- 1Department of Pharmacy, Guangxi Hospital Division of The First Affiliated Hospital, Sun Yat-sen University, Nanning, Guangxi, China
- 2College of Pharmaceutical Science, Guangxi Medical University, Nanning, Guangxi, China
- 3State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, Jiangsu, China
- 4Center for New Drug Safety Evaluation and Research, China Pharmaceutical University, Nanjing, Jiangsu, China
Background: The integration of immune checkpoint inhibitors (ICIs) with chemotherapy (CT) regimens has become a critical focus of clinical investigation in the management of advanced gastric and gastroesophageal junction (G/GEJ) adenocarcinoma over the past several years. Recent phase 3 trials have yielded diverse outcomes, sparking significant debate within the oncological community. In response to these disparate findings, we conducted a meta-analysis to evaluate the therapeutic efficacy and safety profile of this strategy.
Methods: A literature search on PubMed and in major conference proceedings was carried out through December 15, 2024. For efficacy, summary hazard ratios (HRs) for progression-free survival (PFS) and overall survival (OS), odds ratios (ORs) for the objective response rate (ORR) were calculated; for safety, relative risks (RRs) for adverse events (AEs) were assessed.
Results: Nine phase 3 clinical trials, including KEYNOTE-062, CheckMate 649, ATTRACTION-4, ORIENT-16, GEMSTONE-303, KEYNOTE-811, KEYNOTE-859, RATIONALE-305, and COMPASSION-15, which involved a total of 7,825 patients, were analyzed. The addition of ICIs to CT was associated with better PFS (HR, 0.71; 95% CI, 0.65-0.79), OS (HR, 0.79; 95% CI, 0.75-0.83), and a higher ORR (OR, 1.57; 95% CI, 1.43-1.72) compared with CT standalone treatment. However, this combination therapy increased the risk of grade 3–5 AEs (RR, 1.15; 95% CI, 1.09-1.22) and serious AEs (RR, 1.44; 95% CI, 1.21-1.70).
Conclusion: For patients with advanced G/GEJ adenocarcinoma, the addition of ICIs to CT regimens as a first-line treatment offers superior efficacy compared to CT alone, though it comes with an increased risk of toxicity. In the context where multiple strategies are accessible, the pharmacological safety profile can guide practitioners in identifying the most suitable intervention for patients with a higher likelihood of deriving benefits from specific treatment strategies.
Introduction
Gastric and gastroesophageal junction (G/GEJ) adenocarcinoma pose a major global health challenge, ranking among the most frequently diagnosed and lethal forms of cancer across the globe (1, 2). Platinum-based chemotherapy (CT) and its combination with HER-2/VEGFR-targeted therapy were the standard first-line systemic treatment options for patients with advanced G/GEJ adenocarcinoma (3–6). However, these therapeutic strategies offered only limited clinical benefits, underscoring the urgent need for innovative approaches to improve patient prognosis. Immune checkpoint inhibitors (ICIs) have demonstrated potential in treating a variety of cancers and are progressively being investigated as a therapeutic option for advanced G/GEJ adenocarcinoma (7–10).
Nine randomized controlled trials (RCTs) have evaluated first-line ICIs combinations with CT for this indication (11–22). Studies like CheckMate 649, ORIENT-16, and COMPASSION-15 reported improved overall survival (OS) and progression-free survival (PFS) with ICI-CT vs. CT alone, particularly in PD-L1-positive subgroups (12–14, 16, 22). Conversely, ATTRACTION-4 and KEYNOTE-811 only demonstrated PFS benefits, and KEYNOTE-062 showed no survival advantage (11, 15, 19). These discrepancies highlight unresolved questions about subgroup-specific efficacy, such as the role of PD-L1 status, HER2 positivity, or bispecific antibody use (e.g., cadonilimab in COMPASSION-15) (22). Critically, individual trials lacked statistical power to rigorously assess heterogeneous patient subgroups, leaving clinicians uncertain about optimal patient selection for ICI-CT regimens.
Against this backdrop, we conducted a meta-analysis to synthesize data from phase 3 RCTs comparing ICI-CT vs. CT alone in advanced G/GEJ adenocarcinoma. Our analysis aims to clarify the survival benefit of this strategy and quantify efficacy across clinically relevant subgroups—addressing the critical knowledge gap in personalized treatment decisions and guiding evidence-based guidelines for ICI integration.
Methods
Protocol and reporting guidelines
The research protocol was registered on the International Prospective Register of Systematic Reviews (CRD42024620712) and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 checklist (23).
Information sources and search strategy
A meticulously designed systematic search strategy was implemented in PubMed to thoroughly identify all pertinent phase 3 clinical trials published up to December 15, 2024. The search formulas were constructed by combining Medical Subject Headings with free terms. The detailed queries of the search strategies are depicted in Supplementary Table S1. Additionally, to ensure comprehensive coverage of relevant studies, the associated conference abstracts, Supplementary Materials, as well as ClinicalTrials.gov were reviewed.
Selection criteria
For inclusion in the meta-analysis, studies had to meet the following criteria: (i) Trials had to be phase 3 RCTs comparing the combination of ICIs and CT with CT alone. (ii) Participants were required to be patients with previously untreated advanced G/GEJ adenocarcinoma. (iii) Data on survival outcomes, including hazard ratios (HRs) with 95% confidence intervals (CIs), had to be available. Conversely, studies were excluded if: (i) They were not phase 3 RCTs. (ii) CT was not used as the control arm. (iii) ICIs were not used in the experimental arm. (iv) They were ongoing studies without published results as of the literature review date. Only studies meeting the inclusion standards were included in the meta-analysis.
Data collection and assessment of risk of bias
The data from all included studies were extracted and summarized by one investigator and independently verified a second reviewer. The following data were collected where possible: the name of the clinical trial, the year of its publication, the size of the study sample, the treatment protocols, subgroups based on PD-L1 status, the HR along with corresponding 95% CI for PFS and OS, the odds ratio (OR) along with its associated 95% CIs for the objective response rate (ORR), and the incidence of grade 3–5 adverse events (AEs) and serious AEs. Moreover, details regarding the study design were gathered to evaluate the risk of bias in each study. The eligible studies’ bias risk was appraised thoroughly in accordance with the Cochrane bias assessment tool (24).
Statistical analysis
The combined estimates were produced utilizing either a fixed-effects model or a random-effects model, contingent upon the level of heterogeneity observed. The I² statistic and Cochrane Q test were used to assess heterogeneity. Heterogeneity was considered significant if I² exceeded 50% and the Q test p-value was below 0.1. The choice of fixed-effects or random-effects models was guided by both statistical heterogeneity and clinical heterogeneity. Random-effects models were prioritized for outcomes with substantial clinical or methodological diversity, while fixed-effects models were used when homogeneity was strongly supported by data. To assess therapeutic efficacy, HRs with 95% CIs for PFS and OS, and ORs with 95% CIs for ORR, were calculated to obtain a pooled estimate. For the assessment of safety, relative risks (RRs) with 95% CIs for AEs were determined on a per-study basis to provide a comprehensive evaluation. Funnel plots and Egger’s tests were used to check for publication bias. Sensitivity analyses using the leave-one-out approach were conducted to validated the robustness of the pooled results. Sensitivity analyses employing the leave-one-out method were performed to assess the robustness of the pooled results. All statistical analyses were conducted using R software (version 4.2.2), with a two-tailed p-value < 0.05 considered statistically significant.
