- Department of Hepatology and Gastroenterology, The First Hospital of Jilin University, Changchun, China
Background: Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic inflammatory condition with rising global incidence. This study examines the association between the triglyceride-glucose (TyG) index, a marker of insulin resistance, and both the risk and prognosis of IBD.
Methods: This study analyzed data from 327,089 participants in the UK Biobank. The TyG index was calculated based on fasting triglyceride and glucose levels, and participants were categorized into quartiles. Cox proportional hazards models, restricted cubic splines (RCS), subgroup analyses, and mediation analyses were employed to explore the association between TyG and the risk of UC and CD. Covariates were adjusted for age, sex, race, socioeconomic and lifestyle factors, physical activity, common chronic diseases, and polygenic risk scores. Multiple sensitivity analyses were conducted to ensure the robustness of the results.
Results: An elevated TyG index was significantly associated with an increased risk of IBD. For each unit increase in TyG, the risk of UC increased by 14.3% (HR: 1.143, 95% CI: 1.053–1.241), while the risk of CD increased by 13.8% (HR: 1.138, 95% CI: 1.006–1.286). Participants in the highest TyG quartile had a 28.0% higher risk of CD (HR: 1.280, 95% CI: 1.050–1.560) and a 17.8% higher risk of UC (HR: 1.178, 95% CI: 1.028–1.351) compared to those in the lowest quartile. Mediation analysis revealed that the association between TyG and the incidence of CD and UC was mediated by multiple factors, including white blood cell count, mean corpuscular hemoglobin, C-reactive protein, total bilirubin, neutrophil count, reticulocyte percentage, and high-light scatter reticulocyte percentage. The proportion of mediation effects ranged from 1.44% to 30.97%.
Conclusion: This study is the first to systematically investigate the relationship between the TyG index and both the risk and prognosis of IBD, using a large cohort from the UK Biobank. The findings reveal a significant positive association between the TyG index and the risk of developing UC and CD, suggesting that a higher TyG index may serve as an independent predictor of IBD risk.
1 Introduction
Inflammatory bowel disease (IBD), encompassing ulcerative colitis (UC) and Crohn’s disease (CD), represents a chronic inflammatory condition of the gastrointestinal tract. In recent years, the incidence of IBD has shown a rising trend globally, affecting millions of individuals. In Europe alone, the number of affected cases exceeds 3.2 million (1, 2). Although the precise etiology of IBD remains incompletely understood, current research suggests that it results from a multifactorial interplay involving genetic susceptibility, immune dysregulation, and dysbiosis of the gut microbiota (3, 4).
In recent years, the potential link between metabolic disturbances and IBD has gained increasing attention. A growing body of evidence suggests that metabolic syndrome and insulin resistance (IR) may play a pivotal role in the onset and progression of IBD (5, 6). Studies have demonstrated that the prevalence of metabolic syndrome is significantly higher in patients with IBD compared to the general population. Additionally, metabolic syndrome may elevate the risk of IBD development and disease activity (7). This bidirectional relationship indicates the involvement of shared pathophysiological mechanisms in the progression of both conditions.
Among the metabolic disturbances linked to IBD, insulin resistance, a core feature of metabolic syndrome, has garnered particular interest. Research has identified a significant state of insulin resistance in IBD patients, which is closely associated with disease activity and levels of inflammatory markers (8). Insulin resistance may not only increase the risk of developing IBD but could also influence disease prognosis (9). These findings provide important insights into the role of metabolic factors in the pathogenesis of IBD.
In this context, the Triglyceride-Glucose Index (TyG), calculated from fasting triglyceride and glucose levels, has emerged as a novel, simple, and effective marker for insulin resistance. In recent years, it has gained widespread application in the assessment of metabolic health (10, 11). Studies have shown that the TyG index not only serves as a tool for measuring insulin resistance but is also associated with the risk of several chronic conditions, including diabetes, hypertension, and cardiovascular diseases (12, 13). However, the relationship between the TyG index and the risk of IBD has not been thoroughly investigated.
Given the critical roles of insulin resistance and chronic inflammation in the pathogenesis of various diseases, we hypothesize that the TyG index may be potentially linked to the risk of developing IBD. To test this hypothesis, the present study aims to explore the association between the TyG index and the risk and prognosis of IBD by utilizing data from the large-scale UK Biobank cohort. We employed a variety of analytical methods, including Cox proportional hazards models, restricted cubic splines (RCS), subgroup analysis to assess interaction effects, and mediation analysis. This study will contribute to a deeper understanding of the complex relationship between metabolic disorders and IBD, offering valuable insights for the development of future intervention strategies and therapeutic approaches.
2 Methods
2.1 Data source
The UK Biobank is a large-scale and detailed prospective study that recruited over 500,000 participants aged 40 to 69 years over a four-year period starting in 2006. This study has collected, and continues to follow up on, a wide range of phenotypic and multi-omics data, including questionnaire data, physical measurements, sample analyses, whole-genome genotyping data, and extensive health-related outcomes through longitudinal tracking. The UK Biobank obtained ethical approval from the North West Multi-centre Research Ethics Committee (REC reference: 11/NW/03820). All participants provided written informed consent for data collection, analysis, and linkage, and the study was conducted in accordance with the principles of the Declaration of Helsinki (14). Access to the UK Biobank data was granted under application ID 84347. This study was reviewed and approved by the Ethics Committee of the First Hospital of Jilin University (Approval No.: Lin Shen 2024-1263). Our dataset comprised information from 502,129 male and female participants. After excluding individuals with pre-existing IBD at recruitment (n = 3,640), those with undefined IBD subtypes (n = 252), and individuals with missing required data (n = 171,148), a total of 327,089 participants were included in the final analysis. Further details are provided in Figure 1.
