- 1Hepatobiliary & Hydatid Disease Department, Digestive & Vascular Surgery Center, First Affiliated Hospital of Xinjiang Medical University, State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia, Urumqi, China
- 2World Health Organization (WHO) Collaborating Center on Prevention and Management of Echinococcosis, Clinical Medicine Institute, First Affiliated Hospital of Xinjiang Medical University, Urumqi, China
- 3State Key Laboratory on Pathogenesis Prevention & Treatment of High Incidence Diseases in Central Asia, Xinjiang Medical University, Urumqi, China
Echinococcus multilocularis larval tapeworm infection in humans is considered a serious public health issue. The immune interaction between parasites and their hosts is extremely important. NK cells are known innate immune cells that play important roles during infection and tumour progression. However, the possible role of NK cells in hepatic alveolar echinococcosis is not completely clear. In this study, we investigated the functional decrease in NK cells in hepatic alveolar echinococcosis (AE) patients.
Methods: Using human liver tissue samples from 10 patients with hepatic AE, flow cytometry was used to detect the expression of NKG2A molecules on the surface of NK cells, and the correlations between NKG2A+ expression and lesion size, alkaline phosphatase (ALP) levels in close lesion tissues (CLTs) and distal lesion tissues (DLTs) in the liver, and the secretion of functional molecules by NKG2A+ NK cells were analysed.
Results: The expression of NKG2A on CD56dim and CD56bright NK cells in DLTs and CLTs revealed that the percentage of NKG2A+CD56dim NK cells in CLTs was significantly greater than that in DLTs. There was a negative correlation between the expression of NKG2A on NK cells in the CLT and alkaline phosphatase. Additionally, we analysed IFN-γ, TNF-α, granzyme B, and perforin production in NK cells. There was a significant reduction in IFN-γ production in CLTs compared with DLTs. There is a negative correlation between IFN-γ production levels and NKG2A expression in NK cells from the CLT. The capacity of NKG2A+ NK cells from CLT regions to produce IFN-γ and granzyme B was also significantly decreased. In contrast, the perforin level produced by NKG2A+ NK cells was much greater than that produced by NKG2A− NK cells. We also analysed the correlation between the ratio of the NKG2A expression area in CLT and DLT tissues and the PET–CT value and found a positive correlation between NKG2A expression and the PET–CT value.
Conclusion: The increased expression of NKG2A in NK cells induced a reduction in IFN-γ production, and the increased expression of NKG2A may improve lesion activity and fibrosis, which may be helpful for treating hepatic alveolar echinococcosis via immunity.
Introduction
Human alveolar echinococcosis (AE) is a potentially lethal zoonosis caused by the cestode Echinococcus multilocularis (1); it is prevalent mainly in Western China, the Middle East, and Central Europe (2, 3). China accounts for 91% of the global AE burden every year; thus, sustained efforts have been made to prevent, control, and manage this disease (2). Humans can contract AE through contaminated food or water, and the liver is the primary infected organ. If not treated in time, the disease infiltrates and consequently leads to the critical involvement of vasculature and to jaundice, cirrhosis, and other clinical symptoms, which can result in liver failure or even death (4). The fatality rate of untreated or inadequately treated patients with human alveolar echinococcosis is 90% at 10–15 years after diagnosis (4, 5). Hence, it is regarded as a “parasitic cancer” (6).
The immune mechanism of E. multilocularis larvae is important for self-healing or persistent chronic infection after they enter the liver. In hepatic AE patients and animal models, long-term parasitism by larvae is mediated mainly by regulatory T cells and related cytokines, such as IL-10 and TGF-β (7). Our recent studies demonstrated the potential importance of the remaining Th subsets, such as Th17, Treg, and Th9, in E. multilocularis infection (8). Our data indicated that E. multilocularis can induce T-cell exhaustion through the inhibitory receptor TIGIT and that blocking this checkpoint may reverse the functional impairment of T cells and represent a possible approach to immunotherapy against AE (9). In addition, E. multilocularis vesicular fluid increases PD-L1 and CTLA-4 expression in T cells (10).
As important innate immune cells, NK cells constitute the first line of defence against infection and tumours. Many related studies have shown its special role in the tumour progression of liver cancer patients (11). The ability of intrahepatic NK cells to secrete IFN-γ and induce cytotoxicity is decreased significantly in hepatocellular carcinoma patients (12). The intracellular parasite Toxoplasma gondii impairs the ability of NK cells to recognize target cells and reduces the secretion of IFN-γ in the host (13). Malaria may increase the ability of NK cells to secrete IFN-γ via KIR/HLA molecules in early-stage infection (14), and Leishmania can inhibit the proliferation of NK cells (15). Extracellular parasites: When the percentage of NK cells decreases, the expression of NKG2D, CD69, and Ly49A is upregulated in individuals infected with Angiostrongylus cantonensis (16), and Schistosoma japonicum inhibits liver fibrosis by activating liver NK cells and increasing IFN-γ secretion (17). Several related studies have shown that in patients with hepatic alveolar echinococcosis, the activity of NK cells in the peripheral blood is significantly downregulated, and E. multilocularis vesicular fluid may inhibit the activation and proliferation of NK cells in mice (18).
NKG2A is specifically expressed on the surface of some lymphocytes, such as NK cells, T cells, and NKT cells, and transduces inhibitory signals (19). High expression of NKG2A induces functional downregulation of NK cells and is related to poor prognosis in patients with hepatic cellular carcinoma (20). Currently, the newest research reports that NKG2A is a new checkpoint, and blocking NKG2A with monalizumab could promote the antitumour immune activity of CD8+ T cells and NK cells, which could be used as a supplement to the first generation of cancer immunotherapy (11). Our recent study demonstrated that a reduction in NK cell frequency and increased NKG2A may result in low cytotoxic activity through decreased IFN-γ secretion during E. multilocularis infection (18). Therefore, studies on the correlation between the expression of NKG2A on NK cells and the pathological and clinical parameters of hepatic AE are very important.
However, some research has focused on immune interactions in E. multilocularis infection. NK cells are involved in E. multilocularis infection both in vitro and in vivo. However, very little is known regarding the possibility of immune dysfunction in hepatic AE patients. In this study, we demonstrated NKG2A expression in hepatic NK cells and its functional exhaustion, such as decreased IFN-γ production and decreased cellular cytotoxicity. These findings may indicate the existence of a negative regulatory mechanism in exhausted NK cells as a result of the increased expression of the inhibitory receptor NKG2A in hepatic AE patients.
