- 1Department of Neurology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
- 2Department of Urology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
- 3Department of Emergency Internal Medicine, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
- 4Department of Critical Care Medicine, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
Atherosclerosis (AS), as the primary pathological basis of cardiovascular and cerebrovascular diseases, is closely associated with chemokines in its occurrence and progression. CXCL16 establishes a new link between chemokines and AS. We briefly introduced the structural characteristics of CXCL16 and its specific receptor CXCR6, as well as related signaling pathways. Furthermore, the significant role of CXCL16 in the progression of AS was elaborated from the perspective of pathological mechanisms and signal pathways. Meanwhile, we objectively evaluated the potential arterial protective effects of CXCL16. Finally, we discussed various novel therapeutic strategies to alleviate AS by targeting the inhibition of CXCL16 and its regulatory pathways. This review systematically summarizes the multifaceted roles of CXCL16 in AS, providing theoretical foundations and research insights for the precise prevention and treatment of AS.
1 Introduction
Cardiovascular and cerebrovascular diseases, as global health issues, are characterized by high mortality, high disability, and high recurrence rates (1). Atherosclerosis (AS) is the main underlying cause of these diseases (2). Major risk factors for AS include hypertension (3), diabetes (4), hyperlipidemia (5), smoking (6), and excessive alcohol consumption (7). These factors synergistically contribute to the occurrence and progression of AS (8). In recent years, numerous studies have demonstrated that chemokines play an important role in the pathological process of AS, influencing all stages of its development (such as endothelial cell injury, inflammatory cell recruitment, and smooth muscle cell proliferation) (9).
Chemokines are small secreted proteins whose core function is to mediate the directional migration of immune cells through chemotaxis (10). According to the arrangement pattern of conserved cysteine residues at the N-terminus, chemokines can be classified into four subfamilies: CXC, CC, CX3C and C (11). Among these, the CXC subfamily is the most diverse. According to whether or not they contain the ELR (glutamate-leucine-arginine) protein sequence, CXC chemokines can be further divided into functional subgroups with either pro-angiogenic (ELR+) or anti-angiogenic (ELR-) activity (12) (Table 1). CXCL16 is a member of the CXC family (13). CXCL16 is generally considered an independent risk factor for AS (14, 15). However, a small number of studies suggest that CXCL16 may have protective effects against AS (16, 17).
This article elucidates the mechanisms of CXCL16 in AS and its associated signaling, while also summarizing relevant targeted therapies.
2 CXCL16 and its receptor
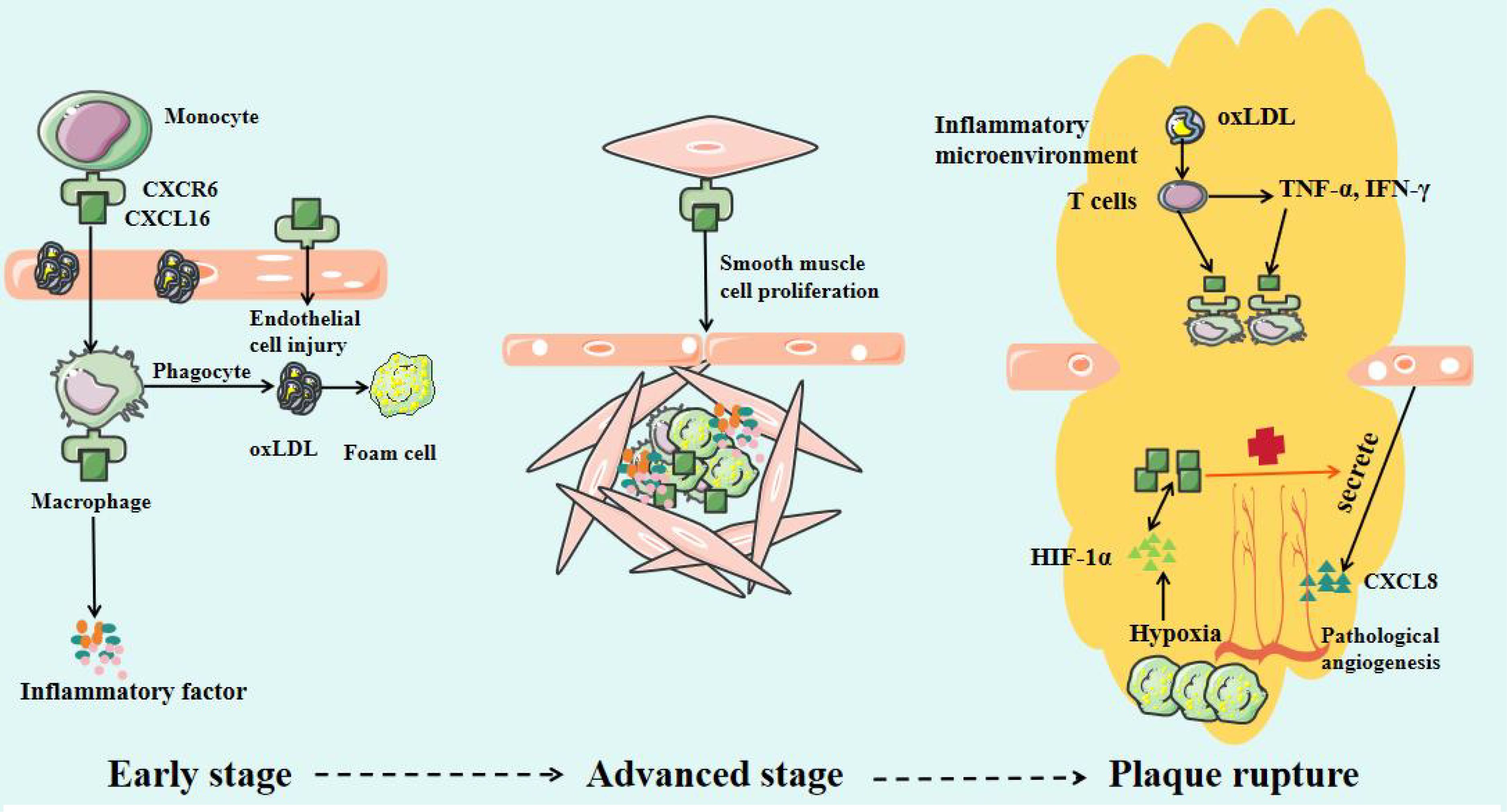
CXCL16 was first cloned in 2000 by Shimaoka et al. (31). Its structure primarily consists of four functional domains: a transmembrane region, a short cytoplasmic domain, an extracellular N-terminal chemokine domain, and a glycosylated mucin-like stalk (32). Along with CX3CL1, it is currently recognized as one of only two known transmembrane chemokines (33). Beyond its classical chemokine functions, CXCL16 exhibits two additional biological roles as an adhesion molecule and a scavenger receptor (34). CXCL16 exists in two different forms: membrane binding (mCXCL16) and soluble CXCL16 (sCXCL16) (35, 36). The generation of these forms depends on proteolytic cleavage of the extracellular domain by the metalloproteinase ADAM10 (37, 38). These two molecular forms demonstrate distinct biological functions (35, 36).
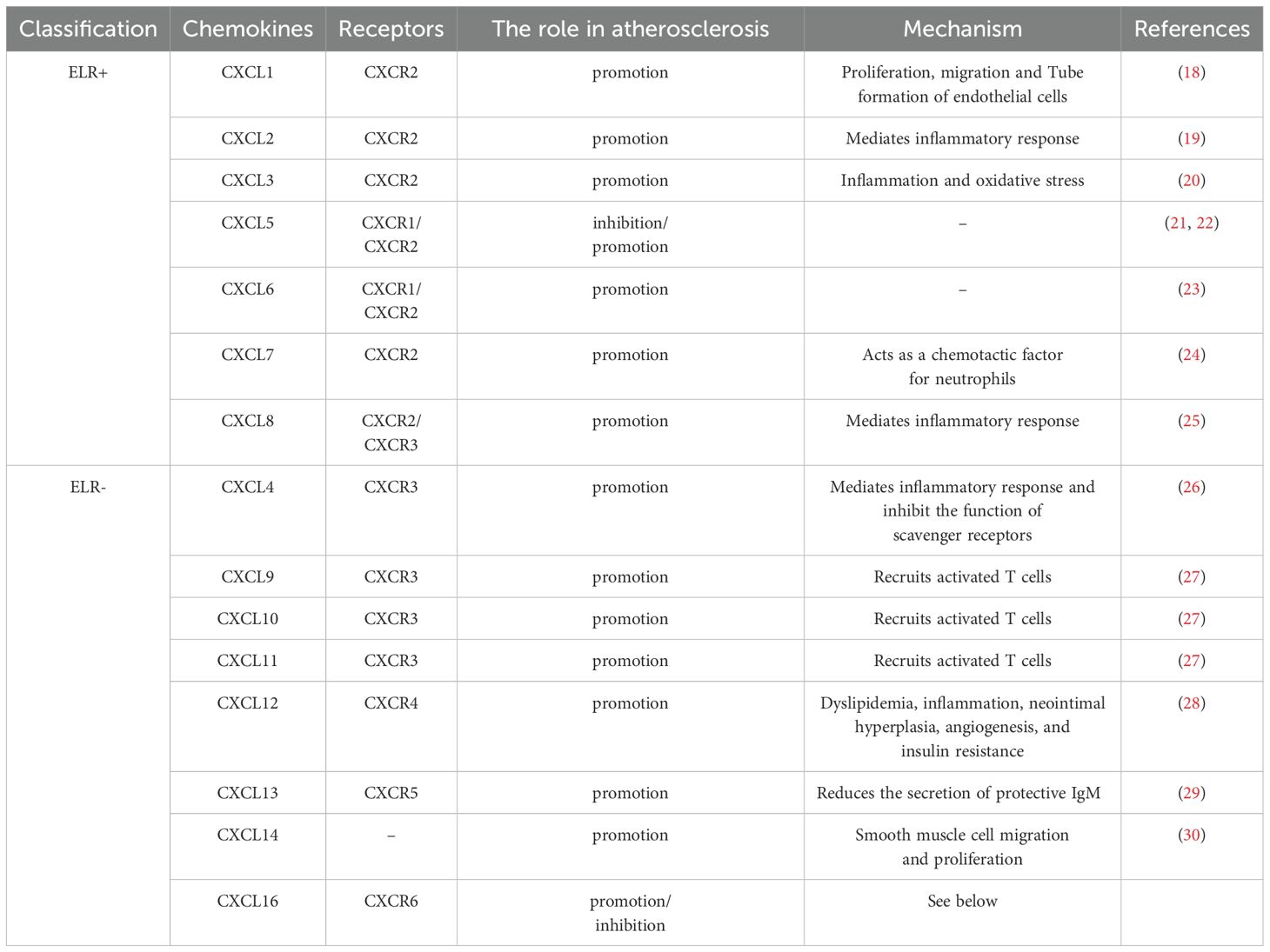
mCXCL16 functions as both an adhesion molecule and a scavenger receptor (39). It primarily mediates the recognition and uptake of oxidized low-density lipoproteins (oxLDL) and phosphatidylserine (PS) (40). Therefore, it was initially named the scavenger receptor for phosphatidylserine and oxidized lipoproteins (SR-PSOX) (41). CXCR6 is a seven-transmembrane G protein-coupled receptor (GPCR) (42). It was originally identified as a co-receptor for human immunodeficiency virus (HIV) in CD4+ and CD8+ T cells (43, 44). It is also known as CD186, Bonzo, STRL, or TYMSTR (42, 45). CXCR6 is the specific receptor for sCXCL16. Through their specific binding, sCXCL16 induces proliferation and migration of CXCR6-expressing cells, while activating downstream signaling pathways (including NF-κB, PI3K/AKT, MAPK pathways) to regulate the occurrence and progression of AS (32, 46). (Figure 1).
