- 1Division of Hematology, Department of Internal Medicine, College of Medicine, The Ohio State University, Columbus, OH, United States
- 2Institute of Medical Engineering & Translational Medicine, Tianjin University, Tianjin, China
- 3Department of Pediatric and Adolescent Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 4Centre of Paediatric Hematology and Oncology, Hong Kong Sanatorium and Hospital, Hong Kong, Hong Kong SAR, China
Natural killer group 2 D (NKG2D) receptor, one of the activation receptors on NK cells, has gained increasing attention in recent years because its ligands are widely expressed in most cancers. Naturally, NKG2D reacts to 8 different stress-induced ligands, MICA/B, and ULBP1-6. Despite being genomically conserved between human and mouse, NKG2D transcripts have splice variants that can differentiate the two. hNKG2D or mNKG2D (both long and short transcripts) interacts with DAP10 only in human but DAP10/12 in mouse, switching on different effector functions such as IFN-γ production and cytotoxicity. Full-length, extracellular or cytoplasmic domains have been used to construct chimeric antigen receptors (CAR) or implement into the antibody structures including bispecific antibodies. Interestingly, most of the NKG2D CARs, either on T cells or NK cells are investigated in preclinical models of solid tumors. In this article, we reviewed the majority of published NKG2D-based CAR and antibody designs, comparing their respective advantages and disadvantages. We also elaborated how these CARs and antibodies were tested in preclinical cancer models and clinical trials in this review article.
1 Introduction
NKG2D (gene name: klrk1) is one of the key activation receptors on NK cells and CD8+ T cells targeting cancer cells and infections (1–4). It is expressed on nearly all human NK and CD8+ T cells and the expression level can be upregulated by IL-2, 7, 12, 15 and negatively regulated by TGF-β, IFN-β1 and IL-21 (5, 6). Studies show NKG2D activation signals are sufficient to activate NK cell function in cytokine-primed NK cells and synergizes with other NK cell activation receptors including NKp46 and co-receptor 2B4 (5, 7). NKG2D recognizes eight stress-induced NKG2D ligands (NKG2DLs) including the MHC class I chain-related proteins A and B (MICA, MICB), and the structurally diverse UL16-binding proteins 1 to 6 (ULBP1-6) as shown in Figure 1 (5, 8). A comparison of human and mouse NKG2D receptors reveals several distinctive features. Mice express two alternatively spliced isoforms of NKG2D: NKG2D-L (long) and NKG2D-S (short) (5). NKG2D-L is expressed on the surface of both resting and activated mouse NK cells and CD8 T cells as a disulfide-bonded homodimer that can interact with DAP10 homodimers. NKG2D-S is expressed on activated mouse NK cells, which can associate with homodimers of either DNAX activating protein (DAP) 10 or DAP12. DAP10 contains a tyrosine-based signaling motif, YINM, which is capable of recruiting a p85 PI3 kinase and Vav-1 signaling complex whereas DAP12 contains a canonical immunoreceptor tyrosine-based activation motif (ITAM), which can recruit Syk and ZAP70 tyrosine kinases (9, 10). In humans, NKG2D-L is the only one expressed on NK, CD8+, γΔ T and CD4+ iNKT cells (11). Upon engagement, the dimerized NKG2D leads to the phosphorylation of YINM motif of DAP10. Phosphorylated DAP10 will then activate both PI3K/AKT and Grb2/Vav1 axis resulting in the upregulations of survival signals, expansion, cytotoxicity and T cell differentiation (9).
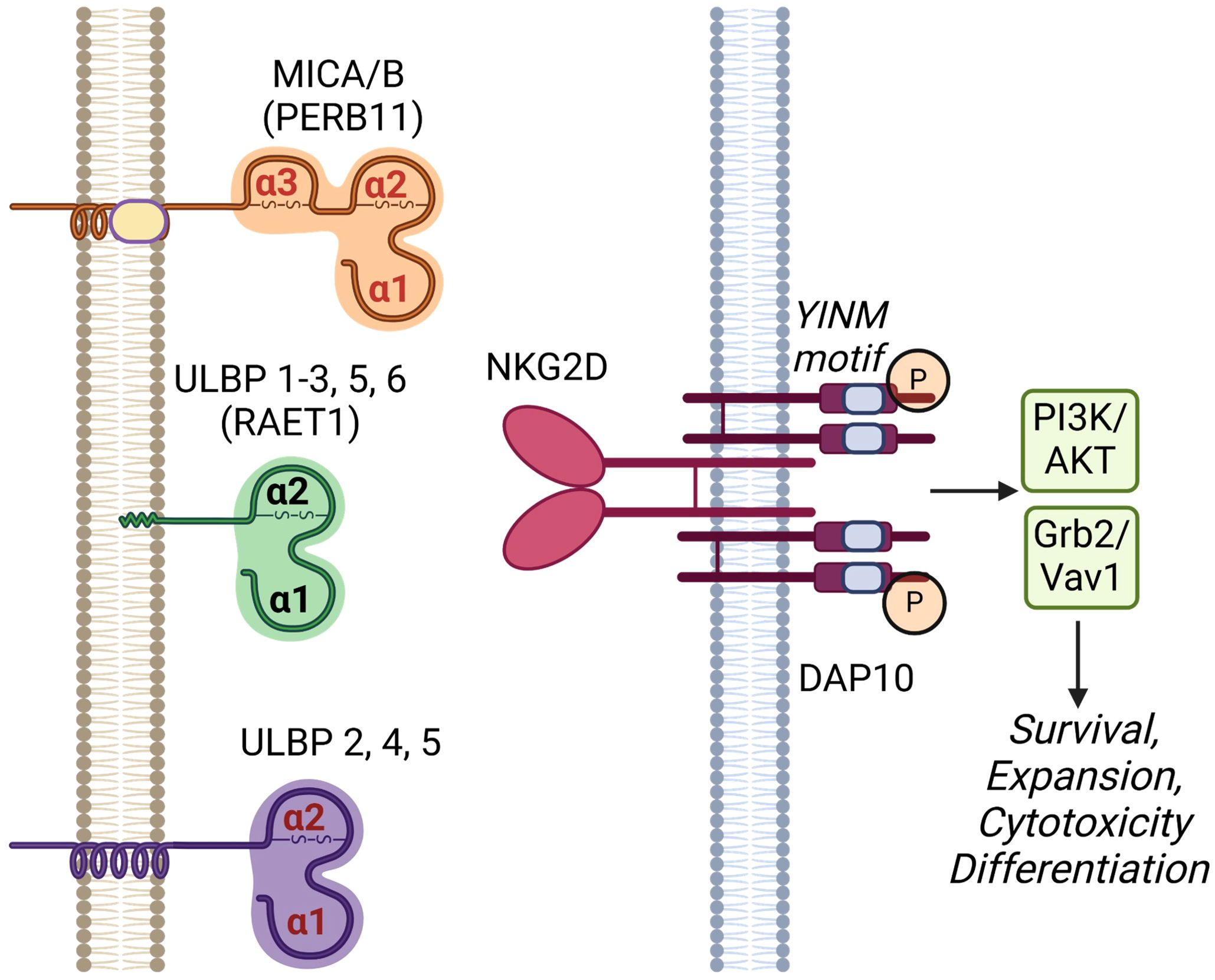
Figure 1. Human NKG2D receptor and its cognate ligands. NKG2D receptor is a C-type lectin-like molecule expressed primarily on NK cells and subsets of T cells. It has a disulfide-linked homodimer that is associated with four DNAX-activating protein 10 (DAP10) forming a hexameric complex. Upon phosphorylation, the YINM motifs of DAP10 recruit and activate phosphatidyl-inositol 3-kinase (PI3K)/Akt and Grb2/Vav1 molecules leading to cell survival, expansion, cytotoxicity and differentiation. NKG2D ligands include proteins in MHC class-I polypeptide-related sequence (MIC) and UL16-binding protein (ULBP) families. MICA and B (other names PERB11.1/11.2) have three MHC-class I related α1, α2 and α3 domains anchored on cell surface by a transmembrane domain. ULBP 1-6 (other name RAET1I/H/N/E/G/L) consists of only α1 and α2 domains and are attached to the cell membrane via either a glycosylphosphatidylinositol (GPI)-anchor (ULBP1-3, 5 and 6) or transmembrane domains (ULBP 2, 4, and 5). Created in BioRender. Chan, W. (2025) https://BioRender.com/n95p848.
The regulation of NKG2D ligands is tightly controlled and typically absent on healthy tissues, but can be upregulated due to DNA damage, infection, and cellular transformation under stress. In hematological malignancies and solid tumors, MICA or MICB is expressed in 100% of colorectal tumors, 97% of breast cancers, 95% of renal cell carcinomas, 81% of ovarian cancer, 77% of primary cutaneous melanomas, and 50% of primary uveal melanomas (4, 5, 8, 12). Given their unique overexpression pattern in tumors, NKG2DLs are promising targets for anticancer therapies (4). The widespread presence of NKG2DLs in human cancer indicates that NKG2D-based CAR-T cells have significant therapeutic potential for a wide range of tumor types and broad oncologic applications. This review focuses on cancer immunotherapy harnessing NKG2DR-NKG2DRL axis, including NKG2D-based CAR-T or NK cell therapy and NKG2D-based antibody therapy.
2 NKG2D-based CAR-T/NK therapies
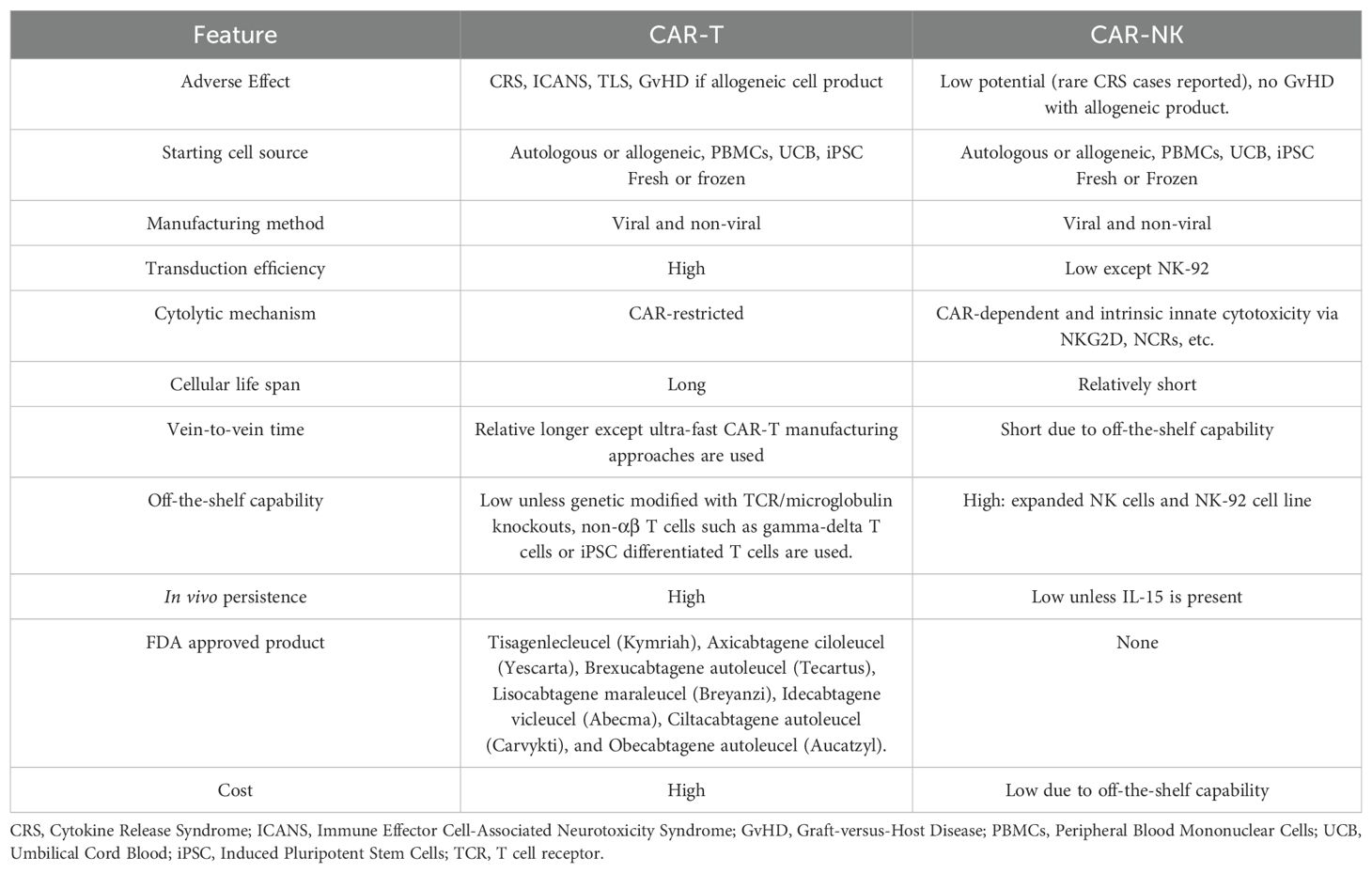
Among all cell therapies with NKG2D-based CARs, most of them used T cells rather than NK cells (around 80% of reviewed literature is about CAR-T cells). Although there is no direct comparison on the properties of NKG2D-based CAR-T versus CAR-NK, we can extrapolate from existing knowledge on CAR-T and CAR-NK cells. In general, CAR-T cells have a higher manufacturing efficiency, longer half-life and longer clinical track record. On the contrary, CAR-NK, especially allogeneic off-the-shelf product, offers a more timely and less costly option with additional secondary cytotoxic mechanisms against cancer cells. (Table 1). Currently, most of the NKG2D-based CAR constructs are primarily in the 2nd generation design which includes a co-stimulatory domain, either 4-1BB or CD28 plus the intracellular signaling region CD3ζ, although some of those are in the 3rd or above generation with more than 2 co-stimulatory domains (13, 14). Different domains of NKG2D receptor molecules have been utilized in the design of NKG2D CARs and some of the designs are beyond 2nd generation (Figure 2). Full-length (1–216) (15–21) or extracellular domain (Uniprot, amino acid from 73 to 216) (1, 22–52) is used as the target binding domain for engaging the NKG2DL-expressing tumor cells including MICA/B and ULBP1–6 for various tumors. Other CARs use transmembrane (Uniprot, amino acid from 7 to 52) (25, 29, 53, 54) of NKG2D. No cytoplasmic domain (Uniprot, amino acid from 1 to 51) of NKG2D was used as a standalone construct in the NKG2D CARs. We elaborate further in the following sections on the use of different NKG2D domains in the CAR design and their application in both preclinical and clinical settings for hematological malignancies and solid tumors.
