- 1Department of Rheumatology and Immunology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 2Department of Endocrinology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 3Department of Gastroenterology and Hepatology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 4Animal Center, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
Purpose: Immune checkpoint inhibitors (ICIs) significantly prolong the survival of cancer patients. including gastric adenocarcinoma (GAC) and esophageal squamous cell carcinoma (ESCC) patients. Immune-related adverse events (irAEs) are inevitably involved in ICIs treatment sometimes with severe consequences. Extreme caution is necessary for predicting irAEs and precisely screening of appropriate patients. We evaluated the association of interleukin-6 (IL-6) with irAEs and their impacts on ICIs treatment effectiveness in advanced GAC and ESCC patients.
Methods: This retrospective study analyzed 121 patients treated with ICIs between March 1, 2020 and August 31, 2023 to evaluate the association between serum IL-6 and ICIs treatment effectiveness. The occurrence of irAEs, including grade and category, and effectiveness of immunotherapy, including objective remission rate (ORR), disease control rate (DCR), progression-free survival (PFS) and overall survival (OS), was evaluated. Categorical count data were tested by chi-square test. Nonparametric rank sum tests were performed using Wilcoxon and Kruskal-Wallis test. Survival rate estimation and survival curves were generated using Kaplan–Meier curve and Log-rank test. Univariate and multivariable COX regression analyses were performed to identify independent prognostic factors.
Results: A total of 121 patients including 79 with GAC and 42 with ESCC patients were randomly divided into TC (n=81) and VC (n=40) groups. Higher serum IL-6 levels were associated with increased incidence of irAEs, the outcome analysis also indicated its association with lower DCR, shorter PFS and shorter OS in TC group. The higher IL-6 related irAEs occurrence and poor prognosis (DCR, PFS) was confirmed in the VC group. Individual tumor analysis showed that higher IL-6 was associated with both irAEs occurrence and poor prognosis (DCR, PFS, OS) in ESCC patients, and with irAEs occurrence and poor prognosis (DCR, PFS) in GAC patients. No statistically significant associations were observed between pathological biomarkers including programmed cell death ligand 1 (PD-L1), mismatch repair (MMR) and human epidermal growth factor receptor 2 (HER2) and either IL-6 levels or irAEs occurrence in both GC and ESCC patients.
Conclusion: Elevated serum IL-6 levels were associated with the incidence of irAEs, and higher IL-6 levels predicted worse prognosis in GAC and ESCC patients with ICIs treatment.
1 Introduction
Immune checkpoint inhibitors (ICIs) blocking cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1) or its ligand of programmed cell death ligand 1(PD-L1) to enhance anti-tumor immunity has made a major breakthrough in cancer treatment. PD-1 on the surface of various immunocyte can bind with PD-L1 on the tumor cell to inhibit T cell activation (1, 2). ICIs combining with chemotherapy for advanced gastric cancer (GC) have moved from the third-line treatment to the first-line treatment due to their efficiency to improve overall survival (OS), progression-free survival (PFS) and objective response rate (ORR) when compared with those of chemotherapy alone (3, 4). Similarly, ICIs combining with chemotherapy for advanced esophageal cancer (EC) also transformed from the second line treatment to the first line treatment with significant improved outcomes (5–7).
Despite these breakthroughs, the overall ORR for immunotherapy in advanced EC and GC remains below 50%. Moreover, the immune system’s natural defense against cancer through ICIs regulation unavoidably results in the damage of normal tissues via abnormal stimulation of the immune system, which is called as immune-related adverse events (irAEs) (8). The occurrence of irAEs is related to the unbalance of immune homeostasis, generation of autoantibodies and autoantigens, dysbacteriosis and cytokines release (9). The types of irAEs also vary with different organs and tissues involved upon different ICIs treatment (10, 11). IrAEs seem to be associated with better effectiveness referring to ORR, PFS and OS (12–15). But serious adverse events may lead to discontinuation treatment, frequent hospitalization with immunosuppressant treatment, and even fatal (16). Therefore, identifying reliable biomarkers for precisely predicting both therapeutic efficacy and irAEs represents a critical challenge in current gastrointestinal cancer immunotherapy.
Interleukin-6 (IL-6) is involved in cell growth, survival, inflammation and immune regulation (17). It could initiate both carcinogenesis and tumor progress via various signaling pathways in tumor microenvironment (18, 19). IL-6 can increase the vascular endothelial growth factor (VEGF) expression via the Janus tyrosine kinase/signal transducer and activator of transcription 3 (JAK/STAT3) signaling pathway, thereby promoting growth, invasion and lymphangiogenesis in GC patients (20). In addition, IL-6 interacts with both epidermal growth factor receptor (EGFR) to promote immunosuppressive microenvironment by inducing myeloid-derived suppressor cells (MDSC) and the vascular endothelial growth factor receptor (VEGFR) to drive tumor-induced angiogenesis in EC (21, 22). Emerging evidence demonstrates a significant association between IL-6 levels and ICIs outcomes. In advanced lung cancer patients receiving anti-PD-1 therapy, the low baseline IL-6 levels in peripheral blood correlated with better treatment effectiveness (23, 24). IL-6 blockade could improve ICIs induced antitumor efficacy in melanoma patients (25).
IL-6 expression had been proved to be associated with irAEs occurrence in some types of cancers. IL-6 levels displayed a positive correlation with irAEs related intestinal toxicity during immunotherapy, IL-6 pathway blockade significantly reduced intestinal damage whereas improved therapeutic outcomes in liver cancer patients (26). Anti-IL-6 receptor (anti-IL-6R) antibody, such as tocilizumab or sarilumab, achieved symptom resolution in approximately 73% irAEs cases among patients with melanoma, genitourinary cancer, or lung cancer in a retrospective analysis (27). Animal model studies of immune-related enterocolitis further showed that the IL-6 levels in intestinal tissues could initiate irAEs related colitis, while IL-6 inhibition simultaneously ameliorated neurotoxicity and enhanced antitumor immunity (28).
These consistent findings across clinical and experimental settings strongly suggest that IL-6 is involved in mediating both therapeutic response and irAEs development in cancer patients. However, the role of IL-6 on ICIs treatment for gastric adenocarcinoma (GAC) and esophageal squamous cell carcinoma (ESCC) — two of the most prevalent and aggressive upper gastrointestinal malignancies — remains unclear. To explore the relationship between IL-6 and irAEs occurrence in GAC and ESCC patients, we conducted the present analysis.
2 Methods
2.1 Patients
A total of 121 patients (87 male and 34 female) with a mean age of 64.1 ± 9.74 years were included in this study. The cohort comprised 79 patients with GAC and 42 with ESCC, who received anti-PD-1 therapy at the Fourth Hospital of Hebei Medical University between March 1, 2020, and August 31, 2023 were retrospectively reviewed. Inclusion criteria were as follows: (i) pathologically confirmed GAC or ESCC; (ii) unresectable patients with stage III or IV; (iii) completion of ≥2 cycles of PD-1 ICIs. Exclusion criteria included: (i) Missing clinical information; (ii) Prior receipt of other immunotherapies; (iii) Patients with successful conversion of neoadjuvant therapy to surgery; (iv) suffering from infection or rheumatic immune disease.
