- 1Department of Hematology, Shanghai Sino United Hospital, Shanghai, China
- 2Department of Medicine, The Chinese University of Hong Kong (CUHK), Hong Kong, Hong Kong SAR, China
- 3State Key Laboratory of Medical Genomics, National Research Center for Translational Medicine, Shanghai Institute of Hematology, Ruijin Hospital Affiliated with Shanghai Jiao Tong University School of Medicine, Shanghai, China
Plasmablastic multiple myeloma (PBM) is an aggressive multiple myeloma (MM) form, identified by a high risk of recurrence and poor prognosis, with limited effective treatment options. Present study reports a case initially diagnosed with IgG-kappa MM with double-hit genetics. Following induction chemotherapy with bortezomib, doxorubicin and dexamethasone (VAD), and subsequent consolidation therapy with ixazomib, lenalidomide, and dexamethasone, the disease progressed, manifesting as a plasmoblastic tumor in the right pelvic cavity. After two cycles of carfezomib, daratumumab, cyclophosphamide, cisplatin, etoposide and dexamethasone (KD-DECP), the patient achieved partial response. She declined autologous stem cell transplantation (ASCT) and instead received radiotherapy as bridging therapy, followed by B-cell maturation antigen (BCMA)-targeting chimeric antigen receptor (CAR) T-cell therapy with pomalidomide as maintenance therapy. She achieved complete response (CR) at 3 months and has remained disease-free for over 15 months based on the latest follow-up. Although grade 2 cytokine release syndrome (CRS) and other adverse events were observed, they were manageable. BCMA CAR-T cell accompanied with bridging radiotherapy and pomalidomide as maintenance therapy provided a promising therapy treatment for PBM, which is more aggressive and with shorter survival. Further studies are demanded to assess the efficiency and long-term benefits for this challenging subtype.
Introduction
In rare cases, the plasma cells infiltrate other tissues outside the bone marrow (BM) including lymph nodes, soft tissue, the central nervous system, etc., a condition known as extramedullary disease (EMD) (1), which usually correlates to a significantly poorer prognosis compared to those without EMD (2). Plasmablastic multiple myeloma (PBM) accounts for approximately 8.2% all multiple myeloma (MM) cases (3) and is often challenging to distinguish from plasmablastic lymphoma (PBL) (4). PBM represents a highly proliferative disease, characterized by unfavorable clinical features, evaluated proliferation index, a high proportion of plasma cell infiltration in BM, abnormal karyotypes, and del (13q) as seen in karyotyping (5). It is also associated with shorter survival (3, 6), particularly during the first 6 months (6).
The incidence of PBM with EMD is very low, but it tends to be more aggressive and is associated with shorter survival (3, 6). Currently, no consensus exists upon the management of patients diagnosed with this condition, and research on treatment options is limited. This article presents the case of a patient with double-hit refractory/relapsed (R/R) MM, accompanied by an extramedullary plasmoblastic tumor. After undergoing BCMA-targeting CAR-T cell therapy, she achieved a CR and has remained disease-free for an extended period. This case provides valuable insights and highlights BCMA CAR-T cell therapy as a promising treatment option regarding this challenging subtype.
