- 1Henan Province Engineering Technology Research Center of Intelligent Diagnosis and Treatment, The First Affiliated Hospital, School of Basic Medicine Sciences, Henan University, Kaifeng, China
- 2Institute of Traditional Chinese Medicine, School of Pharmacy, Henan University, Kaifeng, China
- 3Changchun Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Changchun, China
- 4Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou University, Yangzhou, China
COVID-19 and seasonal influenza have taken a huge toll on the global economy and global health. Given the potential of COVID-19 to transform into a chronic epidemic akin to seasonal influenza, the influenza virus and SARS-CoV-2 will continue to be a significant threat to healthcare for some time to come. Coinfection involving the two viruses has been proven to worsen the severity of the illness, as evidenced by clinical observational data. Vaccination remains the most effective measure in the prevention and treatment of infectious diseases. In addition, the coadministration of influenza virus and SARS-CoV-2 vaccines offered greater benefits than either vaccine alone. Combination vaccines are also a major hotspot in novel vaccine development. This review highlights the advancements in the development of combined vaccines for COVID-19 and seasonal influenza, as demonstrated in animal studies and clinical trials, and emphasizes the importance of a combined vaccine.
1 Introduction
In the past few years, the annual seasonal influenza outbreaks and the worldwide COVID-19 pandemic have inflicted substantial harm on the global economy and human health (1, 2). Despite both being respiratory viruses that primarily manifest as respiratory symptoms, such as nasal discharge, sore throat, cough, and headache, among others, there exist numerous distinctions between SARS-CoV-2 and the influenza virus (3–12). Initially, it was hypothesized that SARS-CoV-2 would supplant influenza viruses as it spread worldwide (13). However, subsequent pandemics have revealed the emergence of novel virus-virus interactions, wherein the co-circulation of influenza viruses and SARS-CoV-2 strains has been reported (14). The coinfection of SARS-CoV-2 with the influenza virus has been found to exacerbate the severity of disease manifestation in humans (15), but the implementation of maintaining social distancing measures appeared to mitigate the impact of such co-infections (16). However, the relaxation of public gathering restrictions and travel limitations was likely to result in a significant increase in co-infection involving the influenza virus.
A reduction in the occurrence of SARS-CoV-2 infection has been noted with influenza vaccination (17). Furthermore, the combination of influenza and SARS-CoV-2 vaccinations has been validated to have an enhanced immunizing effect against SARS-CoV-2 infection, hospitalizations, and overall mortality (18). Clinical trials have additionally confirmed the feasibility of simultaneous vaccination as an effective immunization approach (18). Consequently, the development of combined vaccines targeting both SARS-CoV-2 and influenza may present a more advantageous alternative. Nearly 30 different combination vaccines for SARS-CoV-2 and the influenza virus have been documented, including recombinant live attenuated virus vaccines, mRNA-based vaccines, recombinant protein vaccines, and virus-like particle (VLP) vaccines. All the combination vaccines that have been proven in animal studies could produce robust neutralizing antibodies and protect from both SARS-CoV-2 and influenza infection (Figure 1) (1). Some combination vaccines are already in clinical trials. The good news is that one clinical trial on a nasally administered combination vaccine against influenza and COVID-19, based on a live-attenuated influenza virus vector, has been shown to be tolerable in adults. Here, we review the characteristics and research progress of these combination vaccines.
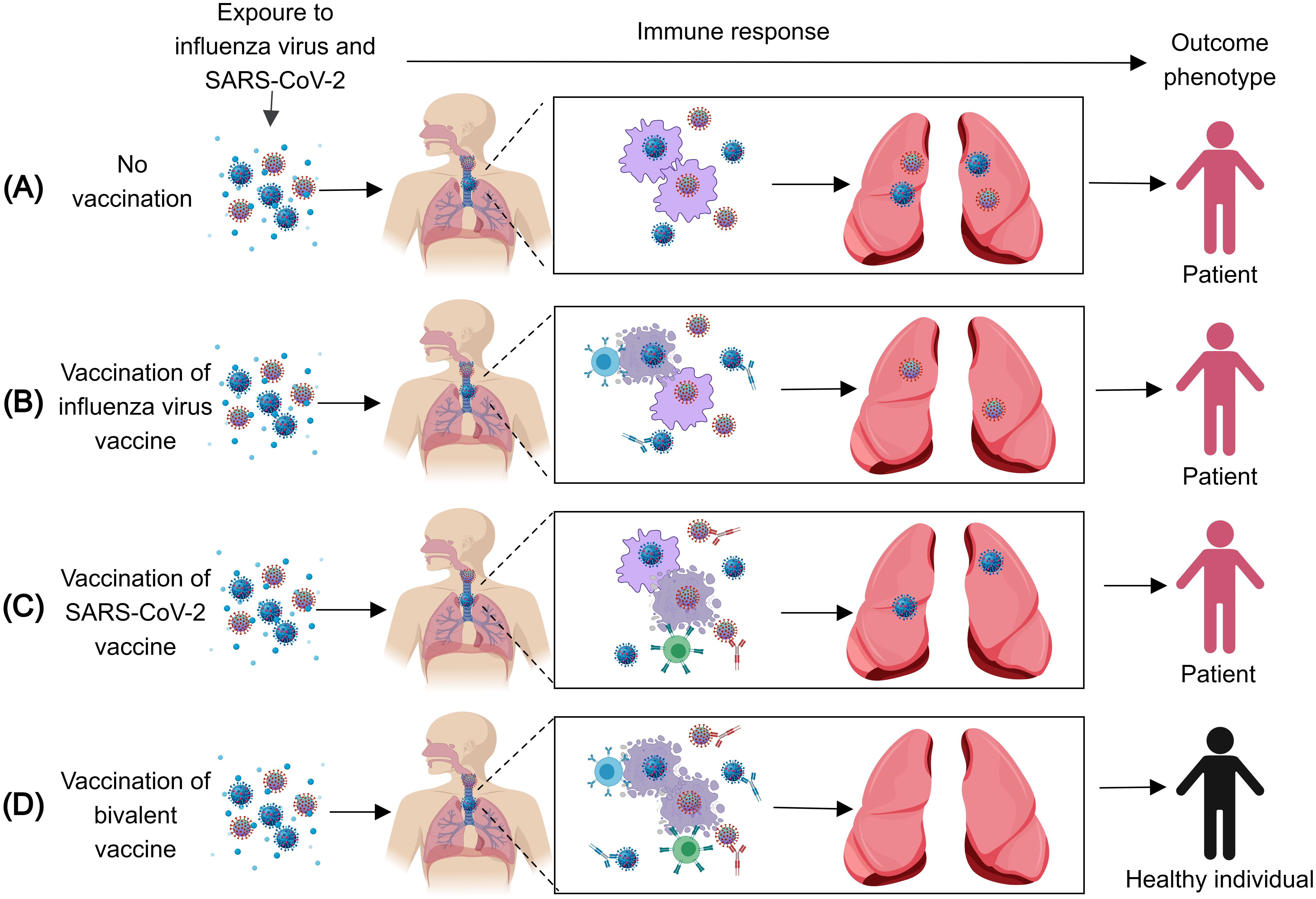
Figure 1. The immune response after infection with the influenza virus and SARS-CoV-2. (A) When exposed to the influenza virus and SARS-CoV-2, unvaccinated Individuals cannot produce the corresponding antibodies and effector T cells against the influenza virus and SARS-CoV-2, thus, they will be infected with the influenza virus and SARS-CoV-2. (B) When exposed to the influenza virus and SARS-CoV-2, individuals vaccinated with the influenza virus vaccine can produce the corresponding antibodies and effector T cells against the influenza virus, thus, they will be infected with SARS-CoV-2. (C) When exposed to the influenza virus and SARS-CoV-2, individuals vaccinated with the SARS-CoV-2 vaccine can produce the corresponding antibodies and effector T cells against SARS-CoV-2, thus, they will be infected with the influenza virus. (D) When exposed to the influenza virus and SARS-CoV-2, individuals vaccinated with the influenza virus vaccine and SARS-CoV-2 vaccine can produce the corresponding antibodies and effector T cells against the influenza virus and SARS-CoV-2, thus, the individuals cannot be infected by either virus.
2 Influenza virus and SARS-CoV-2
2.1 Influenza virus
According to the World Health Organization (WHO), worldwide, annual epidemics of influenza are estimated to result in approximately 3 to 5 million cases of severe illness and about 290,000 to 650,000 respiratory deaths (2). All age groups are affected, but some groups are more at risk than others, as people at greater risk of severe disease or complications when infected are pregnant women, children under 59 months, the elderly, individuals with chronic medical conditions (such as established cardiac, pulmonary, renal, metabolic, neurodevelopmental, liver, or hematologic diseases), and individuals with immunosuppressive conditions (such as HIV/AIDS, receiving chemotherapy or steroids, or malignancy) (2). In industrialized countries, most influenza-related deaths occur among people aged 65 or older (3). The adoption of measures fighting against the threat posed by COVID-19 since the first months of 2020 produced a remarkable decrease in the severity of seasonal influenza-like illnesses during the 2020/21 season (4–6). The COVID-19 control measures helped to prevent influenza from circulating during the 2020/21 season (7). The decrease in the severity of influenza-like illnesses may imply a lower population immunity for the season 2021/22 since most people had not been exposed to the influenza virus for over a year (8, 9). In the past 2 years, there was a more severe influenza outbreak compared to the pre-COVID-19 pandemic period (10).
Influenza viruses are categorized into types A, B, C, and D based on their core proteins, with only types A and B being associated with human diseases of concern. Type A viruses are further subdivided according to their hemagglutinin (HA) and neuraminidase (NA) proteins (11, 12). Despite the availability of effective antiviral drugs, vaccination remains a crucial preventive strategy. Since most of the neutralizing antibody targets are in the HA globular head domain, this region is an essential target for conventional influenza vaccines (13, 14). However, the high mutability of this domain facilitates antigenic drift (15), resulting in variable vaccine effectiveness (VE) ranging from 10% to 60% over the past decade, depending on the match between the vaccine and circulating strains (16). Creating a universal influenza vaccine is a major scientific priority. The vaccine needs to improve the scope and durability of protection against seasonal influenza. Furthermore, it should safeguard against pandemic strains (17, 18). Current research focuses on three key strategies to overcome existing limitations: targeting conserved viral epitopes, developing novel vaccine platforms, and utilizing advanced adjuvants (19). Promising conserved targets for universal vaccines include the HA stem domain (20, 21), Matrix Protein 2 Ectodomain (M2e) (22, 23), NP (24, 25), and NA (26, 27). These conserved epitopes, central to universal vaccine development, have shown enhanced immunogenicity and protection when delivered through innovative platforms (28). For instance, VLP vaccines mimic native viral structures to induce robust immune responses (29–32), nanoparticle vaccines present high-density antigen arrays with intrinsic adjuvant properties (33–35), viral vector vaccines enable mucosal delivery for local immunity (36, 37), and nucleic acid vaccines offer rapid adaptability to emerging outbreaks (38, 39). In conclusion, the development of broadly protective influenza vaccines remains an urgent unmet need in global health.
2.2 SARS-COV-2
As of 10 November 2024, the WHO had documented over 776 million confirmed COVID-19 cases and 7 million deaths worldwide (40). However, the reported COVID-19 data were likely undercounted compared to actual cases due to various reasons (41). In addition to acute symptoms including fever, headache, and cough, patients may develop Long COVID (Post-Acute Sequelae of SARS-CoV-2 infection), a condition characterized by persistent multisystem symptoms lasting weeks to months after initial infection (42, 43). Given their multisystem involvement and established association with psychosocial sequelae, these persistent symptoms can lead to substantial long-term morbidity (44). For two years, people endured the impact of COVID-19 (45). As more and more regions stopped testing for COVID-19, it appeared as if the disease had faded away. While the WHO (40) and Chinese Center for Disease Control and Prevention (CCDC) (46) surveillance data demonstrate a sustained decline in confirmed cases since January 2023, and Wang et al.’s clinical study found predominantly mild acute disease in hospitalized Omicron-infected patients (47), emerging evidence suggests significant life-altering consequences among Long COVID patients following SARS-CoV-2 infection (48).
SARS-CoV-2 is a type of coronavirus, similar to SARS and MERS (49). The virus particle surface is covered with spike proteins (S protein), which are arranged as trimers. During viral entry, the receptor-binding domain (RBD) located in the S1 subunit of the S protein specifically interacts with receptors [angiotensin-converting enzyme (ACE2)] on human cells (50); then, the S2 subunit of the S protein facilitates the fusion between the virus and the cell (51, 52). The RBD’s role in stimulating protective antibodies during immune defense highlights the spike protein’s importance as an antigen in vaccine development (53).
Demonstrated to cause severe multisystem disease in human populations (40, 54), SARS-CoV-2 exhibits high transmissibility via respiratory droplets and aerosols, facilitating its rapid global dissemination (55). Similar to influenza prevention strategies, vaccination remains a cornerstone of COVID-19 pandemic control measures (56). In vaccine target design, while the M protein (57) and NSP3, NSP4, and NSP6 (58, 59) demonstrate immunogenicity, the S and N proteins remain the predominant immunodominant epitopes (60, 61). As of 25 November 2024, global vaccine development efforts included 183 candidates in clinical trials and 199 in preclinical studies, spanning multiple platform technologies: inactivated vaccines, nucleic acid (RNA/DNA) vaccines, viral vector vaccines, VLP vaccines, and protein subunit vaccines. Several of these have received WHO emergency use authorization (62). Compared to conventional vaccine approaches, modern molecular-based platforms demonstrate distinct advantages for addressing both emerging and established viral threats, such as recombinant viral vector-based vaccines (63, 64), VLP-based vaccines (65–69), recombinant protein-based vaccines (70, 71), and nucleic acid-based technologies (72, 73). These innovative platforms enable more adaptable, scalable, and cost-effective vaccine development while maintaining rapid production timelines. Collectively, these technological advances have played a pivotal role in COVID-19 pandemic control efforts.