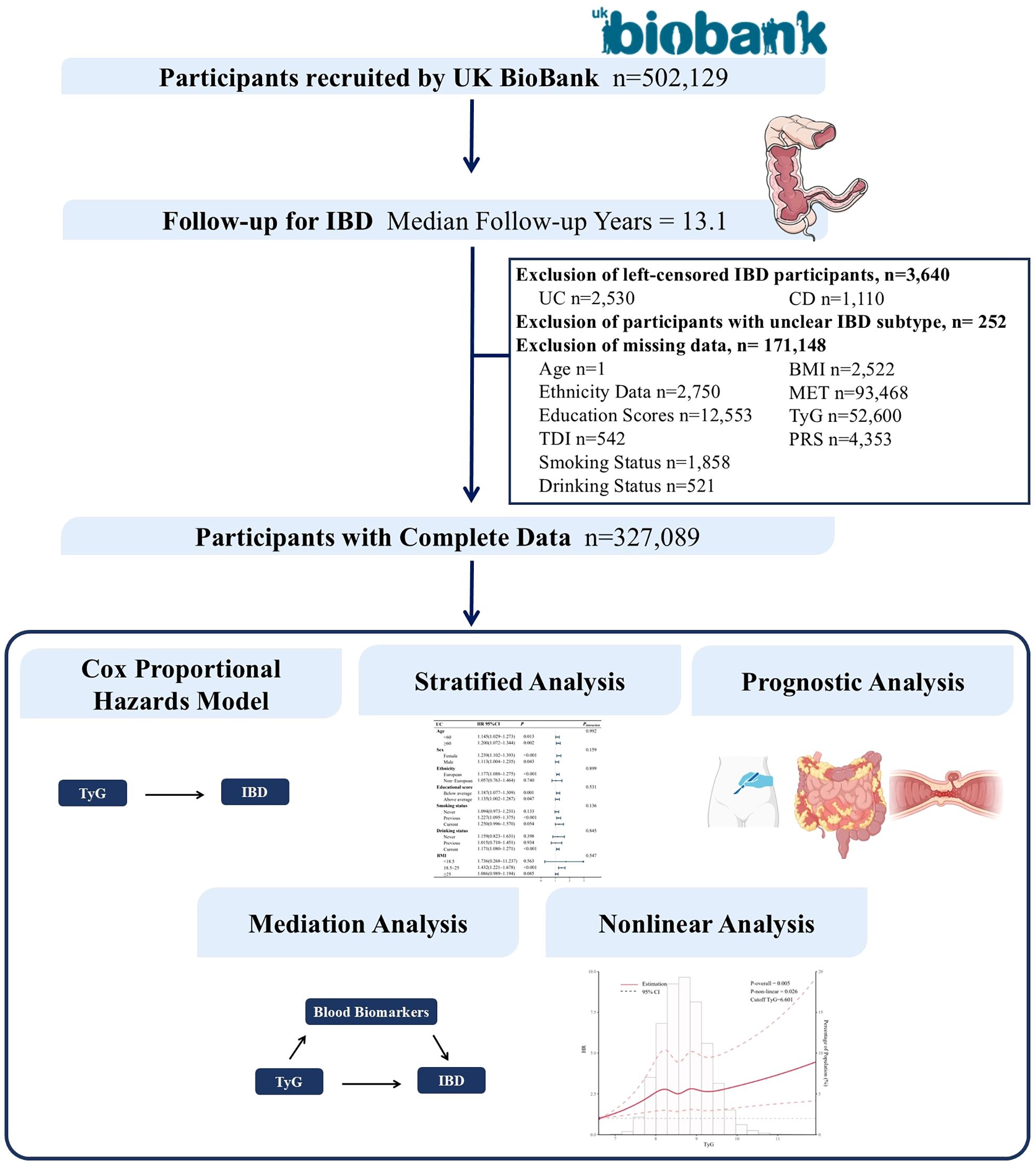
Figure 1. Flowchart of the study design. IBD, inflammatory bowel disease; TDI, Townsend Deprivation Index; BMI, body mass index.
2.2 Selection of variables, covariates, and outcomes
Peripheral venous blood samples were collected from all participants at baseline, and the collection procedures for the UK Biobank study were verified accordingly (15). The TyG index was calculated using the formula (16):
where triglycerides and fasting glucose were both measured in mg/dL. Participants were then categorized into four groups (G1–G4) based on the quartiles of the TyG index (5.865, 8.311, 8.681, 9.078, 12.459).
All diseases, medication prescriptions, and participant deaths were recorded in the database for use and management. Participants were followed from the time of recruitment until the diagnosis of IBD, withdrawal from the study, death, or the last follow-up date, whichever occurred first. The diagnosis of IBD was based on hospital admission codes from the International Classification of Diseases, 10th and 9th editions (ICD-10, ICD-9). Specifically, “K50” or “555” indicated Crohn’s disease, while “K51” or “556” indicated ulcerative colitis.
In the models developed for this study, covariates were adjusted based on potential risk factors for IBD identified from previous research. The adjusted covariates included age, race (European, Asian, African, Chinese, mixed-race, and other), Townsend deprivation index, education level indicator, BMI, smoking status (current, former, never), alcohol consumption (current, former, never), metabolic equivalent of task (MET), hypertension, diabetes, coronary heart disease, malignancy, polygenic risk score for UC, and polygenic risk score for CD. Education attainment indicators were derived from data in the “Children and Youth” and “Adult Skills” domains, representing the “flow” and “stock” of educational disadvantage within a region. The Townsend Deprivation Index, introduced in 1987, is a tool used to measure socioeconomic deprivation, reflecting the level of material and social deprivation in a region. BMI was calculated from measured weight and height and categorized into underweight (BMI < 18.5 kg/m²), normal weight (18.5 kg/m² ≤ BMI < 25.0 kg/m²), and overweight/obesity (BMI ≥ 25.0 kg/m²) (17). Metabolic equivalent of task (MET) was used as an indicator to assess the intensity of participants’ daily physical activity based on a standardized questionnaire. It was calculated relative to resting metabolic rate (1 MET = 3.5 mL O2/kg/min) and incorporated activity type, duration, and frequency to estimate total energy expenditure. According to official recommendations, the weighting factors for walking, moderate activity, and vigorous activity were set at 3, 4, and 8, respectively. Relevant diseases such as hypertension and IBD complications (intestinal obstruction, intra-abdominal abscess, bowel perforation, fistula formation, toxic megacolon, Clostridium difficile infection, sepsis), IBD-related surgeries (small bowel resection, colectomy, anal fistula surgery), and all-cause mortality were extracted using the corresponding ICD-10 and ICD-9 codes. Detailed information on UK Biobank data extraction can be found in Supplementary Table 1. Polygenic risk score data were obtained from calculations published by Thompson et al., with the methodology described in a previous study (18).
The Dietary Inflammatory Index (DII) is a literature- and population-based tool designed to compare the inflammatory potential of diets across different populations (19). It has been widely applied in numerous studies (20, 21). In the UK Biobank, 29 eligible food or nutrient parameters were identified (Supplementary Table 2). Therefore, the DII used in this study was derived based on these 29 food and nutrient parameters. For the calculation of the energy-adjusted DII (E-DII), we followed the methodology outlined in previous research (22). First, food and nutrient intake was adjusted for total energy intake using the density method (nutrient or food intake/total energy intake × 1000 kcal). Then, the steps for DII calculation were repeated to obtain the E-DII.
2.3 Statistical methods and software
All analyses were pre-specified according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines prior to data inspection (23), as detailed in Supplementary Table 3. For the baseline characteristics, continuous variables were presented as means (± standard deviation), and statistical differences were assessed using the Student’s t-test. Categorical variables were reported as counts (percentages) and compared using Pearson’s Chi-squared test. In this study, we not only analyzed TyG as a continuous variable but also divided it into four groups for further analysis. A Cox proportional hazards model was employed to investigate the association between TyG and the incidence of IBD subtypes (UC, CD).
We applied three models to progressively adjust for covariates. Model 1 was adjusted for sex, age, race, Townsend deprivation index, and education score. Model 2 was further adjusted for smoking, alcohol consumption, BMI, and MET based on Model 1. Model 3 was additionally adjusted for hypertension, diabetes, coronary heart disease, malignancy, polygenic risk score for UC, and polygenic risk score for CD based on Model 2. The proportional hazards (PH) assumption for the Cox models was tested using the Schoenfeld residuals method, and all models met the PH assumption. Additionally, we performed subgroup analyses based on age, sex, race, education score, smoking status, alcohol consumption, and BMI to examine potential interactions between TyG and these variables.
