- 1College of Food and Bioengineering, Zhengzhou University of Light Industry, Zhengzhou, China
- 2Henan Key Laboratory of Cold Chain Food Quality and Safety Control, Zhengzhou University of Light Industry, Zhengzhou, China
- 3Institute of Life and Health, Zhengzhou University of Light Industry, Zhengzhou, China
- 4Institute for Regenerative Medicine Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
- 5Basic Medical Department, Graduate School, Chinese People’s Liberation Army General Hospital, Beijing, China
This article presents a comprehensive literature review on the role of the Notch signaling pathway in cancer and its molecular mechanisms. The Notch signaling pathway plays an important role in various types of cancer by regulating key biological processes such as epithelial-endothelial transition, angiogenesis, apoptosis and metabolic reprogramming. This article reviews the relevant mechanisms of the Notch signaling pathway in colorectal cancer, ovarian cancer, oral squamous cell carcinoma, gastric cancer and chronic lymphocytic leukemia, highlighting its dual roles in promoting tumor growth, inhibiting tumor progression and potential therapeutic applications in oncology. Meanwhile, the key roles of the Notch signaling pathway in regulating tumor drug resistance and shaping the tumor microenvironment are discussed, highlighting its importance in clinical applications. Through this review, some ideas and hints for future research directions can be provided to the readers of Notch pathway related research.
1 Introduction
The Notch gene was first described and named in the 20th century by the American scientist Dexter J. Colman through the study of the Notch wing mutant in Drosophila melanogaster (1). Subsequently, scientists have gradually recognized the key role of Notch genes in regulating complex physiological processes in a wide range of organisms, and their major functions are thought to be involved in the development of organ morphology, the coordination of tissue function and repair after tissue injury (2). In recent years, due to the rapid development of tumor therapeutic technologies, gene editing tools and bioinformatics methods, there are significant differences in the degree of dependence of different tumor types on the Notch signaling pathway, and the treatment of tumors by the Notch signaling pathway is still unable to overcome the problems of drug resistance and the impact on the function of healthy tissues, which call for an in-depth exploration of the specific mechanisms of the Notch signaling pathway in the development and progression of tumors. These issues call for an in-depth exploration of its specific mechanism of action in tumor development and progression. Therefore, the study of how the Notch pathway regulates tumors may provide a deeper understanding of the complexity and potential therapeutic applications of the Notch pathway and provide a new approach for innovative tumor therapeutic strategies.
2 Introduction to the notch signaling pathway
The mammalian Notch signaling pathway consists mainly of four Notch receptors (Notch1-4) (3), five Notch ligands (δ-like ligand 1 (DLL1), δ-like ligand 3 (DLL3), δ-like ligand 4 (DLL4), Jagged-1 (JAG1) and Jagged-2 (JAG2)), and a large number of DNA-binding proteins and downstream target genes (4). Each Notch receptor has a number of DNA-binding proteins and downstream target genes (4), of which alterations in any one can affect Notch signaling, including cell proliferation and differentiation (5).Dysregulation of Notch activity can lead to a variety of diseases, including genetic disorders, cardiovascular disease and cancer (6). Among these, each Notch receptor has its own unique function, of which Notch1 is involved in the regulation of processes such as embryonic development, adult tissue maintenance and immune cell development, and plays a critical role in a wide range of cells (7).Notch2 has a similar role in cell signaling to Notch1, but Notch2 may have a specific role in the regulation of angiogenesis (8). Notch3, on the other hand, has a tumor suppressor role (9). Notch4 function is relatively poorly understood and has been implicated in carcinogenesis (10).
3 Molecular mechanism of the notch signaling pathway
The Notch signaling pathways include the classical Notch signaling pathway, which is activated by ligand-receptor binding (11), and the non-classical Notch signaling pathway, which interacts with other signaling pathways in the Notch intracellular structural domain (NICD) and in the nucleus and directly intervenes in the regulation of transcription of target genes (12).
The classical Notch signaling pathway involves the formation of Notch precursors in the endoplasmic reticulum following transcription and translation, leading to the glycosylation of the Notch receptor (13), which is then transported to the Golgi apparatus where it is cleaved into a mature heterodimer in a process also known as S1 cleavage (14). The extracellular structural domain of the Notch receptor and ligand is then ubiquitinated by the ligand, initiating endocytosis and inducing a conformational change in the receptor, exposing the S2 cleavage site of the receptor, which cleaves the extracellular structural domain of Notch and initiates signaling via the metalloproteinases ADAM10 or ADAM17 (15) in a process known as S2 cleavage (16). This is followed by S3 cleavage of the Notch receptor by γ-secretase (17), a process that results in the release of soluble NICD from Notch. NICD is then degraded in the cytoplasm or translocated to the nucleus where it binds to the DNA-binding protein RBP-Jκ, which recruits mastermind-like transcriptional coactivator 1 (MAML1) to form a multiprotein DNA complex. This complex initiates Notch target genes (18) (Figure 1).
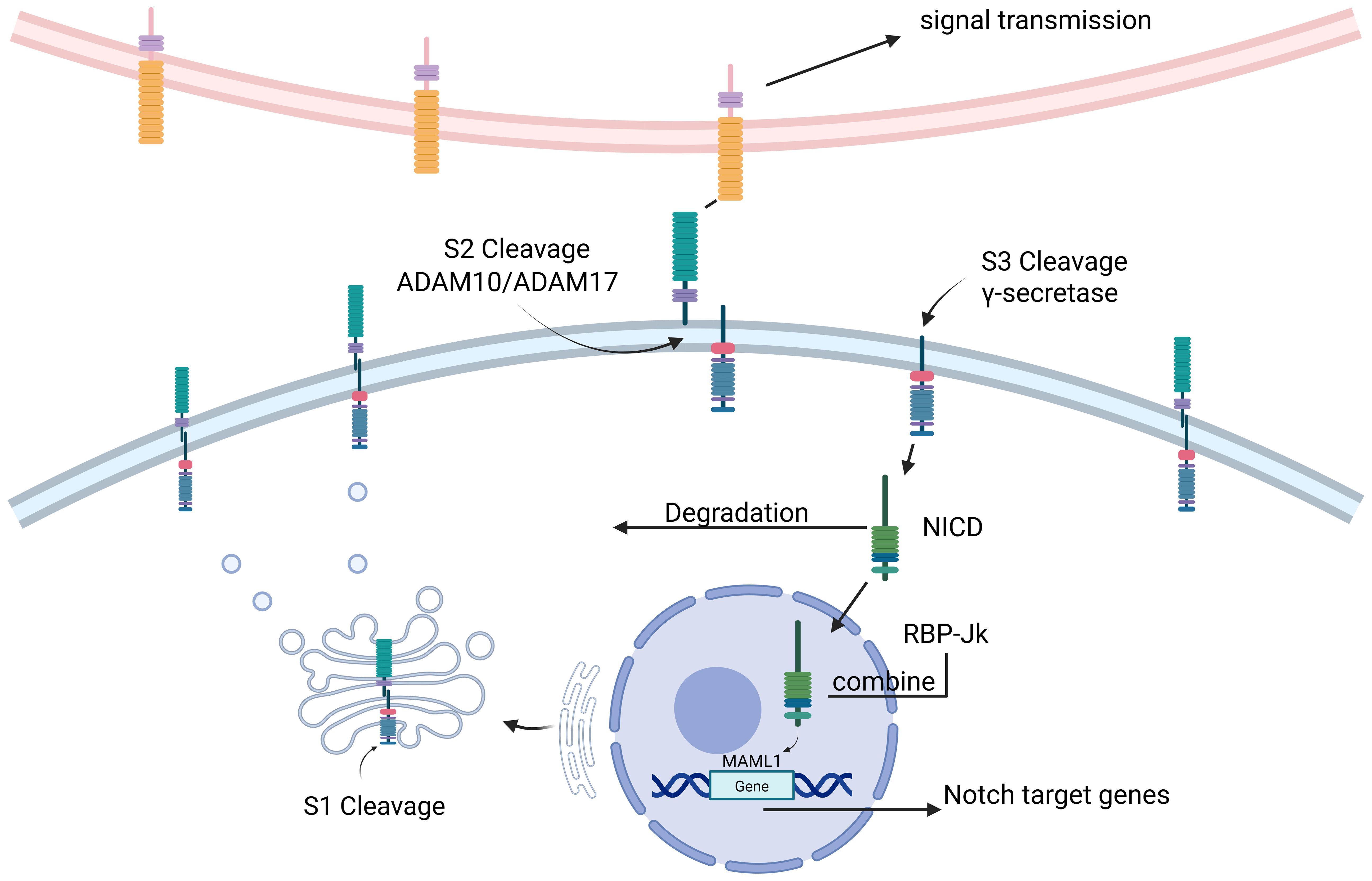
Figure 1. (Figure created using BioRender.com). NICD Notch intracellular domain, ADAM a disintegrin and metalloprotease, MAML mastermind-like. The Notch signaling pathway is where Notch precursors are formed in the endoplasmic reticulum after transcription and translation, translocated to the Golgi for S1 cleavage. The extracellular structural domains of the Notch receptor and ligand are then ubiquitinated by the ligand and initiate endocytosis for S2 cleavage. The Notch receptor then undergoes S3 cleavage by γ-secretase, a process that leads to the release of soluble NICD from Notch. Subsequently, NICD is degraded in the cytoplasm or translocated to the nucleus where it binds to the DNA-binding protein RBP-Jκ, which recruits Mastermind-like transcriptional co-activator 1 (MAML1) to form a multiprotein-DNA complex. This complex initiates Notch target genes.
In addition to the classical Notch pathway, mature Notch receptors on the cell membrane are endocytosed intracellularly under certain circumstances that are not dependent on ligand binding, thereby activating intracellular transduction of Notch signaling, the so-called non-classical Notch pathway (19). Activation of the non-classical pathway has also received increasing attention in cancer research.
