- 1Multiorgan Transplant Centre of Excellence, Liver Transplantation Department, King Fahad Specialist Hospital, Dammam, Saudi Arabia
- 2Internal Medicine Department, School of Medicine, Zamzam University College, Khartoum, Sudan
- 3Department of Medical Virology, Public Health Authority, Southern Sector, Jazan, Saudi Arabia
- 4Department of Internal Medicine, Stockport Hospital National Health Sevices (NHS) Foundation Trust, Manchester, United Kingdom
- 5Department of Gastroenterology and Hepatology, Victor Babes University of Medicine and Pharmacy, Timisoara, Romania
- 6Advanced Regional Research Centre in Gastroenterology and Hepatology, Victor Babes University of Medicine and Pharmacy, Timisoara, Romania
Introduction: The gut microbiota plays a crucial role in regulating immune responses and maintaining a balance within the gut-liver axis. In patients with chronic liver disease (CLD), alterations in gut microbiota have been linked to disease progression and impaired immune function. This study aimed to evaluate the impact of gut-modulating therapies on the immune responses of patients with CLD.
Method: Two independent authors conducted a comprehensive literature search using complementary strategies to identify relevant articles published until March 2025. Review Manager Software (RevMan 5.4) was used for data analysis, and the results were presented using forest plots.
Results: Of the 373 identified studies, 16 were included in the analysis. The findings revealed that gut microbiota-modulating therapies significantly reduced tumor necrosis factor-α (TNF-α) levels compared to control interventions (standardized mean difference [SMD], -0.60; 95% confidence interval [CI] [-0.93, -0.23] p = 0.001), with similar results observed at the 6-month follow-up (SMD -1.3; 95% CI [-2.1, -0.4] p = 0.004). Interleukin-6 (IL-6) levels showed no significant change between the groups (SMD, -0.67; 95% CI [-1.5, 0.12) p = 0.09). C-reactive protein (CRP) levels were significantly reduced by gut-modulating therapies (SMD -1.057; 95% CI [-1.493, -0.621] p = 0.0005), with consistent results at 1- and 6-month follow-up. Changes in interferon-gamma (IFN-γ) and IL-18 levels and cellular immunity were also assessed.
Conclusion: This study highlights the importance of gut microbiota in modulating immune responses in patients with CLD and demonstrates the effectiveness of long-term gut-modulating therapies in reducing inflammatory markers. While CRP and TNF-α levels decreased, changes in IL-6 levels were inconsistent, warranting further research to elucidate the impact of gut microbiota-modulating therapies on this biomarker.
1 Introduction
Chronic liver disease (CLD) is defined by a progressive decline in liver function persisting for more than six months, with cirrhosis representing its terminal stage. The principal etiologies of CLD encompass alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), chronic viral hepatitis, genetic predispositions, autoimmune disorders, and certain pharmacological agents (1). CLD is among the leading causes of mortality worldwide, accounting for 2.2% of deaths, with 1.32 million fatalities reported in 2017 (2). While alcohol consumption is commonly linked to CLD in numerous developed countries, the hepatitis B virus (HBV) is the predominant cause in sub-Saharan Africa and Asia, and NAFLD is becoming increasingly prevalent globally (3). CLD is characterized by persistent inflammation, which plays a critical role in the disease’s progression and associated complications. The inflammatory response in CLD is intricately linked to an imbalance in gut microbiota, resulting in a continuous cycle of hepatic injury and immune system dysfunction. This relationship is particularly evident in the gut-liver axis, where alterations in gut permeability and bacterial translocation contribute to hepatic inflammation.
Gut microbiota plays an important role in the human body because it is linked to the overall good health of an individual, essentially maintaining the structural integrity of the gut and immune regulation, with the main types, including Bacteroidetes and Firmicutes (4). Disrupted gut bacterial composition has been associated with the occurrence of various inflammatory conditions, including CLD (5). Intestinal barrier integrity is essential, as exposure of the immune system to the gut microbiota causes disease and inflammation (5). The interaction is achieved through the portal vein, connecting the liver and the intestines, creating a network (gut–liver axis) of exchange of bile and intestinal products, such as nutrients (6).
Research has increasingly associated gut microbiota with modulating the immune response in CLD, as these bacterial components signal the toll-like receptors (TLRs), activating inflammation. TLR overstimulation leads to tolerance, inhibiting immunity, persistent inflammation, and potentiating CLD (7). Changes in interferon-gamma (IFN-γ) levels have been observed in CLD patients, with studies showing altered production of this cytokine in response to gut microbiota dysbiosis. Similarly, changes in IL-18 levels have been reported in CLD, reflecting the complex interplay between the gut microbiome and the immune system. Furthermore, changes in cellular immunity, including alterations in the number and function of various immune cell populations such as T cells and natural killer cells, have been documented in CLD patients.
Additionally, patients with NAFLD have less microbiota, fewer CD4 and CD8+ lymphocytes, and higher TNF and IFN expression than the healthy cohort. In chronic HBV, intestinal integrity and bacterial composition alteration are the genesis of systemic immune activation TLR activation due to systemic endotoxin presence, catalyzing the inflammatory cascade, leading to CLD (8). Furthermore, patients with HBV-CLD have a decreased number of beneficial bacteria and an increased number of bacteria associated with inflammation (9). Subsequently, changes in peripheral blood mononuclear cells (PBMCs) were observed after interaction with HBV-CLD, revealing that the microbiome and metabolome showed marked alterations in the gut bacteria in HBV-CLD caused by disease progression (10).