We further employed RCS to assess potential nonlinear dose-response relationships between TyG and IBD. Beyond the etiological exploration, we also used the Cox proportional hazards model to investigate the effect of TyG on various IBD complications, IBD-related surgeries, and all-cause mortality in both the UC and CD populations.
Finally, multiple sensitivity analyses were conducted. First, E-DII was added to the model to account for potential dietary influences on the conclusions. Second, IBD cases diagnosed within 1 and 3 years after baseline assessment, as well as those diagnosed before the first follow-up, were excluded to further clarify the long-term impact of baseline TyG levels. Third, participants with cardiovascular disease or cancer were excluded. Fourth, multiple imputation was performed under the assumption that missing values were completely missing at random. The multiple imputation method was conducted using the Fully Conditional Specification (FCS) approach to generate five imputed datasets. The imputation model incorporated all variables used in the analyses, with a maximum of 40 iterations to ensure model convergence. Following imputation, statistical analyses were performed separately on each imputed dataset, and the final results were combined using Rubin’s rules.
All statistical analyses and plots were performed using R Project for Statistical Computing (version 4.3.3). All statistical tests were two-sided, and a p-value < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics
The baseline characteristics of participants, stratified by IBD subtype, are presented in Supplementary Table 4. Among the participants included in the study, females accounted for a higher proportion (52%), and the average age of participants was 56.3 ± 8.1 years. In the group of CD patients, the proportion of females was higher, with their average age being 0.53 years older than that of the non-IBD participants. Compared to non-IBD participants, CD patients tended to have higher education scores, higher Townsend Deprivation Index (TDI) scores, more current and past smoking habits, higher alcohol consumption, lower BMI, and higher TyG scores. In patients with UC, the proportion of males was higher, and their average age was 0.9 year older than that of non-IBD participants. Compared to non-IBD participants, UC patients generally had higher education scores, higher TDI scores, a greater proportion of past smokers, less alcohol consumption, higher BMI, and higher TyG scores. A total of 2,367 IBD patients were included in this study, comprising 740 patients with CD and 1,627 patients with UC. Baseline data stratified by TyG scores can be found in Supplementary Table 5.
3.2 Association between TyG and IBD subtypes
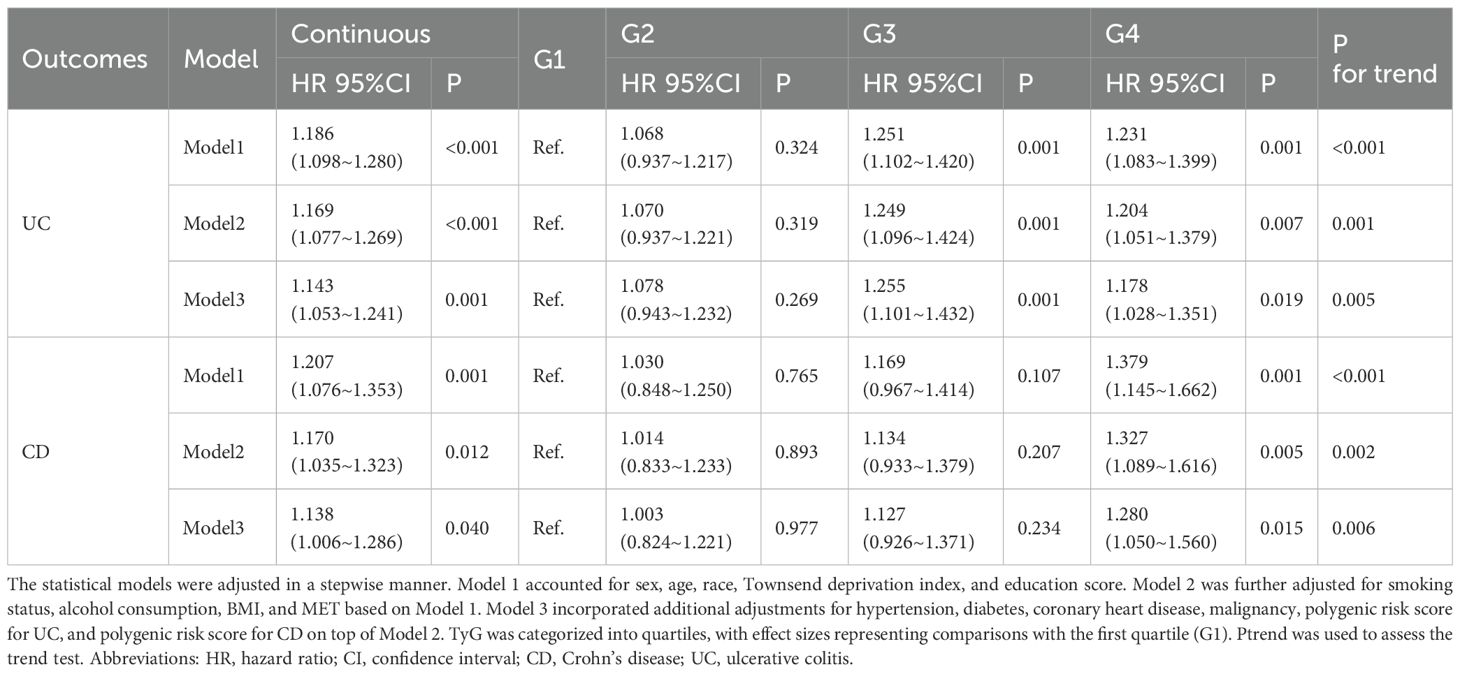
Cox proportional hazards model (Table 1) showed that an elevated TyG index was associated with an increased risk of developing IBD. In Model 3, continuously increasing TyG was significantly associated with the incidence of CD (HR, 95% CI: 1.138 [1.006–1.286], P = 0.040). Compared with the lowest TyG group (G1), the highest TyG group (G4) had a 28.0% higher risk of developing CD (HR, 95% CI: 1.280 [1.050–1.560], P = 0.015). Similarly, in Model 3, continuously increasing TyG was significantly associated with UC (HR, 95% CI: 1.143 [1.053–1.241], P = 0.001). Compared with G1, the G3 group had a 25.5% increased risk of UC (HR, 95% CI: 1.255 [1.101–1.432], P = 0.001), while the G4 group had a 17.8% increased risk (HR, 95% CI: 1.178 [1.028–1.351], P = 0.019).
As shown in Figure 2, after adjusting for covariates, the impact of TyG on UC incidence was more pronounced among former smokers, current alcohol consumers, and individuals with normal weight (BMI between 18.5 and 25). For CD, after covariate adjustment, individuals under 60 years old, females, those with lower education levels, current smokers, current alcohol consumers, and those who were overweight or obese (BMI >25) were more susceptible to the influence of TyG on CD development. Additionally, a significant interaction between TyG and BMI was observed in relation to CD risk.
