- 1Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 2Department of Surgery, Cooper University Hospital, Cooper Medical School of Rowan University, Camden, NJ, United States
- 3Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 4Department of Sarcoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 5Department of Translational Molecular Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 6Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 7Department of Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 8Department of Investigational Cancer Therapeutics, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
Background: Dedifferentiated liposarcoma (DDLPS) is one of the most common types of soft tissue sarcoma (STS) characterized by liposarcomatous differentiation and a predilection for the retroperitoneum. Despite the growing number of histology-specific immune checkpoint blockade (ICB) trials in STS, it is still difficult to identify the radiographic objective response rate (ORR) for DDLPS in the real world setting. This study aimed to evaluate the ORR and survival of patients with DDLPS treated with ICB at a single center.
Methods: We conducted a retrospective study of 31 patients with pathologically confirmed DDLPS treated with ICB at MD Anderson Cancer Center between 2018 and 2023. Patient demographics, disease characteristics, treatment history, and response to ICB were analyzed. Immunohistochemical analysis was performed on tumor samples to assess immune-related markers.
Results: ORR by RECIST 1.1 was 3.2% (n=1/31). Among all patients (n=31), 6% achieved partial radiographic response, while 39% had stable disease, and 55% showed progressive disease. Median progression-free survival (PFS) was 3.5 (95%CI:1.9, 4.7) months, and overall survival (OS) after ICB initiation was 19.7 (95%CI: 8.8, not reached) months. Patients without prior systemic therapy demonstrated better OS (p=0.004). Immunohistochemistry revealed no relationship between pre- or post-ICB expression of CD8, CD20, CD21 and PDL-1 and response.
Conclusion: While the response to ICB in DDLPS remains limited, specific immune markers may influence treatment outcomes. CD20/21 post-ICB appear more important for prognosis. Further research is warranted to identify predictive factors for ICB efficacy in DDLPS.
1 Background
Dedifferentiated liposarcoma (DDLPS) is a subtype of liposarcoma, and the 4th most common soft tissue sarcoma (STS), making up approximately 7.2% of all STS (1). In the 5th edition of the WHO Classification of Tumours of Soft Tissue and Bone, STS are categorized based on histogenesis and molecular characteristics into major groups, including adipocytic, fibroblastic/myofibroblastic, so-called fibrohistiocytic, smooth muscle, skeletal muscle, vascular, peripheral nerve sheath, uncertain differentiation, and undifferentiated sarcomas, reflecting advances in molecular pathology and diagnostic precision (2). Histologically, DDLPS falls under the adipocytic tumor category and tends to be moderate to high-grade with non-lipogenic, undifferentiated cells. DDLPS can arise de novo or from well differentiated liposarcoma (WDLPS) (3). Like WDLPS, DDLPS is characterized by 12q3-q15 amplification, associated with MDM2, HMGA2 and CDK4 gene amplification (3, 4). Primary DDLPS has a 44% 5-year disease specific survival, with microscopic margin negative (R0) surgery, which is the mainstay of treatment for localized extremity and retroperitoneal DDLPS, with radiotherapy recommended for high grade extremity tumors (4–6). Preoperative radiotherapy is not routinely recommended at initial presentation in high grade retroperitoneal DDLPS as it appears to have limited benefit based on a multicenter randomized trial (5, 7, 8). The role of systemic chemotherapy in the localized setting is under investigation in an international multicenter randomized trial, but recommendations of an anthracycline-based therapy is the standard first line therapy for patients with unresectable, metastatic disease (5, 9–12). Despite the recommendation for systemic therapy in metastatic disease, response rates are low ranging from 12% to 24% in the first and second line setting (9, 11, 13).
Therapy with immune checkpoint blockade (ICB) has gained traction in the treatment of STS given the success in other solid tumors such as melanoma and non-small cell lung cancer (14). Furthermore, certain STS subtypes, such as undifferentiated pleomorphic sarcoma and DDLPS, exhibit features suggesting potential responsiveness to ICB, including the presence of tumor-infiltrating lymphocytes, and Programmed Death-Ligand 1(PD-L1) expression (15–17). Several non-histology specific trials have reported 5% to 20% objective response rate (ORR) in patients with metastatic STS treated with ICB (18–22). Initially, SARC028, a multicenter trial of pembrolizumab in select STS histologies showed an objective response rate (ORR) of 20% (n=2/10) for patients with DDLPS but this ORR dropped to 10% (n=4/39) in the expansion cohort with a total of 40 patients (20, 22). Specific to DDLPS, the Alliance A091401 trial reported an 8% and 14% ORR for patients treated with nivolumab and nivolumab/ipilimumab, respectively (18, 20). Furthermore, Italiano and colleagues reported a 7.3% ORR and a 54.5% non-progression rate (NPR) in a pooled analysis of several sarcoma-specific ICB trials (21). Most recently, in a non-comparative phase 2 trial of neoadjuvant ICB in patients with resectable retroperitoneal DDLPS, Roland et al. reported an 8.8% median pathologic response with 38% relapse free survival (RFS) at 24 months (23).
Despite the growing number of histology specific ICB trials in STS, it is still difficult to identify the true response rate for DDLPS outside of a clinical trial. The primary objective of this study was to determine the radiographic ORR and survival with ICB treatment in patients with DDLPS. Secondary objectives include identifying clinicopathologic and histopathologic factors that may predict response to ICB-based therapy.
2 Methods
2.1 Study design
Data were retrospectively extracted from the institutional pharmacy database of 47 patients (≥ 18 years of age) with documented DDLPS treated with ICB at the University of Texas MD Anderson Cancer Center (MDACC) between January 1, 2018, and January 1, 2023. Patients who received ICB at an outside facility are not captured by this database, even if treatment is given based on MD Anderson physician’s recommendation. Inclusion criteria required pathological confirmation of DDLPS, diagnostic confirmation with MDM2 amplification by fluorescent in situ hybridization (FISH), and receipt of at least one ICB dose. Patients were excluded if they received ICB as part of an ongoing clinical trial without published results or received ICB for the treatment of a second malignancy. In total, twelve patients were excluded due to enrollment in ongoing clinical trials with unpublished results and an additional four were excluded for receiving ICB as treatment for a second malignancy.
Patient demographics including age, sex, race, ethnicity, and European Cooperative Oncology Group (ECOG) performance status at the start of ICB were collected. Disease and treatment specific characteristics such as disease stage at receipt of ICB, location of primary tumor, treatment history, clinical trial participation and treatment with combination ICB were obtained. Additional data collected were duration of treatment with ICB, last known follow-up or date of death, best response to ICB treatment and time to best response. The best radiographic response in patients was determined by two methods: the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) and clinical assessment as documented by the treating physician’s notes in the medical record (24, 25). ORR was defined as the percentage of patients who achieved either a complete response (CR) or a partial response (PR; at least a 30% decrease in the sum of the longest diameter (LD) of target lesions, taking as reference the baseline sum LD) as per treating physician notes or when available with precise measurements following RECIST 1.1. Data on treatment toxicity was also collected as documented by each patient’s treating physician.
2.2 Immunohistochemistry
All available hematoxylin and eosin (H&E) stains from a given surgical timepoint were reviewed by an experienced sarcoma pathologist to select a single formalin-fixed, paraffin-embedded sample for immunohistochemical (IHC) staining. Blocks were sectioned onto charged slides at 4-micron thickness and stained on a Leica BOND RX autostainer using the BOND Polymer Refine Detection kit. Primary antibody clones, suppliers, and dilutions used were as follows: PD-L1 (28-8, Dako, Ready-to-Use), CD8 (C8/144B, Life Technologies, 1:100), CD20 (L26, Dako, 1:1400), CD21 (2G9, Leica, 1:20). All IHC stains were evaluated by eye, except for CD8, which was scanned at 20x on an Aperio AT2 whole-slide scanner (Leica) and analyzed with HALO v3.5 image analysis platform (Indica Labs).
Assessing these specific markers provides deeper insight into the tumor microenvironment and its immune landscape, which directly impacts ICB efficacy (15). CD8+ T-cell infiltration reflects cytotoxic immune activity, with higher levels suggesting a greater likelihood of ICB response. PD-L1 expression serves as a checkpoint for immune evasion, with higher levels potentially predicting better responsiveness to PD-L1 blockade. CD20 and CD21 indicate the presence of B cells and tertiary lymphoid structures, which support adaptive immune responses. Together, these markers help define the immune phenotype of DDLPS, guiding therapeutic strategies and identifying patients who may benefit from immunotherapy.
This retrospective study was approved by the MDACC Institutional Review Board.
2.3 Statistical analysis
Categorical variables were reported as percentages and continuous variables as medians and interquartile ranges (IQR). Progression-free survival (PFS) was defined as the time from ICB start to progression, death, whichever occurred first, or last follow-up. Overall survival (OS) was defined as the time from start of ICB to death of any type or last follow-up (OSICB) and as the time from diagnosis to death or last follow up (OSDx). Landmark analysis at best response for OS and PFS was estimated by the Kaplan-Meier method (26). Log-rank test was used to compare the survival distribution by baseline characteristics. The hazard ratio was estimated in Cox proportional hazard regression model. All analyses were performed in SAS 9.4 and R 4.2.3.