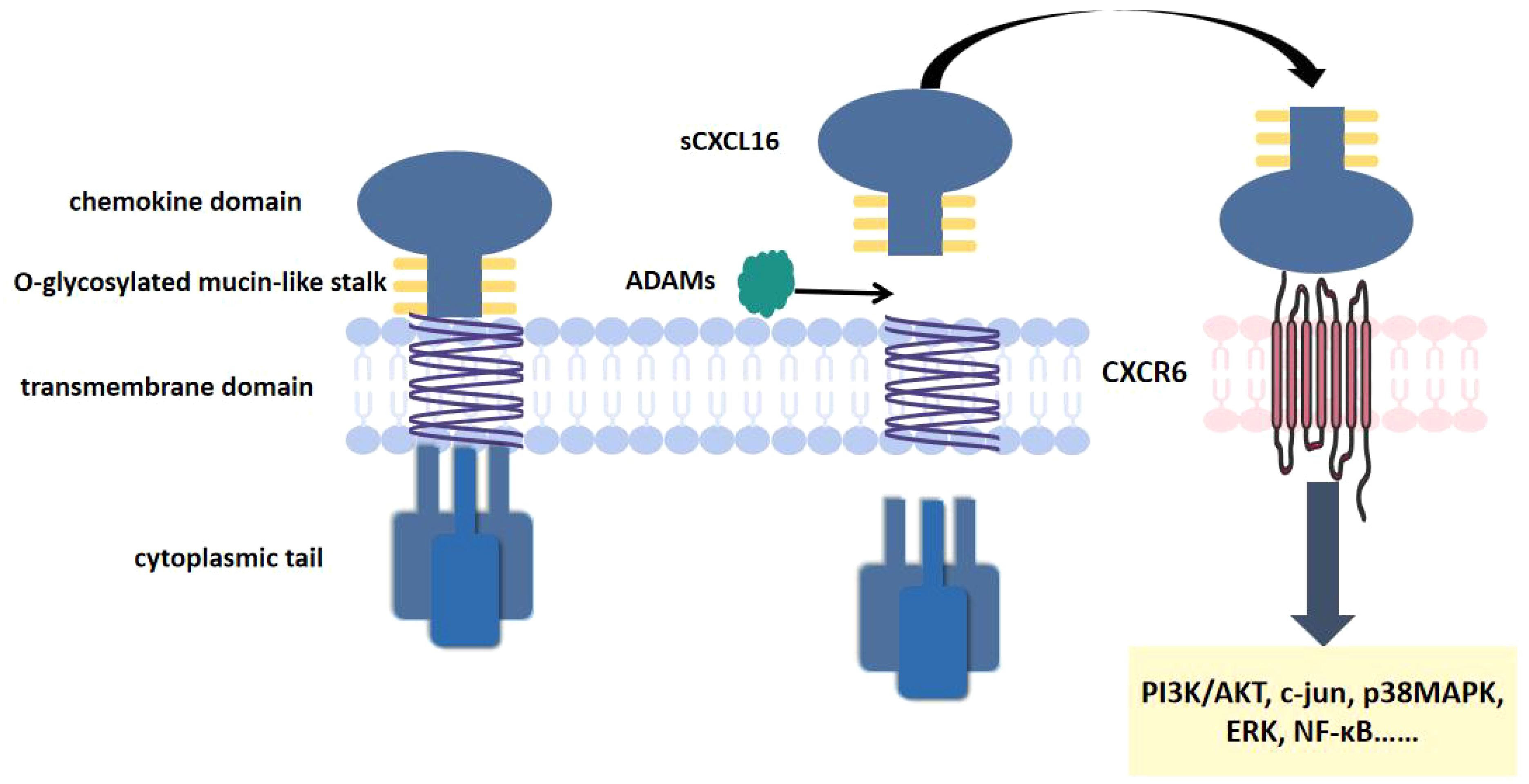
Figure 1. CXCL16 and CXCR6 structure. CXCL16 consists of a transmembrane region, a short cytoplasmic domain, an extracellular N-terminal chemokine domain. CXCR6 is a seven-transmembrane GPCR. CXCL16 binds to CXCR6, activating downstream signaling pathways.
2 The pro-atherosclerotic effect of CXCL16
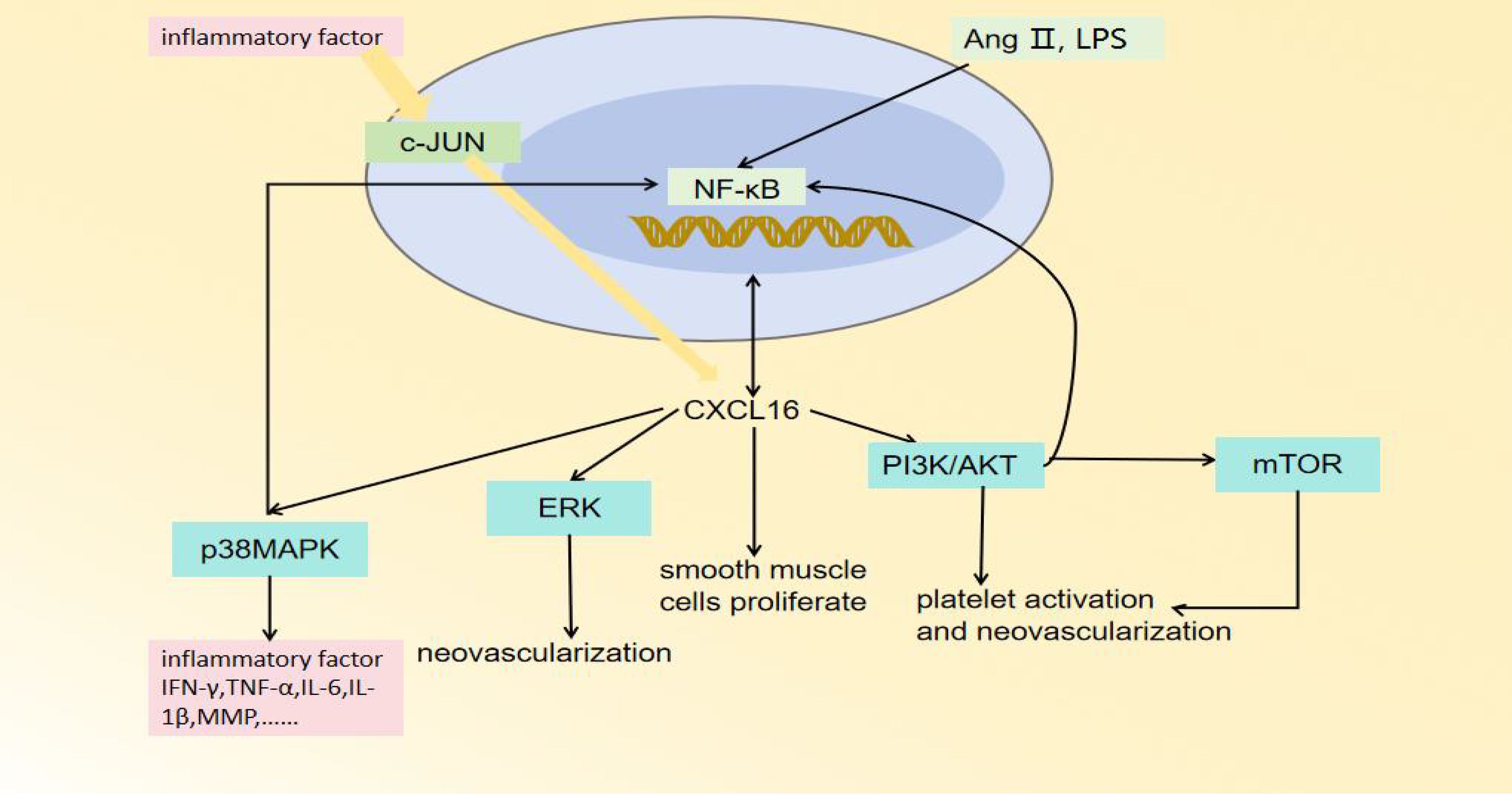
CXCL16 plays an important role in the occurrence, progression, and plaque destabilization of AS. During the early disease stage, CXCL16 promotes inflammatory cell infiltration and foam cell formation, accelerating lipid deposition and vascular endothelial injury (47). In the advanced stage, it further stimulates smooth muscle cell proliferation and migration, inducing intimal thickening and fibrous cap formation (48). At the late stage, sustained inflammatory responses, blood cell aggregation, and pathological neovascularization can lead to fibrous cap thinning, increasing plaque rupture risk (Figure 2) (49). Mechanistic studies reveal that CXCL16 mediates vascular inflammatory responses, intimal remodeling, and angiogenesis through regulation of PI3K/AKT, MAPK, and NF-κB signaling pathways, thereby multi-dimensionally promoting AS progression (Figure 3) (50). Thus, a comprehensive understanding of CXCL16’s pathological role (Figure 4) and molecular mechanisms in AS will provide crucial theoretical foundations for developing targeted intervention strategies for AS.
