- 1Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Thoracic Surgery, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, China
Lung cancer continues to be the primary cause of cancer-related deaths globally, with non-small cell lung cancer (NSCLC) accounting for approximately 85% of all instances. Recently, immune checkpoint inhibitors (ICIs) have transformed the treatment approach for NSCLC, however, only a subset of patients experiences significant benefits. Therefore, identifying reliable biomarkers to forecast the efficacy of ICIs is crucial for ensuring the safety and effectiveness of treatments, becoming a major focus of current research efforts. This review highlights the recent advances in predictive biomarkers for the efficacy of ICIs in the treatment of NSCLC, including PD-L1 expression, tertiary lymphoid structures (TLS), tumor-infiltrating lymphocytes (TILs), tumor genomic alterations, transcriptional signatures, circulating biomarkers, and the microbiome. Furthermore, it underscores the pivotal roles of liquid biopsy, sequencing technologies, and digital pathology in biomarker discovery. Special attention is given to the predictive value of TLS, circulating biomarkers, and transcriptional signatures. The review concludes that the integration of multiple biomarkers holds promise for achieving more accurate efficacy predictions and optimizing personalized immunotherapy strategies. By providing a comprehensive overview of the current progress, this review offers valuable insights into biomarker-based precision medicine for NSCLC and outlines future research directions.
1 Introduction
Lung cancer is a major cause of cancer-related mortality globally, with NSCLC accounting for approximately 85% of all lung cancer cases (1). In recent years, immunotherapy, particularly ICIs, has become central to treating many lung cancers, including advanced or metastatic cases. Additionally, ICIs are increasingly utilized for early-stage tumors in both neoadjuvant and adjuvant settings. The Food and Drug Administration’s (FDA’s) approval of the anti-CTLA-4 monoclonal antibody ipilimumab in 2011 marked the onset of the ICIs immunotherapy era (2). In 2015, the FDA approved the PD-1 inhibitor nivolumab for treating NSCLC after the failure of first-line platinum-based doublet chemotherapy. In 2016, pembrolizumab was authorized by the FDA as a first-line treatment for patients with high PD-L1 expression (≥50%) (3, 4). Tumor cells evade recognition by and attacks from the immune system by expressing immune checkpoint proteins. ICIs act as antibodies against immune checkpoints such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) or programmed cell death ligand 1 (PD-L1) and programmed cell death protein 1 (PD-1), thereby restoring the ability of immune cells to kill tumor cells. However, the mechanisms of immunotherapy are extremely complex and multifactorial. The complex immune microenvironment of tumors and other characteristics of tumors also contribute to the uncertainty regarding the efficacy of ICIs treatment. Despite advancements in ICIs development for cancer treatment, the majority of patients still do not benefit from these medications. Consequently, discovering biomarkers that can predict the effectiveness of ICIs has become a primary research focus (5). Predictive biomarkers refer to biological characteristics that can predict a patient’s response to immune checkpoint inhibitor treatment, including PD-L1 expression, immune cell infiltration, tumor mutation burden (TMB), circulating markers, and microbiome composition. Identifying these markers not only aids in selecting patients who are most likely to benefit from ICIs – thereby achieving personalized treatment – but also improves the safety and effectiveness of the treatment. Furthermore, by understanding the mechanisms of these markers, researchers can develop new combination therapies to overcome resistance. Therefore, the study of predictive biomarkers predictive biomarkers not only advances the clinical application of ICIs but also lays the foundation for the future development of cancer immunotherapy, which has significant clinical and scientific importance. Additionally, screening for predictive biomarkers can reduce treatment costs for patients receiving ICIs and help optimize the allocation of medical resources.
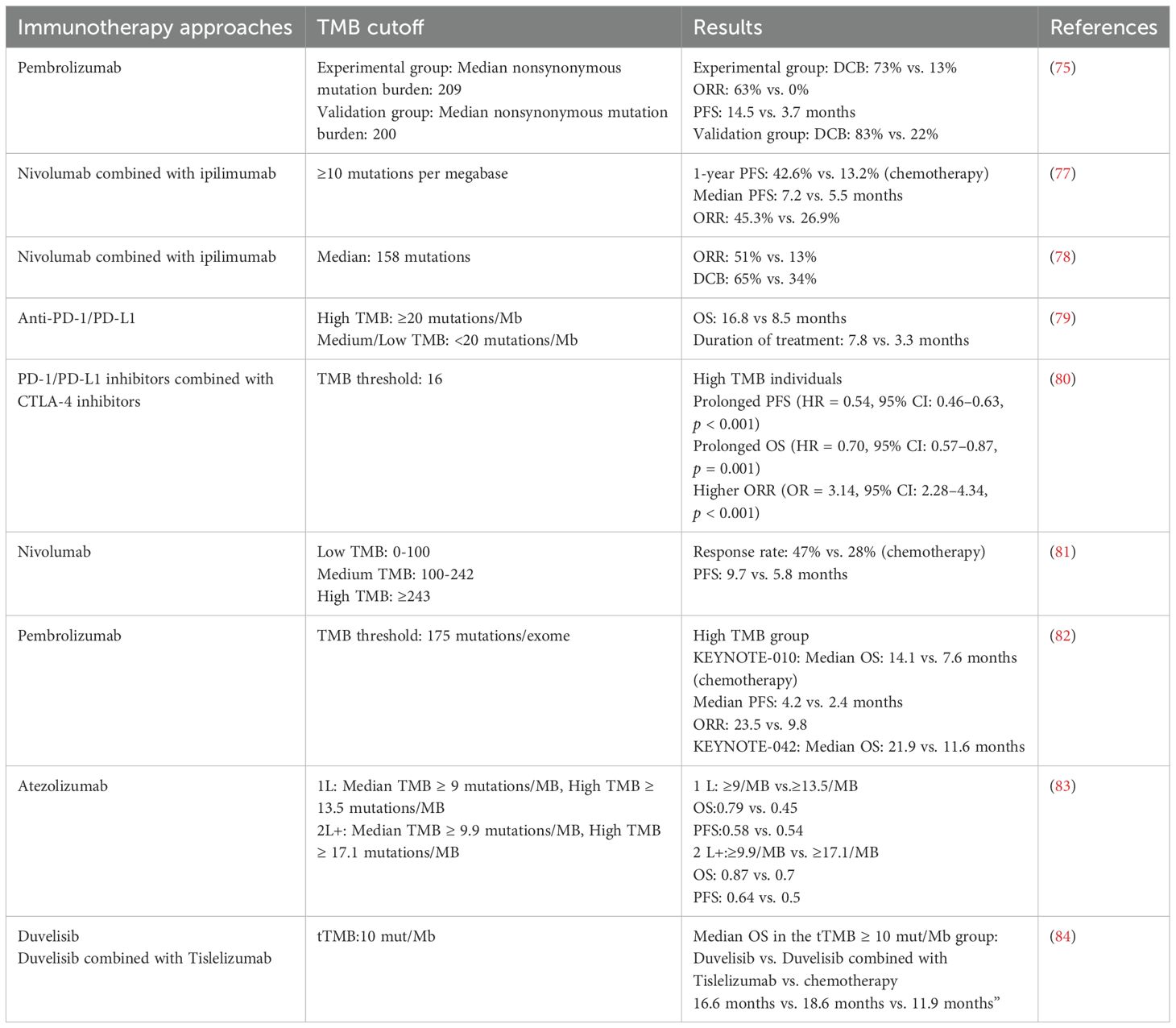
Currently, big data is being applied more widely in the medical field; sequencing technologies are continually being popularized and optimized; the applications of digital pathology and machine learning are becoming increasingly widespread; and our understanding of various biomarkers has increased. The advancement of sequencing technologies has offered robust tools for discovering and applying biological predictive biomarkers. Genomic sequencing of tumor samples can reveal specific gene mutations, whereas transcriptomic sequencing can reflect gene expression levels. Data from epigenomics, proteomics, and metabolomics can also provide support for the discovery of biomarkers. Single-cell sequencing technology allows researchers to study the tumor microenvironment at the level of individual cells, and spatial transcriptomics preserves the spatial information of tumor tissues, thus revealing the relative positions and interactions of different cells within the microenvironment. The application of these new technologies has deepened researchers’ understanding of tumors. Digital pathology analyzes tumor tissue sections via high-resolution images, thereby allowing for a quantitative evaluation of immune cell infiltration within the TME. Machine learning algorithms can process and analyze large amounts of genomic, transcriptomic, and proteomic data to construct predictive models and improve prediction efficiency. The advancement of these new technologies has led to groundbreaking progress in the research of biological predictive biomarkers. The tumor microenvironment provides the immediate context for tumor cells and is essential in their development and progression. The interactions between lymphocytes and stromal cells that form TLSs have become a current research hotspot. TLSs are sites of immune cell accumulation and activation that provide an appropriate microenvironment to promote the maturation and activation of immune cells such as T cells and B cells. Their presence allows immune cells to quickly initiate immune responses near tumor tissue, thus significantly reducing the time required to activate the immune response. The composition, maturation status, and location of TLSs can reflect the immune characteristics of the tumor microenvironment and play crucial roles in predicting the efficacy of ICIs treatment. This review highlights the predictive role of TLSs in the treatment of NSCLC with ICIs. This review also summarizes the latest research advancements on predictive biomarkers, including PD-L1, tumor-infiltrating lymphocytes, TMB, neoantigens, transcriptomic information, circulating tumor DNA, extracellular vesicles, circulating immune cells, circulating tumor cells, specific proteins in peripheral blood, and the microbiome. We discuss the latest research on predictive biomarkers for ICIs efficacy that are under development or in the validation stage, which will play an important role in distinguishing between ICIs responders and nonresponders in the future (Figure 1).
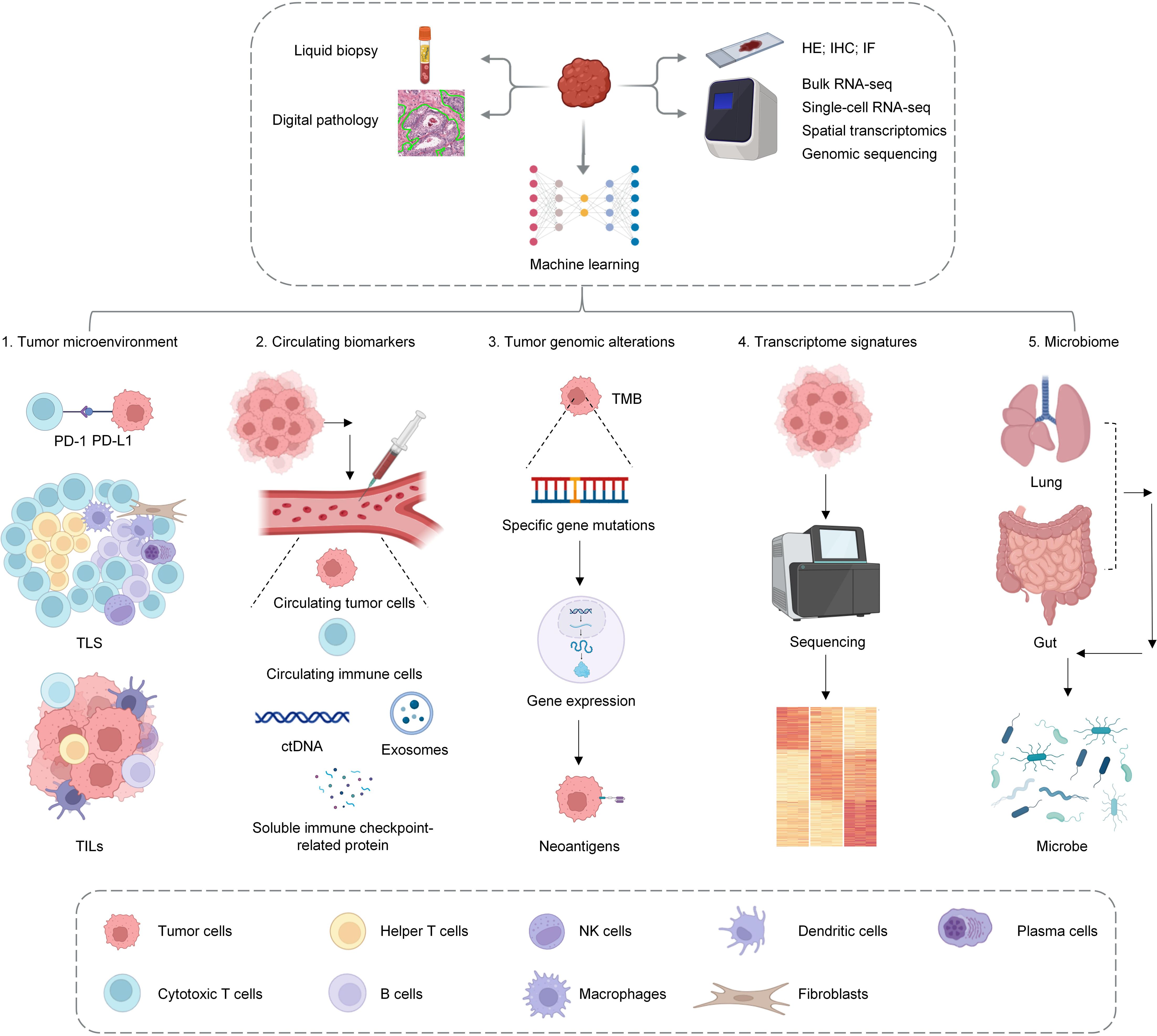
Figure 1. This figure serves as a summary of the main review, illustrating how various advanced techniques, including sequencing technologies, pathological analysis, liquid biopsy, and machine learning, are used to elucidate biomarkers for predicting the efficacy of ICIs. The research focuses on the analysis of the tumor microenvironment (TME), circulating biomarkers, tumor genomic alterations, transcriptional characteristics, and the microbiome.
2 PD-L1
PD-L1 is an immune checkpoint protein that suppresses T-cell activity by interacting with its receptor, PD-1. ICIs (such as PD-1 inhibitors or PD-L1 inhibitors) restore T-cell immune activity against tumors by blocking the interaction between PD-L1 and PD-1. Therefore, high PD-L1 expression may indicate tumor cell sensitivity to ICIs, and methods for detecting PD-L1 are relatively well established, thus making PD-L1 a more practical clinical predictive factor. Consequently, PD-L1 expression levels are currently the most widely accepted and commonly used biomarkers for predicting the efficacy of ICIs, regardless of smoking status (6). PD-L1 can be quantified via the TPS, TC, IC, or CPS (7). The TPS calculation method refers to the proportion of partially or completely membrane PD-L1-stained active tumor cells among all active tumor cells in the sample, and the CPS accounts for the PD-L1 expression of tumor cells and immune cells in the tumor microenvironment. Combining the TPS with the CPS may yield better predictive outcomes (8). Research that was conducted as early as 2012 indicated that a PD-L1 expression level greater than 5% was associated with the efficacy of nivolumab treatment (9). Among NSCLC patients receiving treatment with pembrolizumab, those with a PD-L1 TPS ≥ 50% had an objective response rate (ORR) of 45.2%, whereas the ORR was 16.5% for those with a TPS between 1% and 49% and only 10.7% for those with a TPS < 1% (10). Compared with those receiving chemotherapy, patients with a PD-L1 TPS ≥ 50% who received treatment with pembrolizumab had significantly improved OS and PFS. Additionally, the incidence of grade 3–5 treatment-related adverse events was also significantly lower in the pembrolizumab treatment group (11, 12). In 2016, the FDA approved pembrolizumab as a first-line or second-line treatment for NSCLC patients and indicated the role of PD-L1 expression levels in treatment (13). The CheckMate 227 trial used 1% as the cutoff for PD-L1 expression; in patients with PD-L1 expression levels ≥ 1% and PD-L1 expression levels < 1%, the 5-year survival rates for combination immunotherapy compared with chemotherapy were 24% vs. 14% and 19% vs. 7%, respectively (14). In NSCLC patients receiving CRT combined with durvalumab, those with PD-L1 expression levels ≥ 1% experienced an overall survival benefit (15). However, PD-L1 expression was temporally and spatially specific, and there may be differences between primary tumors and metastatic lesions. The optimal cutoff value for PD-L1 expression also remains unclear (16). Therefore, combining PD-L1 with other predictive indicators may provide a more accurate prediction of the efficacy of ICIs.