Currently, treatment approaches target different causes of CLD. CLD has a wide range of treatment regimens, with probiotics and symbiotic therapy improving alanine aminotransferase (ALT) levels, reducing the immune response through inflammation (11). In another study, using Bifidobacterium with FoS plus lifestyle modification decreased ALT levels and NASH activity (12). Mofidi et al. also concluded that treatment supplementation of patients with NAFLD with symbiotics improved hepatic function (13). Moreover, research has also led to the identification of yogurt probiotics in managing NAFLD, as its supplementation led to a reversal of minimal hepatic encephalopathy (MHE) and increased adherence. Subsequently, Bajaj et al. reported that the simultaneous administration of symbiotics and vitamin E led to good outcomes in patients with NAFLD (14).
Through our diverse approach, this study aimed to analyze the alterations in gut microbiota composition in CLD and their relationship to the immune response by summarizing the evidence of changes in the immune response as a result of therapies that aim to restore the normal gut microbiota composition.
2 Methodology
2.1 Protocol and registration
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. However, the study protocol was not registered in any publicly accessible database.
2.2 Literature search
Two independent researchers conducted a literature review employing two complementary methodologies to identify all articles published up to March 2025. Initially, a comprehensive electronic search was executed using predefined criteria across three databases: Google Scholar, Science Direct, and PubMed. This search utilized the Boolean operators “AND” and “OR” to effectively combine keywords. The complete search strategy for PubMed included: (Gut microbiota OR Gut microbiome) AND (Probiotics OR Synbiotics OR Antibiotics) AND (Chronic liver disease OR Alcoholic hepatitis OR Viral hepatitis OR Cirrhosis OR Alcoholic steatohepatitis OR Non-alcoholic fatty liver disease) AND (Immune response OR Inflammatory markers OR Inflammatory cytokines OR neutrophil function). Furthermore, the reviewers manually examined the reference lists of selected articles to identify any studies that might have been overlooked, thereby ensuring comprehensive coverage of relevant literature.
2.3 Eligibility criteria
After retrieving all the articles from the three databases, they were assessed independently based on the predefined eligibility criteria. A study was included if it meets the following inclusion criteria:
1. Studies published in English.
2. Studies including patients with CLD (including those with cirrhosis, NAFLD, ALD, and chronic hepatitis).
3. Studies investigating the efficacy of different gut-modulating therapies, such as symbiotics, antibiotics, and probiotics.
4. Studies with a comparator, including placebos or other control interventions.
5. Studies designed as either randomized controlled trials (RCTs), observation cohort studies, or case-control studies.
6. Studies reporting changes in immune function, e.g., through changes in inflammatory cytokines or neutrophil activities.
We excluded the studies meeting the following exclusion criteria:
1. Studies not investigating any of the gut microbiota-altering therapies.
2. Studies without any comparator arm to the interventional group.
3. Studies not reporting any of the outcomes of the immune response after gut microbiota modulation.
4. Other secondary studies, case reports, and letters to the editor.
2.4 Study selection and data extraction
Independent reviewers conducted the study selection through a multi-stage process, which involved the removal of duplicate articles, the evaluation of titles and abstracts, and the examination of full texts. Initially, the abstracts of the articles remaining after the elimination of duplicates were assessed against the inclusion criteria. Articles that satisfied these criteria were included, while those with uncertain eligibility underwent a full-text review. Following the selection process, the reviewers independently extracted relevant data from the included studies using extraction forms that had been pilot-tested. Data were collected for all time points and utilized in the analysis, including the first author’s last name and publication year, study setting, design, study inclusion criteria, sample size, average ages, and reported outcomes.
2.5 Statistical analysis
We used the statistical software Review manager (RevMan 5.4) for the meta-analysis, The following outcomes were assessed: changes in the serum levels of interleukin-6 (IL-6), C-reactive proteins (CRPs), IL-18, interferon-γ, and tumor necrosis factor-α (TNF-α), which were analyzed using the standardized mean difference (SMD). Subsequently, the results were presented using forest plots. Statistical significance was determined at p ≤ 0.05. Due to the expected high heterogeneity of the outcomes, we used the random-effects model for the analysis. I2 statistics was used to assess heterogeneity.
2.6 Risk of bias assessment
ROB 2 tool (Cochrane Collaboration) was used to assess the risk of bias (ROB). Using this tool, two authors independently analyzed the ROB in each of the studies. They analyzed the bias that may arise from each of the five key domains, including randomization of the study participants, blinding of participants and investigators, reporting of the study outcomes, and missing data. Using the five domains, the authors assigned the overall ROB for each of the domains as either “high,” “low,” or “some concerns” based on various factors. In case of any disagreement between the reviewers on the ROB in a particular domain, a consensus was reached through the intervention of a third reviewer not involved in the ROB appraisal method. The Newcastle–Ottawa scale was utilized to analyze the methodological quality of observational studies.