3 Results
3.1 Patient characteristics
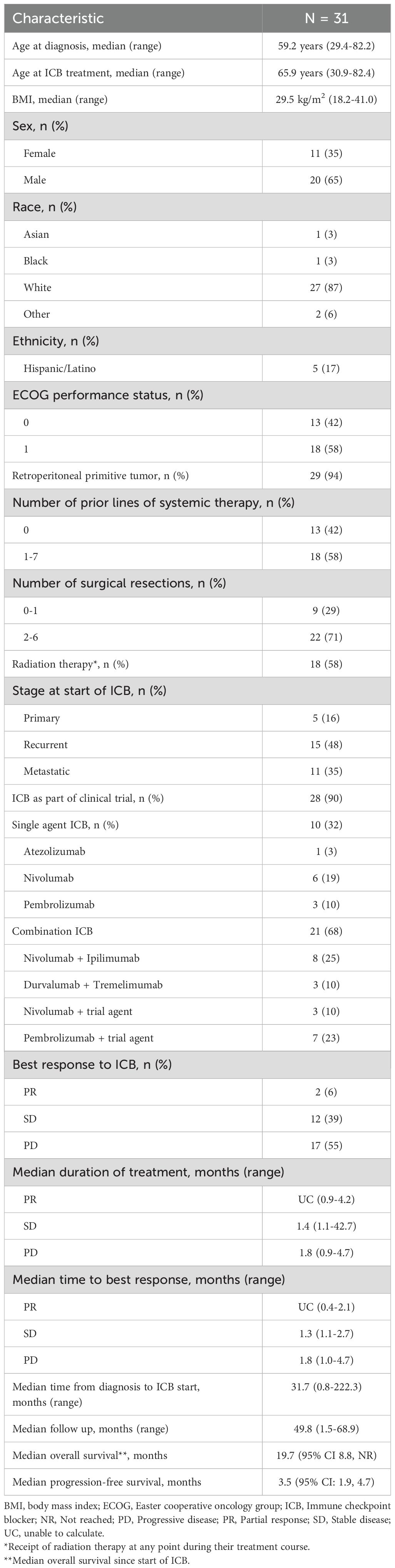
Thirty-one patients were eligible for final analysis. Most patients of the total cohort (n=28/31, 90%) received ICB as part of a clinical trial, 20 (65%) received single-agent ICB and 11 (35%) received combination ICB. Specific ICB agents are listed in Table 1. The patient demographic and clinical characteristics are listed in Table 1.
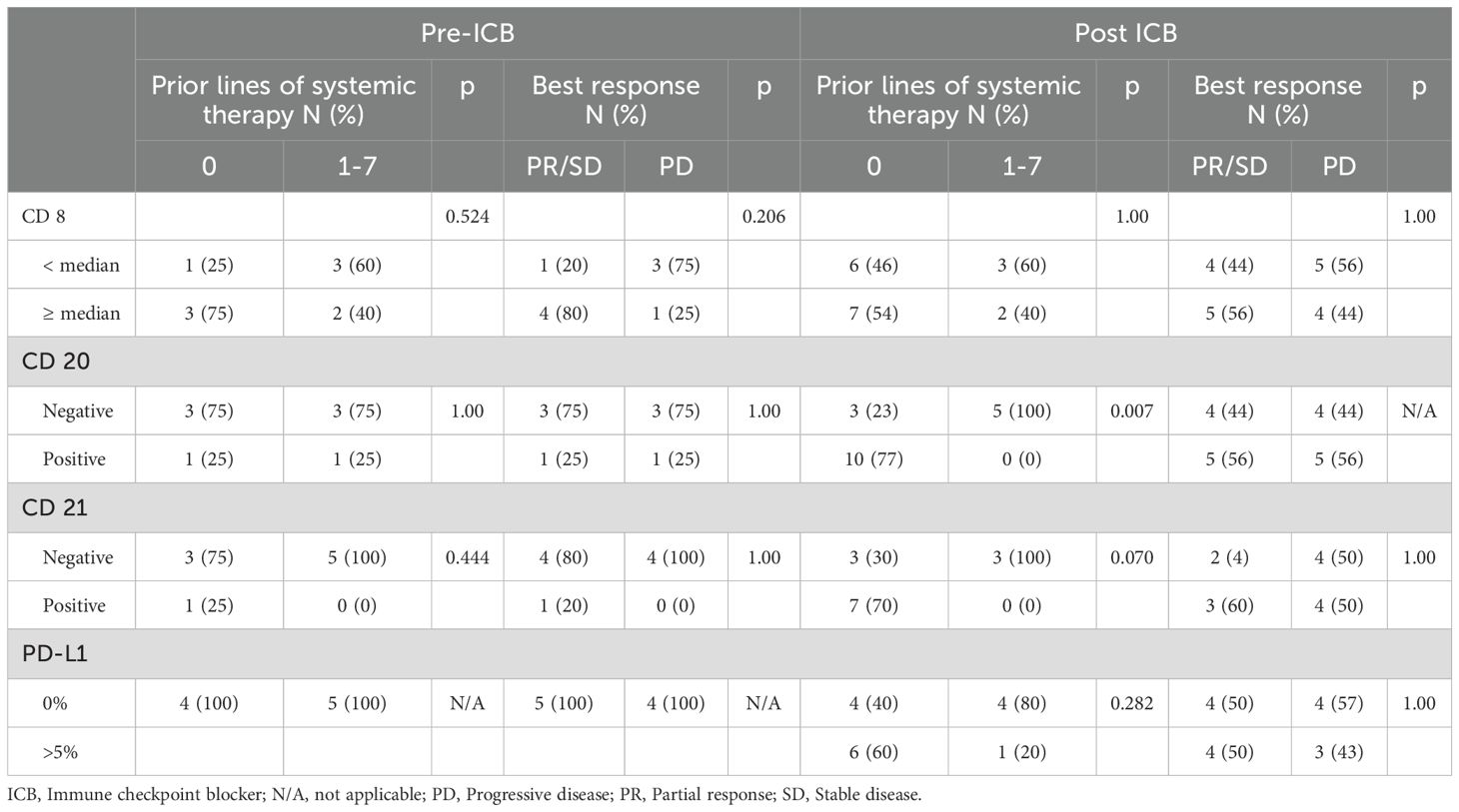
Thirteen (41.9%) patients received ICB as first-line treatment, among those 12 had primary or recurrent disease, and one had metastatic disease. Patients with primary or recurrent disease had a higher proportion of ICB as first-line treatment compared to metastatic patients (60% [n=12/20] versus 9% [n=1/11], p=0.008). Fifty-eight percent (n=18/31) of the patients received at least one line of systemic therapy prior to the start of ICB.
Of those who received systemic therapy prior to ICB (n=18), 28% (n=5) received a gemcitabine-based regimen and 67% (n=12) an anthracycline-based regimen as first-line therapy. There were 71% of patients (n=22/31) who had at least two surgical resections prior to treatment with ICB, while 26% (n=8/31) had one prior resection, and only one (3%) did not have surgery prior to the receipt of ICB. Among all patients (n=31), 58% (n=18) received radiation therapy (RT) as a component of their treatment course and 39% (n=12) received it prior to ICB. The population flow chart is represented in Supplementary Figure S1.
Regarding the pathologic characteristics of the tumors, due to the heterogeneity of pathological reporting over the years, the final pathology reports did not systematically mention the grade and percentage of the dedifferentiated component. However, tumor necrosis was recorded in 20 out of 31 patients in our total cohort. This information was obtained from the first confirmed DDLPS sample before any ICB treatment. In total, 7 patients had no tumor necrosis identified, 5 had less or equal than 10%, and 8 had more than 10% necrosis.
3.2 Treatment and response to ICB
In the whole cohort, looking at response per treating physician’s notes at time of evaluation, two (6%) patients were recorded to have a PR with duration of treatment of 1.2 and 4.2 months, 12/31 (39%) were reported to have stable disease (SD) with median duration of treatment of 1.4 months (range 1.1-42.7), and 17/31 (55%) had progressive disease (PD) with median duration of treatment of 1.8 months (range 0.9-4.7). Time to best response was 1.3 months (range 1.1-2.7) for patients with SD (Table 1). The short response durations mentioned above may be attributable to clinical hyper-progressive disease. The median duration of treatment was 3.8 months (range 1-42.7) in patients with SD who received ICB in the metastatic setting, and 4 patients had SD as best response in the metastatic cohort. In patients who received RT before ICB (n=12), 17% (n=2) had PR, 33% (n=4) had SD, and 50% (n=6) had PD.
Measurable responses per RECIST1.1 for the total cohort are demonstrated in the waterfall plot in Figure 1. The ORR per RESIST 1.1 was 3.2% (n=1/31).