3 Tumor microenvironment
Tumors consist of both tumor and non-tumor cells, and the diverse environment created by their interactions is known as the tumor microenvironment (TME).The TME is the local environment with which tumor cells directly interact and consists of tumor cells, immune cells, stromal cells, blood vessels, and extracellular matrix components. The TME is vital in different phases of tumor development and progression (17). Furthermore, the TME can influence tumor growth and metastasis through various mechanisms, as its immunosuppressive effects potentially enable tumor cells to evade immune surveillance, thereby increasing treatment resistance. Previous studies have shown that the composition and characteristics of the TME can significantly affect the response of tumors to immunotherapy. In predicting patient survival, the quantity, density, and location of immune cells within the tumor may be more advantageous than traditional TNM staging (18). In recent years, due to advancements of high-throughput technologies, researchers have made significant progress in understanding the TME. Furthermore, the role of the TME in predicting the efficacy of ICIs has also attracted an increasing amount of attention.
3.1 Tumor-infiltrating lymphocytes
3.1.1 CD8+ T cells
In tumor-infiltrating lymphocytes, CD8+ T cells play a crucial role as the primary effector cells in antitumor immunity. ICIs can reactivate CD8+ T cells. The efficacy of ICIs might be associated with the status of CD8+ T cells. Assessing the presence and functionality of CD8+ T cells in the TME could help identify patients who are most likely to benefit from ICIs treatment. Single-cell RNA sequencing (scRNA-seq) of NSCLC samples, both before and after anti-PD-1 treatment, showed that patients who respond to treatment have a higher number of CD8+ T cells, suggesting their potential as predictors of anti-PD-1 therapy success. Researchers have discovered two subsets of Texp cells: one with high GZMK expression and another with low expression of genes associated with exhaustion. These Texp cells significantly increase in the number of reactive tumors following anti-PD-1 treatment (19). Another study that used scRNA-seq revealed that following the use of ICIs combined with chemotherapy, the major pathological response (MPR) group presented more pronounced increases in Tem (CD8_GZMK), Trm (CD8_GZMB), and circulating effector T cells (CD8_STMN1) than the nonresponders (TN) (20). A previous meta-analysis evaluated the prognostic role of CD8+ tumor-infiltrating lymphocytes (TILs) among cancer patients receiving ICIs, including those with NSCLC. The results indicated that patients with high CD8+ T-cell infiltration had improved OS, PFS, and ORRs compared with those with lower levels of CD8+ T-cell infiltration. The quantity of CD8+ T cells in both the tumor and stroma was associated with OS and PFS in patients receiving ICIs treatment (21). In a retrospective study, the authors assessed the impact of baseline CD8+ TILs on patient prognosis. The results indicated that in the CRT + ICIs treatment group, a high density of CD8+ TILs (TIL high ≥ 100/mm²) was significantly associated with longer PFS (NR vs. 9.5 months; p = 0.002), whereas in the CRT alone group, CD8+ TILs had no effect on prognosis (22). In metastatic NSCLC patients receiving anti-PD-1 treatment, the response rate was only 16.7% when the number of CD8+ TILs was < 886/mm²; the response rate increased to 60% when the number of CD8+ TILs ranged from 886–1899/mm² (23). However, the thresholds for the level of CD8+ T-cell infiltration vary across different studies, which limits the application of CD8+ T cells in predicting the efficacy of ICIs. Additionally, not all CD8+ TILs can be reactivated by ICIs; some CD8+ T cells do not contribute to antitumor immunity (24, 25). In some studies, total CD8+ T cells could not predict the efficacy of ICI treatment. Furthermore, researchers have explored the ability of cells coexpressing CD8+ T cells and other cell surface markers to predict ICIs efficacy. A previous study revealed that CD39 can serve as a tumor-specific T-cell marker and that CD39+ CD8+ T cells can predict the response of NSCLC patients to PD-1 or PD-L1 blockade treatment, thus serving as an independent predictive indicator. The proportion of CD39+ CD8+ T cells was significantly higher in responders (partial response) than in nonresponders (stable disease or disease progression) (20). CD8+ T cells with high PD-1 expression reflect a high affinity for tumor antigens, indicating the important role of these cells in antitumor immunity. Compared with nonresponders, ICIs-treated responders have significantly higher levels of PD-1 expression on their CD8+ T cells (26). The finding that CD8+ T cells express PD-1 typically indicates that the cytotoxic function of these cells is suppressed. PD-1+ CD8+ TILs can continuously secrete CXCL13, thereby recruiting CXCR5-expressing B cells and Tfh cells into the TME, thus playing an important role in the formation of TLSs. The level of PD-1 expression in CD8+ T cells that exceeded that of healthy donor PBMCs was defined as PD-1T. In NSCLC patients, the proportion of PD-1T cells among responders to PD-1 blockade therapy was significantly greater than that among nonresponders, and PD-1 T cells were associated with improved OS (HR 0.16 (95% CI 0.05–0.52),p < 0.05) (27). Granzyme B (GZB) and Ki-67 expression in T cells represents T-cell activation and proliferation, respectively. Low expression of GZB and Ki-67 suggests that T cells are in a dormant state. PD-1 blockade can restore the effector function of these T cells. Among TILs, those with high CD3 expression and low GZB and Ki-67 expression benefit significantly from PD-1 blockade therapy (28).
3.1.2 Immune-inhibitory cells
The tumor-suppressive microenvironment is influenced by various inhibitory immune cells, including myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), regulatory T cells (Tregs), tumor-associated neutrophils (TANs), and cancer-associated fibroblasts (CAFs), along with inhibitory cytokines like IL-6, IL-8, and TGF-β. This suppressive environment may be linked to unfavorable outcomes in immunotherapy (16, 29). Immunosuppressive cells in peripheral blood (such as MDSCs) can predict the efficacy of ICIs therapy (29).Within the TME, inhibitory immune cells also contribute to forecasting the effectiveness of ICIs therapy. In one previous study, the authors used scRNA-seq to analyze the changes in T cells before and after PD-1 blockade treatment in NSCLC. They reported that the frequency of Tregs slightly reduced in responders, whereas the frequency of Tregs significantly increased in nonresponders. Furthermore, in Tregs, the expression levels of genes associated with immunosuppression, such as IL1R2, REL, and LAYN, are increased (19).ICIs therapy can reactivate CD8+ T cells by stimulating TCR and CD28 pathways and can similarly enhance the function of inhibitory immune cells like Tregs using the same mechanisms. Thus, the balance between these two cell types may impact the effectiveness of ICIs treatment. In NSCLC patients who respond to PD-1 blockade therapy, CD8+ T cells show high levels of PD-1 expression, whereas non-responders have Tregs with high PD-1 expression. The ratio of PD-1 high-expressing CD8+ T cells to PD-1 high-expressing Tregs serves as a predictor for the efficacy of ICIs therapy. Optimal results are observed when CD8+ T cells exhibit high PD-1 expression while Tregs display low PD-1 expression (26). Using scRNA-seq to analyze changes in macrophages before and after ICIs treatment, Macro_SPP1 macrophages were shown to promote tumor angiogenesis, In contrast, Macro_SELENOP is noted for its anti-inflammatory effects. Both subtypes of macrophages exhibit strong M2 (anti-inflammatory or tumor-promoting) characteristics and are classified as tumor-associated macrophages (TAMs). After ICIs treatment, the number of Macro_SPP1 macrophages decreased, whereas the number of Macro_SELENOP macrophages increased in NMPR patients. Senescent Neu_CCL3 neutrophils can recruit Macro_SPP1 macrophages, thereby forming an inhibitory TME. After ICIs treatment, the number of senescent neutrophils decreased in MPR patients, with the most severe depletion observed in senescent Neu_CCL3 cells, whereas the number of senescent neutrophils increased in NMPR patients. After receiving anti-PD1 treatment, CAF-related gene signatures were significantly more prevalent in patients with disease progression (PD) compared to those who achieved a complete response (CR) or partial response (PR) (30).
3.1.3 Tumor-infiltrating B cells
Tumor-infiltrating T cells are often directly distributed within the tumor and make direct contact with tumor cells. In contrast, tumor-infiltrating B (TIB) cells are located primarily in TLSs (31).B cells located in TLSs are linked to the response of antitumor antibodies and the proliferation of CD4+ T cell clones. These B cells from TLSs boost the activity of effector T cells through cytokine production. In NSCLC patients with high-density TLS B cells, the proportion of activated and memory CD4+ T cells is greater, whereas the proportion of Tregs is lower. These findings suggest that TLS B cells are crucial for the prognosis of NSCLC patients and the antitumor T-cell response (32, 33). Researchers have reported that B cells and TLS-related genes (such as CXCL13) are associated with the efficacy of immunotherapy among patients with NSCLC receiving neoadjuvant immunochemotherapy (34). After neoadjuvant treatment with pembrolizumab combined with chemotherapy, tumors from major pathological response (MPR) patients presented a greater abundance of B cells than non-MPR patients did. Additionally, neoadjuvant immunotherapy can induce B-cell class switching and antibody responses (35).
Further research using sc-RNA seq was performed to analyze the TME of NSCLC patients after neoadjuvant anti-PD-1 therapy combined with chemotherapy. The authors reported that FCRL4+FCRL5+ B cells (atypical memory B cells) were located in TLSs, and the abundance of these B cells was significantly greater in MPR patients than in non-MPR patients. These B cells not only increase the efficacy of immunotherapy but also serve as predictive factors for the response of NSCLC patients to ICIs treatment (36). Additionally, studies on mouse models and human lung adenocarcinoma have shown that B cells present in TLSs can produce antibodies that target endogenous retroviruses (ERVs), thereby inhibiting tumor progression. ICIs treatment can expand the B-cell response to target ERVs, thereby increasing the antitumor capacity of ERV-reactive antibodies and prolonging survival in mouse models (37). Furthermore, previous research has indicated that effective immunotherapy relies on CXCL13-mediated TLS formation and that therapeutic administration of CXCL13 can be combined with ICIs to enhance antitumor immune responses (37).
3.2 Tertiary lymphoid structures
3.2.1 Introduction to TLS
Secondary lymphoid organs (SLOs) are essential in triggering adaptive immune responses against tumors. During this process, dendritic cells with tumor antigens travel from the tumor site to the SLO. There, they present these antigens to T cells and B cells through the major histocompatibility complex (MHC), which activates effector T cells and memory B cells to inhibit tumor growth (38). Under the persistent influence of chronic inflammatory factors, TLSs with secondary lymphoid organ-like structures and functions may form in nonlymphoid tissues (39, 40). Guided by chemokines such as CXCL13 and cytokines such as IL-7, lymphocytes and myeloid cells gradually accumulate, thus forming TLSs that include components such as follicular dendritic cells, follicular helper T cells, B cells, T cells, mature dendritic cells, and fibroblastic reticular cells (40). TLSs can exist in the stroma (extratumoral), parenchyma (intratumoral), or infiltrative margins (peritumoral) of tumor tissues. They gradually develop from initial lymphocyte aggregation into mature TLSs. The formation of TLSs within tumor tissues has significant biological implications, as TLSs bypass the process of dendritic cells and lymphocytes migrating from the tumor site to secondary lymphoid organs (SLOs). This enables T cells and B cells to rapidly initiate immune responses near the tumor tissue, significantly shortening the time required for the immune response to activate (41).
TLSs encompass several components, such as adjacent zones for T cells and B cells, the presence of PNAd+ high endothelial venules (HEVs), B cell class switching, mature DC-Lamp+ dendritic cells located in the T-cell zone, and chemokine expression. However, it can sometimes be challenging to determine whether a TLS fully meets all of these criteria, and the structure of the TLS may vary depending on location and inflammatory stimuli. Therefore, some researchers have suggested that these structures with specific tissue characteristics can be collectively referred to as TLSs (42, 43). TLSs play a positive role in promoting antitumor immune responses. Generally, the presence of TLSs within tumor tissues is associated with better treatment responses and lower recurrence rates (40, 43). Given the potential impact of TLSs on the TME, researchers suggest that TLSs might be linked to the prognosis of patients undergoing tumor immunotherapy. Furthermore, inducing the formation of TLSs as an independent therapeutic approach or in combination with other (immune) therapies could enhance tumor treatment efficacy. Studies have shown that ICIs treatment can promote the development of TLSs within tumor tissues (44).
3.2.2 Methods for evaluating TLSs
In-depth exploration of TLSs requires accurate assessment of TLSs. Hematoxylin and eosin (H&E) staining and immunohistochemical (IHC) staining are two commonly used methods for TLS evaluation. H&E staining of tumor tissue sections enables the morphological evaluation of the presence and localization of TLSs. In H&E-stained sections, TLSs appear as clusters of T cells surrounding clusters of B cells. When mature TLSs are present, germinal centers can be clearly observed, thus enabling the accurate identification of mature TLSs in H&E-stained sections. Although H&E staining provides an overview of tissue structure, it may not fully reveal the fine details of TLSs, and immature or early TLSs may be overlooked because of the lack of clear boundaries between B and T-cell regions, potentially leading to an underestimation of TLS quantity. In contrast, IHC staining can more accurately identify the presence and distribution of TLSs when specific antibodies are used to mark particular cell types and molecules. For example, B cells can be marked with CD20, T cells can be marked with CD3, and follicular dendritic cells (FDCs) can be marked with CD23 (Table 1). Methods such as H-DAB and H&E-DAB provide specific labeling of target antigens, thus offering practical grounds for a more accurate identification of TLS presence and maturity. IHC staining can accurately identify early TLSs on the basis of the aggregation of CD20 B cells and CD3 T cells. Furthermore, IHC staining can clearly reveal the formation of CD21+CD23+ FDC networks in secondary TLSs, as well as the follicular structure formed by CD20 B cells and CD23 FDCs, thus distinguishing these cells from early or primary TLSs. While IHC staining partially compensates for the shortcomings of H&E staining, traditional IHC typically enables the detection of only 1–2 markers on a single section. To enable the simultaneous observation of multiple markers on a single section, multiplex immunohistochemistry (mIHC) plays an important role in the assessment of TLSs (39, 40, 45, 46). Whole-slide imaging (WSI) technology combined with image analysis software provides the possibility for automated quantitative analysis of stained slices, thus enhancing the efficiency and accuracy of TLS assessment (47).
Gene expression profiles can also be used for TLS assessment. By using RNA-seq to detect gene expression related to cell populations or chemokines, a comprehensive evaluation of TLS status can be achieved. In earlier studies, researchers reported that the expression profiles of 12 chemokines could predict the presence of ectopic lymphoid structures in invasive colorectal cancer and melanoma (48). In public databases, grouping these 12 chemokines to obtain TLS+ and TLS- cells can predict early recurrence of hepatocellular carcinoma (49). Other studies have also indicated that TLS-related gene expression profiles are positively correlated with the number of TLSs detected by IHC (50). Single-cell RNA sequencing (scRNA-seq) and spatial transcriptomics technologies can provide more in-depth transcriptomic data for TLS research. These methods can accurately assess TLSs and reveal the complex interactions and dynamic changes in TLSs within the tumor microenvironment, thus offering new perspectives for understanding the role of TLSs in tumor immunity (40).
Additionally, radiomics imaging methods, as novel approaches for assessing TLSs, are currently under active exploration. Combining imaging with advanced learning models and digital pathology can increase the visualization potential of TLSs. By analyzing images, TLS-related information can be obtained directly. However, this technology is still in the research stage (51) (Figure 2).
Figure 2. This figure presents a comprehensive overview of the diverse methodologies employed for the detection of TLS. The techniques highlighted include hematoxylin and eosin (H&E) staining for basic structural visualization, immunohistochemistry (IHC) staining for identifying specific cellular markers, bulk RNA sequencing for understanding gene expression profiles, single-cell sequencing for detailed cellular insights, spatial transcriptomics for mapping gene expression in situ, and radiomics analysis for non-invasive imaging assessments. These advanced techniques collectively enhance our ability to study and understand the complex role of TLS in various biological contexts.