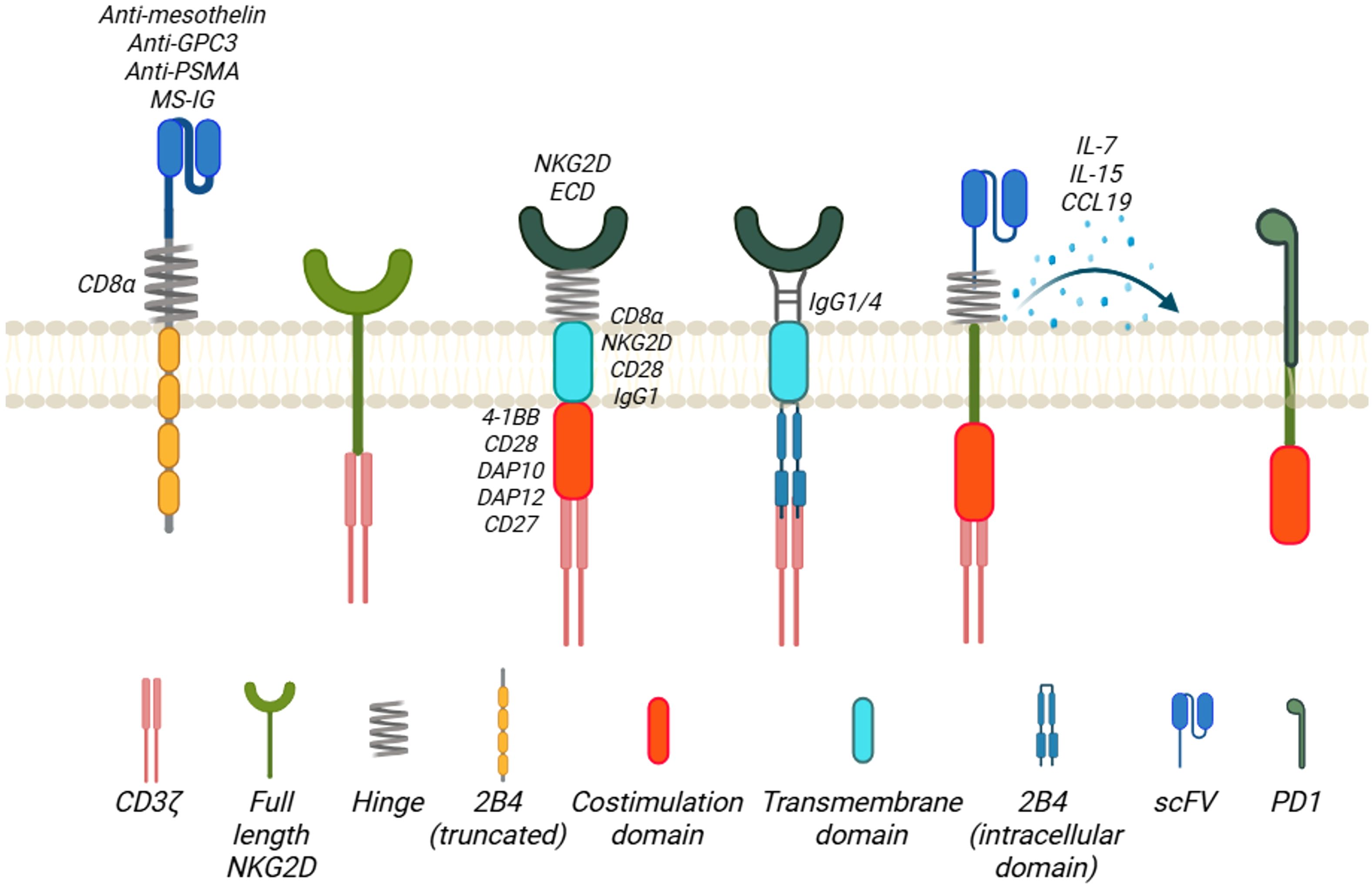
Figure 2. Family of NKG2D-based CAR designs. The antigen-binding domains of the NKG2D-based CARs include extracellular domains of NKG2D receptor molecule (NKG2D ECD) and scFv targeting tumor antigens. The hinge region of the CARs is mostly derived from CD8α molecule, and others are from NKG2D, IgG1/4, or PD1. Except full-length NKG2D or truncated 2B4 is employed in the CAR design, the transmembrane domain of the CARs is CD8α, CD28, or IgG1. For the co-stimulation domains, almost all the NKG2D-based CARs contain CD3ζ, with 4-1BB, DAP12, CD28, DAP10, or CD27 as the second/third co-stimulation domain. Created in BioRender. Chan, W. (2025) https://BioRender.com/777ditl.
3 CAR targeting NKG2DL
3.1 Full length NKG2D
NKG2D receptor as a NK cell activation receptor can lead to NK cell induced cytotoxicity and cytokine production. One of the strategies to harness the intrinsic activation mechanism is to use the full length of NKG2D and studies have attempted to fuse the full length of NKG2D directly to CD3ζ intracellular signaling domains (Figure 2). This design has been tested in the preclinical model of ovarian (15), glioma (55, 56), and leukemia models (16–19).
3.2 Ectodomain
Most of the NKG2D-based CAR designs replace the single chain variable fragment (scFv) domain of the CAR to the ectodomain of NKG2D, which will bind to NKG2DLs. These designs have been studied across various types of cancers and will be discussed below.
3.3 Hinge and transmembrane domains
For the hinge region of all these CARs, they mainly use the region from CD8α (23, 25–28, 30, 31, 33, 35, 39–43, 45, 51, 52, 57–62), CD28 (1, 32, 34, 37, 38, 63, 64), IgG1/4 (24, 32, 34, 37, 38, 46), or NKG2D molecules (25, 29, 53, 54, 65, 66). Notably, 2B4 has been used as a hinge region because it, also known as CD244, is a potent stimulatory co-receptor of NK cell activation and is found to synergize with the NKG2D activation (67). Three different CAR intracellular regions CAR1 (CD244), CAR2 (CD244, NKG2D) and CAR3 (CD244, NKG2D, and CD3ζ) were constructed and compared for their respective functions. CAR2 was found to have a stronger tumoricidal ability on CAR-NK92MI cells and was chosen as the design. PD1, a checkpoint protein in regulating T cell functions and exhaustion, has been used as hinge region of the NKG2D CAR (54). For transmembrane domain, most of the CAR designs adopt CD8α. Others include NKG2D, IgG1 and CD28.
3.4 Co-stimulatory domains
All the NKG2D CARs contain the ITAM from the CD3ζ and most of them contain 4-1BB as co-stimulatory domain as well. Other co-stimulatory domains being used include CD28 (25, 34, 40, 64, 68), 2B4 (25, 44, 68), CD27 (44, 68), NKG2D cytoplasmic domain (67) and DAP10/DAP12 (24, 25, 37–39, 47, 66). DAP10, as included in the CAR design (9, 21, 63), is a small transmembrane protein (93 amino acid long) with minimal extracellular domains (63, 69). The cytoplasmic domain of DAP10 contains a short amino acid sequence of YINM (10, 69). Upon activation and tyrosine phosphorylation, the motif will allow the binding of phosphatidylinositol (PI3K) and a Grb2-Vav1-son of sevenless (SOS1) complex (9). This YINM motif is similar to that of CD28, a co-receptor for the co-stimulation of T cell activation with TCR. DAP12 is a small 12-kDa transmembrane protein that consists of 113 amino acids and a single ITAM within its 48-amino acid cytoplasmic domain (69). It shares less than 25% homology with the ITAM motifs found in the human CD3ζ chain and FcϵRI-γ chain. When a DAP12-associated receptor is engaged, it triggers the activation of SRC-family kinases, leading to the phosphorylation of paired tyrosine residues in the ITAM of DAP12 (5, 69). This in turn recruits cytoplasmic ZAP70 tyrosine kinases and initiates downstream signaling and cytokine production. In human immune cells, DAP12 does not associate with NKG2D, but it does form noncovalent complexes with other receptors, such as killer immunoglobulin-like receptors (KIRs) in both human T cells and NK cells. CD27 is a Traf-linked tumor necrosis factor receptor family member and functions as a T cell costimulatory molecule (70). CD27 is required for generation and long-term maintenance of T cell immunity. Instead of using 4-1BB, CD27 cytoplasmic domain was used along with CD3ζ (NKG2D-27z) (44). NKG2D CAR-T cells with the addition of CD27 as a co-stimulatory domain have anti-tumor activity against triple negative breast cancer in vitro and in vivo MDA-MB-231 fLuc xenograft NOD-SCIDIL2γc-/- (NSG) mouse model. The NKG2D-27z T cells show a persistent phenotype and form a long-term memory in the presence of IL-2.
3.5 Armored NKG2D CARs
Beyond 2nd generation of CARs, the NKG2D CARs can partner with other cytokine-induced receptors such as ectodomain of IL-4 receptor and IL-15 receptor (IL-15R) transmembrane+ICD, namely 4/15NKG2D-CAR-T cells (42). IL-15R is a pro-inflammatory cytokine that stimulates NK cell proliferation and expansion, while the IL-4 receptor extracellular domain (ECD) responds to IL-4 in the tumor microenvironment, where inhibitory signals from IL-4R are converted into IL-15R activation signals downstream. C-C motif chemokine ligand 19 (CCL19), a chemokine receptor has also been engineered into the NKG2D CAR design for better trafficking of the CAR cells to the target cells (64). 15×19 NKG2D CAR-T cells, which incorporate the secretion of interleukin (IL)-15 and CCL19, have an augmented cell expansion, promotion of central memory T (TCM) cell production, and increased cytotoxicity against gastric cell lines compared to conventional CAR T cells. These CAR-T cells also have reduced expression of T cell exhaustion markers, providing longer cancer surveillance in zebra fish model of gastric cancer. IL-21 connected to the NKG2D CAR constructed with NKG2D ECD, 4-1BB and CD3ζ domains augmented the CAR NK-92 cytolytic functions against lung cancer cell lines A549 and H1975 with increased CD107a, IFN-γ and cell proliferation via activation of PI3K/Akt pathway. This armored CARs also reduce the tumor size in a subcutaneous lung cancer mouse model (71).
4 Non-NKG2DL targeting CAR using NKG2D receptor components
Besides targeting NKG2DLs, a group of CAR designs does not use NKG2D ectodomains but uses scFv instead such as anti-mesothelin (25, 63, 67), prostate-specific membrane antigen (PSMA) (67), and glypican 3 protein (GPC3) (63), programmed cell death-1 (PD1) (53, 54) and NKG2D receptor or associated components such as NKG2D transmembrane and DAP10 to diversify the tumor targeting to other tumor-associated antigens.
