- 1Hepatopancreatobiliary Center, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua Medicine, Tsinghua University, Beijing, China
- 2Department of Pathology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua Medicine, Tsinghua University, Beijing, China
- 3Department of General Surgery, Lhasa People’s Hospital, Lhasa, China
Adenosquamous cell carcinoma (ASC) is a rare and aggressive malignant tumor which consists of both adenocarcinoma (AC) and squamous cell carcinoma (SCC) component types. Although ASC can sometimes develop in the stomach, pancreas, gallbladder and thyroid, it rarely occurs in the liver. As such, primary ASC of the liver remains a poorly understood malignancy due to both the paucity of reported cases and scarcity of available published data. As such, while the use of immune checkpoint inhibitors (ICIs), including PD-1 and PD-L1 antagonists, has profoundly changed the treatment paradigm and outcomes in most tumors, there is virtually no previous documentation for the application of ICIs in the treatment of primary hepatic adenosquamous carcinoma. Herein, we report a clinical case of a 54-year-old woman with metachronous double primary tumors, one of which was dMMR ASC of the liver and received 8 cycles of single-agent immunotherapy using sintilimab. The post-treatment response was evaluated as a pathological complete response (pCR).
1 Introduction
Adenosquamous cell carcinoma (ASC) of the liver is a rare variant of cholangiocarcinoma and an even less commonly encountered malignancy (1, 2). Owing to the paucity of ASC of the liver, the diagnosis and treatment of this rare tumor remains elusive, the treatment response using immunotherapy has previously never been documented in the literature (3). Here, we report a case of a 54-year-old female whose diagnosis of ASC of the liver was confirmed through biopsy pathology and whose immunohistochemistry reveals a mismatch repair deficiency (dMMR). After receiving 8 cycles of single-agent immunotherapy, the patient underwent laparoscopic partial hepatectomy, the postoperative pathology of which indicated a pathological complete response (pCR).
2 Case report
The patient is a 54-year-old female who was admitted to the hospital due to the presence of a liver mass that had been discovered six months prior. In reviewing the patient family history, it was found that the patient’s father had passed away due to colon cancer. In addition, the patient was previously diagnosed with atypical endometrial hyperplasia in 2021, due to having an increased menstrual flow which led to receiving a curettage biopsy at another hospital. On August 8th 2021, the patient underwent a radical hysterectomy for uterine cancer, which included a complete removal of the uterus and its accessory organs. Pathological findings confirmed a moderately differentiated endometrioid carcinoma of the uterus, FIGO Grade II. Immunohistochemistry (IHC) showed negative results for MLH1 and PMS2, and positive results for MSH2 and MSH6, indicating a mismatch repair deficiency (dMMR) in the tumor tissue. The patient was then discharged after recovery and has since underwent regular follow-up exams post-surgery.
During a routine follow-up in January 2023, elevated CA19–9 levels (276.9 U/ml) along with abdominal distension was noted. An abdominal CT scan (as shown in Figure 1) revealed an irregular low-density lesion in the left liver, measuring approximately 47mm×33mm×27mm with multiple satellite lesions, and lymph node metastasis in the hepatogastric ligament which could not be ruled out (Figures 1A, C, E). The outpatient multidisciplinary team (MDT), including experts from oncology, hepatobiliary and pancreatic surgery, and pathology, recommended a liver biopsy be performed so as to clarify the origin of the liver mass along with additional immunohistochemical testing to determine its MMR status.
