- 1Department of Medical Oncology, Huzhou Central Hospital, Affiliated Central Hospital of Huzhou University, Huzhou, Zhejiang, China
- 2Clinical Laboratory, Zhejiang Xinda Hospital, Huzhou, Zhejiang, China
Background: The systemic inflammation response index (SIRI) has been investigated for its prognostic relevance in patients with glioma; however, findings remain inconsistent. Therefore, this meta-analysis aimed to clarify the prognostic value of SIRI in glioma.
Methods: PubMed, Web of Science, Embase, Cochrane Library, and CNKI were systematically searched through December 28, 2024. Pooled hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated to assess the association between SIRI and glioma prognosis.
Results: A total of 10 studies including 1,942 participants were analyzed. Elevated SIRI was significantly associated with poorer overall survival (OS) (HR=1.67, 95% CI=1.46–1.91, p<0.001) and shorter progression-free survival (PFS) (HR=1.80, 95% CI=1.29–2.52, p=0.001). Subgroup analyses indicated that the prognostic value of SIRI for OS and PFS was consistent regardless of sample size, pathological subtype, cutoff value, or type of survival analysis (p<0.05). Sensitivity and publication bias analyses confirmed the robustness of the results.
Conclusion: This meta-analysis demonstrates that high SIRI is a significant predictor of OS and PFS in patients with glioma. SIRI may serve as a promising prognostic biomarker in glioma-related clinical practice.
Introduction
Gliomas, tumors of the central nervous system (CNS) originating in glial cells, represent the most prevalent type of neurological tumor, accounting for approximately 80% of primary brain cancers (1). According to updated GLOBOCAN estimates, there were 321,476 new glioma cases and 248,305 related deaths worldwide in 2022 (2). Gliomas are classified into four grades based on genetic profiles and histopathological characteristics: grades I–II are considered low-grade gliomas, while grades III–IV are classified as malignant or high-grade gliomas (3, 4). Despite advances in glioma diagnosis and treatment, clinical outcomes remain poor, with a median survival of 14.5 months (1). Glioblastoma (GBM), a grade IV glioma, is the most prevalent, aggressive, and malignant primary brain tumor in adults (5). GBM accounts for 57.3% of all glioma cases and represents the most common histological subtype. Its prognosis is especially poor, with a 5-year survival rate of less than 6.9% (6). The identification of prognostic biomarkers is critical for improving clinical outcomes in patients with glioma (7). Therefore, there is an urgent need to identify novel, reliable biomarkers to predict glioma prognosis.
Over the past few decades, numerous studies have emphasized the pivotal role of the immune system and cancer-related inflammation in tumor initiation, progression, and metastasis (8, 9). The systemic inflammation response index (SIRI) has emerged as a prognostic tool that incorporates routine hematological parameters (10). Initially proposed in 2016, SIRI is calculated using the following formula: SIRI = neutrophil × monocyte/lymphocyte (10). SIRI has demonstrated significant prognostic value in various cancers, including colorectal cancer (11), gastric cancer (12), cholangiocarcinoma (13), thyroid cancer (14), and non-small cell lung cancer (NSCLC) (15). Its prognostic value in patients with glioma has also been explored (16–25); however, findings have been inconsistent. Some studies have reported that elevated SIRI is significantly associated with poor prognosis in patients with glioma (16–18, 20, 21). Whereas others have found no significant association between SIRI and glioma survival outcomes (19, 24). Consequently, the present study aimed to clarify the prognostic value of SIRI in patients with glioma.
Materials and methods
Study guideline
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (26).
Literature search
We systematically searched PubMed, Web of Science, Embase, Cochrane Library, and CNKI databases through December 28, 2024. The search strategy included the following terms: (systemic inflammation response index OR system inflammation response index OR SIRI OR systemic inflammatory response index) AND (glioma OR gliomas OR glioblastoma). No language restrictions were applied. Additionally, we manually reviewed the references of all included articles to ensure comprehensive coverage.
Inclusion and exclusion criteria
Studies were included if they met the following criteria (1): glioma was pathologically confirmed (2); the study evaluated the association between SIRI and glioma prognosis (3); hazard ratios (HRs) with 95% confidence intervals (CIs) were reported (4); a defined threshold for stratifying high vs. low SIRI was provided; and (5) there were no language restrictions. Exclusion criteria were as follows (1): reviews, case reports, conference abstracts, letters, or commentaries (2); studies lacking survival outcome data (3); studies with duplicate participant cohorts; and (4) animal studies.
Data extraction and quality assessment
Two independent reviewers (Y.J. and L.Z.) extracted data from eligible studies. Discrepancies were resolved through discussion. Extracted data included: first author, publication year, country, sample size, sex distribution, age, study design, study center type, WHO tumor grade, study period, tumor pathology, treatment modality, SIRI threshold and its determination method, follow-up duration, survival endpoints, type of survival analysis, HRs, and 95% CIs. The primary and secondary outcomes were overall survival (OS) and progression-free survival (PFS), respectively. Study quality was assessed using the Newcastle-Ottawa Scale (NOS) (27), with scores ranging from 0 to 9. Studies scoring ≥6 were considered high quality.
Statistical analysis
To assess the prognostic value of SIRI in patients with glioma, pooled HRs and corresponding 95% CIs were calculated. Between-study heterogeneity was evaluated using Cochran’s Q test and the I2 statistics. An I2 > 50% and p < 0.10 indicated significant heterogeneity, in which case a random-effects model was applied; otherwise, a fixed-effects model was used. Subgroup analyses were performed based on various study characteristics to explore the prognostic relevance of SIRI. Sensitivity analyses were conducted by sequentially removing each study and recalculating the overall effect size to evaluate the robustness of the results. Publication bias was assessed using funnel plots as well as Begg’s and Egger’s tests. All statistical analyses were performed using Stata version 12.0 (StataCorp, College Station, TX, USA). A p-value <0.05 is considered statistically significant.