5 Targeting NKG2DL on both solid tumors and hematological malignancies
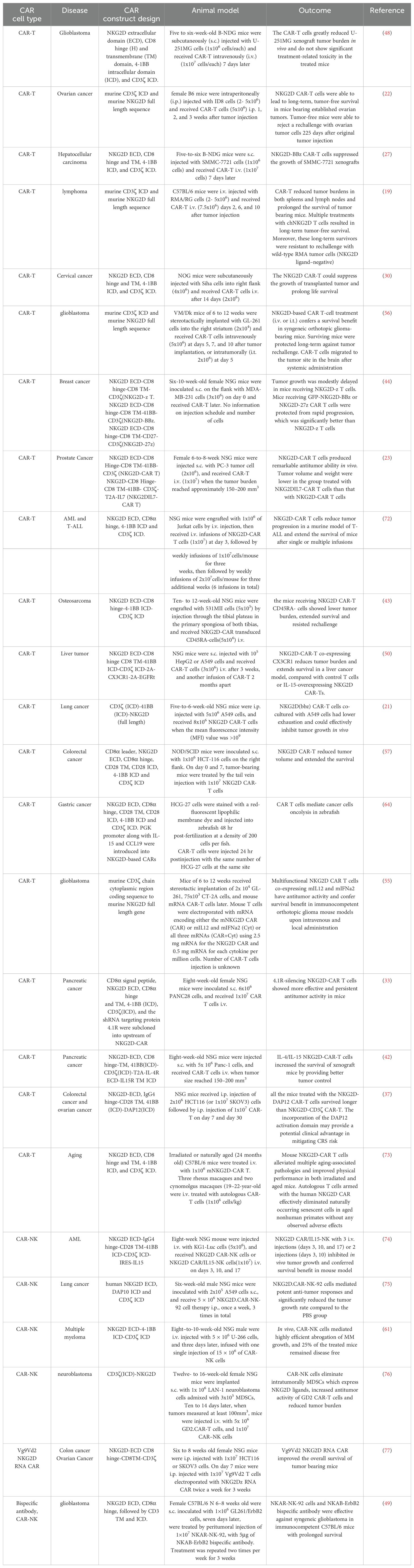
It is noteworthy that most of the published studies on NKG2D CAR-T/NK therapy have been focused on solid tumors, particularly brain tumors, breast cancer, lung cancers, gastrointestinal tracts, reproductive system, and sarcomas. This trend underscores the significant research interest and potential therapeutic applications of NKG2D CAR-T/NK due to the fact that NKG2DLs are broadly and highly expressed on these solid tumors (12). We summarize and discuss the preclinical studies of NKG2D-based CAR-T or NK cells categorized by cancer types (Table 2).
6 NKG2D CAR-T cell targeting solid tumors
6.1 Anti-glioma and neuroblastoma
Traditional therapies including chemotherapy, radiation, and surgical resection fail to cure most glioblastoma patients and the median overall survival of GBM patients is only 14.6 months, highlighting an urgent need for new therapeutic options (78). NKG2DLs are highly expressed in GBM and are considered promising targets for CAR-T cell therapy. Dong et al. confirmed that NKG2DLs are highly expressed in human glioblastoma cells, cancer stem cells and tumor samples (3). Besides, the NKG2D-BBz CAR-T cells efficiently kill glioblastoma cells and glioma stem cells in vitro and produce high levels of TNF-α, perforin, and granzyme B. The CAR-T cells greatly reduce xenograft tumor burden in vivo and do not show significant treatment-related toxicity in the treated mice. As CAR-T cells can pass through blood brain barrier, this study supports the potential of CAR-T therapy as a promising glioblastoma therapeutic strategy.
Neuroblastoma, an extracranial neuroendocrine tumor in pediatric patients, carries a tumor-specific glycolipid antigen GD2 (79). Besides GD2, neuroblastoma cells exhibit high expression levels of NKG2DLs including MICA/B and ULBPs1-3 (80). This could suggest neuroblastoma cells may be susceptible to NKG2D-based CAR-T or CAR-NK cells. Despite this potential, preclinical data evaluating the efficacy of NKG2D-based CAR products against neuroblastoma models remains scarce. Currently, no clinical trials are investigating NKG2D-based CAR-T/NK cell therapies for neuroblastoma treatment.
6.2 Anti-liver cancer
A novel NKG2D CAR-T comprising human NKG2D extracellular domain, 4-1BB, and CD3ζ signaling domains (BBz) has been developed to treat hepatocellular carcinoma (HCC) (27). NKG2D CAR-T cells with 4-1BB and CD3ζ efficiently lysed the HCC cell lines SMMC-7721 and MHCC97H in vitro in an NKG2DL-dependent manner. The NKG2D CAR-T cells effectively suppressed the growth of SMMC-7721 HCC xenografts. These results indicate that NKG2DBBz CAR-T cells could provide a promising therapeutic option for patients with NKG2DL-positive HCC.
6.3 Anti-lung cancers
The introduction of the cytoplasmic domain of DAP10 into second-generation CARs M28z and G28z to create M28z10 and G28z10, targeting mesothelin (MSLN) and glypican 3 (GPC3) respectively, resulted in enhanced and prolonged effector function against MSLN+ lung cancer cell lines (63). In addition, T cells expressing M28z10 or G28z10 exhibited elevated levels of cytokines and show greater anti-tumor activity compared to those expressing M28z. The study demonstrates that DAP10 signaling enhances the function of CAR-T cells in lung cancer cells, indicating its potential to improve the efficacy of CAR-T cell therapies for solid tumors. Jiang J et al. evaluated the therapeutic potential of NKG2D CAR-T cells on non-small cell lung cancer (NSCLC), obtained from diverse human autologous T cell sources (45). The results demonstrated that NKG2D CAR-T cells exhibit significant toxicity with elevated secretion of effector and memory function-related cytokines when compared to non-transduced control T cells. Furthermore, NKG2D CAR-T cells from healthy donors or NSCLC patients’ peripheral blood induced tumor shrinkage, improved survival, increased body weight, increased tumor-infiltrating capacity, and elevated serum IFN-γ levels in mice. This highlights the robust efficacy of NKG2D CAR-T cells in eradicating NSCLC in a NKG2DL-dependent manner, positioning them as a promising therapeutic option for NSCLC patients. An investigation of early cytotoxic lymphocyte infiltration in solid tumors led to the discovery that reduction in C-X3-C Motif Chemokine Ligand 1 & receptor 1 (CX3CL1-CX3CR1) restricts cytotoxic cells from the solid-tumor bed, contributing to tumor evasion (50). To address this, a CAR-T construct was designed, incorporating CX3CR1 overexpression to enhance infiltration. These engineered CAR-T cells demonstrate increased tumor infiltration rates compared to control-activated T cells or IL-15-overexpressing NKG2D CAR-T cells. Furthermore, these CAR-T cells are promising in a liver-cancer model, indicating their potential applicability in various solid malignancies. The combination treatment of NKG2D CAR-NK cells with CD73 targeting demonstrates enhanced anti-tumor cytotoxicity in vitro and in vivo, indicating a potential alleviation from adenosinergic immune-metabolic suppression (47). Furthermore, the blockade of CD73 improves the intra-tumoral homing of CD56+ CAR-NK cells in human lung cancer xenograft models. This approach represents a pioneering effort to modulate purinergic signaling and enhance adoptive NK cell immunotherapy, shedding light on potential autocrine tumor control and adenosinergic signaling.
6.4 Anti-gastrointestinal cancers
Gastric cancer ranks as the fourth leading cause of cancer-related deaths globally and presents a significant challenge in terms of treatment (81). The widespread expression of NKG2D ligands in gastric cancer cells makes them suitable targets for therapy (28). T cells engineered with an NKG2D-based second-generation CAR exhibit significantly enhanced cytolytic activity against gastric cancer cells compared to untransduced T cells. In vivo, these engineered cells effectively suppressed the growth of established gastric cancer xenografts either as a standalone therapy or in combination with chemotherapy cisplatin, a chemotherapy drug for treating gastric cancer (28). Another 2nd generation NKG2D-CAR-T cells with Dickkopf WNT Signaling Pathway Inhibitor 1 (DKK1) inhibition on gastric cancers showed the reversal of the suppressive tumor immune environment, increased NKG2DL expression, and significantly enhanced the immune-activating and tumor-killing capabilities of NKG2D-CAR-T cells in vitro and in vivo (41).
A non-virally engineered NKG2D CAR-T cell showed dose-dependent cytotoxicity against colorectal cancer cells in vitro, along with elevated secretion of IL-2 and IFN-γ compared to untransduced T cells (57). In a xenograft model, these cells effectively suppressed tumor growth, reduced tumor sizes, and prolonged overall survival of mice. Importantly, this study demonstrated the infiltration of human NKG2D-positive lymphocytes in tumor sections of treated mice, without severe pathological changes in vital organs, highlighting the safety and potential of NKG2D CAR-T cells as an immunotherapeutic strategy for human colorectal cancer. An mRNA based transient NKG2D CAR-NK cells without DAP10 but CD27/28, enhance NK cell tumor responses significantly against various solid tumor cell lines in vitro and demonstrating therapeutic benefits in mice with established colorectal cancer (68). Furthermore, in a clinical trial involving patients with metastatic colorectal cancer, local infusion of the CAR-NK cells resulted in reduced ascites generation, a marked decrease in tumor cell numbers, and rapid tumor regression in the liver region, highlighting the promising therapeutic potential of mRNA based NKG2D CAR-modified NK cells in treating metastatic colorectal cancer (39). Lenalidomide, a 4-amino-glutamyl analogue of thalidomide used as an immunomodulatory drug with potent clinical anti-neoplastic efficacy in solid tumors, significantly increases cytotoxic activity of a second-generation NKG2D-CAR-T cells against colorectal cancer cell lines, HCT116 and SW480 (40).
6.5 Anti-pancreatic cancer
Several NKG2D-based CAR-T cells have been investigated in preclinical models of pancreatic carcinoma (PC), which remains a clinical challenge. Cytoskeletal protein 4.1R (4.1R) dampens T cell signaling through inhibiting the phosphorylation of ZAP-70 (82). Knocking down 4.1R activates ERK signaling pathway in NKG2D CAR-T cells, which in turn induces higher cytotoxicity against PC cells in vitro and in a xenograft model (33). The NKG2D CAR-T cells with 4.1R knocked down have an increased proliferation rate and reduced expression of inhibitory receptors PD-1 and T-cell immunoglobulin and mucin domain 3 (TIM-3). With the effort to delineate the role of the G-Protein Coupled Receptor 116 (GPR116) receptor on NK cell function and on enhancing antitumor activity, GPR116-/- mice efficiently eliminated pancreatic cancer by enhancing NK cell proportion and targeting PC tumor through the Gαq/HIF1α/NF-κB signaling pathway (83). Furthermore, the study demonstrated that the downregulation of the GPR116 receptor in NKG2D-CAR-NK92 cells enhances their antitumor activity, presenting a novel approach to improve the efficiency of CAR-NK cell therapy. In an orthotopic implantation model for syngeneic pancreatic ductal adenocarcinoma (PDAC) tissue slices which maintains the immunosuppressive microenvironment, NKG2D CAR (chimeric NKG2D full length and CD3ζ)-T cells successfully eliminate myeloid derived suppressor cells (MDSC) and enhance the antitumor activity of subsequently infused CAR-T cells against primary PDAC cells (29). This emphasizes a potential rescue strategy against mechanisms which may impair NKG2D CAR-T cell activity in tumor microenvironment by eliminating MDSCs.
The effectiveness of CAR-T cell therapy is impeded by intrinsic factors within the tumor microenvironment. To address the immunosuppressive signals mediated by interleukin (IL)-4, a novel inverted cytokine receptor (ICR) was designed to convert IL-4R inhibitory signals into IL-15R activation signals downstream (42). This innovative CAR construct, 4/15NKG2D-CAR, co-expresses IL-4R as an extracellular domain and IL-15R as a transmembrane and intracellular domain. This approach augments NKG2D-CAR-T cell efficacy within the pancreatic tumor microenvironment, enhancing their activation, degranulation, cytokine production, and cytotoxicity against IL-4-expressing pancreatic cancer cells. Notably, 4/15NKG2D-CAR-T cells have higher activation, degranulation, cytokine release, and cytotoxic ability against IL-4+ pancreatic cell lines. They also display increased expansion, reduced exhaustion, and a higher proportion of less differentiated T cell phenotypes in vitro compared to conventional NKG2D-CAR-T cells. This novel NKG2D-CAR-T cell approach effectively overcomes IL-4-mediated immunosuppression in solid tumors, demonstrating superior tumor eradication compared to conventional NKG2D-CAR-T cells.
