- 1Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Guangzhou Hospital of Integrated Traditional and Western Medicine, Guangzhou, China
Rheumatoid arthritis (RA) is an autoimmune disease characterized by synovial inflammation, joint injury, and deformity in the limbs. This disease is widely distributed, with a large number of patients, and there is a possibility of further increasing the number of patients in the current aging society. RA can cause complications in multiple systems, many of which can seriously affect patients’ quality of life and even significantly increase the risk of death. The unclear mechanism of RA makes it difficult to achieve accurate prediction and treatment of its complications. Therefore, this article provides an overview of the research progress on the common or extensively studied complications of RA, such as joint injury, interstitial lung disease, vasculitis, osteoporosis, Sjogren's syndrome, malignant tumors, etc. It identifies the problems and shortcomings in research and proposes some suggestions for future measures, in order to raise awareness among medical professionals and the public about actively controlling these complications and provide some assistance.
Introduction
Rheumatoid arthritis is an autoimmune disease based on synovitis whose mechanism has not yet been identified. This disease not only has the characteristic symptoms of joint pain and swelling, but also can further lead to a variety of extra articular diseases (1).At present, the treatment of rheumatoid arthritis is still mainly to control the disease progression, and there are still a large number of patients with the disease whose condition is difficult to be effectively controlled (2), so most patients will suffer from the disease for life. In North America and Western Europe, the prevalence of rheumatoid arthritis can reach 0.5% to 1%, and studies have shown that the prevalence of this disease can increase with age (3), so with the progress of the aging of the world population, the risk of suffering from this disease will continue to increase. It is obvious that, similar to diabetes, actively controlling the complications of rheumatoid arthritis can effectively reduce the social burden of the disease. However, most of the current studies on the complications of rheumatoid arthritis only focus on a single complication and lack systematic understanding. Therefore, this review summarizes the research on the complications of the disease so far to provide some reference for relevant researchers.
Joint damage
As a disease based on synovial inflammation, the most direct complication of rheumatoid arthritis is joint damage caused by persistent inflammation. Studies have shown that 39%-73% of patients with early rheumatoid arthritis have joint erosion of the hands within 5 years. In addition (4), joint destruction has been considered as one of its characteristics (5), which shows the high probability of this complication in rheumatoid arthritis. At present, the mechanism of joint destruction in rheumatoid arthritis is not clear, but research has found that synovial mesenchymal cells driven by transcription factor c-Fos/AP-1 (6), fibroblast like synovial cells (7), osteoclasts, cytokines including TNF, IL1, IL17, rheumatoid factors, anti CCP antibodies and other autoantibodies all play a role in the occurrence of joint destruction in rheumatoid arthritis (8). Specific to the clinical manifestations, the joint destruction caused by rheumatoid arthritis can be manifested as joint swelling, pain and tenderness (9), bone destruction such as periarticular bone erosion, cartilage degradation as well as joint destruction commonly seen in hands and feet,etc. (10, 11) In terms of treatment, in addition to the basic treatment of rheumatoid arthritis such as oral Disease-modifying anti-rheumatic drugs(DMARDs) and non steroidal anti-inflammatory drugs(NSAIDs), there are also some more specific research on the treatment of rheumatoid arthritis joint destruction, such as denosumab and rituximab, which have obvious inhibitory effect on RA joint destruction (12, 13); TNF blockers that have a profound and sustained inhibitory effect on RA joint bone erosion (14); Histone-modifying inhibitors can remodel RA synovial fibroblasts cells that promote osteoclast bone erosion to inhibit joint bone destruction (15); Small molecule inhibitor of cathepsin K that can directly inhibit the absorption of osteoclasts (16), and joint protection surgery that can effectively alleviate joint destruction (17). In terms of future treatment prospects, ideas such as reducing bone destruction by regulating the metabolic pathway of osteoclasts have also been proposed (15), but it is still important to clarify the best treatment method with sufficient evidence and the treatment with better effect.
Interstitial lung disease
Lung disease has been recognized as one of the important extra articular complications of rheumatoid arthritis (18).Although the complications of RA in the lung are diverse, interstitial lung disease has a high prevalence and is often one of the main complications that lead to the death of RA patients (19).Specific to the number, some studies believe that the prevalence of RA complicated with interstitial lung disease is 3.6% (20), while others believe that the prevalence is between 3.7% and 7.7%, or even higher (21). The differences may be due to the regional and ethnic differences of the experimental population, as well as the inevitable deviation in the collection of patient information. However, these findings vividly prove the high risk of this complication and deserve the attention of medical workers. At present, the clear etiology and pathogenesis of rheumatoid arthritis related interstitial lung disease are not yet clear. However, according to reports, some factors such as ACPA, gene mutations, smoking, environment, autoimmunity and other factors are involved in the pathogenesis of rheumatoid arthritis associated interstitial lung disease (19, 22),and these factors lead to abnormal tissue reaction between alveolar wall and lung parenchyma (19).Some studies have interpreted the mechanism of lung involvement as the damage of airway and alveolar epithelial cells leading to the activation of immune cells and the excessive accumulation of extracellular matrix in lung tissue cells, but the current evidence is still difficult to fully define it (23).In terms of clinical manifestations, rheumatoid arthritis related interstitial lung disease can often have the manifestations of exertional dyspnea and dry cough in the early stage (24). Shortness of breath and bibasilar inspiratory crackles are also considered to be common (25, 26). In the late stage of this complication, clubbing often occurs (27). However, it should be noted that these symptoms are also common in other respiratory diseases. Clinical attention should be paid to the identification to minimize misdiagnosis and omissions. In the diagnosis of this complication, on the basis of the above symptoms, combined with higher inflammatory activity, higher ACPA value, MCP-1/CCL2, IL-18, SDF-1 and other inflammatory factor levels (28), Pulmonary Function Tests(PFTs), bronchoalveolar lavage, HRCT examination (29), lung ultrasound(LUS), serum KL-6 antigen level (30), usually can achieve a more accurate diagnosis, but whether patients need to do all the above-mentioned auxiliary examinations in order to make a clear diagnosis is still unclear. It may be a better choice to consider the clinical and patients’ comprehensive situation. In terms of treatment, the situation is more complex. On the one hand, immunosuppressants are often used as the first choice for rheumatoid arthritis complicated with interstitial lung disease (31); On the other hand, the use of immunosuppressive agents such as methotrexate is limited because the disease is common in the elderly (32), so the use of other drugs should also be paid attention to. Compared with TNF inhibitors, studies have shown that non TNF inhibitors such as abatacept, tocilizumab and rituximab have better efficacy in the treatment of this disease (33). However, antifibrotic drugs, such as nintedanib, have shown good effects in preventing and delaying the progression of the disease, and can be used as adjuvant drugs (34). In the acute exacerbation of the disease, no targeted treatment has yet been produced. At present, high-dose glucocorticoids combined with immunosuppressants are still used to control the disease (35), and a better treatment scheme remains to be studied. If the above drug regimens fail to effectively control the disease, there are also studies showing that lung transplantation can improve dyspnea and quality of life in changing play (36).
