- 1Division of Rheumatology, Center for Autoimmune Diseases, Japanese Red Cross Okayama Hospital, Okayama, Japan
- 2Department of Nephrology, Rheumatology, Endocrinology and Metabolism, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
- 3DNA Chip Research Inc., Medical Laboratory, Kawasaki, Japan
- 4Division of Rheumatology and Nephrology, Department of Internal Medicine, Nagaoka Red Cross Hospital, Niigata, Japan
- 5Division of Dermatology, Center for Autoimmune Diseases, Japanese Red Cross Okayama Hospital, Okayama, Japan
Background: Anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis (MDA5-DM) is associated with severe outcomes, primarily due to rapidly progressive interstitial lung disease (RP-ILD), which is often refractory to standard therapies such as calcineurin inhibitors (e.g., tacrolimus) combined with cyclophosphamide (TC-Tx). This study evaluated the efficacy of a novel multitargeted regimen combining baricitinib, rituximab, and tacrolimus (BRT-Tx) in improving survival outcomes for MDA5-DM patients with poor prognostic factors.
Methods: Fourteen MDA5-DM patients with multiple adverse prognostic factors were studied. Seven received the BRT-Tx regimen, and the remaining seven, previously treated with TC-Tx, served as historical controls. Twelve-month survival was assessed. Transcriptome analysis was performed for six patients (BRT=3, TC=3), beginning with cluster analysis to evaluate whether changes in peripheral blood gene expression varied according to treatment or prognosis. Gene ontology analysis characterized expression profiles in survivors and distinguished treatment effects. Alterations in the type I, II, and III interferon signatures were also assessed.
Results: In the TC-Tx group, four of seven patients succumbed to RP-ILD, whereas all seven BRT-Tx patients survived the 12-month observation period. Only one BRT-Tx patient required combined rescue therapies, including plasma exchange, and one case of unexplained limbic encephalitis (LE) occurred. Cytomegalovirus reactivation was observed in both groups (BRT: 5/7; TC: 6/7). Transcriptomic analysis revealed no treatment-specific clustering of differentially expressed genes (DEGs) before and after therapy. However, survivors and nonsurvivors formed distinct clusters, with survivors showing significant posttreatment suppression of B-cell-related gene expression. Moreover, interferon signature scores were significantly lower after treatment in survivors than in nonsurvivors. BRT-Tx effectively suppressed B-cell-mediated immune responses and maintained a low interferon signature, while TC-Tx resulted in nonspecific gene suppression, and in nonsurvivors, an elevated interferon signature was observed.
Conclusion: BRT-Tx has the potential to improve survival in MDA5-DM patients by effectively targeting hyperactive immune pathways. The combination of rituximab and tacrolimus is expected to disrupt B-cell–T-cell interactions and reduce autoantibody production, whereas baricitinib may suppress both IFN and GM-CSF signaling, regulating excessive autoimmunity mediated by cells such as macrophages. Unlike TC-Tx, BRT-Tx avoids cyclophosphamide-associated risks such as infertility and secondary malignancies. Future randomized controlled trials are warranted to validate its efficacy and safety.
Introduction
Anti-melanoma differentiation-associated gene 5 (MDA5)-positive DM/CADM is known to be fatal when complicated by rapid progressive interstitial lung disease (RP-ILD), with a prevalence of 39–100% (1).
MDA5 is a cytoplasmic RNA virus sensor that belongs to the RIG-I-like receptor family (2). Upon activation, it transmits signals downstream, leading to the production of inflammatory cytokines such as type I IFN and inducing the death of infected cells and antigen-specific immune responses (3).
In 2005, Sato et al. identified antibodies specific to clinically amyopathic dermatomyositis (CADM), termed anti-CADM140 antibodies (4). Subsequent research revealed MDA5 as the CADM140 autoantigen, leading to its designation as the anti-MDA5 antibody (5, 6). However, the mechanisms underlying the production of antibodies against cytoplasmic MDA5 remain unclear.
MDA5 recognizes RNA from viruses (7), such as picornaviruses or coronaviruses (1). In the case of COVID-19, rapid RNA replication occurs in the epithelial cells of the lungs and skin, which is detected by MDA5, a cytoplasmic RNA virus sensor (8). Interestingly, chest HRCT findings and blood cytokine profiles in COVID-19 patients closely resemble those observed in MDA5-DM patients (9–11).
In typical viral infections, IFN serves as a defense mechanism against viral replication.
However, in cases of severe pneumonia caused by COVID-19 and the pathogenesis of MDA5-DM, an aberrantly amplified immune response, alongside dysregulated interferon (IFN) signaling pathways, is postulated to play a critical role. This pathological dysregulation not only intensifies inflammatory processes but also contributes to the autoimmune manifestations characteristic of these conditions (1). In a previous study, we conducted transcriptome analysis of peripheral blood samples obtained prior to treatment from twelve patients with anti-ARS antibody-positive dermatomyositis and seven with MDA5-DM. Hierarchical clustering analysis clearly delineated the two disease groups, with MDA5-DM patients exhibiting increased expression of genes related to innate immune responses, antiviral defense mechanisms, and Fcγ receptor–mediated phagocytosis. Among a subset of five MDA5-DM patients with matched pre- and posttreatment samples (three survivors and two nonsurvivors), a marked posttreatment downregulation of genes involved in antiviral immunity—including type I and II interferon signaling as well as RIG-I-like receptor pathways—was observed exclusively in survivors (12).
Several poor prognostic factors are known to be related to MDA5-DM, including the presence of ILDs (13), an anti-MDA5 antibody titer ≥500 U/L (14), ferritin ≥636 ng/mL, LDH ≥355 U/L, CRP ≥0.8 mg/dL, KL-6 ≥1000 IU/mL (15), age>50 (16) and anti-SSA/Ro52 antibody positivity (17, 18). Additionally, a cluster analysis of MDA5-DM patients (n=121) on the basis of clinical symptoms revealed three subgroups. Among these patients, 18.1% were classified into the poor prognostic group; these patients had a high probability (93.3%) of having rapidly progressive ILD, and their 3-month survival rate was only 20%. In a prognostic prediction model based on clinical manifestations, the absence of Raynaud’s phenomenon and arthritis has been identified as a strong predictor of poor prognosis, with patients lacking both symptoms exhibiting the highest likelihood of belonging to the most unfavorable prognostic group (19).
Recently, several treatment options for MDA5-DM patients with ILD have been reported. Initial treatment with TC-Tx [high-dose glucocorticoids (GCs), calcineurin inhibitors such as tacrolimus (TAC), and intravenous cyclophosphamide (CYC)] was shown to improve 6-month survival compared with that of patients receiving conventional stepwise therapy (89% vs. 33%, P<0.0001) (20). In 2019, treatment with the Janus kinase (JAK) inhibitor tofacitinib (TOF) resulted in a 100% survival rate at 6 months, compared with 78% with conventional treatment (21); however, another group later conducted a similar regimen with a 6-month survival rate of 61.5% (22). There are also reports of increased survival rates with the combination of rituximab (RTX) (23, 24) and plasma exchange (PE) (25, 26) in patients who are resistant to conventional therapy. There is no established treatment for this disease, and although several reports of outcomes with treatment regimens exist, no studies have targeted patients who are predicted to have a poor prognosis.
We administered a combination therapy comprising high-dose GC, the JAK1/2 inhibitor baricitinib (BAR), RTX, and TAC (BRT-Tx) to patients with MDA5-DM, which presented multiple poor prognostic factors. In this study, alongside historical cases treated with TC-Tx, we explored changes in peripheral blood gene expression to elucidate how these alterations varied between survivors and nonsurvivors as well as between the BRT and TC therapeutic approaches.
Patients and methods
Patients
Fourteen Japanese patients who were diagnosed with MDA5-DM at Okayama Red Cross Hospital and Nagaoka Red Cross Hospital were included in this retrospective study. Seven patients received BRT-Tx (after February 2021), while seven historical patients were assigned to the TC-Tx group (after June 2015). The diagnosis of DM was made according to the 2017 Eular/ACR classification criteria for idiopathic inflammatory myopathies (IIMs) (27), and MDA5-DM was diagnosed in patients with characteristic skin symptoms (heliotrope sign, Gottron sign, periungual erythema, etc.) and positive anti-MDA5 antibodies (28). ILD was detected via high-resolution computed tomography (HRCT).
