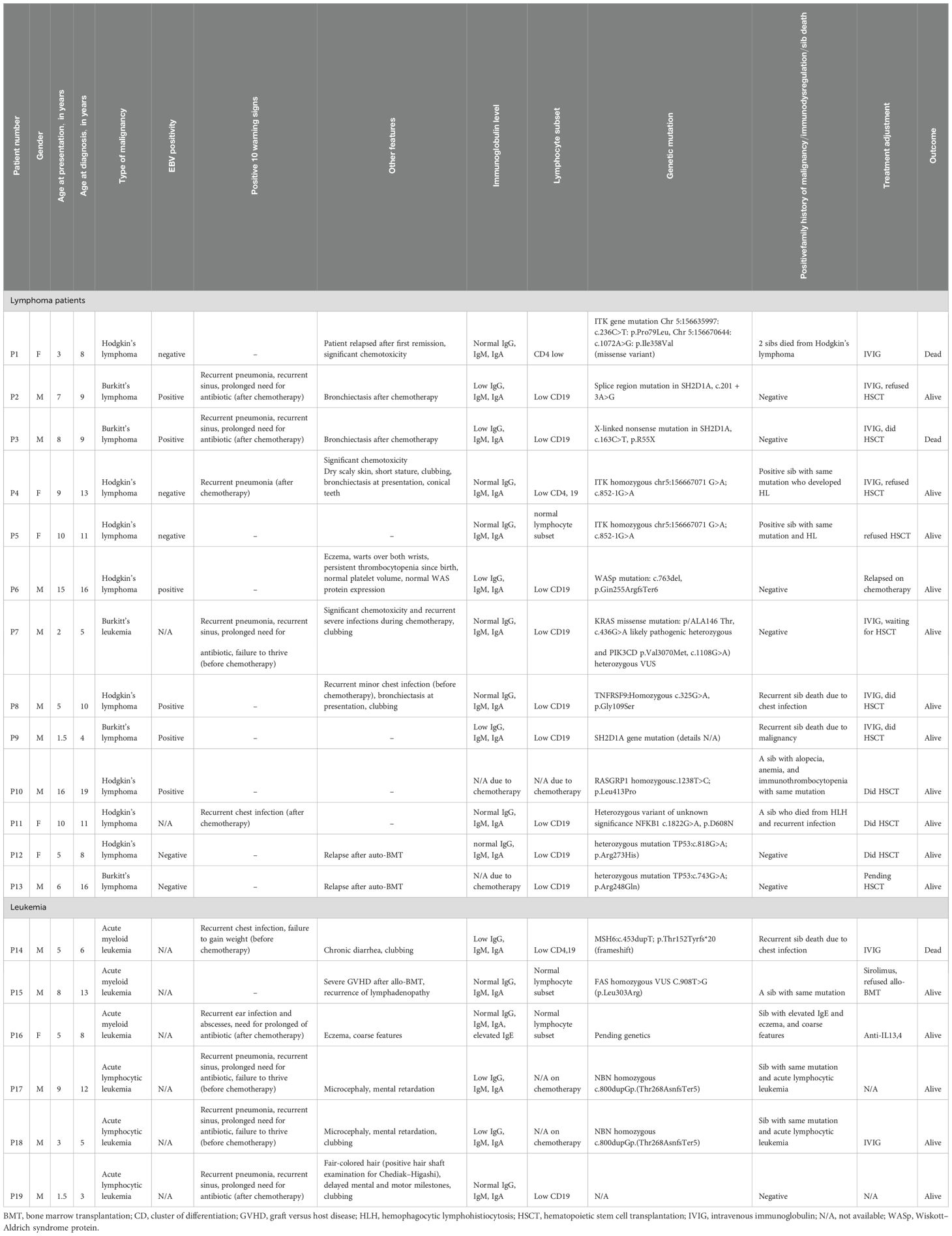
- 1Pediatric Allergy, Immunology & Rheumatology unit, Children’s hospital, Ain Shams University, Cairo, Egypt
- 2Pediatric department, Children Cancer Hospital Egypt (CCHE-57357), Cairo, Egypt
- 3Pediatric Oncology Department, National Cancer Institute, Cairo University, Cairo, Egypt
- 4Clinical Pathology Department, National Cancer Institute, Cairo University, Cairo, Egypt
Background: Inborn errors of immunity (IEI) are a heterogeneous group of different disorders characterized by a defect in the function and/or components of the immune system. Malignancy is the second common cause of death following recurrent infections.
Aim: We present our experience in Children Cancer Hospital Egypt (CCHE-57357) in diagnosing IEI patients who first presented with malignancy rather than infections.
Methods: Data of 19 IEI patients with malignancy referred to the immunology clinic was collected. The reasons for referral were stunted growth or presence of bronchiectasis at presentation, persistent eczema, significant chemotoxicity, history of recurrent infection either during or after stoppage of chemotherapy, and relapse of lymphoid malignancy after auto-BMT.
Results: The patients comprised 14/5 men/women. Their median age at diagnosis with malignancy was 7 years (1.5–16 years). In addition, 13/19 had lymphoma (Hodgkin’s/non-Hodgkin’s) and 6/19 patients had leukemia. Moreover, 9/19 had history of repeated infections, 4/19 had failure to thrive, 5/19 had clubbing, 4/19 had bronchiectasis, 3/19 had significant chemotoxicity, 8/19 had low immunoglobulin, 12/19 had abnormal lymphocyte subsets, and 3/19 had a relapse of the original disease. Genetic testing was done to 18/19. The diagnoses based on genetic and/or immunological investigation according to the IUIS classification were 7/19 (37%) immune-dysregulation, 4/19 (21%) combined immunodeficiency with syndromic features, 3/19 (15.7%), combined immunodeficiency, 3/19 (15.7%) predominantly antibody defect, and 2/19 (10.5%) bone marrow failure defect.
