- 1Canadian Center for Vaccinology, Dalhousie University, Izaak Walton Killam (IWK) Health Centre and the Nova Scotia Health Authority, Halifax, NS, Canada
- 2Department of Pediatrics, Dalhousie University, Halifax, NS, Canada
- 3Department of Microbiology and Immunology, Dalhousie University, Halifax, NS, Canada
- 4BioNet-Asia, Bangkok, Thailand
- 5Center for Vaccine Innovation and Access, Seattle, WA, United States
Background: Both the quantity and quality of circulating anti-pertussis toxin antibodies are important for protection against severe pertussis. We compared the avidity of PT-IgG antibodies in pregnant women and their infants following vaccination during pregnancy with pertussis vaccines containing genetically-detoxified pertussis toxin (PTgen) or chemically-detoxified PT (PTchem).
Methods: We analyzed serum samples collected earlier from pregnant women (at delivery) and their infants (at birth and 2 months of age) participating in a clinical trial where pregnant women had been vaccinated during pregnancy with recombinant acellular pertussis vaccine containing 1 µg PTgen (standalone, ap1gen, [n=37], or combined to tetanus and diphtheria, Tdap1gen [n=34]), 2 µg PTgen (Tdap2gen, n=35), or 5 µg PTgen (TdaP5gen, n=34), or acellular pertussis vaccine containing 8 µg PTchem (Tdap8chem, n=35). Avidity was assessed by adding increasing concentrations (0.25, 0.5, 1, 1.5, 2, and 3 M) of NH4SCN as a bond-breaking agent and measuring PT-IgG levels by ELISA.
Findings: Compared with Tdap8chem, TdaP5gen vaccination was associated with significantly higher total absolute avidity (p<0.001) and medium-high to very-high avidity PT-IgG levels (p≤0.02) in mothers at delivery, infants at birth and infants at 2 months of age. Avidity was comparable to Tdap8chem after vaccination with the low-dose PTgen formulations (ap1gen, Tdap1gen or Tdap2gen). There were no differences for vaccination during the 2nd or 3rd trimester of pregnancy.
Interpretation: Compared with chemically detoxified vaccines, vaccination during pregnancy with recombinant genetically detoxified acellular pertussis vaccine at lower PT concentration provides infants with at least similar or higher quality PT-IgG antibodies. Consequently, recombinant pertussis vaccines may offer comparable or better protection against pertussis.
1 Introduction
Pertussis is a highly contagious human respiratory infection caused by the bacterium Bordetella pertussis. Despite high vaccination coverage, the incidence of pertussis has been increasing globally with cyclic epidemics occurring every 2 to 5 years (1). In 2024, many countries reported the largest pertussis outbreaks since decades (2–5). Pertussis is most severe in young infants who are too young to be vaccinated (6, 7). Pertussis vaccination during pregnancy is a safe and effective strategy to protect vulnerable young infants from severe pertussis (8–11).
Pertussis toxin (PT) plays a fundamental role in the pathogenesis of pertussis (12–14) and is a component of all acellular pertussis vaccines (15–17). Especially in young infants, anti-PT antibodies are an important mechanism of protection against severe disease, which depends on both the quantity and quality of the antibody response (18–21).
Pertussis toxin must be inactivated before it can be safely administered to humans. In most acellular pertussis vaccines, PT has been chemically detoxified; however, chemical treatment can cause conformational changes that lead to dominant immunity against nonprotective epitopes (22–26). Recombinant acellular pertussis vaccines using DNA technologies introducing substitutions in the S1 subunit of wild type PT to inactivate PT were successfully developed and used in childhood immunization programs (27, 28). Genetically detoxified PT (PTgen) retains an antigenic conformation similar to native PT with preservation of epitopes involved in toxin-neutralization (26, 27, 29). In recent years several programs for the development of recombinant acellular pertussis booster vaccines have been initiated (30–33). Results from various clinical trials involving adolescents, adults, and pregnant women and their infants, consistently show that vaccination with PTgen elicits higher PT-IgG antibody titers compared with chemically detoxified PT (PTchem) (30, 31, 34–37).
Avidity, which is a measure of the binding strength between an epitope and an antibody’s binding site, is an important parameter of the functionality of antibodies. Higher PT-IgG avidity may contribute to a higher capacity to neutralize pertussis toxin and protect against severe disease (38, 39). PT-IgG avidity following vaccination with chemically inactivated acellular pertussis vaccines has been studied in different populations including in infants born to mothers who were vaccinated during pregnancy (40–42), but to our knowledge has never been studied for recombinant pertussis vaccines containing PTgen.
In this study we compared PT-IgG avidity in pregnant women and their infants following vaccination during pregnancy (20–33 weeks gestation) with one of four different formulations of a recombinant acellular pertussis vaccine containing variable amounts of PTgen compared with chemically inactivated acellular pertussis booster vaccine. A wide range of concentrations of chaotropic (bond-breaking) agent was used to allow a comprehensive analysis of PT-IgG avidity (43).
2 Materials and methods
2.1 Study design
As an exploratory objective of a phase 2 randomized controlled trial of pertussis vaccination during pregnancy, the avidity of PT-IgG antibodies was assessed in serum samples collected from a pre-selected subset of participating maternal-infant pairs. The study design, safety and immunogenicity outcomes have been reported previously (Thai Clinical Trials Registry, TCTR20180725004) (36, 37). Briefly, a total of 400 healthy pregnant women (18–40 years old) living in Bangkok, Thailand, were enrolled between February and October 2019. Participating pregnant women were randomized 1:1:1:1:1 to receive during pregnancy (at 20–33 weeks gestation) one dose of one of five study vaccines, including four recombinant acellular pertussis vaccine formulations (see: Study vaccines). Individual vaccination histories were not available, but assuming participants followed the Thai national immunization program that has had a 99% coverage for 3 childhood doses since 1996, participants likely received 3 doses of whole cell pertussis containing vaccine during childhood (44). Women who had received diphtheria, tetanus or pertussis-containing vaccine(s) within 1 year prior to enrolment were excluded.
2.2 Study vaccines
Recombinant pertussis vaccines were produced by BioNet-Asia (Thailand). PTgen was produced from a recombinant B. pertussis strain containing a substitution of two amino acids (R9K and E129G) at the enzymatic active site in sub-unit S1 in the PT operon (29). Formulations included: ap1gen containing 1 µg PTgen and 1 µg filamentous hemagglutinin (FHA); Tdap1gen containing tetanus toxoid (7.5 Lf) and reduced-dose diphtheria toxoid (2 Lf) (Td) combined with ap1gen; Tdap2gen (BoostagenRED®) containing 2 µg PTgen and 5 µg FHA combined with Td; a licensed TdaP5gen (Boostagen®) 5 µg PTgen and 5 µg FHA combined with Td. The licensed Tdap8chem comparator (Boostrix™, GlaxoSmithKline) contained 8 µg PTchem, 8 µg FHA and 2.5 μg pertactin combined with 5 Lf tetanus toxoid and 2.5 Lf diphtheria toxoid.
2.3 Ethical consideration
The clinical study was conducted in compliance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and Good Clinical Practice (GCP), the Declaration of Helsinki, and local ethical guidelines. Ethical approval was obtained from the Institutional Review Boards of the Faculty of Medicine Siriraj Hospital at Mahidol University, Faculty of Medicine at Chulalongkorn University, Bangkok, Thailand and Western Institutional Review Board (now known as WIRB-Copernicus Group), Washington, USA. Written informed consent was obtained from all pregnant women before recruitment, including consent for follow-up of their newborns.
2.4 Samples collection and processing
Venous blood samples were randomly selected as a subpopulation of cohort samples obtained from pregnant women at the time of delivery, and from their infants at the time of birth (cord blood or from newborn within 72 hours after birth) and at 2 months of age. Avidity was assessed for a randomly selected subset of mother-infant pairs: ap1gen, n=37, Tdap1gen, n=34, Tdap2gen, n=35, TdaP5gen, n=34 (n=33 for infants at 2 months), and Tdap8chem, n=35 (n=34 for infants at 2 months). Sera were stored at ≤ -20 °C before being shipped on dry ice to the University of British Columbia (Vancouver, British Columbia, Canada) for avidity testing.