Results
Literature search process
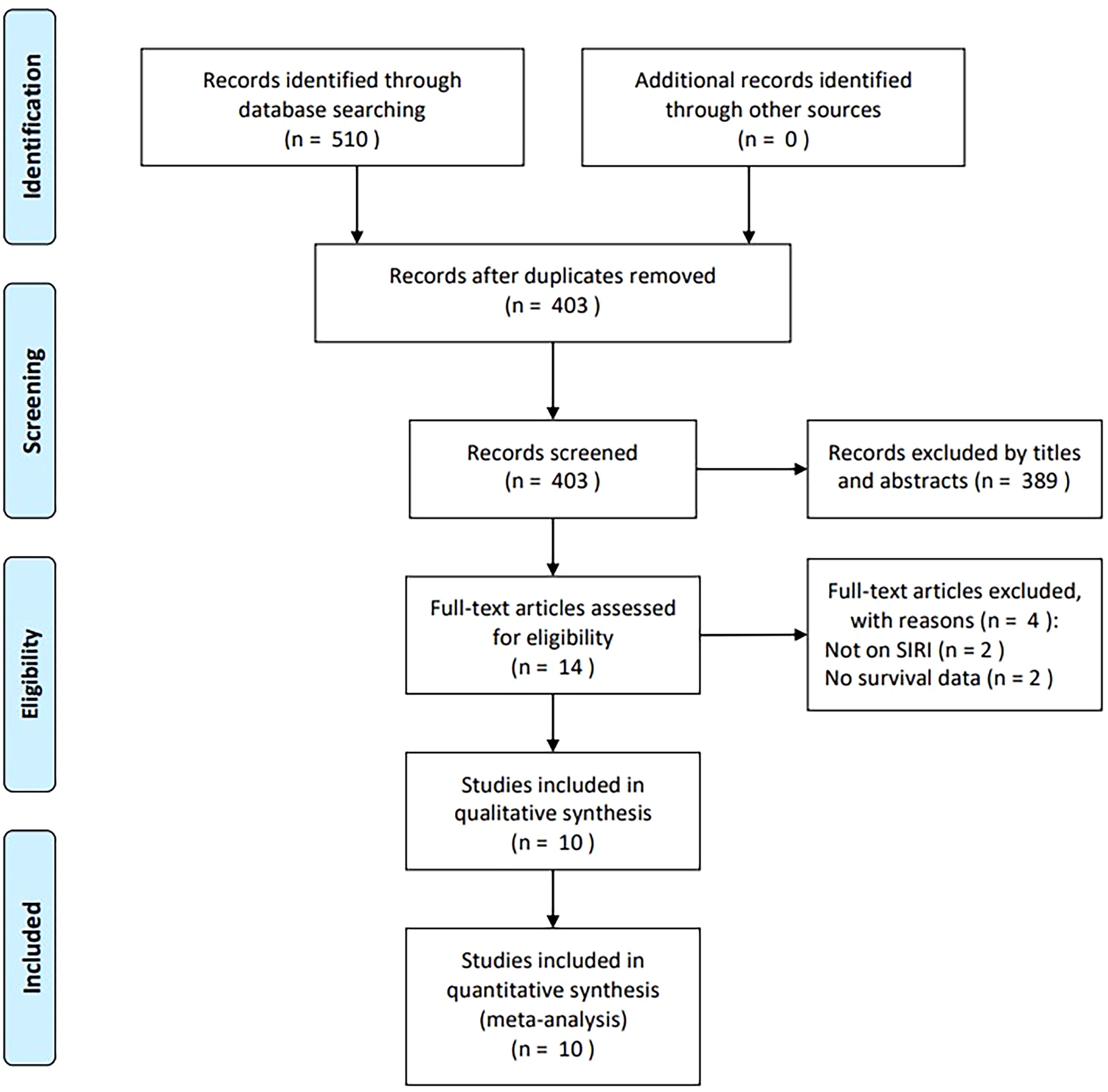
The initial database search identified 510 articles, of which 403 remained after the removal of duplicates (Figure 1). After screening titles and abstracts, 389 records were excluded due to irrelevance or being animal studies. Full texts of 14 articles were assessed for eligibility, and four were excluded—two for not evaluating SIRI and two for lacking survival data. Ultimately, 10 studies comprising 1,942 participants were included in the meta-analysis (16–25) (Figure 1).
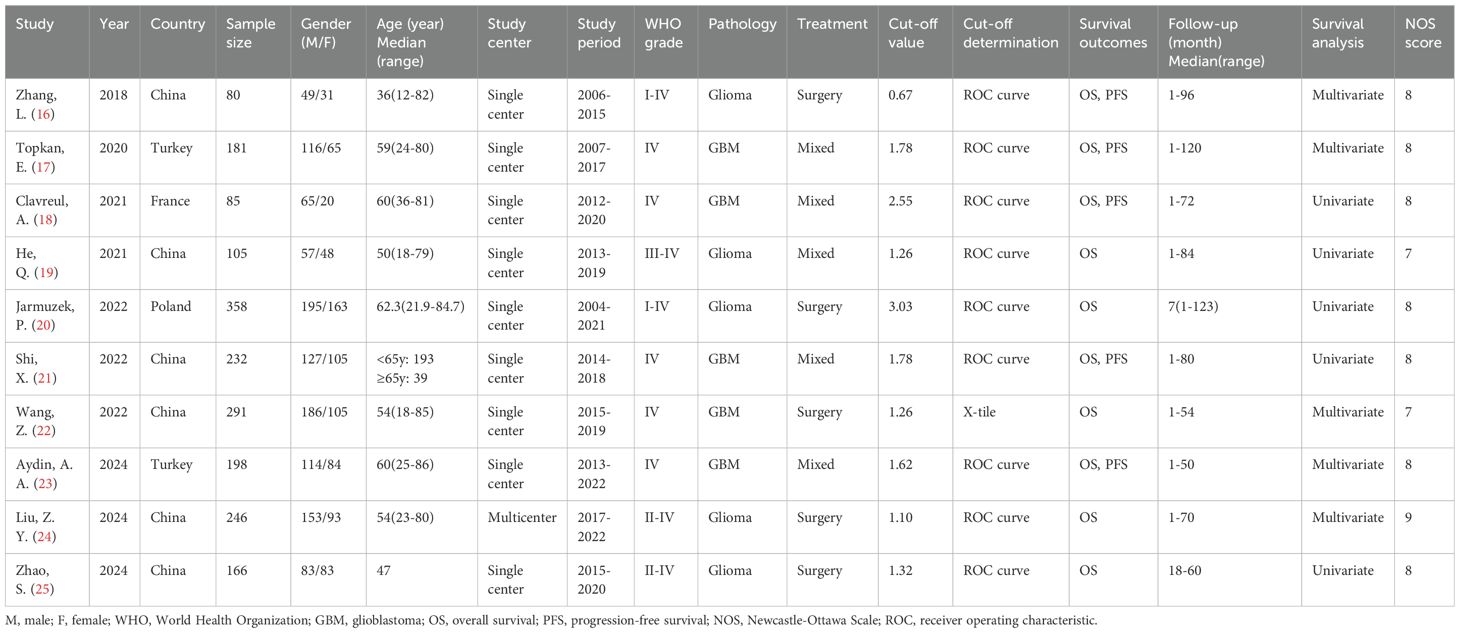
Characteristics of included studies
The 10 included studies were published between 2018 and 2024 and all employed retrospective designs (16–25) (Table 1). Six studies were conducted in China (16, 19, 21, 22, 24, 25), two in Turkey (17, 23), one in France (18) and one in Poland (20). Eight articles were published in English (17–24) and two in Chinese (16, 25). Sample sizes ranged from 80 to 358 participants (median: 189.5). Nine studies were single-center investigations (16–23, 25), while one was a multicenter study (24). Five studies enrolled participants with glioma (16, 19, 20, 24, 25), and five focused on participants with GBM (17, 18, 21–23). Treatment approaches included surgery in five studies (16, 20, 22, 24, 25) and multimodal therapy in five others (17–19, 21, 23). Reported SIRI thresholds ranged from 0.67 to 3.03 (median, 1.47). Nine studies determined cut-off values using receiver operating characteristic (ROC) curve analysis (16–21, 23–25), while one used X-tile software (22). HRs and 95% CIs were derived via univariate regression in five studies (18–21, 25) and multivariate regression in the remaining five (16, 17, 22–24). NOS scores ranged from 7 to 9, indicating high methodological quality (Table 1).