6.6 Anti-gynecological cancers
Ovarian cancer, an aggressive gynecologic malignancy disease, is ranked the fifth most common cause of women’s cancer deaths in North American, Australian, and Western European populations (84). Novel therapies are needed to either complement or even replace chemotherapy and irradiation. Various NKG2DLs, such as MICA/B and ULBP-1, -2, -3, and -4, are expressed across established ovarian cancer cell lines and primary ovarian cancer samples (30). A study profiling high-grade serous ovarian cancer revealed uniform expression of NKG2DLs on tumor cells, suggesting potential for NK cell-based therapies (85). However, the immunosuppressive tumor microenvironment (TME) is dominated by tumor-associated macrophages (TAMs), MDSCs, and regulatory T cells (TREG) (85, 86). These immunosuppressive cells negatively impact the anti-tumor immunity including therapeutic CAR-T or CAR-NK cells via direct cell contact or cytokines such as IL-10 or TGF-β (86). Barber et al. showed adoptive transfer of syngeneic mouse T cells with a CAR which contains only the NKG2D ectodomain and CD3ζ domain, leads to long-term tumor-free survival in ID8 ovarian tumor-bearing mice, generation of both CD4+ and CD8+ ID8-specific T cells, and protection against ID8 tumor challenge and rechallenges (15). These chNKG2D T cells transform the role of myeloid cells within the tumor site and convert them from being immunosuppressive to immune-stimulatory and hence enhance T cell responses. Following chNKG2D T cell treatment, cells isolated from the tumor exhibit increased production of IFN-γ, NO, and other proinflammatory cytokines. The complete response of chNKG2D T cells at the tumor site is dependent on perforin, IFN-γ, and GM-CSF. Spear P et al., reported that a delayed NKG2D CAR-T cell expansion occurs during manufacturing due to the fratricide of NKG2DL expression on activated T cells, but the NKG2D CAR-T eventually expands (26). CD4+ and CD8+ NKG2D CAR-T cells specifically recognize and kill NKG2DL-expressing ovarian cancer cell lines, but not NKG2DL-negative cells. Importantly, this study demonstrates that ovarian cancer cells, which exhibit moderate to low expression levels of NKG2DLs, can be pharmacologically modulated to enhance ligand expression. By using epigenetic agent such as histone deacetylase inhibitor, i.e., sodium valproate (VPA), it can upregulate NKG2DL surface expression and enhance immune recognition by the NKG2D CAR-T cells (31). Administration of these CAR-T cells augments antigen presentation to host CD4+ T cells at tumor site in a CXCR3-dependent manner. These host CD4+ T cells are found to be adequate for optimal tumor protection mediated by NKG2D CAR-expressing T cells but are not necessary if CD4+ T cells are adoptively co-transferred. Besides, there is no obvious off-target toxicity after NKG2D CAR-T infusion. Therefore, NKG2D CAR-T cells become a novel therapeutic approach for treating cervical cancer (30). Target-stimulated secretion of IL-7 from NKG2DIL7-CAR T cells demonstrated increased CAR-T cell number and viability compared to conventional NKG2D-CAR T cells by day 7 due to elevated expression of B-cell lymphoma-2 (Bcl-2), an anti-apoptotic protein, and glucose transporter 1 (Glut1) in NKG2DIL7-CAR T cells (23). This suggests that NKG2D-CAR-T cells expressing IL-7 could potentially persist in the immunosuppressive microenvironment of prostate cancer tissues and induce potent antitumor immunity. A systematic testing on a panel of NK CAR constructs identifies the one with NKG2D transmembrane domain, the 2B4 co-stimulatory domain, and the CD3ζ signaling domain with effective and strong antigen-specific NK cell signaling. Human iPSC-derived NK cells expressing this CAR (NK-CAR-iPSC-NK cells) display a typical NK cell phenotype and demonstrate enhanced anti-tumor activity compared to T-CAR-expressing iPSC-derived NK cells and non-CAR-expressing cells (25). In an ovarian cancer xenograft model, NK-CAR-iPSC-NK cells significantly inhibited tumor growth and prolonged survival, providing a promising “off-the-shelf” standardized lymphocyte therapy for anti-cancer immunotherapy. Similarly, CAR2 (CD244, NKG2D) is chosen to confer a stronger tumoricidal ability on CAR-NK92MI cells (67). p-PSMA-CAR-NK92MI cells are generated by expressing a CAR construct with a polypeptide-based antigen-binding region, an intracellular CD244, and a NKG2D costimulatory domain. They kill PSMA+ target cells selectively and successfully. Additionally, p-PSMA-CAR-NK92MI cells have significantly higher concentrations of IFN-γ, TNF-α, and granzyme B than NK92MI cells. In a CRPC cancer xenograft model, p-PSMA-CAR-NK92MI cells significantly inhibited tumor growth and exerted a more consistent killing effect than NK92MI cells. Ferroptosis is found to be a potential mechanism through which CAR-NK92MI cells utilize to attack cancer cells, which is triggered by IFN-γ.
7 NKG2D CAR-T cell targeting hematological malignancies
NKG2D-based CAR-T or CAR-NK cells have been investigated for the treatment of multiple hematological malignancies including mainly acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS) (16–18, 60, 62, 74), T-acute lymphoblastic leukemia (ALL) (17), lymphoma including B (35) or T cell lymphoma (19), and multiple myeloma (MM) (16, 19, 61). An artificial engineered CAR-T cells with an inert NKG2D receptor that can only be activated by specially designed “MicAbodies”, which is an antibody-ULBP2 fusion proteins binding to specific tumor antigens (35). By changing the MicAbody rather than re-engineering the T cells, this CAR-T platform enables the same immune cells to be redirected against different cancer targets as needed. With Burkitt’s lymphoma Raji cells implanted as a solid tumor model, the CAR-T cells could control the tumor burden with significant tumor shrinkages at the dose of 7x106 and 3.5x107. Moreover, high level of NKG2DL is detected on AML cell lines and primary AML cells (87). CAR-T cell products using either NKG2D full length/CD3ζ or NKG2D ectodomain/CD3ζ effectively kill the AML and T-ALL cell lines and primary patient cells (17). The cytotoxicity of NKG2D CAR-T cells is further enhanced by HDAC inhibitor treatment due to the induction of NKG2D-ligand expression in low-expressing AML cells and primary blasts. NKG2D CAR-NK cells generated from primary human NK cells successfully eradicated AML cells both in vitro and in a preclinical KG-1 cell-line derived mouse model, with their persistence enhanced by IL-15 co-expression (32). Robust engineering CAR-NK cells using optimized virus-free (88), feeder-free protocols (89) improves the feasibility of translating the CAR-NK cells in clinic (89, 90). A phase I clinical trial on the use of NKG2D CAR-T cells in AML/MDS and MM demonstrated that NKG2D CAR-T cells are safe, with no dose-limiting toxicities, cytokine release syndrome, CAR-T cell-related neurotoxicity, or autoimmune reactions (16). However, clinical benefit was modest, with only temporary responses in AML patients at the highest dose, likely due to limited CAR-T cell persistence and low target density. In a multicenter trial THINK, 16 of 25 AML, MDS, or MM patients were treated with CYAD-01 CAR-T cells at dose-escalation regime (18). At a median follow-up of 118 days, among the 12 evaluable patients with AML or MDS, three (25%) achieved an objective response. Two responding patients with AML subsequently underwent allogeneic hematopoietic stem-cell transplantation after CYAD-01 treatment, achieving durable ongoing remissions of 5 and 61 months. 7 patients (44%) had grade 3 or 4 treatment-related adverse events including cytokine release syndrome in 5 patients, with 1 dose-limiting toxicity reported at dose level three, though no treatment-related deaths occurred. While some anti-leukemic activities are shown in clinical trials, further investigation will focus on the combination strategies to enhance target expression and CAR-T cell persistence.
8 Multi-specific NKG2D CAR-T
NKG2D-based CAR-T or CAR-NK cells have been designed to recognize other oncotargets besides NKG2DLs. For AML, dual targeting CAR-T cells have been reported. CD123NK CAR-T cells targeting both CD123 and NKG2DLs shows effective anti-leukemia activity against AML cell lines and in a systemic cell-line derived AML mouse model (60). These NKG2D CAR-T cells also exhibit specific cytotoxicity against primary blasts, myeloid-derived suppressor cells (M-MDSCs), and alternatively activated macrophages (M2 cells), all of which express CD123 or NKG2DLs on over 50% of their cell surface. A novel NKG2D ectodomain/4-1BB/CD3ζ CAR design co-expressing anti-FLT3/4-1BB/CD3ζ CAR has been proposed to target the relapsed/refractory AML patients with FMS-like tyrosine kinase 3-internal tandem duplication (FLT3-ITD), who have limited treatment options and poor prognosis (62). Preclinical studies demonstrated that NKG2D CAR-T cells achieve specific lysis of AML cells both in vitro and in a MOLM-13 derived AML xenograft mouse model. Importantly, the efficacy was further enhanced when combined with the FLT3 inhibitor gilteritinib, which upregulates NKG2D ligand expression on AML cells via NFκB2/Rel B signaling pathway. Celyad have developed CD19/NKG2DL, BCMA/NKG2DL and PSMA/NKG2DL multi-specific CAR T-cells to overcome antigen escape and improve anti-tumor efficacy (18, 91, 92). Their products utilize both tandem constructs that comprise the human NKG2D extracellular domain fused to a scFv targeting CD19, BCMA or PSMA, or dual constructs where NKG2D CAR co-expresses anti-CD19, anti-BCMA or anti-PSMA CAR. Celyad showed CD19/NKG2DL multi-specific CAR T-cells are effective in vitro against CD19+ and CD19− cell lines and against CD19+ primary B-ALL cells. CD19/NKG2DL tandem CAR T-cells outperform CD19 single CAR-T cells in efficiently controlling tumor cells in a relapsed B-ALL in vivo model (93). Similarly, the same study showed BCMA/NKG2DL and PSMA/NKG2DL multi-specific CAR-T cells are efficient even in the absence of BCMA or PSMA. Kaedi Biotherapeutics designs a novel bispecific tandem CAR-T cells (KD-496), which targets both NKG2D ligands and Claudin 18.2 (CLDN18.2) to treat gastric cancer in vitro and in vivo (94). The bispecific CAR-T cell KD-496 has a CD8 hinge region and transmembrane region, 4-1BB costimulatory region and CD3ζ region. Co-incubation of KD-496 CAR-T cells with double positive NUGC4 and MKN-28-18.2 cells specifically lyse tumor cells even at low effector-to-target (E:T) ratio with elevated IFN-γ secretion. Besides, KD-496 CAR- T cells efficiently eliminate xenograft tumors in vivo than single CAR with no obvious safety issue in the treated mice. No obvious pathological changes are observed in the tested organs. Future clinical development of KD-496 CAR-T is warranted with gastric cancer patients. A 2nd generation NKG2D CAR system has been developed recently to have two independent chimeric receptors: One receptor consists of the NKG2D extracellular domain linked with DAP12 for T cell activation, while the other uses the PD-1 extracellular domain linked with 4-1BB for costimulatory signal 2 input (24). The dual NKG2D PDL1 CAR-T cells, generated through electroporation of non-viral piggyBac transposon plasmids, effectively eliminate target cancer cells and eradicate established peritoneal metastasis of both colorectal cancer and ovarian cancer using in vivo mouse model.
9 Clinical application of NKG2D CAR-T
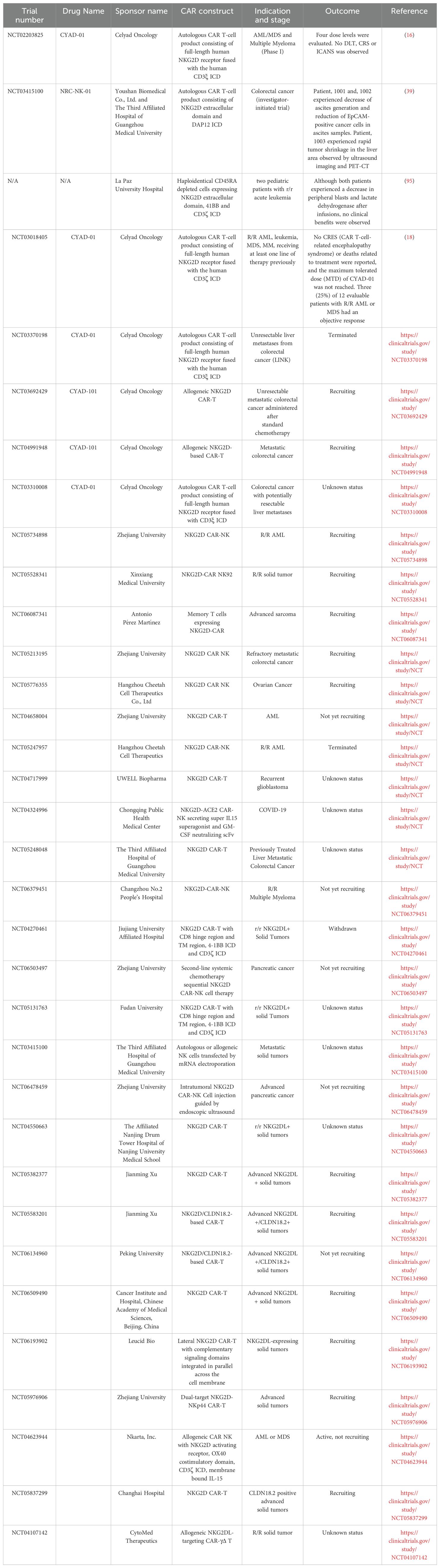
Based on the information from clinicaltrial.gov and open access public domains, there are 21 clinical trials focusing on various NKG2D CAR targets (Table 3). These targets encompass a wide spectrum of hematological conditions, including relapsed/refractory AML, MDS, MM, as well as many types of solid tumors such as refractory metastatic colorectal cancer, ovarian cancer, gastric cancer, hepatocellular carcinoma, glioblastoma, medulloblastoma, triple-negative breast cancer, sarcoma, nasopharyngeal carcinoma, and prostate cancer. We summarize those with clinical data reported.