2.5 Measurement of total PT-IgG, PT-IgG avidity, avidity indices and calculations
Avidity of PT-IgG antibodies was assessed by measuring PT-specific IgG antibody binding in the presence of a range of chaotrope concentrations (NH4SCN at 0.25 molar (M), 0.5M, 1M, 1.5M, 2M, 3M]) using a commercial ELISA kit (EURIMMUN) coated with native highly purified Bordetella pertussis toxin, as published previously (41, 43). Total PT-IgG was measured in PBS (0M NH4SCN) in the same ELISA kit at the same time for avidity assay. PT-IgG levels were calculated against the calibration serums quantified based on the WHO International Standard Pertussis Antiserum, human (1st IS NIBSC code 06/140) according to the instruction. Different avidity indices for PT-IgG were calculated as published previously (41, 43) and as described in 2.5.1, 2.5.2 and 2.5.3, and summarized in Table 1.
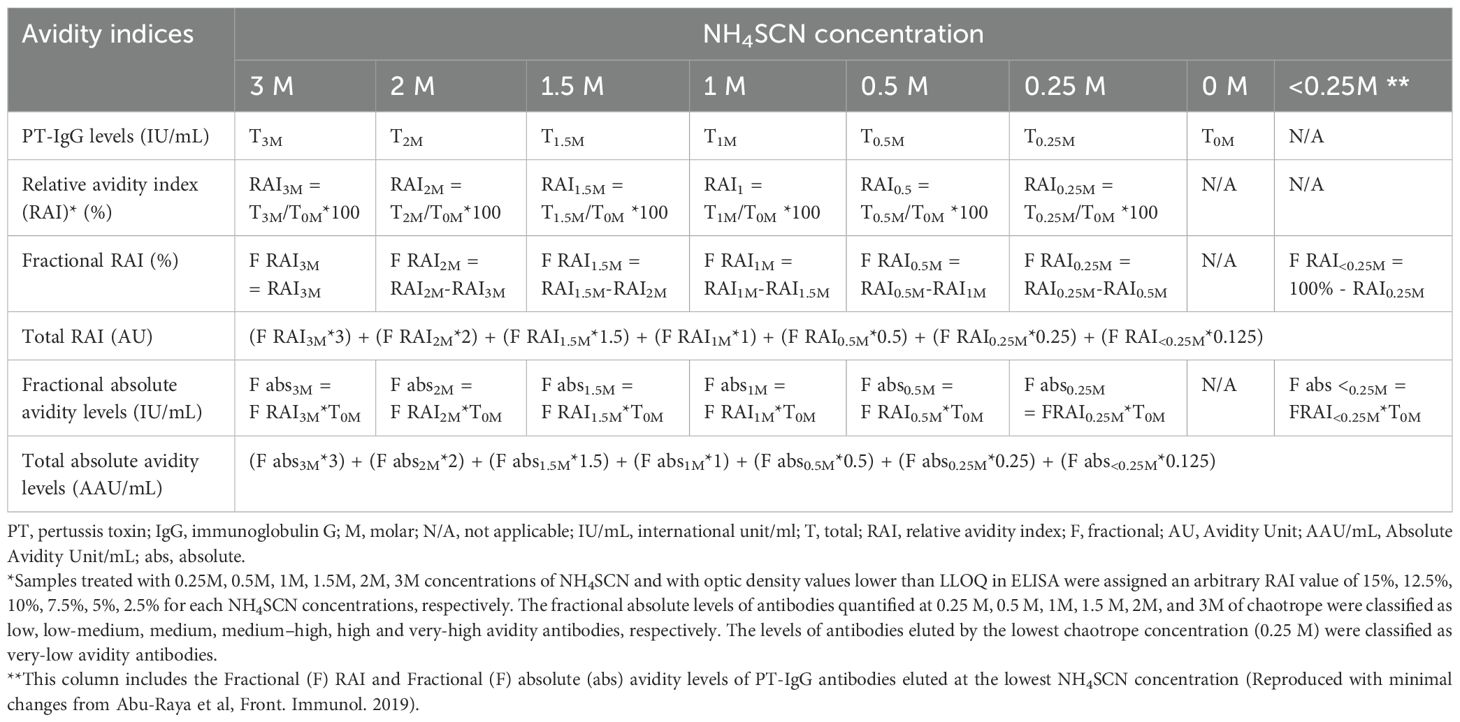
Table 1. Calculation of relative avidity index, fractional relative avidity index, total relative avidity index and quantification of fractional and absolute avidity levels of anti-PT IgG.
2.5.1 Total relative avidity index
The total relative avidity index (total RAI) of PT-IgG antibodies was calculated for each sample. First, a relative avidity index (RAI) was calculated for each NH4SCN concentration as the proportion (%) of PT-IgG concentration in samples treated versus not treated with NH4SCN (for example, RAI3M=T3M/T0M*100 where T3M is PT-IgG concentrations in the presence of 3M NH4SCN and T0M is PT-IgG concentrations in the absence of NH4SCN). Next, a fractional RAI (F RAI) (%), defined as the RAI achieved at a specific NH4SCN concentration, was calculated as the RAI at a specific concentration minus the RAI achieved at the next higher concentration of NH4SCN (for example, RAI1M=RAI1M-RAI1.5M=70%-30%=40%, where 1M and 1.5M represent increasing NH4SCN concentrations). Finally, for each sample the total RAI (AU), reflecting the weighted contribution of the fractional RAIs achieved at different NH4SCN concentrations, was calculated by applying a factor to each fractional RAI corresponding to the respective concentration of NH4SCN giving higher weight to antibodies with higher avidity (e.g. fractional RAI at 2M given a weight of 2): (F RAI3M*3) + (F RAI2M*2) + (F RAI1.5M*1.5) + (F RAI1M*1) + (F RAI0.5M*0.5) + (F RAI0.25M*0.25) + (F RAI<0.25M*0.125) as published previously (41, 43).
2.5.2 Fractional absolute avidity levels
As indices involving RAI are relative measures, fractional and total absolute avidity levels were calculated. The fractional absolute avidity level (F abs) of PT-IgG (IU/mL) reflects the level of PT-IgG that is still bound to the antigen at a specific NH4SCN concentration and calculated as the fractional RAI at a specific NH4SCN concentration multiplied by the anti-PT IgG concentration in the absence of NH4SCN (for example, F abs3M = F RAI3M*T0M). F abs quantified (bound to PT) at 0.25M, 0.5M, 1M, 1.5M, 2M, and 3M of NH4SCN were classified as low, low-medium, medium, medium-high, high and very-high avidity antibodies, respectively. The levels of antibodies eluted (i.e. not bound to the plate) at the lowest NH4SCN concentration (0.25M) were classified as ‘very-low’ avidity antibodies.
2.5.3 Total absolute avidity levels
Total absolute avidity levels (AAU/mL) reflect the weighted contribution of the F abs, and higher weight was given to antibodies with higher avidity by applying a factor to each fractional absolute avidity levels corresponding to the respective concentration of NH4SCN: (F abs3M*3) + (F abs2M*2) + (F abs1.5M*1.5) + (F abs1M*1)+ (F abs0.5M*0.5) + (F abs0.25M*0.25) + (F abs<0.25M*0.125) as published previously (41, 43).
2.6 Statistical analysis
Statistical analyses were performed by the Center of Excellence for Biomedical and Public Health Informatics (BIOPHICS), Bangkok, Thailand, using Statistical Analysis System (SAS) version 9.4. Data was analyzed per protocol. As this was an exploratory analysis of the main clinical study, no formal hypothesis was generated for this study.