Conclusion: Collaborative work between immunologist and oncologist helped in diagnosing patients with IEI who first presented with malignancy.
1 Introduction
Inborn errors of immunity (IEIs) are a group of inherited diseases that are caused by damaging germline variants in single genes affecting the immune cell function and/or number. IEIs have a wide range of clinical picture. Patients usually present with an increased susceptibility to infections (1), and they can also present with autoimmune manifestation, autoinflammation, allergy, bone marrow failure, and/or malignancy (1). This broad clinical picture causes the patients to present to a wide array of providers ranging from primary care physicians to various pediatric subspecialists (2).
Malignancy in IEI patients is a leading cause of mortality following infections. There is a relatively higher risk of mortality due to either disease-related or treatment-related causes in comparison to other patients, and this risk varies between different malignancy subtypes (3–5). It is reported that malignancy can develop in 1.5%–25% of IEI patients, which is considered a relatively high incidence (6, 7). Any type of malignancy can occur, but the most common is lymphoma, representing around 60% of cases, with non-Hodgkin’s lymphoma (NHL) being the greatest contributor (8–10).
This high incidence of malignancy is attributed to both intrinsic and extrinsic factors that initiate and progress the malignant transformation (11–14). Intrinsic factors include various factors such as errors in cell apoptosis, accelerated immune senescence, abnormalities involving cell development and/or signaling, actin cytoskeleton, cytotoxicity, DNA repair, chromosome instability, and telomere maintenance (11, 12, 14, 15). Extrinsic factors include chronic tissue inflammation and numerous infectious agents associated with oncogenic viruses such as Epstein–Barr virus (EBV) in lymphoproliferative conditions and soft tissue tumors, human papillomavirus (HPV) in epithelial tumors, and Helicobacter pylori in stomach cancer (13, 16).
Recurrent infections are usually the main presentation of IEIs. However, some patients may present with malignancy as a first clinically noted presentation, especially in patients with delayed onset or mild IEI phenotypes. The early onset of cancer or a recurrence of lymphoma should raise the suspicion of an underlying IEI (17–20). In a French national registry that retrospectively followed 1375 IEI patients for 10 years. At the beginning of the study, 37% of patients suffered from noninfectious complications, and it was increased by an additional 20%. Malignancy occurred in 7% of patients. A total of 14% of the patients died during the study; 43% of those deaths were due to noninfectious events, of which one quarter was due to malignancy (21).
Treatment of malignancy in IEI patients is challenging as they respond differently to cancer treatment. They can experience increased toxicity and/or decreased efficacy of standard cancer treatment, and unfortunately it is very difficult to predict such events, even within a single IEI subtype. They also suffer from an increased risk of malignancy recurrence (3). Albeit it was found that some children with co-existent IEIs and malignancy might benefit from adjustment in the treatment plan such as dose modifications of chemotherapy, avoidance of radiotherapy, use of antimicrobial prophylaxis, or immunoglobulin replacement therapy (5). But there are no clear guidelines for that. Conversely, cancer recurrence poses the greatest threat in some IEI patients, and accordingly it may not be desirable for dose reductions to be uniformly applied. Hematopoietic stem cell transplantation (HSCT) should be considered in some patients based on diagnosis and medical condition. The aim of HSCT is to achieve both malignant disease control and treatment of the underlying IEI. However, before any of these treatment modifications can be implemented, the diagnosis of IEI must have been considered and confirmed by clinical, laboratory, and genetic testing (3, 21).
Data are rare about patients who first present with malignancy, and the presence of a diagnostic workflow for those patients is still not well developed. Bosch JVWT et al. (3) suggested a diagnostic workflow to be used to help in the diagnosis of those patients. It included a careful review of the patient’s history and physical examination, combined with careful use of routine laboratory investigations and pathological review aiming at identifying patients who warrant additional investigation.
In this study, we aimed to present our experience in Children Cancer Hospital Egypt (CCHE-57357) in diagnosing IEI patients who first presented and were diagnosed with malignancy rather than recurrent infections.
2 Methodology
This is a retrospective descriptive study conducted on patients having malignancy and referred to the immunology clinic in Children Cancer Hospital Egypt (CCHE-57357) for suspicion of an underlying IEI. The causes of referral were either one or more of the following: history of repeated severe infections during or after chemotherapy, presence of bronchiectasis, clubbing or stunted growth at presentation with malignancy, and recurrence of lymphoid malignancy after auto-bone marrow transplantation. The inclusion criteria refer to patients who presented with malignancy and were found to have an underlying IEI by genetic sequencing and/or specific confirmatory laboratory tests. The exclusion criteria refer to patients known to be with IEI and who developed malignancy as patients with ataxia telangiectasia.
All patients were subjected to the following: (1) provide a detailed history, which included age at recruitment, age at diagnosis with malignancy, parental consanguinity, living town, family history of malignancy or recurrent infection specifically the siblings, and history of any of the 10 warning signs before and after chemotherapy; (2) clinical examination was done, and it included anthropometric measures and general examination with stress on the presence of clubbing, organomegaly, eczema, or any peculiar facies; and (3) laboratory examination was done at CCHE-57357, and it included complete blood count with differential and serum immunoglobulin levels (IgG, IgM, and IgA) by nephelometry and flow cytometry assessment for lymphocyte subsets that included both absolute and lymphocyte count. Immunological labs were done before starting chemotherapy and at least after 6 months of stopping the treatment. The lymphocyte subsets included CD3, CD4, CD8, CD19, CD56, CD45RO, CD45RA, CD19+ CD27+ Ig D- (class-switched memory B cells), and CD19+ Ig D+ CD27- (naïve B cells). The flow cytometry specimens include fresh EDTA and heparin blood used for lymphocyte subset enumeration. Mononuclear cell separation was done using Ficol separation technique. It includes analysis for CD3, CD4, CD8, CD16, CD19, CD20, CD45, CD56, CD45RA, CD45RO, CD27, and IgD; the fluorochromes used for staining are as per panel. The samples were analyzed by using multicolor flow cytometry (Coulter Navios EX). Then, Kaloza software was applied for analysis. Whole exome sequencing (WES) was done either in commercial labs or institutional labs.
