- 1Department of Internal Medicine, Erasmus Medical Center Transplant Institute, University Medical Center, Rotterdam, Netherlands
- 2Department of Immunology, Leiden University Medical Center, Leiden, Netherlands
Background and hypothesis: The presence of donor-specific anti-HLA antibodies before kidney transplantation (preDSAs) is associated with decreased graft survival. The hypothesis that increasing donor kidney age is negatively associated with the impact of preDSA on graft survival was investigated.
Methods: Outcome of kidney transplantation in a single center cohort of 2,024 patients transplanted between 2010 and 2020 with a follow-up of at least 3 years was analyzed to assess this relation.
Results: DSAs before transplantation were present in 14% of recipients and showed an independent association with graft loss. The preDSA against HLA class I (2%) or class II (7%) had an adjusted hazard ratio (HR) for death censored graft failure of 5.8 (95% CI 4.4–7.7), while the combination (5%) had an HR of 18.6 (95% CI 13.8–25.1). The preDSA-associated increase in graft failure was caused primarily by an increase in the incidence of antibody-mediated rejection (ABMR), intragraft thrombosis, and primary non-function. These effects were observed more frequently in the deceased donor kidney transplantations compared to living donor kidney transplantations. The incidence of ABMR was not associated with donor kidney age. However, increasing donor kidney age significantly aggravated the negative effect of preDSA on graft survival. For instance, recipients aged ≥65 years transplanted with a deceased donor kidney aged ≥65 years had an uncensored 1- and 3-year graft survival of 83% and 67%, respectively, if transplanted without DSA. This decreased to 56% and 35% if transplanted in the presence of DSA. For comparison, recipients aged ≥65 years of a deceased donor kidney aged <65 years had an uncensored 1- and 3-year graft survival of 92% and 78%, respectively, without preDSA, and if transplanted with preDSA, this decreased to 77% and 69%, respectively.
Conclusions: The negative effect of circulating DSA at the time of transplantation on both early and late death-censored graft survival is heavily influenced by donor age.
1 Introduction
The presence of donor-specific anti-HLA antibodies (preDSAs) before kidney transplantation is associated with an increased risk of antibody-mediated rejection (ABMR) and graft loss (1–3). In two large multicenter studies, the effect of DSAs, measured by Luminex single antigen bead assay, was approximately a 10% difference in a 10-year graft survival (4, 5). In the multicenter Dutch PROCARE study, an increased deleterious effect was observed when both DSAs against HLA class I and class II were present (5). Importantly, in these studies, the level of antibodies, which is semi-quantitively expressed as the mean fluorescence intensity (MFI) of the bead signal, was not a discriminating factor for an increased risk of graft loss, and there seems to be no “safe” MFI threshold for preDSAs (4–6). However, the impact of preDSAs on kidney graft survival differs substantially between studies and within different periods of transplantation (7, 8). This may, in part, be explained by DSA-related factors such as complement binding properties and subtype of IgG (9). In addition, donor-related factors, like HLA expression on the allograft (10, 11), an aged immune system in the elderly recipient (12, 13), Fc-receptor genotype (14), and type of immune suppressive regimen, are variable factors, which may determine the incidence of ABMR.
Another contributing factor could be the age of the donor kidney, which has increased substantially over time in Dutch transplant centers. In particular, the European Senior Program (ESP) of Eurotransplant preferentially allocates kidneys from donors aged 65 years and older to recipients of 65 years and older. The results of the latter program in the Netherlands showed that 1- and 3-year graft survival become increasingly poor as the donor age increases (15). Also, donor kidneys aged above 60 years are particularly vulnerable for graft failure due to a variety of causes such as hypovolemic shock, sepsis, and nephrotoxic medication like calcineurin inhibitors (16, 17).
For the recipient, early graft loss in the first years after transplantation has detrimental effects both in terms of increased mortality and a low likelihood of retransplantation (18–20). The impact of preDSAs on graft survival in relation to donor kidney age has not been documented in detail. We hypothesized that older donor kidneys are more vulnerable for immune activation and endothelial inflammation, which can be triggered by circulating preDSAs. Such an effect could lead to increased graft loss with subsequent important clinical consequences, in particular for the elderly recipients.
In this study, we investigated the effect of preDSA on early and late graft failure with a focus on the interaction with donor kidney age using data from a large single-center cohort with a uniform immune suppressive medication regimen in a recent era.
2 Patients and methods
This study included all 2,124 consecutive kidney transplantations performed between January 2010 and December 2020 at the Erasmus Medical Center in the Netherlands. The last follow-up date for data analysis was June 2024. Recipients were seen at least once a year in our out-patient clinic, and clinical data were registered in a national database (Netherlands Organ Transplant Registry). All transplantation across the ABO blood group barrier (n = 88) or a current positive complement-dependent cytotoxicity cross-match (n = 12) were excluded from analysis.
Induction therapy was basiliximab in the vast majority of patients. The standard immune suppressive medication protocol was based on tacrolimus (aiming for predose concentrations of 10–15 ng/ml in weeks 1–2, 8–12 ng/ml in weeks 3–4, and 5–10 ng/ml, thereafter) combined with mycophenolate mofetil (starting dose of 1 g b.i.d., aiming for predose concentrations of 1.5–3.0 mg/l) and glucocorticoids. All patients received 50 mg of prednisolone b.i.d. intravenously on days 0–3. Thereafter, 20 mg of oral prednisolone was started and subsequently tapered to 5 mg at month 3 and thereafter stopped within 3 months.
The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism” and in accordance with the Declaration of Helsinki. All patients gave written informed consent for participating in the Netherlands Organ Transplant Registry database. Approval for assessing additional clinical information was obtained by the institutional review board of the Erasmus Medical Center (MEC-2021-0357 and MEC-2024-0193). Study design and analysis was done in accordance with the STROBE statement.
All renal biopsies were for cause and were performed in case of progressive loss of graft function. The initial biopsy reviews were rescored following the 2018 Banff Reference Guide (21). ABMR was treated with pulse methylprednisolone and intravenous immunoglobulins (1–2 g/kg bodyweight) with additional plasmapheresis in acute ABMR. Alemtuzumab was administered as second-line treatment in a small number of patients (22).
2.1 Outcomes and variables
For data analysis, the outcome of kidney biopsy was further categorized as previously published (23): rejection, recurrence of primary kidney disease, diagnosis of de novo kidney disease, and interstitial fibrosis with tubulus atrophy (IFTA). In case of graft failure, the diagnosis of for cause kidney biopsies was used to categorize the type of graft failure if no other clinical event could explain the loss of kidney function.
The other graft loss categories were a clinical event leading to irreversible graft failure (e.g., circulatory shock, pyelonephritis, graft thrombosis) and “unknown” if no biopsy was performed and a clinical diagnosis for allograft failure could not be made. Primary non-function is the category of grafts that never had function after transplantation with no other diagnosis than acute tubular necrosis (ATN) as shown by kidney biopsy.
