- 1Key Laboratory of Vascular Biology and Translational Medicine of Hunan Province, Medical School, Hunan University of Chinese Medicine, Changsha, China
- 2Department of Anatomy, Anatomy Teaching Center of Hunan University of Chinese Medicine, Changsha, China
- 3Xiangya Hospital, Central South University, Changsha, China
- 4Department of Integrated Traditional Chinese and Western Medicine, Qian Rongkang Clinic, Loudi, China
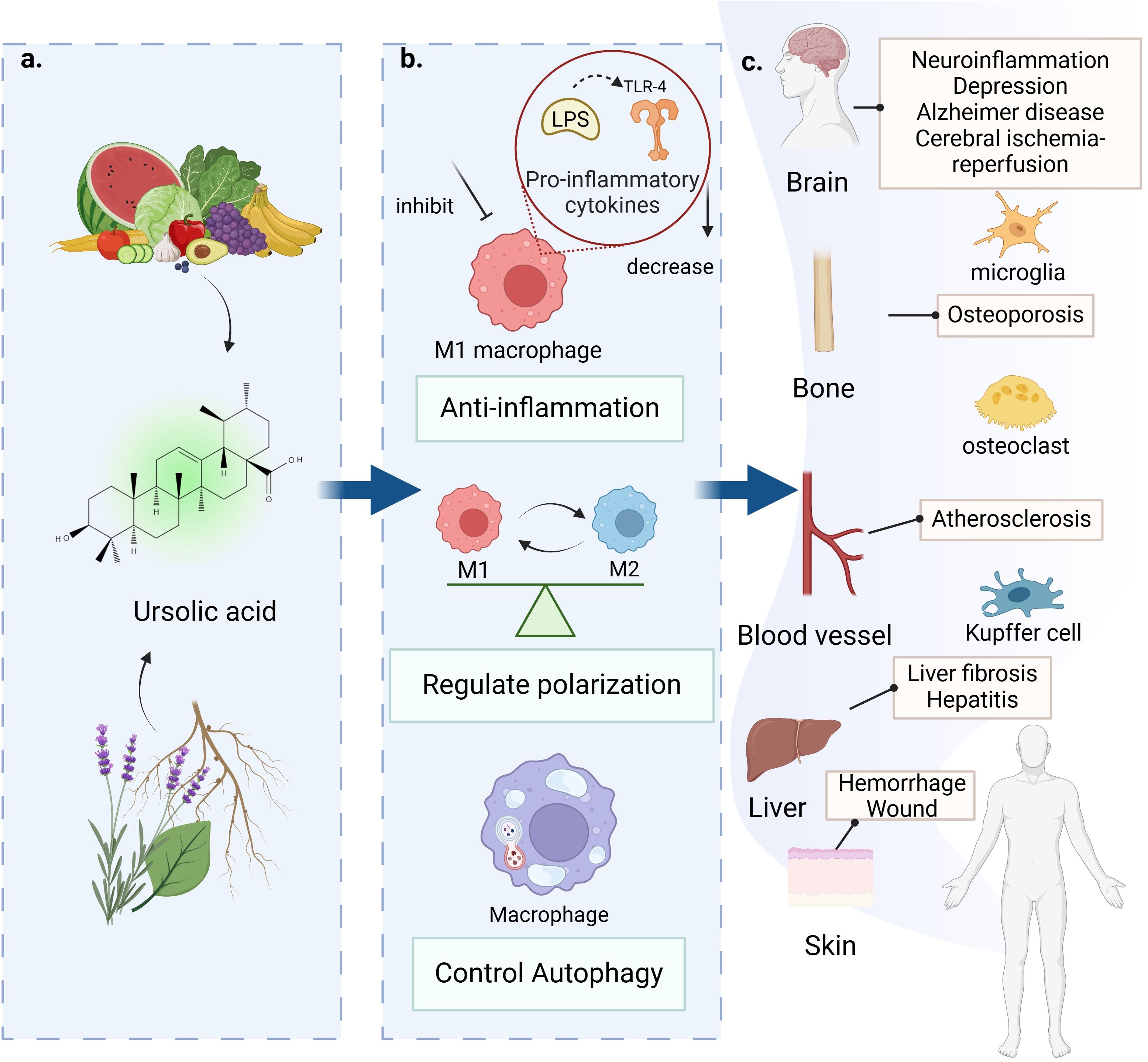
Ursolic acid (UA), a prevalent pentacyclic triterpenoid found in numerous fruits and herbs, has garnered significant attention for its vital role in anti-inflammatory processes and immune regulation. The study of immune cells has consistently been a focal point, particularly regarding macrophages, which play crucial roles in antigen presentation, immunomodulation, the inflammatory response, and pathogen phagocytosis. This paper reveals the underlying regulatory effects of UA on the function of macrophages and the specific therapeutic effects of UA on a variety of diseases. Owing to the superior effect of UA on macrophages, different types of macrophages in different tissues have been described. Through the multifaceted regulation of macrophage function, UA may provide new ideas for the development of novel anti-inflammatory and immunomodulatory drugs. However, to facilitate its translation into actual medical means, the specific mechanism of UA in macrophages and its clinical application still need to be further studied.
1 Introduction
The human immune system is a complicated defense network designed to protect the body against various pathogens, including bacteria, fungi, viruses, parasites, and aberrant cells such as cancer cells (1, 2).
Macrophages, essential cells of the innate immune system, are present in virtually every tissue throughout the human body (3). The origin of macrophages is a sophisticated process. During embryonic development, the first macrophages originate from mesenchymal progenitor cells within the yolk sac. Erythromyeloid progenitors (EMPs) subsequently colonize the fetal liver (4). They eventually differentiate into tissue specific macrophages that colonize embryonic tissue, and these cells are long-lived and self-sustaining. The production of bone marrow-derived monocytes begins after birth. Bone marrow-derived macrophages typically have a shorter lifespan and are constantly being replaced, suggesting that yolk sac derived macrophages are different from bone marrow-derived macrophages (5–7). Macrophages, which are called highly heterogeneous cell populations, exhibit different phenotypes under different stimuli and have high plasticity (8). Bacterial components such as lipopolysaccharide (LPS) and interferon-gamma (IFN-γ) polarize macrophages toward M1 macrophages, which play critical roles in acute inflammatory responses through the production of various proinflammatory cytokines that function in clearing pathogens and causing tissue damage, resulting in proinflammatory and antitumor properties. Conversely, IL-4 and IL-13 induce polarization toward M2 macrophages, which exhibit proinflammatory properties and enhance immune function (9, 10). Notably, with the advancement of omics technologies, the classification of macrophages has extended beyond two categories, revealing a more complex situation (11).
Ursolic acid (UA), a natural compound, can be extracted from the stems, peels, and leaves of a variety of fruits and herbs (12) (Figure 1). With advancements in extraction technology, ultrasonic (13) and microwave-assisted (14) extraction of UA has gained widespread application, significantly increasing the extraction efficiency. In addition to these two techniques, methods such as accelerated solvent extraction and supercritical fluid extraction are also employed (15). The effective extraction of UA is advantageous for investigating its biological activity. UA is widely known as a pentacyclic triterpenoid compound with antitumor (16), anti-inflammatory (17), and regulatory effects on metabolic diseases, as well as cardiovascular diseases (18) but exhibits poor bioavailability. Despite the robust scientific support for UA’s pharmacological properties in vitro and in vivo, its clinical application remains constrained by inherent limitations, prompting researchers to develop multiple strategies such as solid lipid microparticles (SLMs), nanostructured lipid carriers (NLCs), and structural derivatization of UA to enhance its bioavailability. In the animal model of pulmonary tuberculosis, SLM, as a delivery carrier of UA, can increase the biological activity of UA and effectively reduce the load of Mycobacterium tuberculosis burden (Mtb) in infected alveolar macrophages (19). The water insolubility of UA limits its transport and delivery in the human body, resulting in a decreased fraction of UA available for intestinal absorption (20). The application of NLCs significantly enhances the oral bioavailability of UA, with experimental studies demonstrating that UA-loaded NLCs achieve 98.75% inhibition of parasitic infections in standardized vivo models (21). Both increased leishmanicidal activity and reduced inflammatory processes observed in the spleen and liver of animals treated with UA-NLCs can be associated with the uptake of nanoparticles by macrophages (22). Nanostructured lipid carriers loaded with Ocimum sanctum L. leaf extract (OLE-NLCs) were developed for improved transdermal delivery of UA for anti-arthritic therapy (23). Additionally, the anticancer potential of UA-loaded NLCs was evaluated by assessing their cytotoxic effects against the human leukemic K562 and melanoma B16 cell lines (24). Recent research on UA demonstrates that structural derivatization through the introduction of an indole ring at the C-3 position and an amide group at the C-17 position, aiming to enhance pharmacological potential, can significantly suppress LPS-induced pro-inflammatory cytokines in RAW 264.7 macrophages (25).

Figure 1. Ursolic acid sources, extraction, and delivery. UA is a pentacyclic triterpenoid compound that is widely distributed in the fruits and roots or leaves of various plants. At present, the main extraction methods for UA are ultrasonic, microwave, accelerated solvent extraction, and supercritical fluid extraction. Two common delivery methods for UA are nanostructured lipid carriers (NLCs) and solid lipid microparticles (SLMs).
2 Effect of UA on inflammation
Macrophages serve as pivotal mediators and coordinators in the pathogenesis of chronic inflammatory disorders (26). Classically activated M1 macrophages orchestrate host defense mechanisms against bacterial, protozoan, and viral pathogens while contributing to antitumor immunity (27). These macrophages execute critical functions during acute inflammation through the release of proinflammatory mediators (28). Under homeostatic conditions, IL-10 modulates colonic macrophage activity by suppressing inflammatory responses toward gut microbiota-derived signals. This establishes IL-10 as a master anti-inflammatory cytokine. Experimental administration of UA to IL-10-/- murine peritoneal macrophages demonstrated significant suppression of proinflammatory cytokine production (29), suggesting UA mimics IL-10-mediated immunoregulation. Furthermore, dichloromethane extracts from Salvia connivens leaves (DESC) exhibit anti-inflammatory properties through dual mechanisms: enhancing IL-10 biosynthesis and attenuating LPS-induced macrophage activation, with UA being identified as a principal bioactive constituent mediating these effects (30).
