- 1Department of Emergency Medicine, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
- 2Department of Rehabilitation Medicine, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
- 3The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
The process of nerve injury is accompanied by the change of inflammatory microenvironment, which is not conducive to axonal regeneration and hinders the repair of injured nerve. Therefore, looking for a way to improve the inflammatory attack and immune state around the injured nerve is beneficial to the progress of nerve injury repair. In recent years, cell transplantation strategy has played a foreground role in the repair of nerve injury. Olfactory ensheathing cells (OECs) are a special kind of glial cells, which have the characteristics of continuous renewal and survival, antigenic characteristics, variability and promoting the repair of nerve injury. OECs have been recognized in different injury models, including clinical trials, which has become a dominant cell in cell replacement therapy. An important feature of OECs lies in their anti-inflammatory and immunomodulatory functions. They are transplanted into the host to improve the catastrophic inflammatory microenvironment caused by injured nerves, thus promoting the repair and regeneration of injured nerves. The transplantation of OECs into the host can provide good groundwork and support for the repair and regeneration of nerve injury by regulating the activity and infiltration of immune cells, the secretion of inflammatory cytokines and phagocytosis. Therefore, this paper discusses the anti-inflammatory and immunomodulatory mechanisms of OECs transplantation in the repair of nerve injury and the functional role of OECs as an ideal substitute in the treatment of nerve injury.
1 Introduction
Nerve injury is a common problem at present, which can be induced by entrapment, trauma, ischemia, inflammation and immunity, which can lead to a variety of defects, including loss of sensation, loss of movement and chronic pain. These not only bring devastating blows and psychosomatic injuries to patients, but also bring huge economic burden to the family and society (1–3). The ability of injury and regeneration of central nervous system in adult mammals is limited. Although the ability of peripheral nerve regeneration is stronger than that of central nervous system, repair and regeneration cannot be completed after injury. After nerve injury, ischemia, swelling, and bleeding may occur, accompanied by a series of adverse factors, including activation of inflammatory cells, hindering nerve regeneration and functional recovery (4, 5). Although inflammation is a protective mechanism after nerve injury, excessive inflammation can lead to widespread damage and exacerbate disease progression. In these processes, what is more important is that the activity of immune cells around the nerve injury increases and penetrates, thus releasing a variety of inflammatory cytokines and forming an inflammatory microenvironment that is not conducive to the repair of the damaged nerve. During the progression of nerve injury, these inflammatory cells, such as microglia and macrophages, can be activated. These activated cells can migrate and gather at the injury site and by changing their phenotypic changes (pro-inflammatory phenotype), release a variety of pro-inflammatory cytokines and chemokines, induce neuroinflammation, produce neurotoxic effects, and hinder neuron survival and axon regeneration (6, 7).
Central nervous system damage can trigger microglia activation within minutes, and then immune cells in the blood are attracted to chemokines and infiltrate under the influence of the destruction of the blood-brain barrier. Blood-borne monocytes differentiate into phagocytic macrophages, which, together with microglia, constitute innate immunity and are responsible for clearing debris and providing a source of nutritional and anti-inflammatory factors to promote tissue repair, but they also release inflammatory cytokines to promote secondary damage (8, 9). During the secondary injury stage, it can cause vascular damage, ischemia and hypoxia, cell necrosis, neuroinflammation, etc., which can lead to aggravation of tissue damage. Neuroinflammation is an important aspect of the pathological process, which usually involves the aggregation of resident microglia, astrocytes, macrophages and neutrophils. At the beginning of inflammation, the levels of cytokines such as macrophage inflammatory protein-1a (MIP-1a), IL-1, IL-6 and tumor necrosis factor (TNF-a) are u-regulated at the injury site, thereby triggering a cascade amplification reaction through receptor action, prolonging and amplifying nerve damage, causing a vicious cycle and seriously damaging the microenvironment at the nerve injury site (10, 11). In this catastrophic environment, nerve regeneration can be challenging. Therefore, this change in the inflammatory microenvironment during nerve injury increases or hinders the nerve repair process, greatly hindering axon regeneration, nerve bridging, and myelin formation (12–14). Different studies have confirmed that inflammation plays an important role in tissue repair and functional recovery (15). Therefore, it is of great significance for the repair of nerve injury and functional recovery to explore foreground methods to improve the inflammatory microenvironment of nerve injury.
As people continue to explore in this field, in recent years, cell transplantation technology has entered people’s field of vision. The researchers can repair the injured nerve by obtaining functional cells and transplanting them into the host (16, 17). For example, Schwann cells (SCs) implantation combined with electroacupuncture can promote axonal regeneration and myelin regeneration of corticospinal tract after spinal cord injury by up-regulating the expression of neuRegin1 type III in SCs and their downstream signal media (18). OECs are a special kind of glial cells, which can survive and renew for life in the central and peripheral nervous system. The primary olfactory system is characterized by its ability to produce new neurons in adult animals. This special ability is due in part to the presence of OECs, which creates a favorable microenvironment for neurogenesis. OECs promote the continuous regeneration of neurons in the olfactory system (19). OECs can be directly, quickly and effectively reprogrammed into neurons via the single transcription factor Neurogenin 2 (NGN2). These inducing cells exhibit typical neuronal morphology, express multiple neuron-specific markers, generate action potentials, and form functional synapses. Genome-wide RNA sequencing analysis shows that OECs transcriptome profiles can be effectively reprogrammed into neuronal lineages (20). Moreover, OECs are rich in sources and can be derived from olfactory bulb tissue and olfactory mucosa. Primary OECs are easier to obtain from embryonic animals and early postnatal animals, but it is more difficult to obtain purified OECs due to the large number of non-OECs in the embryonic and early postnatal olfactory bulbs and olfactory mucosa. In contrast, OECs can be easily purified and cultured from adult olfactory bulbs and olfactory mucosa to obtain highly viable and highly pure OECs (19). Because of these capabilities, people are exploring the use of OECs in the treatment of nerve injury repair (such as spinal cord injuries) and making significant progress. The mechanisms of OECs transplantation in promoting the repair and functional recovery of injured nerve include promoting axonal regeneration and myelination, secreting a variety of neurotrophic factors and neuroprotection (19, 21, 22). In addition, another important mechanism of OECs promoting the repair of nerve injury can improve this inflammatory microenvironment and exert the characteristics of anti-inflammation and immune regulation. OECs can regulate the activity of immune cells, inhibit the release of inflammatory mediators, and provide a good local microenvironment for axonal regeneration and neuronal regeneration (23, 24). After OECs transplantation, the expression of interleukin-1 receptor antagonist (IL-1ra) in spinal cord tissue was up-regulated, and the expression of many chemokines, including proinflammatory chemokine IL-1α and IL-1β, was down-regulated, which played an anti-inflammatory role (25). OECs can also significantly inhibit the activity of microglia stimulated by lipopolysaccharide and reduce neuroinflammatory response (25). In recent years, OECs have been used in clinical trials, which has confirmed the feasibility and safety of OECs transplantation. Therefore, we explored the anti-inflammatory and immunomodulatory effects of OECs in nerve injury repair, so as to provide a favorable microenvironment for nerve regeneration.
2 Origin of OECs
OECs are glial cells located in the primary olfactory nervous system, which originate from the neural crest. and exist in the nerve fiber layer of the olfactory nerve, olfactory mucosa and olfactory bulb of the system (26). Neural crest cells shed from the dorsal nerve canal through epidermal-mesenchymal transformation (EMT), migrate along the olfactory nerve to the lamina propria of the olfactory mucosa, and pass through the epithelium to the basal margin. Then, neural crest cells-derived OECs can still extend along the olfactory nerve to the telencephalon of the central nervous system, wrapped around the olfactory axon bundle. The olfactory axon of the olfactory nerve ends close to the olfactory bulb, and finally reaches the olfactory nerve layer (ONL) of the olfactory bulb and matures here (22, 27). When the axon grows through the basal layer of the epithelium, it passes through the basement membrane and enters the lamina propria, and combines to form an axonal bundle, which is surrounded by OECs (22). The primary olfactory nervous system is unique in that it can constantly regenerate, even after an injury, as long as the deeper olfactory bulb remains intact. The olfactory bulb tissue contains abundant OECs, which can grow and regenerate olfactory neurons and maintain the function of the olfactory system. This also shows the important role of OECs in the development of the primary olfactory nervous system (28, 29). OECs have unique growth and migration promoting properties, as well as biological functions directly related to neuron survival and axon extension. OECs in the olfactory nerve promote axon bundle formation, while OECs from the olfactory bulb nerve fiber layer mediate unbundling, sequencing, and rebundling of axons and guide axons to their appropriate bulb targets (30, 31). OECs wrap bundles of olfactory axons around the olfactory nerves and in the olfactory nerve layer of the olfactory bulb, and these primary olfactory axon bundles surrounded by OECs support the regeneration of primary olfactory axons from the peripheral to the central nervous system and from the olfactory epithelium to the bulb (32, 33). The Sox10 transcription factor exists in the olfactory nerve and can directly regulate the differentiation and expression of OECs (31). When OECs migrate to the basal edge of the development site, OECs are formed. There are many OECs around the olfactory bulb and in the ONL. ONL is divided into inner layer and outer layer, the inner layer cannot express p75NTR, and the outer layer can express p75NTR. OECs play a key role in neurite growth and olfactory nerve axis function, and can be completely regenerated to maintain sensory function even after extensive injury (31).