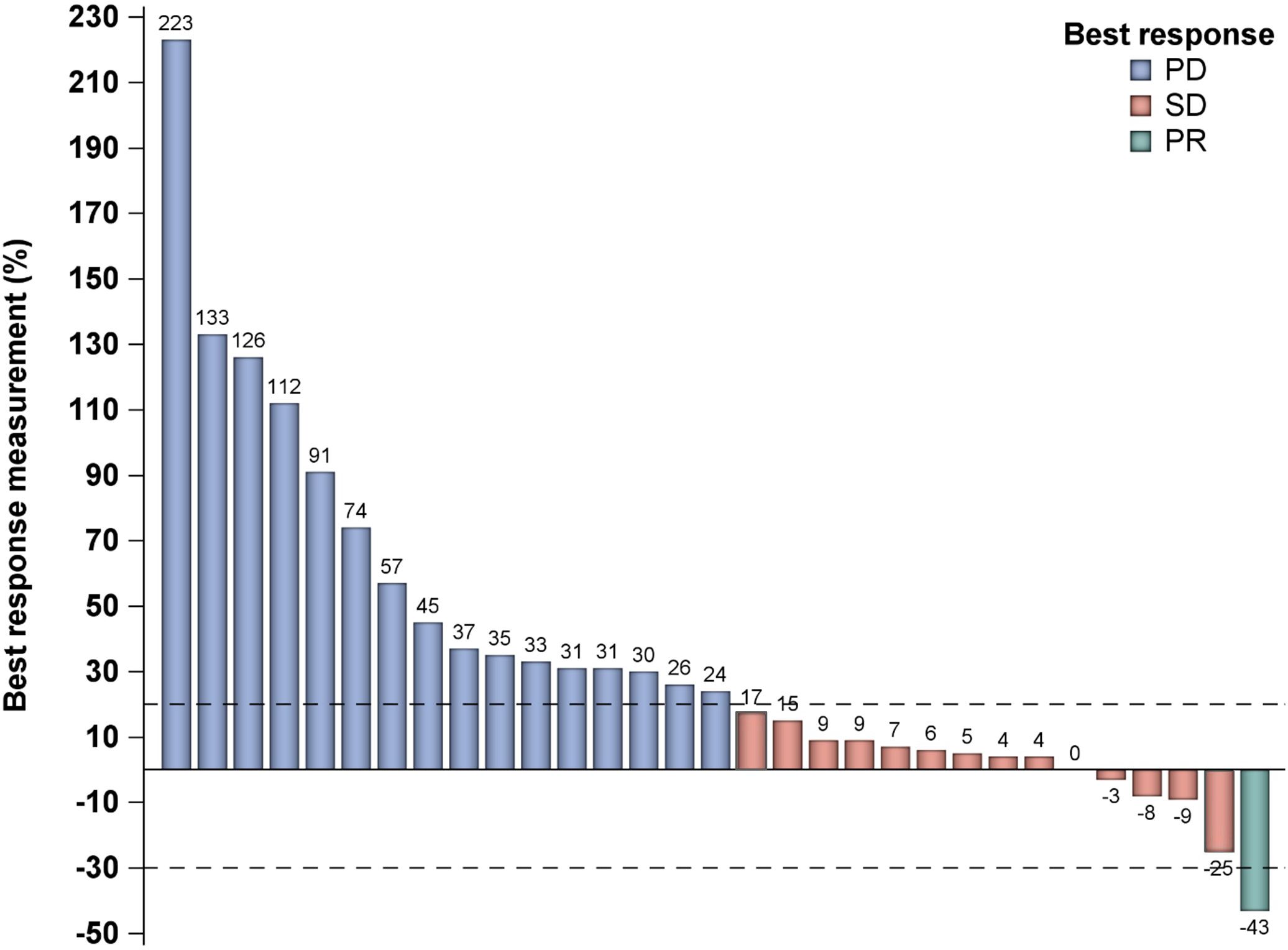
Figure 1. Waterfall plot demonstrating best response measurement. PD, progressive disease; PR, partial response; SD, stable disease.
3.3 Progression-free survival with ICB
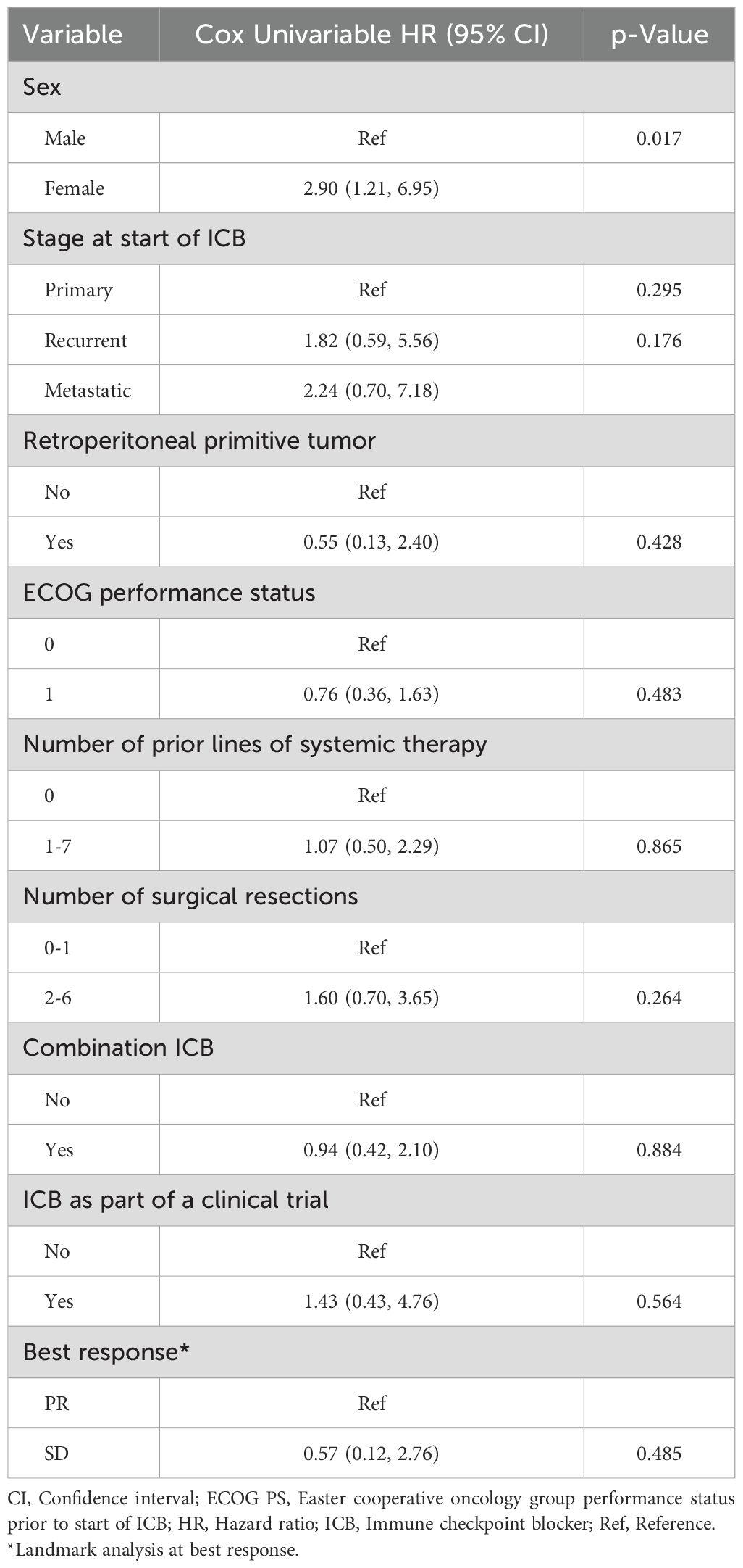
The median follow-up since treatment was 49.8 months (range 1.5-68.9). Among all patients, twenty-nine (94%) had disease progression with a median PFS of 3.5 months (95% CI: 1.9, 4.7). The 3-month PFS rate was 58.1% (95% CI: 39.0, 73.1). The median PFS in patients with PR could not be calculated as there were only two patients in this cohort and their PFS since response were 4.2 months and 15.4 months. The median PFS since response in patients with SD as best response was 9.0 months (95% CI: 2.1, 25.1) (Figure 2). Notably, among patients who had SD as best response, 7/12 had surgery after ICB, whereas for patients who had PR as best response, 1/2 had surgery after ICB. On univariate Cox regression analysis, best response and prior receipt of systemic therapy did not appear to affect PFS (HR 0.57, 95% CI 0.12, 2.76, p = 0.485 and HR 1.07, 95% CI 0.50, 2.29, p = 0.865, respectively, Table 2). The risk of progression among female patients was 2.90 (95% CI: 1.21, 6.95) times the risk among male patients (p=0.017, Table 2). Disease stage at start of ICB (recurrent: HR 1.82, 95% CI 0.59, 5.56, p =0.295; metastatic: HR 2.24, 95% CI 0.70, 7.18, p = 0.176), number of previous surgical resections (HR 1.60, 95% CI 0.70, 3.65, p = 0.264), receipt of ICB as part of a clinical trial (HR 1.43, 95% CI 0.43, 4.76, p = 0.564) and receipt of combination ICB (HR 0.94, 95% CI 0.42, 2.10, p = 0.884) were not associated with PFS on univariate analysis (Table 2). In addition, ECOG performance status 0 or 1 (HR 0.76, 95% CI 0.36,1.63, p = 0.48) and retroperitoneal location of primitive tumor (HR 0.55, 95% CI 0.13,2.40, p = 0.42) did not appear to be associated with PFS (Table 2). The median PFS in patients who received RT before ICB was 3.4 months (95% CI: 1.4, 15.4) whereas the median PFS for those who did not receive RT before ICB was 3.8 months (95% CI: 1.3, 8.0, p=0.79; Supplementary Figure S2).