The innate immune system was originally considered to exhibit nonspecific microbial recognition; however, the identification of Toll-like receptors (TLRs) in the mid-1990s revealed that innate immunity possesses pathogen-specific recognition capabilities (31). Dysregulated TLR signaling may drive acute or chronic inflammatory responses and precipitate systemic autoimmune disorders (32). Specifically, TLR4 serves as the primary receptor for bacterial LPS. LPS-TLR4 binding activates the downstream transcription factor nuclear factor kappa B cells (NF-κB), subsequently inducing the release of proinflammatory mediators including TNF-α, IL-1β, IL-6, chemokines, proteolytic enzymes, and reactive oxygen species (33) (Figure 2). The activation of NF-κB plays a critical role in the release of inflammatory mediators by macrophages. Triterpenic acid extract from Eriobotrya japonica leaves (TAL), with UA as its primary component, alleviated chronic bronchitis by suppressing the nuclear translocation of the NF-κB p65 subunit in alveolar macrophages to downregulate the expression of TNF-α, interleukin-1β (IL-1β), prostaglandin E2 (PGE2), and leukotriene B4 (LTB4) (34). UA can be isolated from the seeds of Cornus officinalis and inhibits the NF-κB and MAPK signaling pathways by inhibiting the binding of TLR4 to LPS on macrophages (35). Mechanistic studies on Sonchus oleraceus aqueous extract revealed concurrent downregulation of TLR4 and COX-2 expression in RAW 264.7 macrophages, with UA being characterized as a principal anti-inflammatory constituent (36). 23-hydroxy-UA exhibits superior inhibitory potency against macrophage-derived NO generation, displaying concentration-dependent suppression of both COX-2 protein abundance and transcriptional output (37, 38). Psidium guajava-derived UA further attenuates intracellular ROS accumulation (39). The inflammatory cascade fundamentally involves immune cell activation and tissue infiltration, where innate immune effectors mediate tissue inflammation through phagocytic clearance or paracrine secretion of bioactive mediators (40). Macrophages uniquely orchestrate chronic inflammatory processes, particularly “metainflammation” - a metabolic inflammation continuum (41). The regulation of inflammation by UA is dynamic, likely mediated through stimulus-dependent macrophage polarization states.
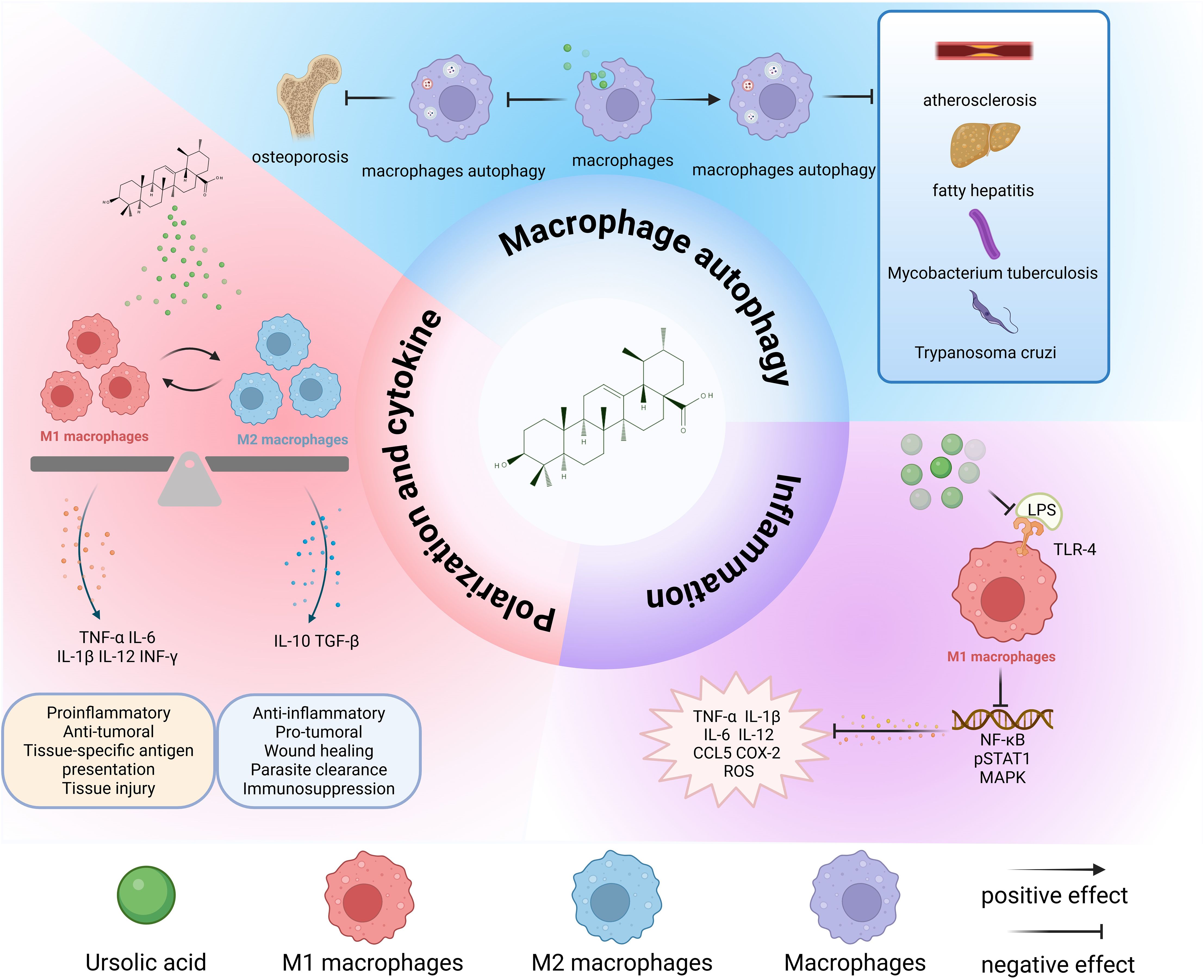
Figure 2. Overall effects of UA on macrophages. UA transforms M1 and M2 macrophages to each other and performs different physiological functions. Promoting macrophage autophagy is beneficial for inhibiting the pathological progression of atherosclerosis, fatty hepatitis, Mycobacterium tuberculosis infection, and parasites. The inhibition of autophagy is beneficial for the treatment of osteoporosis. UA exerts anti-inflammatory effects mainly by inhibiting the binding of LPS to TLR-4, and then inhibiting the conduction of downstream signaling pathways and reducing the release of inflammatory cytokines. TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-1β, interleukin-1β; IL-12, interleukin-12; IL-10, interleukin-10; LPS, lipopolysaccharide; IFN-γ, interferon-gamma; TGF-β, transforming growth factor-β; TLR4, Toll-like receptor 4; CCL5, C-C chemokine ligand 5; ROS, reactive oxygen species; COX-2, cyclooxygenase-2.
3 Effect of UA on macrophage polarization and cytokine release
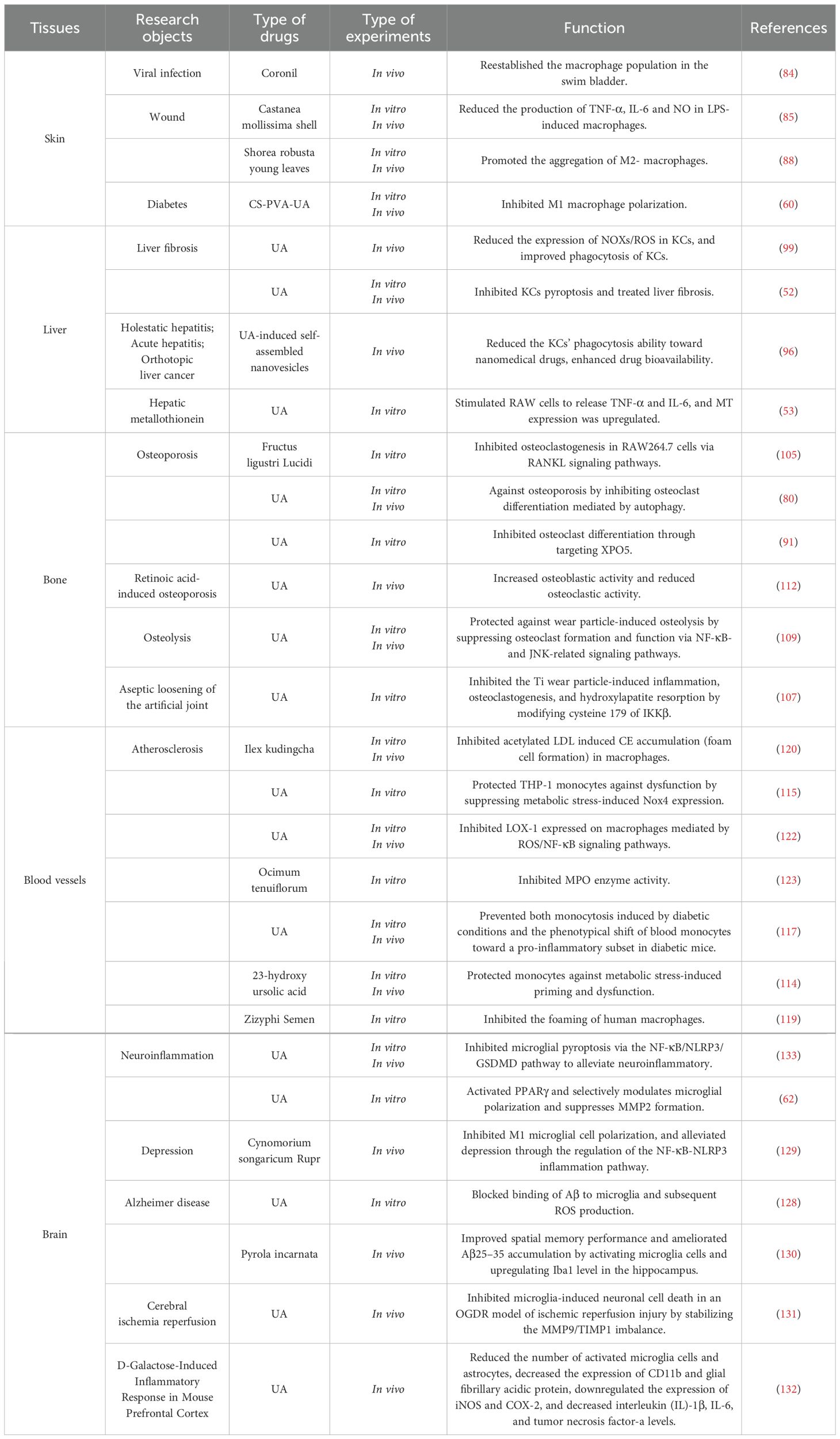
Notably, studies have demonstrated that UA participates in a dynamic regulatory process of cytokine release from macrophages, potentially associated with macrophage polarization (Table 1). The two primary macrophage subtypes, classically activated M1 and alternatively activated M2, represent polarized extremes within a spectrum of activation states (42). Further in vitro investigations revealed that M2-type macrophages can differentiate into distinct subsets: M2a, M2b, M2c, and M2d. M2a macrophages are commonly referred to as “wound healing” macrophages. M2c macrophages, along with M2b, are collectively termed “regulatory macrophages.” In contrast, M2d macrophages exhibit elevated expression of IL-10 and vascular endothelial growth factor (VEGF), both of which demonstrate immunosuppressive properties (43–45). Recent studies have demonstrated that TRIM29 serves as a critical regulator of macrophage polarization by modulating type I interferon (IFN) production (46, 47), PERK-mediated endoplasmic reticulum (ER) stress (48), inflammasome activation (49), and LPS-induced pro-inflammatory cytokine release (50). Notably, pathways such as ER stress (51) and inflammasome activation (52) are closely linked to the anti-inflammatory and immunomodulatory effects of UA. However, whether TRIM29 directly participates in UA-mediated regulation of macrophage polarization remains unclear. Future investigations should explore the interplay between UA and TRIM29-associated molecular networks to clarify its mechanistic role.