2.2 Identification of anti-HLA donor-specific antibodies
Anti-HLA donor-specific antibodies were measured in this study as previously reported (6). A, B, C, DRB1, DRB3, DRB4, DRB5, DQA, DQB, DPA, and DPB were considered for DSA testing. If needed (e.g., in case of anti-DP antibodies), retyping of recipients was performed. During the study period, the methods to detect HLA antibodies have changed from ELISA screening to Luminex screening and subsequently Luminex Single Antigen Bead (SAB) testing. In case of a positive screening, this was followed by antibody identification by SAB assay of either Lifecodes or OneLambda. For the Lifecodes SAB test, data were analyzed using MatchIt. Antibody software version 1.3.1 (Immucor) and results were shown as mean fluorescence intensity (MFI) values, background corrected. Cut-offs were bead specific in combination with a raw MFI of more than 750. For OneLambda, data were analyzed using HLA FUSION antibody software version 3.4.18 (One Lambda) using an MFI of 750 as a cut-off. The percentage of panel reactive antibodies (PRA) at time of transplantation, as determined by complement-dependent cytotoxicity assay, was considered positive when >5%.
2.3 Statistical analysis
Differences in patient, donor, and transplant characteristics were assessed by the Fisher’s exact test for categorical variables and Mann–Whitney U test for continuous variables. All p-values were two-tailed. Death-censored graft survival was initially assessed by Kaplan–Meier survival analysis with log-rank statistics for difference between strata of pretransplant DSA. Donor age was categorized as <45 years (n = 487), 45–54 years (n = 464), 55–64 years (n = 595), and 65 years or older (n = 478). Univariate Cox proportional hazards analysis was used to identify clinical and demographic variables associated with rejection and graft survival. Variables considered for analysis were as follows: donor kidney age, age of recipient, re-transplantation, pre-emptive transplantation, positive PRA, pretransplant DSA, type of DSA (anti-HLA class I and/or II), number of HLA mismatches, type of kidney donor (LD, DCD, DBD), male/female, and cold ischemia time. Variables with a p-value of <0.1 in a univariate analysis were subsequently used in the multivariate Cox proportional hazard analysis with stepwise forward regression to calculate adjusted hazard ratios for the outcome (e.g., graft failure) within the categories of pre-DSA compared to no preDSA. For analysis of the incidence of ABMR, the cases with ABMR only and the cases with ABMR mixed with cellular rejection were combined. Interaction terms that met statistical significance (p < 0.05) were included in the multivariate model. All adjusted hazard ratio’s as shown in the results were calculated with the multivariate Cox proportional hazard analysis. Statistical analysis was performed with the software IBM SPSS statistics 21. Statistical significance was met if the p-value was <0.05.
3 Results
3.1 Baseline characteristics
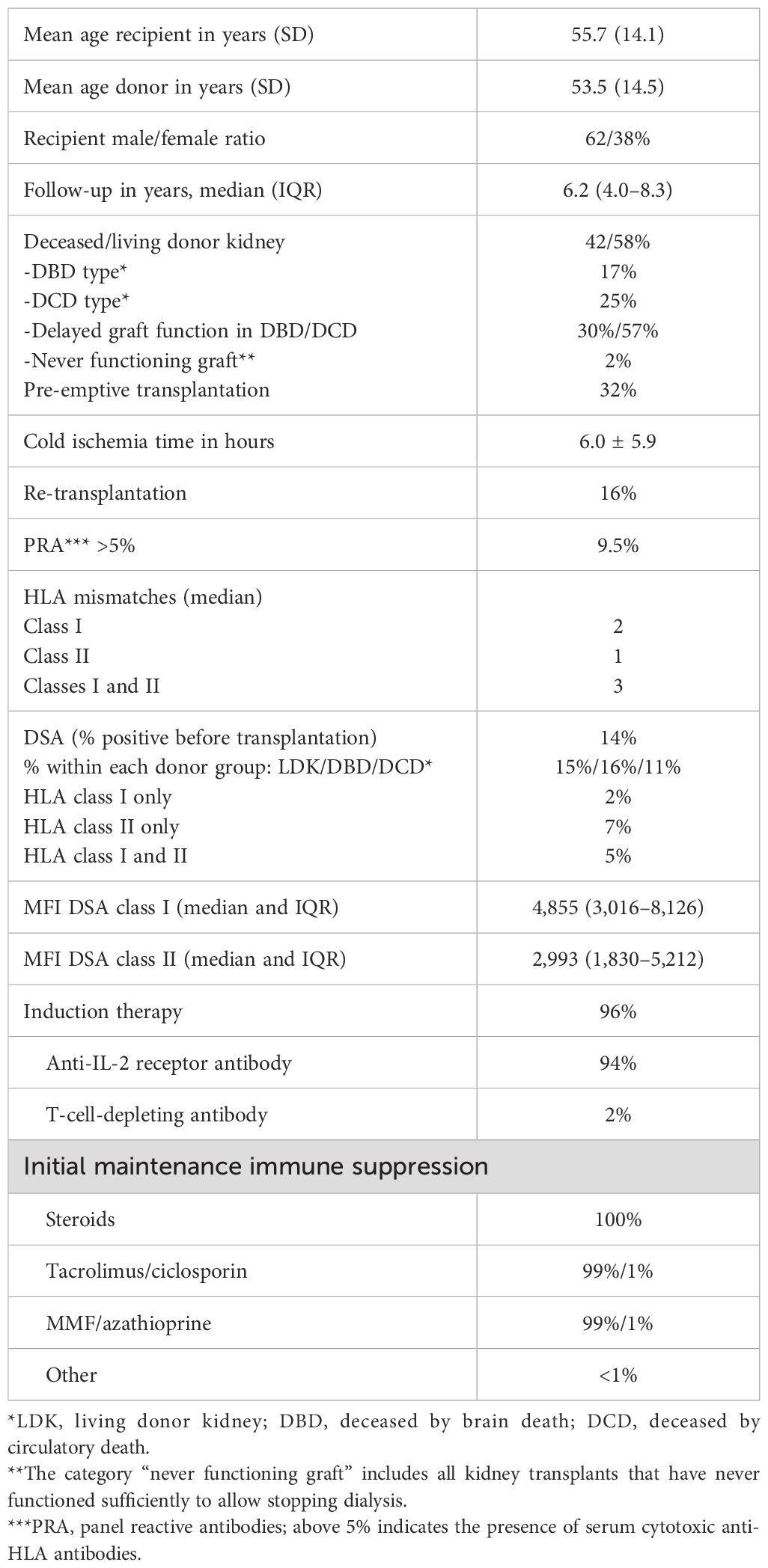
Baseline characteristics are shown in Table 1. The average age of recipients was 56 years, and the average age of donors was 53 years (for LDK 52 years, DBD 56 years, and DCD 56 years), in line with the general trend of increasing numbers of elderly patients receiving a kidney transplant. Over 96% of the recipients were treated with anti-CD25 antibody induction therapy, and 99% continued with the standard protocol of triple immune suppression with tacrolimus as calcineurin inhibitor of choice. The majority of recipients (58%) received a kidney from a living donor, and pre-emptive transplantation was performed in a third of cases. Pretransplant DSAs were present in 14% of transplantations with the majority belonging to anti-HLA class II antibodies, with or without anti-HLA class I antibodies. Of note, the frequency of transplantations with preDSAs decreased from an average of 20% in 2010 to 2014 to 10% in the period 2016–2020. In particular, preDSAs against both classes I and II decreased over the years from an average of 7% to <1% after 2019. The frequency of re-transplantation (14%) was relatively low within this cohort, as was immunization for HLA (positive PRA in 9.5% of cases).