BRT-Tx was administered to patients who met two or more of the following eight poor prognostic factors: 1) the presence of ILD on chest HRCT consistent with MDA5-DM-related lung lesions (mandatory criterion) (13), 2) anti-MDA5 antibody titer ≥500 Index (14), 3) ferritin ≥636 ng/mL (13), 4) LDH≥355 units/L (13), 5) CRP≥0.8 mg/dL (15), 6) KL-6≥1000 IU/mL (15), 7) age>50 years (16) and 8) positivity for anti-Ro-52/SS-A antibodies (17, 18).
The data from MDA5-DM patients treated with BRT therapy were compared with those from historical patients treated with TC-Tx (TC: n = 7, BRT: n = 7). Among these, six patients were included in the gene expression analysis (TC-1 (fatal), TC-2, TC-3 (fatal), BRT-1, BRT-2, and BRT-3). The observation period spanned 12 months, with survival rates assessed at 12 months following the initiation of treatment.
BRT treatment regimen
High-dose GC: In principle, the treatment regimen includes administering 1000 mg/day of methylprednisolone (mPSL) for the first three days, beginning on day 1, followed by a transition to prednisolone (PSL) at a dosage of 1 mg/kg/day. The dose is then gradually reduced by 10–20% every 2–4 weeks on the basis of disease activity.
Baricitinib (BAR): BAR is typically initiated at a dose of 4 mg starting on day 1. If the estimated glomerular filtration rate (eGFR) is <60 ml/min, the starting dose is reduced to 2 mg. In cases where the ferritin concentration is <500 ng/mL and the disease is clinically controlled, the dose is halved, and treatment is discontinued upon normalization of ferritin levels. In instances of concomitant iron deficiency anemia, where ferritin levels may not reliably reflect the disease status, dose adjustments should be made on the basis of other clinical parameters, such as LDH or KL-6, via a comprehensive approach.
Rituximab (RTX): RTX is typically initiated on day 4, with a dose of 375mg/m2 per infusion once weekly for four consecutive weeks, followed by up to four additional infusions every 6 months, in principle. Subsequent dosing should be discontinued once a negative anti-MDA5 antibody status is confirmed.
Tacrolimus (TAC): The initial dose of TAC is 0.0375 mg/kg, which is administered orally twice daily. The target 12-hour trough blood concentration is subsequently maintained within the range of 5–10 ng/mL, with dose adjustments made on the basis of regular monitoring of the trough concentration (Table 1).
TC treatment regimen
High-dose GC: In principle, the treatment regimen includes the administration of 1000 mg/day mPSL for the first three days, beginning on day 1, followed by a transition to prednisolone (PSL) at a dosage of 1 mg/kg/day. The dose is then gradually reduced by 10–20% every 2–4 weeks on the basis of disease activity.
Cyclophosphamide (CYC): CYC is administered intravenously at a dose of 500 to 1000 mg/m², for a total of up to 10 times. Initially, it is given once every 2–4 weeks for six cycles, followed by administration every 4–8 weeks.
Tacrolimus (TAC): The initial dose of TAC is 0.0375 mg/kg, which is administered orally twice daily. The target 12-hour trough blood concentration is subsequently maintained within the range of 5–10 ng/mL, with dose adjustments made on the basis of regular monitoring of the trough concentration.
Combined rescue treatment
During the course of treatment, if disease relapse was observed, adjuvant therapies, such as (1) mPSL pulse therapy or increased dosages of steroids (2), plasma exchange (PE) (5–7 sessions), and (3) intravenous immunoglobulin therapy (IVIG), were added.
Statistical analysis
Statistical analyses were performed to evaluate differences in patient backgrounds between the TC-Tx and BRT-Tx groups. Statistical tests used include the Mann–Whitney U test, Fisher’s exact test, chi-square test, and log-rank test (for prognosis based on 1-year follow-up).
Gene analysis: RNA extraction
Peripheral blood samples were collected at baseline and three months after treatment, or—if applicable—within two weeks prior to death (i.e., approximately 2 to 2.5 months posttreatment) via PAXgene® Blood RNA tubes (Nippon Becton Dickinson, Tokyo, Japan). Total RNA was extracted via the PAXgene® Blood miRNA Kit (with DNase treatment) (QIAGEN K.K., Tokyo, Japan) following the manufacturer’s protocol. The quantity and quality of the total RNA were assessed via the SureSelect Strand-Specific RNA Kit (Agilent Technologies, Inc., CA, USA).
Next-generation sequencing
The RNA-Seq libraries were constructed via the Strand-Specific mRNA-Seq PolyA Kit (Agilent Technologies, Inc., CA, USA) according to the manufacturer’s protocol. For the constructed sequencing libraries, sequencing data were generated (75 bp single-end) via the NextSeq500 platform (Illumina, CA, USA). To process the sequencing reads, Illumina adapter sequences were removed via Trimmomatic v0.33. The trimmed reads were then mapped to the human genome (hg38) and reference transcripts via STAR v2.7.9. The reads aligned to the Ensembl v78 reference genes were quantified with Subread 2.0.3. The average number of reads per sample was set to approximately 20 million.
Transcriptome analysis of the peripheral blood
Differentially expressed genes (DEGs) were defined as those exhibiting an absolute log2 fold change ≥ 1 and a P-value < 0.05 between pre- and posttreatment samples. These DEGs were subsequently subjected to clustering analysis (29) to determine whether their expression patterns were influenced primarily by differences in treatment regimens or by clinical outcomes, such as survival or death. Next, to investigate the gene expression changes associated with differences in treatment regimens or outcomes, we performed Gene Ontology (GO) analysis (30) on the genes that were downregulated after treatment among the DEGs. Additionally, gene set variation analysis (GSVA) (31) was performed to explore the characteristics of the DEGs that differed between the BRT and TC treatment groups.
Interferon signatures
The IFN signature refers to the elevated gene expression induced by IFN. Among the IFN-stimulated genes (ISGs) associated with type 1 IFNs, 73 genes (gene set enrichment analysis (GSEA) C2 reactome dataset), type 2 IFN ISGs, 94 genes (GSEA C2 reactome dataset), and type 3 IFN ISGs, 10 genes (GSEA wikipathway gene set), were analyzed and scored (32).
For the scoring method, the gene expression of 6 patients (TC therapy: n=3, BRT therapy: n=3) before and 2–3 months after treatment was z scored (if the cohort/batch was different, the z score was obtained after correcting for batch by mean centering). A coefficient of +1 for high expression and -1 for low expression was assigned to each gene. The z score was multiplied by the coefficient and summed to obtain the IFN signature score.