SIRI and OS
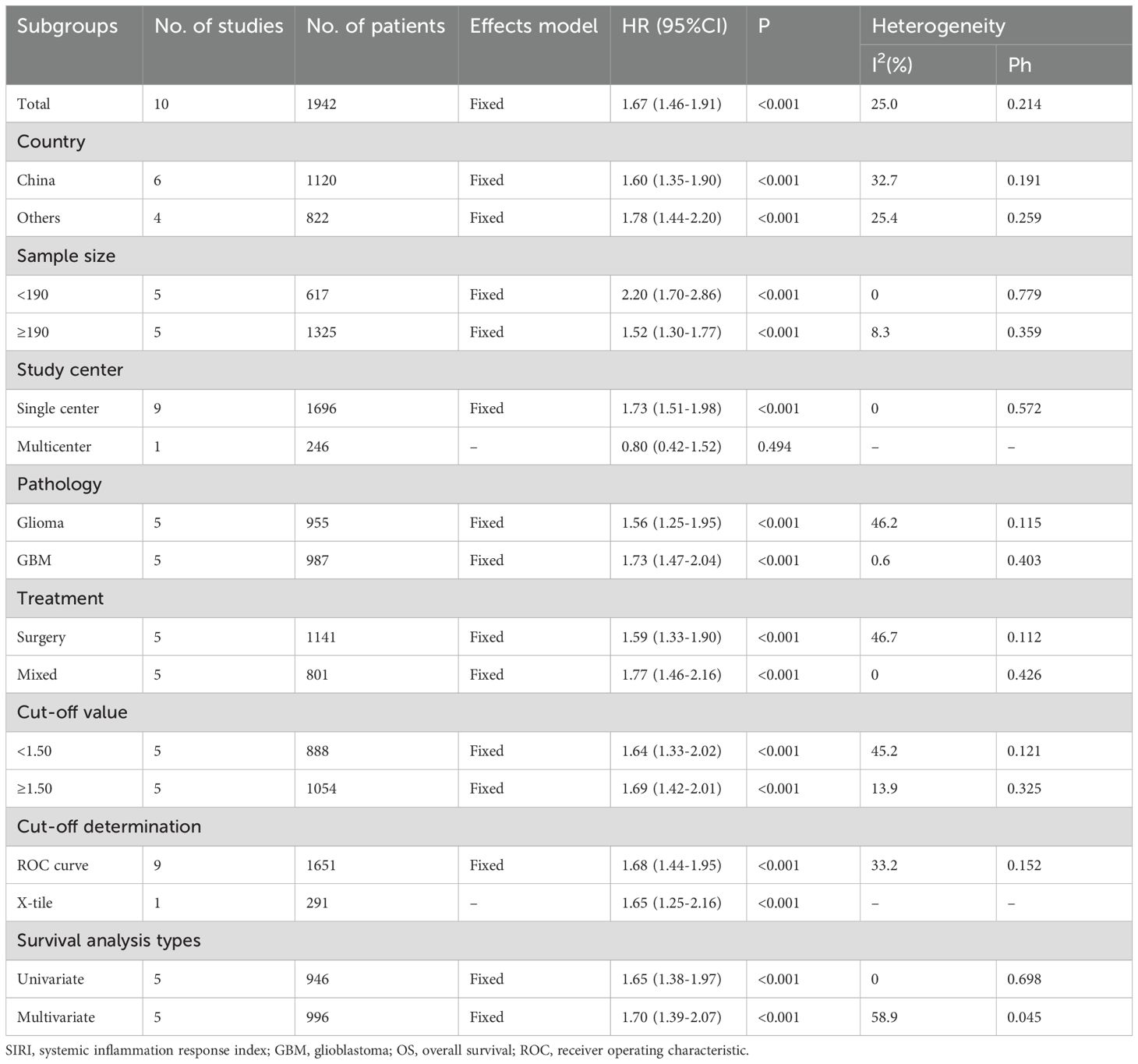
All 10 studies (n=1,942 participants) examined the association between SIRI and OS in patients with glioma (16–25). Given the low heterogeneity among studies (I2 = 25.0%, p = 0.214), a fixed-effects model was applied. Pooled results revealed that elevated SIRI was significantly associated with worse OS (HR = 1.67, 95% CI = 1.46–1.91, p < 0.001) (Figure 2; Table 2). Subgroup analyses confirmed that SIRI was a significant prognostic factor for OS regardless of country, sample size, pathology, treatment modality, threshold, threshold determination method, or type of survival analysis (p < 0.05 for all; Table 2).