A consent form was obtained from the children’s legal guardian, and ethical approval was obtained from the Institutional Review Board (IRB) at the Children’s Cancer Hospital—Egypt (CCHE/57357), IRB #N-0059–015-025.
3 Results
The immunology clinic was established in CCHE-57357 in November 2018. During the period 2018–2023, 283 patients were referred to the immunology clinic because of one of the following reasons: (a) known IEI patients who developed malignancy and under chemotherapy treatment for regular immunological follow-up, (b) patients with non-malignant lymphoproliferation for possibility of an underlying immunological disease, (c) patients diagnosed with malignancy who developed recurrent infections during chemotherapy or after stoppage of chemotherapy, (d) patients who had recurrence of lymphoid malignancy after auto-BMT, (e) those who had peculiar presentations such as clubbing, stunted growth, and bronchiectasis at the time of malignancy diagnosis for possibility of underlying IEIs, and (f) patients with a history of sib death due to either malignancy or recurrent infections.
Out of the 283 patients, 49 patients were suspected to have an underlying IEI based on a history of recurrent infections during their follow-up or peculiar presentation at diagnosis of malignancy or positive family history. The confirmation of IEI diagnosis in 19 patients was based on mainly at least two of the following conditions: genetic sequencing along with clinical phenotype and immunological laboratory. Three patients were suspected to have IEI based on a history of recurrent severe infections during chemotherapy, low CD19, and low IgG, but genetic sequencing did not show any mutation. A total of 19 patients were confirmed to have an underlying IEI. The diagnosis of IEI was not confirmed in the remaining 27 patients as the clinical phenotype and basic immunological labs done were not enough to confirm a final diagnosis on whether it was a primary or a secondary immunodeficiency (Figure 1).
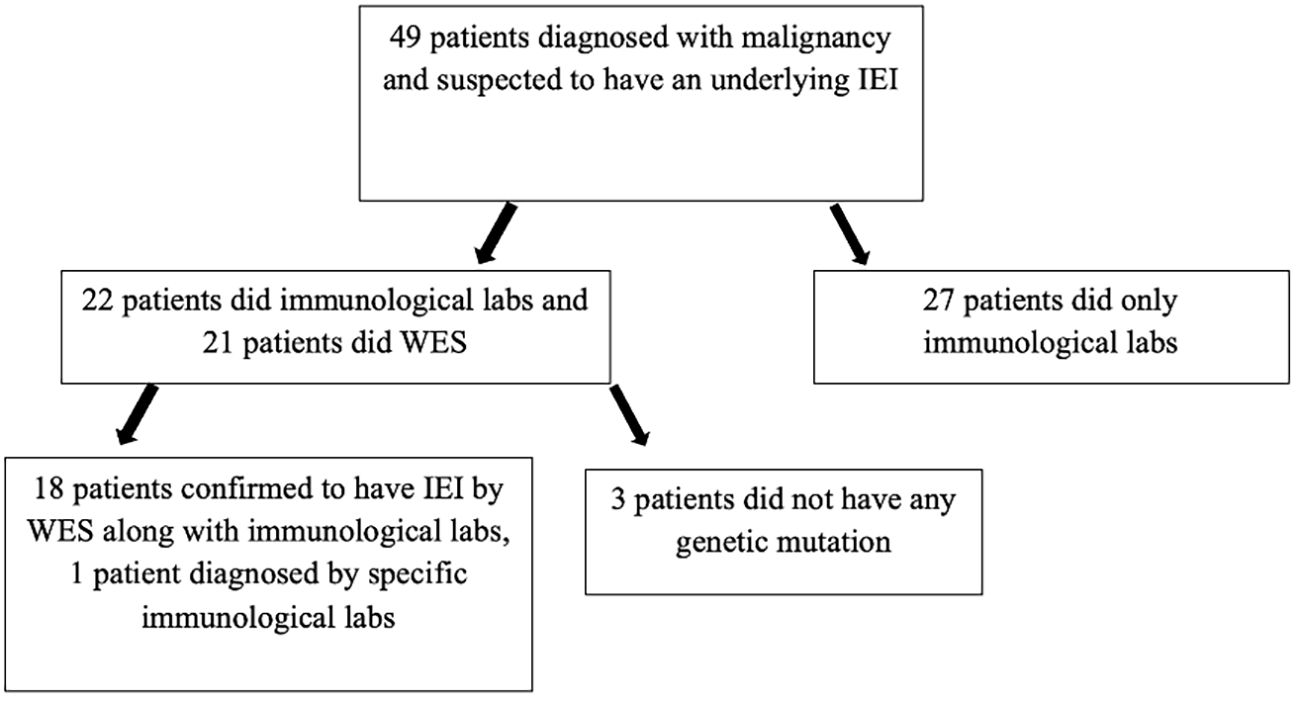
Figure 1. Patients diagnosed with malignancy and who were suspected to have an underlying IEI. WES, whole exome sequencing.
Those 27 patients had the following clinical phenotypes: (a) recurrent infections (recurrent abscesses, recurrent bronchopneumonia, and invasive fungal infections), (b) autoimmune manifestation following chemotherapy as vasculitis, immune thrombocytopenia, nephritis, and arthritis, and (c) peculiar clinical and radiological findings at presentation with malignancy (bronchiectasis, eczema, clubbing, and failure to thrive). The immunological labs showed the following: 10 patients had abnormal lymphocyte subsets, and eight patients had low immunoglobulin levels. A family history of recurrent infections in one of the family members was established in four patients, one patient had sib death early in life due to infection, and two patients had multiple family members diagnosed with malignancy.
