- 1Department of Pharmacy, Chengdu Second People’s Hospital, Chengdu, Sichuan, China
- 2Department of Pharmacy, The First Affiliated Hospital of Xiamen University, Xiamen, Fujian, China
- 3Department of Pharmacy, Nanan People's Hospital of Chongqing, Chongqing, China
- 4Department of Respiratory and Critical Care Medicine, Nanjing Drum Tower Hospital, Nanjing, Jiangsu, China
- 5Department of Pharmacy, Nanjing Drum Tower Hospital, Nanjing, Jiangsu, China
- 6School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu, China
Objectives: Toripalimab combined with chemotherapy is a clinically valuable and important regimen in the treatment of extensive−stage small−cell lung cancer (ES-SCLC). However, there are no studies on the cost-effectiveness of this regimen, so this study was designed to evaluate the cost-effectiveness of Toripalimab regimen for the treatment of ES-SCLC from the perspectives of the Chinese health system and the U.S. health system, respectively.
Methods: A partitioned survival model was developed to simulate the clinical progression and cost consumption of ES-SCLC using the results of the EXTENTORCH study as a source of survival data and incorporating direct medical costs. Model output metrics included incremental cost-effectiveness ratio (ICER), quality-adjusted life-years (QALYs), incremental QALYs, total costs, and incremental costs. The cost-effectiveness of the Toripalimab scheme was judged by comparing the ICER with the willingness to pay (WTP). The robustness of the model was verified by sensitivity analysis and scenario analysis.
Results: The results of the basic analysis showed that from the perspective of the Chinese health system, the Toripalimab group gained 0.18 QALYs more at a cost of $5,204, with an ICER of $29,139/QALY (<WTP). From the standpoint of the U.S. health system, the Toripalimab group spent $156,923 more and also gained 0.17 QALYs more, but the ICER ($915,965/QALY) was much higher than the WTP. Sensitivity and scenario analyses showed the model to be generally stable.
Conclusions: Compared with chemotherapy, the Toripalimab regimen for the treatment of ES-SCLC is cost-effective from the perspective of the Chinese health system, but not from the perspective of the US health system.
1 Introduction
Global cancer statistics show that in 2022, there were 2.48 million new cases of lung cancer, accounting for 12.4% of the total number of new cancer cases, and 1.8 million new lung cancer deaths, accounting for 18.7% of the total cancer deaths, which makes lung cancer the malignant tumor with the highest morbidity and mortality rate in the world (1). In China, lung cancer is also the top malignant tumor in terms of incidence and mortality (1, 2). In the US, lung cancer is the third most common cancer and the first in terms of mortality (1). Small-cell lung cancer (SCLC) is a highly aggressive subtype of lung cancer, accounting for 15%-17% of the total incidence of lung cancer (3, 4). SCLC exhibits rapid growth, a high degree of malignancy, and a propensity for metastasis. Nearly 70% of patients are in extensive stage when diagnosed, and are identified as having extensive-stage small-cell lung cancer (ES-SCLC), with a 5-year survival rate of less than 7% (5–7).
Before 2019, platinum-based DNA cross-linking agents (such as cisplatin or carboplatin) in combination with topoisomerase inhibitors (such as etoposide or irinotecan) is the preferred chemotherapy regimens for ES-SCLC (5). Although the short-term efficacy of this combination therapy is remarkable, due to the biological characteristics of SCLC, patients are highly susceptible to drug resistance leading to tumor recurrence, with a median overall survival (mOS) of 9–11 months (6, 8, 9). In recent years, the role of immunotherapy in the treatment of ES-SCLC has become increasingly prominent. Several studies have shown that immunotherapy represented by programmed death-1 (PD-1) inhibitor and programmed death- ligand 1 (PD-L1) inhibitors significantly prolonged the mOS and median progression-free survival (mPFS) of ES-SCLC patients (10–13). Toripalimab is a PD-1 inhibitor developed in China and approved for marketing in China in December 2018 and in the US in October 2023. The EXTENTORCH study compared the efficacy and safety of Toripalimab combined with chemotherapy (Etoposide + Carboplatin/Cisplatin, EC) versus chemotherapy in the treatment of ES-SCLC (14). The results showed that compared with chemotherapy, Toripalimab plus chemotherapy prolonged the mOS (14.6 vs 13.3 months, hazard ratio [HR] = 0.8, 95% confidence interval [CI] 0.65-0.98) and mPFS (5.8 vs 5.6 months, HR = 0.67, 95%CI: 0.54-0.82), and the security is controllable.
Although Toripalimab combination chemotherapy extended the survival time of ES-SCLC patients compared with conventional chemotherapy, there is a lack of economic evidence to support its use. Choosing a safe, effective and relatively inexpensive drug not only reduces the economic burden on patients but also facilitates the rational allocation of healthcare resources. Therefore, this study is aims to evaluate the cost-effectiveness of Toripalimab in combination with chemotherapy in the field of first-line treatment of ES-SCLC based on the perspective of the health system in China and the US.
2 Methods
2.1 Study design
The study was designed and conducted in accordance with the Consolidated Health Economic Evaluation Report Standards (CHEERS) checklist (Supplementary Table S1) (15). Study data were obtained from the EXTENTORCH study (ClinicalTrials.gov Identifier: NCT04012606), the Menet (https://www.menet.com.cn/), the Drug. price guide (https://www.drugs.com/price-guide/) and published literature.
The target patients included in this study were consistent with the EXTENTORCH study, that is, age ≥ 18 years and diagnosis of ES-SCLC confirmed by pathology or histology, and more detailed clinical characteristics of the patients are shown in Supplementary Table S2 (14). The target patients received drug therapy in two groups (1). Toripalimab group:first received 4 cycles (1 cycle = 3 weeks = 21 days) of Toripalimab (240mg, day1) + Etoposide (100mg/m2, day1-day3) + Carboplatin (AUC=5mg/mL/min, day1)/Cisplatin (75mg/m2, day1), followed by Toripalimab (240mg, day1) until disease progression (2); EC group: first received 4 cycles of Placebo (day1) + Etoposide (100mg/m2, day1-day3) + Carboplatin (AUC=5mg/mL/min, day1)/Cisplatin (75mg/m2, day1), followed by Placebo (day1) until disease progression. The treatment plan after disease progression provided by the EXTENTORCH study was not detailed, so Topotecan (1.25 mg/m2/day, day1-day5) was chosen as the second-line treatment according to the NCCN guideline and the CSCO guideline (16, 17). And best supportive care (BSC) was given to patients not receiving Topotecan. All drugs were given by intravenous route.
2.2 Model overview
A partitioned survival model was developed to simulate the progression of ES-SCLC by TreeAge Pro software (Version:2022). The model starts from the progression-free survival (PFS) state, and patients in the PFS state can transfer to the PFS state, progressive disease (PD) or the death state, and patients in the PD state can also transfer to the PD state or the death state. The model structure is shown in Figure 1. Considering the survival of ES-SCLC patients and the treatment cycle of the EXTENTORCH study, a time horizon (TH) of 10 years with a 21-day modeling cycle was set in this study. The model output metrics included incremental cost-effectiveness ratio (ICER), quality-adjusted life-years (QALYs), incremental QALYs, total cost, and incremental cost.