Vasculitis
Rheumatoid vasculitis(RV) is a complication that directly affects the blood vessels of patients with rheumatoid arthritis and causes vascular inflammation. It can occur in blood vessels of almost all sizes, and it is a rare complication of rheumatoid arthritis with poor prognosis and high mortality (37). Although new treatments with good curative effects such as biotherapy have reduced the incidence of RV to less than 3.9 per million people today (38), the highest five-year mortality rate of more than 60% suggests that we should not ignore this disease (39). The pathogenesis of this complication is not completely clear, but many studies have shown the important role of genetic factors in this mechanism (40–42), and some studies have suggested that the formation of immune complexes promotes the recruitment of neutrophils (43), endothelial cell activation, inflammatory factor production,etc. (44), and then produces vascular inflammation, leading to the damage of vascular wall. In terms of specific symptoms, the skin manifestations of RV are relatively common (45). If it is mainly small vessel inflammation, it can often cause skin infarct papules of fingers, as well as skin purpura, congestion, maculopapular rash; If inflammation occurs in larger blood vessels, it can cause nodules, racemose moss, necrotic ulcers, etc. (45) Vasculitis can also cause peripheral nerve infarction, causing neuritis, distal symmetric sensory or motor neuropathy, in which the lower limbs are more prone to occur than the upper limbs (46); The heart can also have pericarditis, tamponade and other critical symptoms due to RV (39); In the eyes, RV can cause scleritis, keratitis, etc (47, 48); In addition, RV can also cause fatigue, fever, weight loss, muscle pain and other difficult to identify symptoms (49).At present, when diagnosing RV, one of the above clinical manifestations and skin or muscle vasculitis related tissue biopsy is often selected (50), but because the biopsy may be false negative, there are also studies suggesting that the combination of serum rheumatoid factor IgA and C3 complement test results can be considered to assist the diagnosis (51), which also enlightens that the current diagnostic methods still need to be improved. For the moment, there is no unified standard for the treatment of RV (39). Glucocorticoids, DMARDs, cyclophosphamide, cyclosporine, biological agents and other drugs are used in the treatment of the disease (52, 53). Tnfi and rituximab are used as the first-line drugs for the disease because of their good remission effect (54–56), but the formulation of more standardized treatment and management standards is still very important.
Osteoporosis
Rheumatoid arthritis is also an important factor that causes bone mineral density reduction, osteoporosis, and further fractures (57).Studies have shown that compared with the general population, the probability of osteoporosis in patients with rheumatoid arthritis is significantly increased by about 2 times (58). If patients with RA do not receive glucocorticoid treatment, the incidence rate can reach 7.5 per 1000 people per year (59), and the prevalence rate can reach 30% (60). If women with RA are postmenopausal, about one-third of the population can suffer from osteoporosis (58). Considering the significantly elevated fracture risk of patients with osteoporosis, it is urgent to control this complication. In terms of pathogenesis, studies have shown that RA leads to osteoporosis mainly through its effects on osteoclasts and osteoblasts (61). RA can promote the formation of osteoclasts by affecting RANKL, T cells, B cells, etc. (62, 63), while abnormally formed osteoclasts can cause bone resorption and erosion by releasing catalytic decomposition enzymes and acid enzymes (64). On the other hand, RA can also affect the normal formation and development of osteoblasts by affecting bone morphogenetic protein (BMP), resulting in insufficient sources of bone formation (65). In terms of clinical manifestations, as a relatively hidden disease, RA related osteoporosis may not show obvious symptoms in the early stage, so the study of symptoms has not attracted enough attention. However, with the development of the disease, fatigue, pain (66), spinal deformation (67), and fracture may occur in severe cases (68), which often makes the disease get due attention at this time. In terms of diagnosis, a unified standard for the diagnosis of this complication has not yet been formulated, but studies have suggested that combining the gold standard examination of osteoporosis - bone mineral density examination with serum 25 hydroxyvitamin D and bone turnover marker β-CROSSL level examination can accurately predict the occurrence and development of the disease (69), which is worthy of our reference, but more relevant studies are still needed to confirm and enrich the diagnostic methods. In terms of treatment, the current research basically believes that the treatment of this complication is achieved through the basic medication of RA combined with anti osteoporosis drugs (70–72), but the specific usage will be different. Glucocorticoids have the risk of aggravating the progression of osteoporosis, so we should pay attention to control the dosage of glucocorticoids and actively use anti osteoporosis drugs at the same time (70). A variety of biological agents have shown protective effects on bone mass while inhibiting RA inflammation, such as Tofacitinib and Iguratimod (73). However, currently, anti osteoporosis drugs represented by bisphosphonates are still used as first-line drugs, because they can show better effects (70). In addition, non drug treatment has also attracted attention. Adequate intake of calcium and vitamin D, smoking cessation, reduction of alcohol consumption, assessment of fall risk, maintenance of normal weight, avoidance of bad living habits such as sitting for a long time, and active physical exercise are all recommended to patients with RA complicated with osteoporosis to better ensure the curative effect (70, 71). However, the current scheme is still not the best, and the role of treatment is still biased towards maintenance rather than cure (71, 72). Therefore, a better treatment scheme for this complication still needs our efforts.
Sjogren’s syndrome
Rheumatoid arthritis can also cause the same type of disease, Sjogren’s syndrome is one of them (74).In patients with RA, this complication can occur in about 6% to 9% of patients (75). Studies have shown that secondary Sjogren’s syndrome will worsen the condition and increase the mortality of RA patients (76). At present, the pathogenesis of this complication is not clear, but studies have found that as many as 322 genes are commonly associated with RA and Sjogren’s syndrome, among which CXCL10, GZMA, IGTA4, PSMB9 and other genes are closely related to the activity of these two diseases (77), and megakaryocytes may also play an important role in the occurrence of these two diseases (78). Although there is still a lack of research on the mechanism of directly linking the two diseases, the above research may also suggest that the two are linked through gene and cell pathways. Of course, this still needs further research and demonstration. In terms of clinical manifestations, the manifestations of Sjogren’s syndrome secondary to RA are not significantly different from those of primary Sjogren’s syndrome. They are dry eyes caused by lymphoid infiltration of exocrine glands and epithelial cells involving lacrimal and salivary glands and salivary dysfunction with dry mouth as a common manifestation (79), but secondary Sjogren’s syndrome will also show symptoms of RA.In terms of diagnosis, the diagnostic criteria for Sjogren’s syndrome secondary to RA have not yet been formulated, so at present, the classification criteria for primary Sjogren’s syndrome formulated by the American College of Rheumatology/European League Against Rheumatism in 2016 are still mostly referred to (80), such as lymphocytic lip salivary gland inflammation, anti ssa/ro positivity, higher eye stain score, Schirmer test, unstimulated salivary flow, combined with the above symptoms of dry eyes, dry mouth, and earlier diagnosis of RA (81). In addition, some studies have also found the good effect of serum chemokine CCL28 in the diagnosis and evaluation of Sjogren’s syndrome (82), which also suggests that the methods for the diagnosis of this disease still have development potential. In terms of treatment, studies have suggested that abatacept has a good effect on improving the glandular and extraglandular involvement, systemic disease activity and patient reported outcomes in patients with RA related Sjogren’s syndrome, but more high-quality RCTs are still needed to support it (83); Some studies have also found that human chorionic gonadotropin has shown good remission effect on RA and Sjogren’s syndrome, which can be used as an adjuvant treatment option (84). However, in general, the research on the treatment of RA related Sjogren’s syndrome is insufficient, so the current treatment plan for a separate Sjogren’s syndrome is also worthy of reference. The stability of lysosomes found in relevant studies, interferons such as MHV370, RSLV-132, CAR-T cell therapy, epalizumab, seletalisib and other therapies have shown good efficacy and therapeutic potential for Sjogren’s syndrome. What is more worthy of expectation is that a large number of drugs for the treatment of Sjogren’s syndrome are under clinical research at different stages (85), which may suggest that the formulation of standard treatment regimens for this complication will benefit in the near future.