Results
Characteristics of patients at baseline
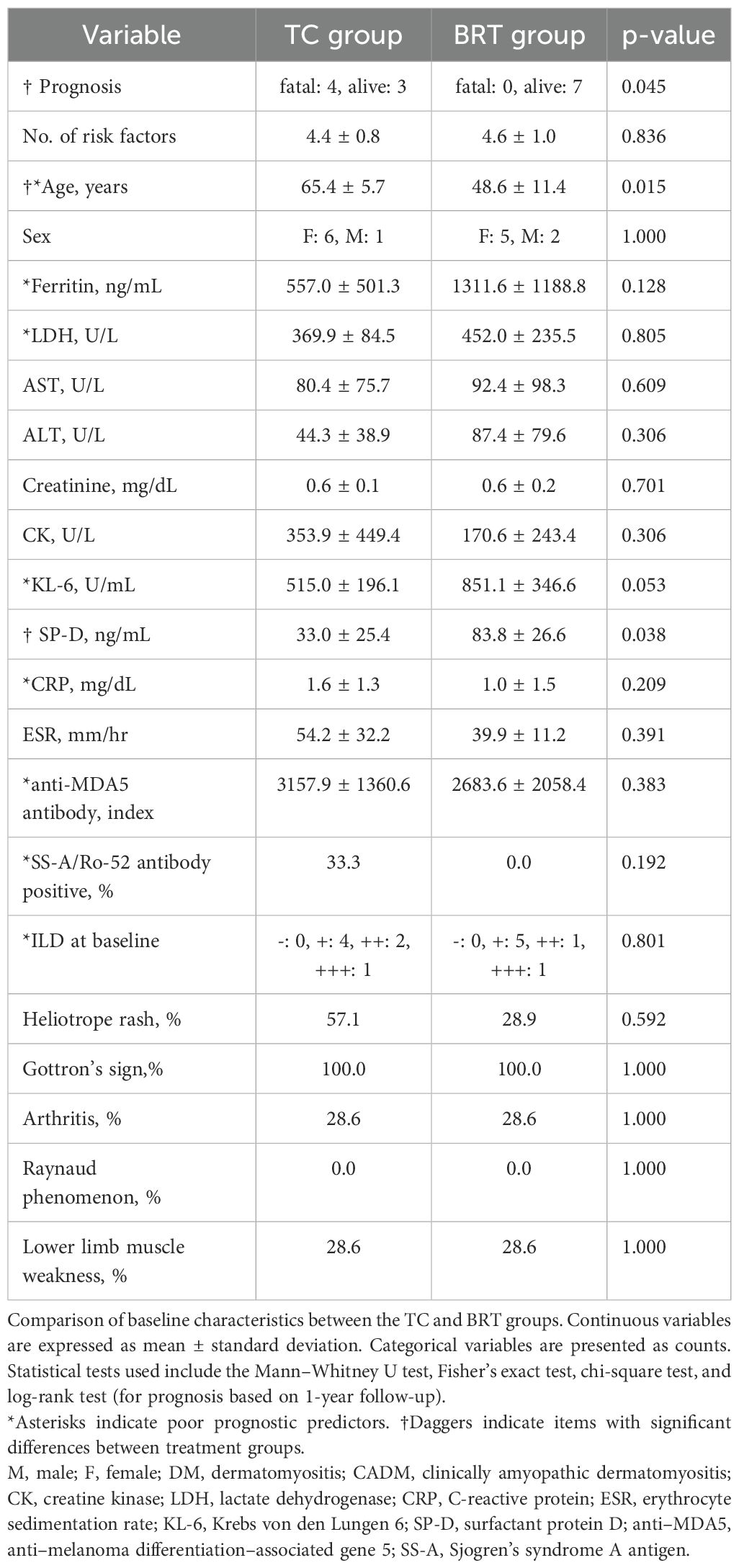
Table 2 shows the background characteristics of patients treated with TC (n=7) and patients treated with BRT (n=7). The number of risk factors was 4.43 ± 0.79 in the TC group and 4.57 ± 0.98 in the BRT group, with no significant difference. Welch’s t test revealed that age was significantly greater in the TC group than in the BRT group (TC: 65.4 ± 5.7; BRT: 48.6 ± 11.4; p = 0.015). In contrast, SP-D (human surfactant protein D) levels were significantly elevated in the BRT group relative to those in the TC group (TC: 33.0 ± 25.4; BRT: 83.8 ± 26.6; p = 0.038). Although not statistically significant, KL-6 levels tended to be higher in the BRT group (TC: 515.0 ± 196.1; BRT: 851.1 ± 346.6; p = 0.053). For qualitative data, a chi-square test was performed. No significant differences were observed in the other items.
Treatment course of each patient
In the TC-Tx group, the survival rate decreased progressively with the number of poor prognostic factors: 100% with three factors, 50% with four, and 25% with five. In contrast, the BRT-Tx group exhibited a consistent 100% survival rate regardless of the number of poor prognostic factors (ranging from three to six). This difference in survival trends between the two groups was statistically significant (log-rank test, P = 0.045), suggesting that BRT-Tx may offer superior survival benefits particularly in patients with high-risk features.
In the BRT group, patient BRT-1 had previously undergone two steroid pulse therapies prior to the initiation of BRT treatment. Despite these efforts, the disease remained completely uncontrolled, and the patient exhibited severe ILD complicated by mediastinal pneumothorax. The chest CT findings closely resembled those observed in severe COVID-19 pneumonia patients. For patient BRT-4, mild worsening of ILD and an increase in the anti-MDA5 antibody titer were observed three months after treatment initiation. In response, combined rescue therapy—including mPSL pulse therapy, PE, and IVIG—was administered, leading to recovery without recurrence. The remaining six BRT patients did not require combined rescue therapy.
No serious adverse events leading to death were observed in any of the patients. In the BRT-1 patient, however, fever and seizures developed on day 91, while the patient received PSL 40 mg and TAC 5 mg. On the basis of the analysis of cerebrospinal fluid (CSF) and findings from head magnetic resonance imaging (MRI), the patient was diagnosed with limbic encephalitis, a condition associated with profound brain dysfunction. Although viral involvement was suspected, definitive confirmation could not be established. Notably, this was the sole serious adverse event reported in BRT patients.
Cytomegalovirus (CMV) reactivation was monitored regularly. Out of 14 patients, 11 experienced reactivation. This included 5 out of 7 patients in the BRT group and 6 out of 7 patients in the TC group. All affected patients received appropriate treatment and achieved recovery. One patient in the BRT group recovered without experiencing CMV reactivation due to prophylactic administration of acyclovir.
Gene expression differences observed before and after treatment are more closely linked to prognosis than to the specific treatment modality
Genetic analyses were conducted on the six patients (TC: n=3, BRT: n=3). DEGs identified before and after treatment were subjected to clustering analysis, which revealed that the DEGs did not stratify according to treatment group (TC vs. BRT) but instead aligned with patient outcomes (survival vs. mortality). These results suggest that variations in gene expression before and after treatment are not treatment dependent but are profoundly influenced by whether the patient survives or succumbs to the disease (Figure 1).
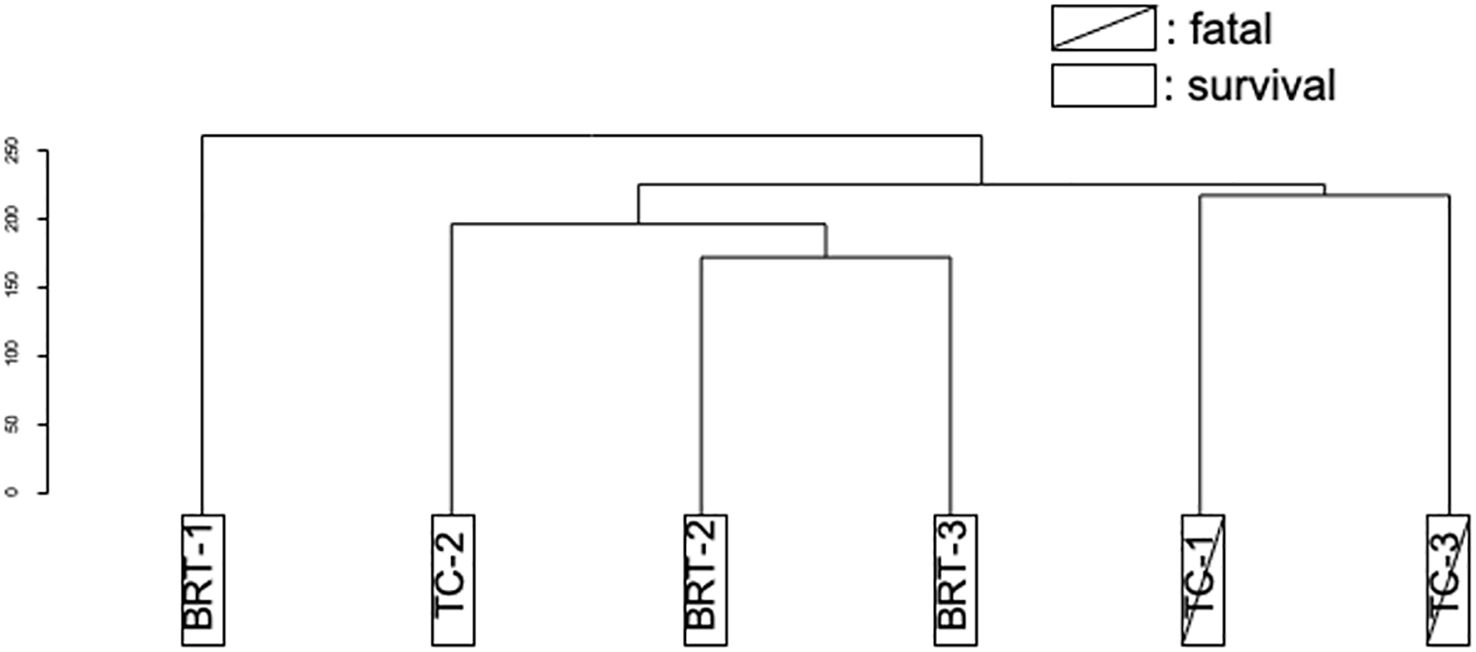
Figure 1. Clustering of DEGs (pre- vs. posttreatment). Cluster analysis of DEGs distinguished deceased patients from survivors, irrespective of the type of treatment administered.