3.2.3 TLSs’ capability to forecast the effectiveness of ICIs
TLSs are essential in the tumor immune microenvironment of NSCLC, as they create a vital local setting for activating immune cells and modulating immune responses. The presence of TLSs is closely associated with the outcomes for NSCLC patients. Tumors that are TLS-positive typically exhibit higher levels of tumor-infiltrating B cells, CD8+ T cells, and Th cells, indicating a more favorable immune environment (52). TLSs are closely related to the efficacy of ICIs treatment because they promote antitumor immune responses and enhance local immune responses.
The maturity of TLSs reflects their functional effectiveness. Mature TLSs can effectively promote interactions between T cells and B cells, thereby triggering a stronger antitumor immune response. Additionally, immunotherapy can enhance the maturation of TLSs, thereby increasing tumor sensitivity to treatment. Therefore, assessing the maturity of TLSs may provide an important basis for optimizing immunotherapy regimens. The presence and density of TLSs are closely related to the patient’s immune response. Studies have shown that higher TLS density is often associated with better prognosis. The location of TLSs has been confirmed to be related to treatment efficacy and disease prognosis in various tumors; however, more in-depth research is needed to further elucidate the relationship between TLS location and NSCLC immunotherapy. Immunotherapy may improve treatment effectiveness by activating TLSs and enhancing local immune responses. This provides a potential biomarker for clinical practice, helping to identify patients who are likely to respond well to immunotherapy and improving treatment success rates (Figure 3, Table 2).
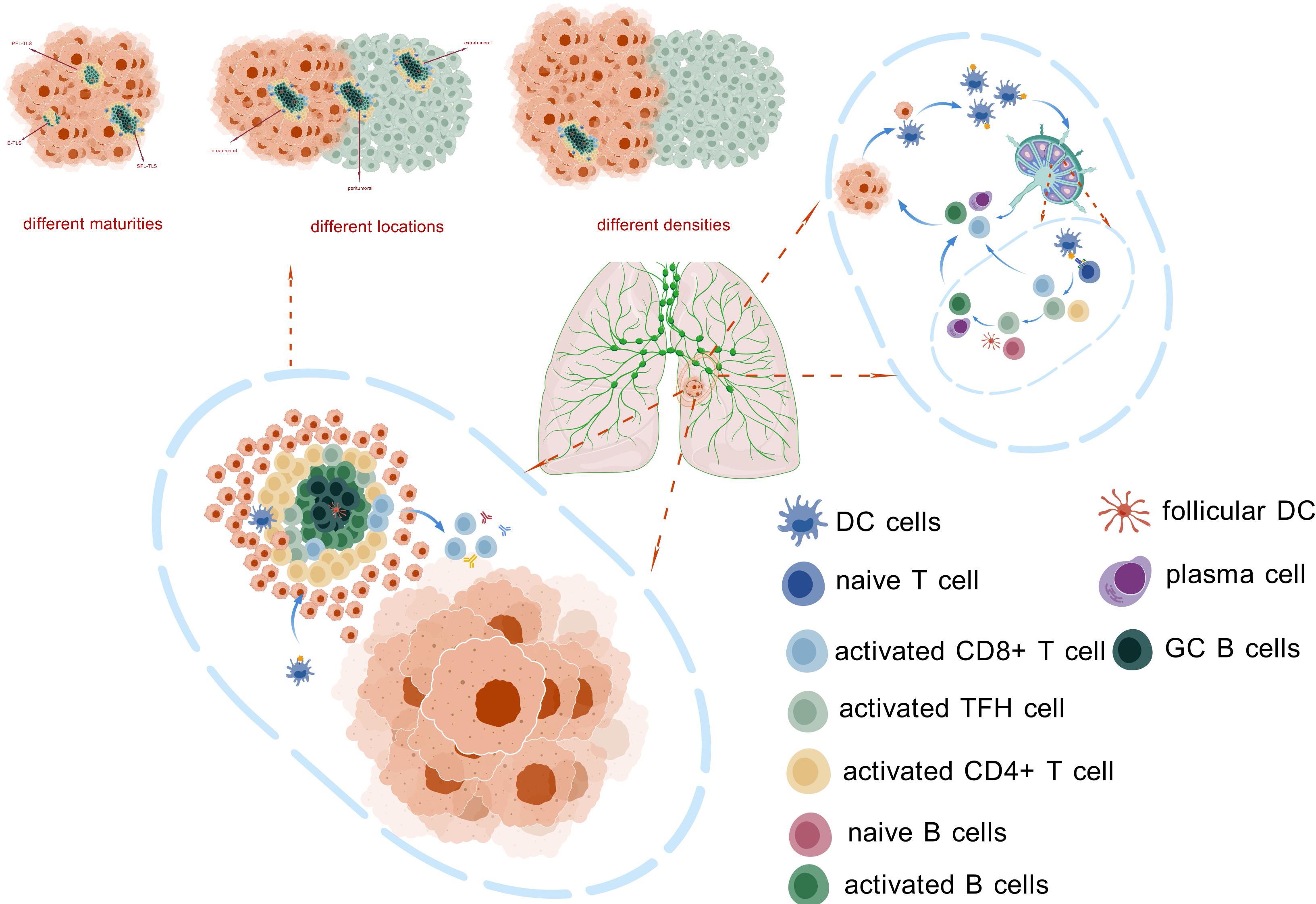
Figure 3. This figure illustrates the composition and function of TLS. These structures can be found at various locations within tumor tissues, exhibiting different levels of maturity and density. The presence of TLS facilitates the activation of antigen-presenting cells (APCs) and anti-tumor immune cells, enabling them to bypass the traditional migration process between tumor sites and draining lymph nodes. This allows for the rapid initiation of immune responses in close proximity to the tumor, potentially enhancing the effectiveness of anti-tumor immunity. The figure highlights the crucial role of TLS in modulating the tumor microenvironment and contributing to local immune regulation.
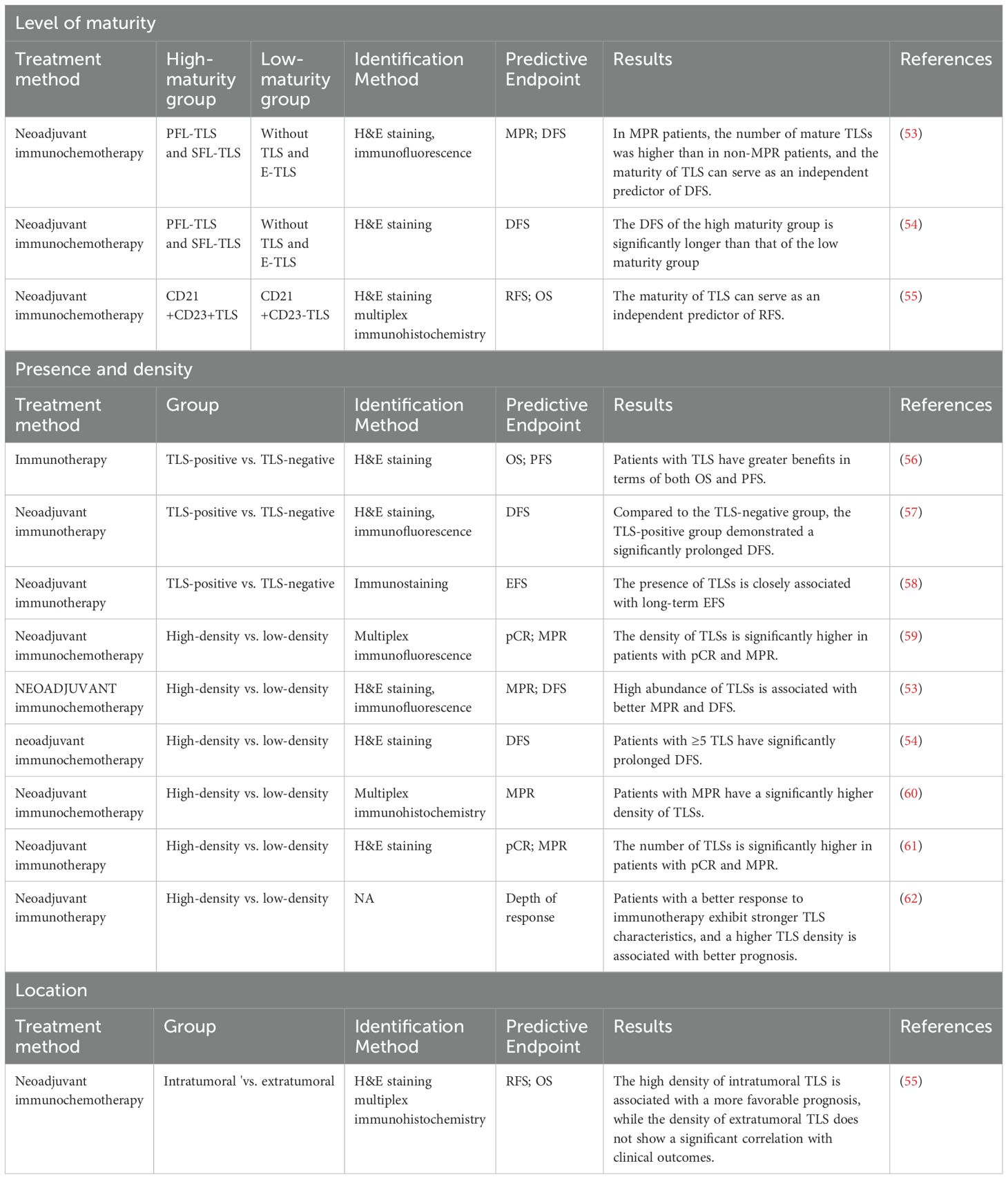
Table 2. The role of TLS with different maturation levels, locations, and densities in ICIs efficacy.
3.2.3.1 The ability of TLSs with varying degrees of maturity to predict the efficacy of ICIs
In NSCLC treatment, the maturity of TLSs is strongly linked to the pathological response and effectiveness of neoadjuvant therapy. The maturation of TLSs starts with the development of HEVs and the clustering of T cells and B cells, which gradually develop into mature structures. This process is typically divided into three stages: early TLS (E-TLS), primary follicle-like TLS (PFL-TLS), and secondary follicle-like TLS (SFL-TLS). The E-TLS stage is characterized by the accumulation of T cells and B cells, but germinal centers and follicular dendritic cells have not yet formed. During the PFL-TLS stage, a network structure of CD21+ follicular dendritic cells (FDCs) is established within the B-cell region, although mature germinal centers have not yet appeared. The SFL-TLS stage marks the maturation of TLSs, featuring a complete CD21+CD23+ FDC network structure and active germinal centers, with a clear separation between the B-cell and T-cell zones. This structure is capable of supporting a comprehensive local immune response, including B-cell activation and antibody production (44, 63, 64).
In patients with lung adenocarcinoma, the presence of mature TLSs is closely associated with improved OS and DFS, and the presence of mature TLSs serves as an independent low-risk factor for lymph node (LN) metastasis (65). Research on lung squamous cell carcinoma has revealed that neoadjuvant chemotherapy significantly reduces the proportion of SFL-TLS and the size of germinal centers, suggesting that TLS maturation is suppressed. At this time, the predictive value of TLS density for survival in patients is lost (64).
Based on the maturity of TLSs, a previous study divided NSCLC patients into a low-maturity group (no TLSs and E-TLSs) and a high-maturity group (PFL-TLSs and SFL-TLSs). Among the 40 patients in the neoadjuvant immunochemotherapy group, 30 exhibited high-maturity TLSs; in the chemotherapy-only group of 41 patients, only 13 had high-maturity TLSs; and in the untreated control group of 40 patients, 25 displayed high-maturity TLSs. The data suggest that neoadjuvant chemotherapy may suppress the maturation process of TLSs, whereas ICIs treatment may induce TLS maturation (53). Further analysis revealed that in the neoadjuvant immunochemotherapy group, 45.0% (n=18) of patients achieved major pathological response (MPR), with 14 patients reaching pathologic complete response (pCR). In the neoadjuvant chemotherapy group, only 17.1% (n=7) of patients achieved MPR, with 2 patients reaching pCR. Patients with MPRs had higher levels of mature TLSs than non-MPR patients did, and TLS maturity was an independent predictor of disease-free survival (DFS) in the neoadjuvant immunochemotherapy group (53). The disease-free survival (DFS) of the high-maturity group (PFL-TLS and SFL-TLS) was significantly longer than that of the low-maturity group (no TLS and E-TLS), with median DFS durations of 34.07 months and 22.30 months, respectively (p = 0.024). These findings indicate that the maturity of TLSs is an important factor in predicting DFS after neoadjuvant therapy (54). Additionally, both the number of immature TLSs and mature TLSs was greater in the neoadjuvant immunochemotherapy cohort than in the neoadjuvant chemotherapy cohort. In the neoadjuvant immunochemotherapy group, the maturity of TLSs was identified as an independent predictor of recurrence-free survival (RFS), a finding that was not observed in the neoadjuvant chemotherapy group (55). After neoadjuvant immunotherapy, the presence of mature TLSs was associated with an increase in CD8+ T-cell density and enhanced infiltration of these cells into the tumor epithelial region, indicating an increase in immune activation within the tumor microenvironment (58). These observations suggest that, compared with the inhibitory effect of neoadjuvant chemotherapy on TLS maturation, neoadjuvant immunochemotherapy enhances TLS maturity. Furthermore, high-maturity TLSs are associated with an improved immune response in the tumor microenvironment and are correlated with better prognosis in patients receiving immunotherapy.
3.2.3.2 The presence and density of TLSs as predictors of ICIs efficacy
In some tumor tissues, lymphocytes and myeloid cells aggregate and interact to form TLSs; however, TLSs are not present in all tumor samples, and their density can vary significantly. TLSs are significantly linked to tumor prognosis and are crucial in antitumor immune responses and the tumor microenvironment. A high TLS density often correlates with a stronger adaptive immune response within the tumor. Consequently, the presence and density of TLSs can predict the effectiveness of ICIs.
The IMpower110 trial was a clinical study that focused on patients with NSCLC and primarily evaluated the efficacy and safety of atezolizumab as a single-agent immunotherapy. The main endpoints of the trial included PFS and OS. In this study, TLSs were defined as lymphoid aggregates (LAs) containing one or more germinal centers. The results indicated that patients with TLSs experienced greater benefits in terms of both OS and PFS, suggesting that the presence of TLSs may be closely associated with the effectiveness of neoadjuvant immunotherapy (56). Additionally, TLS formation was more frequently observed in patients who responded to neoadjuvant immunochemotherapy, whereas it was rarely detected in nonresponders. Compared with the TLS-negative group, the TLS-positive group demonstrated significantly longer DFS, further emphasizing the importance of TLSs in neoadjuvant immunotherapy (57). Changes in the number and size of TLSs were observed after neoadjuvant immunotherapy, and the presence of TLSs was closely associated with long-term event-free survival (EFS) (p < 0.001) (58).
A greater density of TLSs was found to be associated with achieving pCR and MPR after neoadjuvant treatment among NSCLC patients who received neoadjuvant immunochemotherapy (66). In some studies, the authors explored TLSs in trials of neoadjuvant immunochemotherapy and neoadjuvant chemotherapy. Specifically, samples without TLSs were scored as 0; those with 1 to 2 TLSs were scored as 1; those with at least three TLSs were scored as 2; and samples with a high density of TLSs were scored as 3. The results indicated that the number of TLSs was significantly greater in the immunochemotherapy group than in the chemotherapy group. More importantly, a high number of TLSs was found to be associated with better MPR and longer DFS, further validating the potential value of TLSs in assessing the effectiveness of neoadjuvant treatment (53). Researchers have categorized samples into high-expression and low-expression groups on the basis of the number of TLSs observed in the field among NSCLC patients receiving neoadjuvant anti-PD-1/PD-L1 therapy combined with chemotherapy. Specifically, samples with fewer than 5 TLSs in 10 fields were defined as having low expression, whereas samples with ≥5 TLSs were defined as having high expression. The results revealed that patients with high expression of TLSs had significantly prolonged DFS (34.07 vs. 22.30 months, p = 0.041) (54). In studies of lung squamous cell carcinoma, patients who received neoadjuvant immunotherapy had a greater TLS density than did those who did not receive neoadjuvant treatment. In patients who respond more effectively to immunotherapy, TLS features are more evident, and a greater TLS density is linked to a more favorable prognosis (62).