Results
Study selection and characteristics of included studies
The literature search yielded 42 records. Of these, nine trials satisfied the eligibility criteria and were incorporated into the analysis (11–22). The PRISMA flow diagram of identifying the eligible studies is shown in Supplementary Figure S1.
Among the nine included trials, one was an open-label RCT, while the remaining eight were double-blind RCTs. A total of 7,825 patients with advanced G/GEJ adenocarcinoma were included, of whom 3,922 (50.1%) received ICIs plus CT and 3,903 (49.9%) CT alone. The KEYNOTE-062 trial exclusively enrolled patients with a PD-L1 combined positive score (CPS) of ≥ 1. Separately, the KEYNOTE-811 study is designed to evaluate combination therapies in the context of HER2-positive patients. The types of ICIs used in experimental treatment regimens comprised pembrolizumab, nivolumab, sintilimab, sugemalimab, tislelizumab, and cadonilimab. The control arm regimens mainly included cisplatin plus 5-fluorouracil/capecitabine and capecitabine plus oxaliplatin. The characteristics of each trial are listed in Table 1.
Efficacy in intention-to-treat population
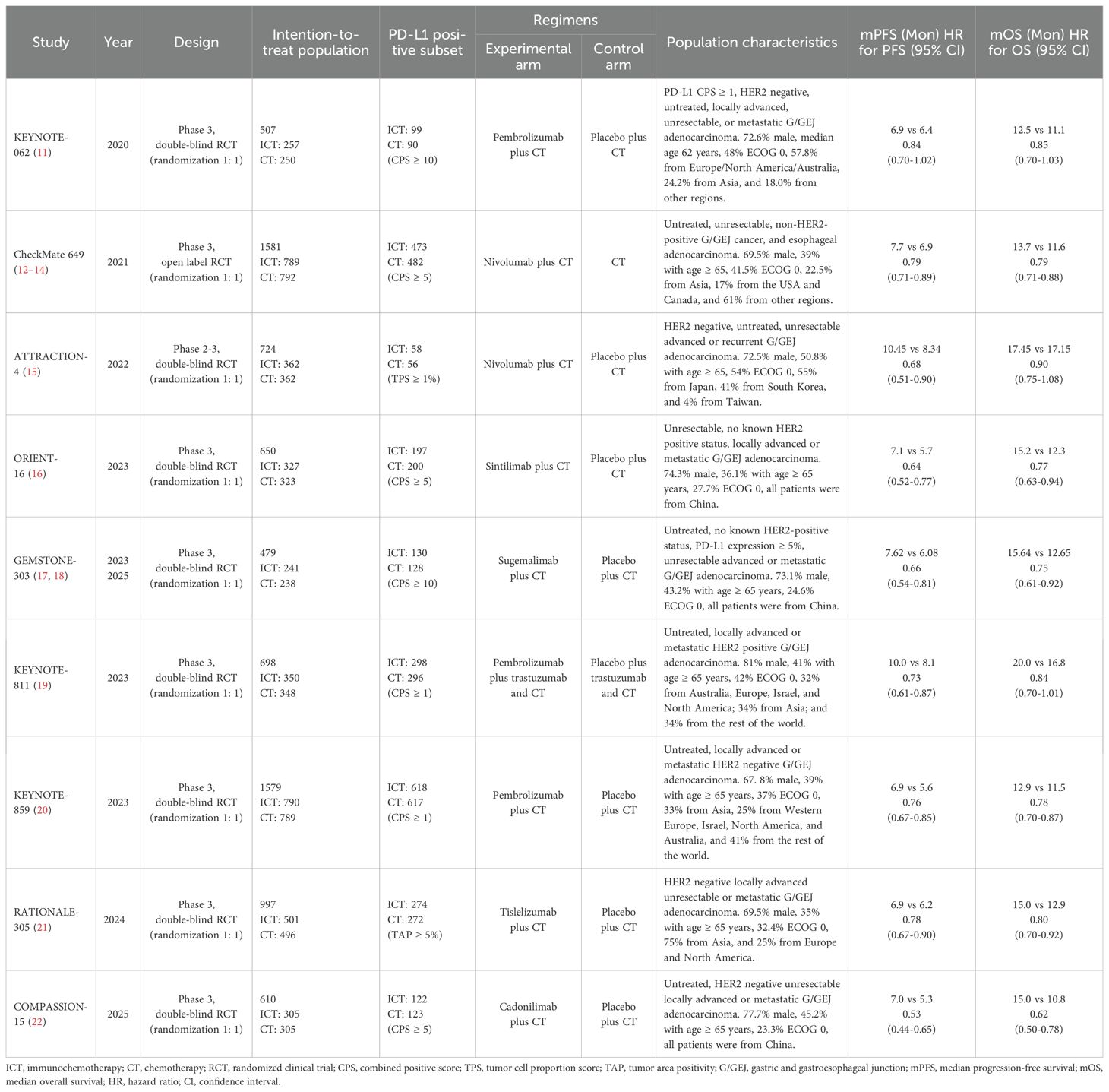
Data for PFS and OS were available from all nine trials involving 7,825 patients. The combined HR for PFS suggested a significant improvement with ICI-CT compared to CT alone (HR, 0.71; 95% CI, 0.65-0.79; Figure 1A), corresponding to a 29% relative reduction in disease progression risk. For OS, the combined HR indicated a 21% relative reduction in mortality risk with ICI-CT (HR, 0.79; 95% CI, 0.75-0.83; Figure 1B). ORR data from 7,249 patients showed a 57% increase in response rate with ICI-CT (OR, 1.57; 95% CI, 1.43-1.72; Figure 1C). These findings demonstrate consistent efficacy of ICI-CT across survival and response endpoints.