Patients and methods
Liver tissues from 36 hepatic AE patients and fresh hepatic AE liver tissue samples were obtained from 10 hepatic AE patients who underwent radical hepatectomy between 2012 and 2015 in the Department of Hepatobiliary and Echinococcosis Surgery Department of the First Affiliated Hospital of Xinjiang Medical University (21–23). In accordance with the location of surgical samples from patients with hepatic AE, the liver tissue adjacent to the lesion (0.5 cm) and its volume of approximately 2 cm × 2 cm × 2 cm were selected as the liver close lesion tissue (CLT), and the corresponding normal liver tissue approximately 3–5 cm away from the lesion was taken as the liver distal lesion tissue (DLT). All fresh tissues were used for phenotypic analysis, and most of them were also used for intracellular cytokine analysis if they had a sufficient number of cells. All patients provided written informed consent in accordance with the Declaration of Helsinki. The protocols for all study cohorts were approved by the Ethics Board of Xinjiang Medical University.
Flow cytometry
Liver tissue-infiltrating leukocytes were obtained as previously described (9). To digest the samples, they were cut into small pieces and digested in RPMI 1640 (HyClone Laboratories, Logan, UT, USA) supplemented with collagenase IV (10 mg/mL, Sigma-Aldrich Corp., St. Louis, MO, USA) and DNase I (33.3 mg/mL, Sigma-Aldrich, USA) at 37°C for 1–2 h. The peripheral lymphocytes, liver tissue-infiltrating leukocytes, and NK cells from the in vitro cultures were stained with fluorochrome-conjugated Abs and then analysed through flow cytometry (24). Abs against the following proteins were used for staining: CD3 (UCHT1), CD56 (HCD56), CD16 (3G8), CD69 (FN50), NKG2D (1D11), IFN-γ (4S.B3), TNF-α (MAb11), perforin (dG9), and granzyme B (GB11) (BioLegend, USA) and NKG2A (131411) (R&D Systems, Minneapolis, MN, USA). The stained cells were analysed using an LSRFortessa flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), and the data were analysed using the FlowJo analysis software V10 (Treestar, Woodburn, OR, USA).
Immunohistochemistry
The paraffin-embedded sections were dewaxed in xylene and rehydrated with distilled water. Following incubation with antibodies against human NKG2A (PA5-21949, Thermo Fisher, Waltham, MA, USA), adjacent sections were stained with the DAB Peroxidase Substrate Kit (SK-4100) (Vector Laboratories, Burlingame, CA, USA). Positive and negative controls were tested before formal staining. The Olympus Optical Microscope cellSens scanning software (Olympus, Tokyo, Japan) was used to perform scanning analysis, and the positive staining surface around the lesion was calculated (25).
NK cell purification
Peripheral blood was obtained from healthy controls, and purified NK cells were enriched from whole blood via RosetteSep™ Human NK Cell Cocktail (50 μL/mL) (STEMCELL Technologies, Vancouver, BC, Canada). The cells were incubated in medium alone or with recombinant human IL-12 p40 (200-12p40) (10 ng/mL; PeproTech, Cranbury, NJ, USA, USA) plus recombinant human IL-15 (200-15) (10 ng/mL; PeproTech, USA), with IL-12+IL-15 plus recombinant human TGF-β1 (1 ng/mL; PeproTech, USA), or with IL-12+IL-15 plus EMP (E. multilocularis protein). The protein was extracted and quantified after grinding E. multilocularis. NK cells were stimulated for 24 h at 37°C in an atmosphere of 5% CO2. NK cell viability was evaluated via the trypan blue exclusion method and analysed via flow cytometry after incubation.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA). The Wilcoxon non-parametric statistical test or Mann–Whitney non-parametric statistical test was used when there were more than two groups. p < 0.05 was considered statistically significant. p-Values are presented as * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
Increased NKG2A expression in NK cells from the close lesion tissue of hepatic AE patients