Overall, the TLS density in the neoadjuvant immunochemotherapy cohort was generally greater than that in the neoadjuvant chemotherapy cohort. Neoadjuvant immunotherapy has the potential to promote TLS formation, suggesting that TLSs play an active role in enhancing patients’ immune responses. In NSCLC patients receiving neoadjuvant immunochemotherapy, those who achieved pCR and MPR were defined as the response group, whereas patients with poor treatment response were defined as the nonresponse group. The results indicated that after neoadjuvant treatment, TLS infiltration increased, and this increase was more pronounced in the response group than in the nonresponse group (59).
Combined analysis of inflammatory biomarkers and TLS results revealed that both TLSs and the PLR can be used to predict the MPR rate in NSCLC patients receiving neoadjuvant immunochemotherapy. Notably, using the combination of the PLR and TLS to assess the MPR in NSCLC patients is more accurate than using either indicator alone. These findings suggest that the combined use of TLSs and traditional biomarkers may increase the accuracy of prognostic assessment (67). In patients who achieved the MPR after neoadjuvant immunochemotherapy, the TLS density was significantly greater (60). In a study of patients with multiple primary lung cancers receiving neoadjuvant pembrolizumab treatment, researchers examined lesions at three sites. After neoadjuvant therapy, TLS formation was observed in all three types of nodules, and compared with nonresponsive nodules, responsive nodules exhibited a greater extent of TLS formation (68).
In a phase II clinical trial (NCT02259621) investigating neoadjuvant therapy, researchers reported that the number of TLSs was significantly greater in pCR and MPR patients than in NR patients (with ≥90% residual RVT in posttreatment samples), suggesting that TLS formation is positively correlated with a favorable treatment response (61). Furthermore, in patients receiving neoadjuvant durvalumab treatment, an analysis of surgical resection tumor samples posttreatment revealed a significant increase in TLS gene expression in MPR patients (69). After neoadjuvant treatment with pembrolizumab combined with chemotherapy, the gene expression of CXCL13 and 12 other chemokines was significantly elevated in tumor tissue. Additionally, mIHC observations revealed a marked increase in TLS density in tumor lesions following neoadjuvant immunotherapy (35). In summary, TLSs play a crucial role in the treatment of NSCLC with ICIs. Their density is significantly associated with pathological responses, such as pCR and MPR, and a high TLS density typically indicates a better prognosis. These findings suggest that TLSs are not only effective biomarkers for assessing immune therapy responses but also potential predictors of long-term survival in patients.
3.2.3.3 The spatial location of TLSs predicts the efficacy of ICIs
TLSs are highly heterogeneously distributed in NSCLC, as they can form in multiple regions of the tumor tissue, including the stroma (extratumoral), parenchyma (intratumoral), and invasive margins (peritumoral) (41). This variation in spatial distribution not only indicates the tumor’s biological behavior but may also significantly impact the treatment response.
In studies of NSCLC, the positivity rate of TLSs within the tumor is usually significantly higher than that in the peritumoral and extratumoral regions, and the average TLS density within the tumor is also markedly greater than that in other areas. The location of TLSs within tumor tissue is not only related to tumor growth and metastatic potential but also closely associated with the clinical and pathological features of patients. For example, a high density of TLSs in the peritumoral or extratumoral regions may be linked to tumor invasiveness and unfavorable prognostic factors. In other types of cancer, such as liver cancer and cholangiocarcinoma, the different locations of TLSs also have been shown to exert various impacts on tumor growth and treatment response (70). The localization of TLSs suggests that they play complex roles in the tumor microenvironment. In patients receiving neoadjuvant immunochemotherapy for NSCLC, a high density of intratumoral TLSs was associated with a more favorable prognosis, whereas the density of extratumoral TLSs is not significantly correlated with clinical outcomes in the two treatment cohorts (55). In the context of immunotherapy, the localization of TLSs may have significant implications for the selection of treatment strategies and the prediction of treatment efficacy. For example, immunomodulatory therapies targeting intratumoral TLSs may more effectively enhance local immune responses, thereby improving the efficacy of neoadjuvant treatment. The localization of TLSs could help identify patients who are most likely to benefit from specific therapies. However, research on the localization of TLSs in NSCLC is still relatively scarce, thus highlighting the urgent need for more systematic research to further elucidate their specific roles in tumor progression and treatment response.
4 Tumor genomic alterations
The tumor cell genome can undergo changes, and during DNA replication, a DNA damage repair (DDR) system exists that identifies and corrects replication errors. Defects in the DDR system can lead to the continuous accumulation of genomic abnormalities, which is a significant cause of tumor development (71, 72). Among them, mismatch repair deficiency (dMMR) can lead to microsatellite instability (MSI) (5, 73); TMB quantifies somatic mutations in tumor cells. Typically, TMB counts nonsynonymous single-nucleotide mutations, but in some cases, TMB may also include synonymous mutations as well as insertions and deletions (indels) (73, 74). All of the above genomic changes can generate neoantigens, and peptide segments containing these neoantigens can be presented on the cell surface, where they may be recognized by T cells (74). The recognition of neoantigens generated by mutations in T cells is an important reason for the efficacy of anti-PD-1 therapy (75). Therefore, tumor genomic alterations and neoantigens are hypothesized to be predictive markers for the efficacy of ICIs among NSCLC patients. In NSCLC, mismatch repair deficiency and high microsatellite instability are relatively rare, but it is one of the tumors with the highest TMB (74). The somatic mutation differences between smokers and nonsmokers are significant, resulting in a wide range of TMBs in NSCLC (75, 76). Multiple studies have confirmed that TMB serves as a predictive marker for ICIs treatment efficacy in NSCLC patients (Table 3), independent of PD-L1 expression levels (81).
Detection of the average copy number variation (CNVA) of chromosome fragments reflects genomic instability by measuring the average copy number changes in small chromosome segments. In studies of NSCLC patients receiving immunotherapy, the high-CNVA group presented elevated PD-L1, CD39, and CD19 expression levels, as well as increased infiltration levels of CD8+ T cells and CD3+ T cells. These findings indicate a high immune infiltration status in the tumor tissue and suggest that the CNVA may serve as a potential alternative indicator for predicting the efficacy of immunotherapy in patients with NSCLC (85).
Additionally, specific gene mutations in NSCLC influence the effectiveness of ICIs. Different gene mutations correspond to varying levels of tumor PD-L1 expression and TMB. For instance, EGFR-mutant NSCLC generally has lower levels of both PD-L1 expression and TMB, while KRAS-mutant NSCLC often shows higher levels. EGFR mutations are common in NSCLC, with the most prevalent forms being exon 19 in-frame deletions and the L858R point mutation in exon 21, accounting for over 90% of all EGFR mutations. EGFR mutations can influence the components of the TME. For example, they can upregulate chemokines such as CXCL10 and CCL2 through the PI3K-AKT-mTOR pathway, further recruiting Tregs and M2 macrophages while reducing the infiltration of CD8+ T cells, Th cells, NK cells, and M1 macrophages. This process promotes the formation of an immunosuppressive microenvironment, which is detrimental to the efficacy of ICIs (86, 87). In NSCLC subtypes with changes in EGFR, HER2, ALK, ROS1, or RET, solely targeting the PD-L1–PD-1 axis provides limited benefits. In contrast, those with KRAS and TP53 co-mutations tend to have better responses to PD-L1-PD-1 blockade, whereas patients with KRAS mutations alongside STK11 and/or KEAP1 often experience poorer outcomes with ICIs treatment (86).
Epigenetic modifications can influence gene expression without altering the DNA sequence itself. These modifications not only play a crucial role in the development and progression of tumors but also have the potential to predict the efficacy of ICIs to some extent. Through mechanisms such as DNA methylation and histone modifications, epigenetic processes can impact the expression of genes associated with immune checkpoints and tumor-associated antigens, thereby affecting the efficacy of ICIs (16, 88).For instance, a study shows that genome-wide methylation patterns characterized by promoter hypermethylation can predict the efficacy of immunotherapy response in NSCLC (89). In another study involving stage IV NSCLC patients treated with anti-PD-1 agents, a DNA methylation signature known as EPIMMUNE was found to correlate with both PFS and OS (90). Histone deacetylases (HDACs) are a group of enzymes that remove acetyl groups from histones, playing a significant role in regulating gene expression, which is crucial for cellular processes such as proliferation, differentiation, and apoptosis. HDAC8 (histone deacetylase 8) has been shown to restore the effector functions of CD8+ T cells, thereby enhancing the therapeutic effects of anti-PD-1 treatment in NSCLC (91). Additionally, research has indicated that the expression of HDAC6 can serve as a prognostic marker for NSCLC patients undergoing ICIs treatment. Furthermore, the combination of HDAC inhibitors with PD-1 inhibitors has demonstrated the potential to reduce tumor growth rates and create a more favorable TME for cytotoxic T lymphocytes (92). Moreover, the integration of epigenetic alterations with liquid biopsy technologies presents new avenues for predicting the efficacy of ICIs in NSCLC. Biomarkers based on DNA methylation in ctDNA show great promise for applications in screening, early diagnosis, and predicting as well as monitoring responses to specific therapies (93). While existing studies have highlighted the relationship between ctDNA methylation and targeted therapies, the predictive role of ctDNA methylation regarding ICIs efficacy warrants further investigation (93). In summary, epigenetic modifications are closely related to anti-tumor immune responses, and ongoing research in this field may lead to the discovery of novel biomarkers for predicting the efficacy of ICIs.
5 Transcriptome signatures
Biomarkers based on gene expression are extensively utilized in oncology. The use of transcriptomic signals to reflect the expression levels of specific genes can predict the efficacy of ICIs treatment. This can be achieved through a comprehensive analysis of transcriptional signals related to T-cell activation, antigen presentation, and the IFNγ pathway. IFNγ is a key regulator of the immune system and plays a crucial role in antitumor immunity. It is also closely linked to PD-L1 expression levels. Research on different tumors has demonstrated that mRNA levels related to IFNγ can predict the effectiveness of PD-1 blockade therapy (94). In a group of NSCLC patients undergoing treatment with durvalumab, those with increased expression levels of genes encoding IFNγ, CD274, LAG3, and CXCL9 were defined as IFN-γ+. Compared with IFN-γ− patients, IFN-γ+ patients exhibited significant improvements in the ORR, median OS, and median PFS (95)., Mutations in genes associated with the IFNγ signaling pathway can lead to poor efficacy of ICIs treatment (5). In NSCLC patients receiving atezolizumab treatment, those with higher levels of T-cell effector and IFNγ-related gene expression experienced prolonged overall survival (96). The IFNG gene encodes IFN-γ, and NSCLC patients treatment with receiving nivolumab treatment who had a high expression of IFNG demonstrated significantly prolonged PFS (97). The GDPLichi score, which is composed of seven DNA damage repair-related genes (DUT, MGMT, POLH, RAD1, RAD17, TYMS, and YWHAG), categorizes patients into high-risk and low-risk groups. Compared with the low-risk group, the high-risk group presented significantly greater TMB, higher neoantigen levels, and higher expression levels of PD-L1, PDCD1, and CTLA4. Therefore, patients in the high-risk group are more sensitive to immunotherapy, and the GDPLichi model can be used to predict the efficacy of ICIs in patients with lung adenocarcinoma (72). In NSCLC patients undergoing anti-PD-L1 therapy, CSF1R and HCST expression levels showed a positive correlation with PD-L1 levels and a high presence of CD8+ T cells, indicating their potential predictive value for prognosis (98). Additionally, TCR coexpressed gene signatures have also been confirmed as predictive factors for ICIs treatment in NSCLC, with elevated expression levels indicating a better prognosis (99).
6 Circulating biomarkers
Circulating biomarkers refer to biomolecules that are present in the blood or other bodily fluids, such as ctDNA and exosomes, that can reflect tumor characteristics or treatment responses (Figure 4). They provide a noninvasive method for early diagnosis, prognosis, and monitoring treatment efficacy, thus representing a significant advancement in clinical practice (100).Circulating tumor DNA (ctDNA) is composed of fragments shed into the bloodstream by tumor cells. Analyzing ctDNA can reveal tumor-specific genetic changes, and tracking ctDNA levels or modifications (like ctDNA methylation) can indicate the effectiveness of ICIs therapy in NSCLC. The blood tumor mutational burden (bTMB) is assessed through ctDNA analysis. In the MYSTIC trial, NSCLC patients with bTMB ≥ 20 mut/Mb who received combination immunotherapy showed enhanced overall survival (84). Previous research indicated that a high ctDNA mutational burden was associated with better efficacy of ICIs treatment across various cancer types, including NSCLC (101). Moreover, ctDNA levels can also serve as a predictive biomarker. In the B-F1RST trial, NSCLC patients receiving atezolizumab treatment with low levels of ctDNA (max somatic allele fraction (MSAF) <1%) had ctDNA amounts that were insufficient for accurate bTMB assessment. However, compared with patients with an MSAF ≥1%, the ORR was significantly better among those with an MSAF <1% (34.5% vs. 10.1%) (102). In a study on ICIs treatment for metastatic NSCLC, the authors quantified ctDNA by measuring the allele fraction of cancer-associated somatic mutations in plasma, defining a ctDNA response as a reduction of more than 50% in the mutant allele fraction. The results indicated that the ctDNA response was associated with improved PFS and OS, and it could predict treatment efficacy at an earlier stage (103). NSCLC patients with molecular residual disease (MRD) following chemoradiotherapy received consolidation ICIs treatment. During the treatment period, two different ctDNA response types were observed: an increase in ctDNA or a decrease in ctDNA. Patients with elevated ctDNA concentrations showed poor response to ICIs treatment, with all of them experiencing disease progression within 4.5 months after the initiation of consolidation therapy. Conversely, patients with decreased ctDNA concentrations benefited from consolidation ICIs treatment (104). In NSCLC patients undergoing immunochemotherapy, alterations in the ctDNA allele fraction (AF) correlated with imaging changes and long-term clinical outcomes. Patients with a reduction in AF had a significantly higher response rate compared to those with an increased AF (60.7% vs. 5.8%, p = 0.0003). Moreover, both median PFS and OS were improved (105). The abovementioned study demonstrated the value of ctDNA for predicting the efficacy of ICIs treatment in NSCLC patients. However, currently, there is no standard process for the collection and handling of ctDNA, and there are limited clinical application data, which restricts its use in efficacy prediction (106).