3.2 Pretransplant DSAs and graft survival
The presence of preDSAs was highly associated with decreased graft survival and ABMR-free survival (Figures 1A, B). The effect of preDSAs of either class I or II was similar, but the combination of preDSA classes I and II had the largest effect. In particular, early graft loss (Figure 1A) and ABMR (Tables 2, 3) shortly after transplantation were observed in the preDSA groups. Multivariable Cox proportional hazard analysis showed an HR for graft failure of 5.8 (95% CI 4.4–7.7) for preDSA class I or II, while the combination had an HR of 18.6 (95% CI 13.8–25.1). Similar results were obtained for the incidence of ABMR.
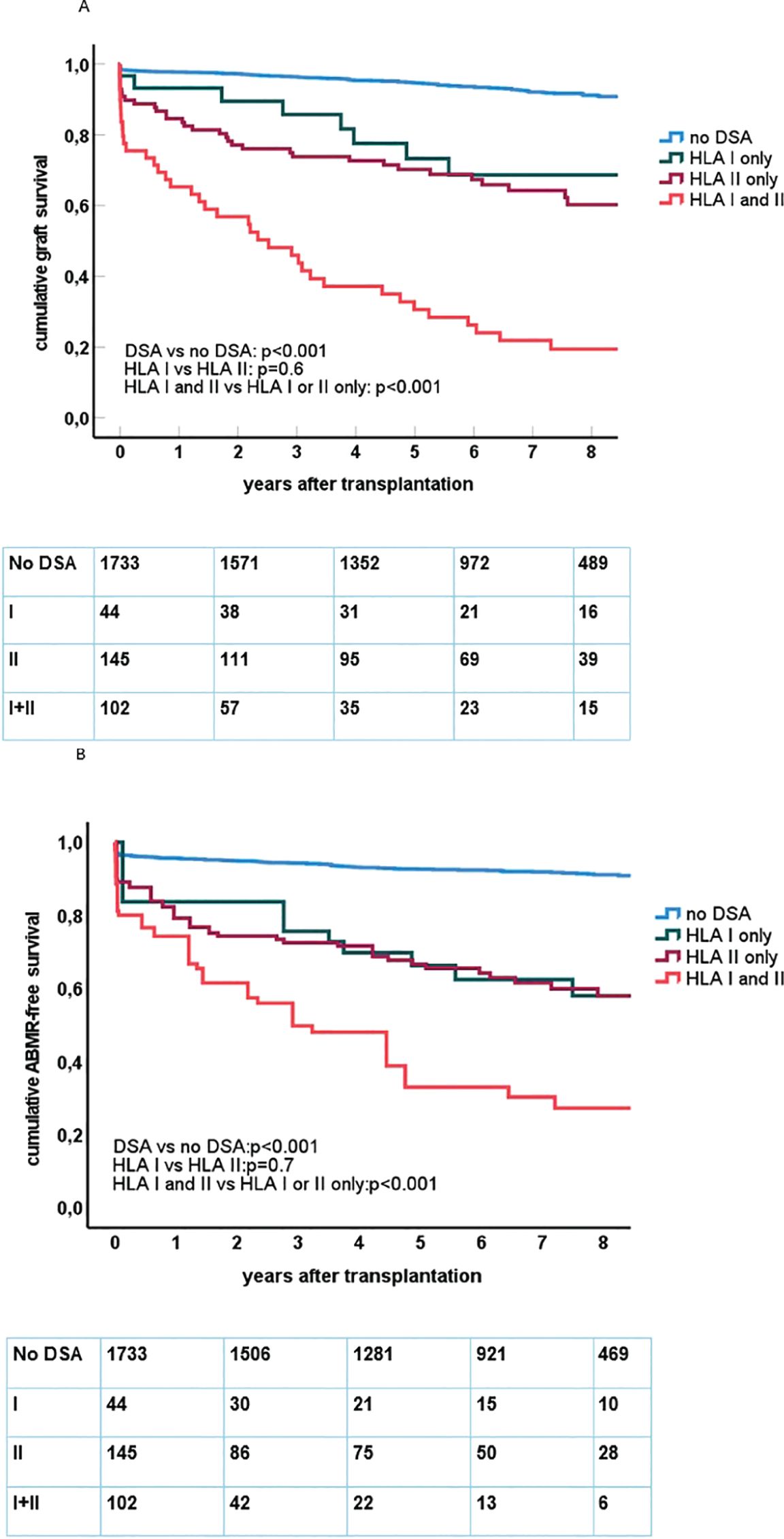
Figure 1. (A) Kaplan–Meier curves for death-censored graft survival after kidney transplantation in relation to the presence and type of pretransplant DSAs. Below the figure, the number of at risk at follow-up is shown within each category. Log rank test for comparing different groups of recipients is shown within the figure. (B) Kaplan–Meier curves for antibody-mediated rejection (ABMR)-free survival after kidney transplantation in relation to the presence and type of pretransplant DSAs. Below the figure, the number of at risk at follow-up is shown within each category. Log rank test for comparing different groups of recipients is shown within the figure.
