- 1Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Debremarkos University, Debre Markos, Ethiopia
- 2Department of Pharmacy, College of Medicine and Health Sciences, Debremarkos University, Debre Markos, Ethiopia
- 3Department of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 4Department of Internal Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Background: Hepatitis B virus (HBV) is a major global public health issue and the most common etiology of chronic liver disease (CLD). The relationship between Helicobacter pylori and HBsAg+ patients was not well investigated and has attracted much scientific and clinical interest, although the relationship remains controversial.
Objective: This study aimed to assess the clinical and biochemical characteristics of HBsAg+ liver disease patients with and without H. pylori infection.
Methods: From April 1, 2021, to March 30, 2022, a hospital-based cross-sectional study was done at the University of Gondar Comprehensive Specialized Hospital on 384 known HBsAg+ liver disease patients recruited using a convenient sampling technique. All the HBsAg+ patients were tested for fecal H. pylori antigen, and blood specimens were analyzed for ALT, AST, ALP, ALB, TP, BILT, TG, and TChol tests using an automated biochemistry analyzer. GraphPad Prism 8.02 and SPSS 25 were used for data analysis, considering a statistically significant P-value of 0.05.
Results: H. pylori co-infection was found in 153 (39.8%) of HBsAg+ study participants. ALT, AST, and total cholesterol mean levels were significantly higher in patients co-infected with H. pylori (p<0.04). Portal hypertension (47.8%), variceal bleeding (60.7%), and hepatocellular carcinoma (HCC) (57.5%) were more common (p< 0.01) in patients with HBV and H. pylori co-infection.
Conclusions: ALT, AST, and TChol mean levels were higher in H. pylori co-infected HBsAg+ patients. Our findings showed that H. pylori has a role in the elevation of clinical and biochemical parameters in HBsAg+ liver diseases.
Introduction
Hepatitis B virus (HBV) is a partially double-stranded hepatotropic DNA virus (1). It is an enveloped DNA virus that belongs to the family Hepadnaviridae, genus Orthohepadnavirus (2). The envelope surrounds an icosahedral nucleocapsid, which encloses a partially double-stranded, relaxed circular DNA (rcDNA) genome of ~3.2 kilobases (3). Four partially overlapping open reading frames (ORFs), termed P (polymerase), S (surface), C (core), and X (HBx protein), define the coding capacity of the HBV genome (4). Mutations in the HBV genome have been observed in all four ORFs in both acute and chronic HBV-infected individuals (5). A total of ten different genotypes of HBV (A-J) have been reported worldwide (6).
Acute HBV infection in healthy and immune-competent adults causes self-restrictive disease with less than 2-3% progression to serum hepatitis B surface antigen-positive (HBsAg +) chronic hepatitis B (CHB) infections (7). The reason behind the adaptive replication of HBV in hepatocytes is mainly due to a lack of functional innate DNA sensing pathways to recognize, control, and clear the virus (8). The adaptive immune response mediates both viral clearance and liver damage, but HBV appears to cause little or no innate immune activation (9). Both the inability of the immune system to resolve CHB and the unique replication strategy of HBV to form a stable covalently closed circular DNA (cccDNA) minichromosome in the hepatocyte nucleus enable infection persistence (10).
The natural course of CHB infection can be divided into four chronological phases based on virus-host interactions: The immune-tolerance phase is characterized by active replication of HBV, HBV e antigen (HBeAg) positivity, and normal alanine transferase (ALT) levels. In the immune clearance phase, HBeAg-positive patients have elevated serum ALT levels and fluctuating HBV-DNA levels. The third stage is the inactive carrier state, in which patients clear HBeAg and develop the corresponding antibody to HBeAg (HBeAg seroconversion), with the remission of liver disease (11). Approximately 20 −30% of individuals in the inactive carrier state may experience a viral relapse and enter the reactivation phase (fourth stage) during follow-up (12, 13).
HBV is not directly cytopathic for hepatocytes. Unlike other viruses, such as hepatitis C virus (HCV) or human immunodeficiency virus (HIV), which enter a rapid phase of propagation after infection, acute HBV infection is preceded by low HBV DNA and antigens in serum and liver for many weeks before the subsequent amplification and spreading phase of HBV infection (14). The virus adapts to different mechanisms for persistent infection and is thought to be a ‘stealth virus’, which poorly induces the expression of type 1 interferon (15).
Liver cirrhosis is a diffuse condition characterized by fibrosis and nodule formation, with CHB virus infection as one of the causes (16). The dysfunctional immune responses play an essential role in persistent HBV infection as well as liver inflammation (17). When comparing the characteristics of immune responses in acute and chronic hepatitis B, Chronic HBV infection develops due to the failure of HBV-specific immune responses (18). Chronic infection of HBV revealed impaired dendritic cell (DC) function, which is reflected by the weakness of both T-cell and B-cell virus-specific immune responses in CHB patients (19).
Persistent exposure of T cells to HBV antigens is crucial for maintaining depressed T cell functionality (20). The quantitative and functional deficiencies of the HBV-specific T-cell response are well-acknowledged as a primary contributor to viral persistence (21). The HBV-specific T cell response is modulated during HBV infection by multi-factorial mechanisms, including programmed cell death 1 (PD-1) expression, IL-10, arginase, myeloid suppressor cells, and T regulatory cells (22).
The humoral immune responses will hand over the virus after release from hepatocytes (23). The released antigens (HBsAg, HBeAg, and HBcAg) will induce the production of their respective antibodies. Hepatitis B core antigen (HBcAg) will not circulate in the blood as it is found in the core of the virus. Anti-HBc antibodies are used to differentiate between acute and chronic HBV infections (24).
H. pylori causes both active and chronic infection and can lead to several disorders, from chronic gastritis to gastric adenocarcinoma, and activates both innate and adaptive immune responses, but the response fails to eradicate the infection (25). Although it is believed that H. pylori is a type of ‘commensal bacterium”, it cannot be classified as normal flora because all patients with gastro-duodenal H. pylori colonization show histological gastroenteritis (26). To complete the colonization process and cause harm to the gastric mucosa, the bacterium must overcome the stomach acid barrier and infiltrate the mucus layer (27). Gastric epithelial cells (GECs) are a primary target for H. pylori infection and actively contribute to the development of acute and chronic inflammation (28).