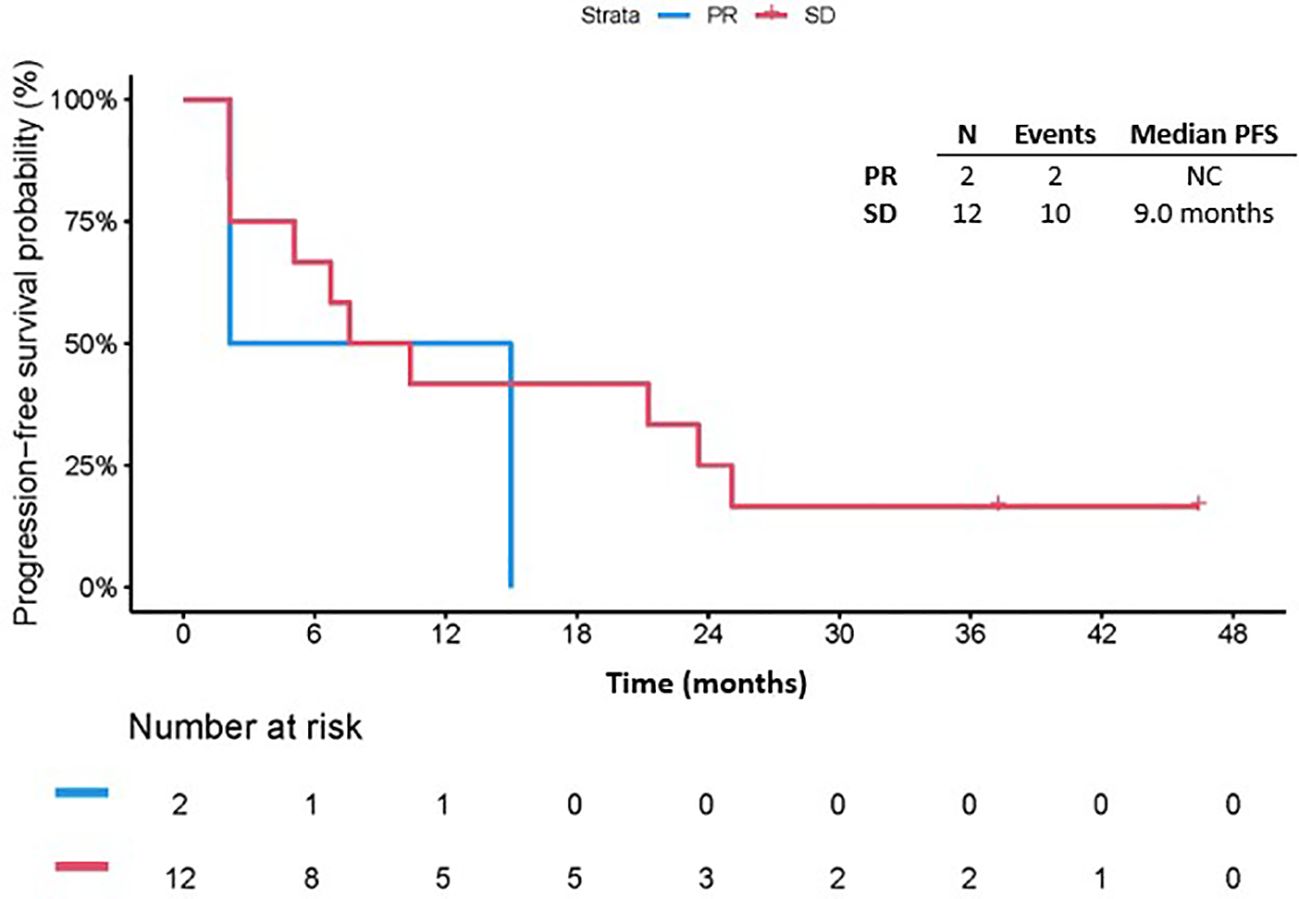
Figure 2. Progression free survival by number of best response. PFS, Progression free survival; PR, partial response; SD, stable disease; NC, not calculable.
Eight out of 17 patients with PD experienced clinical rapid progression, with PD at or prior to first planned imaging evaluation. Of these 8 patients, only 2 had received prior lines of systemic therapy along with surgery and radiation. Both patients had undergone 2 prior lines of systemic therapy, with their last line before ICB being Gemcitabine and Docetaxel for 47 and 27 days, compared to duration on ICB of 5 weeks for both patients. Therefore, none of the patients in this cohort met criteria for hyperprogressive disease. Five patients had only surgery before ICB, and one patient did not receive any treatment prior to ICB.
3.4 Overall survival with ICB
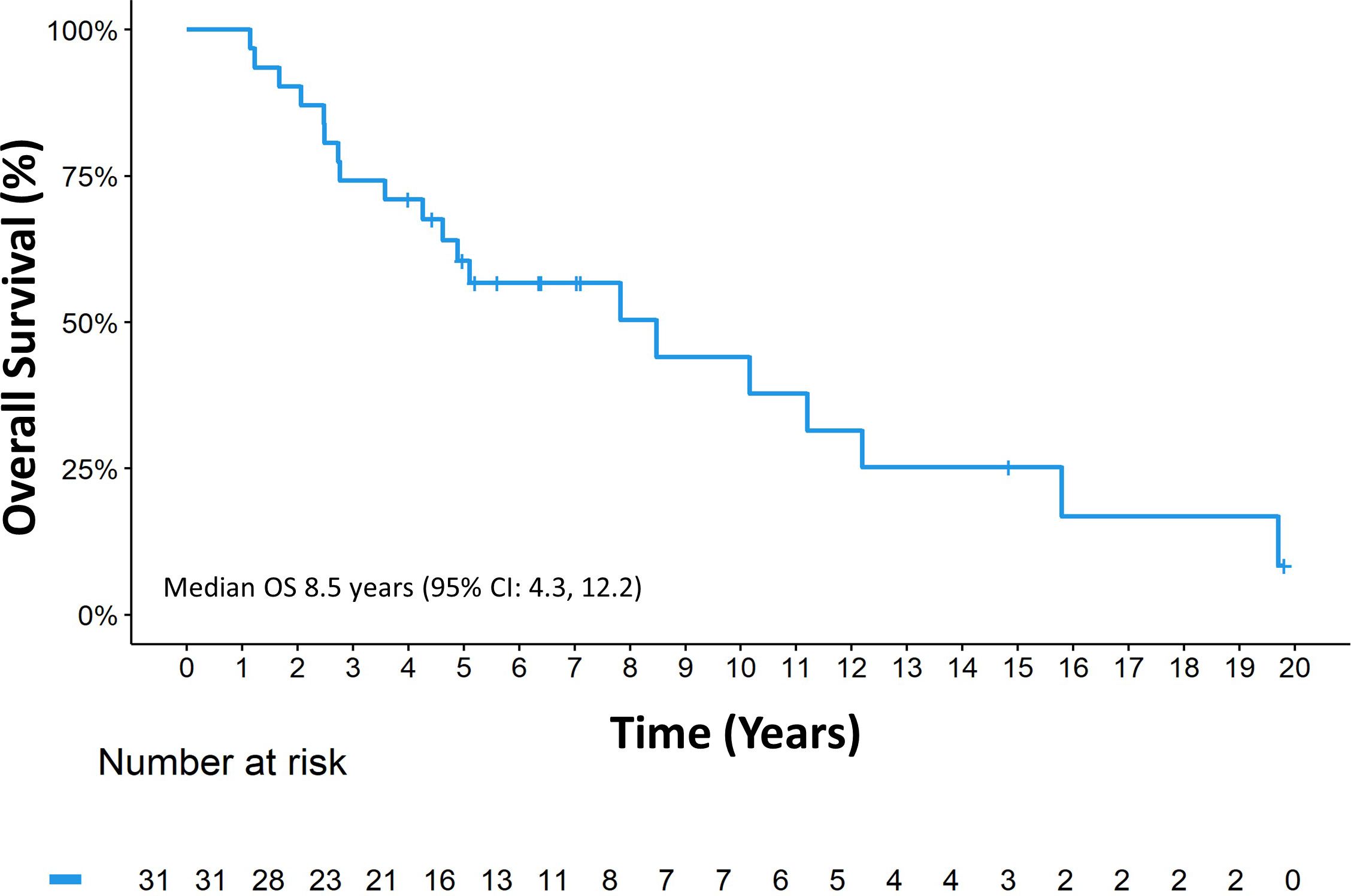
The median follow-up since diagnosis was 14.8 years (range 1.1, 19.8), the median OSDx was 8.5 years (95% CI: 4.3, 12.2) (Figure 3).
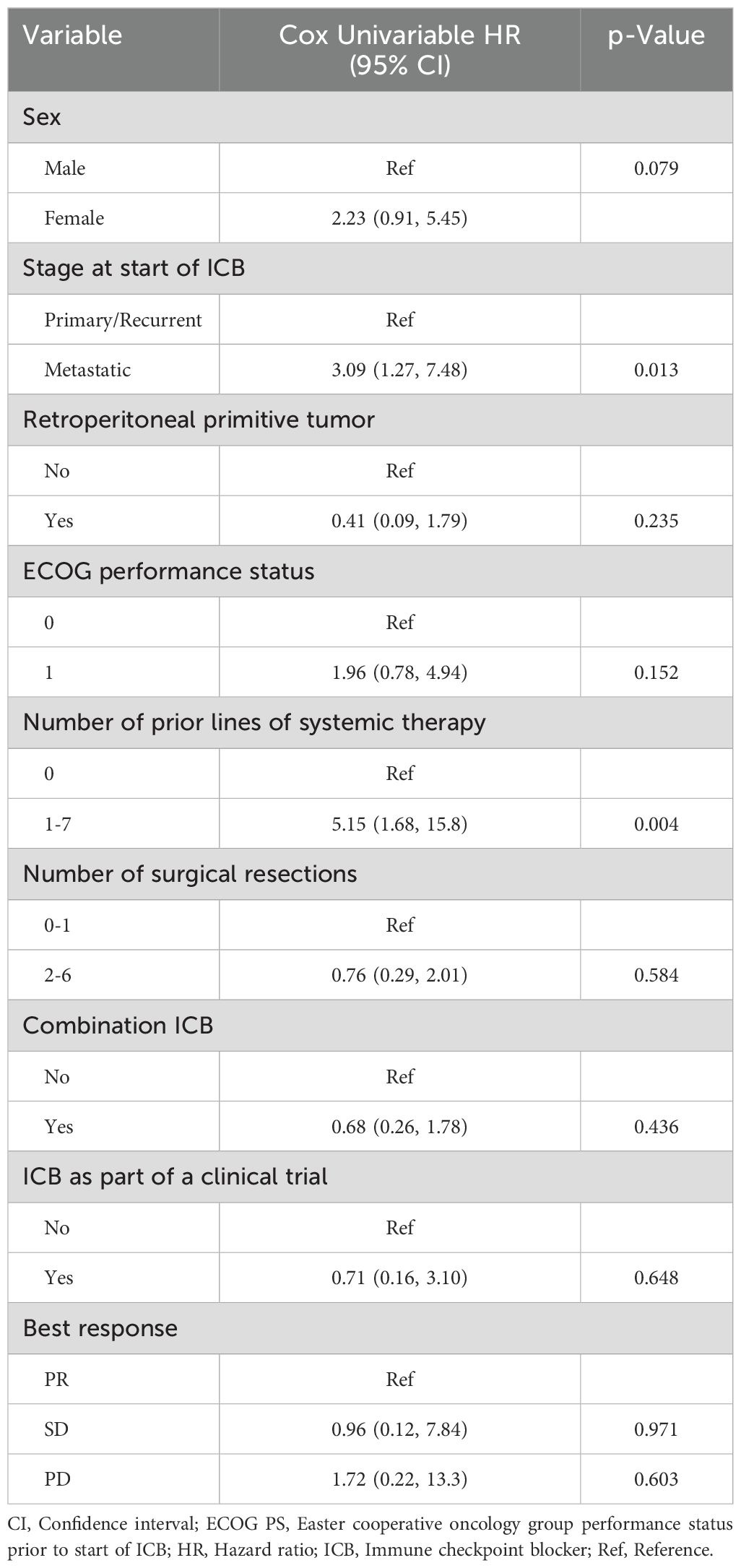
The median OSICB was 19.7 months (95% CI: 8.8, not reached). Receipt of ICB prior to any systemic therapy appeared to impact OSICB (p=0.0015) (Figure 4). In univariate Cox regression analysis for OSICB, patients who received at least one line of systemic therapy prior to ICB was associated with decreased survival, HR 5.15 (95% CI 1.68, 15.8. p = 0.004, Table 3) compared to patients who had no previous systemic therapy prior to ICB. Furthermore, there was no difference in OSDx between patients who received ICB as their first line of treatment versus later lines (p=0.86, Supplementary Figure S3).