4 Research on the notch signaling pathway in cancer
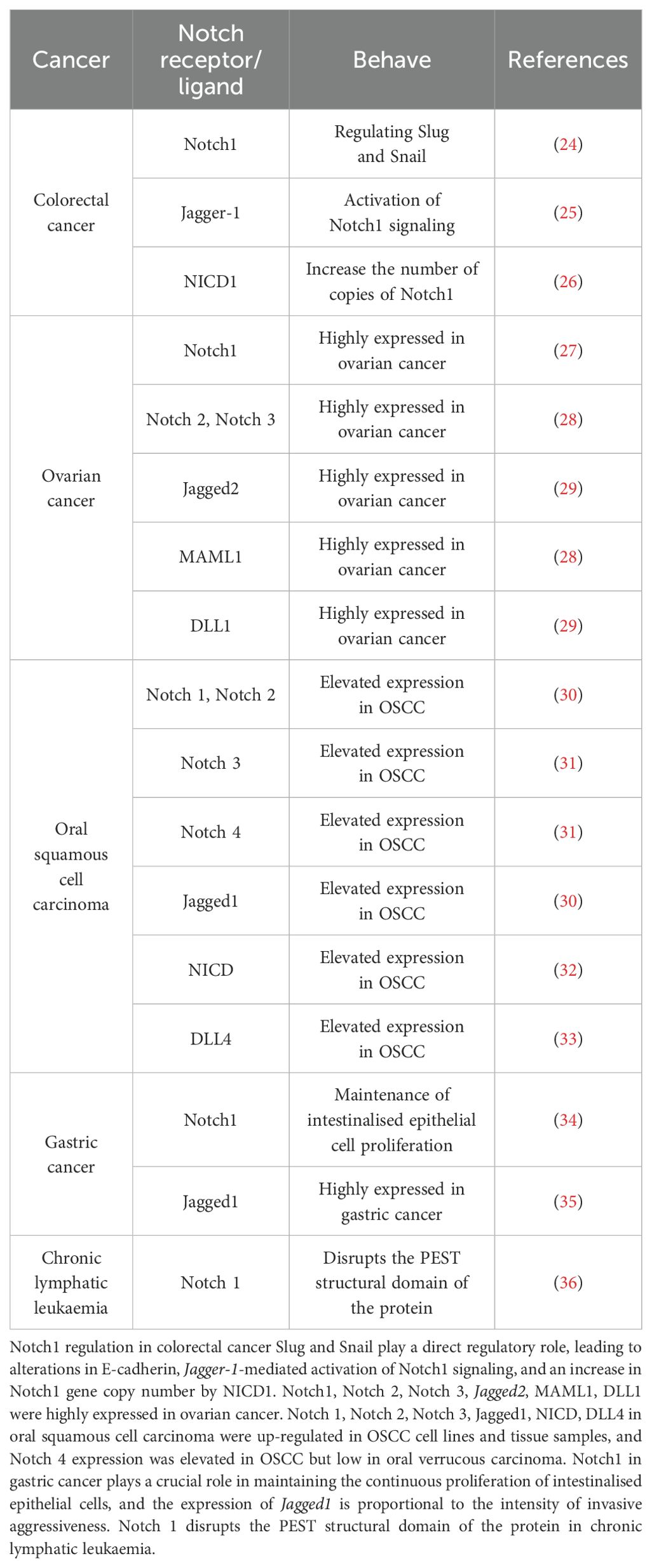
As mentioned above, the Notch signaling pathway plays a crucial role in regulating the physiological functions of cells and is also closely linked to the development of cancer. It can either promote tumor development or play an oncogenic role in some cancers. In the study of many human cancers, Notch gene mutation or signaling dysregulation can trigger various diseases, such as colorectal cancer (20), prostate cancer (21), glioblastoma and other malignant tumors in which Notch signaling has been found to be downregulated or inactivated. However, Notch plays opposite roles in the same type of cancer. Notch is oncogenic in T-lymphocyte malignancies (e.g. T-ALL) and chronic lymphocytic leukemia, but monostatic in B-cell acute lymphoblastic leukemia, chronic granulomonocytic leukemia and acute myeloid leukemia (22, 23). Notch signaling plays different roles in different cancers and needs to be studied according to the type of cancer(Figure 2, Table 1).
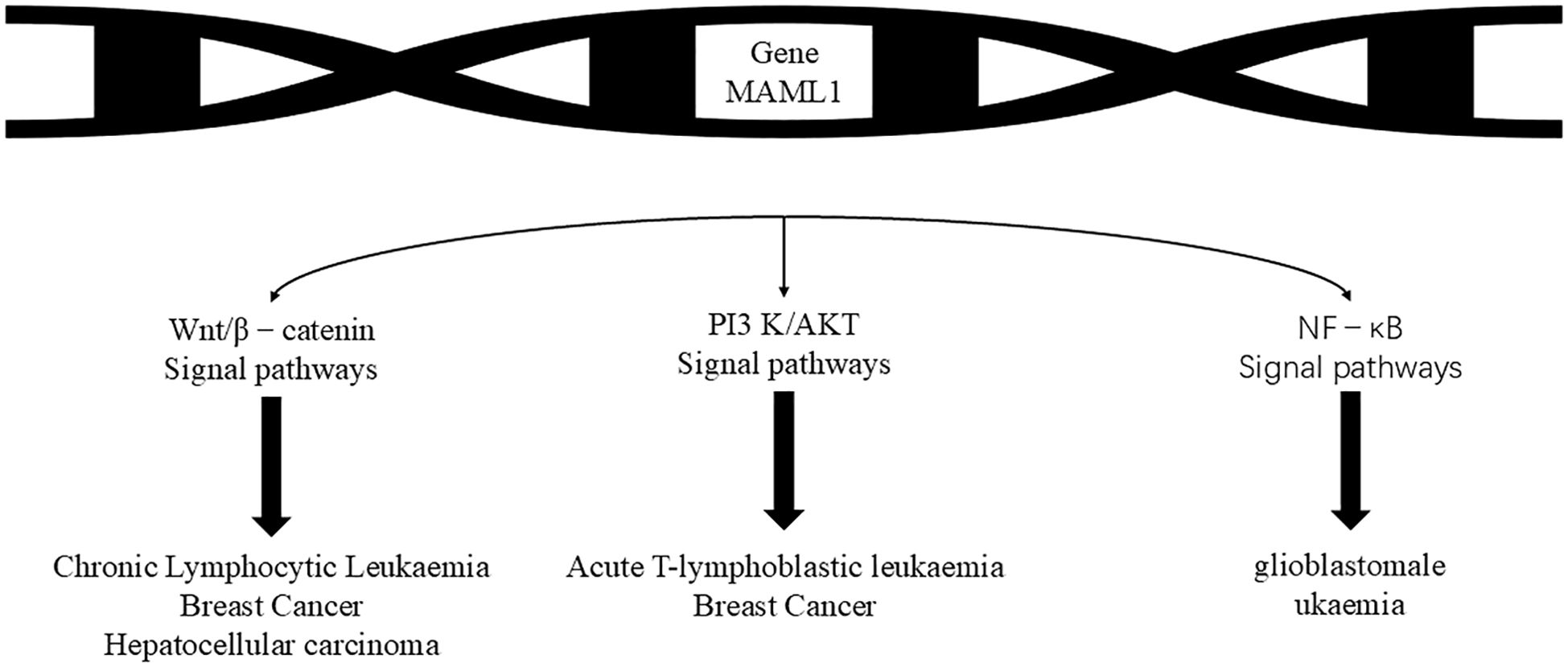
Figure 2. The Notch pathway regulates the following cancer-associated pathways, which then have different functions in different cancers. The MAML1 gene can be used to prevent the development of leukaemia by affecting the Wnt/β-catenin signalling pathway, the PI3 K/AKTS signalling pathway and the NF- kB signaling pathway, leading to chronic lymphocytic leukaemia, breast cancer, hepatocellular carcinoma, acute T-lymphocytic leukaemia, breast cancer and glioblastoma leukaemia.
4.1 The role of notch signaling in colorectal cancer
Colorectal cancer accounts for 1/10 of all cancer cases and deaths and is the third most common cancer in men and the second most common cancer in women, with 1.9 million new colorectal cancer cases and 935,000 deaths in 2020 (37).
In colorectal cancer, the Notch1 signaling pathway plays an important role in the EMT process. In inflammation-induced carcinogenesis, activation of MMP9 can lead to activation of Notch1, which exerts a direct regulatory effect on Slug and Snail, leading to changes in E-cadherin and promoting the EMT process (24). In addition, binding of the Jagged-1 ligand and Notch1 receptor is thought to induce similar mesenchymal transformations, suggesting that Jagged-1-mediated activation of Notch1 signaling plays an important role in inducing the EMT process (25). Colon cancer cells knocked out of Jagged-1 grew much slower than controls, but Jagged-1 deletion is important in promoting colorectal carcinogenesis, a process that induces tumors by inhibiting tumor cell differentiation and promoting angiogenesis (38). Increased Notch1 gene copy number has been found to be significantly associated with poorer survival in patients with advanced colorectal cancer, and tumors with upregulated Notch1 expression in a tumor xenograft model derived from patients with colorectal cancer show significant elevation of Notch1, JAG1 and NICD1, which may be ameliorated by targeting this patient population with Notch1 antibodies (26).
4.2 The role of notch signaling in ovarian cancer
The mortality rate for ovarian cancer patients has been 5% in recent years (39). Such a high mortality rate is due to the insidious nature of the disease and the difficulty of early diagnosis, and more than 70% of patients are already at an advanced stage of the disease when they are diagnosed, missing the best time for treatment.