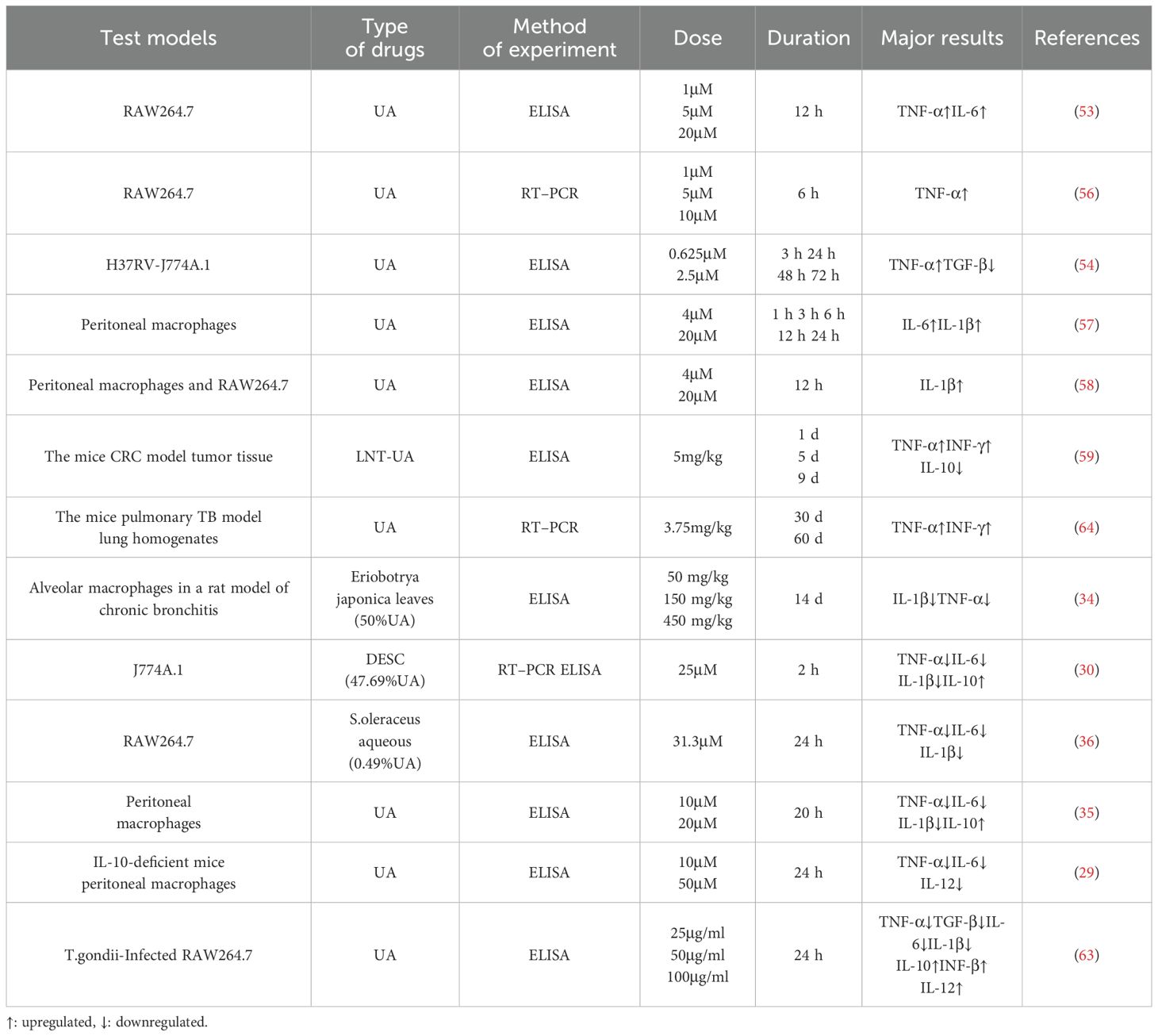
Table 1. Effects of different concentrations of UA at different durations on the release of cytokines from macrophages.
Unlike the common anti-inflammatory effects of UA, experimental studies have revealed that UA upregulates the expression of pro-inflammatory cytokines and enhances M1 macrophage activation. Using RAW 264.7 cells as a model system, researchers demonstrated that UA enhances TNF-α and IL-6 mRNA expression in liver macrophages (53). This phenomenon may be associated with UA-induced upregulation of CD36 receptor expression on macrophages (54). UA inhibits the polarization of M2 macrophages via downregulation of the Wnt pathway, thereby exerting anti-liver cancer effects (55). Moreover, UA enhances iNOS and TNF-α gene expression through NF-κB-dependent transcriptional regulation in macrophages (56). IL-1β exhibits critical involvement in the pathogenesis of inflammatory conditions. UA activates the Raf-1/MEK/ERK and MKK3/6/p38 MAPK pathways in macrophages, which promotes IL-1β gene transcription and leads to IL-1β mRNA expression for intracellular proIL-1β production (57, 58). For colorectal cancer (CRC), we developed a self-assembled nanomedicine (LNT-UA) through a simple nanoprecipitation method, consisting of natural bioactive components UA and lentinan (LNT). LNT-UA treatment significantly enhanced the secretion of antitumor-related cytokines IFN-γ and TNF-α, while concurrently suppressing the production of the immunosuppressive cytokine IL-10 (59).
Concomitantly, UA promotes M2 macrophage polarization, thereby exerting anti-inflammatory effects. A novel dressing design, designated as CS-PVA-UA, comprises electrospun nanofibers fabricated from a chitosan (CS) and polyvinyl alcohol (PVA) blend surface-functionalized with UA for diabetic wound management. Experimental data demonstrated that CS-PVA-UA nanofiber dressings significantly inhibit LPS-induced M1 macrophage polarization, effectively restore M2 phenotypic commitment, and accelerate inflammatory resolution (60). Microglia, the resident macrophages of the central nervous system, exhibit bidirectional plasticity between neurotoxic M1 and neuroprotective M2 states (61). UA confers neuroprotection through PPARγ-mediated selective polarization of microglia toward the M2 phenotype, attenuating neuroinflammatory responses (62). Furthermore, UA manifests dual immunomodulatory activity in Toxoplasma gondii-infected macrophages by augmenting anti-inflammatory cytokine secretion while suppressing proinflammatory mediators (63). Collectively, macrophages orchestrate immune responses via regulation of cytokine/chemokine networks. These signaling molecules mediate immune cell recruitment to inflammatory foci while dynamically modulating the activation states of macrophages. (Figure 2)
4 Effect of UA on macrophage autophagy
Autophagy critically regulates macrophage polarization dynamics (65–69). Tumor-associated macrophages (TAMs), a specialized macrophage population recruited to tumor microenvironments, predominantly exhibit M2-like phenotypes with minor M1 subpopulations, and are mechanistically implicated in facilitating tumor progression through angiogenesis promotion and metastatic niche formation (70). Paradoxically, autophagy-mediated ferroptosis was reported by Dai et al. to drive KRAS oncoprotein internalization in macrophages, concurrently enhancing M2 polarization and macrophage cell death, thereby fostering tumor immune evasion (71). Consequently, pharmacological autophagy inhibition demonstrates therapeutic potential through dual mechanisms: suppressing M2-mediated immune escape and enhancing T cell infiltrate-mediated tumor immunoediting (72). This functional dichotomy positions autophagy as a therapeutic double-edged sword (Figure 2). Notably, autophagy suppression may elicit contradictory effects – inducing pro-inflammatory M1 polarization while exacerbating inflammation. In inflammatory disease contexts, UA modulates macrophage plasticity by enhancing autophagic to attenuate M1 polarization and associated inflammatory cascades.
Macrophage autophagy has been demonstrated to mitigate chronic inflammation progression and organ fibrosis through attenuation of M1 polarization (73). Mechanically, UA enhances autophagy in macrophages via upregulation of autophagy related genes Atg5 and Atg16L1, consequently modulating macrophage functionality and ameliorating murine atherosclerosis (74). Age-related decline in macrophage autophagic capacity may underlie the elevated incidence of senile steatohepatitis (75) and metabolic syndrome (76) in elderly populations, highlighting the therapeutic potential of autophagy-targeted interventions for obesity-related hepatic pathologies (77). In the context of Chagas disease, UA was investigated for its modulatory effects on T. cruzi-infected macrophages and cardiomyocytes in vitro. During late stage infection characterized by intracellular parasite nest formation, UA-induced autophagy activation in both macrophages and cardiac cells was shown to mitigate parasitosis-induced tissue damage (68). Macrophage autophagy has an inhibitory effect on inflammation. UA primarily mediates its anti-inflammatory activity by suppressing the TLR4/MyD88 signaling pathway, whereas pharmacological inhibition of autophagy using 3-methyladenine (3-MA) significantly abrogates this UA-mediated suppression (78). In Mycobacterium tuberculosis (Mtb)-induced tuberculosis (TB), UA enhances autophagy to mitigate macrophage hyperinflammatory responses, thereby improving clinical outcomes (79). UA demonstrates bidirectional regulation of macrophage autophagy. While basal autophagy activates NFATc1 and c-Fos to promote osteoporosis pathogenesis, UA suppresses c-Fos/NFATc1 induction, inhibits osteoclastogenesis, and attenuates pathological autophagy (80).