Our cohort of 19 patients proven to have an underlying IEI after presenting with malignancy included 13 (69%) men and six (31%) women. Their median age at diagnosis with malignancy was 7 years (1.5–16 years), and the median age of referral to the immunology clinic was 8 years (4–19 years). A total of 11 patients had positive parents’ consanguinity. A history of repeated infections was present in 9/19, 4/19 had failure to thrive, 5/19 had clubbing, 4/19 had bronchiectasis, 3/19 had significant chemotoxicity, and 3/19 had a relapse of the original disease (detailed clinical and laboratory data are shown in Table 1).
Genetic testing was done on 18/19 patients, and the results were out for 17/19 patients. The diagnosis based on genetic and/or immunological investigation and clinical findings according to the IUIS 2024 classification (22) were as follows: 7/19 (37%) immune-dysregulation, 4/19 (21%) combined immunodeficiency with syndromic features, 3/19 (15.7%) combined immunodeficiency, 3/19 (15.7%) predominantly antibody defect, and 2/19 (10.5%) bone marrow failure defect.
As recurrent infection was the main cause of referral, history for the 10 warning signs for primary immunodeficiency was assessed before and after chemotherapy (Figure 2). The most frequent warning sign was recurrent pneumonia, followed by recurrent sinus infections and prolonged use of antibiotics. Those infections were either during or after chemotherapy. However, five patients had a significant history of infections before being diagnosed with malignancy, and interestingly, it was not alerting to both parents and their physicians. Those patients had the following diagnosis: P17,18 (combined immunodeficiency and syndromic features (NBN gene mutation) and P4 (immunodysregulation) ITK gene mutation) and P7,14 (predominantly antibody defect (PI3KCD gene mutation) (detailed clinical and laboratory data are shown in Table 1).
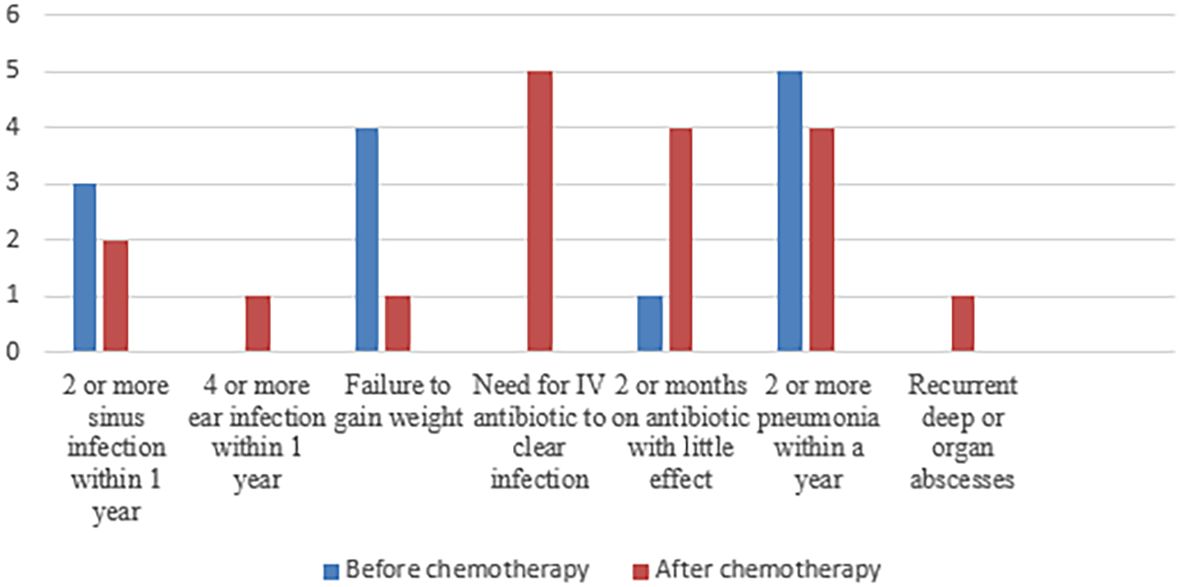
Figure 2. Frequency of the different items of positive 10 warning signs before and after chemotherapy.
Regarding the type of malignancy: six (31%) patients had leukemia, either acute myeloid leukemia or acute lymphoblastic leukemia, and 13 (69%) patients had lymphoma, whether Hodgkin’s or non-Hodgkin’s lymphoma (detailed clinical and laboratory data are shown in Table 2). All patients received their regular chemotherapy protocol except that P1,4,7 had a dose reduction because of significant chemotoxicity. Two of our patients are still on chemotherapy. One patient died during chemotherapy, 16/19 patients had an initial response to chemotherapy, but unfortunately seven patients relapsed, including P12,13 who had a second relapse after auto-BMT. This makes the overall survival 84% (16/19). As for the adjustment in the treatment plan, nine patients started IVIG, six patients did allo-BMT with a success rate of 83%, three patients were on the BMT list, and four patients refused BMT.