Hopfer et al. (29) published the first literature on the expression of the Notch signaling pathway in ovarian cancer in 2005. The literature found that Jagged2, DLL1, Manic Fringe and TSL were more frequently expressed in ovarian adenocarcinomas, while Deltex and Mastermind were more frequently expressed in adenomas, as detected by RT-PCR.Wang et al. (27) used immunohistochemistry, Western blot and RT-PCR to detect Notch1 expression. It was found that the expression of Notch1 in ovarian cancer tissues was significantly higher than that in matched normal ovarian tissues, while Notch1 was highly expressed in ovarian cancer cell lines A2780 and HO-8910, and the expression of Notch1 gradually increased with the poor differentiation of ovarian cancer tissues and the increase of FIGO stage. It can be seen that for the same factor, the expression level is different in different tumor tissues. High expression of NICD in primary ovarian cancer tumors is an independent poor prognostic factor for overall survival (40). Defreitas et al. (28) used bioinformatics methods to analyses multiple databases from CSIOVDB, PRECOG, GENT and CCLE and systematically showed that Notch2, Notch3 and MAML1 were highly expressed in ovarian cancer and were critical for overall patient survival. In conclusion, Notch1-3, Jagged1 and Hes1 are more highly expressed in highly malignant ovarian cancers, and upregulation of Notch pathway component protein expression in ovarian cancer is associated with shortened overall and disease-free survival, especially in patients with advanced tumors. It is therefore hypothesized that Notch may be used as a marker of poor prognosis.
4.3 The role of notch signaling in oral squamous cell carcinoma
Oral squamous cell carcinoma (OSCC) accounts for more than 90% of all oral cancers and is the most common oral and maxillofacial tumor (41). As the ninth most common malignant tumor in the world, OSCC has a high morbidity and mortality rate, and the 5-year survival rate of patients is less than 50% (42). Although large-scale genomic and proteomic analyses in recent years have largely elucidated the molecular biological background of OSCC occurrence and development, few genes or proteins have been identified that can be used for targeted therapy (43).
H et al. (30) found that Notch1, Notch2, Jagged1, HES1 and HEY1 were upregulated in both OSCC cell lines and tissue samples, while NICD accumulated in the nucleus of OSCC cells and Jagged1 was expressed in the cytoplasm. This study also showed that inhibition of the Notch pathway using a γ-secretase inhibitor suppressed OSCC cell growth. Y et al. also found that Notch1 and NICD were specifically upregulated in the invasive front of OSCC, Notch1 expression correlated with the clinical staging of OSCC patients, and TNF-α, an inflammatory cytokine, significantly activated the Notch signaling pathway. The absence of Notch1 expression was associated with cell proliferation in OSCC cell lines and TNF-α-dependent inhibition of invasiveness (32). Whole exome sequencing of OSCC tissue showed that 29.2% of the exome of patients had Notch1 mutations, which were predicted to be inactivating mutations in truncated proteins (44). It has also been shown that mutations in the Abruptex structural domain (C1133Y) of Notch1 promote a cellular oncogenic phenotype by increasing cell proliferation and inducing EMT in OSCC cells (45).The expression of Notch1 was significantly increased in poorly differentiated, stage III, stage IV and lymph node metastasis positive OSCC patients, and overexpression of Notch1, NICD, HES1 and DLL4 predicts poor overall survival in OSCC patients (33). Notch3 and Notch4 have also been found to have increased expression in OSCC, but Notch4 has low expression in oral verrucous carcinoma, so Notch4 has been considered as a suitable prognostic marker to differentiate oral verrucous carcinoma from OSCC (31).
4.4 The role of notch signaling in gastric cancer
Gastric cancer is the fourth most common malignant tumour of the digestive tract with global incidence rate, and at the same time, China is one of the high incidence areas of gastric cancer, with 400,000 incidence cases every year, accounting for 42% of the total number of gastric cancer cases in the world. As late stage or postoperative recurrence of gastric cancer cannot be treated by surgery, chemotherapy is the main treatment for patients at present. However, chemotherapy has obvious side effects such as nausea and vomiting, and the effect of chemotherapy is unsatisfactory (46).
Studies have shown that the Notch signalling pathway is involved in the differentiation of normal gastric mucosa and epithelium into small concave glands. However, the Notch pathway and glandular differentiation are also closely related to gastric cancer cells, Notch1, Notch2, Notch3 in Notch receptors and Jagged1 and Jagged2 in Notch ligands have been found in human gastric cancer tissue samples, and each of them is expressed with different significance in physiological activities (47). For example, the expression of Notch1 in precancerous lesions and gastric cancer tissues, such as intestinal epithelial chemotaxis and hyperdifferentiated intestinal-type gastric cancer, was significantly higher than that in normal tissues, suggesting that Notch1 plays a crucial role in promoting the transformation of epithelial chemotaxis in the gastric mucosa and maintaining the continuous proliferation of intestinal chemo epithelial cells (34, 48). In addition, both Notch1 and Jagged1 proteins are considered to be independent prognostic predictors for gastric cancer, and their positive expression suggests an association with poor prognosis (35).Yeh et al. (49) reported that Jagged1 is closely related to the pathogenesis of gastric cancer, and its expression is directly proportional to the strength of invasive aggressiveness, implying that patients with high Jagged1 expression have a lower survival rate.
4.5 The role of notch signaling in chronic lymphocytic leukemia
Chronic lymphocytic leukemia (CLL) is a blood disorder characterized by clonal proliferation of mature B lymphocytes in the peripheral blood, bone marrow, spleen and lymph nodes, and is common in middle-aged and older people (50). The incidence of CLL is higher in men than in women, and the clinical course of patients is highly variable (51).
Notch1 is one of the most frequently mutated genes in CLL and is a marker of poor prognosis. Most mutations in Notch1 disrupt the structural domains of the protein’s proline-, glutamate-, serine- and threonine-rich polypeptide sequence (PEST), rendering Notch1 unable to maintain its original ubiquitination and leading to proteasomal degradation of the Notch1 receptor (36). In addition, Notch signaling is enhanced by mutations in the gene encoding the Notch1 ubiquitinate, FBXW7, which affects the ubiquitination of the Notch1 protein (52). Point mutations in the untranslated region at the 3’ end of the Notch1 mRNA (3’UTR) often undergo an aberrant splicing event, which also results in the loss of the Notch1 PEST structural domain (53). Clinically, patients with Notch1 mutations develop a more aggressive disease course and the investigation of drugs targeting the Notch pathway is a major advance in changing the prognosis of patients.
5 Investigating the specific mechanisms of notch signaling in cancer
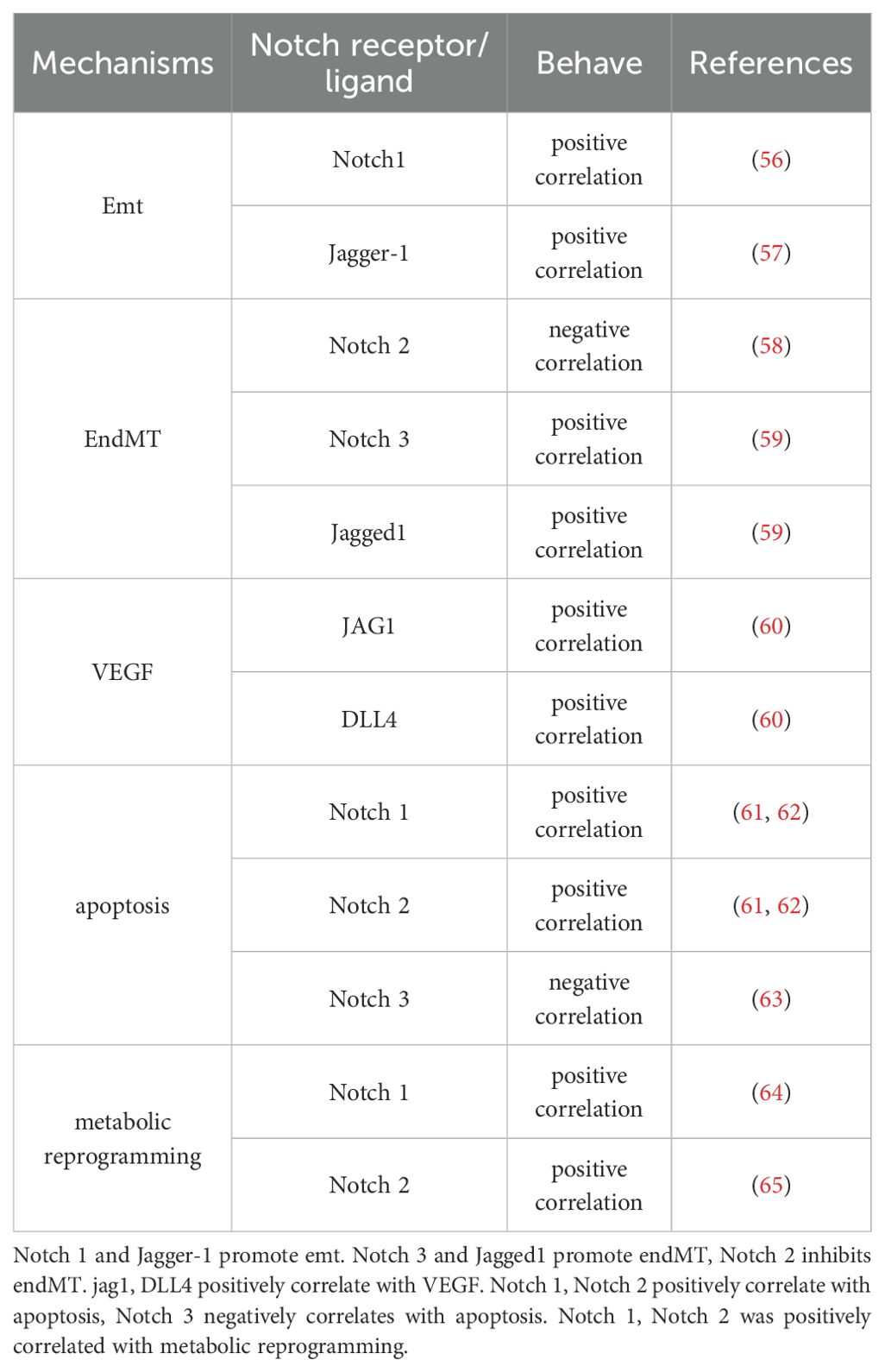
The Notch signaling pathway has a complex role in many aspects of cancer (54). For example, the Notch pathway can play a regulatory role in cancer metabolic reprogramming and the tumor microenvironment (55). In the following sections, we will summaries and highlight the specific mechanisms of Notch signaling mediated tumor initiation and progression in terms of epithelial-mesenchymal transition, endothelial-mesenchymal transition, angiogenesis, apoptosis and metabolic reprogramming (Table 2).