H. pylori may induce immunosuppressive Tregs, dampening antiviral immune responses against HBV and facilitating viral persistence. Chronic H. pylori infection could also exacerbate T-cell exhaustion, reducing control of HBV replication and increasing liver damage (29, 30). The greatest feature of H. pylori is its ability to last for years in the gastric epithelium of the host (31). This adaptive property occurs due to a complex mechanism of H. pylori persistence mediated by proteins, glycoconjugates, and lipids exposed on the surface of this bacterium (32). Gastric innate immune effectors can either eliminate the bacteria or mobilize adaptive immune responses (e.g., Toll-like receptors (TLRs) and cytosolic DNA sensor/adaptor proteins) (33). Recent studies have shown that H. pylori infection most often results in M1 (Inflammatory) and Mreg (Regulatory) macrophage activation (34). The recruited macrophages at the site of infection can produce IL-12, which stimulates T-helper 1(Th1) cells and the production of cytokines such as IFN-γ (35).
H. pylori escapes identification by pattern recognition receptors (PRR) by multiple methods, including avoidance of recognition by TLRs and inhibition of c-type lectin and Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) mediated signaling (36). Although the acquired immune response to H. pylori is composed of both Th1- and Th2-type cells, cytokine profiles indicate a predominance of a Th1 response (37). Although H. pylori is an extracellular pathogen, its immune response is biased to the Th1 type (38).
In recent years, H. pylori has been reported to be associated with the development of a variety of extra-digestive manifestations, including type 2 diabetes, and cardiovascular and liver diseases (39). Chronic H. pylori infection induces a Th1-mediated inflammatory response, potentially contributing to systemic inflammation that could exacerbate liver injury. Virulence factors (e.g., CagA, VacA) may enter the bloodstream, promoting oxidative stress and pro-inflammatory cytokine release, which might accelerate liver fibrosis or carcinogenesis. This study tried to evaluate the burden and clinical contribution of H. pylori infection on the outcome of HBV-related liver diseases in Northwest Ethiopia.
The relationship between H. pylori and liver diseases remains controversial. Recent studies have been trying to investigate the association of H. pylori in the progression of diseases other than gastrointestinal diseases (40). The presence of Helicobacter species has been reported in the hepatic tissues of patients with different hepatic disorders. Chronic inflammation from H. pylori might promote fibrosis progression to cirrhosis and HCC. However, confounders like viral hepatitis or environmental toxins complicate these associations (41). H. pylori has been implicated in some extra-digestive diseases such as cardiovascular, neurologic, and hepatobiliary conditions (42). H. pylori has the induction potential of regulatory B cells, suggesting its role in diminishing the response of effector B cells during HBV infections (43).
H. pylori infection in patients with liver cirrhosis may impact the exacerbation of inflammatory injuries in the stomach, which could directly or indirectly lead to a deficiency of liver function (44). Research conducted in China also showed that the H. pylori positivity rate is high among patients with CHB, and H. pylori is a putative risk factor in the development of HBV-related complications. This is because H. pylori reaches the liver via the bloodstream or the biliary system and then becomes an independent etiological factor causing inflammation (45).
In H. pylori-infected individuals, a Th2 cell-related response induces IgG1 production while a Th1-related response contributes to IgG2 production through IL-2 and IFN-γ secretion (46). Helicobacter species are regarded as important in the development of hepatobiliary illnesses because they can produce inflammatory, fibrotic, and necrotic damage to the liver, which can progress to HCC (47). In the present study, we tried to assess the interplay of HBV and H. pylori infections on CHB-related liver disease progression.
Materials and methods
Study area, design, and period
The study was conducted at the University of Gondar Comprehensive Specialized Hospital, located in Gondar city, Amhara National Regional State, Northwest Ethiopia. Gondar City is 738km Northwest of Addis Ababa, the capital city of Ethiopia. Based on the Central Statistical Agency of Ethiopia (CSA), Gondar City had a total population of 500,788, of whom 300,000 were men and 200,788 were women (48). The University of Gondar Comprehensive Specialized Hospital is a tertiary care center serving approximately 7 million people in Northwest Ethiopia. A hospital-based cross-sectional study was conducted from April 1, 2021, to March 30, 2022. It was a single-center institution-based study.
Source and study populations
All HBsAg+ liver disease patients attending the University of Gondar Comprehensive Specialized Hospital. HBsAg+ patients presented at the University of Gondar Comprehensive Specialized Hospital during the study period and were eligible to study.
Inclusion criteria exclusion criteria
Patients who were positive for HBsAg and with the age of above 18 years old were included in the current study. We excluded patients with clinically confirmed schistosomiasis, and viral hepatitis other than HBV, HIV, and pregnancy.
Sample size and sampling technique
The sample size was determined using the single population proportion formula by considering the prevalence of H. pylori in HBsAg+ patients =50% (p=0.5), Z /2 = 1.96, and margin of error =5% (d=0.05). Finally, the total sample size became 384 HBsAg+ individuals who were recruited using a convenient sampling technique.
Data collection and laboratory methods
Demographic and clinical data collection
Demographic and clinical data were collected following the approval of the study protocol by the ethical review committee of the University of Gondar. At each data collection unit, trained nurses collected all relevant information (demographic, clinical) using a structured questionnaire in a face-to-face interview with the patient and from the patient’s medical records. During clinical data collection, the patients were enrolled from the inpatient and outpatient departments of the gastroenterology clinic at the University of Gondar Comprehensive Specialized Hospital. The clinical parameters, such as portal hypertension, hepatocellular carcinoma (HCC), variceal bleeding, and hepatic decompensation, were defined based on the radiological (ultrasound, endoscopy/colonoscopy) and pathology (biopsy) findings.
