- Department of Dermatology, Hebei Medical University Third Hospital, Shijiazhuang, China
Alopecia areata (AA) is an autoimmune disease characterized by inflammatory and non-scarring hair loss, mediated by CD8+ T cells and primarily affecting hair follicles. Janus kinase (JAK) inhibitors selectively inhibit JAK, block the signal transducer and activator of transcription pathway, and often interfere with T-cell-mediated inflammatory cytokine pathways. They are a class of targeted anti-inflammatory drugs that can promote the activation of hair follicle stem cells. Studies have shown that JAK inhibitors exhibited good efficacy and safety in the treatment of AA, with fewer serious side effects. This article reviews the mechanism of action of JAK inhibitors in the treatment of AA and the effects and side effects of representative drugs.
1 Introduction
Although alopecia areata (AA) is not life threatening, it does affect physical appearance, psychology, and daily life, as it can recur or even progress in most patients. From 1990 to 2021, global AA incidence has increased in absolute terms (1). The lifetime prevalence of AA is 0.7%–3.8%, and 7%–12% of patients progress to total or general alopecia (2). The estimated lifetime prevalence of AA in the overall population is 0.10% worldwide, with 0.12% of adults and 0.03% of children affected (3).
AA is caused by a number of factors, and the common theory is the breakdown of the immune privilege of the hair follicle. Hair follicle atrophy and hair loss occur when follicular stem cells, dermal papilla cells, and related proteins undergo progressive damage via CD8+ T-cell-mediated inflammatory pathways and cytokine cascades. This pathological process mainly involves immunological recognition abnormalities, inflammatory amplification signaling, and microenvironmental dysregulation within the hair follicle. As a type of special CD8+ T cell, cytotoxic CD8+NKG2D+ T cells carry the NKG2D receptor (product of the KLRK1 gene), which is one of the natural killer immune receptors (4, 5). Stimulatory factors in patients with AA produce interferon-gamma (IFN-γ) through a positive feedback loop between follicular epithelial and CD8+NKG2D+ T cells via the Janus kinase (JAK)-signal transducer activator of transcription (STAT) pathway, which promotes the loss of follicular immune privilege (6). In patients with AA, hair loss results from T-cell-induced inflammation in the hair follicle regions, which disrupts the growth cycle and impairs its function (7, 8). Lymphocytic infiltration and several pro-inflammatory cytokines, such as interleukin (IL)-15 and IFN-γ, also contribute to hair loss (9, 10). As shown in Figure 1, JAK inhibitors specifically inhibit JAK1/2 in hair follicle epithelial cells and JAK1/3 in T cells, thereby inhibiting CD8+NKG2D+ T-cell activity and STAT phosphorylation and reducing IL-15 and IFN-γ secretion to slow the loss of hair follicle immune privilege. This pathway is initiated when follicular epithelial cells present self-antigens to CD8+NKG2D+ T cells via major histocompatibility complex I antigen and NKG2D/NKG2D ligand complexes and activate CD8+NKG2D+ T cells. Activated CD8+NKG2D+ T cells produce IFN-γ, which binds to the corresponding IFN-γ receptor on follicular epithelial cells, triggering the downstream JAK1/2-STAT pathway. This leads to the upregulation of IL-15 and IL-15R α, which in turn binds to activated CD8+NKG2D+ T cells and triggers further upregulation of IFN-γ through the JAK1/3-STAT pathway. These molecular interactions create a positive feedback loop between the two cell types to amplify the inflammatory immune response.
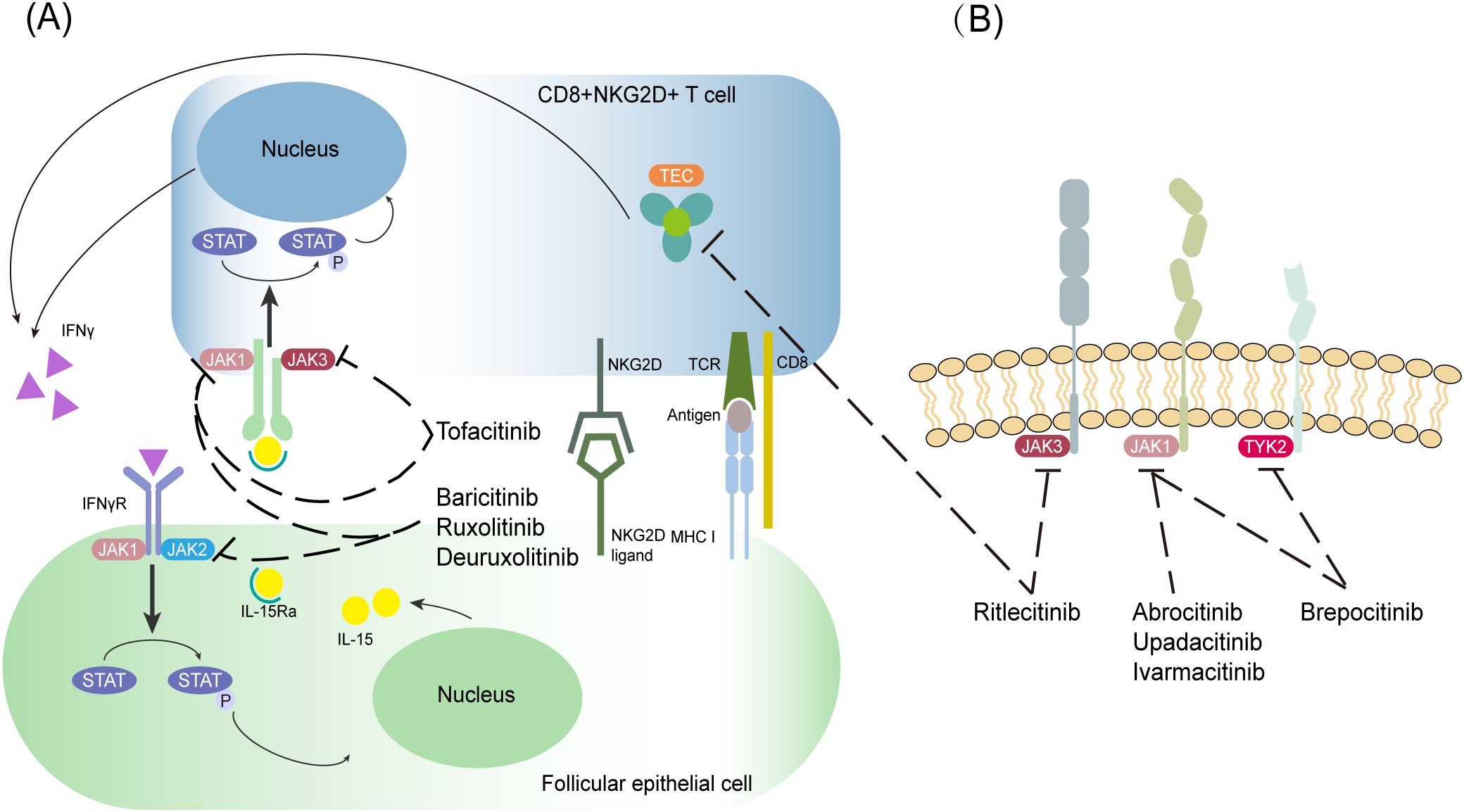
Figure 1. Schematic diagram of the mechanism of JAK inhibitors for the treatment of AA. (A, B) JAK-STAT-dependent immune pathway in AA. The targets of various JAK inhibitors are indicated in the figure, and the black dashed line with a blunt tip indicates inhibition.
