- 1Department of Rheumatology and Immunology, First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, Anhui, China
- 2Institute of Rheumatology, Anhui Academy of Chinese Medicine, Hefei, Anhui, China
- 3Anhui Province Key Laboratory of Modern Chinese Medicine, Department of Internal Medicine Application Foundation Research and Development, Hefei, Anhui, China
Rheumatic disease is a chronic inflammatory disease that imposes significant societal and economic burdens. Accumulating evidence has demonstrated that cellular senescence plays an important role in inflammation-induced rheumatic diseases. Due to the lack of effective therapies, there is an urgent need for a deeper understanding of the etiopathogenesis of rheumatic diseases. In this review, we systematically summarized the role of cellular senescence in rheumatic diseases. We first focused on the mechanisms and hallmarks of cellular senescence, and then summarized evidence that can induce or aggravate cellular senescence, as well as related signaling pathways. Next, we discussed the mechanisms of interaction between cellular senescence and rheumatic diseases. Additionally, we focused on and elucidated the mechanisms and impacts of chondrocyte senescence and mesenchymal stem cell (MSC) senescence in osteoarthritis (OA) and systemic lupus erythematosus (SLE), respectively. Finally, we highlighted the potential of therapies targeting senescent cells in rheumatic diseases as a strategy, especially the multi-target effect of traditional Chinese medicine.
1 Introduction
Rheumatic diseases are a class of chronic, multi-system autoimmune diseases with a complex and multifactorial etiology, including rheumatoid arthritis (RA), osteoarthritis (OA), ankylosing spondylitis (AS), systemic lupus erythematosus (SLE), systemic sclerosis (SS), etc. (1, 2). Besides, dysfunctional joints and skin, and the extra-articular manifestations include cardiac, ocular, lung, cutaneous, gastrointestinal, neurological, and renal involvement in rheumatic diseases (3, 4). Furthermore, long-term oral treatment can exacerbate liver and kidney function damage and significantly affect patient’s quality of life (5, 6). The clinical treatment of rheumatic diseases is limited because of its complex pathogenesis.
Senescence is a cellular state marked by irreversible cell cycle arrest, accompanied by distinct secretory phenotypes and metabolic reprogramming, which critically contribute to aging and chronic disease progression. Chronic inflammation is a core feature of the pathogenesis of rheumatic diseases (7, 8). Cellular senescence is a process accompanied by low-grade chronic inflammation (9). Senescent cells secrete pro-inflammatory mediators, which in turn affect cellular senescence (10, 11). Conversely, inflammation plays a pivotal role in driving cellular senescence. This phenomenon occurs when inflammation is excessively regulated and is known as inflammageing (12–14). Growing studies have shown that cellular senescence affects cell function through the secretion of senescence-associated secretory phenotypes (SASP), which exacerbate inflammatory responses and tissue damage; additionally, cellular senescence’s significant role in rheumatic disease pathogenesis is also elucidated (15).
Recent advancements in the study of cellular senescence and rheumatic diseases underscore the necessity of a comprehensive review in this area. In this review, we explored the history, mechanism, and hallmarks of cellular senescence, as well as its role in the pathology of rheumatic diseases; additionally, we summarized evidence that can induce or aggravate cellular senescence, and related signaling pathways. By clarifying the connection between cellular senescence and rheumatic diseases, this review aimed to provide valuable insights into the treatment of rheumatic diseases, as targeting this process holds promise for improving the outcomes of rheumatic disease patients.
2 Cellular senescence
2.1 Hallmarks of cellular senescence
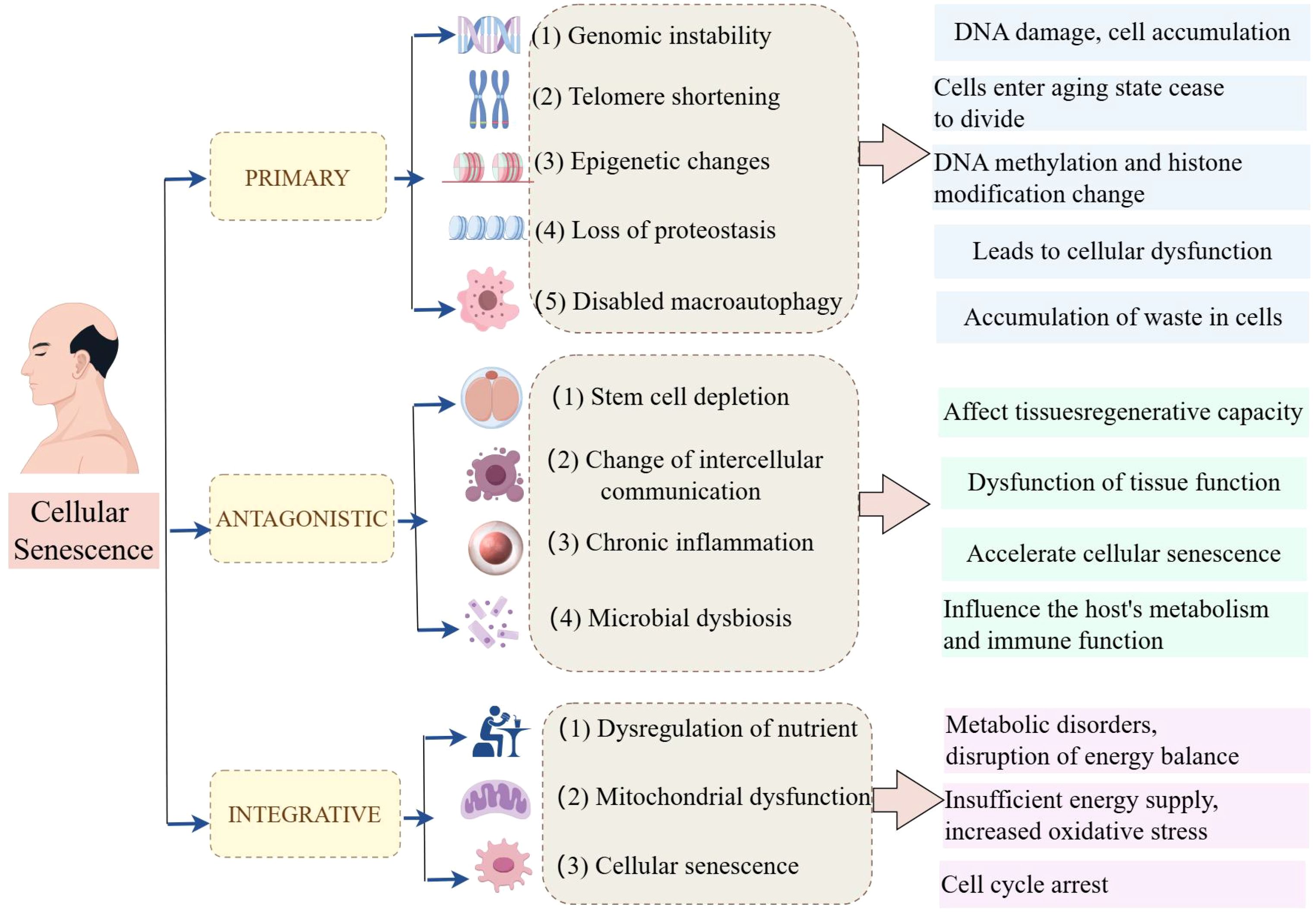
In 1961, Leonard Hayflick and Paul Moorhead first described the phenomenon known as “cellular senescence”, a form of “senescence at the cellular level”; additionally, they stated that primary human fibroblasts have a restricted lifespan in culture, with approximately 50 cell divisions (16). Cellular senescence is a complex biological process involving multiple cellular and molecular mechanisms (17, 18). In recent years, researchers have summarized twelve major characteristics of cellular senescence, which not only help us understand the biological basis of senescence but also provide new perspectives for developing anti-senescence therapies (Figure 1).
The primary characteristics of hallmarks of cellular senescence are shown as follows. (1) Genomic instability is an important characteristic of cellular senescence. As cells age, DNA damage gradually accumulates within the cell, leading to genomic instability. This instability may result in the loss of cellular function and even contribute to the development of diseases such as cancer. (2) Telomere shortening (TS) is another significant characteristic of cellular senescence. Telomeres are protective structures at the ends of chromosomes whose length gradually decreases during cell division. When telomeres become too short, cells enter a senescence state and cease to divide. (3) Epigenetic change is also an important sign of cell senescence. As age increases, epigenetic markers (such as DNA methylation and histone modification) change, affecting gene expression and cellular function. (4) The loss of protein homeostasis, characterized by an increase in protein misfolding and aggregation, is also a hallmark of cellular senescence. This phenomenon leads to cellular dysfunction and is associated with various age-related diseases. (5) Dysfunction of macroautophagy also plays an important role in cell senescence. Macroautophagy is a process in which cells clear damaged or excess organelles, and its dysfunction may lead to the accumulation of waste in cells.
The main antagonistic hallmarks were shown as follows. (1) Stem cell depletion is an important manifestation of cellular senescence. As age increases, the number and function of stem cells gradually decrease, affecting the regenerative capacity of tissues. (2) The change in intercellular communication is also a characteristic of cell senescence. Changes in intercellular signaling may lead to the dysfunction of tissue function. (3) Chronic inflammation, also known as inflammation senescence, is an important characteristic of cell senescence. Chronic inflammation state accelerates cell senescence and is associated with various age-related diseases. (4) Microbial dysbiosis is also considered a hallmark of cellular senescence. Changes in the gut microbiome may affect the host’s metabolism and immune function, thus accelerating the senescence process.
Furthermore, the integrative factors mainly included 3 items. (1) Dysregulation of nutrient sensing is a feature of cellular senescence. Decreased responsiveness to nutritional signals may lead to metabolic disorders and disruption of energy balance. (2) Mitochondrial dysfunction plays a key role in cellular senescence. Mitochondria are the energy factories of cells, and their dysfunction leads to insufficient energy supply and increased oxidative stress. (3) After being subjected to various stresses, cells will enter an irreversible state of growth arrest, known as cellular senescence. These features provide a framework for understanding cellular senescence and developing potential targets for new anti-senescence strategies.
Senescence is a cellular state characterized by growth arrest, anti-apoptosis, SASP, and DNA damage response (DDR) (19–21). These characteristics are expressed and mechanized differently in different biological contexts. First, cells enter an irreversible cell cycle arrest, typically accompanied by activation of DDR. DDR is the mechanism by which cells recognize and repair DNA damage; if the damage cannot be repaired, the cells will enter a senescent state. Additionally, the sustained activation of DDR is associated with TS, and telomere damage leads to cellular senescence while remaining active during the senescence process (22). Second, compared to apoptotic cells, senescent cells indeed exhibit significant anti-apoptotic characteristics. This anti-apoptotic property is mainly due to the significant increase of anti-apoptotic proteins (such as BCL-2) in senescent cells. The increase in this protein leads to cell resistance to apoptosis, thereby promoting the accumulation of senescent cells. Additionally, senescent cells avoid cell death by modulating the expression of apoptosis-related genes, thereby accumulating in the body. Senescent cells still maintain metabolic activity and exhibit a characteristic known as SASP even after experiencing cell cycle arrest. SASP leads to the secretion of various pro-inflammatory cytokines, including IL-1, IL-6, IL-8, proteases, and matrix metalloproteinases (23). SASP production is closely related to the continuous activation of DDR and plays an important regulatory role in the senescence process. Finally, the role of DDR in cellular senescence is not limited to DNA repair but also involves the regulation of the cell cycle and signaling (24). Abnormal activation of DDR can lead to genomic instability, which is critical in senescence and cancer development. These characteristics interact with each other through complex molecular mechanisms, which jointly affect the fate of cells and the health of organisms.
Biological markers that reflect direct evidence of cellular senescence have not yet been identified, but several markers have been used to indirectly detect senescent cells (25). The most widely used marker of senescent cells is senescence-associated beta-galactosidase (SA-beta-Gal), a lysosomal hydrolase whose activity is significantly increased in senescent cells (26). The activity of SA-β-Gal is typically detected under conditions of pH 6.0, making it one of the classic methods for identifying senescent cells. However, the expression of SA-β-Gal is not always directly related to cellular senescence. For example, some studies have found that SA-β-Gal activity also appears in certain healthy non-proliferative cells. Therefore, although is an important senescence marker, it should be used in conjunction with other indicators to more accurately assess cellular senescence status (27). Histone γ-H2AX (a replacement histone), the second most common marker of cellular senescence after SA-β-Gal, is phosphorylated at the C-terminal serine-139 of the H2AX histone (28). Histone γ-H2AX is the most sensitive marker of double-stranded DNA breaks (DSBs) and TS. The number of γ-H2AX foci increases in damaged and senescent cells in most tissues and species both in vivo and in vitro (29). As an important marker in the early cellular response to DNA DSBs, γ-H2AX’s role in cellular senescence has attracted significant attention. Studies have shown that γH2AX formation is associated with cell cycle arrest and apoptosis, which may be a protective mechanism adopted by cells when confronted with irreversible DNA damage. Additionally, γH2AX may also participate in regulating senescence-related signaling pathways, thereby affecting cell fate (30).