3 Results
3.1 Search results
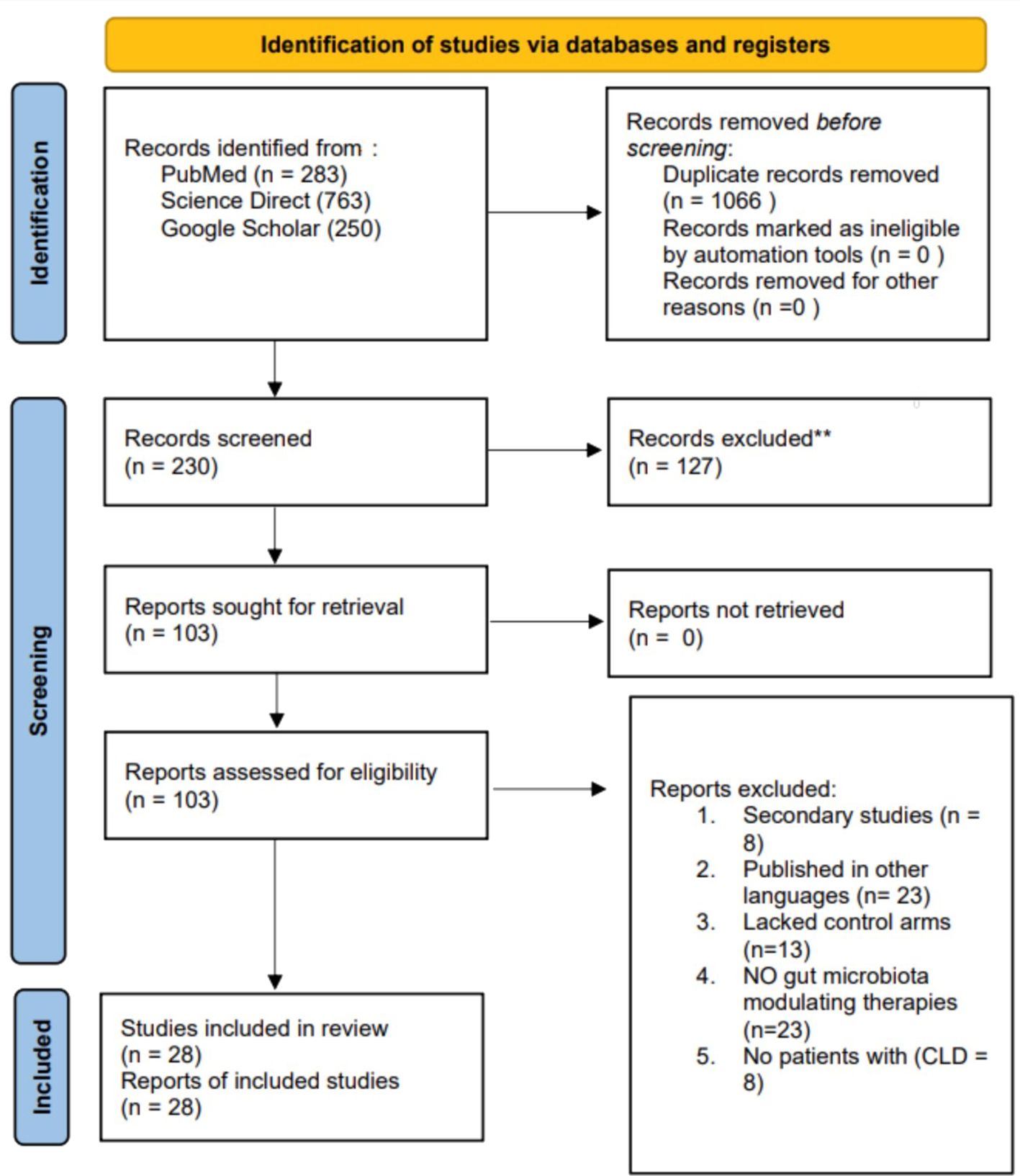
An extensive literature search yielded 1,296 articles from the databases. Following the assessment for duplicates, 1,066 redundant articles were eliminated. Subsequently, 230 abstracts were reviewed for their relevance to the study topic, resulting in the exclusion of 127 studies. The remaining 103 articles were retrieved and evaluated based on predetermined eligibility criteria, of which only 28 met the inclusion criteria and were incorporated into the review. The remaining 75 articles were excluded for the following reasons: 23 were not published in English, 13 lacked control arms, 8 were secondary studies such as reviews, 23 did not include microbiota-modulating therapies as one of their interventions, and 8 excluded patients with CLD. Figure 1 presents a PRISMA diagram that summarizes the search strategy.
3.2 Characteristics of the included studies
This study includes 28 studies conducted in different countries, including the United Kingdom, Austria, Malaysia, Iran, the United States of America, Japan, Spain, Italy, India, and Ukraine. Among the included studies, 27 were RCTs (12–38), whereas one was an observational study (11). The various interventions investigated across the studies included probiotics and symbiotics. Table 1 shows the characteristics of the included studies.
3.3 Methodological quality and ROB of the included studies
The methodological quality of the included observational study was fair (Table 1). Most included studies showed low ROB (Figure 2). A study by Dhiman et al. (24) had high ROB contributed by the high ROB under outcome measurement (Table 2).
3.4 TNF-α levels
13 studies reported the outcome of changes in TNF-alpha levels. A pooled analysis of the outcomes showed that gut microbiota-modulating therapies significantly decreased the TNF-α levels compared to placebo (SMD -0.60; 95% CI [-0.93, -0.23] p = 0.001). A subgroup analysis of the outcome found similar results during the 2- months follow-up (SMD -1.2; 95% CI [-2.3, 0.13] p = 0.03) and 6-month follow-up periods (SMD -1.3; 95% CI [-2.1, -0.4] p = 0.004). However, no significant difference was observed in the 1-month and 3-month follow-up period (SMD -0.23; 95% CI [-0.94, 0.48] p = 0.53) and (SMD -0.45; 95% CI [-0.91, 0.02] p = 0.06), respectively (Figure 3).