Moreover, genetic factors (11), hypersensitivity (12–14), and gut microorganisms (15) are also strongly associated with the development of AA. Immune-related genes, such as MBL-associated serine protease 2, toll-like receptor 1, and chromosome 11 open reading frame 30, are significantly associated with susceptibility to AA. Alternatively, patients with atopic AA exhibiting elevated IgE and IL-4 levels and mast cells/eosinophils count in lesions may benefit from adjunctive allergy therapy to enhance standard AA treatment efficacy (13). Furthermore, gut microbiomes, including Parabacteroides distasonis and Clostridiales vadin BB60, are associated with alopecia universalis (16). AA cannot be cured immediately; its treatment mainly includes drug, physical, and surgical treatments, such as laser and microneedling, which are less effective for severe and special AA types. Since 2014, scientists have been using JAK inhibitors to treat AA, atopic dermatitis (AD), and other chronic inflammatory diseases, and they have shown promising efficacy (5). Targeted small molecule inhibitors are more precise, with fewer side effects than conventional immunological drugs. In this article, we reviewed the research progress on JAK inhibitors in AA treatment.
2 Research progress on JAK inhibitors in AA treatment
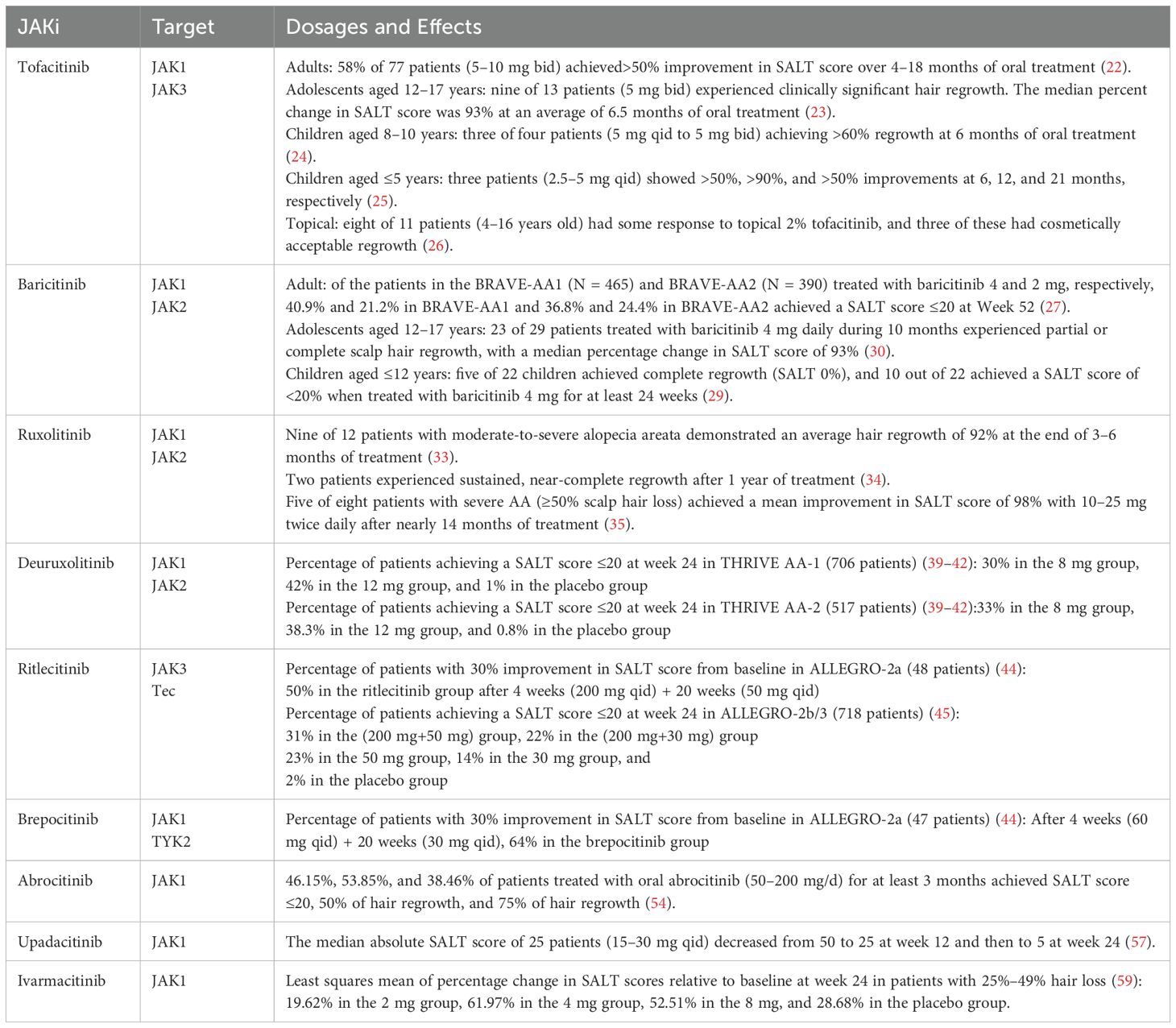
Currently, the first-generation JAK inhibitors, such as tofacitinib, baricitinib, and ruxolitinib, are mostly the non-selective types, with a wide inhibition but poor selectivity. Second-generation JAK inhibitors are more precise, with a narrow inhibition window but good selectivity: ritlecitinib and abrocitinib specifically inhibit JAK3 and JAK1, as shown in Table 1.