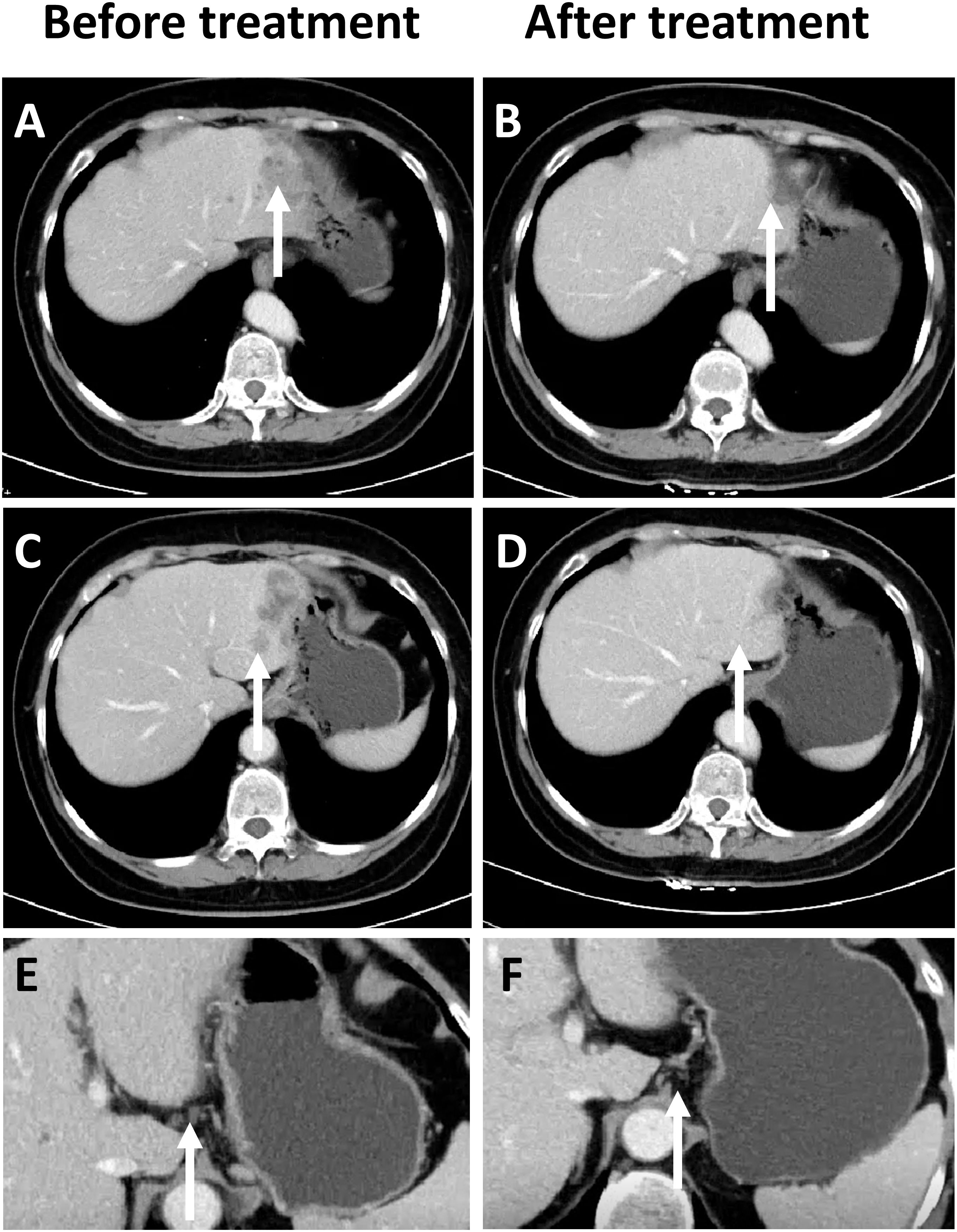
Figure 1. Comparison of CT Imaging changes, prior to and following immunotherapy in patients. (A) Prior to treatment, a low-density mass can be observed in the left lobe of the liver at the site indicated by the arrow (white). (B) After treatment, tumor regression can be observed in the left lobe of the liver. (C) Prior to treatment, satellite lesions can be observed at the edge of the main tumor in the left lobe of the liver. (D) After treatment, the satellite lesions have completely regressed. (E) Enlarged lymph nodes can be observed in the hepatogastric ligament. (F) The size of the previously enlarged lymph nodes are completely reduced.
On March 15th 2023, an ultrasound-guided liver mass biopsy was performed, and the pathology confirmed a diagnosis of ASC of the liver. Immunohistochemistry results showed: P40 (+), CK19 (+), CK7 (+), Ki67 (40%), P53(mutant expression), MLH1 (+), MSH2 (-), MSH6 (-), PMS2 (+) (Figures 2A–E). Given the patient’s family history of colorectal cancer, the presence of two metachronous primary tumors (endometrial carcinoma and hepatic ASC), and immunohistochemical evidence of mismatch repair deficiency in both tumors, the multidisciplinary team (MDT) made a clinical inference of Lynch syndrome. However, due to financial reasons, the patient refused further genetic testing. Given the presence of multifocal lesions and enlarged lymph nodes indicating a high risk of recurrence following surgery, the dMMR status, and likelihood of Lynch Syndrome, the MDT experts concurred that immunotherapy would be the patient’s best treatment choice. The recommended treatment plan included 200 mg of sintilimab every 3 weeks intravenously and 8 mg of lenvatinib every day orally. Sintilimab is an engineered PD-1 inhibitor which has shown greater PD-1 binding affinity in vitro than either nivolumab and pembrolizumab, and superior PD-1 occupancy and antitumor effects in humanized mouse models (4). Furthermore, it has exhibited efficacy in both adenocarcinoma and squamous cell carcinoma pathological types and therapeutic potential in a wide range of malignant liver and biliary tract tumors (5, 6). Treatment using Sintilimab was ultimately chosen based on several factors such as its relatively lower cost compared to other ICIs and its demonstrated efficacy in cases of biliary tract malignancies (7, 8). However, due to gastric discomfort in the first week of neoadjuvant therapy, oral lenvatinib use was discontinued. Ultimately, the patient only received 8 cycles of single-agent sintilimab neoadjuvant therapy prior to surgery. Post-treatment, serum CA19–9 levels decreased from 276.9 U/ml to 26.34 U/ml (normal range). As shown in Figures 1A, C, a low-density shadow and satellite lesions can be observed in the left lateral lobe of the liver prior to treatment. Both the tumor lesions and satellite lesions had almost completely disappeared after the neoadjuvant therapy (Figures 1B, D). Even the previously suspected metastatic lymph nodes in the hepatogastric ligament showed significant shrinkage in comparison to prior (Figures 1E, F). Therefore, the patient underwent laparoscopic partial liver resection and regional lymph node dissection. As shown in Figure 2F, the postoperative pathology results revealed diffuse and complete necrosis of the neoplasm, indicating a pathological complete response (pCR) to treatment. A 16-month follow up found no tumor recurrence. The patient’s clinical workup, including medical history, diagnostic and therapeutic procedures, and follow-up, is illustrated in Supplementary Figure 1.