Malignant tumor
Rheumatoid arthritis has also been recognized as a risk factor for a variety of malignancies (86). Malignant tumor, as we all know, is a disease with poor prognosis, which is still one of the main diseases leading to death until now (87). Many studies have suggested that RA can increase the risk of malignant tumors. Although the specific data are still unclear, the increase can reach 50% (88, 89), which makes us not ignore the existence of RA in the occurrence and treatment of malignant tumors. In the pathogenesis, some studies have shown that the mechanism of RA leading to malignant tumors may be due to long-term chronic inflammatory stimuli (90). Some studies also believe that common triggers such as smoking and airway inflammation may induce RA and malignant tumors at the same time (91–94). On the other hand, genetic and environmental factors have also attracted some attention (95), but in general, these mechanisms are not clear enough and need more experimental proof.In terms of symptoms and diagnosis, there is not enough research to explain the specific symptoms and diagnostic characteristics of RA related malignant tumors. Interleukin-6 is considered a potential biomarker linking rheumatoid arthritis and malignant tumors, with the potential to monitor RA associated malignant tumors, but further experimental evidence is needed (96).Time has been considered by some studies as the method to determine the complications (89), but there are inevitably many interfering factors, making time not the best diagnostic method. Therefore, enough attention and a large number of experiments are needed to support the research on the special symptoms and diagnostic basis of this complication. In terms of treatment, there is no unified and objective guidance plan for the treatment of RA related malignant tumors, but some good suggestions can still be found from the existing expert consensus. Compared to biological Disease-Modifying Anti-Rheumatic Drugs(bDMARDs),conventional synthetic Disease-Modifying Antirheumatic Drugs(csDMARDs) are considered safer for active cancer. Compared to targeted synthetic Disease-Modifying Anti-Rheumatic Drugs(tsDMARDs), conventional synthetic Disease-Modifying Antirheumatic Drugs are also considered a more reliable choice. However, at the same time, individual factors such as the patient’s age, cancer type, stage, prognosis, and values should also be considered when choosing medication (95); The use of corticosteroids is controversial as they may have both positive and negative effects on cancer, and therefore should be used with caution (97, 98); If the patient’s cancer requires surgical treatment, non steroidal anti-inflammatory drugs, immunosuppressants, and other medications used to treat RA may be discontinued to prevent risks such as bleeding and infection (99, 100). In terms of radiation therapy, no significant effect of radiation therapy on RA has been found yet, but caution should still be exercised when using it (95). When facing chemotherapy, targeted drug therapy, immune checkpoint inhibitors, and other treatments, attention should be paid to the interaction with anti rheumatic drugs and the possible adverse consequences (95, 101). In summary, RA related malignant tumors can usually be treated with both RA and malignant tumors simultaneously, but monitoring of efficacy, adverse reactions, and more comprehensive and universal treatment guidelines are still needed.
Cardiovascular diseases
Given that vasculitis has been taken into consideration, the risk of cardiovascular disease cannot be ignored. From an epidemiological perspective, RA can increase the probability of cardiovascular disease in the human body by 48% and increase the mortality rate related to cardiovascular disease by 50%. Among RA patients, 4.68% may experience cardiovascular outcomes. Cardiovascular disease accounts for the largest proportion of excess mortality in RA, at approximately 39.6% (102, 103). In terms of pathogenesis, current research suggests that the cardiovascular disease outcome of rheumatoid arthritis is mainly caused by inflammation. Rheumatoid arthritis leads to the abundant presence of inflammatory factors such as interleukin(IL)-1,IL-6 and tumor necrosis factor (TNF) in the systemic circulation. These inflammatory factors can cause vascular endothelial dysfunction, and affect lipid metabolism, causing abnormal activity and expression of lipoprotein esterase, reducing high-density lipoprotein cholesterol, increasing low-density lipoprotein cholesterol, decreasing levels of apolipoprotein A1 and increasing levels of apolipoprotein B. Disruptive lipid metabolism can further promote inflammatory reactions and increase blood viscosity, and then lead to atherosclerosis and more serious cardiovascular diseases. At the same time, the increased secretion of proteolytic enzymes and the expression of interferon - γ (IFN - γ) in rheumatoid arthritis also promote plaque instability, which increases the risk of severe cardiovascular disease (104).The clinical manifestations of rheumatoid arthritis can include pericarditis, cardiomyopathy, arrhythmia, atherosclerosis, heart failure, valvular heart disease, of which the most common is considered to be ischemic heart disease represented by atherosclerosis and congestive heart failure (105, 106).In terms of diagnosis, current research mainly focuses on predicting and early detecting cardiovascular complications in patients with rheumatoid arthritis, including monitoring blood lipids, arterial ultrasound examination, cardiovascular magnetic resonance imaging, and other methods to detect cardiovascular lesions as early as possible (107–109).In addition, biomarkers such serological asasymmetric dimethylarginine(ADMA),interleukin-17A and osteoprotegerin that have the potential to predict cardiovascular complications in patients with rheumatoid arthritis are also being studied (110, 111).In terms of treatment, current research still focuses on controlling the inflammation and disease activity of rheumatoid arthritis to reduce the probability of cardiovascular complications or alleviate existing cardiovascular complications. The main therapeutic drugs are still traditional nonsteroidal anti-inflammatory drugs, glucocorticoids, conventional synthetic anti rheumatic drugs, biologics, targeted synthetic anti rheumatic drugs (104).However, some studies have also found the potential of JAK inhibitors in the treatment of rheumatoid arthritis combined with cardiovascular disease, which may indicate the emergence of new and more targeted therapeutic drugs in the near future (112).
Advanced therapy
Advanced therapy refers to a new type of treatment that utilizes cutting-edge biological technologies such as cells and genes, which is a new choice for current and future difficult diseases. At present, the mechanism and treatment research of rheumatoid arthritis is not sufficient, and the current drugs for treating rheumatoid arthritis are still not perfect. Therefore, advanced treatment may be a promising method. Due to the lack of targeted strategies for the treatment of complications of rheumatoid arthritis, most of them still focus on treating the inflammation itself. Therefore, the current advanced treatment for its complications is also based on the study of treating rheumatoid arthritis itself.
MicroRNAs are a class of non coding single stranded DNA molecules that exist in eukaryotes and are involved in post transcriptional regulation of gene expression. When their regulatory ability is disrupted, immune inflammations including RA will further develop (113).Based on this mechanism, researchers currently studying interventions targeting microRNAs such as hsa-miR-21-5p and miR-340-5p that are highly associated with rheumatoid arthritis to control the condition of rheumatoid arthritis (114).Perhaps we will soon see the emergence of specific drugs and their expected effects.
Genome editing technology is a cutting-edge technology that precisely modifies specific target genes in the genome of organisms. The Clustered Regularly Interspaced Short Palindromic Repeats(CRISPR)-Cas9 system is a genome editing technology developed based on bacterial natural immune systems that can accurately identify and cleave DNA sequences, its precise characteristics make it a good tool for gene editing therapy for rheumatoid arthritis (115).Another condition that makes gene editing technology possible for treating rheumatoid arthritis is the numerous genetic related studies that have been completed, such as large-scale genome wide association studies (GWAS).The genes IL-20RA, OLIG3, miR-155, and others discovered in current research are considered suitable choices for treating rheumatoid arthritis through CRISPR-Cas9 technology (116).However, considering the off target risks and ethical concerns that still exist in the technology, the specific application of treatment may take some time.
Mesenchymal stem cell (MSC) therapy is a cutting-edge therapy that utilizes the self-renewal and differentiation abilities of mesenchymal stem cells to treat diseases. Due to the fact that mesenchymal stem cells can participate in regulating the activity of the immune system through various pathways to achieve inhibitory effects on inflammatory responses, these cells have certain potential for controlling the condition of rheumatoid arthritis (117).Some completed studies on mesenchymal stem cell therapy for rheumatoid arthritis have shown that this treatment is effective and relatively safe for rheumatoid arthritis, but the current high cost of this treatment makes it not yet recommended as one of the top priority treatments. When mesenchymal stem cell therapy becomes an alternative treatment for traditional treatments such as DMARD or biologics, the greater risks of infection, thrombosis, tumors, etc. will also plague the choice of treatment, and these issues are urgent to be addressed (118).But overall, mesenchymal stem cells are a promising approach for treating rheumatoid arthritis, and improving medication methods and reducing treatment costs will promote further promotion of this method.
Adoptive regulatory T cell therapy is a treatment method that involves modifying regulatory T cells extracted from healthy individuals or patients in vitro and reintroducing them into the patient’s body to enhance the immune system’s regulatory function. Regulatory T cells(Tregs) refer to a subset of T cells that can control the immune response in the human body, and have inhibitory and anti-inflammatory effects on the body’s immune response. Existing research suggests that this therapy has a significant inhibitory effect on inflammation, but currently there are not many studies on this treatment for rheumatoid arthritis, and the risks of regulatory T cells being converted into pathogenic cells and easily contaminated during extraction still need to be addressed (119). Therefore, more relevant experiments are still needed to further confirm the effectiveness and safety of this treatment for rheumatoid arthritis.