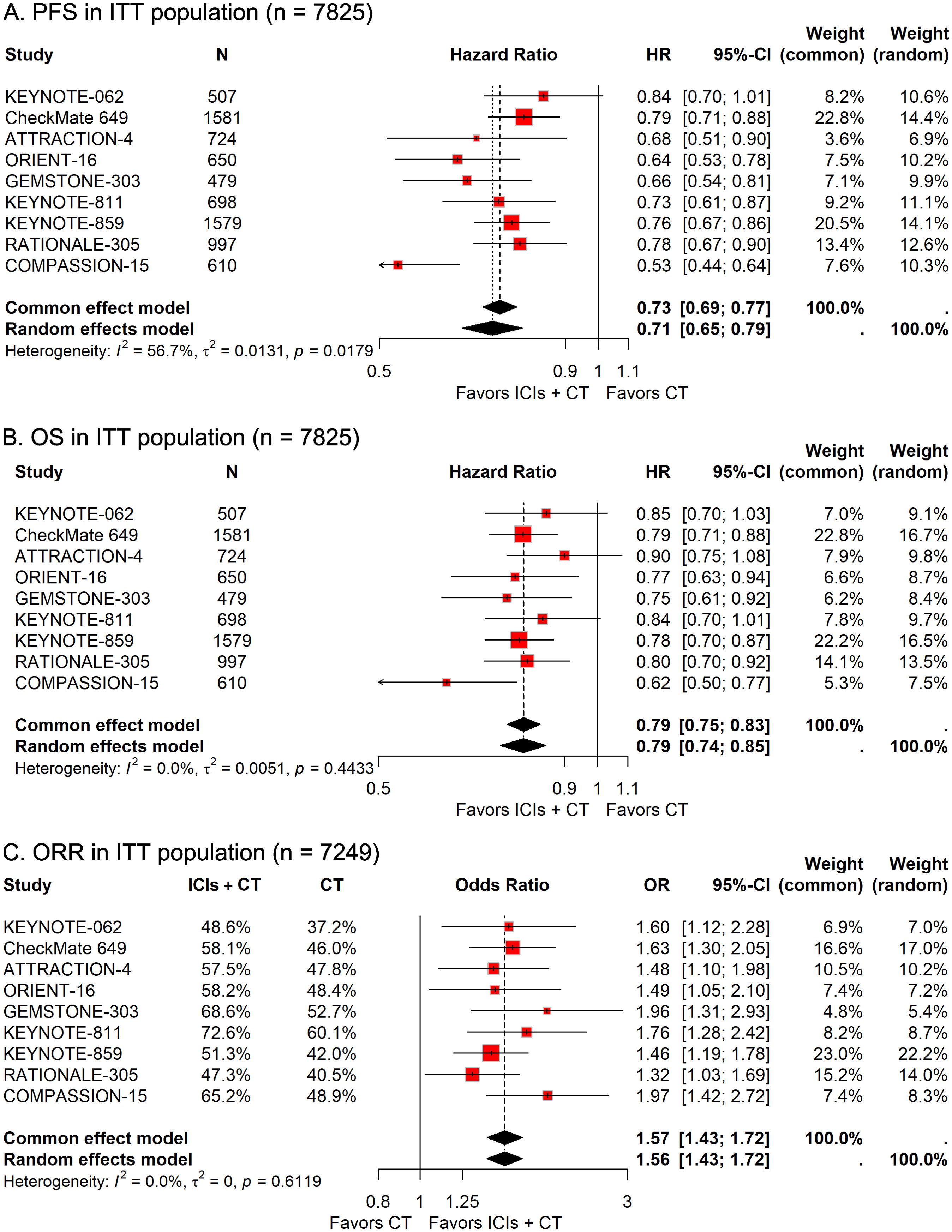
Figure 1. Forest plots depicting pooled efficacy outcomes in the intention-to-treat (ITT) population: (A) progression-free survival (PFS), (B) overall survival (OS), and (C) objective response rate (ORR) for immune checkpoint inhibitors plus chemotherapy (ICIs + CT) versus chemotherapy (CT) alone.
Safety in ITT population
In the group of 3,898 patients receiving ICIs combined with CT, 2,369 individuals (60.8%) suffered from grade 3–5 AEs, compared to 2,013 of the 3,857 patients (52.2%) treated only with CT. The pooled RR revealed that the addition of ICIs to CT significantly elevated the risk of grade 3–5 AEs (RR, 1.15; 95% CI, 1.09-1.22; Figure 2A). Safety data regarding serious AEs of ICIs plus CT versus CT alone were available in five trials. The prevalence of serious AEs was 32.6% (665/2,037) in the ICIs plus CT group and 22.5% (452/2,012) in the CT-alone group. The pooled analysis results indicated that patients receiving the combination treatment had a significantly increased risk of experiencing serious AEs (RR, 1.44; 95%CI, 1.21-1.70; Figure 2B).
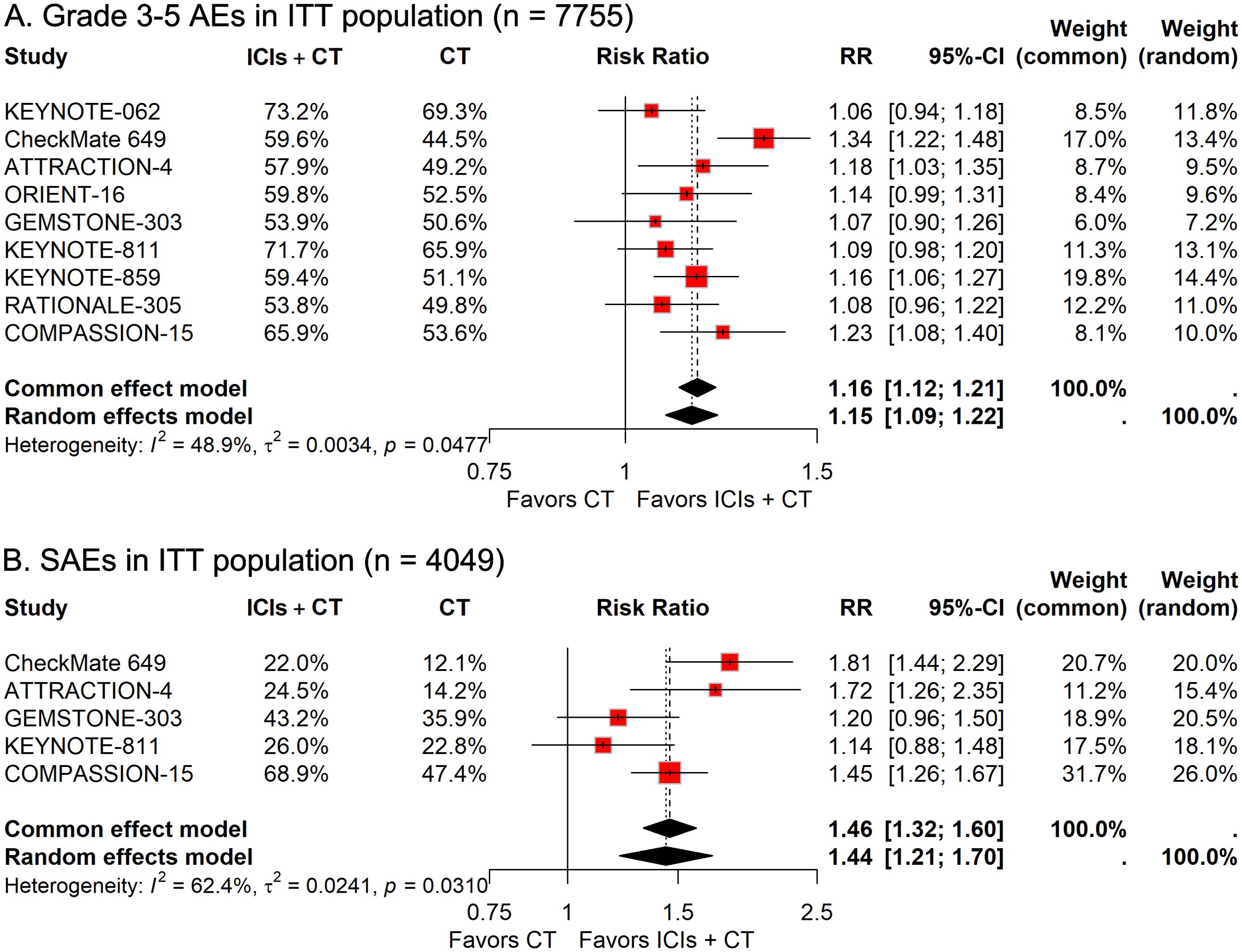
Figure 2. Forest plots illustrating safety outcomes in the intention-to-treat (ITT) population: (A) grade 3–5 adverse events (AEs) and (B) serious AEs, reported as relative risk (RR) for immune checkpoint inhibitors plus chemotherapy (ICIs + CT) versus chemotherapy (CT) alone.