A study team based in in Guangdong, China reported a phase 1 clinical trial results on three metastatic colorectal cancer patients received adoptive transfer of NKG2D CAR mRNA-engineered natural killer cells (39). CAR-NK cells are prepared by electroporation of in vitro transcribed mRNA NKG2D CAR with NKG2D ectodomain fused to DAP12 (39). These NKG2D mRNA CAR-NK shows strong cytotoxicity against tumor cells in vitro and in mouse models. A patient received two intraperitoneal injections (i.p.) of autologous CAR-NK cells (2x107 and 1x108 cells), while another patient was treated with four i.p. infusions of allogeneic haploidentical CAR-NK cells (1x108, 3x108, 5x108, and 7x108 cells). Both patients had decreased ascitic fluid production with reduced EpCAM-positive cancer cells in ascites samples. The 3rd Patient was treated with six infusions of allogeneic haploidentical CAR-NK cells (5x108 cells twice, 1x109 cells twice, 2x109 cells twice), with ultrasound-guided percutaneous injection, followed by intraperitoneal infusion of the CAR-NK cells. Rapid tumor shrinkage in the hepatic area was demonstrated by ultrasound imaging and positron emission tomography (PET)-computed tomographic (CT), which was confirmed by immunohistochemistry staining.
In a study performed on two pediatric patients with advanced relapsed and refractory acute leukemia, haploidentical CD45RA depleted cells which expressed NKG2D-41BB-CD3ζ CAR were infused (58). Patient 1 was a three-year-old female who received 3 weekly infusions of NKG2D CAR-TCD45RA- (1x107 cells/kg), and the only adverse effect was fever. Then the patient underwent lymphodepletion prior to two weekly infusions of the same number of CAR-T cells, with only skin rash observed. However, the patient died on day +60 post-infusion due to disease progression. Patient 2 was a 15-year-old female, who received a single dose of CAR-T CD45RA- (1x107 cells/kg), experienced grade 2 cytokine release syndrome (CRS), and died on day +14. Patients 2 had an invasive fungal infection, which might impact on the outcome of the patient. Although a decrease in peripheral blasts and lactate dehydrogenase was observed after infusions in both patients, no clinical benefits were observed.
CYAD-01 from Celyad Oncology (formerly known as NKR-2) is an autologous NKG2D-CAR T cell therapy being tested alone or in combination with chemotherapy for hematological and solid cancers. An enhanced version of CYAD-01, CYAD-02, incorporates the shRNA targeting NKG2D ligands on CAR-T cells to improve its efficacy and is currently under clinical trials in patients with acute myeloid leukemia and myelodysplastic syndrome (13, 96). Additionally, Celyad Oncology has developed CYAD-101, an allogeneic NKG2D-CAR-T cell therapy for patients with unresectable metastatic colorectal cancer. A single infusion of low-dose CYAD-01 in the absence of preconditioning chemotherapy was evaluated in a first-in-human clinical study (NCT02203825) (10). Autologous T cells were transfected with a γ-retroviral vector encoding NKG2D CAR with CD3ζ signaling domain. Four dose levels (1×106, 3×106, 1×107, 3×107 total viable T cells) were evaluated. No dose limiting toxicity (DLT), CRS or Immune effector cell-associated neurotoxicity syndrome (ICANS) was observed, none of the grade 3 and 4 adverse events were attributed to the NKG2D-CAR-T infusion. No objective tumor responses were observed in the low dose group. Only one patient with AML at dose level 4 experienced improvement in hematologic parameters without further treatment. The expansion and persistence of NKG2D-CAR T cell was limited according to preclinical study, suggesting higher dose and multiple infusions of CYAD-01 might be necessary (16).
Celyad’s allogeneic CAR-T pipeline, CYAD-101, featured the incorporation of the TCR Inhibitory Molecule (TIM) to mitigate the risk of graft-versus-host disease (GvHD). TIM, a truncated CD3ζ peptide, is co-expressed with the NKG2D-CAR construct and integrated into the T cell receptor (TCR) complex to dampen TCR responses. Notably, CYAD-101 showed CAR-driven antitumor activity both in vitro and in vivo with signs of GvHD in mouse models (92). A significant aspect of the CYAD-101 pipeline is the production of two clinical grade independent batches from a single donor, resulting in 4.8×1010 CAR-T cells, an ample quantity for the entire dose-escalation phase of the planned alloSHRINK clinical trial (NCT03692429). These two batches exhibited high consistency, predominantly comprising a CD4+ T-cell population that maintains a similar effector memory/central memory phenotype with minimal expression of exhaustion markers (over 99% LAG3-PD-1- population). Furthermore, these clinical grade CYAD-101 cells demonstrated specific in vitro anti-tumor activity with minimal response to TCR stimulation.
10 NKG2D-based antibodies as cancer treatment
The anti-tumor role of NKG2D has been demonstrated in various studies using different anti-NKG2D antibodies either as a neutralizing antibody or activation antibody. Using two novel anti-mouse NKGD monoclonal antibodies (derived from hamster), stimulation with anti-NKG2D mAb redirected and enhanced lysis of tumor targets expressing NKG2D ligand (97). Notably, NKG2D alone did not induce cytokine release, but in conjunction with other NK activation receptors, cytokine release can then be enhanced. This supports NKG2D’s ability to co-stimulate multiple NK activation receptors. Cross-linking NKG2D with an anti-NKG2D antibody to simulate ligand binding shows an increase in the production of soluble TRAIL (sTRAIL) by γδ T cells (98). This sTRAIL induces apoptosis in lung cancer cells through TRAIL R2. Neutralizing these sTRAIL or blocking lung cancer cell TRAIL R2 leads to a significant reduction in γδ T-cell-mediated cytotoxicity to lung cancer cells, suggesting the unresolved mechanism of anticancer immunity through the NKG2D-regulated production of sTRAIL. Talebian L et al. also proved the role of NKG2D by demonstrating that via blocking the NKG2D receptor through monoclonal antibodies or siRNAs on cytotoxic T cells will reverse their cytotoxicity on autologous myeloma cell (99). In the same study, the T cell population of NKG2D+CD3+CD8+ can be expanded ex vivo, and these cells identify and destroy autologous and allogeneic myeloma cells independently of T-cell receptor or MHC-I expression. NKG2D+CD3+CD8+ T cells provide anti-myeloma activity in a NKG2D-dependent manner and trigger the release of proinflammatory IFN-γ and TNF-α.
Two bispecific antibodies have attempted to target NKG2D ligand negative tumor cells. NKAB-ErbB2 significantly enhanced the lysis of ErbB2-positive breast carcinoma cells by NKG2D-expressing NK cells from peripheral blood, surpassing the effectiveness of an ErbB2-specific IgG1 mini-antibody that induces cytotoxicity via CD16 activation (49). Additionally, NKAB-ErbB2 demonstrates synergy with NK-92 cells or primary T cells engineered with an NKG2D-CD3ζ chimeric antigen receptor (NKAR), leading to targeted cell killing and notably improved anti-tumor activity. Importantly, these effects are not hindered by soluble MICA, which is known to inhibit NKG2D-mediated natural cytotoxicity. In an immunocompetent mouse model of glioblastoma with low or absent NKG2DL expression, the combination of NKAR-NK-92 cells and NKAB-ErbB2 effectively suppressed the growth of ErbB2-positive tumors, resulting in treatment-induced endogenous antitumor immunity and cures majority of the animals. A novel NKG2D bispecific antibody (CS1-NKG2D biAb) is designed to bridge CS1 (other name SLAMF7) positive human MM cell lines and all NKG2D+ cytolytic cells including NK and cytotoxic T cells (6). The cytotoxicity was specific to both CS1 and NKG2D with a specific triggering on the phosphorylation of AKT, a downstream protein kinase of the activated NKG2D-DAP10 complex on effectors cells. In vivo, the survival is significantly extended using the CS1-NKG2D biAb in a xenograft NSG mouse model engrafted with both human PBMCs and MM cell lines.
11 Synergism with OTHER CANCER therapy or antibodies
The effect of anti-NKG2D antibody has been tested also in the presence of chemotherapy or other antibodies targeting immune checkpoint protein. Using A549 lung cancer and murine Lewis lung carcinoma models, applying either anti-PD-1 or anti-NKG2D antibodies in combination with oxaliplatin (OXA) synergistically suppresses tumor growth and prolongs mouse survival, offering a promising treatment benefit (100). OXA’s role in promoting T cells and NK cells infiltration through the CXCL9/10/11-CXCR3 axis can enhance anti-PD1 or anti-NKG2D immunotherapy in lung cancer. Ionizing radiation (IR) induced NKG2D ligand RAE-1 expression in vivo, and the combination therapy of IR and anti-CTLA-4 mAb resulted in tumor-infiltrating lymphocyte (TIL) motility arrest. However, the addition of anti-NKG2D mAb blocked this TIL arrest induced by the IR/9H10 combination therapy.
12 Challenge facing NKG2D-based immunotherapeutics
While early results are promising, potential challenges remain to be resolved. For instance, the presence of surface NKG2DLs does not always translate into an enhanced cytolytic immune response against cancer due to NKG2DLs shedding and tumor microenvironment. Soluble NKG2DLs in the serum of patients with leukemias, MM, and lymphomas have been shown to have prognostic significance (101–104). Tumors shed NKG2DLs from their surface to evade the immune surveillance, resulting in high levels of soluble NKG2DLs (5, 10). These soluble NKG2DLs bind to NKG2D, leading to internalization and systemic desensitization of NKG2D in effector cells and impaired anti-tumor function. Therefore, although targeting NKG2DLs represents a promising treatment strategy for cancer therapy, it is crucial to address the potential impact of soluble NKG2DLs on effector cell responsiveness by downregulating the NKG2D. Strategies including pharmacological modulation have been proposed to mitigate the effect of shedding. Inhibitors of ADAM10 and ADAM17, which function as ectodomain sheddases to cleave the NKG2D from effector membrane, have been proposed to block and restore the NKG2D activation (105, 106). Nutlin-3a, a small molecule inhibitor targeting the p53-antagonist MDM2, reverses the dysregulation of p53 functions in neuroblastoma cell lines and patient cells (107). This treatment stimulates the surface expression of ULBPs and NK cell coreceptor DNAM-1 ligands (PVR and Nectin-2). As a result, Nutlin-3a induces augmented NK cell cytotoxicity against neuroblastoma both in vitro and in vivo. This evidence also suggests the potential integration of different pathways regulating NKG2DL and DNAM-1 ligand expression. Dual-targeting CAR designs incorporating both NKG2D and DNAM-1 (108) warrant further clinical investigation.
During CAR-T expansion, CD3/CD28 and cytokine-induced activation creates stress and transiently induces NKG2DL on T cells (18). Consequently, NKG2D CAR-T cells may kill each other during culture and expansion, preventing the large-scale manufacturing of CAR-T for clinical development. Potential strategies to avoid fratricide have been proposed in the large-scale manufacturing of NKG2D CAR-T (109). PI-3K inhibitor LY294002 was included in the manufacturing process. LY294002 is shown to reversibly reduce NKG2D expression on the CAR-T surface and partially controls fratricide during manufacturing and enhanced viability post-thawing as well. But the level of manufacturing failure increases, as the trial moved through the dose-escalation phase towards the upper dose level. This is largely attributed to the effect of LY294002 which can suppress T cell proliferation (110). Alternatively, NKG2D blocking antibody was added during the expansion phase of cell culture to further prevent fratricide (109). These two strategies enable the efficient manufacture of CYAD-01 T cells to the levels required for the THINK clinical trial. NKG2D CAR-NK instead of CAR-T cells are reported to be resistant to the soluble NKG2DLs and self-fratricide because NKG2DLs expresses on T cells upon activation and CAR-T manufacturing under cytokine and CD3/CD28 stimulations (76).