OECs culture are a source of cells needed for nerve injury treatment. This is due to its advantages, such as high migration ability, easy source (can be derived from nasal olfactory mucosa or olfactory bulb tissue), simple in vitro culture, and non-tumorigenicity (34). At present, OECs from autologous or allogeneic origin can be obtained through in vitro culture systems. In basic research, olfactory bulb tissue or olfactory mucosa (the lamina propria of the olfactory mucosa consists of OECs covering olfactory nerve fibers) can be taken from animals (such as dogs, rats and mice) and can be obtained by culture in vitro. These cultured OECs are further identified by using the OECs-specific marker p75NTR (35–37). The human nasal mucosa is located in the middle and superior turbinate and nasal septum of the nasal cavity, while the olfactory mucosa plays an important role in smell and is the only nerve tissue exposed to the external environment. This feature allows people to easily access the olfactory mucosa (34). It is worth mentioning that the production of OECs in young patients is relatively high. Higher yields of OECs can be obtained from specimens collected from the tail of the superior turbinate, while cell culture is poor in patients with severe mucosal disease or elderly patients (38–41). This also means that the culture rich in human OECs and the cells with high viability and yield can be obtained by selecting the olfactory mucosa of appropriate age and location. Although the in vitro culture method of OECs is relatively mature and stable, for clinical trials, the source of cells and the ethical issues are a major challenge hindering clinical development.
3 Heterogeneity and antigenicity of OECs
Another important feature of OECs is their antigenicity, morphological and functional heterogeneity. In the early stage, it is shown that there are different antigenic OECs subsets in the olfactory system, which is closely related to their location and development. Now studies have shown that OECs in the olfactory nerve and olfactory bulb express a series of antigens, which can be detected and identified by various cellular and molecular techniques (23, 24). OECs in the lamina propria can strongly express S100 β and weakly express glial fibrillary acidic protein (GFAP) and nerve cell adhesion molecules. OECs in the olfactory nerve layer express low neurotrophic receptors p75, neural cell adhesion molecule (NCAM) and GFAP, while the OECs in the olfactory bulb do not express these molecules (23). Due to the heterogeneity and anatomical location of OECs, there are differences in the expression of some antigenic factors. For example, neuropeptide Y is expressed in OECs during the development of olfactory nerve layer, but not in OECs of olfactory nerve layer and peripheral olfactory system in adulthood, which is closely related to the heterogeneity of OECs (23, 24).
There are also differences in morphology of OECs, which reflects the functional role of OECs in the repair of nerve injury. OECs can be similar to SCs and astrocytes and undergo morphological changes due to external and internal environmental changes (42). Although OECs express many molecular and cellular characteristics of SCs and astrocytes, there are significant differences in molecular expression between OECs and these two kinds of cells (43). OECs are mainly protuberant under pro-inflammatory conditions and flat under anti-inflammatory conditions (43). Compared with the flat OECs under anti-inflammatory conditions, the OECs carried by processes showed higher cell metabolic activity and higher mobility, and significantly promoted the growth and extension of axons under anti-inflammatory conditions. Studies have shown that transcriptional coactivator related protein (YAP) downstream of ROCK pathway mediates the morphological transformation of OECs and enhances their ability to promote axonal growth by up-regulating the expression of L1 cell adhesion molecule (L1-CAM) (43). Inhibitors of RhoA pathway, toxin B, C3, Ymur27632 or overexpression of dN-RhoA blocked the morphological changes of OECs from processes to flattening induced by serum, while lysophosphatidic acid activated RhoA pathway promoted the morphological changes of OECs from processes to flattening (44). These studies have revealed the plasticity and heterogeneity of OECs.
In addition, OECs derived from olfactory mucosa (OM) can regulate inflammatory process and the formation of extracellular matrix, but have poor ability of regeneration, while OECs derived from olfactory bulb (OB) can promote functional recovery by inducing targeted axonal regeneration (19). OM-OECs overexpress genes characteristic of wound healing and extracellular matrix regulation. In contrast, the genetic profile of OB-OECs has been shown to play an important role in nervous system development (45). OECs were extracted from OB and OM, and gene and protein expression profiles of cells were compared using transcriptomics and non-quantitative proteomic techniques. The results showed that both OB-OECs and OM-OECs highly express genes and proteins that regulate cell growth, reproduction, cell death, and vascular endothelial cell regeneration. Differentially expressed genes and proteins in OB-OECs play a key role in regulating nerve regeneration and axon regeneration and extension, nerve impulse transmission and response to axon injury. The differentially expressed genes and proteins in OM-OECs are mainly involved in the positive regulation of inflammatory responses, defense responses, cytokine binding, cell migration and wound healing (46). There are 2164 differentially expressed genes in OB-OECs in patients with Parkinson’s disease, of which 1090 are up-regulated and 1074 are down-regulated. The most significantly expressed genes include down-regulated genes CHRNA3, SNCG, OR7D2 and up-regulated genes TOP2A, RM2 and IGLC2. Important pathways rich in down-regulated genes include neurodegeneration, oxidative phosphorylation, and olfactory conduction. Pathways rich in upregulated genes include several pathways involved in neuroinflammation, immune and inflammatory responses, such as NF-kB, Jak-STAT, toll-like signaling pathways, and pathways involving pro-inflammatory mediators (47). These findings suggest that differentially expressed genes and proteins can explain why OB-OECs and OM-OECs exhibit different therapeutic effects.
4 Phagocytosis of OECs in the repair of nerve injury
Nervous system injury is characterized by neuronal degeneration and death, persistent cell and apoptotic fragments, as well as inflammatory response and immune damage, which create an adverse environment for nerve survival and axonal regeneration (23). Therefore, in the process of nerve repair, timely cleaning/removal of denatured/apoptotic cells and fragments and inhibition of inflammatory response are of great significance for the repair of injured nerve. Glial cells play an important role in the maintenance and stability of nervous system function. Glial cells can respond quickly to nerve injury by removing fragments from the injured site and providing necessary growth factors and structural support, all of which provide support for neuronal regeneration (48, 49). During the development of the primary olfactory system, the axons are inaccurately located, and the axons inappropriately project to the target layer or over-project to the deep layer of the olfactory bulb. In addition, there is a large number of apoptosis of primary olfactory neurons during embryonic and postnatal development, and axons of degenerated neurons need to be removed (50). The olfactory nerve is constantly renewed throughout life, and the OECs maintain the stability of the olfactory system and constantly stimulate nerve regeneration (48). The phagocytosis of OECs to clear neuronal fragments has been shown to contribute to the growth of neurons. OECs can exert phagocytosis by secreting immunomodulatory molecules, thus maintaining microenvironmental homeostasis and supporting neuronal survival and axonal growth (32, 51). In fact, OECs can remove axonal fragments and bacteria through their morphological and phenotypic changes, including cytoskeleton hypertrophy and rearrangement, transition from resting state to phagocytic phenotype, and protect olfactory nerves from microbial infection (24, 51). Studies have shown that the phagocytosis of primary olfactory axon fragments by OECs occurs at 14.5 days of the embryo, and the phagocytosis of axonal fragments continues to enter postnatal animals during the period of widespread mispositioning of olfactory axons (52).
OECs respond to phagocytosis of bacteria, which may be essential for responding to microbes invading the central nervous system through the peripheral nerve. SCs can help remove fragments by attracting macrophages to the injured site during the repair of peripheral nerve injury (52). But unlike SCs, OECs in the olfactory system do not seem to attract macrophages (52). Studies have shown that OECs have higher phagocytosis and transport capacity than SCs and produce lower amounts of proinflammatory cytokines (50). Macrophage migration inhibitory factor (MIF) is an important innate immune regulator, which plays a key role in many functions such as nerve regeneration and response to pathogens, except the primary olfactory nervous system. MIF participates in the fact that a large number of macrophages are missing in the olfactory nerve bundle and strongly stimulates the phagocytosis of OECs (53). It has been found that macrophages often appear near OECs after nerve injury, but they only play a small role in clearing axonal fragments (33). These mean that OECs are the primary phagocytes of the primary olfactory nerve from the early stage of embryonic development. Interestingly, the conditioned medium of macrophages co-incubated with platelet-derived growth factor (PDGF) can promote the phagocytosis of OECs and regulate the expression of myelin sheath genes related to nerve repair in OECs under inflammatory conditions (54).