Subgroup analysis
Due to the fact that individual studies lacked sufficient power to analyze diverse clinically related subgroups, to better understand the efficacy of ICIs combined with CT in specific subsets and guide individualized precision treatment, we performed a series of subgroup analyses according to patient demographics, disease pathology, and therapeutic protocols
Subgroup analysis stratified by patient demographics
To investigate how differences in demographic features affect the therapeutic efficacy of ICIs plus CT in G/GEJ adenocarcinoma patients, we carried out various subgroup evaluations taking into account relevant factors such as age, sex, Eastern Cooperative Oncology Group (ECOG) Performance Status, and residential area. The pooled HRs revealed that the addition of ICIs to CT significantly prolonged survival regardless of age, sex, ECOG status, and residential area (Figures 3, 4).
Figure 3. Forest plots displaying the results of progression-free survival (PFS) and overall survival (OS) stratified by (A, B) age and (C, D) sex in patients receiving immune checkpoint inhibitors plus chemotherapy (ICIs + CT) versus chemotherapy (CT) alone.
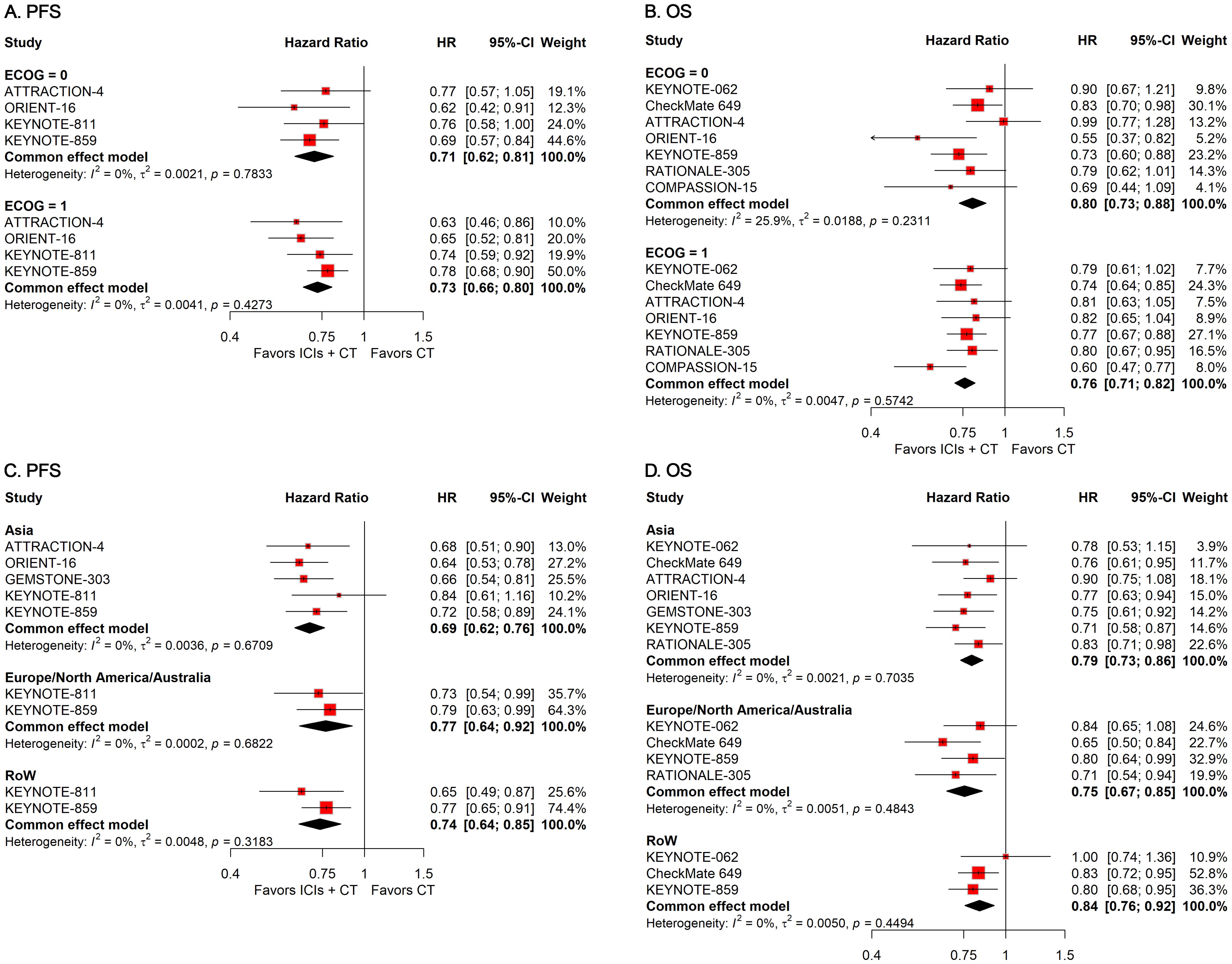
Figure 4. Forest plots displaying the results of progression-free survival (PFS) and overall survival (OS) stratified by (A, B) Eastern Cooperative Oncology Group (ECOG) performance status and (C, D) residential area.
Subgroup analysis stratified by disease pathology
To explore the influence of diverse pathological attributes on the therapeutic outcome of ICIs in conjunction with CT for G/GEJ adenocarcinoma, we performed a series of subgroup assessments based on various factors such as PD-L1 status, microsatellite status, tumor site, subtype, history of previous gastrectomy or esophagectomy, and number of metastasis sites. The combined HRs indicated a substantial enhancement in survival with the integration of ICIs and CT regardless of PD-L1 status, microsatellite status, tumor site, subtype, and history of previous gastrectomy or esophagectomy (Figures 5, 6). However, while the combination of ICIs and CT significantly improved PFS regardless of the number of metastasis sites, it only improved OS in patients with a few metastasis sites rather than a large number of them (Figures 6E, F).