Case presentation
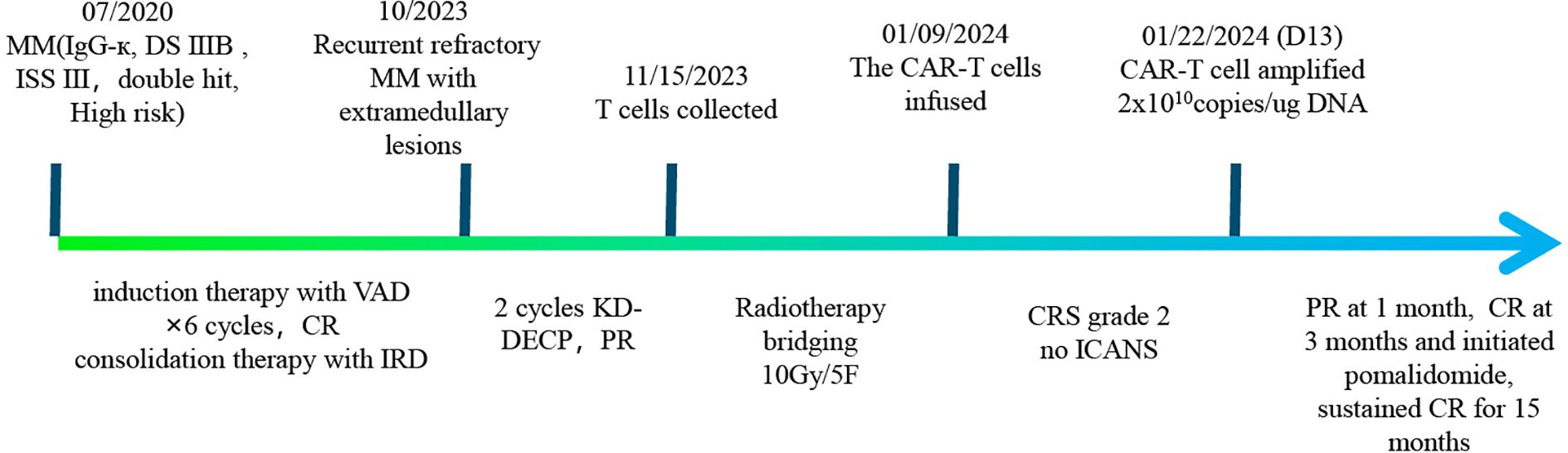
A 61-year-old female patient having pain in chest, back and ribs, with general good condition and had no positive signs, without abdominal pain, abdominal distension, constipation, diarrhea, fever or pain, but with fatigue, weight loss and anemia. Physical examination of the abdomen reveals a mass with a tough texture and without tenderness. Subsequently, a series of laboratory tests were underwent, which led to a diagnosis of IgG-kappa MM with Durie-Salmon stage IIIB and International Staging System (ISS) stage III in July 2020. Fluorescence in situ hybridization (FISH) of BM aspirate was positive for 1q21 gene amplification, IGH gene rearrangement, (t(4;14)) IGH/FGFR3 fusion, and RB1 gene deletion, indicating a double-hit disease according to the Mayo Clinic’s mSMART 3.0 program (6). Chromosome karyotype analysis revealed that 43, X, -X, -6, -10, del(12)(p11.2), -13, der(17)t(1;17)(q21; q25), -20, +mar1, +mar2[2]/46, XX [16].
As the patient refused autologous hematopoietic stem cell transplantation (ASCT), she underwent induction therapy of six cycles bortezomib, doxorubicin, and dexamethasone (VAD), which resulted in complete remission. This was followed by four cycles of ixazomib, lenalidomide and dexamethasone (IRD) as consolidation chemotherapy, which was later switched to lenalidomide and dexamethasone alone, or lenalidomide monotherapy, due to poor tolerance of ixazomib and dexamethasone.
The M protein became positive in May 2023 with 1.5%, and the free light chain ratio progressively increased, suggesting biochemical relapse. The patient then noticed a mass in right lower abdomen. Abdominal computed tomography (CT) showcased a mass in the right pelvic cavity, and PET/CT revealed hypermetabolic uptake in the right lower abdomen with 12.3*6.6*12.9 cm, SUVmax=20.3. Pathological analysis indicated a plasmoblastic tumor, with high proliferative activity and MYC overexpression, both of which are associated with high risk, and a ki-67 index of about 90%. BM aspirate showcased 1% plasma cell infiltration, and following immunophenotypic markers were observed: CD38(bright+), CD56+, CD138+, cKappa+, BCMA(100%)+, CD19-, CD27-, CD28- and cLambda-.
Then, she received two cycles of KD-DECP with halved dosage due to poor tolerance: carfilzomib 20mg/m2 on days 1-2, 27mg/m2 on days 8-9, 15-16; daratumumab 16 mg/kg weekly, 700 mg on days 1, 8, 15, and 22; cyclophosphamide 400 mg, cisplatin 10 mg, and etoposide 40 mg on days 1-4; and dexamethasone 20 mg on days 1-4, 8-9, and 15-16. During this regimen, the patient developed grade 4 neutropenia and febrile neutropenia. After two cycles of treatment, she was assessed as having a partial response with tumor size 4.0*2.4 cm, but progressed rapidly with free light chain rises after a week.