Specimen collection and processing
We collected about eight milliliters (8 mL) of venous blood by serum separator tubes (SST) from the forearms of all eligible patients. The serum was isolated from the collected blood samples by centrifuging at 3500 rpm for 5 minutes in the serology laboratory and separated into different serum test tubes. The first serum tube (4 ml) was transported to the clinical chemistry lab for biochemical testing. For further serological and virological examination, the second serum was transferred to cryotubes and stored at -80°C.
In addition, a stool sample was collected from each CLD patient included in this study for the identification of the H. pylori fecal antigen (RAPID Hp StAR test).
Biochemical assays
The mean concentrations of alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), serum albumin (ALB), total protein (TP), total bilirubin (BILT), total cholesterol (TChol) and triglycerides (TG) were analyzed by using automated DxC AU 700 Chemiluminescence assay (BECKMAN COULTER, Ireland Inc., Lismeehan, O’Callaghan’s Mills, Co. Clare, Ireland) following the manufacturer’s instructions. Both enzymatic and non-enzymatic assays were performed by the same automated biochemistry analyzer.
Data quality control
Study site assessment and pretest of the tools and assays were done before data collection to optimize the experimental setup. Data collectors were given appropriate training about data collection and related procedures. Positive and negative controls were run against HBsAg and H. pylori tests. Patient samples were collected, shipped, and stored under appropriate conditions and strict supervision. Standard operating procedures (SOPs) and optimization protocols were followed during serological and virological assays.
Data analysis
Data entry was done via Microsoft Excel 2013. Normally distributed data were expressed as mean ± standard deviation, and skewed data were expressed as median (interquartile range, IQR). Statistical analyses were performed using SPSS 25.0 (IBM, New York) or GraphPad Prism 8.0.2. Categorical variables were analyzed by the Chi-square test, and nonparametric tests (Mann-Whitney test) were used for non-normal continuous data, by taking a P-value of<0.05 as statistically significant.
Ethical considerations
The study protocol was reviewed ethically and approved by the ethical committee of the School of Biomedical and Laboratory Sciences (SBLS) at the University of Gondar. Ethical clearance with a reference number (Ref. No. SBMLS/2759) was obtained. Study participants were asked for their consent to be included in the study. Study participants who tested positive for HBsAg and/or H. pylori during screening were linked to healthcare providers of the UoG hospital. All the information obtained from the study participants was coded to maintain confidentiality.
Results
Demographic characteristics of the study participants
From a total of 384 HBsAg+ adults enrolled in this study, 153 participants were H. pylori co-infected (39.8%). 259 (67.4%) subjects were males, and 268 (69.8%) of them were rural residents. Overall, 47.1% of subjects were unable to read and write, and 221 (57.6%) of them were farmers. Overall, 317 (82.6%) participants were married. Three hundred thirty-nine (88.3%) of participants were not alcohol users (Table 1).
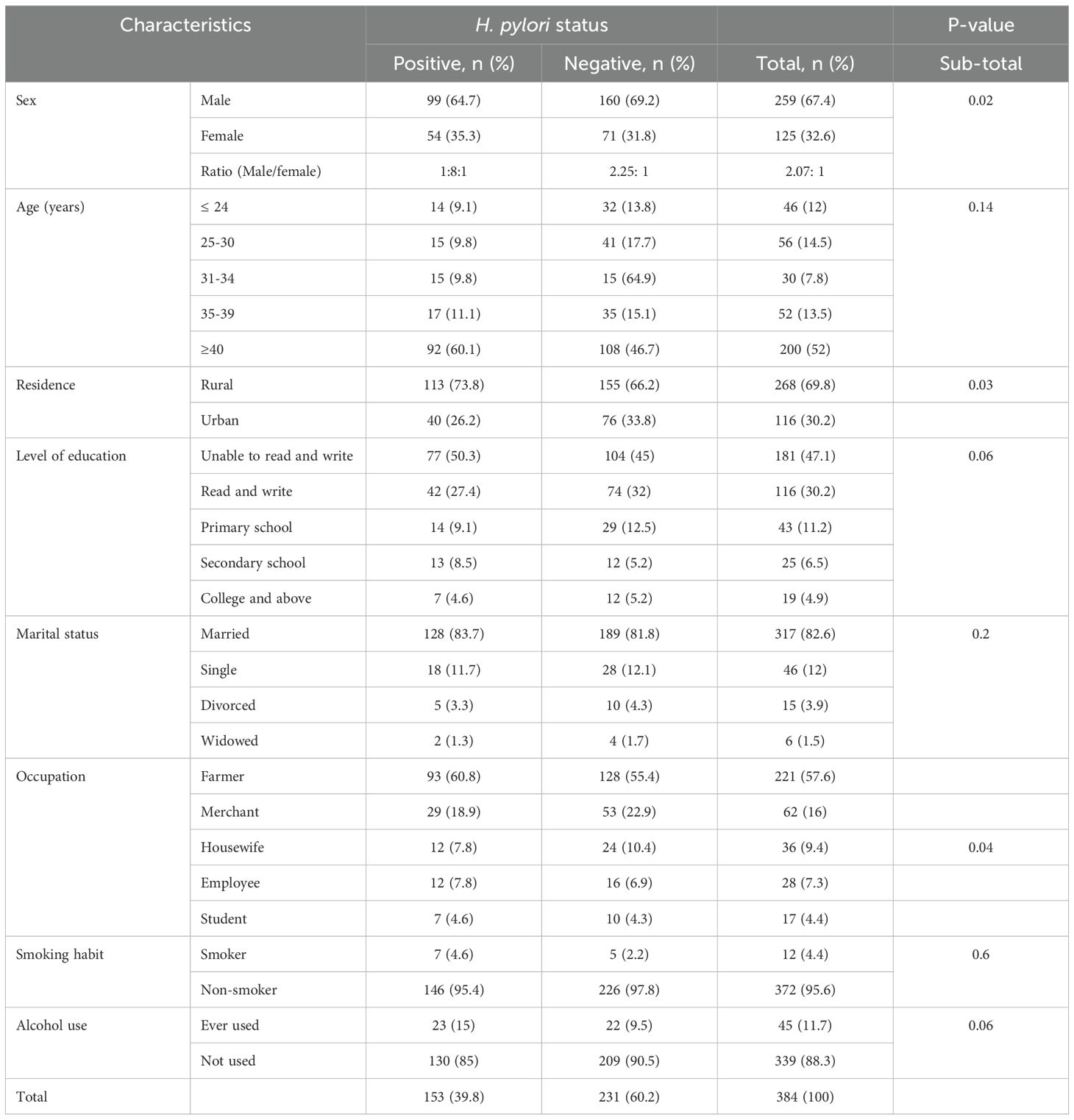
Table 1. Socio-demographic characteristics of HBsAg+ study participants with and without H. pylori at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2022.