5.1 The role of notch pathway in EMT
EMT stands for epithelial mesenchymal transition, a biological process originally discovered by Elizabeth Hays in the 1980s (66). In this process, epithelial cells lose their cellular polarity and tight junctions and gain the ability to migrate and invade, transforming into cells with mesenchymal properties (67). EMT plays an important role in embryonic development, tissue repair, organ fibrosis, and tumor invasion and metastasis (68). Regulation of the EMT pathway is a complex molecular event involving a variety of molecular mechanisms, including the Notch signaling pathway.
Studies have shown that Notch1 can regulate the EMT process in tumor cells by cross talking with several EMT-related transcription and growth factors (Snail, Slug, Twist1, and FGF, etc.). Timerman et al. (56) found that: in endothelial cells, Notch1 can promote EMT by upregulating the expression of Snail, resulting in the downregulation of E-calmodulin expression. Chen et al. (57) found that in colorectal cancer, miR-598 inhibits the Notch signaling pathway by suppressing the downstream target gene JAG1, thereby regulating the occurrence of EMT. A detailed study found that the relationship between the Notch pathway and EMT is reflected in its effect on cell adhesion molecules such as E-cadherin (69). Overexpression of Notch ICD increases Snail expression and leads to loss of E-cadherin. Downregulation of intercellular adhesion molecules is one of the hallmark changes during EMT, whereas inhibition of the Notch pathway counteracts the decrease in E-cadherin and Snail expression (70). Overexpression of Notch-1 in prostate cancer promotes prostate cancer cell migration and invasion, whereas downregulation of Notch-1 expression inhibits prostate cancer cell migration and invasion that occur through induction of EMT (71). Thus, the Notch signaling pathway may play a role in regulating the expression of EMT-related genes, which promotes the process of EMT and thus affects the invasive and metastatic behavior of tumors.
5.2 The role of notch pathway in endothelial-mesenchymal transition
Endothelial-mesenchymal transition (EndMT), similar to EMT, is a process in which endothelial cells lose their endothelial characteristics and transform into mesenchymal cells when exposed to external stimuli. In recent years, EndMT has been shown to play an important role in disease pathogenesis and has been implicated in the development of cardiovascular disease, organ fibrosis and cancer. While Notch signaling is the main signaling pathway regulating the EndMT process, others include TGF-β/Samd3 signaling pathway, BMP signaling pathway, Wnt signaling pathway, etc., and these signaling pathways cross and influence each other to promote the development of EndMT (72).
RLX inhibited the transformation of cardiac fibroblasts into cardiac myofibroblasts, and the mechanism of action was the Notch-mediated TGF-β signaling pathway (73). In endothelial cells, Sahoo et al. (58) found that Notch2, mRNA and protein expression were downregulated in human pulmonary artery endothelial cells stimulated by hypoxia, and that Notch2 inhibition resulted in an increase in the proliferative and migratory capacity of endothelial cells, and Wang et al. (59) found that hypoxia activated the Notch3 signaling pathway and induced high expression of Notch3, Jagged-1 and HES1, which promoted the transformation of pulmonary artery endothelial cells into mesenchymal cells. Thus, the Notch signaling pathway may play a role in regulating the expression of EndMT-related genes and, by affecting its downstream proteins, may inhibit the mesenchymal transdifferentiating of endothelial cells, leading to a decrease in the expression of epithelial cell markers and an increase in the expression of mesenchymal markers.
5.3 The role of notch pathway in angiogenesis
Blood vessels are one of the largest organs in the human body, and vascular endothelial growth factor (VEGF) and its receptor are essential for angiogenesis. Two ligands of the Notch signaling pathway (JAG1 and Dll4) play important roles in angiogenesis. In particular, Dll4, which finely regulates the selection of endothelial cells towards tip versus stem cell differentiation (74), is fundamentally involved in the control of VEGF-induced vascular sprouting. Regulation of the Notch signaling pathway has been shown to promote angiogenesis (75) and this process of Notch signaling is critical for tumor angiogenesis.
Qiu et al. (76) showed that Dll4 was positively correlated with VEGF expression in primary glioblastoma, and Fraser et al. (77) demonstrated that in vivo blockade of Dll4 with anti-Dll4 monoclonal antibody in primate ovaries increased luteal vasculature and micro vessel density. Wang et al. (60) found that the expression of Dll4 was positively correlated with VEGFR1; while in ovarian cancer tissues, the expression of Notch1 and VEGFR2 was associated with micro vessel density, with Notch1 expression increased in ovarian tumor tissues. correlation; while in ovarian cancer tissues, the expression of Notch1 and VEGFR2 was correlated with micro vessel density, with Notch1 expression increased in ovarian tumor tissues. In malignant tumors such as lung cancer, the relationship between the Notch pathway and vascular endothelial growth factor (VEGF) is particularly close.Jagged1, as a ligand of the Notch pathway, can significantly upregulate the expression of VEGF and promote tumor neovascularization upon binding to Notch1, which in turn promotes tumor proliferation and metastasis (60). In some cases, activation of Notch signaling can also inhibit VEGF expression, thereby reducing angiogenesis (78). The existence of this bidirectional regulatory mechanism complicates the role of the Notch signaling pathway in tumor angiogenesis, and future studies will further explore the molecular mechanisms behind this balance.
5.4 The role of notch pathway in apoptosis
Apoptosis consists mainly of extrinsic and intrinsic pathways. The exogenous pathway, also known as the death receptor pathway, induces apoptosis through transmembrane receptor-mediated activation of the caspase family of aspartate protein hydrolases. The endogenous pathway, also known as the mitochondrial pathway, stimulates the direct generation of intracellular signals that cause structural changes in the mitochondrial membrane, releasing pro-apoptotic substances that induce apoptosis (79). The Notch signaling pathway plays an important role in the regulation of apoptosis in tumor cells. Studies have shown that the role of Notch signaling in apoptosis is different for different types of tumor cells.
In hepatocellular carcinoma, QI et al. (61) found that activation of Notch signaling promoted apoptosis in SMMC7721 hepatocellular carcinoma cells, and WANG et al. (62) found that overexpression of Notch signaling enhanced TEAIL-induced apoptosis in hepatocytes. In lung cancer, KONISHI et al. (63) also reported that inhibition of Notch3 expression promoted apoptosis in NSCLC, and LIN et al. (80) further demonstrated the critical role of the Notch3-Jagged1 axis in lung cancer cell apoptosis. In breast cancer, ZHU et al. (81) reported that Linc-OIP5 affects apoptosis in breast cancer MDA-MB-231 cells. The mechanism of apoptosis is complex, and although more and more studies have elucidated the mechanism of the Notch pathway in regulating apoptosis, the diversity and variability of tumor cells still require further exploration of the key nodes of the Notch pathway affecting apoptosis in tumor cells, so as to effectively utilize the Notch pathway for the treatment of tumors.
5.5 The role of notch pathway in metabolic reprogramming
The phenomenon of metabolic reprogramming was first discovered in cancer cells in 1927, referring to the phenomenon that tumor cells abandon oxidative phosphorylation in favor of glycolysis for energy supply when using glucose in an aerobic environment. Today, some scientists refer to the change in cellular energy supply as “metabolic reprogramming” (82).
Notch signaling plays a key role in the metabolic reprogramming of cancer cells. Notch signaling has been reported to regulate reverse electron transfer (RET) through interaction with specific respiratory chain complex I (RC-I) proteins containing electron transport Fe-S clusters and NAD(H)-binding sites, which in turn affects energy metabolism in brain cancer stem cells (64). In a mouse model of renal fibrosis, the Notch2 signaling pathway was found to affect the metabolism of mouse kidney cells by regulating the expression of the mitochondrial transcription factor (ATfam), which in turn affects the development of renal fibrosis in mice (65). Particularly during the glycolytic transition. During this transition in cancer cells, the Notch pathway is active and several genes are directly regulated as transcriptional targets. This regulation mediates the shift in cellular metabolism towards the Warburg effect (83, 84). All of the above studies have demonstrated that the Notch signaling pathway can be involved in the metabolic reprogramming of cells in various models.
6 Therapeutic potential of the notch signaling pathway
The Notch signaling pathway is a conserved signaling pathway that plays an important role in the regulation of cell fate, tissue development and tissue homeostasis in multicellular organisms (85). Accumulating evidence suggests that the Notch signaling pathway acts as both an oncogenic factor (86) and a tumor suppressor in a variety of cancers (87). Dysregulation of this pathway promotes epithelial-mesenchymal transition and angiogenesis in malignant tumors, which are closely associated with cancer proliferation, invasion and metastasis. In addition, the Notch signaling pathway is involved in tumor drug resistance and tumor suppression. Therefore, a comprehensive understanding of these biological processes is essential for the development of innovative therapeutic strategies targeting the Notch signaling pathway.
6.1 The role of notch pathway in tumor drug resistance
Chemotherapy is one of the commonly used treatments for malignant tumors, but acquired resistance is the main reason that hinders the clinical efficacy of patients and leads to treatment failure. Abnormal expression of the Notch signaling pathway is closely related to the formation of tumor chemoresistance (88). It is related to the inherent heterogeneity of tumor cells and genetic background or genetic changes after chemotherapy, while the specific mechanism of Notch signaling pathway involved in the formation of tumor drug resistance is not very clear, Notch signaling pathway may play a role in tumor drug resistance by participating in the process of CSCs phenotyping, EMT and crosstalk with other signaling pathways.