Senescence-associated heterochromatic foci (SAHF) are densely packed nuclear structures, which contribute to the maintenance of cellular senescence by silencing proliferation-related genes (31, 32). Their formation involves key chromatin remodeling factors, notably the retinoblastoma (Rb) protein and BRG1, whose interaction is essential for SAHF assembly (33). Moreover, the p16INK4a gene, also known as CDKN2a, encodes a cyclin-dependent kinase inhibitor that induces cell cycle arrest, contributing to senescent cell accumulation (34, 35). Similarly, p21, a downstream effector of p53, inhibits CDK activity during G1/S and G2/M transitions (36, 37). Sustained expression of p21 promotes senescence as a protective response to chronic stress, with its expression level and localization influencing its regulatory function (38). These pathways are highly relevant to the pathogenesis of rheumatic diseases and may offer potential targets for therapy.
2.2 Stressors and cellular senescence
2.2.1 Inflammation induces cellular senescence
Inflammation is a critical factor that can induce cellular senescence, which is a state of stable cell cycle arrest typically accompanied by a pro-inflammatory phenotype (39, 40) (Figure 2). Inflammatory signals can trigger senescence, and senescent cells can in turn promote inflammation through SASP. This creates a feedback loop that can lead to chronic inflammation, commonly referred to as “inflammaging”, which is a hallmark of senescence (41). For instance, the cGAS-STING pathway is activated by cytoplasmic chromatin fragments in senescent cells, leading to the production of type I interferons and other inflammatory mediators, thereby linking DNA damage responses to inflammation and senescence (42). Moreover, senescent cell accumulation in tissues maintains a pro-inflammatory environment, contributing to cellular senescence-related pathologies. Clearing these cells has been shown to alleviate inflammation and restore tissue homeostasis, highlighting the potential therapeutic benefits of targeting senescent cells to mitigate inflammation-induced tissue damage (43).
2.2.2 Reactive oxygen species induces cellular senescence
The ROS generated during oxidative stress is a potent trigger for cellular senescence whereby it irreversibly limits cell proliferation and these senescent cells secrete various SASP, further inducing the senescence of adjacent cells (44). The accumulation of ROS can lead to oxidative damage to cellular components (such as DNA, proteins, and lipids), thereby triggering the senescence program (45). This process is often associated with the activation of specific signaling pathways and the expression of senescence-associated markers. Specifically, one of the key pathways involved in ROS-induced cellular senescence is the p53/p21 signaling. The presence of ROS can lead to the activation of p53, a tumor suppressor protein that regulates the expression of cyclin-dependent kinase inhibitor p21 (46). Upregulation of p21 results in cell cycle arrest. Additionally, ROS can activate other signaling pathways, such as the IL-6/STAT3 pathway, which is implicated in the induction of cellular senescence in nerve cells (47). ROS accumulation promotes the activation of this pathway, contributing to senescence. Furthermore, ROS is involved in the regulation of autophagy and lysosomal function, which are critical processes in the maintenance of cellular homeostasis. In the context of bleomycin-induced cellular senescence, ROS has been shown to mediate lysosomal membrane permeabilization and inhibit autophagy, leading to the accumulation of damaged cellular components and the promotion of senescence (48). These findings highlight the complex interplay between ROS, cellular senescence, and various cellular pathways.
2.2.3 High glucose induces cellular senescence
Both extracellular and intracellular metabolic disturbances significantly promote cellular senescence. For example, extracellular high glucose refers to an increase in glucose levels in the extracellular environment, which can induce cellular senescence through oxidative stress and inflammatory pathways. Conversely, intracellular high glucose refers to intracellular metabolic dysregulation, which independently contributes to senescence via mechanisms such as hexosamine pathway activation or advanced glycation end-products. Numerous studies have demonstrated that high glucose stimulation induces early cellular senescence (49, 50). For example, it has been shown that high glucose microenvironment markedly decreases miR-30a-5p expression in HMEC-1, elevates SA-β-gal and p21 levels, fosters cellular senescence, and hinders cell proliferation, migration, and vasculogenesis (51). Moreover, studies have shown that high glucose can induce cellular senescence through multiple signaling pathways. For example, high glucose promotes the senescence of mesenchymal stem cells (MSCs) via the Akt/mammalian target of rapamycin (mTOR) signaling pathway (52). During the process of high glucose-induced cellular senescence, the expression and activity of SIRT1 protein are also significantly affected. Studies have found that high sugar leads to the downregulation of SIRT1 expression, thereby accelerating the senescence of endothelial cells. Additionally, compounds such as Rutaecarpine, an indoloquinazoline alkaloid from Evodia rutaecarpa, could attenuate high glucose-induced endothelial senescence by activating SIRT1, which deacetylates p53 and suppresses ROS generation (53). As has been evidenced previously, under conditions of high glucose, the level of ROS in cells significantly increases, leading to oxidative damage and cellular senescence (54). Additionally, high glucose induces cells to secrete inflammatory factors (such as IL-6 and TNF-α), exacerbating cellular senescence and dysfunction (55). The above studies provide important theoretical foundations for understanding the mechanisms of high glucose-induced cellular senescence.
3 The relationship between different types of rheumatic diseases and cellular senescence
3.1 RA and cellular senescence
3.1.1 Overview of RA pathogenesis
RA is a chronic arthritic disease characterized by synovial proliferation, joint swelling and pain, and cartilage destruction, eventually leading to joint stiffness, deformity, and dysfunction, affecting about 12% of the global adult population (56, 57). Previous studies have suggested that oxidative stress, inflammation, hypercoagulable state, and apoptosis are closely related to RA pathogenesis (58–60). Growing evidence has suggested that there is a link between cellular senescence and RA pathogenesis (61).
3.1.2 TS and genomic instability
TS is a hallmark of cellular senescence; although telomeres shorten physiologically with each cell division, this shortening rate can be increased by allostatic load and inflammatory insults, thus affecting the cellular senescence process (62). In a previous study of early and follow-up changes in leukocyte telomere length (rLTL) in RA patients, it has been found that rLTL shortening is associated with age, disease activity score (DET), and natural rLTL (63). In another multinational cohort study, researchers have demonstrated that TL shortening is closely associated with functional mutations of telomere-associated genes (TRGs) and senescence. Additionally, rTL is linked to baseline disease severity in RA-related interstitial lung disease (RA-ILD) (64). These observations are very similar to RA-ILD among U.S. Veterans; short TL is strongly associated with prevalent but not incident RA-ILD (65). These findings collectively support the view that TL shortening acts as a marker of accelerated RA senescence and underscore the importance of further research into TL variations.
3.1.3 Bone cell senescence and joint destruction
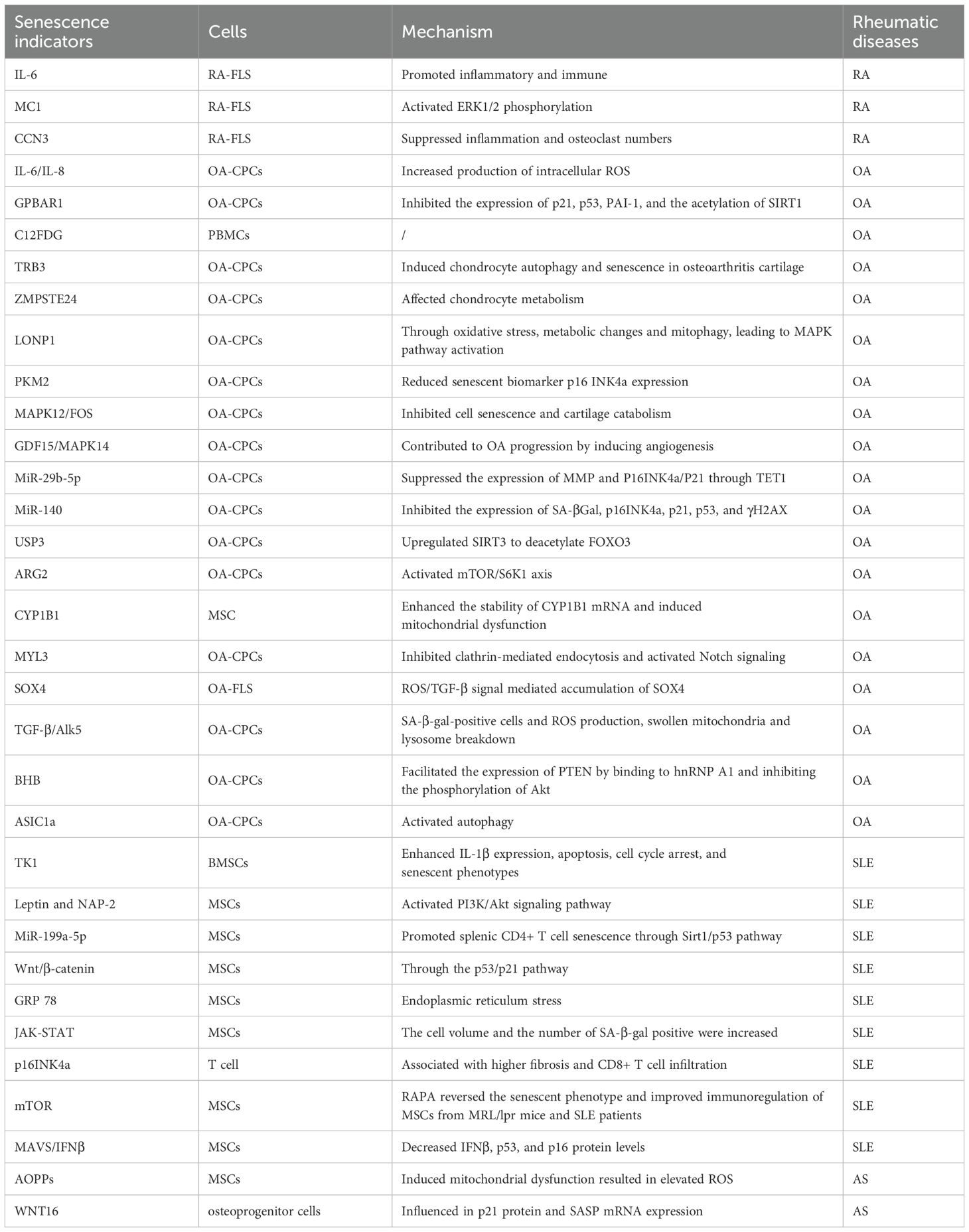
In the process of bone resorption and metabolism, osteoclasts are mainly responsible for bone resorption and osteoblasts are responsible for bone formation (66). Osteoclasts are the major mediators of bone destruction, which are regarded as the main cell type of bone destruction in RA under pathological state (67). Bone destruction is the main feature of RA, which can result in osteoporosis. A previous study has shown that osteoblast replicative senescence in periarticular bones occurs more rapidly with senescence in RA than in OA patients, contributing to periarticular osteopenia in RA (68). This demonstrates that the replicative senescence of osteoblasts occurs more rapidly, exacerbating bone loss in RA. Cellular communication network factor 3 (CCN3), previously known as nephroblastoma overexpressed (NOV), is a secreted multifunctional protein involved in various cellular processes (69). A previous study has suggested that CCN3 is a new senescence marker of chondrocytes, and CCN3 overexpression in cartilage may partially promote chondrocyte senescence, leading to articular cartilage degeneration through the induction of p53 and p21 (70) (Table 1). Taiki et al. have manifested that CCN3 is highly expressed in RA joints and its level is associated with the severity of the disease (71). Further in-vitro assay demonstrated that stimulation with CCN3 resulted in the activation of the senescence pathway in fibroblast-like synoviocytes (FLS) and osteoclast differentiation in monocytes. These findings provide new potential targets for RA treatment, and future research can further explore the specific mechanisms of CCN3’s role in RA and its potential as a therapeutic target for preventing cellular senescence.
3.1.4 Synovial fibroblast senescence
The basic pathology of RA has been identified as a disorder of inflammation in the rheumatoid synovium, in which the predominant cell type is FLS (72, 73). The melanocortin 1 receptor (MC1R) is a member of the G-protein coupled receptor (GPCR) superfamily and belongs to the melanocortin (MC) subfamily of receptors (74). It has been shown that RA-FLS acquire a senescence phenotype after activating the MC1; selective activation of the GPCR MC1 may prevent the vicious cycle of reciprocal activation between FLS and macrophages within the inflamed joint environment, and then arise from the exploitation of senescence to target FLS that in RA fail to switch off (75). Moreover, through the GEO database, seven central genes (including IL-6) were identified, which are primarily associated with immune inflammation (76). Further experiments revealed that the inhibition of IL-6 significantly decreased the degree of FLS senescence. These findings demonstrated that RA-FLS senescence may promote RA development via inflammatory and immune mechanisms.
3.1.5 Immune cell senescence
Many other senescent immune cells were found in RA lesions, contributing to the development or progression of RA. For example, it has been recently suggested that chronic inflammation-induced senescence impairs immunomodulatory properties of synovial fluid MSCs in RA, such as stemness, proliferation, cellular senescence, in-vitro differentiation, and in-vivo immunomodulatory properties (77). A previous study has proven that CD56(+) T cells with features of senescence in RA contribute to maladaptive immune responses, indicating that CD56(+) T cells are potential targets for therapy (78).