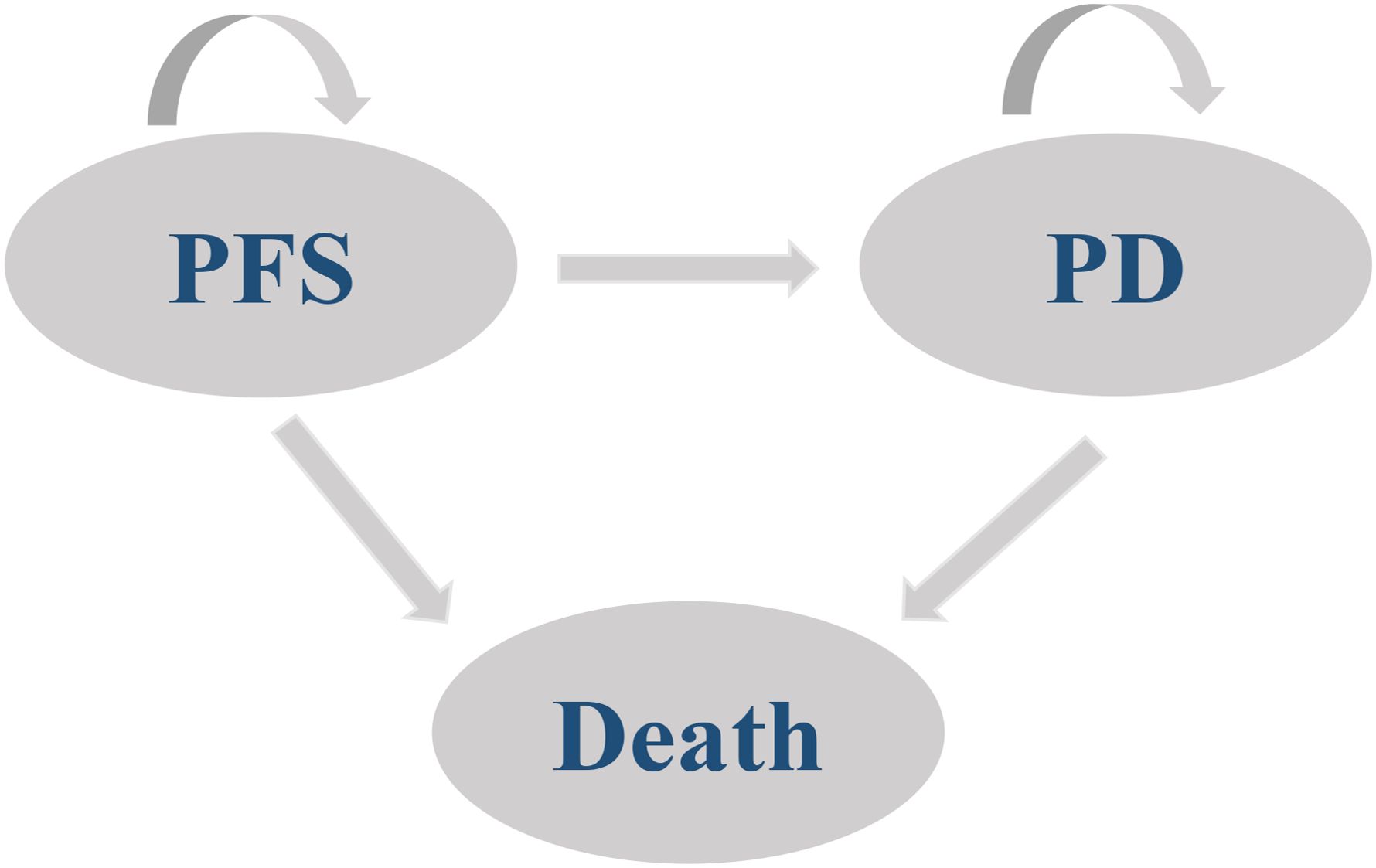
Figure 1. Schematic of the model structure. PFS, Progression-free survival; PD, Progressive disease.
2.3 Survival estimate
The EXTENTORCH study provided survival-related data (14). Firstly, survival information from PFS curves and OS curves in the EXTENTORCH study was digitally extracted using Engauge Digitizer software. The individual data were then reconstructed according to the method by Guyot P et al. (18). The survHE package in R software (4.3.1) (https://www.r-project.org/) was then invoked to fit the parameter distributions of the survival curves. The optimal fitting model was selected by combining the visual inspection with Akaike information criterion (AIC) and Bayesian information criterion (BIC). Smaller values of AIC and BIC indicate better fitting. Supplementary Table S3 shows the AIC and BIC values of each model, and it can be seen that Log-logistic distribution is the optimal fitting model for all curves. The relevant fitting parameters are shown in Supplementary Table S4, and the fitting curve graphs are shown in Supplementary Figure S1.
2.4 Cost and utility estimate
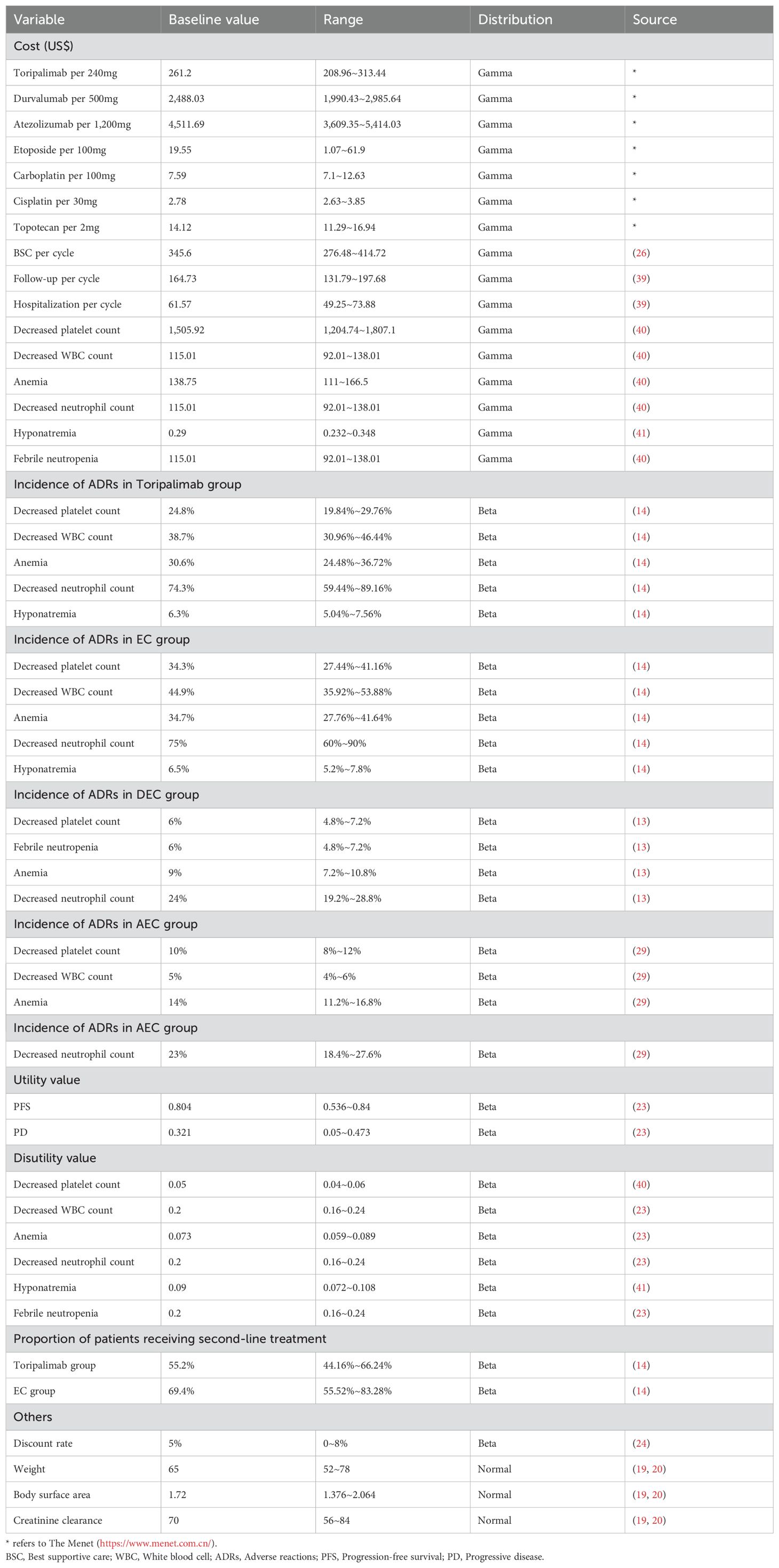
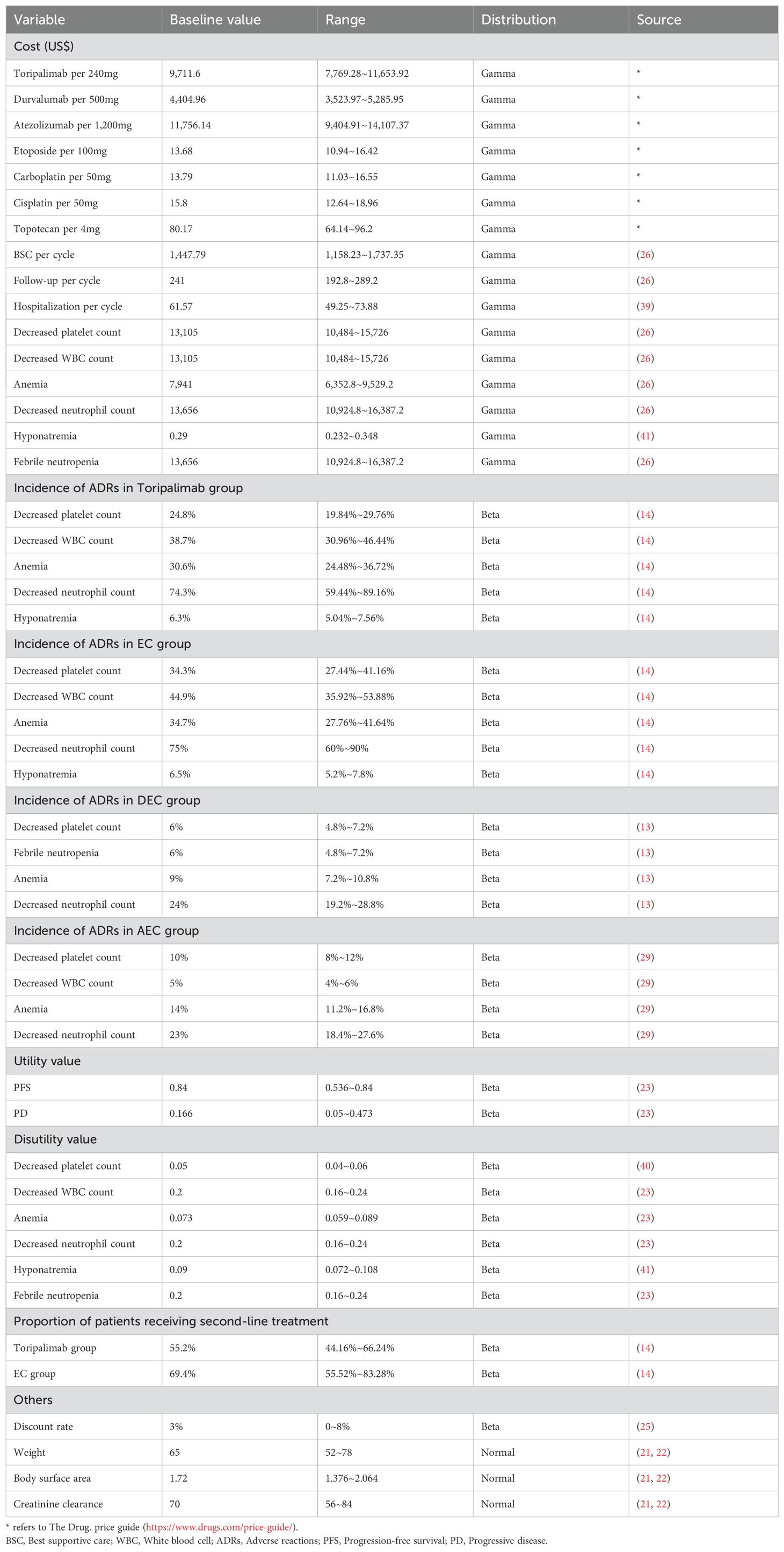
The perspective was the health system, so only direct healthcare costs were considered, including drug costs, BSC costs, adverse reactions (ADRs) handling costs, hospitalization costs and follow-up costs. Drug cost information was obtained from the Menet and Drug. price guide, and all other costs were obtained from the relevant literature. ADRs treatment costs were only considered for grade 3–4 ADRs with an incidence rate greater than 5%, and it was assumed that ADRs treatment costs were one-time costs. To calculate the drug cycle cost, it was assumed that the body weight of the Chinese patient was 65Kg, the body surface area (BSA) was 1.72m2, and the creatinine clearance (CCR) was 70ml/min (19, 20); American patients had a body weight of 70Kg, a BSA of 1.82m2, and a CCR of 70ml/min (21, 22). Tables 1, 2 provide detailed cost information.