To investigate the expression of NKG2A in intrahepatic NK cells, we analysed the CLT and DLT of liver AE lesions. As shown in Figures 1A–C, the percentage of NKG2A+ NK cells in the CLT was significantly greater than that in the DLT. As previously demonstrated (26), we analysed the expression of NKG2A on CD56dim and CD56bright NK cells in DLT and CLT and reported that the percentage of NKG2A+CD56dim NK cells in CLTs was significantly greater than that in DLTs. There was a negative correlation between the expression of NKG2A on NK cells in the CLT and alkaline phosphatase (ALP) (p = 0.0234, r = −0.7212) (Figure 1D), but there was no correlation between alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels (Figures 1E–G). These results suggest that the percentage of NKG2A+ NK cells in the CLT of hepatic AE patients was significantly increased, which was mainly manifested by the upregulation of NKG2A expression on CD56dim NK cells.
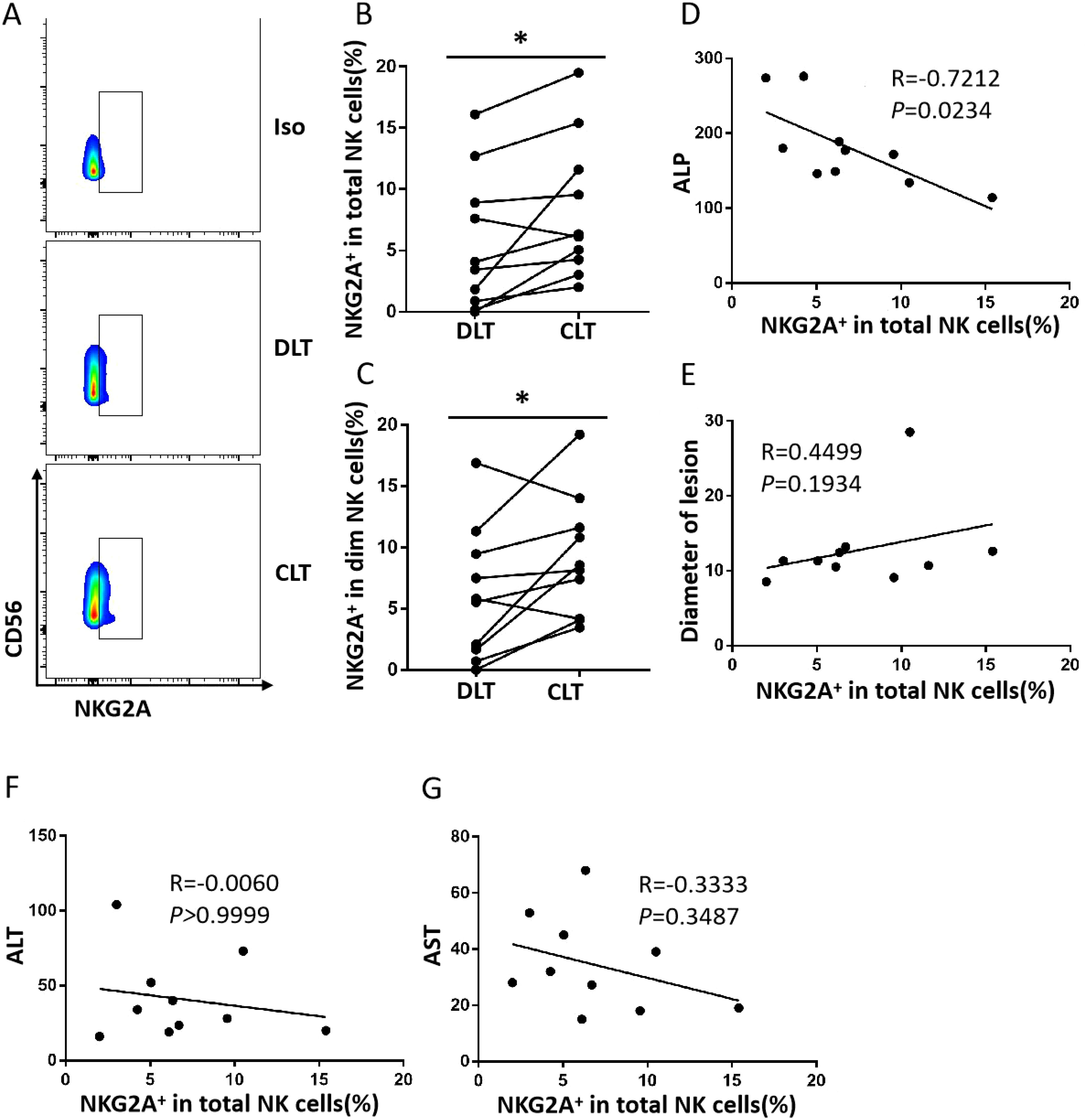
Figure 1. The frequency of close lesion tissue NKG2A+ NK cells is increased in hepatic AE. (A) Representative NKG2A expression in different liver NK cell subsets from DLTs and CLTs from hepatic AE patients. (B) NKG2A expression in NK cells from paired DLTs and CLTs from hepatic AE patients (N = 10, Wilcoxon non-parametric statistical test). (C) Percentages of NKG2A+CD56dim NK cells in paired DLTs and CLTs from patients with hepatic AE (n = 10, Wilcoxon non-parametric statistical test). (D–G) Correlations between NKG2A expression in hepatic NK cells from CLTs and the serum Alkaline Phosphatase (ALP), ALT, and AST levels and lesion diameters in hepatic AE patients. Spearman’s correlation coefficients are shown. (P values are presented as *P < 0.05). AE, alveolar echinococcosis; DLTs, distal lesion tissues; CLTs, close lesion tissues; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Increased expression of NKG2A in NK cells induced a significant reduction in IFN-γ production in close lesion tissue compared with distal lesion tissue from hepatic AE patients
The function of NK cells is affected by combining both activating receptors and inhibitory receptors. According to previous studies (27), NKG2A-induced phenotypic changes may be accompanied by functional alterations in NK cells. Therefore, we analysed IFN-γ, TNF-α, granzyme B, and perforin production in NK cells (Figure 2). There was a significant reduction in IFN-γ production in the CLT compared with DLT (Figure 2A). However, there was no significant difference in granzyme B, perforin, or TNF-α levels (Figures 2B–D). A negative correlation was detected between IFN-γ production and NKG2A expression in NK cells from the CLT (Figure 2E). In addition, the capacity of NKG2A+ NK cells from CLT regions to produce IFN-γ and granzyme B was also significantly decreased (Figures 2F, G). However, the ability of NKG2A+ NK cells to produce TNF-α and perforin was not significantly different between the CLT and the DLT (Figures 2H, I). In addition, the ability of NKG2A+ NK cells to secrete IFN-γ and granzyme B was, surprisingly, significantly lower than that of NKG2A− NK cells (Figures 3A, B). In contrast, NKG2A+ NK cells produced much higher perforin levels than NKG2A+ NK cells did (Figure 3C), but there is there was no significant difference produced TNF-α levels (Figure 3D). These findings suggest that the increased expression of NKG2A on NK cells in the CLT decreased the ability of NK cells to secrete IFN-γ.
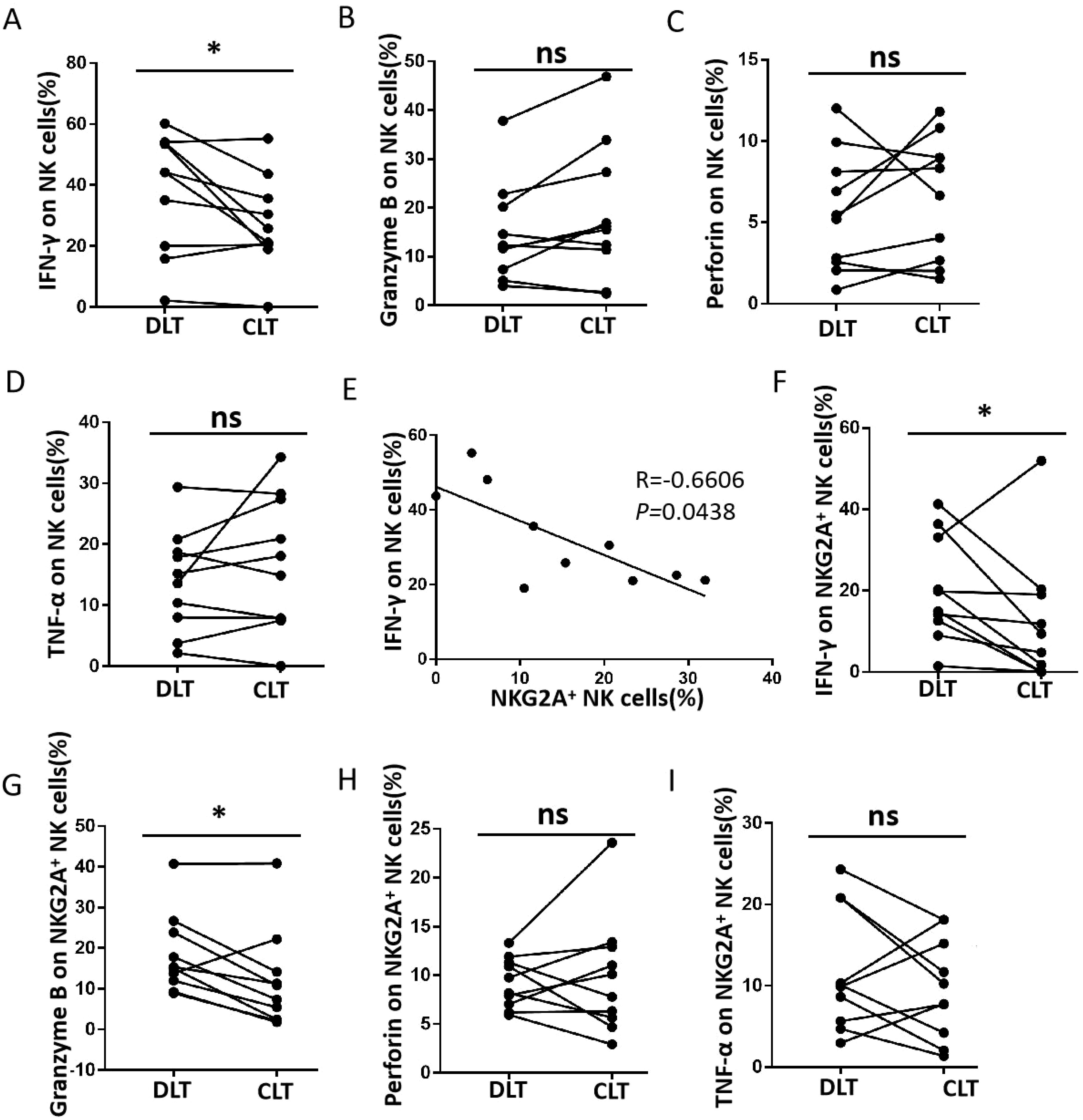
Figure 2. Functional impairment of CLT NK cells from hepatic AE patients. (A–D) The levels of IFN-γ, granzyme B, perforin, and TNF-α produced by NK cells in the CLT and DLT regions in hepatic AE patients (n = 10, Wilcoxon non-parametric statistical test). (E) Analysis of the correlation between IFN-γ levels and the percentage of NKG2A+ NK cells in the tissue samples. Each dot represents a single region CLT from a hepatic AE patient (Spearman’s correlation test). (F–I) The levels of IFN-γ, granzyme B, perforin, and TNF-α produced by NKG2A+ NK cells in CLT regions in hepatic AE patients (n = 10, Wilcoxon non-parametric statistical test). (P values are presented as *P < 0.05), no significant different (ns). CLT, close lesion tissue; AE, alveolar echinococcosis; DLT, distal lesion tissue.