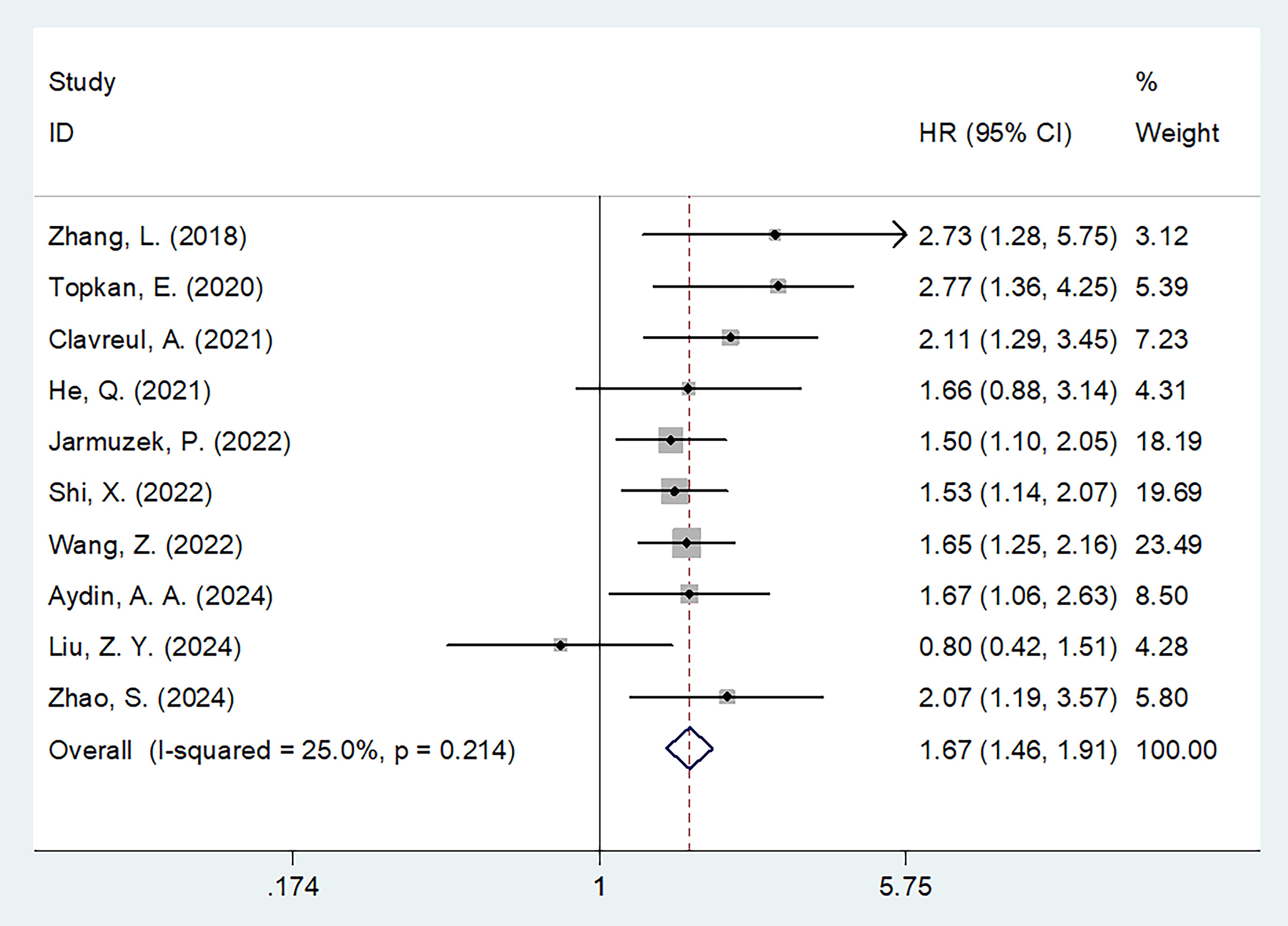
Figure 2. Forest plots of HR with 95% CI for correlation between SIRI and OS in patients with glioma.
SIRI and PFS
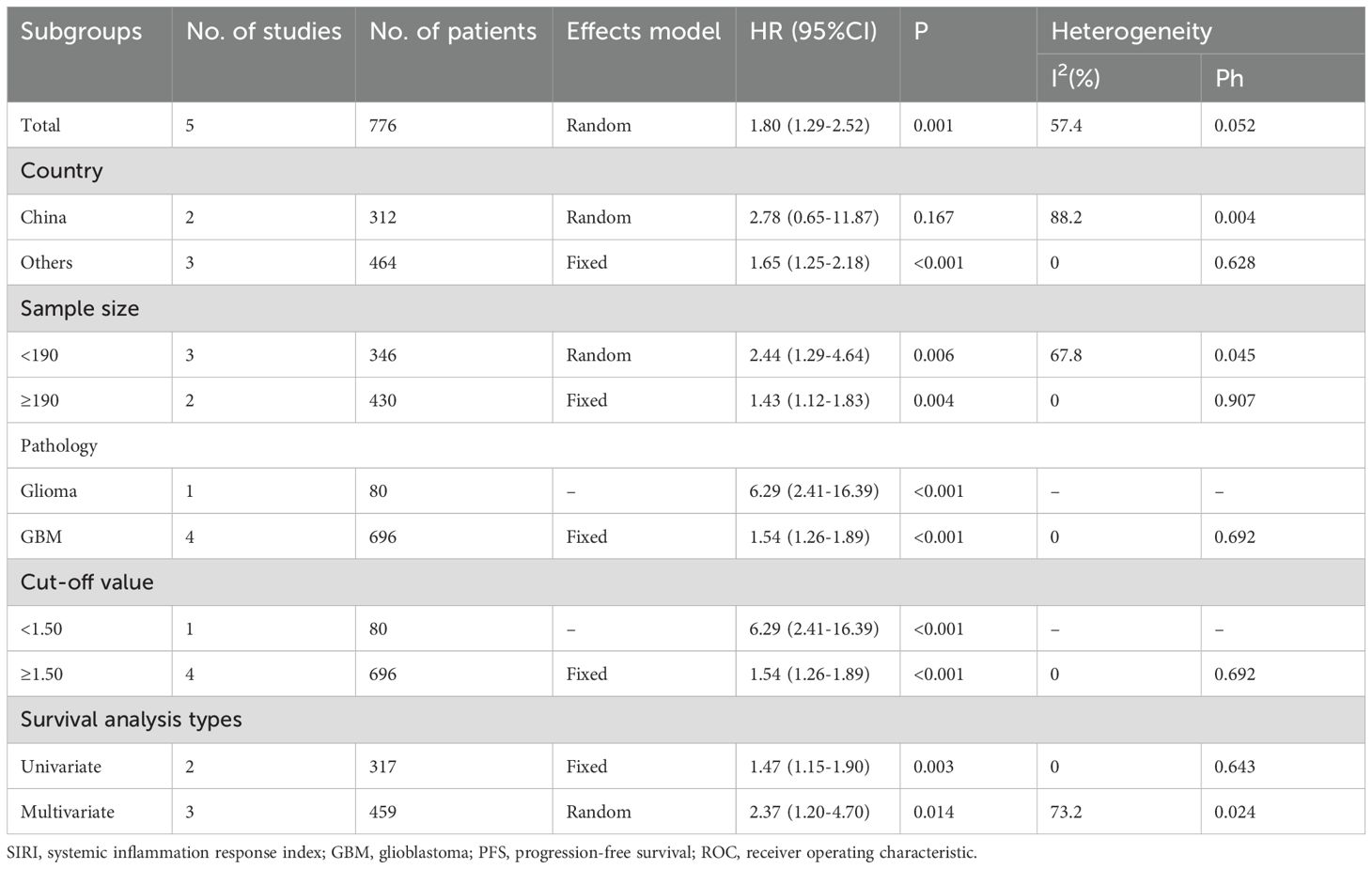
Five studies involving 776 participants assessed the relationship between SIRI and PFS (16–18, 21, 23). A random-effects model was used due to substantial heterogeneity (I2 = 57.4%, p = 0.052) (Table 3), likely arising from variations in sample size, pathology type, and treatment protocols across studies (Table 1). The meta-analysis showed that high SIRI was significantly associated with poorer PFS (HR = 1.80, 95% CI = 1.29–2.52, p = 0.001) (Figure 3; Table 3). Subgroup analyses demonstrated that this association remained significant regardless of sample size, pathology, threshold, or analysis method (p < 0.05 for all; Table 3).