2.3 Coinfection of SARS-CoV-2 and influenza virus
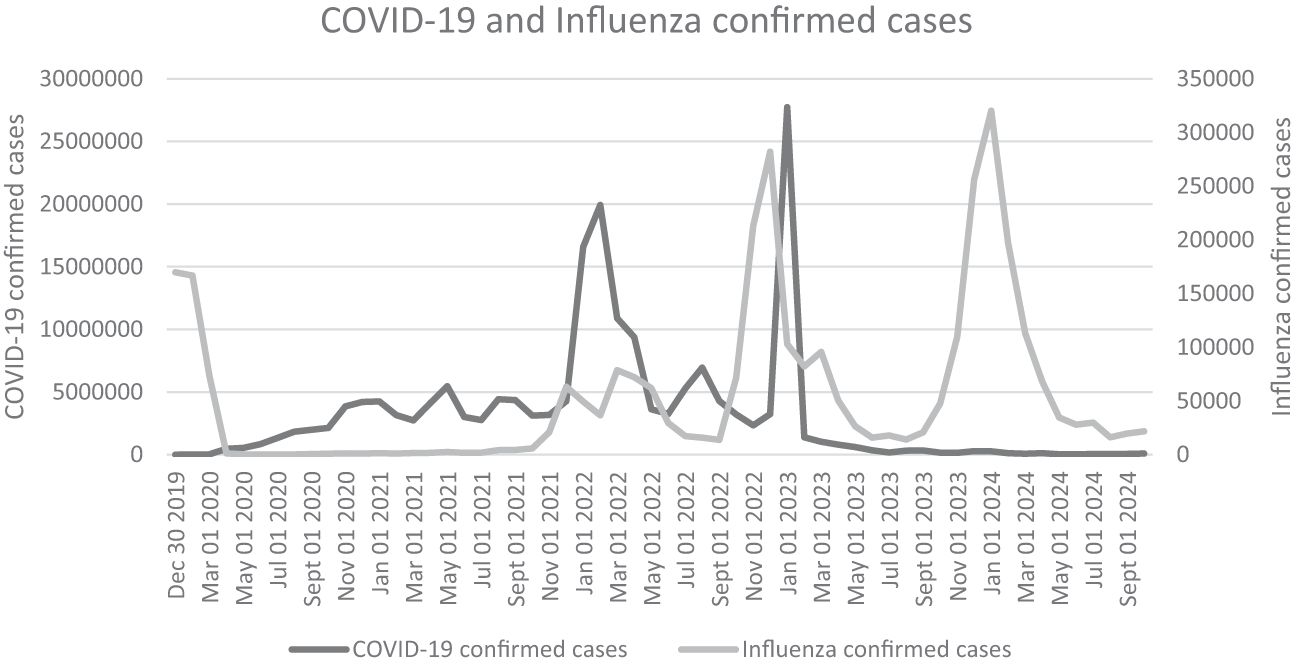
As airborne pathogens, both the influenza virus and SARS-CoV-2 primarily target the human respiratory system, including the nasal mucosa, trachea, bronchi, and alveoli (74, 75). Effective preventive measures such as washing hands, social distancing, and mask-wearing can significantly reduce transmission risks for these pathogens (76). Notably, public health interventions implemented during COVID-19 outbreaks not only controlled SARS-CoV-2 spread but also suppressed seasonal influenza epidemics (77). However, following the relaxation of COVID-19 containment measures, influenza A virus (IAV) circulation rebounded to pre-pandemic levels, and in some cases exceeded them, while SARS-CoV-2 continued to circulate endemically (Graph 1) (78–80). Coinfection with IAV and SARS-CoV-2 has been reported in many regions (81–83), which may be facilitated by the co-localization of ACE2 and sialic acid receptors in respiratory tissues (Figure 2) (74, 75). While social distancing measures initially reduced coinfection risks (84), their subsequent removal, particularly travel restrictions, led to a marked increase in coinfection rates (85, 86). Importantly, pre-existing IAV infection had been shown to enhance SARS-CoV-2 infectivity (87), and clinical studies confirm that coinfected patients experience more severe disease manifestations (88, 89). A systematic review by Xiang et al. (2023) concluded that IAV-SARS-CoV-2 coinfection leads to worse clinical outcomes (90). Supporting this, Hashemi et al. (2023) reported a striking 22.3% coinfection rate with H1N1 influenza and SARS-CoV-2 among deceased patients in northeastern Iran (82). Collectively, these findings suggest that viral coinfections may contribute to elevated morbidity and mortality in patients with acute respiratory infections.
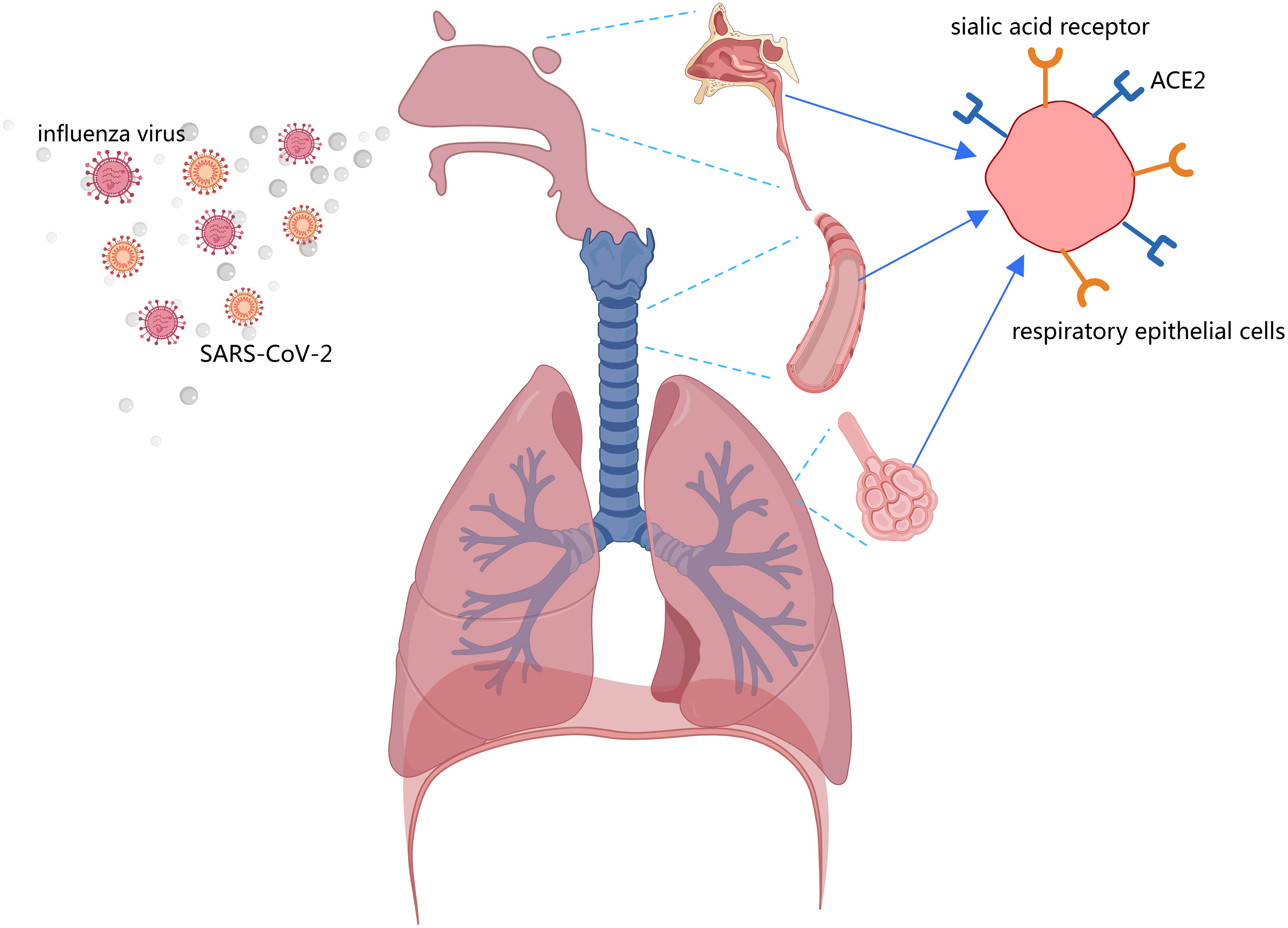
Figure 2. Both the sialic acid receptor (influenza virus receptor) and ACE2 are present in respiratory epithelial cells, including the nasal mucosa, trachea, bronchi, and alveoli.
Furthermore, animal studies demonstrated that coinfection with the influenza virus and SARS-CoV-2 also resulted in more severe disease outcomes (91–93). In murine models, simultaneous infection with SARS-CoV-2 and H1N1 (compared to single-virus infection) prolonged the acute viral phase, exacerbated immune cell infiltration, and elevated inflammatory cytokine levels in bronchoalveolar lavage fluid, ultimately causing more severe pneumonia and pulmonary damage (93). Similar findings were observed in ferret models, where coinfected animals showed significantly greater weight loss and more pronounced inflammatory pathology in both upper (nasal turbinate) and lower (lung) respiratory tracts compared to single-virus infections (92). Most critically, Bao et al. (2021) reported that H1N1/SARS-CoV-2 coinfection in mice not only extended symptom duration and worsened pulmonary lesions but also resulted in significantly higher mortality rates than either monoinfection (91).
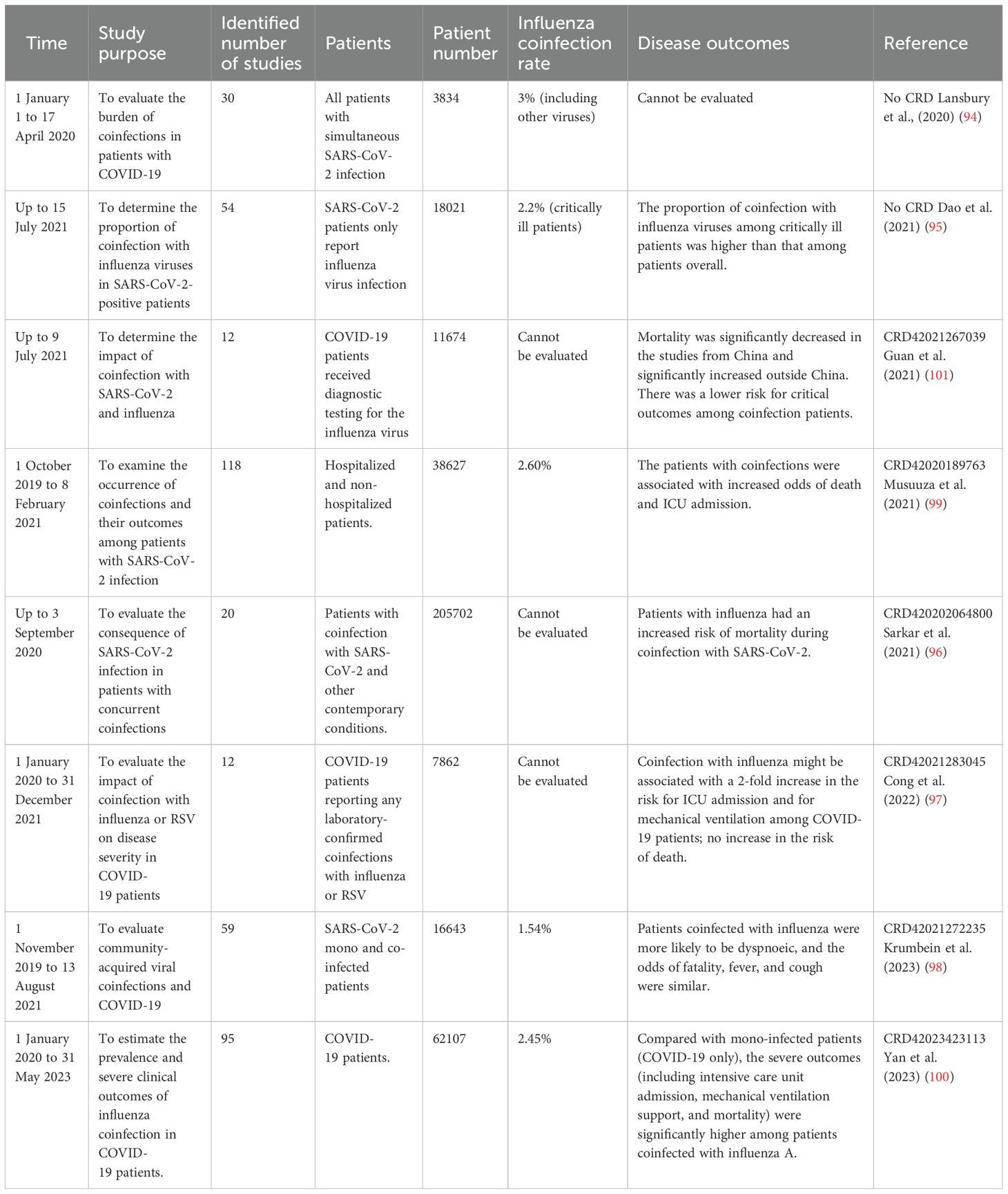
To confirm the coinfection rate of influenza and COVID-19, we analyzed all meta-analyses associated with coinfection of influenza and COVID-19 from PubMed up to 25 November 2024 (Table 1). The inaugural review in this field (94), which evaluated coinfection prevalence among SARS-CoV-2-confirmed patients, reported an overall viral coinfection rate of 3% (including influenza and other respiratory viruses). As shown in Table 1, the majority of the meta-analyses demonstrated that influenza coinfection was significantly correlated with worse clinical outcomes, particularly increased risks of ICU admission, mechanical ventilation requirement, and mortality (95–100). However, regional variations in disease severity and fatality rates were observed, likely attributable to differences in local public health policies (101). These findings were further corroborated by a large-scale UK study (n=7,000) that, through multivariable regression analysis, identified influenza coinfection as an independent risk factor for invasive mechanical ventilation in COVID-19 patients (102). During the peak COVID-19 pandemic period, reported coinfection rates ranged from 0.6% to 2.6% globally (95, 98, 99), potentially reflecting the combined effects of international containment measures (5, 7) and virus interference (103).