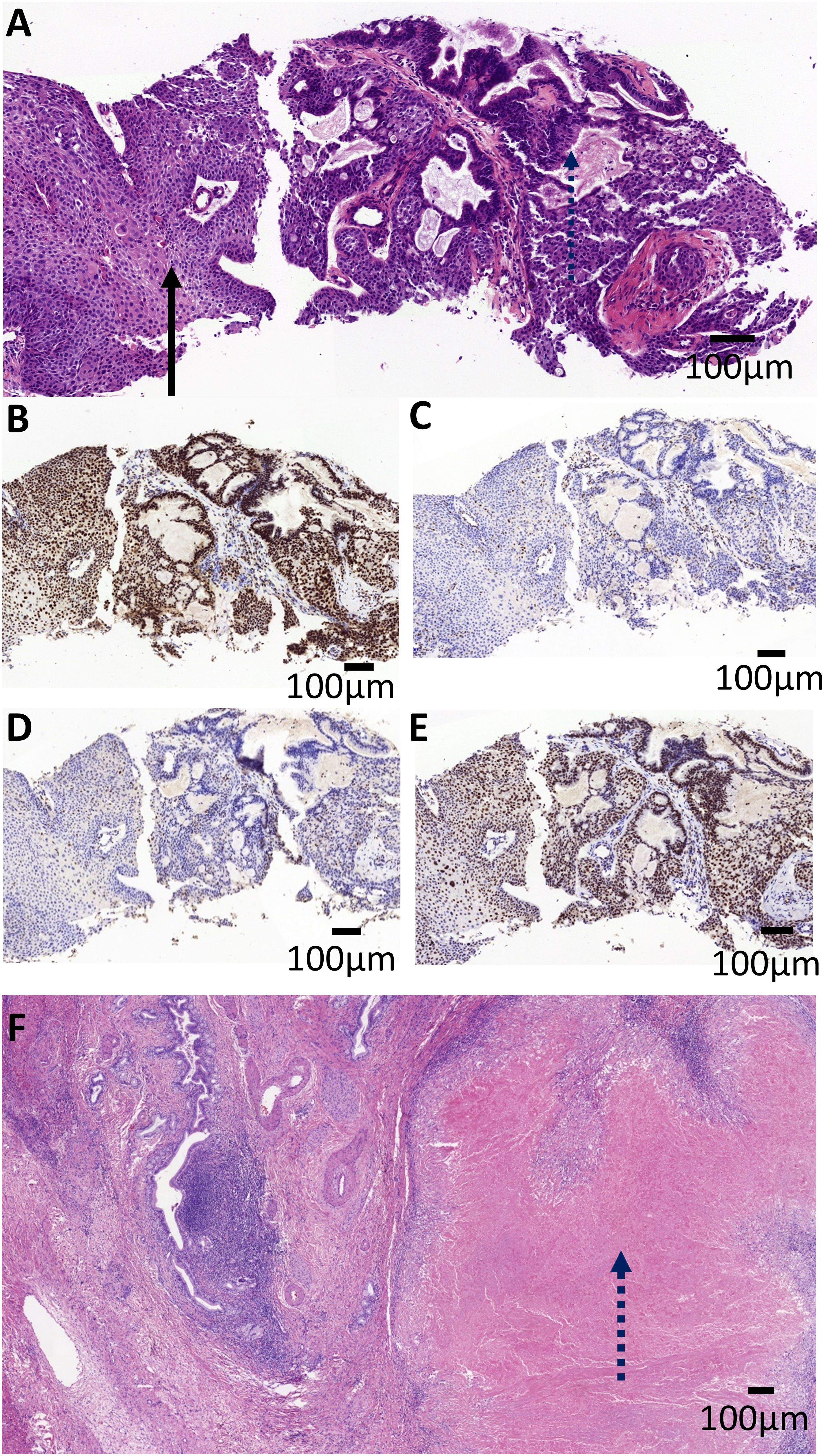
Figure 2. Pathological imaging of the patient tumor. (A) The pre-operative biopsy revealed the tumor was composed of adenocarcinoma with tubular pattern (the dashed arrow) and squamous cell carcinoma with nest architecture (the solid arrow). The tumor cells were positive for MLH1 (B) and PMS2 (E), and negative for MLH2 (C) and MSH6 (D). (F) Microscopic examination of the post-operative specimens revealed diffuse and complete necrosis of the neoplasm (the dashed arrow), indicating a pathological complete response (pCR). Scale bar, 100 mm.
3 Discussion
The current consensus dictates that ASC of the liver is a rare variant of intrahepatic cholangiocarcinoma (9). It was reported by the National Cancer Center Hospital in Japan that from 2016 to 2017, the incidence of rare intrahepatic bile duct tumors was 0.736%. ASC of the liver accounted for 4.96% (30/605) of rare intrahepatic bile duct tumors (10). Likewise, according to the SEER (Surveillance Epidemiology and End Results) database, the average annual incidence of ASC in the United States was only 1.77/100,000 from 1973 to 2015. Among these, the total cases of ASC of liver was 18 or 0.6% of all cholangiocarcinoma cases, thus confirming the rarity of primary ASC of liver occurrence on a multinational scale (11).