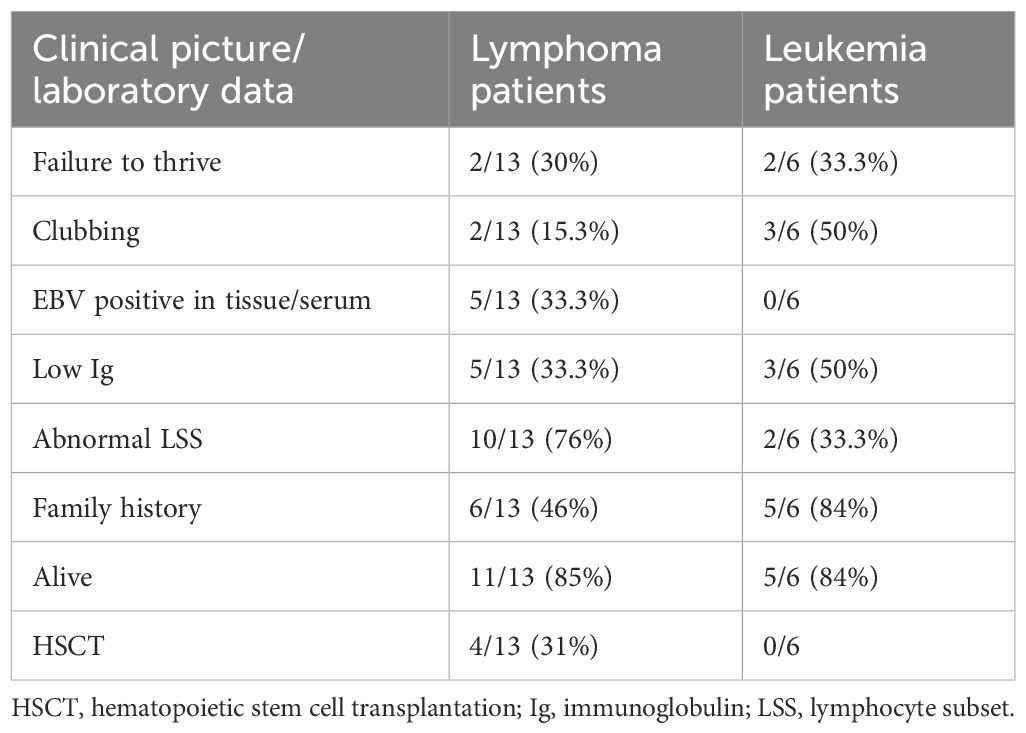
Table 2. Comparison between clinical and laboratory data for patients with IEI and leukemia or lymphoma.
Lymphoma patients: In our cohort, the patients with lymphoma were nine (69%) men and four (31%) women with a median age of diagnosis of 10 years (2–17 years). Nine patients had Hodgkin’s lymphoma, and five had non-Hodgkin’s lymphoma. Six patients had positive Epstein–Barr virus (EBV) in tissue (three patients in each group). A history of repeated infections was present in five (38.4%) patients. Three patients had a relapse of their original disease, and three patients had significant chemotoxicity. A family history of a sib death due to infection or malignancy or presence of symptoms of immunodysregulation was positive in seven (54%) patients. Interestingly, two sibs with exact ITK mutation (chr5:156667071 G>A; c.852-1G>A) had different clinical and laboratory phenotypes. Seven patients started intravenous immunoglobulin (IVIG) replacement therapy to combat recurrent infections, six patients did HSCT, only one patient died during HSCT from cytomegalovirus (CMV) viremia, and three patients refused HSCT. The overall survival is 11/13 patients.
Leukemia patients: Six patients were diagnosed with leukemia, and they were five (83%) men and one (17%) woman with a median age at diagnosis with malignancy of 5 years (2–9 years). A history of repeated infections was present in five (38.4%) patients. P16 suffered from extensive eczema following treatment with chemotherapy and allo-HSCT from her sib. A family history of a sib who died due to infection or malignancy or presence of malignancy or presence of symptoms of immunodysregulation was positive in five (83%) patients. None of our patients had adjustment in the chemotherapy plan. Only one patient (P15) who did allo-HSCT followed by severe graft-versus-host disease and recurrence of lymphadenopathy is currently controlled on sirolimus. Two patients started IVIG replacement therapy to combat recurrent infections. P14 died from recurrent severe uncontrolled infection during chemotherapy. The overall survival is 5/6 patients.
4 Discussion
IEIs have been increasingly recognized in association with hematologic malignancies (23), with a higher incidence of malignancy occurrence early in life (12). Malignancy in IEI patients is considered the second most common cause of death in patients with IEI after infections (24). Knowledge of the fact of increased risk of malignancy in IEI patients highlights the importance of a synergistic effort by immunologists and oncologists in tracking down the potential development of cancer in known IEI patients as well as the possibility of an underlying IEI in patients with newly diagnosed cancers (19).
The possibility of an underlying IEI in patients with newly diagnosed malignancy can be suggested by a medical history of severe infections or a family history of IEI, the type of cancer, age of the patient, or a high rate of therapy-related toxicity (3, 19). Based on our cohort, recurrent infections during the patients’ follow-up along with a positive family history of malignancy or recurrent infection were the most frequent alerting signs for an oncologist to refer the patients for further diagnosis. Adding to that are some clinical clues at first presentation to the oncologist such as clubbing, failure to thrive, persistent eczema, and facies. Other causes were significant chemotoxicity and relapse of malignancy after auto-BMT.
Being a rare disease with a broad spectrum of clinical phenotype, the exact incidence rates for malignancies occurring are difficult to ascertain and confirm (7). The risk of malignancy in IEI has been individually mentioned in different studies, and it showed a range of 1.42–2.3 relatively increased risk in comparison to the general population (10, 16, 18). Unfortunately, we could not screen each patient coming to the hospital for the possibility of IEI, and accordingly the true incidence could not be assessed.
The malignancies most identified in IEI are those related to the lymphoreticular system such as lymphomas, leukemias, malignant histiocytosis, and thymus tumors corresponding up to 96% of the identified malignancies (18). Non-Hodgkin’s lymphoma (NHL) and Hodgkin’s disease (HD) account for 48.6% and 10%, respectively, of the malignancies seen in patients with IEI (25). The presence of a persistent lymphoproliferation in IEI patients makes the diagnosis of lymphoid malignancies very challenging. In addition to the challenge in poor response to treatment protocols, there is an increased risk of toxicity and the contraindication of radiotherapy in some of them such as in DNA repair defect (26). It is also worth mentioning that both NHL and HL are diagnosed at younger ages in patients with IEI, and NHL is more common in men with IEI (5, 6, 27, 28). This went in alignment with our study, where all patients with NHL were male, and the median age of diagnosis was 6 years (1.5–9 years).