5 Effect of UA on tissue macrophages
5.1 Skin
Hemostasis, inflammation, proliferation, and remodeling are four sophisticated and finely orchestrated physiological stages of wound healing (81). The inflammatory response is thought to involve wound healing in the first phase (82). Macrophages play pivotal roles in inflammation and wound healing by releasing cytokines such as epidermal growth factor and tumor growth factor-β(TGF-β), stimulating the proliferation of fibroblasts and keratinocytes (83), and simultaneously, they can suppress the release of inflammatory cells, facilitate the infiltration of M2 macrophages, and exert an anti-inflammatory effect (Figure 3).

Figure 3. Mechanisms of the effects of UA on macrophages in various tissue diseases. UA affects macrophages, Kupffer cells, osteoclasts, and microglia to treat diabetes, parasitic infection, viral infection, liver fibrosis, atherosclerosis, osteoporosis, Alzheimer’s disease, depression, and neuritis through a variety of signaling pathways and targets. NO, nitric oxide; ROS, reactive oxygen species; COX-2, cyclooxygenase-2; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-1β, interleukin-1β; NOX2, oxidase 2; NOX4, oxidase 4; NLRP3, receptors containing pyrin domain 3; GSDMD, gasdermin D; TLR4, Toll-like receptor 4; LPS, lipopolysaccharide; MPK-1, MAPK phosphatases-1; MCP-1, monocyte chemoattractant protein-1; ACAT, Acyl-coenzyme A: cholesterol acyltransferase; LOX-1, lectin-like oxidized LDL receptor-1; MPO, myeloperoxidase; Iba1, ionized calcium binding adapter molecule 1; MMP9, matrix metalloproteinases 9; PPARγ, peroxisome proliferator-activated receptor gamma; TIMP1, tissue inhibitor matrix metalloproteinase 1; MMP2, matrix metalloproteinases 2.
Researchers have used a zebrafish model to xenograft the human lung epithelial cell line A549 to study the protective effect of herb-based drug named Coronil against SARS-CoV-2 infection. One of the active ingredients of Coronil is UA, which can effectively prevent bleeding in the pelvic, dorsal, and other parts of the fish and increase the number of neutrophils and macrophages in the swim bladder to normal levels (84). Castanea mollissima shell (C. mollissima shell) is a traditional Chinese medicine used for wound healing and anti-inflammatory purposes. The first compound of the ethanol extracts of C. mollissima shell was identified as UA, which can reduce the number of macrophages induced by LPS, has a basic anti-inflammatory effect, and promotes wound healing (85). A new type of wound therapy for diabetes, namely, CS-PVA-UA dressings, has good shape similarity to the natural extracellular matrix (ECM) of skin collagen fibers. This type of nanofiber can promote cell adhesion and accelerate wound healing and skin regeneration in vivo (60). The number of M1 macrophages is greater in diabetic wounds than in normal wounds (86), instead of M2 phenotypes, which inhibits the inflammation stage to the proliferation stage and promotes the healing of wounds (87). In a diabetic wound mouse model, the CS-PVA-UA nanofiber dressing was used to investigate its impact on skin regeneration, the results indicated that it effectively promoted revascularization, reepithelialization, collagen matrix deposition and remodeling, as well as hair follicle regeneration in diabetic wounds. This approach facilitates rapid and high quality skin wound healing in diabetic mice (44). In India, the Shorea robusta plant was found to treat skin injuries, including wounds and burns, and UA was identified as an effective compound of Shorea robusta plant with strong anti-inflammatory activity (88). It has been reported that in Leishmania, UA components extracted from the leaves of Baccharis uncinella upregulate Th1 cytokines, such as IL-12, can induce the differentiation and activation of IFN-γ-secreting CD4+T lymphocyte subpopulations, activate infected macrophages, and clear intracellular parasites (89–91). Experiments have shown that UA inhibits the development of skin lesions and reduces skin parasitics in BALB/c infected mice (92). Although many studies have demonstrated the effects of UA on wound healing and inflammation inhibition (Table 2) (Figure 4), some questions still need answers. Through Ikeda et al.’s finding that UA enhances iNOS and TNF-α expression in macrophages, UA-induced increases in proinflammatory mediator levels play a role in promoting the development of skin tumor formation in mice (93).
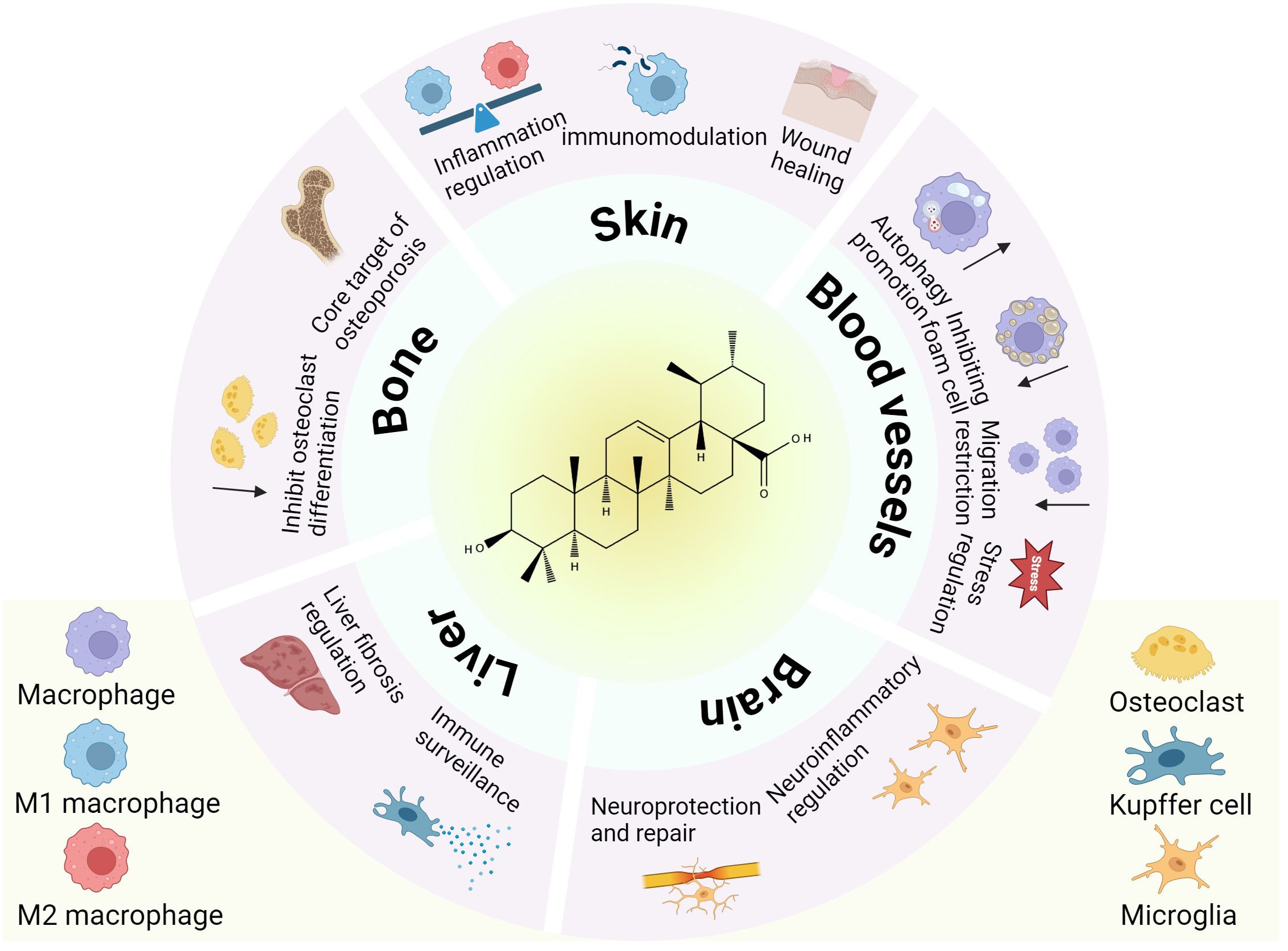
Figure 4. The role of tissue macrophages in disease under the influence of UA. This schematic illustrates the pleiotropic effects of UA on macrophage subtypes across diverse tissues (skin, bone, liver, brain, and blood vessels).
5.2 Liver
Kupffer cells, a type of macrophage located in the hepatic venous sinuses, exhibit robust phagocytic activity(Figure 4). It has a strong phagocytic capacity for particulate matter, including nanoparticles (94, 95). Yuan et al. reported that liver cells are the predominant cells for the uptake of UA-induced self-assembled nanovesicles (V-UAs) and can escape the phagocytosis of Kupffer cells (96). As the early stage of cirrhosis, liver fibrosis is a complex process of fibrosis and inflammation caused by chronic liver injury (97). An imbalance between M1 and M2 macrophages has an important effect on the progression of this disease, while UA also has therapeutic effects on liver fibrosis (98). Wan et al. reported that UA alleviated liver fibrosis in mice by inhibiting the NOX2/NLRP3 inflammasome signaling pathway and thereby restraining Kupffer cell pyroptosis (52)(Figure 3). Another in vitro study demonstrated that UA can alleviate CCL4-induced liver fibrosis, meanwhile, the phagocytosis of Kupffer cells is not affected (99). Furthermore, UA has been reported to induce liver metallothionein (MT) activity (100). The underlying mechanism may be that UA acts indirectly on the liver through mediators released by Kupffer cells or the UA stimulates immune-active cells, leading to the upregulation of MT (53). Current research suggests that the mechanisms underlying the influence of UA on Kupffer cells warrant further investigation.
5.3 Bone
Osteoclasts and osteoblasts are important components of bone remodeling, and osteoclasts are multinucleated cells (101–103). In addition, osteoclasts are the only bone-absorbing cells in the body and play a pivotal role in the remodeling of the skeletal system (104).
