- 1Department of Emergency Surgery, the Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
- 2Department of Neurosurgery, the Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
Tregs play a crucial role in maintaining immune homeostasis, but their involvement in cancer and other diseases has made them a focus of intense research. Tregs contribute to immune evasion by tumors and can affect responses to therapies. Understanding their mechanisms and the potential to manipulate them therapeutically is critical for improving cancer treatment strategies. This review aims to provide an updated perspective on the role of Tregs in cancer and beyond, with a focus on their immunological control mechanisms and therapeutic potential. We examine the recent advances in understanding Treg biology, their interaction with the tumor microenvironment, and the strategies developed to target Tregs for cancer immunotherapy. The review highlights the dual role of Tregs in promoting immune tolerance and in facilitating tumor progression. It discusses the various markers, transcription factors, and signaling pathways involved in Treg differentiation and function. Moreover, we explore the potential of targeting Tregs using novel therapeutic approaches, including monoclonal antibodies, checkpoint inhibitors, and gene editing. The review emphasizes emerging strategies for modulating Treg function in a way that enhances anti-tumor immunity while minimizing systemic autoimmunity.
Introduction
Regulatory T cells (Tregs) are a specialized subset of T cells essential for maintaining immune homeostasis and preventing autoimmune responses. These cells are characterized by the expression of the transcription factor Foxp3 (Forkhead box P3), which is crucial for their development and function (1). Tregs originate from precursor cells in the thymus and are marked by high levels of the interleukin-2 receptor alpha chain (CD25) and CD4 expression (2). Their primary function is to suppress aberrant immune responses that could lead to autoimmunity, thereby maintaining the delicate balance of the immune system. Tregs can be broadly classified into natural Tregs (nTregs), which develop in the thymus, and induced Tregs (iTregs), which differentiate from conventional CD4+ T cells in the periphery under specific conditions, such as the presence of transforming growth factor-beta (TGF-β) and IL-2 (3).
The role of Tregs in cancer is complex, involving both tumor-promoting and tumor-suppressing activities (4). In the tumor microenvironment, Tregs often accumulate and suppress anti-tumor immune responses, aiding in tumor survival and progression (5). This immunosuppressive environment enables tumor cells to evade immune surveillance. Recent therapeutic advancements have focused on modulating Treg activity to enhance cancer immunotherapy (5–7). Strategies include selectively depleting Tregs within tumors using agents targeting CD25 or cytotoxic T-lymphocyte associated protein 4 (CTLA-4), and engineering Tregs with chimeric antigen receptors (CARs) to improve their specificity and function against tumors (8–12). These approaches aim to disrupt the immunosuppressive shield around tumors while preserving the overall immune balance, thereby enhancing the efficacy of immune checkpoint inhibitors and other cancer therapies.
Beyond their immunosuppressive roles, Tregs exhibit several non-canonical functions that are critical for various physiological processes. Tregs play a significant role in tissue repair and regeneration by secreting cytokines and growth factors like amphiregulin, which aid in wound healing and the restoration of tissue integrity (13, 14). In metabolic regulation, Tregs influence lipid metabolism and insulin sensitivity, particularly in adipose tissues where they help maintain metabolic homeostasis (15, 16). Tregs are also crucial in supporting stem cell niches, such as those in the bone marrow, skin, and intestines, where they maintain stem cell quiescence and function through direct interactions and cytokine secretion (17). Additionally, Tregs regulate angiogenesis, the formation of new blood vessels, by balancing pro- and anti-angiogenic factors, which is also essential for both tissue repair and tumor progression (18).
This review aims to provide a comprehensive overview of the diverse roles of Tregs in immune regulation, cancer therapy, and beyond. By exploring the multifaceted functions of Tregs, we seek to highlight their potential as therapeutic targets in various diseases, including cancer, autoimmune disorders, and conditions like tissue regeneration. Understanding the complex interplay between Tregs and their environment is crucial for developing innovative therapies that can modulate these cells to achieve desired clinical outcomes. This synthesis of current knowledge underscores the importance of Tregs in both health and disease, offering insights into future research directions and therapeutic strategies.