Samples treated with 0.25M, 0.5M, 1M, 1.5M, 2M, 3M concentrations of NH4SCN and with optic density values lower than LLOQ were assigned an arbitrary RAI value of 15%, 12.5%, 10%, 7.5%, 5%, 2.5% for each NH4SCN concentrations, respectively. Total PT-IgG levels, total absolute avidity levels of PT-IgG, and F abs levels of PT-IgG did not follow a normal distribution and were log-transformed to calculate geometric mean concentrations (GMCs) and 95% confidence intervals (95% CI). Total RAI of PT-IgG followed a normal distribution and means with 95% CI were calculated. Outcomes were compared for statistical differences between the five different vaccine groups using the Kruskal-Wallis test. In addition, differences between an individual recombinant vaccine group and Tdap8chem were compared using an Independent t-test. Correlations between total PT-IgG and total RAI were assessed by calculating the Spearman correlation coefficient rho. A p-value of ≤ 0.05 was considered statistically significant.
3 Results
3.1 Study population
Demographics and baseline characteristics of pregnant women and their infants included in the avidity analysis are presented in Supplementary Table S1. Vaccination during the 2nd (13–26 weeks gestation) vs. 3rd trimester of pregnancy (≥ 27 weeks gestation) was evenly distributed amongst the vaccine groups.
3.2 Correlation between avidity and PT-IgG levels
Overall correlations between PT-IgG levels and total RAI across (for all vaccine groups combined) were moderate in pregnant women at delivery (Spearman rho = 0.620, p<0.0001), in infants at birth (rho = 0.526, p < 0.0001) and at 2 months of age, rho = 0.724, p<0.0001) (Supplementary Figure S1). This indicates that the avidity of anti-PT IgG measures a function that is not entirely dependent on anti-PT IgG levels.
3.3 Total relative avidity index
PT-IgG total RAIs were comparable in pregnant women at delivery and in infants at birth for each of the recombinant pertussis vaccine formulations compared with Tdap8chem (Figure 1A) (Table 2). However, at 2 months of age, PT-IgG total RAI was significantly higher in infants whose mothers had received TdaP5gen as compared with Tdap8chem (p < 0.001) (Figure 1A; Table 2).
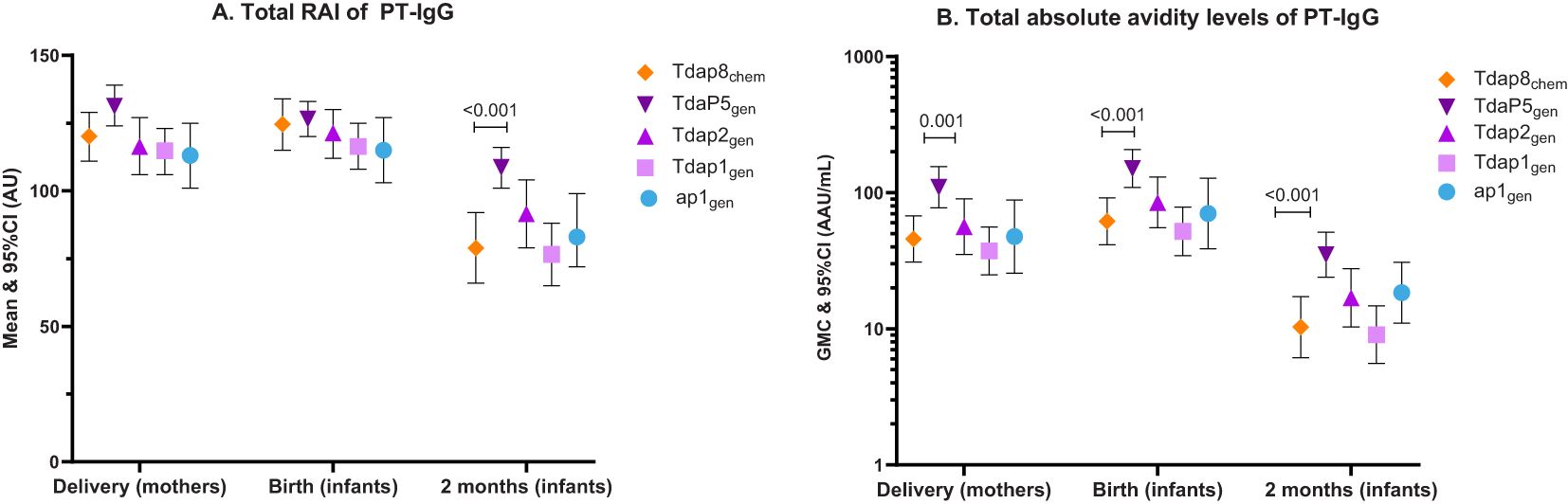
Figure 1. PT-IgG total relative avidity index and total absolute avidity in women at delivery, newborns at birth and infants at 2 months of age. The figure shows (A) means and 95% confidence intervals (CI) of PT-IgG total relative avidity (total RAI), and (B) geometric mean concentrations (GMC) and 95% CI of PT-IgG total absolute avidity levels in pregnant women at delivery and their infants at birth and 2 months of age after vaccination during pregnancy with Tdap8chem (orange; diamond);TdaP5gen (dark purple; downward triangle); Tdap2gen (purple; upward triangle); Tdap1gen (pink; square); or ap1gen (blue; circle). For each individual recombinant vaccine group responses were compared with responses for Tdap8chem using an Independent t-test: when significant (p-value ≤ 0.05), the p-value is noted.
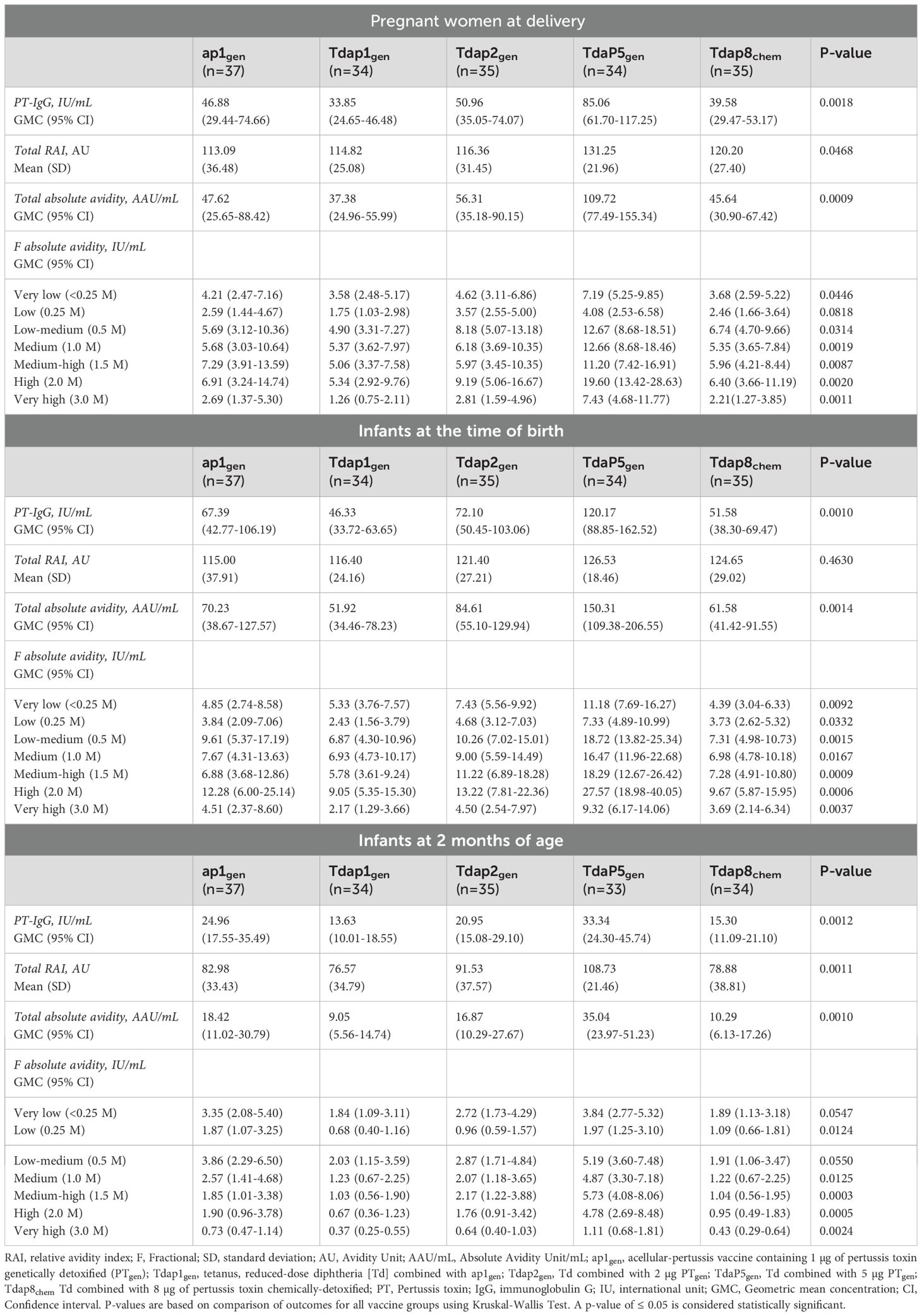
Table 2. Levels of total PT-IgG, and PT-IgG total relative avidity index, total absolute avidity, and fractional absolute avidity levels of very low to very high avidities in pregnant women at the time of delivery, and infants at the time of birth and at 2 months of age after vaccination during pregnancy with different formulations of recombinant pertussis vaccines or chemically detoxified pertussis vaccine.