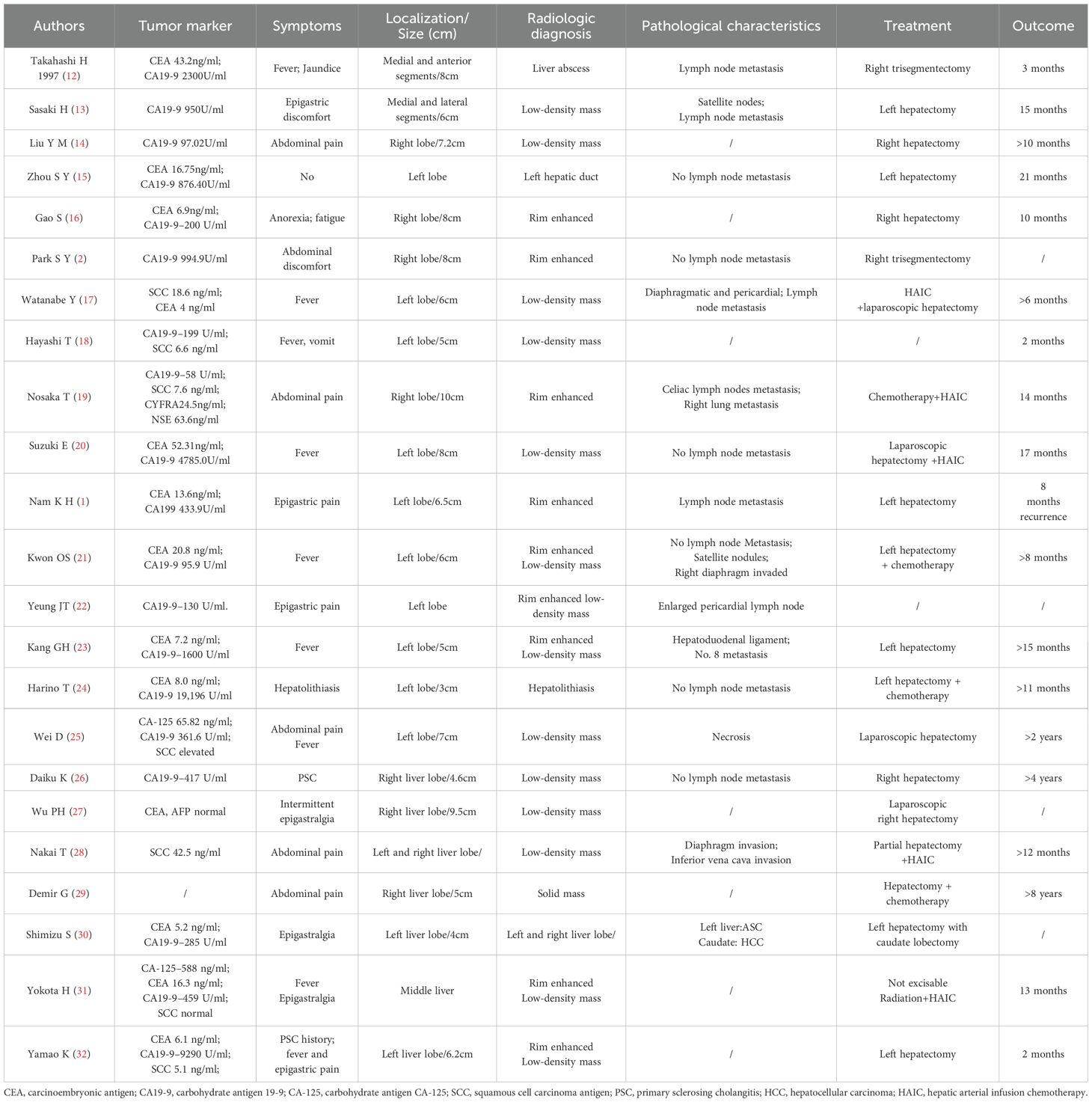
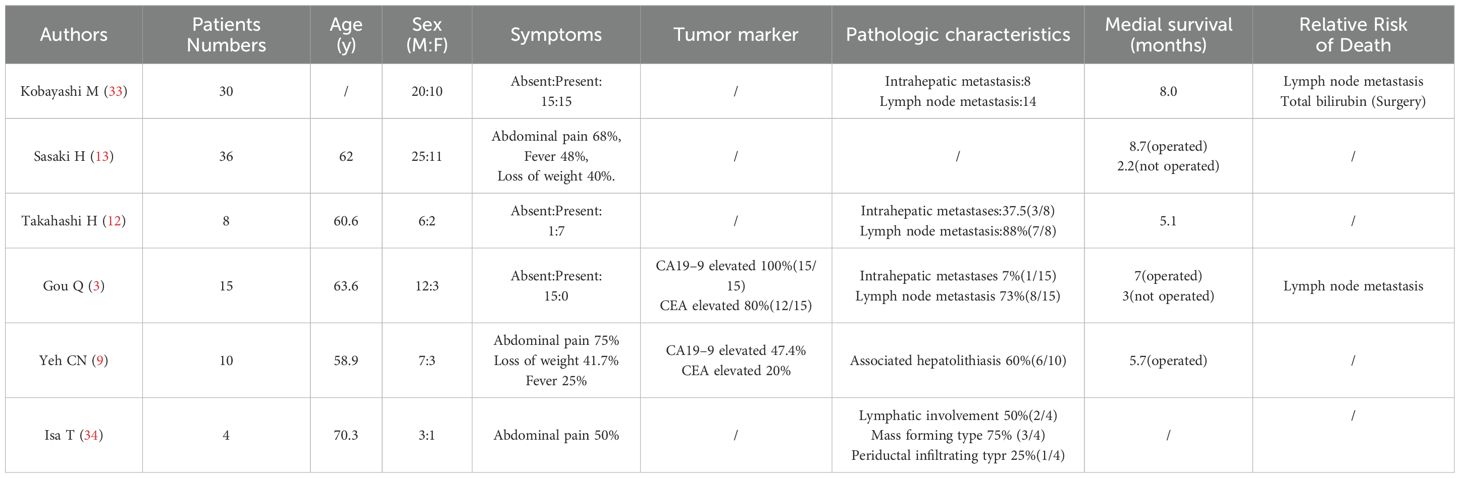
While most published reports have been presented as either singular case reports or a series of small scale studies, we have provided detailed tables consisting of comprehensive patient information obtained from case reports conducted in the last 30 years (Table 1) and which also summarize the results of a series of small case studies (Table 2).