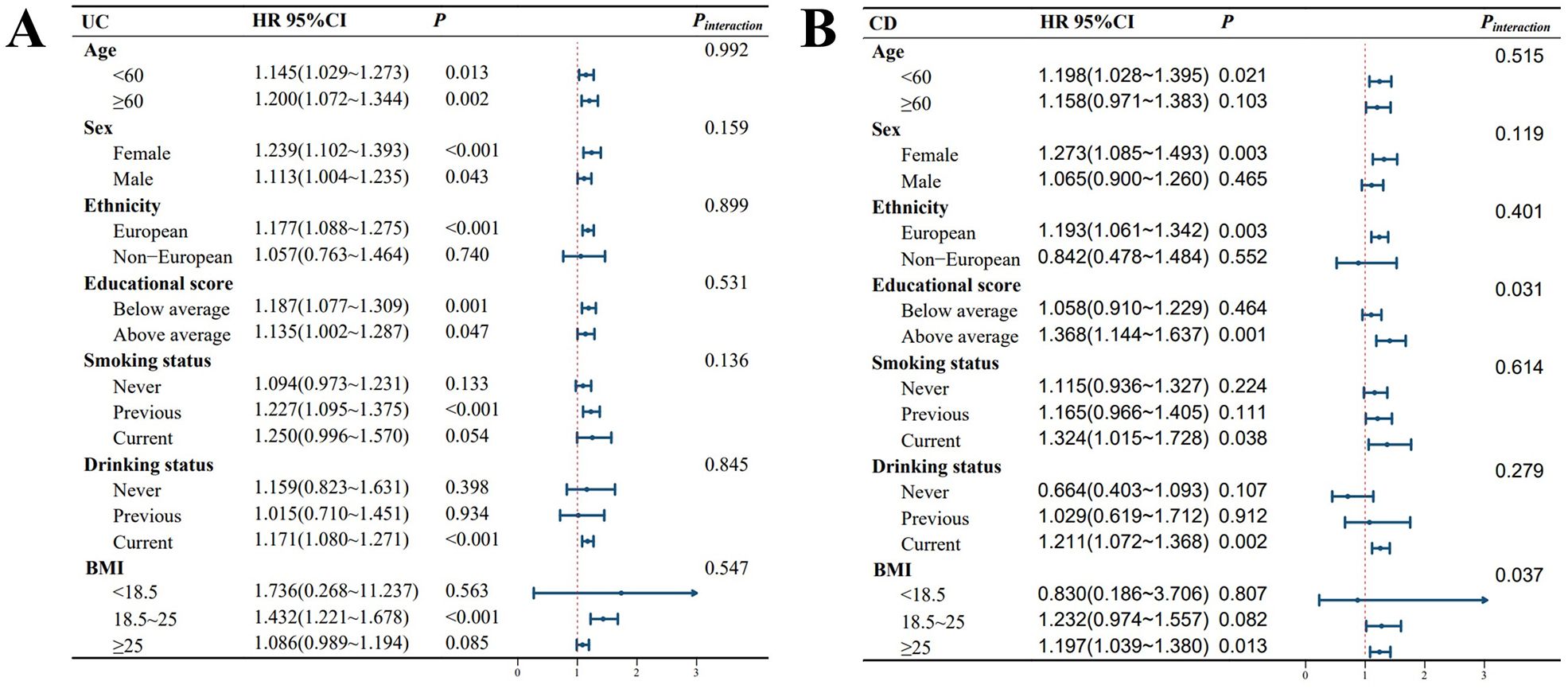
Figure 2. Forest plot of stratified analyses. P-interaction represents the P value for the significance of the interaction effect. The average education score was 14.31. Abbreviations: BMI, body mass index; HR, hazard ratio; CI, confidence interval. (A) presents the TyG-stratified analysis for UC, while (B) illustrates the TyG-stratified analysis for CD. All models used in the analysis were adjusted for the following covariates: sex, age, race, Townsend deprivation index, education score, smoking status, alcohol consumption, BMI, MET, hypertension, diabetes, coronary heart disease, malignancy, polygenic risk score for UC, and polygenic risk score for CD.
3.3 Study on mediators of the association between TyG and IBD
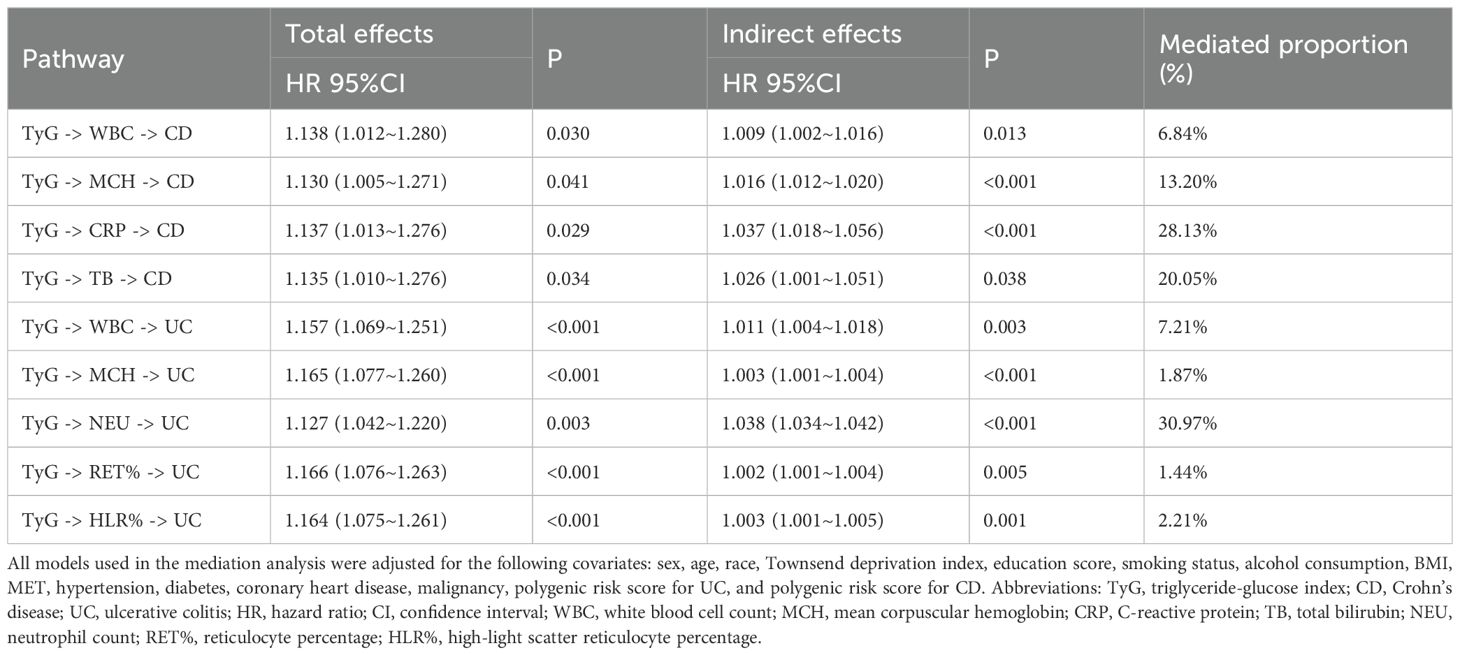
As shown in Table 2, mediation analysis indicated that the association between TyG and CD incidence was mediated by multiple factors, including white blood cell count, mean corpuscular hemoglobin, C-reactive protein (CRP), and total bilirubin. The top mediating factor was CRP, with a mediation effect proportion of 28.13% (P < 0.001). Similarly, the association between TyG and UC incidence was mediated by several factors, including white blood cell count, neutrophil count, reticulocyte percentage, and high-light scatter reticulocyte percentage. The top mediating factor was neutrophil count, with a mediation effect proportion of 30.97% (P < 0.001).