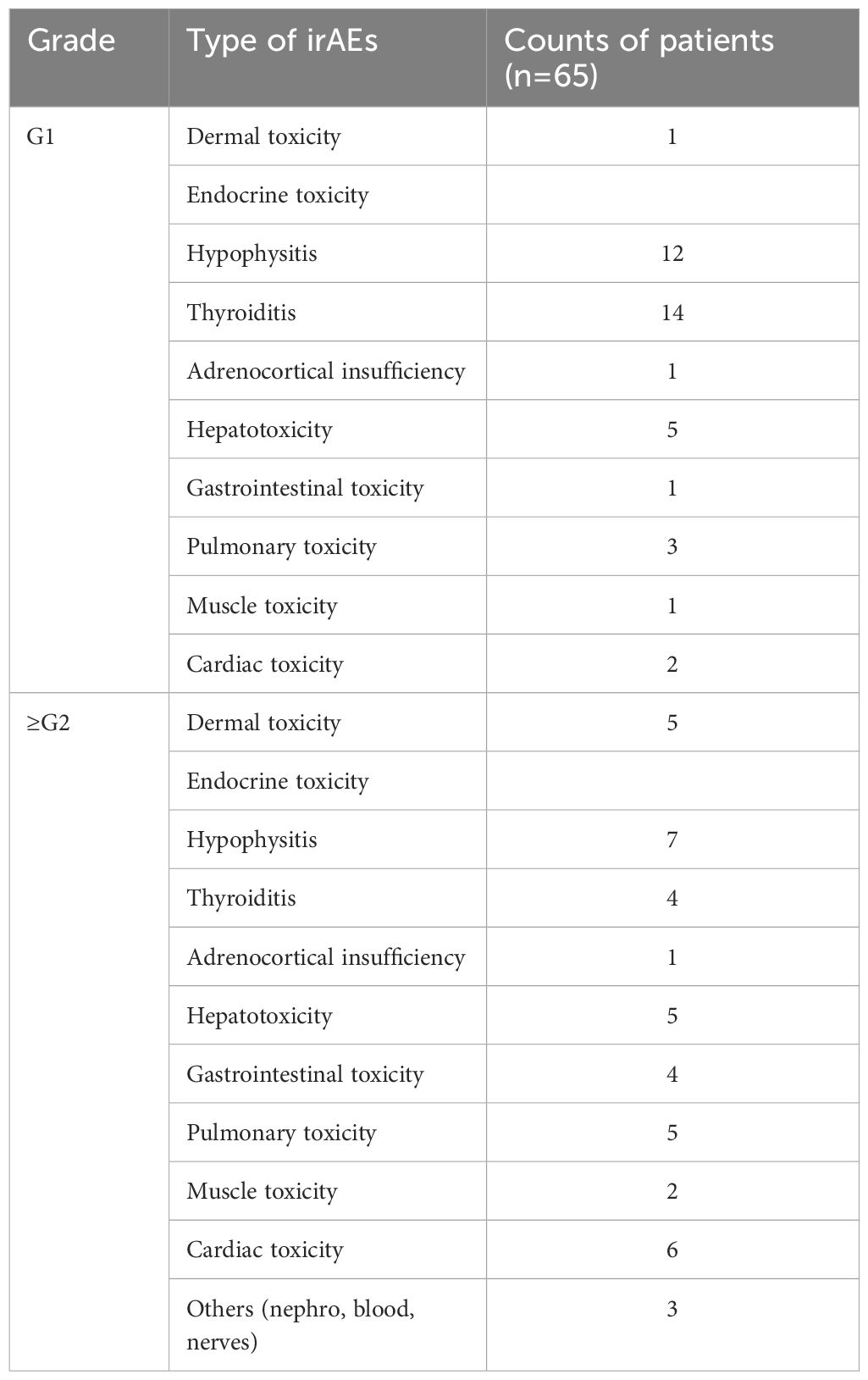
The entire cohort was initially divided into a training cohort (TC, n=81) and a validation cohort (VC, n=40) at a 2:1 ratio. Subsequently stratification was performed according to tumor type (GAC, n=79; ESCC, n=42) for individual tumor analysis. The following clinical parameters were systematically evaluated: gender, age, IL-6 levels included baseline or posttreatment, Eastern Cooperative Oncology Group performance status (ECOG PS), TNM stage (III/IV), surgery history (defined as cases of postoperative recurrence or metastasis), ICIs regimen, treatment lines, irAEs and cancer type. The flow chart of the analysis design is shown in Figure 1. IrAEs were defined as inflammatory toxicity caused by immune tolerance imbalance due to ICIs. The National Cancer Institute Common Terminology Criteria for Adverse Events ver.4.03 (http://ctep.cancer.gov/protocolDevelopment/elec-tronic_applications/ctc.htm#ctc_40) was used for the irAEs assessment. Given that grade 1 irAEs are generally asymptomatic, while grade ≥ 2 irAEs may cause symptoms and even lead to suspension or permanent discontinuation of ICIs, we stratified the irAEs cohort into grade 1 and grade ≥ 2 groups.
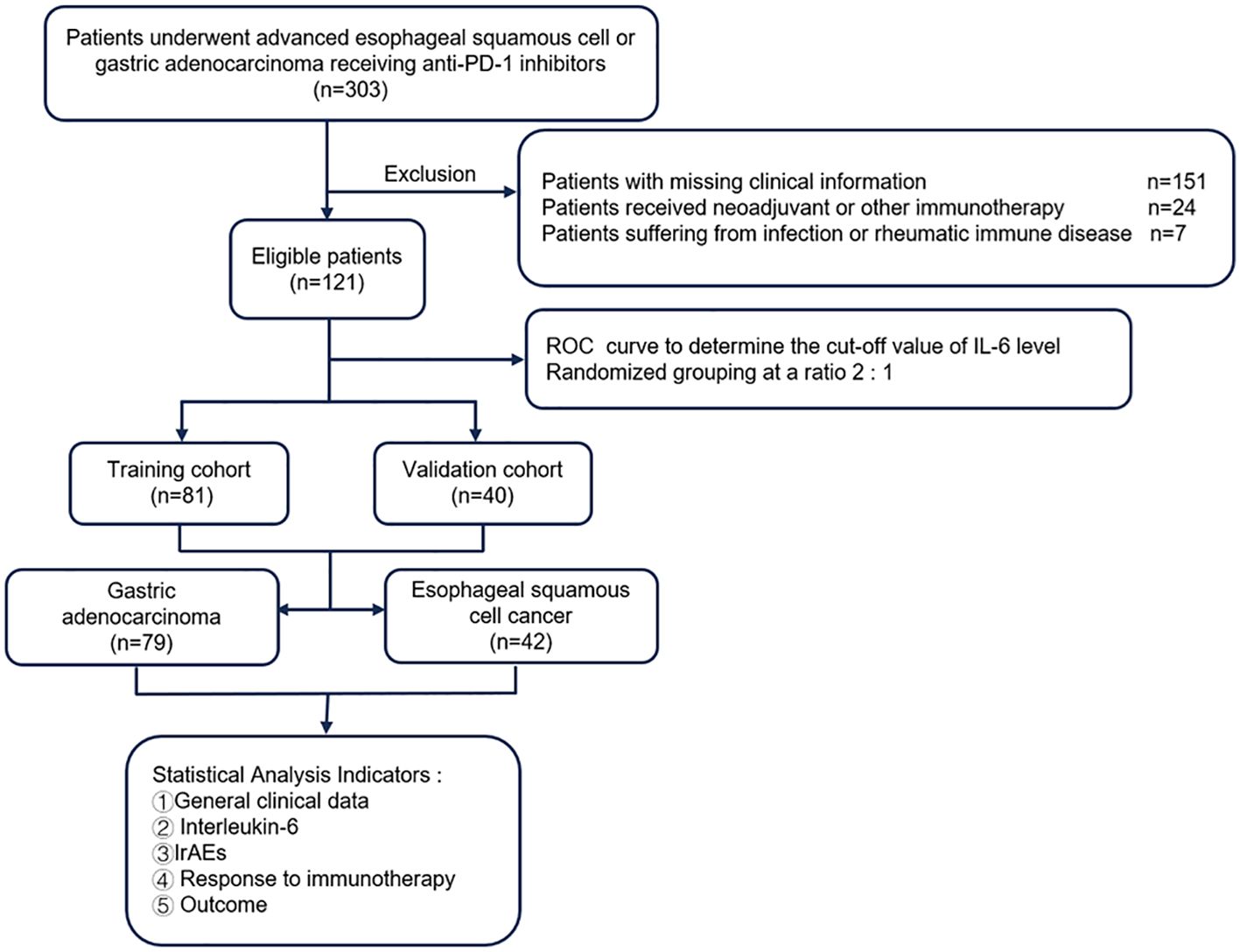
Figure 1. Flow diagram of the study. PD-1, programmed cell death 1; IL-6, interleukin-6; ROC, operating characteristic curve.
The timing of serum IL-6 measurements varied among participants, with some samples collected at baseline, others during treatment, and some at both baseline and post-treatment, including instances when irAEs occurred. For analysis purpose, we categorized the IL-6 level fluctuations as follows: if the overall change in IL-6 levels from baseline to post-treatment was no more than half of the initial value, we recorded the average of these levels. Conversely, if the fluctuation exceeded half of the initial level, we documented the highest value observed. Therefore, an increase in IL-6 either at the beginning or during the treatment process was defined as high IL-6. Boxplot analysis revealed 5 outliers in IL-6 levels among 121 patients, all of which were confirmed to be clinically relevant and thus retained. Specifically, 4 patients with GAC exhibited elevated IL-6 levels, concurrently presenting with irAEs. The types of irAEs observed included hepatotoxicity (n = 4), cardiotoxicity (n = 3), endocrine toxicity (n = 3), and dermatologic toxicity (n = 1). Additionally, 1 patient with ESCC demonstrated multi-organ endocrine toxicity involving the pituitary, thyroid, and adrenal glands. Comprehensive clinical profiles were available for all cases, confirming the biological significance of these outliers. These data points were preserved to ensure both clinical relevance and analytical rigor.
