health Labour Sciences Research Grant for Research on Intractable Diseases from the Ministry of Health, Labour and Welfare (MHLW) of Japan (Grant Numbers JPMH23FC1016 to RN and HO, and JPMH23FC1023 to HO); and a MEXT/JSPS KAKENHI grant (JP 25K11104 to HO and JP 24K18463 to SK).
Acknowledgments
The authors appreciate the invaluable assistance of Shinya Sato, who obtained permission to study his patients. We also thank Michelle Kahmeyer-Gabbe, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
The Department of Early Diagnosis and Preventive Medicine for Rare Intractable Pediatric Diseases, Graduate School of Medicine, Gifu University is an endowed department supported by an unrestricted grant from the Gifu Research Center for Public Health. KNi received honoraria for lectures from Novartis Pharma, Abbvie and AstraZeneca, and his department received corporate scholarship grants from Chugai Pharmaceutical and Mitsubishi-Tanabe. DKi received honoraria for lectures from Novartis Pharma, Abbvie, Mitsubishi-Tanabe, Chugai Pharmaceutical, AstraZeneca, Pfizer, Eli Lilly, Asahi KASEI, GSK, Eisai, Taisho Pharmaceutical, UCB Japan and Amgen. YI received grants from Pfizer Japan Inc. and honoraria for lectures from Abbvie Japan GK and AstraZeneca K.K. TE received honoraria for lectures from Abbvie and Novartis Pharma. SS received grants from Chugai Pharmaceutical and Asahi Kasei. DKo received honoraria for lectures from Novartis Pharma, Abbvie, Janssen, Mitsubishi-Tanabe, Chugai Pharmaceutical, AstraZeneca, Pfizer and Eli Lilly. EHa received honoraria for lectures from Abbvie. UK received honoraria for lectures from Novartis Pharma and Abbvie. MO received honoraria for lectures from Chugai Pharmaceutical, Teijin Pharma, Astellas Pharma Inc., Novartis Pharma, Takeda pharmaceuticals and BioMarin Pharmaceutical Japan. HU received honoraria for lectures from Novartis Pharma, Chugai Pharmaceutical, GlaxoSmithKline, Abbvie and Pfizer. DA received honoraria for lectures from Abbvie and Mitsubishi-Tanabe. MI received honoraria for lectures from Mitsubishi-Tanabe and Chugai Pharmaceutical and participated in a clinical trial conducted by Novartis Pharma. TakuI received payment or honoraria for lectures and presentations from Mitsubishi Tanabe Pharma Corporation, EA Pharma, Abbvie GK, Janssen Pharma, Nihon Kayaku, Alfressa Pharma, Takeda Pharmaceuticals, JIMRO, Eisai, Sandoz, and Miyarisan. HS received honoraria for presentations from Novartis Pharma. MasS received honoraria for lectures from Novartis Pharma, Abbvie, Chugai, AstraZeneca, Pfizer and Eli Lilly. HK received honoraria for lectures from Takeda pharmaceuticals. MH received research grants and/or speaker fees from Abbvie, Asahi Kasei, Astellas, Bristol Meyers, Chugai, EA Pharma, Eisai, Daiichi Sankyo, Eli Lilly, Novartis Pharma, Taisho Toyama, and Mitsubishi Tanabe Pharma Corporation. NN received honoraria for educational events from Abbvie. YW received honoraria for lectures from Chugai Pharmaceutical, Pfizer and Eli Lilly. KI received honoraria for lectures and presentations from Novartis and consulting fees from SOBI. TYa received honoraria for lectures and presentations from Novartis Pharma. HO received honoraria for lectures and presentations from Novartis and Abbvie, and participated in a clinical trial conducted by SOBI, and his department received scholarship grants from Chugai, Asahi Kasei, Abbott Japan, Kyowa Kirin, Otsuka Pharmaceutical, Sumitomo Pharma, maruho, JCR, Japan Blood Products Organization, Mitsubishi-Tanabe and Shionogi.