3.4 Total absolute avidity levels
PT-IgG total absolute avidity was significantly higher for TdaP5gen compared with Tdap8chem in pregnant women at the time of delivery (p = 0.0011), in infants at the time of birth (p = 0.0006), and in infants at 2 months of age (p = 0.0002) (Figure 1B) (Table 2). PT-IgG total absolute avidity after vaccination with the lower-dose recombinant pertussis vaccines (ap1gen, Tdap1gen or Tdap2gen) was comparable with Tdap8chem (Figure 1B; Table 2).
3.5 Fractional absolute avidity (very-low to very-high avidity)
PT-IgG antibodies of absolute very-low to very-high avidity were comparable at the time of delivery in women vaccinated with ap1gen, Tdap1gen or Tdap2gen as compared with Tdap8chem, except for significantly higher levels of very-low avidity antibodies in infants at birth after vaccination in pregnancy with Tdap2gen versus Tdap8chem (Figures 2A-C; Table 2). Vaccination with TdaP5gen was associated with significantly higher fractional absolute avidity of PT-IgG of all strengths including medium-high, high and very-high binding strength, in pregnant women at delivery, infants at birth, and infants at 2 months of age as compared with Tdap8chem vaccination, with the exception of PT-IgG of low avidity which was comparable in women at delivery and infants at 2 months of age (Figures 2A-C; Table 2).
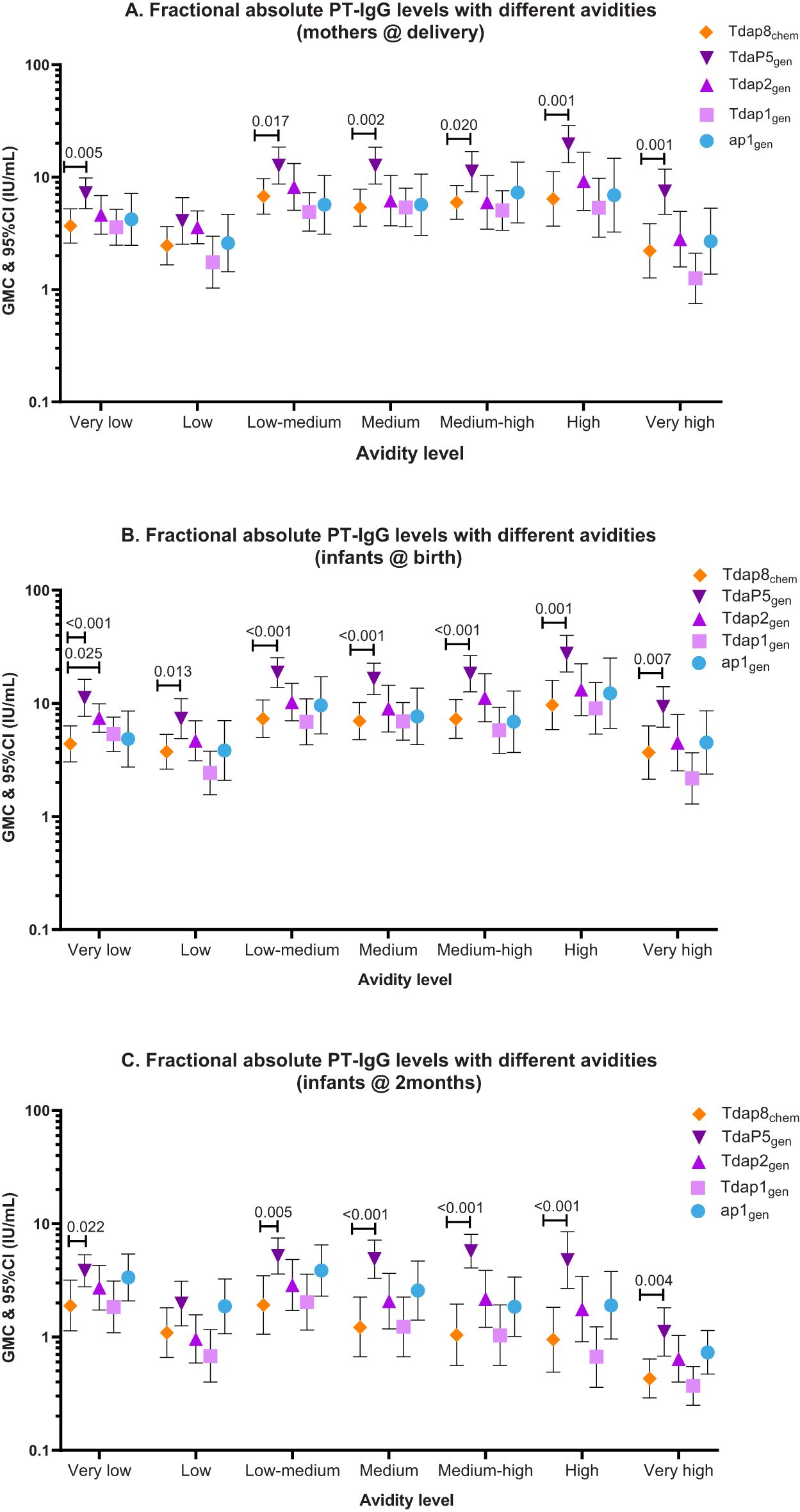
Figure 2. PT-IgG fractional absolute avidity (F abs) levels in women at delivery, newborns at birth and infants at 2 months of age. The figure shows geometric mean concentrations (GMC) and 95% confidence intervals (CI) for PT-IgG fractional absolute levels (F abs) with different avidities in (A) pregnant women at delivery, and (B) their infants at birth and (C) 2 months of age after vaccination during pregnancy with Tdap8chem (orange; diamond); TdaP5gen (dark purple; downward triangle); Tdap2gen (purple; upward triangle); Tdap1gen (pink; square)); or ap1gen (blue; circle). F abs quantified at 0.25M, 0.5M, 1M, 1.5M, 2M, and 3M of NH4SCN were classified as low, low-medium, medium, medium-high, high and very-high avidity, respectively. Responses were compared For each individual recombinant vaccine group with responses for Tdap8chem using an Independent t-test: when significant (p-value ≤ 0.05), the p-value is noted.
3.6 Effect of gestation age at the time vaccination on PT-IgG avidity
No differences in PT-IgG avidity were observed in any of the vaccine groups when comparing vaccination in the 2nd versus 3rd trimester of pregnancy. This includes total RAI, total absolute avidity levels, and fractional absolute PT-IgG levels at delivery, in infants at birth and 2 months of age (Figures 3A-C; Supplementary Table S2).