To ascertain the optimal cut-off value for serum interleukin-6 (IL-6) levels, we utilized the receiver operating characteristic (ROC) curve analysis, which included OS as a parameter. Before ROC analysis, we performed Z-score normalization on the raw data using SPSS 25.0 software (IBM SPSS, NY, USA). Following transformation, the normalized data exhibited a mean of 0 and a standard deviation of 1, conforming to a standard normal distribution. We then generated the ROC curve (Figure 2A). The area under the curve (AUC) was calculated to be 0.672 (95% CI: 0.546–0.798, p=0.005). At the maximum Youden index (0.4), the optimal IL-6 cutoff was determined to be 17.16 pg/mL, which corresponded to a sensitivity of 75.9% and specificity of 64.1%. Thereby, the optimal cut-off value of IL-6 with 17.16 pg/ml was applied to distinguish patients as low IL-6 expression group (Low IL-6) and high IL-6 expression group (High IL-6).
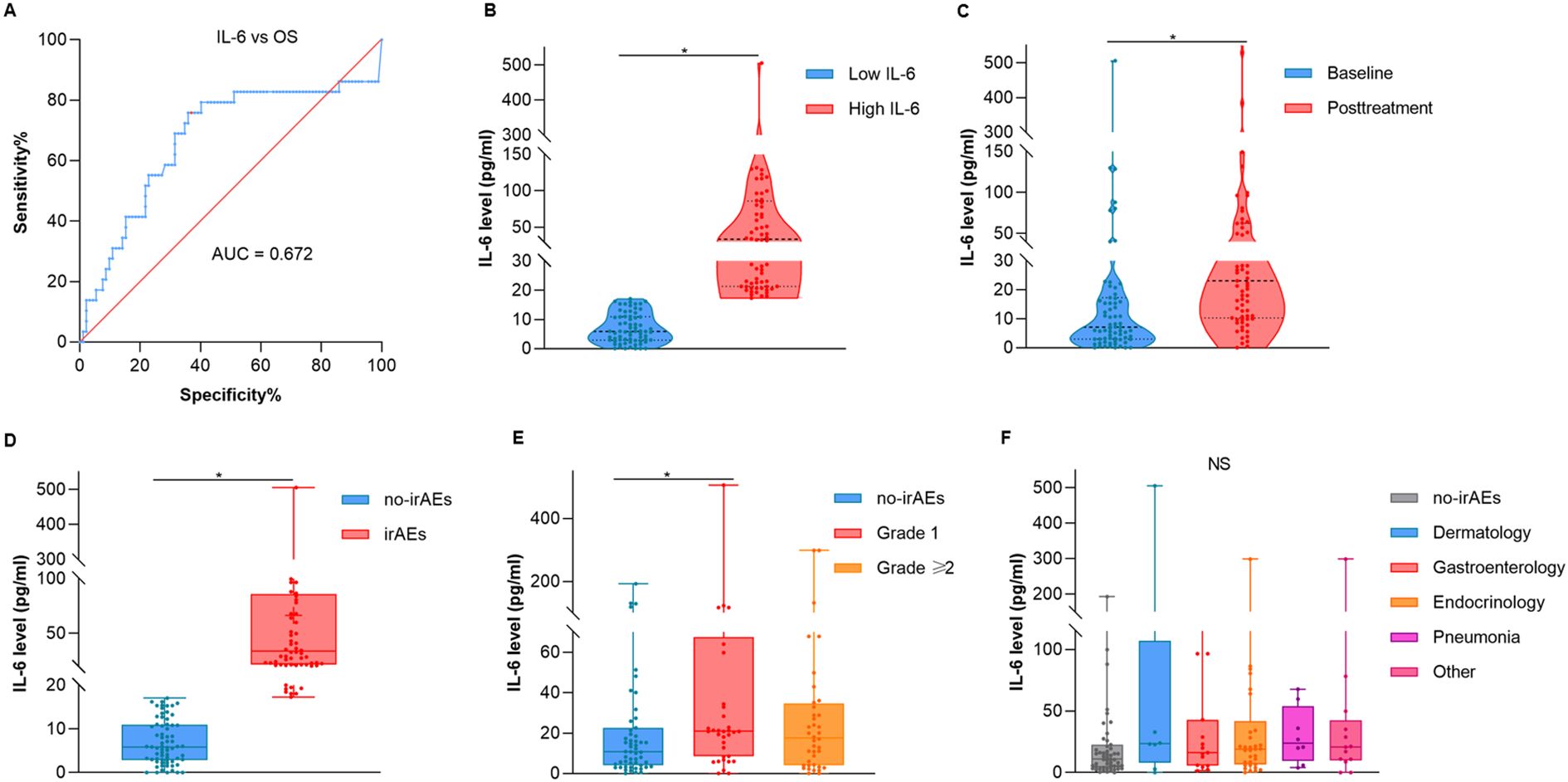
Figure 2. The correlation between the overall distribution of interleukin-6 and irAEs. (A) ROC curves for the serum IL-6 levels and OS. (B) Violin plot of the Low IL-6 and High IL-6 group. (C) Violin plot of the IL-6 at baseline and posttreatment. (D) Box and whisker diagram of IL-6 between no-irAEs and irAEs. (E) Box and whisker diagram of IL-6 across different grades. (F) Box and whisker diagram of IL-6 across different types of irAEs. OS, overall survival; AUC, area under curve. *p < 0.05.
Due to this retrospective study only utilized the existing anonymously information for analysis, a waiver of informed consent was applied for patients involved. All procedures performed in this study were in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2024KS059).
2.2 Multiple microsphere flow immunofluorescence luminescence method
Serum IL-6 levels were determined using the Cytokine Detection Kit (Risker Biological Technology Co., Ltd. Qingdao, China), operated strictly according to the instructions by the laboratory department of our hospital. The cytokine antibody with fluorescent microsphere was combined with both the biotin-labeled cytokine pairing antibody and the cytokines in the sample to form a “sandwich” complex, which was subsequently reacted with the phycoerythrin-labeled streptavidin. The fluorescence intensity was detected by Navios flow cytometry (Beckman Coulter, Inc. Bria, California, USA). An eight points curve was drawn based the mean fluorescence intensity of the standard IL-6 values, which were diluted four times in sequence from 10000pg/ml (2500pg/ml, 625pg/ml, 156.3pg/ml, 39.1pg/ml, 9.8pg/ml, 2.4pg/ml, 0pg/ml). The IL-6 concentration of the samples was obtained by the position of their fluorescence intensity on the eight-points standard curve. IL-6 levels were measured before the treatment as well as after at least twice cycles of the treatment, with additional measurements taken depending on the treatment duration.
2.3 Treatment assessment
Patients received standard anti-PD-1 antibodies (mono-immunotherapy, in combination with chemotherapy or targeted drugs, or as triple therapy for combining ICIs with both chemotherapy and targeted therapy every 3 weeks until disease progression, clinical deterioration, intolerable toxicity or patient rejection. The types of immunotherapy drugs used included camrelizumab, sintilimab, pembrolizumab, toripalimab, serplulimab and tislelizumab, while the targeted were apatinib, regorafenib, trastuzumab and lenvatinib. Objective tumor response was assessed according to the “Response Evaluation Criteria in Solid Tumors” (RECIST) version 1.1 (29), using repeated computed tomography (CT) or magnetic resonance imaging (MRI) scans every 2 or 3 cycles.
2.4 Statistical analysis
Tumor effectiveness was evaluated based on ORR and (disease control rate) DCR according to RECIST version 1.1. PFS was defined as the time from first beginning of anti-PD-1 therapy to progression, death or study cutoff. OS was defined as the time from commencement of ICIs-based systemic therapy to death or study cutoff. GraphPad Prism version 8.0 (GraphPad Software, San Diego, California USA) was used to draw the graphs. All collected data were statistically analyzed by SPSS 25.0 software (IBM SPSS, NY, USA). Wilcoxon and Kruskal-Wallis tests were used for nonparametric rank sum test to explore differences between two dependent samples and among multiple groups. Clinical categorical count data were analyzed by chi-square test or Fisher’s exact test. Survival rates were estimated by Kaplan–Meier curve and Log-rank test. Univariate and multivariable COX regression analyses were performed to identify potential prognostic factors, with p < 0.05 considered statistically significant.
3 Results
3.1 The overall description of IL-6 and irAEs
A total of 121 patients were enrolled in the study. As shown in Figure 2, violin plots were employed to delineate the mean and density distribution disparities of IL-6 levels across all participants (Figure 2B, p = 0.022). A statistically significant elevation in IL-6 levels was observed post anti-PD-1 therapy when compared to baseline values (Figure 2C, p = 0.019).