OECs express growth-promoting adhesion molecules and extracellular matrix molecules, and bind closely to olfactory axons in space, which is consistent with the involvement of OECs in promoting and guiding olfactory axon growth (42). OECs secrete anti-inflammatory cytokine transforming growth factor β1 (TGF-β1) in the process of phagocytosis and removal of neuronal fragments. TGF-β1 enhances phagocytosis of OECs by regulating integrin/MFG-E8 signal pathway (55). TGF-β1 makes OECs move to flat direction and increase cell area, which may also participate in the enhancement of phagocytosis of OECs, promote OECs to clear neuronal fragments and increase neuronal survival rate (55). In the model of primary cultured spinal cord neurons in vitro, it was found that OECs could phagocytize a large number of degenerated neuronal fragments, which further showed that OECs increased the length of neuronal axons and enriched the number of neurons with axonal branches (32). In addition, the phagocytic activity of OECs does not affect the production of bioactive molecules, which provides a favorable environment for the survival of neurons (32). Through the co-culture model in vitro, it was found that the combination of lipopolysaccharide and curcumin could significantly enhance the activity of OECs, up-regulate the expression of chemokine (C-X-C motif) ligand 1, C-X-C motif ligand 2, TNF-α and Toll-like receptor 4 in OECs, enhance the phagocytosis of OECs and greatly promote the growth of neurons (56). These studies have revealed the key role of OECs in maintaining and stabilizing the function of the nervous system, creating an environment conducive to the growth of neurons by swallowing apoptotic and degenerative cells. Therefore, this functional characteristic of OECs may play an important role in the mechanism of nerve injury repair. However, more studies need to reveal the phagocytic ability in the repair of nerve injury.
5 Neuroinflammation mediated by immune regulation of OECs
Neuroinflammation mediated by immune cell activation and infiltration and the release of cytokines play an important role in nerve injury (49, 57, 58). The polarization of microglia/macrophages shows pro-or anti-inflammatory phenotypes, which can change the local microenvironment, process and severity of injury. These processes include the release of pro-inflammatory and anti-inflammatory cytokines, such as IL-1 α, IL-6, IL-8, IL-13, TNF-a and chemokine granulocyte colony stimulating factor and granulocyte macrophage colony stimulating factor (59, 60). Resident microglia near the injury site are activated, and neutrophils, macrophages, lymphocytes and natural killer cells are recruited to gather and infiltrate to the injury site, causing inflammatory damage by releasing cytokines such as free radicals, reactive oxygen species (ROS) and nitric oxide (NO) (23, 24). Therefore, it is necessary to stimulate nerve regeneration and find effective ways to regulate the transition of microglia/macrophages to neuroprotective phenotypes.
OECs have been shown to be beneficial to nerve injury by regulating immune cell activity and nerve inflammation, produce anti-inflammatory effects, support neuronal survival and promote angiogenesis (61, 62). OECs transplantation can improve the immune microenvironment at the transplant site by inhibiting the activity of immune cells, including macrophages and microglia, and the release of inflammatory cytokines, and create a favorable microenvironment for nerve regeneration (63). OECs can delay the activation of microglia/macrophages and reduce the peak of multiphasic inflammation and immune response in a time-dependent manner, resulting in neuroprotection and prevention of further inflammatory injury (64). In addition, studies have shown that OECs-derived exosomes (OECs-Exo) can inhibit the polarization of proinflammatory macrophages/microglia and increase the number of anti-inflammatory cells after spinal cord injury (64). OECs-Exo can be phagocytized by microglia and partially reverse endotoxin-induced pro-inflammatory polarization by inhibiting NF–κB and c-jun signal pathways in vitro (61). OECs-Exo can inhibit the changes of pro-inflammatory phenotype of macrophages/microglia, reduce neuronal death and protect neuronal survival and axons (61). OECs-Exo enriched in hsa-miRNA-6780-5p reduce polyglutamine (PolyQ) expression and increase autophagy levels, and inhibit Spinocerebellar Ataxia type 3 (SCA3) expression (65). Moreover, OECs prevent TNF-a induced cell death in neurons, in part through exosome-derived brain-derived neurotrophic factor (BDNF) (66). OECs overexpressed by nuclear receptor-associated factor 1 (Nurr1) and neuron 2 (Ngn2) increase the vitality of PC12 cells, inhibit oxidative stress and cell death, and show significant neuroprotective, antioxidant and anti-cell death effects (67). Aβ (25-35) triggers oxidative damage mediated by ROS, changes the structure and function of mitochondria, and leads to the activation of the intrinsic cell death pathway in mitochondria. Significant changes in cyclin D1 intensity and increases in total ROS levels are particularly observed in OECs exposed to toxic fragments of Aβ (25-35) (68).
OECs regulate the polarization of microglia from pro-inflammatory phenotype (M1) to anti-inflammatory phenotype (M2) through APOE/TREM2/NF-κB pathway, which effectively reduces the malignant inflammatory response and promotes the functional recovery after spinal cord injury in rats (69). Further studies have shown that curcumin-treated OECs can effectively promote nerve survival and axonal growth. The transplanted OECs can improve the neurological prognosis of spinal cord injury in SD male rats. Its curative effect is largely attributed to the anti-inflammatory activity of OECs by regulating the polarization of microglia from M1 phenotype to M2 phenotype (70). Intravenous transplantation of OECs after spinal cord injury regulates microglia activation in the brain and spinal cord of SD rats by upregulating REV-ERBα (71). Moreover, IL-4 plays a leading role in triggering the phenotype of M1 to M2 microglia, significantly reducing the levels of M1 markers IL-1β, IL-6, TNF-α and inducible nitric oxide synthase, while increasing M2 markers Arg-1, TNF-β, IL-10 and CD206. This process coordinates the microglial polarization event regulated by IL-4 with the crosstalk between JAK1/STAT1/3/6 signal and NF-κB/SOCS1/3 signal (70). Studies have shown that curcumin activation of transplanted OECs can promote nerve regeneration and functional recovery after spinal cord injury in rats, and its mechanism is closely related to the production of neurotrophic and anti-inflammatory factors by OECs, reduction of pro-inflammatory cytokines, and significant reduction of cavity and glial scar formation (72) (Figure 1). These studies have revealed the immunomodulatory function of OECs in the process of nerve injury and regeneration, which can reduce nerve inflammation and promote regeneration by changing the activity of immune cells.
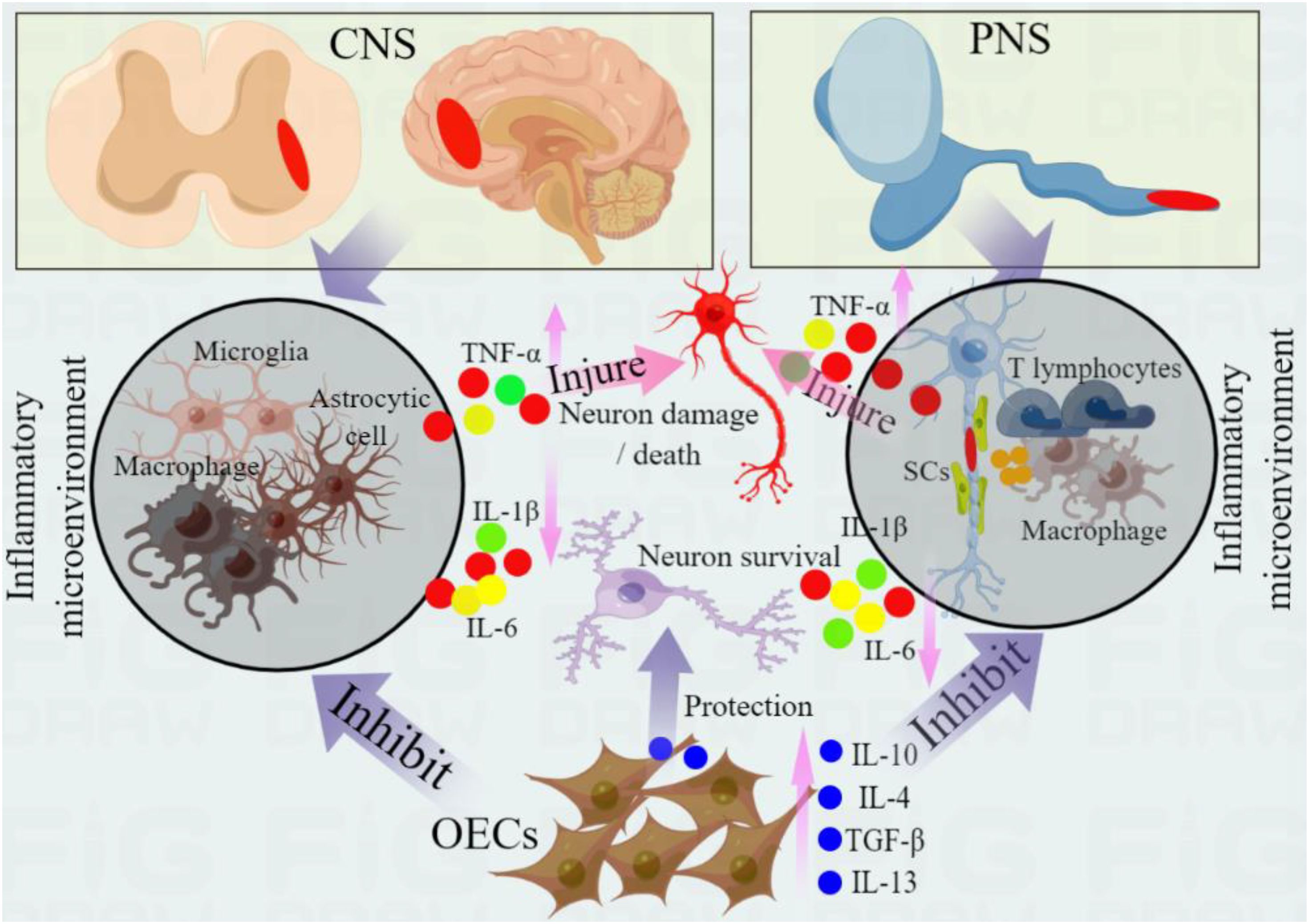
Figure 1. Immunomodulatory and anti-inflammatory effects of OECs in nerve repair. Central nervous system injury (such as spinal cord injury) can induce the activation and infiltration of immune cells (microglia, macrophages and astrocytes), while peripheral nerve injury (PNS) induces the activation of SCs, and activated SCs induce the migration and infiltration of inflammatory cells (such as macrophages and T lymphocytes) by releasing chemokines. These immune cells activation can release pro-inflammatory cytokines (such as IL-1β, IL-6 and TNF-α), further aggravate secondary injury, promote neuronal injury and apoptosis, and hinder nerve regeneration. OECs can regulate the activity of immune cells, change their polarization, promote the transformation of anti-inflammatory phenotype and reduce the release of proinflammatory cytokines. In addition, OECs express a variety of cytokines, which can improve the inflammatory environment, protect injured nerves and promote nerve regeneration and repair by secreting anti-inflammatory cytokines (such as IL-10, IL-4 and TGF- β). These anti-inflammatory cytokines produced by OECs can further inhibit the level of pro-inflammatory cytokines and protect the survival of neurons.