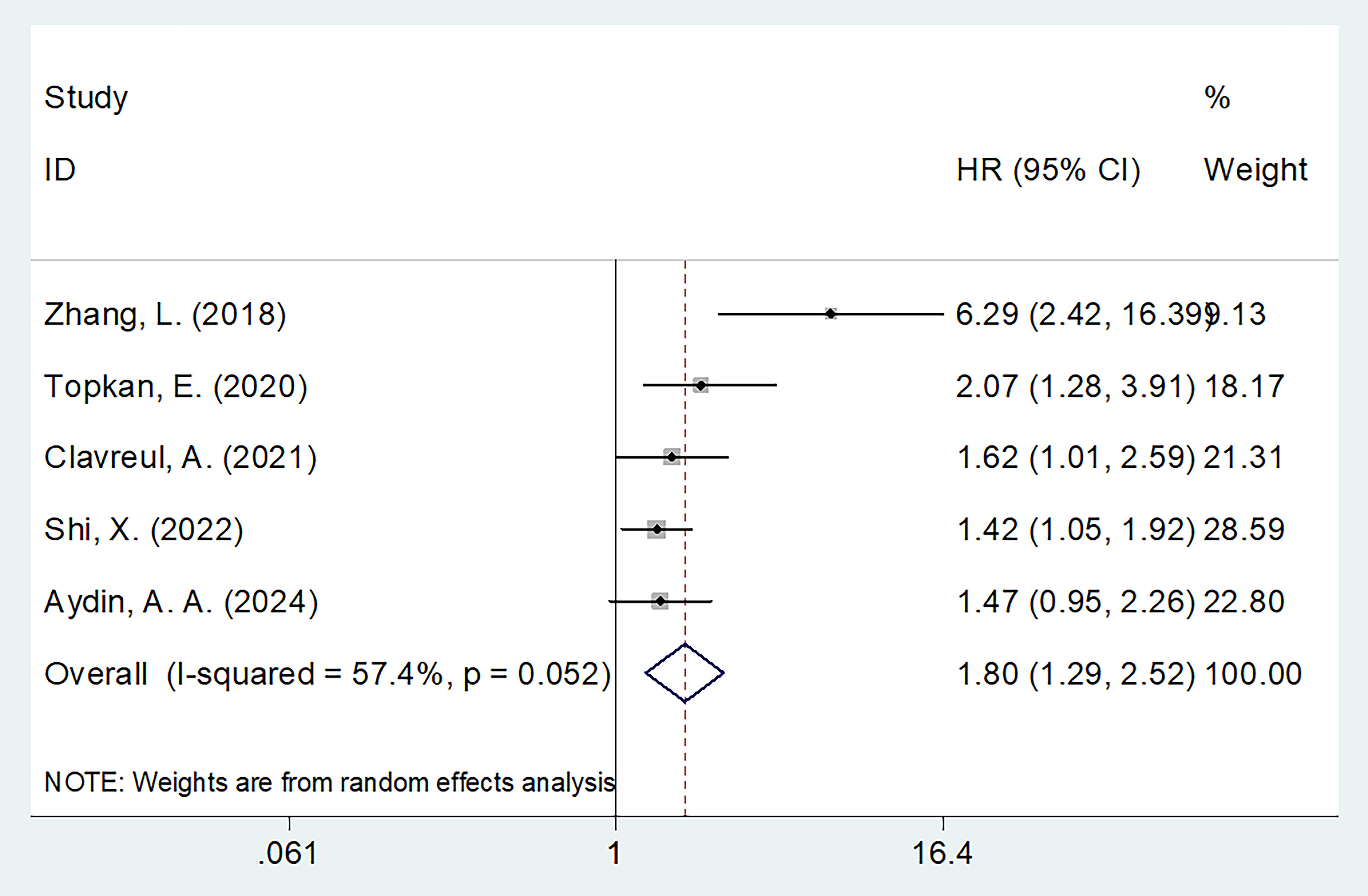
Figure 3. Forest plots of HR with 95% CI for correlation between SIRI and PFS in patients with glioma.
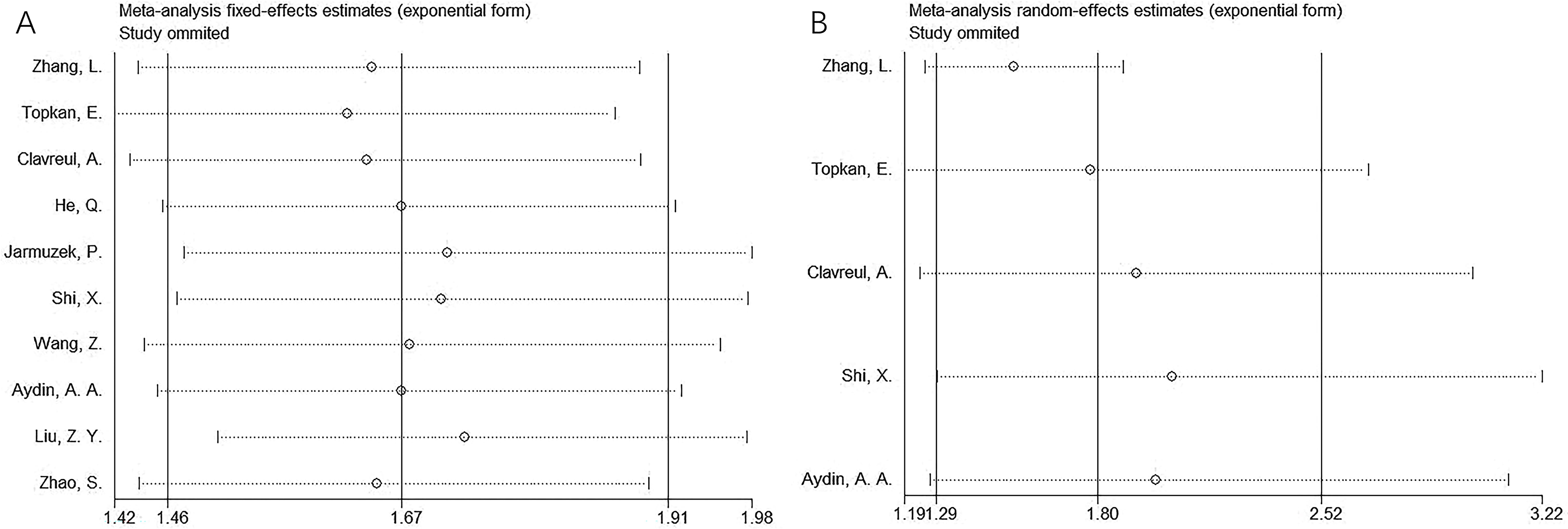
Sensitivity analysis
Sensitivity analyses were conducted by sequentially excluding each study to evaluate the stability of the pooled estimates. Results indicated that no single study significantly influenced the overall findings, confirming the robustness of the results for both OS and PFS (Figure 4).