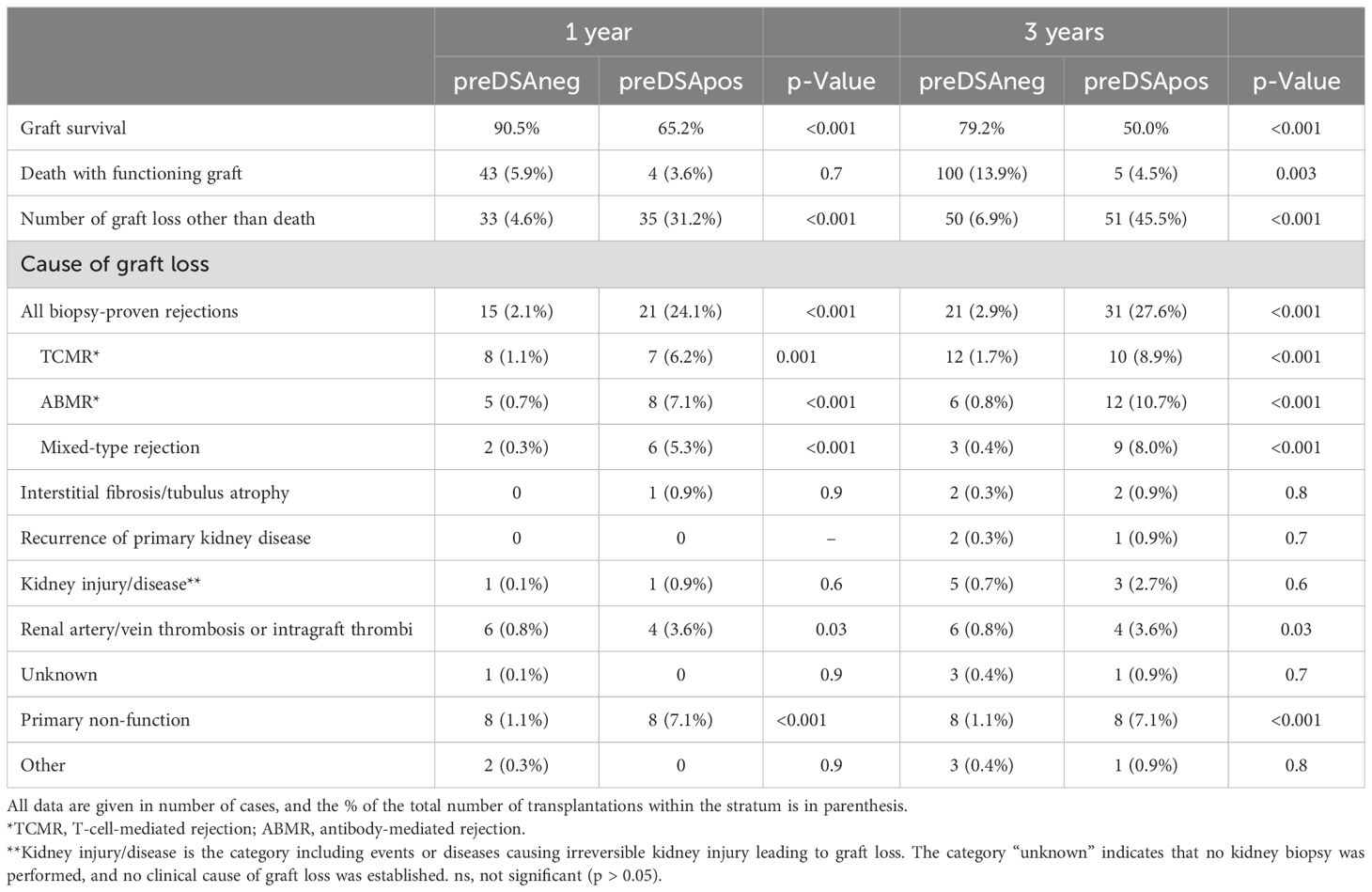
Table 2. Graft loss of deceased donor kidney transplantations (n = 832) at 1 and 3 years after transplantation stratified for the presence of DSAs at time of transplantation in preDSA negative (n = 720) or preDSA positive (n = 112).
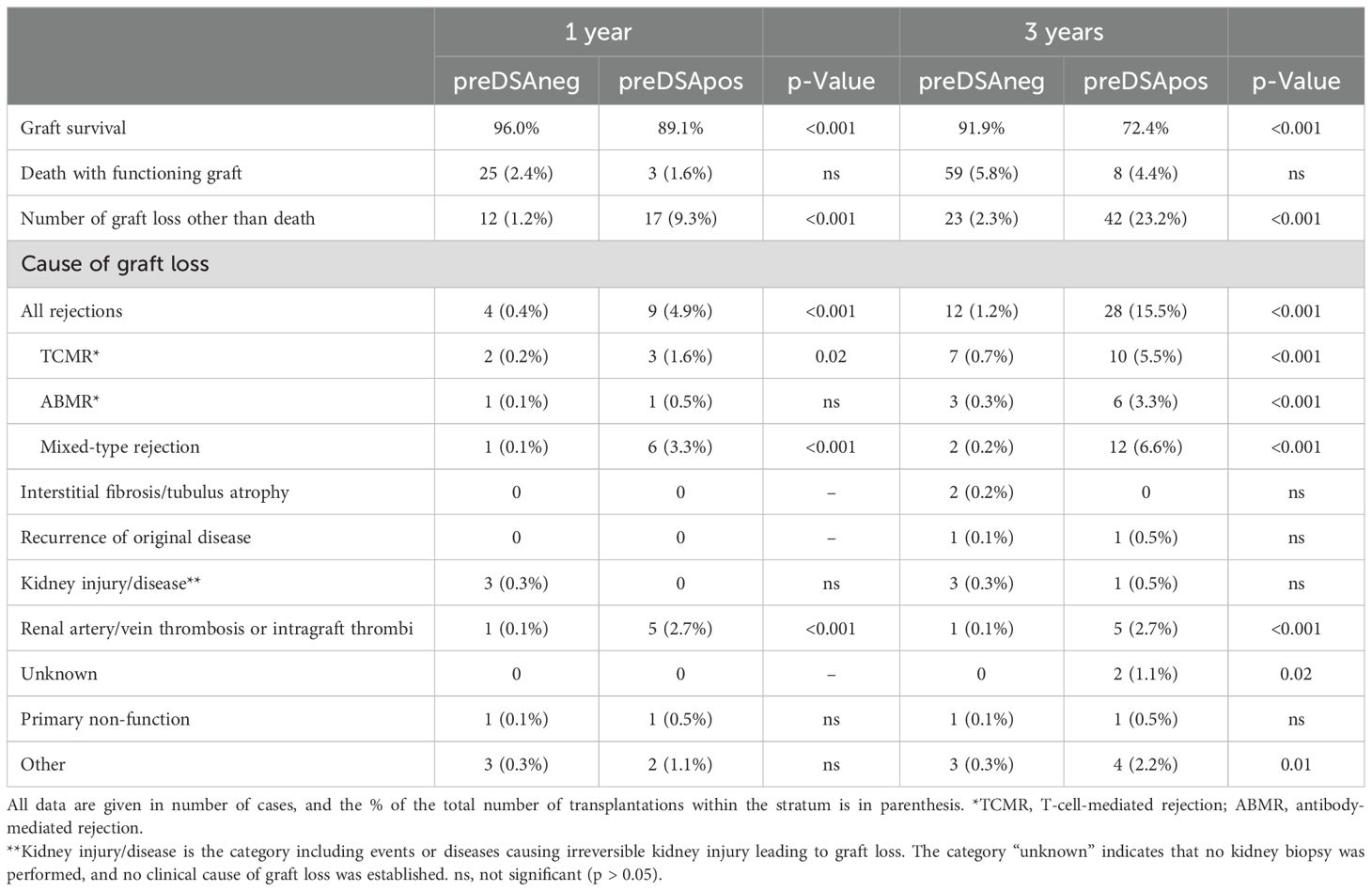
Table 3. Graft loss of living donor kidney transplantations (n = 1,192) at 1 and 3 years after transplantation stratified for the presence of DSA at time of transplantation in preDSA negative (n = 1,011) or preDSA positive (n = 181).