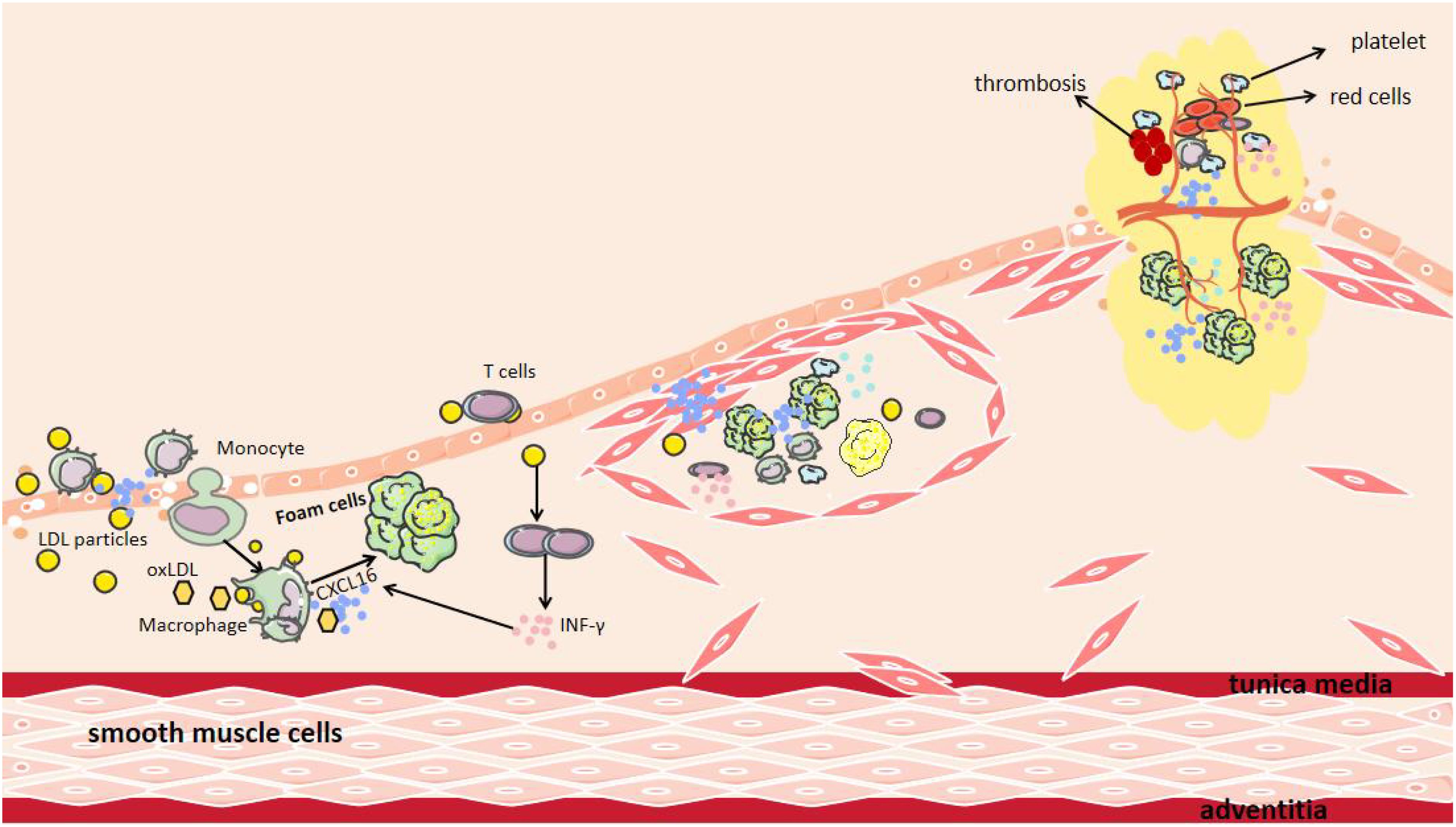
Figure 2. The Role of CXCL16 in AS. After endothelial cell injury, CXCL16 on the endothelial cells surface mediates the recruitment and adhesion of both inflammatory cells and pro-inflammatory cytokines to the damaged surface. Recruited macrophages subsequently infiltrate the intimal layer, where CXCL16 facilitates their phagocytosis of LDL and oxidized LDL (oxLDL). Additionally, CXCL16 acts as a scavenger receptor, providing receptors for oxLDL. INF-γ produced by T cells increases the production of CXCL16. Furthermore, CXCL16 can promote the aggregation of other blood cells and the formation of new blood vessels, leading to plaque instability.
2.1 CXCL16 induces vascular inflammatory response
Chemokines drive sustained low-level chronic inflammatory responses by recruiting inflammatory cells and factors to the lesion sites (51). This persistent inflammatory state not only facilitates atherosclerotic plaque formation but also leads to plaque destabilization and rupture (52) (Table 2).
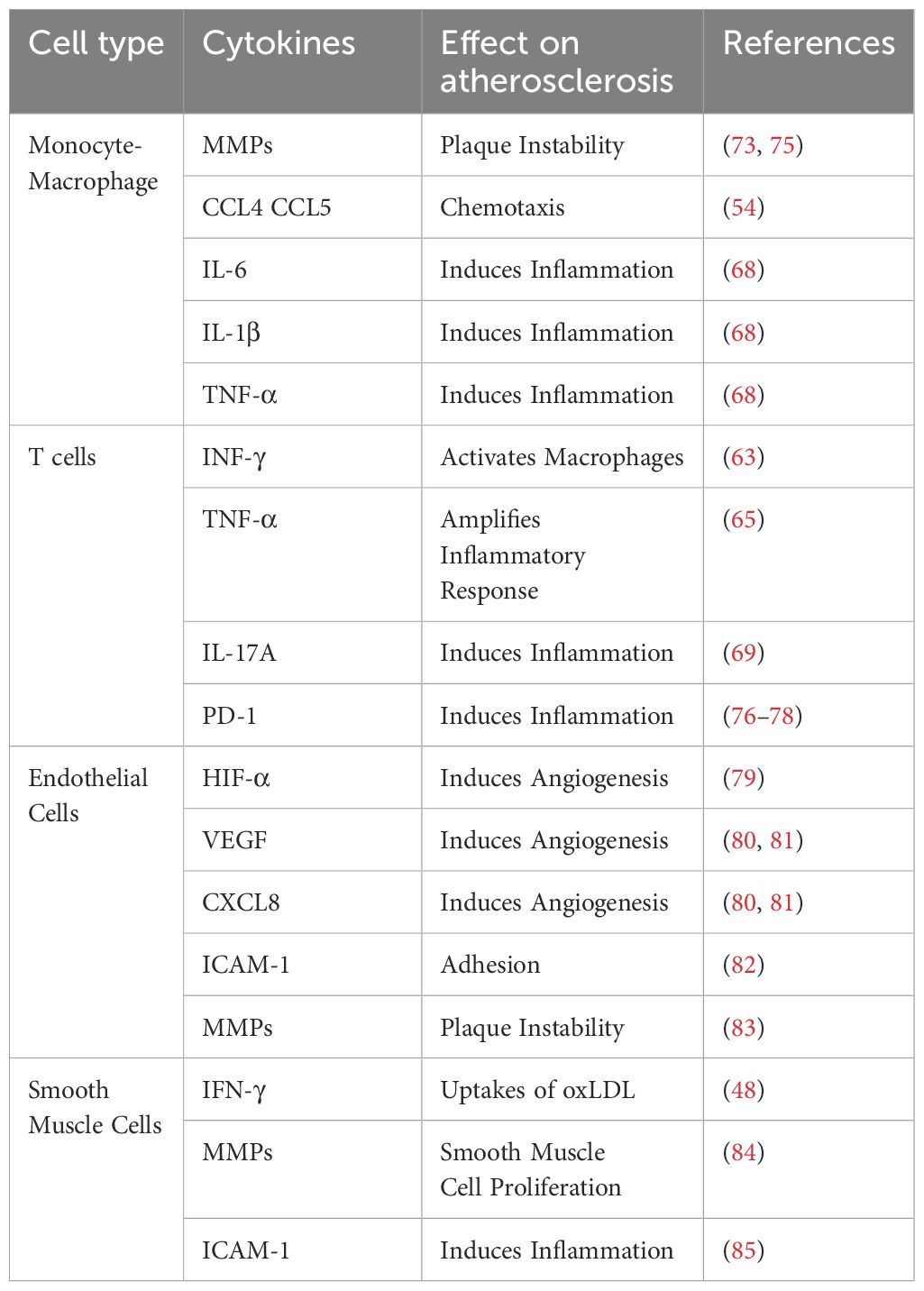
Table 2. The Mechanism of CXCL16 Chemotaxis of Various Cells, Cytokine Secretion, and Involvement in Atherosclerosis.
CXCL16 specifically chemoattracts various CXCR6-expressing immune cells, including monocytes/macrophages, T cells, NK cells, invariant natural killer T cells and plasma cells (46). In the early stages of AS, when the vascular endothelial barrier is destroyed, CXCL16 recruits monocytes from circulation to the subendothelial space through chemotaxis, facilitating their differentiation into macrophages (53). In the murine model of myocardial infarction, CXCL16-mediated activation of NF-κB and p38MAPK pathways drives upregulation of CCL4 and CCL5, resulting in amplified monocyte recruitment and subsequent aggravation of cardiac injury (54). In human umbilical vein endothelial cells (HUVECs) and macrophages, LPS and the proinflammatory cytokine TWEAK can induce CXCL16 production through NF-κB signaling pathways (55–57). It is noteworthy that CXCL16 serves not only as a chemokine but also plays an equally important role as a scavenger receptor (58). It can recognize and mediate the phagocytosis of oxLDL by macrophages, promoting the formation of foam cells, which is a critical step in the development of AS (59, 60).
With the continuous development of AS, the continuous accumulation of oxLDL can activate T cells, promoting their secretion of pro-inflammatory factors such as IFN-γ and TNF-α (61, 62). Notably, these cytokines (particularly TNF-α and IFN-γ) can further stimulate T cells to produce CXCL16, continuously recruiting macrophages, and maintaining inflammatory microenvironment (63–65). Once T cells infiltrate the plaques, they may undergo clonal proliferation and secrete large amounts of inflammatory cytokines (66). This process ultimately leads to plaque destabilization and even rupture (67). CXCL16 can promote the secretion of inflammatory factors (including IL-6, IL-1β, VCAM-1, ICAM-1 and IL-17A) in macrophages and T cells of plaques through activation of the p38 MAPK signaling pathway, thereby exacerbating plaque instability and contributing to adverse cardiovascular events (68–72). Furthermore, excessive activation of the CXCL16/CXCR6 axis upregulates the expression of matrix metalloproteinases (MMPs), leading to the abnormal degradation of elastin and collagen in the vascular extracellular matrix and promoting calcium salt deposition. These pathological changes ultimately result in fibrous cap thinning and rupture (68, 73). This finding has been experimentally validated in ApoE-/- mice: lentivirus-mediated CXCL16 overexpression significantly upregulated inflammatory mediators (including MMPs, CCL2, VCAM-1, and TNF-α) and markedly aggravated plaque instability (74).