Today’s standard of care for patients with clinically diagnosed or suspected IEI involves genetic testing. The different genetic testing methods available include Sanger sequencing of single genes, targeted gene sequencing panels (targeted next-generation sequencing, tNGS), whole exome sequencing (WES), and whole genome sequencing (WGS), which can all be expanded to trio- or whole-family analyses. The choice of the appropriate method depends on the clinical presentation, the suspected type of IEI, and access to resources (29, 30). The goal of genetic testing in IEI is to confirm the clinical diagnosis and accordingly improve the patients’ management. A genetic diagnosis can inform about prognosis, guide in treatment decisions, enable genetic counseling, and provide the opportunity for predictive family testing for relatives at risk. In our cohort, we did WES for 21 patients, and only 18 patients had a confirmatory IEI genetic mutation.
Unlike our study where the most prevalent IEI diagnosis was immunodysregulation, several studies showed a higher prevalence of malignancy in predominantly antibody production deficiencies (31, 32), while others showed more prevalence in the combined deficiencies of T and B cells (ataxia telangiectasia mainly, hyper-IgE syndromes, Wiskott–Aldrich syndrome), activated phosphoinositide 3-kinase delta syndrome (APDS), and autoimmune lymphoproliferative syndrome (ALPS) (19, 33–35).
The treatment of IEI patients with malignancy is often challenging as it requires balancing between increased susceptibility to infection and the additional suppression of the immune system (33). However, to date, the treatment of malignancies in IEI generally is similar to that of the non-IEI patients. Treatment modalities must be tailored on an individual basis (5). In patients with DNA repair defects, radiomimetic agents should not be a first choice, and alkylating substances, such as daunorubicin, etoposide and methotrexate, should be used with dose reduction (14). In B cell lymphomas, regimens that include anti-CD20 monoclonal antibody (rituximab) for short intervals can yield a better advantageous outcome with less toxicity such as those of infections, mucositis, and bone marrow suppression, which are highly observed in IEI patients (36). Importantly, infectious complications should be prevented by regular antimicrobial prophylaxis and monthly intravenous immunoglobulin (IVIG). HSCT seems to be the ultimate curative therapy for many IEI patients (5, 14). In our cohort the addition of monthly IVIG for those with recurrent infections was beneficial in reducing the rate and severity of infection, along with bronchiectasis progression in those who had it. HSCT was discussed with all patients, and only four did a transplantation with a success rate of 75%.
Screening methods for IEI patients presenting with sporadic infections, autoimmunity, autoinflammation, and malignancy are absent, as the 10 warning signs are only useful in screening patients with infections (37). Using this tool as a screening method excluded 20% of patients with IEI, in whom their clinical presentation did not involve infectious diseases. In addition, only three points of the 10 warning signs were found to be beneficial in suspicious cases, and they were a positive family history, need for intravenous antibiotics, and failure to thrive (38). However, in our cohort, the 10 warning signs for primary immunodeficiency were assessed before and after chemotherapy. They were beneficial in considering IEI as an underlying etiology in some patients. The most frequent warning sign was recurrent pneumonia, followed by recurrent sinus infections and the prolonged use of antibiotics during or after ending chemotherapy.
Finally, raising awareness among oncologists and hematologists about the possibility of IEI as a cause of malignancy was a successful point in our center that helped in diagnosing patients with IEI and adjusting their management plan. This model extended to other oncology centers in Egypt. However, larger studies are needed to develop solid warning signs for IEI in patients with immunodysregulation. International efforts which are needed to create an international registry of IEI cases with detailed information on the occurrence of cancer is fundamental to optimizing the diagnostic process and to evaluating the outcomes of new therapeutic options, aiming to improve prognosis and reduce comorbidities (18).
4.1 Study limitations
We could not screen every patient diagnosed with malignancy for an underlying IEI, and we could not provide confirmatory genetic sequencing for all patients suspected with IEI.
5 Conclusion
Our data present an example for non-infectious manifestation of IEI and the warning signs we used in our center to suspect IEI as a cause of malignancy. The recommended workup to be done upon suspicion includes immunoglobulin and lymphocyte subset at the time of diagnosis before chemotherapy or after 6 months of chemotherapy with close follow-up of infections. Peculiar presentation and family history are very important points in the suspicion of IEI. A history of infections following the termination of chemotherapy should be taken seriously. Genetic sequencing is an important confirmatory test for the diagnosis of IEI in case of an absence of solid criteria for diagnosing IEI.