The utility value information was extracted from an international study, which showed that Chinese patients had a utility value of 0.804 in the PFS stage and 0.321 in the PD stage, whereas American patients had a utility value of 0.84 in the PFS stage and 0.166 in the PD stage (23). The reduction in the utility value due to ADRs was also obtained from the published literature, and the specific information on the utility value is shown in Tables 1, 2.
When the TH is more than 1 year, the costs and health outputs occurring in the future need to be discounted. The TH of this study is 10 years, so an annual discount rate of 5% was taken for China and 3% for the United States based on relevant guidelines and literature recommendations (24, 25).
2.5 Basic analysis
The economy of Toripalimab regimen was judged by comparing the ICER with the willingness to pay (WTP), if the ICER is greater than the WTP, it is considered that Toripalimab regimen does not have cost-effective advantage, and conversely, it is cost-effective. According to the literature and guideline recommendations, the WTP for China was set to be 3 times the gross domestic product (GDP) per capita in 2023 (WTP = 3*GDP = 3*$12,291 = $36,874/QALY) for the basic analysis, whereas that for the US was set to be $150,000/QALY (24, 26)
2.6 Sensitivity analysis
One-way sensitivity analysis (OWSA) was performed to investigate the effect of single parameter changes on the model, and the results was presented as a tornado plot. The Menet provides a range of values for the cost parameter, published literature provides a range of values for the utility value, the discount rate is recommended to be set at 0-8% according to the guideline, and the ranges of the other parameters are set at± 20% of their base values (24, 26).