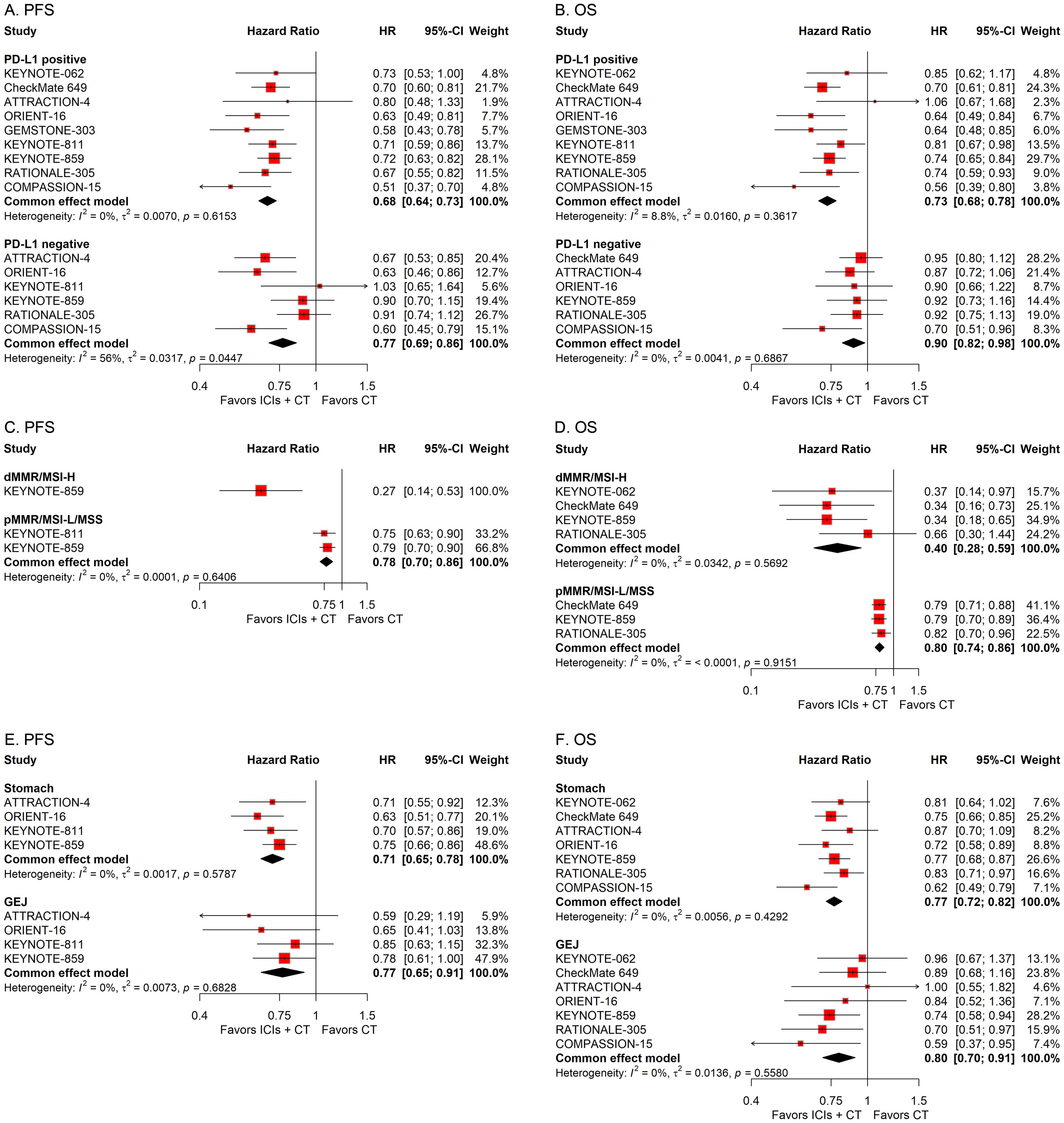
Figure 5. Forest plots displaying the results of progression-free survival (PFS) and overall survival (OS) stratified by PD-L1 status (A, B), microsatellite status (C, D), and tumor site (E, F).
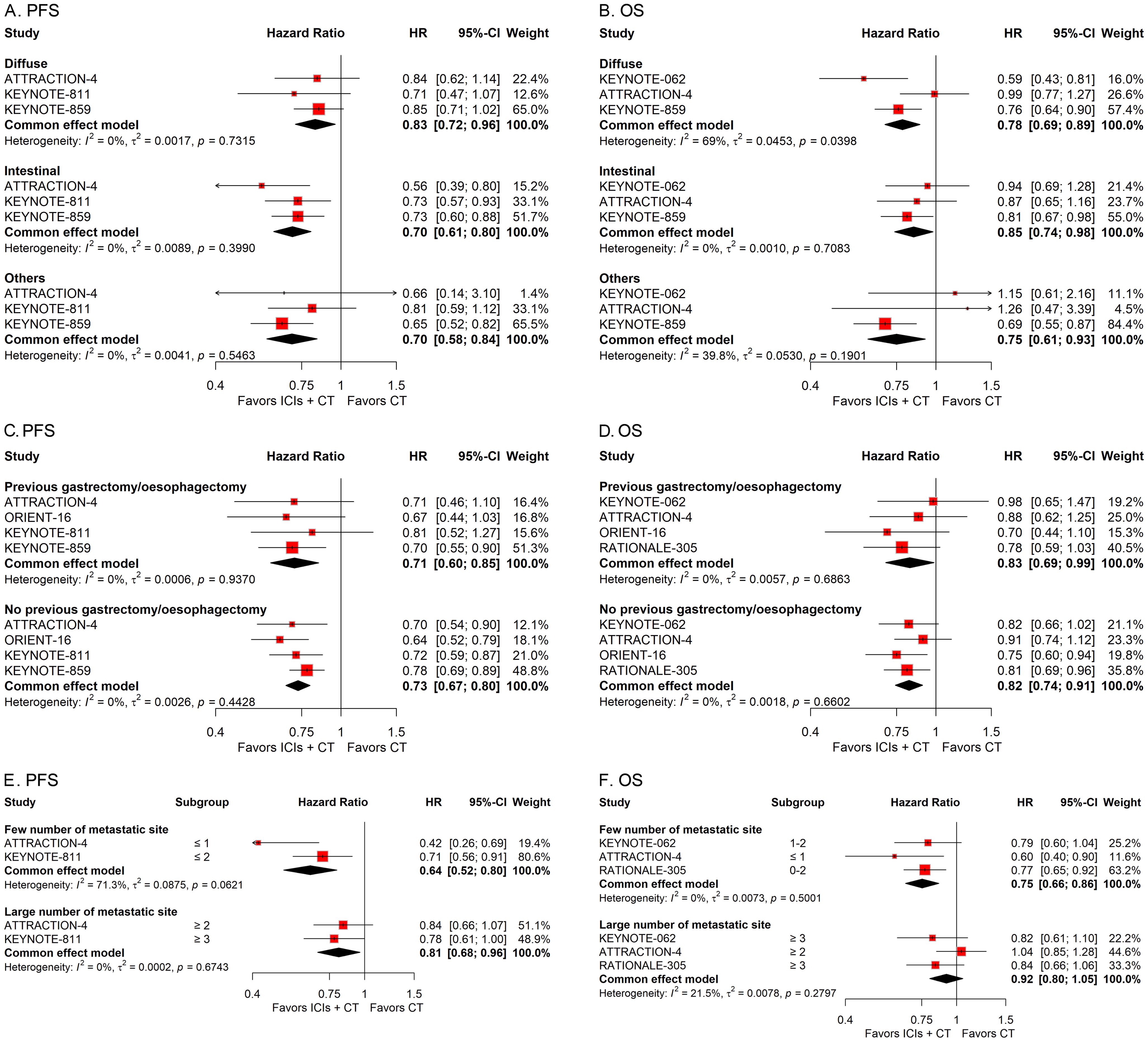
Figure 6. Forest plots displaying the results of progression-free survival (PFS) and overall survival (OS) stratified by subtype (A, B), history of previous gastrectomy or esophagectomy (C, D), and number of metastasis sites (E, F).