Characteristics of gene expression changes following BRT and TC
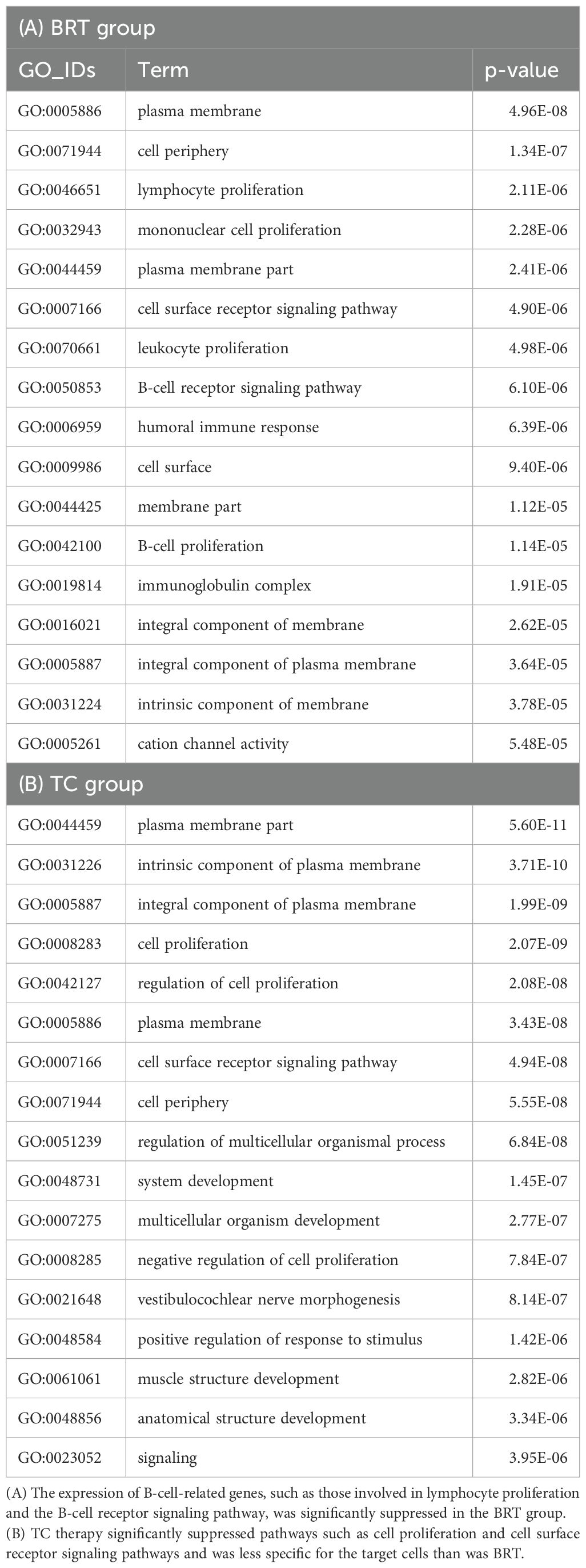
To explore the distinctive characteristics of the effects of the TC and BRT therapies, GSVA and GO analyses were performed on the DEGs identified before and after treatment in the BRT (n = 3) and TC (n = 3) groups, respectively. GSVA revealed that, compared with TC-Tx, BRT-Tx significantly suppressed “leukocyte transendothelial migration” (p = 0.0198). Additionally, GO analysis of the genes that were downregulated after treatment among the DEGs revealed a significant reduction in the expression of genes related to B-cell functions in the BRT group, including those involved in “lymphocyte/B-cell proliferation” and the “B-cell receptor signaling pathway” (Table 3A). In contrast, GO analysis of the TC group indicated significant suppression of genes associated with nonspecific cellular processes, such as “cell proliferation” and “cell surface receptor signaling pathways” (Table 3B).
Characteristics of the surviving and fatal patients
GO analyses were conducted on the DEGs identified before and after treatment in surviving patients (n = 4) and fatal patients (n = 2). These analyses revealed significant suppression of the expression of B-cell-related genes, including those associated with the “immunoglobulin complex,” “B-cell receptor signaling pathway,” and “immunoglobulin receptor binding” (Table 4A). In our prior analysis involving a smaller cohort (12), the type I and II IFN signaling and RIG-I-like receptor pathways were ranked among the most significantly downregulated GO terms in survivors (n = 3) posttreatment. The lack of such findings in the current study appears to result from the inclusion of one additional survivor (BRT-3) whose IFN-related gene expression changes did not exceed the DEG threshold (|log2-fold change| ≥ 1).
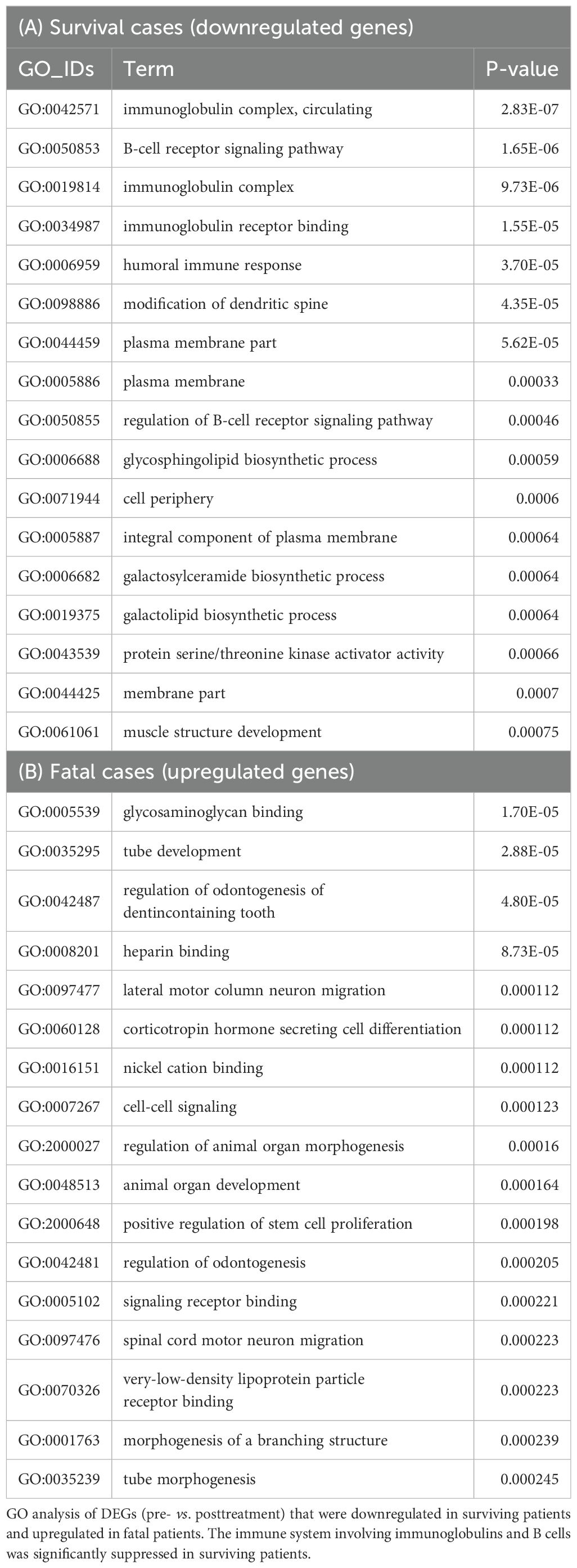
Table 4. Characteristics of surviving patients (n=4) and fatal patients (n=2) according to GO analysis.