Data on graft survival and cause of graft loss was performed separately for living (LD) and deceased donor (DD) kidney transplantations because of significant differences in effect size of preDSA (Tables 2, 3). At 1 year after transplantation, the graft survival in the preDSA-positive DD versus the preDSA-negative DD group was significantly lower (65.2% vs. 89.5%). This was mainly caused by an increased incidence of AMBR (whether or not “mixed” with TCMR) from 1% to 12.4%, primary non-function (1.1% vs. 7.1%), and thrombosis (0.8% vs. 3.6%), usually of the renal vein or artery. At 3 years post-transplantation, the uncensored graft survival in the preDSA DD group was 50% (79% in the DD group without preDSA), largely attributable to a further increase in ABMR-related graft loss to 18.7%. Within the LD group, similar negative effects, although to lesser extent than within the DD group, were seen in the preDSA-positive group. At 1-year, graft loss other than death was increased (9.3% vs. 1.2%). Specifically, ABMR-related graft loss (3.8% vs. 0.2%) and kidney thrombosis were increased (2.7% vs. 0.1%). At year 3, the cumulative incidence of graft loss other than death was 10 times higher in the preDSA group (23.2 vs. 2.3%).
3.3 The negative impact of preDSAs on graft survival is largely determined by older age of the donor kidney
The age category of the donor kidney was significantly associated with the impact of preDSA on graft survival (Figure 2). Multivariate Cox proportional hazard analysis was performed to assess the effect of preDSA in different age groups of donor kidneys (Table 4; Supplementary Table 1 for all significant variables). Within every age group, the hazard ratio of preDSAs against HLA class I or II only or the combination thereof was significantly associated with an increase in the hazard ratio for graft loss. The hazard ratio of preDSA was roughly similar in all donor age categories, but this resulted in a much higher absolute % of graft loss in the older donor age categories as can be appreciated from Figure 2. Specifically, recipients aged ≥65 years transplanted with a DD kidney within the Eurotransplant Senior Program (n = 213) had an uncensored 1- and 3-year graft survival of 83% and 67%, respectively, without preDSAs, but if transplanted with preDSAs, this decreased to 56% and 35%, respectively. If recipients aged ≥65 years received a DD kidney of <65 years of age (n = 131), the uncensored 1- and 3-year graft survival was 92% and 78%, respectively, without preDSAs, and if transplanted with preDSAs, this decreased to 77% and 69%, respectively. In both LD and DD groups the donor age itself was not associated with an increase in the incidence of ABMR (data not shown). Thus, it is the age and the type (LD or DD) of donor kidney, which are the main determinants of the impact of preDSA on graft survival.
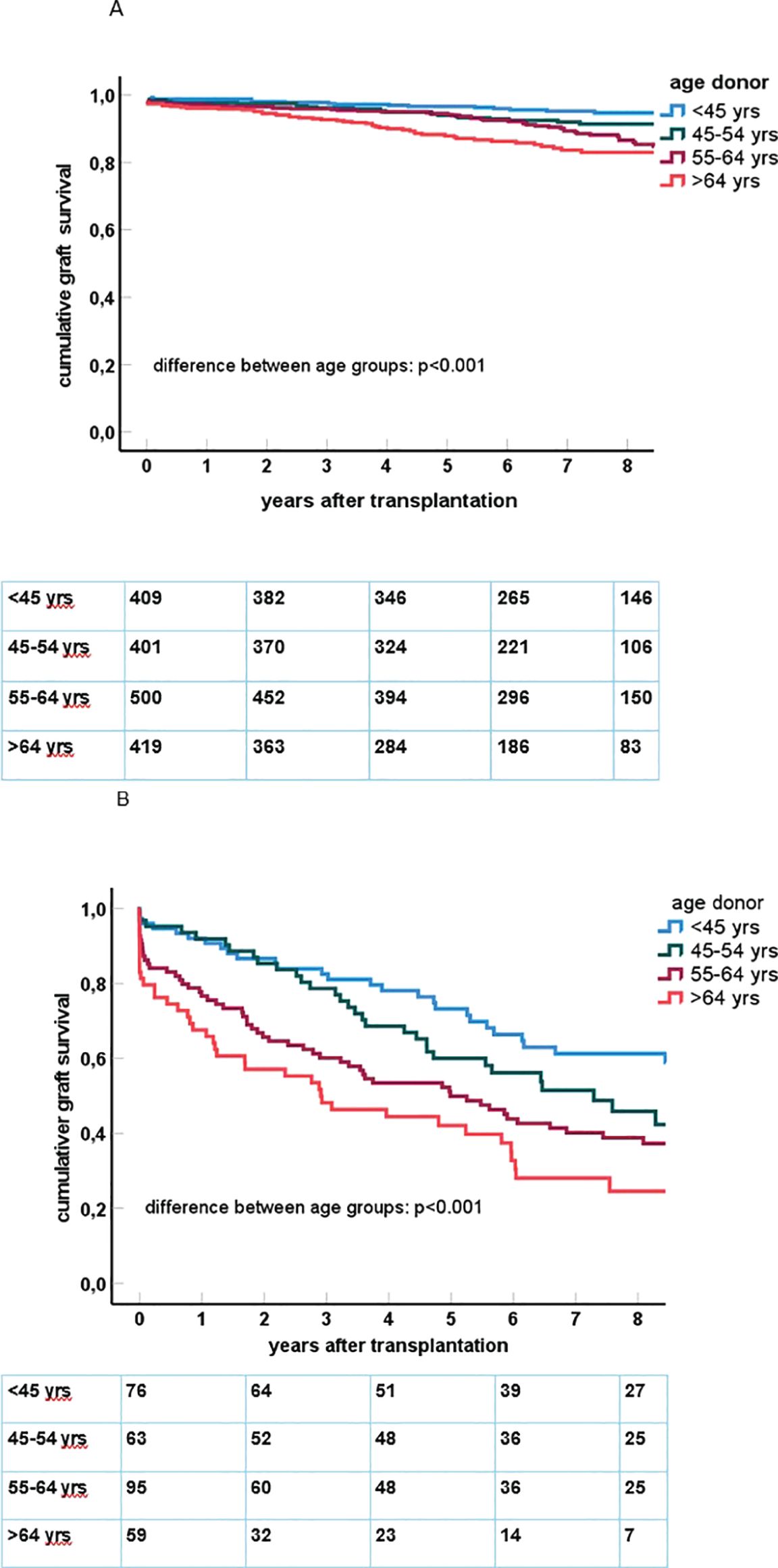
Figure 2. (A) In the upper figure, Kaplan–Meier curves for death-censored graft survival after kidney transplantation in the group of recipients of a donor kidney without pretransplant DSA are shown. The number of cases within each age category is n = 409 (age <45 years), n = 401 (45–54 years), n = 500 (55–64 years), n = 419 (65 years and older). (B) In the lower figure, Kaplan–Meier curves for death-censored graft survival after kidney transplantation in the group of recipients of a donor kidney with pretransplant DSA are shown. The number of cases within each age category is n = 76 (age <45 years), n = 63 (45–54 years), n = 95 (55–64 years), n = 59 (65 years and older).