Regarding the involvement of the Notch signaling pathway in the transformation of drug-resistant EMT in tumors, Wang et al. (89) found that the drug-resistant cell line GR had lower expression of E-cadherin, an epithelial adhesion molecule, and higher expression of mesenchymal markers such as vimentin compared to gemcitabine-sensitive cells of GS from pancreatic cancer. Downregulation of Notch-2 and Jagged-1 by siRNA resulted in increased expression of E-cadherin and downregulation of vimentin and ZEB1 expression, reversing EMT. In a study on the involvement of the Notch signaling pathway in regulating CSC formation in tumor resistance, Capodanno et al. (90) found that the expression of inactive and active forms of the Notch2 receptor and its downstream target gene Hes1 was increased in a 5-FU-resistant human insulinoma cell line CM tumor stem cell microspheres. In vitro treatment with DAPT alone did not inhibit the clonogenic ability of insulinoma tumor stem-like cells, whereas the combination of DAPT and 5-FU significantly inhibited their clonogenicity. McAuliffe et al. (91) found that the Notch signaling pathway, in particular the Notch-3 signaling pathway, is particularly important for the maintenance of ovarian cancer CSCs and platinum drug resistance. Overexpression of NICD3 significantly increased the expression of CD44, a key stem cell marker, suggesting that there may be cross-talk between Notch-3 and CD44 to maintain the ovarian cancer stem cell phenotype.
6.2 The role of notch pathway in tumor suppression
The theoretical basis for the Notch signaling pathway as a target for cancer therapy stems from the fact that Notch can interact with a variety of signaling pathways to achieve regulation of tumor cell proliferation, apoptosis and differentiation. The Notch signaling pathway not only regulates the differentiation, proliferation and apoptosis of normal cells, but its aberrant activation is also closely associated with the development of a variety of cancers (92). Therefore, it is important to investigate the role of the Notch signaling pathway in tumor suppression.
Lewis et al. (93) found that Notch signaling is activated in T-ALL, promotes the expression of the target gene HES, which inhibits the expression of PTEN, which is downregulated and promotes the activation of PI3K/Akt, thus inhibiting the apoptosis of tumor cells, and when a Notch signaling inhibitor is applied, it promotes the expression of PTEN and reverses the anti-apoptotic effect of PI3K/Akt and induces apoptosis. Studies have shown (94) that activation of the Notch signaling pathway can inhibit the expression of INK4a/ARF gene, promote the degradation of p53 by MDM2, down-regulate the oncogenic effect of p53 and inhibit the apoptosis of tumor cells. Khan et al (95) reported that downregulation of Notch signaling promotes the expression of pro-apoptotic protein Bax, reduces the expression of anti-apoptotic protein Bcl-2, and induces apoptosis in prostate cancer cells. Sustained inhibition of Notch1 activity and activation of Bcl-2, Bcl-XL and caspase-3 in breast cancer MCF-7 and MDA-MB-231 cells reduced and promoted tumor cell apoptosis (96).
7 Clinical progress related to the notch signaling pathway
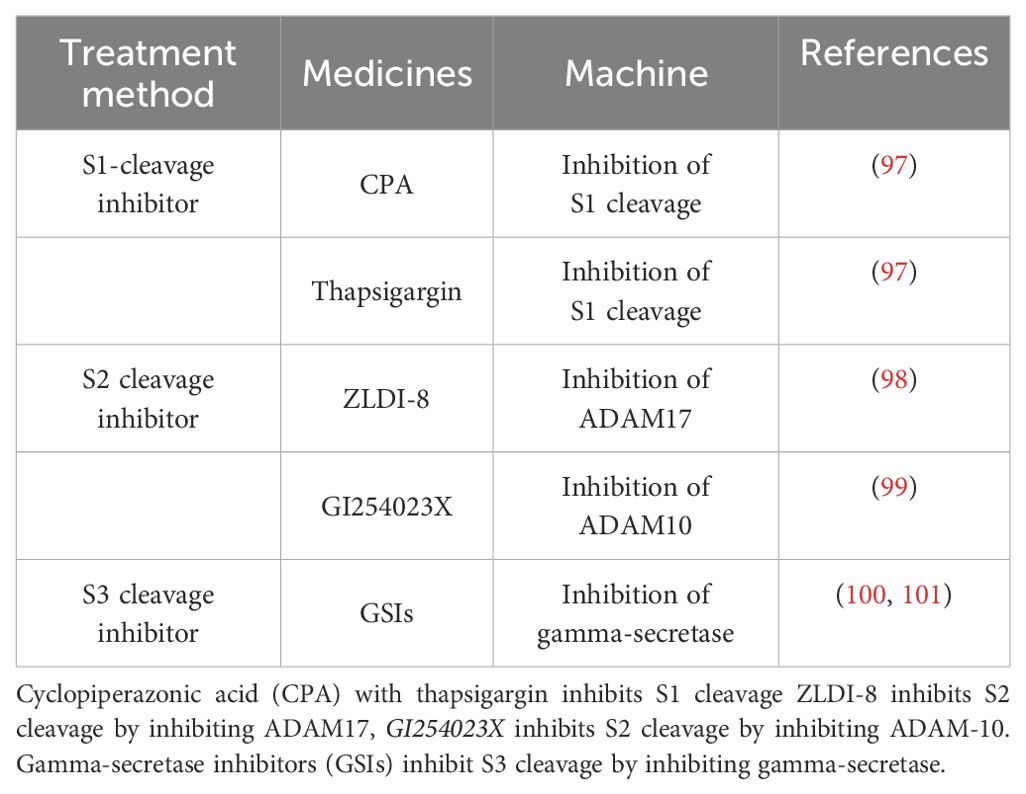
As a highly conserved signaling pathway, Notch signaling plays an important role in various biological processes such as growth, development and tissue repair. Notch signaling can produce different or even opposite biological effects with changes in time and cell type. Therefore, researchers have developed a variety of Notch-targeted therapeutics to target the different stages of the Notch pathway (Table 3).
Currently, therapies targeting the Notch pathway are being evaluated in clinical trials, with the main drugs targeting S1 cleavage being cycloheximide (CPA) and thapsigargin, which block Notch signaling by inhibiting the activity of myoendoplasmic reticulum Ca2+-ATPases (SERCAs) to prevent translocation of mutant Notch1 to the cell surface (97). Since Notch precursors generated in the ER are glycosylated and translocated to the Golgi for S1 cleavage, a process that requires the involvement of Ca2+, SERCAs are critical for Notch signaling as key cofactors that regulate ATP-dependent calcium pumping (102). Notch ligand-receptor binding in S2 cleavage can be blocked by the metalloproteinase ADAM10/TACE (also known as ADAM17) through the known proteolytic cleavage of Notch extracellular structural domains regulates the rate of Notch1 signaling (S2 cleavage). As a key enzyme in S2 cleavage, inhibition of ADAM targets the entire Notch pathway (103). For example, the Notch/ADAM17 inhibitor ZLDI-8 induces apoptosis in chemotherapy-resistant NSCLC and inhibits migration, invasion and EMT of drug-resistant lung cancer cells (98). The ADAM-10 inhibitor GI254023X may be used as a potential treatment for glioblastoma to inhibit cancer cell growth (99). The extracellular-to-intracellular transduction of Notch signaling in S3 cleavage is dependent on γ-secretase complex-mediated S3 cleavage, suggesting that γ-secretase function is closely related to Notch signaling (100). γ-Secretase inhibitors (GSIs) were first used in clinical trials for the treatment of Alzheimer’s disease (AD), but the trial was discontinued before completion of the phase III trial due to severe Notch-related adverse events (101). However, GSIs are still being widely investigated as cancer therapies in preclinical studies and have shown antitumor activity in a variety of cancers, such as hepatocellular carcinoma, where the use of the γ-secretase inhibitor DAPT or the combination of DAPT with the CD147-directed antibody HAb18 has been shown to be effective in the treatment of human HCC tumors transplanted in situ (104).
7.1 Therapeutic potential of notch signaling pathway-targeted inhibitors
The use of Notch signaling pathway inhibitors in clinical trials has shown great therapeutic potential, such as inhibition of S1, which reduces Ca2+ in the cellular endoplasmic reticulum, thereby inhibiting protein trafficking and altering translation, translocation and maturation of Notch1 and thus bepridil (105). Inhibition of S2 in HCC reduces Notch NICD release and improves the efficacy of anticancer drugs (106). Inhibition of the Notch pathway by miR-3163 targeting ADAM-17 increases the sensitivity of HCC cells to molecularly targeted drugs (107). The γ-secretase inhibitor DAPT in S3 can effectively treat human HCC tumors transplanted in situ (104). Researchers can design a series of Notch-targeted therapeutics for each stage of the Notch signaling pathway, and inhibition of the Notch signaling pathway can effectively control the progression of many cancers. However, because the Notch pathway also plays an important role in normal cells (108), the search for drugs that can effectively inhibit tumors without causing health effects has become a focus of future research. Scientists are currently working to develop more tumor-selective Notch inhibitors to improve therapeutic efficacy and reduce side effects. This will also be a future goal of Notch pathway inhibitors for clinical treatment.
8 The application prospect of notch signaling pathway in clinical progress
In summary, this review systematically elucidates the molecular mechanism of the Notch signaling pathway and its characterization in cancer. By integrating and analyzing the existing research, we demonstrate the diversity of the pathway and the complexity of its regulatory mechanisms, which can be exerted in different tissues or in the same tissue at different times. These features highlight the need to focus on key factors such as tissue specificity, prophase changes, downstream molecular effects and impact on normal tissues when studying the Notch pathway in cancer.
With the rapid development of genomics and bioinformatics technologies, scientists are not only able to more accurately identify aberrant expression patterns in the Notch pathway, but also to combine multi-omics data for quantitative analysis, which provides the theoretical basis for personalized clinical treatment strategies. However, overactivation of the Notch pathway is not a single hazard, and its intervention can lead to complex pathological responses, so efficacy must be carefully balanced against toxicity and side effects in practical application.