Conversely, in fatal cases, the upregulation of genes associated with “glycosaminoglycan binding” and “heparin binding”—molecular functions implicated in the coagulation system—was observed (Table 4B).
Comparison of IFN signatures before and after treatment
Although the present GO analysis did not reveal significant suppression of type I interferon (IFN)-related genes in survivors, prior research has consistently implicated type I IFNs as key mediators in the pathogenesis of MDA5-DM (12, 33, 34). To investigate this further, IFN signature scores for type I, type II, and type III IFN pathways were calculated, and changes before and after treatment were analyzed for each patient (Figure 2A). When comparing BRT and TC therapies, no significant differences were observed in the trends of IFN signature scores before and after treatment. However, a distinct pattern was observed when surviving and deceased patients were compared. In surviving patients, the IFN signature score either decreased or remained low following treatment. In contrast, deceased patients presented increased IFN signature scores posttreatment. Notably, one of the two deceased patients presented an increase in type I IFN signatures, whereas both deceased patients presented elevated type II and type III IFN signatures after treatment. As shown in Figure 2B, the expression levels of type I, II, and III interferon-related genes before and after treatment were normalized via genewise Z scores. The distribution of expression changes was illustrated via violin plots. Notably, for all interferon types, gene expression was significantly lower in surviving patients than in deceased patients.
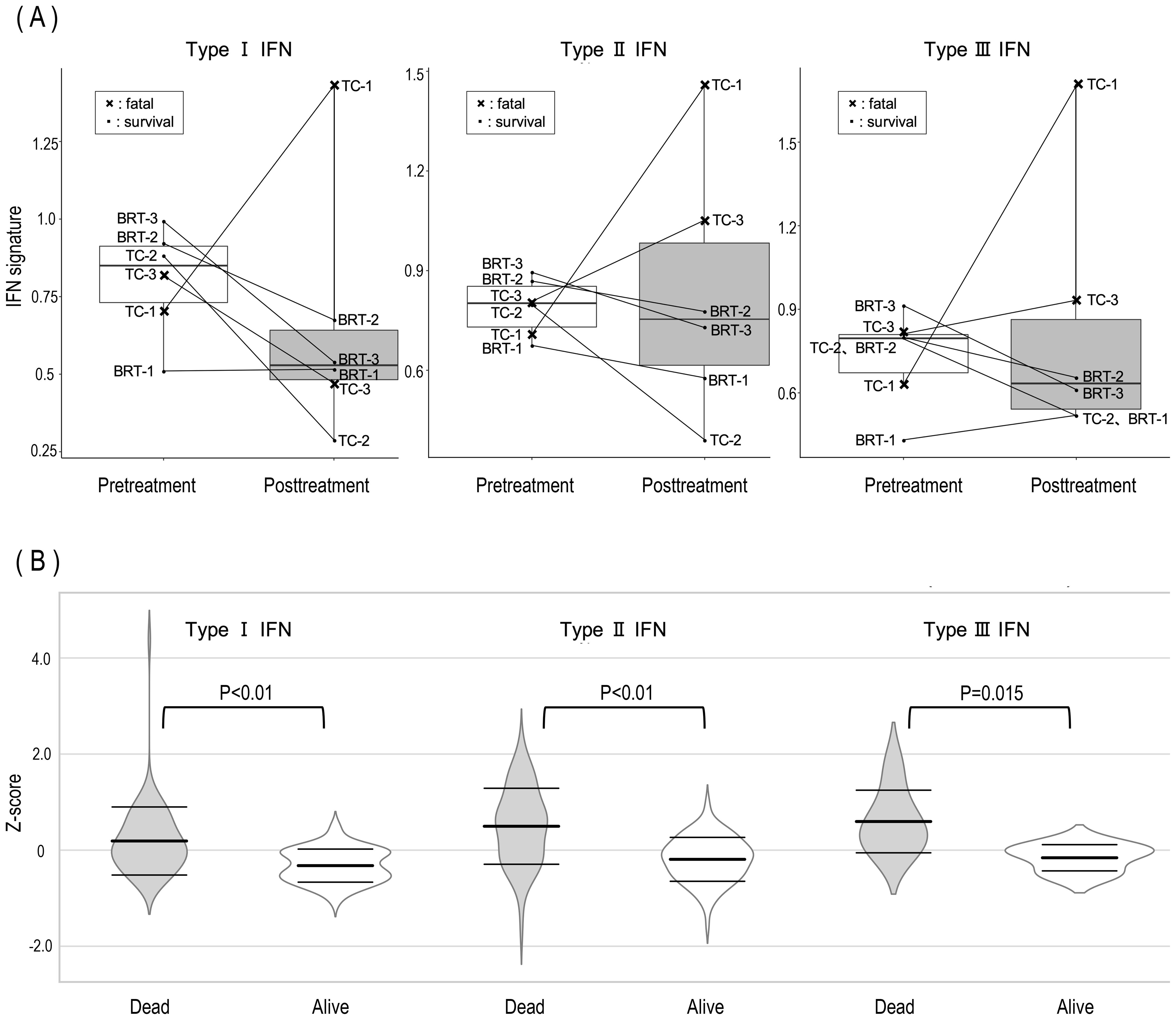
Figure 2. Posttreatment dynamics of IFN signature scores and gene expression in surviving and deceased MDA5-DM patients. (A) Posttreatment alterations in IFN signature scores revealed consistent increases in type II and type III IFN scores among all deceased patients, alongside an elevation in the type I IFN score in one deceased patient. The X symbols on the graph indicate deceased patients (TC-1 and TC-3). (B) Violin plots illustrating the distribution of log2 fold changes in type I, II, and III IFN-related gene expression between surviving and deceased patients. Central bold horizontal bars represent mean values, whereas upper and lower whiskers denote standard deviations. Statistical comparisons were performed via Welch’s t test.
Discussion
MDA5-DM is a rare autoimmune disease with high mortality rates, particularly when associated with ILD. Among these factors, RP-ILD is a well-recognized poor prognostic factor. A recent study reported various immunological abnormalities in active MDA5-DM patients, including hyperactivation of antibody-producing cells, CD8+ T-cell activation, and elevated levels of type I and II IFNs (33). While macrophages were not a primary focus of these studies, our autopsy findings suggest that in patients who succumb to RP-ILD associated with MDA5-DM, the majority of immune cells present in the alveoli are macrophages, implying that their dysregulation might represent a central pathological mechanism. The known correlation between elevated ferritin levels and macrophage activation (35), as well as the recognition of hyperferritinemia as a critical marker of disease activity and poor prognosis (15), further support this hypothesis.
The management of severe anti-MDA5 antibody-positive dermatomyositis (MDA5-DM) remains challenging, with no standardized therapy established yet. The rarity and rapid progression of this disease limit the feasibility of randomized controlled trials, thereby restricting high-level evidence. Studies have shown that TC-Tx, a combination of tacrolimus (TAC), cyclophosphamide (CYC) and high-dose glucocorticoids (GCs), is effective in newly diagnosed MDA5-DM patients with ILD (20, 36). Consequently, TC-Tx is widely used initially, especially for rapidly progressive ILD (RP-ILD) (37). However, some cases remain refractory.
Mycophenolate mofetil (MMF), an immunosuppressant that inhibits DNA replication in lymphocytes via inosine monophosphate dehydrogenase, has shown efficacy in refractory cases of MDA5-DM with ILD (38). Alternative combinations using MMF instead of CYC show potential, although evidence is currently limited.