3.4 Nonlinear analysis of TyG and IBD
As shown in Figure 3, a nonlinear relationship was observed between the TyG index and UC incidence. The pattern was characterized by a sharp initial increase (TyG between 6.6 and 8.2), followed by a plateau phase (TyG between 8.2 and 9.2), and then another increase when TyG exceeded 9.2 (P for nonlinearity = 60.041). Although no significant nonlinear effect was observed between the TyG index and CD incidence (P = 0.210), the trend in the figure suggests an initial increase (TyG between 8.2 and 9.2), followed by a plateau phase when TyG exceeded 9.2.
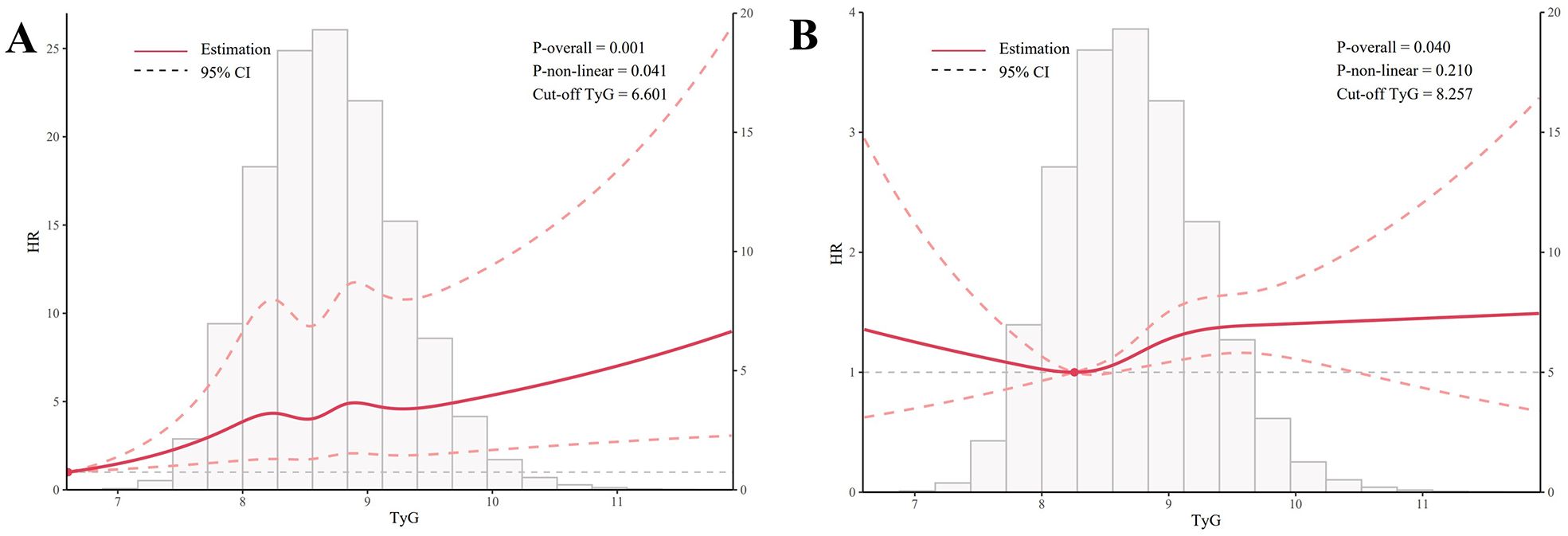
Figure 3. Graphical representation of non-linear relationships (Restricted Cubic Spline). (A) illustrates the nonlinear relationship between TyG and UC, while (B) depicts the nonlinear relationship between TyG and CD. All models used in the analysis were adjusted for the following covariates: sex, age, race, Townsend deprivation index, education score, smoking status, alcohol consumption, BMI, MET, hypertension, diabetes, coronary heart disease, malignancy, polygenic risk score for UC, and polygenic risk score for CD.
3.5 Association of TyG with prognosis of IBD
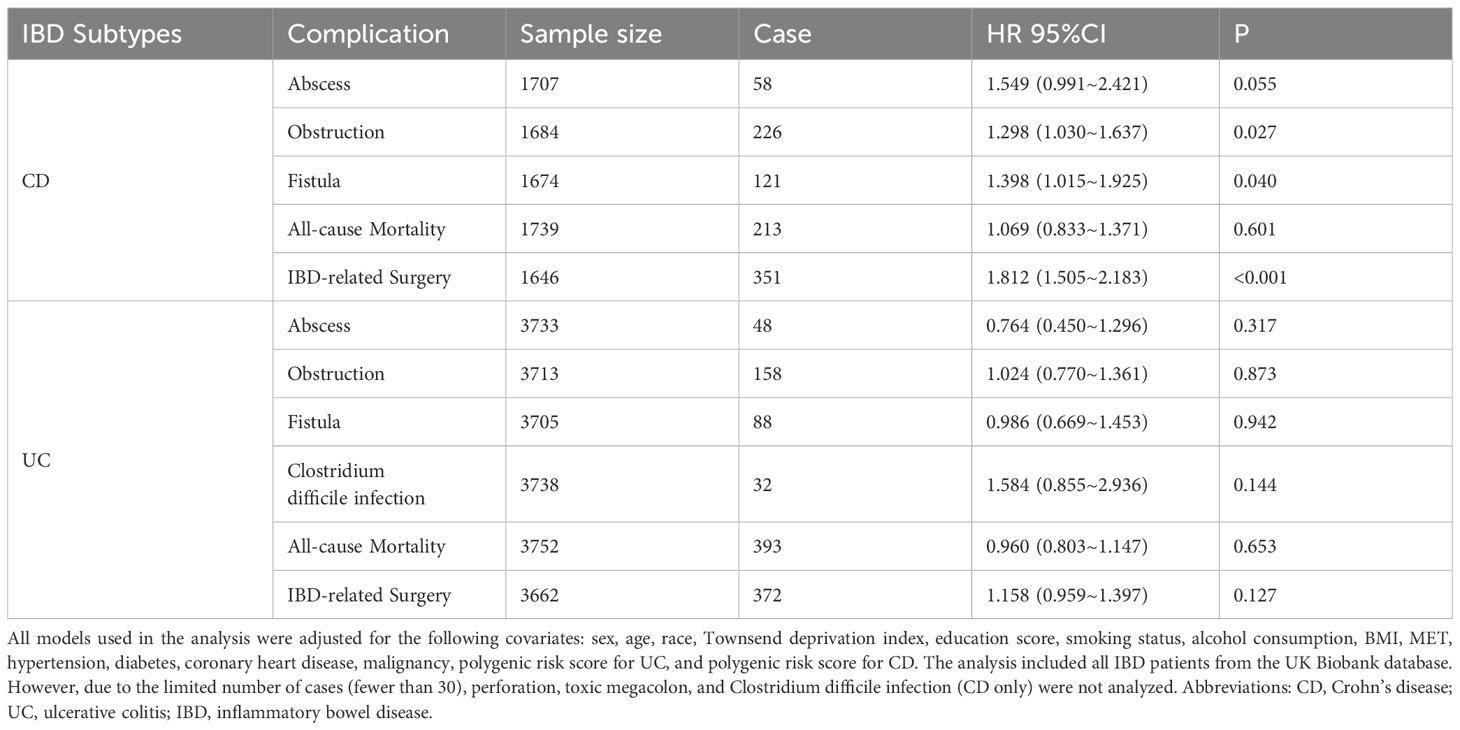
We also analyzed the prognostic factors of UC and CD, including complications, infections, IBD-related surgeries, and all-cause mortality, using the TyG index. In the fully adjusted Cox regression model, a higher TyG index was associated with an increased risk of intestinal obstruction complications in CD patients (HR, 95% CI: 1.298(1.030~1.637); P = 0.027). Additionally, a higher TyG index was linked to a higher incidence of fistula complications in CD patients (HR, 95% CI: 1.398(1.015~1.925); P = 0.040) and an increased likelihood of IBD-related surgeries in CD patients (HR, 95% CI: 1.812(1.505~2.183); P < 0.001). No significant associations were found between the TyG index and other prognostic indicators in IBD patients (Table 3).