Chimeric antigen receptor(CAR) T-cell therapy is a cutting-edge treatment method that has recently received a lot of attention. Similar to the adoptive regulatory T-cell therapy mentioned above, its method is to modify and extract T cells from patients’ bodies in vitro, making them more powerful in treating specific pathogens. This therapy was initially researched around treating tumors, but as some researchers attempted to use it to treat immune diseases such as systemic sclerosis and achieved good results, researchers believe that it may also have great potential for rheumatoid arthritis (120).Given the complex characteristics of rheumatoid arthritis, current research on CAR-T cell therapy for the treatment of rheumatoid arthritis includes precise treatment through various methods, such as labeling rheumatoid arthritis specific serum markers with fluorescein isothiocyanate (FITC), rheumatoid arthritis specific associated HLA DR alleles and their self antigen peptides, and using chimeric autoantibody receptor T cells (CAAR-T) and chimeric antigen receptors in regulatory T cells (CAR Tregs), but the most suitable method has not been determined yet, and more experiments are still needed to confirm the efficacy and avoid risks (121–123).CAR-T cell therapy is considered a highly promising treatment for rheumatoid arthritis, but its promotion and application are still greatly limited until the risks of severe inflammation and high costs are resolved.
Overall, there is a wealth of research on advanced treatment methods for rheumatoid arthritis, but there is generally a lack of sufficient clinical trials to prove their efficacy and safety. If considering the treatment of complications of rheumatoid arthritis, the specificity of these treatments is still not precise enough. However, as therapies that are very different from traditional treatments for rheumatoid arthritis and its complications, we have reason to believe that they may replace existing therapies in the future to better control the condition of rheumatoid arthritis and its complications with higher efficacy and safety.
Summary
As a disease whose mechanism cannot be elucidated yet, rheumatoid arthritis can not only affect local joints, but also multiple human systems or organs such as the respiratory system, cardiovascular system, and bones, and may induce other autoimmune diseases, malignant tumors, etc., as shown in Figure 1. These complications increase the risk of life-threatening situations, which warns us not to ignore further understanding and active treatment of rheumatoid arthritis complications. Unfortunately, although this disease has been discovered for over a hundred years, the mechanism, characteristics, and treatment of its complications have not been fully understood, which is a significant obstacle to achieving the goal of improving disease prognosis. However, at the same time, understanding the complex mechanisms of rheumatoid arthritis may require more time and manpower to determine the characteristics of its complications, as well as diagnosis and treatment. Although the diagnosis and treatment guidelines for complications of rheumatoid arthritis have not been formulated yet, these diseases are gradually attracting more and more attention and research. It is also evident that rheumatoid arthritis patients have greatly reduced the risk of deterioration due to the use of anti rheumatic drugs, the advanced treatment for it has also shown good results and has great potential. Therefore, we should still believe that a bright future is not far away.
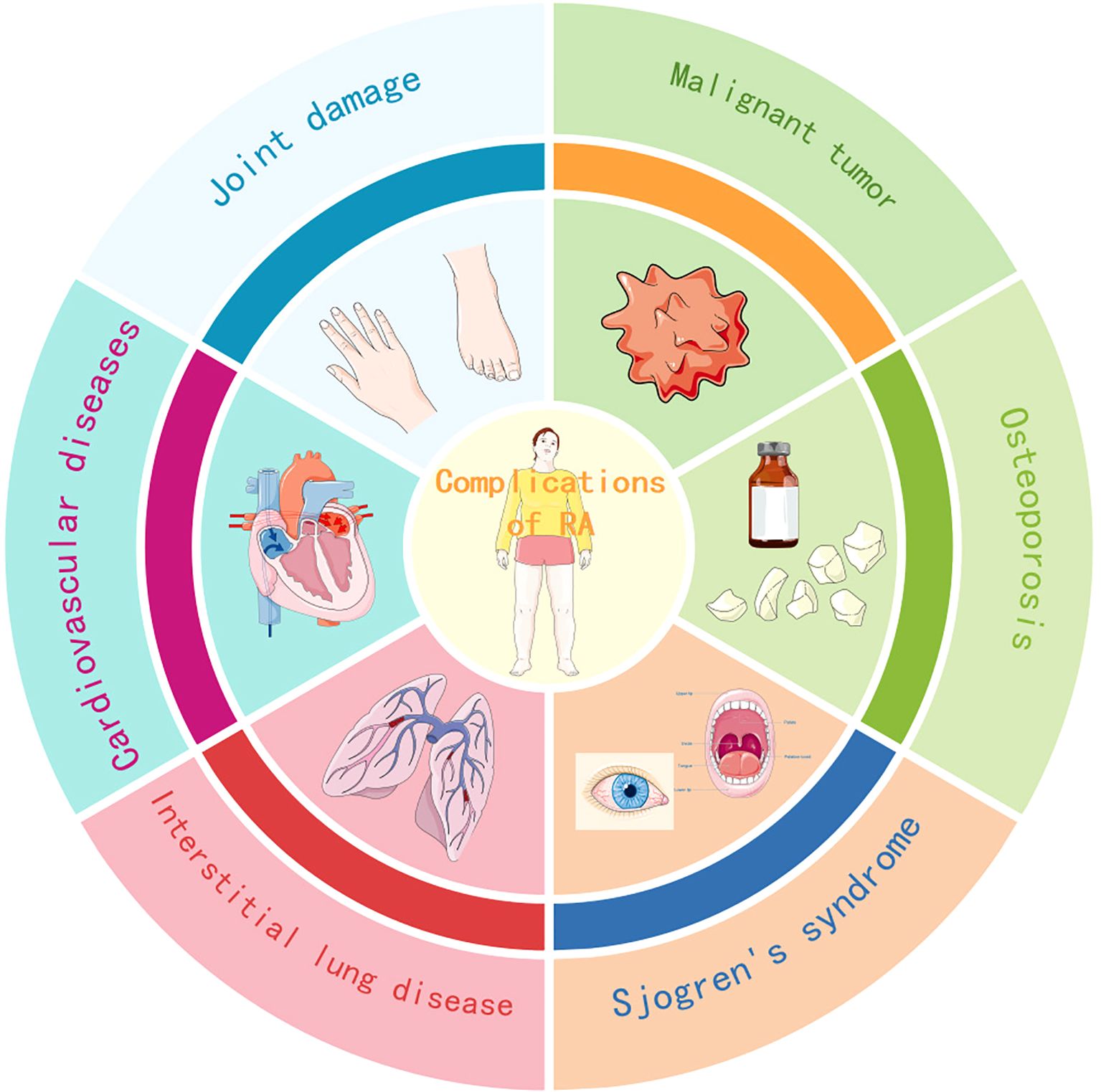
Figure 1. Common and highly concerned complications of rheumatoid arthritis. This study mainly discusses the complications of six common and widely studied types of rheumatoid arthritis, including joint injury, interstitial lung disease, osteoporosis, Sjogren’s syndrome, cardiovascular disease (including vasculitis), and malignant tumors.
Author contributions
HZ: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. ZY: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. R-TW: Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. ZH: Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Basic and Applied Basic Research Project in Huadu District, Guangzhou City(24HDQYLH24).
Acknowledgments
This study is grateful for the funding from the Science, Technology, Industry, Commerce and Information Technology Bureau of Huadu District, Huadu District People’s Hospital, Guangzhou Hospital Of Integrated Traditional And Western Medicine, and Huadu Maternal and Child Health Hospital (Hu Zhong Hospital) in Huadu District, Guangzhou. Fund Number:24HDQYLH24.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gravallese EM and Firestein GS. Rheumatoid arthritis - common origins, divergent mechanisms. N Engl J Med. (2023) 388:529–42. doi: 10.1056/NEJMra2103726
2. Brown P, Pratt AG, and Hyrich KL. Therapeutic advances in rheumatoid arthritis. BMJ. (2024) 384:e070856. doi: 10.1136/bmj-2022-070856
3. Venetsanopoulou AI, Alamanos Y, Voulgari PV, and Drosos AA. Epidemiology of rheumatoid arthritis: genetic and environmental influences. Expert Rev Clin Immunol. (2022) 18:923–31. doi: 10.1080/1744666X.2022.2106970
4. Scott DL, Smith C, and Kingsley G. Joint damage and disability in rheumatoid arthritis: an updated systematic review. Clin Exp Rheumatol. (2003) 21:S20–7.