The Janus kinase (JAK) inhibitor tofacitinib (TOF), the first approved JAK inhibitor, which is commonly used in MDA5-DM, has shown benefits in refractory cases (39). Reports on other JAK inhibitors are sparse. Additionally, the biologic agent rituximab (RTX) has shown promise, with survival benefits reported in combination with TOF and plasma exchange (PE) (40). Further research is needed to confirm these therapeutic options.
This study investigated the efficacy and underlying mechanisms of a novel multitarget combination therapy, BRT-Tx (baricitinib [BAR], rituximab [RTX], tacrolimus [TAC], and high-dose glucocorticoids [GCs]), in seven patients with MDA5-DM-associated ILD, including patients who exhibited rapid progression. These findings were compared with those of seven historical patients treated with TC-Tx (tacrolimus [TAC], cyclophosphamide [CYC], and high-dose glucocorticoids [GCs]).
Our findings demonstrated the superiority of BRT-Tx over TC-Tx in improving survival outcomes. All seven patients treated with BRT-Tx survived, and only one required combined rescue therapy to manage disease relapse. In contrast, four of the seven TC-Tx cases resulted in fatal outcomes, underscoring the limitations of conventional regimens.
Transcriptome analyses further highlighted the mechanistic differences between the therapies: BRT-Tx effectively suppressed lymphocyte-related genes, including those involved in the B-cell receptor signaling pathway, whereas TC-Tx predominantly suppressed genes associated with nonspecific cell proliferation.
Gene ontology analysis comparing survivors and nonsurvivors revealed that excessive B-cell activation and antibody-mediated effector responses were suppressed in the surviving group. Although IFN-related GO terms were not enriched, this was likely due to the modest expression changes in many IFN-related genes, which did not meet the DEG threshold (|log2-fold change| ≥ 1). When focusing specifically on IFN-related genes, posttreatment expression of the type I, II, and III IFN pathways was significantly downregulated in survivors. These findings underscore the pivotal role of suppressing B-cell–driven signaling and further suggest that attenuation of interferon responses may also contribute to favorable therapeutic outcomes in MDA5-DM patients.
These results suggest that BRT-Tx provides a targeted approach to modulating immune dysregulation in MDA5-DM patients, offering promising outcomes even in high-risk patients with poor prognostic factors.
Mechanisms of BRT therapy
BRT-Tx was developed as a multitarget therapeutic regimen designed to address the complex immunopathology of MDA5-DM. Each component was selected for its ability to modulate specific immune pathways:
1. Baricitinib (BAR): Suppressing the JAK-STAT pathway.
BAR, a JAK1/2 inhibitor, suppresses type I and II IFN signaling and the granulocyte–macrophage colony–stimulating factor (GM–CSF) pathway. These pathways are critical for macrophage activation and inflammatory cascades in the pathogenesis of MDA5-DM. Compared with tofacitinib (TOF), BAR more strongly inhibits JAK2, resulting in superior suppression of GM-CSF and IFN-γ signaling (41). Furthermore, the antifibrotic properties of BAR, as demonstrated in a murine model of bleomycin-induced pulmonary fibrosis (42), suggest its potential to reduce lung injury and fibrosis in human diseases such as MDA5-DM-associated ILD. Its clinical efficacy in severe COVID-19 pneumonia, which shares pathological features with MDA5-DM, further supports its use (43, 44). On the other hand, it suppresses the IFN signaling pathway, necessitating heightened vigilance to prevent the reactivation of latent viral infections. Particularly during the period of BAR administration, regular monitoring for CMV reactivation is essential, with assessments recommended at least once every 1–2 weeks, and prophylactic administration of acyclovir is also recommended.
2. Rituximab (RTX): Targeting B cells and autoantibody production.
RTX offers a targeted approach to suppressing B-cell-mediated immune responses by depleting CD20-positive B cells, ultimately leading to a reduction in the production of anti-MDA5 autoantibodies. This mechanism is particularly important in managing MDA5-DM, where autoantibody titers are strongly correlated with disease prognosis (14).
Compared with cyclophosphamide (CYC), RTX is a safer alternative. CYC is effective in reducing autoantibody production but is associated with significant long-term risks, including infertility and carcinogenesis (45), making its use less favorable, particularly in younger patients. Additionally, RTX has demonstrated the ability to deplete B cells rapidly, providing earlier modulation of immune responses than CYC does.
The safety profile of RTX is further supported by studies indicating fewer adverse events than CYC in the treatment of collagen disease-associated ILD while maintaining comparable efficacy in controlling disease activity (46). These characteristics position RTX as a preferred option for patients with MDA5-DM and poor prognostic factors, where rapid and targeted immunomodulation is critical.
3. Tacrolimus (TAC): Suppressing T-cell activation.
TAC inhibits calcineurin-mediated T-cell activation, addressing the hyperactivated CD8+ T cells implicated in MDA5-DM pathogenesis (20, 33). Its established safety and efficacy in autoimmune diseases complement the actions of BAR and RTX, ensuring comprehensive immune modulation.
Prophylactic management of opportunistic infections
Potent immunosuppressive therapies inherently increase susceptibility to opportunistic infections, which can lead to life-threatening complications if not adequately addressed. Accordingly, rigorous infection prophylaxis is essential. To mitigate the risk of Pneumocystis jirovecii pneumonia (PCP) and fungal infections, sulfamethoxazole-trimethoprim (ST combination) and antifungal agents—such as oral amphotericin B suspension—are routinely administered to high-risk patients receiving intensive immunosuppression.
In the context of the BRT-Tx regimen, baricitinib (BAR), a JAK inhibitor, suppresses IFN signaling and consequently elevates the risk of reactivation of latent viral infection. Prophylactic antiviral administration and close monitoring are thus warranted. Acyclovir is widely used for its established efficacy against herpes simplex virus types 1 and 2 (HSV-1/2), varicella-zoster virus (VZV), and Epstein–Barr virus (EBV) and has also been reported to exhibit modest activity against cytomegalovirus (CMV). Given the relatively high incidence of CMV reactivation during immunosuppressive therapy, regular virological monitoring is indispensable. In patients receiving BAR, CMV DNAemia/antigenemia should be assessed at intervals of 1 to 2 weeks. Although ganciclovir possesses greater intrinsic activity against CMV, acyclovir is selected for prophylaxis because CMV reactivation can be readily surveilled and treated preemptively, whereas HSV reactivation is difficult to detect and, on occasion, culminates in severe encephalitis.
Combined rescue therapy
One patient who underwent BRT-Tx required combined rescue therapy, which included high-dose glucocorticoids (GCs), plasma exchange (PE), and intravenous immunoglobulin (IVIG), to manage disease relapse. PE facilitates a rapid reduction in circulating autoantibody levels, thereby creating a critical window for RTX-induced B-cell depletion to exert therapeutic effects (25, 26). In the present study, antibody-mediated effector responses were significantly suppressed in the survivor group, suggesting that PE-mediated autoantibody clearance may represent a useful adjunctive strategy in the treatment of MDA5-positive dermatomyositis. Additionally, IVIG contributes to the modulation of excessive immune responses, further stabilizing the patient’s condition (47, 48). This case highlights that while BRT-Tx may not serve as a universal solution for all MDA5-DM patients, it holds significant potential to achieve life-saving outcomes when strategically combined with adjunctive therapies.
The protocol and management considerations for BRT therapy are listed in Table 1.
Limitations and future directions
The small sample size and retrospective nature of this study impose limitations on the generalizability of its findings. Moreover, while there was no significant difference in the number of poor prognostic factors among the patients, variability in the severity of ILD at the initiation of treatment introduces a potential confounding factor that may influence the interpretation of survival outcomes. To address these limitations, a randomized controlled trial (RCT) comparing BRT-Tx and TC-Tx in MDA5-DM patients with poor prognostic factors is planned. This trial aims to provide more robust and definitive evidence to substantiate the findings of this study.