The remaining author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1548042/full#supplementary-material
Abbreviations
ADA, adalimumab; AIH, autoimmune hepatitis; ANI, Anifrolumab; BD, Behcet’s disease; CAN, Canakinumab; CNS, Central nervous system; HA20, A20 haploinsufficiency; HCT, hematopoietic cell transplantation; HLA, Human leukocyte antigen; IFN, interferon; IFX, infliximab; IL, interleukin; JAK, Janus kinase; MTDs, molecular target drugs; MRI, magnetic resonance imaging; NF-κB, nuclear factor κ light-chain enhancer of activated B cells; PFAPA, periodic fever, aphthous stomatitis, pharyngitis and adenitis; PIDJ, Primary Immunodeficiency Database in Japan; PSL, prednisolone; TNF, tumor necrosis factor; TOF, Tofacitinib.
References
1. Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet. (2016) 48:67–73. doi: 10.1038/ng.3459
2. Catrysse L, Vereecke L, Beyaert R, and van Loo G. A20 in inflammation and autoimmunity. Trends Immunol. (2014) 35:22–31. doi: 10.1016/j.it.2013.10.005
3. Kadowaki T, Ohnishi H, Kawamoto N, Hori T, Nishimura K, Kobayashi C, et al. Haploinsufficiency of A20 causes autoinflammatory and autoimmune disorders. J Allergy Clin Immunol. (2018) 141:1485–8.e11. doi: 10.1016/j.jaci.2017.10.039
4. Aeschlimann FA, Batu ED, Canna SW, Go E, Gul A, Hoffmann P, et al. A20 haploinsufficiency (HA20): clinical phenotypes and disease course of patients with a newly recognised NF-kB-mediated autoinflammatory disease. Ann Rheum Dis. (2018) 77:728–35. doi: 10.1136/annrheumdis-2017-212403
5. Shiraki M, Williams E, Yokoyama N, Shinoda K, Nademi Z, Matsumoto K, et al. Hematopoietic cell transplantation ameliorates autoinflammation in A20 haploinsufficiency. J Clin Immunol. (2021) 41:1954–6. doi: 10.1007/s10875-021-01124-1
6. Mitsui-Sekinaka K, Sekinaka Y, Endo A, Imai K, and Nonoyama S. The primary immunodeficiency database in Japan. Front Immunol. (2021) 12:805766. doi: 10.3389/fimmu.2021.805766
7. Ye Z, Wang Y, Tang Z, Wang X, Sun L, Wang L, et al. Understanding endoscopic and clinicopathological features of patients with very early onset inflammatory bowel disease: Results from a decade of study. Dig Liver Dis. (2024) 56:50–4. doi: 10.1016/j.dld.2023.08.041
8. Mulhern CM, Hong Y, Omoyinmi E, Jacques TS, D’Arco F, Hemingway C, et al. Janus kinase 1/2 inhibition for the treatment of autoinflammation associated with heterozygous TNFAIP3 mutation. J Allergy Clin Immunol. (2019) 144:863–6 e5. doi: 10.1016/j.jaci.2019.05.026
9. He T, Huang Y, Luo Y, Xia Y, Wang L, Zhang H, et al. Haploinsufficiency of A20 due to novel mutations in TNFAIP3. J Clin Immunol. (2020) 40:741–51. doi: 10.1007/s10875-020-00792-9
10. Zhan H, Cheng L, and Li Y. Neuro-Behçet’s disease: an update of clinical diagnosis, biomarkers, and immunopathogenesis. Clin Exp Immunol. (2025) 219:1–19. doi: 10.1093/cei/uxae123
11. Guedes RP, Csizmadia E, Moll HP, Ma A, Ferran C, and da Silva CG. A20 deficiency causes spontaneous neuroinflammation in mice. J Neuroinflammation. (2014) 11:122. doi: 10.1186/1742-2094-11-122
12. Takagi M, Ogata S, Ueno H, Yoshida K, Yeh T, Hoshino A, et al. Haploinsufficiency of TNFAIP3 (A20) by germline mutation is involved in autoimmune lymphoproliferative syndrome. J Allergy Clin Immunol. (2017) 139:1914–22. doi: 10.1016/j.jaci.2016.09.038