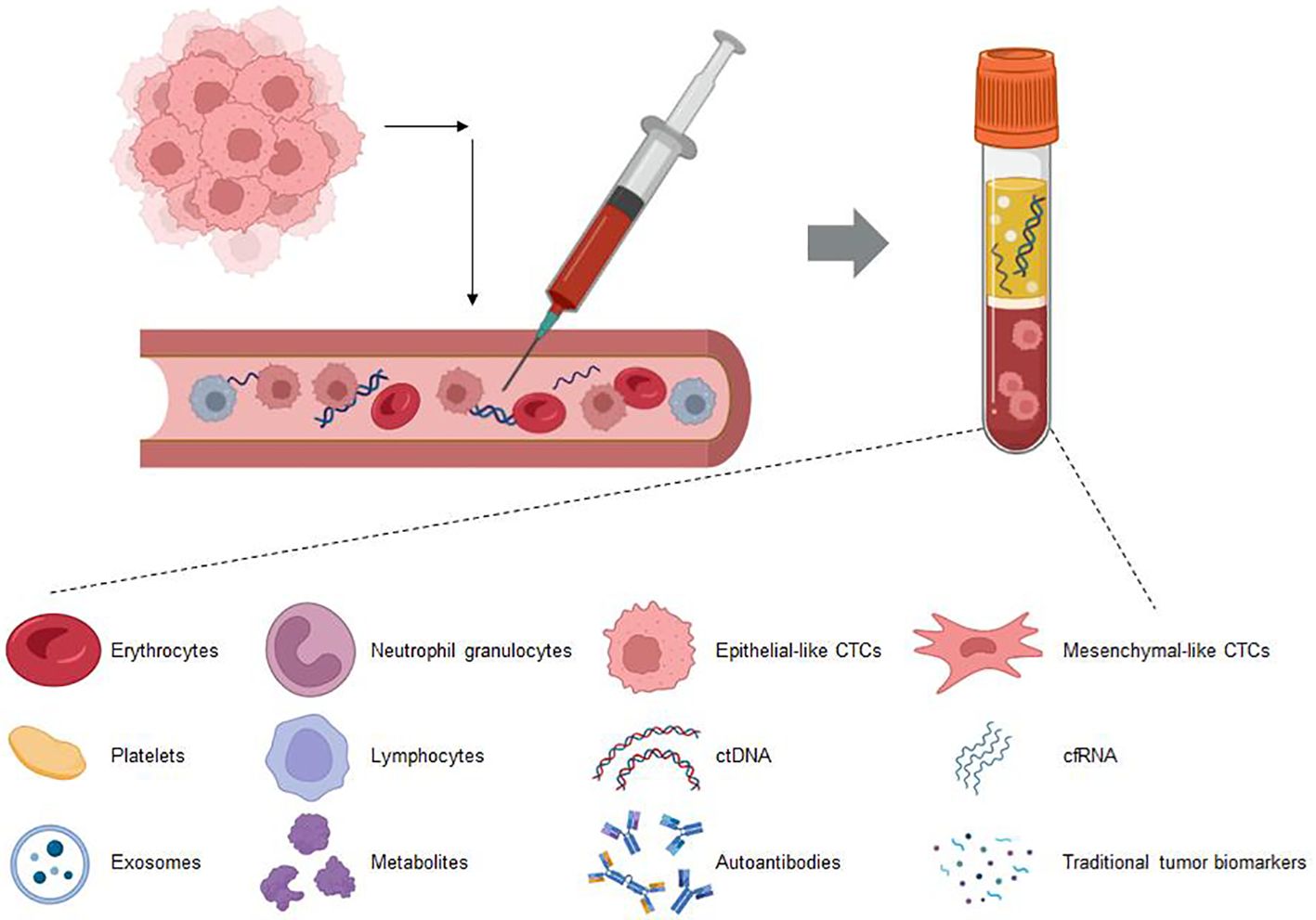
Figure 4. This figure showcases the various biomarkers detectable in blood circulation through liquid biopsy techniques. These biomarkers include circulating tumor DNA (ctDNA), exosomes, circulating immune cells, circulating tumor cells (CTCs), and soluble proteins. Liquid biopsy provides a minimally invasive method to capture these diverse components, offering valuable insights into the molecular and cellular dynamics within the body. This approach holds significant promise for early diagnosis, monitoring disease progression, and tailoring personalized treatment strategies in oncology.
Circulating immune cells also have a certain predictive function for the efficacy of ICIs treatment. For example, in NSCLC patients treated with ICIs, the number of CD4+CD25+CD127loFoxP3+ Treg cells significantly decreased compared with that at baseline in patients with pseudoprogression one week after treatment, whereas the number of these cells markedly increased in patients with hyperprogression. In the responder group, both CD4+CD25+CD127loFoxP3+ Treg cells and PD-1+CD4+CD25+CD127loFoxP3+ Treg cells were significantly reduced (107).In NSCLC patients treated with anti-PD-1 antibodies, elevated baseline levels of circulating CD4+CCR9+, CD4+CCR10+, or CD8+CXCR4+ T cells were linked to poorer overall survival (15.7 vs. 35.9 months, HR 0.16, p = 0.003; 22.0 vs. not reached, HR 0.10, p = 0.003; and 22.0 vs. not reached, HR 0.29, p = 0.02) (108). In a cohort of advanced NSCLC patients treated with atezolizumab, an increase in the lymphocyte ratio was observed in patients with disease control, whereas a significant decrease was noted in patients with disease progression. Additionally, a decrease in circulating CD4+ and CD8+ T cells and an increase in Tregs and MDSCs were observed in the disease progression group, whereas the opposite changes in circulating immune cells were observed in the disease control group (109)In NSCLC patients undergoing nivolumab treatment, an elevated central memory/effector T-cell ratio was linked to better PFS, indicating higher tumor PD-L1 expression levels. Conversely, an increase in exhausted cells and a reduction in memory effector CD8+ T cells were associated with disease progression (110, 111). The neutrophil-to-lymphocyte ratio (NLR) is determined by comparing the absolute numbers of circulating neutrophils to lymphocytes. The NLR is closely related to the innate immune system and can reflect the tumor status (112). In NSCLC patients receiving neoadjuvant ICIs therapy, a decrease in the NLR of more than 10% after four weeks of treatment has been shown to be associated with tumor regression and the MPR. Additionally, patients with a decreased NLR had improved PFS and OS (113–115). In NSCLC patients with a PD-L1 TPS ≥50% and without EGFR or ALK mutations treated with pembrolizumab, those with a dNLR <2.6 had a significantly improved ORR, median PFS, and median OS compared with those with a dNLR ≥2.6 (116). During the first six weeks of nivolumab treatment, early increases in cfDNA and the NLR indicate poorer survival outcomes in advanced NSCLC patients, suggesting the potential role of these biomarkers in real-time monitoring of immunotherapy resistance (117).Moreover, a higher myeloid-to-lymphoid cell ratio (M:L) and elevated absolute neutrophil count were linked to shorter PFS and OS (118). A low platelet-to-lymphocyte ratio (PLR) and a low monocyte-to-lymphocyte ratio (MLR) are associated with better PFS (119).Furthermore, there are additional potential predictive biomarkers in circulation. Blood-based soluble immune checkpoint-related proteins, like soluble PD-L1 (sPD-L1), are linked to the prognosis of advanced lung cancer and might serve as potential indicators for predicting the effectiveness of ICIs (120).The potential of sPD-L1 as an alternative marker for PD-L1 TPS is still being studied. After treatment with nivolumab, either an increase or stabilization in plasma sPD-L1 levels has been associated with a more favorable prognosis (121). Increased preoperative sPD-L1 levels were found to be associated with poor prognosis (122). Serum granzyme B is released by cytotoxic CD8+ T cells and NK cells. An analysis of stage IV NSCLC patients receiving nivolumab treatment revealed that patients with lower baseline serum granzyme B levels had poorer PFS and OS than those with higher levels (123). Circulating tumor cells (CTCs) refer to cancer cells released from the primary tumor that enter the bloodstream or lymphatic system and may form metastases in other parts of the body. An analysis of CTCs in advanced NSCLC patients receiving nivolumab treatment revealed that the presence of CTCs or the expression of PD-L1 on the surface of CTCs could reflect treatment efficacy: patients with PD-L1+ CTCs were more likely to experience disease progression. However, owing to the small sample size of the study, further experiments are needed for validation (124).Extracellular vesicles (EVs), such as exosomes and microvesicles, carry bioactive molecules and are crucial in cellular communication (125). Previous research has indicated that EVs derived from tumor tissue can serve as noninvasive biomarkers. Higher levels of costimulatory molecules such as CD9, CD81, and CD63 in EVs are associated with an ICIs response and a better ORR (126). EVs can carry small molecular substances such as miRNAs, which have also been confirmed to be associated with ICIs efficacy.EV-miR-625-5p has been recognized as an independent biomarker for predicting the response to ICIs in NSCLC patients with PD-L1 expression levels of 50% or higher (127, 128).EVs offer a novel method for detecting PD-L1, where higher levels of exosomal PD-L1 are linked to improved ORR and OS (129).Research on patients with malignant melanoma and NSCLC undergoing anti-PD-1 antibody therapy showed that two months post-treatment, the PD-L1 mRNA levels in plasma exosomes significantly decreased in responders. In contrast, there was no notable change in patients with stable disease, while those with disease progression experienced a significant increase (130). Researchers have proposed a biosensor for the quantitative detection of the EVs PD-1/PD-L1 mRNA (Au SERP); this method achieved an accuracy of 72.2% in distinguishing between ICIs responders and nonresponders (131). EVs have broad application prospects in the exploration of noninvasive biomarkers.
7 Microbiome
The microorganisms that reside in the human gut, skin, and other mucosal surfaces are collectively referred to as the microbiome. They engage with the human immune system and contribute to the establishment and progression of both innate and adaptive immunity (132). Recent studies have uncovered a connection between the microbiome and the effectiveness of ICIs therapy. Research on the microbiome in lung cancer has focused primarily on gut microorganisms. Currently, studies have also revealed the impact of lower respiratory tract microbes on lung cancer and their potential ability to predict lung cancer treatment outcomes (133–135). Pulmonary microorganisms in the human body are associated with various diseases, and the composition of the lung microbiome differs across lung cancer patients. Research has shown that Veillonella dispar predominates in lung cancer patients with high PD-L1 expression, whereas the abundance of Neisseria species is significantly greater in patients with low PD-L1 expression. Furthermore, V. dispar is prevalent among those who respond to immunotherapy, while Haemophilus influenzae and Neisseria perflava are more frequently found in non-responders (136). The microbiome is linked to immune-related adverse events (iRAEs) in lung cancer patients receiving immunotherapy. Those who did not encounter iRAEs had a more diverse gut microbiota, including Bifidobacterium and Desulfovibrio. In patients who respond to chemotherapy combined with immunotherapy, there is an increase in Clostridiales and a decrease in Rikenellaceae (137). In NSCLC patients receiving ICIs treatment, the abundance of Akkermansiaceae is greater in those with stable disease and partial response than in those with disease progression (138, 139). In patients receiving ICIs treatment who never use antibiotics, the α diversity of the gut microbiome is associated with OS. In patients with a good ORR and PFS greater than 6 months, Ruminococcaceae UCG 13 and Agathobacter were more abundant. Additionally, there are differences in the gut microbiota between patients with high-grade and low-grade iRAEs (140). In NSCLC patients receiving anti-PD-1 treatment, those with higher microbial diversity have prolonged PFS compared with those with low microbial diversity. There are also differences in the gut microbiota composition between responders and nonresponders (7). However, the microbiome exhibits significant interindividual variability and is easily influenced by environmental factors, which limits its application in predicting the efficacy of ICIs.Advancements in detection technologies are necessary to assess the potential of the microbiome in personalized treatment.
8 Discussion
NSCLC, the most common form of lung cancer, significantly impacts global health. Immunotherapy is becoming an increasingly vital part of NSCLC treatment. However, a majority of patients still fail to respond favorably to immunotherapy. As a result, ongoing research is largely concentrated on discovering biomarkers that can forecast the effectiveness of immunotherapy. This helps in selecting patients who are more likely to benefit, thereby improving the overall success rate.
The TME is the local environment in which tumor cells are directly embedded, and its components and functions play crucial roles at various stages of tumorigenesis. Immunotherapy can cause localized alterations in the TME, and the components of this microenvironment may also play important roles in predicting the efficacy of immunotherapy. Tumor-infiltrating lymphocytes are immune cells that infiltrate tumor tissues, and the interactions between these immune cells can regulate the immune state of the tumor microenvironment, which is closely correlated with patient prognosis. As the main cell type involved in antitumor immunity, high infiltration of CD8+ T cells is associated with better outcomes in immunotherapy. Additionally, the expression of other surface markers on CD8+ T cells may reflect changes in their function, and certain types of CD8+ T cells (such as CD39+CD8+ T cells and PD-1+CD8+ T cells) may predict benefits from immunotherapy more effectively than total CD8+ T-cell infiltration alone. There are also immunosuppressive cells in the TME, such as Tregs, TANs, TAMs, and CAFs. Typically, CAFs are considered to have multiple tumor-promoting functions, including promoting tumor cell proliferation and angiogenesis in tumor tissues, and are associated with tumor invasion, metastasis, and drug resistance (141). However, recent results from scRNA-seq and spatial transcriptomics analyses have shown that certain subtypes of CAFs are closely associated with the formation of TLSs, which can recruit B cells and T cells, thus playing a significant role in antitumor immunity (142, 143). Owing to the significant heterogeneity of CAFs, more in-depth studies of CAFs based on single-cell and spatial transcriptomics analyses are needed. Tumor-infiltrating B cells are found mainly in TLSs, where they can produce antitumor antibodies and enhance effector T-cell responses. Higher B-cell infiltration correlates with better prognosis. Moreover, studies have shown that in patients with advanced NSCLC who have liver metastases undergoing immunotherapy with ICIs, the organ-specific response rate (OSRR) and organ-specific disease control rate (OSDCR) of liver metastases are lower than those of lung tumors. Further research has found that compared to lung tumors, the infiltration ratios of CD8+ T cells and CD56dim+ NK cells in liver metastases are significantly reduced (p = 0.036 and p = 0.016). Additionally, in liver metastases, the expression of immune activation-related genes (such as CD8A, LCK, and ICOS) is downregulated, and liver metastases exhibit more pronounced immunosuppressive characteristics, such as a higher proportion of Tregs and a lower capacity for effector T cell recruitment. These findings further emphasize the important role of the TME components in the efficacy of ICIs. The immunosuppressive microenvironment of liver metastases may limit the effectiveness of ICIs therapy. For example, the lower infiltration of CD8+ T cells and NK cells in liver metastases, along with the downregulation of immune activation-related genes, may hinder the effective activation of anti-tumor immune responses through immunotherapy. Given the immunosuppressive features of liver metastases, the research suggests that combination therapies (such as ICIs with anti-angiogenic agents or adoptive cell therapy) may be more beneficial in improving the effects of immunotherapy. Immune treatments targeting liver metastases need to consider their unique immunological microenvironment characteristics and develop more precise therapeutic strategies (144). TLSs are aggregates of immune cells that form within tumor tissues and exhibit structures and functions similar to those of secondary lymphoid organs. The formation of these structures indicates a more favorable immune state in the tumor, allowing immune cells to quickly initiate immune responses near the tumor and play a significant role in antitumor immunity. Their maturation status, presence, density, and location can partially predict the efficacy of immunotherapy. Compared with immature TLSs, mature TLSs have active germinal centers that support comprehensive local immune responses, and the presence of mature TLSs in tumor tissues can better predict the efficacy of immunotherapy. Not all tumor tissues can develop TLSs, and a high density of these structures suggests a better response to immunotherapy. TLSs can be found within the tumor, in the surrounding tissue, or outside the tumor; this spatial distribution reflects the biological behavior of the tumor and may also influence the treatment response. A high density of TLSs within the tumor is associated with better outcomes in immunotherapy. As an immune checkpoint molecule, PD-L1 binds to its ligand PD-1 to inhibit T-cell activity, and the expression level of PD-L1 is currently the most widely accepted and utilized biomarker for predicting the efficacy of ICIs. When PD-L1 is highly expressed, patients tend to have better outcomes following immunotherapy. Although the TPS is widely used to predict ICIs response, PD-L1 detection is typically based on IHC, which relies on the quality of tumor samples. Additionally, different laboratories use varying staining standards, and establishing a cutoff for PD-L1 is also a challenge. Therefore, the use of PD-L1 alone as a predictive indicator may not yield satisfactory results (145, 146). In squamous cell carcinoma, IHC detection of PD-L1 levels does not effectively predict ICIs efficacy (147). In recent years, studies have attempted to combine PET imaging with specific radiolabeled tracers to detect immune checkpoint proteins such as PD-L1 (148–150). PD-L1 expression levels can also be detected in CTCs; however, whether this indicator can serve as a predictive biomarker for ICIs efficacy remains unclear. Some studies have suggested that high PD-L1 expression in CTCs indicates a better prognosis (151–153); however, there are also results indicating no correlation between the two factors (154).