2.2 CXCL16 induces intimal thickening
In the advanced stages of AS, vascular intimal hyperplasia not only exacerbates the retention of lipoprotein in the intima but also accelerates significantly the progression of AS (86). Vascular smooth muscle cells (VSMCs) play a crucial role in phenotypic switching and functional dysregulation (87). Current studies have confirmed that CXCL16 promotes VSMCs proliferation and migration by multiple mechanisms (48, 60, 88). Bysani Chandrasekar et al. demonstrated that CXCL16 enhanced aortic smooth muscle cell proliferation and migration in a PI3K/AKT-dependent manner (88). Similarly, Chandrasekar, B. et al. found that CXCL16 increased intercellular adhesion and stimulated VSMC proliferation by activating the NF-κB signaling pathway (60). Additionally, the uptake of oxLDL induced by IFN-γ depends on the upregulation of CXCL16 expression in VSMCs (48). Inflammatory cytokines boost CXCL16 expression by inducing c-Jun binding to the CXCL16 promoter, thereby promoting VSMC proliferation and contributing to AS (88, 89). In addition, aging VSMCs exhibit increased expression of chemokines (such as CXCL16), adhesion molecules (such as ICAM-1), and innate immune receptors (such as Toll-like receptors 4) (82, 85). These changes collectively establish a persistent pro-inflammatory microenvironment that further promotes the progression of AS. Although the precise mechanisms of CXCL16 in VSMCs remain unclear, targeting this chemokine may offer a new therapeutic strategy to mitigate post-angioplasty restenosis (88).
2.3 CXCL16 induces angiogenesis
As the lesion progresses, pathological thickening of the vascular wall leads to luminal stenosis, significantly reducing local tissue perfusion and inducing a hypoxic state (90). The abnormal accumulation of lipids and the formation of necrotic core in plaques create a vicious cycle, continuously stimulating the upregulation of hypoxia-inducible factor-1α (HIF-1α) expression (91). This chronic hypoxic microenvironment activates pro-angiogenic signaling pathways, inducing the formation of pathological neovascularization within plaques. These structurally fragile neovessels not only increase the risk of intraplaque hemorrhage but also significantly elevate the potential for plaque rupture (92).
In 2009, Zhuge, X. et al. revealed that CXCL16 is a angiogenic factor with multifunctional regulatory properties (93). CXCL16 promotes pathological neovascularization in a dose-dependent manner (79). Immunohistochemical analysis found that CXCL16 was strongly expressed in endothelial cells of pathological neovascularization (94). CXCL16 promotes angiogenesis through multiple mechanisms. Firstly, the hypoxic microenvironment of atherosclerotic plaques induces HIF-1α, which in turn upregulates CXCL16 expression (95). Conversely, CXCL16 secreted by HUVECs further enhances HIF-1α-mediated vascular endothelial growth factor (VEGF) production by activating the PI3K/AKT signaling pathway, forming a pro-angiogenic vicious cycle (96, 97). Additionally, prior studies confirmed that CXCL16 markedly enhanced the proliferative capacity, chemotactic motility, and vascular network formation of HUVECs in vitro by activating the ERK pathway (93). Secondly, CXCL16 can induce endothelial cells to produce the potent pro-angiogenic factor CXCL8/IL-8 (98), indirectly promoting angiogenesis through this paracrine mechanism (80, 81). Thirdly, CXCL16 also induces the expression of MMPs in endothelial cells. These enzymes degrade the extracellular matrix and release stored pro-angiogenic factors, ultimately contributing to plaque instability (99).
3 The protective effect of CXCL16 against AS
A few studies have reported that CXCL16 has a protective effect against AS. Aslanian, A. M. et al. found that atherosclerotic plaque burden was unexpectedly increased in LDLR-/- mouse models with CXCL16 gene knockout. The authors attributed this phenomenon primarily to impaired scavenger receptor function caused by CXCL16 deficiency, which subsequently reduced the efficiency of apoptotic cell clearance. Additionally, this study demonstrated that CXCL16 knockout significantly reduced oxLDL binding and internalization by macrophages in vitro (16). These findings markedly contradict previous research conclusions regarding the pro-atherogenic roles of classical scavenger receptors such as SR-A and CD36 (100, 101). We speculate that the bidirectional regulatory effects of CXCL16 stem from its dual functional properties (chemokine vs scavenger receptor). In different pathological microenvironments, when one function becomes dominant, corresponding phenotypic characteristics emerge (16). In addition, the choice of animal models and the impact of gene knockout may affect further investigations. Similarly, a study reported that the level of CXCL16 were reduced in patients of coronary AS (17). However, this study only included a small number of patients with stable or unstable angina. And the results of this study were overturned in a larger cohort study (102). Van Lieshout A. W.,et al. questioned the results of this study (103). Based on current research, we believe that the conclusions of this study require further exploration.
4 Targeted therapy
Atherosclerotic cardiovascular disease currently stands as the leading global cause of mortality (104). Although lipid-lowering medications can effectively reduce blood lipid and inflammatory cytokine levels, some individuals still exhibit residual cardiovascular risk, highlighting the need for further therapeutic interventions (105). As key regulators of leukocyte migration, chemokines play a central role in immune surveillance and inflammatory responses. In recent years, drug development targeting the chemokine system has become a major focus in the treatment of inflammatory diseases (106). Throughout the entire process of AS development, the chemokine network participates in regulating multiple pathological pathways, making it a highly promising therapeutic target (9). Among these, CXCL16 and its signaling pathway play significant roles by mediating inflammatory cell infiltration, regulating the proliferation of vascular endothelial and smooth muscle cells, and influencing angiogenesis (47–49). Although early studies suggested that CXCL16 may possess dual regulatory properties, recent evidence consistently indicated that its pro-atherogenic effect dominated. Considering the limitations of previous studies proposing a “protective role” hypothesis, we propose that selective inhibition of CXCL16 and its signaling pathway may serve as an effective strategy to delay the progression of AS (Table 3).
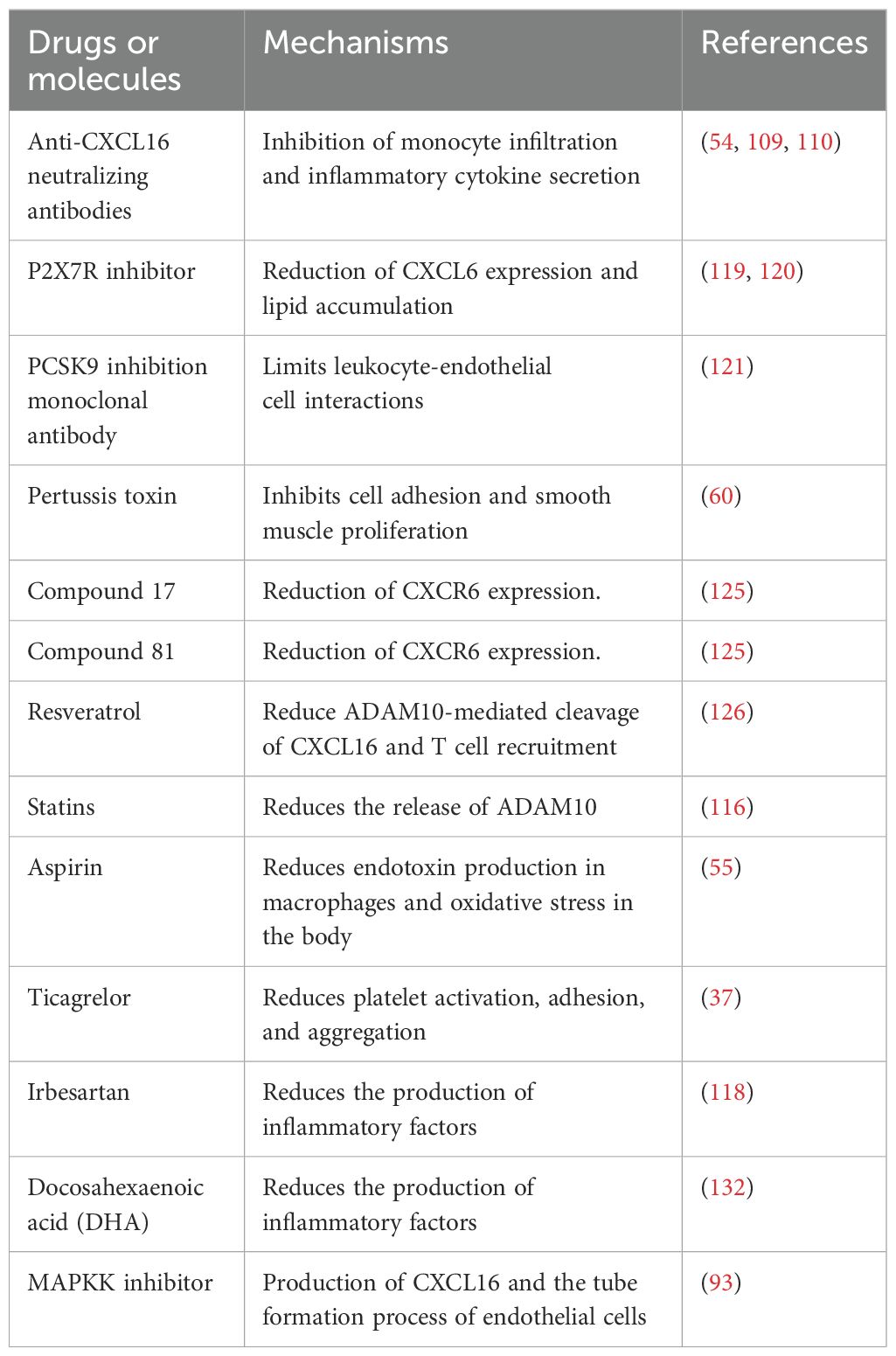
Table 3. The anti-atherosclerotic effects of various drugs and compounds at the chemokine molecular level.