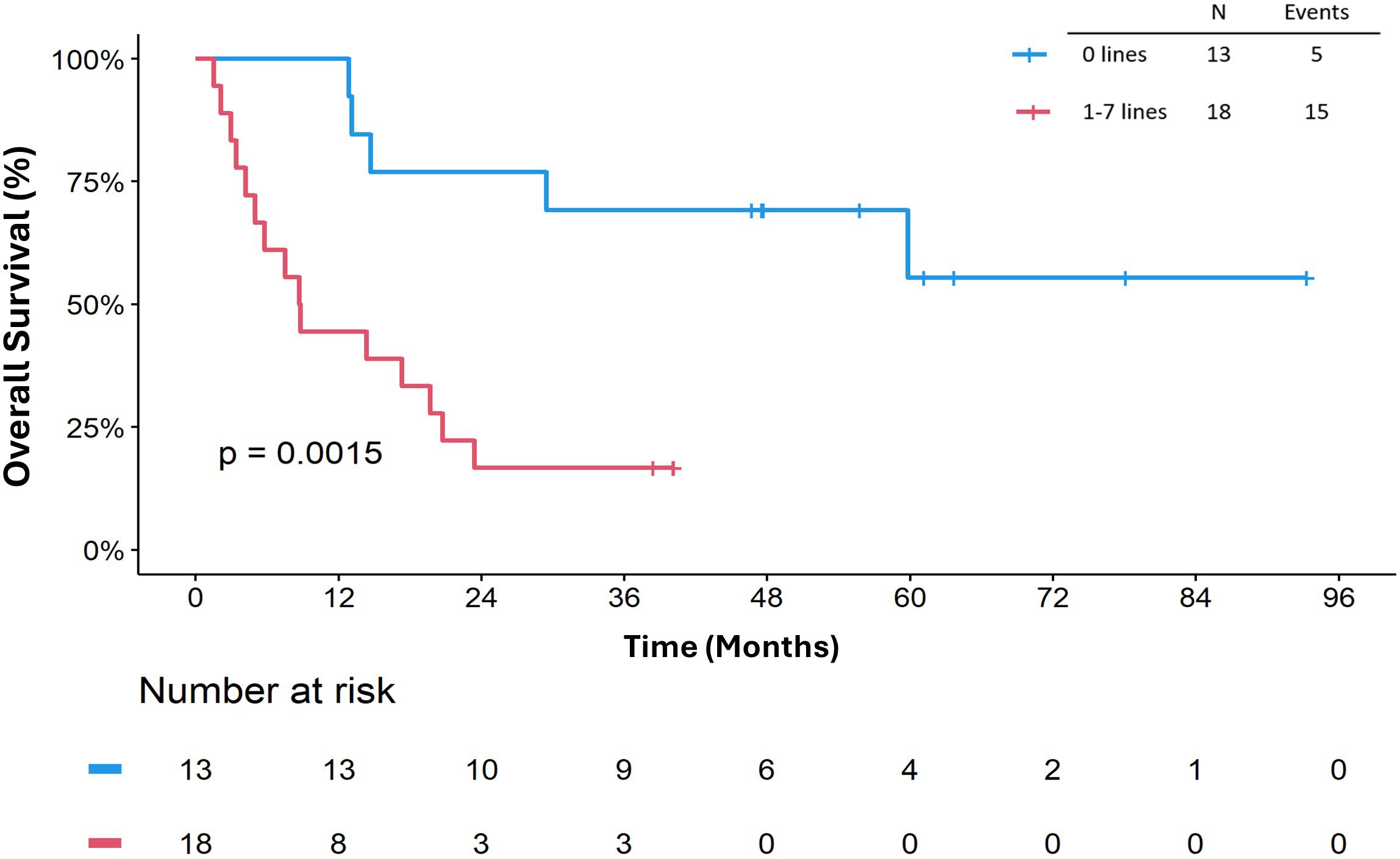
Figure 4. Overall survival (OSICB) by number of prior lines of systemic therapy. OSICB is defined as start from immune checkpoint blocker to death or last follow-up.
The risk of death among metastatic patients was 3.09 (95% CI: 1.27 7.48) times the risk of death among primary/recurrent patients (p = 0.013, Table 3). Sex (HR. 2.23, 95% CI 0.91, 5.45, p = 0.079), number of surgical resections (HR 0.76, 95% CI 0.29, 2.01, p = 0.584), receipt of ICB as part of a clinical trial (HR 0.71, 95% CI 0.16, 3.10, p = 0.648), and receipt of combination ICB (HR 0.68, 95% CI 0.26, 1.78, p = 0.436) did not appear to impact OSICB (Table 3). In addition, ECOG performance status 0 or 1 (HR 1.96, 95% CI 0.78, 4.94, p = 0.15) and retroperitoneal location of primitive tumor (HR 0.41, 95% CI 0.09, 1.79, p = 0.23) were not associated with OSICB (Table 3). Furthermore, best response to ICB did not appear to be associated with OSICB (p=0.48; Figure 5). When we stratified OSICB by response, the median OS since response was 43.5 months (95% CI: 11.6, not reached) in patients with SD, and 12.1 months (95% CI: 1.6, not reached) for those with PD (Figure 5).
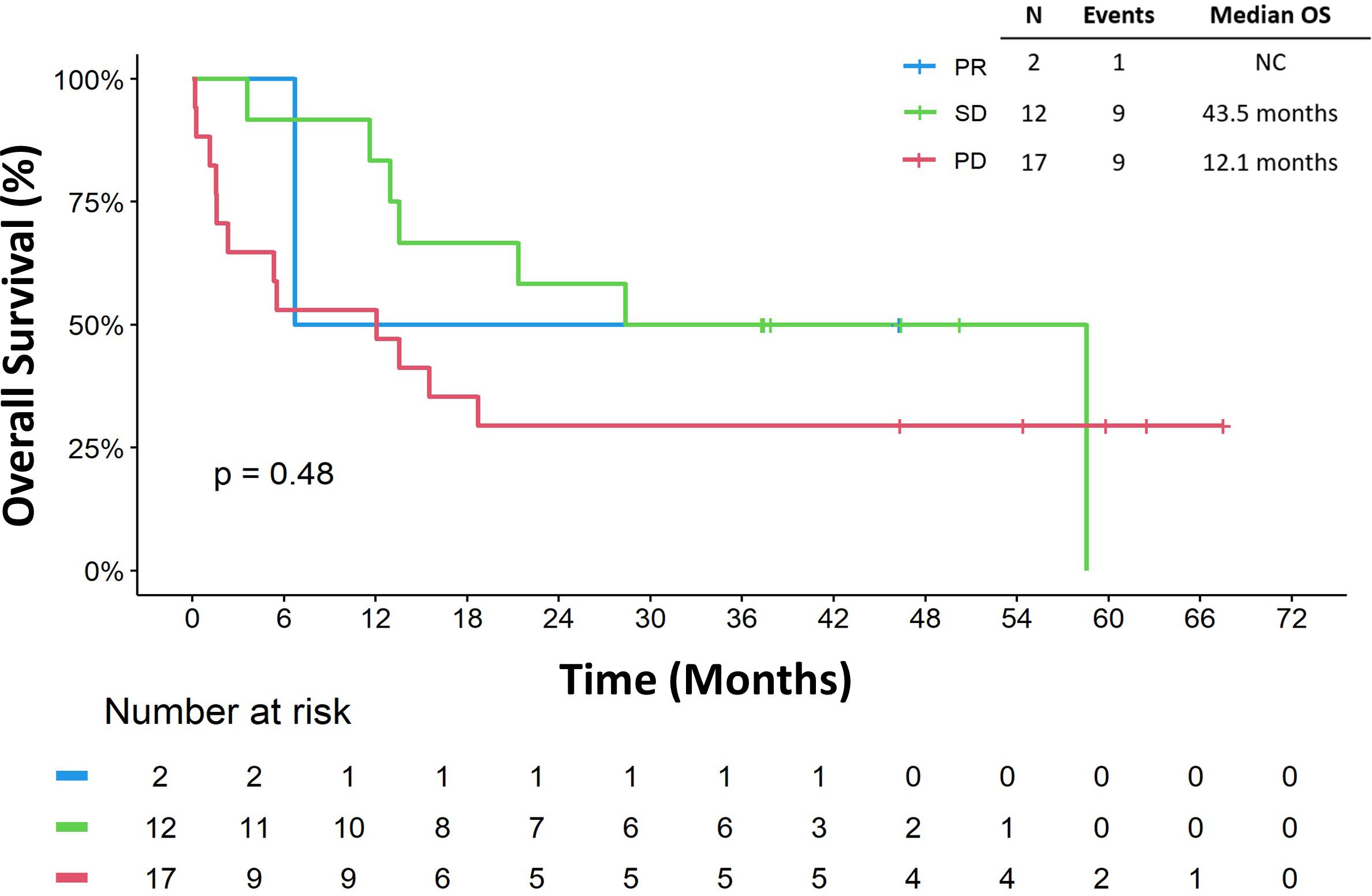
Figure 5. Overall survival by best response. Overall survival is defined as start from immune checkpoint blocker to death or last follow-up. NC, not calculable; OS, overall survival; PD, progressive disease; PR, partial response; SD, stable disease.