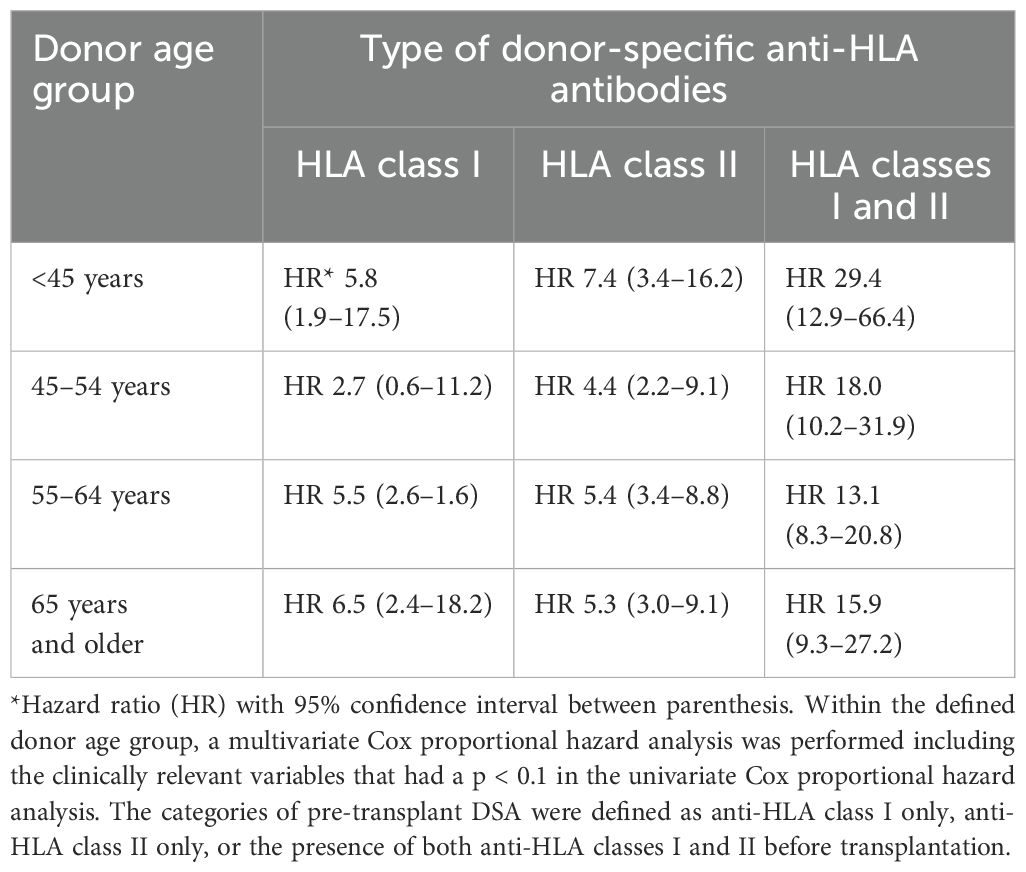
Table 4. The hazard ratios for graft failure within different categories of pre-transplant DSA compared to those of recipients without pre-transplant DSA, analyzed per donor age group.
4 Discussion
The current study shows that transplantation with a negative CDC-XM but in the presence of DSAs is associated with increased early and late graft loss. This is primarily because of ABMR, but an increased risk for primary non-function (in case of DD kidneys) and thrombosis was also observed. In particular, the combination of preDSAs against both HLA classes I and II carried a high risk for graft loss. These findings regarding the negative effects of preDSA are not consistent in the literature, but they have been observed in three large cohort studies (4, 5, 24) with a separate analysis of cause of graft failure when DSAs are present (2, 6). In the current study, the combination of preDSA against both HLA classes I and II carried the highest risk for graft loss, while preDSAs for either class I or II had a similar lower risk. This finding is in agreement with the results of the Swiss Transplant Cohort Study (24) and of the Dutch National PROCARE study involving 4,724 transplantations performed between 1995 and 2006 (5). After publication of these results, transplantations with this combination of preDSAs dropped sharply in our center. Of note, a German cohort study did not observe such an effect of combined preDSAs for classes I and II on graft survival, which may be related to differences in type of maintenance immune suppressive drugs and the use of desensitization procedures (4).
In our center, basiliximab instead of lymphocyte depleting induction is given. This differs from practices in, for example, the United States, where over 60% of recipients receive T-cell depleting induction therapy (25). As discussed in a previous report (6), approaches to induction therapy vary substantially, and in many European countries, basiliximab is the first-line therapy in the vast majority of recipients. Although the KDIGO guidelines suggest using a lymphocyte-depleting agent for kidney transplant recipients at high immunologic risk, this strategy was not adopted in the past in many (European) centers, as the evidence level of this recommendation was modest, and most data were from deceased donor transplantation. In addition, the negative effects on graft survival of preDSAs was and is a matter of debate, and others reported a general favorable outcome of transplantations with preDSAs using non-depleting antibody induction (26, 27).
Currently, we avoid transplantation across preDSAs as much as possible by the use of an effective computer algorithm-supported national cross-over program in case of a living donor (28, 29) and a desensitization program involving imlifidase for deceased donor kidney transplantations (30).
The age of older kidney donors has increased by almost 10 years in our center compared to that of 20 years ago (23). Furthermore, within the ESP, DD kidneys aged 65 years or older are preferentially allocated to recipients aged 65 years or older, and the number of older recipients has been growing over the years. An analysis of the results of kidney transplantation in the elderly recipients within the Netherlands in the period 2005 to 2015 demonstrated that the ESP resulted in a 1-year graft survival of 77% and a death-censored 1-year graft survival of 87% (15). These results are in accordance with our study, and based on the abovementioned PROCARE study, it is likely that a similar percentage of transplantations within ESP was performed in the presence of preDSAs and thus substantially influenced the results of that study.
It is a consistent finding that the age of the donor kidney is related to the risk for graft failure, especially in deceased donor transplantation (16, 17). This may be multifactorial as older kidneys are more vulnerable for any form of acute kidney injury including cold ischemia time. In addition, data from the ESP showed that the older deceased donor kidneys reached an eGFR <30 ml/min at 1 year in >50% of recipients vs. 26% in recipients of a young donor kidney (15) Thus, the functional reserve capacity of the older donor kidneys is frequently low with the consequence of reaching graft loss at an earlier time point in case of adverse events such as ABMR. Older donor kidneys are known to be more immunogenic and associated with a higher risk for TCMR (23, 31), but in this cohort, we could not find an independent association between the age of the donor kidney and the risk for clinically relevant ABMR. However, as no per protocol biopsies were performed, we cannot rule out the possibility that subclinical ABMR may be more frequently seen in kidneys of older age donors. Nevertheless, preDSAs significantly increased the risk for ABMR, which led to a substantial graft loss within the first year in every donor kidney age group. In particular, the combination of old donor age and deceased donation led to a much-shortened graft survival for reasons mentioned above, but the hazard ratio was in a similar range for the different donor kidney age groups. Transplantation without preDSAs, using a triple immune suppression regime with anti-CD25 induction, shows a death-censored graft loss of <5% at 1 year, even when older DD kidneys were used (of which half were DCD type kidneys). Also, long-term survival is remarkably good in kidney transplantations without preDSAs. Although relatively few transplantations are performed with preDSAs, the negative impact on graft survival is of such magnitude (specifically in the older DD kidney group) that graft survival of the whole group is significantly affected. Comparing results between transplantation centers or over time should therefore always be considered in the context of the frequency of transplantations with preDSAs and donor kidney age.