13. Deshayes S, Bazille C, El Khouri E, Kone-Paut I, Giurgea I, Georgin-Lavialle S, et al. Chronic hepatic involvement in the clinical spectrum of A20 haploinsufficiency. Liver Int. (2021) 41:1894–900. doi: 10.1111/liv.14935
14. Pandurangi S, Malik A, Owens J, Valencia CA, Miethke AG, and Center for Autoimmune Liver Disease Research G. Deleterious variants in TNFAIP3 are associated with type II and seronegative pediatric autoimmune hepatitis. J Hepatol. (2024) 80:e26–e8. doi: 10.1016/j.jhep.2023.09.028
15. Iwasa T, Miwa T, Unome S, Hanai T, Imai K, Takai K, et al. A case of A20 haploinsufficiency complicated by autoimmune hepatitis. Hepatol Res. (2024) 54:606–11. doi: 10.1111/hepr.14003
16. Hautala T, Vahasalo P, Kuismin O, Keskitalo S, Rajamaki K, Vaananen A, et al. A family with A20 haploinsufficiency presenting with novel clinical manifestations and challenges for treatment. J Clin Rheumatol. (2021) 27:e583–e7. doi: 10.1097/RHU.0000000000001268
17. Higuchi T, Oka S, Furukawa H, Nakamura M, Komori A, Abiru S, et al. Role of deleterious single nucleotide variants in the coding regions of TNFAIP3 for Japanese autoimmune hepatitis with cirrhosis. Sci Rep. (2019) 9:7925. doi: 10.1038/s41598-019-44524-5
18. Li GM, Liu HM, Guan WZ, Xu H, Wu BB, and Sun L. Expanding the spectrum of A20 haploinsufficiency in two Chinese families: cases report. BMC Med Genet. (2019) 20:124. doi: 10.1186/s12881-019-0856-1
19. Shaheen ZR, Williams SJA, and Binstadt BA. Case report: A novel TNFAIP3 mutation causing haploinsufficiency of A20 with a lupus-like phenotype. Front Immunol. (2021) 12:629457. doi: 10.3389/fimmu.2021.629457
20. Endo Y, Funakoshi Y, Koga T, Ohashi H, Takao M, Miura K, et al. Large deletion in 6q containing the TNFAIP3 gene associated with autoimmune lymphoproliferative syndrome. Clin Immunol. (2022) 235:108853. doi: 10.1016/j.clim.2021.108853
21. Schultheiß C, Paschold L, Mohebiany AN, Escher M, Kattimani YM, Müller M, et al. A20 haploinsufficiency disturbs immune homeostasis and drives the transformation of lymphocytes with permissive antigen receptors. Sci Adv. (2024) 10:eadl3975. doi: 10.1126/sciadv.adl3975
22. Manthiram K, Preite S, Dedeoglu F, Demir S, Ozen S, Edwards KM, et al. Common genetic susceptibility loci link PFAPA syndrome, Behçet’s disease, and recurrent aphthous stomatitis. Proc Natl Acad Sci U.S.A. (2020) 117:14405–11. doi: 10.1073/pnas.2002051117
23. Karri U, Harasimowicz M, Carpio Tumba M, and Schwartz DM. The complexity of being A20: from biological functions to genetic associations. J Clin Immunol. (2024) 44:76. doi: 10.1007/s10875-024-01681-1
24. Bots SJ, Parker CE, Brandse JF, Lowenberg M, Feagan BG, Sandborn WJ, et al. Anti-drug antibody formation against biologic agents in inflammatory bowel disease: A systematic review and meta-analysis. Biodrugs. (2021) 35:715–33. doi: 10.1007/s40259-021-00507-5
25. Balsa A, Sanmarti R, Rosas J, Martin V, Cabez A, Gomez S, et al. Drug immunogenicity in patients with inflammatory arthritis and secondary failure to tumour necrosis factor inhibitor therapies: the REASON study. Rheumatol (Oxford). (2018) 57:688–93. doi: 10.1093/rheumatology/kex474
26. Curci D, Lucafo M, Cifu A, Fabris M, Bramuzzo M, Martelossi S, et al. Pharmacogenetic variants of infliximab response in young patients with inflammatory bowel disease. Clin Transl Sci. (2021) 14:2184–92. doi: 10.1111/cts.13075