3 Co-administration of influenza virus and SARS-CoV-2 vaccines
During the COVID-19 pandemic, numerous studies documented co-infections of IAV and SARS-CoV-2 across diverse geographical regions (82, 83, 104). These dual infections were associated with significantly worse clinical outcomes, including increased disease severity, higher mortality rates, and greater strain on healthcare infrastructure (95–99). Concomitant vaccination against both COVID-19 and influenza can be used as a preventive measure.
3.1 Association between influenza vaccination and SARS-CoV-2 infection
Influenza vaccines are designed to protect from influenza virus infection, but growing evidence suggests they may also confer protective benefits against SARS-CoV-2 infection (105, 106). As early as October 2020, a systematic review conducted by Del Riccio et al. (2020) demonstrated significant inverse associations between influenza vaccination and COVID-19 severity outcomes, including reduced hospitalization rates and mortality among COVID-19 patients (Figure 3) (107).
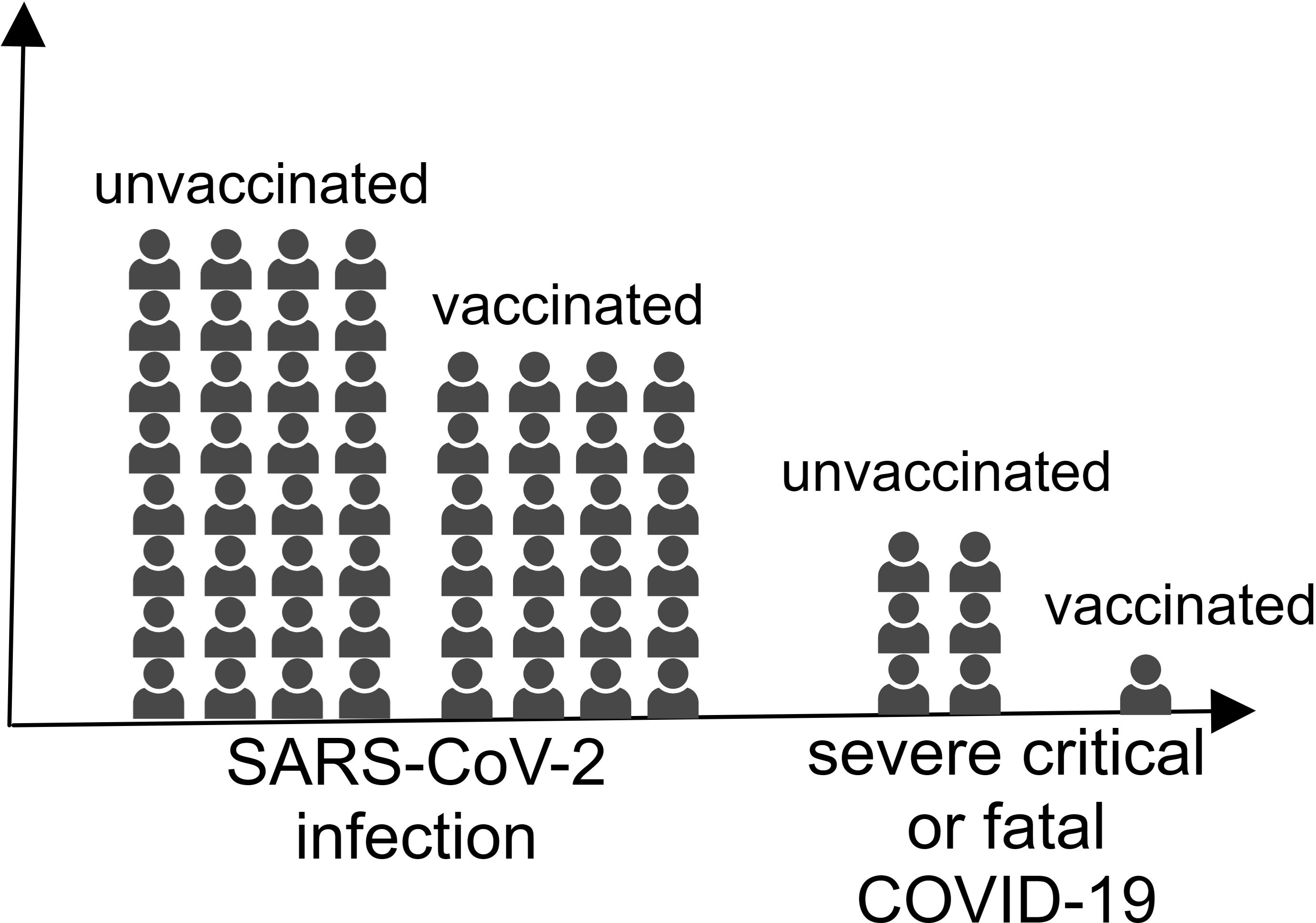
Figure 3. The individuals vaccinated with the influenza virus vaccine were less likely to be infected with SARS-CoV-2 and develop severe COVID-19.
Multiple epidemiological studies across diverse regions documented reduced SARS-CoV-2 infection rates among influenza-vaccinated populations (108–110). A striking example came from Italian healthcare workers, where influenza vaccination was associated with a 57% relative reduction in SARS-CoV-2 positivity (12.4% vs. 6.1%) compared to their unvaccinated counterparts (109). Comparable protective effects were observed among healthcare personnel in Qatar (29.7% effectiveness) (110) and Bahrain (54.3% effectiveness) (111). This protective association extends to high-risk populations, including elderly individuals, chronic disease patients, and healthcare workers (112, 113). A large-scale study of over 2 million seniors (eni years) revealed that influenza vaccination during the first 2 pandemic years was associated with a 22%–24% reduction in SARS-CoV-2 infection risk (114). Population-level evidence from meta-analyses encompassing 55,996,841 subjects further confirmed that influenza vaccination significantly reduced both SARS-CoV-2 infection rates and severe clinical outcomes (106). Notably, these observational findings were supported by randomized controlled trial data, demonstrating a 51% reduction in coronavirus infection risk among influenza vaccine recipients (115).
Beyond reducing SARS-CoV-2 infection rates, influenza vaccination demonstrated significant associations with mitigated disease severity in patients with COVID-19. Clinical studies revealed that vaccinated individuals experienced lower 60-day mortality rates and reduced ventilator requirements (116, 117). These protective effects were particularly pronounced in high-risk populations: among adults ≥66 years, influenza vaccination was associated with 17%–32% lower hospitalization rates and 27%–42% reduced mortality risk (114). Even among inpatients, unvaccinated hospitalized patients faced twice the ICU admission risk compared to their vaccinated counterparts, with enhanced protection observed in patients <65 years and non-obese individuals (118). Furthermore, influenza-vaccinated patients with COVID-19 exhibited decreased incidence of severe complications, including sepsis, stroke, and deep vein thrombosis, throughout disease progression (105). Current evidence strongly supports an inverse relationship between influenza vaccination and both SARS-CoV-2 infection risk and COVID-19 severity.
The protective mechanism of influenza vaccination against SARS-CoV-2 infection primarily involves the stimulation of innate immune defenses. One animal study demonstrated that prior influenza vaccination reduced viral shedding and mitigated pulmonary pathology upon SARS-CoV-2 challenge (119). This protection appears to be mediated through enhanced innate immune priming as the influenza vaccines activated key immune sentinels, including neutrophils, dendritic cells, and macrophages via Toll-like receptor (TLR) pathways, before viral exposure (120). Notably, TLR7-mediated recognition of SARS-CoV-2 may be particularly affected by this pre-activation (121). The vaccine-induced immune modulation extends to natural killer (NK) cells (122) and γδ T cells (123), with cross-reactive responses potentially attenuating SARS-CoV-2 pathogenicity (124). These innate immune cells that had been immunized generated a stronger targeted immune response that can be observed through epigenetic and metabolic changes after being exposed to immunostimulants (125). It is likely that interferons also played a role in this process after vaccination (126), while the MF59 adjuvant itself may provide non-specific protection when administered pre-exposure (127). The influenza vaccine primarily mediates protective immune responses against SARS-CoV-2 through non-specific immune mechanisms.
3.2 Co-administration of influenza and SARS-CoV-2 vaccination
As early as fall 2021, the WHO encouraged the simultaneous administration of the seasonal influenza vaccine and the SARS-CoV-2 vaccine at separate sites (128). Subsequently, acknowledging the compounded public health impact of concurrent influenza and COVID-19 threats, several nations, including the United Kingdom, adopted policies promoting dual vaccination (129). Thankfully, subsequent evidence has substantiated these policy decisions. Current clinical data now provide robust evidence for evaluating the outcomes of co-administration of influenza and SARS-CoV-2 vaccines.
According to the 2021 National Health Interview Survey in the US, a statistical study on COVID-19 infection status showed that the concurrent administration of both vaccines provided 35% infection protection, 6 percentage points higher than COVID-19 vaccination alone (130). A large-scale cohort study (n=600,000) demonstrated that dual influenza and COVID-19 vaccination was associated with a 27% reduced risk of SARS-CoV-2 infection and 45% lower all-cause mortality compared to COVID-19 vaccination alone (131). Experimental evidence from murine models corroborated these clinical findings, demonstrating that co-vaccination conferred complete protection against both SARS-CoV-2 and H1N1 challenge, with 100% survival maintenance and preservation of baseline body mass in all vaccinated subjects (91).
To systematically evaluate the safety and immunogenicity of concomitant vaccination, we conducted a PubMed search for clinical trials investigating coadministration of influenza and SARS-CoV-2 vaccines, identifying eight relevant studies published before 25 November 2024 (132–139) (Table 2). No significant safety issues regarding co-administration were reported. All age groups and all influenza and COVID-19 vaccines tested in the trials showed a high level of safety (132–136). Adverse events were usually mild in both the co-administration and mono-administration groups. Severe complications, while observed, exhibited low incidence rates with no significant differences compared to the control groups (134, 136). In all the included studies, co-administration of COVID-19 vaccines (including mRNA-1273, ChAdOx1, BNT162b2, CoronaVac, BBIBP-CorV, and NVX-CoV2373) and seasonal influenza vaccines (including HD-QIV, QIVc, QIVr, IIV4, and aTIV) had no significant negative impact on the immunogenicity of influenza or COVID-19 vaccines (132–136). Given the satisfactory immunogenicity and safety of coadministration of seasonal influenza vaccines and SARS-CoV-2 vaccines (140), public health institutions should play a central role in providing specific and tailored strategies.
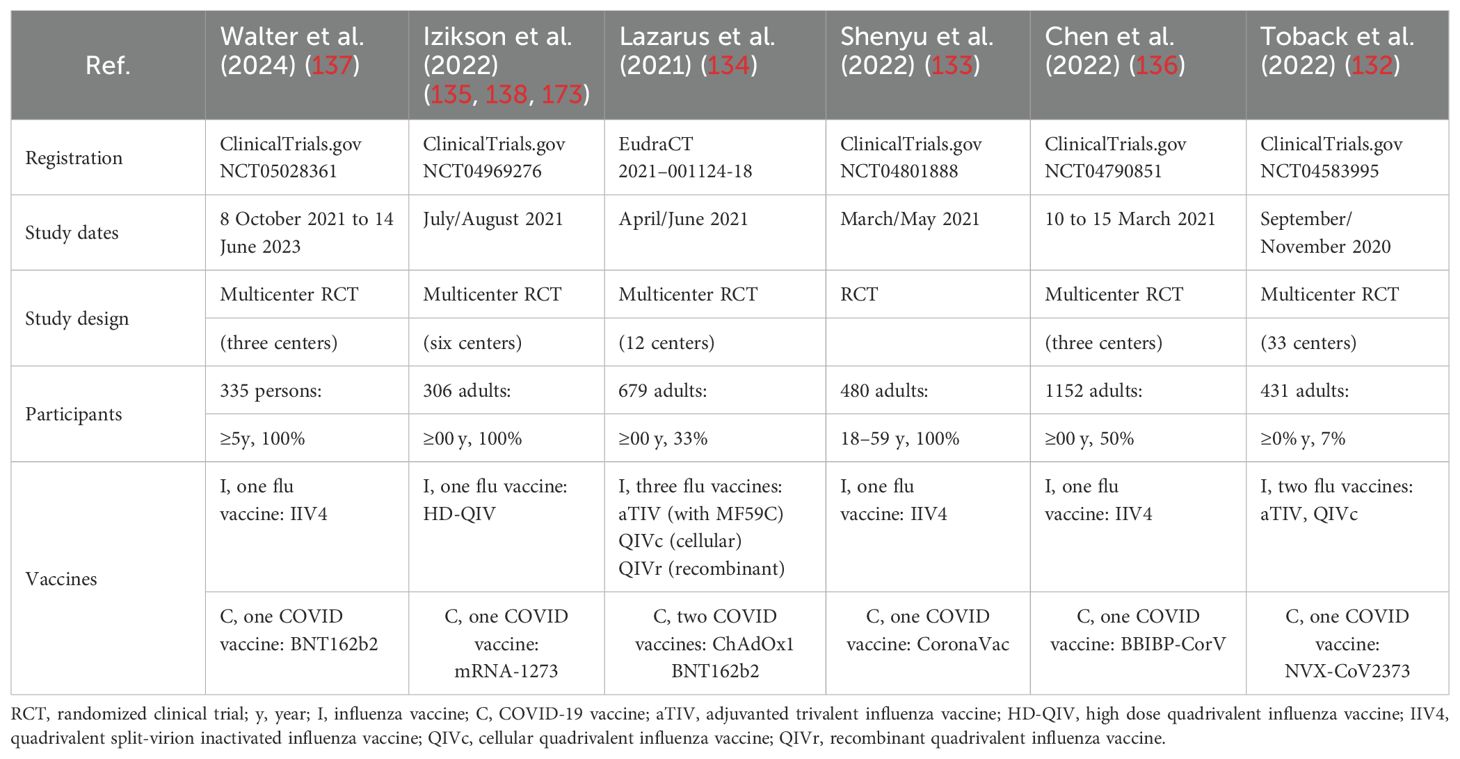
Table 2. Safety and immunogenicity clinical trial studies on the co-administration of influenza and SARS-CoV-2 vaccinations.