UA is the main active ingredient in Fructus Ligustri Lucidi (FLL), an effective and well-known Chinese medicine used to treat osteoporosis. FLL ethanol extract suppresses RANKL-induced osteoclast differentiation in RAW264.7 macrophage-derived osteoclast precursors by inhibiting NF-κB signaling (105). UA mitigates LPS-induced inflammatory bone loss in mice by inhibiting RANKL-induced activation of key osteoclastogenic transcription factors, including c-Fos and NFATc1 (106). This may be one of the mechanisms by which UA inhibits osteoclast formation. And UA inhibited Ti particles induced inflammation and osteoclastogenesis by inhibiting IKKβcys-179 (107). UA was obtained via bioactivity guided fractionation of loquat leaves and was found to inhibit osteoclast differentiation at concentrations of 4 and 10 μg/mL (108). By inhibiting the NF-kB and JNK signaling pathways, UA decreases the number of tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts (109). Through network pharmacological analysis, UA was shown to target osteoclasts mainly via a variety of signaling pathways, namely, the MAPK and tumor necrosis factor α (TNF-α) signaling pathways (110). Osteoclasts are important targets for osteoporosis (111), and osteoclast differentiation plays a key role in osteoporosis (Figure 3). UA may improve osteoporosis by inhibiting autophagy-mediated osteoclast differentiation (80). Additionally, UA can suppress the activity of osteoclasts while enhancing the activity of osteoblasts to facilitate bone formation, which constitutes another merit in the treatment of osteoporosis (112) (Figure 4). Researchers Tan et al. investigated why UA inhibits osteoclast differentiation at the molecular structure level and reported that it is likely related to C-29 and C-30 methyl groups (113).
5.4 Blood vessels
Macrophage autophagy exerts a protective role against early stage atherosclerosis, whereas its functional impairment in advanced disease phases exacerbates vascular inflammation, oxidative stress, and plaque necrosis, thereby accelerating disease progression (66). In the early stages of atherosclerosis, cholesterol crystals can promote the polarization of M1 macrophages and produce inflammatory responses (43).
23-Hydroxyursolic acid (23-OHUA) was identified as a potential phytochemical for the prevention and treatment of atherosclerosis. MKP-1 is a key anti-regulatory factor controlling monocyte adhesion and chemotaxis. 23-OHUA enhances MKP-1 activity in blood monocytes to a certain extent, suggesting that UA protects monocytes from metabolic priming and their transformation into a hyperchemotactic pro-atherosclerotic phenotype (114). UA exerts its anti-atherosclerotic effects by protecting blood monocytes from the effects and reprogramming induced by metabolic stress instead of lowering glucose and lipids (Figure 3). This situation was also mentioned in another study. UA preserves THP-1 monocyte functionality under metabolic stress through suppression of Nox4-mediated oxidative pathways (115). During lesion development, macrophages maintain a chronic inflammatory state (116). In animal experiments, UA may safeguard against the progression of atherosclerotic lesions in diabetic mice by restricting macrophage migratory capacity, and the reactivity of oxidative stress THP-1 monocytes to chemoattractant protein-1 (MCP-1) was increased; however, the surface expression of the MCP-1 receptor (CCR2) was not changed. UA can inhibit the chemotactic effect of oxidative stress on MCP-1 in a dose-dependent manner (117). The formation of macrophage-derived foam cells in atherosclerotic lesions is due to the transfer of free cholesterol to cholesterol esters (118). UA has been shown to inhibit the foam cell formation (119). Therefore, UA extracted from Ilex kudingcha significantly improved hyperlipidemia and atherosclerosis in APOE-deficient mice (120). LOX-1, a highly expressed transmembrane protein, is present in macrophages and is essential for the pathogenesis of atherosclerosis (121). UA decreases the mRNA and protein expression of LOX-1 (122). UA has been identified as an important component of basil. Myeloperoxidase (MPO) is an oxidase that is related to the pathogenesis of atherosclerosis. Basil extract can be used as an MPO inhibitor and as a nondrug treatment for atherosclerosis (123) (Table 2). Considering the pivotal role of macrophages in atherosclerotic diseases (Figure 4), UA has been a popular research topic, and UA has been shown to significantly reverse the abnormal activation of macrophages in atherosclerosis and to play a role in protecting blood vessels.
5.5 Brain
A series of neurological disorders are linked to oxidative damage and excessive inflammation, which are prevalent mechanisms by which UA affects these brain diseases (124) (Figure 4). UA has shown strong therapeutic potential in a variety of neurological diseases (125) and has a strong effect on microglial polarization, and the release of cytokines and inflammatory mediators (Table 2). The accumulation of amyloid beta (Aβ) in the brain represents a hallmark pathological characteristic of Alzheimer’s disease (AD). Aβ binds to receptor complexes (such as CD36) via microglia, triggering the release of proinflammatory cytokines and the production of neurotoxic reactive oxygen species, which in turn leads to neuronal degeneration (126). Microglia are closely related to the deposition site of Aβ in the brain, which activates microglia and produces a range of neurotoxins (127). UA reduces the ability of microglia to bind Aβ; however, it has no effect on the uptake capacity of microglia (128). Toll-like receptor 4 (TLR4) is expressed on the surface of microglia and mainly mediates the activation and inflammation of microglia induced by binding with lipopolysaccharide (LPS) (32). UA can inhibit LPS, and the combination of TLR4 with immune cells such as macrophages has been confirmed (35, 78). Perhaps through this route, effects on microglial function occur, and experiments are needed to confirm this hypothesis. Zhang et al. reported that UA, which has significant antidepressant activity, was one of the extracts of Cynomorium songaricum Rupr via LC-MS/MS analysis (129). Moreover, Li et al. reported that UA, a bioactive phytochemical of wintergreen, improves spatial memory performance by activating microglia and increasing Iba1 levels in the hippocampus. These findings suggest that UA improves cognitive performance in mice and holds promise as a natural treatment for neurodegenerative diseases (130). Furthermore, UA inhibits neuronal death in oxygen and glucose deprivation–reoxygenation (OGDR) models of microglia-induced ischemia–reperfusion injury (131). In the inflammatory response of brain tissue, UA can significantly reduce the number of activated microglia and reduce the level of inflammatory factors in the prefrontal cortex of D-galactose (D-gal)-treated mice, which can alleviate the brain inflammatory response (132). Both in vivo and in vitro studies have shown that UA can inhibit microglial polarization from the M2 phenotype to the M1 phenotype, significantly inhibit related pathways, reduce cytokine levels, and thus reduce the neuroinflammatory response induced by intracerebral hemorrhage (133). In LPS/IFN-γ activated microglia, UA modulates M1/M2 polarization through the PPARγ/MMP2 pathway, suggesting a potential mechanism underlying its neuroinflammatory regulatory effects (62)(Figure 3).
6 Discussion and prospects
This review details the effects of UA on the functions of macrophages, including polarization, cytokine release, and autophagy, as well as the role of ursolic acid-mediated macrophages in various diseases. UA is a potential drug in inflammatory diseases, and its internal mechanism has always been one of the research hotspots. Macrophages are an emerging target in immunotherapy. The combination of UA and macrophages is closely related to the immune escape of various inflammation-mediated diseases and tumors, which is worthy of further study.
In summary, UA strongly affects macrophages, but many questions need to be answered. Accurately controlling the regulatory effects of UA on cytokine release from macrophages for clinical treatment is one of the most important issues. Numerous studies on UA help develop immune cell medications that are more effective and less harmful, as well as new targets and avenues for expanding immunotherapy applications.
Author contributions
WF: Data curation, Writing – original draft, Writing – review & editing. KY: Investigation, Resources, Software, Writing – review & editing. YZ: Resources, Supervision, Writing – review & editing. ZX: Project administration, Writing – review & editing. RKQ: Writing – review & editing, Resources, Project administration. RHQ: Writing – review & editing, Conceptualization, Funding acquisition, Methodology, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Department of Science and Technology of Hunan Province (NO. 2014FJ3085), the Education Department of Hunan Province (NO. 14C0858), the Hunan University of Chinese Medicine (NO. 2021XJJJ001).