27. Billiet T, Dreesen E, Cleynen I, Wollants WJ, Ferrante M, Van Assche G, et al. A genetic variation in the neonatal fc-receptor affects anti-TNF drug concentrations in inflammatory bowel disease. Am J Gastroenterol. (2016) 111:1438–45. doi: 10.1038/ajg.2016.306
28. Kadowaki T, Kadowaki S, and Ohnishi H. A20 haploinsufficiency in East Asia. Front Immunol. (2021) 12:780689. doi: 10.3389/fimmu.2021.780689
29. Elhani I, Riller Q, Boursier G, Hentgen V, Rieux-Laucat F, and Georgin-Lavialle S. A20 haploinsufficiency: A systematic review of 177 cases. J Invest Dermatol. (2024) 144:1282–94.e8. doi: 10.1016/j.jid.2023.12.007
30. Doroudchi MA, Thauland TJ, Patel BA, and Butte MJ. Anifrolumab to treat a monogenic interferonopathy. J Allergy Clin Immunol Pract. (2024) 12:1374–6.e1. doi: 10.1016/j.jaip.2024.02.013
31. Philip R, Elhani I, Gallou S, Boysson H, Martin Silva N, Georgin-Lavialle S, et al. A20 haploinsufficiency diagnosis beyond systemic lupus erythematosus: A systematic review of the literature. Autoimmun Rev. (2025) 24:103722. doi: 10.1016/j.autrev.2024.103722
32. Gisbert JP, Marin AC, McNicholl AG, and Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. (2015) 41:613–23. doi: 10.1111/apt.13083
33. Lecoutour A, Dupont C, Caldari D, Dumant C, Vanrenterghem A, Ruiz M, et al. Efficacy of infliximab after loss of response of/intolerance to adalimumab in pediatric Crohn’s disease: A retrospective multicenter cohort study of the “GETAID pediatrique. J Pediatr Gastroenterol Nutr. (2024) 78:1116–25. doi: 10.1002/jpn3.12044
34. Schwartz DM, Blackstone SA, Sampaio-Moura N, Rosenzweig S, Burma AM, Stone D, et al. Type I interferon signature predicts response to JAK inhibition in haploinsufficiency of A20. Ann Rheum Dis. (2020) 79:429–31. doi: 10.1136/annrheumdis-2019-215918
35. Boyadzhieva Z, Ruffer N, Burmester G, Pankow A, and Krusche M. Effectiveness and safety of JAK inhibitors in autoinflammatory diseases: A systematic review. Front Med (Lausanne). (2022) 9:930071. doi: 10.3389/fmed.2022.930071
36. Hatemi I, Hatemi G, Pamuk ON, Erzin Y, and Celik AF. TNF-alpha antagonists and thalidomide for the management of gastrointestinal Behçet’s syndrome refractory to the conventional treatment modalities: a case series and review of the literature. Clin Exp Rheumatol. (2015) 33:S129–37.
37. Qiu T, Li H, Sun T, Men P, Cui X, Liu C, et al. Thalidomide as a treatment for inflammatory bowel disease in children and adolescents: A systematic review. J Clin Pharm Ther. (2020) 45:1134–42. doi: 10.1111/jcpt.13196
38. Bramuzzo M, Giudici F, Arrigo S, Lionetti P, Zuin G, Romano C, et al. Efficacy and tolerance of thalidomide in patients with very early onset inflammatory bowel disease. Inflammation Bowel Dis. (2024) 30:20–8. doi: 10.1093/ibd/izad018
39. Zhang C, Yu Z, Gao S, Ma M, Gou L, Wang C, et al. Efficacy and safety of thalidomide in children with monogenic autoinflammatory diseases: a single-center, real-world-evidence study. Pediatr Rheumatol Online J. (2023) 21:124. doi: 10.1186/s12969-023-00881-0
40. Mitsunaga K, Inoue Y, Naito C, Ogata H, Itoh Y, Natsui Y, et al. A case of A20 haploinsufficiency in which intestinal inflammation improved with thalidomide. Rheumatol (Oxford). (2023) 62:e193–e5. doi: 10.1093/rheumatology/keac634