4.1 Anti-atherosclerotic effects by inhibiting CXCL16, CXCR6 and proteases
Compared with non-atherosclerotic tissues, atherosclerotic plaques contain significantly higher levels of inflammatory cells, inflammatory factors, and lipid deposits (107, 108). Studies showed that targeting CXCL16 could effectively modulate this pathological process. For instance, in murine models of myocardial infarction, administration of anti-CXCL16 neutralizing antibodies inhibits monocyte infiltration and improve cardiac function after myocardial infarction (54). Similarly, in murine models of glomerulonephritis, anti-CXCL16 neutralizing antibodies reduced the expression of IL-4 and IL-10 (109). In a murine model of Salmonella enterica serovar Enteritidis infection, anti-CXCL16 neutralizing antibodies reduced the expression of IFN-γ (110). Additionally, lentivirus-mediated CXCL16 knockdown can inhibit macrophage transformation into foam cells and reduce lipid deposition in the arterial walls of ApoE-/- mice (111, 112). These findings collectively indicated that targeting CXCL16 could reduce the accumulation of inflammatory cells, cytokines, and lipids, thereby mitigating the development of AS.
In addition to the direct inhibition of CXCL16, studies have found that the promoter region of CXCL16 contains the binding site of FOXO3, and targeting FOXO3 could reduce the expression of CXCL16 (113). In addition, the basic amino acid residues in the CXCL16 chemokine domain are critical for its function. Point mutation of basic amino acid plays a key role in CXCL16 function. Disrupting these key residues can attenuate the pro-atherogenic effects of CXCL16 (114, 115). The presence of these molecular recognition elements demonstrates the druggability of this target. Currently, some available drugs (including aspirin, ticagrelor, irbesartan, PCSK9-blocking monoclonal antibody, P2X7R inhibitor A438079) can reduce the release of CXCL16 and inflammatory responses (37, 55, 116–121).
Targeting the specific receptor CXCR6 of CXCL16 can also attenuate the development of AS. Studies found that CXCR6 knockout in mice inhibited monocyte infiltration into vascular walls, reduced inflammatory response and myocardial ischemia-reperfusion injury (122). Elena Galkina et al. revealed that CXCR6-deficient ApoE-/- mice reduced T cell and macrophage infiltration in aortic walls along with suppressed production of pro-inflammatory cytokine IFN-γ (123). The Gi receptor inhibitors (pertussis toxin) exert protective effects by both blocking cell adhesion and inhibiting aortic smooth muscle proliferation (60). These findings collectively indicated that targeting CXCR6 produced effects comparable to those observed with CXCL16 inhibition. Postea, O. et al. found that homocysteine could enhance CXCR6-mediated lymphocyte recruitment, thereby promoting the progression of AS (124). This suggests that homocysteine-lowering medications potentially reduce inflammatory cell accumulation. Some compounds (such as compound 81 and compound 17) have been found to reduce the expression of CXCR6 (125). With their favorable oral bioavailability, these compounds represent promising candidates for next-generation anti-atherosclerotic therapies.
ADAM10 serves as the key protease mediating the conversion of mCXCL16 to sCXCL16 (126). Gough, P. J. et al. showed that knocking down ADAM10 reduced this constitutive shedding of CXCL16. ADAM10 inhibitors, such as resveratrol, can effectively block the proteolytic processing of CXCL16 (116, 126). A small molecule compound (GI254023X) has been identified as ADAM10 inhibitor that reduces the release of sCXCL16 (127). Simvastatin, a commonly used clinical drug, has also been found to inhibit the activity of ADAM10 (116).
4.2 Anti-atherosclerotic effects by inhibiting the regulatory mechanisms of CXCL16
CXCL16 exerts anti-inflammatory effects by modulating multiple signaling pathways including NF-κB, PI3K/AKT, and MAPK, thereby attenuating AS progression (50). Various pharmacological agents, such as NF-κB inhibitors (JSH-23, SN50), aspirin, rapamycin, PI3K/AKT inhibitors, and irbesartan, have been demonstrated to downregulate CXCL16 expression (55, 118, 128, 129). Various NF-κB inhibitors have proven effective in reducing inflammatory cell infiltration and suppressing atherosclerotic plaque formation (54, 55). Aspirin can inhibit the nuclear translocation of the NF-κB p65 subunit, thus reducing the progression of AS (130). The PI3K inhibitor (LY294002 or wortmannin), AKT inhibitor (SH-6) and JNK inhibitor (SP600125) can reduce platelet adhesion and smooth muscle cell proliferation (60, 79, 131). The p38 inhibitors and ERK inhibitors (PD98059) inhibit HUVEC proliferation, migration, tube formation and HIF-1α expression. Notably, the HIF-1α selective inhibitor (PX-12) not only inhibits these biological processes but also suppresses CXCL16 production (79).
In this section, we have highlighted that numerous existing drugs can effectively reduce CXCL16 or modulate its receptor, protease, and related signaling pathways. This drug repurposing strategy offers the advantage of accelerating clinical validation while significantly reducing development costs. However, it should be noted that currently there are no drugs specifically targeting CXCL16. Therefore, the development of CXCL16-specific probes and advanced nanodelivery technologies has become an imperative research direction.
5 Conclusion
CXCL16 is an important chemokine and immune regulator, which is widely expressed in various cells such as endothelial cells, monocytes, macrophages, and T cells. Previous studies showed that CXCL16 played a complex dual regulatory role in the development of AS. In this review, we summarize the key pathological mechanisms and related signaling pathways of CXCL16 in promoting AS, while also providing an objective evaluation of its potential protective effects. Based on current evidence, we propose that the pro-inflammatory and pro-atherogenic effects of CXCL16 dominate in AS. Therefore, targeted inhibition of CXCL16 represents a promising therapeutic approach for AS. Although the development of CXCL16-targeted drugs still faces numerous challenges such as target selectivity and optimization of drug delivery methods, advances in both mechanistic understanding and novel drug delivery technologies are expected to lead to breakthrough progress. These studies may not only yield more effective treatments but also uncover new intervention targets, thereby opening new avenues for the prevention and treatment of AS.
Author contributions
YL: Writing – original draft. XT: Writing – original draft. CJ: Writing – review & editing. XC: Writing – review & editing. CC: Writing – review & editing. CL: Writing – review & editing. SY: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (Grant Nos.82001251).
Acknowledgments
Thanks for every author for efforts and the National Natural Science Foundation of China (Grant Nos.82001251) for help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Collaborators, G.B.D.S. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Wang Y, Zhao Y, Ye T, Yang L, Shen Y, Li H, et al. Ferroptosis signaling and regulators in atherosclerosis. Front Cell Dev Biol. (2021) 9:809457. doi: 10.3389/fcell.2021.809457
3. Gonzalez-Guerra A, Roche-Molina M, García-Quintáns N, Sánchez-Ramos C, Martín-Pérez D, Lytvyn M, et al. Sustained elevated blood pressure accelerates atherosclerosis development in a preclinical model of disease. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22168448
4. Sinha A, Ning H, Cameron N, Bancks M, Carnethon MR, Allen NB, et al. Atherosclerotic cardiovascular disease or heart failure: first cardiovascular event in adults with prediabetes and diabetes. J Card Fail. (2023) 29:246–54. doi: 10.1016/j.cardfail.2022.10.426
5. Zhao H, Liu M, Liu H, Suo R, and Lu C. Naringin protects endothelial cells from apoptosis and inflammation by regulating the Hippo-YAP Pathway. Biosci Rep. (2020) 40. doi: 10.1042/BSR20193431
6. Messner B and Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. (2014) 34:509–15. doi: 10.1161/ATVBAHA.113.300156
7. Kiechl S, Willeit J, Rungger G, Egger G, Oberhollenzer F, Bonora E, et al. Alcohol consumption and atherosclerosis: what is the relation? Prospective results from the Bruneck Study. Stroke. (1998) 29:900–7. doi: 10.1161/01.STR.29.5.900
8. Guzik TJ and Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. (2017) 70:660–7. doi: 10.1161/HYPERTENSIONAHA.117.07802