3.6 Sensitivity analysis
In the sensitivity analysis, after adding E-DII to the model, the conclusions remained consistent with previous findings (Supplementary Table 6), indicating that the effect of TyG was independent of individual dietary differences. Similarly, after excluding IBD cases diagnosed within 1 and 3 years after baseline assessment, as well as those diagnosed before the first follow-up, and after removing participants with cardiovascular disease or cancer, the conclusions remained unchanged (Supplementary Tables 7–8). Finally, after performing multiple imputations for missing covariates, the previously established conclusions remained robust (Supplementary Table 9).
4 Discussion
This study, based on the large-scale cohort data from the UK Biobank, is the first to systematically investigate the relationship between the TyG index and the risk and prognosis of IBD. It reveals a significant association between the TyG index and IBD, providing crucial evidence for the link between metabolic health and the pathogenesis of IBD. The findings indicate that, after adjusting for relevant covariates, the TyG index is positively associated with the risk of UC and CD. Specifically, for each 1-unit increase in the TyG index, the risk of UC and CD increases by 14.3% and 13.8%, respectively. This relationship was consistently observed not only in the analysis of continuous variables but also in the quartile and trend analyses, highlighting the potential value of the TyG index as a tool for assessing IBD risk.
The TyG index, calculated from fasting glucose and triglyceride levels, is commonly regarded as a biomarker of insulin resistance (24, 25). Our findings align with the recent research trends regarding the relationship between insulin resistance and IBD. A large cohort study reported an association between insulin resistance and an increased risk of IBD (9), which supports the context of our study. Moreover, the TyG index, as a simple and effective marker, has demonstrated significant utility in predicting and assessing MetS (26). Relevant studies suggest that MetS and IBD share similar pathophysiological characteristics, including inflammation, adipose tissue metabolism dysregulation, and immune response disorders (27, 28). Our research further extends this understanding, suggesting that even in individuals who do not meet the diagnostic criteria for MetS, subtle metabolic changes, as reflected by an elevated TyG index, may increase the risk of developing IBD. This finding is also of significant importance for the early prevention and risk management of IBD (29).
Some fundamental studies have indicated that insulin resistance is closely associated with an increase in the release of inflammatory factors, such as TNF-α and IL-6. It is believed that insulin resistance may alter gene expression within the NF-κB signaling pathway, leading to the upregulation of pro-inflammatory genes, thereby promoting a robust inflammatory response and immune activation (30). Furthermore, the enhanced release of inflammatory factors directly affects the inflammatory status of the intestinal mucosa, impacting the metabolism and function of intestinal epithelial cells, resulting in compromised intestinal barrier function and increased intestinal permeability, which may exacerbate the development of IBD (31). Moreover, chronic inflammation in IBD patients has been shown to exacerbate insulin resistance, creating a vicious cycle (32).
The mediation analysis in our study elucidated potential biological mechanisms underlying the association between the TyG and IBD. For CD, CRP exhibited the strongest mediating effect (28.13%, P < 0.001), highlighting the central role of systemic inflammation in the TyG-CD relationship, while white blood cell count, mean corpuscular hemoglobin, and total bilirubin mediation suggested the involvement of immune regulation, erythrocyte function, and oxidative balance in the pathophysiological link between TyG and CD (33, 34). For UC, neutrophil count emerged as the most significant mediator (30.97%, P < 0.001), aligning closely with the hallmark features of intestinal inflammation in UC, and the mediating roles of reticulocyte-related parameters indicated that erythropoietic abnormalities might represent another important pathway through which TyG influences UC pathogenesis. Notably, white blood cell count mediated associations of TyG with both CD and UC, suggesting immune system activation as a common pathway connecting insulin resistance to IBD (35), while the differences in primary mediators between CD and UC reflected subtype-specific pathological mechanisms. These findings not only deepen our understanding of the TyG-IBD association but also reveal a complex interaction network between metabolic dysregulation and intestinal inflammation, supporting the clinical value of monitoring and intervening in metabolic abnormalities among high-risk IBD populations, while simultaneously establishing a theoretical foundation for developing novel therapeutic strategies targeting these mediating pathways (32, 36).
This stratified analysis revealed that older age, female gender, and higher BMI populations exhibit a stronger correlation between UC and CD incidence and the TyG index. Moreover, a significant interaction was observed between the TyG index and gender in the onset of UC and CD. The following sections will elucidate the potential mechanisms underlying these associations. First, with increasing age, insulin sensitivity declines and metabolic function deteriorates. Older individuals are more prone to insulin resistance, which is closely associated with an elevated TyG index (37). Concurrently, immune function also decreases with age, potentially increasing susceptibility to IBD. Therefore, the impact of the TyG index on UC and CD is more pronounced in the elderly population. In addition, metabolic and immune responses differ between males and females. Estrogen plays a regulatory role in insulin sensitivity and lipid metabolism, potentially influencing the TyG index. Estrogen can enhance insulin signaling by activating the phosphoinositide 3-kinase (PI3K) and protein kinase B (Akt) pathways, which in turn promotes the phosphorylation of insulin receptor substrates (IRS) (38). Moreover, estrogen can activate AMP-activated protein kinase (AMPK), thereby increasing fatty acid oxidation and inhibiting the expression of lipogenic genes such as fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) (39). Estrogen also affects the gut microbiota and immune responses (40). These complex interactions may explain the significant gender-related interaction between the TyG index and the incidence of UC and CD.