The effects of simultaneous changes in multiple parameters on the model were examined by probabilistic sensitivity analysis (PSA), and the results were presented as a cost-effectiveness scatterplot and a cost-effectiveness acceptability curve (CEAC). Second-order Monte Carlo simulations were used to perform PSA for 1,000 random repeated samples of parameters conforming to different probability distributions. In this study, the costs obeyed the Gamma distribution, and the utility values, the incidence of ADRs, and the discount rate obeyed the Beta distribution.
2.7 Scenario analysis
Scenario Analysis 1:In order to explore the effect of different TH on the results, the TH was set to 5 and 8 years for the scenario analysis, respectively.
Scenario Analysis 2:Health utility values are often one of the most important causes of variation in the results of pharmacoeconomic evaluations (27). To assess the impact of utility values on outcomes, Scenario Analysis 2 used the results of a real-world study for a cost-effectiveness analysis with utility values of 0.7 for the PFS stage and 0.6 for the PD stage (28).
2.8 Exploratory analysis
The two internationally recognized first-line immunotherapy regimens for ES-SCLC are Durvalumab plus EC (DEC) and Atezolizumab plus EC (AEC). This exploratory analysis aims to compare the cost-effectiveness of DEC/AEC vs Toripalimab combined with EC (TEC) in the treatment of ES-SCLC. The CASPIAN and IMpower133 trials provided therapeutic and survival information for ES-SCLC patients (13, 29). Firstly, given the absence of head-to-head clinical trials of DEC/AEC vs TEC, this study designated TEC as the control group and employed network meta-analysis (NMA) to construct a comparative framework. This approach enabled the estimation of hazard ratios (HRs) of PFS and OS for DEC/AEC vs TEC, and the detailed results are presented in Supplementary Table S5. Subsequently, referencing the methodology proposed by Hoyle et al. in the pharmacoeconomic evaluation of advanced renal cancer, the following parametric transformation formula was applied to adjust and calibrate the survival data of DEC group and AEC group (30).
Finally, the costs of DEC group and AEC group were calculated in accordance with the methodology outlined in Section 2.4 (“Cost and Utility Estimate”). By integrating costs and survival information, models can be established to perform a comprehensive cost-effectiveness of TEC vs DEC/AEC in the treatment of ES-SCLC.
3 Results
3.1 Results of basic analysis
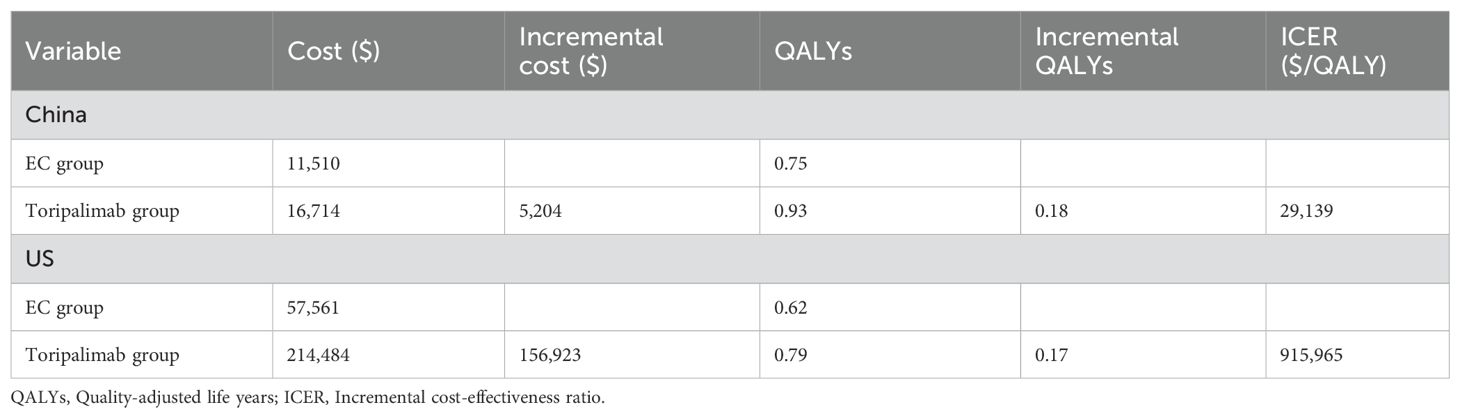
The results of the basic analysis showed that not only were the total costs higher in the Toripalimab group than in the EC group, both in China and in the US ($16,714 vs $11,510, $57,561 vs $ 214,484), but the health outputs were also more than in the EC group (0.93 QALYs vs 0.75 QALYs, 0.79 QALYs vs 0.62 QALYs). Toripalimab regimen was cost-effective in China (ICER: $29,139/QALY< WTP: $36,874/QALY) but not in the US (ICER: $915,965/QALY > WTP: $150,000/QALY). See Table 3 for details of the results.
3.2 Results of sensitivity analysis
The results of OWSA are shown in Figure 2. As can be seen from the tornado plot, in China, the utility value of the PFS stage had the greatest impact on ICER, and the proportion of patients in the EC group receiving second-line therapy, the cost of Toripalimab and Etoposide, and the utility value of the PD stage also had a moderate impact on ICER. In the US, the parameters that had the greatest impact on ICER included the utility value of the PFS stage and the cost of Toripalimab, and the other parameters had a small impact on ICER.