Acknowledgments
All images were drawn via Biorender.com, and the authors would like to express their gratitude to Biorender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zuo B, Zhang Y, Zhao K, Wu L, Qi H, Yang R, et al. Universal immunotherapeutic strategy for hepatocellular carcinoma with exosome vaccines that engage adaptive and innate immune responses. J Hematol Oncol. (2022) 15:46. doi: 10.1186/s13045-022-01266-8
2. van Duijn A, van der Burg SH, Scheeren FA. CD47/SIRPα Axis: bridging innate and adaptive immunity. J Immunother Cancer. (2022) 10:e004589. doi: 10.1136/jitc-2022-004589
3. Yan J, Horng T. Lipid metabolism in regulation of macrophage functions. Trends Cell Biol. (2020) 30:979–89. doi: 10.1016/j.tcb.2020.09.006
4. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. (2013) 14:986–95. doi: 10.1038/ni.2705
5. Zheng X, Turkowski K, Mora J, Brüne B, Seeger W, Weigert A, et al. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget. (2017) 8:48436–52. doi: 10.18632/oncotarget.17061
6. Bian Z, Gong Y, Huang T, Lee C, Bian L, Bai Z, et al. Deciphering human macrophage development at single-cell resolution. Nature. (2020) 582:582. doi: 10.1038/s41586-020-2316-7
7. Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
8. Zhu S, Yi M, Wu Y, Dong B, Wu K. Roles of tumor-associated macrophages in tumor progression: implications on therapeutic strategies. Exp Hematol Oncol. (2021) 10:60. doi: 10.1186/s40164-021-00252-z
9. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. (2010) 11:889–96. doi: 10.1038/ni.1937
10. Yuan Y, Wu D, Hou Y, Zhang Y, Tan C, Nie X, et al. Wnt signaling: modulating tumor-associated macrophages and related immunotherapeutic insights. Biochem Pharmacol. (2024) 223:116154. doi: 10.1016/j.bcp.2024.116154
11. Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. (2021) 184:792–809.e23. doi: 10.1016/j.cell.2021.01.010
12. Hussain H, Green IR, Ali I, Khan IA, Ali Z, Al-Sadi AM, et al. Ursolic acid derivatives for pharmaceutical use: A patent review (2012-2016). Expert Opin Ther Pat. (2017) 27:1061–72. doi: 10.1080/13543776.2017.1344219
13. Xia E-Q, Yu Y-Y, Xu X-R, Deng G-F, Guo Y-J, Li H-B. Ultrasound-assisted extraction of oleanolic acid and ursolic acid from ligustrum lucidum ait. Ultrason. Sonochemistry. (2012) 19:772–6. doi: 10.1016/j.ultsonch.2011.11.014
14. Xia E-Q, Wang B-W, Xu X-R, Zhu L, Song Y, Li H-B. Microwave-assisted extraction of oleanolic acid and ursolic acid from ligustrum lucidum ait. Int J Mol Sci. (2011) 12:5319–29. doi: 10.3390/ijms12085319
15. López-Hortas L, Pérez-Larrán P, González-Muñoz MJ, Falqué E, Domínguez H. Recent developments on the extraction and application of ursolic acid. A review. Food Res Int. (2018) 103:130–49. doi: 10.1016/j.foodres.2017.10.028
16. Yin R, Li T, Tian JX, Xi P, Liu RH. Ursolic acid, a potential anticancer compound for breast cancer therapy. Crit Rev Food Sci Nutr. (2018) 58:568–74. doi: 10.1080/10408398.2016.1203755
17. Al-Kuraishy HM, Al-Gareeb AI, Negm WA, Alexiou A, Batiha GE-S. Ursolic acid and SARS-coV-2 infection: A new horizon and perspective. Inflammopharmacology. (2022) 30:1493–501. doi: 10.1007/s10787-022-01038-3
18. Nguyen HN, Ullevig SL, Short JD, Wang L, Ahn YJ, Asmis R. Ursolic acid and related analogues: triterpenoids with broad health benefits. Antioxidants (Basel). (2021) 10:1161. doi: 10.3390/antiox10081161
19. Saini V, Mata Espinosa D, Pandey A, Dighe V, Barrios Payán J, Prasad Myneedu V, et al. Antimycobacterial activity of solid lipid microparticles loaded with ursolic acid and oleanolic acid: in vitro, in vivo, and toxicity assessments. Microorganisms. (2024) 12:2140. doi: 10.3390/microorganisms12112140
20. Martin D, Navarro Del Hierro J, Villanueva Bermejo D, Fernández-Ruiz R, Fornari T, Reglero G. Bioaccessibility and antioxidant activity of calendula officinalis supercritical extract as affected by in vitro codigestion with olive oil. J Agric Food Chem. (2016) 64:8828–37. doi: 10.1021/acs.jafc.6b04313
21. Das S, Ghosh S, De AK, Bera T. Oral delivery of ursolic acid-loaded nanostructured lipid carrier coated with chitosan oligosaccharides: development, characterization, in vitro and in vivo assessment for the therapy of leishmaniasis. Int J Biol Macromol. (2017) 102:996–1008. doi: 10.1016/j.ijbiomac.2017.04.098
22. Ja J, Imo S. Preclinical assessment of ursolic acid loaded into nanostructured lipid carriers in experimental visceral leishmaniasis. Pharmaceutics. (2021) 13:908. doi: 10.3390/pharmaceutics13060908
23. Ahmad A, Abuzinadah MF, Alkreathy HM, Banaganapalli B, Mujeeb M. Ursolic acid rich ocimum sanctum L leaf extract loaded nanostructured lipid carriers ameliorate adjuvant induced arthritis in rats by inhibition of COX-1, COX-2, TNF-α and IL-1: pharmacological and docking studies. PloS One. (2018) 13:e0193451. doi: 10.1371/journal.pone.0193451
24. Nahak P, Karmakar G, Chettri P, Roy B, Guha P, Besra SE. Influence of lipid core material on physicochemical characteristics of an ursolic acid-loaded nanostructured lipid carrier: an attempt to enhance anticancer activity. Langmuir : ACS J Surf. Colloids. (2016) 32:9816–25. doi: 10.1021/acs.langmuir.6b02402
25. Choudhary R, Kumar P, Shukla SK, Bhagat A, Anal JMH, Kour G, et al. Synthesis and Potential Anti-Inflammatory Response of Indole and Amide Derivatives of Ursolic Acid in LPS-Induced RAW 264.7 Cells and Systemic Inflammation Mice Model: Insights into iNOS, COX2 and NF-κB. Bioorg. Chem. (2025) 155:108091. doi: 10.1016/j.bioorg.2024.108091
26. Netea MG, Balkwill F, Chonchol M, Cominelli F, Donath MY, Giamarellos-Bourboulis EJ, et al. A guiding map for inflammation. Nat Immunol. (2017) 18:826–31. doi: 10.1038/ni.3790
27. Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. (2020) 877:173090. doi: 10.1016/j.ejphar.2020.173090
28. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. (2011) 11:723–37. doi: 10.1038/nri3073
29. Chun J, Lee C, Hwang SW, Im JP, Kim JS. Ursolic acid inhibits nuclear factor-κB signaling in intestinal epithelial cells and macrophages, and attenuates experimental colitis in mice. Life Sci. (2014) 110:23–34. doi: 10.1016/j.lfs.2014.06.018
30. González-Chávez MM, Ramos-Velázquez CS, Serrano-Vega R, Pérez-González C, Sánchez-Mendoza E, Pérez-Gutiérrez S. Anti-inflammatory activity of standardized dichloromethane extract of salvia connivens on macrophages stimulated by LPS. Pharm Biol. (2017) 55:1467–72. doi: 10.1080/13880209.2017.1305423
31. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. (2010) 11:373–84. doi: 10.1038/ni.1863
32. Zusso M, Lunardi V, Franceschini D, Pagetta A, Lo R, Stifani S, et al. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J Neuroinflammation. (2019) 16:148. doi: 10.1186/s12974-019-1538-9
33. Bryant CE, Spring DR, Gangloff M, Gay NJ. The molecular basis of the host response to lipopolysaccharide. Nat Rev Microbiol. (2010) 8:8–14. doi: 10.1038/nrmicro2266
34. Huang Y, Li J, Wang R, Wu Q, Li Y, Yu S, et al. Effect of triterpene acids of eriobotrya japonica (Thunb.) lindl. Leaf on inflammatory cytokine and mediator induction from alveolar macrophages of chronic bronchitic rats. Inflamm Res. : Off J Eur Histamine Res Soc. (2007) 56:76–82. doi: 10.1007/s00011-006-5185-0
35. Jang S-E, Jeong J-J, Hyam SR, Han MJ, Kim D-H. Ursolic acid isolated from the seed of cornus officinalis ameliorates colitis in mice by inhibiting the binding of lipopolysaccharide to toll-like receptor 4 on macrophages. J Agric Food Chem. (2014) 62:9711–21. doi: 10.1021/jf501487v
36. Li Q, Dong D-D, Huang Q-P, Li J, Du Y-Y, Li B, et al. The anti-inflammatory effect of sonchus oleraceus aqueous extract on lipopolysaccharide stimulated RAW 264.7 cells and mice. Pharm Biol. (2017) 55:799–809. doi: 10.1080/13880209.2017.1280514
37. Shin K-M, Kim R-K, Azefack TL, David L, Luc SB, Choudhary MI, et al. In vitro anti-inflammatory activity of 23-hydroxyursolic acid isolated from cussonia bancoensis in murine macrophage RAW 264.7 cells. Planta Med. (2004) 70:803–7. doi: 10.1055/s-2004-827226
38. Ruan J, Sun F, Hao M, Han L, Yu H, Lin F, et al. Structurally diverse triterpenes obtained from the fruits of ziziphus jujuba mill. as inflammation inhibitors by NF-κB signaling pathway. Food Funct. (2021) 12:4496–503. doi: 10.1039/d1fo00117e
39. Kim M-H, Kim JN, Han SN, Kim H-K. Ursolic acid isolated from guava leaves inhibits inflammatory mediators and reactive oxygen species in LPS-stimulated macrophages. Immunopharmacol Immunotoxicol. (2015) 37:228–35. doi: 10.3109/08923973.2015.1021355
40. Sun L, Wang X, Saredy J, Yuan Z, Yang X, Wang H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. (2020) 37:101759. doi: 10.1016/j.redox.2020.101759
41. Li C, Xu MM, Wang K, Adler AJ, Vella AT, Zhou B. Macrophage polarization and meta-inflammation. Transl Res. (2018) 191:29–44. doi: 10.1016/j.trsl.2017.10.004
42. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. (2012) 122:787–95. doi: 10.1172/JCI59643
43. Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. (2014) 262:153–66. doi: 10.1111/imr.12218
44. Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. (2021) 22:6995. doi: 10.3390/ijms22136995
45. Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front Immunol. (2019) 10:1084. doi: 10.3389/fimmu.2019.01084
46. Xing J, Zhang A, Zhang H, Wang J, Li X, Zeng M, et al. TRIM29 promotes DNA virus infections by inhibiting innate immune response. Nat Commun. (2017) 8:945. doi: 10.1038/s41467-017-00101-w
47. Xing J, Zhang A, Minze L, Li X, Zhang Z. TRIM29 negatively regulates the type I IFN production in response to RNA virus. J Immunol (baltim. Md, : 1950). (2018) 201:183–92. doi: 10.4049/jimmunol.1701569