Further analysis using the Wilcoxon test revealed that patients experiencing irAEs exhibited higher IL-6 levels than those without irAEs (Figure 2D, p = 0.005). Additionally, the Kruskal-Wallis test indicated that serum IL-6 levels were markedly elevated in patients with grade 1 irAEs compared to those without any irAEs (Figure 2E, p = 0.034). However, no significant correlation was observed between IL-6 levels and the specific types of irAEs encountered (Figure 2F, p = 0.321). Table 1 presents a breakdown of the various irAEs, with each entry representing the count of patients affected by particular irAEs. It is noteworthy that the total count of irAEs types (n = 82) exceeds the total number of patients with irAEs (n = 65), as some individuals presented with multiple types of irAEs. These findings suggested that elevated serum IL-6 levels serve as a biomarker for the development of adverse events in patients receiving anti-PD-1 therapy, rather than being indicative of a specific type of irAEs.
3.2 Internal validation of the correlation between IL-6 and the irAEs occurrence and patients’ prognosis
A total of 121 patients, including 79 with GAC and 42 with ESCC, were randomly divided into TC (n = 81) and VC (n = 40) groups at a 2:1 ratio. Baseline characteristics, including gender, age, ECOG score, tumor stage, surgery history, therapy regimen, and treatment lines, were well balanced between groups (Table 2).
In TC group, higher IL-6 levels were associated with a higher incidence of irAEs (p = 0.011, Table 2). Outcome analysis also indicated high IL-6 were associated with lower DCR (29.3%, 95% CI: 14.7% - 43.8% vs. 67.5%, 95% CI: 52.3% - 82.7%, p = 0.001), shorter PFS (p = 0.004) and shorter OS (p = 0.007) when compared with those of low IL-6 group (Figures 3A, 4A, B). Univariate COX regression analysis revealed the clinical characteristics, including higher IL-6, later TNM stage and later treatment lines were associated with worse outcome, including shorter PFS and shorter OS, in TC group (Table 3). Multivariable analysis indicated that high IL-6 levels and late treatment lines were independent risk factors modifying both PFS (HR = 2.102, 95% CI: 1.077 - 4.103, p = 0. 029; HR = 6.601, 95% CI: 3.042 - 14.321, p = 0. 000) and OS (HR = 3.309, 95% CI: 1.027 - 10.660, p = 0. 045; HR = 13.468, 95% CI: 3.752 - 48.342, p = 0. 000) (Table 3).
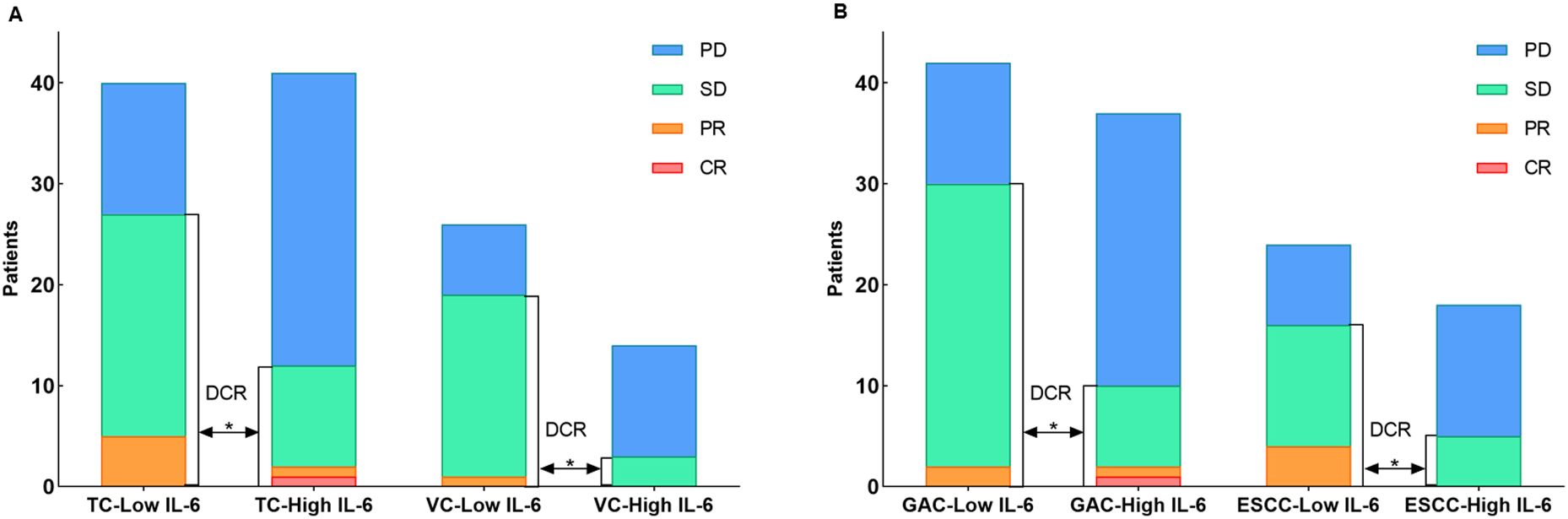
Figure 3. (A) The distribution of immunotherapy responses in the TC and VC groups. (B) The distribution of immunotherapy responses in the GAC and ESCC groups.
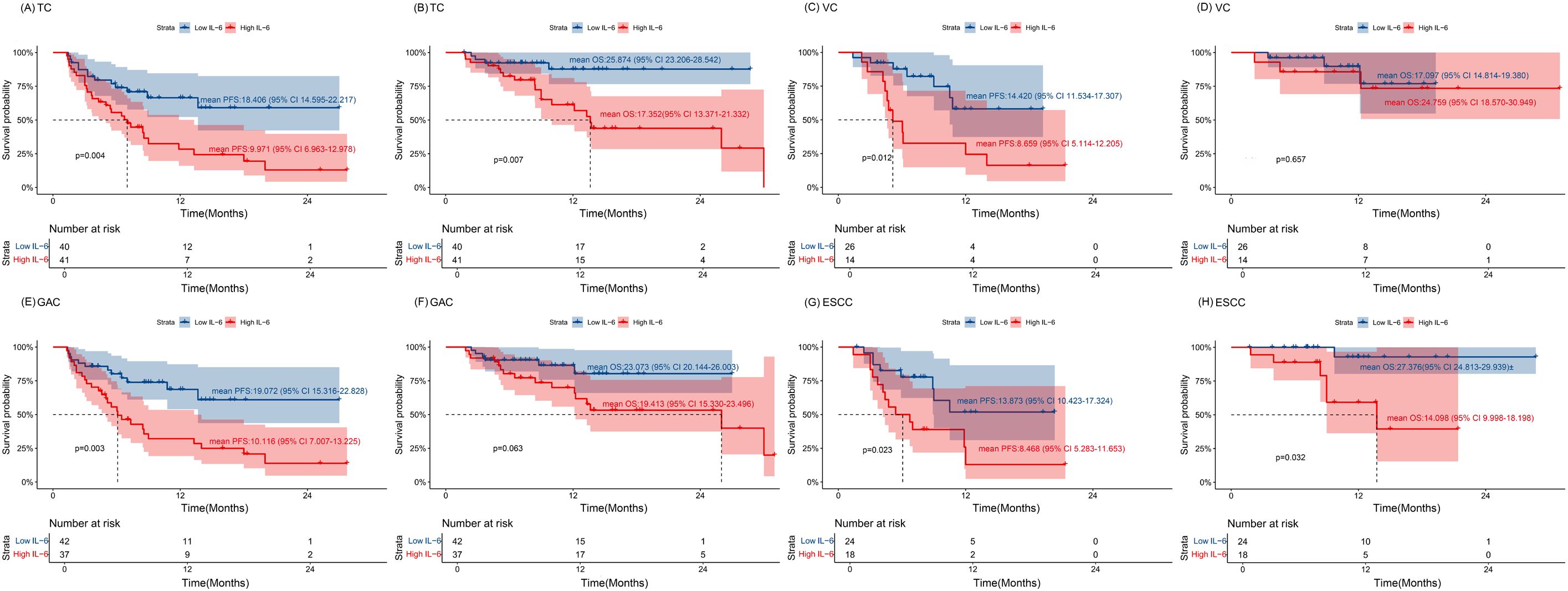
Figure 4. The association of IL-6 with the prognosis of overall patients (A, B) The Kaplan–Meier curve of PFS and OS for the TC cohort. (C, D) The Kaplan–Meier curve of PFS and OS for the VC cohort. (E, F) The Kaplan–Meier curve of PFS and OS for the GAC group. (G, H) The Kaplan–Meier curve of PFS and OS for the ESCC group. Time: Months; TC, training cohort; VC, validation cohort; ESCC, esophageal squamous cell carcinoma; GAC, gastric adenocarcinoma.