6 OECs produce cytokines conducive to the repair of nerve injury
The process of nerve regeneration is accompanied by the production of a variety of cytokines, and some inflammatory cytokines and chemokine-mediated inflammatory cascades aggravate nerve injury (73, 74). Therefore, improving this change, reducing the release of pro-inflammatory cytokines and increasing the production of anti-inflammatory cytokines are beneficial to nerve regeneration and functional recovery. OECs can regulate the injured area and promote the regeneration of injured nerve by producing growth factors and cytokines. OECs can produce anti-inflammatory cytokines, such as IL-4, IL-10, TGF-β and IL-13, which prevent cell degeneration or death by regulating the production of inducible nitric oxide synthase and nitric oxide under the stimulation of lipopolysaccharide/interferon-γ (64). These anti-inflammatory cytokines inhibit the release of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-2 and IL-6. Most anti-inflammatory factors (IL-4, IL-10, IL-13 and TGF-β) derived from OECs are involved in regulating cell survival, proliferation and migration, thus promoting nerve regeneration after nerve injury (51, 75). OECs not only have strong innate immune regulation, but also can remove cell fragments under the mediation of cytokines (IL-10 and TGF-β) (51). There is also the expression of phosphatidyl serine (PS) receptor in OECs, which enables OECs to recognize apoptotic OECs and phagocytize apoptotic fragments by binding to phosphatidylserine (51). OECs express p75, S100β, and GFAP, which are characteristic markers of globular OECs (activated state). OECs also express a group of unique developmental important proteins-CD44, β1 integrin, P200, Notch3, NG2, vascular endothelial growth factor (VEGF), pituitary adenylate cyclase-activating polypeptide (PACAP) and CREB binding protein (CBP/p300), promote and facilitate nerve regeneration in the adult nervous system according to environmental stimuli and changes (76). OECs attenuate the pro-inflammatory response of human brain microvascular endothelial cells under hypoxia, reduce the HIF-1α/VEGFA signal activation pathway, reduce IL-8 levels, and produce anti-inflammatory effects (77).
Astrocytes play a key role in central nervous system inflammation and nerve regeneration (53, 54). Activation of glial cells leads to the release of specific chemokines and pro-inflammatory cytokines, including IL-1, IL-6 and tumor necrosis factor. These cytokines prevent axonal regeneration by activating their respective cascade reactions, amplifying the inflammatory response, changing the microenvironment, and promoting cell death, thereby preventing axonal regeneration (78, 79). OECs can regulate the response of astrocytes and create an environment conducive to regeneration. Studies have shown that IL-10-mediated upregulation of Matrix metalloproteinase-13 (MMP-13) reduced the interaction between OECs and reactive astrocytes and inhibited glial scars (80). In addition, NF-κB is a key transcriptional regulator of inflammatory genes. Stimulation of p65 NF-κB translocation to the nucleus provides a basis for inflammatory activation of astrocytes (81, 82). Molecules released by OECs can inhibit the activation of NF-κB and play a neuroprotective role after central nervous system injury. Astrocytes cultured in OECs conditioned medium showed a decrease in nuclear translocation of NF-κB, which is a pro-inflammatory protein that induces neurotoxicity of astrocytes (83). Soluble factors released by OECs significantly inhibit NF-κB translocation in astrocytes induced by PMA/calcium carrier or microglial derived factor, which may be related to the expression of insulin-like growth factor-1 in OECs to significantly slow down NF-κB translocation in astrocytes (84). In addition, OECs can produce α B-crystallin (CryAB) through paracrine, which is an anti-inflammatory protein that coordinates the immune response between OECs and astrocytes (83). This suggests that CryAB and other factors secreted by OECs are potential drugs that can improve or even reverse the growth inhibitory environment created by neurotoxic reactive astrocytes after nervous system injury. Moreover, OECs significantly inhibit the transcription of proinflammatory cytokines and granulocyte-macrophage colony-stimulating factor in activated astrocytes (84).
OECs can regulate the microglia-astrocyte response by secreting anti-inflammatory cytokines such as IL-4, IL-10, IL-13 and TGF-β, thus down-regulating pro-inflammatory factors IL-1β, tumor necrosis factor and IL-6 (23). After OECs transplantation, the expression of interleukin-1 receptor antagonist (IL-1ra) in spinal cord tissue was up-regulated, while the expression of many chemokines, including proinflammatory chemokine IL-1 α and IL-1 β, was down-regulated (25). In vitro studies have confirmed that lipopolysaccharide stimulates OECs to secrete IL-1ra, while IL-1ra gene knockout significantly reduces its ability to regulate the activity of microglia, thus reducing neuroinflammation (25). In addition, OECs express chemokine and their homologous receptors, such as chemokine (CXC motif) ligand 1 (CXCL1), which may play a role in embryonic development or after transplantation of OECs at injured sites. CXCL12, CXCL4, and chemokine (CX3C motif) ligand 1 (CX3CL1) have been shown to play a key role in neuroinflammation as a signal factor for neutrophil and various leukocyte recruitment (23, 85). These studies reveal a fact that OECs can express and secrete a variety of anti-inflammatory cytokines and inhibit the inflammatory response after nerve injury, which is beneficial to the repair of injured nerve. However, the cytokines produced by OECs are still complex in improving the process of neuroinflammation, and more studies are needed to clarify this mechanism. If we can fully understand the anti-inflammatory function of OECs, making use of this feature can bring certain value to the repair of nerve injury and functional recovery.
7 Present situation and challenge of immune characteristics of OECs in clinical trials of nerve injury
Based on the reliable results of the basic research on the application of OECs in the repair of nerve injury. In recent years, researchers have begun to explore the rise from the basis to the clinical trial stage. Different studies have confirmed the feasibility and safety of clinical trials of OECs (86, 87). A clinical trial reported the therapeutic effect of OECs transplantation in 108 patients with chronic spinal cord injury. The results showed that 14 cases changed from American Spinal Injury Association (ASIA) A to ASIA B, 18 cases changed from ASIA A to ASIA C, 9 cases improved their walking ability or made them walk again with or without walking assistance, and 12 of 84 males improved their sexual function. 31 patients underwent MRI examination, and no pathological changes such as tumor, hemorrhage, swelling, cyst, nerve tissue destruction or infection were found in or around OECs transplant sites (88). Electromyography was performed in 31 patients, 29 cases were improved and 2 cases had no change. Paravertebral sensory evoked potential (PVSEP) test was performed in 31 patients, of which 28 cases were improved and the other 3 cases had no change. In addition, no deterioration or complications were found in all patients during the follow-up period (88). This clinical trial reveals that OECs treatment is safe, improves neurological function and improves quality of life in patients with fully chronic SCI. Another clinical trial reported that six patients with chronic complete spinal cord injury were treated with autologous OECs transplantation. After 24 months of follow-up, the standard neural classification scores of ASIA and International Society of Neurorepair Spinal Cord injury rating scale (IANR-SCIFRS) were significantly improved. No clinical complications occurred (89). This suggests that OECs transplantation appear to be clinically safe and may promote neurological recovery in SCI.