Clinicopathological characteristics: Based on the summary of the above literature, clinicopathological information profiling of patients with ASC of the liver reveals a higher incidence in males and that the most commonly presented symptoms are upper abdominal discomfort, fever, and other (3, 9, 12, 13, 33, 34). However, it is difficult to distinguish ASC of the liver from other primary liver tumors like hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma (ICC) solely through analysis of clinical symptoms, laboratory tests, and imaging examinations. Similarly, due to the limited sample size, the reported elevated levels of CA19–9 and CEA in ASC of the liver patients tend to vary across different studies. In spite of this, it was found that the elevation rate of CA19–9 in ASC of the liver patients to be relatively high, based on series of small case studies by various researchers such as Gou Q (3), and Yeh CN (9). Although this seems promising, CA19–9 alone cannot distinguish ASC of the liver from ICC. A few other reports have indicated elevated SCC antigen levels in patients with ASC of the liver (18, 19, 28, 32). SCC antigen can be theoretically used as a potential marker to distinguish ASC of liver from HCC/ICC. However, whether or not low detection rate of SCC antigen is due to its inherently low levels in ASC patients or is simply due to insufficient testing remains inconclusive. In other words, it is highly recommended that SCC antigens are simultaneously tested to further support differential diagnosis.
Pathogenesis and treatment strategies: A few studies have reported that ASC of the liver can be associated with conditions such as primary sclerosing cholangitis (PSC) (26), and hepatolithiasis (24). As shown in Table 1, ASC of the liver typically has a tumor diameter which exceeds 5 cm and imaging results including rim-enhanced, low-density masses sometimes accompanied by satellite lesions (13, 21), regional (12, 13, 17) and distant (17, 22, 28) lymph node metastasis, and invasion of adjacent organs or distant metastases (19, 21, 28). Since ASC of the liver is a rare condition, there is a limited understanding of its pathogenesis and optimal treatment strategies. The prognosis of ASC of the liver is commonly known to be poor and surgery is the preferred treatment. Sasaki et al. (13) reported a median survival of 8.7 months for patients who underwent surgery and 2.2 months for those who did not. The basic surgical principles for ASC should be similar to the treatment algorithm for ICC given their similar rapid proliferation and high invasiveness. The best treatment strategy for most ASC patients should be established by a multidisciplinary team. Similarly to ICC, the first step is assessment of tumor resectability, typically evaluated using CT and/or MRI with MRCP. PET and/or EUS-guided fine-needle aspiration/biopsy is often necessary to confirm or exclude metastasis, given the high incidence of lymph node metastases (35). Surgical resection is preferred specifically when there is only one tumor and no regional lymph node metastases are present. In addition, regional lymphadenectomy should be a standard procedure during liver resection (36).
Similarly to ICC, if regional LNM or multiple tumors are present in ASC patients, the choice between resection and drug therapy should depend on the extent of metastasis and the number of tumors. In spite of this, systemic and local treatment effects for ASC of the liver remain uncertain. Nosaka T (19). reported a case of ASC of the liver with distant metastasis that initially responded to gemcitabine-cisplatin therapy, but tumor marker levels rebounded once again after only six months. Hepatic arterial infusion chemotherapy (HAIC) with cisplatin (CDDP) and 5-fluorouracil (5-FU) was then administered, leading to tumor shrinkage. The patient ultimately died 14 months following initial treatment. Demir G (29) reported an ASC patient who underwent segmentectomy followed by cisplatin-based chemotherapy and survived for more than eight years. Suzuki E (20) described another ASC patient who experienced rapid postoperative recurrence with multiple metastases within three months. This patient’s disease was controlled with cisplatin and 5-fluorouracil and passed away 14 months after surgery. Reports by Nakai T (28), Yokota H (31) also demonstrated that HAIC could effectively control tumor progression and improve patients’ quality of life. Based on these case reports, which provide detailed descriptions of treatment strategies and outcomes, chemotherapy or HAIC are also potentially effective treatment options for ASC of the liver. However, past treatment strategies for ASC of the liver have predominantly involved systemic chemotherapy or local therapies such as HAIC and radiotherapy, while the efficacy of targeted therapy and immunotherapy has been rarely reported in the literature. Sintilimab, a type of ICIs, has demonstrated significant therapeutic potential in the management of malignant hepatobiliary tumors (37–40). The therapeutic effect of sintilimab in this case reveals the potential of ICIs in the treatment of ASC of the liver. In the management of ASC of the liver with immune checkpoint inhibitors (ICIs), it is essential to monitor not only treatment efficacy but also the occurrence of immune-related adverse events (irAEs), including immune-mediated pneumonitis, colitis, and hepatitis. As far as we know, this is the first reported case in which one of the two primary malignancies of the patient is ASC of the liver. This is also the first report on the application of ICIs in primary ASC of the liver, although there are some recent studies which show that ICIs can achieve good results in metastatic hepatic ASC (41, 42). The precise mechanism underlying the response of hepatic ASC to immune checkpoint inhibitors (ICIs) remains to be elucidated—particularly whether such efficacy is driven by tumor-intrinsic mismatch repair deficiency or a germline predisposition such as Lynch syndrome. While sporadic case reports have described adenosquamous carcinoma in patients with Lynch syndrome (43, 44), there is currently no established evidence indicating a common association between ASC and Lynch syndrome. To optimize treatment strategies, prospective studies are essential to identify predictive biomarkers and define which patients are most likely to benefit from immunotherapy. In addition, Hong et al. (45) has recently published a case report of a woman with ASC of the extrahepatic biliary tract with multiple lymph node metastases which is characterized by HER-2 amplification. This patient received a chemotherapy regimen consisting of gemcitabine, cisplatin, and trastuzumab, displaying a progression-free survival (PFS) of 5 months. Gou et al. reported an inoperable ASC patient with intrahepatic metastasis who received sorafenib-targeted therapy and survived for 9 months (3).