Author contributions
NR: Writing – original draft, Writing – review & editing. YM: Investigation, Writing – original draft. HR: Writing – review & editing. AEl: Supervision, Writing – original draft. MH: Writing – review & editing. HAR: Writing – review & editing. MF: Validation, Writing – original draft. NM: Writing – review & editing. AM: Data curation, Writing – original draft. ME: Writing – original draft. MFS: Writing – review & editing. NA: Writing – review & editing. ST: Writing – original draft. SG: Writing – review & editing. AEm: Writing – review & editing. IS: Writing – review & editing. NE: Investigation, Writing – original draft. AE-H: Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to acknowledge Sophei Hambelton (Children’s Stem Cell Transplant Unit, Great North Children’s Hospital, Newcastle upon Tyne, UK; Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, UK) and Karin Englandtart (Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, UK) for providing diagnostic genetic sequencing for our patients at their labs.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang Q, Frange P, Blanche S, and Casanova JL. Pathogenesis of infections in HIV-infected individuals: insights from primary immunodeficiencies. Curr Opin Immunol. (2017) 48:122–33. doi: 10.1016/j.coi.2017.09.002
2. Campbell E, Shaker MS, and Williams KW. Clinical updates in inborn errors of immunity: a focus on the noninfectious clinical manifestations. Curr Opin Pediatr. (2024) 36:228–36. doi: 10.1097/MOP.0000000000001331
3. Bosch JVWT, Hlaváčková E, Derpoorter C, Fischer U, Saettini F, Ghosh S, et al. How to recognize inborn errors of immunity in a child presenting with a Malignancy: guidelines for the pediatric hemato-oncologist. Pediatr Hematol Oncol. (2023) 40:131–46. doi: 10.1080/08880018.2022.2085830
4. Bogaert DJ, Laureys G, Naesens L, Mazure D, De Bruyne M, Hsu AP, et al. GATA2 deficiency and haematopoietic stem cell transplantation: challenges for the clinical practitioner. Br J Haematol. (2020) 188:768–73. doi: 10.1111/bjh.16247
5. Bomken S, van der Werff Ten Bosch J, Attarbaschi A, Bacon CM, Borkhardt A, Boztug K, et al. Current understanding and future research priorities in Malignancy associated with inborn errors of immunity and DNA repair disorders: the perspective of an interdisciplinary working group. Front Immunol. (2018) 9:2912. doi: 10.3389/fimmu.2018.02912
6. Cekic S, Metin A, Aytekin C, Edeer Karaca N, Baris S, Karali Y, et al. The evaluation of Malignancies in Turkish primary immunodeficiency patients; a multicenter study. Pediatr Allergy Immunol. (2020) 31:528–36. doi: 10.1111/pai.13231
7. Mellemkjaer L, Hammarstrom L, Andersen V, Yuen J, Heilmann C, Barington T, et al. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin Exp Immunol. (2002) 130:495–500. doi: 10.1046/j.1365-2249.2002.02004.x
8. Mueller BU and Pizzo PA. Cancer in children with primary or secondary immunodeficiencies. J Pediatr. (1995) 126:1–10. doi: 10.1016/S0022-3476(95)70491-4
9. Filipovich AH, Mathur A, Kamat D, and Shapiro RS. Primary immunodeficiencies: genetic risk factors for lymphoma. Cancer Res. (1992) 52:5465s–7s.
10. Jonkman-Berk BM, van den Berg JM, Ten Berge IJ, Bredius RG, Driessen GJ, Dalm VA, et al. Primary immunodeficiencies in the Netherlands: national patient data demonstrate the increased risk of Malignancy. Clin Immunol. (2015) 156:154–62. doi: 10.1016/j.clim.2014.10.003
11. Mortaz E, Tabarsi P, Mansouri D, Khosravi A, Garssen J, Velayati A, et al. Cancers related to immunodeficiencies: update and perspectives. Front Immunol. (2016) 7:365. doi: 10.3389/fimmu.2016.00365
12. Hauck F, Voss R, Urban C, and Seidel MG. Intrinsic and extrinsic causes of Malignancies in patients with primary immunodeficiency disorders. J Allergy Clin Immunol. (2018) 141:59–68.e4. doi: 10.1016/j.jaci.2017.06.009
13. Satge D and Tumor A. Profile in primary immune deficiencies challenges the cancer immune surveillance concept. Front Immunol. (2018) 9:1149. doi: 10.3389/fimmu.2018.01149
14. Baris S and Kolukisa B. Immune dysfunction in inborn errors of immunity causing Malignancies. Expert Rev Clin Immunol. (2021) 17:695–9. doi: 10.1080/1744666X.2021.1925542
15. Ogulur I, Ertuzun T, Kocamis B, Kendir Demirkol Y, Uyar E, Kiykim A, et al. Parents of ataxia-telangiectasia patients display a distinct cellular immune phenotype mimicking ATM-mutated patients. Pediatr Allergy Immunol. (2021) 32:349–57. doi: 10.1111/pai.13387
16. Vajdic CM, Mao L, Van Leeuwen MT, Kirkpatrick P, Grulich AE, and Riminton S. Are antibody deficiency disorders associated with a narrower range of cancers than other forms of immunodeficiency? Blood. (2010) 116:1228–34. doi: 10.1182/blood-2010-03-272351
17. Goudouris ES, Felix MMR, Kuschnir FC, and Solé D. Malignancies in the inborn errors of immunity. Rev Assoc Med Bras (1992). (2024) 70:e2024S104. doi: 10.1590/1806-9282.2024S104
18. Mayor PC, Eng KH, Singel KL, Abrams SI, Odunsi K, Moysich KB, et al. Cancer in primary immunodeficiency diseases: cancer incidence in the United States immune deficiency network registry. J Allergy Clin Immunol. (2018) 141:1028–35. doi: 10.1016/j.jaci.2017.05.024
19. Tiri A, Masetti R, Conti F, Tignanelli A, Turrini E, Bertolini P, et al. Inborn errors of immunity and cancer. Biol (Basel). (2021) 10:313. doi: 10.3390/biology10040313
20. Wang Y and Abolhassani H. Updates of cancer hallmarks in patients with inborn errors of immunity. Curr Opin Allergy Clin Immunol. (2022) 22:352–63. doi: 10.1097/ACI.0000000000000863
21. Alligon M, Mahlaoui N, Courteille V, Costes L, Afonso V, Randrianomenjanahary P, et al. An appraisal of the frequency and severity of noninfectious manifestations in primary immunodeficiencies: A study of a national retrospective cohort of 1375 patients over 10 years. J Allergy Clin Immunol. (2022) 149:2116–25. doi: 10.1016/j.jaci.2021.12.790
22. Bousfiha AA, Jeddane L, Moundir A, Poli MC, Aksentijevich I, Cunningham-Rundles C, et al. The 2024 update of IUIS phenotypic classification of human inborn errors of immunity. J Hum Immun. (2025) 1:e20250002. doi: 10.70962/jhi.20250002
23. Duan L and Grunebaum E. Hematological Malignancies associated with primary immunodeficiency disorders. Clin Immunol. (2018) 194:46–59. doi: 10.1016/j.clim.2018.06.011
24. Müller J and Kovács G. Primer immundefektus esetén előforduló onkohematológiai kórképek [Primary immunodeficiencies and haemato-oncology. Orv Hetil. (2018) 159:2073–8. doi: 10.1556/650.2018.31240
25. Gross TG and Shiramizu B. Lymphoproliferative disorders and Malignancies related to immunodeficiencies. In: Pizzo PA and Poplack DG, editors. Principles and practice of pediatric oncology. Lippincott Williams & Wilkins, Philadelphia, PA, USA (2006). p. 748–67.