A recent case reported the effect of OECs transplantation in a patient who received OECs transplantation one year ago for complete traumatic spinal cord injury in the C6-C7 segment. Within a few days after cell therapy, the patient began to show clinical improvement. Six-year follow-up showed that his ASIA changed from ASIA-A to ASIA-C (90). The score of the International Society of Neurorepair Spinal Cord Injury Functional Rating Scale changed from 14 (prior to cell therapy) to 31+/-3 (six years after cell therapy). The main improvements in his daily life activities include eating, dressing and writing, standing and walking, urine control or urination. His sexual function returned to normal (90). Although this report reflects the beneficial effects of OECs transplantation, but more data are needed to support the reliability of the treatment effect. Other clinical trials have reported the transplantation of fetal OECs derived from olfactory bulb tissue via injection into the upper and lower ends of spinal cord injury sites in 300 patients (222 of whom had complete chronic SCI and 78 had incomplete chronic SCI). All patients were evaluated using ASIA criteria before and 2–8 weeks after transplantation. The partially-improved neurological functions assessed by the ASIA standard were indicated by the motor scores increasing from 39.1 +/- 20.6 to 45.9 +/- 20.3, the light touch scores from 51.7 +/- 24.9 to 63.4 +/- 23.0, and the pin prick scores from 53.0 +/- 24.2 to 65.3 +/- 22.7. There was no significant difference in the functional improvement of the motor, light touch, and pin brick when compared with the age, sex, duration after the injury, and the injury degrees and levels. The motor scores and light touch scores at the cervical level were higher than the scores at the thoracic level (91). This also suggests that OECs transplantation in the treatment of chronic spinal cord injury can quickly and partially improve neurological function. In addition, some studies have shown that co-transplantation of OECs with other cells (such as SCs and MSCs) into patients has good tolerance, which can improve the function and corresponding symptoms of patients after nerve injury (90, 92). For example, a phase one clinical trial evaluated the safety of cell transplantation by implanting autologous OEC and MSC into three patients with thoracic traumatic spinal cord injury through lumbar puncture. After 2 years follow-up, all adverse events and possible functional outcomes were recorded through general clinical examinations before and after surgery, magnetic resonance imaging (MRI), neurological assessment based on the SCI International Standards for Neural Classification, and functional assessment using the Spinal Cord Independence Measurement Version III (SCIM III). The results showed that no serious security issues were found. There was no change in MRI and no tumor tissue formation was found. ASIA improved from A to B in one of the participants. SCIM III evaluation also showed that the participant’s function had improved to some extent (92). The other two participants had negligible or no improvement in sensory scores, while there were no changes in AISA and SCIM III scores. No recovery from exercise was observed by any of the participants (92). This clinical trial did not produce any adverse results, which may indicate that a combination of autologous OEC and MSC is safe to treat SCI in humans. These studies have revealed the safety and feasibility of OECs in clinical trials. However, the immune and anti-inflammatory effects of OECs transplantation in patients have not been reported. There is no related literature to detect the changes of serum inflammatory and anti-inflammatory cytokines and the degree of local inflammatory cell infiltration after OECs transplantation. Therefore, more basic research is needed to fully clarify the immunomodulatory and anti-inflammatory characteristics of OECs, and only on the basis of a full understanding of this mechanism can be better over-applied to clinical trials. promote nerve injury repair and functional recovery by improving or enhancing the immune characteristics and anti-inflammatory ability of OECs.
8 Conclusion and prospect
Both basic and clinical trials have shown the advantages of OECs in the repair of nerve injury, but the mechanism of OECs in promoting nerve regeneration and nerve repair is still unclear. However, the immunogenicity and immunomodulatory function of OECs have been understood. First. OECs express a variety of cytokines, which can produce a variety of nutritional factors, growth factors and anti-inflammatory factors through paracrine, and can inhibit or reduce the expression level of pro-inflammatory cytokines and inhibitory factors, which provides a favorable microenvironment for nerves. Secondly, OECs can promote the transformation of immune cells to anti-inflammatory phenotype by regulating the activity and infiltration of immune cells, including macrophages, microglia and astrocytes, and by changing the polarization of these cells. reduce secondary tissue injury and nerve degeneration, protect neuron survival and promote axonal regeneration. Finally, OECs can guide the long-distance extension of newborn axons and bridge distal damaged nerves, provide strong support for immune function.
The application of OECs in the field of basic research in nerve injury treatment has achieved exciting and encouraging results, and the possibility of gradually transitioning to clinical trial applications. Although these current studies have revealed that OECs are feasible and safe in clinical trials, their therapeutic effects are not ideal and need to be verified by more data. This may be related to the existence of some key issues that need to be solved urgently. The origin of OECs is the first problem to be solved. The imperfect in vitro culture system, including the culture cycle, cell vitality and quality, and the possible potential factors that may affect the stability of OECs genes during the culture process, can lead to limited widespread access and application of OECs. Therefore, improving the in vitro culture system and obtaining a sustainable source of cells are the first problems to be solved, which may allow obtaining a sustainable source of OECs through somatic cell gene reprogramming technology to provide new hope for nerve repair. Secondly, significant progress has been made in the therapeutic application of OECs in animal models of nerve injury, but the therapeutic effect in clinical trials is controversial, which may be related to differences between species. Therefore, results obtained from basic research cannot be directly applied to clinical trials. More basic research is needed to understand the working mechanism of OECs in the body, fully grasp their nerve regeneration, anti-inflammatory and immunoregulatory functions to achieve effective therapeutic results. Thirdly, issues such as OECs transplantation method, transplantation time, transplantation cell dose, and transplantation cell localization and tracking in vivo are also important factors leading to differences in treatment effects. OECs can be transplanted into the host through a variety of methods (including venous transplantation, intrathecal transplantation, and local transplantation), but neither method can ensure whether the transplanted cells can colonize and survive the injury site for a long time. Transplanted cells may also have negative effects through blood circulation or penetration and metabolic problems in the body. There is currently no unified standard for the optimal time for cell transplantation, and it is unclear at which time point after nerve injury can be transplanted to achieve the best therapeutic effect. Whether higher doses of transplanted cells produce better therapeutic effects remains unclear. Therefore, these problems require more time and research to explore and solve them in the future. Although the application of OECs in the treatment of nerve injury is in the primary stage, the potential of OECs in the treatment of nerve injury cannot be denied. In short, OECs are a promising candidate for treatment.