3.5 Toxicity
Twenty-five patients experienced treatment toxicity: 17 (54.8%) grade 1, 4 (12.9%) grade 2, and 4 (12.9%) grade 3 toxicities. No grade four toxicities were reported. Five (16.1%) patients stopped ICB treatment due to toxicity. The distribution of toxicities is reported in Supplementary Table S1.
3.6 Immunohistochemistry
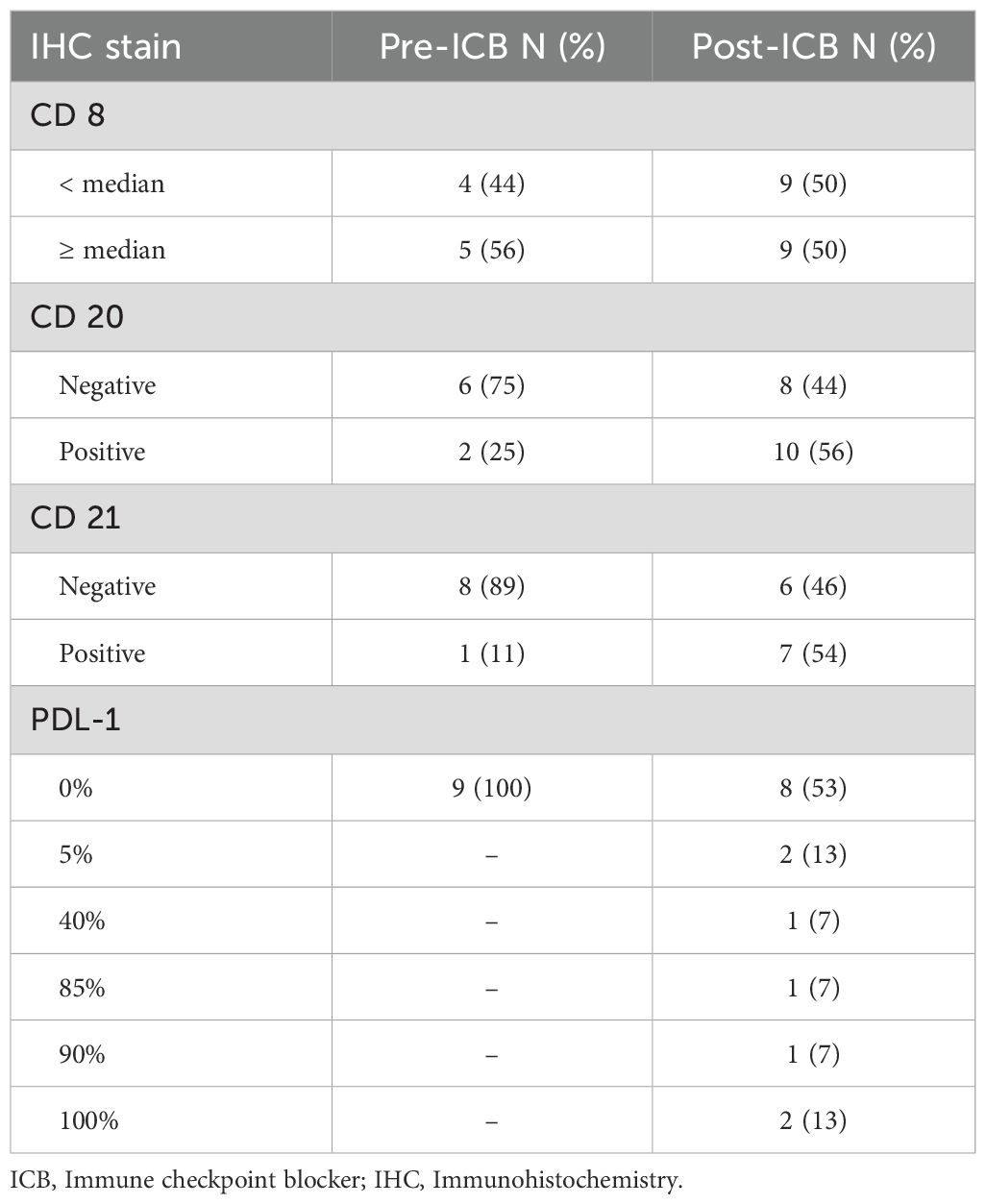
A total of 19 patients had tissue available for IHC staining for CD8, CD20, CD21, and PD-L1 (Table 4). Representative immunohistochemistry images of tumors are available in the Supplementary Material (Supplementary Figure S4).
Of the 19 patients with available IHC staining, nine had adequate specimens prior to ICB treatment (CD20 n = 8, CD21/CD8/PD-L1 n = 9) and 18 patients had specimens available after ICB treatment for analysis (CD8/CD20 n = 18, CD21 n= 13, PD-L1 n = 15). Among these 19 patients, 4 (21%) had primary disease, 12 (63%) had recurrent disease, and 3 (16%) had metastatic disease. Eight patients had both pre and post ICB tissue available for IHC stains. Among these patients, 50% (n=4/8) had PD as best response and 50% (n=4/8) had SD as best response. Half of these patients had one or more prior line of therapy and the other half had none. Half of these patients received ICB combination and 37.5% (n=3/8) received therapy between biospecimen collection. Due to the limited number of samples, the description below is purely numerical without statistical analyses to limit the false discovery.
Before ICB, patients without prior systemic therapy more often had higher CD8 densities (n = 3/4) compared to those previously treated (n = 2/5). CD20 was negative in 75% of patients with (n = 3/4) and without (n = 3/4) prior therapy. CD21 was absent in all tumors from previously treated patients (n = 5/5) and in 75% of untreated cases (n = 3/4). All nine tumors were PD-L1 negative pre-ICB. Tumors with CD8 densities below the cohort median had progressive disease (PD) in 75% of cases (n = 3/4), versus 20% (n = 1/5) in those with higher CD8 densities (Table 5).
Post-ICB, CD20 and CD21 were expressed in 56% (n = 10/18) and 54% (n = 7/13) of tumors, respectively, and PD-L1 ≥ 5% was observed in 47% (n = 7/15) of patients. Among patients initially negative for PD-L1, 50% (n = 4/8) became positive after ICB. CD20 was newly expressed in 37.5% (n = 3/8) and lost in 12.5% (n = 1/8); CD21 was newly expressed in 25% (n = 2/8; Table 6).
Table 6. Characteristics of patients in whom pre/post ICB tissue was available for Immunohistochemistry stains.
CD20 expression post-ICB was found in 77% (n = 10/13) of patients without prior systemic therapy and in none (n = 0/5) of those with prior treatment. CD8 ≥ median post-ICB was seen in 54% (n = 7/13) without prior therapy vs. 40% (n = 2/5) with prior therapy. CD21 was expressed in 70% (n = 7/10) of untreated and 0% (n = 0/3) of previously treated patients. PD-L1 ≥ 5% post-ICB was seen in 60% (n = 6/10) of untreated and 20% (n = 1/5) of treated patients (Table 5).
Patients with CD21+ tumors post-ICB had numerically better OS and PFS (Supplementary Figure S5). In exploratory survival analyses, CD21+ was associated with lower risk of death (HR = 0.03, 95% CI: 0.001–0.72, p = 0.031), as was CD8 ≥ median post-ICB (HR = 0.08, 95% CI: 0.01–0.67, p = 0.019).
4 Discussion
Despite the success of immunotherapy for treatment of many solid tumors, DDLPS continues to be a challenge. In this study we report our institutional experience treating 31 patients with DDLPS with ICB. Regarding the evaluation of patients’ responses, RESIST has not been reliably associated with PFS and OS in the sarcoma field. Therefore, we have used two methods of evaluation, including the physician’s assessment, which is often different from RECIST (27–29). Strictly according to RESIST, we noted an ORR of 3.2% (n=1/31, patient was treated with nivolumab single-agent), which is lower than what was reported in SARC028 (ORR of 10%) and Alliance A091401 (ORR of 8% with nivolumab and 14% with nivolumab/ipilimumab) (20, 22, 30). Our findings suggest that the ORR to ICB in DDLPS is overall low. Additionally, we report a median PFS of 3.4 months (95% CI: 1.6, 4.2) and a median OS of 20 months, similar to what has been previously reported (17, 18, 20, 22, 31).
Similar to data from SARC028, Alliance A091401, and their expansion cohorts, the majority (n = 20, 65%) of patients in our cohort were treated with single-agent ICB, with limited impact on OS and RFS (17, 18, 20, 22). In the pooled analysis of phase II trials by Italiano et al.,the authors reported better ORR across all histologies with PD1/PD-L1 single agent treatment (ORR 18.7, 95% CI 2.1 - 71.6.), vs. 11.4 (95% CI 3.5 - 31.4) with combination ICB and 14 (95% CI 0.5 - 84.2.) in those treated with combination ICB with non-immunological (21). The reported ORR for patients with DDLPS with single and combination ICB was 7.3 (95% CI 1.2 - 33.7) similar to our report (21).