Given the patient was diagnosed with PBM with high Ki-67 and rapid progression after KD-DECP, which suggested she might not benefit from or tolerate further chemotherapy, and the fact that she refused ASCT, combined with the abundant BCMA expressions on her BM cells and the reported anti-myeloma effects of BCMA CAR-T cell therapy, our group decided to proceed with CAR-T as the next treatment option. Peripheral blood samples were collected to generate the engineered CAR-T cells. The patient underwent radiotherapy for the residual tumor in the abdomen as bridging therapy, with a dose of 10Gy/5 fractions. There was no distinct change in the tumor size after radiotherapy. After 3 days of lymphodepletion with fludarabine and cyclophosphamide, BCMA CAR-T cells (Equecabtagene Autoleucel, Eque-cel) from IASO Biotherapeutics were administered at 1.0×106 CAR-T cells/kg, considering the extremely high risk disease, pomadomide was initiated after 3-month of CAR-T infusion as long-term maintenance therapy.
Continuous expansion regarding BCMA CAR-T cells was observed, having the peak copy number in peripheral blood reaching 2×1010 copies/ug genomic DNA 13 days post-infusion (Figure 1). She developed grade II cytokine release syndrome (CRS) for 5 days with IL-6 >5000 pg/ml, IL-8 287.61 pg/ml, IL-10 130.62 pg/ml and IFN-γ517.1 pg/ml, along with neutropenia and thrombocytopenia, which resolved after three months. Two doses of tocilizumab (8mg/kg every 8 hours), steroid 10mg once and ibuprofen were used to manage CRS. No immune effector cell-associated neurotoxicity syndrome (ICANS) happened. She achieved partial response (PR) at 1 month with tumor size 3.7 * 6.4cm (Figure 2) and complete remission (CR) at month 3 (Figure 3) post CAR-T-cell infusion. Approximately 15 months after CART-cell therapy, she remained sCR (strict complete response) and MRD negative (Figure 4).
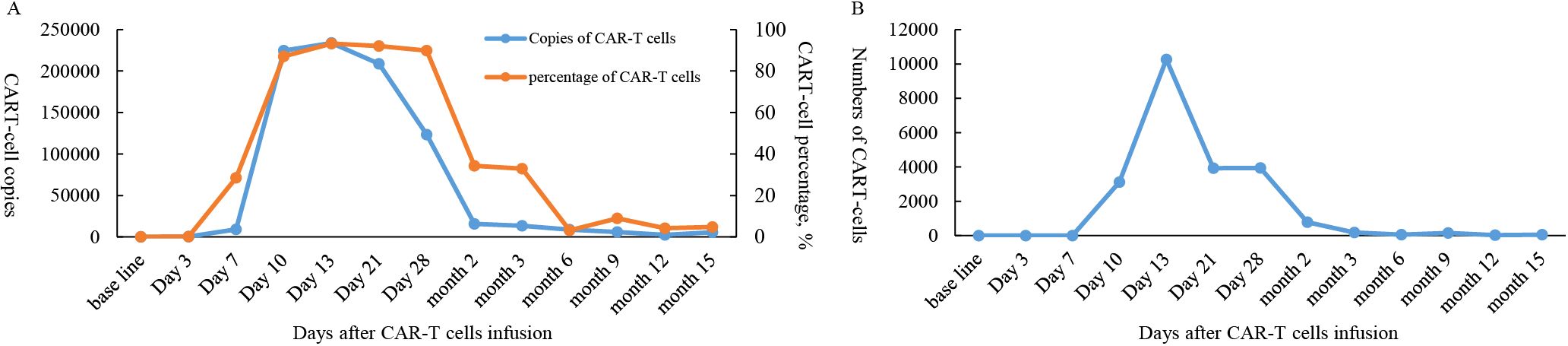
Figure 1. Cell expansion and persistence in peripheral blood following BCMA CAR-T cell infusion. (A) Copies and percentage of CAR-T cells. (B) Numbers of CAR-T cells.