In conclusion, ASC patients may benefit greatly from systemic chemotherapy, HAIC therapy, targeted and immune therapy. We should make greater efforts to explore these treatment approaches in order to improve patients’ quality of life or extend their survival in order to further develop the most optimal treatment of ASC of the liver.
4 Conclusions
This case presents the first reported instance of a patient with primary hepatic adenosquamous carcinoma (ASC) that achieved a pathological complete response (pCR) following immunotherapy. This outcome highlights the potential of immune checkpoint inhibitor use in treating ASC of the liver, particularly in tumors with dMMR status, providing many valuable insights for the future management of this rare malignancy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Beijing Tsinghua Changgung Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZB: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. Y-AC: Conceptualization, Data curation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YX: Conceptualization, Supervision, Validation, Writing – original draft. JPS: Formal analysis, Investigation, Methodology, Writing – review & editing. JWS: Investigation, Writing – review & editing. CX: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1578368/full#supplementary-material
Supplementary Figure 1 | Timeline of treatment and follow-up. Clinical timeline of diagnosis, treatment, and follow-up. The patient underwent a radical hysterectomy for uterine cancer in August 2021. In January 2023, a low-density liver lesion was detected during routine follow-up. A liver mass biopsy in March 2023 confirmed adenosquamous carcinoma (ASC) of the liver. The patient received eight cycles of sintilimab as neoadjuvant immunotherapy from March to October 2023, followed by laparoscopic partial liver resection. As of March 2025, no evidence of tumor recurrence has been observed.
References
1. Nam KH, Kim JY. Primary adenosquamous carcinoma of the liver: A case report. Clin Mol Hepatol. (2016) 22:503–8. doi: 10.3350/cmh.2016.0077
2. Park SY, Cha EJ, Moon WS. Adenosquamous carcinoma of the liver. Clin Mol Hepatol. (2012) 18:326–9. doi: 10.3350/cmh.2012.18.3.326
3. Gou Q, Fu S, Xie Y, Zhang M, Shen Y. Treatment and survival patterns of primary adenosquamous carcinoma of the liver: A retrospective analysis. Front Oncol. (2021) 11:621594. doi: 10.3389/fonc.2021.621594
4. Wang J, Fei K, Jing H, Wu Z, Wu W, Zhou S, et al. Durable blockade of pd-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. mAbs. (2019) 11:1443–51. doi: 10.1080/19420862.2019.1654303
5. Liu X, Yi Y. Recent updates on sintilimab in solid tumor immunotherapy. Biomark Res. (2020) 8:69. doi: 10.1186/s40364-020-00250-z
6. Zhou N, Li X, Yang Y, Tan S, Zhang S, Huang Q, et al. Sintilimab plus nab-paclitaxel as second-line treatment for advanced biliary tract cancer: Study protocol for an investigator-initiated phase 2 trial (Napasinti trial). BMC Cancer. (2023) 23:729. doi: 10.1186/s12885-023-11188-4
7. Zeng TM, Yang G, Lou C, Wei W, Tao CJ, Chen XY, et al. Clinical and biomarker analyses of sintilimab plus gemcitabine and cisplatin as first-line treatment for patients with advanced biliary tract cancer. Nat Commun. (2023) 14:1340. doi: 10.1038/s41467-023-37030-w
8. Jin S, Zhao R, Zhou C, Zhong Q, Shi J, Su C, et al. Feasibility and tolerability of sintilimab plus anlotinib as the second-line therapy for patients with advanced biliary tract cancers: an open-label, single-arm, phase ii clinical trial. Int J Cancer. (2023) 152:1648–58. doi: 10.1002/ijc.34372
9. Yeh CN, Jan YY, Chen MF. Adenosquamous carcinoma of the liver: Clinicopathologic study of 10 surgically treated cases. World J Surg. (2003) 27:168–72. doi: 10.1007/s00268-002-6585-0
10. Satake T, Morizane C, Rikitake R, Higashi T, Okusaka T, Kawai A. The epidemiology of rare types of hepatobiliary and pancreatic cancer from national cancer registry. J Gastroenterol. (2022) 57:890–901. doi: 10.1007/s00535-022-01920-5
11. Li HS, He T, Yang LL. Adenosquamous carcinoma of the digestive system: A literature review. Scand J Gastroenterol. (2020) 55:1268–76. doi: 10.1080/00365521.2020.1832571
12. Takahashi H, Hayakawa H, Tanaka M, Okamura K, Kosaka A, Mizumoto R, et al. Primary adenosquamous carcinoma of liver resected by right trisegmentectomy: Report of a case and review of the literature. J Gastroenterol. (1997) 32:843–7. doi: 10.1007/bf02936966
13. Sasaki H, Hayashi M, Miyahara S, Shimomura M, Takahashi H, Kawarada Y. Adenosquamous carcinoma of the liver: Case report. J Bepato-Biliary-Pancreatic Surg. (1994) 1:179–83. doi: 10.1007/BF01222246
14. Liu YM, Lei YL, Liu F. Adenosquamous carcinoma of the liver: the challenge of diagnosis. Liver Int. (2023) 43:2320–2. doi: 10.1111/liv.15708
15. Zhou SY, Qiao ZG, Li CL, Chen TB. Primary adenosquamous carcinoma of the liver. Kaohsiung J Med Sci. (2020) 36:857–8. doi: 10.1002/kjm2.12252
16. Gao S, Chen D, Huang L, Wu L, Dai R, Shan Y. Primary adenosquamous carcinoma of the liver: A case report and review of the literature. Int J Clin Exp Pathol. (2015) 8:9687–92.