Clinical and biochemical features of study participants
Among 384 HBsAg+ study participants, 83 (21.6%) patients used traditional medicine in different forms or preparations. Regarding their HBV treatment status, 106 (27.6%) patients were treatment-naïve. The treatment status and traditional medicine use of HBsAg+ patients showed no significant differences between the two groups of participants. Portal hypertension (47.8%), variceal bleeding (60.7%), and hepatocellular carcinoma (HCC) (57.5%) had a significant association with H. pylori co-infection (p<0.01) (Table 2).
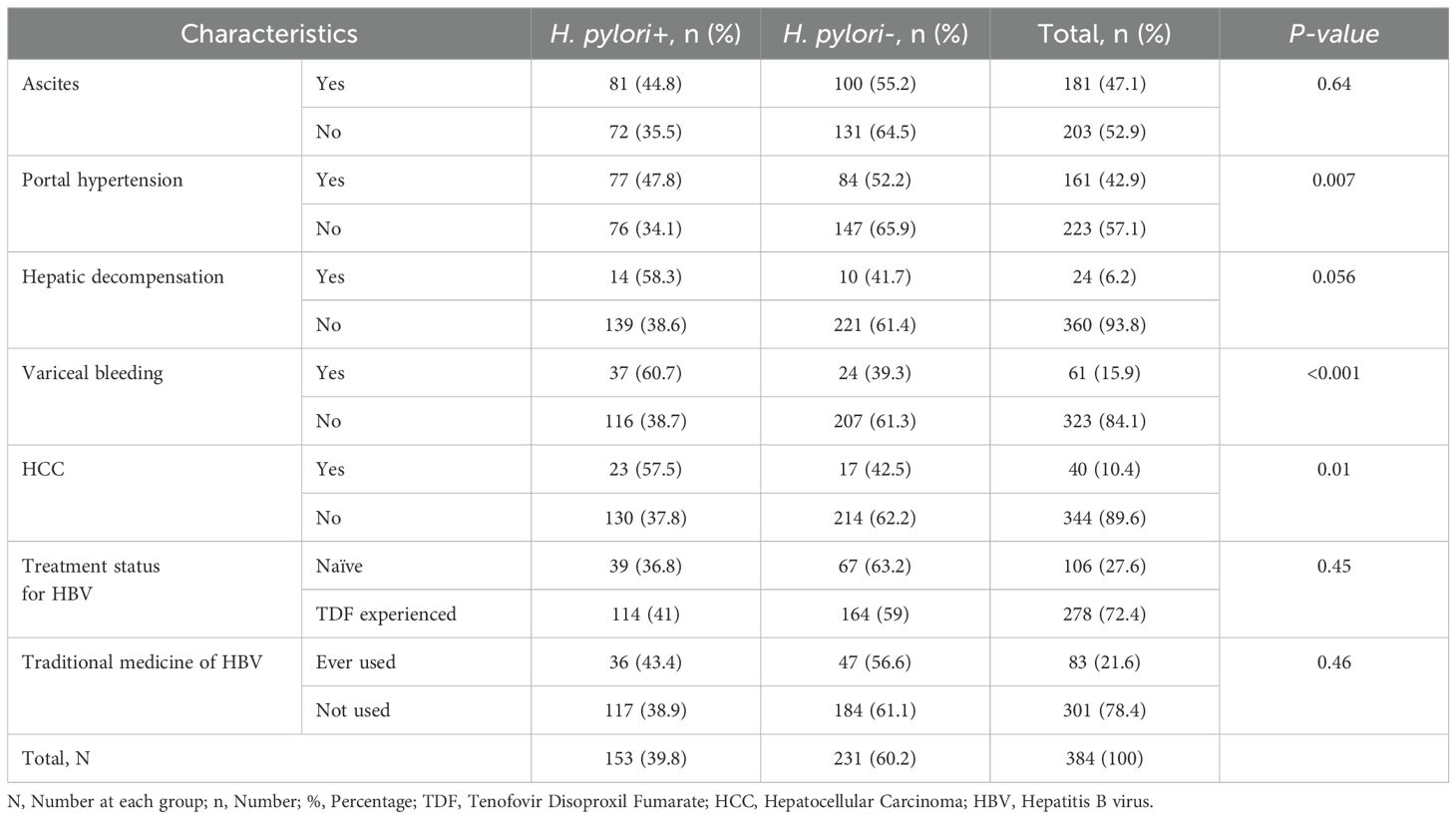
Table 2. Clinical characteristics of 384 HBsAg+ study participants with and without H. pylori at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2022.
Among the biochemical parameters, ALT, AST, and TChol levels were significantly higher in the H. pylori co-infected patient group (p<0.01). Although there was a difference in the mean value of ALP, ALB, TP, and BILT in the two groups, the difference was not statistically significant. Overall, the mean concentrations of liver enzymes and proteins were higher in the H. pylori co-infected group of patients (Table 3).