2.1 Tofacitinib
Tofacitinib selectively inhibits JAK1/JAK3 within the JAK-STAT signaling pathway, blocking IFN-γ receptor-mediated cytokine signaling and suppressing T-cell activation (17, 18). By disrupting the positive feedback loop between CD8+NKG2D+ T and follicular epithelial cells, it reduces IL-15 and IFN-γ secretion, thereby preserving hair follicle immune privilege.
A total of 80 patients treated with tofacitinib for >6 months had a remission rate of 33.8% after 24 weeks of treatment (19). In a retrospective study that included 35 patients with AA, 10 patients (32.3%) achieved almost complete or total scalp hair regrowth (20). The study population primarily involved treatment-refractory patients with advanced alopecia areata, including severe, totalis, and universalis subtypes, with tofacitinib demonstrating therapeutic efficacy for these challenging cases. Based on the retrospective data on 32 long-term Korean patients with moderate to severe AA, Park et al. found that 56.3% of the patients had a 50% improvement in the Severity of Alopecia Tool (SALT) scores from baseline with a daily dose of 10 mg tofacitinib. They found that the duration of tofacitinib (rather than the total dose) was associated with a favorable response (21). In addition, Liu et al. studied the effect of tofacitinib in the treatment of 90 patients with AA, alopecia totalis, or alopecia universalis, with the majority of patients relapsing within 2–3 months of stopping the medication, suggesting that long-term treatment may be required to keep the disease quiescent (22). Adult patients (5–10 mg bid) (22), adolescent patients (5 mg bid) (23), preadolescent children (5 mg qid to 5 mg bid) (24), and children aged ≤5 years (2.5–5 mg qid) (25) had a favorable systemic response to tofacitinib. Colleen et al. found that eight out of 11 pediatric patients responded to topical treatment with 2% tofacitinib, and hair regrowth was cosmetically effective in three of these cases (26).
Although tofacitinib has not been formally approved for AA, accumulating clinical trial evidence and therapeutic experience support its efficacy in patients with AA of different ages and refractory subtypes (1).
2.2 Baricitinib
Baricitinib, a reversible JAK1/JAK2 inhibitor, was approved by the US Food and Drug Administration in June 2022 and the Chinese National Drug Administration in March 2023 for the treatment of adults with severe AA.
In one Phase 2 trial and two Phase 3 trials (BRAVE AA-1 and BRAVE AA-2), baricitinib showed an improved efficacy, which continued for over 52 weeks. The two Phase 3 trials enrolled 654 and 546 patients treated with baricitinib 4 and 2 mg, respectively; 40.9% and 21.2% in the BRAVE AA-1 group and 36.8% and 24.4% in the BRAVE AA-2 group achieved a SALT score ≤20 at week 52 (27). Baricitinib is more likely to achieve ≥80% scalp hair coverage compared with placebo, and common adverse events included infection, headache, acne, and elevated blood creatine phosphokinase level (28). In addition, a retrospective longitudinal cohort study was conducted to evaluate the safety and efficacy of baricitinib in the treatment of patients with AA aged <12 years treated for at least 24 weeks, 19 of whom showed a mean reduction in SALT scores of 68% after treatment with a mean daily dose of 4 mg baricitinib (29). A total of 29 adolescent patients (aged 12–17 years) with moderate to severe AA were evaluated, and 23 patients (79%) achieved partial or complete hair regrowth after treatment with baricitinib for at least 3 months with an average final baricitinib dose of 4 mg daily (30). Baricitinib currently appears to have a more favorable risk–benefit ratio compared with available treatment options (particularly systemic immunosuppressants) for the treatment of AA (31, 32).
Clinical trial data indicate that baricitinib demonstrates efficacy and favorable tolerability across diverse age groups with moderate to severe AA. As a promising novel therapeutic agent, additional investigations are necessary to establish long-term safety profiles and efficacy outcomes within this patient population (27, 29–31).
2.3 Ruxolitinib and deuruxolitinib
Ruxolitinib specifically inhibits the JAK1/JAK2-related pathway, and although not formally approved for the treatment of AA, there have been a number of successful case studies and reports in recent years.
Ruxolitinib showed efficacy in nine out of 12 patients with moderately severe AA in the high-dose group (20 mg bid) (33), two patients with severe AA (one patient with chronic AA and one patient with acute episodes of AA) in the medium-dose group (30 mg qid) (34), and five out of eight patients with severe AA in the low-dose group (10–25 mg bid) (35). In a double-blind, controlled Phase 2 study, there was no statistically significant difference in the trend of SALT scores between the experimental and control groups after the topical application of ruxolitinib cream (36), suggesting that oral ruxolitinib is effective; however, topical application has no significant effect in patients with AA. To some extent, the relative systemic bioavailability of topical ruxolitinib was significantly lower than that of oral ruxolitinib, possibly due to insufficient penetration. Hair regrowth was not maintained after ruxolitinib was discontinued, and some patients even lost hair. Delaying the end of treatment and tapering the dose may be helpful in maintaining hair growth (34). In addition, the role of ruxolitinib in protecting hair follicles by acting on immune effectors and inhibiting the downstream effects of IFN-γ signaling has been demonstrated by an in vitro hair follicle culture model (37).
Recently, deuruxolitinib, a deuterated form of ruxolitinib, has been approved by the US Food and Drug Administration for the treatment of severe AA, following the approval of baricitinib and ritlecitinib for the treatment of AA. Deuterium-substituted drugs are made by replacing one or more carbon–hydrogen (C–H) bonds with carbon–deuterium (C–D) bonds at specific metabolic sites in the drug molecule. This substitution prolongs the half-life of the drug, reduces the production of toxic metabolites and drug–drug interactions, lowers the administered dose, improves safety, and results in improved efficacy. In a double-blind, placebo-controlled Phase 2 study of 149 patients, those treated with 8 mg or 12 mg deuruxolitinib experienced a significant reduction in the severity of AA. One of these patients experienced a serious adverse event of grade 3 cellulitis with sinus congestion, sinus infection, and influenza (38). To further evaluate the efficacy and safety of deuruxolitinib, two large studies involving 1,223 patients, THRIVE AA-1 and THRIVE AA-2, were conducted. In the THRIVE AA-1 study, the proportions of achieving a SALT score of ≤20 in the 8 mg group, 12 mg, and placebo groups were 30%, 42%, and 1%, respectively; in the THRIVE AA-2 study, these proportions were 33%, 38.3%, and 0.8%, respectively. Both doses of deuruxolitinib resulted in significant scalp hair regrowth from 8 weeks. They continued for 24 weeks and were generally well tolerated, with their overall safety profile in patients with AA suggesting their suitability for clinical use (39–42).