3.2 OA and cellular senescence
3.2.1 Pathogenesis overview
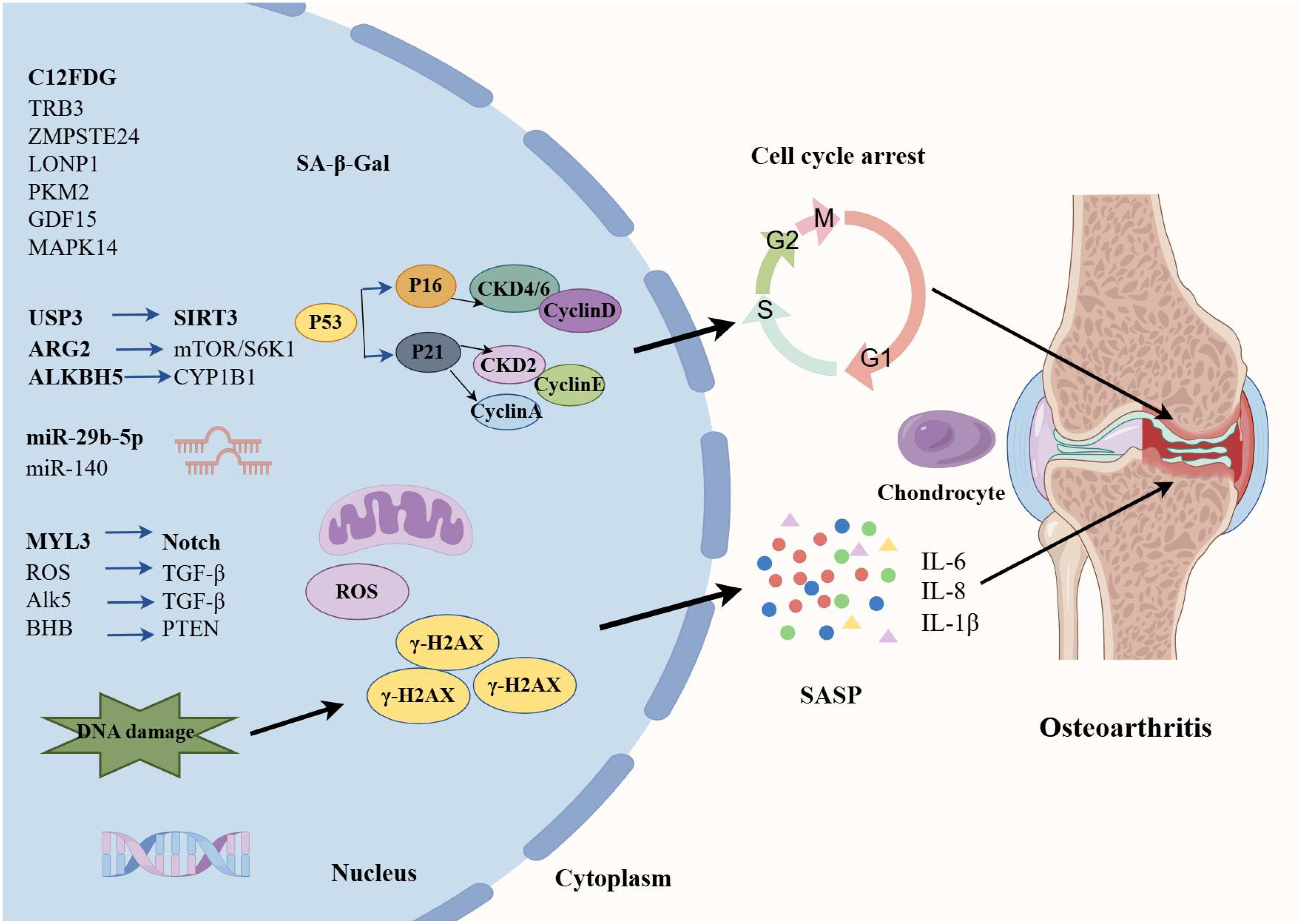
OA is a chronic inflammatory disease closely related to cartilage degeneration characterized by joint pain, stiffness, joint swelling, and deformity, which significantly affect the quality of life of patients (79). OA has a complex pathogenesis involving multiple factors. Senescence is one of the primary causes of OA (80, 81) (Figure 3). As age advances, articular cartilage gradually degenerates, and the function of chondrocytes declines, leading to wear and destruction of cartilage. Additionally, obesity is also a significant risk factor for OA (82). Excessive body weight increases the burden on joints, especially the knee joint, thereby accelerating the degenerative process of cartilage. Joint injury is also a significant factor in the development of OA. Mechanical damage to joints can trigger local inflammatory responses, leading to further cartilage damage and degeneration (83).
3.2.2 Senescence-associated secretory phenotype
The senescent cells increase the expression of cytokines, namely SASP (such as IL-β, IL-6, and TNF-α (84)), chemokines (such as MCP-1 and IL-8), cell adhesion molecules (such as E-selectin, ICAM-1, and VCAM-1), and MMP (such as MMP-2). A study about chondrogenic progenitor cells (CPCs) in OA has revealed that OA-CPCs exhibit elevated ROS levels along with a relatively high percentage of senescent cells, and senescence in OA-CPCs is mediated by the release of pro-inflammatory cytokines (such as IL-6 and IL-8) (85).
3.2.3 Diagnostic biomarkers
With the advancement of cellular and molecular sciences, various molecules have been identified as cellular senescence-related molecules in OA, providing promising biomarkers for early diagnosis of OA and monitoring of OA progression and efficacy of treatments. For example, C12FDG+ PBMCs and T cells are elevated in OA patients, which may provide additional quantifiable clinical tools to assess healthy senescence and potential indicators for acute and chronic senescence-associated diseases in OA (86). Autophagy is a metabolic process that degrades damaged cells and proteins, and the defects in autophagy are closely associated with senescence (87). A previous study has evaluated the potential involvement of TRB3 in cartilage autophagy and cellular senescence in OA (88). They have concluded that TRB3 knockdown significantly rescues the reduced autophagy and elevated cell senescence in human chondrocytes. Therefore, interfering with TRB3 expression in cartilage may serve as a target for the prevention and treatment of OA cellular senescence. The deficiency of ZMPSTE24 could induce Hutchinson-Gilford progeria syndrome (HGPS), and it has been found that the expression of ZMPSTE24 is decreased in the articular cartilage of OA (89). Further transcriptome sequencing resulted in the deletion of ZMPSTE24 or affects chondrocyte metabolism, inhibits cell proliferation, and promotes cell senescence.
3.2.4 Mitochondrial dysfunction
Mitochondrial dysfunction is strongly implicated in the cellular senescence mechanism, which is considered to be the culprit of cell senescence. Additionally, changes in mitochondrial function during senescence are considered to have a controlling role in cell fate (90, 91). A study has found that the expression of Lon protease 1 (LONP1), a mitochondrial protease, is decreased in human OA cartilage and senescent rat chondrocytes (92). LONP1 knockdown causes the imbalance of oxidative stress, metabolic changes, and mitophagy, leading to downstream MAPK pathway activation. These findings demonstrate that LONP1 is a central regulator of mitochondrial function in chondrocytes; downregulation of LONP1 with cellular senescence contributes to OA. Pyruvate kinase isoform M2 (PKM2) is one of the isoenzymes of pyruvate kinase and a key glycolytic enzyme. The role of PKM2 in senescence has also attracted significant attention. A recent study has indicated that the glycolytic enzyme PMK2 modulates chondrocyte senescence in OA but does not participate in the regulation of inflammation (93). MAPK12 and FOS have attracted widespread attention as potential senescence-related biomarkers. Through the analysis of OA-related datasets, researchers have identified significant expression changes in these two genes in the cartilage tissue of OA patients. Specifically, overexpression of MAPK12 and downregulation of FOS can promote cell proliferation and chondrocyte synthesis metabolism, and inhibit cell senescence and chondrocyte degradation metabolism, thereby alleviating the symptoms of OA (94). In addition, Weng et al. have shown that the GDF15/MAPK14 axis is a driver of senescent chondrocytes and can contribute to OA progression by inducing angiogenesis (95).
3.2.5 Epigenetic regulation
microRNA (miR) expression involving epigenetic changes is one of the hallmarks of cellular senescence (96). Recently, it has been reported that senescence‐associated miR‐140 can effectively attenuate the progression of early-stage OA by retarding chondrocyte senescence (97). Specifically, pre-transfection with miR-140 could effectively inhibit the expression of cell senescence biomarkers (SA-β-Gal, p16INK4a, p21, p53, and γH2AX). Taken together, this study contributed new evidence for the involvement of miR-mediated epigenetic regulation of chondrocyte senescence in OA pathogenesis. Elsewhere, an experimental study has found that cellular senescence-related miRNA, miR-29b-5p, is markedly downregulated in OA cartilage, and their upregulation suppresses the expression of matrix metalloproteinases and senescence-associated genes (P16INK4a/P21) via TET1 (98).
Cellular senescence is characterized by various epigenetic changes, of which the mechanisms are mainly protein modification, such as acetylation, ubiquitination, and lactylation (99). m6A is a common RNA modification that plays an important role in regulating cell biological functions, and ALKBH5 is one of the key m6A demethylases (100). Recent research has demonstrated that downregulation of ALKBH5 expression facilitates MSC senescence by enhancing the stability of CYP1B1 mRNA and inducing mitochondrial dysfunction, revealing a novel mechanism of m6A in MSC senescence (101). Ubiquitination is a post-translational modification and a fundamental mechanism of proteostasis; ubiquitin-specific protease 3 (USP3) serves as a deubiquitinating enzyme to upregulate SIRT3 expression. A previous study has demonstrated that USP3 probably attenuates IL-1β-mediated chondrocyte senescence by deacetylating FOXO3 through SIRT3 (102). Protein lactylation, a novel PTM, is a new contributor to the epigenetic landscape and governs diverse physiological conditions (103). As has been evidenced previously, lactate accumulates in the pathological environment of OA, significantly upregulating the expression of arginase 2 (ARG2) and promoting the senescence of OA-FLS by activating the mTOR/S6K1 signaling pathway (104). This senescence is characterized by features such as G1/S phase arrest, increased ROS, and β-galactosidase production. These results suggested that the ARG2-mTOR/S6K1 axis plays an important role in lactate-induced OA-FLS senescence and that regulating this signaling pathway can provide new targets and therapeutic strategies for treating OA and other senescence-related diseases.
3.2.6 Main signaling pathways
Various signaling pathways participate in the cellular senescence process. For example, it has been shown that the Notch signaling pathway plays a crucial role in cell fate determination (105). In the Notch signaling process, the endocytosis mediated by clathrin is an important regulatory step. A previous study has shown that the absence of MYL3 leads to enhanced iridium-mediated endocytosis, which further promotes Notch internalization and activation of the Notch signaling (106), thereby accelerating the cellular senescence process of OA chondrocytes. Moreover, SOX4 acts as a key transcription factor in the senescence process of OA- FLS. It has been shown that the activation of SOX4 promotes cellular senescence and the formation of SASP in OA-FLS, a process regulated by the ROS/TGF-β signaling (107). Therefore, in-depth research on the regulatory mechanisms and functions of SOX4 in cellular senescence is expected to provide new insights and targets for the treatment of diseases (such as OA). Another study has determined that the TGF-β/Alk5 signaling prevents OA initiation by regulating the senescence of articular cartilage stem cells (108). Similarly, it has been found that β-hydroxybutyrate (BHB) exhibits anti-senescence effects in various cellular senescence-related diseases. A study has shown that BHB could alleviate the senescence of OA-Chos and slow down the progression of OA (109). GO enrichment analysis revealed significant changes in cell cycle genes, with PTEN being the most significantly differentially expressed gene. BHB promotes PTEN expression by binding to hnRNP A1 and inhibiting Akt phosphorylation, thereby alleviating the senescence of chondrocytes.
3.2.7 Autophagy-senescence crosstalk
Autophagy plays a crucial role in cellular senescence, especially under oxidative stress conditions (110, 111). Oxidative stress can influence cellular autophagy activities through multiple pathways, thereby affecting the senescence process of cells (112). A previous study has shown that acid-sensitive ion channel 1a (ASIC1a) participates in acid-induced chondrocyte autophagy, and also inhibits senescence-related markers, suggesting that ASIC1a may be involved in acid-induced OA articular chondrocyte senescence by activating autophagy (113). A study has directly compared the different effects of oxidative and inflammatory stresses on the senescence of rat chondrocytes, and they have found that intra-articular injections of H2O2 cause no change in senescent markers; however, IL/TNF injections increase the proportion of p16- and SASP factor-positive OA chondrocytes (114).