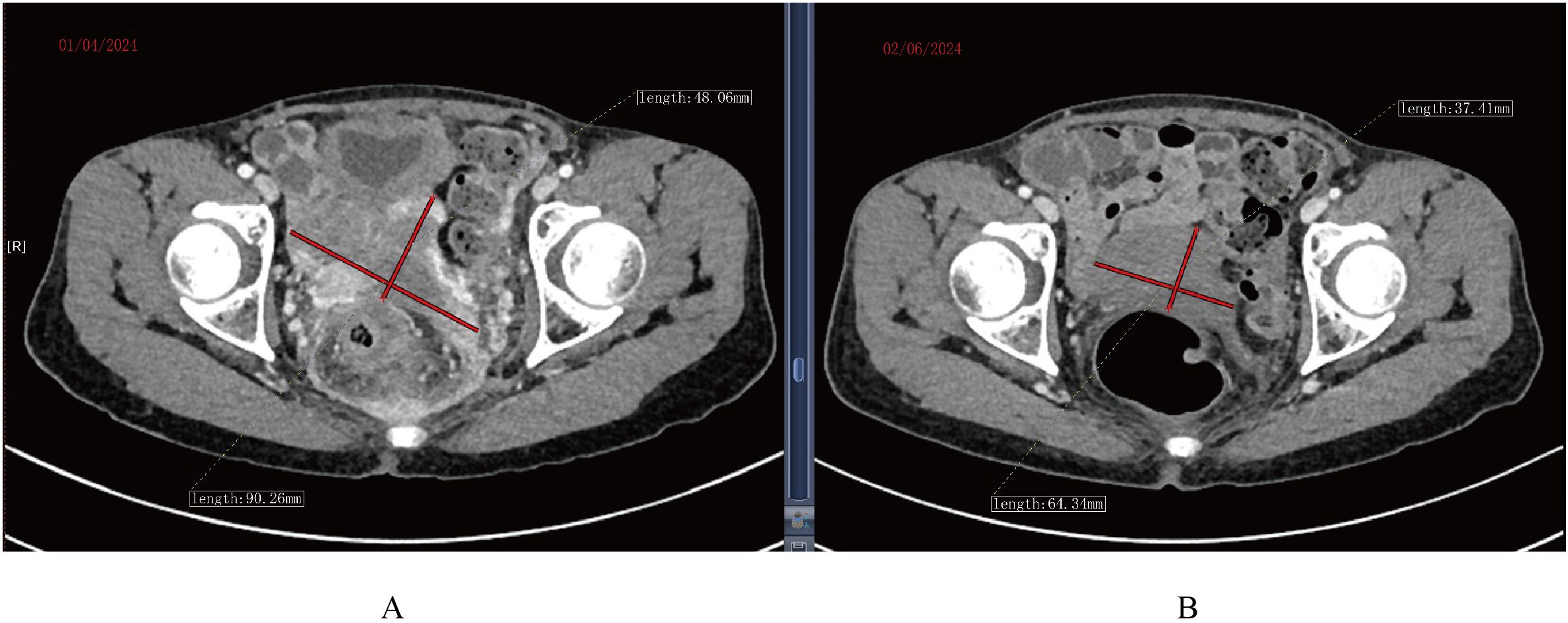
Figure 2. CT scan of tumor mass changes showing partial response 1 month post CAR-T cell infusion. (A) CT scan before infusion of CAR-T infusion. (B) CT scan 1 month post CAR-T cell infusion.