5. Li N, Jiang L, Cai Y, Liu JY, Zhao T, Kong N, et al. The correlation between interleukin-34 and bone erosion under ultrasound in rheumatoid arthritis. Mod Rheumatol. (2020) 30:269–75. doi: 10.1080/14397595.2019.1593576
6. Shiozawa S, Tsumiyama K, Yoshida K, and Hashiramoto A. Pathogenesis of joint destruction in rheumatoid arthritis. Arch Immunol Ther Exp (Warsz). (2011) 59:89–95. doi: 10.1007/s00005-011-0116-3
7. Mor A, Abramson SB, and Pillinger MH. The fibroblast-like synovialcell in rheumatoid arthritis: a key player in inflammation and joint destruction. ClinImmunol. (2005) 115:118–28. doi: 10.1016/j.clim.2004.12.009
8. van den Berg WB, van Lent PL, Joosten LA, Abdollahi-Roodsaz S, and Koenders MI. Amplifying elements of arthritis and joint destruction. Ann RheumDis. (2007) 66Suppl3:iii45–8. doi: 10.1136/ard.2007.079830
9. Drosos AA, Pelechas E, and Voulgari PV. Conventional radiography of the hands and wrists in rheumatoid arthritis. What a rheumatologist should know and how to interpret the radiological findings. Rheumatol Int. (2019) 39:1331–41. doi: 10.1007/s00296-019-04326-4
10. Yue J, Wu D, and Tam LS. The role of imaging in early diagnosis and prevention of joint damage in inflammatory arthritis. Expert Rev Clin Immunol. (2018) 14:499–511. doi: 10.1080/1744666X.2018.1476849
11. Hamamoto Y, Ito H, Furu M, Hashimoto M, Fujii T, Ishikawa M, et al. Serological and progression differences of joint destruction in the wrist and the feet in rheumatoid arthritis - A cross-sectional cohort study. PloS One. (2015) 10:e0136611. doi: 10.1371/journal.pone.0136611
12. Mochizuki T, Yano K, Ikari K, Kawakami K, Hiroshima R, Koenuma N, et al. Effects of denosumab treatment on bone mineral density and joint destruction in patients with rheumatoid arthritis. J Bone Miner Metab. (2018) 36:431–8. doi: 10.1007/s00774-017-0848-1
13. Boumans MJ, Thurlings RM, Yeo L, Scheel-Toellner D, Vos K, Gerlag DM, et al. Rituximab abrogates joint destruction in rheumatoid arthritis by inhibiting osteoclastogenesis. Ann Rheum Dis. (2012) 71:108–13. doi: 10.1136/annrheumdis-2011-200198
14. Schett G, Coates LC, Ash ZR, Finzel S, and Conaghan PG. Structural damage in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: traditional views, novel insights gained from TNF blockade, and concepts for the future. Arthritis Res Ther. (2011) 13 Suppl 1:S4. doi: 10.1186/1478-6354-13-S1-S4
15. Yan M, Su J, and Li Y. Rheumatoid arthritis-associated bone erosions: evolving insights and promising therapeutic strategies. Biosci Trends. (2020) 14:342–8. doi: 10.5582/bst.2020.03253
16. Drake MT, Clarke BL, Oursler MJ, and Khosla S. Cathepsin K inhibitors for osteoporosis: biology, potential clinical utility, and lessons learned. Endocr Rev. (2017) 38:325–50. doi: 10.1210/er.2015-1114
17. Yano K, Ikari K, Tobimatsu H, Tominaga A, and Okazaki K. Joint-preserving surgery for forefoot deformities in patients with rheumatoid arthritis: A literature review. Int J Environ Res Public Health. (2021) 18:4093. doi: 10.3390/ijerph18084093
18. Wilsher M, Voight L, Milne D, Teh M, Good N, Kolbe J, et al. Prevalence of airway and parenchymal abnormalities in newly diagnosed rheumatoid arthritis. Respir Med. (2012) 106:1441–6. doi: 10.1016/j.rmed.2012.06.020
19. Kadura S and Raghu G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur Respir Rev. (2021) 30:210011. doi: 10.1183/16000617.0011-2021
20. Richman NC, Yazdany J, Graf J, Chernitskiy V, and Imboden JB. Extraarticular manifestations of rheumatoid arthritis in a multiethnic cohort of predominantly Hispanic and Asian patients. Med (Baltimore). (2013) 92:92–7. doi: 10.1097/MD.0b013e318289ce01
21. Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheumatol. (2010) 62:1583–91. doi: 10.1002/art.27405
22. Dai Y, Wang W, Yu Y, and Hu S. Rheumatoid arthritis-associated interstitial lung disease: an overview of epidemiology, pathogenesis and management. Clin Rheumatol. (2021) 40:1211–20. doi: 10.1007/s10067-020-05320-z
23. Lee H, Lee SI, and Kim HO. Recent advances in basic and clinical aspects of rheumatoid arthritis-associated interstitial lung diseases. J Rheum Dis. (2022) 29:61–70. doi: 10.4078/jrd.2022.29.2.61
24. Manfredi A, Cassone G, Luppi F, Atienza-Mateo B, Cavazza A, Sverzellati N, et al. Rheumatoid arthritis related interstitial lung disease. Expert Rev Clin Immunol. (2021) 17:485–97. doi: 10.1080/1744666X.2021.1905524
25. Manfredi A, Cassone G, Cerri S, Venerito V, Fedele AL, Trevisani M, et al. Diagnostic accuracy of a velcro sound detector (VECTOR) for interstitial lung disease in rheumatoid arthritis patients: the InSPIRAtE validation study (INterStitial pneumonia in rheumatoid ArThritis with an electronic device). BMC Pulm Med. (2019) 19:111. doi: 10.1186/s12890-019-0875-x
26. Sgalla G, Walsh SLF, Sverzellati N, Fletcher S, Cerri S, Dimitrov B, et al. Velcro-type” crackles predict specific radiologic features of fibrotic interstitial lung disease. BMC Pulm Med. (2018) 18:103. doi: 10.1186/s12890-018-0670-0
27. Messina R, Guggino G, Benfante A, and Scichilone N. Interstitial lung disease in elderly rheumatoid arthritis patients. Drugs Aging. (2020) 37:11–8. doi: 10.1007/s40266-019-00727-z
28. Mena-Vázquez N, Godoy-Navarrete FJ, Lisbona-Montañez JM, Redondo-Rodriguez R, Manrique-Arija S, Rioja J, et al. Inflammatory biomarkers in the diagnosis and prognosis of rheumatoid arthritis-associated interstitial lung disease. Int J Mol Sci. (2023) 24:6800. doi: 10.3390/ijms24076800
29. Koduri G and Solomon JJ. Identification, monitoring, and management of rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol. (2023) 75:2067–77. doi: 10.1002/art.42640
30. Wang Y, Chen S, Zheng S, Lin J, Hu S, Zhuang J, et al. The role of lung ultrasound B-lines and serum KL-6 in the screening and follow-up of rheumatoid arthritis patients for an identification of interstitial lung disease: review of the literature, proposal for a preliminary algorithm, and clinical application to cases. Arthritis Res Ther. (2021) 23:212. doi: 10.1186/s13075-021-02586-9
31. Pugashetti JV and Lee JS. Overview of rheumatoid arthritis-associated interstitial lung disease and its treatment. Semin Respir Crit Care Med. (2024) 45:329–41. doi: 10.1055/s-0044-1782218
32. Akiyama M and Kaneko Y. Pathogenesis, clinical features, and treatment strategy for rheumatoid arthritis-associated interstitial lung disease. Autoimmun Rev. (2022) 21:103056. doi: 10.1016/j.autrev.2022.103056
33. Mena-Vázquez N, Godoy-Navarrete FJ, Manrique-Arija S, Aguilar-Hurtado MC, Romero-Barco CM, Ureña-Garnica I, et al. Non-anti-TNF biologic agents are associated with slower worsening of interstitial lung disease secondary to rheumatoid arthritis. Clin Rheumatol. (2021) 40:133–42. doi: 10.1007/s10067-020-05227-9
34. Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. (2019) 381:1718–27. doi: 10.1056/NEJMoa1908681
35. Ota M, Iwasaki Y, Harada H, Sasaki O, Nagafuchi Y, Nakachi S, et al. Efficacy of intensive immunosuppression in exacerbated rheumatoid arthritis-associated interstitial lung disease. Mod Rheumatol. (2017) 27:22–8. doi: 10.3109/14397595.2016.1173816
36. Yazdani A, Singer LG, Strand V, Gelber AC, Williams L, and Mittoo S. Survival and quality of life in rheumatoid arthritis-associated interstitial lung disease after lung transplantation. J Heart Lung Transplant. (2014) 33:514–20. doi: 10.1016/j.healun.2014.01.858
37. Kishore S, Maher L, and Majithia V. Rheumatoid vasculitis: A diminishing yet devastating menace. Curr Rheumatol Rep. (2017) 19:39. doi: 10.1007/s11926-017-0667-3
38. Ntatsaki E, Mooney J, Scott DG, and Watts RA. Systemic rheumatoid vasculitis in the era of modern immunosuppressive therapy. Rheumatol (Oxford). (2014) 53:145–52. doi: 10.1093/rheumatology/ket326
39. Mertz P, Wollenschlaeger C, Chasset F, Dima A, and Arnaud L. Rheumatoid vasculitis in 2023: Changes and challenges since the biologics era. Autoimmun Rev. (2023) 22:103391. doi: 10.1016/j.autrev.2023.103391
40. Turesson C, Schaid DJ, Weyand CM, Jacobsson LT, Goronzy JJ, Petersson IF, et al. The impact of HLA-DRB1 genes on extra-articular disease manifestations in rheumatoid arthritis. Arthritis Res Ther. (2005) 7:R1386–93. doi: 10.1186/ar1837
41. Nishimura WE, Sachetto Z, Costallat LT, Yazbek MA, Londe AC, Guariento EG, et al. The role of KIR2DL3/HLA-C*0802 in Brazilian patients with rheumatoid vasculitis. Clinics (Sao Paulo). (2015) 70:408–12. doi: 10.6061/clinics/2015(06)04
42. Nishimura WE, Costallat LT, Fernandes SR, Conde RA, and Bertolo MB. Association of HLA-DRB5*01 with protection against cutaneous manifestations of rheumatoid vasculitis in Brazilian patients. Rev Bras Reumatol. (2012) 52:366–74.
43. Wang L, Luqmani R, and Udalova IA. The role of neutrophils in rheumatic disease-associated vascular inflammation. Nat Rev Rheumatol. (2022) 18:158–70. doi: 10.1038/s41584-021-00738-4
44. Matsunawa M, Isozaki T, Odai T, Yajima N, Takeuchi HT, Negishi M, et al. Increased serum levels of soluble fractalkine (CX3CL1) correlate with disease activity in rheumatoid vasculitis. Arthritis Rheumatol. (2006) 54:3408–16. doi: 10.1002/art.22208
45. Chen KR, Toyohara A, Suzuki A, and Miyakawa S. Clinical and histopathological spectrum of cutaneous vasculitis in rheumatoid arthritis. Br J Dermatol. (2002) 147:905–13. doi: 10.1046/j.1365-2133.2002.04933.x
46. Puéchal X, Said G, Hilliquin P, Coste J, Job-Deslandre C, Lacroix C, et al. Peripheral neuropathy with necrotizing vasculitis in rheumatoid arthritis. A clinicopathologic and prognostic study of thirty-two patients. Arthritis Rheumatol. (1995) 38:1618–29. doi: 10.1002/art.1780381114
47. Squirrell DM, Winfield J, and Amos RS. Peripheral ulcerative keratitis ‘corneal melt’ and rheumatoid arthritis: a case series. Rheumatol (Oxford). (1999) 38:1245–8. doi: 10.1093/rheumatology/38.12.1245
48. Okhravi N, Odufuwa B, McCluskey P, and Lightman S. Scleritis. Surv Ophthalmol. (2005) 50:351–63. doi: 10.1016/j.survophthal.2005.04.001
49. Makol A, Crowson CS, Wetter DA, Sokumbi O, Matteson EL, and Warrington KJ. Vasculitis associated with rheumatoid arthritis: a case-control study. Rheumatol (Oxford). (2014) 53:890–9. doi: 10.1093/rheumatology/ket475
50. Scott DG and Bacon PA. Intravenous cyclophosphamide plus methylprednisolone in treatment of systemic rheumatoid vasculitis. Am J Med. (1984) 76:377–84. doi: 10.1016/0002-9343(84)90654-5
51. Voskuyl AE, Hazes JM, Zwinderman AH, Paleolog EM, van der Meer FJ, Daha MR, et al. Diagnostic strategy for the assessment of rheumatoid vasculitis. Ann Rheum Dis. (2003) 62:407–13. doi: 10.1136/ard.62.5.407
52. Coffey CM, Richter MD, Crowson CS, Koster MJ, Warrington KJ, Ytterberg SR, et al. Rituximab therapy for systemic rheumatoid vasculitis: indications, outcomes, and adverse events. J Rheumatol. (2020) 47:518–23. doi: 10.3899/jrheum.181397
53. Johns K and Littlejohn G. Clinical experience with combination disease-modifying antirheumatic drug therapy with cyclosporine. Clin Exp Rheumatol. (1999) 17:S91–4.
54. Assmann G, Pfreundschuh M, and Voswinkel J. Rituximab in patients with rheumatoid arthritis and vasculitis-associated cutaneous ulcers. Clin Exp Rheumatol. (2010) 28:81–3.
55. Josselin L, Mahr A, Cohen P, Pagnoux C, Guaydier-Souquières G, Hayem G, et al. Infliximab efficacy and safety against refractory systemic necrotising vasculitides: long-term follow-up of 15 patients. Ann Rheum Dis. (2008) 67:1343–6. doi: 10.1136/ard.2007.083584
56. Bartolucci P, Ramanoelina J, Cohen P, Mahr A, Godmer P, Le Hello C, et al. Efficacy of the anti-TNF-alpha antibody infliximab against refractory systemic vasculitides: an open pilot study on 10 patients. Rheumatol (Oxford). (2002) 41:1126–32. doi: 10.1093/rheumatology/41.10.1126
57. Wysham KD, Baker JF, and Shoback DM. Osteoporosis and fractures in rheumatoid arthritis. Curr Opin Rheumatol. (2021) 33:270–6. doi: 10.1097/BOR.0000000000000789
58. Haugeberg G, Uhlig T, Falch JA, Halse JI, and Kvien TK. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheumatol. (2000) 43:522–30. doi: 10.1002/1529-0131(200003)43:3<522::AID-ANR7>3.0.CO;2-Y
59. Wilson JC, Sarsour K, Gale S, Pethö-Schramm A, Jick SS, and Meier CR. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). (2019) 71:498–511. doi: 10.1002/acr.23611
60. Hauser B, Riches PL, Wilson JF, Horne AE, and Ralston SH. Prevalence and clinical prediction of osteoporosis in a contemporary cohort of patients with rheumatoid arthritis. Rheumatol (Oxford). (2014) 53:1759–66. doi: 10.1093/rheumatology/keu162
61. Gupta N, Kanwar N, Arora A, Khatri K, and Kanwal A. The interplay of rheumatoid arthritis and osteoporosis: exploring the pathogenesis and pharmacological approaches. Clin Rheumatol. (2024) 43:1421–33. doi: 10.1007/s10067-024-06932-5
62. Kotake S, Udagawa N, Hakoda M, Mogi M, Yano K, Tsuda E, et al. Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. (2001) 44:1003–12. doi: 10.1002/1529-0131(200105)44:5<1003::AID-ANR179>3.0.CO;2-
63. Komatsu N and Takayanagi H. Immune-bone interplay in the structural damage in rheumatoid arthritis. Clin Exp Immunol. (2018) 194:1–8. doi: 10.1111/cei.13188
64. Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, and Goldring SR. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. (1998) 152:943–51.