9. Yan Y, Thakur M, van der Vorst EPC, Weber C, and Döring Y. Targeting the chemokine network in atherosclerosis. Atherosclerosis. (2021) 330:95–106. doi: 10.1016/j.atherosclerosis.2021.06.912
10. Zhou C, Gao Y, Ding P, Wu T, and Ji G. The role of CXCL family members in different diseases. Cell Death Discov. (2023) 9:212. doi: 10.1038/s41420-023-01524-9
11. Miller MC and Mayo KH. Chemokines from a structural perspective. Int J Mol Sci. (2017) 18. doi: 10.3390/ijms18102088
12. Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, and Kasper J. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. (1995) 270:27348–57. doi: 10.1074/jbc.270.45.27348
13. Bakogiannis C, Sachse M, Stamatelopoulos K, and Stellos K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine. (2019) 122:154157. doi: 10.1016/j.cyto.2017.09.013
14. Ma A, Pan X, Xing Y, Wu M, Wang Y, and Ma C. Elevation of serum CXCL16 level correlates well with atherosclerotic ischemic stroke. Arch Med Sci. (2014) 10:47–52. doi: 10.5114/aoms.2013.39200
15. Ma A, Yang S, Wang Y, Wang X, and Pan X. Increase of serum CXCL16 level correlates well to microembolic signals in acute stroke patients with carotid artery stenosis. Clin Chim Acta. (2016) 460:67–71. doi: 10.1016/j.cca.2016.06.026
16. Aslanian AM and Charo IF. Targeted disruption of the scavenger receptor and chemokine CXCL16 accelerates atherosclerosis. Circulation. (2006) 114:583–90. doi: 10.1161/CIRCULATIONAHA.105.540583
17. Sheikine Y, Bang CS, Nilsson L, Samnegård A, Hamsten A, Jonasson L, et al. Decreased plasma CXCL16/SR-PSOX concentration is associated with coronary artery disease. Atherosclerosis. (2006) 188:462–6. doi: 10.1016/j.atherosclerosis.2005.11.025
18. Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. (2000) 165:5269–77. doi: 10.4049/jimmunol.165.9.5269
19. Guo LY, Yang F, Peng LJ, Li YB, and Wang AP. CXCL2, a new critical factor and therapeutic target for cardiovascular diseases. Clin Exp Hypertens. (2020) 42:428–37. doi: 10.1080/10641963.2019.1693585
20. Qin W, Gan F, Liang R, Li J, Lai X, Dai Y, et al. Identification of monocyte-associated genes related to the instability of atherosclerosis plaque. Oxid Med Cell Longev. (2022) 2022:3972272. doi: 10.1155/2022/3972272
21. Ravi S, Schuck RN, Hilliard E, Lee CR, Dai X, Lenhart K, et al. Clinical evidence supports a protective role for CXCL5 in coronary artery disease. Am J Pathol. (2017) 187:2895–911. doi: 10.1016/j.ajpath.2017.08.006
22. Chen L, Yang Z, Lu B, Li Q, Ye Z, He M, et al. Serum CXC ligand 5 is a new marker of subclinical atherosclerosis in type 2 diabetes. Clin Endocrinol (Oxf). (2011) 75:766–70. doi: 10.1111/j.1365-2265.2011.04119.x
23. Ryabov VV, Vorobeva DA, Kologrivova IV, and Suslova TE. Pro-inflammatory biomarkers and progression of atherosclerosis in patients with myocardial infarction with non-obstructive coronary artery disease: 1-year follow-up. J Pers Med. (2023) 13. doi: 10.3390/jpm13121669
24. Ku EJ, Cho KC, Lim C, Kang JW, Oh JW, Choi YR, et al. Discovery of plasma biomarkers for predicting the severity of coronary artery atherosclerosis by quantitative proteomics. BMJ Open Diabetes Res Care. (2020) 8. doi: 10.1136/bmjdrc-2019-001152
25. An Z, Li J, Yu J, Wang X, Gao H, Zhang W, et al. Neutrophil extracellular traps induced by IL-8 aggravate atherosclerosis via activation NF-κB signaling in macrophages. Cell Cycle. (2019) 18:2928–38. doi: 10.1080/15384101.2019.1662678
26. Gleissner CA. Macrophage phenotype modulation by CXCL4 in atherosclerosis. Front Physiol. (2012) 3:1. doi: 10.3389/fphys.2012.00001
27. Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, et al. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest. (1999) 104:1041–50. doi: 10.1172/JCI6993
28. Gao JH, Yu XH, and Tang CK. CXC chemokine ligand 12 (CXCL12) in atherosclerosis: An underlying therapeutic target. Clin Chim Acta. (2019) 495:538–44. doi: 10.1016/j.cca.2019.05.022
29. van der Vorst EPC, Daissormont I, Aslani M, Seijkens T, Wijnands E, Lutgens E, et al. Interruption of the CXCL13/CXCR5 chemokine axis enhances plasma igM levels and attenuates atherosclerosis development. Thromb Haemost. (2020) 120:344–7. doi: 10.1055/s-0039-3400746
30. Tong W, Duan Y, Yang R, Wang Y, Peng C, Huo Z, et al. Foam cell-derived CXCL14 muti-functionally promotes atherogenesis and is a potent therapeutic target in atherosclerosis. J Cardiovasc Transl Res. (2020) 13:215–24. doi: 10.1007/s12265-019-09915-z
31. Matloubian M, David A, Engel S, Ryan JE, and Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol. (2000) 1:298–304. doi: 10.1038/79738
32. Mei J, Yan Y, Li SY, Zhou WJ, Zhang Q, Li MQ, et al. CXCL16/CXCR6 interaction promotes endometrial decidualization via the PI3K/AKT pathway. Reproduction. (2019) 157:273–82. doi: 10.1530/REP-18-0417
33. Abu El-Asrar AM, Nawaz MI, Ahmad A, De Zutter A, Siddiquei MM, Blanter M, et al. Evaluation of proteoforms of the transmembrane chemokines CXCL16 and CX3CL1, their receptors, and their processing metalloproteinases ADAM10 and ADAM17 in proliferative diabetic retinopathy. Front Immunol. (2020) 11:601639. doi: 10.3389/fimmu.2020.601639
34. Jovanović I, Zivković M, Djurić T, Popović M, Alavantić D, Stanković A, et al. CXCL16 in vascular pathology research: from macro effects to microRNAs. J Atheroscler Thromb. (2015) 22:1012–24. doi: 10.5551/jat.29942
35. Gough PJ, Garton KJ, Wille PT, Rychlewski M, Dempsey PJ, Raines EW, et al. A disintegrin and metalloproteinase 10-mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. J Immunol. (2004) 172:3678–85. doi: 10.4049/jimmunol.172.6.3678
36. Abel S, Hundhausen C, Mentlein R, Schulte A, Berkhout TA, Broadway N, et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol. (2004) 172:6362–72. doi: 10.4049/jimmunol.172.10.6362
37. Guan T, Emschermann F, Schories C, Groga-Bada P, Martus P, Borst O, et al. Platelet SR-PSOX/CXCL16-CXCR6 axis influences thrombotic propensity and prognosis in coronary artery disease. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms231911066
38. Aboyoussef AM, Abdel-Sattar AR, Abdel-Bakky MS, and Messiha BAS. Enoxaparin prevents CXCL16/ADAM10-mediated cisplatin renal toxicity: Role of the coagulation system and the transcriptional factor NF-κB. Life Sci. (2021) 270:119120. doi: 10.1016/j.lfs.2021.119120
39. Wang FT, Wu TQ, Lin Y, Jiao YR, Li JY, Ruan Y, et al. The role of the CXCR6/CXCL16 axis in the pathogenesis of fibrotic disease. Int Immunopharmacol. (2024) 132:112015. doi: 10.1016/j.intimp.2024.112015
40. Shimaoka T, Nakayama T, Fukumoto N, Kume N, Takahashi S, Yamaguchi J, et al. Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J Leukoc Biol. (2004) 75:267–74. doi: 10.1189/jlb.1003465
41. Shimaoka T, Kume N, Minami M, Hayashida K, Kataoka H, Kita T, et al. Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR-PSOX, on macrophages. J Biol Chem. (2000) 275:40663–6. doi: 10.1074/jbc.C000761200
42. Li T, Pan J, Chen H, Fang Y, and Sun Y. CXCR6-based immunotherapy in autoimmune, cancer and inflammatory infliction. Acta Pharm Sin B. (2022) 12:3255–62. doi: 10.1016/j.apsb.2022.03.012
43. Clapham PR and Weiss RA. Immunodeficiency viruses. Spoilt for choice of co-receptors. Nature. (1997) 388:230–1. doi: 10.1038/40758
44. Alkhatib G, Liao F, Berger EA, Farber JM, and Peden KW. A new SIV co-receptor, STRL33. Nature. (1997) 388:238. doi: 10.1038/40789
45. Ignatius R, Wei Y, Beaulieu S, Gettie A, Steinman RM, Pope M, et al. The immunodeficiency virus coreceptor, Bonzo/STRL33/TYMSTR, is expressed by macaque and human skin- and blood-derived dendritic cells. AIDS Res Hum Retroviruses. (2000) 16:1055–9. doi: 10.1089/08892220050075318
46. Bao N, Fu B, Zhong X, Jia S, Ren Z, Wang H, et al. Role of the CXCR6/CXCL16 axis in autoimmune diseases. Int Immunopharmacol. (2023) 121:110530. doi: 10.1016/j.intimp.2023.110530
47. Hu ZB, Chen Y, Gong YX, Gao M, Zhang Y, Wang GH, et al. Activation of the CXCL16/CXCR6 pathway by inflammation contributes to atherosclerosis in patients with end-stage renal disease. Int J Med Sci. (2016) 13:858–67. doi: 10.7150/ijms.16724
48. Wågsäter D, Olofsson PS, Norgren L, Stenberg B, and Sirsjö A. The chemokine and scavenger receptor CXCL16/SR-PSOX is expressed in human vascular smooth muscle cells and is induced by interferon gamma. Biochem Biophys Res Commun. (2004) 325:1187–93. doi: 10.1016/j.bbrc.2004.10.160
49. Jin G. The relationship between serum CXCL16 level and carotid vulnerable plaque in patients with ischemic stroke. Eur Rev Med Pharmacol Sci. (2017) 21:3911–5.