The results of the non-linear analysis revealed a significant non-linear relationship between the TyG index and the risk of UC, characterized by an initial steep increase followed by a plateau. Although the non-linear relationship between the TyG index and CD risk did not reach statistical significance, graphical analysis suggested a trend of a gradual increase at first, followed by a sharper rise. These findings indicate that the impact of the TyG index on the risk of IBD may involve a threshold effect, where the risk becomes more pronounced within certain ranges of the TyG index. The identification of this non-linear relationship holds important clinical implications, as it suggests that the complex non-linearity should be taken into account when using the TyG index to assess IBD risk. This approach could facilitate more accurate identification of high-risk individuals and provide a foundation for developing personalized preventive strategies.
Research has demonstrated that higher TyG index levels are associated with an increased incidence of intestinal obstruction, fistula complications, and IBD-related surgeries in CD patients. This may result from the combined effects of exacerbated chronic inflammation, heightened oxidative stress, and accelerated fibrosis. Under conditions of insulin resistance, the secretion of pro-inflammatory cytokines, such as TNF-α and IL-6, is elevated, which may worsen chronic intestinal inflammation (41). Simultaneously, oxidative stress induced by hyperglycemia and hyperlipidemia may impair intestinal epithelial cells, disrupting the intestinal barrier function (42). Additionally, overexpression of insulin-like growth factor-1 (IGF-1) under insulin-resistant conditions may contribute to intestinal fibrosis (43). Therefore, in clinical practice, regular assessment of the metabolic status of IBD patients, including calculation of the TyG index, encouraging patients to improve their metabolic health through diet and exercise, and developing individualized treatment plans based on their metabolic status, may help prevent complications, reduce the need for surgery, and improve overall prognosis. Nevertheless, further prospective studies are required to validate these findings and to explore the specific effects of interventions targeting metabolic abnormalities in the management of IBD.
The primary strength of this study lies in its use of data from the UK Biobank, a large-scale, high-quality population-based cohort, which provides a substantial sample size and long follow-up period, offering robust statistical support for the findings. We employed a variety of statistical methods, including Cox regression, stratified analysis, interaction analysis, mediation analysis, and non-linear analysis, to comprehensively explore the relationship between the TyG index and IBD, as well as its related complications, from multiple perspectives. However, this study also has several limitations. As an observational study, although we identified a significant association between the TyG index and IBD risk, we are unable to establish a causal relationship between the two. Despite our efforts to adjust for multiple potential confounders, residual confounding factors such as unmeasured dietary patterns, environmental exposures, and genetic predispositions could still influence the observed association. The bidirectional relationship between metabolic dysregulation and inflammation also complicates causal inference in this context. Additionally, the UK Biobank primarily includes individuals of European descent, which limits the generalizability of the findings to other racial or ethnic groups. Finally, this study only considered the baseline TyG index, and thus did not capture the potential impact of temporal changes in the TyG index on IBD risk.
Based on the findings and limitations of this study, future research directions should include: conducting prospective cohort studies or randomized controlled trials to further validate the causal relationship between the TyG index and IBD risk; investigating the molecular and cellular mechanisms underlying the influence of the TyG index on IBD risk, particularly focusing on identified potential mediators such as CRP and inflammatory cell counts; assessing whether interventions that improve metabolic status (lowering the TyG index) can reduce IBD risk or improve disease outcomes; and evaluating the clinical utility of TyG index in IBD management through: (1) integration into risk prediction models for early IBD screening in high-risk populations, (2) use as a stratification tool for personalized treatment approaches, and (3) potential adoption as a monitoring parameter to assess treatment efficacy. These applications should be validated across diverse racial and ethnic populations, with longitudinal assessments capturing dynamic TyG changes. Implementation studies examining the feasibility and cost-effectiveness of incorporating TyG assessment into routine clinical practice would translate these findings into practical clinical protocols while maintaining the advantages of this simple, cost-effective biomarker derived from standard laboratory tests.
5 Conclusion
In summary, this study is the first to confirm a significant association between the TyG index and the risk of IBD in a large population. It also highlights the complexity and potential mechanisms underlying this relationship. These findings offer new insights into the pathogenesis of IBD, underscoring the importance of metabolic health in its prevention and management. Future research should further explore the causal relationship and clinical relevance of these findings, providing a scientific basis for the prevention, early diagnosis, and development of personalized treatment strategies for IBD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the North West Multi-center Research Ethics Committee, Manchester, U.K.(REC reference for UK Biobank 11/NW/0382). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
MZ: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. FH: Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. LM: Investigation, Methodology, Validation, Writing – original draft. FL: Data curation, Formal analysis, Methodology, Writing – original draft. MR: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The authors sincerely expressed their gratitude to all members who took part in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1548444/full#supplementary-material
References
1. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet (London England). (2017) 390:2769–78. doi: 10.1016/s0140-6736(17)32448-0
2. Burisch J and Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol. (2015) 50:942–51. doi: 10.3109/00365521.2015.1014407
3. White Z, Cabrera I, Kapustka I, and Sano T. Microbiota as key factors in inflammatory bowel disease. Front Microbiol. (2023) 14:1155388. doi: 10.3389/fmicb.2023.1155388
4. Santana PT, Rosas SLB, Ribeiro BE, Marinho Y, and de Souza HSP. Dysbiosis in inflammatory bowel disease: pathogenic role and potential therapeutic targets. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23073464
5. Mahmoud M and Syn WK. Impact of obesity and metabolic syndrome on IBD outcomes. Digest Dis Sci. (2024) 69:2741–53. doi: 10.1007/s10620-024-08504-8
6. Shen Z, Zhang M, Liu Y, Ge C, Lu Y, Shen H, et al. Prevalence of metabolic syndrome in patients with inflammatory bowel disease: a systematic review and meta-analysis. BMJ Open. (2024) 14:e074659. doi: 10.1136/bmjopen-2023-074659
7. Nagahori M, Hyun SB, Totsuka T, Okamoto R, Kuwahara E, Takebayashi T, et al. Prevalence of metabolic syndrome is comparable between inflammatory bowel disease patients and the general population. J Gastroenterol. (2010) 45:1008–13. doi: 10.1007/s00535-010-0247-z
8. Hyun CK. Molecular and pathophysiological links between metabolic disorders and inflammatory bowel diseases. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22179139
9. Doğan AN, Kahraman R, and Akar T. Evaluation of insulin resistance and beta cell activity in patients with inflammatory bowel disease. Eur Rev Med Pharmacol Sci. (2022) 26:3989–94. doi: 10.26355/eurrev_202206_28969
10. Alizargar J, Bai CH, Hsieh NC, and Wu SV. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. (2020) 19:8. doi: 10.1186/s12933-019-0982-2
11. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1210/jc.2010-0288
12. Zhang M, Wang B, Liu Y, Sun X, Luo X, Wang C, et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: The Rural Chinese Cohort Study. Cardiovasc Diabetol. (2017) 16:30. doi: 10.1186/s12933-017-0514-x
13. Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, and Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. (2016) 46:189–97. doi: 10.1111/eci.12583
14. Trehearne A. Genetics, lifestyle and environment. UK Biobank is an open access resource following the lives of 500,000 participants to improve the health of future generations. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. (2016) 59:361–7. doi: 10.1007/s00103-015-2297-0
15. Elliott P and Peakman TC. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. (2008) 37:234–44. doi: 10.1093/ije/dym276
16. Tao LC, Xu JN, Wang TT, Hua F, and Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. (2022) 21:68. doi: 10.1186/s12933-022-01511-x
17. Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. BioMed Environ Sci. (2002) 15:83–96.