Anthracycline-based chemotherapy remains the standard-of-care first-line therapy for DDLPS, with an ORR around 26% and in some series as high as 40% reported (13, 32, 33). Gemcitabine-based chemotherapy (34–36) has a PFS and OS of 9.2 and 18.8 months, respectively (37). Later lines of therapy are generally reported to have around 3 months PFS with ORR of around 10% (38–40), and thus ICB in DDLPS remains a third-line and beyond therapeutic option, when compared with current standards. However, there is biological and clinical rational for introducing ICB in earlier lines of treatment, as it has been reported to be more effective in this setting (23, 41, 42). Our study included 42% (n=13) of chemotherapy-naïve patients, but these patients did not seem to have improved outcomes on ICB compared to the rest of the cohort.
Growing evidence indicates that the use of ICB in the neoadjuvant setting enhances the systemic T-cell response to tumor antigens (41) and promotes complex changes in the immune microenvironment (23, 43, 44). DDLPS are amongst the STS with higher immune infiltration making ICB therapy a promising strategy (44–47). In the phase 2 SARC032 trial, investigators evaluated the efficacy of adding pembrolizumab to the standard of care for STS (48). The study demonstrated a notable improvement in disease-free survival (DFS) with neoadjuvant immunotherapy. Specifically, patients with DDLPS who received neoadjuvant immunotherapy alongside the standard of care had significantly higher DFS compared to the control group, which did not receive immunotherapy (HR 0.55, 95% CI: 0.13–2.35) (48). However, this was in the specific setting of extremity DDLPS, and it remains unclear whether the benefit from ICB is the same in retroperitoneal DDLPS or extremity DDLPS. We have previously reported a trial of nivolumab +/- ipilimumab in the neoadjuvant setting for retroperitoneal DDLPS and the ORR and DFS were less encouraging (23). Thus, the biggest challenge in the field remains the identification of biomarkers of response to ICB for DDLPS.
Our results suggest that tumor specimens obtained after ICB are more informative than specimens before ICB in terms of prognosis, a finding that our group has already reported in our Durvalumab and Tremelimumab trial in the advanced setting (49). CD20 and CD21 seem to be amongst the most important for prognosis, in fact, patients whose tumors did not express CD21 post-ICB tended to have worse survival. This is very consistent with findings of tertiary lymphoid structures and intra-tumoral B-cells as important drivers of response to ICB, although the mechanism driving this response remains to be identified (50).
Limitations to our study include the single institution, retrospective study design with a small number of participants leading to bias in the analysis. Additionally, this cohort remains heterogeneous in clinical characteristics such as previous lines of therapy and number of previous surgeries. Our translational studies are also limited by the availability of samples, with most analyses lacking statistical power to be considered significant. Furthermore, while the response of DDLPS to immune ICB has been evaluated in only a few patients, our study reflects similar limitations, making it too early to standardize or draw definitive conclusions regarding ICB efficacy. In our study, we chose to highlight clinical variables and won’t be focusing on IHC variables. We did not run a Cox multivariable analysis for the molecular markers due to the small sample size. CD21 has shown promise as a surrogate marker, but its utility remains limited due to the small sample size and lack of validation. Given these challenges, reliable biomarkers should be further assessed, and PD-L1 evaluation could be considered to enhance future analyses.
5 Conclusions
While the response to ICB in DDLPS remains limited, specific expression of immune markers may influence treatment outcomes. B-cell markers after treatment may be biomarkers of treatment benefit but more mechanistic insights and larger datasets are needed. More collaborative efforts are needed to pool data in patients with DDLPS to assess benefit of ICB and identify reliable markers of response.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by MD Anderson Cancer Center institutional review board (IRB 2023-0005). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because A waiver of informed consent was used for this study due to this being a retrospective chart review that involves no diagnostic or therapeutic intervention, as well as no direct patient contact. Study staff are unable to obtain consent from study subjects because due to the international patient population of MD Anderson, it would be impractical to obtain informed consent from individual patients prior to beginning the protocol. Additionally, it would bias the study if it were restricted to only patients whom we were able to contact. It would not be practical to conduct this research without this waiver if the status of the patient were unknown, i.e., whether they are alive or deceased, and it is difficult to trace the whereabouts of all patients.
Author contributions
MT: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. CL: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. MZ: Writing – original draft, Writing – review & editing. RL: Writing – review & editing. DI: Writing – review & editing. KW: Writing – review & editing. EK: Writing – review & editing. MZ: Writing – review & editing. CS: Writing – review & editing. KH: Writing – review & editing. AC: Writing – review & editing. AB: Writing – review & editing. BG: Writing – review & editing. AF: Writing – review & editing. DM: Writing – review & editing. AY: Writing – review & editing. MN: Writing – review & editing. DA: Writing – review & editing. AL: Writing – review & editing. RR: Writing – review & editing. SP: Writing – review & editing. VR: Writing – review & editing. AJL: Writing – review & editing. CR: Writing – review & editing. NS: Writing – review & editing. EN: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. EN acknowledges grant funding from Fondation pour la Recherche Medicale, Fondation Nuovo Soldati, QuadW Foundation, K12 Paul Calabresi (grant 5K12CA088084), Robert Winn Career Development Award 2024 and the BMS foundation. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P50CA272170, P30CA016672 and used the Biostatistics Resource Group. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1567736/full#supplementary-material
References
1. Blay JY, Honoré C, Stoeckle E, Meeus P, Jafari M, Gouin F, et al. Surgery in reference centers improves survival of sarcoma patients: a nationwide study. Ann Oncol. (2019) 30:1143–53. doi: 10.1093/annonc/mdz124
2. Board WCoTE. WHO Classification of Tumours of Soft Tissue and Bone. 5th. Lyon, France: IARC Press (2020).
3. Watson S, Gruel N, Le Loarer F. New developments in the pathology and molecular biology of retroperitoneal sarcomas. Eur J Surg Oncol. (2023) 49:1053–60. doi: 10.1016/j.ejso.2022.02.005
4. Crago AM, Singer S. Clinical and molecular approaches to well differentiated and dedifferentiated liposarcoma. Curr Opin Oncol. (2011) 23:373–8. doi: 10.1097/CCO.0b013e32834796e6
5. Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Ann Oncol. (2021) 32:1348–65. doi: 10.1016/j.annonc.2021.07.006
6. Crago AM, Dickson MA. Liposarcoma: multimodality management and future targeted therapies. Surg Oncol Clin N Am. (2016) 25:761–73. doi: 10.1016/j.soc.2016.05.007
7. Bonvalot S, Gronchi A, Le Péchoux C, Swallow CJ, Strauss D, Meeus P, et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. (2020) 21:1366–77. doi: 10.1016/S1470-2045(20)30446-0
8. Salerno KE, Alektiar KM, Baldini EH, Bedi M, Bishop AJ, Bradfield L, et al. Radiation therapy for treatment of soft tissue sarcoma in adults: executive summary of an ASTRO clinical practice guideline. Pract Radiat Oncol. (2021) 11. doi: 10.1016/j.prro.2021.04.005
9. Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. (2020) 70:200–29. doi: 10.3322/caac.21605
10. Seddon BM, Whelan J, Strauss SJ, Leahy MG, Woll PJ, Cowie F, et al. GeDDiS: A prospective randomised controlled phase III trial of gemcitabine and docetaxel compared with doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft tissue sarcomas (EudraCT 2009-014907-29). J Clin Oncol. (2015) 33:10500. doi: 10.1200/jco.2015.33.15_suppl.10500
11. Frezza AM, Stacchiotti S, Gronchi A. Systemic treatment in advanced soft tissue sarcoma: what is standard, what is new. BMC Med. (2017) 15:109. doi: 10.1186/s12916-017-0872-y
12. Surgery with or without neoadjuvant chemotherapy in high risk retroperitoneal sarcoma (STRASS2): ClinicalTrials.gov identifier: NCT04031677 (2023). Available online at: https://clinicaltrials.gov/study/NCT04031677?tab=tabletrial-contacts (Accessed July 22, 2024).