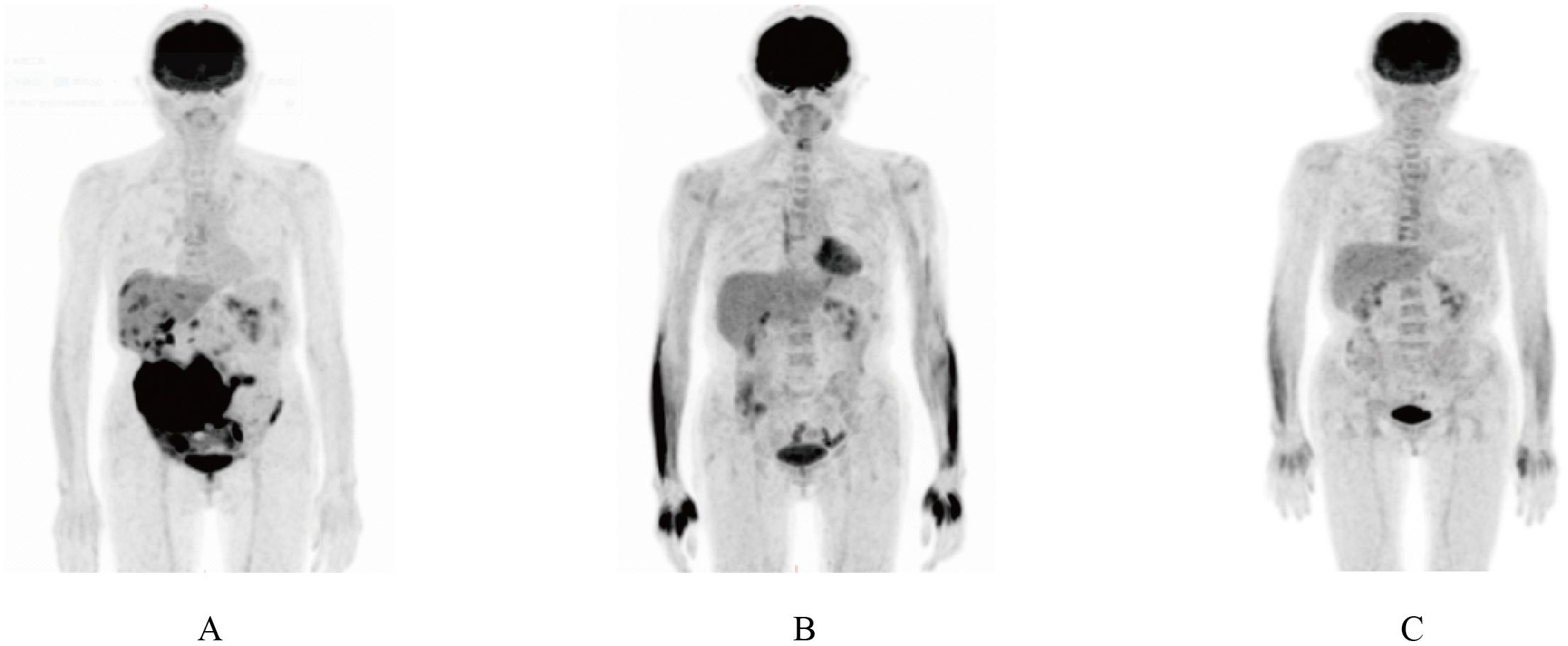
Figure 3. PET/CT scan showing complete remission 3 months after CAR-T therapy. (A) PET/CT scan showing a mass in lower right abdomen after induction and consolidation therapy. (B) PET/CT scan before CAR-T cell therapy. (C) PET/CT scan 3 months after CAR-T cell infusion.
Discussion and conclusions
Discussion
A meta-analysis indicated that high-risk cytogenetic profile significantly correlated to worse overall survival (OS), progression-free survival (PFS), and overall response rate (ORR). Additionally, EMD presence linked to significantly worse OS and PFS comparing to standard-risk genetics (7). The prognosis of PBM was poor than that of other types of MM (6). PBM may appear in BM and/or extramedullary tissues, but PBM with EMD is associated with worse OS than those without extramedullary involvement, with median OS of 17 and 28 months, respectively (8). CAR-T cell therapy may emerge as a potential therapeutic option towards high-risk PBM with EMD. In particular, Eque-cel has shown promising results in MM patients. The FUMANBA-1 trial illustrated that Eque-cel caused durable responses in patients of heavily pretreated refractory/relapsed multiple myeloma (RRMM), with 96.0% ORR and a 12-month PFS rate 78.8% (9). Another study unraveled that the median PFS and OS of Eque-cel in RRMM was 22.6 and 50.2 months, respectively (10). FUMANBA-2 trail showed favorable results, evaluating Eque-cel in newly diagnosed high-risk MM, with a 100% ORR and 71.4% minimal residual disease (MRD) negativity (11).