4 Combination vaccines for SARS-CoV-2 and the influenza virus
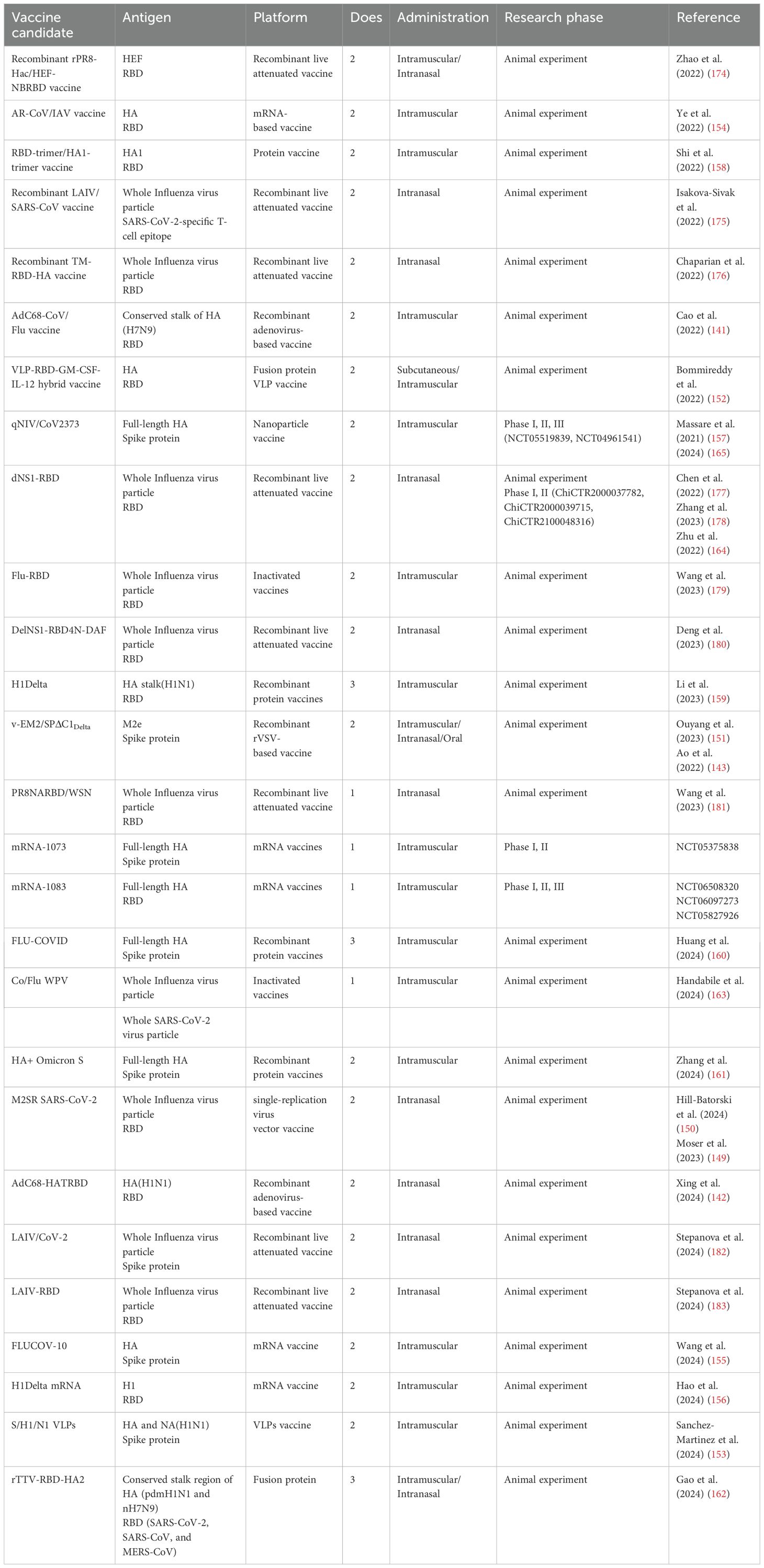
As of 25 November 2024, there were 27 successfully developed combination vaccine candidates employing diverse platform technologies documented in PubMed (Figure 4, Table 3). Among the developed combination vaccines, 12 utilized recombinant live-attenuated viral vectors, which included nine influenza virus vector vaccines, two adenovirus 68 (AdC68)-based vaccines (141, 142), and one recombinant vesicular stomatitis virus (rVSV)-based vaccine (143). In the design of influenza-vectored combination vaccines, SARS-CoV-2 antigenic sequences (e.g., RBD, spike protein, or T-cell epitopes) were inserted into the open reading frames (ORFs) of influenza viral proteins (e.g., NA or HA) in attenuated strains. This strategy enables dual antigen presentation, preserving influenza virion epitopes while expressing SARS-CoV-2 immunogens for robust immune recognition. To enhance immunogenicity, gene modifications and functional editing were employed to optimize fusion immunogen design. In contrast, AdC68 (141, 142) and rVSV (143) vector-based combination vaccines incorporated SARS-CoV-2 antigen sequences directly into the viral ORFs, leading to endogenous antigen expression in host cells. Unlike influenza vectors, which display antigens on virion surfaces, these systems presented antigens as fusion proteins, altering immune cell recognition mechanisms.
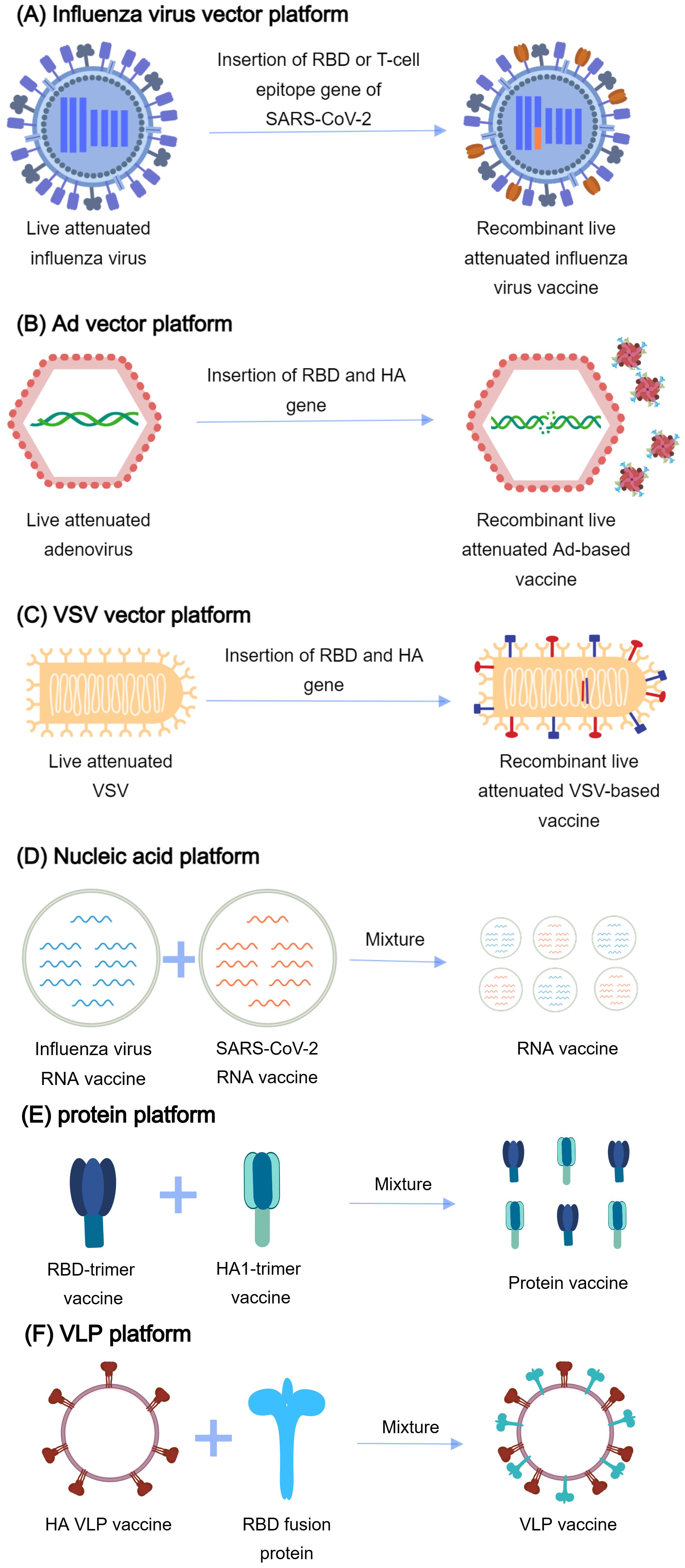
Figure 4. The platforms for vaccine development. (A) Influenza virus vector platform: The gene of RBD (receptor binding domain) or T-cell epitope of SARS-CoV-2 is inserted into the influenza virus genome. RBD or T-cell epitope is expressed on the surface of influenza virus particles. (B) Ad vector platform: The fusion gene of RBD of SARS-CoV-2, the stalk domain of hemagglutinin (HA) of influenza virus, and human ferritin is inserted into the adenovirus genome. The gene is delivered into the body by the recombinant adenovirus. The fusion immunogen self-assembles into nanoparticles. (C) VSV vector platform: The fusion gene of RBD of SARS-CoV-2 and Matrix Protein 2 Ectodomain (M2e) of the influenza virus are inserted into the vesicular stomatitis virus (VSV) genome. The gene is delivered into the body and expressed. (D) Nucleic acid platforms: A mixture of influenza virus RNA vaccine and SARS-CoV-2 RNA vaccine generates the combined vaccines. (E) Protein platforms: A mixture of the RBD-trimer vaccine and the HA1-trimer vaccine generates the combined vaccines. (F) VLP platform: Incorporation of GPI-RBD-GM-CSF (glycosylphosphatidylinositol, RBD, and granulocyte-macrophage colony-stimulating factor) fusion protein and virus-like particles (VLPs) generates the combined vaccines.
Among existing combination vaccine platforms, viral vectors—particularly influenza-based systems—represented the predominant strategy. Leveraging well-established live-attenuated influenza vaccine (LAIV) technology, these vectors effectively induced T-cell immunity (144) and have been successfully engineered to elicit robust responses against incorporated SARS-CoV-2 epitopes (145, 146). However, pre-existing influenza immunity may attenuate their efficacy, prompting the use of alternative vectors such as AdC68 and rVSV (147, 148). To address potential virulence reversion risks in LAIV platforms, single-replication influenza vectors (e.g., M2SR SARS-CoV-2) offered enhanced safety (149, 150). Viral vector vaccines inherently mimic natural infection, enabling potent immunogenicity even without adjuvants. Their compatibility with mucosal delivery [e.g., intranasal/oral administration (151)] further enhanced mucosal immunity—a critical advantage over conventional platforms.
Among the developed combination vaccine candidates, two utilized VLP technology, incorporating fusion antigens from both influenza and SARS-CoV-2 into self-assembling VLPs (152, 153). Other platforms included mRNA vaccines (154–156), nanoparticle vaccines (157), and recombinant protein vaccines (158–162). These were typically developed through physical mixing of validated monovalent vaccines. The latter approach enabled quicker clinical translation, as pre-approved monovalent components already had established safety and efficacy profiles. Notably, only one inactivated vaccine candidate was developed (163), created by mixing two separate inactivated vaccines. This limited representation suggests that traditional inactivated vaccine strategies may be less suitable for developing broad-spectrum, multivalent vaccines compared to more modular platforms.
All combination vaccine candidates demonstrated the capacity to elicit robust neutralizing antibodies and confer dual protection against influenza and SARS-CoV-2 infections. However, their immunological profiles exhibited significant variations in the durability of antibodies, the strength of cellular and humoral immunity, the presence or absence of mucosal immunity, and the level of protection against different viral subtypes. These functional differences stem primarily from divergent epitope selection strategies for influenza (e.g., HA/NA variants) and SARS-CoV-2 (e.g., RBD/spike variants) antigens across platforms. The modular design and manufacturing flexibility of these bivalent systems (VLP, mRNA, nanoparticle, etc.) are accelerating the development of next-generation multivalent vaccines with broader spectrum coverage.