Author contributions
W-JZ: Writing – original draft, Writing – review & editing. CZ: Writing – original draft, Writing – review & editing. Y-SX: Writing – review & editing. D-YC: Data curation, Formal Analysis, Supervision, Conceptualization, Software, Writing – original draft, Writing – review & editing. L-XF: Data curation, Formal Analysis, Supervision, Conceptualization, Software, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. These studies were supported by grants from the National Natural Science Foundation project (82460233),Youth Science Foundation of Jiangxi Province (20224BAB216030), Natural Science Foundation of Jiangxi Province (20232BAB206048), Jiangxi Province Traditional Chinese Medicine Science and Technology Plan Project (2023B1213), National Natural incubation Project of the Second Affiliated Hospital of Nanchang University (2022YNFY12006). Doctor of the second affiliated Hospital of Nanchang University starts the project (B3091). Youth Project of Science and Technology Plan of the Health Commission of Jiangxi Province (202510038).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang WJ, Liu SC, Ming LG, Yu JW, Zuo C, Hu DX, et al. Potential role of Schwann cells in neuropathic pain. Eur J Pharmacol. (2023) 956:175955. doi: 10.1016/j.ejphar.2023.175955
2. Wang S, Wen X, Fan Z, Ding X, Wang Q, Liu Z, et al. Research advancements on nerve guide conduits for nerve injury repair. Rev Neurosci. (2024) 35:627–37. doi: 10.1515/revneuro-2023-0093
3. Zhang WJ, Li X, Liao JX, Hu DX, and Huang S. Schwann cells transplantation improves nerve injury and alleviates neuropathic pain in rats. Purinergic Signal. (2024). doi: 10.1007/s11302-024-10046-7
4. Hu X, Xu W, Ren Y, Wang Z, He X, Huang R, et al. Spinal cord injury: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. (2023) 8:245. doi: 10.1038/s41392-023-01477-6
5. Lee CY, Chooi WH, Ng SY, and Chew SY. Modulating neuroinflammation through molecular, cellular and biomaterial-based approaches to treat spinal cord injury. Bioeng Transl Med. (2022) 8:e10389. doi: 10.1002/btm2.10389
6. Gu D, Xia Y, Ding Z, Qian J, Gu X, Bai H, et al. Inflammation in the peripheral nervous system after injury. Biomedicines. (2024) 12:1256. doi: 10.3390/biomedicines12061256
7. Liu F, Cheng X, Zhong S, Liu C, Jolkkonen J, Zhang X, et al. Communications between peripheral and the brain-resident immune system in neuronal regeneration after stroke. Front Immunol. (2020) 11:1931. doi: 10.3389/fimmu.2020.01931
8. Zhou X, Wahane S, Friedl MS, Kluge M, Friedel CC, Avrampou K, et al. Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2. Nat Neurosci. (2020) 23:337–50. doi: 10.1038/s41593-020-0597-7
9. Li Y, Wei B, Liu X, Shen XZ, and Shi P. Microglia, autonomic nervous system, immunity and hypertension: Is there a link? Pharmacol Res. (2020) 155:104451. doi: 10.1016/j.phrs.2019.104451
10. Mukhamedshina YO, Akhmetzyanova ER, Martynova EV, Khaiboullina SF, Galieva LR, and Rizvanov AA. Systemic and local cytokine profile following spinal cord injury in rats: A multiplex analysis. Front Neurol. (2017) 8:581. doi: 10.3389/fneur.2017.00581
11. Chio JCT, Xu KJ, Popovich P, David S, and Fehlings MG. Neuroimmunological therapies for treating spinal cord injury: Evidence and future perspectives. Exp Neurol. (2021) 341:113704. doi: 10.1016/j.expneurol.2021.113704
12. Du J, Cheng N, Deng Y, Xiang P, Liang J, Zhang Z, et al. Astrocyte senescence-like response related to peripheral nerve injury-induced neuropathic pain. Cell Mol Biol Lett. (2023) 28:65. doi: 10.1186/s11658-023-00474-5
13. Wong KA and Benowitz LI. Retinal ganglion cell survival and axon regeneration after optic nerve injury: role of inflammation and other factors. Int J Mol Sci. (2022) 23:10179. doi: 10.3390/ijms231710179
14. Su X, Jing X, Jiang W, Li M, Liu K, Teng M, et al. Curcumin-Containing polyphosphazene nanodrug for Anti-Inflammation and nerve regeneration to improve functional recovery after spinal cord injury. Int J Pharm. (2023) 642:123197. doi: 10.1016/j.ijpharm.2023.123197
15. Li M, Yin H, Yan Z, Li H, Wu J, Wang Y, et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. (2022) 140:23–42. doi: 10.1016/j.actbio.2021.12.006
16. Deng K, Hu DX, and Zhang WJ. Application of cell transplantation in the treatment of neuropathic pain. Neuroscience. (2024) 554:43–51. doi: 10.1016/j.neuroscience.2024.06.035
17. Zhao M, Li J, Gao Z, Guo D, Yang Y, Wang F, et al. miR-145a-5p/Plexin-A2 promotes the migration of OECs and transplantation of miR-145a-5p engineered OECs enhances the functional recovery in rats with SCI. Neurobiol Dis. (2023) 182:106129. doi: 10.1016/j.nbd.2023.106129
18. Tan C, Yang C, Liu H, Tang C, and Huang S. Effect of Schwann cell transplantation combined with electroacupuncture on axonal regeneration and remyelination in rats with spinal cord injury. Anat Rec (Hoboken). (2021) 304:2506–20. doi: 10.1002/ar.v304.11
19. Liu JP, Wang JL, Hu BE, Zou FL, Wu CL, Shen J, et al. Olfactory ensheathing cells and neuropathic pain. Front Cell Dev Biol. (2023) 11:1147242. doi: 10.3389/fcell.2023.1147242
20. Sun X, Tan Z, Huang X, Cheng X, Yuan Y, Qin S, et al. Direct neuronal reprogramming of olfactory ensheathing cells for CNS repair. Cell Death Dis. (2019) 10:646. doi: 10.1038/s41419-019-1887-4
21. Ding L, Hu DX, Yang L, and Zhang WJ. Application of olfactory ensheathing cells in peripheral nerve injury and its complication with pathological pain. Neuroscience. (2024) 560:120–9. doi: 10.1016/j.neuroscience.2024.09.037
22. Liao JX, Zhu FQ, Liu YY, Liu SC, Liu ZX, and Zhang WJ. The role of olfactory ensheathing cells in the repair of nerve injury. Eur J Pharmacol. (2024) 966:176346. doi: 10.1016/j.ejphar.2024.176346
23. Martin-Lopez E, Brennan B, Mao T, Spence N, Meller SJ, Han K, et al. Inflammatory response and defects on myelin integrity in the olfactory system of K18hACE2 mice infected with SARS-CoV-2. eNeuro. (2024) 11. doi: 10.1523/ENEURO.0106-24.2024
24. Jiang Y, Guo J, Tang X, Wang X, Hao D, and Yang H. The immunological roles of olfactory ensheathing cells in the treatment of spinal cord injury. Front Immunol. (2022) 13:881162. doi: 10.3389/fimmu.2022.881162
25. Zhang L, Zhuang X, Kotitalo P, Keller T, Krzyczmonik A, Haaparanta-Solin M, et al. Intravenous transplantation of olfactory ensheathing cells reduces neuroinflammation after spinal cord injury via interleukin-1 receptor antagonist. Theranostics. (2021) 11:1147–61. doi: 10.7150/thno.52197
26. Zhang LP, Liao JX, Liu YY, Luo HL, and Zhang WJ. Potential therapeutic effect of olfactory ensheathing cells in neurological diseases: neurodegenerative diseases and peripheral nerve injuries. Front Immunol. (2023) 14:1280186. doi: 10.3389/fimmu.2023.1280186
27. Barraud P, Seferiadis AA, Tyson LD, Zwart MF, Szabo-Rogers HL, Ruhrberg C, et al. Neural crest origin of olfactory ensheathing glia. Proc Natl Acad Sci U S A. (2010) 107:21040–5. doi: 10.1073/pnas.1012248107
28. Radtke C and Wewetzer K. Translating basic research into clinical practice or what else do we have to learn about olfactory ensheathing cells? Neurosci Lett. (2009) 456:133–6. doi: 10.1016/j.neulet.2008.07.097
29. Graziadei PP and Monti Graziadei GA. Neurogenesis and plasticity of the olfactory sensory neurons. Ann N Y Acad Sci. (1985) 457:127–42. doi: 10.1111/j.1749-6632.1985.tb20802.x
30. Ekberg JA and St John JA. Crucial roles for olfactory ensheathing cells and olfactory mucosal cells in the repair of damaged neural tracts. Anat Rec (Hoboken). (2014) 297:121–8. doi: 10.1002/ar.v297.1
31. Rich CA, Perera SN, Andratschke J, Stolt CC, Buehler DP, Southard-Smith EM, et al. Olfactory ensheathing cells abutting the embryonic olfactory bulb express Frzb, whose deletion disrupts olfactory axon targeting. Glia. (2018) 66:2617–31. doi: 10.1002/glia.v66.12
32. Liu MC, Guo QF, Zhang WW, Luo HL, Zhang WJ, and Hu HJ. Olfactory ensheathing cells as candidate cells for chronic pain treatment. J Chem Neuroanat. (2024) 137:102413. doi: 10.1016/j.jchemneu.2024.102413
33. Huang HY, Xiong MJ, Pu FQ, Liao JX, Zhu FQ, and Zhang WJ. Application and challenges of olfactory ensheathing cells in clinical trials of spinal cord injury. Eur J Pharmacol. (2024) 963:176238. doi: 10.1016/j.ejphar.2023.176238
34. Hashemi M and Hadjighassem M. Primary olfactory ensheathing cell culture from human olfactory mucosa specimen. Bio Protoc. (2017) 7:e2275. doi: 10.21769/BioProtoc.2275
35. Guerout N, Paviot A, Bon-Mardion N, Honoré A, Obongo R, Duclos C, et al. Transplantation of olfactory ensheathing cells to evaluate functional recovery after peripheral nerve injury. J Vis Exp. (2014):e50590. doi: 10.3791/50590
36. Ito D, Carwardine D, Prager J, Wong LF, Kitagawa M, Jeffery N, et al. Methods of olfactory ensheathing cell harvesting from the olfactory mucosa in dogs. PloS One. (2019) 14:e0213252. doi: 10.1371/journal.pone.0213252
37. Zhang WJ, Luo C, Huang C, Liu SC, and Luo HL. Microencapsulated neural stem cells inhibit sciatic nerve injury-induced pain by reducing P2 × 4 receptor expression. Front Cell Dev Biol. (2021) 9:656780. doi: 10.3389/fcell.2021.656780
38. Kachramanoglou C, Law S, Andrews P, Li D, and Choi D. Culture of olfactory ensheathing cells for central nerve repair: the limitations and potential of endoscopic olfactory mucosal biopsy. Neurosurgery. (2013) 72:170–8. doi: 10.1227/NEU.0b013e31827b99be
39. Choi D, Li D, Law S, Powell M, and Raisman G. A prospective observational study of the yield of olfactory ensheathing cells cultured from biopsies of septal nasal mucosa. Neurosurgery. (2008) 62:1140–4. doi: 10.1227/01.NEU.0000313571.44795.49
40. Zhu Q, Liu H, Lang J, Peng H, and Zhao S. The cultivation and migration in vitro of olfactory ensheathing cells from human olfactory mucosa. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2015) 29:410–5.