3.2.8 Comparison of immunosenescence between RA and OA
RA and OA are two distinct joint disorders that differ significantly in etiology, pathogenesis, and immunological characteristics, especially in the context of immunosenescence. RA is an autoimmune disease marked by systemic immune activation, involving aberrant lymphocyte responses, persistent inflammation, and features of accelerated immunosenescence (such as the expansion of senescent T cells, TS, and excessive production of SASP factors). In contrast, OA is primarily a degenerative joint disease characterized by localized cartilage degradation and comparatively mild immune involvement, whereas low-grade inflammation is mainly mediated by macrophages and selected cytokines. Despite these differences, both RA and OA share some overlapping features, including infiltration of immune cells (such as macrophages and T cells) into affected joints, and engagement of common inflammatory pathways. However, the magnitude and nature of immune responses differ significantly, with RA involving more pronounced lymphocyte activation and systemic immune dysfunction. It’s critical to understand these distinctions, as OA is often used as a comparative model in RA studies. Clarifying the shared and unique aspects of immunosenescence in these diseases provides deeper insights into their respective pathophysiologies and may aid in identifying disease-specific therapeutic targets.
3.3 SLE and cellular senescence
3.3.1 Disease overview and pathogenic mechanisms
SLE is a systemic autoimmune disease with various pathogenic factors and involvement of multiple organs, as well as diverse clinical manifestations and a relapsing and remitting course, among which the kidney is one of the most commonly involved organs (115). SLE predominantly affects young women, and its pathogenesis involves various factors, including genetic predispositions, environmental triggers (such as ultraviolet light), and immune system abnormalities (116). SLE patients have various autoantibodies in their bodies, which not only target nuclear antigens but may also target other cellular components. These autoantibodies bind to antigens to form immune complexes, which deposit in tissues and trigger inflammatory responses and tissue damage (117). The complement system also plays a crucial role in the pathogenesis of SLE. Deficiencies in the early components of the complement system can lead to improper clearance of immune complexes and apoptotic cell debris, thereby promoting the onset of SLE. Additionally, excessive activation of the complement system may also result in organ damage (118). Moreover, cellular senescence plays a crucial role in the pathogenesis of SLE (119). During the natural course of SLE, the ongoing process of chronic immune activation and pro-inflammatory phenotypes can lead to immunosenescence (120). Studies have shown that premature senescence of immune cells occurs in SLE patients, which may be associated with mitochondrial dysfunction, abnormal immune metabolism, and telomere/telomerase imbalance. For example, in a cohort study by Sahena et al., it has been concluded that TL is shortened in SLE patients, which is significantly associated with Ro antibody positivity (121). Therefore, in-depth research on the mechanisms of cellular senescence in SLE is not only helpful for understanding its pathogenesis but may also provide clues for developing new therapeutic strategies.
3.3.2 T cell senescence and dysfunction
The activation of T cells plays a crucial role in the pathogenesis of SLE. Studies have shown that there are abnormalities in T cell signaling in SLE patients, which can lead to hyperactivation of T cells and disruption of self-tolerance, thereby promoting disease progression (122). Additionally, the number of Th17 cells increases while that of regulatory T cells (Tregs) decreases in SLE patients, and this imbalance between Th17/Tregs is also considered an important factor in the pathogenesis of SLE (123). The CD57 antigen and killer cell lectin-like receptor G1 (KLRG1) are markers commonly defined by the terminally differentiated T cells, which are also senescence markers. As has been evidenced previously, SLE patients show notably higher senescence T cell markers compared to the controls, and the increase of T cell senescence, especially in the CD8 compartment, is correlated with the disease activity and severity of SLE (124). The other two studies have respectively mentioned that T cellular senescence is related to early arteriosclerosis and angiogenesis in SLE. A study has shown that T cell cellular senescence markers (such as CD4+CD57+, CD8+CD57+, CD4+CD28null, and CD8+CD28null) are also significantly higher in SLE patients, showing a significant positive correlation with the level of atherosclerotic markers (cIMT, FMD, and soluble adhesion molecules) (125). These results suggested that T cell cellular senescence may be a significant factor in early atherosclerosis in SLE patients. Another study has revealed the existence of an aberrant CD28null-angiogenic T cell population in SLE patients, which is characterized by a senescent and cytotoxic phenotype, and is associated with anti-dsDNA titer and increased production of pro-inflammatory mediators (126). Wang et al. have shown that the frequency of CD4+CD57+ senescent T cells is notably elevated and is positively correlated with disease activity, indicating that CD4+CD57+ senescent CD4+ T cells play a dominant role in the pathogenesis of SLE and hold promise for developing a potential treatment for ameliorating lupus phenotypes (127). Induction of senescence in T cells in SLE is a potential therapeutic approach. SIRT1 is a NAD-dependent deacetylase that protects against cell senescence by deacetylating p53. It has been shown that miR-199a-5p can promote the senescence of CD4+ T cells in the spleen by regulating the SIRT1/p53 pathway, thereby alleviating lupus symptoms in MRL/lpr mice. The core of this mechanism lies in the crucial role of the SIRT1/p53 pathway in cellular senescence and immune regulation (128). These findings provide new insights and potential targets for the treatment of SLE.
3.3.3 MSC senescence
MSCs have shown significant potential in the treatment of SLE and other autoimmune diseases, especially in cellular senescence terms (129). Studies have demonstrated that MSCs can modulate the immune system through various mechanisms, thereby suppressing the autoimmune response in SLE patients. Firstly, MSCs possess potent immune regulatory capabilities, as they can reduce the production of pro-inflammatory cytokines by inhibiting the proliferation of T cells and B cells, thus alleviating inflammatory responses. Additionally, MSCs can induce the generation of regulatory Tregs and restore immune balance, thereby mitigating the pathological progression of SLE. In clinical application, the transplantation of MSCs has been proven to improve renal function and other organ damage in SLE patients (130). Chen et al. have identified that TK1 expression is remarkably elevated in SLE bone marrow-derived MSCs (BM-MSCs), and silencing TK1 could alleviate inflammation, growth arrest, and senescence in BM-MSCs of SLE, highlighting TK1’s considerable promise as a therapeutic target against SLE (131). The Wnt/β-catenin signaling plays an important role in stem cell senescence. A study has also demonstrated that the Wnt/β-catenin signaling may play a critical role in the senescence of SLE BM-MSCs through the p53/p21 pathway (132). Therefore, elucidating the mechanisms of the Wnt/β-catenin signaling involved in the senescence of SLE BM-MSCs will help improve the transplantation efficacy of BM-MSCs in SLE. Furthermore, another study has underscored the pivotal role of the JAK-STAT signaling in regulating the senescence of BM-MSCs in SLE (133). They have found that AG490, the inhibitor of JAK2, and knockdown of STAT3 in BM-MSCs, could significantly reverse the senescence. Interestingly, the PI3K/AKT signaling also emerges as a key factor in the implementation of cellular senescence. It has been discovered that leptin and neutrophil-activating peptide 2 (NAP-2) act synergistically to promote MSC senescence through enhancement of the PI3K/Akt signaling pathway in SLE patients. Additionally, the mTOR pathways and inhibition of the mTOR signaling pathways of rapamycin, are closely related to SLE cellular senescence (134). Gu et al. have demonstrated that RAPA reverses the senescent phenotype and improves immunoregulation of MSCs in MRL/lpr mice and SLE patients through inhibition of the mTOR signaling pathway (135). Additionally, the role of the IFNβ signaling in systemic SLE is increasingly recognized, especially in cellular senescence. It has been evidenced that IFNβ signaling forms a positive feedback loop with mitochondrial antiviral signaling (MAVS), a mechanism that plays a crucial role in the senescence phenotype of SLE BM-MSCs (136). These findings suggest that the IFNβ signaling pathway may become a new target for SLE treatment. The activation of endoplasmic reticulum stress (ERS) is involved in the growth arrest in the G1 phase of the cell cycle. Research has revealed that ERS-mediated senescence is a critical determinant of BM-MSCs in SLE patients (137).
3.3.4 Organ-specific senescence manifestations
SLE can lead to damage to multiple organs and systems, such as lupus nephritis and lupus encephalopathy. Neuropsychiatric manifestations (NP) are common in SLE patients, which is closely related to cellular senescence (138). A previous study has considered that SLE patients develop an accelerated immunosenescence which contributes to cognitive dysfunction, especially in attention, recall, and visuospatial domains (136). Lupus nephritis is characterized by inflammation in the kidney, proteinuria, and progressive kidney damage. Hence, there is a need to better understand the mechanisms underlying disease progression. The increase in p16INK4a-positive cells has been shown to be closely related to renal fibrosis and CD8+ T cell infiltration, suggesting that cellular senescence may play a crucial role in the pathogenesis of LN (139).
3.4 AS and cellular senescence
AS is a chronic inflammatory disease primarily characterized by sacroiliac joint and spinal erosion. Patients may experience joint pain, stiffness, and limited range of motion, and in severe cases, spinal deformity and joint fusion (140). Moreover, AS is strongly associated with human leukocyte antigen B27, which predominantly affects young and middle-aged males (141). Currently, there is no specific curative medication for AS, and biological agents are the primary drugs for AS treatment. However, these biologics are often expensive and may have numerous side effects. The introduction of multiple biologics targeting TNF-α and IL-17 marks a significant advancement in the management of AS, providing relief for many patients who have not responded to conventional therapies (such as NSAIDs and physiotherapy) (142, 143). Despite their effectiveness, biologics drugs still have some limitations. Hence, it’s necessary to explore alternative therapies.
It has been pointed out that oxidative stress exists in the serum environment of AS patients, which can lead to the senescence of MSCs. Oxidative stress promotes the senescence of MSCs by inducing mitochondrial dysfunction and excessive ROS production (144). This process not only exacerbates the decline in cell function but may also trigger inflammatory responses, thereby contributing to the pathological progression of AS. WNT16 is critical for bone homeostasis, which is pivotal for the differentiation and bone formation of osteoblasts. It has been revealed that WNT16 is highly expressed in AS, and WNT16 treatment facilitates cellular senescence in AS-osteoprogenitor cells during osteoblast differentiation accompanied by suppression of bone formation (145). In addition, premature senescence of T-cell subsets in axial spondyloarthritis has been demonstrated in a prospective study (146). Specifically, this study demonstrates an age-inappropriate shrinkage of thymic output, and an inappropriate shortening of telomeres in young patients with aSpA. All the above studies evidence that cellular senescence is closely related to the development of AS.
3.5 SS and cellular senescence
SS is a systemic autoimmune disease characterized by vasculopathy, inflammation, and fibrosis of the skin and internal organs, often resulting in severe disability and high mortality (147). The pathogenesis of SS is complex, and many mechanisms have not yet been clarified. Although it is inappropriate to consider SS as a disease of senescence, the possible role of cellular senescence in SS pathogenesis should be considered as an important factor (148). There is strong evidence that inflammation and oxidative stress may be involved in this process (149, 150).
Accumulating studies have indicated that the alterations in TL play a role in the development of various autoimmune diseases. There are very few studies on TL in SS. Artlett et al. have found that the average loss of telomeric DNA in SS patients and family members is 3 kb; however, this loss is not related to age, but is caused by chromosomal instability (151). In another study, Tarhan et al. have demonstrated a very low telomerase activity in SS compared with that in other connective tissue diseases. These results indicated that the important role of cellular senescence in SS has been emerging (152). A cross-sectional study supports the significant association between cellular senescence and SS; they have found that endothelial-to-mesenchymal transition (EndMT) and fibroblast senescence are more abundant in skin biopsies of SSc patients. Specifically, cellular senescence may accelerate the fibrosis process by promoting the activation of fibroblasts and excessive deposition of extracellular matrix (ECM) (153). These findings indicate that both senescence and EndMT are involved in the pathway leading to skin fibrosis in SS, and may be valuable biomarkers and/or possible targets for novel therapeutic interventions.
ILD is the most common pulmonary manifestation in SS patients and is the most frequent cause of SSc disease-related death (154). A recent gene expression meta-analysis has revealed cellular senescence signatures in SS-ILD (155). They have suggested that markers (GDF15, COMP, and CDKN2A) and pathways (p53) of senescence are significantly increased in SS-ILD; moreover, TL in SSc-ILD is comparable to idiopathic pulmonary fibrosis. This study represents an important step forward toward a better understanding of the cellular senescence association of SS, particularly with lung involvement. In SS patients, MSCs not only maintain their pluripotency and proliferative capacity but also normally induce regulatory Tregs with functional phenotypes. Tregs play crucial roles in maintaining immune tolerance and suppressing excessive immune responses, which is of great significance for cell-based therapeutic strategies. As previously revealed, SS-MSCs show an increase in senescence biomarkers; moreover, the increased activation of the IL-6 pathway observed in our cells may represent an adaptive mechanism to senescence, while preserving Tregs functions, including immunosuppression (156).
4 Treatment strategy for rheumatic diseases from the perspective of cellular senescence
In recent years, therapeutic strategies targeting cellular senescence have become a focal point in anti-senescence research, including drugs that selectively clear senescent cells (known as senolytics) and drugs that inhibit SASP and other senescent markers (known as senomorphics). In preclinical studies, senolytics have shown significant potential. For example, research has found that senolytics can induce apoptosis in senescent cells by inhibiting specific proteasome pathways, thereby reducing SASP secretion and improving tissue function (157). Additionally, researchers have developed senolytic prodrugs that can be specifically activated in vivo through interaction with senescence biomarkers, thereby reducing drug side effects and enhancing therapeutic efficacy (158).