Genomic alterations can occur in tumor cells, and the TMB can be used to quantify somatic mutations in these cells. An increased TMB is linked to the production of additional neoantigens that can be identified by T cells. NSCLC is one of the tumor types with the highest TMB, suggesting that a high TMB is correlated with better immunotherapy efficacy, independent of PD-L1 expression levels. Importantly, less than 10% of nonsynonymous mutations induce immunogenicity, and currently, there is no definitive threshold for TMB to accurately predict the efficacy of ICIs, thereby limiting the clinical application of TMB as a biomarker (155). Nevertheless, the TMB combined with other predictive biomarkers (such as PD-L1 expression and the NLR) still has strong predictive power (156). The average copy number variation of chromosomal fragments reflects genomic instability and may serve as a potential surrogate marker for TMB. Specific types of genetic mutations in tumor cells are also linked to the efficacy of immunotherapy. A comprehensive analysis of transcriptional signals related to T-cell activation, antigen presentation, and the IFNγ pathway can also serve the purpose of predicting therapeutic efficacy. Currently, researchers are working to identify gene expression-based features that can accurately predict the efficacy of ICIs; however, the existing studies are in the early stages and require further investigation for clinical application (157).
Certain circulating biomarkers can also act as indicators predicting the effectiveness of immunotherapy. Circulating tumor DNA (ctDNA) consists of fragments released by tumor cells into the bloodstream, and ctDNA analysis can identify tumor-specific genetic alterations. The bTMB can be determined through an analysis of ctDNA, with a higher bTMB suggesting better outcomes following immunotherapy. Additionally, monitoring the dynamic changes in ctDNA levels can provide insights, as a decrease in ctDNA concentration during immunotherapy is associated with a favorable treatment response. Specific types of immune cells in the circulation are also linked to the efficacy of immunotherapy; for example, a decrease in the NLR may indicate better treatment outcomes. Other biomarkers, such as soluble PD-L1 (sPD-L1) and serum granzyme B, can also predict responses to immunotherapy to some extent. However, some studies have indicated that the levels of plasma sPD-L1 may not be significantly correlated with patient overall survival (158). Therefore, further exploration is needed. Peripheral blood biomarkers represent a significant advancement in clinical practice; however, owing to their relatively late development, their clinical utility still has certain limitations. The microbiome contributes to the development of innate and adaptive immunity and is linked to the effectiveness of immunotherapy. Studies have shown that specific types of microorganisms residing in the gut or lungs can predict the effectiveness of immunotherapy. However, the microbiome exhibits significant variability among individuals and is easily influenced by environmental factors, which limits its application in predicting the efficacy of immunotherapy. Future advancements in detection technologies are needed to determine the potential of the microbiome in personalized treatment.
TILs and TLSs reflect the immune status of the TME, while PD-L1 expression levels can indicate the immune evasion of tumor cells to some extent. The occurrence of mutations in tumor cells and the generation of neoantigens facilitate T-cell recognition and are crucial mechanisms for the efficacy of immunotherapy. Liquid biopsy technology can be used to detect substances released from tumor tissue into the circulation, allowing researchers to conveniently analyze specific characteristics of tumor tissue and monitor dynamic changes in real time. The microbiome can influence both the innate and adaptive immune responses of the host. The various predictive biomarkers for immunotherapy efficacy mentioned above affect antitumor immune responses through different mechanisms and can predict efficacy to some extent. However, the structural complexity of tumor tissues and their interactions with the host lead to challenges in predicting the efficacy of treatment based on a single biomarker. Currently, the only biomarker that has been approved and widely used for predicting the efficacy of immunotherapy in NSCLC is PD-L1. Nevertheless, as mentioned earlier, there are several issues with the use of PD-L1 alone to predict treatment efficacy. Other biomarkers, such as the TMB, have shown potential in predicting response in some clinical trials but have not yet been approved as standalone biomarkers for immunotherapy. A comprehensive analysis involving multiple biomarkers may better reveal the intrinsic characteristics of tumor tissues in different individuals. By leveraging new technologies such as machine learning and artificial intelligence, a holistic assessment of various biomarkers could yield improved predictive ability.
The widespread adoption of new technologies – particularly sequencing technologies – has led to a deeper understanding of biological predictive markers. However, a single marker often fails to provide ideal predictive value. The combined use of multiple markers may offer a better solution. By integrating different markers, researchers can capture disease mechanisms more comprehensively, leading to improved patient stratification and more effective treatment strategies. The application of multiple biomarkers represents an important advancement in personalized medicine, ensuring that treatment decisions are based on a more comprehensive and detailed understanding of each patient’s unique biological characteristics.
9 Conclusions
Precision medicine, as an important direction for the future development of healthcare, aims to provide individualized treatment plans for different patients. In the field of NSCLC, ICIs represent a significant breakthrough, making the in-depth study of biomarkers for predicting ICIs efficacy particularly important. Currently, research on biomarkers for ICIs efficacy mainly focuses on several aspects, including PD-L1 expression levels, tumor-infiltrating lymphocytes, TLS, Tumor genomic alterations, Transcriptome Signatures, circulating biomarkers, and microbiomes. However, there are still many limitations in current biomarker research. First, the detection methods for biomarkers have not yet been standardized, and different laboratories may use varying techniques and standards, leading to inconsistent results. Additionally, many studies primarily focus on the evaluation of single biomarkers, lacking comprehensive research on the combined analysis of multiple biomarkers, which may overlook complex biological mechanisms. Therefore, further research is urgently needed to clarify the true value of these biomarkers and explore their applicability in different patient populations. In future research, we should continue to explore biomarkers that can effectively distinguish ICIs responders from non-responders and promote the combined application of multiple biomarkers, as this will be a key direction for future development.
Author contributions
JZ: Writing – original draft, Writing – review & editing. ZS: Writing – original draft, Writing – review & editing. YZ: Writing – review & editing. CZ: Writing – review & editing. QX: Writing – review & editing. GZ: Writing – original draft, Writing – review & editing. FT: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Beijing Natural Science Foundation (7232134); National High-Level Hospital Clinical Research Funding (2022-PUMCH-C-043); National Key R&D Program of China (2022YFC2407406); CAMS Initiative for Innovative Medicine (2021-1-I2M-012); Beijing Hope Run Special Fund of Cancer Foundation of China (LC2021L01); Beijing Municipal Science & Technology Commission (Z211100002921058).
Acknowledgments
Some of the image materials are sourced from the Generic Diagramming Platform (159) and BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. (2018) 553:446–54. doi: 10.1038/nature25183
2. Wang Y, Wang M, Wu HX, Xu RH. Advancing to the era of cancer immunotherapy. Cancer Commun. (2021) 41:803–29. doi: 10.1002/cac2.12178
3. Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. (2023) 22:37. doi: 10.1186/s12943-023-01740-y
4. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. (2015) 33:1974–U161. doi: 10.1200/JCO.2014.59.4358
5. Bai RL, Lv Z, Xu DS, Cui JW. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. biomark Res. (2020) 8:17. doi: 10.1186/s40364-020-00209-0
6. Gainor JF, Rizvi H, Aguilar EJ, Skoulidis F, Yeap BY, Naidoo J, et al. Clinical activity of programmed cell death 1 (PD-1) blockade in never, light, and heavy smokers with non-small-cell lung cancer and PD-L1 expression. Ann Oncol. (2020) 31:404–11. doi: 10.1016/j.annonc.2019.11.015
7. Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. (2021) 18:345–62. doi: 10.1038/s41571-021-00473-5
8. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. (2014) 515:563–7. doi: 10.1038/nature14011
9. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
10. Garon EB, Rizvi NA, Hui RN, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. (2015) 372:2018–28. doi: 10.1056/NEJMoa1501824
11. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7
12. Reck M, Rodr D, Robinson AG, Hui RN, Csoszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
13. Pai-Scherf L, Blumenthal GM, Li HS, Subramaniam S, Mishra-Kalyani PS, He K, et al. FDA approval summary: pembrolizumab for treatment of metastatic non-small cell lung cancer: first-line therapy and beyond. Oncologist. (2017) 22:1392–9. doi: 10.1634/theoncologist.2017-0078
14. Brahmer JR, Lee JS, Ciuleanu TE, Caro RB, Nishio M, Urban L, et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in checkMate 227. J Clin Oncol. (2023) 41:1200–12. doi: 10.1200/JCO.22.01503
15. Paz-Ares L, Spira A, Raben D, Planchard D, Cho BC, Özgüroglu M, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. (2020) 31:798–806. doi: 10.1016/j.annonc.2020.03.287
16. Mino-Kenudson M, Schalper K, Cooper W, Dacic S, Hirsch FR, Jain D, et al. Predictive biomarkers for immunotherapy in lung cancer: perspective from the international association for the study of lung cancer pathology committee. J Thorac Oncol. (2022) 17:1335–54. doi: 10.1016/j.jtho.2022.09.109
17. de Visser KE, Joyce JA. The evolving tumor microenvironment From cancer initiation to metastatic outgrowth. Cancer Cell. (2023) 41:374–403. doi: 10.1016/j.ccell.2023.02.016
18. Rad HS, Monkman J, Warkiani ME, Ladwa R, O’Byrne K, Rezaei N, et al. Understanding the tumor microenvironment for effective immunotherapy. Med Res Rev. (2021) 41:1474–98. doi: 10.1002/med.21765
19. Liu BL, Hu XD, Feng KC, Gao RR, Xue ZQ, Zhang SJ, et al. Temporal single-cell tracing reveals clonal revival and expansion of precursor exhausted T cells during anti-PD-1 therapy in lung cancer. Nat Cancer. (2022) 3:108–21. doi: 10.1038/s43018-021-00292-8
20. Yeong J, Suteja L, Simoni Y, Lau KW, Tan AC, Li HH, et al. Intratumoral CD39+CD8+ T cells predict response to programmed cell death protein-1 or programmed death ligand-1 blockade in patients with NSCLC. J Thorac Oncol. (2021) 16:1349–58. doi: 10.1016/j.jtho.2021.04.016
21. Li F, Li CC, Cai XY, Xie ZH, Zhou LQ, Cheng B, et al. The association between CD8+tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: A systematic review and meta-analysis. EClinicalMedicine. (2021) 41:12. doi: 10.1016/j.eclinm.2021.101134
22. Shirasawa M, Yoshida T, Imabayashi T, Okuma K, Matsumoto Y, Masuda K, et al. Baseline PD-L1 expression and tumour-infiltrated lymphocyte status predict the efficacy of durvalumab consolidation therapy after chemoradiotherapy in unresectable locally advanced patients with non-small-cell lung cancer. Eur J Cancer. (2022) 162:1–10. doi: 10.1016/j.ejca.2021.11.013
23. Uryvaev A, Passhak M, Hershkovits D, Sabo E, Bar-Sela G. The role of tumor-infiltrating lymphocytes (TILs) as a predictive biomarker of response to anti-PD1 therapy in patients with metastatic non-small cell lung cancer or metastatic melanoma. Med Oncol. (2018) 35:9. doi: 10.1007/s12032-018-1080-0
24. Horton BL, Morgan DM, Momin N, Zagorulya M, Torres-Mejia E, Bhandarkar V, et al. Lack of CD8+ T cell effector differentiation during priming mediates checkpoint blockade resistance in non-small cell lung cancer. Sci Immunol. (2021) 6:18. doi: 10.1126/sciimmunol.abi8800
25. Gueguen P, Metoikidou C, Dupic T, Lawand M, Goudot C, Baulande S, et al. Contribution of resident and circulating precursors to tumor-infiltrating CD8+ T cell populations in lung cancer. Sci Immunol. (2021) 6:15. doi: 10.1126/sciimmunol.abd5778
26. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol. (2020) 21:1346–58. doi: 10.1038/s41590-020-0769-3
27. Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. (2018) 24:994–1004. doi: 10.1038/s41591-018-0057-z
28. Gettinger SN, Choi J, Mani N, Sanmamed MF, Datar I, Sowell R, et al. A dormant TIL phenotype defines non-small cell lung carcinomas sensitive to immune checkpoint blockers. Nat Commun. (2018) 9:15. doi: 10.1038/s41467-018-05032-8
29. Koh J, Kim Y, Lee KY, Hur JY, Kim MS, Kim B, et al. MDSC subtypes and CD39 expression on CD8+ T cells predict the efficacy of anti-PD-1 immunotherapy in patients with advanced NSCLC. Eur J Immunol. (2020) 50:1810–9. doi: 10.1002/eji.202048534
30. Galbo PM, Zang XX, Zheng DY. Molecular features of cancer-associated fibroblast subtypes and their implication on cancer pathogenesis, prognosis, and immunotherapy resistance. Clin Cancer Res. (2021) 27:2636–47. doi: 10.1158/1078-0432.CCR-20-4226
31. Wang SS, Liu W, Ly D, Xu H, Qu LM, Zhang L. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol. (2019) 16:6–18. doi: 10.1038/s41423-018-0027-x
32. Germain C, Devi-Marulkar P, Knockaert S, Biton J, Kaplon H, Letaïef L, et al. Tertiary lymphoid structure-B cells narrow regulatory T cells impact in lung cancer patients. Front Immunol. (2021) 12:626776. doi: 10.3389/fimmu.2021.626776
33. Pradhan M, Chocry M, Gibbons DL, Sepesi B, Cascone T. Emerging biomarkers for neoadjuvant immune checkpoint inhibitors in operable non-small cell lung cancer. Transl Lung Cancer Res. (2021) 10:17. doi: 10.21037/tlcr-20-573
34. Cascone T, Leung CH, Weissferdt A, Pataer A, Carter BW, Godoy MCB, et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat Med. (2023) 29(3):593–604. doi: 10.1038/s41591-022-02189-0