65. Bramlage CP, Häupl T, Kaps C, Ungethüm U, Krenn V, Pruss A, et al. Decrease in expression of bone morphogenetic proteins 4 and 5 in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. (2006) 8:R58. doi: 10.1186/ar1923
66. Iwamoto J, Makita K, Sato Y, Takeda T, and Matsumoto H. Alendronate is more effective than elcatonin in improving pain and quality of life in postmenopausal women with osteoporosis. Osteoporos Int. (2011) 22:2735–42. doi: 10.1007/s00198-010-1495-8
67. Ishikawa Y, Miyakoshi N, Kasukawa Y, Hongo M, and Shimada Y. Spinal curvature and postural balance in patients with osteoporosis. Osteoporos Int. (2009) 20:2049–53. doi: 10.1007/s00198-009-0919-9
68. Coughlan T and Dockery F. Osteoporosis and fracture risk in older people. Clin Med (Lond). (2014) 14:187–91. doi: 10.7861/clinmedicine.14-2-187
69. Tan LM, Long TT, Guan XL, Wu SF, Zheng W, Fu HY, et al. Diagnostic value of vitamin D status and bone turnover markers in rheumatoid arthritis complicated by osteoporosis. Ann Clin Lab Sci. (2018) 48:197–204.
70. Raterman HG and Lems WF. Pharmacological management of osteoporosis in rheumatoid arthritis patients: A review of the literature and practical guide. Drugs Aging. (2019) 36:1061–72. doi: 10.1007/s40266-019-00714-4
71. Raterman HG, Bultink IE, and Lems WF. Osteoporosis in patients with rheumatoid arthritis: an update in epidemiology, pathogenesis, and fracture prevention. Expert Opin Pharmacother. (2020) 21:1725–37. doi: 10.1080/14656566.2020.1787381
72. Zerbini CAF, Clark P, Mendez-Sanchez L, Pereira RMR, Messina OD, Uña CR, et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int. (2017) 28:429–46. doi: 10.1007/s00198-016-3769-2
73. Chen J, Che Q, Kou Y, Rong X, Zhang X, Li M, et al. A novel drug combination of Tofacitinib and Iguratimod alleviates rheumatoid arthritis and secondary osteoporosis. IntImmunopharmacol. (2023) 124:110913. doi: 10.1016/j.intimp.2023.110913
74. Conforti A, Di Cola I, Pavlych V, Ruscitti P, Berardicurti O, Ursini F, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev. (2021) 20:102735. doi: 10.1016/j.autrev.2020.102735
75. Kim H, Cho SK, Kim HW, Han J, Kim Y, Hwang KG, et al. The prevalence of sjögren’s syndrome in rheumatoid arthritis patients and their clinical features. J Korean Med Sci. (2020) 35:e369. doi: 10.3346/jkms.2020.35.e369
76. Hajiabbasi A, Shenavar Masooleh I, Alizadeh Y, Banikarimi AS, and Ghavidel Parsa P. Secondary sjogren’s syndrome in 83 patients with rheumatoid arthritis. Acta Med Iran. (2016) 54:448–53.
77. Wu L, Wang Q, Gao QC, Shi GX, Li J, Fan FR, et al. Potential mechanisms and drug prediction of Rheumatoid Arthritis and primary Sjögren’s Syndrome: A public databases-based study. PloS One. (2024) 19:e0298447. doi: 10.1371/journal.pone.0298447
78. Wang Y, Xie X, Zhang C, Su M, Gao S, Wang J, et al. Rheumatoid arthritis, systemic lupus erythematosus and primary Sjögren’s syndrome shared megakaryocyte expansion in peripheral blood. Ann Rheum Dis. (2022) 81:379–85. doi: 10.1136/annrheumdis-2021-220066
79. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. (2002) 61:554–8. doi: 10.1136/ard.61.6.554
80. Zehrfeld N, Witte T, and Ernst D. Das Sjögren-Syndrom im Fokus [Focus on Sjögren’s syndrome - Diagnosis and treatment. Dtsch Med Wochenschr. (2024) 149:734–9. doi: 10.1055/a-2136-3498
81. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American college of rheumatology/European league against rheumatism classification criteria for primary sjögren’s syndrome: A consensus and data-Driven methodology involving three international patient cohorts. Arthritis Rheumatol. (2017) 69:35–45. doi: 10.1002/art.39859
82. Yu X, Zhu F, Yu X, Wang J, Wu B, and Li C. Serum CCL28 as a biomarker for diagnosis and evaluation of Sjögren’s syndrome. Scand J Rheumatol. (2023) 52:200–7. doi: 10.1080/03009742.2021.2001930
83. Tsuboi H, Toko H, Honda F, Abe S, Takahashi H, Yagishita M, et al. Abatacept ameliorates both glandular and extraglandular involvements in patients with Sjögren’s syndrome associated with rheumatoid arthritis: Findings from an open-label, multicentre, 1-year, prospective study: The ROSE (Rheumatoid Arthritis with Orencia Trial Toward Sjögren’s Syndrome Endocrinopathy) and ROSE II trials. Mod Rheumatol. (2023) 33:160–8. doi: 10.1093/mr/roac011
84. Rao CV. Potential therapy for rheumatoid arthritis and sjögren syndrome with human chorionic gonadotropin. Reprod Sci. (2016) 23:566–71. doi: 10.1177/1933719115597765
85. Baldini C, Fulvio G, La Rocca G, and Ferro F. Update on the pathophysiology and treatment of primary Sjögren syndrome. Nat Rev Rheumatol. (2024) 20:473–91. doi: 10.1038/s41584-024-01135-3
86. Yang TO, Floud S, and Reeves GK. Million Women Study Collaborators. Rheumatoid arthritis and cancer risk in the Million Women Study. Int J Epidemiol. (2024) 53:dyae006. doi: 10.1093/ije/dyae006
87. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024 [published correction appears in CA Cancer J Clin. 2024 Mar-Apr;74(2):203. doi: 10.3322/caac.21830. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
88. Klein A, Polliack A, and Gafter-Gvili A. Rheumatoid arthritis and lymphoma: Incidence, pathogenesis, biology, and outcome. Hematol Oncol. (2018) 36:733–9. doi: 10.1002/hon.2525
89. Cho MH, Cho JH, Eun Y, Han K, Jung J, Cho IY, et al. Rheumatoid arthritis and risk of lung cancer: A nationwide cohort study. J Thorac Oncol. (2024) 19:216–26. doi: 10.1016/j.jtho.2023.10.006
90. Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheumatol. (2006) 54:692–701. doi: 10.1002/art.21675
91. Warren GW and Cummings KM. Tobacco and lung cancer: risks, trends, and outcomes in patients with cancer. Am Soc Clin Oncol Educ Book. (2013) 33:359–64. doi: 10.1200/EdBook_AM.2013.33.359
92. Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. (2010) 69:70–81. doi: 10.1136/ard.2008.096487
93. Gibiot Q, Monnet I, Levy P, Brun AL, Antoine M, Chouaïd C, et al. Interstitial lung disease associated with lung cancer: a case-control study. J Clin Med. (2020) 9:700. doi: 10.3390/jcm9030700
94. Naccache JM, Gibiot Q, Monnet I, Antoine M, Wislez M, Chouaid C, et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis. (2018) 10:3829–44. doi: 10.21037/jtd.2018.05.75
95. Pundole X and Suarez-Almazor ME. Cancer and rheumatoid arthritis. Rheum Dis Clin North Am. (2020) 46:445–62. doi: 10.1016/j.rdc.2020.05.003
96. Morand S, Staats H, Creeden JF, Iqbal A, Kahaleh B, Stanbery L, et al. Molecular mechanisms underlying rheumatoid arthritis and cancer development and treatment. Future Oncol. (2020) 16:483–95. doi: 10.2217/fon-2019-0722