Four vaccine platforms had advanced to clinical trials: two RNA vaccines (NCT05375838, NCT06097273), one influenza virus vector vaccine (ChiCTR2000037782, ChiCTR2000039715, ChiCTR2100048316) (164), and one nanoparticle vaccine (NCT05519839, NCT04961541) (165). All the platforms had demonstrated acceptable safety profiles in early-phase trials. mRNA-1073, mRNA-1083, and qNIV/CoV2373 were developed by monovalent vaccines that had been validated in clinical trials. While preliminary clinical data support the safety profiles of these four vaccine candidates, their efficacy in human populations remains to be established through large-scale Phase III trials.
Despite the proven efficacy of combination vaccines against both influenza and COVID-19, vaccine hesitancy remains a significant challenge in immunization campaigns. Multiple factors contribute to vaccination reluctance, including a limited understanding of communicable diseases (166), insufficient awareness of vaccine importance (167), socioeconomic disparities, and variable public health infrastructure (168). First and foremost, the government must ensure the safety of vaccines, as this is a matter of paramount importance (169). Educational interventions can enhance public awareness regarding infections and vaccines, thereby increasing individuals’ willingness to undergo vaccination (170). Furthermore, offering vaccination services in public venues such as pharmacies not only provides greater convenience but also has the potential to boost vaccination rates (171). In a questionnaire survey, it was found that compared with receiving the flu vaccine and the COVID-19 vaccine alone, the majority of American consumers and healthcare providers may be more inclined to receive a single-dose flu + COVID combined vaccine, an advantage of a combined vaccine (172).
5 Conclusion and future prospects
During the COVID-19 pandemic, influenza cases remained at low levels due to implemented containment measures. However, following the conclusion of COVID-19 restrictions, influenza incidence has rebounded to pre-pandemic levels. The decline in COVID-19 case numbers and the WHO’s declaration that COVID-19 no longer constitutes a Public Health Emergency of International Concern (PHEIC) have inadvertently fostered public misperceptions that SARS-CoV-2 has been eradicated. Unfortunately, the statement only means that COVID-19 is no longer an emergency and no longer requires international coordination. COVID-19 is also a persistent health issue. According to the 2023–2025 COVID-19 Strategic Preparedness and Response Plan released by WHO, millions of people each week continue to be confirmed as infected/reinfected and thousands of people are dying around the world. Given the ongoing uncertainties posed by the potential evolution of SARS-CoV-2, WHO committee members emphasized during the deliberative session that it is time to transition to long-term management rather than neglect ongoing surveillance despite the significant reduction in COVID-19 severity.
Over the past 3 years, social distancing and isolation measures proved to be the most effective but ultimately unsustainable strategy for containing viral transmission, as they significantly disrupted socioeconomic functioning. Against both SARS-CoV-2 and influenza, vaccines remain the most viable solution for interrupting transmission chains. To date, over 13 billion doses of COVID-19 vaccines have been administered globally. According to an Imperial College London study on excess mortality (2022), emergency-authorized vaccines saved an estimated 20 million lives between December 2020 and December 2021 alone. As previously discussed, co-administration of influenza and COVID-19 vaccines provided superior protection compared to monovalent vaccination. Animal studies demonstrated that combination vaccines not only enhanced efficacy but also reduced immunization frequency and adverse events. Although human clinical validation is ongoing, this approach represents a promising strategy for pandemic containment. New mutated strains and immune escape drive scientists to adopt new technologies and platforms in constructing more effective and broad-spectrum combination vaccines.
The combination vaccines mentioned above can provide protection against a variety of subtypes of influenza viruses and SARS-CoV-2. However, the range of virus subtypes that vaccines can target is restricted, and their efficacy against newly emerging subtypes cannot be assured. It is imperative to allocate additional resources toward vaccine research and development. Furthermore, public health authorities should enhance promotional efforts to raise public awareness regarding the significance of vaccination and encourage greater acceptance. Simultaneously, measures should be implemented to improve the accessibility and convenience of vaccination services.
Combination vaccines offer governments significant cost-containment advantages through streamlined production and distribution, while simultaneously improving population-level vaccine uptake by reducing required immunization visits. Combination vaccine design platforms provide valuable foundational experience for future vaccine development. The focus of our discussion in this article is combination vaccines for influenza and COVID-19, but the vaccine platform can be used for any two or more pathogens, even cancer. Even if efforts to develop new combination vaccine technologies and platforms do not yield direct benefits against COVID-19 and influenza, they could still contribute to combating other diseases, like malignant tumors or other infectious diseases, or even a new pandemic. The flexibility and scalability of the vaccine strategies mentioned above provide more opportunities for experimentation in vaccines and greater potential for creating broad-spectrum, multivalent vaccines. Although there is no vaccine that can simultaneously protect people against annual seasonal influenza and SARS-CoV-2 variants, it may be on the way.
Author contributions
CH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. CN: Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. XHL: Supervision, Data curation, Formal analysis, Funding acquisition, Writing – review & editing. KH: Data curation, Investigation, Methodology, Project administration, Software, Writing – original draft. ML: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. XG: Investigation, Methodology, Software, Writing – original draft. QW: Data curation, Investigation, Software, Writing – original draft. WS: Formal analysis, Resources, Software, Writing – original draft. YZ: Formal analysis, Methodology, Project administration, Writing – original draft. YL: Investigation, Project administration, Resources, Writing – original draft. ZR: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. XDL: Supervision, Data curation, Formal analysis, Funding acquisition, Writing – review & editing. TW: Funding acquisition, Resources, Supervision, Writing – review & editing. XX: Project administration, Funding acquisition, Conceptualization, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Youth Fund of the National Natural Science Foundation of China (No. 31902287), Key Research Projects of Higher Education Institutions in Henan Province (No.25A310004), Henan Province Key Research and Promotion Special Project (Science and Technology Tackling) (No.242102310467), Henan Medical Education Research Project (No.WJLX2023104), the open project research of the First Affiliated Hospital of Henan University (No.KFZD24001), Science and Technology Tackling Program of Henan Province (No. 242102310190), Joint Construction Project of Henan Medical Science and Technology Research Program (No. LHJG20240389), Kaifeng Science and Technology Development Plan Project (No. 2303020), the open project research of the first Affiliated Hospital of Henan University (No. KFZD24002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stepanova E, Isakova-Sivak I, and Rudenko L. Options for the development of a bivalent vaccine against SARS-CoV-2 and influenza. Expert Rev Vaccines. (2022) . 21:1533–5. doi: 10.1080/14760584.2022.2117692
2. World Health Organization. Influenza (seasonal) . Available online at: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal) (Accessed November 25, 2024).
3. Thompson WW, Weintraub E, Dhankhar P, Cheng PY, Brammer L, Meltzer MI, et al. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respir Viruses. (2009) . 3:37–49. doi: 10.1111/j.1750-2659.2009.00073.x
4. Soo RJJ, Chiew CJ, Ma S, Pung R, and Lee V. Decreased influenza incidence under COVID-19 control measures, Singapore. Emerg Infect Dis. (2020) . 26:1933–5. doi: 10.3201/eid2608.201229
5. Feng L, Zhang T, Wang Q, Xie Y, Peng Z, Zheng J, et al. Impact of COVID-19 outbreaks and interventions on influenza in China and the United States. Nat Commun. (2021) . 12:3249. doi: 10.1038/s41467-021-23440-1
6. Organization WH. FluNet summary. World Health Organization. (2024). Available online at: https://www.who.int/tools/flunet/flunet-summary (Accessed November 25, 2024).
7. Cowling BJ, Ali ST, Ng TWY, Tsang TK, Li JCM, Fong MW, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. (2020) . 5:e279–88. doi: 10.1016/s2468-2667(20)30090-6
8. Solomon DA, Sherman AC, and Kanjilal S. Influenza in the COVID-19 era. Jama. (2020) . 324:1342–3. doi: 10.1001/jama.2020.14661
9. Lee K, Jalal H, Raviotta JM, Krauland MG, Zimmerman RK, Burke DS, et al. Estimating the impact of low influenza activity in 2020 on population immunity and future influenza seasons in the United States. Open Forum Infect Dis. (2022) 9:ofab607. doi: 10.1093/ofid/ofab607
10. Zhu W and Gu L. Resurgence of seasonal influenza driven by A/H3N2 and B/Victoria in succession during the 2023–2024 season in Beijing showing increased population susceptibility. J Med Virol. (2024) . 96:e29751. doi: 10.1002/jmv.29751
11. Javanian M, Barary M, Ghebrehewet S, Koppolu V, Vasigala V, and Ebrahimpour S. A brief review of influenza virus infection. J Med Virol. (2021) . 93:4638–46. doi: 10.1002/jmv.26990
12. Hu L, Lao G, Liu R, Feng J, Long F, and Peng T. The race toward a universal influenza vaccine: Front runners and the future directions. Antiviral Res. (2023) . 210:105505. doi: 10.1016/j.antiviral.2022.105505
13. Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, and Wilson IA. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. (2006) . 312:404–10. doi: 10.1126/science.1124513
14. Zost SJ, Wu NC, Hensley SE, and Wilson IA. Immunodominance and antigenic variation of influenza virus hemagglutinin: implications for design of universal vaccine immunogens. J Infect Dis. (2019) 219:S38–s45. doi: 10.1093/infdis/jiy696
15. Kim H, Webster RG, and Webby RJ. Influenza virus: dealing with a drifting and shifting pathogen. Viral Immunol. (2018) . 31:174–83. doi: 10.1089/vim.2017.0141
16. Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. (2016) . 16:942–51. doi: 10.1016/s1473-3099(16)00129-8
17. Coughlan L and Palese P. Overcoming barriers in the path to a universal influenza virus vaccine. Cell Host Microbe. (2018) . 24:18–24. doi: 10.1016/j.chom.2018.06.016
18. Nachbagauer R and Palese P. Is a universal influenza virus vaccine possible? Annu Rev Med. (2020) . 71:315–27. doi: 10.1146/annurev-med-120617-041310
19. Erbelding EJ, Post DJ, Stemmy EJ, Roberts PC, Augustine AD, Ferguson S, et al. A universal influenza vaccine: the strategic plan for the national institute of allergy and infectious diseases. J Infect Dis. (2018) . 218:347–54. doi: 10.1093/infdis/jiy103
20. Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. (2011) . 333:843–50. doi: 10.1126/science.1204839
21. Throsby M, van den Brink E, Jongeneelen M, Poon LL, Alard P, Cornelissen L, et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PloS One. (2008) . 3:e3942. doi: 10.1371/journal.pone.0003942
22. Zebedee SL and Lamb RA. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. (1988) . 62:2762–72. doi: 10.1128/jvi.62.8.2762-2772.1988
23. Lee YN, Kim MC, Lee YT, Kim YJ, and Kang SM. Mechanisms of cross-protection by influenza virus M2-based vaccines. Immune Netw. (2015) . 15:213–21. doi: 10.4110/in.2015.15.5.213
24. Voeten JT, Bestebroer TM, Nieuwkoop NJ, Fouchier RA, Osterhaus AD, and Rimmelzwaan GF. Antigenic drift in the influenza A virus (H3N2) nucleoprotein and escape from recognition by cytotoxic T lymphocytes. J Virol. (2000) . 74:6800–7. doi: 10.1128/jvi.74.15.6800-6807.2000
25. Brown LE and Kelso A. Prospects for an influenza vaccine that induces cross-protective cytotoxic T lymphocytes. Immunol Cell Biol. (2009) . 87:300–8. doi: 10.1038/icb.2009.16
26. Monto AS, Petrie JG, Cross RT, Johnson E, Liu M, Zhong W, et al. Antibody to influenza virus neuraminidase: an independent correlate of protection. J Infect Dis. (2015) . 212:1191–9. doi: 10.1093/infdis/jiv195
27. Wohlbold TJ and Krammer F. In the shadow of hemagglutinin: a growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses. (2014) . 6:2465–94. doi: 10.3390/v6062465
28. Wang WC, Sayedahmed EE, Sambhara S, and Mittal SK. Progress towards the development of a universal influenza vaccine. Viruses. (2022) 14:73. doi: 10.3390/v14081684
29. Huang Y, França MS, Allen JD, Shi H, and Ross TM. Next generation of computationally optimized broadly reactive HA vaccines elicited cross-reactive immune responses and provided protection against H1N1 virus infection. Vaccines (Basel). (2021) 9:107. doi: 10.3390/vaccines9070793
30. Allen JD and Ross TM. Next generation methodology for updating HA vaccines against emerging human seasonal influenza A(H3N2) viruses. Sci Rep. (2021) . 11:4554. doi: 10.1038/s41598-020-79590-7
31. Kim MC, Song JM, O E, Kwon YM, Lee YJ, Compans RW, et al. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther. (2013) . 21:485–92. doi: 10.1038/mt.2012.246
32. Kirsteina A, Akopjana I, Bogans J, Lieknina I, Jansons J, Skrastina D, et al. Construction and immunogenicity of a novel multivalent vaccine prototype based on conserved influenza virus antigens. Vaccines (Basel). (2020) 8:197. doi: 10.3390/vaccines8020197
33. Kanekiyo M, Joyce MG, Gillespie RA, Gallagher JR, Andrews SF, Yassine HM, et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat Immunol. (2019) . 20:362–72. doi: 10.1038/s41590-018-0305-x
34. Wang Y, Deng L, Gonzalez GX, Luthra L, Dong C, Ma Y, et al. Double-layered M2e-NA protein nanoparticle immunization induces broad cross-protection against different influenza viruses in mice. Adv Healthc Mater. (2020) . 9:e1901176. doi: 10.1002/adhm.201901176
35. Hiremath J, Kang KI, Xia M, Elaish M, Binjawadagi B, Ouyang K, et al. Entrapment of H1N1 influenza virus derived conserved peptides in PLGA nanoparticles enhances T cell response and vaccine efficacy in pigs. PloS One. (2016) . 11:e0151922. doi: 10.1371/journal.pone.0151922
36. Coughlan L, Mullarkey C, and Gilbert S. Adenoviral vectors as novel vaccines for influenza. J Pharm Pharmacol. (2015) . 67:382–99. doi: 10.1111/jphp.12350
37. Asthagiri Arunkumar G, McMahon M, Pavot V, Aramouni M, Ioannou A, Lambe T, et al. Vaccination with viral vectors expressing NP, M1 and chimeric hemagglutinin induces broad protection against influenza virus challenge in mice. Vaccine. (2019) . 37:5567–77. doi: 10.1016/j.vaccine.2019.07.095
38. Yan J, Morrow MP, Chu JS, Racine T, Reed CC, Khan AS, et al. Broad cross-protective anti-hemagglutination responses elicited by influenza microconsensus DNA vaccine. Vaccine. (2018) 36:3079–89. doi: 10.1016/j.vaccine.2017.09.086
39. Freyn AW, Ramos da Silva J, Rosado VC, Bliss CM, Pine M, Mui BL, et al. A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice. Mol Ther. (2020) . 28:1569–84. doi: 10.1016/j.ymthe.2020.04.018