Currently, BCMA CAR-T is mainly used in patients with RRMM, particularly those with multiple prior lines of therapy, often accompanied by compromised organ function. In this setting, disease progression can be rapid. Following review of PBM data and shared decision-making discussions, the patient demonstrated full comprehension of the poor prognosis, constrained treatment alternatives, and lack of standard therapy for it. Given her documented resistance to and poor tolerance of prior chemotherapy regimens, the patient declined ASCT and elected to proceed with anti-BCMA CAR-T cell therapy.
It has been reported that EMD (12, 13) and high disease burden (14–16) of CAR-T infusion correlate to high risk of higher-grade CRS and ICANS. Bridging therapy is used to control cancer progression as well as reduce the severe CRS and ICANS risks. Amongst potential bridging treatments, radiation therapy (RT) is an efficient one, especially if the tumor is limited. Preclinical evidence demonstrates that RT potentiates CAR-T cell efficacy by increasing tumor antigen exposure, thereby mitigating antigen-negative relapse (17). RT is shown to be effective, which is safe bridging therapy for local cancer control (18–20). A retrospective study reported that patients receiving RT as bridging therapy had significantly better 1-year PFS and OS comparing to patients who received chemotherapy or no bridging therapy. The 1-year PFS was 51.2% in RT group, 28.2% in chemotherapy group, and 47.6% in no-bridging group. The 1-year OS was 86.7%, 52.7% and 69%, respectively (21). Bridging radiotherapy reduces tumor burden in extramedullary multiple myeloma patients without compromising subsequent CAR-T cell infusion, demonstrating significant clinical implications of the interplay between radiation and immunotherapy.
The role of maintenance therapy after CAR T-Cell therapy in relapsed/refractory multiple myeloma (RRMM) remains unclear and investigational. While CAR-T induces deep responses (including MRD negativity in some patients), relapse remains common due to CAR-T cell exhaustion, antigen escape, or tumor microenvironment resistance. Maintenance therapy aims to prolong remission, but standardized guidelines are lacking. Potential Maintenance Strategies including Immunomodulatory Drugs (IMiDs), Anti-CD38 Monoclonal Antibodies (e.g., daratumumab), Anti-BCMA bispecific antibodies (e.g., teclistamab) or ADC (belantamab mafodotin). IMiDs exert multiple anti-MM effects, including anti-angiogenic, anti-proliferative, and immunomodulatory effects, and has been demonstrated to promote T-cell proliferation in an IL-2-dependent manner (22). Investigations have shown that maintenance therapy with pomalidomide and lenalidomide may assist in promoting CAR-T cell re-expansion in patients with high-risk MM (23, 24). Most of the patients who received ciltacabtagene autoleucel lost detectable CAR T cells by 6 months after infusion (25). Maintenance may be initiated after CAR-T recovery (e.g., ≥3 months post-infusion). Overlapping immunosuppression (e.g., infections) must be balanced. BCMA CAR-T cell therapy with long-term pomalidomide resulted in low recurrence rate and manageable adverse effects (24). In patients that had pomalidomide as maintenance therapy post CAR-T cell infusions, median time to progression (TTP) was 13 months, though OS was not achieved. After a median follow-up of 27 months, the median OS and TTP was 10.7 and 5.85 months, respectively, in patients without receiving pomalidomide (24).In this case, considering PBM combined with multiple high-risk factors, we opted for pomalidomide maintenance therapy. At 12 months post-infusion, we detected 2,296 copies of CAR-T cells/μg gDNA. Remarkably, at 15 months, 5,670 copies/μg gDNA were still detectable (Figure 1), indicating that the CAR-T persistence duration in this patient has already exceeded the median persistence duration of 419 days reported for Eque-cel (26) The observed increase in expansion during this phase might potentially be attributed to the therapeutic effects of pomalidomide maintenance. We anticipate that this treatment approach may enable the patient to achieve prolonged disease-free survival (DFS).There is currently no established standard maintenance regimen, and participation in clinical trials should be prioritized (e.g., NCT05257083, NCT04832854).Outside trials, individualized decisions based on patient risk, prior therapies, and response depth may include IMiDs, anti-CD38 antibodies, or observation. Long-term data are needed to define optimal strategies.