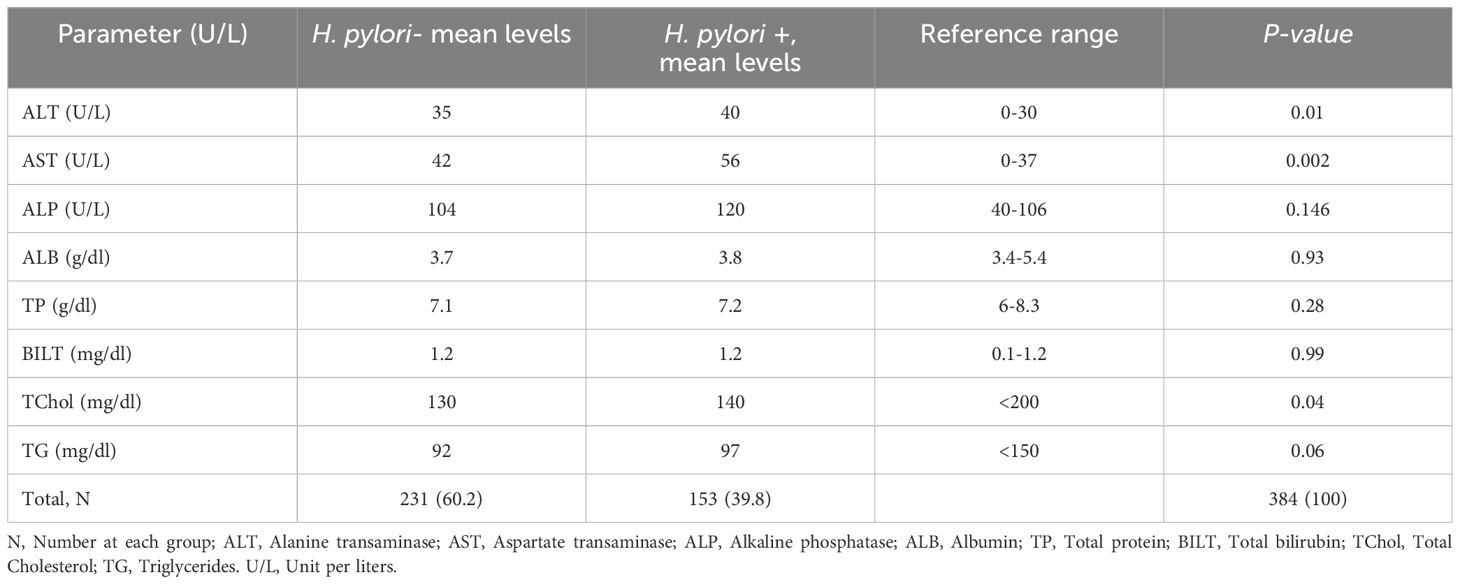
Table 3. Comparison of Biochemical Tests in 384 HBsAg+ Study Participants with and without H. pylori co-infection at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2022.
Discussions
Infections with HBV and H. pylori are two major public health issues in Ethiopia. Epidemiological studies in Ethiopia have reported a high prevalence of both infections, but there were no previously published reports on the relationship between HBV and H. pylori in liver disease patients and the contribution of H. pylori co-infection, particularly in Northwestern Ethiopia. Assessing the clinical and laboratory features of liver disease patients infected with these important pathogens is crucial for understanding and managing the disease progression in HBV-liver disease. In the present study, we tried to characterize the clinical, serological, and virological features of HBsAg+ liver disease patients with and without H. pylori co-infection.
In the current study, the prevalence of H. pylori infection (39.8%) among HBsAg+ patients appeared lower than the overall pooled prevalence in the general Ethiopian population (52.2%) (49). However, the prevalence of H. pylori infection in the current study population was comparable to similar studies on HBV-related liver disease patients (40, 50). Previous studies reported a higher H. pylori prevalence in the general population than in liver disease patients. This discrepancy could be due to the repeated exposure of patients to different antibiotics during hospital visits, variations in the sensitivity of fecal H. pylori antigen tests, and the time trend of studies showing a decreasing pattern of H. pylori infection.
H. pylori colonization of the liver may occur through bacterial translocation from the stomach through the portal system, especially in the advanced stages of liver disease when portal hypertension develops. Furthermore, the bacteria can reach the liver via circulating phagocytes and macrophages. The present study was in agreement with previous studies that demonstrated that post-inflammatory liver cirrhosis complications were more frequent in H. pylori-infected patients than in the H. pylori-negative patient group, as H. pylori could upregulate the expression of various inflammatory factors (50–52). In our study population, HBV-induced complications of liver cirrhosis, such as portal hypertension, variceal bleeding, and HCC, were more prevalent in H. pylori co-infected patients than in the HBV-mono-infected group of patients. In this study, hepatic decompensation was also higher among the co-infected group, although it showed no significant difference. These clinical findings confirm our hypothesis that H. pylori co-infection has a synergistic effect on the progression of CHB-related liver diseases (50).
Liver enzymes and non-enzymatic biochemical markers of liver function had been increased in our study subjects who had H. pylori co-infection, in line with similar studies (53). In this study, ALT, AST, and TChol levels were significantly higher in the H. pylori co-infected group of patients, while TG and ALP levels were slightly elevated in the same group of patients. Our results support the theory that liver colonization with H. pylori bacteria promotes liver function deterioration via toxic injury and autoimmune inflammation. These findings align with studies reporting the role of H. pylori in CHB disease progression (54, 55).
There were several limitations in this study. First thing, we had no access to molecular, experimental, and immunological assays. We just used a cross-sectional study design with convenience sampling techniques. We did not use quantitative assays to measure the level of fecal antigen for the diagnosis of H. pylori infection. Consequently, the exact change of values might not be revealed in the HBV/H. pylori co-infected and HBV mono-infected patients. Saying all these, this study provided insight into the contribution of H. pylori infection among HBV-infected liver disease patients.