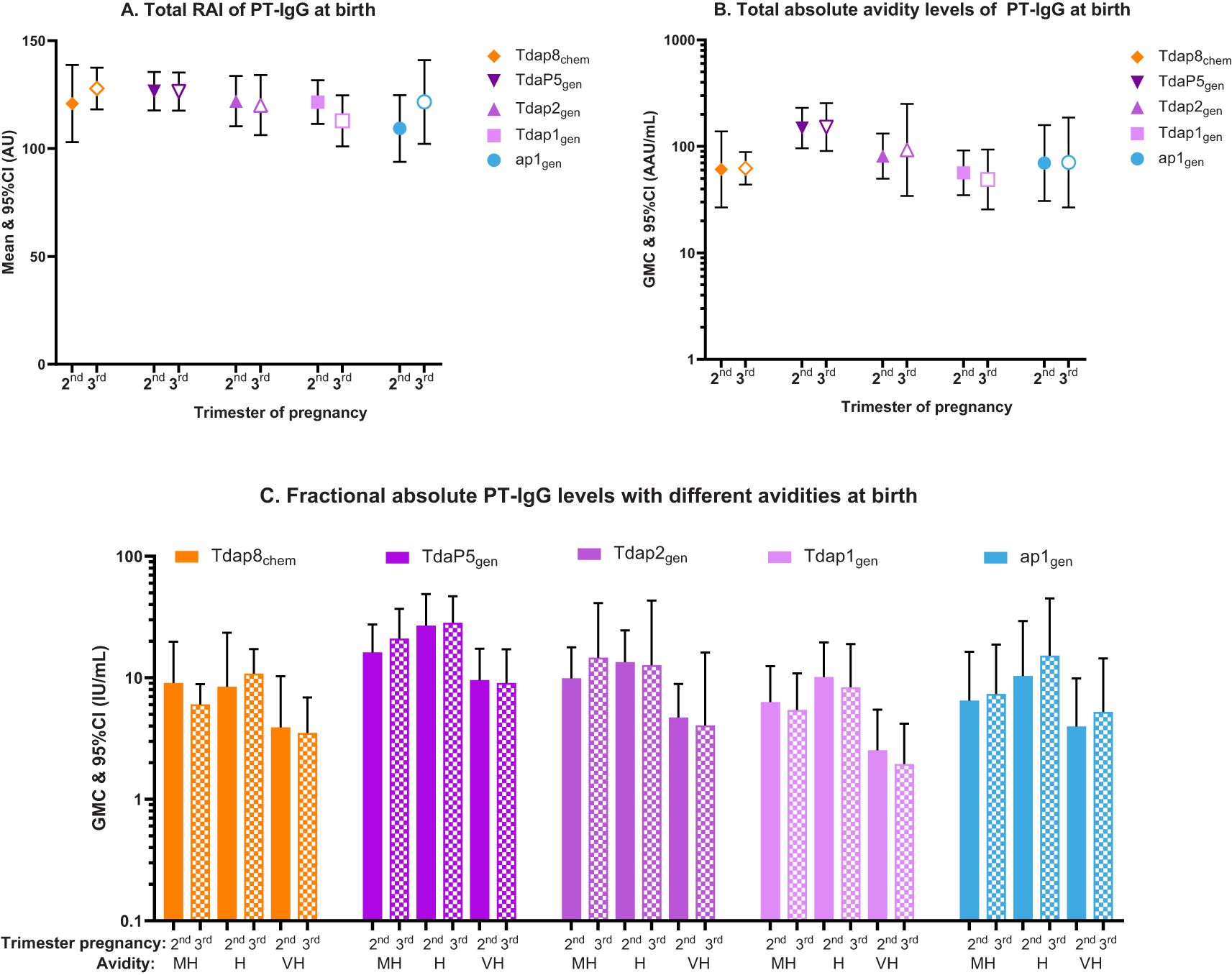
Figure 3. Total relative avidity index (Total RAI), total absolute avidity levels, and fractional absolute PT-IgG levels measured in newborns at the time of birth according to vaccination in the 2nd or 3rd trimester of pregnancy. The figure shows (A) means and 95% confidence intervals (CI) of PT-IgG total relative avidity (total RAI), (B) geometric mean concentrations (GMC) and 95% CI of PT-IgG total absolute levels, and (C) GMCs and 95% CI of fractional absolute levels (F abs) in infants at the time of birth after vaccination with Tdap8chem (orange; diamond); TdaP5gen (dark purple; downward triangle); Tdap2gen (purple; upward triangle); Tdap1gen (pink; square); or ap1gen (blue; circle) during the 2nd trimester (closed symbols and bars) or 3rd trimester (open symbols and pattern bars). Only F abs quantified at 1.5M, 2M, and 3M of NH4SCN and classified as medium-high (MH), high (H) and very-high (VH) avidity, are presented. Responses were compared for vaccination during the 2nd versus. 3rd trimester using an Independent t-test, but no statistical differences were found.
4 Discussion
To our best knowledge, this is the first study describing avidity for PT-IgG antibodies induced by recombinant acellular pertussis vaccine containing genetically inactivated PT. TdaP5gen, a licensed recombinant acellular pertussis vaccine containing 5 µg genetically inactivated PT, induced significantly higher PT-IgG avidity in pregnant women and transferred to their infants than a widely used Tdap booster containing 8 µg chemically detoxified PT. Vaccination with lower-dose recombinant acellular pertussis vaccines induced comparable PT-IgG avidity in pregnant women and infants than the comparator Tdapchem vaccine while containing 4- or 8- times less PT.
Several studies have reported on PT-IgG avidity after vaccination with conventional chemically detoxified acellular pertussis vaccines, including three studies analysing cord blood samples from cohorts following vaccination during pregnancy (40, 41, 45). These studies did not follow infants prospectively to investigate the persistence of PT-IgG avidity during the first months of life. In our study, we assessed PT-IgG avidity in cord but also in corresponding samples of mothers at the time of delivery, and longitudinally in infants at 2 months of age. We demonstrated the effective transplacental transfer of PT-IgG avidity from vaccinated mothers to newborns for all pertussis vaccines. Subsequent follow-up of infants at 2 months of age demonstrated that PT-IgG avidity remained significantly higher when mothers had been vaccinated with recombinant TdaP5gen.
A plausible explanation why genetically inactivated PT is associated with higher PT-IgG avidity is that in contrast to chemical detoxification of PT that leads to varying degrees of denaturation and loss of important protective conformational epitopes, site-specific genetic detoxification maintains the three-dimensional structure of the toxin (22, 26, 33). The crystal structure of PTgen R9K/E129G (included in the recombinant pertussis vaccines studied here) is nearly identical to that of native PT and antigen stimulation of human whole blood indicated broader immunogenicity of PTgen R9K/E129G compared with PTchem (33). Furthermore, using cryo-electron microscopy it was recently shown that two potently neutralizing anti-PT antibodies with complementary mechanisms, hu11E6 and hu1B7, bind to PTgen R9K/E129G, thereby confirming the preservation of these neutralizing binding sites in PTgen (26).
Analysis of epitope binding, PT-neutralizing antibodies, memory-B cells, and avidity are all parameters of the quality of the anti-PT immune response. It has been demonstrated in multiple clinical trials that vaccination with genetically detoxified PT induces higher PT-neutralizing antibody titers compared with licensed Tdapchem vaccines (31, 34, 35, 46). Longitudinal follow-up studies of participants vaccinated with recombinant acellular pertussis vaccine containing 5 µg PTgen have shown that PT-neutralizing antibody levels remain elevated for at least 5 years (47, 48), and following vaccination during pregnancy PT-neutralizing antibodies are effectively transferred to infants in whom they remain elevated for at least 2 months at significantly higher levels compared with Tdapchem (36, 37). Vaccination with PTgen but not PTchem also elicits robust memory B-cell responses to PT as demonstrated in a clinical trial of booster vaccination in adolescents (34). The current observations of higher PT-IgG avidity add further evidence to the higher quality of the immune response induced by genetically as compared with chemically detoxified PT (38, 49, 50).