41. Voronova АD, Stepanova OV, Valikhov MP, Chadin AV, Dvornikov АS, Reshetov IV, et al. Preparation of human olfactory ensheathing cells for the therapy of spinal cord injuries. Bull Exp Biol Med. (2018) 164:523–7. doi: 10.1007/s10517-018-4025-x
42. Vincent AJ, West AK, and Chuah MI. Morphological and functional plasticity of olfactory ensheathing cells. J Neurocytol. (2005) 34:65–80. doi: 10.1007/s11068-005-5048-6
43. Li Y, Huo S, Fang Y, Zou T, Gu X, Tao Q, et al. ROCK inhibitor Y27632 induced morphological shift and enhanced neurite outgrowth-promoting property of olfactory ensheathing cells via YAP-dependent up-regulation of L1-CAM. Front Cell Neurosci. (2018) 12:489. doi: 10.3389/fncel.2018.00489
44. Huang ZH, Wang Y, Yuan XB, and He C. RhoA-ROCK-Myosin pathway regulates morphological plasticity of cultured olfactory ensheathing cells. Exp Cell Res. (2011) 317:2823–34. doi: 10.1016/j.yexcr.2011.09.004
45. Guérout N, Derambure C, Drouot L, Bon-Mardion N, Duclos C, Boyer O, et al. Comparative gene expression profiling of olfactory ensheathing cells from olfactory bulb and olfactory mucosa. Glia. (2010) 58:1570–80. doi: 10.1002/glia.v58:13
46. Lan YX, Yang P, Zeng Z, Yadav N, Zhang LJ, Wang LB, et al. Gene and protein expression profiles of olfactory ensheathing cells from olfactory bulb versus olfactory mucosa. Neural Regener Res. (2022) 17:440–9. doi: 10.4103/1673-5374.317986
47. Tremblay C, Aslam S, Walker JE, Lorenzini I, Intorcia AJ, Arce RA, et al. RNA sequencing of olfactory bulb in Parkinson’s disease reveals gene alterations associated with olfactory dysfunction. Neurobiol Dis. (2024) 196:106514. doi: 10.1016/j.nbd.2024.106514
48. Barton MJ, John JS, Clarke M, Wright A, and Ekberg J. The glia response after peripheral nerve injury: A comparison between schwann cells and olfactory ensheathing cells and their uses for neural regenerative therapies. Int J Mol Sci. (2017) 18:287. doi: 10.3390/ijms18020287
49. Clifford T, Finkel Z, Rodriguez B, Joseph A, and Cai L. Current advancements in spinal cord injury research-glial scar formation and neural regeneration. Cells. (2023) 12:853. doi: 10.3390/cells12060853
50. Nazareth L, Lineburg KE, Chuah MI, Tello Velasquez J, Chehrehasa F, St John JA, et al. Olfactory ensheathing cells are the main phagocytic cells that remove axon debris during early development of the olfactory system. J Comp Neurol. (2015) 523:479–94. doi: 10.1002/cne.v523.3
51. Su Z, Chen J, Qiu Y, Yuan Y, Zhu F, Zhu Y, et al. Olfactory ensheathing cells: the primary innate immunocytes in the olfactory pathway to engulf apoptotic olfactory nerve debris. Glia. (2013) 61:490–503. doi: 10.1002/glia.22450
52. Nazareth L, St John J, Murtaza M, and Ekberg J. Phagocytosis by peripheral glia: importance for nervous system functions and implications in injury and disease. Front Cell Dev Biol. (2021) 9:660259. doi: 10.3389/fcell.2021.660259
53. Wright AA, Todorovic M, Murtaza M, St John JA, and Ekberg JA. Macrophage migration inhibitory factor and its binding partner HTRA1 are expressed by olfactory ensheathing cells. Mol Cell Neurosci. (2020) 102:103450. doi: 10.1016/j.mcn.2019.103450
54. Basu S, Choudhury IN, Lee JYP, Chacko A, Ekberg JAK, and St John JA. Macrophages treated with VEGF and PDGF exert paracrine effects on olfactory ensheathing cell function. Cells. (2022) 11:2408. doi: 10.3390/cells11152408
55. Li Y, Zou T, Xue L, Yin ZQ, Huo S, and Xu H. TGF-β1 enhances phagocytic removal of neuron debris and neuronal survival by olfactory ensheathing cells via integrin/MFG-E8 signaling pathway. Mol Cell Neurosci. (2017) 85:45–56. doi: 10.1016/j.mcn.2017.08.006
56. Hao DJ, Liu C, Zhang L, Chen B, Zhang Q, Zhang R, et al. Lipopolysaccharide and curcumin co-stimulation potentiates olfactory ensheathing cell phagocytosis via enhancing their activation. Neurotherapeutics. (2017) 14:502–18. doi: 10.1007/s13311-016-0485-8
57. Tong D, Zhao Y, Tang Y, Ma J, Wang M, Li B, et al. MiR-487b suppressed inflammation and neuronal apoptosis in spinal cord injury by targeted Ifitm3. Metab Brain Dis. (2022) 37:2405–15. doi: 10.1007/s11011-022-01015-3
58. Chang YH, Wu KC, Hsu CJ, Tu TC, Liu MC, Chiang RY, et al. Therapeutic potential of olfactory ensheathing cells and adipose-derived stem cells in osteoarthritis: insights from preclinical studies. Cells. (2024) 13:1250. doi: 10.3390/cells13151250
59. Akhmetzyanova E, Kletenkov K, Mukhamedshina Y, and Rizvanov A. Different approaches to modulation of microglia phenotypes after spinal cord injury. Front Syst Neurosci. (2019) 13:37. doi: 10.3389/fnsys.2019.00037
60. Zhang H, Xiang L, Yuan H, and Yu H. PTPRO inhibition ameliorates spinal cord injury through shifting microglial M1/M2 polarization via the NF-κB/STAT6 signaling pathway. Biochim Biophys Acta Mol Basis Dis. (2024) 1870:167141. doi: 10.1016/j.bbadis.2024.167141
61. Delarue Q, Robac A, Semprez F, Duclos C, Pileyre B, Neveu P, et al. Brain inflammation and cognitive decline induced by spinal cord injury can be reversed by spinal cord cell transplants. Brain Behav Immun. (2025) 125:388–97. doi: 10.1016/j.bbi.2025.01.014
62. Pellitteri R, La Cognata V, Russo C, Patti A, and Sanfilippo C. Protective role of eicosapentaenoic and docosahexaenoic and their N-ethanolamide derivatives in olfactory glial cells affected by lipopolysaccharide-induced neuroinflammation. Molecules. (2024) 29:4821. doi: 10.3390/molecules29204821
63. Liao JX, Huang QM, Pan ZC, Wu J, and Zhang WJ. The anti-inflammatory and immunomodulatory effects of olfactory ensheathing cells transplantation in spinal cord injury and concomitant pathological pain. Eur J Pharmacol. (2024) 982:176950. doi: 10.1016/j.ejphar.2024.176950
64. Fan H, Chen Z, Tang HB, Shan LQ, Chen ZY, Wang XH, et al. Exosomes derived from olfactory ensheathing cells provided neuroprotection for spinal cord injury by switching the phenotype of macrophages/microglia. Bioeng Transl Med. (2021) 7:e10287. doi: 10.1002/btm2.10287
65. Chen YS, Harn HJ, Hong ZX, Huang YC, Lin YT, Zheng HX, et al. Preconditioning of exosomes derived from human olfactory ensheathing cells improved motor coordination and balance in an SCA3/MJD mouse model: A new therapeutic approach. Eur J Pharm Sci. (2023) 191:106608. doi: 10.1016/j.ejps.2023.106608
66. Chen Z, Fan H, Chen ZY, Jiang C, Feng MZ, Guo XY, et al. OECs prevented neuronal cells from apoptosis partially through exosome-derived BDNF. J Mol Neurosci. (2022) 72:2497–506. doi: 10.1007/s12031-022-02097-5
67. Liu Q, Qin Q, Sun H, Zhong D, An R, Tian Y, et al. Neuroprotective effect of olfactory ensheathing cells co-transfected with Nurr1 and Ngn2 in both in vitro and in vivo models of Parkinson’s disease. Life Sci. (2018) 194:168–76. doi: 10.1016/j.lfs.2017.12.038
68. Campisi A, Sposito G, Grasso R, Bisicchia J, Spatuzza M, Raciti G, et al. Effect of astaxanthin on tissue transglutaminase and cytoskeletal protein expression in amyloid-beta stressed olfactory ensheathing cells: molecular and delayed luminescence studies. Antioxidants (Basel). (2023) 12:750. doi: 10.3390/antiox12030750