Subgroup analysis stratified by treatment protocols
To examine the effects of different treatment protocols on the efficacy of ICIs combined with CT for G/GEJ adenocarcinoma, we undertook two separate subgroup studies, focusing on variables including the ICIs classification and the CT treatment scheme. The pooled HRs revealed that the addition of ICIs to CT notably extended survival regardless of the type of ICIs and regimen of CT used (Figure 7).
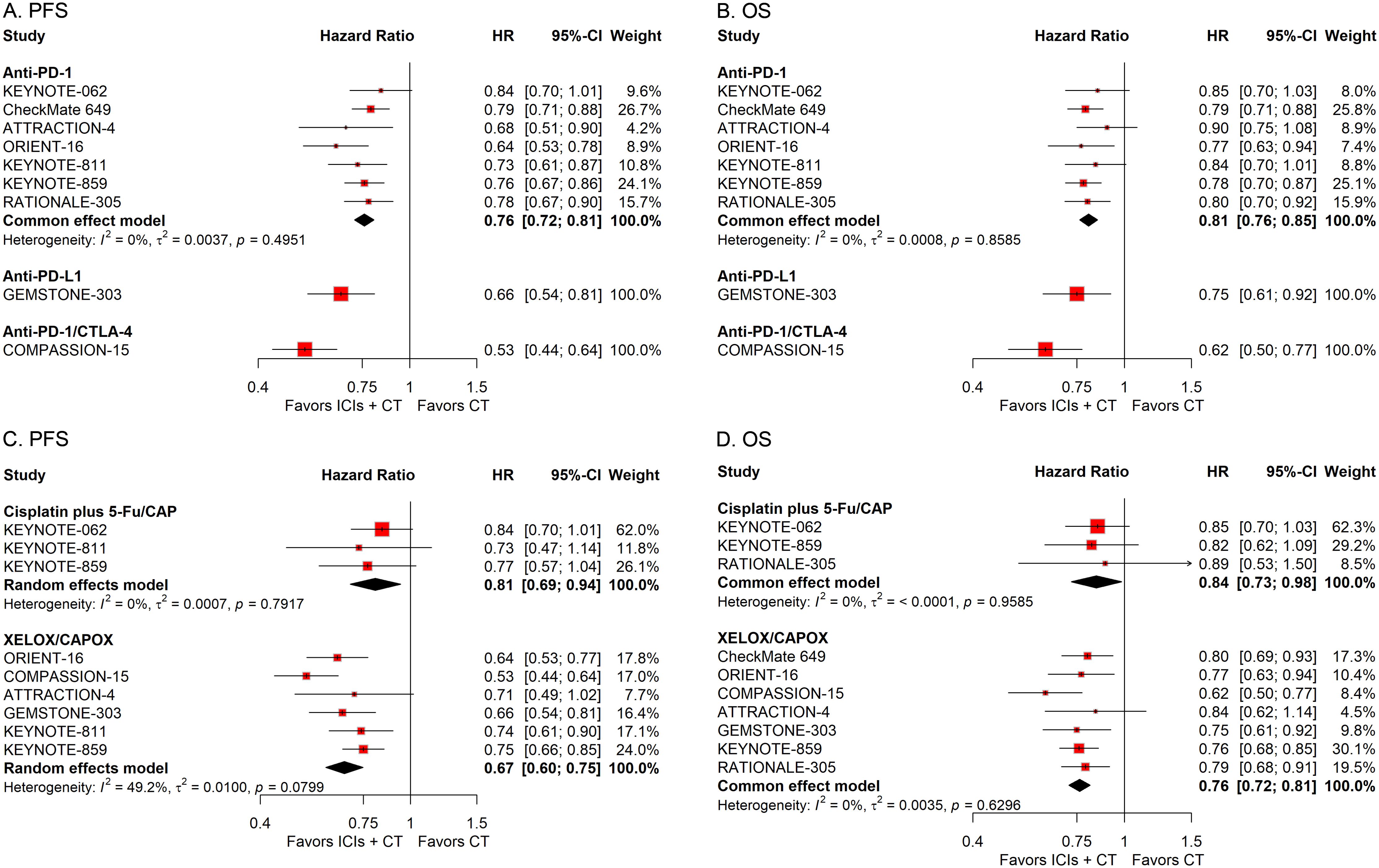
Figure 7. Forest plots displaying the results of progression-free survival (PFS) and overall survival (OS) stratified by the type of ICIs (A, B) and the regimen of CT (C, D).
Risk of bias and sensitivity analysis
Details of the bias risk assessment for each study were presented in Supplementary Figures S2, S3. No substantial publication bias was detected (Supplementary Figure S4). Sensitivity analyses verified the stability of the combined outcomes. (Supplementary Figure S5).
Discussion
The addition of ICIs to CT regimens for advanced cancers has emerged as a pivotal area of investigation within oncology (10, 25), with its role in G/GEJ adenocarcinoma garnering significant attention. Several phase 3 clinical trials have previously explored the efficacy of ICIs in combination with CT for advanced G/GEJ adenocarcinoma. While individual studies presented variable results, particularly in terms of OS, the overall trend points towards a benefit with the addition of ICIs. This meta-analysis aimed to integrate data from multiple phase 3 clinical trials to deeply explore the efficacy and safety of this combined treatment strategy, providing a strong basis for clinical decision-making.
The results of our meta-analysis unequivocally demonstrate the significant enhancement in PFS, OS, and ORR when ICIs are added to CT compared to CT alone. These results not only reinforce the therapeutic potential of ICIs in advanced G/GEJ adenocarcinoma but also suggest that ICIs combined with CT have the potential to emerge as a hopeful new benchmark in the treatment of this difficult-to-treat and frequently resistant cancer. However, while the efficacy benefits are clear, the significant increase in toxicity must be carefully considered. The higher risk of grade 3–5 AEs and serious AEs associated with the ICIs plus CT regimen calls for heightened vigilance in monitoring patients. The toxicity profile of this combination highlights the need for individualized management strategies. Strategies to manage these AEs, such as early detection and prompt intervention, will be essential in maximizing the therapeutic benefits without compromising patient safety. In clinical practice, patients’ individual situations should be fully considered, the benefits and risks of treatment should be balanced, and reasonable treatment plans should be formulated. At the same time, multidisciplinary collaboration should be strengthened to provide comprehensive treatment and management for patients. In addition, continuous attention should be paid to research progress, and new research achievements should be timely applied to clinical practice to continuously optimize treatment strategies and improve patients’ treatment outcomes and quality of life.