This study further showed that recombinant vaccine containing lower quantities of PTgen (1 µg and 2 µg) elicited PT-IgG avidity comparable to Tdapchem containing 4- or 8- fold more PT. This provides further evidence that the inactivation process of PT is a critical determinant of PT-IgG avidity and that genetic versus chemical detoxification leads to higher avidity.
We also studied whether vaccination at various stages of gestation of pregnancy might affect the avidity of antibodies transferred to infants. No differences in PT-IgG avidity were observed when mothers were vaccinated during the 2nd or 3rd trimester of pregnancy for any of the studied vaccines. This is consistent with a Swiss study that analyzed cord blood samples from infants born to mothers vaccinated with Tdapchem during pregnancy, and did not find PT-IgG avidity when comparing second versus third trimester vaccination, or different intervals between vaccination and birth (51). A difference with our study is that in the Swiss study PT-IgG avidity was assessed using three concentrations of NH4SCN (1M, 2M and 3M) as compared with six in our study (0.25M, 0.5M, 1M, 1.5M, 2M and 3M) and results were presented in relative avidity indices that do not incorporate absolute antibody levels. In another study using a series of chaotropic concentrations similar to our study, PT-IgG avidity was found to be higher in newborns when mothers had been vaccinated with Tdapchem during 28–32 weeks of gestation as compared with 33–36 weeks of gestation, or when vaccinated 5–12 weeks before delivery versus within 4 weeks before delivery (41). It is plausible that using a broader range of chaotropic concentrations provides deeper insights into avidity development, thereby increasing the likelihood of detecting differential avidity responses. Other factors that may explain discrepancies in reported results include but are not limited to differences in pertussis epidemiology and vaccination history and the small sample size in this study.
Although there is no direct evidence confirming the clinical relevance of PT-IgG avidity, there is evidence from other respiratory bacterial infections supporting the notion that higher avidity provides higher protection. For example, in mice the levels of anti-pneumococcal serotype 6B-specific antibodies needed to prevent lethal bacteremia from the same serotype were found to be lower for high avidity antibodies (38). For Haemophilus influenzae type b (Hib) the avidity of antibodies induced following vaccination with Hib conjugate vaccine was shown to be a surrogate for protective immunity (49). Therefore, it may be assumed that the higher PT-IgG avidity response induced by vaccination with PTgen containing vaccine contributes to improved protection compared to PTchem. While there are no efficacy trials for the current new generation of recombinant acellular pertussis vaccines, it has previously been reported that the efficacy of a former pediatric recombinant acellular pertussis vaccine was comparable to that of chemically detoxified acellular pertussis vaccine whilst containing 5-times less PT (50). In our study, formulations of recombinant pertussis vaccine with 4-to-8-times less PT content than the chemically detoxified comparator induced similar PT-IgG avidity, which may translate into similar efficacy.
Our study has strengths and limitations. Our study is unique in that it provides detailed characterization of a full spectrum of avidity of PT-IgG for different PTgen doses and formulations of recombinant acellular pertussis vaccine. Using a dilution series of NH4SCN to provide the whole spectrum of avidity is essential considering the lack of knowledge of a clinically relevant levels avidity (42). In addition, antibody avidity was not only assessed in infants at birth, but also at 2 months old, and in the vaccinated mothers. This makes it one of the most comprehensive studies on PT-IgG antibody avidity following pertussis vaccination in pregnancy. Longer follow-up of infants beyond 2 months of age would have enabled demonstrating the persistence of elevated PT-IgG avidity and potential longer-lasting protection offered by maternal Tdap5gen vaccination in infants; however, infants received childhood DTP vaccines starting at 2 months of age and assessing PT-IgG avidity in children following primary immunization was out of the scope of this study. In the main clinical trial, however, it was demonstrated that at 5 months of age (1 month after infants had completed the 2nd priming dose), PT-IgG levels remained significantly higher in infants whose mothers had received TdaP5gen versus Tdap8chem: a difference that may be explained by the persistence of higher maternal PT-IgG levels in the maternal recombinant TdaP5gen vaccine group (52).Assessing PT-IgG avidity in infants where the local recommendation is to start the first priming dose at 3 months of age or older, may be something to consider for a future study. Other limitations include the relatively small sample size which could affect the generalizability of the results and limit statistical power, and that we did not analyze PT-IgG avidity in baseline samples in pregnant women before vaccination. Pre-vaccination PT-IgG levels had been assessed earlier and found to be low in all study groups (36): measurement of avidity would not have yielded quantifiable levels that can be analyzed. It is also yet to be studied how vaccination history may impact avidity responses. Like most pregnant women worldwide, including in countries that changed to priming with acellular pertussis vaccines, pregnant women participating in our study were vaccinated in childhood with whole cell pertussis vaccines and are unlikely to have received pertussis booster vaccines after priming in infancy (44). Studies in forthcoming years, when relatively more pregnant women will have vaccinated exclusively with acellular pertussis vaccines, may show how this affects PT-IgG antibody avidity in infants of mothers vaccinated in pregnancy.
In conclusion, the method that is used to inactivate PT for immunization influences PT-IgG avidity. Vaccination during pregnancy with recombinant acellular pertussis vaccines containing genetically detoxified PT at lower content than acellular pertussis vaccines containing chemically detoxified PT results in efficient transplacental transfer of at least similar or higher quantity and quality anti-PT antibodies. Vaccination with recombinant acellular pertussis vaccine may therefore provide infants with highly efficient and longer-lasting immune protection during the first most vulnerable months in life, but this remains to be studied.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon request.
Ethics statement
The studies involving humans were approved by the Institutional Review Boards of the Faculty of Medicine Siriraj Hospital at Mahidol University, Faculty of Medicine at Chulalongkorn University, Bangkok, Thailand and Western Institutional Review Board (now known as WIRB-Copernicus Group), Washington, USA. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
BA-R: Formal analysis, Writing – original draft, Writing – review & editing, Conceptualization, Investigation, Methodology, Supervision. GG: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AB: Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. YT: Formal analysis, Writing – original draft, Writing – review & editing. NB: Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. HTP: Funding acquisition, Investigation, Resources, Supervision, Writing – original draft, Writing – review & editing. WW: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by a grant from the Bill & Melinda Gates Foundation Seattle, USA (grant number OPP1120084). The findings and conclusions contained within are those of the authors and do not necessarily reflect the positions or policies of the Bill & Melinda Gates Foundation.
Acknowledgments
We thank all study participants (pregnant women and their infants), study investigators, and clinical staff at the Faculty of Medicine Siriraj Hospital, Mahidol University and Center of Excellence in Pediatric Infectious Diseases and Vaccines, Chulalongkorn University, for their valuable contributions to the clinical trial that provided the serum samples of this analysis. We also thank BioNet-Asia’s clinical operations team for their significant contributions to the clinical trials, and ethical approval and sample arrangement for this supply chain team for sample shipment, and the members of BioNet scientific advisory board for their advice. We also thank BIOPHICS for data management and statistical analysis, and the PATH team.
Conflict of interest
Author BA-R received honoraria for participation in live meetings from Sanofi Pasteur France and Canada related to pertussis and RSV. BA-R received nominal payment as a reviewer for ELSEVIER and as a member of a data and safety monitoring board for a study conducted by Chulalongkorn University (Bangkok, Thailand). BA-R is co-investigator on studies funded by GSK, Pfizer, Merck, Moderna, Vaccitech and Inventprise. All funds have been paid to his institute, and he has not received any personal payments. Authors GG and AB received consultancy honoraria from BioNet-Asia including for the published work. Authors WW and HTP are employees of BioNet-Asia.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1569151/full#supplementary-material
References
1. Decker MD and Edwards KM. Pertussis (Whooping cough). J Infect Dis. (2021) 224:S310–s20. doi: 10.1093/infdis/jiaa469
2. European Centre for Disease Prevention and Control E. Increase of pertussis cases in the EU/EEA. Stockholm: ECDC (2024).