2.4 Ritlecitinib and brepocitinib
Ritlecitinib is an irreversible, selective JAK3/TEC kinase inhibitor. It selectively inhibits JAK3 by irreversibly covalently binding to the Cys residue at position 909 of the catalytic structural domain. In contrast, in other JAKs, the residue at this position is replaced by a serine. Members of the TEC kinase family have a Cys residue at the same position as JAK3 and are, therefore, also inhibited by ritlecitinib. In the cellular environment, ritlecitinib inhibits the cytolytic activity of natural killer and CD8+ T cells and the production of IFN-γ by inhibiting members of the TEC kinase family (43).
Ritlecitinib was approved by the US Food and Drug Administration in June 2023 and by the Chinese National Drug Administration in October 2023 for the treatment of severe AA in adolescents and adults aged ≥12 years. It is the second JAK inhibitor approved for the treatment of AA after baricitinib and the first approved for the treatment of adolescents with AA. Compared with other JAK inhibitors, ritlecitinib offers a novel mechanism of action with a rapid onset of action and a high safety profile. In the ALLEGRO-2a study, about half of the 48 patients with AA who had more than 50% scalp hair loss showed a 30% improvement in SALT scores from baseline after 24 weeks of oral ritlecitinib, demonstrating good efficacy and safety of ritlectinib (44). In the randomized, double-blind ALLEGRO-2b/3 study, ritlecitinib was well tolerated in 718 adult and adolescent patients with severe AA, with approximately 22–31% achieving a SALT score ≤20 at week 24 (45). Piliang et al. found that hair regrowth was maintained up to week 48 in patients with an initial response at week 24. Approximately one-third of patients who did not initially meet the efficacy target at week 24 achieved a response with continued ritlecitinib treatment (46). Blair et al. analyzed data from selected ALLEGRO-2b/3 and ALLEGRO-LT trials and concluded that ritlecitinib has clinically meaningful and sustained long-term efficacy in patients with AA (47). King et al. reviewed the ALLEGRO trial series and found that ritlecitinib was well tolerated in patients with AA (48). Common adverse reactions included headache, positive new coronavirus tests, nasopharyngitis, acne, and upper respiratory tract infections, and milder symptoms.
Brepocitinib (PF-06700841) selectively inhibits TYK2 and JAK1 channels. Although brepocitinib is not yet approved, studies have shown that it has a significant efficacy. King et al. found that 64% and 50% of patients treated with brepocitinib and ritlecitinib, respectively, had a 30% improvement in their SALT scores compared with baseline (44). Peeva et al. found in the phase 2a ALLEGRO-2a trial of brepocitinib and ritlecitinib that patients who had a poor response to ritlecitinib may benefit from treatment with brepocitinib (49). Guttman et al. found that at week 24, patients in both the brepocitinib and ritlecitinib arms had significant improvements in AA lesion areas that were close to or exceeded the level of non-lesion areas at baseline (50). At week 12, brepocitinib showed greater improvement in scalp hair than ritlecitinib; however, at week 24, ritlecitinib showed more significant improvement.
2.5 Abrocitinib and upadacitinib
As shown in Figure 2, abrocitinib showed greater selectivity for JAK1 than JAK2 (28-fold), JAK3 (>340-fold), and TYK2 (43-fold) in biochemical assays (51). One patient with both AD and AA experienced complete regrowth of scalp hair and significant improvement in AD after 1 year of abrocitinib treatment (52). Significant hair regrowth was reported after prolonged abrocitinib treatment in two patients with AD with severe AA (53). In a retrospective study of 13 patients with AA treated with oral abrocitinib (50–200 mg/d) for at least 3 months, 46.15%, 53.85%, and 38.46% of patients achieved SALT score ≤20, 50% of hair regrowth, and 75% of hair regrowth, respectively (54).

Figure 2. Comparison of JAK1 selectivity profile. Grouped bar graph showing the fold selectivity of JAK1 compared with other JAK subtypes (JAK2, JAK3, and TYK2) for abrocitinib, upadacitinib, and ivarmacitinib in biochemical assays. Numerical labels indicate specific fold differences, with “>40” and “>340” denoting values exceeding the upper detection limit. This figure demonstrates the high specificity of abrocitinib, upadacitinib, and ivarmacitinib for JAK1 inhibition.
Upadacitinib showed greater selectivity for JAK1 than JAK2 (>40-fold), JAK3 (130-fold), and TYK2 (190-fold) in cellular assays and was initially shown to be effective in the treatment of AD (55). Cantelli et al. reported that switching to upadacitinib in a patient with both AD and AA after failures of multiple therapies resulted in a significant improvement in both AD and AA (56). Flora et al. included 25 patients with AA between October 2021 and July 2022 (four patients had a history of AD) and found that the median absolute SALT score decreased from 50 to 25 at week 12 and then to 5 at week 24 (57).
These studies suggest that abrocitinib and upadacitinib may be effective treatment options for patients with both AD and AA.
2.6 Ivarmacitinib
Ivarmacitinib (formerly SHR0302), a highly selective JAK1 inhibitor, exhibits potency and selectivity for JAK1 that is over 10-fold for JAK2, 77-fold for JAK3, and 420-fold for TYK2 in cellular assays (58). Zhou et al. set up three doses of 2, 4, and 8 mg/day to test the appropriate dose in a phase 2 clinical trial (59). The results showed dose dependence at the 2 and 4 mg/day doses but not at the 8 mg/day dose. It was hypothesized that the drug may have reached its maximum therapeutic effect at the 4 mg/day dose. At week 24, the least square mean percentage change from baseline in SALT scores was 19.62%, 61.97%, 52.51%, and 28.68% in the 2, 4, 8 mg/day, and placebo groups, respectively, in patients with 25%–49% hair loss. The drug is currently in phase 3 clinical trials in China. Additionally, in a systematic review, baricitinib was likely the most effective therapy, followed by ritlecitinib and ivarmacitinib. However, both of the former showed a dose-dependent effect but not in ivarmacitinib (60).