3.5 Changes in 1L-6 levels
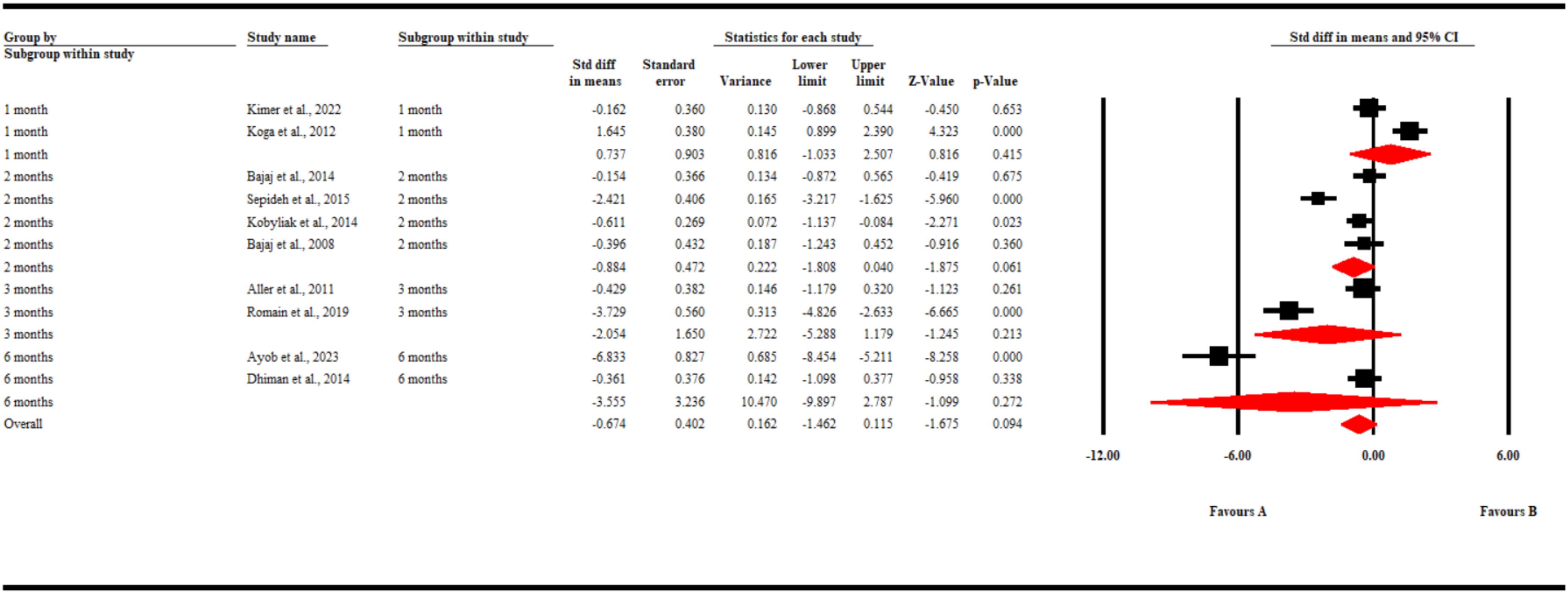
Nine studies reported changes in IL-6 levels. Our pooled analysis found no significant difference in the changes in IL-6 levels in both groups of patients (SMD -0.67; 95% CI [-1.5, 0.12) p = 0.09). Similarly, a subgroup analysis found that during the different time points in follow-up, gut microbiota-modulating therapies failed to significantly decrease the levels of Il-6 compared to placebo (SMD0.74; 95% CI [-1.03, 2.51] p = 0.42) at 1 month, (SMD -0.88; 95% CI [-1.81, 0.04] p = 0.06) at 2 months, (SMD -2.05; 95% CI [-5.29, 1.18] p = 0.21) at 3 months, and (SMD -3.56; 95% CI [-9.9, 2.79] p = 0.27) at 6 months (Figure 4).