In conclusion, this case demonstrates that BCMA CAR-T cell therapy, accompanied with RT as bridging therapy and pomalidomide as maintenance therapy, is efficient to treat MM with extramedullary plasmablastic disease, with manageable adverse events. However, further prospective investigations having larger sample sizes are demanded to validate superiority of the therapeutic strategies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because this is a case report in routine care, without ethical approval, but with the informed consent of the patient. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LZ: Writing – original draft. PC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. J-QM: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – review & editing
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bansal R, Rakshit S, and Kumar S. Extramedullary disease in multiple myeloma. Blood Cancer J. (2021) 11:161. doi: 10.1038/s41408-021-00527-y
2. Nakamoto-Matsubara R, Nardi V, Horick N, Fukushima T, Han RS, Shome R, et al. Integration of clinical outcomes and molecular features in extramedullary disease in multiple myeloma. Blood Cancer J. (2024) 14:224. doi: 10.1038/s41408-024-01190-9
3. Greipp PR, Leong T, Bennett JM, Gaillard JP, Klein B, Stewart JA, et al. Plasmablastic morphology—an independent prognostic factor with clinical and laboratory correlates: Eastern Cooperative Oncology Group (ECOG) myeloma trial E9486 report by the ECOG Myeloma Laboratory Group. Blood. (1998) 91:2501–7. doi: 10.1182/blood.V91.7.2501
4. Zhou J and Nassiri M. Lymphoproliferative neoplasms with plasmablastic morphology: an overview and diagnostic approach. Arch Pathol Lab Med. (2022) 146:407–14. doi: 10.5858/arpa.2021-0117-RA
5. Møller HE, Preiss BS, Pedersen P, B Kristensen I, Hansen CT, Frederiksen M, et al. Clinicopathological features of plasmablastic multiple myeloma: a population-based cohort. APMIS. (2015) 123:652–8. doi: 10.1111/apm.12411
6. Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. (2022) 97:1086–107. doi: 10.1002/ajh.26590
7. Gagelmann N, Ayuk FA, Klyuchnikov E, Wolschke C, Berger SC, and Kröger N. Impact of high-risk disease on efficacy of CAR-T cell therapy for multiple my-eloma: A meta-analysis of 723 patients. Haematologica. (2023) 108:2799–802. doi: 10.3324/haematol.2022.282510
8. Chen B, Yuan CT, Yang CF, Ho C, Lin YK, Su YZ, et al. Plasmablastic myeloma in Taiwan frequently presents with extramedullary and extranodal mass mimicking plasmablastic lymphoma. Virchows Arch. (2022) 481:283–93. doi: 10.1007/s00428-022-03342-3
9. Li CR, Zhou KS, Hu YX, Zou DH, Chen LJ, Chen B, et al. Equecabtagene autoleucel in patients with relapsed or refractory multiple myeloma: the FUMANBA-1 nonrandomized clinical trial. JAMA Oncol. (2024) 10:1681–8. doi: 10.1001/jamaoncol.2024.4879
10. Yu QX, Wang D, Li Z, An N, Li CH, Bao YH, et al. Long-term safety and efficacy of the fully human CAR-T therapy CT103A in relapsed/refractory multiple myeloma. Mol Ther. (2024) 8:S1525-0016(24)00740-8. doi: 10.1016/j.ymthe.2024.11.013
11. Chen LJ, Shao XY, Jin YY, Li P, Zhang R, Xu Y, et al. Eque-cel, a novel fully human BCMA-targeting CAR-T therapy in patients with high risk newly diagnosed multiple myeloma. EHA Library. (2024) 422310:S206. doi: 10.1002/hem3.104
12. Li W, Liu M, Yuan T, Yan L, Cui R, and Deng Q. Efficacy and follow-up of humanized anti-BCMA CAR-T cell therapy in relapsed/refractory multiple myeloma patients with extramedullary-extraosseous, extramedullary-bone related, and without extramedullary disease. Hematol Oncol. (2022) 40:223–32. doi: 10.1002/hon.2958