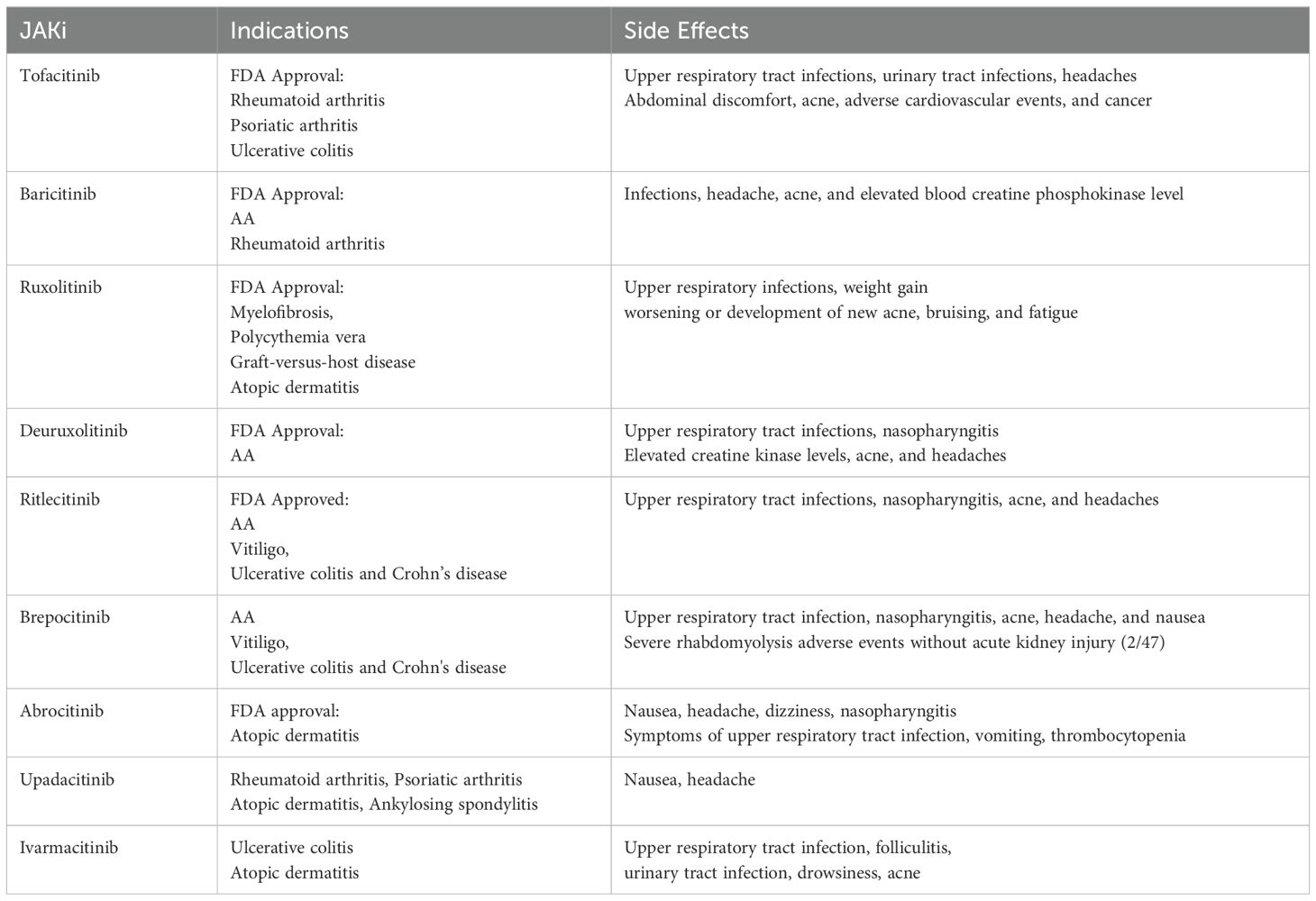
3 Side effects
Systemic JAK inhibitors are well tolerated, with most side effects being mild with a very low discontinuation rate of 1.6% compared with 2.2% in the control group (61). The most common side effect of JAK inhibitors is infection, especially viral (herpes and influenza), fungal, and mycobacterial infectious disorders (62). The most commonly reported serious adverse reactions include varicella-zoster, pneumonia, tuberculosis, sepsis, and non-melanoma skin cancer (63). These characteristics are shown in Table 2.
Most first-generation JAK inhibitors are pan-JAK inhibitors with more adverse events such as upper respiratory tract infections, urinary tract infections, headaches, cardiovascular events, and cancer (64). A study comparing tofacitinib with tumor necrosis factor inhibitors showed that tofacitinib was associated with a higher risk of adverse cardiovascular events and cancer in patients with rheumatoid arthritis (65). Patients on JAK inhibitors also often have elevated levels of low-density lipoprotein, a known risk factor for cardiovascular disease, which, in most cases, declines during treatment (63, 66).
Second-generation JAK inhibitors are more selective and have significantly fewer side effects than first-generation JAK inhibitors. The most common adverse events in patients treated with abrocitinib were nasopharyngitis, nausea, and acne (67). Flora et al. found that during the first 24 weeks of upadacitinib treatment, no one experienced significant laboratory abnormalities or infectious complications, and no participants discontinued treatment due to adverse events (57). In a randomized, placebo-controlled phase 2a study, ritlecitinib had the least number of adverse events (82,105, 124) and the lowest proportion of participants experiencing adverse events (67%,74%, and 77%) compared with placebo and brepocitinib, with nasopharyngitis and headache more common (44).
For serious adverse events, ruxolitinib, tofacitinib, and baricitinib were associated with infectious adverse events [IC025 1.7, especially viral infection (herpes and influenza)] and thrombosis (IC025 0.4), tofacitinib was associated with gastrointestinal perforation events (IC025 1.5). There is not a significant increase in the reporting of major cardiovascular events, where adverse events were considered significant if the lower end of the 95% credibility interval of the IC (IC025) was positive (62). However, isolated cases of adverse effects have been reported, including myocardial infarction with baricitinib, hypertensive urgency with tofacitinib, neutropenia with baricitinib, brepocitinib, and deuruxolitinib (61).
There is currently a lack of post-marketing surveillance data, and much attention needs to be paid to individual patient outcomes with JAK inhibitors.
4 Conclusion
Compared with the traditional treatments, the effect of JAK inhibitors on severe AA is promising. JAK inhibitors can bring significant improvement to patients who do not respond to corticosteroids or traditional immunosuppressants, patients whose disease has lasted for more than 10 years, and patients whose eyebrows and eyelashes are affected. There is a possibility that JAK inhibitors may be beneficial for patients with AA with other comorbidities such as psoriasis, vitiligo, and AD. In severe AA, oral JAK inhibitors have shown more significant effects, and topical medications that act like intralesional steroids are ideal for children and patients with localized disease because of their limited area of action and ability to minimize the side effects associated with systemic medications. However, there may be a high relapse rate with short-term use or in more severely affected patients; therefore, long-term maintenance therapy may be required to achieve sustained remission. In China, baricitinib and ritlecitinib have been approved for the treatment of AA. Overall, JAK inhibitors have significantly changed the challenging landscape for the treatment of moderate to severe AA, and their efficacy is now widely recognized. Now, more attention needs to be paid to the conditions of use, safety, and tailoring of JAK inhibitors to individual patient characteristics to ensure that they can deliver the best outcomes.