TME influences NKG2D-based CAR-T or CAR-NK efficacy through multiple mechanisms such as immunosuppressive cells like TAMs, MDSCs and TREG, which express NKG2DLs, enabling CAR-mediated targeting of both malignant and stromal components (13, 14). In a preclinical neuroblastoma model, NKG2Dζ NK cells effectively kill both ex vivo generated MDSCs in vitro and tumor-infiltrating MDSCs in vivo. This elimination of immunosuppressive MDSCs indirectly reduces tumor burden and prolongs survival (76). However, the TME also poses challenges through soluble NKG2D ligands (e.g., MICA/B) and exosomal factors that downregulate receptor expression or induce exhaustion (13), although NKG2Dζ NK are reported to be resistant to the immunosuppressive TGF-β and soluble NKG2DL in TME of neuroblastoma (76). Metabolic constraints and inhibitory checkpoints (e.g., PD-1, LAG-3) further dampen functionality, though combination strategies with radiation therapy, HDAC inhibitors or checkpoint blockade show potential to enhance ligand expression and sustain effector responses (13, 17, 111, 112). While off-tumor toxicity remains a concern due to ligand expression on stressed non-malignant tissues, current clinical approaches aim to balance efficacy with safety through controlled CAR designs and microenvironment modulation.
NKG2DL, as a stress ligand, expresses in cells under stress response and cell senescence. The on-target-off-tumor effect of NKG2D CAR-T cells on non-malignant cells under stress or senescence during stressful cancer treatment remains unknown. Evidence shows NKG2D CAR-T cells could eliminate human cells undergoing senescence induced by replicative stress, oncogenic stress, DNA damage in vitro in a selective and effective way (73).
13 Targeting NKG2D using blocking or activating antibodies
A set of highly specific anti-human NKG2D single-domain antibodies targeting different epitopes has been developed over the years (113, 114). These single-domain antibodies are incorporated into bivalent and bispecific antibodies using a versatile plug-and-play Fab-like format. Depending on the context, these Fab-like antibodies display activating or neutralizing effects on the immune response mediated by the NKG2DL/NKG2D axis. In solution, the bivalent anti-NKG2D antibodies, which compete with NKG2DL, effectively block the activation of NK cells seeded on immobilized MICA, making them potential antagonists. Additionally, a bispecific anti-NKG2DxHER2 antibody that simultaneously engages HER2 on tumor cells and NKG2D on NK cells induces cytotoxicity of unstimulated NK cells in a tumor-specific manner, regardless of their apparent affinities and epitopes (49). Crucially, the bispecific antibody that does not compete with ligand binding retains its full cytotoxic activity in the presence of ligands, which is a valuable attribute for overcoming immunosuppressive effects induced by soluble ligands in the tumor microenvironment. Recent studies have highlighted the importance of 2:1 stoichiometry, distinct binding epitopes from natural ligand, and optimal-affinity interactions (nM range) in the design of agonistic anti-NKG2D antibodies (115, 116).
14 NKG2D as AN activation receptor or coreceptor depending on effector cell types
Although NKG2D is primarily recognized as an activation receptor in NK cells, it also demonstrates versatility by functioning as a co-receptor depending on the cell type and context. As an activating receptor, NKG2D is predominantly expressed on cytolytic cells in the immune system, where its engagement can directly stimulate the production of cytokines and cytotoxic molecules in NK cells. On activated NK cells primed by pro-inflammatory cytokines such as IL-2 and IL-15, NKG2D provides direct stimulatory signals. However, NKG2D’s role extends beyond just activation. In resting NK cells, it acts as a co-activator, synergizing with other receptors such as NKp46 and 2B4 (117). In αβ T cells such as CD8+ T cells, NKG2D typically provides a co-stimulatory signal, promoting T cell receptor (TCR)-dependent cytotoxicity, production of pro-inflammatory cytokines, and memory differentiation (99). In invariant NKT cells, it facilitates direct target cell lysis and provides co-stimulatory activation, whereas in mucosal-associated invariant T (MAIT) cells, particularly CD8+ subsets, it functions primarily as a co-stimulatory molecule. This dual functionality underscores NKG2D’s status as one of the most versatile and widely distributed activating/co-stimulatory NK-related receptors. The activation signals mounted on effector cells depend on the intensity and duration of ligand engagement, further highlights its adaptability. Ultimately, NKG2D’s role can vary based on the cell type, activation state, and surrounding cytokine environment, making it a crucial and flexible component of the immune system’s regulatory network.
15 Conclusions and future directions
NKG2D has been harnessed in numerous CAR and antibody designs for cancer immunotherapy, incorporating full-length receptors, extracellular domains, or cytoplasmic components, and applying these constructs to both T and NK cells. With the known intrinsic functions in NKG2D-NKG2DL axis, further investigations should focus on how these NKG2D-based CARs and antibodies may affect the intrinsic signaling pathways inside the effector cells. Utilizing NKG2D-knockout cellular models in NK cell lines such as NK-92 and KHYG-1, as well as in primary NK cells, represents a promising strategy to investigate the intrinsic effects of NKG2D-CAR expression and antibody engagement on NK cell function. While emerging evidence supports the therapeutic potential of NKG2D-based CARs and antibodies in cancer immunotherapy, their application will be particularly promising for solid tumors, where NKG2D ligands are often highly expressed.
Author contributions
JH: Conceptualization, Data curation, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing, Formal analysis. YW: Writing – original draft, Writing – review & editing, Conceptualization. GC: Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization. WC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the OSU Department of Internal Medicine Junior Investigator Award (GR13032), Division Sponsored Research Program (2024-GR135047), Pelotonia Idea Award (2023-2024), and Elsa Pardee Foundation Cancer Research Award (20321).
Acknowledgments
We thank all the supports from Dr. Lapo Alinari and Pelotonia Research Center.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Deng W, Gowen BG, Zhang L, Wang L, Lau S, Iannello A, et al. Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science. (2015) 348:136–9. doi: 10.1126/science.1258867
2. Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. (2003) 3:781–90. doi: 10.1038/nri1199
3. Spear P, Barber A, Rynda-Apple A, and Sentman CL. NKG2D CAR T-cell therapy inhibits the growth of NKG2D ligand heterogeneous tumors. Immunol Cell Biol. (2013) 91:435–40. doi: 10.1038/icb.2013.17
4. Spear P, Wu MR, Sentman ML, and Sentman CL. NKG2D ligands as therapeutic targets. Cancer Immun. (2013) 13:8.
5. Lanier LL. NKG2D receptor and its ligands in host defense. Cancer Immunol Res. (2015) 3:575–82. doi: 10.1158/2326-6066.CIR-15-0098
6. Chan WK, Kang S, Youssef Y, Glankler EN, Barrett ER, Carter AM, et al. A CS1-NKG2D bispecific antibody collectively activates cytolytic immune cells against multiple myeloma. Cancer Immunol Res. (2018) 6:776–87. doi: 10.1158/2326-6066.CIR-17-0649
7. Bryceson YT, March ME, Ljunggren HG, and Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. (2006) 107:159–66. doi: 10.1182/blood-2005-04-1351
8. López-Larrea C, Suárez-Alvarez B, López-Soto A, López-Vázquez A, and Gonzalez S. The NKG2D receptor: sensing stressed cells. Trends Mol Med. (2008) 14:179–89. doi: 10.1016/j.molmed.2008.02.004
9. Upshaw JL, Arneson LN, Schoon RA, Dick CJ, Billadeau DD, and Leibson PJ. NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nat Immunol. (2006) 7:524–32. doi: 10.1038/ni1325
10. Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. (1999) 285:730–2. doi: 10.1126/science.285.5428.730
11. Stojanovic A, Correia MP, and Cerwenka A. The NKG2D/NKG2DL axis in the crosstalk between lymphoid and myeloid cells in health and disease. Front Immunol. (2018) 9:827. doi: 10.3389/fimmu.2018.00827
12. Le Bert N and Gasser S. Advances in NKG2D ligand recognition and responses by NK cells. Immunol Cell Biol. (2014) 92:230–6. doi: 10.1038/icb.2013.111
13. Curio S, Jonsson G, and Marinović S. A summary of current NKG2D-based CAR clinical trials. Immunother Adv. (2021) 1:ltab018. doi: 10.1093/immadv/ltab018
14. Sentman CL and Meehan KR. NKG2D CARs as cell therapy for cancer. Cancer J. (2014) 20:156–9. doi: 10.1097/PPO.0000000000000029
15. Barber A, Rynda A, and Sentman CL. Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol. (2009) 183:6939–47. doi: 10.4049/jimmunol.0902000
16. Baumeister SH, Murad J, Werner L, Daley H, Trebeden-Negre H, Gicobi JK, et al. Phase I trial of autologous CAR T cells targeting NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer Immunol Res. (2019) 7:100–12. doi: 10.1158/2326-6066.CIR-18-0307
17. Driouk L, Gicobi JK, Kamihara Y, Rutherford K, Dranoff G, Ritz J, et al. Chimeric antigen receptor T cells targeting NKG2D-ligands show robust efficacy against acute myeloid leukemia and T-cell acute lymphoblastic leukemia. Front Immunol. (2020) 11:580328. doi: 10.3389/fimmu.2020.580328
18. Sallman DA, Kerre T, Havelange V, Poiré X, Lewalle P, Wang ES, et al. CYAD-01, an autologous NKG2D-based CAR T-cell therapy, in relapsed or refractory acute myeloid leukaemia and myelodysplastic syndromes or multiple myeloma (THINK): haematological cohorts of the dose escalation segment of a phase 1 trial. Lancet Haematol. (2023) 10:e191–202. doi: 10.1016/S2352-3026(22)00378-7
19. Zhang T, Barber A, and Sentman CL. Chimeric NKG2D modified T cells inhibit systemic T-cell lymphoma growth in a manner involving multiple cytokines and cytotoxic pathways. Cancer Res. (2007) 67:11029–36. doi: 10.1158/0008-5472.CAN-07-2251
20. VanSeggelen H, Hammill JA, Dvorkin-Gheva A, Tantalo DG, Kwiecien JM, Denisova GF, et al. T cells engineered with chimeric antigen receptors targeting NKG2D ligands display lethal toxicity in mice. Mol Ther. (2015) 23:1600–10. doi: 10.1038/mt.2015.119
21. Wei C, Xia K, Xie Y, Ye S, Ding Y, Liu Z, et al. Combination of 4-1BB and DAP10 promotes proliferation and persistence of NKG2D(bbz) CAR-T cells. Front Oncol. (2022) 12:893124. doi: 10.3389/fonc.2022.893124
22. Barber A, Zhang T, and Sentman CL. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J Immunol. (2008) 180:72–8. doi: 10.4049/jimmunol.180.1.72
23. He C, Zhou Y, Li Z, Farooq MA, Ajmal I, Zhang H, et al. Co-expression of IL-7 improves NKG2D-based CAR T cell therapy on prostate cancer by enhancing the expansion and inhibiting the apoptosis and exhaustion. Cancers (Basel). (2020) 12(7):1969. doi: 10.3390/cancers12071969
24. Jiang G, Ng YY, Tay JCK, Du Z, Xiao L, Wang S, et al. Dual CAR-T cells to treat cancers co-expressing NKG2D and PD1 ligands in xenograft models of peritoneal metastasis. Cancer Immunol Immunother. (2023) 72:223–34. doi: 10.1007/s00262-022-03247-9
25. Li Y, Hermanson DL, Moriarity BS, and Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. (2018) 23:181–192.e5. doi: 10.1016/j.stem.2018.06.002
26. Spear P, Barber A, and Sentman CL. Collaboration of chimeric antigen receptor (CAR)-expressing T cells and host T cells for optimal elimination of established ovarian tumors. Oncoimmunology. (2013) 2:e23564. doi: 10.4161/onci.23564
27. Sun B, Yang D, Dai H, Liu X, Jia R, Cui X, et al. Eradication of hepatocellular carcinoma by NKG2D-based CAR-T cells. Cancer Immunol Res. (2019) 7:1813–23. doi: 10.1158/2326-6066.CIR-19-0026
28. Tao K, He M, Tao F, Xu G, Ye M, Zheng Y, et al. Development of NKG2D-based chimeric antigen receptor-T cells for gastric cancer treatment. Cancer Chemother Pharmacol. (2018) 82:815–27. doi: 10.1007/s00280-018-3670-0
29. Wang J, Liu X, Ji J, Luo J, Zhao Y, Zhou X, et al. Orthotopic and heterotopic murine models of pancreatic cancer exhibit different immunological microenvironments and different responses to immunotherapy. Front Immunol. (2022) 13:863346. doi: 10.3389/fimmu.2022.863346