69. Jiang C, Chen Z, Wang X, Zhang Y, Guo X, Fan H, et al. Curcumin-activated olfactory ensheathing cells improve functional recovery after spinal cord injury by modulating microglia polarization through APOE/TREM2/NF-κB signaling pathway. J Neuroimmune Pharmacol. (2023) 18:476–94. doi: 10.1007/s11481-023-10081-y
70. Guo J, Tang X, Deng P, Hui H, Chen B, An J, et al. Interleukin-4 from curcumin-activated OECs emerges as a central modulator for increasing M2 polarization of microglia/macrophage in OEC anti-inflammatory activity for functional repair of spinal cord injury. Cell Commun Signal. (2024) 22:162. doi: 10.1186/s12964-024-01539-4
71. Zhang L, Wang L, Tan Y, Li C, and Fang C. Olfactory ensheathing cell ameliorate neuroinflammation following spinal cord injury through upregulating REV-ERBα in microglia. Cell Transplant. (2024) 33:9636897241261234. doi: 10.1177/09636897241261234
72. Guo J, Cao G, Yang G, Zhang Y, Wang Y, Song W, et al. Transplantation of activated olfactory ensheathing cells by curcumin strengthens regeneration and recovery of function after spinal cord injury in rats. Cytotherapy. (2020) 22:301–12. doi: 10.1016/j.jcyt.2020.03.002
73. Amanollahi M, Jameie M, Heidari A, and Rezaei N. The dialogue between neuroinflammation and adult neurogenesis: mechanisms involved and alterations in neurological diseases. Mol Neurobiol. (2023) 60:923–59. doi: 10.1007/s12035-022-03102-z
74. Jiang S, Li W, Song M, Liang J, Liu G, Du Q, et al. CXCL1-CXCR2 axis mediates inflammatory response after sciatic nerve injury by regulating macrophage infiltration. Mol Immunol. (2024) 169:50–65. doi: 10.1016/j.molimm.2024.03.006
75. Gómez RM, Sánchez MY, Portela-Lomba M, Ghotme K, Barreto GE, Sierra J, et al. Cell therapy for spinal cord injury with olfactory ensheathing glia cells (OECs). Glia. (2018) 66:1267–301. doi: 10.1002/glia.23282
76. Wang J, Cheng C, Liu Z, Lin Y, Yang L, Zhang Z, et al. Inhibition of A1 astrocytes and activation of A2 astrocytes for the treatment of spinal cord injury. Neurochem Res. (2023) 48:767–80. doi: 10.1007/s11064-022-03820-9
77. Agafonova A, Cosentino A, Musso N, Prinzi C, Russo C, Pellitteri R, et al. Hypoxia-induced inflammation in in vitro model of human blood-brain barrier: modulatory effects of the olfactory ensheathing cell-conditioned medium. Mol Neurobiol. (2025) 62:4008–22. doi: 10.1007/s12035-024-04517-6
78. Ramesh G, MacLean AG, and Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. (2013) 2013:480739. doi: 10.1155/2013/480739
79. Xu X, Wen S, Zhang Y, Cao W, Yue P, Kong J, et al. A key protein from Borrelia burgdorferi could stimulate cytokines in human microglial cells and inhibitory effects of Cucurbitacin IIa. IBRO Neurosci Rep. (2023) 15:376–85. doi: 10.1016/j.ibneur.2023.11.004
80. Khankan RR, Griffis KG, Haggerty-Skeans JR, Zhong H, Roy RR, Edgerton VR, et al. Olfactory ensheathing cell transplantation after a complete spinal cord transection mediates neuroprotective and immunomodulatory mechanisms to facilitate regeneration. J Neurosci. (2016) 36:6269–86. doi: 10.1523/JNEUROSCI.0085-16.2016
81. Dong X, Shu L, Zhang J, Yang X, Cheng X, Zhao X, et al. Ogt-mediated O-GlcNAcylation inhibits astrocytes activation through modulating NF-κB signaling pathway. J Neuroinflammation. (2023) 20:146. doi: 10.1186/s12974-023-02824-8
82. Liu C, Zhao XM, Wang Q, Du TT, Zhang MX, Wang HZ, et al. Astrocyte-derived SerpinA3N promotes neuroinflammation and epileptic seizures by activating the NF-κB signaling pathway in mice with temporal lobe epilepsy. J Neuroinflammation. (2023) 20:161. doi: 10.1186/s12974-023-02840-8
83. Saglam A, Calof AL, and Wray S. Novel factor in olfactory ensheathing cell-astrocyte crosstalk: Anti-inflammatory protein α-crystallin B. Glia. (2021) 69:1022–36. doi: 10.1002/glia.23946
84. Hale DM, Ray S, Leung JY, Holloway AF, Chung RS, West AK, et al. Olfactory ensheathing cells moderate nuclear factor kappaB translocation in astrocytes. Mol Cell Neurosci. (2011) 46:213–21. doi: 10.1016/j.mcn.2010.09.004
85. Yan Y, Su J, and Zhang Z. The CXCL12/CXCR4/ACKR3 response axis in chronic neurodegenerative disorders of the central nervous system: therapeutic target and biomarker. Cell Mol Neurobiol. (2022) 42:2147–56. doi: 10.1007/s10571-021-01115-1
86. Lima C, Escada P, Pratas-Vital J, Branco C, Arcangeli CA, Lazzeri G, et al. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil Neural Repair. (2010) 24:10–22. doi: 10.1177/1545968309347685
87. Huang H, Chen L, Wang H, Xi H, Gou C, Zhang J, et al. Safety of fetal olfactory ensheathing cell transplantation in patients with chronic spinal cord injury. A 38-month follow-up MRI. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2006) 20:439–43.
88. Huang H, Xi H, Chen L, Zhang F, and Liu Y. Long-term outcome of olfactory ensheathing cell therapy for patients with complete chronic spinal cord injury. Cell Transplant. (2012) 21 Suppl 1:S23–31. doi: 10.3727/096368912X633734
89. Rao Y, Zhu W, Guo Y, Jia C, Qi R, Qiao R, et al. Long-term outcome of olfactory ensheathing cell transplantation in six patients with chronic complete spinal cord injury. Cell Transplant. (2013) 22 Suppl 1:S21–5. doi: 10.3727/096368913X672127
90. Chen D, Xi H, Tan K, and Huang H. Recovering voiding and sex function in a patient with chronic complete spinal cord injury by olfactory ensheathing cell transplantation. Case Rep Neurol Med. (2022) 2022:9496652. doi: 10.1155/2022/9496652
91. Huang H, Wang H, Chen L, Gu Z, Zhang J, Zhang F, et al. Influence factors for functional improvement after olfactory ensheathing cell transplantation for chronic spinal cord injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2006) 20:434–8.
92. Zamani H, Soufizomorrod M, Oraee-Yazdani S, Naviafar D, Akhlaghpasand M, Seddighi A, et al. Safety and feasibility of autologous olfactory ensheathing cell and bone marrow mesenchymal stem cell co-transplantation in chronic human spinal cord injury: a clinical trial. Spinal Cord. (2022) 60:63–70. doi: 10.1038/s41393-021-00687-5
Keywords: OECS, immune regulation, anti-inflammation, nerve injury repair, regeneration
Citation: Chen D-y, Zhang W-j, Zuo C, Xu Y-s and Fu L-x (2025) Immune characteristics of olfactory ensheathing cells and repair of nerve injury. Front. Immunol. 16:1571573. doi: 10.3389/fimmu.2025.1571573
Received: 05 February 2025; Accepted: 25 April 2025;
Published: 15 May 2025.
Edited by:
Yasser M El-Sherbiny, Nottingham Trent University, United KingdomReviewed by:
Vanina Usach, Universidad de Buenos Aires, ArgentinaJames St John, Griffith University, Australia
Xiaohui Wang, Xi’an Honghui Hospital, China
Copyright © 2025 Chen, Zhang, Zuo, Xu and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liu-xiang Fu, Zmx4MTk5MDA5QDE2My5jb20=
 Ding-yi Chen1
Ding-yi Chen1 Wen-jun Zhang
Wen-jun Zhang Yong-sheng Xu
Yong-sheng Xu