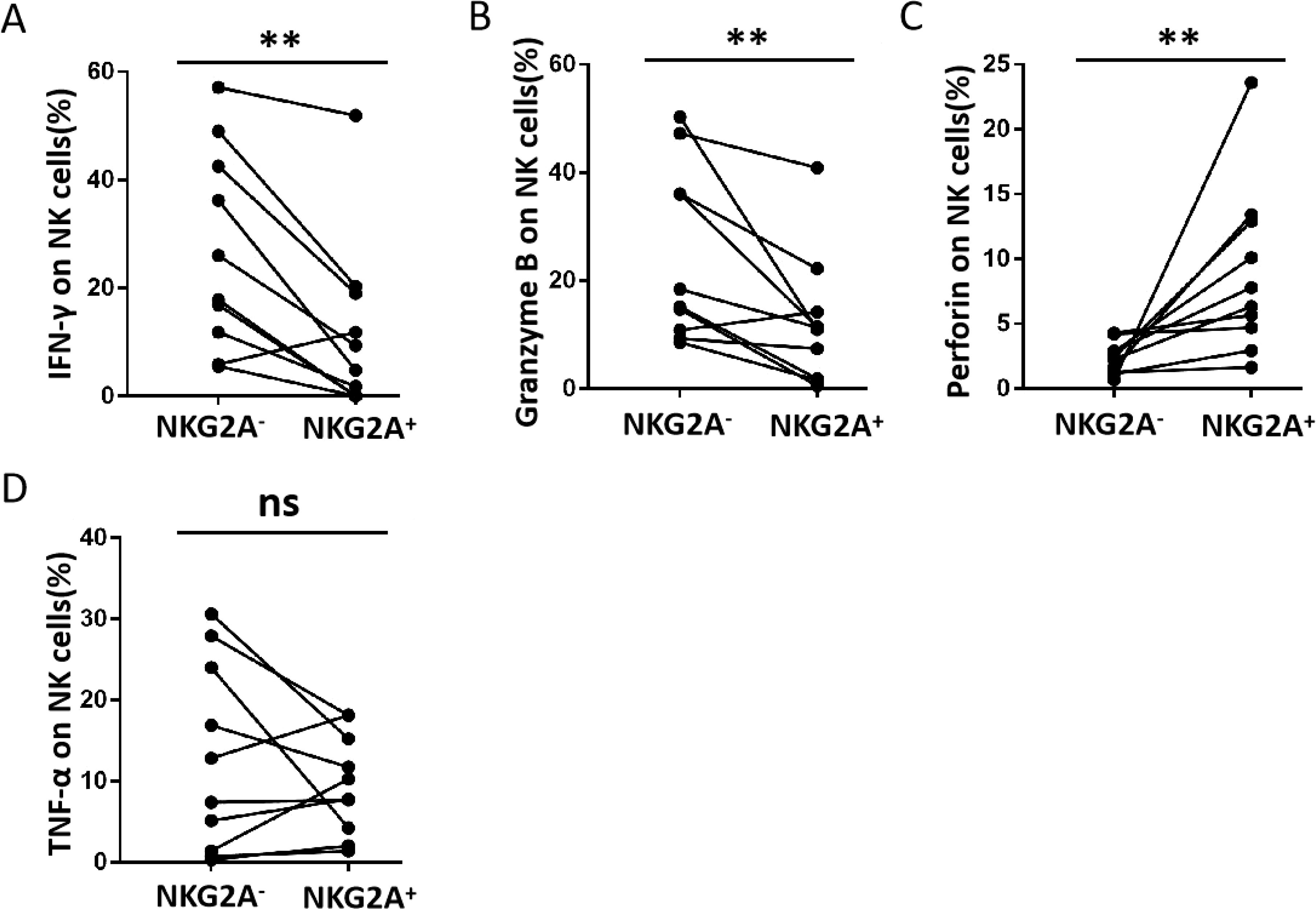
Figure 3. The ability of NKG2A+ and NKG2A− NK cells in CLT from hepatic AE patients to produce cytokines. (A–D) The levels of IFN-γ, granzyme B, perforin, and TNF-α produced by NKG2A+ NK and NKG2A− NK cells in CLT regions in hepatic AE patients (n = 10, Wilcoxon non-parametric statistical test). (P values are presented as **P < 0.01), no significant different (ns). CLT, close lesion tissue; AE, alveolar echinococcosis.