30. Zhang Y, Li X, Zhang J, and Mao L. Novel cellular immunotherapy using NKG2D CAR-T for the treatment of cervical cancer. BioMed Pharmacother. (2020) 131:110562. doi: 10.1016/j.biopha.2020.110562
31. Song DG, Ye Q, Santoro S, Fang C, Best A, and Powell DJ. Chimeric NKG2D CAR-expressing T cell-mediated attack of human ovarian cancer is enhanced by histone deacetylase inhibition. Hum Gene Ther. (2013) 24:295–305. doi: 10.1089/hum.2012.143
32. Du Z, Ng YY, Zha S, and Wang S. piggyBac system to co-express NKG2D CAR and IL-15 to augment the. Mol Ther Methods Clin Dev. (2021) 23:582–96. doi: 10.1016/j.omtm.2021.10.014
33. Gao Y, Lin H, Guo D, Cheng S, Zhou Y, Zhang L, et al. Suppression of 4.1R enhances the potency of NKG2D-CAR T cells against pancreatic carcinoma via activating ERK signaling pathway. Oncogenesis. (2021) 10:62. doi: 10.1038/s41389-021-00353-8
34. Guo C, Chen H, Yu J, Lu H, Xia Q, Li X, et al. Engagement of an optimized lentiviral vector enhances the expression and cytotoxicity of CAR in human NK cells. Mol Immunol. (2023) 155:91–9. doi: 10.1016/j.molimm.2023.01.010
35. Landgraf KE, Williams SR, Steiger D, Gebhart D, Lok S, Martin DW, et al. convertibleCARs: A chimeric antigen receptor system for flexible control of activity and antigen targeting. Commun Biol. (2020) 3:296. doi: 10.1038/s42003-020-1021-2
36. Liu R, Luo Q, Luo W, Wan L, Zhu Q, Yin X, et al. A soluble NK-CAR mediates the specific cytotoxicity of NK cells toward the target CD20. Aging Dis. (2022) 13:1576–88. doi: 10.14336/AD.2022.0415
37. Ng YY, Tay JCK, Li Z, Wang J, Zhu J, and Wang S. T cells expressing NKG2D CAR with a DAP12 signaling domain stimulate lower cytokine production while effective in tumor eradication. Mol Ther. (2021) 29:75–85. doi: 10.1016/j.ymthe.2020.08.016
38. Tay JCK, Wang J, Du Z, Ng YY, Li Z, Ren Y, et al. Manufacturing NKG2D CAR-T cells with piggyBac transposon vectors and K562 artificial antigen-presenting cells. Mol Ther Methods Clin Dev. (2021) 21:107–20. doi: 10.1016/j.omtm.2021.02.023
39. Xiao L, Cen D, Gan H, Sun Y, Huang N, Xiong H, et al. Adoptive transfer of NKG2D CAR mRNA-engineered natural killer cells in colorectal cancer patients. Mol Ther. (2019) 27:1114–25. doi: 10.1016/j.ymthe.2019.03.011
40. Zarei M, Abdoli S, Farazmandfar T, and Shahbazi M. Lenalidomide improves NKG2D-based CAR-T cell activity against colorectal cancer cells invitro. Heliyon. (2023) 9:e20460. doi: 10.1016/j.heliyon.2023.e20460
41. Zhang Y, Liang K, Zhou X, Zhang X, Xu H, Dai H, et al. Combination therapy of DKK1 inhibition and NKG2D chimeric antigen receptor T cells for the treatment of gastric cancer. Cancer Sci. (2023) 114:2798–809. doi: 10.1111/cas.15828
42. Zhou Y, Farooq MA, Ajmal I, He C, Gao Y, Guo D, et al. Co-expression of IL-4/IL-15-based inverted cytokine receptor in CAR-T cells overcomes IL-4 signaling in immunosuppressive pancreatic tumor microenvironment. BioMed Pharmacother. (2023) 168:115740. doi: 10.1016/j.biopha.2023.115740
43. Fernández L, Metais JY, Escudero A, Vela M, Valentín J, Vallcorba I, et al. Memory T cells expressing an NKG2D-CAR efficiently target osteosarcoma cells. Clin Cancer Res. (2017) 23:5824–35. doi: 10.1158/1078-0432.CCR-17-0075
44. Han Y, Xie W, Song DG, and Powell DJ. Control of triple-negative breast cancer using ex vivo self-enriched, costimulated NKG2D CAR T cells. J Hematol Oncol. (2018) 11:92. doi: 10.1186/s13045-018-0635-z
45. Jiang J, Liu Y, Zeng Y, Fang B, and Chen Y. Annihilation of non-small cell lung cancer by NKG2D CAR-T cells produced from T cells from peripheral blood of healthy donors. J Interferon Cytokine Res. (2023) 43:445–54. doi: 10.1089/jir.2023.0043
46. Lehner M, Götz G, Proff J, Schaft N, Dörrie J, Full F, et al. Redirecting T cells to Ewing's sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PloS One. (2012) 7:e31210. doi: 10.1371/journal.pone.0031210
47. Wang J, Lupo KB, Chambers AM, and Matosevic S. Purinergic targeting enhances immunotherapy of CD73. J Immunother Cancer. (2018) 6:136. doi: 10.1186/s40425-018-0441-8
48. Yang D, Sun B, Dai H, Li W, Shi L, Zhang P, et al. T cells expressing NKG2D chimeric antigen receptors efficiently eliminate glioblastoma and cancer stem cells. J Immunother Cancer. (2019) 7:171. doi: 10.1186/s40425-019-0642-9
49. Zhang C, Röder J, Scherer A, Bodden M, Pfeifer Serrahima J, Bhatti A, et al. Bispecific antibody-mediated redirection of NKG2D-CAR natural killer cells facilitates dual targeting and enhances antitumor activity. J Immunother Cancer. (2021) 9(10):e002980. doi: 10.1136/jitc-2021-002980
50. Trinh T, Adams WA, Calescibetta A, Tu N, Dalton R, So T, et al. CX3CR1 deficiency-induced TIL tumor restriction as a novel addition for CAR-T design in solid Malignancies. iScience. (2023) 26:106443. doi: 10.1016/j.isci.2023.106443
51. Teng X, Rong Z, Li S, Yang W, and Lu Z. T cells engineered with full-length NKG2D linked to signaling domains of 4-1BB and CD3ζ show enhanced antitumor activity. Immunol Cell Biol. (2023) 101:458–64. doi: 10.1111/imcb.12634
52. Ang WX, Ng YY, Xiao L, Chen C, Li Z, Chi Z, et al. Electroporation of NKG2D RNA CAR improves Vγ9Vδ2 T cell responses against human solid tumor xenografts. Mol Ther Oncolytics. (2020) 17:421–30. doi: 10.1016/j.omto.2020.04.013
53. Guo C, Wang X, Zhang H, Zhi L, Lv T, Li M, et al. Structure-based rational design of a novel chimeric PD1-NKG2D receptor for natural killer cells. Mol Immunol. (2019) 114:108–13. doi: 10.1016/j.molimm.2019.07.009
54. Lu C, Guo C, Chen H, Zhang H, Zhi L, Lv T, et al. A novel chimeric PD1-NKG2D-41BB receptor enhances antitumor activity of NK92 cells against human lung cancer H1299 cells by triggering pyroptosis. Mol Immunol. (2020) 122:200–6. doi: 10.1016/j.molimm.2020.04.016
55. Meister H, Look T, Roth P, Pascolo S, Sahin U, Lee S, et al. Multifunctional mRNA-based CAR T cells display promising antitumor activity against glioblastoma. Clin Cancer Res. (2022) 28:4747–56. doi: 10.1158/1078-0432.CCR-21-4384
56. Weiss T, Weller M, Guckenberger M, Sentman CL, and Roth P. NKG2D-based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Cancer Res. (2018) 78:1031–43. doi: 10.1158/0008-5472.CAN-17-1788
57. Deng X, Gao F, Li N, Li Q, Zhou Y, Yang T, et al. Antitumor activity of NKG2D CAR-T cells against human colorectal cancer cells in vitro and in vivo. Am J Cancer Res. (2019) 9:945–58.
58. Fernández A, Pérez-Martínez A, Escudero A, Mirones I, González B, de Paz R, et al. Infusion of haploidentical NKG2D-CAR-T. Leuk Lymphoma. (2022) 63:1970–4. doi: 10.1080/10428194.2022.2057490
59. Ibáñez-Navarro M, Fernández A, Escudero A, Esteso G, Campos-Silva C, Navarro-Aguadero M, et al. NKG2D-CAR memory T cells target pediatric T-cell acute lymphoblastic leukemia. Front Immunol. (2023) 14:1187665. doi: 10.3389/fimmu.2023.1187665
60. Jin X, Xie D, Sun R, Lu W, Xiao X, Yu Y, et al. CAR-T cells dual-target CD123 and NKG2DLs to eradicate AML cells and selectively target immunosuppressive cells. Oncoimmunology. (2023) 12:2248826. doi: 10.1080/2162402X.2023.2248826
61. Leivas A, Valeri A, Córdoba L, García-Ortiz A, Ortiz A, Sánchez-Vega L, et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J. (2021) 11:146. doi: 10.1038/s41408-021-00537-w
62. Li KX, Wu HY, Pan WY, Guo MQ, Qiu DZ, He YJ, et al. A novel approach for relapsed/refractory FLT3. Mol Cancer. (2022) 21:66. doi: 10.1186/s12943-022-01541-9
63. Zhao R, Cheng L, Jiang Z, Wei X, Li B, Wu Q, et al. DNAX-activating protein 10 co-stimulation enhances the anti-tumor efficacy of chimeric antigen receptor T cells. Oncoimmunology. (2019) 8:e1509173. doi: 10.1080/2162402X.2018.1509173
64. Zhou Z, Li J, Hong J, Chen S, Chen M, Wang L, et al. Interleukin-15 and chemokine ligand 19 enhance cytotoxic effects of chimeric antigen receptor T cells using zebrafish xenograft model of gastric cancer. Front Immunol. (2022) 13:1002361. doi: 10.3389/fimmu.2022.1002361
65. Lonez C, Verma B, Hendlisz A, Aftimos P, Awada A, Van Den Neste E, et al. Study protocol for THINK: a multinational open-label phase I study to assess the safety and clinical activity of multiple administrations of NKR-2 in patients with different metastatic tumour types. BMJ Open. (2017) 7:e017075. doi: 10.1136/bmjopen-2017-017075
66. Sun L, Jiang G, Ng YY, Xiao L, Du Z, Wang S, et al. T cells with split CARs specific for NKG2D ligands and PD-L1 exhibit improved selectivity towards monocyte-derived cells while effective in eliminating acute myeloid leukaemia in vivo. J Cancer Res Clin Oncol. (2023) 149:10189–201. doi: 10.1007/s00432-023-04865-1
67. Wu L, Liu F, Yin L, Wang F, Shi H, Zhao Q, et al. The establishment of polypeptide PSMA-targeted chimeric antigen receptor-engineered natural killer cells for castration-resistant prostate cancer and the induction of ferroptosis-related cell death. Cancer Commun (Lond). (2022) 42:768–83. doi: 10.1002/cac2.12321
68. Li Z, Chi Z, Ang WX, Chen C, Tay JC, Ng YY, et al. Experimental treatment of colorectal cancer in mice with human T cells electroporated with NKG2D RNA CAR. Immunotherapy. (2020) 12:733–48. doi: 10.2217/imt-2019-0137
69. Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunol Rev. (2009) 227:150–60. doi: 10.1111/j.1600-065X.2008.00720.x
70. Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, and Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. (2000) 1:433–40. doi: 10.1038/80877
71. Zhang Y, Zhang C, He M, Xing W, Hou R, and Zhang H. Co-expression of IL-21-Enhanced NKG2D CAR-NK cell therapy for lung cancer. BMC Cancer. (2024) 24:119. doi: 10.1186/s12885-023-11806-1
72. Ibanez-Navarro M, Fernandez A, Escudero A, Esteso G, Campos-Silva C, Navarro-Aguadero MA, et al. NKG2D-CAR memory T cells target pediatric T-cell acute lymphoblastic leukemia in vitro and in vivo but fail to eliminate leukemia initiating cells. Front Immunol. (2023) 14:1187665. doi: 10.3389/fimmu.2023.1187665
73. Yang D, Sun B, Li S, Wei W, Liu X, Cui X, et al. NKG2D-CAR T cells eliminate senescent cells in aged mice and nonhuman primates. Sci Transl Med. (2023) 15:eadd1951. doi: 10.1126/scitranslmed.add1951
74. Du Z, Ng YY, Zha S, and Wang S. piggyBac system to co-express NKG2D CAR and IL-15 to augment the in vivo persistence and anti-AML activity of human peripheral blood NK cells. Mol Ther Methods Clin Dev. (2021) 23:582–96. doi: 10.1016/j.omtm.2021.10.014
75. Wang J, Lupo KB, Chambers AM, and Matosevic S. Purinergic targeting enhances immunotherapy of CD73(+) solid tumors with piggyBac-engineered chimeric antigen receptor natural killer cells. J Immunother Cancer. (2018) 6:136. doi: 10.1186/s40425-018-0441-8
76. Parihar R, Rivas C, Huynh M, Omer B, Lapteva N, Metelitsa LS, et al. NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol Res. (2019) 7:363–75. doi: 10.1158/2326-6066.CIR-18-0572
77. Ang WX, Ng YY, Xiao L, Chen C, Li Z, Chi Z, et al. Electroporation of NKG2D RNA CAR improves vgamma9Vdelta2 T cell responses against human solid tumor xenografts. Mol Ther Oncolytics. (2020) 17:421–30. doi: 10.1016/j.omto.2020.04.013
78. Nakano I. Stem cell signature in glioblastoma: therapeutic development for a moving target. J Neurosurg. (2015) 122(2):324–30. doi: 10.3171/2014.9.JNS132253
79. Chan GC and Chan CM. Anti-GD2 directed immunotherapy for high-risk and metastatic neuroblastoma. Biomolecules. (2022) 12(3):358. doi: 10.3390/biom12030358
80. Brandetti E, Veneziani I, Melaiu O, Pezzolo A, Castellano A, Boldrini R, et al. MYCN is an immunosuppressive oncogene dampening the expression of ligands for NK-cell-activating receptors in human high-risk neuroblastoma. Oncoimmunology. (2017) 6:e1316439. doi: 10.1080/2162402X.2017.1316439
81. Thrift AP, Wenker TN, and El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. (2023) 20:338–49. doi: 10.1038/s41571-023-00747-0
82. Chen L, Hughes RA, Baines AJ, Conboy J, Mohandas N, and An X. Protein 4.1R regulates cell adhesion, spreading, migration and motility of mouse keratinocytes by modulating surface expression of beta1 integrin. J Cell Sci. (2011) 124:2478–87. doi: 10.1242/jcs.078170
83. Guo D, Jin C, Gao Y, Lin H, Zhang L, Zhou Y, et al. GPR116 receptor regulates the antitumor function of NK cells via Gαq/HIF1α/NF-κB signaling pathway as a potential immune checkpoint. Cell Biosci. (2023) 13:51. doi: 10.1186/s13578-023-01005-7
84. Wild C, Weiderpass E, and Stewart B. World cancer report 2020. Lyon: International Agency for Research on Cancer (2020) p. 199–512. C W, E W, B S.