35. Hui ZZ, Zhang JL, Ren YL, Li XL, Yan CH, Yu WW, et al. Single-cell profiling of immune cells after neoadjuvant pembrolizumab and chemotherapy in IIIA non-small cell lung cancer (NSCLC). Cell Death Dis. (2022) 13:18. doi: 10.1038/s41419-022-05057-4
36. Hu JJ, Zhang LL, Xia HR, Yan YL, Zhu XS, Sun FH, et al. Tumor microenvironment remodeling after neoadjuvant immunotherapy in non-small cell lung cancer revealed by single-cell RNA sequencing. Genome Med. (2023) 15:25. doi: 10.1186/s13073-023-01164-9
37. Ng KW, Boumelha J, Enfield KSS, Almagro J, Cha HG, Pich O, et al. Antibodies against endogenous retroviruses promote lung cancer immunotherapy. Nature. (2023) 616:563–73. doi: 10.1038/s41586-023-05771-9
38. Rosenberg SA. Cancer immunotherapy comes of age. Nat Clin Pract Oncol. (2005) 2:115–5. doi: 10.1038/ncponc0101
39. Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. (2019) 19:307–25. doi: 10.1038/s41568-019-0144-6
40. Munoz-Erazo L, Rhodes JL, Marion VC, Kemp RA. Tertiary lymphoid structures in cancer - considerations for patient prognosis. Cell Mol Immunol. (2020) 17:570–5. doi: 10.1038/s41423-020-0457-0
41. Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science. (2022) 375:eabf9419. doi: 10.1126/science.abf9419
42. Dieu-Nosjean MC, Goc J, Giraldo NA, Sautès-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. (2014) 35:571–80. doi: 10.1016/j.it.2014.09.006
43. da Graça CG, van Baarsen LGM, Mebius RE. Tertiary lymphoid structures: diversity in their development, composition, and role. J Immunol. (2021) 206:273–81. doi: 10.4049/jimmunol.2000873
44. Sato Y, Silina K, van den Broek M, Hirahara K, Yanagita M. The roles of tertiary lymphoid structures in chronic diseases. Nat Rev Nephrol. (2023) 19:525–37. doi: 10.1038/s41581-023-00706-z
45. Barmpoutis P, Di Capite M, Kayhanian H, Waddingham W, Alexander DC, Jansen M, et al. Tertiary lymphoid structures (TLS) identification and density assessment on H&E-stained digital slides of lung cancer. PloS One. (2021) 16:19. doi: 10.1371/journal.pone.0256907
46. Shang TY, Jiang TY, Lu T, Wang H, Cui XW, Pan YF, et al. Tertiary lymphoid structures predict the prognosis and immunotherapy response of cholangiocarcinoma. Front Immunol. (2023) 14:1166497. doi: 10.3389/fimmu.2023.1166497
47. Kumar N, Gupta R, Gupta S. Whole slide imaging (WSI) in pathology: current perspectives and future directions. J Digit Imaging. (2020) 33:1034–40. doi: 10.1007/s10278-020-00351-z
48. Messina JL, Fenstermacher DA, Eschrich S, Qu XT, Berglund AE, Lloyd MC, et al. 12-chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep. (2012) 2:6. doi: 10.1038/srep00765
49. Calderaro J, Petitprez F, Becht E, Laurent A, Hirsch TZ, Rousseau B, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. (2019) 70:58–65. doi: 10.1016/j.jhep.2018.09.003
50. Shen YC, Hsu CL, Jeng YM, Ho MC, Ho CM, Yeh CP, et al. Reliability of a single-region sample to evaluate tumor immune microenvironment in hepatocellular carcinoma. J Hepatol. (2020) 72:489–97. doi: 10.1016/j.jhep.2019.09.032
51. Zhang YY, Xu MJ, Ren YQ, Ba YH, Liu ST, Zuo AN, et al. Tertiary lymphoid structural heterogeneity determines tumour immunity and prospects for clinical application. Mol Cancer. (2024) 23:20. doi: 10.1186/s12943-024-01980-6
52. Weng YM, Yuan JP, Cui X, Wang JS, Chen HL, Xu L, et al. The impact of tertiary lymphoid structures on tumor prognosis and the immune microenvironment in non-small cell lung cancer. Sci Rep. (2024) 14:16. doi: 10.1038/s41598-024-64980-y
53. Sun XY, Liu WR, Sun LA, Mo HL, Feng YN, Wu XY, et al. Maturation and abundance of tertiary lymphoid structures are associated with the efficacy of neoadjuvant chemoimmunotherapy in resectable non-small cell lung cancer. J Immunother Cancer. (2022) 10:8. doi: 10.1136/jitc-2022-005531
54. Liu Y, Xiong L, Chen Y, Cai RX, Xu XY, Wang T, et al. Complete pathological remission and tertiary lymphoid structures are associated with the efficacy of resectable NSCLC receiving neoadjuvant chemoimmunotherapy: A double-center retrospective study. Hum Vaccines Immunother. (2023) 19:12. doi: 10.1080/21645515.2023.2285902
55. Xie M, Jing Y, Xue X. EP06.02–07 tertiary lymphoid structures predict clinical outcomes of neoadjuvant chemoimmunotherapy in resectable NSCLC. J Thorac Oncol. (2023) 18:S481–18. doi: 10.1016/j.jtho.2023.09.892
56. Srivastava MK, Gayevskiy V, Ma V, Estay I, Rodas M, Rajendran BK, et al. 606 IMpower110: Tertiary lymphoid structures (TLS) and clinical outcomes in advanced non-small cell lung cancer (NSCLC) treated with first-line atezolizumab or chemotherapy. J Immunother Cancer. (2023) 11:A690. doi: 10.1136/jitc-2023-SITC2023.0606
57. Xu FH, Zhu H, Dong YJ, Li L, Liu N, Yuan SH. Combined inflammatory parameters and tertiary lymphoid structure predict prognosis in patients with resectable non-small cell lung cancer treated with neoadjuvant chemoimmunotherapy. Front Immunol. (2023) 14:1244256. doi: 10.3389/fimmu.2023.1244256
58. Rothschild SI, Zippelius A, Eboulet EI, Prince SS, Betticher D, Bettini A, et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer-A multicenter single-arm phase II trial. J Clin Oncol. (2021) 39:2872–80. doi: 10.1200/JCO.21.00276
59. Chen TX, Cao ZQ, Sun YJ, Huang J, Shen SP, Jin YP, et al. Neoadjuvant chemoimmunotherapy increases tumor immune lymphocytes infiltration in resectable non-small cell lung cancer. Ann Surg Oncol. (2023) 30:7549–60. doi: 10.1245/s10434-023-14123-w
60. Cai WH, Jing M, Wen JX, Gu YJ, Zhao XC, Xue ZQ. Immune cell infiltrative characteristics reveals the superior efficacy of neoadjuvant immunochemotherapy versus chemotherapy alone in a real-world cohort of resectable non-small cell lung cancer. Cancer Res. (2022) 82:1. doi: 10.1158/1538-7445.AM2022-6110
61. Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol. (2018) 29:1853–60. doi: 10.1093/annonc/mdy218
62. Zhai J, Mao Y, Li J, Dong Z, Wang L, Zheng X, et al. Characteristics of tertiary lymphatic structures in lung squamous cell carcinoma and their impact on prognosis. Acad J Chin Pla Med School. (2023) 44:725–32, 49. doi: 10.12435/j.issn.2095-5227.2023.006
63. Xie M, Lin X, Bao X, Liang Y, Deng H, Song J, et al. Tertiary lymphoid structure in tumor microenvironment and immunotherapy of lung cancer. Archivos Bronconeumol. (2024) 60 Suppl 2:S77–85. doi: 10.1016/j.arbres.2024.07.020
64. Silina K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. (2018) 78:1308–20. doi: 10.1158/0008-5472.CAN-17-1987
65. Wakasu S, Tagawa T, Haratake N, Kinoshita F, Oku Y, Ono Y, et al. Preventive effect of tertiary lymphoid structures on lymph node metastasis of lung adenocarcinoma. Cancer Immunol Immunother. (2023) 72:1823–34. doi: 10.1007/s00262-022-03353-8
66. Cai WH, Jing M, Gu YJ, Bei T, Zhao XC, Chen SQ, et al. Tumor microenvironment features decipher the outperformance of neoadjuvant immunochemotherapy over chemotherapy in resectable non-small cell lung cancer. Front Immunol. (2022) 13:984666. doi: 10.3389/fimmu.2022.984666
67. Xu FH, Zhu H, Xiong DL, Wang K, Dong YJ, Li L, et al. Tertiary lymphoid structures combined with biomarkers of inflammation are associated with the efficacy of neoadjuvant immunochemotherapy in resectable non-small cell lung cancer: A retrospective study. Thorac Cancer. (2024) 15:172–81. doi: 10.1111/1759-7714.15175
68. Zhang C, Yin K, Liu SY, Yan LX, Su J, Wu YL, et al. Multiomics analysis reveals a distinct response mechanism in multiple primary lung adenocarcinoma after neoadjuvant immunotherapy. J Immunother Cancer. (2021) 9:6. doi: 10.1136/jitc-2020-002312
69. Cascone T, Kar G, Spicer JD, Garcia-Campelo R, Weder W, Daniel DB, et al. Neoadjuvant durvalumab alone or combined with novel immuno-oncology agents in resectable lung cancer: the phase II neoCOAST platform trial. Cancer Discov. (2023) 13:2394–411. doi: 10.1158/2159-8290.CD-23-0436
70. Xin SY, Wen S, He PP, Zhao YL, Zhao H. Density of tertiary lymphoid structures and their correlation with prognosis in non-small cell lung cancer. Front Immunol. (2024) 15:1423775. doi: 10.3389/fimmu.2024.1423775
71. Leng Y, Dang SY, Yin F, Gao TS, Xiao X, Zhang Y, et al. GDPLichi: a DNA damage repair-related gene classifier for predicting lung adenocarcinoma immune checkpoint inhibitors response. Front Oncol. (2021) 11:733533. doi: 10.3389/fonc.2021.733533
72. Lei YY, Zhang GC, Zhang CQ, Xue LY, Yang ZL, Lu ZL, et al. The average copy number variation (CNVA) of chromosome fragments is a potential surrogate for tumor mutational burden in predicting responses to immunotherapy in non-small-cell lung cancer. Clin Transl Immunol. (2021) 10:16. doi: 10.1002/cti2.1231
73. Cormedi MCV, Van Allen EM, Colli LM. Predicting immunotherapy response through genomics. Curr Opin Genet Dev. (2021) 66:1–9. doi: 10.1016/j.gde.2020.11.004
74. Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. (2019) 30:44–56. doi: 10.1093/annonc/mdy495
75. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. (2015) 348:124–8. doi: 10.1126/science.aaa1348
76. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio S, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. (2013) 500:415–21. doi: 10.1038/nature12477
77. Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. New Engl J Med. (2018) 378:2093–104. doi: 10.1056/NEJMoa1801946
78. Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. (2018) 33:843–852. doi: 10.1016/j.ccell.2018.03.018
79. Singal G, Miller PG, Agarwala V, Li G, Kaushik G, Backenroth D, et al. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non–small cell lung cancer using a clinicogenomic database. JAMA. (2019) 321:1391–9. doi: 10.1001/jama.2019.3241
80. Meng GX, Liu XW, Ma T, Lv DS, Sun G. Predictive value of tumor mutational burden for immunotherapy in non-small cell lung cancer: A systematic review and meta-analysis. PloS One. (2022) 17:19. doi: 10.1371/journal.pone.0263629
81. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. (2017) 376:2415–26. doi: 10.1056/NEJMoa1613493
82. Herbst RS, Lopes G, Kowalski DM, Nishio M, Wu YL, de Castro G, et al. 3570 - association between tissue TMB (tTMB) and clinical outcomes with Pembrolizumab monotherapy (Pembro) in PD-L1-positive advanced NSCLC in the KEYNOTE-010 and -042 trials. Ann Oncol. (2019) 30:v916–30. doi: 10.1093/annonc/mdz394.077
83. Kowanetz M, Zou W, Shames D, Cummings C, Rizvi N, Spira A, et al. OA20.01 tumor mutation burden (TMB) is associated with improved efficacy of atezolizumab in 1L and 2L+ NSCLC patients. J Thorac Oncol. (2017) 12:S321–2. doi: 10.1016/j.jtho.2016.11.343
84. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. (2020) 6:661–74. doi: 10.1001/jamaoncol.2020.0237
85. Goldberg SB, Narayan A, Kole AJ, Decker RH, Teysir J, Carriero NJ, et al. Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin Cancer Res. (2018) 24:1872–80. doi: 10.1158/1078-0432.CCR-17-1341
86. Otano I, Ucero AC, Zugazagoitia J, Paz-Ares L. At the crossroads of immunotherapy for oncogene-addicted subsets of NSCLC. Nat Rev Clin Oncol. (2023) 20:143–59. doi: 10.1038/s41571-022-00718-x
87. Ramos-Ramírez M, Caballe-Pérez E, Lucio-Lozada J, Romero-Nuñez E, Castillo-Ruiz C, Dorantes-Sánchez L, et al. Immunomodulatory role of oncogenic alterations in non-small cell lung cancer: a review of implications for immunotherapy. Cancer Metastasis Rev. (2025) 44:30. doi: 10.1007/s10555-025-10245-7
88. Yao X, Gao C, Sun C, Chen Z-S, Zhuang J. Epigenetic code underlying EGFR-TKI resistance in non-small cell lung cancer: Elucidation of mechanisms and perspectives on therapeutic strategies. Drug Discov Today. (2025) 30:104321. doi: 10.1016/j.drudis.2025.104321
89. Kim JY, Choi JK, Jung H. Genome-wide methylation patterns predict clinical benefit of immunotherapy in lung cancer. Clin Epigenet. (2020) 12:119. doi: 10.1186/s13148-020-00907-4
90. Duruisseaux M, Martínez-Cardús A, Calleja-Cervantes ME, Moran S, Castro de Moura M, Davalos V, et al. Epigenetic prediction of response to anti-PD-1 treatment in non-small-cell lung cancer: a multicentre, retrospective analysis. Lancet Respir Med. (2018) 6:771–81:10. doi: 10.1016/S2213-2600(18)30284–4
91. Liu S, Ma SY, Liu G, Hou LJ, Guan Y, Liu L, et al. CK2B induces CD8+ T-cell exhaustion through HDAC8-mediated epigenetic reprogramming to limit the efficacy of anti-PD-1 therapy in non-small-cell lung cancer. Adv Sci. (2025) 12(16):e2411053. doi: 10.1002/advs.202411053
92. Shin HS, Choi J, Lee J, Lee SY. Histone deacetylase as a valuable predictive biomarker and therapeutic target in immunotherapy for non-small cell lung cancer. Cancer Res Treat. (2022) 54:458–68. doi: 10.4143/crt.2021.425
93. Lianidou E. Detection and relevance of epigenetic markers on ctDNA: recent advances and future outlook. Mol Oncol. (2021) 15:1683–700. doi: 10.1002/1878-0261.12978
94. Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. (2017) 127:2930–40. doi: 10.1172/JCI91190
95. Higgs BW, Morehouse CA, Streicher K, Brohawn PZ, Pilataxi F, Gupta A, et al. Interferon gamma messenger RNA signature in tumor biopsies predicts outcomes in patients with non-small cell lung carcinoma or urothelial cancer treated with durvalumab. Clin Cancer Res. (2018) 24:3857–66. doi: 10.1158/1078-0432.CCR-17-3451
96. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. (2016) 387:1837–46. doi: 10.1016/S0140-6736(16)00587-0
97. Karachaliou N, Gonzalez-Cao M, Crespo G, Drozdowskyj A, Aldeguer E, Gimenez-Capitan A, et al. Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther Adv Med Oncol. (2018) 10:1758834017749748. doi: 10.1177/1758834017749748
98. Qi XG, Qi CY, Wu T, Hu Y. CSF1R and HCST: novel candidate biomarkers predicting the response to immunotherapy in non-small cell lung cancer. Technol Cancer Res Treat. (2020) 19:12. doi: 10.1177/1533033820970663
99. Wang YT, Liu Y, Li XH, Li WM, Xue ZH, He XQ, et al. TCR coexpression signature predicts immunotherapy resistance in NSCLC. Front Pharmacol. (2022) 13:875149. doi: 10.3389/fphar.2022.875149
100. You WJ, Shang B, Sun J, Liu XQ, Su LL, Jiang SJ. Mechanistic insight of predictive biomarkers for antitumor PD-1/PD-L1 blockade: A paradigm shift towards immunome evaluation. Oncol Rep. (2020) 44:424–37. doi: 10.3892/or.2020.7643
101. Socinski MA, Paul SM, Yun C, Hu S, Shen V, Velcheti V, et al. Abstract CT194: exploratory subgroup analysis of atezolizumab (atezo) clinical characteristics in patients (PTS) with low circulating tumor DNA (ctDNA) in B-F1RSTti phase II trial evaluating blood-based tumor mutational burden (bTMB) in NSCLC. Cancer Res. (2019) 79:CT194. doi: 10.1158/1538-7445.AM2019-CT194
102. Kang D, Chung C, Sun P, Lee D, Lee SI, Park D, et al. Circulating regulatory T cells predict efficacy and atypical responses in lung cancer patients treated with PD-1/PD-L1 inhibitors. Cancer Immunol Immunother. (2022) 71:579–88. doi: 10.1007/s00262-021-03018-y