In addition, current research into the application of the Notch signaling pathway in cancer still faces many challenges, the most prominent of which are how to develop targeted drugs with high specificity and low toxicity, and how to effectively respond to the complex regulatory needs of different cancer types and pathological stages. In the future, we will combine gene editing technology, artificial intelligence algorithms and clinical data to further explore and analyses the potential of the Notch pathway in cancer therapy and provide new ideas for the development of targeted drugs and personalized therapeutic strategies.
Author contributions
HZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition. WH: Writing – review & editing, Conceptualization. ZJ: Writing – review & editing, Conceptualization. BL: Writing – review & editing, Data curation. YL: Writing – review & editing, Methodology. XW: Supervision, Writing – review & editing. HML: Investigation, Writing – review & editing. HLL: Software, Writing – review & editing. XT: Validation, Writing – review & editing. PL: Funding acquisition, Resources, Supervision, Validation, Writing – review & editing. PT: Writing – review & editing, Supervision, Conceptualization, Project administration. LY: Writing – review & editing, Supervision, Conceptualization, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Research funding provided by faculty member Peifeng Li.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dexter JS. The analysis of a case of continuous variation inDrosophila by a study of its linkage relations. Am Nat. (1914) 48:712–58. doi: 10.1086/279446
2. Zhou B, Lin W, Long Y, Yang Y, Zhang H, Wu K, et al. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct Tar. (2022) 7:95. doi: 10.1038/s41392-022-00934-y
3. Suresh S and Irvine AE. The Notch signaling pathway in nor mal and Malignant blood cell production. J Cell Commun Signal. (2015) 9:5–13. doi: 10.1007/s12079-015-0271-0
4. Falo-Sanjuan J and Bray SJ. Decoding the Notch signal. Dev Growth Diffe. (2020) 62:4–14. doi: 10.1111/dgd.12644
5. Gratton R, Tricarico PM, d'Adamo AP, Bianco AM, Moura R, Agrelli A, et al. Notch signaling regulation in autoinflammatory diseases. Int J Mol Sci. (2020) 21:8847–54. doi: 10.3390/ijms21228847
6. Wang W, Okajima T, and Takeuchi H. Significant roles of notch O-glycosylation in cancer. Molecules. (2022) 27:1783. doi: 10.3390/molecules27061783
7. Li X, Ji M, Zhong S, Zha Q, Xu J, Zhao J, et al. MicroRNA-34a modulates chemosensitivity of breast cancer cells to adriamycin by targeting notch1. Arch Med Res. (2012) 43:514–21. doi: 10.1016/j.arcmed.2012.09.007
8. Mazur PK, Einwächter H, Lee M, Sipos B, Nakhai H, Rad R, et al. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc Natl Acad Sci United States America. (2010) 107:13438–43. doi: 10.1073/pnas.1002423107
9. Jeong JY, Kang H, Kim TH, Kim G, Heo JH, Kwon AY, et al. MicroRNA-136 inhibits cancer stem cell activity and enhances the anti-tumor effect of paclitaxel against chemoresistant ovarian cancer cells by targeting Notch3. Cancer Lett. (2017) 386:168–78. doi: 10.1016/j.canlet.2016.11.017
10. Xiu M, Zeng X, Shan R, Wen W, Li J, Wan R, et al. Targeting Notch4 in cancer: molecular mechanisms and therapeutic perspectives. Cancer Manag Res. (2021) 13:7033–45. doi: 10.2147/CMAR.S315511
11. Siebel C and Lendahl U. Notch signaling in development, tissue homeostasis, and disease. J Physiol Rev. (2017) 97:1235–94. doi: 10.1152/physrev.00005.2017
12. Mu X, Agarwal R, March D, Rothenberg A, Voigt C, Tebbets J, et al. Notch signaling mediates skeletal muscle atrophy in cancer cachexia caused by osteosarcoma. J Sarcoma. (2016) 2016:3758162. doi: 10.1155/2016/3758162
13. Matsumoto K, Luther KB, and Haltiwanger RS. Diseases related to Notch glycosylation. Mol Asp. Med. (2021) 79:100938. doi: 10.1016/j.mam.2020.100938
14. Lake RJ, Grimm LM, Veraksa A, Banos A, and Artavanis-Tsakonas S. In vivo analysis of the Notch receptor S1 cleavage. PLoS One. (2009) 4:e6728. doi: 10.1371/journal.pone.0006728
15. Zolkiewska A. ADAM proteases: ligand processing and modulation of the Notch pathway. Cell Mol Life Sci. (2008) 65:2056–68. doi: 10.1007/s00018-008-7586-4
16. Adam J and Jafar-Nejad H. The roles of Notch signaling in liver development and disease. Biomolecules. (2019) 9:608. doi: 10.3390/biom9100608
17. De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. (1999) 398:518–22. doi: 10.1038/19083
18. Kim GS, Park HS, and Lee YC. OPTHiS identifies the molecular basis of the direct interaction between CSL and SMRT corepressor. Mol Cells. (2018) 41:842–52. doi: 10.14348/molcells.2018.0196
19. Kwon C, Cheng P, King IN, Andersen P, Shenje L, Nigam V, et al. Notch post-translationally regulates β-catenin protein in stem and progenitor cells. Nat Cell Biol. (2011) 13:1244–51. doi: 10.1038/ncb2313
20. Vinson KE, George DC, Fender AW, Bertrand FE, and Sigounas G. The Notch pathway in colorectal cancer. Int J Cancer. (2016) 138:1835–42. doi: 10.1002/ijc.v138.8
21. Kwon OJ, Zhang L, Wang J, Su Q, Feng Q, Zhang XH, et al. Notch promotes tumor metastasis in a prostate-specific pten-null mouse model. J Clin Invest. (2016) 126:2626–41. doi: 10.1172/JCI84637
22. Bazzoni R and Bentivegna A. Role of notch signaling pathway in glioblastoma pathogenesis. Cancers (Basel). (2019) 11:292. doi: 10.3390/cancers11030292
23. Hernandez Tejada FN, Galvez Silva JR, and Zweidler-Mckay PA. The challenge of targeting Notch in hematologic Malignancies. Front Pediatr. (2014) 2:54. doi: 10.3389/fped.2014.00054
24. Ren C, Yao Y, Han R, Huang Q, Li H, Wang B, et al. Cerebral ischemia induces angiogenesis in the peri-infarct regions via Notch1 signaling activation. Exp Neurol. (2018) 304:30–40. doi: 10.1016/j.expneurol.2018.02.013
25. Pal D, Tyagi A, Chandrasekaran B, Alattasi H, Ankem MK, Sharma AK, et al. Suppression of Notch1 and AKT mediated epithelial to mesenchymal transition by Verrucarin J in metastatic colon cancer. Cell Death Dis. (2018) 9:798. doi: 10.1038/s41419-018-0810-8
26. Arcaroli JJ, Tai WM, McWilliams R, Bagby S, Blatchford PJ, Varella-Garcia M, et al. A Notch 1 gene copy number gain is a prognostic indicator of worse survival and a predictive biomarker to a Notch1 targeting antibody in colorectal cancer. Int J Cancer. (2016) 138:195–205. doi: 10.1002/ijc.29676
27. Wang M, Wang J, Wang L, Wu L, and Xin X. Notch1 expression correlates with tumor differentiation status in ovarian carcinoma. Med Oncol. (2010) 27:1329–35. doi: 10.1007/s12032-009-9384-8
28. Defreitas S, Rowe M, Paculis L, and Jia D. Integration of bioinformatics approaches and experimental validations to understand the role of Notch signaling in ovarian cancer. J Vis Exp. (2020) 12(155). doi: 10.3791/60502
29. Hopfer O, Zwahlen D, Fey MF, and Aebi S. The Notch pathway in ovarian carcinomas and adenomas. Br J Cancer. (2005) 93:709–18. doi: 10.1038/sj.bjc.6602719
30. Hijioka H, Setoguchi T, Miyawaki A, et al. Upregulation of Notch pathway molecules in oral squamous cell carcinoma. Int J Oncol. (2010) 36:817–22. doi: 10.3892/ijo_00000558
31. Harishankar MK, Mohan AM, Krishnan AV, and Devi A. Downregulation of Notch 4- a prognostic marker in distinguishing oral verrucous carcinoma from oral squamous cell carcinoma. Braz J Otorhinolaryngol. (2019) 85:11–6. doi: 10.1016/j.bjorl.2017.09.005
32. Wu-Chou YH, Hsieh CH, Liao CT, Lin YT, Fan WL, Yang CH, et al. The pathological significance of Notch 1 in oral squamous cell carcinoma. Lab Invest. (2013) 93:1068–81. doi: 10.1038/labinvest.2013.95
33. Zhao YY, Yu GT, Xiao T, and Hu J. The Notch signaling pathway in head and neck squamous cell carcinoma:A meta- analysis. Adv Clin Exp Med. (2017) 26:881–7. doi: 10.17219/acem/64000
34. Sun Y, Gao X, Liu J, Kong QY, Wang XW, Chen XY, et al. Differential Notch1 and Notch2 ex pression and frequent activation of Notch signaling in gastric cancers. Arch Pathol Lab Med. (2011) 135:451–8. doi: 10.5858/2009-0665-OA.1
35. Du X, Cheng Z, Wang YH, et al. Role of Notch signaling pathway in gastric cancer: A meta-analysis of the literature. 世界胃肠 病学杂志. (2014) 20:9191–9. doi: 10.3748/wjg.v20.i27.9191
36. Almasri M, Amer M, Ghanej J, Mahmoud AM, Gaidano G, Moia R, et al. Druggable molecular pathways in chronic lymphocytic leukemia. Life. (2022) 12:283. doi: 10.3390/life12020283
37. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
38. Dai Y, Wilson G, Huang B, et al. Silencing of Jagged1 inhibits cell growth and inva sion in colorectal cancer. Cell Death Dis. (2014) 5:e1170. doi: 10.1038/cddis.2014.137
39. Siegel RL, Miller KD, Fuchs HE, and Jemal A Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
40. Alniaimi AN, Demorest-Hayes K, Alexander VM, Seo S, Yang D, Rose S, et al. Increased Notch1 expression is associated with poor overall survival in patients with ovarian cancer. Int J Gynecol Cancer. (2015) 25:208–13. doi: 10.1097/IGC.0000000000000359
41. Sasahira T, Kurihara-Shimomura M, Shimojjukoku Y, Shima K, and Kirita T. Searching for new molecular targets for oral squamous cell careinoma with a view to clinical implementation of precision medicine. J Pers Med. (2022) 12:413. doi: 10.3390/jpm12030413
42. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018:CLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394 –424. doi: 10.3322/caac.21492
43. Jiang YY, Lin DC, Mayakonda A, Hazawa M, Ding LW, Chien WW, et al. Targeting super-en-hancer-associated oncogenes in oesophageal squamous cell carcinoma. Gut. (2017) 66:1358–68. doi: 10.1136/gutjnl-2016-311818
44. Su SC, Lin CW, Liu YF, Fan WL, Chen MK, Yu CP, et al. Exome sequencing of oral squamous cell carcinoma reveals molecular subgroups and novel therapeutic opportunities. Theranostics. (2017) 7:1088–99. doi: 10.7150/thno.18551
45. Zheng Y, Wang Z, Ding X, Zhang W, Li G, Liu L, et al. A novel Notch 1 missense mutation (C1133Y) in the abruptex domain exhibits enhanced proliferation and invasion in oral squamous cell carcinoma. Cancer Cell Int. (2018) 18:6. doi: 10.1186/s12935-017-0496-5
46. Lin Y, Ueda J, Kikuchi S, Totsuka Y, Wei WQ, Qiao YL, et al. Comparative epidemiology of gastric cancer between Japan and China. World J Gastro-enterol. (2011) 17:4421–8. doi: 10.3748/wjg.v17.i39.4421
47. Kang H, An HJ, Song JY, Kim TH, Heo JH, Ahn DH, et al. Notch3 and Jagged2 contribute to gastric cancer development and to glandular differentiation asso ciated with MUC2 and MUC5AC expression. Histopathology. (2012) 61:576–86. doi: 10.1111/j.1365-2559.2012.04274.x
48. Wang Z, Li Y, and Sarkar FH. Notch signaling proteins: legitimate targets for cancer therapy. Curr Protein Pept Sci. (2010) 11:398–408. doi: 10.2174/138920310791824039
49. Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC, Li AF, et al. The activated Notch1 signal pathway is associated with gastric cancer progression through cy clooxygenase-2. Cancer Res. (2009) 69:5039–48. doi: 10.1158/0008-5472.CAN-08-4021
50. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. (2022) 36:1720–48. doi: 10.1038/s41375-022-01620-2
51. Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al. Chronic lymphocytic leukaemia:ESMO Clinical Practice Guidelines for diagnosis,treat⁃mentand follow⁃up. Ann Oncol. (2021) 32:23–33. doi: 10.1016/j.annonc.2020.09.019
52. Pozzo F, Bittolo T, Tissino E, Zucchetto A, Bomben R, Polcik L, et al. Multiple mechanisms of Notch 1 activation in chronic lymphocytic leukemia: Notch1 mutations and beyond. Cancers. (2022) 14:2997. doi: 10.3390/cancers14122997
53. Jelloul FZ, Yang R, Garces S, Kanagal-Shamanna R, Ok CY, Loghavi S, et al. Landscape of Notch 1 mutations and co-occurring biomarker alterations in chronic lymphocytic leukemia. Leuk Res. (2022) 116:106827. doi: 10.1016/j.leukres.2022.106827
54. D’Assoro AB, Leon-Ferre R, Braune EB, and Lendahl U. Roles of Notch signaling in the tumor microenvironment. Int J Mol Sci. (2022) 23:6241. doi: 10.3390/ijms23116241
55. Shi Q, Xue C, Zeng Y, Yuan X, Chu Q, Jiang S, et al. Notch signaling pathway in cancer: from mechanistic insights to targeted therapies. Sig Transduct Target Ther. (2024) 9:128. doi: 10.1038/s41392-024-01828-x
56. Timmerman LA, Grego-Bessa J, Raya A, Bertrán E, Pérez-Pomares JM, Díez J, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. (2004) 18:99–115. doi: 10.1101/gad.276304
57. Chen J, Zhang H, Chen Y, Qiao G, Jiang W, Ni P, et al. miR-598 inhibits metasta sis in colorectal cancer by suppressing JAG1/Notch2 path way stimulating EMT. Expl Cell Res. (2017) 352:104–12. doi: 10.1016/j.yexcr.2017.01.022
58. Sahoo S, Li Y, de Jesus D, Sembrat J, Rojas MM, Goncharova E, et al. Notch2 suppres⁃sion mimicking changes in human pulmonary hyper⁃tension modulates Notch1 and promotes endothelial cell proliferation. Am J Physiol Heart Circ Physiol. (2021) 321:H542–57. doi: 10.1152/ajpheart.00125.2021
59. Wang LL, Zhu XL, Han SH, and Xu L. Hypoxia upregu⁃ lates Notch 3 signaling pathway to promote endo⁃ thelial-mesenchymal transition in pulmonary artery endothelial cellsJ/OL. Evid Based Complement Alternat Med. (2021) 2021:1525619. doi: 10.1155/2021/1525619
60. Wang H, Huang X, Zhang J, Shao N, Chen LO, Ma D, et al. The expression of VEGF and DLL 4/Notch pathway molecules in ovarian cancer. Clin Chim Acta. (2014) 436:243–8. doi: 10.1016/j.cca.2014.06.005
61. Qi R, An H, Yu Y, Zhang M, Liu S, Xu H, et al. Notchl signaling inhibits growth of hu-man hepatocellular carcinoma through induction of cell cycle ar-rest and apoptosis. Cancer Res. (2003) 63:8323–9.
62. Wang C, Qi R, Li N, Wang Z, An H, Zhang Q, et al. Notch1 signaling sensitizes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human hepatocellular carcinoma cells by inhibiting Akt/Hdm2-mediated p53 degradation and up-regulating p53-dependent DR5 expression. J Biol Chem. (2009) 284:16183–90. doi: 10.1074/jbc.M109.002105
63. Konishi J, Yi F, Chen X, Vo H, Carbone DP, Dang TP, et al. Notch3 cooperates with the EGFR pathway to modulate apoptosis through the induction of bim. Oncogene. (2010) 29:589–96. doi: 10.1038/onc.2009.366
64. Ojha R, Tantray I, Rimal S, Mitra S, Cheshier S, Lu B, et al. Regulation of reverse electron transfer at mitochondrial complex i by unconventional notch action in cancer stem cells. Dev Cell. (2022) 57:260–76.e9. doi: 10.1016/j.devcel.2021.12.020
65. Huang S, Park J, Qiu C, Chung KW, Li SY, Sirin Y, et al. Jagged1/Notch2 controls kidney fibrosis via Tfam-mediated metabolic reprogramming. PLoS Biol. (2018) 16:e2005233. doi: 10.1371/journal.pbio.2005233
66. Greenburg G and Hay ED. Cytoskeleton and thyroglobulin expression change during transformation of thyroid epithelium to mesenchyme-like cells. Devel opment. (1988) 102:605–22. doi: 10.1242/dev.102.3.605
67. Das V, Bhattacharya S, Chikkaputtaiah C, Hazra S, and Pal M. The basics of epithelial-mesenchymal transition (EMT): a study from a structure, dynamics, and functional perspective. J Cell Physiol. (2019) 234:14535–55. doi: 10.1002/jcp.v234.9
68. Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. (2020) 21:341–52. doi: 10.1038/s41580-020-0237-9
69. Lu H, Wu C, Jiang XW, and Zhao Q. ZLDI-8 suppresses angiogenesis and vasculogenic mimicry in drug-resistant NSCLC in vitro and in vivo. Lung Cancer. (2023) 182:107279. doi: 10.1016/j.lungcan.2023.107279
70. Saad S, Stanners SR, Yong R, Tang O, and Pollock CA. Notch mediated epithe lial to mesenchymal transformation is associated with in creased expression of the Snail transcription factor. Int J Biochem Cell Biol. (2010) 42:1115–22. doi: 10.1016/j.biocel.2010.03.016
71. Zhang L, Sha J, Yang G, Huang X, Bo J, Huang Y, et al. Activation of Notch pathway is linked with epithelial-mesenchymal transition in prostate cancer cells. Cell Cycle. (2017) 16:999–1007. doi: 10.1080/15384101.2017.1312237
72. Ciszewski WM, Wawro ME, Sacewicz-Hofman I, and Sobierajska K. Cytoskeleton reorganization in endMT—The role in cancer and fibrotic diseases. Int J Mol Sci. (2021) 22:11607–7. doi: 10.3390/ijms222111607
73. Zhou X, Chen X, Cai JJ, Chen LZ, Gong YS, Wang LX, et al. Relaxin inhibits cardiac fibrosis and endothelial-mesenchymal transition via the Notch pathway. Drug Des Devel Ther. (2015) 9:4599–611. doi: 10.2147/DDDT.S85399
74. Ceneri E, De Stefano A, Casalin I, Finelli C, Curti A, Paolini S, et al. Signaling pathways and bone marrow microenvironment in myelodysplastic neoplasms. Adv Biol Regul. (2024) 95:101071. doi: 10.1016/j.jbior.2024.101071
75. Ran QS, Yu YH, Fu XH, and Wen YC. Activation of the Notch signaling pathway promotes neurovascular repair after traumatic brain injury. Neural Regener Res. (2015) 10:1258–64. doi: 10.4103/1673-5374.162758