In kidney transplant programs worldwide, the mean age of recipients is steadily increasing (32); in our center, half of the transplantations are performed in recipients 60 years of age and older with an active approach to list elderly patients for transplantation (16). An aged immune system and frailty can make elderly recipients prone to side effects of immune suppressive drugs and infections, especially after anti-rejection treatment (13, 33). Therefore, the benefits of kidney transplantation need to be carefully balanced against these risks. In addition, early graft loss is a dramatic event in the elderly recipients as it increases mortality after returning to dialysis, and relisting is even more infrequent than for the total population of recipients with early graft loss (18, 19). Instead, if an elderly recipient does not experience early graft loss, the risk of death-censored graft loss beyond 1 year after transplantation is remarkably low (16).
Based on the data presented, preDSAs should be avoided as much as possible, at least in case of an older DD kidney and entirely in case of preDSAs against both HLA classes I and II. Clearly, the results are much better for preDSA and a young LD kidney, but even then, the risk of late graft loss was substantially increased.
The strength of the current study is the large number of kidney transplantations with a uniform immune suppressive regimen based on anti-CD25 induction therapy and maintenance with tacrolimus, MMF, and prednisone with follow-ups for at least 3 years after transplantation. As only for cause biopsies were performed, the cumulative incidence of ABMR may have been even higher than reported. However, the cause of graft failure for virtually all patients was documented, which revealed that primary non-function (with no signs of ABMR in the biopsy) and graft thrombosis were much more frequently seen in association with preDSAs. The deleterious effects of preDSAs may be better predicted if a flow cytometry crossmatch assay is positive (34). However, in a recent analysis in our center, we could not confirm a relation between height of MFI or flow cytometry crossmatch-positive DSAs and graft survival (6).
In conclusion, increasing donor kidney age significantly aggravates the negative effect of pretransplant donor-specific anti-HLA antibodies on graft survival after kidney transplantation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Medical ethical comittee Erasmus MC. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MB: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JK: Methodology, Project administration, Writing – original draft, Writing – review & editing. DR: Investigation, Methodology, Writing – original draft, Writing – review & editing. MK: Investigation, Methodology, Writing – original draft, Writing – review & editing. SH: Investigation, Methodology, Writing – original draft, Writing – review & editing. AD: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JV: Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors wish to acknowledge the contribution of the renal pathology team over the years, in particular, J. von der Thussen and M. Clahsen-van Groningen.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1574324/full#supplementary-material
References
1. Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. (2010) 21:1398–406. doi: 10.1681/ASN.2009101065
2. Betjes MGH, Sablik KS, Otten HG, Roelen DL, Claas FH, de Weerd A. Pretransplant donor-specific anti-HLA antibodies and the risk for rejection-related graft failure of kidney allografts. J Transplant. (2020) 2020:5694670. doi: 10.1155/2020/5694670
3. Aubert O, Loupy A, Hidalgo L, Duong van Huyen JP, Higgins S, Viglietti D, et al. Antibody-mediated rejection due to preexisting versus de novo donor-specific antibodies in kidney allograft recipients. J Am Soc Nephrol. (2017) 28:1912–23. doi: 10.1681/ASN.2016070797
4. Ziemann M, Altermann W, Angert K, Arns W, Bachmann A, Bakchoul T, et al. Preformed donor-specific HLA antibodies in living and deceased donor transplantation: A multicenter study. Clin J Am Soc Nephrol. (2019) 14:1056–66. doi: 10.2215/CJN.13401118
5. Kamburova EG, Wisse BW, Joosten I, Allebes WA, van der Meer A, Hilbrands LB, et al. Differential effects of donor-specific HLA antibodies in living versus deceased donor transplant. Am J Transplant. (2018) 18:2274–84. doi: 10.1111/ajt.14709
6. Tramper T, Roelen DL, Brand-Schaaf SH, Kal-van Gestel JA, Kho MML, Reinders MEJ, et al. The detrimental effect of donor-specific antibodies is irrespective of its level in highly-immunized living donor kidney transplant recipients: A case-control series. Front Immunol. (2022) 13:1093359. doi: 10.3389/fimmu.2022.1093359
7. Higgins R, Lowe D, Hathaway M, Williams C, Lam FT, Kashi H, et al. Human leukocyte antigen antibody-incompatible renal transplantation: excellent medium-term outcomes with negative cytotoxic crossmatch. Transplantation. (2011) 92:900–6. doi: 10.1097/TP.0b013e31822dc38d
8. Orandi BJ, Garonzik-Wang JM, Massie AB, Zachary AA, Montgomery JR, Van Arendonk KJ, et al. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant. (2014) 14:1573–80. doi: 10.1111/ajt.12786
9. Louis K, Lefaucheur C. DSA in solid organ transplantation: is it a matter of specificity, amount, or functional characteristics? Curr Opin Organ Transplant. (2022) 27:392–8. doi: 10.1097/MOT.0000000000001006
10. Hönger G, Krähenbühl N, Dimeloe S, Stern M, Schaub S, Hess C. Inter-individual differences in HLA expression can impact the CDC crossmatch. Tissue Antigens. (2015) 85:260–6. doi: 10.1111/tan.12537
11. Carey BS, Poulton KV, Poles A. Factors affecting HLA expression: A review. Int J Immunogenetics. (2019) 46:307–20. doi: 10.1111/iji.12443
12. Betjes MG, Langerak AW, van der Spek A, de Wit EA, Litjens NH. Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int. (2011) 80:208–17. doi: 10.1038/ki.2011.110
13. Litjens NHR, van der List ACJ, Klepper M, Reijerkerk D, Prevoo F, Betjes MGH. Older age is associated with a distinct and marked reduction of functionality of both alloreactive CD4+ and CD8+ T cells. Front Immunol. (2024) 15:1406716. doi: 10.3389/fimmu.2024.1406716
14. Litjens N, Peeters A, Gestel JK, Klepper M, Betjes M. The FCGR3A 158 V/V-genotype is associated with decreased survival of renal allografts with chronic active antibody-mediated rejection. Sci Rep. (2021) 11:7903. doi: 10.1038/s41598-021-86943-3
15. Peters-Sengers H, Berger SP, Heemskerk MB, Al Arashi D, Homan van der Heide JJ, Hemke AC, et al. Stretching the limits of renal transplantation in elderly recipients of grafts from elderly deceased donors. J Am Soc Nephrol. (2017) 28:621–31. doi: 10.1681/ASN.2015080879
16. Betjes MGH, Kho MML, Roodnat J, de Weerd AE. Transplant candidates of 70+ Years have superior survival if receiving pre-emptively a living donor kidney. J Clin Med. (2024) 13. doi: 10.3390/jcm13071853
17. Foley DP, Patton PR, Meier-Kriesche HU, Li Q, Shenkman B, Fujita S, et al. Long-term outcomes of kidney transplantation in recipients 60 years of age and older at the University of Florida. Clin Transpl. (2005), 101–9.