Conclusions and recommendations
The ALT, AST, and TChol levels were increased during H. pylori co-infection. Our data also showed that clinical parameters, including portal hypertension, variceal bleeding, and hepatocellular carcinoma (HCC), were more frequently detected in HBsAg/H. pylori co-infected patients than those with HBsAg mono-infection. From the findings of the current study, it can be concluded that H. pylori significantly enhances the clinical, biochemical, and serological parameters during the co-infection of H. pylori with HBsAg+ liver disease patients. Physicians who treat liver disease patients are highly recommended to screen and treat patients with H. pylori co-infection. We propose active screening for H. pylori in patients with CHB. Virological markers of HBV should also be closely monitored during the treatment of CHB-related liver diseases. We would also like to recommend that future researchers conduct large-scale studies that include advanced laboratory methods with an adequate sampling of patients to assess the accurate implications of all test parameters of CHB-related liver diseases co-infected with H. pylori bacteria. Besides, it would be imperative that future studies include molecular and immunological methods with a longitudinal cohort of patients and advanced statistical methods.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study protocol was reviewed ethically following the Helsinki Declaration and approved by the ethical committee of the School of Biomedical and Laboratory Sciences (SBLS) at the University of Gondar. Ethical clearance with a reference number (Ref. No. SBMLS/2759) was obtained. Study participants were asked for their consent to be included in the study. Study participants who tested positive for HBsAg and/or H. pylori during screening were linked to healthcare providers of the UoG hospital. All the information obtained from the study participants was coded to maintain confidentiality.
Author contributions
MB: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BT: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ML: Conceptualization, Data curation, Formal Analysis, Resources, Supervision, Writing – original draft, Writing – review & editing. ZA: Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. YB: Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to express our gratitude to the Department of Immunology and Molecular Biology, School of Biomedical and Laboratory Sciences (SBLS), College of Medicine and Health Sciences (CMHS), University of Gondar, for allowing me to perform this study in my field of interest.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALB, Albumin; ALP, Alkaline Phosphatase; ALT, Alanine Transaminase; AST, Aspartate Transaminase; BILT, Bilirubin Total; cccDNA, Covalently Closed Circular DNA; CHB, Chronic Hepatitis B; CLD, Chronic Liver Disease; DBIL, Direct Bilirubin; ELISA, Enzyme-Linked Immunosorbent Assay; GI, Gastrointestinal; HCC, Hepatocellular Carcinoma; H. Pylori, Helicobacter pylori; INF, Interferon; MAMPs, Microbe-associated Molecular Patterns; NAFLD, Non-alcoholic Fatty Liver Disease; PRR, Pathogen Recognition Receptors; PUD, Peptic Ulcer Disease; PCR, Polymerase Chain Reaction; RcDNA, Relaxed Circular DNA; TLR, Toll-like Receptor; Treg, Regulatory T cells; TP, Total Protein.
References
1. Valaydon ZS and Locarnini SA. The virological aspects of hepatitis B. Best Pract Res Clin Gastroenterol. (2017) 31:257–64. doi: 10.1016/j.bpg.2017.04.013
2. Sajid M, Ullah H, Yan K, He M, Feng J, Shereen MA, et al. The functional and antiviral activity of interferon alpha-inducible IFI6 against hepatitis B virus replication and gene expression. Front Immunol. (2021) 12:1028. doi: 10.3389/fimmu.2021.634937
3. Xi J, Luckenbaugh L, and Hu J. Multiple roles of PP2A binding motif in hepatitis B virus core linker and PP2A in regulating core phosphorylation state and viral replication. PloS Pathog. (2021) 17:e1009230. doi: 10.1371/journal.ppat.1009230
4. Yuan S, Liao G, Zhang M, Zhu Y, Wang K, Xiao W, et al. Translatomic profiling reveals novel self-restricting virus-host interactions during HBV infection. J Hepatol. (2021) 75:74–85. doi: 10.1016/j.jhep.2021.02.009
5. Adesina OA, Akanbi OA, Opaleye OO, Japhet MO, Wang B, Oluyege AO, et al. Detection of Q129H immune escape mutation in apparently healthy hepatitis B virus carriers in southwestern Nigeria. Viruses. (2021) 13:1273. doi: 10.3390/v13071273
6. Song H, Xu F, Xiao Q, and Tan G. Hepatitis B virus X protein and its host partners. Cell Mol Immunol. (2021) 18:1345–6. doi: 10.1038/s41423-021-00674-z
7. Joshi SS and Coffin CS. Hepatitis B and pregnancy: virologic and immunologic characteristics. Hepatol Commun. (2020) 4:157–71. doi: 10.1002/hep4.1460
8. Golsaz-Shirazi F and Shokri F. Cross talk between hepatitis B virus and innate immunity of hepatocytes. Rev Med Virol. (2022) 32(1):e2256. doi: 10.1002/rmv.2256
9. Iannacone M and Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol. (2022) 22:19–32. doi: 10.1038/s41577-021-00549-4
10. Dandri M. Epigenetic modulation in chronic hepatitis B virus infection. Semin Immunopathology. (2020) 42:173–85. doi: 10.1007/s00281-020-00780-6
11. Block TM, Chang K-M, and Guo J-T. Prospects for the global elimination of hepatitis B. Annu Rev Virol. (2021) 8:437–58. doi: 10.1146/annurev-virology-091919-062728
12. Cai Y and Yin W. The multiple functions of B cells in chronic HBV infection. Front Immunol. (2020) 11:582292. doi: 10.3389/fimmu.2020.582292
13. Campos-Valdez M, Monroy-Ramírez HC, Armendáriz-Borunda J, and Sánchez-Orozco LV. Molecular mechanisms during hepatitis B infection and the effects of the virus variability. Viruses. (2021) 13:1167. doi: 10.3390/v13061167
14. Cheng X, Xia Y, Serti E, Block PD, Chung M, Chayama K, et al. Hepatitis B virus evades innate immunity of hepatocytes but activates cytokine production by macrophages. Hepatology. (2017) 66:1779–93. doi: 10.1002/hep.29348
15. Megahed FAK, Zhou X, and Sun P. The interactions between HBV and the innate immunity of hepatocytes. Viruses. (2020) 12:285. doi: 10.3390/v12030285
16. Mehrabi S, Genco E, Maturi M, and D’Onofrio M. Chronic hepatitis and liver fibrosis/cirrhosis. In: Imaging of the liver and intra-hepatic biliary tract. United States: Springer (2021). p. 281–93.