48. Wang J, Lu W, Zhang J, Du Y, Fang M, Zhang A, et al. Loss of TRIM29 mitigates viral myocarditis by attenuating PERK-driven ER stress response in male mice. Nat Commun. (2024) 15:3481. doi: 10.1038/s41467-024-44745-x
49. Wang J, Wang L, Lu W, Farhataziz N, Gonzalez A, Xing J, et al. TRIM29 controls enteric RNA virus-induced intestinal inflammation by targeting NLRP6 and NLRP9b signaling pathways. Mucosal Immunol. (2025) 18:135–50. doi: 10.1016/j.mucimm.2024.10.004
50. Xing J, Weng L, Yuan B, Wang Z, Jia L, Jin R, et al. Identification of a role for TRIM29 in the control of innate immunity in the respiratory tract. Nat Immunol. (2016) 17:1373–80. doi: 10.1038/ni.3580
51. Gou W, Luo N, Yu B, Wu H, Wu S, Tian C, et al. Ursolic acid derivative UA232 promotes tumor cell apoptosis by inducing endoplasmic reticulum stress and lysosomal dysfunction. Int J Biol Sci. (2022) 18:2639–51. doi: 10.7150/ijbs.67166
52. Wan Y, Zhang W, Huang C, Jian J, Zhang Y, Liu Q, et al. Ursolic acid alleviates kupffer cells pyroptosis in liver fibrosis by the NOX2/NLRP3 inflammasome signaling pathway. Int Immunopharmacol. (2022) 113:109321. doi: 10.1016/j.intimp.2022.109321
53. Jeong HG, Kim HG, Hwang YP. Involvement of cytokines in the hepatic expression of metallothionein by ursolic acid. Toxicol Lett. (2005) 155:369–76. doi: 10.1016/j.toxlet.2004.11.002
54. López-García S, Castañeda-Sanchez JI, Jiménez-Arellanes A, Domínguez-López L, Castro-Mussot ME, Hernández-Sanchéz J, et al. Macrophage activation by ursolic and oleanolic acids during mycobacterial infection. Molecules. (2015) 20:14348–64. doi: 10.3390/molecules200814348
55. Goyal H, Parwani S, Fatima K, Kaur J. Harnessing the power of calculus bovis: anti-cancer properties and wnt pathway modulation in hepatocellular carcinoma. World J Gastroenterol. (2024) 30:4496–502. doi: 10.3748/wjg.v30.i41.4496
56. You HJ, Choi CY, Kim JY, Park SJ, Hahm KS, Jeong HG. Ursolic acid enhances nitric oxide and tumor necrosis factor-alpha production via nuclear factor-kappaB activation in the resting macrophages. FEBS Lett. (2001) 509:156–60. doi: 10.1016/s0014-5793(01)03161-1
57. Ikeda Y, Murakami A, Fujimura Y, Tachibana H, Yamada K, Masuda D, et al. Aggregated ursolic acid, a natural triterpenoid, induces IL-1beta release from murine peritoneal macrophages: role of CD36. J Immunol. (2007) 178:4854–64. doi: 10.4049/jimmunol.178.8.4854
58. Ikeda Y, Murakami A, Ohigashi H. Strain differences regarding susceptibility to ursolic acid-induced interleukin-1beta release in murine macrophages. Life Sci. (2008) 83:43–9. doi: 10.1016/j.lfs.2008.05.001
59. Mao Q, Min J, Zeng R, Liu H, Li H, Zhang C, et al. Self-assembled traditional chinese nanomedicine modulating tumor immunosuppressive microenvironment for colorectal cancer immunotherapy. Theranostics. (2022) 12:6088–105. doi: 10.7150/thno.72509
60. Lv H, Zhao M, Li Y, Li K, Chen S, Zhao W, et al. Electrospun chitosan-polyvinyl alcohol nanofiber dressings loaded with bioactive ursolic acid promoting diabetic wound healing. Nanomaterials (Basel). (2022) 12:2933. doi: 10.3390/nano12172933
61. Liu L-R, Liu J-C, Bao J-S, Bai Q-Q, Wang G-Q. Interaction of microglia and astrocytes in the neurovascular unit. Front Immunol. (2020) 11:1024. doi: 10.3389/fimmu.2020.01024
62. Wang Y, Qiu L, Deng S, Liu F, He Z, Li M, et al. Ursolic Acid Promotes Microglial Polarization toward the M2 Phenotype via PPARγ Regulation of MMP2 Transcription. Neurotoxicology. (2023) 96:81–91. doi: 10.1016/j.neuro.2023.04.001
63. Choi WH, Lee IA. The mechanism of action of ursolic acid as a potential anti-toxoplasmosis agent, and its immunomodulatory effects. Pathogens. (2019) 8:61. doi: 10.3390/pathogens8020061
64. Jiménez-Arellanes A, Luna-Herrera J, Cornejo-Garrido J, López-García S, Castro-Mussot ME, Meckes-Fischer M, et al. Ursolic and oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complement Altern Med. (2013) 13:258. doi: 10.1186/1472-6882-13-258
65. Liu T, Wang L, Liang P, Wang X, Liu Y, Cai J, et al. USP19 suppresses inflammation and promotes M2-like macrophage polarization by manipulating NLRP3 function via autophagy. Cell Mol Immunol. (2021) 18:2431–42. doi: 10.1038/s41423-020-00567-7
66. Luo Y, Lu S, Gao Y, Yang K, Wu D, Xu X, et al. Araloside C attenuates atherosclerosis by modulating macrophage polarization via sirt1-mediated autophagy. Aging (Albany NY). (2020) 12:1704–24. doi: 10.18632/aging.102708
67. Sanjurjo L, Aran G, Téllez É, Amézaga N, Armengol C, López D, et al. CD5L promotes M2 macrophage polarization through autophagy-mediated upregulation of ID3. Front Immunol. (2018) 9:480. doi: 10.3389/fimmu.2018.00480
68. Vanrell MC, Martinez SJ, Muñoz LI, Salassa BN, Gambarte Tudela J, Romano PS. Induction of autophagy by ursolic acid promotes the elimination of trypanosoma cruzi amastigotes from macrophages and cardiac cells. Front Cell Infect Microbiol. (2022) 12:919096. doi: 10.3389/fcimb.2022.919096
69. Xu J, Zhang J, Zhang Z, Gao Z, Qi Y, Qiu W, et al. Hypoxic glioma-derived exosomes promote M2-like macrophage polarization by enhancing autophagy induction. Cell Death Dis. (2021) 12:373. doi: 10.1038/s41419-021-03664-1
70. Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. (2021) 6:75. doi: 10.1038/s41392-021-00484-9
71. Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, et al. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. (2020) 16:2069–83. doi: 10.1080/15548627.2020.1714209
72. Zhang Z, Chen W-Q, Zhang S-Q, Bai J-X, Lau C-L, Sze SC-W, et al. The human cathelicidin peptide LL-37 inhibits pancreatic cancer growth by suppressing autophagy and reprogramming of the tumor immune microenvironment. Front Pharmacol. (2022) 13:906625. doi: 10.3389/fphar.2022.906625
73. Wen J-H, Li D-Y, Liang S, Yang C, Tang J-X, Liu H-F. Macrophage autophagy in macrophage polarization, chronic inflammation and organ fibrosis. Front Immunol. (2022) 13:946832. doi: 10.3389/fimmu.2022.946832
74. Leng S, Iwanowycz S, Saaoud F, Wang J, Wang Y. Ursolic acid enhances macrophage autophagy and attenuates atherogenesis. J Lipid Res. (2016) 57:1006–16. doi: 10.1194/jlr.M065888
75. Noureddin M, Yates KP, Vaughn IA, Neuschwander-Tetri BA, Sanyal AJ, McCullough A, et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. (2013) 58:1644–54. doi: 10.1002/hep.26465
76. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third national health and nutrition examination survey. JAMA. (2002) 287:356–9. doi: 10.1001/jama.287.3.356
77. Liu K, Zhao E, Ilyas G, Lalazar G, Lin Y, Haseeb M, et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy. (2015) 11:271–84. doi: 10.1080/15548627.2015.1009787
78. Zhao J, Zheng H, Sui Z, Jing F, Quan X, Zhao W, et al. Ursolic acid exhibits anti-inflammatory effects through blocking TLR4-myD88 pathway mediated by autophagy. Cytokine. (2019) 123:154726. doi: 10.1016/j.cyto.2019.05.013
79. Shen J, Fu Y, Liu F, Ning B, Jiang X. Ursolic acid promotes autophagy by inhibiting akt/mTOR and TNF-α/TNFR1 signaling pathways to alleviate pyroptosis and necroptosis in mycobacterium tuberculosis-infected macrophages. Inflammation. (2023) 46:1749–63. doi: 10.1007/s10753-023-01839-w
80. Zheng H, Feng H, Zhang W, Han Y, Zhao W. Targeting autophagy by natural product ursolic acid for prevention and treatment of osteoporosis. Toxicol Appl Pharmacol. (2020) 409:115271. doi: 10.1016/j.taap.2020.115271
81. Hassanshahi A, Hassanshahi M, Khabbazi S, Hosseini-Khah Z, Peymanfar Y, Ghalamkari S, et al. Adipose-derived stem cells for wound healing. J Cell Physiol. (2019) 234:7903–14. doi: 10.1002/jcp.27922
82. Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. (2007) 127:514–25. doi: 10.1038/sj.jid.5700701
83. Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. (2018) 9:419. doi: 10.3389/fphys.2018.00419
84. Balkrishna A, Solleti SK, Verma S, Varshney A. Application of humanized zebrafish model in the suppression of SARS-coV-2 spike protein induced pathology by tri-herbal medicine coronil via cytokine modulation. Molecules. (2020) 25:5091. doi: 10.3390/molecules25215091
85. Luo P, Li X, Ye Y, Shu X, Gong J, Wang J. Castanea mollissima shell prevents an over expression of inflammatory response and accelerates the dermal wound healing. J Ethnopharmacol. (2018) 220:9–15. doi: 10.1016/j.jep.2018.03.020
86. Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. (2020) 11:259. doi: 10.1186/s13287-020-01756-x
87. Aitcheson SM, Frentiu FD, Hurn SE, Edwards K, Murray RZ. Skin wound healing: normal macrophage function and macrophage dysfunction in diabetic wounds. Molecules. (2021) 26:4917. doi: 10.3390/molecules26164917
88. Mukherjee H, Ojha D, Bharitkar YP, Ghosh S, Mondal S, Kaity S, et al. Evaluation of the wound healing activity of shorea robusta, an Indian ethnomedicine, and its isolated constituent(s) in topical formulation. J Ethnopharmacol. (2013) 149:335–43. doi: 10.1016/j.jep.2013.06.045
89. Kaur S, Kaur T, Garg N, Mukherjee S, Raina P, Athokpam V. Effect of dose and route of inoculation on the generation of CD4+ Th1/th2 type of immune response in murine visceral leishmaniasis. Parasitol Res. (2008) 103:1413–9. doi: 10.1007/s00436-008-1150-x
90. Rocha FJS, Schleicher U, Mattner J, Alber G, Bogdan C. Cytokines, signaling pathways, and effector molecules required for the control of leishmania (Viannia) Braziliensis in mice. Infect Immun. (2007) 75:3823–32. doi: 10.1128/IAI.01335-06
91. Ota H, Takashima Y, Matsumoto Y, Hayashi Y, Matsumoto Y. Pretreatment of macrophages with the combination of IFN-gamma and IL-12 induces resistance to leishmania major at the early phase of infection. J Vet Med Sci. (2008) 70:589–93. doi: 10.1292/jvms.70.589