50. Korbecki J, Bajdak-Rusinek K, Kupnicka P, Kapczuk P, Simińska D, Chlubek D, et al. The role of CXCL16 in the pathogenesis of cancer and other diseases. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22073490
51. Terkeltaub R, Boisvert WA, and Curtiss LK. Chemokines and atherosclerosis. Curr Opin Lipidol. (1998) 9:397–405. doi: 10.1097/00041433-199810000-00003
52. Hermans L and O'Sullivan TE. Send it, receive it, quick erase it: A mouse model to decipher chemokine communication. J Exp Med. (2024) 221. doi: 10.1084/jem.20240582
53. Linke B, Meyer Dos Santos S, Picard-Willems B, Keese M, Harder S, Geisslinger G, et al. CXCL16/CXCR6-mediated adhesion of human peripheral blood mononuclear cells to inflamed endothelium. Cytokine. (2019) 122:154081. doi: 10.1016/j.cyto.2017.06.008
54. Zhang J, Hao W, Zhang J, Li T, Ma Y, Wang Y, et al. CXCL16 promotes ly6Chigh monocyte infiltration and impairs heart function after acute myocardial infarction. J Immunol. (2023) 210:820–31. doi: 10.4049/jimmunol.2200249
55. Lehrke M, Millington SC, Lefterova M, Cumaranatunge RG, Szapary P, Wilensky R, et al. CXCL16 is a marker of inflammation, atherosclerosis, and acute coronary syndromes in humans. J Am Coll Cardiol. (2007) 49:442–9. doi: 10.1016/j.jacc.2006.09.034
56. Xiao Q, Zhu X, Yang S, Wang J, Yin R, Song J, et al. LPS induces CXCL16 expression in HUVECs through the miR-146a-mediated TLR4 pathway. Int Immunopharmacol. (2019) 69:143–9. doi: 10.1016/j.intimp.2019.01.011
57. Ruiz-Ortega M, Ortiz A, and Ramos AM. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) and kidney disease. Curr Opin Nephrol Hypertens. (2014) 23:93–100. doi: 10.1097/01.mnh.0000437331.23794.81
58. Wuttge DM, Zhou X, Sheikine Y, Wågsäter D, Stemme V, Hedin U, et al. CXCL16/SR-PSOX is an interferon-gamma-regulated chemokine and scavenger receptor expressed in atherosclerotic lesions. Arterioscler Thromb Vasc Biol. (2004) 24:750–5. doi: 10.1161/01.ATV.0000124102.11472.36
59. Schielke L, Zimmermann N, Hobelsberger S, Steininger J, Strunk A, Blau K, et al. Metabolic syndrome in psoriasis is associated with upregulation of CXCL16 on monocytes and a dysbalance in innate lymphoid cells. Front Immunol. (2022) 13:916701. doi: 10.3389/fimmu.2022.916701
60. Chandrasekar B, Bysani S, and Mummidi S. CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, I kappa B kinase, and nuclear factor-kappa B and induces cell-cell adhesion and aortic smooth muscle cell proliferation. J Biol Chem. (2004) 279:3188–96. doi: 10.1074/jbc.M311660200
61. Poznyak AV, Bezsonov EE, Popkova TV, Starodubova AV, and Orekhov AN. Immunity in atherosclerosis: focusing on T and B cells. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22168379
62. Wu MY, Li CJ, Hou MF, and Chu PY. New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int J Mol Sci. (2017) 18. doi: 10.3390/ijms18102034
63. Wu F, Mao C, Mou X, Xu C, Zheng T, Bu L, et al. Decreased β-catenin expression contributes to IFNγ-induced chemokine secretion and lymphocyte infiltration in Hashimoto's thyroiditis. Endocr Connect. (2022) 11. doi: 10.1530/EC-21-0451
64. van der Voort R, van Lieshout AW, Toonen LW, Slöetjes AW, van den Berg WB, Figdor CG, et al. Elevated CXCL16 expression by synovial macrophages recruits memory T cells into rheumatoid joints. Arthritis Rheum. (2005) 52:1381–91. doi: 10.1002/art.21004
65. Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y, et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-α mediated NF-κB pathway. J Cell Mol Med. (2016) 20:2318–27. doi: 10.1111/jcmm.2016.20.issue-12
66. Paulsson G, Zhou X, Törnquist E, and Hansson GK. Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. (2000) 20:10–7. doi: 10.1161/01.ATV.20.1.10
67. Yu SS and Du JL. Current views on selenoprotein S in the pathophysiological processes of diabetes-induced atherosclerosis: potential therapeutics and underlying biomarkers. Diabetol Metab Syndr. (2024) 16:5. doi: 10.1186/s13098-023-01247-y
68. Wang S, Wu J, Li X, Tan R, Chen L, Yang L, et al. CXCR6 mediates pressure overload-induced aortic stiffness by increasing macrophage recruitment and reducing exosome-miRNA29b. J Cardiovasc Transl Res. (2023) 16:271–86. doi: 10.1007/s12265-022-10304-2
69. Butcher MJ, Wu CI, Waseem T, and Galkina EV. CXCR6 regulates the recruitment of pro-inflammatory IL-17A-producing T cells into atherosclerotic aortas. Int Immunol. (2016) 28:255–61. doi: 10.1093/intimm/dxv068
70. Kwon KH, Ohigashi H, and Murakami A. Dextran sulfate sodium enhances interleukin-1 beta release via activation of p38 MAPK and ERK1/2 pathways in murine peritoneal macrophages. Life Sci. (2007) 81:362–71. doi: 10.1016/j.lfs.2007.05.022
71. Yu Q, Zeng K, Ma X, Song F, Jiang Y, Tu P, et al. Resokaempferol-mediated anti-inflammatory effects on activated macrophages via the inhibition of JAK2/STAT3, NF-κB and JNK/p38 MAPK signaling pathways. Int Immunopharmacol. (2016) 38:104–14. doi: 10.1016/j.intimp.2016.05.010
72. Diegelmann J, Seiderer J, Niess JH, Haller D, Göke B, Reinecker HC, et al. Expression and regulation of the chemokine CXCL16 in Crohn's disease and models of intestinal inflammation. Inflammation Bowel Dis. (2010) 16:1871–81. doi: 10.1002/ibd.21306
73. Mir H, Kaur G, Kapur N, Bae S, Lillard JW, Singh S, et al. Higher CXCL16 exodomain is associated with aggressive ovarian cancer and promotes the disease by CXCR6 activation and MMP modulation. Sci Rep. (2019) 9:2527. doi: 10.1038/s41598-019-38766-6
74. Yi GW, Zeng QT, Mao XB, Cheng M, Yang XF, Liu HT, et al. Overexpression of CXCL16 promotes a vulnerable plaque phenotype in Apolipoprotein E-Knockout Mice. Cytokine. (2011) 53:320–6. doi: 10.1016/j.cyto.2010.11.016
75. Chen Y, Waqar AB, Nishijima K, Ning B, Kitajima S, Matsuhisa F, et al. Macrophage-derived MMP-9 enhances the progression of atherosclerotic lesions and vascular calcification in transgenic rabbits. J Cell Mol Med. (2020) 24:4261–74. doi: 10.1111/jcmm.15087
76. Qiu MK, Wang SC, Dai YX, Wang SQ, Ou JM, Quan ZW, et al. PD-1 and tim-3 pathways regulate CD8+ T cells function in atherosclerosis. PloS One. (2015) 10:e0128523. doi: 10.1371/journal.pone.0128523
77. Cochain C, Chaudhari SM, Koch M, Wiendl H, Eckstein HH, Zernecke A, et al. Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PloS One. (2014) 9:e93280. doi: 10.1371/journal.pone.0093280
78. Wang B, Wang Y, Sun X, Deng G, Huang W, Wu X, et al. CXCR6 is required for antitumor efficacy of intratumoral CD8(+) T cell. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2021-003100
79. Yu X, Zhao R, Lin S, Bai X, Zhang L, Yuan S, et al. CXCL16 induces angiogenesis in autocrine signaling pathway involving hypoxia-inducible factor 1α in human umbilical vein endothelial cells. Oncol Rep. (2016) 35:1557–65. doi: 10.3892/or.2015.4520
80. Isozaki T, Arbab AS, Haas CS, Amin MA, Arendt MD, Koch AE, et al. Evidence that CXCL16 is a potent mediator of angiogenesis and is involved in endothelial progenitor cell chemotaxis: studies in mice with K/BxN serum-induced arthritis. Arthritis Rheum. (2013) 65:1736–46. doi: 10.1002/art.37981
81. Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. (2003) 278:8508–15. doi: 10.1074/jbc.M208231200
82. Zeng M, Xie Z, Zhang J, Li S, Wu Y, Yan X, et al. Arctigenin attenuates vascular inflammation induced by high salt through TMEM16A/ESM1/VCAM-1 pathway. Biomedicines. (2022) 10. doi: 10.3390/biomedicines10112760
83. Dhoke NR, Kaushik K, and Das A. Cxcr6-based mesenchymal stem cell gene therapy potentiates skin regeneration in murine diabetic wounds. Mol Ther. (2020) 28:1314–26. doi: 10.1016/j.ymthe.2020.02.014
84. Johnson JL. Metalloproteinases in atherosclerosis. Eur J Pharmacol. (2017) 816:93–106. doi: 10.1016/j.ejphar.2017.09.007
85. Chistiakov DA, Orekhov AN, and Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf). (2015) 214:33–50. doi: 10.1111/apha.2015.214.issue-1
86. Zhang Y, Fu Y, Zhang C, Jia L, Yao N, Lin Y, et al. MED1 deficiency in macrophages accelerates intimal hyperplasia via ROS generation and inflammation. Oxid Med Cell Longev. (2021) 2021:3010577. doi: 10.1155/2021/3010577
87. Ma Z, Mao C, Chen X, Yang S, Qiu Z, Yu B, et al. Peptide vaccine against ADAMTS-7 ameliorates atherosclerosis and postinjury neointima hyperplasia. Circulation. (2023) 147:728–42. doi: 10.1161/CIRCULATIONAHA.122.061516
88. Chandrasekar B, Mummidi S, Valente AJ, Patel DN, Bailey SR, Freeman GL, et al. The pro-atherogenic cytokine interleukin-18 induces CXCL16 expression in rat aortic smooth muscle cells via MyD88, interleukin-1 receptor-associated kinase, tumor necrosis factor receptor-associated factor 6, c-Src, phosphatidylinositol 3-kinase, Akt, c-Jun N-terminal kinase, and activator protein-1 signaling. J Biol Chem. (2005) 280:26263–77. doi: 10.1074/jbc.M502586200
89. Liu C, Wu M, Qu J, et al. JNK and Jag1/Notch2 co-regulate CXCL16 to facilitate cypermethrin-induced kidney damage. Ecotoxicol Environ Saf. (2022) 238:113582. doi: 10.1016/j.ecoenv.2022.113582
90. Gimbrone MA Jr. and García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. (2016) 118:620–36. doi: 10.1161/CIRCRESAHA.115.306301
91. Parathath S, Mick SL, Feig JE, Joaquin V, Grauer L, Habiel DM, et al. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ Res. (2011) 109:1141–52. doi: 10.1161/CIRCRESAHA.111.246363
92. Higashida T, Kanno H, Nakano M, Funakoshi K, and Yamamoto I. Expression of hypoxia-inducible angiogenic proteins (hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and E26 transformation-specific-1) and plaque hemorrhage in human carotid atherosclerosis. J Neurosurg. (2008) 109:83–91. doi: 10.3171/JNS/2008/109/7/0083
93. Zhuge X, Murayama T, Arai H, Yamauchi R, Tanaka M, Shimaoka T, et al. CXCL16 is a novel angiogenic factor for human umbilical vein endothelial cells. Biochem Biophys Res Commun. (2005) 331:1295–300. doi: 10.1016/j.bbrc.2005.03.200
94. Yamauchi R, Tanaka M, Kume N, Minami M, Kawamoto T, Togi K, et al. Upregulation of SR-PSOX/CXCL16 and recruitment of CD8+ T cells in cardiac valves during inflammatory valvular heart disease. Arterioscler Thromb Vasc Biol. (2004) 24:282–7. doi: 10.1161/01.ATV.0000114565.42679.c6
95. Korbecki J, Kojder K, Kapczuk P, Kupnicka P, Gawrońska-Szklarz B, Gutowska I, et al. The effect of hypoxia on the expression of CXC chemokines and CXC chemokine receptors-A review of literature. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22020843
96. Wang YH, Dong YY, Wang WM, Xie XY, Wang ZM, Chen RX, et al. Vascular endothelial cells facilitated HCC invasion and metastasis through the Akt and NF-κB pathways induced by paracrine cytokines. J Exp Clin Cancer Res. (2013) 32:51. doi: 10.1186/1756-9966-32-51
97. Jiang BH, Zheng JZ, Aoki M, and Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A. (2000) 97:1749–53. doi: 10.1073/pnas.040560897
98. Gao Q, Zhao YJ, Wang XY, Qiu SJ, Shi YH, Sun J, et al. CXCR6 upregulation contributes to a proinflammatory tumor microenvironment that drives metastasis and poor patient outcomes in hepatocellular carcinoma. Cancer Res. (2012) 72:3546–56. doi: 10.1158/0008-5472.CAN-11-4032
99. Hawinkels LJ, Zuidwijk K, Verspaget HW, de Jonge-Muller ES, van Duijn W, Ferreira V, et al. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur J Cancer. (2008) 44:1904–13. doi: 10.1016/j.ejca.2008.06.031
100. de Winther MP, Gijbels MJ, van Dijk KW, van Gorp PJ, suzuki H, Kodama T, et al. Scavenger receptor deficiency leads to more complex atherosclerotic lesions in APOE3Leiden transgenic mice. Atherosclerosis. (1999) 144:315–21. doi: 10.1016/S0021-9150(98)00332-3
101. Sakaguchi H, Takeya M, Suzuki H, Hakamata H, Kodama T, Horiuchi S, et al. Role of macrophage scavenger receptors in diet-induced atherosclerosis in mice. Lab Invest. (1998) 78:423–34.