41. Wu CW, Sasa G, Salih A, Nicholas S, Vogel TP, Cahill G, et al. Complicated Diagnosis and Treatment of HA20 due to Contiguous Gene Deletions involving 6q23.3. J Clin Immunol. (2021) 41:1420–3. doi: 10.1007/s10875-021-01048-w
42. Signa S, Dell’Orso G, Gattorno M, and Faraci M. Hematopoietic stem cell transplantation in systemic autoinflammatory diseases - the first one hundred transplanted patients. Expert Rev Clin Immunol. (2022) 18:667–89. doi: 10.1080/1744666X.2022.2078704
43. Higgs BW, Liu Z, White B, Zhu W, White WI, Morehouse C, et al. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann Rheum Dis. (2011) 70:2029–36. doi: 10.1136/ard.2011.150326
Keywords: A20 haploinsufficiency, TNFAIP3, autoinflammatory disease, molecular target drugs, secondary failure
Citation: Shiraki M, Kadowaki S, Miwa Y, Nishimura K, Maruyama Y, Kishida D, Imagawa K, Kobayashi C, Takada H, Mitsunaga K, Inoue Y, Ebato T, Miyamoto T, Hiejima E, Sato S, Migita K, Matsubayashi T, Kobayashi D, Hasegawa E, Kaneko U, Ishikawa T, Onodera M, Matsushita K, Koike Y, Umebayashi H, Kakuta F, Abukawa D, Funakoshi Y, Ishimura M, Otani Y, Nishizawa T, Ishige T, Hatori R, Tanaka S, Kusunoki S, Nakamura K, Shirai H, Hatai Y, Miyaoka F, Kaneko S, Shimbo A, Shimizu M, Kanegane H, Hashimoto M, Negoro N, Yoshida T, Wada Y, Usami M, Wada T, Izawa K, Yasumi T, Nishikomori R and Ohnishi H (2025) Clinical characteristics and treatment strategies for A20 haploinsufficiency in Japan: a national epidemiological survey. Front. Immunol. 16:1548042. doi: 10.3389/fimmu.2025.1548042
Received: 19 December 2024; Accepted: 23 May 2025;
Published: 12 June 2025.
Edited by:
Piero Ruscitti, University of L’Aquila, ItalyReviewed by:
Katerina Laskari, National and Kapodistrian University of Athens, GreeceZarina T. Zainudeen, Universiti Sains Malaysia, Malaysia
Copyright © 2025 Shiraki, Kadowaki, Miwa, Nishimura, Maruyama, Kishida, Imagawa, Kobayashi, Takada, Mitsunaga, Inoue, Ebato, Miyamoto, Hiejima, Sato, Migita, Matsubayashi, Kobayashi, Hasegawa, Kaneko, Ishikawa, Onodera, Matsushita, Koike, Umebayashi, Kakuta, Abukawa, Funakoshi, Ishimura, Otani, Nishizawa, Ishige, Hatori, Tanaka, Kusunoki, Nakamura, Shirai, Hatai, Miyaoka, Kaneko, Shimbo, Shimizu, Kanegane, Hashimoto, Negoro, Yoshida, Wada, Usami, Wada, Izawa, Yasumi, Nishikomori and Ohnishi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hidenori Ohnishi, b2huaXNoaS5oaWRlbm9yaS5mMUBmLmdpZnUtdS5hYy5qcA==
 Mayuka Shiraki1
Mayuka Shiraki1 Saori Kadowaki
Saori Kadowaki Yuki Miwa
Yuki Miwa Kenichi Nishimura
Kenichi Nishimura Yuta Maruyama
Yuta Maruyama Dai Kishida
Dai Kishida Hidetoshi Takada
Hidetoshi Takada Yuzaburo Inoue
Yuzaburo Inoue Takasuke Ebato
Takasuke Ebato Eitaro Hiejima
Eitaro Hiejima Shuzo Sato
Shuzo Sato Kiyoshi Migita
Kiyoshi Migita Tadashi Matsubayashi
Tadashi Matsubayashi Takashi Ishikawa
Takashi Ishikawa Masafumi Onodera
Masafumi Onodera Masataka Ishimura
Masataka Ishimura Harumi Shirai
Harumi Shirai Masaki Shimizu
Masaki Shimizu Hirokazu Kanegane
Hirokazu Kanegane Motomu Hashimoto
Motomu Hashimoto Taro Yoshida
Taro Yoshida Masaaki Usami
Masaaki Usami Taizo Wada
Taizo Wada Kazushi Izawa
Kazushi Izawa Takahiro Yasumi
Takahiro Yasumi Ryuta Nishikomori
Ryuta Nishikomori Hidenori Ohnishi
Hidenori Ohnishi