Phoenixin-20 (PNX-20) is a peptide targeting G-protein-coupled receptor 173 (GPR173) with promising anti-inflammatory properties. A recent study has found that PNX-20 protects the TNF-α-induced cell senescence in RA-FLSs by downregulating STAT6, suggesting that PNX-20 may be a promising agent for the treatment of RA (159) (Table 2). Apremilast is a phosphodiesterase 4 (PDE4) inhibitor that has therapeutic potential in various inflammatory diseases. SIRT1 is an important deacetylase that plays a crucial role in cellular senescence and inflammatory responses (160). In this study, Apremilast has been found to prevent IL-17-induced cellular senescence in ATDC5 chondrocytes by modulating the SIRT1 pathway, which provides a new idea and potential therapeutic strategy for the treatment of cartilage degeneration-related diseases (161). Navitoclax (ABT263) is a well-known anti-senescence drug that can alleviate symptoms of OA by selectively eliminating senescent cells (162). Using Navitoclax to clear these senescent cells can effectively reduce the levels of inflammatory mediators, decrease the activity of matrix-degrading enzymes, and protect cartilage tissue. Therefore, as a potential therapeutic approach, Navitoclax can alleviate symptoms of OA and promote the regeneration and repair of cartilage by targeting senescent cells.
Traditional Chinese medicine (TCM) has a multi-pathway, multi-target, and multi-level mechanism of action in treating senescence, which makes it highly regarded in anti-senescence research (163). Importantly, TCM can effectively delay the senescence process by modulating some signaling pathways (such as AMPK/mTOR, FOXO, and SIRT1). These signaling pathways play crucial roles in cellular energy metabolism, oxidative stress response, and autophagy, thereby improving the overall senescence state of the organism. Huangqin Qingre Chubi Capsules (HQC), a Chinese patent medicine prepared by the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, strengthens the spleen and resolves dampness, and clears heat and unblocks collaterals (164). The latest research has suggested that HQC modulates SASP factors by regulating the STAG1/TP53/P21 signal transduction axis and decelerating cartilage senescence and degradation in OA patients (165). A previous study has also demonstrated that HQC delays chondrocyte senescence in OA rats, and inhibits inflammatory response and ECM degradation by inhibiting the p53/p21 pathway in experimental animals (166). Quzhi Tang (QZT) is a compound formula composed of six TCM herbs, which has achieved good clinical outcomes in treating KOA. A previous study has shown that QZT can regulate the GPNMB/Nrf2/NF-κB signaling pathway, repair mitochondrial damage, and improve the senescence process of chondrocytes (167). The in-vivo experiments have demonstrated that interfering with GPNMB expression can reduce mitochondrial dysfunction and cellular senescence while increasing Nrf2 expression and decreasing NF-κB expression levels. Treatment with QZT can increase Nrf2 expression, decrease NF-κB expression, repair mitochondrial damage, and improve the degree of chondrocyte senescence. Fruitauin A (FTA) is a phenylethanolide extracted from the fruit of Forsythia suspensa, exhibiting significant anti-inflammatory and antioxidant properties. Furthermore, the in-vitro experiments have revealed that FTA improves mitochondrial function, enhances mitochondrial autophagy, inhibits the activation of NLRP3 inflammatory bodies, and suppresses chondrocyte senescence. Additionally, this study has found that FTA directly binds to Nrf2 and enhances its phosphorylation through PKC (168). Ubiquitin-fold modifier 1-specific E3 ligase 1 (UFL1) has been identified as a novel target of panaxatriol for anti-senescence effects. A previous study has shown that panaxatriol inhibits chondrocyte senescence through the UFL1/forkhead box O1 (FOXO1)/P21 and UFL1/NF-κB/SASPs signaling pathways (169).
Despite the promising performance of these therapeutic strategies in preclinical studies, there are still some challenges in clinical application. Researchers are exploring how to translate these small molecule senolytics and other anti-senescence interventions into clinical practice to play a preventive or therapeutic role in rheumatic diseases.
5 Conclusions and prospects
Overall, this review explored the link between cellular senescence and rheumatic diseases. Despite significant efforts to develop effective treatments for rheumatic diseases, a definitive solution remains elusive. The review highlighted the importance of understanding cellular senescence, senescence-associated secretomes, and their interactions with rheumatic diseases. Targeting cellular senescence has emerged as a significant strategy in the treatment of rheumatic diseases. By reducing the burden of senescent cells, senolytics can mitigate the inflammatory environment and improve tissue function, offering a novel therapeutic avenue for managing rheumatic diseases. However, there are still some limitations, which are elaborated as follows.
First, the lack of specific markers for cellular senescence is a major obstacle. Although some studies have identified some potential markers (such as p16INK4a and β-galactosidase), they are not always specific and may behave differently in different cell types and tissues. Furthermore, the heterogeneity of senescent cells increases the complexity of identification, as different cell types may exhibit distinct characteristics and functions during the senescence process.
Second, the senescent cells may negatively impact the microenvironment of surrounding tissues by secreting pro-inflammatory cytokines and other factors, thereby exacerbating disease progression. Therefore, developing technologies and strategies that can effectively identify and eliminate senescent cells is crucial for slowing the progression of cellular senescence-related diseases. In short, despite some progress in the study of cellular senescence, further exploration and development of specific markers and recognition techniques are needed to better understand the role of senescent cells in physiological and pathological processes and provide new ideas for the treatment of rheumatic diseases.
Third, the challenges in applying nanotechnology to cellular senescence are multifaceted. One major challenge is the need for precise targeting of senescent cells without affecting normal cells. This requires the development of highly specific nanocarriers that can recognize and bind to markers unique to senescent cells. Additionally, the safety and biocompatibility of nanomaterials must be thoroughly evaluated to prevent adverse effects. Additionally, nanoparticle-induced inflammatory responses (such as generation of ROS and activation of the NF-κB signaling pathway) need to be carefully managed to avoid exacerbating cellular damage. Despite these challenges, the potential benefits of nanotechnology in combating cellular senescence are significant. By improving the delivery and efficacy of anti-senescence therapies, nanotechnology could play a crucial role in preventing rheumatic diseases.
An increasing number of studies have provided promising indications of a causal link between cellular senescence and the onset and progression of rheumatic diseases. However, it is crucial to expand research efforts in both basic science and clinical applications to unlock the full potential of targeting senescent cells.
Author contributions
JW: Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing. JL: Funding acquisition, Supervision, Writing – review & editing. LW: Methodology, Resources, Investigation, Writing – review & editing. FW: Data curation, Investigation, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Clinical Research Project of Anhui University of Traditional Chinese Medicine in 2024 (2024YFYLCZX09) and National Key Discipline of Traditional Chinese Medicine - Traditional Chinese Medicine Bi Disease ((2023) No. 85).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Finckh A and Deane KD. Prevention of rheumatic diseases: strategies, caveats, and future directions. Rheum Dis Clin North Am. (2014) 40:771–85. doi: 10.1016/j.rdc.2014.07.010
2. Chen W, Liu D, Li Q-Z, Li Q-Z, and Zhu H. The function of ncRNAs in rheumatic diseases. Epigenomics. (2019) 11:821–33. doi: 10.2217/epi-2018-0135
3. Ouardi NE, Djossou JH, Taoubane L, Ghassem MA, Toufik H, Majjad A, et al. Extra-articular manifestations in patients with ankylosing spondylitis: baseline characteristics from the RBSMR study. Mediterr J Rheumatol. (2022) 33:316–21. doi: 10.31138/mjr.33.3.316
4. Conforti A, Di Cola I, Pavlych V, Ruscitti P, Berardicurti O, Ursini F, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev. (2021) 20:102735. doi: 10.1016/j.autrev.2020.102735
5. Villalobos-Sánchez L, Blanco-Cáceres B, Bachiller-Corral J, Rodríguez-Serrano MT, Vázquez-Díaz M, and Lázaro de Mercado Y P. Quality of life of patients with rheumatic diseases. Reumatol Clin (Engl Ed). (2024) 20:59–66. doi: 10.1016/j.reuma.2023.06.004
6. Ristic B, Carletto A, Fracassi E, Pacenza G, Zanetti G, Pistillo F, et al. Comparison and potential determinants of health-related quality of life among rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis: A cross-sectional study. J Psychosom Res. (2023) 175:111512. doi: 10.1016/j.jpsychores.2023.111512
7. Newgard Christopher B and Sharpless Norman E. Coming of age: molecular drivers of aging and therapeutic opportunities. J Clin Invest. (2013) 123:946–50. doi: 10.1172/JCI68833
8. Childs Bennett G, Durik Matej, Baker Darren J, and van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. (2015) 21:1424–35. doi: 10.1038/nm.4000
9. Joosten Leo AB, Abdollahi-Roodsaz S, Dinarello Charles A, Dinarello CA, O'Neill L, and Netea MG. Toll-like receptors and chronic inflammation in rheumatic diseases: new developments. Nat Rev Rheumatol. (2016) 12:344–57. doi: 10.1038/nrrheum.2016.61
10. Bektas A, Schurman Shepherd H, Sen R, and Ferrucci L. Aging, inflammation and the environment. Exp Gerontol. (2018) 105:10–8. doi: 10.1016/j.exger.2017.12.015
11. Cavanagh Mary M, Weyand Cornelia M, and Goronzy Jörg J. Chronic inflammation and aging: DNA damage tips the balance. Curr Opin Immunol. (2012) 24:488–93. doi: 10.1016/j.coi.2012.04.003
12. Del Pinto R and Ferri C. Inflammation-accelerated senescence and the cardiovascular system: mechanisms and perspectives. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19123701
13. Han Z, Wang K, Ding S, and Zhang M. Cross-talk of inflammation and cellular senescence: a new insight into the occurrence and progression of osteoarthritis. Bone Res. (2024) 12:69. doi: 10.1038/s41413-024-00375-z
14. Sarkar D and Fisher Paul B. Molecular mechanisms of aging-associated inflammation. Cancer Lett. (2006) 236:13–23. doi: 10.1016/j.canlet.2005.04.009
15. Fang C-L, Liu B, and Wan M. Bone-SASP" in skeletal aging. Calcif Tissue Int. (2023) 113:68–82. doi: 10.1007/s00223-023-01100-4
16. Hayflick L and Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. (1961) 25:585–621. doi: 10.1016/0014-4827(61)90192-6
17. López-Otín C, Blasco Maria A, Partridge L, Partridge L, Serrano M, and Kroemer G. The hallmarks of aging. Cell. (2013) 153:1194–217. doi: 10.1016/j.cell.2013.05.039
18. López-Otín C, Blasco Maria A, Partridge L, Partridge L, Serrano M, and Kroemer G. Hallmarks of aging: An expanding universe. Cell. (2023) 186:243–78. doi: 10.1016/j.cell.2022.11.001