17. Watanabe Y, Osaki A, Kimura K, Yakubo S, Takaku K, Sato M, et al. Unresectable primary hepatic adenosquamous carcinoma successfully treated with systemic and transcatheter hepatic arterial injection chemotherapies followed by conversion surgery: A case report and literature review. BMC Gastroenterol. (2021) 21:491. doi: 10.1186/s12876-021-02070-3
18. Hayashi T, Mizuki A, Yamaguchi T, Hasegawa T, Kunihiro T, Tsukada N, et al. Primary adenosquamous carcinoma of the liver which produces granulocyte-colony-stimulating factor and parathyroid hormone related protein: Association with leukocytosis and hypercalcemia. Internal Med (Tokyo JP). (2001) 40:631–4. doi: 10.2169/internalmedicine.40.631
19. Nosaka T, Ohtani M, Namikawa S, Takahashi K, Naito T, Ofuji K, et al. Advanced primary adenosquamous carcinoma of the liver with a small cell carcinoma component: an autopsy case report. Clin J Gastroenterol. (2021) 14:1496–502. doi: 10.1007/s12328-021-01474-8
20. Suzuki E, Hirai R, Ota T, Shimizu N. Primary adenosquamous carcinoma of the liver: Case report. J hepato-biliary-pancreatic Surg. (2002) 9:769–73. doi: 10.1007/s005340200108
21. Kwon OS, Lee HS, Koh DW, Cho YJ, Park YH, Park DK, et al. A case of primary adenosquamous carcinoma of the liver presented with liver abscess. KR J Internal Med. (2001) 16:270–3. doi: 10.3904/kjim.2001.16.4.270
22. Yeung JT, Fan WC, Cheng RL. Adenosquamous carcinoma presenting as liver abscess. Singapore Med J. (2012) 53:e110–3.
23. Kang GH, Lee BS, Kang DY. A case of primary adenosquamous carcinoma of the liver. KR J Hepato-Biliary-Pancreatic Surg. (2013) 17:38–41. doi: 10.14701/kjhbps.2013.17.1.38
24. Harino T, Tomimaru Y, Noguchi K, Nagase H, Ogino T, Hirota M, et al. a rare case of adenosquamous carcinoma in the liver with hepatolithiasis. Gan Kagaku Ryoho Cancer Chemo. (2019) 46:772–4.