The subgroup analyses provide valuable insights into the potential variability in treatment response across different patient subgroups. The consistent improvement in PFS and OS across various demographic and pathological characteristics, including age, sex, ECOG performance status, PD-L1 status, microsatellite status, tumor site, subtype, and history of previous gastrectomy or esophagectomy, suggests that ICI plus CT may benefit a broad spectrum of patients with advanced G/GEJ adenocarcinoma. However, the observed difference in OS benefit based on the number of metastasis sites highlights the need for personalized treatment approaches. Patients with fewer metastasis sites may experience greater OS benefits from ICI plus CT, suggesting a potential role for this combination in earlier-stage disease or in patients with a limited burden of metastatic disease.
While this meta-analysis provides valuable insights into the efficacy and safety of ICIs plus CT in advanced G/GEJ adenocarcinoma, there are several limitations that should be acknowledged. First, the included trials had certain heterogeneity in patient populations, treatment regimens, PD-L1 assessment methods, which might affect the accuracy and universality of the results. Although random-effects model was used to handle heterogeneity in the analysis, the existence of heterogeneity remains a non-negligible issue. The included trials utilized varying PD-L1 evaluation metrics, such as CPS, tumor proportion score (TPS), and tumor area positivity (TAP), with different cutoff values (e.g., CPS ≥ 1 vs. CPS ≥ 10, TPS ≥ 1%) (11, 15, 22). For example, KEYNOTE-062 enrolled patients with CPS ≥ 1, while GEMSTONE-303 required CPS ≥ 10, reflecting divergent criteria for defining PD-L1 positivity. This inconsistency may have introduced bias in subgroup analyses, as PD-L1 expression thresholds are known to correlate with ICIs response rates (12, 19). Notably, our subgroup analysis showed consistent PFS/OS benefits across all PD-L1 strata, but the lack of uniform assessment methods limits the precision of biomarker-driven conclusions. Future trials should standardize PD-L1 evaluation to facilitate comparable subgroup analyses. CT backbones and ICI types varied significantly across trials. CT regimens included cisplatin plus 5-fluorouracil, capecitabine plus oxaliplatin, and other combinations, while ICIs encompassed PD-1 inhibitors (pembrolizumab, nivolumab, sintilimab, tislelizumab), a PD-L1 inhibitor (sugemalimab), and a dual PD-1/CTLA-4 bispecific antibody (cadonilimab in COMPASSION-15) (12, 22). These differences may affect efficacy and toxicity profiles: for instance, dual checkpoint inhibition in COMPASSION-15 showed particularly pronounced OS benefits (HR = 0.62) but was not directly comparable to single-agent PD-1 inhibitors (22). Additionally, HER2-targeted combinations (e.g., trastuzumab in KEYNOTE-811) introduced further complexity, as HER2 positivity itself is a prognostic factor (19). Despite this diversity, our subgroup analysis indicated consistent benefits across ICI classes and CT regimens, suggesting a robust overall effect of chemo-immunotherapy combinations. However, the safety data (e.g., grade 3–5 AEs) may be influenced by regimen intensity, highlighting the need for tailored toxicity management based on specific ICI-CT combinations. The trials enrolled patients from diverse regions, including Asia, Europe, and North America, where gastric cancer epidemiology (e.g., Helicobacter pylori infection rates, tumor subtypes) and treatment practices differ (2, 15). For example, ATTRACTION-4 and ORIENT-16 recruited primarily Asian populations, while CheckMate 649 included a more global cohort (12, 15). Ethnic differences in immune response pathways (e.g., HLA genotypes) and baseline comorbidities may contribute to treatment variability, though our subgroup analysis by residential area showed uniform benefits across regions (Figures 4C, D). Nonetheless, underreporting of geographic-specific data in some trials limits the ability to fully dissect these effects, emphasizing the need for stratified analyses in future multinational studies. These sources of heterogeneity highlight the real-world complexity of treating advanced G/GEJ adenocarcinoma, where patient selection and regimen choice are influenced by multiple factors. While our random-effects model provided conservative estimates applicable to broad populations, clinicians should interpret subgroup results with awareness of biomarker assay variability and treatment context. Moving forward, standardizing PD-L1 assessment (e.g., adopting CPS as a unified metric), reporting detailed geographic/ethnic stratification, and investigating interactions between ICI types and CT backbones will be crucial for optimizing precision oncology in this setting. Additionally, while the analysis was based on phase 3 clinical trials, the duration of follow-up in many of these studies was relatively short. Longitudinal assessments of patient survival, quality of life, and treatment tolerability will be crucial in informing clinical decision-making and refining treatment algorithms for these types of cancers. Finally, although specific biases (e.g., open-label design) exist in individual trials, the predominance of low-risk studies for key bias domains (Supplementary Figures S2, S3), absence of publication bias, and stable results in sensitivity analyses (Supplementary Figures S4, S5) reinforce the robustness of our conclusions.
While our meta-analysis demonstrates significant survival benefits of ICI-CT combinations in controlled clinical trials, their translation into real-world practice requires careful consideration. Recent real-world studies report consistent efficacy but highlight key differences. A multicenter retrospective cohort (n = 573) showed even stronger survival benefits (PFS: HR = 0.45, OS: HR = 0.40), likely due to less post-progression crossover and broader patient inclusion, including elderly (≥ 75 years) and PD-L1-unknown patients (60% of the cohort) (26). Notably, patients with low PD-L1 CPS (1-4) achieved significant OS prolongation (HR = 0.24), filling an evidence gap from RCTs that focus on CPS ≥ 5. However, real-world challenges include higher treatment discontinuation in elderly patients due to toxicity and limited access to specialty care for managing immune-related adverse events. Economic barriers further impact equity, particularly in regions with low PD-L1 testing rates. These insights complement our meta-analysis, advising clinicians to balance efficacy with patient-specific factors—such as comorbidities, testing availability, and resource constraints—when implementing ICI-CT, especially for patients with unclear biomarker status or complex real-world profiles. This integrated evidence supports a pragmatic approach to optimize treatment selection while advocating for standardized toxicity protocols and policy interventions to enhance accessibility.
The promising results of this meta-analysis open several avenues for future research. First, while clinical trials have highlighted the importance of biomarkers such as PD-L1 expression, further refinement in biomarker-guided treatment will be essential for optimizing patient selection and improving therapeutic outcomes. The KEYNOTE-062 trial, for example, demonstrated that pembrolizumab monotherapy provided a significant benefit for patients with PD-L1-positive tumors, yet the combination of pembrolizumab and CT failed to significantly extend PFS or OS over solo CT treatment. This finding suggests the importance of PD-L1 expression in guiding treatment decisions, especially as we continue to explore the efficacy of ICIs in different subsets of G/GEJ adenocarcinoma. Thus, while PD-L1 remains an important biomarker for identifying potential responders to immunotherapy, the role of other biomarkers (e.g., microsatellite instability, tumor mutational burden) and clinical features will need to be further explored. In addition, the exploration of dual immune checkpoint inhibition (e.g., combining PD-1/PD-L1 inhibitors with CTLA-4 inhibitors) in combination with CT is an area of active investigation. The COMPASSION-15 trial, which evaluated the combination of cadonilimab, an anti-PD-1 and anti-CTLA-4 bispecific antibody, with CT, demonstrated promising results in improving PFS both and OS, highlighting the potential for more aggressive immunotherapy combinations in the future. However, the balance between efficacy and toxicity with dual immune checkpoint inhibition requires further investigation. Moreover, future studies should aim to explore precision medicine approaches that integrate genetic, molecular, and immune profiles of patients to better predict responses to ICIs. While current research has identified some potential biomarkers, such as PD-L1 expression and microsatellite instability, more comprehensive profiling of the tumor microenvironment and immune landscape may allow for more precise patient selection. Finally, given the promising results of ICIs in HER2-positive G/GEJ adenocarcinoma in the KEYNOTE-811 trial, further exploration of immunotherapy in combination with targeted therapies, such as HER2 inhibitors (trastuzumab), could represent a future frontier for treating this specific patient subset.