85. Li Y-R, Ochoa CJ, Zhu Y, Kramer A, Wilson M, Fang Y, et al. Profiling ovarian cancer tumor and microenvironment during disease progression for cell-based immunotherapy design. iScience. (2023) 26(10):107952. doi: 10.1016/j.isci.2023.107952
86. Yang Y, Yang J, Zhao X, and Wei X. Tumor microenvironment in ovarian cancer: function and therapeutic strategy. Front Cell Dev Biol. (2020) 8:758. doi: 10.3389/fcell.2020.00758
87. Wu Z, Zhang H, Wu M, Peng G, He Y, Wan N, et al. Targeting the NKG2D/NKG2D-L axis in acute myeloid leukemia. BioMed Pharmacother. (2021) 137:111299. doi: 10.1016/j.biopha.2021.111299
88. Ingegnere T, Mariotti FR, Pelosi A, Quintarelli C, De Angelis B, Tumino N, et al. Human CAR NK cells: A new non-viral method allowing high efficient transfection and strong tumor cell killing. Front Immunol. (2019) 10:957. doi: 10.3389/fimmu.2019.00957
89. Quintarelli C, Sivori S, Caruso S, Carlomagno S, Falco M, Boffa I, et al. Efficacy of third-party chimeric antigen receptor modified peripheral blood natural killer cells for adoptive cell therapy of B-cell precursor acute lymphoblastic leukemia. Leukemia. (2020) 34:1102–15. doi: 10.1038/s41375-019-0613-7
90. Del Bufalo F, Becilli M, Rosignoli C, De Angelis B, Algeri M, Hanssens L, et al. Allogeneic, donor-derived, second-generation, CD19-directed CAR-T cells for the treatment of pediatric relapsed/refractory BCP-ALL. Blood. (2023) 142:146–57. doi: 10.1182/blood.2023020023
91. Lonez C, Hendlisz A, Shaza L, Aftimos P, Vouche M, Donckier V, et al. Celyad's novel CAR T-cell therapy for solid Malignancies. Curr Res Transl Med. (2018) 66:53–6. doi: 10.1016/j.retram.2018.03.001
92. Michaux A, Mauën S, Breman E, Dheur MS, Twyffels L, Saerens L, et al. Clinical grade manufacture of CYAD-101, a NKG2D-based, first in class, non-gene-edited allogeneic CAR T-cell therapy. J Immunother. (2022) 45:150–61. doi: 10.1097/CJI.0000000000000413
93. Bolsée J, Violle B, Jacques-Hespel C, Marijsse J, and Breman E. 232 NKG2D-based multi-specific CAR T-cells to overcome antigen escape and improve anti-tumor efficacy. J ImmunoTherapy Cancer. (2023) 11:A267–7. doi: 10.1136/jitc-2023-SITC2023.0232
94. Hui X, Weiguang L, Huixia L, Dongying G, Xiaowei W, and Hong-jiu D. Tandem CAR-T cells targeting CLDN18.2 and NKG2DL for treatment of gastric cancer. J Clin Oncol. (2023) 40(16)suppl. doi: 10.1200/JCO.2022.40.16_suppl.40
95. Fernandez A, Perez-Martinez A, Escudero A, Mirones I, Gonzalez B, de Paz R, et al. Infusion of haploidentical NKG2D-CAR-T(CD45RA-) cells in two pediatric patients with advanced relapsed and refractory acute leukemia was safe but achieved no clinical benefits. Leuk Lymphoma. (2022) 63:1970–4. doi: 10.1080/10428194.2022.2057490
96. Fontaine M, Demoulin B, Bornschein S, Raitano S, Lenger S, MaChado H, et al. Next Generation NKG2D-based CAR T-cells (CYAD-02): Co-expression of a Single shRNA Targeting MICA and MICB Improves Cell Persistence and Anti-Tumor Efficacy in vivo. Blood. (2019) 134:3931–1. doi: 10.1182/blood-2019-129998
97. Ho EL, Carayannopoulos LN, Poursine-Laurent J, Kinder J, Plougastel B, Smith HR, et al. Costimulation of multiple NK cell activation receptors by NKG2D. J Immunol. (2002) 169:3667–75. doi: 10.4049/jimmunol.169.7.3667
98. Dokouhaki P, Schuh NW, Joe B, Allen CA, Der SD, Tsao MS, et al. NKG2D regulates production of soluble TRAIL by ex vivo expanded human γδ T cells. Eur J Immunol. (2013) 43:3175–82. doi: 10.1002/eji.201243150
99. Talebian L, Fischer DA, Wu J, Channon JY, Sentman CL, Ernstoff MS, et al. The natural killer-activating receptor, NKG2D, on CD3+CD8+ T cells plays a critical role in identifying and killing autologous myeloma cells. Transfusion. (2014) 54:1515–21. doi: 10.1111/trf.12517
100. Xin M, Lin D, Yan N, Li H, Li J, and Huang Z. Oxaliplatin facilitates tumor-infiltration of T cells and natural-killer cells for enhanced tumor immunotherapy in lung cancer model. Anticancer Drugs. (2022) 33:117–23. doi: 10.1097/CAD.0000000000001248
101. Nückel H, Switala M, Sellmann L, Horn PA, Dürig J, Dührsen U, et al. The prognostic significance of soluble NKG2D ligands in B-cell chronic lymphocytic leukemia. Leukemia. (2010) 24:1152–9. doi: 10.1038/leu.2010.74
102. Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J, et al. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A. (2008) 105:1285–90. doi: 10.1073/pnas.0711293105
103. Groh V, Wu J, Yee C, and Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. (2002) 419:734–8. doi: 10.1038/nature01112
104. Zocchi MR, Catellani S, Canevali P, Tavella S, Garuti A, Villaggio B, et al. High ERp5/ADAM10 expression in lymph node microenvironment and impaired NKG2D ligands recognition in Hodgkin lymphomas. Blood. (2012) 119:1479–89. doi: 10.1182/blood-2011-07-370841
105. Zhang Y, Luo F, and Dong K. Soluble NKG2D ligands impair CD8. Oncol Lett. (2023) 26:297. doi: 10.3892/ol.2023.13883
106. Zocchi MR, Camodeca C, Nuti E, Rossello A, Venè R, Tosetti F, et al. ADAM10 new selective inhibitors reduce NKG2D ligand release sensitizing Hodgkin lymphoma cells to NKG2D-mediated killing. Oncoimmunology. (2016) 5:e1123367. doi: 10.1080/2162402X.2015.1123367
107. Veneziani I, Infante P, Ferretti E, Melaiu O, Battistelli C, Lucarini V, et al. Nutlin-3a enhances natural killer cell-mediated killing of neuroblastoma by restoring p53-dependent expression of ligands for NKG2D and DNAM-1 receptors. Cancer Immunol Res. (2021) 9:170–83. doi: 10.1158/2326-6066.CIR-20-0313
108. Focaccetti C, Benvenuto M, Pighi C, Vitelli A, Napolitano F, Cotugno N, et al. DNAM-1-chimeric receptor-engineered NK cells, combined with Nutlin-3a, more effectively fight neuroblastoma cells. Front Immunol. (2022) 13:886319. doi: 10.3389/fimmu.2022.886319
109. Demoulin B, Eytan B, and Gilham D. Tackling fratricide to manufacture clinical grade NKG2D-CAR T cells for cancer therapy. Immunotherapy Cancer. (2018) 8:VIII420. doi: 10.1093/annonc/mdy288.051
110. Breslin EM, White PC, Shore AM, Clement M, and Brennan P. LY294002 and rapamycin co-operate to inhibit T-cell proliferation. Br J Pharmacol. (2005) 144:791–800. doi: 10.1038/sj.bjp.0706061
111. Liu YF, Chiang Y, Hsu FM, Tsai CL, and Cheng JC. Radiosensitization effect by HDAC inhibition improves NKG2D-dependent natural killer cytotoxicity in hepatocellular carcinoma. Front Oncol. (2022) 12:1009089. doi: 10.3389/fonc.2022.1009089
112. Jia H, Yang H, Xiong H, and Luo KQ. NK cell exhaustion in the tumor microenvironment. Front Immunol. (2023) 14:1303605. doi: 10.3389/fimmu.2023.1303605
113. Raynaud A, Desrumeaux K, Vidard L, Termine E, Baty D, Chames P, et al. Anti-NKG2D single domain-based antibodies for the modulation of anti-tumor immune response. Oncoimmunology. (2020) 10:1854529. doi: 10.1080/2162402X.2020.1854529
114. Lutz S, Klausz K, Albici AM, Ebinger L, Sellmer L, Teipel H, et al. Novel NKG2D-directed bispecific antibodies enhance antibody-mediated killing of Malignant B cells by NK cells and T cells. Front Immunol. (2023) 14:1227572. doi: 10.3389/fimmu.2023.1227572
115. Fallon D, Huang CS, Ma J, Morgan C, and Zhou ZS. Agonistic anti-NKG2D antibody structure reveals unique stoichiometry and epitope compared to natural ligands. MAbs. (2024) 16:2433121. doi: 10.1080/19420862.2024.2433121
116. Kwong KY, Baskar S, Zhang H, Mackall CL, and Rader C. Generation, affinity maturation, and characterization of a human anti-human NKG2D monoclonal antibody with dual antagonistic and agonistic activity. J Mol Biol. (2008) 384:1143–56. doi: 10.1016/j.jmb.2008.09.008
Keywords: NKG2D, immunotherapy, CAR-T therapy, NK cell therapy, cancer
Citation: Han J, Wang Y, Chan GC-F and Chan WK (2025) Designs of NKG2D-based immunotherapeutics for cancer. Front. Immunol. 16:1557644. doi: 10.3389/fimmu.2025.1557644
Received: 08 January 2025; Accepted: 03 June 2025;
Published: 19 June 2025.
Edited by:
Emilie Narni-Mancinelli, INSERM U1104 Centre d’Immunologie de Marseille-Luminy (CIML), FranceReviewed by:
Sara Passos, Century Therapeutics, United StatesIrene Veneziani, Bambino Gesù Children’s Hospital (IRCCS), Italy
Copyright © 2025 Han, Wang, Chan and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Godfrey Chi-Fung Chan, Z2NmY2hhbkBoa3UuaGs=; Wing Keung Chan, d2luZy5jaGFuQG9zdW1jLmVkdQ==
†Present address: Jianfeng Han, Chongqing Tianyimei Life Science Co., Ltd, Chongqing, China
‡These authors have contributed equally to this work and share last authorship
 Jianfeng Han
Jianfeng Han Youwei Wang
Youwei Wang Godfrey Chi-Fung Chan
Godfrey Chi-Fung Chan Wing Keung Chan
Wing Keung Chan