40. WHO coronavirus (COVID-19) dashboard . Available online at: https://covid19.who.int/ (Accessed November 25, 2024).
41. Alvarez E, Bielska IA, Hopkins S, Belal AA, Goldstein DM, Slick J, et al. Limitations of COVID-19 testing and case data for evidence-informed health policy and practice. Health Res Policy Syst. (2023) . 21:11. doi: 10.1186/s12961-023-00963-1
42. Greenhalgh T, Knight M, A’Court C, Buxton M, and Husain L. Management of post-acute covid-19 in primary care. Bmj. (2020) 370:m3026. doi: 10.1136/bmj.m3026
43. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. (2021) . 27:601–15. doi: 10.1038/s41591-021-01283-z
44. Dennis A, Cuthbertson DJ, Wootton D, Crooks M, Gabbay M, Eichert N, et al. Multi-organ impairment and long COVID: a 1-year prospective, longitudinal cohort study. J R Soc Med. (2023) . 116:97–112. doi: 10.1177/01410768231154703
45. Khattak S, Faheem M, Nawaz B, Khan M, Khan NH, Ullah N, et al. Knowledge, attitude, and perception of cancer patients towards COVID-19 in Pakistan: A cross-sectional study. Int J Environ Res Public Health. (2022) 19:7926. doi: 10.3390/ijerph19137926
46. The situation of the coronavirus infection in China (2023). Available online at: https://www.Chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202303/t20230318_264368.html (Accessed November 25, 2024).
47. Wang B, Yu Y, Yu Y, Wang N, Chen F, Jiang B, et al. Clinical features and outcomes of hospitalized patients with COVID-19 during the Omicron wave in Shanghai, China. J Infect. (2023) . 86:e27–9. doi: 10.1016/j.jinf.2022.08.001
48. Xu R, Wang W, and Zhang W. As the SARS-CoV-2 virus evolves, should Omicron subvariant BA.2 be subjected to quarantine, or should we learn to live with it? Front Public Health. (2022) . 10:1039123. doi: 10.3389/fpubh.2022.1039123
49. Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. (2020) . 9:221–36. doi: 10.1080/22221751.2020.1719902
50. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. (2020) . 367:1260–3. doi: 10.1126/science.abb2507
51. Chen Y, Guo Y, Pan Y, and Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. (2020) . 525:135–40. doi: 10.1016/j.bbrc.2020.02.071
52. Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. (2020) 117:11727–34. doi: 10.1073/pnas.2003138117
53. Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. (2020) . 17:613–20. doi: 10.1038/s41423-020-0400-4
54. Han YJ, Ren ZG, Li XX, Yan JL, Ma CY, Wu DD, et al. Advances and challenges in the prevention and treatment of COVID-19. Int J Med Sci. (2020) . 17:1803–10. doi: 10.7150/ijms.47836
55. Fehr AR and Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. (2015) . 1282:1–23. doi: 10.1007/978-1-4939-2438-7_1
56. Tregoning JS, Flight KE, Higham SL, Wang Z, and Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. (2021) . 21:626–36. doi: 10.1038/s41577-021-00592-1
57. Hayn M, Hirschenberger M, Koepke L, Nchioua R, Straub JH, Klute S, et al. Systematic functional analysis of SARS-CoV-2 proteins uncovers viral innate immune antagonists and remaining vulnerabilities. Cell Rep. (2021) . 35:109126. doi: 10.1016/j.celrep.2021.109126
58. Benvenuto D, Angeletti S, Giovanetti M, Bianchi M, Pascarella S, Cauda R, et al. Evolutionary analysis of SARS-CoV-2: how mutation of Non-Structural Protein 6 (NSP6) could affect viral autophagy. J Infect. (2020) . 81:e24–7. doi: 10.1016/j.jinf.2020.03.058
59. Ferreira AC, Soares VC, de Azevedo-Quintanilha IG, Dias S, Fintelman-Rodrigues N, Sacramento CQ, et al. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discov. (2021) . 7:43. doi: 10.1038/s41420-021-00428-w
60. Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP, et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N Engl J Med. (2020) . 383:1544–55. doi: 10.1056/NEJMoa2024671
61. Francica JR, Flynn BJ, Foulds KE, Noe AT, Werner AP, Moore IN, et al. Protective antibodies elicited by SARS-CoV-2 spike protein vaccination are boosted in the lung after challenge in nonhuman primates. Sci Transl Med. (2021) 13:4547. doi: 10.1126/scitranslmed.abi4547
62. WHO. COVID-19 vaccine tracker and landscape . Available online at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (Accessed November 25, 2024).
63. Li H, Zhang Y, Li D, Deng YQ, Xu H, Zhao C, et al. Enhanced protective immunity against SARS-CoV-2 elicited by a VSV vector expressing a chimeric spike protein. Signal Transduct Target Ther. (2021) . 6:389. doi: 10.1038/s41392-021-00797-9
64. Matić Z and Šantak M. Current view on novel vaccine technologies to combat human infectious diseases. Appl Microbiol Biotechnol. (2022) . 106:25–56. doi: 10.1007/s00253-021-11713-0
65. Ward BJ, Gobeil P, Séguin A, Atkins J, Boulay I, Charbonneau PY, et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat Med. (2021) . 27:1071–8. doi: 10.1038/s41591-021-01370-1
66. Prates-Syed WA, Chaves LCS, Crema KP, Vuitika L, Lira A, Côrtes N, et al. VLP-based COVID-19 vaccines: an adaptable technology against the threat of new variants. Vaccines (Basel). (2021) 9:1409. doi: 10.3390/vaccines9121409
67. Ren Z, Zhao Y, Liu J, Ji X, Meng L, Wang T, et al. Inclusion of membrane-anchored LTB or flagellin protein in H5N1 virus-like particles enhances protective responses following intramuscular and oral immunization of mice. Vaccine. (2018) . 36:5990–8. doi: 10.1016/j.vaccine.2018.08.053
68. Ren Z, Zhao Y, Liu J, Ji X, Meng L, Wang T, et al. Intramuscular and intranasal immunization with an H7N9 influenza virus-like particle vaccine protects mice against lethal influenza virus challenge. Int Immunopharmacol. (2018) . 58:109–16. doi: 10.1016/j.intimp.2017.12.020
69. Ren Z, Ji X, Meng L, Wei Y, Wang T, Feng N, et al. H5N1 influenza virus-like particle vaccine protects mice from heterologous virus challenge better than whole inactivated virus. Virus Res. (2015) . 200:9–18. doi: 10.1016/j.virusres.2015.01.007
70. Tian JH, Patel N, Haupt R, Zhou H, Weston S, Hammond H, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun. (2021) . 12:372. doi: 10.1038/s41467-020-20653-8
71. Karothia D, Dash PK, Parida M, Bhagyawant S, and Kumar JS. Inhibition of West Nile virus Replication by Bifunctional siRNA Targeting the NS2A and NS5 Conserved Region. Curr Gene Ther. (2018) . 18:180–90. doi: 10.2174/1566523218666180607091311
72. Fang E, Liu X, Li M, Zhang Z, Song L, Zhu B, et al. Advances in COVID-19 mRNA vaccine development. Signal Transduct Target Ther. (2022) . 7:94. doi: 10.1038/s41392-022-00950-y
73. Lim M, Badruddoza AZM, Firdous J, Azad M, Mannan A, Al-Hilal TA, et al. Engineered nanodelivery systems to improve DNA vaccine technologies. Pharmaceutics. (2020) 12:30. doi: 10.3390/pharmaceutics12010030
74. Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, et al. Structural and functional basis of SARS-coV-2 entry by using human ACE2. Cell. (2020) . 181:894–904.e9. doi: 10.1016/j.cell.2020.03.045
75. Shinya K, Ebina M, Yamada S, Ono M, Kasai N, and Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. (2006) . 440:435–6. doi: 10.1038/440435a
76. Traylor ZP, Aeffner F, and Davis IC. Influenza A H1N1 induces declines in alveolar gas exchange in mice consistent with rapid post-infection progression from acute lung injury to ARDS. Influenza Other Respir Viruses. (2013) . 7:472–9. doi: 10.1111/j.1750-2659.2012.00414.x
77. Mezencev R, Klement C, and Dluholucký S. Potential problem of the co-occurrence of pandemic COVID-19 and seasonal influenza. Epidemiol Mikrobiol Imunol. (2021) 70:68–71.
78. Yang J, Gong Y, Zhang C, Sun J, Wong G, Shi W, et al. Co-existence and co-infection of influenza A viruses and coronaviruses: Public health challenges. Innovation (Camb). (2022) . 3:100306. doi: 10.1016/j.xinn.2022.100306
79. confirmed cases of influenza (2024). Available online at: https://ourworldindata.org/explorers/influenza (Accessed November 25, 2024).
80. Number of COVID-19 cases reported to WHO (2024). Available online at: https://data.who.int/dashboards/covid19/cases?n=c (Accessed November 25, 2024).