Higher NKG2A expression is correlated with lesion activity and fibrosis in hepatic AE patients
After the expression of NKG2A in CLT and DLT tissues from 36 patients with hepatic AE was examined, our results suggested that the expression of NKG2A was significantly increased in the CLT (Figures 4A, B). We also analysed the correlation between the ratio of the NKG2A expression area in the CLT and DLT tissues (CLT/DLT) and the PET–CT value (SUVmax) and found a positive correlation between NKG2A expression and the PET–CT value (SUVmax) (p = 0.0065, R = 0.735) (Figure 4C). In this study, according to liver tissue samples from patients with hepatic AE who underwent PET–CT examination, Masson staining was used to detect the collagen area (fibrotic area) around the lesion, and α-SMA immunohistochemical staining was used to detect the positive expression of hepatic stellate cells. A PET–CT (SUVmax) value greater than or equal to 3.0 was included in the high-activity lesion group, and a value less than 3.0 was included in the low-activity lesion group. The results revealed that the area of fibrosis around lesions was significantly lower in high-activity lesions than in low-activity lesions (Figures 4D, E), and a negative correlation existed between the NKG2A+ expression area and the fibrosis prelesion area (Figures 4F, G) (these results need to be further verified by expanding the sample size). These results suggest that the expression of NKG2A in the CLT was upregulated and was positively correlated with the activity of the lesion. Moreover, the greater activity of the lesion and the lower degree of fibrosis around the lesion result in the lesion not being fully limited by the fibrous layer, which induces faster growth of the hepatic AE lesion.
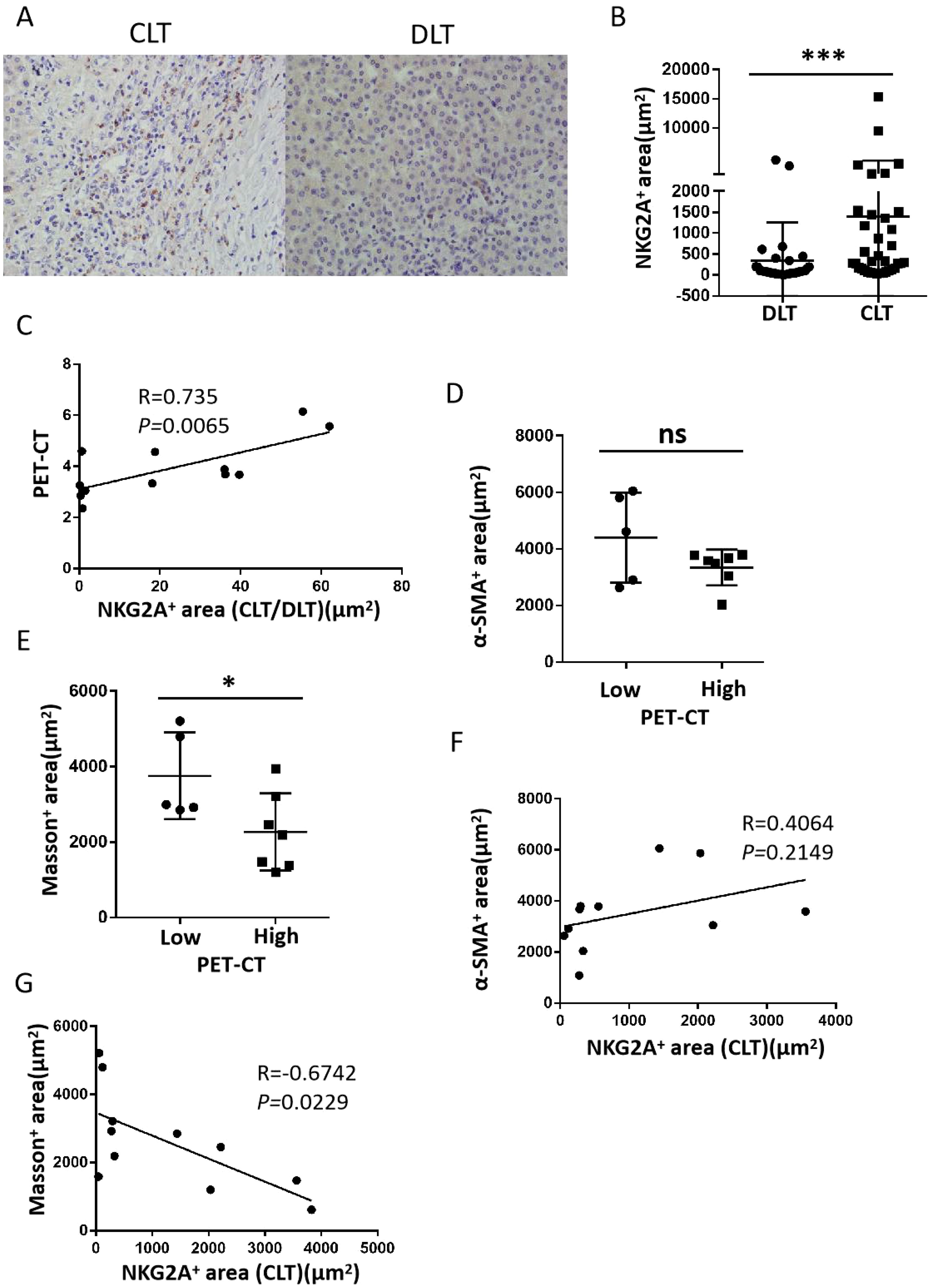
Figure 4. The expression of NKG2A and fibrosis around lesions in CLT regions from hepatic AE patients. (A) Representative micrographs showing NKG2A+ cells in the CLT and DLT of hepatic patients. Original magnifications, ×10, ×40; bar D 100 mm. (B) Cumulative data are shown (Mann–Whitney unpaired non-parametric statistical tests). (C) Correlation between NKG2A+ expression and the PET–CT value (SUVmax) in the CLT region from hepatic AE patients (N = 12) (Spearman’s correlation coefficient (r) and (p) values are shown). (D, E) α-SMA immunohistochemical staining revealed positive expression of hepatic stellate cells, and Masson staining revealed the collagen area (fibrotic area) around the lesion in high-activity and low-activity lesions from the hepatic AE lesion. (F, G) Correlations between positive expression of hepatic stellate cells with NKG2A+ expression area and fibrosis prelesion area with NKG2A+ expression [Spearman’s correlation coefficients (r) and (p) values are shown]. (P values are presented as *P < 0.05; ***P < 0.001), no significant different (ns). CLT, close lesion tissue; AE, alveolar echinococcosis; DLT, distal lesion tissue.
Compared with those of CD56bright NK cells, the percentages of NK cells are predominantly decreased on CD56dim NK cells
Human NK cells are divided into two subsets, and their main functions are not exactly the same: CD56bright NK cells are responsible for cytokine secretion, whereas CD56dim NK cells are responsible for cytotoxicity (28). We analysed the percentage of NKG2A expression in different subsets of NK cells in hepatic AE patients. The gating strategy used to separate the CD56bright and CD56dim NK cells is shown in Figure 5A. We observed that the percentages of total NK cells (Figure 5B) and CD56bright NK cells (Figure 5C) in the CLT and DLT tissues were not significantly different. However, the percentage of CD56dim NK cells among total NK cells decreased significantly in the CLT (Figure 5D). These results further confirmed that the decreased percentage of the NK cell subset was mainly CD56dim NK cells in the CLT tissues of hepatic AE patients.