18. Thompson DJ, Wells D, Selzam S, Peneva I, Moore R, Sharp K, et al. A systematic evaluation of the performance and properties of the UK Biobank Polygenic Risk Score (PRS) Release. PloS One. (2024) 19:e0307270. doi: 10.1371/journal.pone.0307270
19. Shivappa N, Steck SE, Hurley TG, Hussey JR, and Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/s1368980013002115
20. Azarmanesh D, Bertone-Johnson ER, Pearlman J, Liu Z, and Carbone ET. Association of the dietary inflammatory index with depressive symptoms among pre- and post-menopausal women: findings from the national health and nutrition examination survey (NHANES) 2005-2010. Nutrients. (2022) 14. doi: 10.3390/nu14091980
21. de Castro Mendes F, Paciência I, Cavaleiro Rufo J, Silva D, Cunha P, Farraia M, et al. The inflammatory potential of diet impacts the association between air pollution and childhood asthma. Pediatr Allergy Immunol. (2020) 31:290–6. doi: 10.1111/pai.13185
22. Shafiee NH, Razalli NH, Shahril MR, Muhammad Nawawi KN, Mohd Mokhtar N, Abd Rashid AA, et al. Dietary inflammatory index, obesity, and the incidence of colorectal cancer: findings from a hospital-based case-control study in Malaysia. Nutrients> . (2023) 15. doi: 10.3390/nu15040982
23. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, and Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. (2008) 61:344–9. doi: 10.1016/j.jclinepi.2007.11.008
24. Simental-Mendía LE, Rodríguez-Morán M, and Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndrome Relat Disord. (2008) 6:299–304. doi: 10.1089/met.2008.0034
25. Du T, Yuan G, Zhang M, Zhou X, Sun X, and Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. (2014) 13:146. doi: 10.1186/s12933-014-0146-3
26. Wei X, Min Y, Song G, Ye X, and Liu L. Association between triglyceride-glucose related indices with the all-cause and cause-specific mortality among the population with metabolic syndrome. Cardiovasc Diabetol. (2024) 23:134. doi: 10.1186/s12933-024-02215-0
27. Wen Z and Fiocchi C. Inflammatory bowel disease: autoimmune or immune-mediated pathogenesis? Clin Dev Immunol. (2004) 11:195–204. doi: 10.1080/17402520400004201
28. Kredel L, Batra A, and Siegmund B. Role of fat and adipokines in intestinal inflammation. Curr Opin Gastroenterol. (2014) 30:559–65. doi: 10.1097/mog.0000000000000116
29. Son DH, Lee HS, Lee YJ, Lee JH, and Han JH. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutri metabol Cardiovasc Dis: NMCD. (2022) 32:596–604. doi: 10.1016/j.numecd.2021.11.017
30. Slattery ML, Mullany LE, Sakoda L, Samowitz WS, Wolff RK, Stevens JR, et al. The NF-κB signalling pathway in colorectal cancer: associations between dysregulated gene and miRNA expression. J Cancer Res Clin Oncol. (2018) 144:269–83. doi: 10.1007/s00432-017-2548-6
31. Lin CJ, Chu NF, Hung YJ, Chang JB, He CT, Hsiao FC, et al. The association of retinol-binding protein 4 with metabolic syndrome and obesity in adolescents: the effects of gender and sex hormones. Clin Pediatr (Phila). (2013) 52:16–23. doi: 10.1177/0009922812459948
32. Karaahmet F, Basar O, Coban S, and Yuksel I. Dyslipidemia and inflammation in patients with inflammatory bowel disease. Digest Dis Sci. (2013) 58:1806–7. doi: 10.1007/s10620-013-2614-0
33. Demirci-Çekiç S, Özkan G, Avan AN, Uzunboy S, Çapanoğlu E, and Apak R. Biomarkers of oxidative stress and antioxidant defense. J Pharm BioMed Anal. (2022) 209:114477. doi: 10.1016/j.jpba.2021.114477
34. Oustamanolakis P, Koutroubakis IE, and Kouroumalis EA. Diagnosing anemia in inflammatory bowel disease: beyond the established markers. J Crohn’s Colitis. (2011) 5:381–91. doi: 10.1016/j.crohns.2011.03.010
35. Lee CJ, Sears CL, and Maruthur N. Gut microbiome and its role in obesity and insulin resistance. Ann New York Acad Sci. (2020) 1461:37–52. doi: 10.1111/nyas.14107
36. Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, et al. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. (2022) 16:102581. doi: 10.1016/j.dsx.2022.102581
37. Motobayashi M, Matsuoka K, Takenaka K, Fujii T, Nagahori M, Ohtsuka K, et al. Predictors of mucosal healing during induction therapy in patients with acute moderate-to-severe ulcerative colitis. J Gastroenterol Hepatol. (2019) 34:1004–10. doi: 10.1111/jgh.14565
38. Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, et al. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes. (2006) 55:2001–14. doi: 10.2337/db05-1692
39. D’Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, and Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. (2005) 280:35983–91. doi: 10.1074/jbc.M507339200
40. Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. (2016) 7:313–22. doi: 10.1080/19490976.2016.1203502
41. Halim M and Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr. (2019) 13:1165–72. doi: 10.1016/j.dsx.2019.01.040
42. Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. (2007) 282:35279–92. doi: 10.1074/jbc.M706762200
43. Macias-Ceja DC, Mendoza-Ballesteros MT, Ortega-Albiach M, BarraChina MD, and Ortiz-Masià D. Role of the epithelial barrier in intestinal fibrosis associated with inflammatory bowel disease: relevance of the epithelial-to mesenchymal transition. Front Cell Dev Biol. (2023) 11:1258843. doi: 10.3389/fcell.2023.1258843
Keywords: inflammatory bowel disease, triglyceride-glucose index, UK Biobank, cohort study, biomarker
Citation: Zhang M, Hu F, Miao L, Li F and Rao M (2025) Evaluating the triglyceride-glucose index as a biomarker for inflammatory bowel disease risk: insights from a UK Biobank prospective study. Front. Immunol. 16:1548444. doi: 10.3389/fimmu.2025.1548444
Received: 19 December 2024; Accepted: 04 August 2025;
Published: 27 August 2025.
Edited by:
Olga Maria Nardone, Federico II University Hospital, ItalyReviewed by:
Mingming Zhang, Central South University, ChinaShi Xue Dai, Guangdong Provincial People’s Hospital, China
Alessia La Mantia, University of Naples Federico II, Italy
Copyright © 2025 Zhang, Hu, Miao, Li and Rao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Rao, cmFvbWluQGpsdS5lZHUuY24=
 Manli Zhang
Manli Zhang Feng Hu
Feng Hu Ling Miao
Ling Miao Fan Li
Fan Li Min Rao
Min Rao