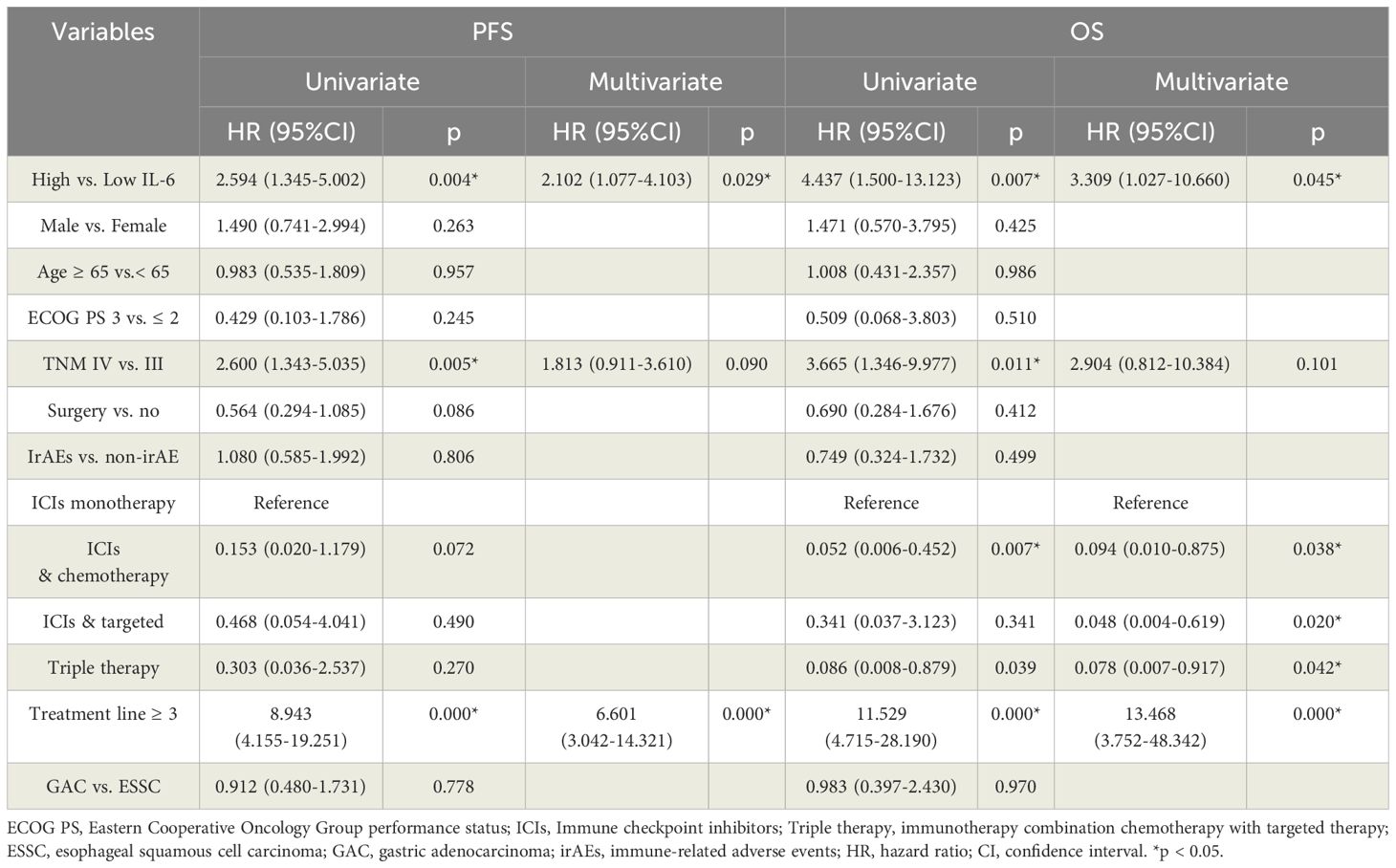
Table 3. Univariate and multivariable Cox proportional hazards model analyses of PFS and OS in TC group.
The association between high IL-6 levels and irAEs was confirmed in the VC group referring to the DCR (21.4%, 95% CI: 3.2% - 46.0% vs. 73.1%, 95% CI: 54.8% - 91.3%, p = 0.002) and PFS (p = 0.012) (Figures 3A, 4C, D). Univariate COX regression (HR = 3.408, 95% CI: 1.303 - 8.911, p = 0. 012) and multivariable analysis (HR = 3.031, 95% CI: 1.110 - 8.276, p = 0. 030) showed that high IL-6 levels was significantly associated with shorter PFS, but no association was observed between IL-6 levels and OS (Supplementary Table S1). These data underscore that high IL-6 levels were not only associated with the occurrence of irAEs, but also the outcomes of ICIs treatment.
3.3 Individual analysis of gastric and esophageal carcinoma
Subsequent individual tumor analysis was performed for all GAC and ESCC patients in the VC and TC groups, and their characteristics are shown in Table 4. High IL-6 levels were confirmed to be linked with irAEs occurrence (p = 0.028), as well as a lower DCR (27.0%, 95% CI: 12.0% - 42.0% vs. 71.4%, 95% CI: 57.2% - 85.7%, p = 0.000), shorter PFS (p = 0.003) and a trend toward shorter OS (p = 0.063) in the GAC group (Figures 3B, 4E, F). After univariate analysis, multivariable analysis identified high IL-6 levels (HR: 2.371, 95%CI: 1.086 - 5.179, p = 0.014) as an independent predictor for shorter PFS in GAC patients (Table 5). Consistent with the findings in GAC patients, higher IL-6 levels were also associated with irAEs occurrence (Table 4, p = 0.026) and lower DCR in ESCC patients (Figure 3B, p = 0.013). Survival analysis revealed associations with shorter PFS (p = 0.023) and OS (p = 0.032), and high IL-6 levels were verified as independent risk factors (Figures 4G, H, Supplementary Table S2). These data confirmed that the serum IL-6 levels were associated with both irAEs occurrence and treatment effectiveness in GAC and ESCC patients. We further evaluated the pathological characteristics, including HER2, MMR and PD-L1 status, for their association with IL-6 or irAEs in GAC and ESCC patients, but no statistically significant difference could be archived (data not shown).
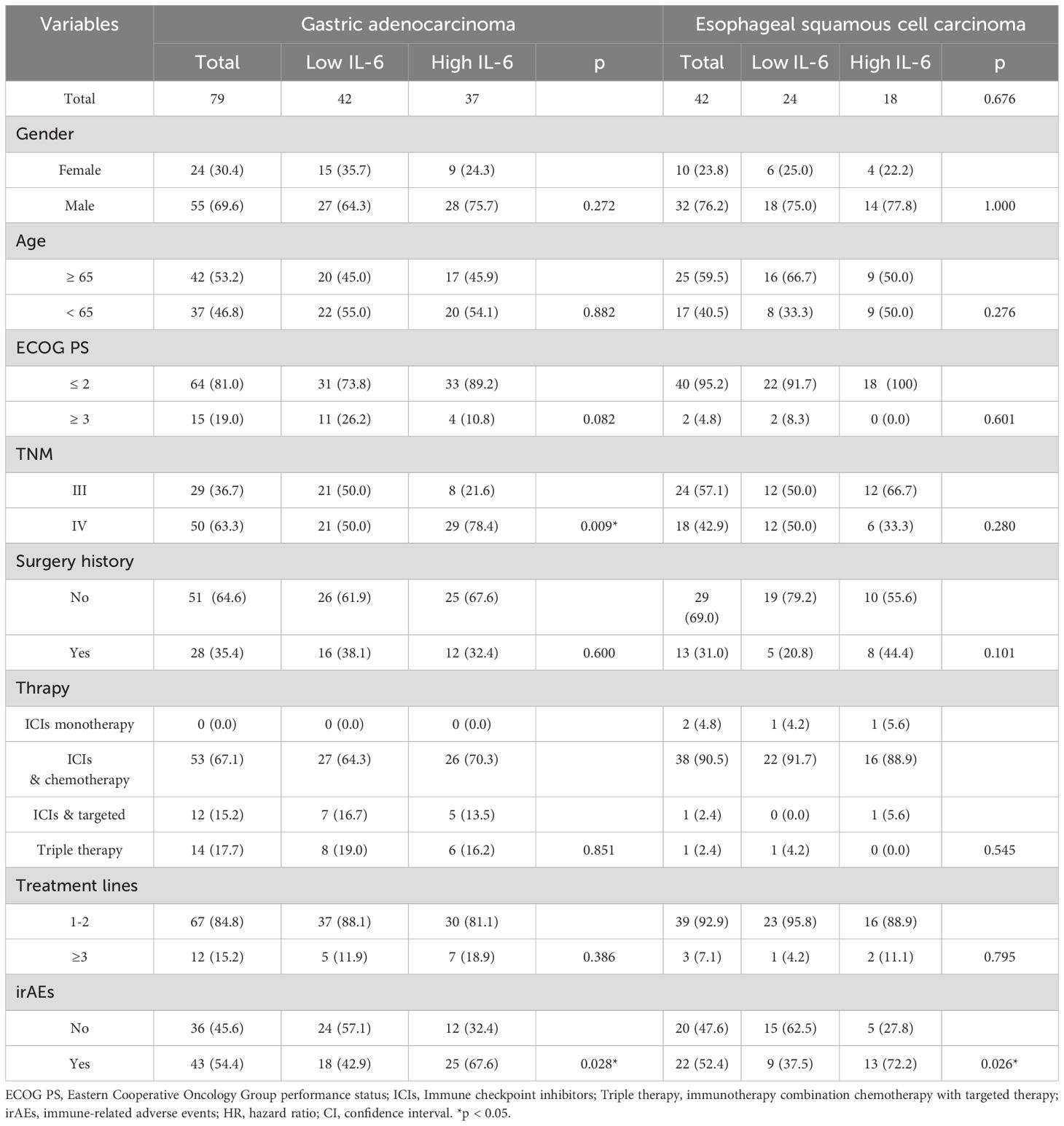
Table 4. Characteristics of the patients with gastric adenocarcinoma and esophageal squamous cell cancer.