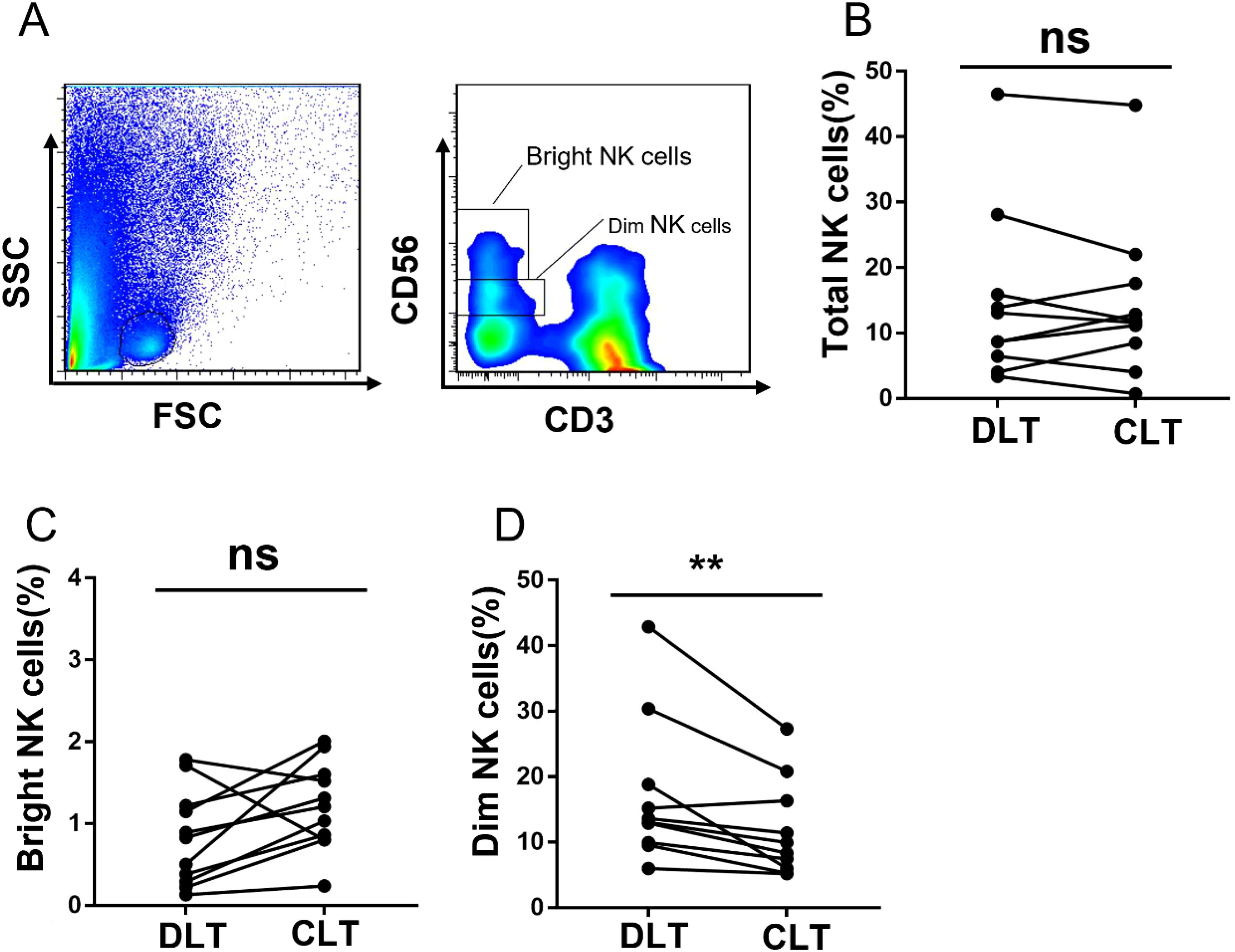
Figure 5. The subset of NK cells in CLT is decreased, and those in hepatic AE patients are CD56dim NK cells. (A) The gating strategy for the CLT used to analyse CD56bright NK cells and CD56dim NK cells via flow cytometry. (B) The percentages of total NK cells in the CLTs and DLTs from hepatic AE patients (N = 10, Wilcoxon non-parametric statistical test). (C, D) Percentages of CD56bright NK cells and CD56dim NK cells in the CLTs and DLTs of hepatic AE patients (n = 10, Wilcoxon non-parametric statistical test). (P values are presented as **P < 0.01), no significant different (ns). CLT, close lesion tissue; AE, alveolar echinococcosis; DLT, distal lesion tissue.
Discussion
Our study demonstrated the obviously increased expression of NKG2A in NK cells in the CLT, which may induce a significant reduction in IFN-γ production from closely related lesion tissue; moreover, increased NKG2A expression is correlated with lesion activity and fibrosis in hepatic AE patients. According to present research reports, this is the first report on hepatic NK cells and their related functions in hepatic AE patients.
The percentage of human liver NK cells among total lymphocytes is approximately 25%–40% (29), and approximately 90% of NK cells in the peripheral blood and spleen belong to the CD56dim subset, whereas only 50% of NK cells in the liver belong to the CD56dim subset. NK cell subsets have different effects on the proliferation response, cytotoxicity, cytokine production, and expression of NK cell receptors and adhesion molecules (29). NK cells, as critical components of the innate immune system, are important effector lymphocyte populations involved in antitumour and anti-infection immunity (30). However, in the context of tumours and chronic infections, NK cells exhibit an exhausted status similar to that of exhausted T cells, resulting in poor effector function and an altered phenotype. In ovarian cancer, the expression of NKG2A is upregulated, which induces NK cell failure (28). The upregulation of NKG2A on NK cells may indicate antitumour immune tolerance and promote tumour metastasis in lung cancer patients (31). Previous studies have shown that the NK toxicity of peripheral blood mononuclear cells (PBMCs) in AE patients is lower than that in non-parasitic biliary disease patients (32). Another recent study revealed that the vesicular fluid of E. multilocularis has an inhibitory effect on the activation and proliferation of NK cells in human PBMCs (10). According to previous relevant studies and our research, the increased expression of NKG2A on NK cells in the CLT of hepatic AE patients may be the main mechanism mediating NK cell dysfunction. Monalizumab treatment can restore CD107 and IFN-γ production in NK cells against various tumour cells (33). When class I MHC ligands of NK cell inhibitory receptors are downregulated, which commonly occurs in tumour cells, the loss of inhibitory signals and the resulting unabated positive signalling also leads to NK cell activation (IFN-γ and TNF-α). This phenomenon is referred to as the “missing-self” response (34). Therefore, we also demonstrated that the ability of NK cells to secrete IFN-γ and granzyme B in the CLT of a lesion is significantly lower than that in the DLT. Moreover, the percentage of NKG2A+ NK cells in the CLT may be negatively correlated with the percentage of IFN-γ secretion. In other words, the inhibitory molecule NKG2A is upregulated on NK cells in the CLT of the lesion, which is the area with the strongest inflammatory immune response, possibly leading to the downregulation of NK cell function.