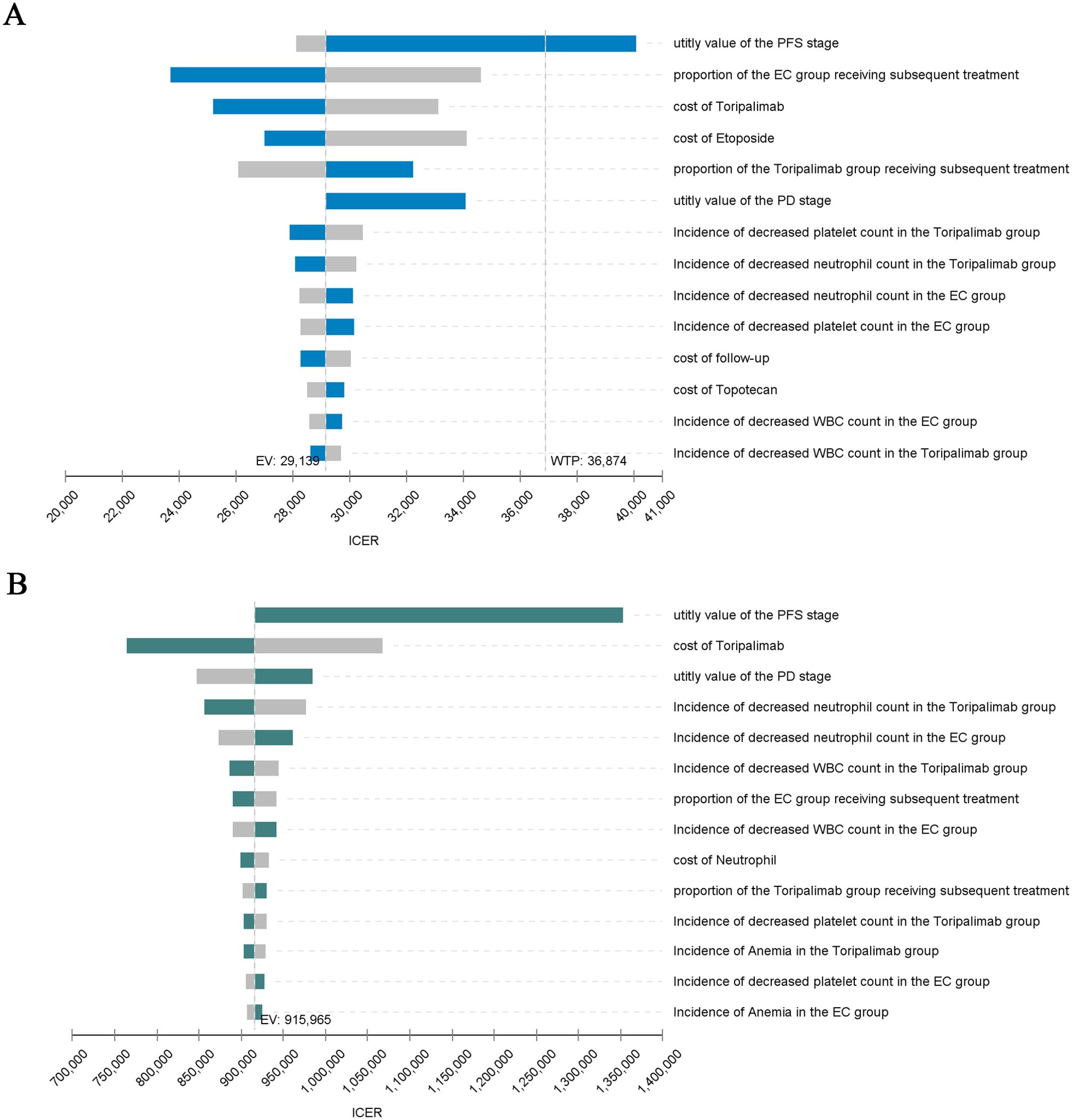
Figure 2. Tornado Diagram. (A) China: Tornado Diagram. (B) America: Tornado Diagram. PFS, Progression-free survival; PD, Progressive disease; ICER, Incremental cost-effectiveness ratio; EV, Expected value; WTP, Willingness to pay; WBC, White blood cell.
The results of PSA are shown in Figures 2, 3, respectively. The cost-effectiveness scatter plot for China shows that when WTP is set to 1*GDP, 2*GDP and 3*GDP, the economic probabilities of Toripalimab scheme are 0.7%, 29.7% and 77.1%, respectively. However, the cost-effectiveness scatter plot for America shows that Toripalimab is unlikely to be economical even if WTP is set at the current high value ($150,000/QALY). The CEAC chart demonstrates that the Toripalimab regimen begins to demonstrate potential economic advantage (probability = 0.1%) at a WTP threshold of $6,667/QALY in the Chinese healthcare context. Conversely, in the U.S. healthcare system, a significantly higher WTP threshold of $540,000/QALY is required for the Toripalimab regimen to demonstrate comparable economic viability. In China, the Toripalimab regimen attains a 50.5% probability of cost-effectiveness at a WTP threshold of $28,400/QALY, thereby surpassing conventional chemotherapy in economic efficiency. By contrast, within the US healthcare paradigm, the Toripalimab regimen necessitates an exceptionally high WTP threshold ($867,000/QALY) to achieve equivalent cost-effectiveness probability (50%) relative to conventional chemotherapy approaches.
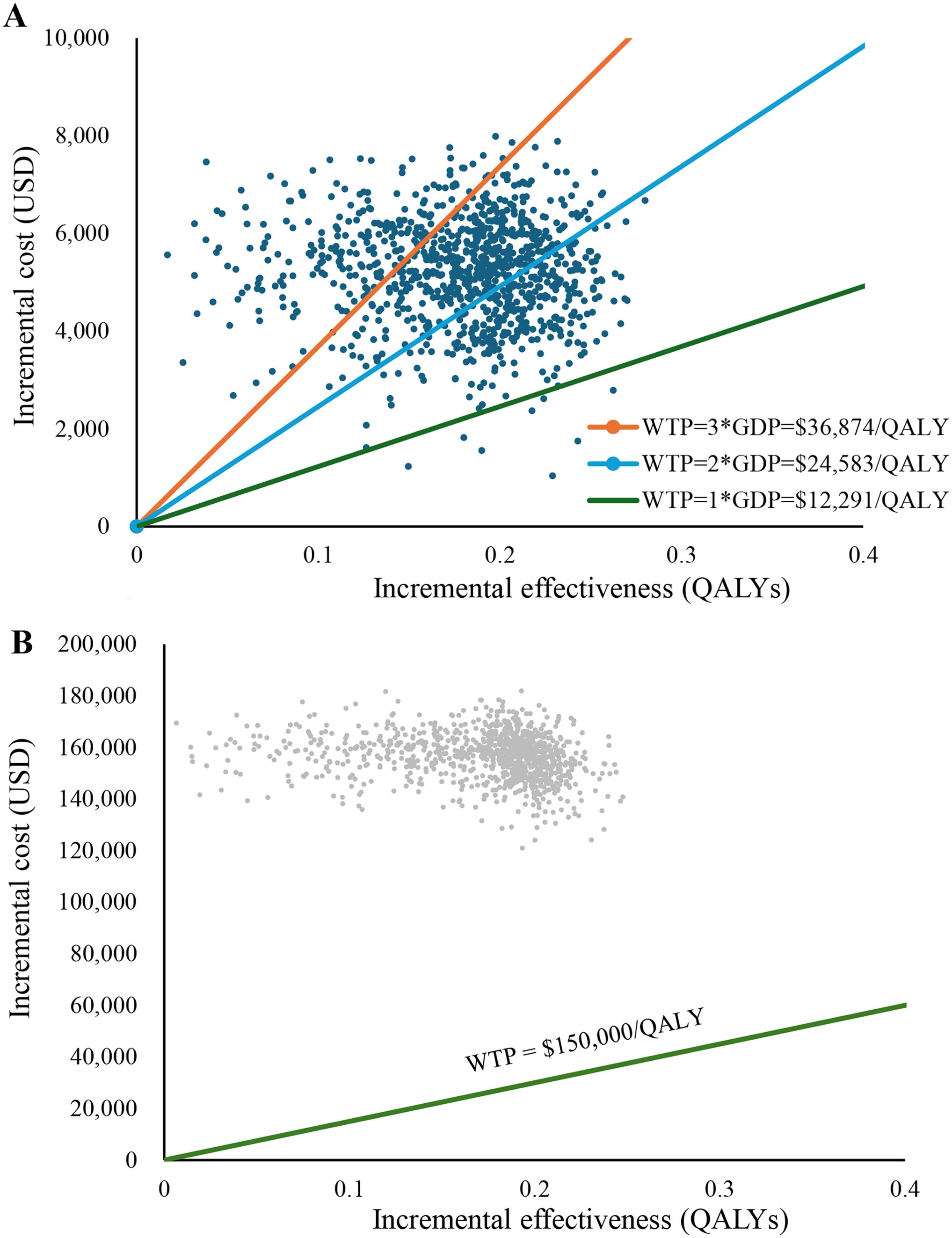
Figure 3. Cost-effectiveness scatter plot. (A) China: Cost-effectiveness scatter plot. (B) America: Cost-effectiveness scatter plot. WTP, Willingness to pay; GDP, gross domestic product; QALY, Quality-adjusted life year.