3. Nordholm AC, Emborg HD, Nørgaard SK, Nygaard U, Ronayne A, Nielsen LB, et al. Pertussis epidemic in Denmark, August 2023 to February 2024. Euro Surveill. (2024) 29. doi: 10.2807/1560-7917.es.2024.29.14.2400160
4. UK Health Security Agency. Confirmed cases of pertussis in England by month . Available online at: https://www.gov.uk/government/publications/pertussis-epidemiology-in-england-2024/confirmed-cases-of-pertussis-in-england-by-month (Accessed December 1, 2024).
5. Holt E. Pertussis outbreak in Czech Republic. Lancet Infect Dis. (2024) 24:e359. doi: 10.1016/s1473-3099(24)00291-3
6. Winter K, Zipprich J, Harriman K, Murray EL, Gornbein J, Hammer SJ, et al. Risk factors associated with infant deaths from pertussis: A case-control study. Clin Infect Dis. (2015) 61:1099–106. doi: 10.1093/cid/civ472
7. Abu-Raya B, Bettinger JA, Vanderkooi OG, Vaudry W, Halperin SA, Sadarangani M, et al. Burden of children hospitalized with pertussis in Canada in the acellular pertussis vaccine era, 1999-2015. J Pediatr Infect Dis Soc. (2020) 9:118–27. doi: 10.1093/jpids/piy128
8. Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. (2014) 384:1521–8. doi: 10.1016/s0140-6736(14)60686-3
9. Baxter R, Bartlett J, Fireman B, Lewis E, and Klein NP. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics. (2017) 139. doi: 10.1542/peds.2016-4091
10. Fernandes EG, Sato APS, Vaz-de-Lima LRA, Rodrigues M, Leite D, de Brito CA, et al. The effectiveness of maternal pertussis vaccination in protecting newborn infants in Brazil: A case-control study. Vaccine. (2019) 37:5481–4. doi: 10.1016/j.vaccine.2019.03.049
11. Kandeil W, van den Ende C, Bunge EM, Jenkins VA, Ceregido MA, and Guignard A. A systematic review of the burden of pertussis disease in infants and the effectiveness of maternal immunization against pertussis. Expert Rev Vaccines. (2020) 19:621–38. doi: 10.1080/14760584.2020.1791092
12. Paddock CD, Sanden GN, Cherry JD, Gal AA, Langston C, Tatti KM, et al. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis. (2008) 47:328–38. doi: 10.1086/589753
13. Halasa NB, Barr FE, Johnson JE, and Edwards KM. Fatal pulmonary hypertension associated with pertussis in infants: does extracorporeal membrane oxygenation have a role? Pediatrics. (2003) 112:1274–8. doi: 10.1542/peds.112.6.1274
14. Scanlon K, Skerry C, and Carbonetti N. Association of pertussis toxin with severe pertussis disease. Toxins (Basel). (2019) 11. doi: 10.3390/toxins11070373
15. Taranger J, Trollfors B, Lagergard T, Sundh V, Bryla DA, Schneerson R, et al. Correlation between pertussis toxin IgG antibodies in postvaccination sera and subsequent protection against pertussis. J Infect Dis. (2000) 181:1010–3. doi: 10.1086/315318
16. Dalby T, Andersen PH, and Hoffmann S. Epidemiology of pertussis in Denmark, 1995 to 2013. Euro Surveill. (2016) 21:30334. doi: 10.2807/1560-7917.es.2016.21.36.30334
17. Gregg KA and Merkel TJ. Pertussis toxin: a key component in pertussis vaccines? Toxins (Basel). (2019) 11:557. doi: 10.3390/toxins11100557
18. Nguyen AW, DiVenere AM, Papin JF, Connelly S, Kaleko M, and Maynard JA. Neutralization of pertussis toxin by a single antibody prevents clinical pertussis in neonatal baboons. Sci Adv. (2020) 6:eaay9258. doi: 10.1126/sciadv.aay9258
19. Healy CM, Rench MA, Swaim LS, Timmins A, Vyas A, Sangi-Haghpeykar H, et al. Kinetics of maternal pertussis-specific antibodies in infants of mothers vaccinated with tetanus, diphtheria and acellular pertussis (Tdap) during pregnancy. Vaccine. (2020) 38:5955–61. doi: 10.1016/j.vaccine.2020.06.050
20. Abu-Raya B, Forsyth K, Halperin SA, Maertens K, Jones CE, Heininger U, et al. Vaccination in pregnancy against pertussis: a consensus statement on behalf of the global pertussis initiative. Vaccines (Basel). (2022) 10:1990. doi: 10.3390/vaccines10121990
21. Barkoff AM, Knuutila A, Mertsola J, and He Q. Evaluation of anti-PT antibody response after pertussis vaccination and infection: the importance of both quantity and quality. Toxins (Basel). (2021) 13. doi: 10.3390/toxins13080508
22. Ibsen PH. The effect of formaldehyde, hydrogen peroxide and genetic detoxification of pertussis toxin on epitope recognition by murine monoclonal antibodies. Vaccine. (1996) 14:359–68. doi: 10.1016/0264-410x(95)00230-x
23. Sutherland JN, Chang C, Yoder SM, Rock MT, and Maynard JA. Antibodies recognizing protective pertussis toxin epitopes are preferentially elicited by natural infection versus acellular immunization. Clin Vaccine Immunol. (2011) 18:954–62. doi: 10.1128/cvi.00561-10
24. Van den Biggelaar AHJ and Poolman JT. Predicting future trends in the burden of pertussis in the 21st century: implications for infant pertussis and the success of maternal immunization. Expert Rev Vaccines. (2016) 15:69–80. doi: 10.1586/14760584.2016.1105136
25. Eberhardt CS and Siegrist CA. What is wrong with pertussis vaccine immunity? Inducing and recalling vaccine-specific immunity. Cold Spring Harb Perspect Biol. (2017) 9. doi: 10.1101/cshperspect.a029629
26. Goldsmith JA, Nguyen AW, Wilen RE, Wijagkanalan W, McLellan JS, and Maynard JA. Structural basis for antibody neutralization of pertussis toxin. PNAS. (2025) 122:e2419457122. doi: 10.1073/pnas.2419457122
28. Del Giudice G and Rappuoli R. Genetically derived toxoids for use as vaccines and adjuvants. Vaccine. (1999) 17 Suppl 2:S44–52. doi: 10.1016/s0264-410x(99)00234-0
29. Buasri W, Impoolsup A, Boonchird C, Luengchaichawange A, Prompiboon P, Petre J, et al. Construction of Bordetella pertussis strains with enhanced production of genetically-inactivated Pertussis Toxin and Pertactin by unmarked allelic exchange. BMC Microbiol. (2012) 12:61. doi: 10.1186/1471-2180-12-61
30. Leroux-Roels G, Lattanzi M, Solis CD, Contorni M, Costantini M, Moraschini L, et al. randomized, controlled, dose-ranging study of investigational acellular pertussis (aP) and reduced tetanus-diphtheria-acellular pertussis (TdaP) booster vaccines in adults. Hum Vaccin Immunother. (2017) 14:45–58. doi: 10.1080/21645515.2017.1385686
31. Sricharoenchai S, Sirivichayakul C, Chokephaibulkit K, Pitisuttithum P, Dhitavat J, Pitisuthitham A, et al. A genetically inactivated two-component acellular pertussis vaccine, alone or combined with tetanus and reduced-dose diphtheria vaccines, in adolescents: a phase 2/3, randomised controlled non-inferiority trial. Lancet Infect Dis. (2018) 18:58–67. doi: 10.1016/s1473-3099(17)30612-6
32. Auderset F, Ballester M, Mastelic-Gavillet B, Fontannaz P, Chabaud-Riou M, Reveneau N, et al. Reactivating immunity primed by acellular pertussis vaccines in the absence of circulating antibodies: enhanced bacterial control by TLR9 rather than TLR4 agonist-including formulation. Front Immunol. (2019) 10:1520. doi: 10.3389/fimmu.2019.01520