26. Ye X, Maglione PJ, Wehr C, Li X, Wang Y, Abolhassani H, et al. Genomic characterization of lymphomas in patients with inborn errors of immunity. Blood Adv. (2022) 6:5403–14. doi: 10.1182/bloodadvances.2021006654
27. Seidemann K, Tiemann M, Henze G, Sauerbrey A, Müller S, and Reiter A. Therapy for non-Hodgkin lymphoma in children with primary immunodeficiency: analysis of 19 patients from the BFM trials. Med Pediatr Oncol. (1999) 33:536–44. doi: 10.1002/(sici)1096-911x(199912)33:6<536::aid-mpo3>3.0.co;2-z
28. Özyörük D, Güzelküçük Z, Metin A, Emir S, Yazal Erdem A, Kacar D, et al. Clinical profile and outcomes of primary immunodeficiency and Malignancy in childhood at a tertiary oncology center in developing country. Pediatr Hematol Oncol. (2022) 39:600–12. doi: 10.1080/08880018.2022.2045408
29. Seidel MG, Kindle G, Gathmann B, Quinti I, Buckland M, van Montfrans J, et al. The European society for immunodeficiencies (ESID) registry working definitions for the clinical diagnosis of inborn errors of immunity. J Allergy Clin Immunol Pract. (2019) 7:1763–70. doi: 10.1016/j.jaip.2019.02.004
30. von Hardenberg S, Klefenz I, Steinemann D, Di Donato N, Baumann U, Auber B, et al. Current genetic diagnostics in inborn errors of immunity. Front Pediatr. (2024) 12:1279112. doi: 10.3389/fped.2024.1279112
31. Fekrvand S, Abolhassani H, Esfahani ZH, Fard NNG, Amiri M, Salehi H, et al. Cancer trends in inborn errors of immunity: A systematic review and meta-analysis. J Clin Immunol. (2024) 45:34. doi: 10.1007/s10875-024-01810-w
32. Kiaee F, Azizi G, Rafiemanesh H, Zainaldain H, Sadaat Rizvi F, Alizadeh M, et al. Malignancy in common variable immunodeficiency: a systematic review and meta-analysis. Expert Rev Clin Immunol. (2019) 15:1105–13. doi: 10.1080/1744666X.2019.1658523
33. Riaz IB, Faridi W, Patnaik MM, and Abraham RS. A systematic review on predisposition to lymphoid (B and T cell) neoplasias in patients with primary immunodeficiencies and immune dysregulatory disorders (inborn errors of immunity. Front Immunol. (2019) 10:777. doi: 10.3389/fimmu.2019.00777
34. Tavakol M, Delavari S, Salami F, Ansari S, Rasouli SE, Chavoshzadeh Z, et al. Diversity of Malignancies in patients with different types of inborn errors of immunity. Allergy Asthma Clin Immunol. (2022) 18:106–6. doi: 10.1186/s13223-022-00747-2
35. Notarangelo LD, Fischer A, Geha RS, Casanova JL, Chapel H, Conley ME, et al. Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol. (2009) 124:1161–78. doi: 10.1016/j.jaci.2009.10.013
36. Delmonte OM, Castagnoli R, Calzoni E, and Notarangelo LD. Inborn errors of immunity with immune dysregulation: from bench to bedside. Front Pediatr. (2019) 7:353. doi: 10.3389/fped.2019.00353
37. Arkwright PD and Gennery AR. Ten warning signs of primary immunodeficiency: a new paradigm is needed for the 21st century. Ann N Y Acad Sci. (2011) 1238:7–14. doi: 10.1111/j.1749-6632.2011.06206.x
Keywords: inborn error of immunity, malignancy, leukemia, lymphoma, immunodysregulation, bone marrow transplantation
Citation: Radwan N, Medany Y, Rashad H, Elhemaly A, Hammad M, Rahman HA, Fakhry M, Marouf NM, Mahdy A, Elsherif M, Salama MF, Ali N, Talaat S, Gohar S, Emad A, Sidhom I, EL-Sharkawy N and El-Haddad A (2025) The unveiled face of IEI: Children Cancer Hospital—Egypt (CCHE-57357) experience. Front. Immunol. 16:1570328. doi: 10.3389/fimmu.2025.1570328
Received: 03 February 2025; Accepted: 02 October 2025;
Published: 30 October 2025.
Edited by:
Eleonora Gambineri, University of Florence, ItalyReviewed by:
Emilia Cirillo, University of Naples Federico II, ItalyEkaterini Simoes Goudouris, Federal University of Rio de Janeiro, Brazil
Copyright © 2025 Radwan, Medany, Rashad, Elhemaly, Hammad, Rahman, Fakhry, Marouf, Mahdy, Elsherif, Salama, Ali, Talaat, Gohar, Emad, Sidhom, EL-Sharkawy and El-Haddad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nesrine Radwan, bmVzcmluZXJhZHdhbkB5YWhvby5jb20=
 Nesrine Radwan
Nesrine Radwan Youssef Medany2,3
Youssef Medany2,3 Mahmoud Hammad
Mahmoud Hammad Mona Fakhry
Mona Fakhry Ahmed Mahdy
Ahmed Mahdy Mariam Elsherif
Mariam Elsherif Nesreen Ali
Nesreen Ali Seham Gohar
Seham Gohar Ahmed Emad
Ahmed Emad Iman Sidhom
Iman Sidhom Alaa El-Haddad
Alaa El-Haddad