3.3 Results of scenario analysis
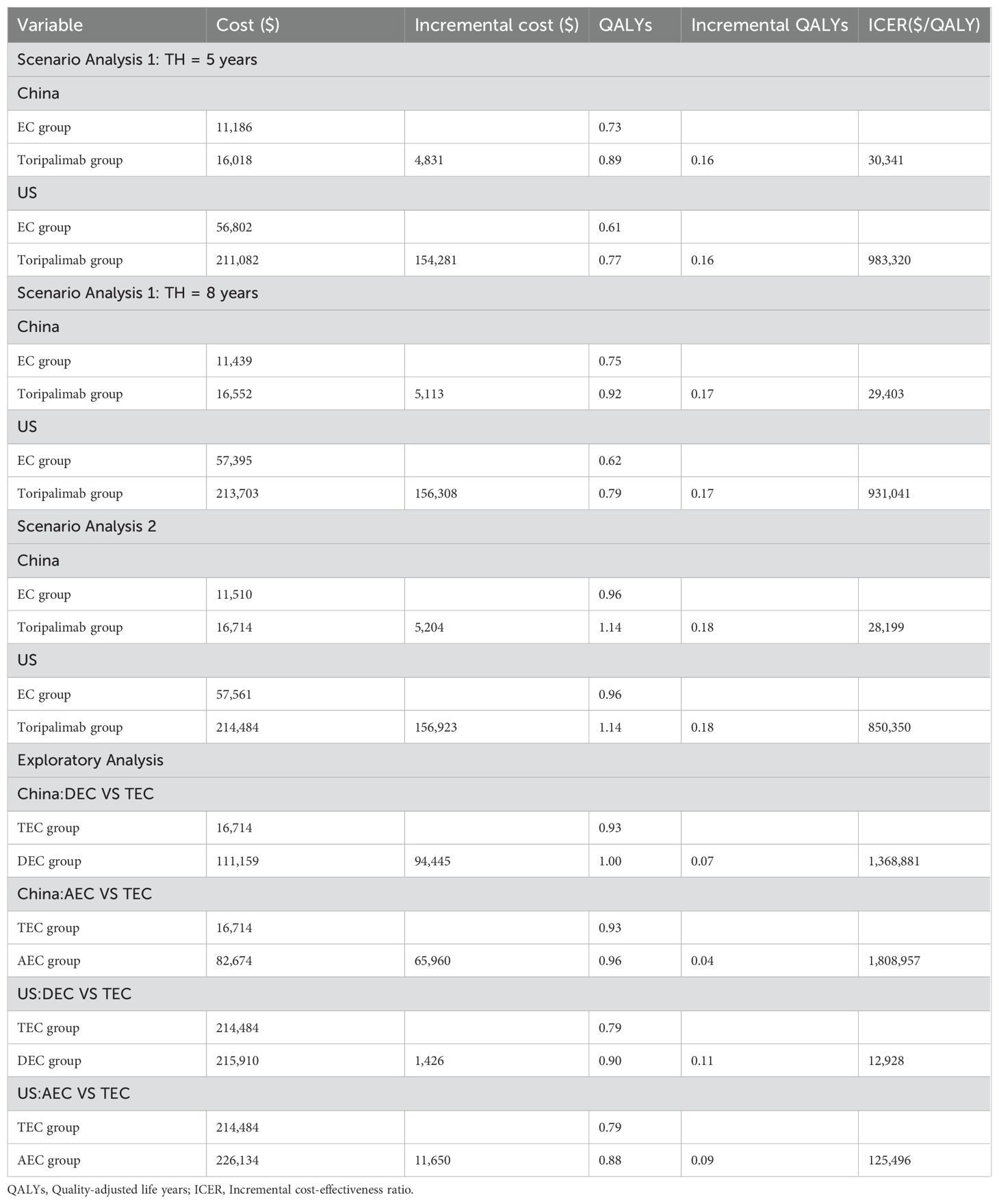
Table 4 provides the results of the detailed scenario analysis. Scenario analysis 1 shows that the Toripalimab regimen is economical in China, but not in the US, regardless of whether the TH is 5 or 8 years. Scenario analysis 2 shows that after changing the utility value, the ICER decreases to $28,199/QALY (<WTP) in China and to $850,350/QALY (> WTP) in the US.
3.4 Results of exploratory analysis
The exploratory analysis (Table 2) indicates that TEC demonstrates cost-effectiveness for ES-SCLC treatment compared with DEC/AEC within the Chinese healthcare system (ICERDEC vs TEC: $1,368,881/QALY, ICERAEC vs TEC: $1,808,957/QALY), whereas it fails to demonstrate economic viability in the US context (ICERDEC vs TEC: $$12,928/QALY, ICERAEC vs TEC: $125,496/QALY).
4 Discussion
Recent years have witnessed significant advancements in the clinical application of immunotherapy for ES-SCLC. Currently, DEC and AEC regimens have been established as internationally recognized first-line immunotherapy standards for ES-SCLC. On the one hand, comparative pricing analysis reveals that Toripalimab demonstrates cost advantages over both Durvalumab and Atezolizumab in pharmaceutical markets across China and the US. T The cost of Toripalimab ($261.20/cycle) was significantly lower than that of Atezolizumab ($4,511.69/cycle) and Durvalumab ($7,464.10/cycle) in the Chinese market. Similarly, Toripalimab ($9,711.60/cycle) also offers a price advantage over Atezolizumab ($11,756.14/cycle) and Durvalumab ($13,214.88/cycle) in the U.S. market. On the other hand, the EXTENTORCH trial demonstrated survival benefits with Toripalimab regimen compared to chemotherapy alone. This combination of clinical efficacy and price advantage strongly suggests that Toripalimab regimen may have a unique cost-effectiveness advantage. Existing studies have consistently demonstrated the AEC and DEC regimens are not cost-effective in the treatment of ES-SCLC in China and the US (31–34). However, no study has evaluated the cost-effectiveness of TEC regimen in the treatment of ES-SCLC. Considering the price advantage and clinical benefits of Toripalimab, a systematic pharmacoeconomic assessment of Toripalimab regimen carries important clinical and policy implications. Therefore, this study evaluated the cost-effectiveness of TEC regimen for ES-SCLC from the perspective of health systems in China and the US. The results showed that Toripalimab regimen was economical in China and uneconomical in the US when compared with either chemotherapy or AEC/DEC regimen.
OWSA showed that the health utility value at the PFS stage was the parameter that had the greatest impact on ICER in both the Chinese and American perspectives. The utility value directly affects the calculation of QALY, which is a key indicator for assessing the effectiveness of medical interventions. The utility value expresses the quality of life between full health and death, and the QALY allows the conversion of life years of different interventions into equivalent years of health status, thus facilitating the comparison of costs and effects between different treatment options. In the cost-effectiveness analysis, too high utility value leads to a relatively low ICER. Conversely, if the utility value is underestimated, the ICER will be higher. Therefore, choosing an appropriate utility value is crucial for calculating ICER. Unfortunately, the EXTENTORCH study did not report the utility value data, and there is a lack of research on the quality of life (i.e., utility value) of patients with SCLC. For the basic analysis, this study referred to published studies and chose to use the results of a large international study on the utility value in non-small cell lung cancer as an alternative to the utility value for SCLC (31, 32). But in scenario analysis 2, this study also changed the health utility values for PFS stage and PD stage based on the results of a study on the quality of life of SCLC patients (28). Considering the small sample size included in that real-world study, the results may not be representative enough to be analyzed as a special scenario only. Fortunately, scenario analysis 2 was consistent with the findings of the basic analysis.