Publication bias
Potential publication bias was assessed using funnel plots, as well as Begg’s and Egger’s tests. The funnel plots were symmetrical (Figure 5), and statistical tests did not indicate significant publication bias for OS or PFS (Begg’s test: p = 0.371/0.408; Egger’s test: p = 0.127/0.225) (Figure 5).
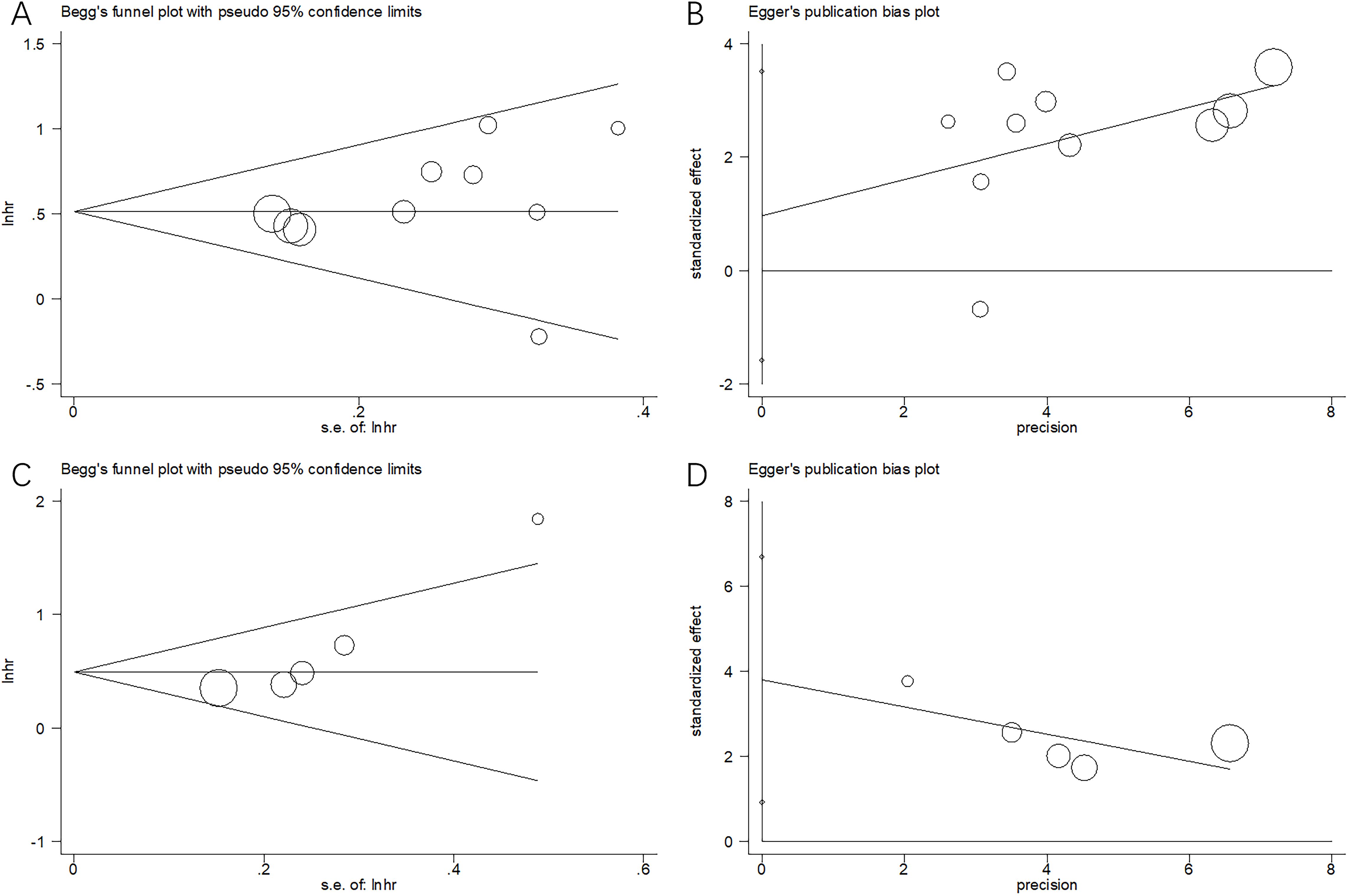
Figure 5. Publication bias by Begg’s test and Egger’s test. (A) Begg’s test for OS, p=0.371; (B) Egger’s test for OS, p=0.408; (C) Begg’s test for PFS, p=0.127; and (D) Egger’s test for PFS, p=0.225.
Discussion
SIRI has been extensively investigated for its prognostic value in glioma; however, previous findings remain inconsistent. The present study synthesized data from 10 studies involving 1,942 participants (16–25) to clarify the prognostic role of SIRI in glioma. Our results demonstrate that elevated SIRI significantly predicts both OS and PFS in individuals with glioma. Moreover, the prognostic performance of SIRI was consistent regardless of sample size, pathological classification, threshold definition, or type of survival analysis employed. Sensitivity analysis and assessments for publication bias confirmed the robustness of our findings. Collectively, these results identify SIRI as a meaningful prognostic marker for both short- and long-term outcomes in patients with glioma. To our knowledge, this study provides the first comprehensive evidence supporting the clinical relevance of SIRI in glioma prognosis.