However, it should be noted that the conclusions are limited by the retrospective nature of some of the studies, the small number of patients, and the lack of a control group, and large-scale clinical trials are needed to further validate these effects. The promising use of various JAK inhibitors in the treatment of AA is currently being investigated intensively.
Author contributions
YS: Writing – original draft. QL: Funding acquisition, Writing – review & editing. YZ: Writing – review & editing. YL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Medical Science Research Program of Hebei Provincial Health Commission (No. 20240028) and Hebei Natural Science Foundation (No. H2022206328).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AA, alopecia areata; IFN-γ, interferon-gamma; JAK, Janus kinase; STAT, signal transducer activator of transcription; IL, interleukin; AD, atopic dermatitis; SALT, Severity of Alopecia Tool.
References
1. Zhou J, Liang L, Zhang H, Liu M, Zhu Z, Leng L, et al. Global burden of alopecia areata and associated diseases: a trend analysis from 1990 to 2021. J Cosmet Dermatol. (2025) 24:e70076. doi: 10.1111/jocd.70076
2. Rudnicka L, Arenbergerova M, Grimalt R, Ioannides D, Katoulis AC, Lazaridou E, et al. European expert consensus statement on the systemic treatment of alopecia areata. J Eur Acad Dermatol Venereol. (2024) 38:687–94. doi: 10.1111/jdv.19768
3. Mostaghimi A, Gao W, Ray M, Bartolome L, Wang T, Carley C, et al. Trends in prevalence and incidence of alopecia Areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. (2023) 159:411–8. doi: 10.1001/jamadermatol.2023.0002
4. Li Q, Wang Y, Guo Q, Cao J, Feng Y, Ke X. Nanostructured lipid carriers promote percutaneous absorption and hair follicle targeting of tofacitinib for treating alopecia areata. J Control Release. (2024) 372:778–94. doi: 10.1016/j.jconrel.2024.06.060
5. Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. (2014) 20:1043–9. doi: 10.1038/nm.3645
6. Gilhar A, Laufer-Britva R, Keren A, Paus R. Frontiers in alopecia areata pathobiology research. J Allergy Clin Immunol. (2019) 144:1478–89. doi: 10.1016/j.jaci.2019.08.035
7. Šutić Udović I, Hlača N, Massari LP, Brajac I, Kaštelan M, Vičić M. Deciphering the complex immunopathogenesis of alopecia areata. Int J Mol Sci. (2024) 25:5652. doi: 10.3390/ijms25115652
8. Rajabi F, Drake LA, Senna MM, Rezaei N. Alopecia areata: a review of disease pathogenesis. Br J Dermatol. (2018) 179:1033–48. doi: 10.1111/bjd.16808
9. Simakou T, Butcher JP, Reid S, Henriquez FL. Alopecia areata: a multifactorial autoimmune condition. J Autoimmun. (2019) 98:74–85. doi: 10.1016/j.jaut.2018.12.001
10. Freitas E, Guttman-Yassky E, Torres T. Baricitinib for the treatment of alopecia areata. Drugs. (2023) 83:761–70. doi: 10.1007/s40265-023-01873-w
11. Al-Eitan LN, Alghamdi MA, Al Momani RO, Aljamal HA, Abdalla AM, Mohammed HM. Genetic predisposition of alopecia areata in Jordanians: a case-control study. Heliyon. (2022) 8:e09184. doi: 10.1016/j.heliyon.2022.e09184
12. Ito T, Kageyama R, Nakazawa S, Honda T. Understanding the significance of cytokines and chemokines in the pathogenesis of alopecia areata. Exp Dermatol. (2020) 29:726–32. doi: 10.1111/exd.14129
13. Zhang X, McElwee KJ. Allergy promotes alopecia areata in a subset of patients. Exp Dermatol. (2019) 29:239–42. doi: 10.1111/exd.14027
14. Carnicle JM, Hendricks AJ, Shi VY. Reactivation of alopecia areata after dupilumab therapy for atopic dermatitis. Dermatitis. (2021) 32:e80–e2. doi: 10.1097/DER.0000000000000512
15. Liu Z, Liu X. Gut microbiome, metabolome, and alopecia areata. Front Microbiol. (2023) 14:1281660. doi: 10.3389/fmicb.2023.1281660
16. Moreno-Arrones OM, Serrano-Villar S, Perez-Brocal V, Saceda-Corralo D, Morales-Raya C, Rodrigues-Barata R, et al. Analysis of the gut microbiota in alopecia areata: identification of bacterial biomarkers. J Eur Acad Dermatol Venereol. (2019) 34:400–5. doi: 10.1111/jdv.15885
17. Dai Z, Chen J, Chang Y, Christiano AM. Selective inhibition of JAK3 signaling is sufficient to reverse alopecia areata. JCI Insight. (2021) 6:e142205. doi: 10.1172/jci.insight.142205
18. Palmroth M, Kuuliala K, Peltomaa R, Virtanen A, Kuuliala A, Kurttila A, et al. Tofacitinib suppresses several JAK-STAT pathways in rheumatoid arthritis in vivo and baseline signaling profile associated with treatment response. Front Immunol. (2021) 12:738481. doi: 10.3389/fimmu.2021.738481
19. Huang J, Deng S, Li J, Tang Y, Liu F, Liu Y, et al. Drug survival and long-term outcome of tofacitinib in patients with alopecia areata: a retrospective study. Acta Derm Venereol. (2023) 103:adv13475. doi: 10.2340/actadv.v103.13475
20. Benton S, Farah R, Freese R, Hordinsky M. Tofacitinib as a pragmatic treatment choice for alopecia areata: A retrospective review. Dermatol Ther. (2022) 35:e15310. doi: 10.1111/dth.15310
21. Park HS, Kim MW, Lee JS, Yoon H-S, Huh C-H, Kwon O, et al. Oral tofacitinib monotherapy in Korean patients with refractory moderate-to-severe alopecia areata: a case series. J Am Acad Dermatol. (2017) 77:978–80. doi: 10.1016/j.jaad.2017.06.027
22. Liu LY, Craiglow BG, Dai F, King BA. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. (2017) 76:22–8. doi: 10.1016/j.jaad.2016.09.007
23. Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. (2017) 76:29–32. doi: 10.1016/j.jaad.2016.09.006
24. Craiglow BG, King BA. Tofacitinib for the treatment of alopecia areata in preadolescent children. J Am Acad Dermatol. (2019) 80:568–70. doi: 10.1016/j.jaad.2018.08.041
25. Dai YX, Chen CC. Tofacitinib therapy for children with severe alopecia areata. J Am Acad Dermatol. (2019) 80:1164–6. doi: 10.1016/j.jaad.2018.12.041
26. Putterman E, Castelo-Soccio L. Topical 2% tofacitinib for children with alopecia areata, alopecia totalis, and alopecia universalis. J Am Acad Dermatol. (2022) 87:1207–1209.e1. doi: 10.1016/j.jaad.2018.02.031
27. Kwon O, Senna MM, Sinclair R, Ito T, Dutronc Y, Lin CY, et al. Efficacy and safety of baricitinib in patients with severe alopecia areata over 52 weeks of continuous therapy in two phase III trials (BRAVE-AA1 and BRAVE-AA2). Am J Clin Dermatol. (2023) 24:443–51. doi: 10.1007/s40257-023-00764-w
28. Fung S, Shirley M. Baricitinib: a review in severe alopecia areata. Am J Clin Dermatol. (2023) 24:661–8. doi: 10.1007/s40257-023-00799-z
29. Asfour L, Bokhari L, Bhoyrul B, Eisman S, Moussa A, Rees H, et al. Treatment of moderate-to-severe alopecia areata in pre-adolescent children with baricitinib. Br J Dermatol. (2023) 189:248–50. doi: 10.1093/bjd/ljad118
30. Moussa A, Eisman S, Kazmi A, Poa J, Chitreddy V, Rathnayake D, et al. Treatment of moderate-to-severe alopecia areata in adolescents with baricitinib: a retrospective review of 29 patients. J Am Acad Dermatol. (2023) 88:1194–6. doi: 10.1016/j.jaad.2022.12.033
31. King B, Mostaghimi A, Shimomura Y, Zlotogorski A, Choi G-S, Blume-Peytavi U, et al. Integrated safety analysis of baricitinib in adults with severe alopecia areata from two randomized clinical trials. Br J Dermatol. (2023) 188:218–27. doi: 10.1093/bjd/ljac059
32. Gupta AK, Wang T, Polla Ravi S, Bamimore MA, Piguet V, Tosti A. Systematic review of newer agents for the management of alopecia areata in adults: Janus kinase inhibitors, biologics and phosphodiesterase-4 inhibitors. J Eur Acad Dermatol Venereol. (2022) 37:666–79. doi: 10.1111/jdv.18810
33. Mackay-Wiggan J, Jabbari A, Nguyen N, Cerise JE, Clark C, Ulerio G, et al. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight. (2016) 1:e89790. doi: 10.1172/jci.insight.89790
34. Vandiver A, Girardi N, Alhariri J, Garza LA. Two cases of alopecia areata treated with ruxolitinib: a discussion of ideal dosing and laboratory monitoring. Int J Dermatol. (2017) 56:833–5. doi: 10.1111/ijd.13598
35. Liu LY, King BA. Ruxolitinib for the treatment of severe alopecia areata. J Am Acad Dermatol. (2019) 80:566–8. doi: 10.1016/j.jaad.2018.08.040
36. Olsen EA, Kornacki D, Sun K, Hordinsky MK. Ruxolitinib cream for the treatment of patients with alopecia areata: A 2-part, double-blind, randomized, vehicle-controlled phase 2 study. J Am Acad Dermatol. (2020) 82:412–9. doi: 10.1016/j.jaad.2019.10.016
37. Kim JE, Lee YJ, Park HR, Lee DG, Jeong KH, Kang H. The effect of JAK inhibitor on the survival, anagen re-entry, and hair follicle immune privilege restoration in human dermal papilla cells. Int J Mol Sci. (2020) 21:5137. doi: 10.3390/ijms21145137
38. King B, Mesinkovska N, Mirmirani P, Bruce S, Kempers S, Guttman-Yassky E, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus Kinase inhibitor, in moderate-to-severe alopecia areata. J Am Acad Dermatol. (2022) 87:306–13. doi: 10.1016/j.jaad.2022.03.045
39. King B, Senna MM, Mesinkovska NA, Lynde C, Zirwas M, Maari C, et al. Efficacy and safety of deuruxolitinib, an oral selective Janus kinase inhibitor, in adults with alopecia areata: Results from the Phase 3 randomized, controlled trial (THRIVE-AA1). J Am Acad Dermatol. (2024) 91:880–8. doi: 10.1016/j.jaad.2024.06.097
40. King B, Mesinkovska NA, Mostaghimi A, Hamilton C, Cassella J. 54022 pooled safety assessments from the multinational phase 3 THRIVE-AA1 and THRIVE-AA2 trials of deuruxolitinib in adult patients with moderate to severe alopecia areata. J Am Acad Dermatol. (2024) 91. doi: 10.1016/j.jaad.2024.07.1117
41. Senna MM, King B, Mesinkovska NA, Mostaghimi A, Hamilton C, Cassella J. 51840 efficacy of the oral JAK1/JAK2 inhibitor deuruxolitinib in adult patients with moderate to severe alopecia areata: pooled results from the multinational double-blind, placebo-controlled THRIVE-AA1 and THRIVE-AA2 phase 3 trials. J Am Acad Dermatol. (2024) 91. doi: 10.1016/j.jaad.2024.07.161
42. Mesinkovska NA, King B, Mostaghimi A, Hamilton C, Cassella J. 54029 pooled patient-reported outcomes from the phase 3 THRIVE-AA1 and THRIVE-AA2 trials of deuruxolitinib in adult patients with moderate to severe alopecia areata. J Am Acad Dermatol. (2024) 91. doi: 10.1016/j.jaad.2024.07.289
43. Xu H, Jesson MI, Seneviratne UI, Lin TH, Sharif MN, Xue L, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. (2019) 14:1235–42. doi: 10.1021/acschembio.9b00188
44. King B, Guttman-Yassky E, Peeva E, Banerjee A, Sinclair R, Pavel AB, et al. A phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral Janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24-week results. J Am Acad Dermatol. (2021) 85:379–87. doi: 10.1016/j.jaad.2021.03.050
45. King B, Zhang X, Harcha WG, Szepietowski JC, Shapiro J, Lynde C, et al. Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: a randomised, double-blind, multicentre, phase 2b–3 trial. Lancet. (2023) 401:1518–29. doi: 10.1016/S0140-6736(23)00222-2