25. Wei D, Lu L, Ying C, Qingsong K, Dongbo L, Feibo L. Primary hepatic adenosquamous carcinoma: A rare case report. Indian J Pathol Microbiol. (2021) 64:S140–s2. doi: 10.4103/ijpm.Ijpm_785_18
26. Daiku K, Fukuda K, Morimoto O, Takiuchi D, Shimakoshi H, Kegasawa T, et al. Primary adenosquamous carcinoma of the liver detected during cancer surveillance in a patient with primary sclerosing cholangitis. Clin J Gastroenterol. (2020) 13:1273–9. doi: 10.1007/s12328-020-01204-6
27. Wu PH, Su YC, Kuo KK. A rare case of primary adenosquamous carcinoma of the liver with gallbladder metastasis. Kaohsiung J Med Sci. (2020) 36:561–2. doi: 10.1002/kjm2.12227
28. Nakai T, Ono K, Terayama K, Yamagami T, Nishimura T. Case report: Adenosquamous carcinoma of the liver successfully treated with repeated transcatheter arterial infusion chemotherapy (Tace) with degradable starch microspheres. Br J Radiol. (2004) 77:516–8. doi: 10.1259/bjr/63282776
29. Demir G, Yanmaz T, Celik AF, Ozbay G, Serdengecti S. Primary adenosquamous carcinoma of the liver: Case with the longest survival. J Clin Gastroenterol. (2005) 39:924–5. doi: 10.1097/01.mcg.0000180805.56952.04
30. Shimizu S, Oshita A, Tashiro H, Amano H, Kobayashi T, Tanaka M, et al. Synchronous double cancers of primary hepatic adenosquamous carcinoma and hepatocellular carcinoma: Report of a case. Surg Today. (2013) 43:418–23. doi: 10.1007/s00595-012-0346-y
31. Yokota H, Matoba M, Tonami H, Hasegawa T, Saito H, Kurose N. Imaging findings in primary adenosquamous carcinoma of the liver: A case report. Clin Imaging. (2007) 31:279–82. doi: 10.1016/j.clinimag.2007.01.007
32. Yamao K, Takenaka M, Imai H, Nakai A, Omoto S, Kamata K, et al. Primary hepatic adenosquamous carcinoma associated with primary sclerosing cholangitis. Oncology. (2017) 93 Suppl 1:76–80. doi: 10.1159/000481236
33. Kobayashi M, Okabayashi T, Okamoto K, Namikawa T, Araki K. A clinicopathologic study of primary adenosquamous carcinoma of the liver. J Clin Gastroenterol. (2005) 39:544–8. doi: 10.1097/01.mcg.0000165705.74079.fc
34. Isa T, Kusano T, Muto Y, Furukawa M, Kiyuna M, Toda T. Clinicopathologic features of resected primary adenosquamous carcinomas of the liver. J Clin Gastroenterol. (1997) 25:623–7. doi: 10.1097/00004836-199712000-00015
35. Alvaro D, Gores GJ, Walicki J, Hassan C, Sapisochin G, Komuta M. EASL-ILCA clinical practice guidelines on the management of intrahepatic cholangiocarcinoma. J Hepatol. (2023) 79:181–208. doi: 10.1016/j.jhep.2023.03.010
36. Kubo S, Shinkawa H, Asaoka Y, Ioka T, Igaki H, Izumi N, et al. Liver cancer study group of Japan clinical practice guidelines for intrahepatic cholangiocarcinoma. Liver Cancer. (2022) 11:290–314. doi: 10.1159/000522403
37. Zeng TM, Pan YF, Yuan ZG, Chen DS, Song YJ, Gao Y. Immune-related rna signature predicts outcome of pd-1 inhibitor-combined gemcis therapy in advanced intrahepatic cholangiocarcinoma. Front Immunol. (2022) 13:943066. doi: 10.3389/fimmu.2022.943066
38. Ding X, Li G, Sun W, Shen Y, Teng Y, Xu Y, et al. Sintilimab combined with lenvatinib for advanced intrahepatic cholangiocarcinoma in second-line setting-a multi-center observational study. Front Oncol. (2022) 12:907055. doi: 10.3389/fonc.2022.907055
39. Wang K, Xiang YJ, Yu HM, Cheng YQ, Liu ZH, Qin YY, et al. Adjuvant sintilimab in resected high-risk hepatocellular carcinoma: A randomized, controlled, phase 2 trial. Nat Med. (2024) 30:708–15. doi: 10.1038/s41591-023-02786-7
40. Zhu M, Liu Z, Chen S, Luo Z, Tu J, Qiao L, et al. Sintilimab plus bevacizumab combined with radiotherapy as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A multicenter, single-arm, phase 2 study. Hepatol (Baltimore Md). (2024) 80:807–15. doi: 10.1097/hep.0000000000000776
41. Liu Q, Li R, Zhu W, Zheng P. Case report: Microsatellite instability-high pancreas adenosquamous carcinoma with postoperative liver metastasis recurrence treated with multimodality therapy achieving complete pathological response. Front Immunol. (2024) 15:1456343. doi: 10.3389/fimmu.2024.1456343
42. Xu Y, Li Q, Zhao J, Ni X, Li P, Hu W. Case report: Complete response to pembrolizumab in a liver metastatic colon adenocarcinoma patient with a novel likely pathogenic germline msh2 mutation. Front Immunol. (2022) 13:1064488. doi: 10.3389/fimmu.2022.1064488
43. Ching D, Amanuel B, Khor TS. Primary adenosquamous carcinoma in a patient with lynch syndrome. Pathology. (2019) 51:534–7. doi: 10.1016/j.pathol.2019.02.007
44. Duncan VE, Harada S, Stevens TM. Primary colon adenosquamous carcinoma in a patient with lynch syndrome: A new histologic subtype associated with microsatellite instability? Int J Surg Pathol. (2016) 24:653–5. doi: 10.1177/1066896916659539
Keywords: adenosquamous carcinoma of liver, immune checkpoint inhibitors, pathological complete response, sintilimab, PD-1
Citation: Bai Z, Chen Y-A, Xiao Y, Song J, Song J and Xiang C (2025) Use of sintilimab in primary adenosquamous carcinoma of the liver results in pathological complete response: a case report and literature review. Front. Immunol. 16:1578368. doi: 10.3389/fimmu.2025.1578368
Received: 17 February 2025; Accepted: 07 April 2025;
Published: 30 April 2025.
Edited by:
Rongxin Zhang, Guangdong Pharmaceutical University, ChinaCopyright © 2025 Bai, Chen, Xiao, Song, Song and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Canhong Xiang, eGNoYTAxMTE0QGJ0Y2guZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Zhiqing Bai
Zhiqing Bai Yu-Ann Chen
Yu-Ann Chen Ying Xiao
Ying Xiao Jianping Song1
Jianping Song1