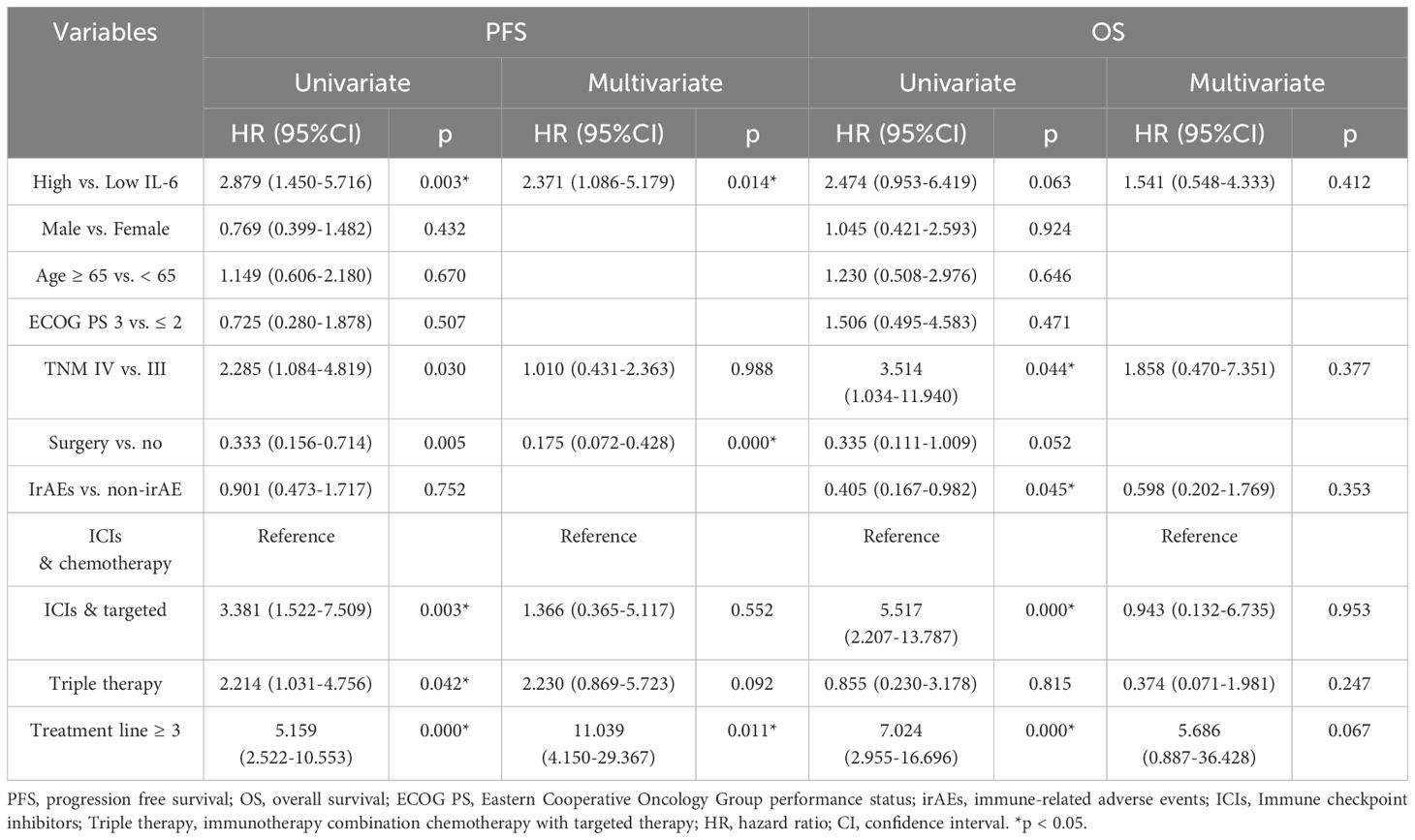
Table 5. Univariate and multivariable Cox proportional hazards model analyses of PFS and OS in gastric adenocarcinoma group.
4 Discussion
Our data demonstrated that higher levels of serum IL-6 were associated with both irAEs occurrence and treatment effectiveness (DCR, PFS and OS) in patients receiving ICIs in TC cohort. These potential associations also confirmed in VC cohort, except for OS. Several factors, including insufficient sample size limited statistical power to detect meaningful differences, potential influence of pathological characteristics, comorbidities and follow-up duration, might have modified the final survival analysis. The consistent statistical significance of PFS across all cohorts strongly suggests that early elevation of IL-6 may serve as a timely biomarker of immunotherapy response. Individual tumor analysis in both GAC and ESCC patients also confirmed the results observed in TC and VC analyses. Our study is consistent with previous reports that higher IL-6 levels are correlated with the worse prognosis in non-small cell lung cancer (NSCLC) and melanoma patients (30–34).
The key signaling pathways of the IL-6/JAK/STAT3 axis promote tumor growth, metastasis, and metabolism (35), IL-6 also activates the Yes-associated protein (YAP) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signaling pathways to promote cell proliferation, migration and invasion as well as mediating activation of transcription factors CCAAT/enhancer-binding protein beta/delta (C/EBPβ/δ) to induce epithelial-mesenchymal transition (EMT) and amplification of cancer stem cells (36, 37). Beyond its pro-tumorigenic roles, IL-6 promotes angiogenesis by the VEGF signal pathway, thereby weakening the effectiveness of ICIs in many types of cancers, including gastrointestinal cancers, prostate cancer, oral squamous cell carcinoma, and hepatocellular carcinoma (38–40). A novel role of tumor-intrinsic PD-L1/JAK/STAT3/IL-6/MDSC axis in both immunosuppression and tumor progression has been recently reported in NSCLC (41). These mechanisms may partly explain the negative impact of IL-6 on ICIs treatment efficacy.
In the tumor microenvironment, cytokines generation is one of the main mechanisms underlying irAEs development (9). Preclinical studies with irAEs model have found that irAEs significantly induce IL-6 production (42). Clinically, elevated IL-6 levels have been associated with the occurrence of psoriatic dermatitis in patients with malignant melanoma receiving nivolumab therapy (43). These findings are consistent with our observations in GAC and ESCC patients, that IL-6 was positively associated with irAEs occurrence. The precise mechanism needs to be fully elucidated to determine which factor initially triggers the others. However, the therapeutic potential of IL-6 inhibition for irAEs has been explored. An anti-IL-6R monoclonal antibody tocilizumab has been applied in clinical treatment for irAEs, including colitis, arthritis and irAEs related cytokine release syndrome (44–47). All of above indicate that IL-6 is not only a target for tumor control but also a contributor to irAEs.