19. Shmulevich R and Krizhanovsky V. Cell senescence, DNA damage, and metabolism. Antioxid Redox Signal. (2021) 34:324–34. doi: 10.1089/ars.2020.8043
20. Kirkland James L and Tchkonia T. Cellular senescence: A translational perspective. EBioMedicine. (2017) 21:21–8. doi: 10.1016/j.ebiom.2017.04.013
21. Sapieha P and Mallette Frédérick A. Cellular senescence in postmitotic cells: beyond growth arrest. Trends Cell Biol. (2018) 28:595–607. doi: 10.1016/j.tcb.2018.03.003
22. Bernadotte A, Mikhelson Victor M, and Spivak Irina M. Markers of cellular senescence. Telomere shortening as A marker Cell senescence Aging (Albany NY). (2016) 8:3–11. doi: 10.18632/aging.100871
23. Panda Amaresh C, Abdelmohsen K, and Gorospe M. SASP regulation by noncoding RNA. Mech Ageing Dev. (2017) 168:37–43. doi: 10.1016/j.mad.2017.05.004
24. Sławińska N and Krupa R. Molecular aspects of senescence and organismal ageing-DNA damage response, telomeres, inflammation and chromatin. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22020590
25. Suryadevara V, Hudgins Adam D, Rajesh A, Pappalardo A, Karpova A, Dey AK, et al. SenNet recommendations for detecting senescent cells in different tissues. Nat Rev Mol Cell Biol. (2024) 25:1001–23. doi: 10.1038/s41580-024-00738-8
26. Valieva Y, Ivanova E, Fayzullin A, Kurkov A, and Igrunkova A. Senescence-associated β-galactosidase detection in pathology. Diagnostics (Basel). (2022) 12. doi: 10.3390/diagnostics12102309
27. de Mera-Rodríguez José A, Álvarez-Hernán G, Gañán Y, Martín-Partido G, Rodríguez-León J, and Francisco-Morcillo J. Is senescence-associated β-galactosidase a reliable in vivo marker of cellular senescence during embryonic development? Front Cell Dev Biol. (2021) 9:623175. doi: 10.3389/fcell.2021.623175
28. Siddiqui MS, François M, Fenech Michael F, and Leifert WR. Persistent γH2AX: A promising molecular marker of DNA damage and aging. Mutat Res Rev Mutat Res. (2015) 766:1–19. doi: 10.1016/j.mrrev.2015.07.001
29. Turinetto V and Giachino C. Multiple facets of histone variant H2AX: a DNA double-strand-break marker with several biological functions. Nucleic Acids Res. (2015) 43:2489–98. doi: 10.1093/nar/gkv061
30. Yoshioka K-I, Atsumi Y, Fukuda H, Masutani M, and Teraoka H. The quiescent cellular state is Arf/p53-dependent and associated with H2AX downregulation and genome stability. Int J Mol Sci. (2012) 13:6492–506. doi: 10.3390/ijms13056492
31. Corpet A and Stucki M. Chromatin maintenance and dynamics in senescence: a spotlight on SAHF formation and the epigenome of senescent cells. Chromosoma. (2014) 123:423–36. doi: 10.1007/s00412-014-0469-6
32. Olan I, Handa T, and Narita M. Beyond SAHF: An integrative view of chromatin compartmentalization during senescence. Curr Opin Cell Biol. (2023) 83:102206. doi: 10.1016/j.ceb.2023.102206
33. Tu Z and Aird Katherine M. Zhang Rugang, Chromatin remodeling, BRCA1, SAHF and cellular senescence. Cell Cycle. (2013) 12:1653–4. doi: 10.4161/cc.24986
34. Ohtani N, Yamakoshi K, Takahashi A, and Hara E. The p16INK4a-RB pathway: molecular link between cellular senescence and tumor suppression. J Med Invest. (2004) 51:146–53. doi: 10.2152/jmi.51.146
35. Bruce JL, Hurford RK, Classon M, Koh J, and Dyson N. Requirements for cell cycle arrest by p16INK4a. Mol Cell. (2000) 6:737–42. doi: 10.1016/S1097-2765(00)00072-1
36. Mijit M, Caracciolo V, Melillo A, Amicarelli F, and Giordano A. Role of p53 in the regulation of cellular senescence. Biomolecules. (2020) 10. doi: 10.3390/biom10030420
37. Rufini A, Tucci P, Celardo I, and Melino G. Senescence and aging: the critical roles of p53. Oncogene. (2013) 32:5129–43. doi: 10.1038/onc.2012.640
38. Yan J, Chen S, Yi Z, Zhao R, Zhu J, Ding S, et al. The role of p21 in cellular senescence and aging-related diseases. Mol Cells. (2024) 47:100113. doi: 10.1016/j.mocell.2024.100113
39. Javali Prashanth S, Sekar M, Kumar A, and Thirumurugan K. Dynamics of redox signaling in aging via autophagy, inflammation, and senescence. Biogerontology. (2023) 24:663–78. doi: 10.1007/s10522-023-10040-3
40. Ponnappan S and Ponnappan U. Aging and immune function: molecular mechanisms to interventions. Antioxid Redox Signal. (2011) 14:1551–85. doi: 10.1089/ars.2010.3228
41. Shive C and Pandiyan P. Inflammation, immune senescence, and dysregulated immune regulation in the elderly. Front Aging. (2022) 3:840827. doi: 10.3389/fragi.2022.840827
42. Chen C and Xu P. Cellular functions of cGAS-STING signaling. Trends Cell Biol. (2023) 33:630–48. doi: 10.1016/j.tcb.2022.11.001
43. Gasek Nathan S, Kuchel George A, Kirkland James L, and Xu M. Strategies for targeting senescent cells in human disease. Nat Aging. (2021) 1:870–9. doi: 10.1038/s43587-021-00121-8
44. Nousis L, Kanavaros P, and Barbouti A. Oxidative stress-induced cellular senescence: is labile iron the connecting link? Antioxidants (Basel). (2023) 12. doi: 10.3390/antiox12061250
45. Lettieri-Barbato D, Aquilano K, Punziano C, Punziano C, Minopoli G, and Faraonio R. MicroRNAs, long non-coding RNAs, and circular RNAs in the redox control of cell senescence. Antioxidants (Basel). (2022), 11(3). doi: 10.3390/antiox11030480
46. Zhou Z, Yin Y, Chang Q, Sun G, Lin J, and Dai Y. Downregulation of B-myb promotes senescence via the ROS-mediated p53/p21 pathway, in vascular endothelial cells. Cell Prolif. (2017) 50. doi: 10.1111/cpr.2017.50.issue-2
47. Yun UnJ, Park SE, Jo YS, Kim J, and Shin DY. DNA damage induces the IL-6/STAT3 signaling pathway, which has anti-senescence and growth-promoting functions in human tumors. Cancer Lett. (2012) 323:155–60. doi: 10.1016/j.canlet.2012.04.003
48. Qi Z, Yang W, Baibing X, Chen T, Lu X, Zhang R, et al. ROS-mediated lysosomal membrane permeabilization and autophagy inhibition regulate bleomycin-induced cellular senescence. Autophagy. (2024) 20:2000–16. doi: 10.1080/15548627.2024.2353548
49. Dong D-Y, Li P-Y, Wang Y-F, Wang P, Wu YH, Gao SG, et al. High glucose-increased miR-200c contributes to cellular senescence and DNA damage in neural stem cells. Birth Defects Res. (2023) 115:1770–9. doi: 10.1002/bdr2.v115.18
50. Ksiazek K, Breborowicz A, Jörres A, and Witowski J. Oxidative stress contributes to accelerated development of the senescent phenotype in human peritoneal mesothelial cells exposed to high glucose. Free Radic Biol Med. (2007) 42:636–41. doi: 10.1016/j.freeradbiomed.2006.12.002
51. Ning Y, Zhou X, Wang G, Zhang L, and Wang J. Exosome miR-30a-5p regulates glomerular endothelial cells' EndMT and angiogenesis by modulating notch1/VEGF signaling pathway. Curr Gene Ther. (2024) 24:159–77. doi: 10.2174/0115665232258527230919071328
52. Jin J, Gong J, Zhao Li, Li Y, and He Q. LncRNA Hoxb3os protects podocytes from high glucose-induced cell injury through autophagy dependent on the Akt-mTOR signaling pathway. Acta Biochim Pol. (2021) 68:619–25. doi: 10.18388/abp.2020_5483
53. Xiong Y, Wang H-X, Yan H, Zhu SL, Guo SW, Peng WJ, et al. Rutaecarpine prevents high glucose-induced endothelial cell senescence through transient receptor potential vanilloid subtype 1/ SIRT1 pathway. J Cardiovasc Pharmacol. (2022) 79:e129–37. doi: 10.1097/FJC.0000000000001166
54. Wang W, Li P, Xu J, Wu XK, Guo ZL, Fan LJ, et al. Resveratrol attenuates high glucose-induced nucleus pulposus cell apoptosis and senescence through activating the ROS-mediated PI3K/Akt pathway. Biosci Rep. (2018) 38. doi: 10.1042/BSR20171454
55. Quan Yi, Jiang C-t, Xue B, Zhu SG, and Wang X. High glucose stimulates TNFα and MCP-1 expression in rat microglia via ROS and NF-κB pathways. Acta Pharmacol Sin. (2011) 32:188–93. doi: 10.1038/aps.2010.174
56. Aletaha D and Smolen Josef S. Diagnosis and management of rheumatoid arthritis: A review. JAMA. (2018) 320:1360–72. doi: 10.1001/jama.2018.13103
57. Sahin D, Di Matteo A, and Emery P. Biomarkers in the diagnosis, prognosis and management of rheumatoid arthritis: A comprehensive review. Ann Clin Biochem. (2024) 62(1). doi: 10.1177/00045632241285843
58. Sun Y, Liu J, Xin L, Wen J, Zhou Q, Chen X, et al. Xinfeng capsule inhibits inflammation and oxidative stress in rheumatoid arthritis by up-regulating LINC00638 and activating Nrf2/HO-1 pathway. J Ethnopharmacol. (2023) 301:115839. doi: 10.1016/j.jep.2022.115839
59. Wen J, Liu J, Wan L, Jiang H, Xin L, Sun Y, et al. mA-mediated lncRNA MAPKAPK5-AS1 induces apoptosis and suppresses inflammation via regulating miR-146a-3p/SIRT1/NF-κB axis in rheumatoid arthritis. Cell Cycle. (2023) 22:2602–21. doi: 10.1080/15384101.2024.2302281
60. Wang F and Liu J. Regulating the lncRNA DSCR9/RPLP2/PI3K/AKT axis: an important mechanism of Xinfeng capsules in improving rheumatoid arthritis. Front Immunol. (2024) 15:1465442. doi: 10.3389/fimmu.2024.1465442
61. De Cauwer A, Mariotte A, Sibilia J, Bahram S, and Georgel P. DICER1: A key player in rheumatoid arthritis, at the crossroads of cellular stress, innate immunity, and chronic inflammation in aging. Front Immunol. (2018) 9:1647. doi: 10.3389/fimmu.2018.01647
62. Arellano MYG, VanHeest M, Emmadi S, Abdul-Hafez A, Ibrahim SA, Thiruvenkataramani RP, et al. Role of mesenchymal stem/stromal cells (MSCs) and MSC-derived extracellular vesicles (EVs) in prevention of telomere length shortening, cellular senescence, and accelerated biological aging. Bioengineering (Basel). (2024) 11. doi: 10.3390/bioengineering11060524
63. Svyryd Y, Pascual-Ramos V, Contreras-Yañez Irazú, Muñoz-Tellez LA, Luna-Muñoz L, López-Hernández MA, et al. Telomeres Length Variations in a Rheumatoid Arthritis Patients Cohort at Early Disease Onset and after Follow-Up. Rev Invest Clin. (2022) 74:202–11. doi: 10.24875/RIC.22000048
64. Doyle Tracy J, Juge P-A, Peljto Anna L, Lee S, Walts AD, Esposito AJ, et al. Short peripheral blood leukocyte telomere length in rheumatoid arthritis-interstitial lung disease. Thorax. (2024) 79:182–5. doi: 10.1136/thorax-2023-220022
65. Natalini Jake G, England Bryant R, Baker JF, Chen Q, Singh N, Mahajan TD, et al. Associations between shortened telomeres and rheumatoid arthritis-associated interstitial lung disease among U.S. Veterans. Respir Med. (2022) 201:106943. doi: 10.1016/j.rmed.2022.106943
66. Wacili Da, Lin T, and Yue Z. The role of osteoclast energy metabolism in the occurrence and development of osteoporosis. Front Endocrinol (Lausanne). (2021) 12:675385. doi: 10.3389/fendo.2021.675385
67. Arnett TR and Orriss IR. Metabolic properties of the osteoclast. Bone. (2017) 115:25–30. doi: 10.1016/j.bone.2017.12.021
68. Yudoh K, Matsuno H, Osada R, Nakazawa F, Katayama R, and Kimura T. Decreased cellular activity and replicative capacity of osteoblastic cells isolated from the periarticular bone of rheumatoid arthritis patients compared with osteoarthritis patients. Arthritis Rheum. (2000) 43:2178–88. doi: 10.1002/1529-0131(200010)43:10<2178::AID-ANR5>3.0.CO;2-Z
69. Satoshi K, Harumi K, Bernard P, Kawata K, Hattori T, and Nishida T. Cellular communication network factor 3 in cartilage development and maintenance. J Cell Commun Signal. (2021) 15:533–43. doi: 10.1007/s12079-021-00629-z
70. Miho K, Koichi K, Sei K, Fu S, Miyake Y, Ogo A, et al. CCN3 (NOV) drives degradative changes in aging articular cartilage. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21207556
71. Tokuhiro T, Matsumae G, Endo T, Ogawa Y, Ogawa T, Liyile C, et al. Cellular communication network factor 3 contributes to the pathological process of rheumatoid arthritis through promoting cell senescence and osteoclastogenesis in the joint. J Autoimmun. (2024) 149:103334. doi: 10.1016/j.jaut.2024.103334