Humans are occasional intermediate hosts, and the severity of liver AE in humans is caused by the continuous asexual reproduction of E. multilocularis and strong inflammatory granuloma infiltration around it, which leads to pathological damage to the liver after parasitic infection. In this study, we analysed the positive expression of NKG2A in the CLT and DLT surrounding liver AE lesions using immunohistochemical staining techniques. The results suggest that the positive expression of NKG2A in the CLT was significantly greater than that in the DLT and that there may be a positive correlation between NKG2A (CLT/DLT) and PET–CT (SUVmax) values. Reuter (35) used positron emission tomography (PET) to track the metabolic changes in 18F-FDG in liver AE lesions, and the active area around the liver AE lesion exhibited differences in energy metabolism. Therefore, at present, PET–CT (SUVmax) values can be used to effectively determine the activity of AE lesions. On the basis of our research results, the greater the expression of NKG2A in the surrounding tissues of the lesion, the greater the activity of the lesion, the significantly lower the degree of fibrosis around the lesion, the weaker the fibrous layer to restrict the lesion, and the greater the speed at which the lesion grows and invades the surrounding normal liver tissue.
In summary, infection of the host with E. multilocularis leads to the infiltration of inflammatory cells, proliferation of fibroblasts, and promotion of organ fibrosis. Moreover, the fibrous layer is involved in the inflammatory response around the larvae. On the one hand, this can effectively prevent the parasite from receiving a sufficient immune response from the host, which is beneficial for parasite growth and continued parasitism. On the other hand, it limits the parasite’s continued growth, invasion, and growth of new metastatic lesions. Our research revealed that after infection with E. multilocularis, the increased expression of NKG2A on NK cells leads to the downregulation of IFN-γ secretion. The increase in NKG2A expression in the tissue near the lesion in patients with liver AE is similar to that observed in animal experiments, leading to a decrease in the function of NK cells, which secrete IFN-γ and granzyme B, and a reduction in fibrosis around the lesion may increase lesion activity and growth. However, further research is needed to explore the detailed interaction mechanism involved, and we need to expand the sample size to further validate the conclusion.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Approval Report of the Animal Experiment Medical Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AA: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. TT: Writing – original draft, Writing – review & editing, Conceptualization, Investigation. PS: Writing – original draft, Writing – review & editing, Conceptualization. CZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TJ: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. QG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RL: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. YS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Tianshan Talents—Science and Technology Innovation Leading Talents Project—high-level leading talents, grant no. 2022TSYCLJ0034; Xinjiang Uygur Autonomous Region Natural Science Foundation key project, grant no. 2022D01D17; Exceptional Professionals at the First Affiliated Hospital of Xinjiang Medical University grant no. zyrc202409; and State Key Laboratory of Pathogenesis, Prevention and Treatment of Central Asian High Incidence Diseases Fund (SKL-HIDCA-2024-22).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fimmu.2025.1638960.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Baumann S, Shi R, Liu W, Bao H, Schmidberger J, Kratzer W, et al. Correction to: Worldwide literature on epidemiology of human alveolar echinococcosis: a systematic review of research published in the twenty-first century. Infection. (2022) 50:287–8. doi: 10.1007/s15010-021-01695-6
2. Wang X, Dai G, Li M, Jia W, Guo Z, Lu J, et al. Prevalence of human alveolar echinococcosis in China: a systematic review and meta-analysis. BMC Public Health. (2020) 20:1105. doi: 10.1186/s12889-020-08989-8
3. Ma T, Wang Q, Hao M, Xue C, Wang X, Han S, et al. Epidemiological characteristics and risk factors for cystic and alveolar echinococcosis in China: an analysis of a national population-based field survey. Parasit Vectors. (2023) 16:181. doi: 10.1186/s13071-023-05788-z
4. Aji T, Dong JH, Shao YM, Zhao JM, Li T, Tuxun T, et al. Ex vivo liver resection and autotransplantation as alternative to allotransplantation for end-stage hepatic alveolar echinococcosis. J Hepatol. (2018) 69:1037–46. doi: 10.1016/j.jhep.2018.07.006
5. Ferrer Inaebnit E, Molina Romero FX, Segura Sampedro JJ, González Argenté X, and Morón Canis JM. A review of the diagnosis and management of liver hydatid cyst. Rev Esp Enferm Dig. (2022) 114:35–41. doi: 10.17235/reed.2021.7896/2021
6. Feng C, Cheng Z, Xu Z, Tian Y, Tian H, Liu F, et al. EmCyclinD-EmCDK4/6 complex is involved in the host EGF-mediated proliferation of Echinococcus multilocularis germinative cells via the EGFR-ERK pathway. Front Microbiol. (2022) 13:968872. doi: 10.3389/fmicb.2022.968872
7. Gao H, Huo L, Mo X, Jiang B, Luo Y, Xu B, et al. Suppressive effect of pseudolaric acid B on Echinococcus multilocularis involving regulation of TGF-β1 signaling in vitro and in vivo. Front Microbiol. (2022) 13:1008274. doi: 10.3389/fmicb.2022.1008274
8. Jiang T, Sun W, Aji T, Shao Y, Guo C, Zhang C, et al. Single-cell heterogeneity of the liver-infiltrating lymphocytes in individuals with chronic echinococcus multilocularis infection. Infect Immun. (2022) 90:e0017722. doi: 10.1128/iai.00177-22
9. Zhang C, Lin R, Li Z, Yang S, Bi X, Wang H, et al. Immune exhaustion of T cells in alveolar echinococcosis patients and its reversal by blocking checkpoint receptor TIGIT in a murine model. Hepatology. (2020) 71:1297–315. doi: 10.1002/hep.30896