33. Ausar SF, Zhu S, Duprez J, Cohen M, Bertrand T, Steier V, et al. Genetically detoxified pertussis toxin displays near identical structure to its wild-type and exhibits robust immunogenicity. Commun Biol. (2020) 3:427. doi: 10.1038/s42003-020-01153-3
34. Blanchard Rohner G, Chatzis O, Chinwangso P, Rohr M, Grillet S, Salomon C, et al. Boosting teenagers with acellular pertussis vaccines containing recombinant or chemically inactivated pertussis toxin: a randomized clinical trial. Clin Infect Dis. (2018) 68:1213–22. doi: 10.1093/cid/ciy594
35. Chokephaibulkit K, Puthanakit T, Bhat N, Mansouri S, Tang Y, Lapphra K, et al. A phase 2 randomized controlled dose-ranging trial of recombinant pertussis booster vaccines containing genetically inactivated pertussis toxin in women of childbearing age. Vaccine. (2022) 40:2352–61. doi: 10.1016/j.vaccine.2021.10.076
36. Puthanakit T, Chokephaibulkit K, Chaithongwongwatthana S, Bhat N, Tang Y, Anugulruengkitt S, et al. A phase 2 randomized controlled dose-ranging trial of recombinant pertussis booster vaccines containing genetically inactivated pertussis toxin in pregnant women. Vaccine. (2023) 31:4541–53. doi: 10.1016/j.vaccine.2023.06.001
37. Chokephaibulkit K, Puthanakit T, Chaithongwongwatthana S, Bhat N, Tang Y, Anugulruengkitt S, et al. Effective and safe transfer of maternal antibodies persisting two months postpartum following maternal immunization with different doses of recombinant pertussis-containing vaccines. Vaccine. (2024) 42:383–95. doi: 10.1016/j.vaccine.2023.11.042
38. Usinger WR and Lucas AH. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun. (1999) 67:2366–70. doi: 10.1128/iai.67.5.2366-2370.1999
39. Oostindie SC, Lazar GA, Schuurman J, and Parren P. Avidity in antibody effector functions and biotherapeutic drug design. Nat Rev Drug Discov. (2022) 21:715–35. doi: 10.1038/s41573-022-00501-8
40. Abu Raya B, Bamberger E, Almog M, Peri R, Srugo I, and Kessel A. Immunization of pregnant women against pertussis: the effect of timing on antibody avidity. Vaccine. (2015) 33:1948–52. doi: 10.1016/j.vaccine.2015.02.059
41. Abu-Raya B, Giles ML, Kollmann TR, and Sadarangani M. The effect of timing of tetanus-diphtheria-acellular pertussis vaccine administration in pregnancy on the avidity of pertussis antibodies. Front Immunol. (2019) 10:2423. doi: 10.3389/fimmu.2019.02423
42. Knuutila A, Dalby T, Ahvenainen N, Barkoff AM, Jørgensen CS, Fuursted K, et al. Antibody avidity to pertussis toxin after acellular pertussis vaccination and infection. Emerg Microbes Infect. (2023) 12:e2174782. doi: 10.1080/22221751.2023.2174782
43. Abu-Raya B, Giles ML, Kollmann TR, and Sadarangani M. Profiling avidity of antibodies elicited by vaccination using enzyme-linked immunosorbent assay-based elution - Insights into a novel experimental and analytical approach. Vaccine. (2020) 38:5389–92. doi: 10.1016/j.vaccine.2020.06.060
44. Blackwood JC, Cummings DA, Broutin H, Iamsirithaworn S, and Rohani P. Deciphering the impacts of vaccination and immunity on pertussis epidemiology in Thailand. Proc Natl Acad Sci U S A. (2013) 110:9595–600. doi: 10.1073/pnas.1220908110
45. Cabore RN, Maertens K, Dobly A, Leuridan E, Van Damme P, and Huygen K. Influence of maternal vaccination against diphtheria, tetanus, and pertussis on the avidity of infant antibody responses to a pertussis containing vaccine in Belgium. Virulence. (2017) 8:1245–54. doi: 10.1080/21505594.2017.1296998
46. Puthanakit T, Tangsathapornpong A, Anugulruengkitt S, Nantanee R, Bunjoungmanee P, Mansouri S, et al. A reduced-dose recombinant pertussis vaccine booster in Thai adolescents: a phase 2/3, observer-blinded, randomised controlled, non-inferiority trial. Lancet Child Adolesc Health. (2024) 8:900–9. doi: 10.1016/s2352-4642(24)00173-1
47. Pitisuttithum P, Dhitavat J, Sirivichayakul C, Pitisuthitham A, Sabmee Y, Chinwangso P, et al. Antibody persistence 2 and 3 years after booster vaccination of adolescents with recombinant acellular pertussis monovalent aP(gen) or combined TdaP(gen) vaccines. EClinicalMedicine. (2021) 37:100976. doi: 10.1016/j.eclinm.2021.100976
48. Pitisuttithum P, Sirivichayakul C, Dhitavat J, Pitisuthitham A, Mansouri S, and Pham HT. Pertussis immunity 5 years after booster vaccination with recombinant pertussis vaccines. JAMA Netw Open. (2024) 7:e2449182. doi: 10.1001/jamanetworkopen.2024.49182
49. Goldblatt D, Vaz AR, and Miller E. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J Infect Dis. (1998) 177:1112–5. doi: 10.1086/517407
50. Rappuoli R. The vaccine containing recombinant pertussis toxin induces early and long-lasting protection. Biologicals. (1999) 27:99–102. doi: 10.1006/biol.1999.0189
51. Sartoretti J, Fontannaz P, Martinez de Tejada B, Othenin-Girard V, Chilin A, Lemaître B, et al. Influence of timing of maternal pertussis immunization on the avidity of transferred antibodies in term and preterm neonates. Clin Infect Dis. (2023) 77:645–8. doi: 10.1093/cid/ciad227
52. Puthanakit T, Chokephaibulkit K, Anugulruengkitt S, Chaithongwongwatthana S, Phongsamart W, Wittawatmongkol O, et al. Infant responses to primary immunization following vaccination in pregnancy with varying doses of recombinant acellular pertussis vaccine alone or combined with tetanus-diphtheria. Pediatr Infect Dis J. (2025) 44:S56–s60. doi: 10.1097/inf.0000000000004609
Keywords: pertussis, avidity, pertussis toxin, genetically inactivated, recombinant vaccine, maternal immunization, vaccination during pregnancy
Citation: Abu-Raya B, Del Giudice G, van den Biggelaar AHJ, Tang Y, Bhat N, Pham HT and Wijagkanalan W (2025) Avidity of pertussis toxin antibodies following vaccination with genetically versus chemically detoxified pertussis toxin-containing vaccines during pregnancy. Front. Immunol. 16:1569151. doi: 10.3389/fimmu.2025.1569151
Received: 31 January 2025; Accepted: 23 April 2025;
Published: 22 May 2025.
Edited by:
Sonia Jangra, The Rockefeller University, United StatesReviewed by:
Alex-Mikael Barkoff, University of Turku, FinlandAapo Knuutila, University of Turku, Finland
Copyright © 2025 Abu-Raya, Del Giudice, van den Biggelaar, Tang, Bhat, Pham and Wijagkanalan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anita H. J. van den Biggelaar, YW5pdGFAYmlvbmV0Lm9uZQ==; Wassana Wijagkanalan, d2Fzc2FuYS53QGJpb25ldC1hc2lhLmNvbQ==
 Bahaa Abu-Raya
Bahaa Abu-Raya Giuseppe Del Giudice4
Giuseppe Del Giudice4 Anita H. J. van den Biggelaar
Anita H. J. van den Biggelaar Wassana Wijagkanalan
Wassana Wijagkanalan