17. Saeidi A, Zandi K, Cheok YY, Saeidi H, Wong WF, Lee CYQ, et al. T-cell exhaustion in chronic infections: reversing the state of exhaustion and reinvigorating optimal protective immune responses. Front Immunol. (2018) 9:2569. doi: 10.3389/fimmu.2018.02569
18. Lebossé F, Testoni B, Fresquet J, Facchetti F, Galmozzi E, Fournier M, et al. Intrahepatic innate immune response pathways are downregulated in untreated chronic hepatitis B. J Hepatol. (2017) 66:897–909. doi: 10.1016/j.jhep.2016.12.024
19. Farag MM, Peschel G, Müller M, and Weigand K. Characterization of the interaction between subviral particles of hepatitis B virus and dendritic cells–in vitro study. Infection Drug resistance. (2019) 12:3125. doi: 10.2147/IDR.S221294
20. Marotel M, Villard M, Drouillard A, Tout I, Besson L, Allatif O, et al. Peripheral natural killer cells in chronic hepatitis B patients display multiple molecular features of T cell exhaustion. Elife. (2021) 10:e60095. doi: 10.7554/eLife.60095.sa2
21. Kräutler NJ, Yermanos A, Pedrioli A, Welten SP, Lorgé D, Greczmiel U, et al. Quantitative and qualitative analysis of humoral immunity reveals continued and personalized evolution in chronic viral infection. Cell Rep. (2020) 30:997–1012. e1016. doi: 10.1016/j.celrep.2019.12.088
22. Sun Y, Yu M, Qu M, Ma Y, Zheng D, Yue Y, et al. Hepatitis B virus-triggered PTEN/β-catenin/c-Myc signaling enhances PD-L1 expression to promote immune evasion. Am J Physiology-Gastrointestinal Liver Physiol. (2020) 318:G162–73. doi: 10.1152/ajpgi.00197.2019
23. Danane J, Allali K, and Hammouch Z. Mathematical analysis of a fractional differential model of HBV infection with antibody immune response. Chaos Solitons Fractals. (2020) 136:109787. doi: 10.1016/j.chaos.2020.109787
24. Fourati S and Pawlotsky J-M. Recent advances in understanding and diagnosing hepatitis B virus infection. Virologie. (2019) 23:23–34. doi: 10.12688/f1000research.8983.1
25. Darmadi D and Ruslie RH. Immunology of helicobacter pylori infection. Rijeka: IntechOpen (2022). doi: 10.5772/intechopen.104592
26. Gu H. Role of flagella in the pathogenesis of helicobacter pylori. Curr Microbiol. (2017) 74:863–9. doi: 10.1007/s00284-017-1256-4
27. Wizenty J, Tacke F, and Sigal M. Responses of gastric epithelial stem cells and their niche to Helicobacter pylori infection. Ann Trans Med. (2020) 8:568. doi: 10.21037/atm.2020.02.178
28. Pachathundikandi SK, Tegtmeyer N, Arnold IC, Lind J, Neddermann M, Falkeis-Veits C, et al. T4SS-dependent TLR5 activation by Helicobacter pylori infection. Nat Commun. (2019) 10:1–11. doi: 10.1038/s41467-019-13506-6
29. Ofoezie EF, Ogbonna CA, Olisakwe SC, Anunobi CJ, George ET, Babarinde S, et al. Alisigwe CV et al: Role of infectious agents in cancer pathogenesis and therapy. Microbe. (2025) 6:100284. doi: 10.1016/j.microb.2025.100284
30. Roetman JJ, Apostolova MKI, and Philip M. Viral and cellular oncogenes promote immune evasion. Oncogene. (2022) 41:921–9. doi: 10.1038/s41388-021-02145-1
31. Ailloud F, Didelot X, Woltemate S, Pfaffinger G, Overmann J, Bader RC, et al. Within-host evolution of Helicobacter pylori shaped by niche-specific adaptation, intragastric migrations and selective sweeps. Nat Commun. (2019) 10:1–13. doi: 10.1038/s41467-019-10050-1
32. Ansari S and Yamaoka Y. Helicobacter pylori virulence factors exploiting gastric colonization and its pathogenicity. Toxins. (2019) 11:677. doi: 10.3390/toxins11110677
33. Dooyema SD, Noto JM, Wroblewski LE, Piazuelo MB, Krishna U, Suarez G, et al. Helicobacter pylori actively suppresses innate immune nucleic acid receptors. Gut Microbes. (2022) 14:2105102. doi: 10.1080/19490976.2022.2105102
34. Bagheri N, Salimzadeh L, and Shirzad H. The role of T helper 1-cell response in Helicobacter pylori-infection. Microbial pathogenesis. (2018) 123:1–8. doi: 10.1016/j.micpath.2018.06.033
35. da Silva EAW, da Silva NMJW, Rodrigues RR, Adad SJ, de Lima Pereira SA, Ribeiro BM, et al. Arginase-1 and treg profile appear to modulate inflammatory process in patients with chronic gastritis: IL-33 may be the alarm cytokine in H. pylori-positive patients. Mediators Inflammation. (2019) 2019:2536781. doi: 10.1155/2019/2536781
36. Mejías-Luque R and Gerhard M. Immune evasion strategies and persistence of Helicobacter pylori. Mol Pathogenesis Signal Transduction by Helicobacter pylori. (2017) 400:53–71. doi: 10.1007/978-3-319-50520-6_3
37. Xie W, Zhao W, Zou Z, Kong L, and Yang L. Oral multivalent epitope vaccine, based on UreB, HpaA, CAT, and LTB, for prevention and treatment of Helicobacter pylori infection in C57BL/6 mice. Helicobacter. (2021) 26(3):e12807. doi: 10.1111/hel.12807
38. Jafarzadeh A, Larussa T, Nemati M, and Jalapour S. T cell subsets play an important role in the determination of the clinical outcome of Helicobacter pylori infection. Microbial pathogenesis. (2018) 116:227–36. doi: 10.1016/j.micpath.2018.01.040
39. Jamali R, Mofid A, Vahedi H, Farzaneh R, and Dowlatshahi S. The effect of helicobacter pylori eradication on liver fat content in subjects with non-alcoholic Fatty liver disease: a randomized open-label clinical trial. Hepatitis monthly. (2013) 13:e14679. doi: 10.5812/hepatmon.14679
40. Séhonou J, Kpossou A, and Kanvi J. Helicobacter pylori infection in hepatitis B virus carriers in Cotonou: epidemiology and socio-demographic factors associated with the co-infection. Gastroenterol Hepatol Open Access. (2019) 10:14–7. doi: 10.15406/ghoa.2019.10.00350
41. Castaño-Rodríguez N, Mitchell HM, and Kaakoush NO. NAFLD, Helicobacter species and the intestinal microbiome. Best Pract Res Clin Gastroenterol. (2017) 31:657–68. doi: 10.1016/j.bpg.2017.09.008
42. Mohammadifard M, Saremi Z, Rastgoo M, and Akbari E. Relevance between helicobacter pylori infection and non-alcoholic fatty liver disease in Birjand, Iran. J Med Life. (2019) 12:168. doi: 10.25122/jml-2019-0012
43. Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, and van de Veen W. Regulatory B cells, A to Z. Allergy. (2021) 76:2699–715. doi: 10.1111/all.14763
44. Al-Amrani M and Al-Zaazaai A. The relation between helicobacter pylori infection, serum ammonia and hepatic encephalopathy in Yemeni cirrhotic patients. Res Rep Gastroenterol. (2020) 111:2. doi: 10.37532/rrg.2020.4(1).111
45. Cui L, Markou A, Stratton CW, and Lianidou E. Diagnosis and assessment of microbial infections with host and microbial microRNA profiles. In: Advanced techniques in diagnostic microbiology. Switzerland: Springer (2018). p. 563–97.