PSA not only elucidated the economic disparities of the Toripalimab regimen across different geographical contexts, but also provided a quantifiable comparison basis for cross-regional investigations through specific WTP thresholds and corresponding economic probabilities. The China Guidelines for Pharmacoeconomic Evaluation (2020) recommend using 1~3 times the national GDP per capita as the WTP (24). When performing PSA, this study sets WTP to 1*GDP, 2*GDP, and 3*GDP respectively. The analytical outcomes revealed that across 1,000 simulations, he Toripalimab regimen demonstrated cost-effectiveness probabilities of 0.7%, 29.7%, and 77.1%, respectively, under these progressive WTP thresholds, thereby indicating substantial sensitivity of the regimen’s economic viability to WTP parameters within the Chinese healthcare context. From the perspective of the US healthcare system, where cost-effectiveness evaluations for oncological interventions typically employ WTP thresholds ranging from $100,000 to $150,000/QALY (35). In this study, the Toripalimab regimen failed to demonstrate economic viability even at a high WTP of $150,000/QALY gained. This observation potentially reflects an incongruity between the pricing structure of Toripalimab and its corresponding clinical benefit profile within the US healthcare framework.
Given that TH also has a significant impact on ICER, scenario analysis 1 calculated ICER under different TH (36). Typically, the TH for pharmacoeconomic evaluation should be sufficiently long to obtain the full impact of the intervention measures on the costs and health outcomes of the subjects (24, 37). When fitting and extrapolating the survival curves, we found that the mortality rate in the Toripalimab group reached 99% when the TH was set to 10 years, so a TH of 10 years was chosen for the basic analysis. We also found that the mortality rate in the EC group had reached 99% when the TH = 8 years, so the ICER at TH = 8 years was also calculated in scenario analysis 1. Considering that the ES-SCLC is extremely malignant and the 5-year survival rate of patients receiving EC therapy is also low, so a TH of 5 years was also set. Previously Kim et al. found that the ICER decreases with increasing TH in most of the pharmacoeconomically evaluated studies (36). Scenario analysis 1 also showed that incremental costs and incremental QALYs increase with increasing TH, but the advantage of increasing incremental QALYs is sufficient to compensate for the disadvantage of increasing incremental costs, so it leads to smaller ICER, which confirms the findings of Kim et al. The costs of immunotherapy are mostly incurred in the short term, but because of the “delayed effect” of immunosuppressants, it takes a relatively long time for their health benefits to be realized (36, 38). Therefore, the longer the TH, the more QALYs are captured, leading to a reduction in ICER.
There are also some limitations of this study (1). There are some inevitable biases when survival curves fitting and extrapolation methods are used to obtain survival information beyond the observation period (2). Due to the lack of studies on health utility values in ES-SCLC patients, the use of alternative utility values may have slightly affected the results (3). Given the limited treatment pathway details reported in the EXTENTORCH trial, this study unified subsequent anticancer therapy, which may be different from clinical practice (4). The data utilized for this exploratory analysis were not derived from “head-to-head” clinical trials. Despite the implementation of methodologically rigorous NMA to adjust and calibrate survival data, the indirect nature of these comparisons introduces inherent methodological uncertainties (5). The enrollment population of the EXTENTORCH study did not include U.S. ES-SCLC patients, which may be somewhat different from the actual survival rate of U.S. ES-SCLC. Despite these limitations, this study also verified the stability of the model through sensitivity and scenario analyses, so the results of the study can still provide economic references for policy makers, clinicians and patients.
5 Conclusions
This study conducted a comprehensive cost-effectiveness analysis comparing Toripalimab plus chemotherapy versus chemotherapy alone as first-line treatment for ES-SCLC from the dual perspectives of Chinese and US healthcare systems through a modeling approach using data from a phase III clinical trial, and validated the stability of the model through a sensitivity analysis with a series of scenario analyses. The findings demonstrated Toripalimab combination chemotherapy was economic in China when compared to chemotherapy alone, while was uneconomic in the US.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
JY: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. FC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JL: Formal Analysis, Methodology, Validation, Visualization, Software, Writing – review & editing. YZ: Formal Analysis, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HW: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YL: Conceptualization, Formal Analysis, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research is supported by the Natural Science Foundation of Fujian Province (number: 2024J011357).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1556100/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
3. Oronsky B, Reid TR, Oronsky A, and Carter CA. What’s new in SCLC? A review. Neoplasia. (2017) 19:842–47. doi: 10.1016/j.neo.2017.07.007
4. Wang S, Tang J, Sun T, Zheng X, Li J, Sun H, et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep. (2017) 7:1339. doi: 10.1038/s41598-017-01571-0
5. Megyesfalvi Z, Gay CM, Popper H, Pirker R, Ostoros G, Heeke S, et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J Clin. (2023) 73:620–52. doi: 10.3322/caac.21785
6. Semenova EA, Nagel R, and Berns A. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes Dev. (2015) 29:1447–62. doi: 10.1101/gad.263145.115
7. Byers LA and Rudin CM. Small cell lung cancer: where do we go from here? Cancer. (2015) 121:664–72. doi: 10.1002/cncr.29098
8. Farago AF and Keane FK. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. (2018) 7:69–79. doi: 10.21037/tlcr.2018.01.16
9. Demedts IK, Vermaelen KY, and van Meerbeeck JP. Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur Respir J. (2010) 35:202–15. doi: 10.1183/09031936.00105009
10. Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. Jama. (2022) 328:1223–32. doi: 10.1001/jama.2022.16464
11. Cheng Y, Chen J, Zhang W, Xie C, Hu Q, Zhou N, et al. Benmelstobart, anlotinib and chemotherapy in extensive-stage small-cell lung cancer: a randomized phase 3 trial. Nat Med. (2024) 30:2967–76. doi: 10.1038/s41591-024-03132-1
12. Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2022) 23:739–47. doi: 10.1016/s1470-2045(22)00224-8
13. Paz-Ares L, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. (2022) 7:100408. doi: 10.1016/j.esmoop.2022.100408
14. Cheng Y, Zhang W, Wu L, Zhou C, Wang D, Xia B, et al. Toripalimab plus chemotherapy as a first-line therapy for extensive-stage small cell lung cancer: the phase 3 EXTENTORCH randomized clinical trial. JAMA Oncol. (2024) 11:16–25. doi: 10.1001/jamaoncol.2024.5019
15. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. (2022) 25:3–9. doi: 10.1016/j.jval.2021.11.1351
16. Ganti AKP, Loo BW, Bassetti M, Blakely C, Chiang A, D’Amico TA, et al. Small cell lung cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:1441–64. doi: 10.6004/jnccn.2021.0058
17. Health Commission Of The People’s Republic Of China N. National guidelines for diagnosis and treatment of lung cancer 2022 in China (English version). Chin J Cancer Res. (2022) 34:176–206. doi: 10.21147/j.issn.1000-9604.2022.03.03
18. Guyot P, Ades AE, Ouwens MJ, and Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2012) 12:9. doi: 10.1186/1471-2288-12-9
19. Qiao L, Zhou Z, Zeng X, and Tan C. Cost-effectiveness of domestic PD-1 inhibitor camrelizumab combined with chemotherapy in the first-line treatment of advanced nonsquamous non-small-cell lung cancer in China. Front Pharmacol. (2021) 12:728440. doi: 10.3389/fphar.2021.728440
20. Luo X, Zhou Z, Zeng X, and Liu Q. The cost-effectiveness of tislelizumab plus chemotherapy for locally advanced or metastatic nonsquamous non-small cell lung cancer. Front Pharmacol. (2022) 13:935581. doi: 10.3389/fphar.2022.935581
21. Lin S, Luo S, Gu D, Li M, Rao X, Wang C, et al. First-line durvalumab in addition to etoposide and platinum for extensive-stage small cell lung cancer: A U.S.-based cost-effectiveness analysis. Oncologist. (2021) 26:e2013–e20. doi: 10.1002/onco.13954
22. Wan X, Luo X, Tan C, Zeng X, Zhang Y, and Peng L. First-line atezolizumab in addition to bevacizumab plus chemotherapy for metastatic, nonsquamous non-small cell lung cancer: A United States-based cost-effectiveness analysis. Cancer. (2019) 125:3526–34. doi: 10.1002/cncr.32368
23. Nafees B, Lloyd AJ, Dewilde S, Rajan N, and Lorenzo M. Health state utilities in non-small cell lung cancer: An international study. Asia Pac J Clin Oncol. (2017) 13:e195–203. doi: 10.1111/ajco.12477
24. Association CP. China guidelines for pharmacoeconomic evaluations (2020). Available online at: https://www.cpa.org.cn/cpadmn/attached/file/20201203/1606977380634185.pdf (Accessed December 31, 2025).