97. Lossignol D. A little help from steroids in oncology. J Transl Int Med. (2016) 4:52–4. doi: 10.1515/jtim-2016-0011
98. Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. (2018) 36:2872–8. doi: 10.1200/JCO.2018.79.0006
99. Franco AS, Iuamoto LR, and Pereira RMR. Perioperative management of drugs commonly used in patients with rheumatic diseases: a review. Clinics (Sao Paulo). (2017) 72:386–90. doi: 10.6061/clinics/2017(06)09
100. Stuck AE, Minder CE, and Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. (1989) 11:954–63. doi: 10.1093/clinids/11.6.954
101. Pundole X, Abdel-Wahab N, and Suarez-Almazor ME. Arthritis risk with immune checkpoint inhibitor therapy for cancer. Curr Opin Rheumatol. (2019) 31:293–9. doi: 10.1097/BOR.0000000000000601
102. England BR, Thiele GM, Anderson DR, and Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ. (2018) 361:k1036. doi: 10.1136/bmj.k1036
103. Gamarski R, Castro FF, Nascimento JASD, and Abreu MM. The Frequency of Cardiovascular Diseases in Rheumatoid Arthritis in Brazil: 10-year Cohort Study with DATASUS Databases. A Frequência de Doenças Cardiovasculares na Artrite Reumatoide no Brasil: Estudo de Coorte de 10 Anos com Bancos de Dados do DATASUS. Arq Bras Cardiol. (2025) 122:e20240313. doi: 10.36660/abc.20240313
104. Fragoulis GE, Panayotidis I, and Nikiphorou E. Cardiovascular risk in rheumatoid arthritis and mechanistic links: from pathophysiology to treatment. Curr Vasc Pharmacol. (2020) 18:431–46. doi: 10.2174/1570161117666190619143842
105. Sanghavi N, Ingrassia JP, Korem S, Ash J, Pan S, and Wasserman A. Cardiovascular manifestations in rheumatoid arthritis. Cardiol Rev. (2024) 32:146–52. doi: 10.1097/CRD.0000000000000486
106. Figus FA, Piga M, Azzolin I, McConnell R, and Iagnocco A. Rheumatoid arthritis: Extra-articular manifestations and comorbidities. Autoimmun Rev. (2021) 20:102776. doi: 10.1016/j.autrev.2021.102776
107. Liao KP. Cardiovascular disease in patients with rheumatoid arthritis. Trends Cardiovasc Med. (2017) 27:136–40. doi: 10.1016/j.tcm.2016.07.006
108. Jamthikar AD, Gupta D, Puvvula A, Johri AM, Khanna NN, Saba L, et al. Cardiovascular risk assessment in patients with rheumatoid arthritis using carotid ultrasound B-mode imaging. Rheumatol Int. (2020) 40:1921–39. doi: 10.1007/s00296-020-04691-5
109. Ntusi NAB, Francis JM, Gumedze F, Karvounis H, Matthews PM, Wordsworth PB, et al. Cardiovascular magnetic resonance characterization of myocardial and vascular function in rheumatoid arthritis patients. Hellenic J Cardiol. (2019) 60:28–35. doi: 10.1016/j.hjc.2018.01.008
110. Rydell E, Jacobsson LTH, and Turesson C. Circulating interleukin 17A and other inflammatory proteins may predict cardiovascular disease in early rheumatoid arthritis. J Rheumatol. (2024) 51:752–8. doi: 10.3899/jrheum.2023-1078
111. Huang Q, Liu Y, Cheng Y, Jia F, Pu C, Yan Q, et al. High-throughput quantitation of serological dimethylarginines by LC/MS/MS: Potential cardiovascular biomarkers for rheumatoid arthritis. J Pharm BioMed Anal. (2023) 232:115336. doi: 10.1016/j.jpba.2023.115336
112. Baldini C, Moriconi FR, Galimberti S, Libby P, and De Caterina R. The JAK-STAT pathway: an emerging target for cardiovascular disease in rheumatoid arthritis and myeloproliferative neoplasms. Eur Heart J. (2021) 42:4389–400. doi: 10.1093/eurheartj/ehab447
113. Gaál Z. Role of microRNAs in immune regulation with translational and clinical applications. Int J Mol Sci. (2024) 25:1942. doi: 10.3390/ijms25031942
114. Nguyen LD, Wei Z, Silva MC, Barberán-Soler S, Zhang J, Rabinovsky R, et al. Small molecule regulators of microRNAs identified by high-throughput screen coupled with high-throughput sequencing. Nat Commun. (2023) 14:7575. doi: 10.1038/s41467-023-43293-0
115. Mirgayazova R, Khadiullina R, Chasov V, Mingaleeva R, Miftakhova R, Rizvanov A, et al. Therapeutic editing of the TP53 gene: is CRISPR/cas9 an option? Genes (Basel). (2020) 11:704. doi: 10.3390/genes11060704
116. Lee MH, Shin JI, Yang JW, Lee KH, Cha DH, Hong JB, et al. Genome editing using CRISPR-cas9 and autoimmune diseases: A comprehensive review. Int J Mol Sci. (2022) 23:1337. doi: 10.3390/ijms23031337
117. Sarsenova M, Issabekova A, Abisheva S, Rutskaya-Moroshan K, Ogay V, and Saparov A. Mesenchymal stem cell-based therapy for rheumatoid arthritis. Int J Mol Sci. (2021) 22:11592. doi: 10.3390/ijms222111592
118. Mehta B, Pedro S, Ozen G, Kalil A, Wolfe F, Mikuls T, et al. Serious infection risk in rheumatoid arthritis compared with non-inflammatory rheumatic and musculoskeletal diseases: a US national cohort study. RMD Open. (2019) 5:e000935. doi: 10.1136/rmdopen-2019-000935
119. Dall’Era M, Pauli ML, Remedios K, Taravati K, Sandova PM, Putnam AL, et al. Adoptive treg cell therapy in a patient with systemic lupus erythematosus. Arthritis Rheumatol. (2019) 71:431–40. doi: 10.1002/art.40737
120. Bergmann C, Müller F, Distler JHW, Györfi AH, Völkl S, Aigner M, et al. Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells. Ann Rheum Dis. (2023) 82:1117–20. doi: 10.1136/ard-2023-223952
121. Zhang B, Wang Y, Yuan Y, Sun J, Liu L, Huang D, et al. In vitro elimination of autoreactive B cells from rheumatoid arthritis patients by universal chimeric antigen receptor T cells. Ann Rheum Dis. (2021) 80:176–84. doi: 10.1136/annrheumdis-2020-217844
122. Whittington KB, Prislovsky A, Beaty J, Albritton L, Radic M, and Rosloniec EF. CD8+ T cells expressing an HLA-DR1 chimeric antigen receptor target autoimmune CD4+ T cells in an antigen-specific manner and inhibit the development of autoimmune arthritis. J Immunol. (2022) 208:16–26. doi: 10.4049/jimmunol.2100643
Keywords: complications, research progress, rheumatism, autoimmune disease, rheumatoid arthritis
Citation: Zeng H, Yuan Z, Wu R-t and Huang Z (2025) Research progress on complications of rheumatoid arthritis. Front. Immunol. 16:1561926. doi: 10.3389/fimmu.2025.1561926
Received: 16 January 2025; Accepted: 05 May 2025;
Published: 27 May 2025.
Edited by:
Marko Radic, University of Tennessee Health Science Center (UTHSC), United StatesReviewed by:
Marco Folci, University of Milan, ItalyArnulfo Hernan Nava-Zavala, Mexican Social Security Institute, Mexico
Copyright © 2025 Zeng, Yuan, Wu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhisheng Huang, Y2dkaHpzQDE2My5jb20=
†These authors have contributed equally to this work
 Haiqing Zeng
Haiqing Zeng Zedongfang Yuan1,2†
Zedongfang Yuan1,2† Zhisheng Huang
Zhisheng Huang