Conclusions
This study highlights the superiority of BRT-Tx over historical TC-Tx in managing MDA5-DM-associated ILD. By targeting key immune pathways through BAR, RTX, and TAC, BRT-Tx offers a comprehensive and effective approach for controlling disease progression, even in high-risk patients. These findings support the inclusion of BRT-Tx as a first-line therapeutic option for MDA5-DM patients with multiple poor prognostic factors, warranting further validation in larger, prospective trials.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ddbj.nig.ac.jp/, DRA020447.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Okayama Red Cross Hospital Ethics Committee (acceptance number R5-002). All the patients included in the study provided written informed consent for treatment. In addition, written informed consent was obtained from all patients in the BRT group for the publication of potentially identifiable information, including clinical data and images.
Author contributions
MT: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. YN: Data curation, Investigation, Writing – review & editing. YS: Data curation, Investigation, Writing – review & editing, Formal analysis, Methodology, Software, Validation. MH: Data curation, Writing – review & editing, Formal analysis, Software, Validation. YM: Writing – review & editing, Supervision. TN: Writing – review & editing, Data curation. TS: Data curation, Writing – review & editing. TU: Data curation, Writing – review & editing, Supervision. JW: Supervision, Writing – review & editing. YK: Supervision, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was partially supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP25ek0109784.
Acknowledgments
The authors would like to sincerely thank Akira Uno, who inspired us to gain valuable insights into the development of treatment methods, and all the patients who participated in this study. Special thanks are also extended to Yumi Komori for her invaluable assistance with the data analysis.
Conflict of interest
Authors YS and MH were employed by the company DNA Chip Research Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nombel A, Fabien N, and Coutant F. Dermatomyositis with anti-mda5 antibodies: bioclinical features, pathogenesis and emerging therapies. Front Immunol. (2021) 12:773352. doi: 10.3389/fimmu.2021.773352
2. Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, et al. Shared and unique functions of the dexd/H-box helicases rig-I, mda5, and lgp2 in antiviral innate immunity. J Immunol. (2005) 175:2851–8. doi: 10.4049/jimmunol.175.5.2851
3. Dias Junior AG, Sampaio NG, and Rehwinkel J. A balancing act: mda5 in antiviral immunity and autoinflammation. Trends Microbiol. (2019) 27:75–85. doi: 10.1016/j.tim.2018.08.007
4. Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, et al. Autoantibodies to a 140-kd polypeptide, cadm-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis rheumatism. (2005) 52:1571–6. doi: 10.1002/art.21023
5. Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, Fujita T, et al. Rna helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis rheumatism. (2009) 60:2193–200. doi: 10.1002/art.24621
6. Nakashima R, Imura Y, Kobayashi S, Yukawa N, Yoshifuji H, Nojima T, et al. The rig-I-like receptor ifih1/mda5 is a dermatomyositis-specific autoantigen identified by the anti-cadm-140 antibody. Rheumatology. (2010) 49:433–40. doi: 10.1093/rheumatology/kep375
7. Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, et al. Differential roles of mda5 and rig-I helicases in the recognition of rna viruses. Nature. (2006) 441:101–5. doi: 10.1038/nature04734
8. Gonzalez D, Gupta L, Murthy V, Gonzalez EB, Williamson KA, Makol A, et al. Anti-mda5 dermatomyositis after covid-19 vaccination: A case-based review. Rheumatol Int. (2022) 42:1629–41. doi: 10.1007/s00296-022-05149-6
9. Tanizawa K, Handa T, Nakashima R, Kubo T, Hosono Y, Watanabe K, et al. Hrct features of interstitial lung disease in dermatomyositis with anti-cadm-140 antibody. Respir Med. (2011) 105:1380–7. doi: 10.1016/j.rmed.2011.05.006
10. Gono T, Kaneko H, Kawaguchi Y, Hanaoka M, Kataoka S, Kuwana M, et al. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology. (2014) 53:2196–203. doi: 10.1093/rheumatology/keu258
11. Yin X, Riva L, Pu Y, Martin-Sancho L, Kanamune J, Yamamoto Y, et al. Mda5 governs the innate immune response to sars-cov-2 in lung epithelial cells. Cell Rep. (2021) 34:108628. doi: 10.1016/j.celrep.2020.108628
12. Koyama Y, Sato Y, Nakai Y, and Sakamoto M. Detecting the critical factors in the pathogenesis of anti-melanoma differentiation-associated gene 5–positive dermatomyositis (Mda5 dm) by gene expression analysis of peripheral blood. Arthritis Rheumatol. (2022) 74((Suppl 9).
13. Lian X, Zou J, Guo Q, Chen S, Lu L, Wang R, et al. Mortality risk prediction in amyopathic dermatomyositis associated with interstitial lung disease: the flair model. Chest. (2020) 158:1535–45. doi: 10.1016/j.chest.2020.04.057
14. Cao H, Pan M, Kang Y, Xia Q, Li X, Zhao X, et al. Clinical manifestations of dermatomyositis and clinically amyopathic dermatomyositis patients with positive expression of anti-melanoma differentiation-associated gene 5 antibody. Arthritis Care Res. (2012) 64:1602–10. doi: 10.1002/acr.21728
15. Gono T, Masui K, Nishina N, Kawaguchi Y, Kawakami A, Ikeda K, et al. Risk prediction modeling based on a combination of initial serum biomarker levels in polymyositis/dermatomyositis-associated interstitial lung disease. Arthritis Rheumatol. (2021) 73:677–86. doi: 10.1002/art.41566
16. Xie H, Zhang D, Wang Y, Shi Y, Yuan Y, Wang L, et al. Risk factors for mortality in patients with anti-mda5 antibody-positive dermatomyositis: A meta-analysis and systematic review. Semin Arthritis rheumatism. (2023) 62:152231. doi: 10.1016/j.semarthrit.2023.152231
17. Xu L, You H, Wang L, Lv C, Yuan F, Li J, et al. Identification of three different phenotypes in anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis patients: implications for prediction of rapidly progressive interstitial lung disease. Arthritis Rheumatol. (2023) 75:609–19. doi: 10.1002/art.42308
18. Temmoku J, Sato S, Fujita Y, Asano T, Suzuki E, Kanno T, et al. Clinical significance of myositis-specific autoantibody profiles in Japanese patients with polymyositis/dermatomyositis. Medicine. (2019) 98:e15578. doi: 10.1097/MD.0000000000015578
19. Allenbach Y, Uzunhan Y, Toquet S, Leroux G, Gallay L, Marquet A, et al. Different phenotypes in dermatomyositis associated with anti-mda5 antibody: study of 121 cases. Neurology. (2020) 95:e70–e8. doi: 10.1212/WNL.0000000000009727
20. Tsuji H, Nakashima R, Hosono Y, Imura Y, Yagita M, Yoshifuji H, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. (2020) 72:488–98. doi: 10.1002/art.41105
21. Chen Z, Wang X, and Ye S. Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease. New Engl J Med. (2019) 381:291–3. doi: 10.1056/NEJMc1900045
22. Fan L, Lyu W, Liu H, Jiang H, Chen L, Liu Y, et al. A retrospective analysis of outcome in melanoma differentiation-associated gene 5-related interstitial lung disease treated with tofacitinib or tacrolimus. J Rheumatol. (2022) 49:1356–64. doi: 10.3899/jrheum.220367
23. Nascimento J, Tenazinha C, Campanilho-Marques R, Cordeiro I, and Salgado S. Rituximab in the treatment of anti-mda5 dermatomyositis-associated interstitial lung disease: A case-based literature review. ARP Rheumatol. (2022) 1:168–73.