103. Moding EJ, Liu YF, Nabet BY, Chabon JJ, Chaudhuri AA, Hui AB, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nat Cancer. (2020) 1:176–83. doi: 10.1038/s43018-019-0011-0
104. Ricciuti B, Jones G, Severgnini M, Alessi JV, Recondo G, Lawrence M, et al. Early plasma circulating tumor DNA (ctDNA) changes predict response to first-line pembrolizumab-based therapy in non-small cell lung cancer (NSCLC). J Immunother Cancer. (2021) 9:9. doi: 10.1136/jitc-2020-001504
105. Snyder A, Morrissey MP, Hellmann MD. Use of circulating tumor DNA for cancer immunotherapy. Clin Cancer Res. (2019) 25:6909–15. doi: 10.1158/1078-0432.CCR-18-2688
106. Khagi Y, Goodman AM, Daniels GA, Patel SP, Sacco AG, Randall JM, et al. Hypermutated circulating tumor DNA: correlation with response to checkpoint inhibitor-based immunotherapy. Clin Cancer Res. (2017) 23:5729–36. doi: 10.1158/1078-0432.CCR-17-1439
107. Rogado J, Pozo F, Troule K, Sánchez-Torres JM, Romero-Laorden N, Mondejar R, et al. Peripheral blood mononuclear cells predict therapeutic efficacy of immunotherapy in NSCLC. Cancers. (2022) 14:11. doi: 10.3390/cancers14122898
108. Zhuo ML, Chen HX, Zhang TZ, Yang X, Zhong J, Wang YY, et al. The potential predictive value of circulating immune cell ratio and tumor marker in atezolizumab treated advanced non-small cell lung cancer patients. Cancer biomark. (2018) 22:467–76. doi: 10.3233/CBM-171089
109. Mosca M, Nigro MC, Pagani R, De Giglio A, Di Federico A. Neutrophil-to-lymphocyte ratio (NLR) in NSCLC, gastrointestinal, and other solid tumors: immunotherapy and beyond. Biomolecules. (2023) 13:15. doi: 10.3390/biom13121803
110. Manjarrez-Orduño N, Menard LC, Kansal S, Fischer P, Kakrecha B, Jiang C, et al. Circulating T cell subpopulations correlate with immune responses at the tumor site and clinical response to PD1 inhibition in non-small cell lung cancer. Front Immunol. (2018) 9:1613. doi: 10.3389/fimmu.2018.01613
111. Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH, Ahn BC, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol: Off J Eur Soc Med Oncol. (2019) 30:1104–13. doi: 10.1093/annonc/mdz123
112. Hwang M, Canzoniero JV, Rosner S, Zhang GF, White JR, Belcaid Z, et al. Peripheral blood immune cell dynamics reflect antitumor immune responses and predict clinical response to immunotherapy. J Immunother Cancer. (2022) 10:12. doi: 10.1136/jitc-2022-004688
113. Alessi JV, Ricciuti B, Alden SL, Bertram AA, Lin JJ, Sakhi M, et al. Low peripheral blood derived neutrophil-to-lymphocyte ratio (dNLR) is associated with increased tumor T-cell infiltration and favorable outcomes to first-line pembrolizumab in non-small cell lung cancer. J Immunother Cancer. (2021) 9:8. doi: 10.1136/jitc-2021-003536
114. Ayers KL, Ma M, Debussche G, Corrigan D, McCafferty J, Lee K, et al. A composite biomarker of neutrophil-lymphocyte ratio and hemoglobin level correlates with clinical response to PD-1 and PD-L1 inhibitors in advanced non-small cell lung cancers. BMC Cancer. (2021) 21:11. doi: 10.1186/s12885-021-08194-9
115. Sánchez-Gastaldo A, Muñoz-Fuentes MA, Molina-Pinelo S, Alonso-García M, Boyero L, Bernabé-Caro R. Correlation of peripheral blood biomarkers with clinical outcomes in NSCLC patients with high PD-L1 expression treated with pembrolizumab. Transl Lung Cancer Res. (2021) 10:2509–22. doi: 10.21037/tlcr-21-156
116. Passiglia F, Galvano A, Castiglia M, Incorvaia L, Calò V, Listì A, et al. Monitoring blood biomarkers to predict nivolumab effectiveness in NSCLC patients. Ther Adv Med Oncol. (2019) 11:11. doi: 10.1177/1758835919839928
117. Del Re M, Marconcini R, Pasquini G, Rofi E, Vivaldi C, Bloise F, et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br J Cancer. (2018) 118:820–4. doi: 10.1038/bjc.2018.9
118. Soyano AE, Dholaria B, Marin-Acevedo JA, Diehl N, Hodge D, Luo Y, et al. Peripheral blood biomarkers correlate with outcomes in advanced non-small cell lung Cancer patients treated with anti-PD-1 antibodies. J Immunother Cancer. (2018) 6:9. doi: 10.1186/s40425-018-0447-2
119. Jiang ML, Peng WY, Pu XX, Chen BL, Li J, Xu F, et al. Peripheral blood biomarkers associated with outcome in non-small cell lung cancer patients treated with nivolumab and durvalumab monotherapy. Front Oncol. (2020) 10:913. doi: 10.3389/fonc.2020.00913
120. Hurkmans DP, Basak EA, Schepers N, Oomen-De Hoop E, van der Leest CH, El Bouazzaoui S, et al. Granzyme B is correlated with clinical outcome after PD-1 blockade in patients with stage IV non-small-cell lung cancer. J Immunother Cancer. (2020) 8:9. doi: 10.1136/jitc-2020-000586
121. Meyo MT, Jouinot A, Giroux-Leprieur E, Fabre E, Wislez M, Alifano M, et al. Predictive value of soluble PD-1, PD-L1, VEGFA, CD40 ligand and CD44 for nivolumab therapy in advanced non-small cell lung cancer: A case-control study. Cancers. (2020) 12:18. doi: 10.3390/cancers12020473
122. Mazzaschi G, Minari R, Zecca A, Cavazzoni A, Ferri V, Mori C, et al. Soluble PD-L1 and circulating CD8+PD-1+and NK cells enclose a prognostic and predictive immune effector score in immunotherapy treated NSCLC patients. Lung Cancer. (2020) 148:1–11. doi: 10.1016/j.lungcan.2020.07.028
123. Nicolazzo C, Raimondi C, Mancini M, Caponnetto S, Gradilone A, Gandini O, et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci Rep. (2016) 6:8. doi: 10.1038/srep31726
124. Zheng DP, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. (2020) 30:492–506. doi: 10.1038/s41422-020-0332-7
125. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. (2016) 30:836–48. doi: 10.1016/j.ccell.2016.10.009
126. Signorelli D, Ghidotti P, Proto C, Brambilla M, De Toma A, Ferrara R, et al. Circulating CD81-expressing extracellular vesicles as biomarkers of response for immune-checkpoint inhibitors in advanced NSCLC. Front Immunol. (2022) 13:987639. doi: 10.3389/fimmu.2022.987639
127. Duréndez-Sáez E, Torres-Martinez S, Calabuig-Fariñas S, Meri-Abad M, Ferrero-Gimeno M, Camps C. Exosomal microRNAs in non-small cell lung cancer. Transl Cancer Res. (2021) 10:3128–39. doi: 10.21037/tcr-20-2815
128. Pantano F, Zalfa F, Iuliani M, Simonetti S, Manca P, Napolitano A, et al. Large-scale profiling of extracellular vesicles identified miR-625-5p as a novel biomarker of immunotherapy response in advanced non-small-cell lung cancer patients. Cancers. (2022) 14:15. doi: 10.3390/cancers14102435
129. Yang Q, Chen M, Gu J, Niu K, Zhao X, Zheng L, et al. Novel biomarkers of dynamic blood PD-L1 expression for immune checkpoint inhibitors in advanced non-small-cell lung cancer patients. Front Immunol. (2021) 12:665133. doi: 10.3389/fimmu.2021.665133
130. Okuma Y, Hosomi Y, Nakahara Y, Watanabe K, Sagawa Y, Homma S. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer. (2017) 104:1–6. doi: 10.1016/j.lungcan.2016.11.023
131. Nguyen LTH, Zhang JJ, Rima XY, Wang XY, Kwak KJ, Okimoto T, et al. An immunogold single extracellular vesicular RNA and protein (AuSERP) biochip to predict responses to immunotherapy in non-small cell lung cancer patients. J Extracell Vesicles. (2022) 11:24. doi: 10.1002/jev2.12258
132. Jang HJ, Choi JY, Kim K, Yong SH, Kim YW, Kim SY, et al. Relationship of the lung microbiome with PD-L1 expression and immunotherapy response in lung cancer. Respir Res. (2021) 22:10. doi: 10.1186/s12931-021-01919-1
133. Jin YP, Dong H, Xia LL, Yang Y, Zhu YQ, Shen Y, et al. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in chinese patients with NSCLC. J Thorac Oncol. (2019) 14:1378–89. doi: 10.1016/j.jtho.2019.04.007
134. Masuhiro K, Tamiya M, Fujimoto K, Koyama S, Naito Y, Osa A, et al. Bronchoalveolar lavage fluid reveals factors contributing to the efficacy of PD-1 blockade in lung cancer. JCI Insight. (2022) 7:15. doi: 10.1172/jci.insight.157915
135. Chu SJ, Cheng ZX, Yin ZY, Xu JJ, Wu F, Jin Y, et al. Airway fusobacterium is associated with poor response to immunotherapy in lung cancer. OncoTargets Ther. (2022) 15:201–13. doi: 10.2147/OTT.S348382
136. Chau J, Yadav M, Liu B, Furqan M, Dai Q, Shahi S, et al. Prospective correlation between the patient microbiome with response to and development of immune-mediated adverse effects to immunotherapy in lung cancer. BMC Cancer. (2021) 39:3. doi: 10.1186/s12885-021-08530-z
137. Grenda A, Iwan E, Chmielewska I, Krawczyk P, Giza A, Bomba A, et al. Presence of Akkermansiaceae in gut microbiome and immunotherapy effectiveness in patients with advanced non-small cell lung cancer. AMB Express. (2022) 12:7. doi: 10.1186/s13568-022-01428-4
138. Hakozaki T, Richard C, Elkrief A, Hosomi Y, Benlaïfaoui M, Mimpen I, et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res. (2020) 8:1243–50. doi: 10.1158/2326-6066.CIR-20-0196
139. Derosa L, Routy B, Thomas AM, Iebba V, Zalcman G, Friard S, et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. (2022) 28:315–24. doi: 10.1038/s41591-021-01655-5
140. Tsay JCJ, Wu BG, Sulaiman I, Gershner K, Schluger R, Li YH, et al. Lower airway dysbiosis affects lung cancer progression. Cancer Discov. (2021) 11:293–307. doi: 10.1158/2159-8290.CD-20-0263
141. Zhang H, Yue XH, Chen Z, Liu C, Wu WT, Zhang N, et al. Define cancer-associated fibroblasts (CAFs) in the tumor microenvironment: new opportunities in cancer immunotherapy and advances in clinical trials. Mol Cancer. (2023) 22:50. doi: 10.1186/s12943-023-01860-5
142. Zhang YF, Liu GJ, Zeng QW, Wu WR, Lei K, Zhang CK, et al. CCL19-producing fibroblasts promote tertiary lymphoid structure formation enhancing anti-tumor IgG response in colorectal cancer liver metastasis. Cancer Cell. (2024) 42:26. doi: 10.1016/j.ccell.2024.07.006
143. Onder L, Papadopoulou C, Lutge A, Cheng H-W, Lutge M, Perez-Shibayama C, et al. Fibroblastic reticular cells generate protective intratumoral Tcell environments in lung cancer. Cell. (2024) 188(2):430–46.e20. doi: 10.1016/j.cell.2024.10.042
144. Deng JY, Gou Q, Yang L, Chen ZH, Yang MY, Yang XR, et al. Immune suppressive microenvironment in liver metastases contributes to organ-specific response of immunotherapy in advanced non-small cell lung cancer. J Immunother Cancer. (2023) 11:e007218. doi: 10.1136/jitc-2023-007218
145. Zou YT, Hu XQ, Zheng SQ, Yang AL, Li X, Tang HL, et al. Discordance of immunotherapy response predictive biomarkers between primary lesions and paired metastases in tumours: A systematic review and meta-analysis. EBioMedicine. (2021) 63:15. doi: 10.1016/j.ebiom.2020.103137
146. Liberini V, Mariniello A, Righi L, Capozza M, Delcuratolo MD, Terreno E, et al. NSCLC biomarkers to predict response to immunotherapy with checkpoint inhibitors (ICI): from the cells to in vivo images. Cancers. (2021) 13:35. doi: 10.3390/cancers13184543
147. Lu S, Yu Y, Zhang Y. Nivolumab versus docetaxel in advanced squamous-cell non-small cell lung cancer. The journal of evidence-based medicine. N Engl J Med. (2015) 15:225. doi: 10.1056/NEJMoa1504627
148. Bensch F, van der Veen EL, Lub-de Hooge MN, Jorritsma-Smit A, Boellaard R, Kok IC, et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. (2018) 24:1852–8. doi: 10.1038/s41591-018-0255-8
149. Dall’Olio FG, Calabró D, Conci N, Argalia G, Marchese PV, Fabbri F, et al. Baseline total metabolic tumour volume on 2-deoxy-2- 18F fluoro-D- GLUCOSE POSITRON EMISSION TOMOGRAPHY-COMPUTED TOMOGRAPHY as a promising biomarker in patients with advanced non-small cell lung cancer treated with first-line pembrolizumab. Eur J Cancer. (2021) 150:99–107. doi: 10.1016/j.ejca.2021.03.020
150. Niemeijer AN, Leung D, Huisman MC, Bahce I, Hoekstra OS, van Dongen G, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun. (2018) 9:5. doi: 10.1038/s41467-018-07131-y
151. Guibert N, Delaunay M, Lusque A, Boubekeur N, Rouquette I, Clermont E, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer. (2018) 120:108–12. doi: 10.1016/j.lungcan.2018.04.001
152. Tamminga M, de Wit S, Hiltermann TJN, Timens W, Schuuring E, Terstappen L, et al. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J Immunother Cancer. (2019) 7:9. doi: 10.1186/s40425-019-0649-2
153. Dall’Olio FG, Gelsomino F, Conci N, Marcolin L, De Giglio A, Grilli G, et al. PD-L1 expression in circulating tumor cells as a promising prognostic biomarker in advanced non-small-cell lung cancer treated with immune checkpoint inhibitors. Clin Lung Cancer. (2021) 22:423–31. doi: 10.1016/j.cllc.2021.03.005
154. Janning M, Kobus F, Babayan A, Wikman H, Velthaus JL, Bergmann S, et al. Determination of PD-L1 expression in circulating tumor cells of NSCLC patients and correlation with response to PD-1/PD-L1 inhibitors. Cancers. (2019) 11:16. doi: 10.3390/cancers11060835
155. Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. (2014) 515:572–6. doi: 10.1038/nature14001
156. Kao C, Powers E, Wu Y, Datto MB, Green MF, Strickler JH, et al. Predictive value of combining biomarkers for clinical outcomes in advanced non-small cell lung cancer patients receiving immune checkpoint inhibitors. Clin Lung Cancer. (2021) 22:500–9. doi: 10.1016/j.cllc.2021.03.017
157. Ma YM, Yang J, Ji TT, Wen FY. Identification of a novel m5C/m6A-related gene signature for predicting prognosis and immunotherapy efficacy in lung adenocarcinoma. Front Genet. (2022) 13:990623. doi: 10.3389/fgene.2022.990623
158. Ando K, Hamada K, Watanabe M, Ohkuma R, Shida M, Onoue R, et al. Plasma levels of soluble PD-L1 correlate with tumor regression in patients with lung and gastric cancer treated with immune checkpoint inhibitors. Anticancer Res. (2019) 39:5195–201. doi: 10.21873/anticanres.13716
Keywords: NSCLC, immune checkpoint inhibitors, biomarker, tertiary lymphoid structures, circulating biomarkers
Citation: Zhang J, Song Z, Zhang Y, Zhang C, Xue Q, Zhang G and Tan F (2025) Recent advances in biomarkers for predicting the efficacy of immunotherapy in non-small cell lung cancer. Front. Immunol. 16:1554871. doi: 10.3389/fimmu.2025.1554871
Received: 03 January 2025; Accepted: 18 April 2025;
Published: 08 May 2025.
Edited by:
Jinghua Pan, Jinan University, ChinaReviewed by:
Kenji Morimoto, Kyoto Prefectural University of Medicine, JapanLuyao Wang, Zhejiang University, China
Yang Yang, National Key Laboratory of Dairy Biotechnology, China
Copyright © 2025 Zhang, Song, Zhang, Zhang, Xue, Zhang and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guochao Zhang, MTg4MDEwMzg3MThAMTYzLmNvbQ==; Fengwei Tan, dGFuZmVuZ3dlaUAxMjYuY29t
†These authors have contributed equally to this work
 Jiacheng Zhang
Jiacheng Zhang Zehao Song1†
Zehao Song1† Qi Xue
Qi Xue Guochao Zhang
Guochao Zhang Fengwei Tan
Fengwei Tan