Our study showed that high serum IL-6 levels were associated with both irAEs occurrence and poor outcomes. However, previous reports and our own findings have indicated that irAEs are correlated with better treatment effectiveness in gastrointestinal tumors (48–50). IL-6 appears to exhibit dual effect on ICIs treatment, with implications for both treatment efficiency and irAEs. The mechanisms underlying irAEs-mediated ICIs effectiveness might be highly complex, involving multiple organs, including the lungs, gastrointestinal tract, thyroid, skin, joint, and so on. The different types and grades of irAEs toxicity might exert different effects on different tumors due to variations in the immune microenvironment (51). Previous reports have suggested that only gastrointestinal tract, thyroid and skin related irAEs are associated with better ICIs treatment efficiency (14, 49, 52, 53). We also found that cardiac, hepatic, and pulmonary irAEs displayed negative or neutral effects on ICIs efficiency. The small sample size limits our ability to further evaluation of which irAEs (positive, negative or neutral effect on ICIs efficiency) are related to IL-6 promotion. Additionally, many clinical characteristics, including age, ECOG PS score, TNM stage, and treatment line appear to contribute to ICIs effectiveness beyond irAEs in gastrointestinal tumors. The different tumor microenvironments of each individual, which are not clearly defined, can also affect the final outcome. All of these factors might contribute to the dual effect of IL-6. Further stratified analyses with larger sample size are needed to evaluate the effects of IL-6 on different types of irAEs and the prognostic correlation of different types of irAEs in cancer treatment (53, 54).
This study had some limitations. Firstly, it is retrospective study conducted in a single medical center with relatively small sample size, making it difficult to collect complete paired serum IL-6 data with standard spatial and temporal differences for statistical analysis. Secondly, this study did not completely rule out the effects of adverse reactions resulted from the combined target therapy and chemotherapy.
To our knowledge, this is one of the few real-world studies to reveal the relationship between IL-6 and the effectiveness of ICIs, focusing on gastric and esophageal cancer. These findings may guide us to identify irAEs as early as possible and minimize their adverse effects of irAEs on tumor treatment. Furthermore, the potential predictive value of IL-6 for irAEs and the effectiveness of ICIs treatment may pave the way for future prospective studies involving larger cohorts. These insights may further motivate other researchers to explore the predictive potential of IL-6 in ICIs treatment across a broader range of tumors. This could facilitate the development of more precise patient screening protocols and ultimately contribute to the optimization of the therapeutic benefits of ICIs.
In conclusion, our findings demonstrate that elevated IL-6 not only correlates with the incidence of irAEs but also serves as a prognostic indicator for poorer outcomes in gastric adenocarcinoma and esophageal squamous cell carcinoma patients receiving ICIs. These associations may extend to other malignancies of similar origin, such as colorectal cancer and hepatobiliary cancers. Further investigations are needed to validate IL-6 as both a predictive marker of irAEs occurrence and a treatment target for irAEs management.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://doi.org/10.5281/zenodo.15332218.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this retrospective study only utilized the existing anonymously information for analysis, waiver of informed consent was applied for patients involved.
Author contributions
HM: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition. SZ: Investigation, Methodology, Writing – review & editing, Data curation. PJ: Formal analysis, Methodology, Writing – review & editing, Data curation. HD: Formal analysis, Investigation, Methodology, Writing – original draft. FW: Conceptualization, Investigation, Supervision, Writing – review & editing. YZ: Formal analysis, Methodology, Validation, Writing – review & editing. JW: Formal Analysis, Project administration, Supervision, Validation, Writing – review & editing. ZG: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Medical Science Research Project of Hebei Province (No. 20230908).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1553882/full#supplementary-material
References
1. Jalili-Nik M, Soltani A, Mashkani B, Rafatpanah H, and Hashemy SI. PD-1 and PD-L1 inhibitors foster the progression of adult T-cell Leukemia/Lymphoma. Int immunopharmacol. (2021) 98:107870. doi: 10.1016/j.intimp.2021.107870
2. Nakamura T, Sato T, Endo R, Sasaki S, Takahashi N, Sato Y, et al. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J immunother Cancer. (2021) 9:e002852. doi: 10.1136/jitc-2021-002852
3. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (London England). (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
4. Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. (2023) 24:1181–95. doi: 10.1016/S1470-2045(23)00515-6
5. Satoh T, Kato K, Ura T, Hamamoto Y, Kojima T, Tsushima T, et al. Five-year follow-up of nivolumab treatment in Japanese patients with esophageal squamous-cell carcinoma (ATTRACTION-1/ONO-4538-07). Esophagus. (2021) 18:835–43. doi: 10.1007/s10388-021-00850-0
6. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet (London England). (2021) 398:759–71. doi: 10.1016/S0140-6736(21)01234-4
7. Kato K, Doki Y, Ogata T, Motoyama S, Kawakami H, Ueno M, et al. First-line nivolumab plus ipilimumab or chemotherapy versus chemotherapy alone in advanced esophageal squamous cell carcinoma: a Japanese subgroup analysis of open-label, phase 3 trial (CheckMate 648/ONO-4538-50). Esophagus: Off J Japan Esophageal Society. (2023) 20:291–301. doi: 10.1007/s10388-022-00970-1
8. Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ (Clinical Res ed). (2018) 360:k793. doi: 10.1136/bmj.k793
9. Postow MA, Sidlow R, and Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. New Engl J med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
10. Khoja L, Day D, Wei-Wu Chen T, Siu LL, and Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. (2017) 28:2377–85. doi: 10.1093/annonc/mdx286
11. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis primers. (2020) 6:38. doi: 10.1038/s41572-020-0160-6
12. Das S and Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J immunother cancer. (2019) 7:306. doi: 10.1186/s40425-019-0805-8
13. Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - A systematic review and meta-analysis. Cancer Treat Rev. (2021) 92:102134. doi: 10.1016/j.ctrv.2020.102134
14. Rogado J, Sánchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Leví A, et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer (Oxford England: 1990). (2019) 109:21–7. doi: 10.1016/j.ejca.2018.10.014
15. Socinski MA, Jotte RM, Cappuzzo F, Nishio M, Mok TSK, Reck M, et al. Association of immune-related adverse events with efficacy of atezolizumab in patients with non-small cell lung cancer: pooled analyses of the phase 3 IMpower130, IMpower132, and IMpower150 randomized clinical trials. JAMA oncol. (2023) 9:527–35. doi: 10.1001/jamaoncol.2022.7711
16. Kalinich M, Murphy W, Wongvibulsin S, Pahalyants V, Yu KH, Lu C, et al. Prediction of severe immune-related adverse events requiring hospital admission in patients on immune checkpoint inhibitors: study of a population level insurance claims database from the USA. J immunother Cancer. (2021) 9:e001935. doi: 10.1136/jitc-2020-001935
17. Tanaka T, Narazaki M, and Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. (2014) 6:a016295. doi: 10.1101/cshperspect.a016295
18. Jones SA and Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. (2018) 18:773–89. doi: 10.1038/s41577-018-0066-7
19. Ma H, Xing N, Hou L, Wu J, Sha Z, Wang F, et al. Dicer inhibits hepatocellular carcinoma by inhibiting the interleukin 6 pathway. (2023) 23:e134381. doi: 10.5812/hepatmon-134381
20. Zhao G, Zhu G, Huang Y, Zheng W, Hua J, Yang S, et al. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol reports. (2016) 35:1787–95. doi: 10.3892/or.2016.4544
21. Qiu JG, Wang L, Liu WJ, Wang JF, Zhao EJ, Zhou FM, et al. Apigenin inhibits IL-6 transcription and suppresses esophageal carcinogenesis. Front Pharmacol. (2019) 10:1002. doi: 10.3389/fphar.2019.01002