10. Bellanger AP, Courquet S, Pallandre JR, and Godet Y. Echinococcus multilocularis vesicular fluid induces the expression of immune checkpoint proteins in vitro. Parasite Immunol. (2020) 42:e12711. doi: 10.1111/pim.12711
11. André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. (2018) 175:1731–1743 e13. doi: 10.1016/j.cell.2018.10.014
12. Lu Z, Chai X, Pan Y, and Li S. The causality between CD8(+) NKT cells and CD16(-) CD56 on NK cells with hepatocellular carcinoma: a Mendelian randomization study. Infect Agent Cancer. (2024) 19:3. doi: 10.1186/s13027-024-00565-8
13. Brito C, Lourenço C, Magalhães J, Reis S, and Borges M. Nanoparticles as a Delivery System of Antigens for the Development of an Effective Vaccine against Toxoplasma gondii. Vaccines (Basel). (2023) 11:733. doi: 10.3390/vaccines11040733
14. Zhao HD, Qian HB, Wang ZK, Ren RK, Yu TB, and Liu HL. Patient with suspected co-infection of hemorrhagic fever with renal syndrome and malaria: a case report. Front Med (Lausanne). (2024) 11:1341015. doi: 10.3389/fmed.2024.1341015
15. Costa-da-Silva AC, Nascimento DO, Ferreira JRM, Guimarães-Pinto K, Freire-de-Lima L, Morrot A, et al. Immune responses in leishmaniasis: an overview. Trop Med Infect Dis. (2022) 7:54. doi: 10.3390/tropicalmed7040054
16. Zhang R, Miao T, Qin M, Zhao C, Wang W, Zhang C, et al. CX(3)CL1 recruits NK cells into the central nervous system and aggravates brain injury of mice caused by angiostrongylus cantonensis infection. Front Cell Infect Microbiol. (2021) 11:672720. doi: 10.3389/fcimb.2021.672720
17. Liu Z, Zhang L, Liang Y, and Lu L. Pathology and molecular mechanisms of Schistosoma japonicum-associated liver fibrosis. Front Cell Infect Microbiol. (2022) 12:1035765. doi: 10.3389/fcimb.2022.1035765
18. Abulizi A, Shao Y, Aji T, Li Z, Zhang C, Aini A, et al. Echinococcus multilocularis inoculation induces NK cell functional decrease through high expression of NKG2A in C57BL/6 mice. BMC Infect Dis. (2019) 19:792. doi: 10.1186/s12879-019-4417-1
19. Jiang H and Jiang J. Balancing act: the complex role of NK cells in immune regulation. Front Immunol. (2023) 14:1275028. doi: 10.3389/fimmu.2023.1275028
20. Hofman T, Ng SW, Garcés-Lázaro I, Heigwer F, Boutros M, Cerwenka A, et al. IFNγ mediates the resistance of tumor cells to distinct NK cell subsets. J Immunother Cancer. (2024) 12:e009410. doi: 10.1136/jitc-2024-009410
21. Kiewisz J, Waśniewski T, Kieżun J, Skowrońska A, Kaczmarek MM, Szóstak B, et al. WNT4 gene and protein expression in endometrial cancer and its significance. Cancers (Basel). (2023) 15:4780. doi: 10.3390/cancers15194780
22. Delgado SL, Perilla PM, Salgado DM, Rojas MC, and Narváez CF. Efficiency of automated viral RNA purification for pediatric studies of dengue and zika in hyperendemic areas. J Trop Med. (2023) 2023:1576481. doi: 10.1155/2023/1576481
23. Vanderheijden C, Yakkioui Y, Vaessen T, Santegoeds R, Temel Y, Hoogland G, et al. Developmental gene expression in skull-base chordomas and chondrosarcomas. J Neurooncol. (2025) 172:249–56. doi: 10.1007/s11060-024-04913-x
24. Wang Z, Liu N, Yang Y, and Tu Z. The novel mechanism facilitating chronic hepatitis B infection: immunometabolism and epigenetic modification reprogramming. Front Immunol. (2024) 15:1349867. doi: 10.3389/fimmu.2024.1349867
25. Hammerich L and Tacke F. Hepatic inflammatory responses in liver fibrosis. Nat Rev Gastroenterol Hepatol. (2023) 20:633–46. doi: 10.1038/s41575-023-00807-x
26. Sendekie B, Gebeyehu B, Mihret A, Kassa T, and Kebede N. Epidemiological study on bovine cystic echinococcosis: Abattoir survey, cyst characterization and its economic impact at Mekaneyesuse municipality abattoir, Northwest Ethiopia. Parasite Epidemiol Control. (2024) 25:e00355. doi: 10.1016/j.parepi.2024.e00355
27. Fisher JG, Doyle ADP, Graham LV, Khakoo SI, and Blunt MD. Disruption of the NKG2A:HLA-E immune checkpoint axis to enhance NK cell activation against cancer. Vaccines (Basel). (2022) 10:1993. doi: 10.3390/vaccines10121993
28. Sun C, Xu J, Huang Q, Huang M, Wen H, Zhang C, et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology. (2017) 6:e1264562. doi: 10.1080/2162402X.2016.1264562
29. Male V, Stegmann KA, Easom N J, and Maini MK. Natural killer cells in liver disease. Semin Liver Dis. (2017) 37:198–209. doi: 10.1055/s-0037-1603946
30. Boersma B, Poinot H, and Pommier A. Stimulating the antitumor immune response using immunocytokines: A preclinical and clinical overview. Pharmaceutics. (2024) 16:974. doi: 10.3390/pharmaceutics16080974
31. Zeng Y, Lv X, and Du J. Natural killer cell−based immunotherapy for lung cancer: Challenges and perspectives (Review). Oncol Rep. (2021) 46:232. doi: 10.3892/or.2021.8183
32. Zhang C, Shao Y, Yang S, Bi X, Li L, Wang H, et al. T-cell tolerance and exhaustion in the clearance of Echinococcus multilocularis: role of inoculum size in a quantitative hepatic experimental model. Sci Rep. (2017) 7:11153. doi: 10.1038/s41598-017-11703-1
33. Zhang C and Liu Y. Targeting NK cell checkpoint receptors or molecules for cancer immunotherapy. Front Immunol. (2020) 11:1295. doi: 10.3389/fimmu.2020.01295
34. Myers JA and Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. (2021) 18:85–100. doi: 10.1038/s41571-020-0426-7
Keywords: hepatic alveolar echinococcosis, NK cell, NKG2A, immune exhaustion, IFN-γ
Citation: Abulizi A, Tuergan T, Shalayiadang P, Zhang C, Zhang R, Jiang T, Guo Q, Wang H, Li L, Lin R, Shao Y and Aji T (2025) Hepatic alveolar echinococcosis infection induces a decrease in NK cell function through high expression of NKG2A in patients. Front. Immunol. 16:1563248. doi: 10.3389/fimmu.2025.1563248
Received: 19 January 2025; Accepted: 21 April 2025;
Published: 12 May 2025; Corrected: 24 October 2025.
Edited by:
Florent Ginhoux, Singapore Immunology Network (A*STAR), SingaporeCopyright © 2025 Abulizi, Tuergan, Shalayiadang, Zhang, Zhang, Jiang, Guo, Wang, Li, Lin, Shao and Aji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tuerganaili Aji, dHVlcmdhbjc4QHNpbmEuY29t
 Abuduaini Abulizi
Abuduaini Abulizi Talaiti Tuergan1,2
Talaiti Tuergan1,2 Chuanshan Zhang
Chuanshan Zhang Liang Li
Liang Li Renyong Lin
Renyong Lin Yingmei Shao
Yingmei Shao Tuerganaili Aji
Tuerganaili Aji