76. Qiu XX, Chen L, Wang CH, Lin ZX, Chen BJ, You N, et al. The vascular Notch ligands Delta-like ligand 4 (DLL4) and Jagged1 (JAG1) have opposing correlations with microvascularization but a uniform prognostic effect in primary glioblastoma: A preliminary study. World Neurosurg. (2016) 88:447–58. doi: 10.1016/j.wneu.2015.10.058
77. Fraser HM, Hastings JM, Allan D, Morris KD, Rudge JS, Wiegand SJ, et al. Inhibition of delta-like ligand 4 induces luteal hypervascularization followed by functional and structural luteolysis in the primate ovary. Endocrinology. (2012) 153:1972–83. doi: 10.1210/en.2011-1688
78. Zhang XX, Zhang LL, Yang HL, and Wang XW Mechanism of Wnt/β-catenin signaling pathway in enhanced Malignant phenotype of non-small cell lung cancer induced by anti-angiogenesis therapy. Asian Pacific J Trop Med. (2016) 9:58–62. doi: 10.1016/j.apjtm.2015.12.012
79. Peter ME. Programmed cell death: Apoptosis meets necrosis. Nature. (2011) 471:310–2. doi: 10.1038/471310a
80. Lin L, Mernaugh R, Yi F, Blum D, Carbone DP, Dang TP, et al. Targeting specifie regions ofthe Notch3 ligand - binding domain induces apoptosis and inhibitstumr growth in lung cancer. Cancer Res. (2010) 70:632–8. doi: 10.1158/0008-5472.CAN-09-3293
81. Zhu Q, Li Y, Dong X, Yang Y, Wang H, Guo S, et al. Line - OlP5 loss regulates migration and invasion in MDA-MB-231 breast cancer cells by inhibiting YAP1/JAG1signaling. 0ncol Lett. (2020) 19:103–12. doi: 10.3892/ol.2019.11071
82. Sellers K, Allen TD, Bousamra M, Tan J, Méndez-Lucas A, Lin W, et al. Progress in the study of inherent im mune cell metabolic reprogramming to regulate immune homeo stasis in sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2019) 31:910–2. doi: 10.1038/s41416-019-0464-z
83. Warburg O The metabolism of carcinoma cells1. J Cancer Res. (1925) 9:148–63. doi: 10.1158/jcr.1925.148
84. Warburg O, Wind F, and Negelein E. The metabolism of tumors in the body. J Gen Physiol. (1927) 8:519–30. doi: 10.1085/jgp.8.6.519
85. Li D, Masiero M, Banham AH, and Harris AL. The Notch ligand Jagged1as a target for anti-tumor therapy. Front Oncol. (2014) 254:1–13. doi: 10.3389/fonc.2014.00254
86. Fu Y, Chang A, Chang L, Niessen K, Eapen S, Setiadi A, et al. Differential regulation of transforming growth factor β signaling pathways by Notch inhuman endothelial cells. J Biol Chem. (2009) 284:19452–62. doi: 10.1074/jbc.M109.011833
87. Zhang P, Li H, Yang B, Yang F, Zhang LL, Kong QY, et al. Biological significance and therapeutic implication of resveratrol-inhibited Wnt, Noteh and STAT3 signaling in cervical cancer cells. Genes Cancer. (2014) 5:154–64. doi: 10.18632/genesandcancer.v5i5-6
88. Pomeroy AE, Schmidt EV, Sorger PK, and Palmer AC. Drug independence and the curability of cancer by combination chemotherapy. Trends Cancer. (2022) 8:915–29. doi: 10.1016/j.trecan.2022.06.009
89. Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, et al. Acquisition of epithelial-mesen chymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the Notch signaling pathway. Cancer Res. (2009) 69:2400−7. doi: 10.1158/0008-5472.CAN-08-4312
90. Capodanno Y, Buishand FO, Pang LY, Kirpensteijn J, Mol JA, Argyle DJ, et al. Notch pathway inhibition targets chemo resistant insulinoma cancer stem cells. Endocr Relat Cancer. (2018) 25:131−44. doi: 10.1530/ERC-17-0415
91. McAuliffe SM, Morgan SL, Wyant GA, Tran LT, Muto KW, Chen YS, et al. Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc Natl Acad Sci USA. (2012) 109:E2939−48. doi: 10.1073/pnas.1206400109
92. Pandey P, Khan F, Singh M, Verma A, Kumar H, Mazumder A, et al. Study deciphering the crucial involvement of notch signaling pathway in human cancers. Endocrine Metab Immune Disord Drug Targets. (2023) 24(11):1241–53. doi: 10.2174/0118715303261691231107113548
93. Lewis HD, Leveridge M, Strack PR, Haldon CD, O'neil J, Kim H, et al. Apoptosis in T cell acute lymphoblastic leukemia cells after cell cycle arrest induced by pharmacological inhibition of Notch signa ling. Chem Biol. (2007) 14:209−19. doi: 10.1016/j.chembiol.2006.12.010
94. Beverly LJ, Felsher DW, and Capobianco AJ. Suppression of p53 by Notch in lymphomagenesis: implications for initiation and regression. Cancer Res. (2005) 65:7159−68. doi: 10.1158/0008-5472.CAN-05-1664
95. Khan F, Singh VK, Saeed M, Kausar MA, and Ansari IA. Carvacrol induced program cell death and cell cycle arrest in androgen−independent human prostate cancer cells via inhibition of Notch signalling. Anticancer Agents Med Chem. (2019) 19:1588−608. doi: 10.2174/1871520619666190731152942
96. Sun Z, Zhou C, Liu F, Zhang W, Chen J, Pan Y, et al. Inhibition of breast cancer cell survival by Xanthohumol via modulation of the Notch signaling pathway in vivo and in vitro. Oncol Lett. (2018) 15:908−16. doi: 10.3892/ol.2017.7434
97. Baldoni S, Del Papa B, Dorillo E, Aureli P, De Falco F, Rompietti C, et al. Bepridil exhibits anti-leukemic activity associated with Notch 1 pathway inhibition in chronic lymphocytic leukemia. Int J Cancer. (2018) 143:958–70. doi: 10.1002/ijc.v143.4
98. Lu HY, Zu YX, Jiang XW, Sun XT, Liu TY, Li RL, et al. Novel ADAM-17 inhibitor ZLDI-8 inhibits the proliferation and metastasis of chemo-resistant non-small-cell lung cancer by reversing Notch and epithelial mesenchymal transition in vitro and in vivo. Pharmacol Res. (2019) 148:104406. doi: 10.1016/j.phrs.2019.104406
99. Liu R, Qin XP, Zhuang Y, Zhang Y, Liao HB, Tang JC, et al. Glioblastoma recurrence correlates with NLGN 3 levels. Cancer Med. (2018) 7:2848–59. doi: 10.1002/cam4.2018.7.issue-7
100. Schroeter EH, Kisslinger JA, and Kopan R. Notch-1 signalling requires ligand induced proteolytic release of intracellular domain. Nature. (1998) 393:382–6. doi: 10.1038/30756
101. Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med. (2013) 369:341–50. doi: 10.1056/NEJMoa1210951
102. Pagliaro L, Marchesini M, and Roti G. Targeting oncogenic Notch signaling with SERCA inhibitors. J Hematol Oncol. (2021) 14:1–17. doi: 10.1186/s13045-020-01015-9
103. Lieber T, Kidd S, and Young MW. Kuzbanian-mediated cleavage of drosophila notch. Genes Dev. (2002) 16:209–21. doi: 10.1101/gad.942302
104. Yong YL, Zhang RY, Liu ZK, Wei D, Shang YK, Wu J, et al. Gamma-secretase complex-dependent intramembrane proteolysis of CD147 regulates the Notch1 signaling pathway in hepatocellular carcinoma. J Pathol. (2019) 249:255–67. doi: 10.1002/path.v249.2
105. Baldoni S, Del Papa B, Dorillo E, Aureli P, De Falco F, Rompietti C, et al. Bepridil exhibits anti-leukemic activity associated with Notch 1 pathway inhibition in chronic lymphocytic leukemia. Int J Cancer. (2018) 143:958–70. doi: 10.1002/ijc.v143.4
106. Lu HY, Chu HX, Tan YX, Qin XC, Liu MY, Li JD, et al. Novel ADAM-17 inhibitor ZLDI-8 inhibits the metastasis of hepatocellular carcinoma by reversing epithelial-mesenchymal transition in vitro and in vivo. Life Sci. (2020) 244:117343. doi: 10.1016/j.lfs.2020.117343
107. Yang B, Wang C, Xie H, Wang Y, Huang J, Rong Y, et al. microRNA-3163 targets ADAM-17 and enhances the sensitivity of hepatocellular carcinoma cells to molecular targeted agents. Cell Death Dis. (2019) 10:784. doi: 10.1038/s41419-019-2023-1
Keywords: notch signaling pathway, cancer, tumor development, therapeutic potential, clinical progress
Citation: Zhang H, Hang W, Jing Z, Liu B, Wang X, Li Y, Luo H, Lv H, Tao X, Timashev P, Li Y and Li P (2025) The role of notch signaling pathway in cancer: mechanistic insights, therapeutic potential, and clinical progress. Front. Immunol. 16:1567524. doi: 10.3389/fimmu.2025.1567524
Received: 27 January 2025; Accepted: 27 May 2025;
Published: 24 June 2025.
Edited by:
Lisa M. Minter, University of Massachusetts Amherst, United StatesReviewed by:
Sonu Kumar Gupta, Augusta University, United StatesSonia Batan, Augusta University, United States
Copyright © 2025 Zhang, Hang, Jing, Liu, Wang, Li, Luo, Lv, Tao, Timashev, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pei-Feng Li, cGVpZmVuZ2xpQHp6dWxpLmVkdS5jbg==
†These authors have contributed equally to this work
 Haokun Zhang
Haokun Zhang Wang Hang
Wang Hang ZhaoHui Jing1,2,3
ZhaoHui Jing1,2,3 Bingyan Liu
Bingyan Liu Xiaoxiao Wang
Xiaoxiao Wang Yilin Li
Yilin Li Huomin Luo
Huomin Luo Huilin Lv
Huilin Lv Peifeng Li
Peifeng Li