SIRI is calculated using neutrophil, monocyte, and lymphocyte counts (10). An elevated SIRI may result from increased neutrophils and/or monocytes and/or decreased lymphocytes. Although the mechanisms underlying SIRI’s prognostic value in glioma remain to be fully elucidated, several plausible biological explanations exist. Neutrophils, as primary mediators of the inflammatory response, release vascular endothelial growth factor (VEGF), proteases, and chemokines that promote angiogenesis, thereby creating a tumor-supportive microenvironment (28). VEGF and interleukin-8 (IL-8), produced by tumor cells, further stimulate neutrophils within the tumor microenvironment, leading to the release of fibroblast growth factor, platelet-derived growth factor, matrix metalloproteinases, and interleukin-6 (IL-6) (29). In addition, neutrophils can suppress antitumor immunity by inhibiting T-cell activation through the production of reactive oxygen species, nitric oxide, and arginase (30). Monocytes, particularly those differentiating into tumor-associated macrophages (TAMs), also contribute to immune suppression and tumor progression. TAMs promote apoptosis of antitumor T cells and support angiogenesis through the release of pro-angiogenic factors (31). They also facilitate extracellular matrix degradation and tumor cell migration, thereby promoting metastasis (32). TAMs are recruited and activated by cytokines and chemokines such as tumor necrosis factor-α and monocyte chemoattractant protein-1 within the tumor microenvironment (33). The interaction between TAMs and cancer cells further enhances tumor angiogenesis, invasion, and migration while suppressing anticancer immune responses, ultimately contributing to disease progression and poor prognosis (34). Conversely, lymphocytes play a central role in host antitumor immunity. They contribute to cytotoxic responses that inhibit tumor growth and metastasis (35). Lymphocytes exert antitumor effects by activating the p53 signaling pathway and secreting IL-17, which induces cancer cell death and suppresses tumor proliferation (36). Furthermore, lymphocytes aid in immune surveillance by promoting cytotoxic cell-mediated destruction of tumor cells and preventing tumor dissemination (37). Therefore, SIRI represents a biologically plausible and clinically relevant prognostic marker derived from neutrophil, monocyte, and lymphocyte counts.
Numerous recent studies have reported the prognostic significance of SIRI in various cancers through meta-analyses (38–42). For instance, Shen et al. found that elevated SIRI significantly predicted both OS and PFS in patients with pancreatic cancer, based on a meta-analysis involving 1,160 participants (38). Similarly, Wu et al. reported that higher SIRI was strongly associated with poorer OS and disease-free survival in patients with gastric cancer, as shown in their meta-analysis of seven studies (39). A recent meta-analysis involving 3,728 participants demonstrated that elevated SIRI was significantly associated with both OS and PFS in NSCLC (40). Gu et al. also reported that high SIRI was markedly correlated with worse OS and PFS in patients with cancer treated with programmed cell death 1/PD-1 ligand 1 immune checkpoint inhibitors, based on their meta-analysis of six studies (41). In another meta-analysis involving 17 studies, Yang and colleagues showed that elevated SIRI was a significant predictor of poor OS in patients with oral cancer (42). These findings are consistent with our meta-analysis, further supporting the prognostic value of SIRI across a range of cancer types.
Despite the promising results, several limitations should be acknowledged. First, only retrospective studies were included, which may introduce heterogeneity. Second, the cut-off values used to define high SIRI were not standardized across studies, potentially leading to selection bias. Third, most included studies were conducted at single centers. Therefore, large-scale, prospective, multicenter studies are warranted to validate our findings.
Conclusions
In summary, this meta-analysis demonstrated that elevated SIRI significantly predicted both OS and PFS in participants with glioma. Moreover, subgroup analyses confirmed the consistency of this prognostic effect across various study characteristics. These findings suggest that SIRI may serve as a promising glioma-related clinical prognostic biomarker.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YJ: Conceptualization, Data curation, Formal Analysis, Investigation, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. LZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Project administration, Resources, Software, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SIRI, systemic inflammation response index; HR, hazard ratio; CI, confidence interval; OS, overall survival; PFS, progression-free survival; CNS, central nervous system; GBM, glioblastoma; NSCLC, non-small cell lung cancer; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NOS, Newcastle-Ottawa Scale; ROC, receiver operating characteristic; VEGF, vascular endothelial growth factor; TAMs, tumor-associated macrophages.
References
1. Weller M, Wen PY, Chang SM, Dirven L, Lim M, Monje M, et al. Glioma. Nat Rev Dis Primers. (2024) 10:33. doi: 10.1038/s41572-024-00516-y
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J clinicians. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta neuropathologica. (2007) 114:97–109. doi: 10.1007/s00401-007-0243-4
4. Komori T. Grading of adult diffuse gliomas according to the 2021 WHO Classification of Tumors of the Central Nervous System. Lab investigation; A J Tech Methods Pathol. (2022) 102:126–33. doi: 10.1038/s41374-021-00667-6
5. Schaff LR and Mellinghoff IK. Glioblastoma and other primary brain Malignancies in adults: A review. Jama. (2023) 329:574–87. doi: 10.1001/jama.2023.0023
6. Grochans S, Cybulska AM, Simińska D, Korbecki J, Kojder K, Chlubek D, et al. Epidemiology of glioblastoma multiforme-literature review. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14102412
7. Śledzińska P, Bebyn MG, Furtak J, Kowalewski J, and Lewandowska MA. Prognostic and predictive biomarkers in gliomas. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms221910373
8. Grivennikov SI, Greten FR, and Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
9. Diakos CI, Charles KA, McMillan DC, and Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. doi: 10.1016/s1470-2045(14)70263-3
10. Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. (2016) 122:2158–67. doi: 10.1002/cncr.30057
11. Hayama T, Ochiai H, Ozawa T, Miyata T, Asako K, Fukushima Y, et al. High systemic inflammation response index (SIRI) level as a prognostic factor for colorectal cancer patients after curative surgery: a single-center retrospective analysis. Sci Rep. (2025) 15:1008. doi: 10.1038/s41598-024-84991-z