3.6 CRP levels
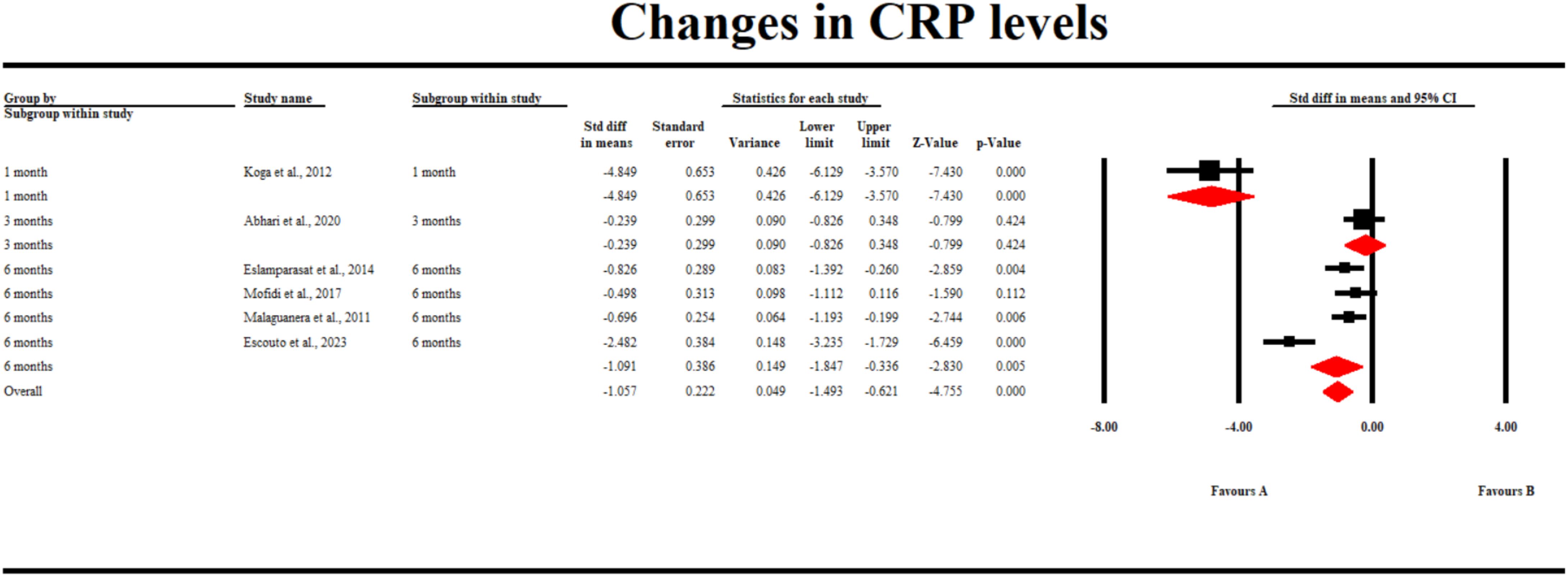
Only 6 studies reported changes in CRP levels. Our pooled analysis found that gut microbiota-modulating therapies significantly reduced serum CRP levels compared to placebo (SMD -1.057; 95% CI [-1.493, -0.621] p = 0.0005). Furthermore, similar results were observed at the 1-month and 6-month follow-up periods (SMD -4.85; 95% CI [-6.13, -3.57] p < 0.001) and (SMD -1.01; 95% CI [-1.85, -0.34] p = 0.005). However, no significant difference was observed after 3 months of interventions (SMD -0.239; 95% CI [-0.83, 0.35] p = 0.424) (Figure 5).
3.7 Changes in interferon-gamma (IFN-γ) levels
Two studies reported changes in interferon-γ. Our analysis found that gut-modulating therapies did not significantly affect the levels of IFN-γ in patients with chronic liver disease (SMD -0.259; 95% CI [-0.677, 0.159], p = 0.225) (Figure 6). Despite some variability across individual studies, the pooled results suggest that the interventions had no statistically significant impact on IFN-γ expression. This indicates that while gut microbiota-targeted treatments may influence other inflammatory markers, their effect on IFN-γ remains inconclusive and warrants further investigation through larger, well-designed clinical trials.
3.8 Changes in IL-18 levels
Two studies reported changes in IL-18 levels. Our pooled analysis showed that gut microbiome-modulating therapies did not significantly affect the levels of IL-18 in patients with chronic liver diseases (SMD -0.06; 95% CI [-0.47, 0.36] p = 079) (Figure 7).Our pooled analysis revealed that gut microbiome-modulating therapies did not significantly alter IL-18 levels in patients with chronic liver diseases (SMD -0.06; 95% CI [-0.47, 0.36] p = 0.79) (Figure 7). This finding was based on two studies that reported changes in IL-18 levels. The lack of significant effect suggests that these therapies may not have a substantial impact on this particular inflammatory marker in the context of chronic liver diseases.
3.9 Changes in cellular immunity
Due to differences in the reporting of cellular immunity changes, we could only do a narrative synthesis of the reported outcomes. Three of the included studies reported on the changes in immunohistochemistry of CLD patients. Romain et al. found that the neutrophil oxidative burst significantly increased in CLD patients treated with probiotics after stimulation with PMA (19). Similar results were observed by Horvath et al. However, they noted that the resting oxidative burs had no significant changes. Horvath et al. also found that the phagocytic activity of the neutrophils in the overall study population decreased with time in all the patients. This was, however, not observed in the phagocytic capacity of the monocytes, which increased after 3 months in the probiotic group (16). Lastly, Nor et al. found that the expression of CD4+ lymphocytes did not change in both groups but observed a decrease in the levels of CD8+ lymphocytes in patients treated with placebo (17).
4 Discussion
Modulation of the gut microbiota is crucial to maintain the gut–liver axis, as it prevents the interaction of the gut microbiomes with inflammatory cells, preventing CLD. The most frequent cytokines include TNF-α, IL-6, and CRP. Therapeutic intervention in patients with CLD aims to decrease the levels of these pro-inflammatory cytokines. Therefore, treatment of patients with CLD includes diet, probiotics, or fecal microbiota to enhance normal gut bacterial growth, may relieve gut dysbiosis, and improve the prognosis of patients with CLD (39).
TNF-α is a pro-inflammatory cytokine causing the immunopathogenesis of various diseases and organs, such as the liver, where it is involved in liver inflammation and apoptosis of hepatocytes, resulting in CLD (40). Generally, TNF-α is a major factor contributing to the onset and prognosis of NAFLD because high TNF-α have been found in patients with NAFLD (41). Subsequently, in patients with ALD, the serum TNF-α levels were elevated, suggesting liver disease. The increased TNF-α levels in the diseased state of patients with CLD indicate its crucial role in the inflammation and pathogenesis of various CLDs.