13. Deng HB, Liu MJ, Yuan T, Zhang H, Cui R, Li JY, et al. Efficacy of humanized anti-BCMA CAR T cell therapy in Relapsed/Refractory multiple myeloma patients with and without extramedullary disease. Front Immunol. (2021) 12:720571. doi: 10.3389/fimmu.2021.720571
14. Cohen AD, Parekh S, Santomasso BD, Pérez-Larraya JG, Donk NW, Arnulf B, et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. (2022) 12:32. doi: 10.1038/s41408-022-00629-1
15. Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Cancer Discov. (2016) 6:664–79. doi: 10.1158/2159-8290.CD-16-0040
16. Santomasso B, Bachier C, Westin J, Rezvani K, and Shpall EJ. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book. (2019) 39:433–44. doi: 10.1200/EDBK_238691
17. DeSelm C, Palomba ML, Yahalom J, Hamieh M, Eyquem J, Rajasekhar VK, et al. Low dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol Ther. (2018) 26:2542–52. doi: 10.1016/j.ymthe.2018.09.008
18. Wright CM, LaRiviere MJ, Baron JA, Uche C, Xiao Y, Arscott WT, et al. Bridging radiation therapy prior to commercial chimeric antigen receptor T-cell therapy for relapsed/refractory aggressive b-cell lymphoma. Int J Radiat Oncol Biol Phys. (2020) 108:178–88. doi: 10.1016/j.ijrobp.2020.05.014
19. Pinnix CC, Gunther JR, Dabaja BS, Strati P, Fang P, Hawkins MC, et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large b-cell lymphoma. Blood Adv. (2020) 4:2871–83. doi: 10.1182/bloodadvances.2020001837
20. Manjunath SH, Cohen AD, Lacey SF, Davis MM, Garfall AL, Melenhorst JJ, et al. The safety of bridging radiation with anti-BCMA CAR T-cell therapy for multiple myeloma. Clin Cancer Res. (2021) 27:6580–90. doi: 10.1158/1078-0432.CCR-21-0308
21. Bramanti S, Mannina D, Chiappella A, Casadei B, Philippis CD, Giordano L, et al. Role of bridging RT in relapsed/refractory diffuse large B-cell lymphoma undergoing CAR-T therapy: a multicenter study. Bone Marrow Transpl. (2025) 60:32–8. doi: 10.1038/s41409-024-02427-8
22. Holstein SA and McCarthy PL. Immunomodulatory drugs in multiple myeloma: mechanisms of action and clinical experience. Drugs. (2017) 77:505–20. doi: 10.1007/s40265-017-0689-1
23. Garfall AL, Cohen AD, Susanibar-Adaniya SP, Hwang WT, Vogl DT, Waxma AJN, et al. Anti-BCMA/CD19 CAR T cells with early immunomodulatory maintenance for multiple myeloma responding to initial or later-line therapy. Blood Cancer Discov. (2023) 4:118–33. doi: 10.1158/2643-3230
24. Yan Y, Tu YX, Cheng Q, Zhang J, Wang EH, Deng ZQ, et al. BCMA CAR-T therapy combined with pomalidomide is a safe and effective treatment for relapsed/refractory multiple Myeloma. J Transl Med. (2024) 22:1087. doi: 10.1186/s12967-024-05772-w
25. Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. (2021) 398:314–24. doi: 10.1016/S0140-6736(21)00933-8
Keywords: BCMA CAR-T, extramedullary, plasmablastic multiple myeloma, Equecabtagene Autoleucel, maintenance
Citation: Zhou L, Cheng PNM and Mi J-q (2025) Case Report: BCMA-targeting CAR T-cell therapy induces complete and durable remission in relapsed extramedullary plasmablastic multiple myeloma. Front. Immunol. 16:1567403. doi: 10.3389/fimmu.2025.1567403
Received: 27 January 2025; Accepted: 29 April 2025;
Published: 26 May 2025.
Edited by:
Shahrzad Jalali, Mayo Clinic, United StatesReviewed by:
Abhinava Mishra, University of California, Santa Barbara, United StatesHujun Li, Xuzhou Medical University, China
Liqiong Liu, The 6th Affiliated Hospital of Shenzhen University Health Science Center, China
Copyright © 2025 Zhou, Cheng and Mi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Zhou, c2lsZW5jZWxpbHlAMTYzLmNvbQ==; Jian-qing Mi, amlhbnFpbmdtaUBzaHNtdS5lZHUuY24=
 Lili Zhou
Lili Zhou Paul Ning Man Cheng2
Paul Ning Man Cheng2