72. Dorra EM, Wajih K, Nadia S, Tarhouni L, Rekik S, Jemmali S, et al. The synovial fluid fibroblast-like synoviocyte: A long-neglected piece in the puzzle of rheumatoid arthritis pathogenesis. Front Immunol. (2022) 13:942417. doi: 10.3389/fimmu.2022.942417
73. Falconer J, Murphy AN, Young SP, Clark AR, Tiziani S, Guma M, et al. Review: synovial cell metabolism and chronic inflammation in rheumatoid arthritis. Arthritis Rheumatol. (2018) 70:984–99. doi: 10.1002/art.40504
74. Fernando Guimarães,Cavatão, Pinto ÉdersonSM, Krause MJ, Alho CS, and Dorn M. Molecular basis of MC1R activation: mutation-induced alterations in structural dynamics. Proteins. (2024) 92:1297–307. doi: 10.1002/prot.26722
75. Montero-Melendez T, Nagano Ai, Chelala C, Filer A, Buckley CD, and Perretti M. Therapeutic senescence via GPCR activation in synovial fibroblasts facilitates resolution of arthritis. Nat Commun. (2020) 11:745. doi: 10.1038/s41467-020-14421-x
76. Xu F, Li Z, Liu T, Pang X, Fan C, and Jiang H. The role of cellular senescence in the pathogenesis of Rheumatoid Arthritis: Focus on IL-6 as a target gene. Cytokine. (2024) 184:156762. doi: 10.1016/j.cyto.2024.156762
77. Lee H-J, Lee W-J, Hwang S-C, Choe Y, Kim S, Bok E, et al. Chronic inflammation-induced senescence impairs immunomodulatory properties of synovial fluid mesenchymal stem cells in rheumatoid arthritis. Stem Cell Res Ther. (2021) 12:502. doi: 10.1186/s13287-021-02453-z
78. Michel Joshua J, Turesson C, Lemster B, Atkins SR, Iclozan C, Bongartz T, et al. CD56-expressing T cells that have features of senescence are expanded in rheumatoid arthritis. Arthritis Rheum. (2007) 56:43–57. doi: 10.1002/art.22310
79. Terkawi MA, Ebata T, Yokota S, Takahashi D, Endo T, Matsumae G, et al. Low-grade inflammation in the pathogenesis of osteoarthritis: cellular and molecular mechanisms and strategies for future therapeutic intervention. Biomedicines. (2022) 10. doi: 10.3390/biomedicines10051109
80. Surmachevska N and Rubio J. Senescence in osteoarthritis: overview of mechanisms and therapeutics. Eur J Rheumatol. (2023) 11:S3–6. doi: 10.5152/eurjrheum.2023.22077
81. Liu Y, Zhang Z, Li T, Xu H, and Zhang H. Senescence in osteoarthritis: from mechanism to potential treatment. Arthritis Res Ther. (2022) 4:174. doi: 10.1186/s13075-022-02859-x
82. Kulkarni K, Karssiens T, Kumar V, and Pandit H. Obesity and osteoarthritis. Maturitas. (2016) 89:22–8. doi: 10.1016/j.maturitas.2016.04.006
83. Francisco V, Pérez T, Pino Jesús, López V, Franco E, Alonso A, et al. Biomechanics, obesity, and osteoarthritis. The role of adipokines: When the levee breaks. J Orthop Res. (2017) 36:594–604. doi: 10.1002/jor.23788
84. Birch J and Gil Jesús. Senescence and the SASP: many therapeutic avenues. Genes Dev. (2020) 34:1565–76. doi: 10.1101/gad.343129.120
85. Jacob J, Aggarwal A, Aggarwal A, Bhattacharyya S, Kumar V, Sharma V, et al. Senescent chondrogenic progenitor cells derived from articular cartilage of knee osteoarthritis patients contributes to senescence-associated secretory phenotype via release of IL-6 and IL-8. Acta Histochem. (2022) 124:151867. doi: 10.1016/j.acthis.2022.151867
86. Hambright William S, Duke Victoria R, Goff Adam D, Goff AW, Minas LT, Kloser H, et al. Clinical validation of CFDG as a marker associated with senescence and osteoarthritic phenotypes. Aging Cell. (2024) 23:e14113. doi: 10.1111/acel.14113
87. Rajendran P, Alzahrani Abdullah M, Hanieh Hamza N, Kumar SA, Ben Ammar R, Rengarajan T, et al. Autophagy and senescence: A new insight in selected human diseases. J Cell Physiol. (2019) 234:21485–92. doi: 10.1002/jcp.v234.12
88. Gu Y, Yan R, Yang W, Zeng Y, and Yao Q. High TRB3 expression induces chondrocyte autophagy and senescence in osteoarthritis cartilage. Aging (Albany NY). (2022) 14:5366–75. doi: 10.18632/aging.204066
89. Suo J, Shao R, Yang R, Wang J, Zhang Z, Wang D , et al. Accelerated aging in articular cartilage by ZMPSTE24 deficiency leads to osteoarthritis with impaired metabolic signaling and epigenetic regulation. Cell Death Dis. (2023) 14:336. doi: 10.1038/s41419-023-05856-3
90. Wang Y, Liu Y, Chen E, and Pan Z. The role of mitochondrial dysfunction in mesenchymal stem cell senescence. Cell Tissue Res. (2020) 382:457–62. doi: 10.1007/s00441-020-03272-z
91. Miwa S, Kashyap S, Chini E, and von Zglinicki T. Mitochondrial dysfunction in cell senescence and aging. J Clin Invest. (2022) 132. doi: 10.1172/JCI158447
92. He Y, Ding Q, Chen W, Lin C, Ge L, Ying C, et al. LONP1 downregulation with ageing contributes to osteoarthritis via mitochondrial dysfunction. Free Radic Biol Med. (2022) 191:176–90. doi: 10.1016/j.freeradbiomed.2022.08.038
93. Liu Bo, Wang C, Weng Z, Yang Y, Zhao H, Zhang Y, et al. Glycolytic enzyme PKM2 regulates cell senescence but not inflammation in the process of osteoarthritis. Acta Biochim Biophys Sin (Shanghai). (2023) 55:1425–33. doi: 10.3724/abbs.2023062
94. Geng N, Xian M, Deng L, Kuang B, Pan Y, Liu K, et al. Targeting the senescence-related genes MAPK12 and FOS to alleviate osteoarthritis. J Orthop Translat. (2024) 47:50–62. doi: 10.1016/j.jot.2024.06.008
95. Weng P-W, Pikatan NW, Setiawan SA, Yadav VK, Fong IH, Hsu CH, et al. Role of GDF15/MAPK14 axis in chondrocyte senescence as a novel senomorphic agent in osteoarthritis. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23137043
96. Hamdan Y, Mazini L, and Malka G. Exosomes and micro-RNAs in aging process. Biomedicines. (2021) 9. doi: 10.3390/biomedicines9080968
97. Si H-B, Yang T-M, Li L, Tian M, Zhou L, Li DP, et al. miR-140 attenuates the progression of early-stage osteoarthritis by retarding chondrocyte senescence. Mol Ther Nucleic Acids. (2020) 19:15–30. doi: 10.1016/j.omtn.2019.10.032
98. Zhu J, Yang S, Qi Y, Gong Z, Zhang H, Liang K, et al. Stem cell-homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model. Sci Adv. (2022) 8:eabk0011. doi: 10.1126/sciadv.abk0011
99. Nacarelli T, Liu P, and Zhang R. Epigenetic basis of cellular senescence and its implications in aging. Genes (Basel). (2017) 8. doi: 10.3390/genes8120343
100. Wang J, Wang J, Gu Q, Ma Y, Yang Y, Zhu J, et al. The biological function of m6A demethylase ALKBH5 and its role in human disease. Cancer Cell Int. (2020) 20:347. doi: 10.1186/s12935-020-01450-1
101. Ye G, Li J, Yu W, Xie Z, Zheng G, Liu W, et al. ALKBH5 facilitates CYP1B1 mRNA degradation via m6A demethylation to alleviate MSC senescence and osteoarthritis progression. Exp Mol Med. (2023) 55:1743–56. doi: 10.1038/s12276-023-01059-0
102. Zhou Qi, Wang W, Wu J, Qiu S, Yuan S, Fu PL, et al. Ubiquitin-specific protease 3 attenuates interleukin-1β-mediated chondrocyte senescence by deacetylating forkhead box O-3 via sirtuin-3. Bioengineered. (2022) 13:2017–27. doi: 10.1080/21655979.2021.2012552
103. He Y, Song T, Ning J, Wang Z, Yin Z, Jiang P, et al. Lactylation in cancer: Mechanisms in tumour biology and therapeutic potentials. Clin Transl Med. (2024) 14:e70070. doi: 10.1002/ctm2.v14.11
104. Huang Y, Yue S, Yan Z, Liu Y, Qiao J, Zhang M, et al. Lactate-upregulated ARG2 expression induces cellular senescence in fibroblast-like synoviocytes of osteoarthritis via activating the mTOR/S6K1 signaling pathway. Int Immunopharmacol. (2024) 142:113071. doi: 10.1016/j.intimp.2024.113071
105. Reicher A, Foßelteder J, Kwong Lawrence N, and Pichler M. Crosstalk between the Notch signaling pathway and long non-coding RNAs. Cancer Lett. (2018) 420:91–6. doi: 10.1016/j.canlet.2018.01.070
106. Cao He, Yang P, Liu J, Shao Y, Li H, Lai P, et al. MYL3 protects chondrocytes from senescence by inhibiting clathrin-mediated endocytosis and activating of Notch signaling. Nat Commun. (2023) 14:6190. doi: 10.1038/s41467-023-41858-7
107. Ye X, Yin C, Huang X, Huang Y, Ding L, Jin M, et al. ROS/TGF-β signal mediated accumulation of SOX4 in OA-FLS promotes cell senescence. Exp Gerontol. (2021) 156:111616. doi: 10.1016/j.exger.2021.111616
108. Tan Q, Wang Q, Kuang L, Zhang J, Peng X, Liang S, et al. TGF-β/Alk5 signaling prevents osteoarthritis initiation via regulating the senescence of articular cartilage stem cells. J Cell Physiol. (2021) 236:5278–92. doi: 10.1002/jcp.v236.7
109. Xia G, Wen Zi, Zhang L, Huang J, Wang X, Liang C, et al. β-Hydroxybutyrate alleviates cartilage senescence through hnRNP A1-mediated up-regulation of PTEN. Exp Gerontol. (2023) 175:112140. doi: 10.1016/j.exger.2023.112140
110. Kang C and Elledge Stephen J. How autophagy both activates and inhibits cellular senescence. Autophagy. (2016) 12:898–9. doi: 10.1080/15548627.2015.1121361
111. Pantelis P, Theocharous G, Lagopati N, Veroutis D, Thanos DF, Lampoglou GP, et al. The dual role of oxidative-stress-induced autophagy in cellular senescence: comprehension and therapeutic approaches. Antioxidants (Basel). (2023) 12. doi: 10.3390/antiox12010169
112. Li Q, Lin Y, Liang G, Xiao N, Zhang H, Yang X, et al. Autophagy and senescence: the molecular mechanisms and implications in liver diseases. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms242316880
113. Yang Y, Ding J, Chen Y, Ma G, Wei X, Zhou R, et al. Blockade of ASIC1a inhibits acid-induced rat articular chondrocyte senescence through regulation of autophagy. Hum Cell. (2022) 35:665–77. doi: 10.1007/s13577-022-00676-7
114. Yagi M, Endo K, Komori K, and Sekiya I. Comparison of the effects of oxidative and inflammatory stresses on rat chondrocyte senescence. Sci Rep. (2023) 13:7697. doi: 10.1038/s41598-023-34825-1
115. Ameer MA, Chaudhry H, Mushtaq J, Khan OS, Babar M, Hashim T, et al. An overview of systemic lupus erythematosus (SLE) pathogenesis, classification, and management. Cureus. (2022) 14:e30330. doi: 10.7759/cureus.30330
116. Sharma U. The SLE conundrum: A comprehensive analysis of pathogenesis, recent developments, and the future of therapeutic interventions. Crit Rev Immunol. (2025) 45:41–54. doi: 10.1615/CritRevImmunol.2024053504
117. Dema B and Charles N. Autoantibodies in SLE: specificities, isotypes and receptors. Antibodies (Basel). (2016) 5. doi: 10.3390/antib5010002
118. Weinstein A, Alexander Roberta V, and Zack Debra J. A review of complement activation in SLE. Curr Rheumatol Rep. (2021) 23:16. doi: 10.1007/s11926-021-00984-1
119. Gao L, Slack M, Barnas Jennifer L, McDavid A, Anolik J, and Looney RJ. Cell senescence in lupus. Curr Rheumatol Rep. (2019) 21:1. doi: 10.1007/s11926-019-0800-6
120. Tsai C-Y, Shen C-Y, Liao H-T, Li KJ, Lee HT, Lu CS, et al. Molecular and cellular bases of immunosenescence, inflammation, and cardiovascular complications mimicking "Inflammaging" in patients with systemic lupus erythematosus. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20163878
121. Haque S, Rakieh C, Marriage F, Ho P, Gorodkin R, Teh LS, et al. Shortened telomere length in patients with systemic lupus erythematosus. Arthritis Rheum. (2013) 65:1319–23. doi: 10.1002/art.37895
122. Katsuyama T, Tsokos George C, and Moulton Vaishali R. Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front Immunol. (2018) 9:1088. doi: 10.3389/fimmu.2018.01088
123. Rother N and van der Vlag J. Disturbed T cell signaling and altered th17 and regulatory T cell subsets in the pathogenesis of systemic lupus erythematosus. Front Immunol. (2015) 6:610. doi: 10.3389/fimmu.2015.00610
124. Kalim H, Wahono CS, Permana BPO, Pratama MZ, and Handono K. Association between senescence of T cells and disease activity in patients with systemic lupus erythematosus. Reumatologia. (2021) 59:292–301. doi: 10.5114/reum.2021.110318