We found that gut microbiota modulation therapies could reduce inflammation and immune response in patients with CLD. The TNF-α levels were significantly reduced in the interventional groups compared with the controls, with a significant effect occurring after treatment for 6 months, concluding that long-term therapy was beneficial compared with short-term. Similar to our study, a previous meta-analysis by Wang et al., which focused on probiotics in patients with NAFLD, TNF-α levels were significantly decreased in the interventional groups compared with the controls (42). The study also highlighted that the clinical benefits of gut-modulating therapies were more apparent with increased treatment time.
The clinical benefit of gut-modulating therapies in reducing pro-inflammatory cytokines was also apparent after analysis of CRP levels. We found that CRP levels were significantly reduced in patients receiving gut-modulating therapies compared with the controls. Similar results were reported by Pan et al., who determined that gut-modulating therapies reduced the inflammation in patients with NAFLD, specifically reducing CRP levels (43). Unlike other inflammatory cytokines, no significant difference was found in the IL-6 levels in both treatment groups. Furthermore, in some subgroup analyses, IL-6 significantly increased in the patients receiving gut-modulating therapies. Similar results were found by Kazimi et al., who established that IL-6 levels were significantly increased in patients receiving gut-modulating therapies (prebiotics and probiotics) compared with the controls (44).
Neutrophils are a critical component of the innate immune system. Over the years, it has been established that the interplay between gut microbiota and neutrophils interact to adjust the magnitude of neutrophil-mediated immunity (45). In liver disease, neutrophils are one of the significant innate immunity cells that have been associated with its pathogenesis (46). Empirical evidence from our included studies indicates that gut microbiota modulation may significantly reduce the pro-inflammatory state of the neutrophils. This, therefore, enables the neutrophils to have oxidative bursts upon stimulation (19). However, the effect on other immune cells, such as CD4+ and CD8+ lymphocytes, has yet to be observed.
5 Limitations
The objective of this review was to assess the efficacy of therapies targeting gut health in modulating the immune response in individuals with CLD. Pro-inflammatory markers are recognized as reliable indicators of inflammation and its variations; however, other measures, such as the activity of neutrophils and macrophages, can also effectively represent the immune response. Although certain studies reported changes in neutrophil activity, the available data were insufficient, precluding the aggregation of results (15) and limiting the ability to draw conclusions regarding the impact of gut microbiota-modulating therapies on cellular and innate immune responses.
6 Conclusion
This research demonstrates the potential of gut microbiota-targeted therapies in chronic liver disease (CLD) treatment. The interventions reduced pro-inflammatory cytokines, particularly TNF-α and CRP, suggesting alleviation of chronic inflammation in CLD. However, variability in IL-6 levels highlights the need for nuanced inflammatory marker monitoring. The findings have important clinical implications, opening possibilities for personalized CLD management strategies. Clinicians may consider these approaches as complementary or alternative treatments to enhance overall effectiveness. Future research should focus on the extended follow-up periods to observe long-term effects on gut microbiota composition and inflammatory markers. Comprehensive assessment of disease progression, including liver function tests and fibrosis markers. Developing personalized approaches based on individual patient characteristics while promising, these results underscore the need for continued investigation to fully exploit the benefits of gut microbiota modulation in managing the immune response in CLD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
EG: Conceptualization, Formal Analysis, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KS: Conceptualization, Investigation, Project administration, Resources, Visualization, Writing – original draft. ZH: Investigation, Methodology, Resources, Software, Writing – review & editing. BM: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sharma A and Nagalli S. (2023). Chronic Liver Disease. In: StatPearls. StatPearls Publishing, Treasure Island (FL. Available at: http://www.ncbi.nlm.nih.gov/books/NBK554597/.
2. Cheemerla S and Balakrishnan M. Global epidemiology of chronic liver disease. Clin Liver Dis. (2021) 17:365–70. doi: 10.1002/cld.1061
3. Ladep NG, Akbar SMF, and Al Mahtab M. Global Epidemiology of Chronic Liver Disease. In: Wong RJ and Gish RG, editors. Clinical Epidemiology of Chronic Liver Diseases. (2021) Springer International Publishing, Cham. p. 41–55.
4. Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, and Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. (2015) 21:8787–803. doi: 10.3748/wjg.v21.i29.8787
5. Thursby E and Juge N. Introduction to the human gut microbiota. Biochem J. (2017) 474:1823–36. doi: 10.1042/BCJ20160510
6. Albillos A, de Gottardi A, and Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. (2020) 72:558–77. doi: 10.1016/j.jhep.2019.10.003
7. Miyake Y and Yamamoto K. Role of gut microbiota in liver diseases. Hepatology Res. (2013) 43:139–46. doi: 10.1111/j.1872-034X.2012.01088.x
8. Kassa Y, Million Y, Gedefie A, and Moges F. Alteration of gut microbiota and its impact on immune response in patients with chronic HBV infection: A review. Infection Drug Resistance. (2021) 14:2571–8. doi: 10.2147/IDR.S305901
9. Yan F, Zhang Q, Shi K, Zhang Y, Zhu B, Bi Y, et al. Gut microbiota dysbiosis with hepatitis B virus liver disease and association with immune response. Front Cell Infect Microbiol. (2023) 13:1152987. doi: 10.3389/fcimb.2023.1152987
10. Shen Y, Wu S-D, Chen Y, Li XY, Zhu Q, Nakayama K, et al. Alterations in gut microbiome and metabolomics in chronic hepatitis B infection-associated liver disease and their impact on peripheral immune response. Gut Microbes. (2023) 15:2155018. doi: 10.1080/19490976.2022.2155018
11. Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, and Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatology. (2008) 48:945–51. doi: 10.1016/j.jhep.2008.02.015
12. Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non-alcoholic steatohepatitis. Dig Dis Sci. (2012) 57:545–53. doi: 10.1007/s10620-011-1887-4
13. Mofidi F, Poustchi H, Yari Z, Nourinayyer B, Merat S, Sharafkhah M, et al. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr. (2017) 117:662–8. doi: 10.1017/S0007114517000204
14. Bajaj JS, Saeian K, Christensen KM, Hafeezullah M, Varma RR, et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterology. (2008) 103:1707–15. doi: 10.1111/j.1572-0241.2008.01861.x
15. Macnaughtan J, Figorilli F, García-López E, Lu H, Jones H, Sawhney R, et al. A double-blind, randomized placebo-controlled trial of probiotic lactobacillus casei shirota in stable cirrhotic patients. Nutrients. (2020) 12:1651. doi: 10.3390/nu12061651
16. Horvath A, Leber B, Schmerboeck B, Tawdrous M, Zettel G, Hartl A, et al. Randomised clinical trial: the effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment Pharmacol Ther. (2016) 44:926–35. doi: 10.1111/apt.2016.44.issue-9
17. Mohamad Nor MH, Ayob N, Mokhtar NM, Raja Ali RA, Tan GC, et al. The effect of probiotics (MCP® BCMC® Strains) on hepatic steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease. Nutrients. (2021) 13:3192. doi: 10.3390/nu13093192
18. Koga H, Tamiya Y, Mitsuyama K, Ishibashi M, Matsumoto S, Imaoka A, et al. Probiotics promote rapid-turnover protein production by restoring gut flora in patients with alcoholic liver cirrhosis. Hepatol Int. (2013) 7:767–74. doi: 10.1007/s12072-012-9408-x
19. Román E, Nieto JC, Gely C, Vidal S, Pozuelo M, Poca M, et al. Effect of a multi-strain probiotic on cognitive function and risk of falls in patients with cirrhosis: A randomized trial. Hepatol Commun. (2019) 3:632–45. doi: 10.1002/hep4.1325
20. Kobyliak N, Abenavoli L, Mykhalchyshyn G, Kononenko L, Boccuto L, Kyriienko D, et al. A multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: evidence from a randomized clinical trial. J Gastrointestinal Liver Dis. (2018) 27:41–9. doi: 10.15403/jgld.2014.1121.271.kby
21. Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, Sterling RK, et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther. (2014) 39:1113–25. doi: 10.1111/apt.2014.39.issue-10
22. Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, and Hekmatdoost A. Synbiotic supplementation in non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. (2014) 99:535–42. doi: 10.3945/ajcn.113.068890
23. Ekhlasi G, Zarrati M, Agah S, Hosseini AF, Hosseini S, Shidfar S, et al. Effects of symbiotic and vitamin E supplementation on blood pressure, nitric oxide and inflammatory factors in non-alcoholic fatty liver disease. EXCLI J. (2017) 16:278–90. doi: 10.17179/excli2016-846
24. Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, et al. Probiotic VSL3 reduces liver disease severity and hospitalization in patients with cirrhosis: A randomized, controlled trial. Gastroenterology. (2014) 147:1327–1337.e3. doi: 10.1053/j.gastro.2014.08.031
25. Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, et al. Effect of a probiotic on liver aminotransferases in non-alcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. (2011) 15:1090–5.
26. Ayob N, Nawawi KNM, Nor MHM, Raja Ali RA, Ahmad HF, Oon SF, et al. The effects of probiotics on small intestinal microbiota composition, inflammatory cytokines and intestinal permeability in patients with non-alcoholic fatty liver disease. Biomedicines. (2023) 11:640. doi: 10.3390/biomedicines11020640
27. Escouto GS, Port GZ, Tovo CV, Fernandes SA, Peres A, Dorneles GP, et al. Probiotic supplementation, hepatic fibrosis, and the microbiota profile in patients with non-alcoholic steatohepatitis: A randomized controlled trial. J Nutr. (2023) 153:1984–93. doi: 10.1016/j.tjnut.2023.05.019