Conclusion
The addition of ICIs to CT demonstrates robust improvements in PFS, OS, and ORR, confirming the efficacy of this combination approach in the treatment of advanced G/GEJ adenocarcinoma. However, the heightened risk of toxicity underscores the importance of meticulous patient selection and tailored management strategies. Subgroup analysis indicates that the combination of ICIs and CT may confer benefits across various patient subgroups. Given the availability of several strategies in this setting, the safety profile of drugs and their costs may assist clinicians in choosing the most suitable treatment for patients with G/GEJ adenocarcinoma who are prone to gain advantages from the treatment modality.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YXL: Writing – original draft, Conceptualization, Methodology. YHL: Writing – original draft, Data curation, Formal Analysis, Visualization. JS: Conceptualization, Writing – original draft, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors expressed their gratitude to the databases that supplied the essential data for this investigation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Polish the manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1564604/full#supplementary-material.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X
4. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. (2014) 15:1224–35. doi: 10.1016/S1470-2045(14)70420-6
5. Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. (2018) 19:1372–84. doi: 10.1016/S1470-2045(18)30481-9
6. Cheng J, Cai M, Shuai X, Gao J, Wang G, Tao K. First-line systemic therapy for advanced gastric cancer: a systematic review and network meta-analysis. Ther Adv Med Oncol. (2019) 11:1758835919877726. doi: 10.1177/1758835919877726
7. Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. JAMA. (2022) 328:1223–32. doi: 10.1001/jama.2022.16464
8. Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2023) 401:1853–65. doi: 10.1016/S0140-6736(23)00727-4
9. Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X, et al. Toripalimab plus chemotherapy for patients with treatment-naive advanced non-small-cell lung cancer: A multicenter randomized phase III trial (CHOICE-01). J Clin Oncol. (2023) 41:651–63. doi: 10.1200/JCO.22.00727
10. Zhang X, Shen J, Huang M, Li R. Efficacy and safety of adding immune checkpoint inhibitors to first-line standard therapy for recurrent or advanced cervical cancer: a meta-analysis of phase 3 clinical trials. Front Immunol. (2024) 15:1507977. doi: 10.3389/fimmu.2024.1507977
11. Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. (2020) 6:1571–80. doi: 10.1001/jamaoncol.2020.3370
12. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
13. Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. (2022) 603:942–8. doi: 10.1038/s41586-022-04508-4
14. Janjigian YY, Ajani JA, Moehler M, Shen L, Garrido M, Gallardo C, et al. First-line nivolumab plus chemotherapy for advanced gastric, gastroesophageal junction, and esophageal adenocarcinoma: 3-year follow-up of the phase III checkMate 649 trial. J Clin Oncol. (2024) 42:2012–20. doi: 10.1200/JCO.23.01601
15. Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2022) 23:234–47. doi: 10.1016/S1470-2045(21)00692-6
16. Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. JAMA. (2023) 330:2064–74. doi: 10.1001/jama.2023.19918
17. Zhang X, Wang J, Wang G, Zhang Y, Fan Q, Chuangxin L, et al. Prespecified progression-free survival (PFS) and overall survival (OS) final analyses of a phase III study of sugemalimab plus chemotherapy vs placebo plus chemotherapy in treatment-naïve advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. Ann Oncol. (2024) 34:S1254–S335. doi: 10.1016/j.annonc.2023.10.080
18. Zhang X, Wang J, Wang G, Zhang Y, Fan Q, Lu C, et al. First-line sugemalimab plus chemotherapy for advanced gastric cancer: the GEMSTONE-303 randomized clinical trial. JAMA. (2025) 333(15):1305–1314. doi: 10.1001/jama.2024.28463
19. Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. (2023) 402:2197–208. doi: 10.1016/S0140-6736(23)02033-0
20. Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. (2023) 24:1181–95. doi: 10.1016/S1470-2045(23)00515-6
21. Qiu MZ, Oh DY, Kato K, Arkenau T, Tabernero J, Correa MC, et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial. BMJ. (2024) 385:e078876. doi: 10.1136/bmj-2023-078876
22. Shen L, Zhang YQ, Li ZY, Zhang XT, Gao XY, Liu B, et al. First-line cadonilimab plus chemotherapy in HER2-negative advanced gastric or gastroesophageal junction adenocarcinoma: a randomized, double-blind, phase 3 trial. Nat Med. (2025) 31(4):1163–1170. doi: 10.1038/s41591-024-03450-4
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
24. Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 18:d5928. doi: 10.1136/bmj.d5928
25. Shen J, Ye X, Hou H, Wang Y. Efficacy and safety of immunochemotherapy in advanced triple-negative breast cancer: A meta-analysis of randomised clinical trials. Clin Oncol (R Coll Radiol). (2025) 40:103783. doi: 10.1016/j.clon.2025.103783
26. Zhang X, Dai X, Liu A, Sun M, Cong L, Liang J, et al. Efficacy, safety, and biomarker analysis of first-line immune checkpoint inhibitors with chemotherapy versus chemotherapy for advanced gastric cancer: a multicenter, retrospective cohort study. BMC Med. (2024) 22:585. doi: 10.1186/s12916-024-03801-5
Keywords: immune checkpoint inhibitors, chemotherapy, gastric cancer, gastroesophageal junction adenocarcinoma, meta-analysis
Citation: Lin Y, Liao Y and Shen J (2025) First-line immune checkpoint inhibitors with chemotherapy in advanced gastric and gastroesophageal junction adenocarcinoma: a meta-analysis of phase 3 trials. Front. Immunol. 16:1564604. doi: 10.3389/fimmu.2025.1564604
Received: 21 January 2025; Accepted: 14 April 2025;
Published: 02 May 2025.
Edited by:
Yayun Wang, Air Force Medical University, ChinaReviewed by:
Vikas Somani, Washington University in St. Louis, United StatesYangfeng Zhang, Southern Medical University, China
Jiawei Wu, Jiangsu University, China
Copyright © 2025 Lin, Liao and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhai Shen, c2hlbmpoX3BoYXJtQDEyNi5jb20=
†ORCID: Jinhai Shen, orcid.org/0000-0001-8673-087X
 Yuxuan Lin
Yuxuan Lin Yonghe Liao
Yonghe Liao Jinhai Shen
Jinhai Shen