125. Pratama MZ, Wahono CS, Aslam ABN, Chilmi S, Sari VP, Abdurrohman A, et al. T-cell senescence was correlated with early atherosclerosis in patients with systemic lupus erythematosus. Int J Rheum Dis. (2024) 27:e15339. doi: 10.1111/1756-185X.15339
126. López P, Rodríguez-Carrio J, Martínez-Zapico A, Caminal-Montero L, and Suarez A. Senescent profile of angiogenic T cells from systemic lupus erythematosus patients. J Leukoc Biol. (2016) 99:405–12. doi: 10.1189/jlb.5HI0215-042R
127. Jiang J, Yang M, Zhu H, Long D, He Z, Liu J, et al. CD4CD57 senescent T cells as promoters of systemic lupus erythematosus pathogenesis and the therapeutic potential of senolytic BCL-2 inhibitor. Eur J Immunol. (2024) 54:e2350603. doi: 10.1002/eji.202350603
128. Cheng T, Ding S, Liu S, Li Y, and Sun L. Human umbilical cord-derived mesenchymal stem cell therapy ameliorates lupus through increasing CD4+ T cell senescence via MiR-199a-5p/Sirt1/p53 axis. Theranostics. (2021) 11:893–905. doi: 10.7150/thno.48080
129. Xu J. Therapeutic applications of mesenchymal stem cells for systemic lupus erythematosus. Adv Exp Med Biol. (2018) 1089:73–85. doi: 10.1007/5584_2018_212
130. Li A, Guo F, Pan Q, Chen S, Chen J, Liu HF, et al. Mesenchymal stem cell therapy: hope for patients with systemic lupus erythematosus. Front Immunol. (2021) 12:728190. doi: 10.3389/fimmu.2021.728190
131. Chen F, Meng J, Yan W, Wang M, Jiang Y, and Gao J. Identification of novel immune cell-relevant therapeutic targets and validation of roles of TK1 in BMSCs of systemic lupus erythematosus. Front Mol Biosci. (2022) 9:848463. doi: 10.3389/fmolb.2022.848463
132. Gu Z, Tan W, Feng G, Meng Y, Shen B, Liu H, et al. Wnt/β-catenin signaling mediates the senescence of bone marrow-mesenchymal stem cells from systemic lupus erythematosus patients through the p53/p21 pathway. Mol Cell Biochem. (2014) 387:27–37. doi: 10.1007/s11010-013-1866-5
133. Ji J, Wu Y, Meng Y, Zhang L, Feng G, Xia Y, et al. JAK-STAT signaling mediates the senescence of bone marrow-mesenchymal stem cells from systemic lupus erythematosus patients. Acta Biochim Biophys Sin (Shanghai). (2017) 49:208–15. doi: 10.1093/abbs/gmw134
134. Chen H, Shi B, Feng X, Kong W, Chen W, Geng L, et al. Leptin and neutrophil-activating peptide 2 promote mesenchymal stem cell senescence through activation of the phosphatidylinositol 3-kinase/akt pathway in patients with systemic lupus erythematosus. Arthritis Rheumatol. (2015) 67:2383–93. doi: 10.1002/art.39196
135. Gu Z, Tan W, Ji J, Feng G, Meng Y, Da Z, et al. Rapamycin reverses the senescent phenotype and improves immunoregulation of mesenchymal stem cells from MRL/lpr mice and systemic lupus erythematosus patients through inhibition of the mTOR signaling pathway. Aging (Albany NY). (2016) 8:1102–14. doi: 10.18632/aging.100925
136. Gao L, Bird AK, Meednu N, Dauenhauer K, Liesveld J, Anolik J, et al. Bone marrow-derived mesenchymal stem cells from patients with systemic lupus erythematosus have a senescence-associated secretory phenotype mediated by a mitochondrial antiviral signaling protein-interferon-β Feedback loop. Arthritis Rheumatol. (2017) 69:1623–35. doi: 10.1002/art.40142
137. Gu Z, Meng Y, Tao T, Guo G, Tan W, Xia Y, et al. Endoplasmic reticulum stress participates in the progress of senescence of bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Cell Tissue Res. (2015) 361:497–508. doi: 10.1007/s00441-015-2131-x
138. Hanly John G. Diagnosis and management of neuropsychiatric SLE. Nat Rev Rheumatol. (2014) 10:338–47. doi: 10.1038/nrrheum.2014.15
139. Kalim H, Pratama MZ, Mahardini E, Winoto ES, Krisna PA, and Handono K. Accelerated immune aging was correlated with lupus-associated brain fog in reproductive-age systemic lupus erythematosus patients. Int J Rheum Dis. (2020) 23:620–6. doi: 10.1111/1756-185X.13816
140. Rezaiemanesh A, Abdolmaleki M, Abdolmohammadi K, Aghaei H, Pakdel FD, Fatahi Y, et al. Immune cells involved in the pathogenesis of ankylosing spondylitis. BioMed Pharmacother. (2018) 100:198–204. doi: 10.1016/j.biopha.2018.01.108
141. Chen B, Li J, He C, Li D, Tong W, Zou Y, et al. Role of HLA-B27 in the pathogenesis of ankylosing spondylitis (Review). Mol Med Rep. (2017) 15:1943–51. doi: 10.3892/mmr.2017.6248
142. Arends S, Spoorenberg A, Brouwer E, and van der Veer E. Clinical studies on bone-related outcome and the effect of TNF-α blocking therapy in ankylosing spondylitis. Curr Opin Rheumatol. (2014) 26:259–68. doi: 10.1097/BOR.0000000000000053
143. Wendling D, Verhoeven F, and Prati Clément. Anti-IL-17 monoclonal antibodies for the treatment of ankylosing spondylitis. Expert Opin Biol Ther. (2019) 19:55–64. doi: 10.1080/14712598.2019.1554053
144. Ye G, Xie Z, Zeng H, Wang P, Li J, Zheng G, et al. Oxidative stress-mediated mitochondrial dysfunction facilitates mesenchymal stem cell senescence in ankylosing spondylitis. Cell Death Dis. (2020) 11:775. doi: 10.1038/s41419-020-02993-x
145. Jo S, Weon S, Nam B, Jang MA, Kang H, Kim TJ, et al. WNT16 elevation induced cell senescence of osteoblasts in ankylosing spondylitis. Arthritis Res Ther. (2021) 23:301. doi: 10.1186/s13075-021-02670-0
146. Fessler J, Raicht A, Husic R, Ficjan A, Duftner C, Schwinger W, et al. Premature senescence of T-cell subsets in axial spondyloarthritis. Ann Rheum Dis. (2016) 75:748–54. doi: 10.1136/annrheumdis-2014-206119
147. Bukiri H and Volkmann Elizabeth R. Current advances in the treatment of systemic sclerosis. Curr Opin Pharmacol. (2022) 64:102211. doi: 10.1016/j.coph.2022.102211
148. Piera-Velazquez S and Jimenez Sergio A. Role of cellular senescence and NOX4-mediated oxidative stress in systemic sclerosis pathogenesis. Curr Rheumatol Rep. (2015) 17:473. doi: 10.1007/s11926-014-0473-0
149. Vona R, Giovannetti A, Gambardella L, Malorni W, Pietraforte D, and Straface E. Oxidative stress in the pathogenesis of systemic scleroderma: An overview. J Cell Mol Med. (2018) 22:3308–14. doi: 10.1111/jcmm.2018.22.issue-7
150. Shima Y. Cytokines involved in the pathogenesis of SSc and problems in the development of anti-cytokine therapy. Cells. (2021) 10. doi: 10.3390/cells10051104
151. Artlett CM, Black CM, Briggs DC, Stevens CO, and Welsh KI. Telomere reduction in scleroderma patients: a possible cause for chromosomal instability. Br J Rheumatol. (1996) 35:732–7. doi: 10.1093/rheumatology/35.8.732
152. Tarhan F, Vural F, Kosova B, Aksu K, Cogulu O, Keser G, et al. Telomerase activity in connective tissue diseases: elevated in rheumatoid arthritis, but markedly decreased in systemic sclerosis. Rheumatol Int. (2008) 28:579–83. doi: 10.1007/s00296-007-0472-9
153. Chiu Y-H, Spierings J, van Laar Jacob M, de Vries-Bouwstra JK, van Dijk M, and Goldschmeding R. Association of endothelial to mesenchymal transition and cellular senescence with fibrosis in skin biopsies of systemic sclerosis patients: a cross-sectional study. Clin Exp Rheumatol. (2023) 41:1612–7. doi: 10.55563/clinexprheumatol/i49d3o
154. Cottin V and Brown Kevin K. Interstitial lung disease associated with systemic sclerosis (SSc-ILD). Respir Res. (2019) 20:13. doi: 10.1186/s12931-019-0980-7
155. Yang Monica M, Lee S, Neely J, Hinchcliff M, Wolters PJ, and Sirota M. Gene expression meta-analysis reveals aging and cellular senescence signatures in scleroderma-associated interstitial lung disease. bioRxiv. (2023) 15:1326922. doi: 10.1101/2023.11.06.565810
156. Cipriani P, Di Benedetto P, Liakouli V, Del Papa B, Di Padova M, Di Ianni M, et al. Mesenchymal stem cells (MSCs) from scleroderma patients (SSc) preserve their immunomodulatory properties although senescent and normally induce T regulatory cells (Tregs) with a functional phenotype: implications for cellular-based therapy. Clin Exp Immunol. (2013) 173:195–206. doi: 10.1111/cei.12111
157. Chang M, Dong Y, Cruickshank-Taylor AB, Gnawali G, Bi F, and Wang W. Senolytic prodrugs: A promising approach to enhancing senescence-targeting intervention. Chembiochem. (2024) 25:e202400355. doi: 10.1002/cbic.202400355
158. Zhang L, Pitcher Louise E, Prahalad V, Niedernhofer LJ, and Robbins PD. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. FEBS J. (2023) 290:1362–83. doi: 10.1111/febs.v290.5
159. Yan J, Yao L, Tan Y, and Wang Y. The protective effects of Phoenixin-20 in tumor necrosis factor α (TNF-α)-induced cell senescence of rheumatoid arthritis fibroblast-like synoviocytes (FLS). Aging (Albany NY). (2023) 15:14607–16. doi: 10.18632/aging.205024
160. Almalki WH and Salman AS. Oxidative stress and senescence in aging kidneys: the protective role of SIRT1. EXCLI J. (2024) 23:1030–67. doi: 10.17179/excli2024-7519
161. Wang B, Sun W, Bi K, Li Y, and Li F. Apremilast prevents IL−17−induced cellular senescence in ATDC5 chondrocytes mediated by SIRT1. Int J Mol Med. (2021) 47. doi: 10.3892/ijmm.2021.4845
162. Yang H, Chen C, Chen H, Duan X, Li J, Zhou Y, et al. Navitoclax (ABT263) reduces inflammation and promotes chondrogenic phenotype by clearing senescent osteoarthritic chondrocytes in osteoarthritis. Aging (Albany NY). (2020) 12:12750–70. doi: 10.18632/aging.103177
163. Liu Y, Yang S, Wang K, Lu J, Bao X, Wang R, et al. Cellular senescence and cancer: Focusing on traditional Chinese medicine and natural products. Cell Prolif. (2020) 53:e12894. doi: 10.1111/cpr.v53.10
164. Hu Y, Liu J, Qi Y, Zhou Q, Li Y, Cong C, et al. Integrating clinical data mining, network analysis and experimental validation reveal the anti-inflammatory mechanism of Huangqin Qingre Chubi Capsule in rheumatoid arthritis treatment. J Ethnopharmacol. (2024) 329:118077. doi: 10.1016/j.jep.2024.118077
165. Zhou Q, Liu J, Qi Y, Hu Y, Li Y, Cong C, et al. Jianpi qingre tongluo prescription alleviates the senescence-associated secretory phenotype with osteoarthritis by regulating STAG1/TP53/P21 signaling pathway. J Ethnopharmacol. (2025) 337:118953. doi: 10.1016/j.jep.2024.118953
166. Zhou Q, Liu J, Zhu Y, Wang Y, Wang GZ, Wan L, et al. Mechanism of Huangqin Qingre Chubi Capsules regulating p53/p21 signaling pathway to delay chondrocyte senescence in osteoarthritic rats. Zhongguo Zhong Yao Za Zhi. (2024) 49:3330–9. doi: 10.19540/j.cnki.cjcmm.20240318.702
167. Jie L, Zhang C, Liu Y, Huang Z, Xu B, Zhu Z, et al. Mechanistic study of the regulation of mitochondrial function by the GPNMB/Nrf2/NF-κB signaling pathway mediated by Quzhi Tang to alleviate chondrocyte senescence. J Ethnopharmacol. (2024) 340:119165. doi: 10.1016/j.jep.2024.119165
168. Li W, Zhong Y, Lin Z, Deng Z, Long D, Li M, et al. Forsythoside A mitigates osteoarthritis and inhibits chondrocyte senescence by promoting mitophagy and suppressing NLRP3 inflammasome via the Nrf2 pathway. Phytomedicine. (2024) 135:156052. doi: 10.1016/j.phymed.2024.156052
Keywords: cellular senescence, rheumatic diseases, chronic inflammation, treatment strategy, TCM
Citation: Wen J, Liu J, Wan L and Wang F (2025) New insights into the role of cellular senescence and rheumatic diseases. Front. Immunol. 16:1557402. doi: 10.3389/fimmu.2025.1557402
Received: 08 January 2025; Accepted: 28 April 2025;
Published: 16 May 2025.
Edited by:
Eva Reali, University of Ferrara, ItalyReviewed by:
Johannes Fessler, Medical University of Graz, AustriaFrancesco Nicoli, University of Ferrara, Italy
Copyright © 2025 Wen, Liu, Wan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianting Wen, d2VuanRhaHp5QDE2My5jb20=; Jian Liu, bGl1amlhbmFoenlAMTI2LmNvbQ==
 Jianting Wen
Jianting Wen Jian Liu
Jian Liu Lei Wan
Lei Wan Fanfan Wang
Fanfan Wang