46. Piliang M, Lynde C, King B, Mirmirani P, Sinclair R, Senna M, et al. Sustained hair regrowth with continued ritlecitinib treatment through week 48 in patients with alopecia areata with or without early target responses: post hoc analysis of the ALLEGRO phase 2b/3 trial. J Am Acad Dermatol. (2025) 92:276–84. doi: 10.1016/j.jaad.2024.09.064
47. Blair HA. Ritlecitinib: first approval. Drugs. (2023) 83:1315–21. doi: 10.1007/s40265-023-01928-y
48. King B, Soung J, Tziotzios C, Rudnicka L, Joly P, Gooderham M, et al. Integrated safety analysis of ritlecitinib for the treatment of alopecia areata (AA) from the phase 2 and phase 3 ALLEGRO clinical trial program. SKIN: J Cutan Med. (2023) 7:s291–1. doi: 10.25251/skin.7.supp.291
49. Peeva E, Guttman-Yassky E, Banerjee A, Sinclair R, Cox LA, Zhu L, et al. Maintenance, withdrawal, and re-treatment with ritlecitinib and brepocitinib in patients with alopecia areata in a single-blind extension of a phase 2a randomized clinical trial. J Am Acad Dermatol. (2022) 87:390–3. doi: 10.1016/j.jaad.2021.12.008
50. Guttman-Yassky E, Pavel AB, Diaz A, Zhang N, Del Duca E, Estrada Y, et al. Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers. J Allergy Clin Immunol. (2022) 149:1318–28. doi: 10.1016/j.jaci.2021.10.036
51. Deeks ED, Duggan S. Abrocitinib: first approval. Drugs. (2021) 81:2149–57. doi: 10.1007/s40265-021-01638-3
52. Zhao J, Liu L. A case of atopic dermatitis with alopecia universalis in a patient treated with abrocitinib. JAAD Case Rep. (2022) 22:99–100. doi: 10.1016/j.jdcr.2022.02.027
53. Bennett M, Moussa A, Sinclair R. Successful treatment of chronic severe alopecia areata with abrocitinib. Australas J Dermatol. (2022) 63:274–6. doi: 10.1111/ajd.13836
54. Zhang Y, Wang F, Li X, Zhao Y, Zhang J, Zhao W, et al. Oral abrocitinib for treatment of refractory alopecia areata: A retrospective study. J Am Acad Dermatol. (2025) 92:153–5. doi: 10.1016/j.jaad.2024.09.025
55. Mohamed MF, Bhatnagar S, Parmentier JM, Nakasato P, Wung P. Upadacitinib: mechanism of action, clinical, and translational science. Clin Transl Sci. (2024) 17:e13688. doi: 10.1111/cts.13688
56. Cantelli M, Martora F, Patruno C, Nappa P, Fabbrocini G, Napolitano M. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: a case report. Dermatol Ther. (2022) 35:e15346. doi: 10.1111/dth.15346
57. Flora A, Kozera E, Frew JW. Treatment of alopecia areata with the janus kinase inhibitor upadacitinib: a retrospective cohort study. J Am Acad Dermatol. (2023) 89:137–8. doi: 10.1016/j.jaad.2022.12.056
58. Liu J, Jiang Y, Zhang S, Liu S, Su J, Lin C, et al. Ivarmacitinib, a selective Janus kinase 1 inhibitor, in patients with moderate-to-severe active rheumatoid arthritis and inadequate response to conventional synthetic DMARDs: results from a phase III randomised clinical trial. Ann Rheum Dis. (2025) 84:188–200. doi: 10.1136/ard-2024-226385
59. Zhou C, Yang X, Yang B, Yan G, Dong X, Ding Y, et al. A randomized, double-blind, placebo-controlled phase II study to evaluate the efficacy and safety of ivarmacitinib (SHR0302) in adult patients with moderate-to-severe alopecia areata. J Am Acad Dermatol. (2023) 89:911–9. doi: 10.1016/j.jaad.2023.02.063
60. Husein-ElAhmed H, Husein-ElAhmed S. Comparative efficacy of oral Janus kinase inhibitors and biologics in adult alopecia areata: a systematic review and Bayesian network meta-analysis. J Eur Acad Dermatol Venereol. (2024) 38:835–43. doi: 10.1111/jdv.19797
61. Sechi A, Song J, Dell’Antonia M, Heidemeyer K, Piraccini BM, Starace M, et al. Adverse events in patients treated with Jak-inhibitors for alopecia areata: a systematic review. J Eur Acad Dermatol Venereol. (2023) 37:1535–46. doi: 10.1111/jdv.19090
62. Hoisnard L, Lebrun-Vignes B, Maury S, Mahevas M, El Karoui K, Roy L, et al. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci Rep. (2022) 12:7140. doi: 10.1038/s41598-022-10777-w
63. Gilhar A, Keren A, Paus R. JAK inhibitors and alopecia areata. Lancet. (2019) 393:318–9. doi: 10.1016/S0140-6736(18)32987-8
64. Zhou C, Li X, Wang C, Zhang J. Alopecia areata: an update on etiopathogenesis, diagnosis, and management. Clin Rev Allergy Immunol. (2021) 61:403–23. doi: 10.1007/s12016-021-08883-0
65. Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. (2022) 386:316–26. doi: 10.1056/NEJMoa2109927
66. Reiner Ž. Hypertriglyceridaemia and risk of coronary artery disease. Nat Rev Cardiol. (2017) 14:401–11. doi: 10.1038/nrcardio.2017.31
Keywords: alopecia areata, Janus kinases, baricitinib, ritlecitinib, deuruxolitinib
Citation: Sun Y, Li Q, Zhang Y and Liu Y (2025) Janus kinase inhibitors for alopecia areata: a review of clinical data. Front. Immunol. 16:1577115. doi: 10.3389/fimmu.2025.1577115
Received: 17 February 2025; Accepted: 10 April 2025;
Published: 13 May 2025.
Edited by:
Maria Giovanna Danieli, Università Politecnica delle Marche, ItalyReviewed by:
Virender Kumar, Pandit Bhagwat Dayal Sharma University of Health Sciences, IndiaEmanuela Martina, Marche University Hospital, Italy
Copyright © 2025 Sun, Li, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanli Zhang, Mzg0MDA3NDBAaGVibXUuZWR1LmNu; Yaling Liu, eXpsaW5nX2xpdTIwMjNAaGVibXUuZWR1LmNu
 Yutong Sun
Yutong Sun Qian Li
Qian Li