22. Wang Y, Liu X, Hu G, Hu C, Gao Y, Huo M, et al. EGFR-IL-6 signaling axis mediated the inhibitory effect of methylseleninic acid on esophageal squamous cell carcinoma. Front Pharmacol. (2021) 12:719785. doi: 10.3389/fphar.2021.719785
23. Chen C, Yin H, Zhang Y, Chen H, Xu J, and Ren L. Plasma D-dimer and interleukin-6 are associated with treatment response and progression-free survival in advanced NSCLC patients on anti-PD-1 therapy. Cancer med. (2023) 12:15831–40. doi: 10.1002/cam4.v12.15
24. Zhao N, Yi Y, Cao W, Fu X, Mei N, and Li C. Serum cytokine levels for predicting immune-related adverse events and the clinical response in lung cancer treated with immunotherapy. Front oncol. (2022) 12:923531. doi: 10.3389/fonc.2022.923531
25. Dimitriou F, Hogan S, Menzies AM, Dummer R, and Long GV. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur J Cancer (Oxford England: 1990). (2021) 157:214–24. doi: 10.1016/j.ejca.2021.08.031
26. Yu Y, Wang S, Su N, Pan S, Tu B, Zhao J, et al. Increased circulating levels of CRP and IL-6 and decreased frequencies of T and B lymphocyte subsets are associated with immune-related adverse events during combination therapy with PD-1 inhibitors for liver cancer. Front oncol. (2022) 12:906824. doi: 10.3389/fonc.2022.906824
27. Fa’ak F, Buni M, Falohun A, Lu H, Song J, Johnson DH, et al. Selective immune suppression using interleukin-6 receptor inhibitors for management of immune-related adverse events. J immunother Cancer. (2023) 11:e006814. doi: 10.1136/jitc-2023-006814
28. Hailemichael Y, Johnson DH, Abdel-Wahab N, Foo WC, Bentebibel SE, Daher M, et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell. (2022) 40:509–23.e6. doi: 10.1016/j.ccell.2022.04.004
29. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford England: 1990). (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
30. An J, Gu Q, Cao L, Yang H, Deng P, Hu C, et al. Serum IL-6 as a vital predictor of severe lung cancer. Ann palliative med. (2021) 10:202–9. doi: 10.21037/apm-20-2229
31. Chang CH, Hsiao CF, Yeh YM, Chang GC, Tsai YH, Chen YM, et al. Circulating interleukin-6 level is a prognostic marker for survival in advanced nonsmall cell lung cancer patients treated with chemotherapy. Int J cancer. (2013) 132:1977–85. doi: 10.1002/ijc.v132.9
32. Laino AS, Woods D, Vassallo M, Qian X, Tang H, Wind-Rotolo M, et al. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J immunother Cancer. (2020) 8:e000842. doi: 10.1136/jitc-2020-000842
33. Liu S, Zhou Y, Zhao L, Wang J, Ji R, Wang Y, et al. IL-6 and miR-1271 expression levels in elderly and young gastric cancer patients and correlation analysis with prognosis. Oncol letters. (2019) 17:5419–24. doi: 10.3892/ol.2019.10230
34. Silva EM, Mariano VS, Pastrez PRA, Pinto MC, Castro AG, Syrjanen KJ, et al. High systemic IL-6 is associated with worse prognosis in patients with non-small cell lung cancer. PloS One. (2017) 12:e0181125. doi: 10.1371/journal.pone.0181125
35. Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia (New York NY). (2013) 15:848–62. doi: 10.1593/neo.13706
36. Azar WJ, Christie EL, Mitchell C, Liu DS, Au-Yeung G, and Bowtell DDL. Noncanonical IL6 signaling-mediated activation of YAP regulates cell migration and invasion in ovarian clear cell cancer. Cancer Res. (2020) 80:4960–71. doi: 10.1158/0008-5472.CAN-19-3044
37. Bharti R, Dey G, and Mandal M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: A snapshot of IL-6 mediated involvement. Cancer letters. (2016) 375:51–61. doi: 10.1016/j.canlet.2016.02.048
38. Ishii K, Sasaki T, Iguchi K, Kajiwara S, Kato M, Kanda H, et al. Interleukin-6 induces VEGF secretion from prostate cancer cells in a manner independent of androgen receptor activation. Prostate. (2018) 78:849–56. doi: 10.1002/pros.v78.11
39. Shinriki S, Jono H, Ota K, Ueda M, Kudo M, Ota T, et al. Humanized anti-interleukin-6 receptor antibody suppresses tumor angiogenesis and in vivo growth of human oral squamous cell carcinoma. Clin Cancer Res. (2009) 15:5426–34. doi: 10.1158/1078-0432.CCR-09-0287
40. Zhuang PY, Zhang KW, Wang JD, Zhou XP, Liu YB, Quan ZW, et al. Effect of TALEN-mediated IL-6 knockout on cell proliferation, apoptosis, invasion and anti-cancer therapy in hepatocellular carcinoma (HCC-LM3) cells. Oncotarget> . (2017) 8:77915–27. doi: 10.18632/oncotarget.20946
41. Jeong H, Koh J, Kim S, Yim J, Song SG, Kim H, et al. Cell-intrinsic PD-L1 signaling drives immunosuppression by myeloid-derived suppressor cells through IL-6/Jak/Stat3 in PD-L1-high lung cancer. J immunother Cancer. (2025) 13:e010612. doi: 10.1136/jitc-2024-010612
42. Li Y, Chen Y, Meng Y, Shen M, Yang F, and Ren X. Osimertinib exacerbates immune checkpoint inhibitor-related severe adverse events by activating the IL-6/JAK/STAT3 pathway in macrophages. Cancer Biol med. (2024) 21:1156–70. doi: 10.20892/j.issn.2095-3941.2024.0269
43. Okiyama N and Tanaka R. Varied immuno-related adverse events induced by immune-check point inhibitors - Nivolumab-associated psoriasiform dermatitis related with increased serum level of interleukin-6. Nihon Rinsho Men’eki Gakkai kaishi = Japanese J Clin Immunol. (2017) 40:95–101. doi: 10.2177/jsci.40.95
44. Kim ST, Tayar J, Trinh VA, Suarez-Almazor M, Garcia S, Hwu P, et al. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann rheumatic diseases. (2017) 76:2061–4. doi: 10.1136/annrheumdis-2017-211560
45. Holmstroem RB, Nielsen OH, Jacobsen S, Riis LB, Theile S, Bjerrum JT, et al. COLAR: open-label clinical study of IL-6 blockade with tocilizumab for the treatment of immune checkpoint inhibitor-induced colitis and arthritis. J immunother Cancer. (2022) 10:e005111. doi: 10.1136/jitc-2022-005111
46. Liu LL, Skribek M, Harmenberg U, and Gerling M. Systemic inflammatory syndromes as life-threatening side effects of immune checkpoint inhibitors: case report and systematic review of the literature. J immunother Cancer. (2023) 11:e005841. doi: 10.1136/jitc-2022-005841
47. Stroud CR, Hegde A, Cherry C, Naqash AR, Sharma N, Addepalli S, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract. (2019) 25:551–7. doi: 10.1177/1078155217745144
48. Wang Y, Zhang S, Zhang F, Wang L, Wu C, Zhang X, et al. Young patients show poor efficacy for immune checkpoint inhibitor combined therapy in metastatic gastrointestinal cancers. Front oncol. (2023) 13:1155019. doi: 10.3389/fonc.2023.1155019
49. Xu S, Lai R, Zhao Q, Zhao P, Zhao R, and Guo Z. Correlation between immune-related adverse events and prognosis in hepatocellular carcinoma patients treated with immune checkpoint inhibitors. Front Immunol. (2021) 12:794099. doi: 10.3389/fimmu.2021.794099
50. Zhang X, Xu S, Wang J, Lv Y, Wang N, Lai R, et al. Are anti-PD-1-associated immune related adverse events a harbinger of favorable clinical prognosis in patients with gastric cancer? BMC Cancer. (2022) 22:1136. doi: 10.1186/s12885-022-10199-x
51. Xin Z, You L, Na F, Li J, Chen M, Song J, et al. Immunogenetic variations predict immune-related adverse events for PD-1/PD-L1 inhibitors. Eur J Cancer (Oxford England: 1990). (2023) 184:124–36. doi: 10.1016/j.ejca.2023.01.034
52. Wang Y, Zou J, Li Y, Jiao X, Wang Y, Zhuo N, et al. Serological biomarkers predict immune-related adverse events and clinical benefit in patients with advanced gastrointestinal cancers. Front Immunol. (2022) 13:987568. doi: 10.3389/fimmu.2022.987568
53. Tang K, Seo J, Tiu BC, Le TK, Pahalyants V, Raval NS, et al. Association of cutaneous immune-related adverse events with increased survival in patients treated with anti-programmed cell death 1 and anti-programmed cell death ligand 1 therapy. JAMA Dermatol. (2022) 158:189–93. doi: 10.1001/jamadermatol.2021.5476
Keywords: gastric cancer, esophageal cancer, immune checkpoint inhibitor, interleukin-6, immune-related adverse events, prognosis
Citation: Ma H, Zhang S, Jiao P, Ding H, Wang F, Zhao Y, Wu J and Guo Z (2025) Serum IL-6 predicts immunotherapy-related adverse and outcome in advanced gastric and esophageal cancer patients with Anti-PD-1 treatment. Front. Immunol. 16:1553882. doi: 10.3389/fimmu.2025.1553882
Received: 31 December 2024; Accepted: 13 May 2025;
Published: 30 May 2025.
Edited by:
Jian Song, University Hospital Münster, GermanyReviewed by:
Matias I. Hepp, Universidad Católica de la Santísima Concepción, ChileDipendra Khadka, NADIANBIO Co. Ltd. Wonkwang University School of Medicine, Republic of Korea
Copyright © 2025 Ma, Zhang, Jiao, Ding, Wang, Zhao, Wu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanjun Guo, empndW81ODg2QGhlYm11LmVkdS5jbg==
 Hongfang Ma
Hongfang Ma Shasha Zhang
Shasha Zhang Pengqing Jiao1
Pengqing Jiao1 Zhanjun Guo
Zhanjun Guo