25. Su D, Wu B, and Shi L. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open. (2021) 4:e210037. doi: 10.1001/jamanetworkopen.2021.0037
26. Shao T, Zhao M, Liang L, and Tang W. Serplulimab plus chemotherapy vs chemotherapy for treatment of US and chinese patients with extensive-stage small-cell lung cancer: A cost-effectiveness analysis to inform drug pricing. BioDrugs. (2023) 37:421–32. doi: 10.1007/s40259-023-00586-6
27. Hatswell AJ, Bullement A, Schlichting M, and Bharmal M. What is the impact of the analysis method used for health state utility values on QALYs in oncology? A simulation study comparing progression-based and time-to-death approaches. Appl Health Econ Health Policy. (2021) 19:389–401. doi: 10.1007/s40258-020-00620-6
28. Vedadi A, Shakik S, Brown MC, Lok BH, Shepherd FA, Leighl NB, et al. The impact of symptoms and comorbidity on health utility scores and health-related quality of life in small cell lung cancer using real world data. Qual Life Res. (2021) 30:445–54. doi: 10.1007/s11136-020-02615-1
29. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. (2018) 379:2220–29. doi: 10.1056/NEJMoa1809064
30. Hoyle M, Green C, Thompson-Coon J, Liu Z, Welch K, Moxham T, et al. Cost-effectiveness of temsirolimus for first line treatment of advanced renal cell carcinoma. Value Health. (2010) 13:61–8. doi: 10.1111/j.1524-4733.2009.00617.x
31. Ding D, Hu H, Li S, Zhu Y, Shi Y, Liao M, et al. Cost-effectiveness analysis of durvalumab plus chemotherapy in the first-line treatment of extensive-stage small cell lung cancer. J Natl Compr Canc Netw. (2021) 19:1141–47. doi: 10.6004/jnccn.2020.7796
32. Zhou K, Zhou J, Huang J, Zhang N, Bai L, Yang Y, et al. Cost-effectiveness analysis of atezolizumab plus chemotherapy in the first-line treatment of extensive-stage small-cell lung cancer. Lung Cancer. (2019) 130:1–4. doi: 10.1016/j.lungcan.2019.01.019
33. Li LY, Wang H, Chen X, Li WQ, and Cui JW. First-line atezolizumab plus chemotherapy in treatment of extensive small cell lung cancer: a cost-effectiveness analysis from China. Chin Med J (Engl). (2019) 132:2790–94. doi: 10.1097/cm9.000000000000053
34. Liu G and Kang S. Cost-effectiveness of adding durvalumab to first-line chemotherapy for extensive-stage small-cell lung cancer in China. Expert Rev Pharmacoecon Outcomes Res. (2022) 22:85–91. doi: 10.1080/14737167.2021.1888717
35. Bae YH and Mullins CD. Do value thresholds for oncology drugs differ from nononcology drugs? J Manag Care Spec Pharm. (2014) 20:1086–92. doi: 10.18553/jmcp.2014.20.11.1086
36. Kim DD, Wilkinson CL, Pope EF, Chambers JD, Cohen JT, and Neumann PJ. The influence of time horizon on results of cost-effectiveness analyses. Expert Rev Pharmacoecon Outcomes Res. (2017) 17:615–23. doi: 10.1080/14737167.2017.1331432
37. Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health. (2015) 18:161–72. doi: 10.1016/j.jval.2015.02.001
38. Adunlin G, Ferreri SP, Dong J, and Freeman MK. Immuno-oncology medicines: policy implications and economic considerations. Innov Pharm. (2019) 10(3):10.24926/iip.v10i3.1799. doi: 10.24926/iip.v10i3.1799
39. Long Y, Wang H, Xie X, Li J, Xu Y, and Zhou Y. Updated cost-effectiveness analysis of adebrelimab plus chemotherapy for extensive-stage small cell lung cancer in China. BMJ Open. (2024) 14:e077090. doi: 10.1136/bmjopen-2023-077090
40. Rui M, Fei Z, Wang Y, Zhang X, Ma A, Sun H, et al. Cost-effectiveness analysis of sintilimab + chemotherapy versus camrelizumab + chemotherapy for the treatment of first-line locally advanced or metastatic nonsquamous NSCLC in China. J Med Econ. (2022) 25:618–29. doi: 10.1080/13696998.2022.2071066
Keywords: cost-effectiveness analysis, extensive-stage small-cell lung cancer, Toripalimab, chemotherapy, EXTENTORCH study
Citation: Yang J, Chen F, Li J, Zhou Y, Wang H and Long Y (2025) Cost-effectiveness analysis of Toripalimab regimen for extensive-stage small-cell lung cancer in China and America. Front. Immunol. 16:1556100. doi: 10.3389/fimmu.2025.1556100
Received: 06 January 2025; Accepted: 28 April 2025;
Published: 16 May 2025.
Edited by:
Kevin Lu, University of South Carolina, United StatesReviewed by:
George Gourzoulidis, Health Through Evidence, GreeceGuanming Chen, University of North America, United States
Copyright © 2025 Yang, Chen, Li, Zhou, Wang and Long. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Wang, aGFsc2V5X2dseXlAMTYzLmNvbQ==; Yunchun Long, Y2hycmljaDk4NTRAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jing Yang1†
Jing Yang1† Fang Chen
Fang Chen Yujie Zhou
Yujie Zhou Hao Wang
Hao Wang Yunchun Long
Yunchun Long