18. de Kok MJ, Schaapherder AF, Mensink JW, de Vries AP, Reinders ME, Konijn C, et al. A nationwide evaluation of deceased donor kidney transplantation indicates detrimental consequences of early graft loss. Kidney Int. (2020) 97:1243–52. doi: 10.1016/j.kint.2020.01.043
19. Hamed MO, Chen Y, Pasea L, Watson CJ, Torpey N, Bradley JA, et al. Early graft loss after kidney transplantation: risk factors and consequences. Am J Transplant. (2015) 15:1632–43. doi: 10.1111/ajt.13162
20. Swinarska JT, Stratta RJ, Rogers J, Chang A, Farney AC, Orlando G, et al. Early graft loss after deceased-donor kidney transplantation: what are the consequences? J Am Coll Surg. (2021) 232:493–502. doi: 10.1016/j.jamcollsurg.2020.12.005
21. Roufosse C, Simmonds N, Clahsen-van Groningen M, Haas M, Henriksen KJ, Horsfield C, et al. A 2018 reference guide to the banff classification of renal allograft pathology. Transplantation. (2018) 102:1795–814. doi: 10.1097/TP.0000000000002366
22. Betjes MGH, Kho MML, Litjens NHR, de Weerd AE, Roodnat JI. Alemtuzumab as second-line treatment for late antibody-mediated rejection of transplanted kidneys. Transplant Proc. (2021) 53:2206–11. doi: 10.1016/j.transproceed.2021.07.005
23. Betjes MGH, Roelen DL, van Agteren M, Kal-van Gestel J. Causes of kidney graft failure in a cohort of recipients with a very long-time follow-up after transplantation. Front Med (Lausanne). (2022) 9:842419. doi: 10.3389/fmed.2022.842419
24. de Rougemont O, Deng Y, Frischknecht L, Wehmeier C, Villard J, Ferrari-Lacraz S, et al. Donation type and the effect of pre-transplant donor specific antibodies - Data from the Swiss Transplant Cohort Study. Front Immunol. (2023) 14:1104371. doi: 10.3389/fimmu.2023.1104371
25. Kidney Disease: Improving Global Outcomes Transplant Work G. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. (2009) 9 Suppl 3:S1–155. doi: 10.1111/j.1600-6143.2009.02834.x
26. Zecher D, Bach C, Staudner C, Böger CA, Bergler T, Banas B, et al. Characteristics of donor-specific anti-HLA antibodies and outcome in renal transplant patients treated with a standardized induction regimen. Nephrol Dial Transplant. (2017) 32:730–7. doi: 10.1093/ndt/gfw445
27. Tambur AR, Campbell P, Chong AS, Feng S, Ford ML, Gebel H, et al. Sensitization in transplantation: Assessment of risk (STAR) 2019 Working Group Meeting Report. Am J Transplant. (2020) 20:2652–68. doi: 10.1111/ajt.15937
28. de Klerk M, Kal-van Gestel JA, van de Wetering J, Kho ML, Middel-de Sterke S, Betjes MGH, et al. Creating options for difficult-to-match kidney transplant candidates. Transplantation. (2021) 105:240–8. doi: 10.1097/TP.0000000000003203
29. de Klerk M, Kal-van Gestel JA, Roelen D, Betjes MGH, de Weerd AE, Reinders MEJ, et al. Increasing kidney-exchange options within the existing living donor pool with CIAT: A pilot implementation study. Transpl Int. (2023) 36:11112. doi: 10.3389/ti.2023.11112
30. de Weerd AE, Roelen DL, van de Wetering J, Betjes MGH, Heidt S, Reinders MEJ. Imlifidase desensitization in HLA-incompatible kidney transplantation: finding the sweet spot. Transplantation. (2024) 108:335–45. doi: 10.1097/TP.0000000000004689
31. Tullius SG, Tran H, Guleria I, Malek SK, Tilney NL, Milford E. The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome. Ann Surg. (2010) 252:662–74. doi: 10.1097/SLA.0b013e3181f65c7d
32. Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S, et al. OPTN/SRTR 2018 annual data report: kidney. Am J Transplant. (2020) 20 Suppl s1:20–130. doi: 10.1111/ajt.15672
33. Betjes MGH, De Weerd A. Lowering maintenance immune suppression in elderly kidney transplant recipients; connecting the immunological and clinical dots. Front Med (Lausanne). (2023) 10:1215167. doi: 10.3389/fmed.2023.1215167
34. Ziemann M, Lindemann M, Hallensleben M, Altermann W, Althaus K, Budde K, et al. Risk stratification before living donor kidney transplantation in patients with preformed donor-specific antibodies by different crossmatch methods. Transplant Direct. (2024) 10:e168. doi: 10.1097/TXD.0000000000001680
Keywords: age donor kidney, antibody-mediated rejection, donor-specific anti-HLA antibodies, graft survival, kidney transplantation
Citation: Betjes MGH, Kal-van Gestel JA, Roelen D, Kho MML, Heidt S, de Weerd AE and van de Wetering J (2025) Increasing donor kidney age significantly aggravates the negative effect of pretransplant donor-specific anti-HLA antibodies on kidney graft survival. Front. Immunol. 16:1574324. doi: 10.3389/fimmu.2025.1574324
Received: 10 February 2025; Accepted: 27 March 2025;
Published: 16 April 2025.
Edited by:
Jakob Nilsson, University Hospital Zürich, SwitzerlandReviewed by:
Caroline Wehmeier, University Hospital Basel, SwitzerlandLukas Frischknecht, University Hospital Zürich, Switzerland
Matthias Niemann, PIRCHE AG, Germany
Copyright © 2025 Betjes, Kal-van Gestel, Roelen, Kho, Heidt, de Weerd and van de Wetering. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michiel G. H. Betjes, bS5nLmguYmV0amVzQGVyYXNtdXNtYy5ubA==
†ORCID: Michiel G. H. Betjes, orcid.org/0000-0001-9435-6208
 Michiel G. H. Betjes
Michiel G. H. Betjes Judith A. Kal-van Gestel1
Judith A. Kal-van Gestel1 Dave Roelen
Dave Roelen Marcia M. L. Kho
Marcia M. L. Kho Sebastian Heidt
Sebastian Heidt Annelies E. de Weerd
Annelies E. de Weerd