24. Koichi Y, Aya Y, Megumi U, Shunichi K, Masafumi S, Hiroaki M, et al. A case of anti-mda5-positive rapidly progressive interstitial lung disease in a patient with clinically amyopathic dermatomyositis ameliorated by rituximab, in addition to standard immunosuppressive treatment. Modern Rheumatol / Japan Rheumatism Assoc. (2017) 27:536–40. doi: 10.3109/14397595.2015.1014140
25. Endo Y, Koga T, Suzuki T, Hara K, Ishida M, Fujita Y, et al. Successful treatment of plasma exchange for rapidly progressive interstitial lung disease with anti-mda5 antibody-positive dermatomyositis: A case report. Medicine. (2018) 97:e0436. doi: 10.1097/MD.0000000000010436
26. Shirakashi M, Nakashima R, Tsuji H, Tanizawa K, Handa T, Hosono Y, et al. Efficacy of plasma exchange in anti-mda5-positive dermatomyositis with interstitial lung disease under combined immunosuppressive treatment. Rheumatol (Oxford). (2020) 59:3284–92. doi: 10.1093/rheumatology/keaa123
27. Lundberg IE, Tjarnlund A, Bottai M, Werth VP, Pilkington C, Visser M, et al. 2017 European league against rheumatism/American college of rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann rheumatic Dis. (2017) 76:1955–64. doi: 10.1136/annrheumdis-2017-211468
28. Lu X, Peng Q, and Wang G. Anti-mda5 antibody-positive dermatomyositis: pathogenesis and clinical progress. Nat Rev Rheumatol. (2024) 20:48–62. doi: 10.1038/s41584-023-01054-9
29. Pagnuco IA, Pastore JI, Abras G, Brun M, and Ballarin VL. Analysis of genetic association using hierarchical clustering and cluster validation indices. Genomics. (2017) 109:438–45. doi: 10.1016/j.ygeno.2017.06.009
30. Mi H, Muruganujan A, Ebert D, Huang X, and Thomas PD. Panther version 14: more genomes, a new panther go-slim and improvements in enrichment analysis tools. Nucleic Acids Res. (2019) 47:D419–D26. doi: 10.1093/nar/gky1038
31. Hanzelmann S, Castelo R, and Guinney J. Gsva: gene set variation analysis for microarray and rna-seq data. BMC Bioinf. (2013) 14:7. doi: 10.1186/1471-2105-14-7
32. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci United States America. (2005) 102:15545–50. doi: 10.1073/pnas.0506580102
33. Ye Y, Chen Z, Jiang S, Jia F, Li T, Lu X, et al. Single-cell profiling reveals distinct adaptive immune hallmarks in mda5+ Dermatomyositis with therapeutic implications. Nat Commun. (2022) 13:6458. doi: 10.1038/s41467-022-34145-4
34. Qian J, Li R, Chen Z, Cao Z, Lu L, and Fu Q. Type I interferon score is associated with the severity and poor prognosis in anti-mda5 antibody-positive dermatomyositis patients. Front Immunol. (2023) 14:1151695. doi: 10.3389/fimmu.2023.1151695
35. Ruscitti P, Di Cola I, Di Muzio C, Italiano N, Ursini F, Giacomelli R, et al. Expanding the spectrum of the hyperferritinemic syndrome, from pathogenic mechanisms to clinical observations, and therapeutic implications. Autoimmun Rev. (2022) 21:103114. doi: 10.1016/j.autrev.2022.103114
36. Gono T, Masui K, Sato S, and Kuwana M. Mortality risk stratification using cluster analysis in patients with myositis-associated interstitial lung disease receiving initial triple-combination therapy. Front Med (Lausanne). (2022) 9:883699. doi: 10.3389/fmed.2022.883699
37. Yasui M, Iwamoto T, and Furuta S. New therapies in anti-mda5 antibody-positive dermatomyositis. Curr Opin Rheumatol. (2024) 36:61–8. doi: 10.1097/BOR.0000000000000979
38. Hayashi M, Kikuchi T, and Takada T. Mycophenolate mofetil for the patients with interstitial lung diseases in amyopathic dermatomyositis with anti-mda-5 antibodies. Clin Rheumatol. (2017) 36:239–40. doi: 10.1007/s10067-016-3443-2
39. Kurasawa K, Arai S, Namiki Y, Tanaka A, Takamura Y, Owada T, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology. (2018) 57:2114–9. doi: 10.1093/rheumatology/key188
40. Shirai T, Machiyama T, Sato H, Ishii T, and Fujii H. Intensive induction therapy combining tofacitinib, rituximab and plasma exchange in severe anti-melanoma differentiation-associatedprotein-5 antibody-positive dermatomyositis. Clin Exp Rheumatol. (2023) 41:291–300. doi: 10.55563/clinexprheumatol/8kulbf
41. Traves PG, Murray B, Campigotto F, Galien R, Meng A, and Di Paolo JA. Jak selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann rheumatic Dis. (2021) 80:865–75. doi: 10.1136/annrheumdis-2020-219012
42. Gu S, Liang J, Zhang J, Liu Z, Miao Y, Wei Y, et al. Baricitinib attenuates bleomycin-induced pulmonary fibrosis in mice by inhibiting tgf-beta1 signaling pathway. Molecules. (2023) 28:2195. doi: 10.3390/molecules28052195
43. Liu C, Kieltyka J, Fleischmann R, Gadina M, and O'Shea JJ. A decade of jak inhibitors: what have we learned and what may be the future? Arthritis Rheumatol. (2021) 73:2166–78. doi: 10.1002/art.41906
44. Hall FC, Cheriyan J, Cope AP, Galloway J, Wilkinson I, Bond S, et al. Efficacy and safety of baricitinib or ravulizumab in adult patients with severe covid-19 (Tactic-R): A randomised, parallel-arm, open-label, phase 4 trial. Lancet Respir Med. (2023) 11:1064–74. doi: 10.1016/S2213-2600(23)00376-4
45. Anderson D, Bishop JB, Garner RC, Ostrosky-Wegman P, and Selby PB. Cyclophosphamide: review of its mutagenicity for an assessment of potential germ cell risks. Mutat Res. (1995) 330:115–81. doi: 10.1016/0027-5107(95)00039-l
46. Maher TM, Tudor VA, Saunders P, Gibbons MA, Fletcher SV, Denton CP, et al. Rituximab versus intravenous cyclophosphamide in patients with connective tissue disease-associated interstitial lung disease in the uk (Recital): A double-blind, double-dummy, randomised, controlled, phase 2b trial. Lancet Respir Med. (2023) 11:45–54. doi: 10.1016/S2213-2600(22)00359-9
47. Koguchi-Yoshioka H, Okiyama N, Iwamoto K, Matsumura Y, Ogawa T, Inoue S, et al. Intravenous immunoglobulin contributes to the control of antimelanoma differentiation-associated protein 5 antibody-associated dermatomyositis with palmar violaceous macules/papules. Br J Dermatol. (2017) 177:1442–6. doi: 10.1111/bjd.15499
Keywords: anti-MDA5 antibody-positive dermatomyositis (MDA5-DM), JAK inhibitor, baricitinib, rituximab, multitargeted treatment, IFN signature, transcriptome analysis
Citation: Tokunaga M, Nakai Y, Sato Y, Hiratsuka M, Matsumoto Y, Nakatsue T, Saeki T, Umayahara T, Wada J and Koyama Y (2025) A pilot transcriptomic study of a novel multitargeted BRT regimen for anti–MDA5 antibody-positive dermatomyositis: improving survival over conventional therapy. Front. Immunol. 16:1568338. doi: 10.3389/fimmu.2025.1568338
Received: 29 January 2025; Accepted: 07 July 2025;
Published: 07 August 2025.
Edited by:
Dennis Niebel, University Medical Center Regensburg, GermanyReviewed by:
Tsuyoshi Shirai, Tohoku University, JapanHiroaki Harada, Mitsui Memorial Hospital, Japan
Copyright © 2025 Tokunaga, Nakai, Sato, Hiratsuka, Matsumoto, Nakatsue, Saeki, Umayahara, Wada and Koyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshinobu Koyama, eWtveWFtYUBnYWVhLm9jbi5uZS5qcA==
 Moe Tokunaga
Moe Tokunaga Yu Nakai1
Yu Nakai1 Yoshiharu Sato
Yoshiharu Sato Yoshinori Matsumoto
Yoshinori Matsumoto Takako Saeki
Takako Saeki Jun Wada
Jun Wada Yoshinobu Koyama
Yoshinobu Koyama