92. Yamamoto ES, Campos BLS, Laurenti MD, Lago JHG, Grecco S, dos S, et al. Treatment with triterpenic fraction purified from baccharis uncinella leaves inhibits leishmania (Leishmania) amazonensis spreading and improves th1 immune response in infected mice. Parasitol Res. (2014) 113:333–9. doi: 10.1007/s00436-013-3659-x
93. Ikeda Y, Murakami A, Nishizawa T, Ohigashi H. Ursolic acid enhances cyclooxygenases and tumor necrosis factor-alpha expression in mouse skin. Biosci Biotechnol Biochem. (2006) 70:1033–7. doi: 10.1271/bbb.70.1033
94. Wei Y, Quan L, Zhou C, Zhan Q. Factors relating to the biodistribution & Clearance of nanoparticles & Their effects on in vivo application. Nanomedicine (Lond). (2018) 13:1495–512. doi: 10.2217/nnm-2018-0040
95. Rogoff TM, Lipsky PE. Role of the kupffer cells in local and systemic immune responses. Gastroenterology. (1981) 80:854–60. doi: 10.1016/0016-5085(81)90152-9
96. Yuan C, Fan W, Zhou T, Sun D, Liu H, He Z, et al. Ligand-free high loading capacity ursolic acid self-carried nanovesicles enable hepatocyte targeting via absorbing apolipoproteins. Int J Pharm. (2023) 638:122931. doi: 10.1016/j.ijpharm.2023.122931
97. Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. (2019) 65:37–55. doi: 10.1016/j.mam.2018.09.002
98. Zhang C-Y, Yuan W-G, He P, Lei J-H, Wang C-X. Liver fibrosis and hepatic stellate cells: etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. (2016) 22:10512–22. doi: 10.3748/wjg.v22.i48.10512
99. Gan D, Zhang W, Huang C, Chen J, He W, Wang A, et al. Ursolic acid ameliorates CCl4-induced liver fibrosis through the NOXs/ROS pathway. J Cell Physiol. (2018) 233:6799–813. doi: 10.1002/jcp.26541
100. Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. (2002) 59:627–47. doi: 10.1007/s00018-002-8454-2
101. Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol. (2013) 9:522–36. doi: 10.1038/nrendo.2013.137
102. Mirza F, Canalis E. Management of endocrine disease: secondary osteoporosis: pathophysiology and management. Eur J Endocrinol. (2015) 173:R131–151. doi: 10.1530/EJE-15-0118
103. Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. (2012) 23:576–81. doi: 10.1016/j.tem.2012.03.008
104. Sun Y, Li J, Xie X, Gu F, Sui Z, Zhang K, et al. Macrophage-osteoclast associations: origin, polarization, and subgroups. Front Immunol. (2021) 12:778078. doi: 10.3389/fimmu.2021.778078
105. Xu D, Lyu Y, Chen X, Zhu X, Feng J, Xu Y. Fructus ligustri lucidi ethanol extract inhibits osteoclastogenesis in RAW264.7 cells via the RANKL signaling pathway. Mol Med Rep. (2016) 14:4767–74. doi: 10.3892/mmr.2016.5849
106. Tran TT, Gal M, Ha MT, Hyun S, Kim O, Lee J-H, et al. Triterpenoids from potentilla chinensis inhibit RANKL-induced osteoclastogenesis in vitro and lipopolysaccharide-induced osteolytic bone loss in vivo. Chem Biodiversity. (2024) 22:e202402011. doi: 10.1002/cbdv.202402011
107. Peng M, Qiang L, Xu Y, Li C, Li T, Wang J. Modification of cysteine 179 in IKKβ by ursolic acid inhibits titanium-wear-particle-induced inflammation, osteoclastogenesis, and hydroxylapatite resorption. Mol Pharm. (2018) 15:5244–51. doi: 10.1021/acs.molpharmaceut.8b00747
108. Tan H, Furuta S, Nagata T, Ohnuki K, Akasaka T, Shirouchi B, et al. Inhibitory effects of the leaves of loquat (Eriobotrya japonica) on bone mineral density loss in ovariectomized mice and osteoclast differentiation. J Agric Food Chem. (2014) 62:836–41. doi: 10.1021/jf402735u
109. Jiang C, Xiao F, Gu X, Zhai Z, Liu X, Wang W, et al. Inhibitory effects of ursolic acid on osteoclastogenesis and titanium particle-induced osteolysis are mediated primarily via suppression of NF-κB signaling. Biochimie. (2015) 111:107–18. doi: 10.1016/j.biochi.2015.02.002
110. Zhang W, Xue K, Gao Y, Huai Y, Wang W, Miao Z, et al. Systems pharmacology dissection of action mechanisms of dipsaci radix for osteoporosis. Life Sci. (2019) 235:116820. doi: 10.1016/j.lfs.2019.116820
111. Tan H, Zhao C, Zhu Q, Katakura Y, Tanaka H, Ohnuki K, et al. Ursolic acid isolated from the leaves of loquat (Eriobotrya japonica) inhibited osteoclast differentiation through targeting exportin 5. J Agric Food Chem. (2019) 67:3333–40. doi: 10.1021/acs.jafc.8b06954
112. Cheng M, Liang X-H, Wang Q-W, Deng Y-T, Zhao Z-X, Liu X-Y. Ursolic acid prevents retinoic acid-induced bone loss in rats. Chin J Integr Med. (2019) 25:210–5. doi: 10.1007/s11655-018-3050-y
113. Tan H, Ashour A, Katakura Y, Shimizu KA. Structure-activity relationship study on antiosteoclastogenesis effect of triterpenoids from the leaves of loquat (Eriobotrya japonica). Phytomedicine. (2015) 22:498–503. doi: 10.1016/j.phymed.2015.03.002
114. Nguyen HN, Ahn YJ, Medina EA, Asmis R. Dietary 23-hydroxy ursolic acid protects against atherosclerosis and obesity by preventing dyslipidemia-induced monocyte priming and dysfunction. Atherosclerosis. (2018) 275:333–41. doi: 10.1016/j.atherosclerosis.2018.06.882
115. Ullevig SL, Kim HS, Nguyen HN, Hambright WS, Robles AJ, Tavakoli S, et al. Ursolic acid protects monocytes against metabolic stress-induced priming and dysfunction by preventing the induction of nox4. Redox Biol. (2014) 2:259–66. doi: 10.1016/j.redox.2014.01.003
116. Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. (2019) 124. doi: 10.1161/CIRCRESAHA.118.313591
117. Ullevig SL, Zhao Q, Zamora D, Asmis R. Ursolic acid protects diabetic mice against monocyte dysfunction and accelerated atherosclerosis. Atherosclerosis. (2011) 219:409–16. doi: 10.1016/j.atherosclerosis.2011.06.013
118. Chang TY, Chang CC, Lin S, Yu C, Li BL, Miyazaki A. Roles of acyl-Coenzyme A:Cholesterol acyltransferase-1 and -2. Curr Opin lipidology. (2001) 12:289–96. doi: 10.1097/00041433-200106000-00008
119. Fujiwara Y, Hayashida A, Tsurushima K, Nagai R, Yoshitomi M, Daiguji N, et al. Triterpenoids isolated from zizyphus jujuba inhibit foam cell formation in macrophages. J Agric Food Chem. (2011) 59:4544–52. doi: 10.1021/jf200193r
120. Fujiwara Y, Okada S, Uryu K, Maru I, Komohara Y. The extract of ilex kudingcha inhibits atherosclerosis in apoE-deficient mice by suppressing cholesterol accumulation in macrophages. Biosci Biotechnol Biochem. (2021) 85:2177–84. doi: 10.1093/bbb/zbab140
121. Xu S, Ogura S, Chen J, Little PJ, Moss J, Liu P. LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cell Mol Life Sci. (2013) 70:2859–72. doi: 10.1007/s00018-012-1194-z
122. Li Q, Zhao W, Zeng X, Hao Z. Ursolic acid attenuates atherosclerosis in apoE-/- mice: role of LOX-1 mediated by ROS/NF-κB pathway. Molecules. (2018) 23:1101. doi: 10.3390/molecules23051101
123. Narasimhulu CA, Vardhan S. Therapeutic potential of ocimum tenuiflorum as MPO inhibitor with implications for atherosclerosis prevention. J Med Food. (2015) 18:507–15. doi: 10.1089/jmf.2014.0125
124. Ramos-Hryb AB, Pazini FL, Kaster MP, Rodrigues ALS. Therapeutic potential of ursolic acid to manage neurodegenerative and psychiatric diseases. CNS Drugs. (2017) 31:1029–41. doi: 10.1007/s40263-017-0474-4
125. Woźniak Ł, Skąpska S, Marszałek K. Ursolic acid–A pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules. (2015) 20:20614–41. doi: 10.3390/molecules201119721
126. Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and alzheimer’s disease. Neurobiol Aging. (2000) 21:383–421. doi: 10.1016/s0197-4580(00)00124-x
127. El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, et al. CD36 mediates the innate host response to beta-amyloid. J Exp Med. (2003) 197:1657–66. doi: 10.1084/jem.20021546
128. Wilkinson K, Boyd JD, Glicksman M, Moore KJ, El Khoury JA. High content drug screen identifies ursolic acid as an inhibitor of amyloid beta protein interactions with its receptor CD36. J Biol Chem. (2011) 286:34914–22. doi: 10.1074/jbc.M111.232116
129. Zhang X, Li L, Chen J, Hu M, Zhang Y, Zhang X, et al. Investigation of anti-depression effects and potential mechanisms of the ethyl acetate extract of cynomorium songaricum rupr. through the integration of in vivo experiments, LC-MS/MS chemical analysis, and a systems biology approach. Front Pharmacol. (2023) 14:1239197. doi: 10.3389/fphar.2023.1239197
130. Li S-J, Liu Q, He X-B, Liu J-P, Liu X-L, Hu J, et al. Pyrola incarnata demonstrates neuroprotective effects against β-amyloid-induced memory impairment in mice. Bioorg Med Chem Lett. (2020) 30:126858. doi: 10.1016/j.bmcl.2019.126858
131. Qiu L, Wang Y, Wang Y, Liu F, Deng S, Xue W, et al. Ursolic acid ameliorated neuronal damage by restoring microglia-activated MMP/TIMP imbalance in vitro. Drug Des Devel Ther. (2023) 17:2481–93. doi: 10.2147/DDDT.S411408
132. Lu J, Wu D, Zheng Y, Hu B, Zhang Z, Ye Q, et al. Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-κB pathway activation. Cereb Cortex. (2010) 20:2540–8. doi: 10.1093/cercor/bhq002
Keywords: ursolic acid, macrophage, immunoregulation, inflammation, cytokine
Citation: Feng W, Yang K, Zou Y, Xiao Z, Qian R and Qian R (2025) Progress of ursolic acid on the regulation of macrophage: summary and prospect. Front. Immunol. 16:1576771. doi: 10.3389/fimmu.2025.1576771
Received: 14 February 2025; Accepted: 15 April 2025;
Published: 12 May 2025.
Edited by:
Junji Xing, Houston Methodist Research Institute, United StatesReviewed by:
Guangchuan Wang, Jinzhou Medical University, ChinaCuncai Guo, Washington University in St. Louis, United States
Copyright © 2025 Feng, Yang, Zou, Xiao, Qian and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ronghua Qian, cmhxaWFuQGhudWNtLmVkdS5jbg==
 Wenjing Feng
Wenjing Feng Kehong Yang
Kehong Yang Ying Zou
Ying Zou Zhaohua Xiao
Zhaohua Xiao Rongkang Qian
Rongkang Qian Ronghua Qian
Ronghua Qian