81. Covin S and Rutherford GW. Coinfection, severe acute respiratory syndrome coronavirus 2 (SARS-coV-2), and influenza: an evolving puzzle. Clin Infect Dis. (2021) . 72:e993–4. doi: 10.1093/cid/ciaa1810
82. Hashemi SA, Safamanesh S, Ghasemzadeh-Moghaddam H, Ghafouri M, and Azimian A. High prevalence of SARS-CoV-2 and influenza A virus (H1N1) coinfection in dead patients in Northeastern Iran. J Med Virol. (2021) . 93:1008–12. doi: 10.1002/jmv.26364
83. Wu X, Cai Y, Huang X, Yu X, Zhao L, Wang F, et al. Co-infection with SARS-coV-2 and influenza A virus in patient with pneumonia, China. Emerg Infect Dis. (2020) . 26:1324–6. doi: 10.3201/eid2606.200299
84. Jones N. How COVID-19 is changing the cold and flu season. Nature. (2020) . 588:388–90. doi: 10.1038/d41586-020-03519-3
85. Zhao P, Zhang Y, Wang J, Li Y, Wang Y, Gao Y, et al. Epidemiology of respiratory pathogens in patients with acute respiratory infections during the COVID-19 pandemic and after easing of COVID-19 restrictions. Microbiol Spectr. (2024) . 12:e0116124. doi: 10.1128/spectrum.01161-24
86. Li H, Yang Y, Tao R, and Shang S. Analyzing infections caused by 11 respiratory pathogens in children: Pre- and post-COVID-19 pandemic trends in China. J Med Virol. (2024) . 96:e29929. doi: 10.1002/jmv.29929
87. Bai L, Zhao Y, Dong J, Liang S, Guo M, Liu X, et al. Coinfection with influenza A virus enhances SARS-CoV-2 infectivity. Cell Res. (2021) . 31:395–403. doi: 10.1038/s41422-021-00473-1
88. Iacobucci G. Covid-19: Risk of death more than doubled in people who also had flu, English data show. Bmj. (2020) 370:m3720. doi: 10.1136/bmj.m3720
89. Stowe J, Tessier E, Zhao H, Guy R, Muller-Pebody B, Zambon M, et al. Interactions between SARS-CoV-2 and influenza, and the impact of coinfection on disease severity: a test-negative design. Int J Epidemiol. (2021) . 50:1124–33. doi: 10.1093/ije/dyab081
90. Xiang X, Wang ZH, Ye LL, He XL, Wei XS, Ma YL, et al. Co-infection of SARS-COV-2 and influenza A virus: A case series and fast review. Curr Med Sci. (2021) . 41:51–7. doi: 10.1007/s11596-021-2317-2
91. Bao L, Deng W, Qi F, Lv Q, Song Z, Liu J, et al. Sequential infection with H1N1 and SARS-CoV-2 aggravated COVID-19 pathogenesis in a mammalian model, and co-vaccination as an effective method of prevention of COVID-19 and influenza. Signal Transduct Target Ther. (2021) . 6:200. doi: 10.1038/s41392-021-00618-z
92. Huang Y, Skarlupka AL, Jang H, Blas-MaChado U, Holladay N, Hogan RJ, et al. SARS-coV-2 and influenza A virus coinfections in ferrets. J Virol. (2022) . 96:e0179121. doi: 10.1128/jvi.01791-21
93. Kim EH, Nguyen TQ, Casel MAB, Rollon R, Kim SM, Kim YI, et al. Coinfection with SARS-coV-2 and influenza A virus increases disease severity and impairs neutralizing antibody and CD4(+) T cell responses. J Virol. (2022) . 96:e0187321. doi: 10.1128/jvi.01873-21
94. Lansbury L, Lim B, Baskaran V, and Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. (2020) . 81:266–75. doi: 10.1016/j.jinf.2020.05.046
95. Dao TL, Hoang VT, Colson P, Million M, and Gautret P. Co-infection of SARS-CoV-2 and influenza viruses: A systematic review and meta-analysis. J Clin Virol Plus. (2021) . 1:100036. doi: 10.1016/j.jcvp.2021.100036
96. Sarkar S, Khanna P, and Singh AK. Impact of COVID-19 in patients with concurrent co-infections: A systematic review and meta-analyses. J Med Virol. (2021) . 93:2385–95. doi: 10.1002/jmv.26740
97. Cong B, Deng S, Wang X, and Li Y. The role of respiratory co-infection with influenza or respiratory syncytial virus in the clinical severity of COVID-19 patients: A systematic review and meta-analysis. J Glob Health. (2022) . 12:5040. doi: 10.7189/jogh.12.05040
98. Krumbein H, Kümmel LS, Fragkou PC, Thölken C, Hünerbein BL, Reiter R, et al. Respiratory viral co-infections in patients with COVID-19 and associated outcomes: A systematic review and meta-analysis. Rev Med Virol. (2023) . 33:e2365. doi: 10.1002/rmv.2365
99. Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, and Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PloS One. (2021) . 16:e0251170. doi: 10.1371/journal.pone.0251170
100. Yan X, Li K, Lei Z, Luo J, Wang Q, and Wei S. Prevalence and associated outcomes of coinfection between SARS-CoV-2 and influenza: a systematic review and meta-analysis. Int J Infect Dis. (2023) . 136:29–36. doi: 10.1016/j.ijid.2023.08.021
101. Guan Z, Chen C, Li Y, Yan D, Zhang X, Jiang D, et al. Impact of coinfection with SARS-coV-2 and influenza on disease severity: A systematic review and meta-analysis. Front Public Health. (2021) . 9:773130. doi: 10.3389/fpubh.2021.773130
102. Swets MC, Russell CD, Harrison EM, Docherty AB, Lone N, Girvan M, et al. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet. (2022) . 399:1463–4. doi: 10.1016/s0140-6736(22)00383-x
103. Piret J and Boivin G. Viral interference between respiratory viruses. Emerg Infect Dis. (2022) . 28:273–81. doi: 10.3201/eid2802.211727
104. Sakamoto H, Ishikane M, and Ueda P. Seasonal influenza activity during the SARS-coV-2 outbreak in Japan. Jama. (2020) . 323:1969–71. doi: 10.1001/jama.2020.6173
105. Taghioff SM, Slavin BR, Holton T, and Singh D. Examining the potential benefits of the influenza vaccine against SARS-CoV-2: A retrospective cohort analysis of 74,754 patients. PloS One. (2021) . 16:e0255541. doi: 10.1371/journal.pone.0255541
106. Jiang B, Huang Q, Jia M, Xue X, Wang Q, Yang W, et al. Association between influenza vaccination and SARS-CoV-2 infection and its outcomes: systematic review and meta-analysis. Chin Med J (Engl). (2022) . 135:2282–93. doi: 10.1097/cm9.0000000000002427
107. Del Riccio M, Lorini C, Bonaccorsi G, Paget J, and Caini S. The association between influenza vaccination and the risk of SARS-coV-2 infection, severe illness, and death: A systematic review of the literature. Int J Environ Res Public Health. (2020) 17:7870. doi: 10.3390/ijerph17217870
108. Sim JY, Wu PS, Cheng CF, Chao YC, and Yu CH. Effectiveness of Booster and Influenza Vaccines against COVID-19 among Healthcare Workers, Taiwan. Emerg Infect Dis. (2022) . 28:2126–30. doi: 10.3201/eid2810.221134
109. Domnich A, Orsi A, Sticchi L, Panatto D, Dini G, Ferrari A, et al. Effect of the 2020/21 season influenza vaccine on SARS-CoV-2 infection in a cohort of Italian healthcare workers. Vaccine. (2022) . 40:1755–60. doi: 10.1016/j.vaccine.2022.02.013
110. Tayar E, Abdeen S, Abed Alah M, Chemaitelly H, Bougmiza I, Ayoub HH, et al. Effectiveness of influenza vaccination against SARS-CoV-2 infection among healthcare workers in Qatar. J Infect Public Health. (2023) . 16:250–6. doi: 10.1016/j.jiph.2022.12.016
111. Shosha SH, Ajlan DI, and Al-Ghatam R. Does influenza vaccination help reduce incidence of COVID-19 infection among hospital employees? Med (Baltimore). (2022) . 101:e28479. doi: 10.1097/md.0000000000028479
112. Cocco P, Meloni F, Coratza A, Schirru D, Campagna M, and De Matteis S. Vaccination against seasonal influenza and socio-economic and environmental factors as determinants of the geographic variation of COVID-19 incidence and mortality in the Italian elderly. Prev Med. (2021) . 143:106351. doi: 10.1016/j.ypmed.2020.106351
113. Green I, Ashkenazi S, Merzon E, Vinker S, and Golan-Cohen A. The association of previous influenza vaccination and coronavirus disease-2019. Hum Vaccin Immunother. (2021) . 17:2169–75. doi: 10.1080/21645515.2020.1852010
114. Hosseini-Moghaddam SM, He S, Calzavara A, Campitelli MA, and Kwong JC. Association of influenza vaccination with SARS-coV-2 infection and associated hospitalization and mortality among patients aged 66 years or older. JAMA Netw Open. (2022) . 5:e2233730. doi: 10.1001/jamanetworkopen.2022.33730
115. Chen AT, Stacey HD, Marzok A, Singh P, Ang J, Miller MS, et al. Effect of inactivated influenza vaccination on human coronavirus infection: Secondary analysis of a randomized trial in Hutterite colonies. Vaccine. (2021) . 39:7058–65. doi: 10.1016/j.vaccine.2021.10.021
116. Conlon A, Ashur C, Washer L, Eagle KA, and Hofmann Bowman MA. Impact of the influenza vaccine on COVID-19 infection rates and severity. Am J Infect Control. (2021) . 49:694–700. doi: 10.1016/j.ajic.2021.02.012
117. Candelli M, Pignataro G, Torelli E, Gullì A, Nista EC, Petrucci M, et al. Effect of influenza vaccine on COVID-19 mortality: a retrospective study. Intern Emerg Med. (2021) . 16:1849–55. doi: 10.1007/s11739-021-02702-2
118. Umasabor-Bubu OQ, Bubu OM, Mbah AK, Nakeshbandi M, and Taylor TN. Association between Influenza Vaccination and severe COVID-19 outcomes at a designated COVID-only hospital in Brooklyn. Am J Infect Control. (2021) . 49:1327–30. doi: 10.1016/j.ajic.2021.04.006
119. Ryan KA, Schewe KE, Crowe J, Fotheringham SA, Hall Y, Humphreys R, et al. Sequential delivery of live attenuated influenza vaccine and severe acute respiratory syndrome coronavirus 2 (SARS-coV-2) in the ferret model can reduce SARS-coV-2 shedding and does not result in enhanced lung pathology. J Infect Dis. (2022) . 225:404–12. doi: 10.1093/infdis/jiab594
120. Debisarun PA, Gössling KL, Bulut O, Kilic G, Zoodsma M, Liu Z, et al. Induction of trained immunity by influenza vaccination - impact on COVID-19. PloS Pathog. (2021) . 17:e1009928. doi: 10.1371/journal.ppat.1009928
121. Poulas K, Farsalinos K, and Zanidis C. Activation of TLR7 and innate immunity as an efficient method against COVID-19 pandemic: imiquimod as a potential therapy. Front Immunol. (2020) . 11:1373. doi: 10.3389/fimmu.2020.01373
122. Jost S and Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. (2013) . 31:163–94. doi: 10.1146/annurev-immunol-032712-100001
123. Kim TS and Shin EC. The activation of bystander CD8(+) T cells and their roles in viral infection. Exp Mol Med. (2019) . 51:1–9. doi: 10.1038/s12276-019-0316-1
124. Jewett A. The potential effect of novel coronavirus SARS-coV-2 on NK cells; A perspective on potential therapeutic interventions. Front Immunol. (2020) . 11:1692. doi: 10.3389/fimmu.2020.01692
125. Benn CS, Netea MG, Selin LK, and Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. (2013) . 34:431–9. doi: 10.1016/j.it.2013.04.004
126. Cao RG, Suarez NM, Obermoser G, Lopez SM, Flano E, Mertz SE, et al. Differences in antibody responses between trivalent inactivated influenza vaccine and live attenuated influenza vaccine correlate with the kinetics and magnitude of interferon signaling in children. J Infect Dis. (2014) . 210:224–33. doi: 10.1093/infdis/jiu079
127. Gupta T and Gupta SK. Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. Int Immunopharmacol. (2020) . 86:106717. doi: 10.1016/j.intimp.2020.106717
128. Organization WH. Coadministration of seasonal inactivated influenza and COVID-19 vaccines: interim guidance . Available online at: https://apps.who.int/iris/handle/10665/346897 (Accessed November 25, 2024).
129. Agency UHS. COVID-19 - SARS-coV-2 . Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1102459/Greenbook-chapter-14a-4September22.pdf (Accessed November 25, 2024).
130. Xie Z, Hamadi HY, Mainous AG, and Hong YR. Association of dual COVID-19 and seasonal influenza vaccination with COVID-19 infection and disease severity. Vaccine. (2023) . 41:875–8. doi: 10.1016/j.vaccine.2022.12.043
131. Russo AG, Faccini M, Decarli A, Cattaneo S, Tunesi S, Murtas R, et al. First SARS-CoV-2 vaccine booster and influenza vaccination: risk assessment of COVID-19 hospitalisation and death. Epidemiol Prev. (2022) . 46:324–32. doi: 10.19191/ep22.5-6.070
132. Toback S, Galiza E, Cosgrove C, Galloway J, Goodman AL, Swift PA, et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: an exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet Respir Med. (2022) . 10:167–79. doi: 10.1016/s2213-2600(21)00409-4
133. Shenyu W, Xiaoqian D, Bo C, Xuan D, Zeng W, Hangjie Z, et al. Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine (CoronaVac) co-administered with an inactivated quadrivalent influenza vaccine: A randomized, open-label, controlled study in healthy adults aged 18 to 59 years in China. Vaccine. (2022) . 40:5356–65. doi: 10.1016/j.vaccine.2022.07.021
134. Lazarus R, Baos S, Cappel-Porter H, Carson-Stevens A, Clout M, Culliford L, et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): a multicentre, randomised, controlled, phase 4 trial. Lancet. (2021) . 398:2277–87. doi: 10.1016/s0140-6736(21)02329-1
135. Izikson R, Brune D, Bolduc JS, Bourron P, Fournier M, Moore TM, et al. Safety and immunogenicity of a high-dose quadrivalent influenza vaccine administered concomitantly with a third dose of the mRNA-1273 SARS-CoV-2 vaccine in adults aged ≥65 years: a phase 2, randomised, open-label study. Lancet Respir Med. (2022) . 10:392–402. doi: 10.1016/s2213-2600(21)00557-9
136. Chen H, Huang Z, Chang S, Hu M, Lu Q, Zhang Y, et al. Immunogenicity and safety of an inactivated SARS-CoV-2 vaccine (Sinopharm BBIBP-CorV) coadministered with quadrivalent split-virion inactivated influenza vaccine and 23-valent pneumococcal polysaccharide vaccine in China: A multicentre, non-inferiority, open-label, randomised, controlled, phase 4 trial. Vaccine. (2022) . 40:5322–32. doi: 10.1016/j.vaccine.2022.07.033