46. Soudi H, Falsafi T, Gharavi S, and Mahboubi M. The role of helicobacter pylori proinflammatory outer membrane protein and propolis in immunomodulation on U937 macrophage cell model. Galen Med J. (2020) 9:1687. doi: 10.31661/gmj.v9i0.1687
47. Fukui H. Role of gut dysbiosis in liver diseases: what have we learned so far? Diseases. (2019) 7:58. doi: 10.3390/diseases7040058
48. Yirsa T. Serological and molecular detection of Toxoplasma gondii among slaughtered domestic ruminants and pregnant women in Gondar town, Northwest Ethiopia. Tsedalu Yirsa. (2020) 15:2403–12. doi: 10.17485/IJST/v15i44.1810
49. Melese A, Genet C, Zeleke B, and Andualem T. Helicobacter pylori infections in Ethiopia; prevalence and associated factors: a systematic review and meta-analysis. BMC Gastroenterol. (2019) 19:1–15. doi: 10.1186/s12876-018-0927-3
50. Wang J, Chen R-C, Zheng Y-X, Zhao S-S, Li N, Zhou R-R, et al. Helicobacter pylori infection may increase the risk of progression of chronic hepatitis B disease among the Chinese population: a meta-analysis. Int J Infect Dis. (2016) 50:30–7. doi: 10.1016/j.ijid.2016.07.014
51. Madala S, MacDougall K, Surapaneni BK, Park R, Girotra M, and Kasi A. Coinfection of helicobacter pylori and hepatitis C virus in the development of hepatocellular carcinoma: A systematic review and meta-analysis. J Clin Med Res. (2021) 13:530. doi: 10.14740/jocmr4637
52. Abdel-Razik A, Mousa N, Elhelaly R, Elzehery R, Hasan AS, Abdelsalam M, et al. Helicobacter pylori as an initiating factor of complications in patients with cirrhosis: a single-center observational study. Front Med. (2020) 7:96. doi: 10.3389/fmed.2020.00096
53. Zhao B, Sheng Q, Qin Y, Wang X, Zhao H, and Zhao N. Correlations of Helicobacter pylori with liver function, inflammatory factors and serum levels of FoxP3 and RORgammat in patients with hepatitis B cirrhosis. Eur Rev Med Pharm Sci. (2021) 25:459–65. doi: 10.26355/eurrev_202101_24415
54. Kazemifar AM, Shafikhani AA, HajiNoormohammadi E, Azarion Z, and Hajiaghamohammadi A. Investigating effect of Helicobacter pylori treatment on improvement of non-alcoholic fatty liver parameters: a randomized trial. Egyptian Liver J. (2019) 9:1–5. doi: 10.1186/s43066-019-0001-z
Keywords: HBV, chronic liver disease, H. pylori, HBV/H. pylori co-infection, Ethiopia
Citation: Belayneh M, Tegegne BA, Lemma M, Abay Z and Belyhun Y (2025) Clinical and biochemical characterization of hepatitis B surface antigen-positive patients with or without Helicobacter pylori co-infection at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Front. Immunol. 16:1553411. doi: 10.3389/fimmu.2025.1553411
Received: 30 December 2024; Accepted: 28 May 2025;
Published: 18 June 2025.
Edited by:
Nar Singh Chauhan, Maharshi Dayanand University, IndiaReviewed by:
Monika Yadav, Sanjay Ghodawat University, IndiaShashank Gupta, Norwegian University of Life Sciences, Norway
Asiya Nazir, Abu Dhabi University, United Arab Emirates
Copyright © 2025 Belayneh, Tegegne, Lemma, Abay and Belyhun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mekuriaw Belayneh, bWVrdXJpYXdiZWxheW5laDgyQGdtYWlsLmNvbQ==; Bantayehu Addis Tegegne, QmFudGF5ZWh1YWRkaXMuOTBAZ21haWwuY29t
†Present address: Yeshambel Belyhun, Institute of Human Virology, School of Medicine, University of Maryland, Baltimore, MD, United States
 Mekuriaw Belayneh
Mekuriaw Belayneh Bantayehu Addis Tegegne
Bantayehu Addis Tegegne Mulualem Lemma3
Mulualem Lemma3 Yeshambel Belyhun
Yeshambel Belyhun