28. Roussel E, Brasse-Lagnel C, Tuech J-J, Montialoux H, Papet E, Tortajada P, et al. Influence of probiotics administration before liver resection in patients with liver disease: A randomized controlled trial. World J Surg. (2022) 46:656–65. doi: 10.1007/s00268-021-06388-7
29. Mitrović M, Dobrosavljević A, Odanović O, Knežević-Ivanovski T, Kralj Đ, Erceg S, et al. The effects of synbiotics on the liver steatosis, inflammation, and gut microbiome of metabolic dysfunction-associated liver disease patients-randomized trial. Rom J Intern Med. (2024) 62:184–93. doi: 10.2478/rjim-2024-0004
30. Laghi L, Ortiz MÀ, Rossi G, Román E, Mengucci C, Cantó E, et al. Biomarkers of frailty in patients with advanced chronic liver disease undergoing a multifactorial intervention consisting of home exercise, branched-chain amino acids, and probiotics. Biomolecules. (2024) 14:1410. doi: 10.3390/biom14111410
31. Zhang J, Li C, Duan M, Qu Z, Wang Y, Dong Y, et al. The improvement effects of weizmannia coagulans BC99 on liver function and gut microbiota of long-term alcohol drinkers: A randomized double-blind clinical trial. Nutrients. (2025) 17:320. doi: 10.3390/nu17020320
32. Manzhalii E, Moyseyenko V, Kondratiuk V, Molochek N, Falalyeyeva T, and Kobyliak N. Effect of a specific Escherichia coli Nissle 1917 strain on minimal/mild hepatic encephalopathy treatment. World J Hepatol. (2022) 14:634–46. doi: 10.4254/wjh.v14.i3.634
33. Sepideh A, Karim P, Hossein A, Leila R, Hamdollah M, Mohammad EG, et al. Effects of multi-strain probiotic supplementation on glycemic and inflammatory indices in patients with non-alcoholic fatty liver disease: A double-blind randomized clinical trial. J Am Coll Nutr. (2016) 35:500–5. doi: 10.1080/07315724.2015.1031355
34. Abhari K, Saadati S, Yari Z, Hosseini H, Hedayati M, Abhari S, et al. The effects of Bacillus coagulans supplementation in patients with non-alcoholic fatty liver disease: A randomized, placebo-controlled, clinical trial. Clin Nutr ESPEN. (2020) 39:53–60. doi: 10.1016/j.clnesp.2020.06.020
35. Jayakumar S, Carbonneau M, Hotte N, Befus AD, St Laurent C, Owen R, et al. VSL3® probiotic therapy does not reduce portal pressures in patients with decompensated cirrhosis. Liver Int. (2013) 33:1470–7. doi: 10.1111/liv.2013.33.issue-10
36. Gupta N, Kumar A, Sharma P, Garg V, Sharma BC, and Sarin SK. Effects of the adjunctive probiotic VSL3 on portal haemodynamics in patients with cirrhosis and large varices: a randomized trial. Liver Int. (2013) 33:1148–57. doi: 10.1111/liv.2013.33.issue-8
37. Patel VC, Lee S, McPhail MJW, Da Silva K, Guilly S, Zamalloa A, et al. Rifaximin-α reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial. J Hepatology. (2022) 76:332–42. doi: 10.1016/j.jhep.2021.09.010
38. Kimer N, Meldgaard M, Hamberg O, Kronborg TM, Lund AM, Møller HJ, et al. The impact of rifaximin on inflammation and metabolism in alcoholic hepatitis: A randomized clinical trial. PloS One. (2022) 17:e0264278. doi: 10.1371/journal.pone.0264278
39. Woodhouse CA, Patel VC, Singanayagam A, and Shawcross D. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther. (2018) 47:192–202. doi: 10.1111/apt.2018.47.issue-2
40. Tiegs G and Horst AK. TNF in the liver: targeting a central player in inflammation. Semin Immunopathol. (2022) 44:445–59. doi: 10.1007/s00281-022-00910-2
41. Vachliotis ID and Polyzos SA. The role of tumor necrosis factor-alpha in the pathogenesis and treatment of non-alcoholic fatty liver disease. Curr Obes Rep. (2023) 12:191–206. doi: 10.1007/s13679-023-00519-y
42. Wang Y, Wang Y, and Sun J. The clinical effect of probiotics on patients with non-alcoholic fatty liver disease: a meta-analysis. Bioengineered. (2022) 13:14960–73. doi: 10.1080/21655979.2023.2185941
43. Pan Y, Yang Y, Wu J, Zhou H, and Yang C. Efficacy of probiotics, prebiotics, and synbiotics on liver enzymes, lipid profiles, and inflammation in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. BMC Gastroenterol. (2024) 24:283. doi: 10.1186/s12876-024-03356-y
44. Kazemi A, Soltani S, Ghorabi S, Keshtkar A, Daneshzad E, Nasri F, et al. Effect of probiotic and synbiotic supplementation on inflammatory markers in health and disease status: A systematic review and meta-analysis of clinical trials. Clin Nutr. (2020) 39:789–819. doi: 10.1016/j.clnu.2019.04.004
45. Zhang D and Frenette PS. Cross talk between neutrophils and the microbiota. Blood. (2019) 133(20):2168–77. doi: 10.1182/blood-2018-11-844555
Keywords: gut microbiota, immunity; immune responses, chronic liver diseases, TNF, liver cirrhos
Citation: Gadour E, Shrwani KJ, Hassan Z and Miutescu B (2025) The role of gut microbiota in modulating immune responses in chronic liver disease: a systematic review and meta-analysis. Front. Immunol. 16:1556576. doi: 10.3389/fimmu.2025.1556576
Received: 07 January 2025; Accepted: 29 April 2025;
Published: 16 May 2025.
Edited by:
Ehsaneh Taheri, Shahid Beheshti University of Medical Sciences, IranReviewed by:
Lei Deng, Chinese Academy of Agricultural Sciences, ChinaAntonio Facciorusso, University of Foggia, Italy
Szymon Suwala, Nicolaus Copernicus University in Toruń, Poland
Copyright © 2025 Gadour, Shrwani, Hassan and Miutescu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eyad Gadour, ZXlhZGdhZG91ckBkb2N0b3JzLm9yZy51aw==
 Eyad Gadour
Eyad Gadour Khalid Jebril Shrwani
Khalid Jebril Shrwani Zeinab Hassan4
Zeinab Hassan4