137. Walter EB, Schlaudecker EP, Talaat KR, Rountree W, Broder KR, Duffy J, et al. Safety of Simultaneous vs Sequential mRNA COVID-19 and Inactivated Influenza Vaccines: A Randomized Clinical Trial. JAMA Netw Open. (2024) . 7:e2443166. doi: 10.1001/jamanetworkopen.2024.43166
138. Baum HE, Thirard R, Halliday A, Baos S, Thomas AC, Harris RA, et al. Detection of SARS-CoV-2-specific mucosal antibodies in saliva following concomitant COVID-19 and influenza vaccination in the ComFluCOV trial. Vaccine. (2024) . 42:2945–50. doi: 10.1016/j.vaccine.2024.03.061
140. Tapia-Calle G, Aguilar G, Vaissiere N, Truyers C, Ylisastigui P, Buntinx E, et al. Safety, reactogenicity, and immunogenicity of Ad26.COV2.S co-administered with a quadrivalent standard-dose or high-dose seasonal influenza vaccine: a non-inferiority randomised controlled trial. EClinicalMedicine. (2025) . 79:103016. doi: 10.1016/j.eclinm.2024.103016
141. Cao K, Wang X, Peng H, Ding L, Wang X, Hu Y, et al. A single vaccine protects against SARS-coV-2 and influenza virus in mice. J Virol. (2022) . 96:e0157821. doi: 10.1128/jvi.01578-21
142. Xing M, Hu G, Wang X, Wang Y, He F, Dai W, et al. An intranasal combination vaccine induces systemic and mucosal immunity against COVID-19 and influenza. NPJ Vaccines. (2024) . 9:64. doi: 10.1038/s41541-024-00857-5
143. Ao Z, Ouyang MJ, Olukitibi TA, Warner B, Vendramelli R, Truong T, et al. A recombinant VSV-based bivalent vaccine effectively protects against both SARS-coV-2 and influenza A virus infection. J Virol. (2022) . 96:e0133722. doi: 10.1128/jvi.01337-22
144. Isakova-Sivak I, Grigorieva E, and Rudenko L. Insights into current clinical research on the immunogenicity of live attenuated influenza vaccines. Expert Rev Vaccines. (2020) . 19:43–55. doi: 10.1080/14760584.2020.1711056
145. Isakova-Sivak I, Matyushenko V, Stepanova E, Matushkina A, Kotomina T, Mezhenskaya D, et al. Recombinant live attenuated influenza vaccine viruses carrying conserved T-cell epitopes of human adenoviruses induce functional cytotoxic T-cell responses and protect mice against both infections. Vaccines (Basel). (2020) 8:196. doi: 10.3390/vaccines8020196
146. Matyushenko V, Kotomina T, Kudryavtsev I, Mezhenskaya D, Prokopenko P, Matushkina A, et al. Conserved T-cell epitopes of respiratory syncytial virus (RSV) delivered by recombinant live attenuated influenza vaccine viruses efficiently induce RSV-specific lung-localized memory T cells and augment influenza-specific resident memory T-cell responses. Antiviral Res. (2020) . 182:104864. doi: 10.1016/j.antiviral.2020.104864
147. Vitelli A, Folgori A, Scarselli E, Colloca S, Capone S, and Nicosia A. Chimpanzee adenoviral vectors as vaccines - challenges to move the technology into the fast lane. Expert Rev Vaccines. (2017) . 16:1241–52. doi: 10.1080/14760584.2017.1394842
148. Roberts A, Buonocore L, Price R, Forman J, and Rose JK. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol. (1999) . 73:3723–32. doi: 10.1128/jvi.73.5.3723-3732.1999
149. Moser MJ, Hill-Batorski L, Bowen RA, Matejka SM, Marshall D, Kawaoka Y, et al. Intranasal single-replication influenza vector induces cross-reactive serum and mucosal antibodies against SARS-coV-2 variants. Vaccines (Basel). (2023) 11:1063. doi: 10.3390/vaccines11061063
150. Hill-Batorski L, Bowen R, Bielefeldt-Ohmann H, Moser MJ, Matejka SM, Marshall D, et al. Mucosal immunization with dual influenza/COVID-19 single-replication virus vector protects hamsters from SARS-CoV-2 challenge. Vaccine. (2024) . 42:2770–80. doi: 10.1016/j.vaccine.2024.03.040
151. Ouyang MJ, Ao Z, Olukitibi TA, Lawrynuik P, Shieh C, Kung SKP, et al. Oral Immunization with rVSV Bivalent Vaccine Elicits Protective Immune Responses, Including ADCC, against Both SARS-CoV-2 and Influenza A Viruses. Vaccines (Basel). (2023) 11:1404. doi: 10.3390/vaccines11091404
152. Bommireddy R, Stone S, Bhatnagar N, Kumari P, Munoz LE, Oh J, et al. Influenza virus-like particle-based hybrid vaccine containing RBD induces immunity against influenza and SARS-coV-2 viruses. Vaccines (Basel). (2022) 10:944. doi: 10.3390/vaccines10060944
153. Sanchez-Martinez ZV, Alpuche-Lazcano SP, Stuible M, Akache B, Renner TM, Deschatelets L, et al. SARS-CoV-2 spike-based virus-like particles incorporate influenza H1/N1 antigens and induce dual immunity in mice. Vaccine. (2024) . 42:126463. doi: 10.1016/j.vaccine.2024.126463
154. Ye Q, Wu M, Zhou C, Lu X, Huang B, Zhang N, et al. Rational development of a combined mRNA vaccine against COVID-19 and influenza. NPJ Vaccines. (2022) . 7:84. doi: 10.1038/s41541-022-00478-w
155. Wang Y, Ma Q, Li M, Mai Q, Ma L, Zhang H, et al. A decavalent composite mRNA vaccine against both influenza and COVID-19. mBio. (2024) . 15:e0066824. doi: 10.1128/mbio.00668-24
156. Hao T, Li Y, Liu P, Wang X, Xu K, Lei W, et al. A chimeric mRNA vaccine of S-RBD with HA conferring broad protection against influenza and COVID-19 variants. PloS Pathog. (2024) . 20:e1012508. doi: 10.1371/journal.ppat.1012508
157. Massare MJ, Patel N, Zhou B, Maciejewski S, Flores R, Guebre-Xabier M, et al. Combination respiratory vaccine containing recombinant SARS-coV-2 spike and quadrivalent seasonal influenza hemagglutinin nanoparticles with matrix-M adjuvant. bioRxiv. (2021). doi: 10.1101/2021.05.05.442782. 2021.05.05.442782.
158. Shi R, Zeng J, Xu L, Wang F, Duan X, Wang Y, et al. A combination vaccine against SARS-CoV-2 and H1N1 influenza based on receptor binding domain trimerized by six-helix bundle fusion core. EBioMedicine. (2022) . 85:104297. doi: 10.1016/j.ebiom.2022.104297
159. Li Y, Liu P, Hao T, Liu S, Wang X, Xie Y, et al. Rational design of an influenza-COVID-19 chimeric protective vaccine with HA-stalk and S-RBD. Emerg Microbes Infect. (2023) . 12:2231573. doi: 10.1080/22221751.2023.2231573
160. Huang Y, Shi H, Forgacs D, and Ross TM. Flu-COVID combo recombinant protein vaccines elicited protective immune responses against both influenza and SARS-CoV-2 viruses infection. Vaccine. (2024) . 42:1184–92. doi: 10.1016/j.vaccine.2023.12.084
161. Zhang N, Ye Z, Li C, Zhou J, Xue W, Xiang L, et al. A subunit-based influenza/SARS-CoV-2 Omicron combined vaccine induced potent protective immunity in BALB/c mice. J Med Virol. (2024) . 96:e29479. doi: 10.1002/jmv.29479
162. Gao N, Yang T, Dong L, Tang W, Cao K, Ding L, et al. A multi-antigen vaccinia vaccine broadly protected mice against SARS-CoV-2 and influenza A virus while also targeting SARS-CoV-1 and MERS-CoV. Front Immunol. (2024) . 15:1473428. doi: 10.3389/fimmu.2024.1473428
163. Handabile C, Ohno M, Sekiya T, Nomura N, Kawakita T, Kawahara M, et al. Immunogenicity and protective efficacy of a co-formulated two-in-one inactivated whole virus particle COVID-19/influenza vaccine. Sci Rep. (2024) . 14:4204. doi: 10.1038/s41598-024-54421-1
164. Zhu F, Zhuang C, Chu K, Zhang L, Zhao H, Huang S, et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir Med. (2022) . 10:749–60. doi: 10.1016/s2213-2600(22)00131-x
165. Drugs A. NanoFlu/NVX CoV 2373 combination vaccine - Novavax . Available online at: https://adis.springer.com/drugs/800062632 (Accessed November 25, 2024).
166. Calzolari M, Gammone M, Cattani D, Ottonello G, Aleo G, Sasso L, et al. Attitudes of the population toward vaccines during the COVID-19 pandemic: the PROACTIVE study. Public Health Nurs. (2025). doi: 10.1111/phn.13561
167. Abdul-Mutakabbir JC, Abdul-Mutakabbir R, and Casey SJ. Utilizing an educational intervention to enhance influenza vaccine literacy and acceptance among minoritized adults in southern californian vulnerable communities in the post-COVID-19 era. Infect Dis Rep. (2025) 17:18. doi: 10.3390/idr17020018
168. Kelley K, Gozzi N, Mazzoli M, and Paolotti D. Exploring influenza vaccination determinants through digital participatory surveillance. BMC Public Health. (2025) . 25:1345. doi: 10.1186/s12889-025-22496-8
169. Kitano T, Motoki T, Onaka M, Murata M, Onishi M, Mori T, et al. A questionnaire survey for Japanese parents on intention to vaccinate their children against COVID-19 and influenza. J Infect Chemother. (2025) . 31:102693. doi: 10.1016/j.jiac.2025.102693
170. Shapiro J, Block A, and Markenson D. Understanding vaccine hesitancy at montefiore medical center: implications for public health preparedness. Disaster Med Public Health Prep. (2025) . 19:e63. doi: 10.1017/dmp.2025.55
171. La EM, Sweeney C, Davenport E, and Bunniran S. Pharmacy and healthcare provider offices as convenient adult vaccination settings in the US: Patient experiences from a survey of recently-vaccinated adults. Vaccine. (2025) . 54:127057. doi: 10.1016/j.vaccine.2025.127057
172. Poulos C, Buck PO, Ghaswalla P, Rudin D, Kent C, and Mehta D. US consumer and healthcare professional preferences for combination COVID-19 and influenza vaccines. J Med Econ. (2025) . 28:279–90. doi: 10.1080/13696998.2025.2462412
173. Baos S, Todd R, Thirard R, Harris R, Kirwan J, Joyce K, et al. Delivering COVID-19 vaccine trials at speed: the implementation of a phase IV UK multi-centre randomised controlled trial to determine safety and immunogenicity of COVID-19 vaccines co-administered with seasonal influenza vaccines (ComFluCOV). Trials. (2024) . 25:39. doi: 10.1186/s13063-023-07862-4
174. Zhao Y, Zhao L, Li Y, Liu Q, Deng L, Lu Y, et al. An influenza virus vector candidate vaccine stably expressing SARS-CoV-2 receptor-binding domain produces high and long-lasting neutralizing antibodies in mice. Vet Microbiol. (2022) . 271:109491. doi: 10.1016/j.vetmic.2022.109491
175. Isakova-Sivak I, Stepanova E, Matyushenko V, Niskanen S, Mezhenskaya D, Bazhenova E, et al. Development of a T cell-based COVID-19 vaccine using a live attenuated influenza vaccine viral vector. Vaccines (Basel). (2022) 10:1142. doi: 10.3390/vaccines10071142
176. Chaparian RR, Harding AT, Hamele CE, Riebe K, Karlsson A, Sempowski GD, et al. A virion-based combination vaccine protects against influenza and SARS-coV-2 disease in mice. J Virol. (2022) . 96:e0068922. doi: 10.1128/jvi.00689-22
177. Chen J, Wang P, Yuan L, Zhang L, Zhang L, Zhao H, et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci Bull (Beijing). (2022) . 67:1372–87. doi: 10.1016/j.scib.2022.05.018
178. Zhang L, Jiang Y, He J, Chen J, Qi R, Yuan L, et al. Intranasal influenza-vectored COVID-19 vaccine restrains the SARS-CoV-2 inflammatory response in hamsters. Nat Commun. (2023) . 14:4117. doi: 10.1038/s41467-023-39560-9
179. Wang Z, Li Z, Shi W, Zhu D, Hu S, Dinh PC, et al. A SARS-CoV-2 and influenza double hit vaccine based on RBD-conjugated inactivated influenza A virus. Sci Adv. (2023) 9:eabo4100. doi: 10.1126/sciadv.abo4100
180. Deng S, Liu Y, Tam RC, Chen P, Zhang AJ, Mok BW, et al. An intranasal influenza virus-vectored vaccine prevents SARS-CoV-2 replication in respiratory tissues of mice and hamsters. Nat Commun. (2023) . 14:2081. doi: 10.1038/s41467-023-37697-1
181. Wang D, Deng Y, Zhou J, Wang W, Huang B, Wang W, et al. Single-dose intranasal immunisation with novel chimeric H1N1 expressing the receptor-binding domain of SARS-coV-2 induces robust mucosal immunity, tissue-resident memory T cells, and heterologous protection in mice. Vaccines (Basel). (2023) 11:1453. doi: 10.3390/vaccines11091453
182. Stepanova E, Isakova-Sivak I, Matyushenko V, Mezhenskaya D, Kudryavtsev I, Kostromitina A, et al. Safety and immunogenicity study of a bivalent vaccine for combined prophylaxis of COVID-19 and influenza in non-human primates. Vaccines (Basel). (2024) 12:1099. doi: 10.3390/vaccines12101099
183. Stepanova E, Isakova-Sivak I, Mezhenskaya D, Niskanen S, Matyushenko V, Bazhenova E, et al. Expression of the SARS-CoV-2 receptor-binding domain by live attenuated influenza vaccine virus as a strategy for designing a bivalent vaccine against COVID-19 and influenza. Virol J. (2024) 21:82. doi: 10.1186/s12985-024-02350-w
Keywords: SARS-CoV-2, influenza virus, coinfection, coadministration, combination vaccines
Citation: Hu C, Niu C, Li X, He K, Li M, Gao X, Wei Q, Sun W, Zhao Y, Li Y, Xia X, Ren Z, Li X and Wang T (2025) Progress in combination vaccines and the co-administration of influenza virus and SARS-CoV-2 vaccines. Front. Immunol. 16:1578733. doi: 10.3389/fimmu.2025.1578733
Received: 18 February 2025; Accepted: 25 April 2025;
Published: 25 June 2025.
Edited by:
Milad Zandi, Lorestan University of Medical Sciences, IranReviewed by:
Zhan Zhang, Sun Yat-sen Memorial Hospital, ChinaSwapna Ponnampalli, Loyola Academy UG and PG college, India
Copyright © 2025 Hu, Niu, Li, He, Li, Gao, Wei, Sun, Zhao, Li, Xia, Ren, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiguang Ren, cmVuemhpZ3Vhbmc2NkBvdXRsb29rLmNvbQ==; Xiaodong Li, aGh5eWx4ZDIwMTNAMTI2LmNvbQ==; Tiecheng Wang, d2djaGFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Chengyu Hu1†
Chengyu Hu1† Chenguang Niu
Chenguang Niu Weiyang Sun
Weiyang Sun Xianzhu Xia
Xianzhu Xia Zhiguang Ren
Zhiguang Ren