12. Pehlevan-Özel H, Dinç T, Okay ND, and Tez M. The relationship between systemic inflammation response index and clinical and histopathological features in gastric cancer. Cir Cir. (2024). doi: 10.24875/ciru.23000234
13. Zhang WH, Zhao Y, Zhang CR, Huang JC, Lyu SC, and Lang R. Preoperative systemic inflammatory response index as a prognostic marker for distal cholangiocarcinoma after pancreatoduodenectomy. World J Gastrointest Surg. (2024) 16:2910–24. doi: 10.4240/wjgs.v16.i9.2910
14. Wang Y, Chang J, Hu B, and Yang S. Systemic immune-inflammation index and systemic inflammation response index predict the response to radioiodine therapy for differentiated thyroid cancer. J Inflammation Res. (2024) 17:8531–41. doi: 10.2147/jir.S493397
15. Tang C, Zhang M, Jia H, Wang T, Wu H, Xu K, et al. The systemic inflammation response index (SIRI) predicts survival in advanced non-small cell lung cancer patients undergoing immunotherapy and the construction of a nomogram model. Front Immunol. (2024) 15:1516737. doi: 10.3389/fimmu.2024.1516737
16. Zhang L, Liu P, Ji H, Li S, and Zhang Z. Effect of systemic inflammation response index on clinical prognosis of patients with glioma and its relationship with IDH1 mutation. Pract Oncol J. (2018) 32:25–32. doi: 10.11904/j.issn.1002-3070.2018.01.005
17. Topkan E, Kucuk A, Ozdemir Y, Mertsoylu H, Besen AA, Sezen D, et al. Systemic inflammation response index predicts survival outcomes in glioblastoma multiforme patients treated with standard stupp protocol. J Immunol Res. (2020) 2020:8628540. doi: 10.1155/2020/8628540
18. Clavreul A, Lemée JM, Soulard G, Rousseau A, and Menei P. A simple preoperative blood count to stratify prognosis in isocitrate dehydrogenase-wildtype glioblastoma patients treated with radiotherapy plus concomitant and adjuvant temozolomide. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13225778
19. He Q, Li L, and Ren Q. The prognostic value of preoperative systemic inflammatory response index (SIRI) in patients with high-grade glioma and the establishment of a nomogram. Front Oncol. (2021) 11:671811. doi: 10.3389/fonc.2021.671811
20. Jarmuzek P, Kot M, Defort P, Stawicki J, Komorzycka J, Nowak K, et al. Prognostic values of combined ratios of white blood cells in glioblastoma: A retrospective study. J Clin Med. (2022) 11. doi: 10.3390/jcm11123397
21. Shi X, Li H, Xu Y, Nyalali AMK, and Li F. The prognostic value of the preoperative inflammatory index on the survival of glioblastoma patients. Neurol Sci. (2022) 43:5523–31. doi: 10.1007/s10072-022-06158-w
22. Wang Z, Li J, Yuan Y, Li T, Zuo M, and Liu Y. Prognostic significance of preoperative systemic inflammation response index in newly diagnosed glioblastoma patients underwent gross total resection: a propensity score matching analysis. World J Surg Oncol. (2022) 20:137. doi: 10.1186/s12957-022-02588-0
23. Aydin AA and Yuceer RO. Unraveling the predictive value of the novel global immune-nutrition-inflammation index (GINI) on survival outcomes in patients with grade 4 adult-type diffuse gliomas. Curr Oncol (Toronto Ont). (2024) 31:5027–39. doi: 10.3390/curroncol31090372
24. Liu ZY, Tang F, Wang J, Yang JZ, Chen X, Wang ZF, et al. Serum beta2-microglobulin acts as a biomarker for severity and prognosis in glioma patients: a preliminary clinical study. BMC Cancer. (2024) 24:692. doi: 10.1186/s12885-024-12441-0
25. Zhao S, Yan Z, Yang J, Zhang W, Pan S, and Xu S. Value of combining radiomics and deep-learning with hematological inflammatory markers in predicting the prognosis of glioma. Chin J Magn Reson Imaging. (2024) 15:88–94 + 100. doi: 10.12015/issn.1674-8034.2024.01.014
26. Moher D, Liberati A, Tetzlaff J, and Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical Res ed). (2009) 339:b2535. doi: 10.1136/bmj.b2535
27. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
28. Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. (2001) 189:197–206. doi: 10.1002/jcp.10014
29. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, and Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. (2020) 20:485–503. doi: 10.1038/s41568-020-0281-y
30. Ocana A, Nieto-Jiménez C, Pandiella A, and Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. (2017) 16:137. doi: 10.1186/s12943-017-0707-7
31. Ugel S, Canè S, De Sanctis F, and Bronte V. Monocytes in the tumor microenvironment. Annu Rev Pathol. (2021) 16:93–122. doi: 10.1146/annurev-pathmechdis-012418-013058
32. Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, and Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. (2013) 218:1402–10. doi: 10.1016/j.imbio.2013.06.003
33. Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. (2004) 4:71–8. doi: 10.1038/nrc1256
34. Qian BZ and Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. (2010) 141:39–51. doi: 10.1016/j.cell.2010.03.014
35. Guven DC, Sahin TK, Erul E, Kilickap S, Gambichler T, and Aksoy S. The association between the pan-immune-inflammation value and cancer prognosis: A systematic review and meta-analysis. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14112675
36. Ostroumov D, Fekete-Drimusz N, Saborowski M, Kühnel F, and Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life sciences: CMLS. (2018) 75:689–713. doi: 10.1007/s00018-017-2686-7
37. Paijens ST, Vledder A, de Bruyn M, and Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. (2021) 18:842–59. doi: 10.1038/s41423-020-00565-9
38. Shen H and Zuo F. Prognostic role of systemic inflammation response index (SIRI) in patients with pancreatic cancer: a meta-analysis. Front Oncol. (2024) 14:1465279. doi: 10.3389/fonc.2024.1465279
39. Wu Q and Zhao H. Prognostic and clinicopathological role of pretreatment systemic inflammation response index (SIRI) in gastric cancer: a systematic review and meta-analysis. World J Surg Oncol. (2024) 22:333. doi: 10.1186/s12957-024-03602-3
40. Ye X, Dai M, and Xiang Z. Prognostic role of systemic inflammation response index in patients with non-small cell lung cancer: a meta-analysis. BMJ Open. (2024) 14:e087841. doi: 10.1136/bmjopen-2024-087841
41. Gu X, Han X, Shen Y, and Shi Y. Prognostic value of systemic inflammation response index in cancer patients treated with PD-1/PD-L1 immune checkpoint inhibitors: a meta-analysis. Ann Med. (2024) 56:2413415. doi: 10.1080/07853890.2024.2413415
Keywords: systemic inflammation response index, glioma, meta-analysis, biomarker, prognosis
Citation: Jiang Y and Zhou L (2025) Prognostic value of systemic inflammation response index in patients with glioma: a meta-analysis. Front. Immunol. 16:1576845. doi: 10.3389/fimmu.2025.1576845
Received: 14 February 2025; Accepted: 06 May 2025;
Published: 23 May 2025.
Edited by:
Anand Rotte, Arcellx Inc, United StatesReviewed by:
Fabio Grizzi, Humanitas Research Hospital, ItalyXiangdong Peng, Central South University, China
Copyright © 2025 Jiang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zhou, Z2hvc3RvZ29nb0AxNjMuY29t
 Yizhen Jiang1
Yizhen Jiang1 Lin Zhou
Lin Zhou