13. Livingston JA, Bugano D, Barbo A, Lin H, Madewell JE, Wang WL, et al. Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: defining the benefit and challenges of the standard. Sci Rep. (2017) 7:11836. doi: 10.1038/s41598-017-12132-w
14. Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. (2018) 62:29–39. doi: 10.1016/j.intimp.2018.06.001
15. Gingrich AA, Nassif EF, Roland CL, Keung EZ. The landscape of immunotherapy for retroperitoneal sarcoma. Curr Oncol (Toronto Ont). (2023) 30:2144–58. doi: 10.3390/curroncol30020165
16. Yiong CS, Lin TP, Lim VY, Toh TB, Yang VS. Biomarkers for immune checkpoint inhibition in sarcomas - are we close to clinical implementation? Biomarker Res. (2023) 11. doi: 10.1186/s40364-023-00513-5
17. Roulleaux Dugage M, Nassif EF, Italiano A, Bahleda R. Improving immunotherapy efficacy in soft-tissue sarcomas: A biomarker driven and histotype tailored review. Front Immunol. (2021) 12. doi: 10.3389/fimmu.2021.775761
18. D’Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. (2018) 19:416–26. doi: 10.1016/S1470-2045(18)30006-8
19. Banks LB, D’Angelo SP. The role of immunotherapy in the management of soft tissue sarcomas: current landscape and future outlook. J Natl Compr Cancer Netw. (2022) 20:834–44. doi: 10.6004/jnccn.2022.7027
20. Burgess MA, Bolejack V, Schuetze S, Tine BAV, Attia S, Riedel RF, et al. Clinical activity of pembrolizumab (P) in undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated/pleomorphic liposarcoma (LPS): Final results of SARC028 expansion cohorts. J Clin Oncol. (2019) 37:11015. doi: 10.1200/JCO.2019.37.15_suppl.11015
21. Italiano A, Bellera C, D’Angelo S. PD1/PD-L1 targeting in advanced soft-tissue sarcomas: a pooled analysis of phase II trials. J Hematol Oncol. (2020) 13:55. doi: 10.1186/s13045-020-00891-5
22. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. (2017) 18:1493–501. doi: 10.1016/S1470-2045(17)30624-1
23. Roland CL, Nassif Haddad EF, Keung EZ, Wang W-L, Lazar AJ, Lin H, et al. A randomized, non-comparative phase 2 study of neoadjuvant immune-checkpoint blockade in retroperitoneal dedifferentiated liposarcoma and extremity/truncal undifferentiated pleomorphic sarcoma. Nat Cancer. (2024) 5:625–41. doi: 10.1038/s43018-024-00726-z
24. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
25. Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. (2017) 18:e143–e52. doi: 10.1016/S1470-2045(17)30074-8
26. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. (1958) 53:457–81. doi: 10.1080/01621459.1958.10501452
27. Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. (2007) 25:2325–31. doi: 10.1200/JCO.2006.07.3411
28. Stacchiotti S, Collini P, Messina A, Morosi C, Barisella M, Bertulli R, et al. High-grade soft-tissue sarcomas: tumor response assessment–pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria - PubMed. Radiology. (2009) 251. doi: 10.1148/radiol.2512081403
29. Taieb S, Saada-Bouzid E, Tresch E, Ryckewaert T, Bompas E, Italiano A, et al. Comparison of response evaluation criteria in solid tumours and Choi criteria for response evaluation in patients with advanced soft tissue sarcoma treated with trabectedin: a retrospective analysis. Eur J Cancer (Oxford England: 1990). (2015) 51:202–9. doi: 10.1016/j.ejca.2014.11.008
30. Chen JL, Mahoney MR, George S, Antonescu CR, Liebner DA, Tine BAV, et al. A multicenter phase II study of nivolumab +/- ipilimumab for patients with metastatic sarcoma (Alliance A091401): Results of expansion cohorts. J Clin Oncol. (2020) 38:11511. doi: 10.1200/JCO.2020.38.15_suppl.11511
31. Lee AQ, Hao C, Pan M, Ganjoo KN, Bui NQ. Histologic and immunologic factors associated with response to immune checkpoint inhibitors in advanced sarcoma. Clin Cancer research1. (2025) 31:678–84. doi: 10.1158/1078-0432.CCR-23-1902
32. Italiano A, Toulmonde M, Cioffi A, Penel N, Isambert N, Bompas E, et al. Advanced well-differentiated/dedifferentiated liposarcomas: role of chemotherapy and survival. Ann Oncol. (2012) 23. doi: 10.1093/annonc/mdr485
33. Stacchiotti S, van der Graaf WTA, Sanfilippo RG, Marreaud SI, Van Houdt WJ, Judson IR, et al. First-line chemotherapy in advanced intra-abdominal well-differentiated/dedifferentiated liposarcoma: An EORTC Soft Tissue and Bone Sarcoma Group retrospective analysis. Cancer. (2022) 128. doi: 10.1002/cncr.v128.15
34. Somaiah N, Van Tine BA, Wahlquist AE, Milhem MM, Hill EG, Garrett-Mayer E, et al. A randomized, open-label, phase 2, multicenter trial of gemcitabine with pazopanib or gemcitabine with docetaxel in patients with advanced soft-tissue sarcoma. Cancer. (2021) 127. doi: 10.1002/cncr.v127.6
35. García-Del-Muro X, López-Pousa A, Maurel J, Martín J, Martínez-Trufero J, Casado A, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol. (2011) 29. doi: 10.1200/JCO.2010.33.6107
36. Martin-Broto J, Redondo A, Valverde C, Vaz MA, Mora J, Garcia Del Muro X, et al. Gemcitabine plus sirolimus for relapsed and progressing osteosarcoma patients after standard chemotherapy: a multicenter, single-arm phase II trial of Spanish Group for Research on Sarcoma (GEIS). Ann Oncol. (2017) 28:894–904. doi: 10.1093/annonc/mdx536
37. Thirasastr P, Lin H, Amini B, Wang WL, Cloutier JM, Nassif EF, et al. Retrospective evaluation of the role of gemcitabine-docetaxel in well-differentiated and dedifferentiated liposarcoma. Cancer Med. (2023) 12. doi: 10.1002/cam4.v12.4
38. Le Cesne A, Blay JY, Judson I, Van Oosterom A, Verweij J, Radford J, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol. (2005) 23. doi: 10.1200/JCO.2005.01.180
39. Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. (2016) 34:4282–93. doi: 10.1200/JCO.2015.62.4734
40. Schöffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, Choy E, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet (London England). (2016) 387. doi: 10.1016/S0140-6736(15)01283-0
41. Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Sci (New York NY). (2020) 367. doi: 10.1126/science.aax0182
42. Zhu N, Hou J. Assessing immune infiltration and the tumor microenvironment for the diagnosis and prognosis of sarcoma. Cancer Cell Int. (2020) 20. doi: 10.1186/s12935-020-01672-3
43. Recine F, Vanni S, Bongiovanni A, Fausti V, Mercatali L, Miserocchi G, et al. Clinical and translational implications of immunotherapy in sarcomas. Front Immunol. (2024) 15. doi: 10.3389/fimmu.2024.1378398
44. Albarrán V, Villamayor ML, Pozas J, Chamorro J, Rosero DI, San Román M, et al. Current landscape of immunotherapy for advanced sarcoma. Cancers. (2023) 15. doi: 10.3390/cancers15082287
45. Dancsok AR, Setsu N, Gao D, Blay JY, Thomas D, Maki RG, et al. Expression of lymphocyte immunoregulatory biomarkers in bone and soft-tissue sarcomas. Modern Pathol. (2019) 32. doi: 10.1038/s41379-019-0312-y
46. Wood GE, Meyer C, Petitprez F, D’Angelo SP. (2024). “Immunotherapy in sarcoma: current data and promising strategies”. In: American Society of Clinical Oncology Educational Book. Alexandria, Virginia, USA: American Society of Clinical Oncology (ASCO)., Vol. 44.
47. Tazzari M, Bergamaschi L, De Vita A, Collini P, Barisella M, Bertolotti A, et al. Molecular determinants of soft tissue sarcoma immunity: targets for immune intervention. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22147518
48. Mowery YM, Ballman KV, Hong AM, Schuetze SM, Wagner AJ, Monga V, et al. Safety and efficacy of pembrolizumab, radiation therapy, and surgery versus radiation therapy and surgery for stage III soft tissue sarcoma of the extremity (SU2C-SARC032): an open-label, randomised clinical trial. Lancet. (2024) 404. doi: 10.1016/S0140-6736(24)01812-9
49. Somaiah N, Conley AP, Parra ER, Lin H, Amini B, Solis Soto L, et al. Durvalumab plus tremelimumab in advanced or metastatic soft tissue and bone sarcomas: a single-centre phase 2 trial. Lancet Oncol. (2022) 23:1156–66. doi: 10.1016/S1470-2045(22)00392-8
Keywords: sarcoma, dedifferentiated liposarcoma, Anti-PD1, immunotherapy, immune-checkpoint inhibitors, survival
Citation: Torres MB, Leung CH, Zoghbi M, Lazcano R, Ingram D, Wani K, Keung EZ, Zarzour MA, Scally CP, Hunt KK, Conley A, Bishop AJ, Guadagnolo BA, Farooqi A, Mitra D, Yoder AK, Nakazawa MS, Araujo D, Livingston A, Ratan R, Patel S, Ravi V, Lazar AJ, Roland CL, Somaiah N and Nassif Haddad EF (2025) Dedifferentiated liposarcomas treated with immune checkpoint blockade: the MD Anderson experience. Front. Immunol. 16:1567736. doi: 10.3389/fimmu.2025.1567736
Received: 28 January 2025; Accepted: 11 April 2025;
Published: 30 April 2025.
Edited by:
Silvia Vanni, IRST, ItalyReviewed by:
Alessandro De Vita, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyZhuang Aobo, Xiamen University, China
Won Jin Jeon, Loma Linda University, United States
Bodil Elisabeth Engelmann, Herlev Hospital, Denmark
Natalia Georgantzoglou, Mass General Brigham, United States
Copyright © 2025 Torres, Leung, Zoghbi, Lazcano, Ingram, Wani, Keung, Zarzour, Scally, Hunt, Conley, Bishop, Guadagnolo, Farooqi, Mitra, Yoder, Nakazawa, Araujo, Livingston, Ratan, Patel, Ravi, Lazar, Roland, Somaiah and Nassif Haddad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elise F. Nassif Haddad, RUZOYXNzaWZAbWRhbmRlcnNvbi5vcmc=
 Madeline B. Torres1,2
Madeline B. Torres1,2 Cheuk Hong Leung
Cheuk Hong Leung Marianne Zoghbi
Marianne Zoghbi Emily Z. Keung
Emily Z. Keung Anthony Conley
Anthony Conley B. Ashleigh Guadagnolo
B. Ashleigh Guadagnolo Ahsan Farooqi
Ahsan Farooqi Devarati Mitra
Devarati Mitra Michael S. Nakazawa
Michael S. Nakazawa Andrew Livingston
Andrew Livingston Vinod Ravi
Vinod Ravi Alexander J. Lazar
Alexander J. Lazar Christina L. Roland
Christina L. Roland Neeta Somaiah
Neeta Somaiah Elise F. Nassif Haddad
Elise F. Nassif Haddad