102. Mitsuoka H, Toyohara M, Kume N, Hayashida K, Jinnai T, Tanaka M, et al. Circulating soluble SR-PSOX/CXCL16 as a biomarker for acute coronary syndrome -comparison with high-sensitivity C-reactive protein. J Atheroscler Thromb. (2009) 16:586–93. doi: 10.5551/jat.1081
103. van Lieshout AW, Popa C, van Riel PL, and Radstake TR. Can CXCL16 be linked to coronary vascular disease? Comment on the article by Sheikine et al. Atherosclerosis. (2006) 189:470–1; author reply 472-3.
104. Camacho-Encina M, Booth LK, Redgrave RE, Folaranmi O, Spyridopoulos I, Richardson GD, et al. Cellular senescence, mitochondrial dysfunction, and their link to cardiovascular disease. Cells. (2024) 13. doi: 10.3390/cells13040353
105. Deroissart J, Porsch F, Koller T, and Binder CJ. Anti-inflammatory and immunomodulatory therapies in atherosclerosis. Handb Exp Pharmacol. (2022) 270:359–404. doi: 10.1007/164_2021_505
106. Koenen RR and Weber C. Chemokines: established and novel targets in atherosclerosis. EMBO Mol Med. (2011) 3:713–25. doi: 10.1002/emmm.201100183
107. Tabas I and Bornfeldt KE. Intracellular and intercellular aspects of macrophage immunometabolism in atherosclerosis. Circ Res. (2020) 126:1209–27. doi: 10.1161/CIRCRESAHA.119.315939
108. De Meyer GRY, Zurek M, Puylaert P, and Martinet W. Programmed death of macrophages in atherosclerosis: mechanisms and therapeutic targets. Nat Rev Cardiol. (2024) 21:312–25. doi: 10.1038/s41569-023-00957-0
109. Yang SH, Kim SJ, Kim N, Oh JE, Lee JG, Chung NH, et al. NKT cells inhibit the development of experimental crescentic glomerulonephritis. J Am Soc Nephrol. (2008) 19:1663–71. doi: 10.1681/ASN.2007101117
110. Fahy OL, Townley SL, and McColl SR. CXCL16 regulates cell-mediated immunity to Salmonella enterica serovar Enteritidis via promotion of gamma interferon production. Infect Immun. (2006) 74:6885–94. doi: 10.1128/IAI.01065-06
111. Zhang L, Liu HJ, Li TJ, Yang Y, Guo XL, Wu MC, et al. Lentiviral vector-mediated siRNA knockdown of SR-PSOX inhibits foam cell formation in vitro. Acta Pharmacol Sin. (2008) 29:847–52. doi: 10.1111/j.1745-7254.2008.00823.x
112. Ma KL, Wu Y, Zhang Y, Wang GH, Hu ZB, Ruan XZ, et al. Activation of the CXCL16/CXCR6 pathway promotes lipid deposition in fatty livers of apolipoprotein E knockout mice and HepG2 cells. Am J Transl Res. (2018) 10:1802–16.
113. Zhao G, Zhang H, Zhu S, Wang S, Zhu K, Zhao Y, et al. Interleukin-18 accelerates cardiac inflammation and dysfunction during ischemia/reperfusion injury by transcriptional activation of CXCL16. Cell Signal. (2021) 87:110141. doi: 10.1016/j.cellsig.2021.110141
114. Liu W, Yin L, and Dai Y. Modification of SR-PSOX functions by multi-point mutations of basic amino acid residues. Biochimie. (2013) 95:224–30. doi: 10.1016/j.biochi.2012.09.016
115. Liu W, Yin L, Chen C, and Dai Y. Function modification of SR-PSOX by point mutations of basic amino acids. Lipids Health Dis. (2011) 10:59. doi: 10.1186/1476-511X-10-59
116. Smith C, Halvorsen B, Otterdal K, Waehre T, Yndestad A, Fevang B, et al. High levels and inflammatory effects of soluble CXC ligand 16 (CXCL16) in coronary artery disease: down-regulatory effects of statins. Cardiovasc Res. (2008) 79:195–203. doi: 10.1093/cvr/cvn071
117. Crookenden MA, Lake AVR, Burke CR, Pratt JT, Mitchell MD, Phyn CVC, et al. Effect of nonsteroidal anti-inflammatory drugs on the inflammatory response of bovine endometrial epithelial cells in vitro. J Dairy Sci. (2023) 106:2651–66. doi: 10.3168/jds.2021-21742
118. Clancy P, Koblar SA, and Golledge J. Angiotensin receptor 1 blockade reduces secretion of inflammation associated cytokines from cultured human carotid atheroma and vascular cells in association with reduced extracellular signal regulated kinase expression and activation. Atherosclerosis. (2014) 236:108–15. doi: 10.1016/j.atherosclerosis.2014.06.011
119. Zhu Y, Li Q, Chen Y, Tian M, Xun W, and Sun S. P2X7 receptor inhibition attenuates podocyte injury by oxLDL through deregulating CXCL16. Cell Biol Int. (2022) 46:454–61. doi: 10.1002/cbin.11742
120. Pupovac A, Foster CM, and Sluyter R. Human P2X7 receptor activation induces the rapid shedding of CXCL16. Biochem Biophys Res Commun. (2013) 432:626–31. doi: 10.1016/j.bbrc.2013.01.134
121. Marques P, Domingo E, Rubio A, Martinez-Hervás S, Ascaso JF, Piqueras L, et al. Beneficial effects of PCSK9 inhibition with alirocumab in familial hypercholesterolemia involve modulation of new immune players. BioMed Pharmacother. (2022) 145:112460. doi: 10.1016/j.biopha.2021.112460
122. Zhao G, Wang S, Wang Z, Sun A, Yang X, Qiu Z, et al. CXCR6 deficiency ameliorated myocardial ischemia/reperfusion injury by inhibiting infiltration of monocytes and IFN-γ-dependent autophagy. Int J Cardiol. (2013) 168:853–62. doi: 10.1016/j.ijcard.2012.10.022
123. Galkina E, Harry BL, Ludwig A, Liehn EA, Sanders JM, Bruce A, et al. CXCR6 promotes atherosclerosis by supporting T-cell homing, interferon-gamma production, and macrophage accumulation in the aortic wall. Circulation. (2007) 116:1801–11. doi: 10.1161/CIRCULATIONAHA.106.678474
124. Postea O, Koenen RR, Hristov M, Weber C, and Ludwig A. Homocysteine up-regulates vascular transmembrane chemokine CXCL16 and induces CXCR6+ lymphocyte recruitment in vitro and in vivo. J Cell Mol Med. (2008) 12:1700–9. doi: 10.1111/j.1582-4934.2008.00223.x
125. Peddibhotla S, Hershberger PM, Jason Kirby R, et al. Discovery of small molecule antagonists of chemokine receptor CXCR6 that arrest tumor growth in SK-HEP-1 mouse xenografts as a model of hepatocellular carcinoma. Bioorg Med Chem Lett. (2020) 30:126899. doi: 10.1016/j.bmcl.2019.126899
126. Abdel-Bakky MS, Alqasoumi A, Altowayan WM, Amin E, and Darwish MA. Resveratrol inhibited ADAM10 mediated CXCL16-cleavage and T-cells recruitment to pancreatic β-cells in type 1 diabetes mellitus in mice. Pharmaceutics. (2022) 14. doi: 10.3390/pharmaceutics14030594
127. Ludwig A, Hundhausen C, Lambert MH, Broadway N, Andrews RC, Bickett DM, et al. Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb Chem High Throughput Screen. (2005) 8:161–71. doi: 10.2174/1386207053258488
128. Ma Z, Yu R, Zhu Q, Sun L, Jian L, Wang X, et al. CXCL16/CXCR6 axis promotes bleomycin-induced fibrotic process in MRC-5 cells via the PI3K/AKT/FOXO3a pathway. Int Immunopharmacol. (2020) 81:106035. doi: 10.1016/j.intimp.2019.106035
129. Hattermann K, Ludwig A, Gieselmann V, Held-Feindt J, and Mentlein R. The chemokine CXCL16 induces migration and invasion of glial precursor cells via its receptor CXCR6. Mol Cell Neurosci. (2008) 39:133–41. doi: 10.1016/j.mcn.2008.03.009
130. Lu L, Liu H, Peng J, Gan L, Shen L, Zhang Q, et al. Regulations of the key mediators in inflammation and atherosclerosis by aspirin in human macrophages. Lipids Health Dis. (2010) 9:16. doi: 10.1186/1476-511X-9-16
131. Borst O, Münzer P, Gatidis S, Schmidt EM, Schönberger T, Schmid E, et al. The inflammatory chemokine CXC motif ligand 16 triggers platelet activation and adhesion via CXC motif receptor 6-dependent phosphatidylinositide 3-kinase/Akt signaling. Circ Res. (2012) 111:1297–307. doi: 10.1161/CIRCRESAHA.112.276444
Keywords: CXCL16, chemokine, CXCR6, atherosclerosis, mechanism
Citation: Liu Y, Tian X, Jia C, Cheng X, Cui C, Li C and Yang S (2025) The role of CXCL16 in atherosclerosis: from mechanisms to therapy. Front. Immunol. 16:1555438. doi: 10.3389/fimmu.2025.1555438
Received: 07 February 2025; Accepted: 05 May 2025;
Published: 26 May 2025.
Edited by:
Yves Delneste, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Sergio M. Pontejo, National Institute of Allergy and Infectious Diseases (NIH), United StatesAntoine Caillon, McGill University, Canada
Copyright © 2025 Liu, Tian, Jia, Cheng, Cui, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaonan Yang, eWFuZ3NuMjYxNTA0QDEyNi5jb20=; Cuiping Li, cXlmeTExMTI1NUBxZHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yue Liu1†
Yue Liu1† Shaonan Yang
Shaonan Yang