Treg origin and identity
Tregs are a specialized subset of T cells that play a crucial role in maintaining immune homeostasis and preventing excessive immune responses. Like other T cell subsets, Tregs develop in the thymus from precursor cells. They undergo specific differentiation processes, including the expression of the transcription factor Foxp3, which is considered a master regulator of Treg development and function (19–21). Tregs are characterized by the expression of certain surface markers, of which the most used marker for identifying is CD4. Additionally, Tregs express high levels of CD25 and the transcription factor Foxp3, which is considered a key marker of Treg identity.
Current research revealed that the gut microbiota is pivotal in shaping immune development early in life. Sanidad et al. revealed that the microbiota in the small intestine of neonatal mice significantly differs from that in adults, notably enriched with neurotransmitters such as serotonin (22). Both human and mouse neonates exhibit a higher concentration of bacteria that produce 5-HT (5-Hydroxytryptamine) in their small intestines. In neonates, gut bacteria not only boost 5-HT production by activating the Tryptophan Hydroxylase 1 enzyme, which transforms tryptophan into 5-HT, but also enhance its availability by suppressing MAO-A (Monoami ne Oxidase A), an enzyme that breaks down 5-HT (22). This increase in serotonin uniquely influences T cell metabolism in neonates by raising the levels of indole-3-acetaldehyde (I3A), which suppresses the mTOR pathway and facilitates the development of Tregs (22, 23). Administering 5-HT orally to neonatal mice, but not to adults, fosters systemic and enduring immune tolerance to dietary antigens and commensal bacteria, illustrating a mechanism through which gut bacteria promote oral tolerance in early life (22).
In our discussion on Tregs, we now explicitly differentiate between nTregs and iTregs. nTregs are characterized by their development in the thymus as a distinct lineage of T cells, where the expression of the transcription factor Foxp3 plays a pivotal role in their differentiation and function. nTregs are essential for maintaining self-tolerance and immune homeostasis, preventing autoimmune diseases by suppressing aberrant immune responses against self-antigens (3).
Conversely, iTregs are generated from conventional CD4+ T cells in the periphery under certain conditions, notably in the presence of TGF-β and IL-2. iTregs also express Foxp3 and share suppressive functions like nTregs; however, their induction, stability, and roles in immune responses, especially in the context of inflammation and tolerance to non-self-antigens, distinguish them from nTregs. The plasticity of iTregs and their ability to arise from effector T cells under specific microenvironmental cues underscore their versatility in immune regulation (24).
By elaborating on these differences, we aim to provide a comprehensive understanding of the diverse regulatory mechanisms mediated by these two Treg subsets, further enriching the discussion on their significance in immune tolerance and potential therapeutic implications.
Canonical mechanisms of immune regulation by Tregs
Tregs exert their immunosuppressive and regulatory functions through various mechanisms (Figure 1): i. Consumption of IL-2: One of the central mechanisms by which Tregs exert their immunosuppressive function is through the high-affinity CD25, which enables them to outcompete conventional T cells for IL-2 (3). By consuming IL-2 in a CD25-dependent manner, Tregs effectively deprive the surrounding effector T cell populations of this critical growth factor, thereby limiting their expansion and activity. This IL-2 consumption mechanism represents a non-cytolytic, contact-independent strategy that is particularly relevant in maintaining peripheral tolerance and preventing excessive immune activation. ii. Direct Cell-to-Cell Suppression: Tregs can directly interact with other immune cells, such as effector T cells, DCs (dendritic cells), and B cells, through cell-to-cell contact. This interaction leads to the suppression of immune responses and the inhibition of the activation and proliferation of effector T cells (25). iii. Secretion of Suppressive Molecules: Treg cells exhibit the production of various cytokines, including IL-10, TGF-β, IL-35 and growth differentiation factor 15 (GDF15), which play pivotal roles in immune regulation and tolerance maintenance (26, 27). These cytokines contribute to the suppression of effector T cell responses and the promotion of immune homeostasis, highlighting the multifaceted regulatory functions of Treg cells in modulating immune responses. iv. Metabolic Regulation: Tregs can modulate the metabolism of effector T cells and alter their nutrient availability, thereby suppressing their proliferation and effector functions (28).
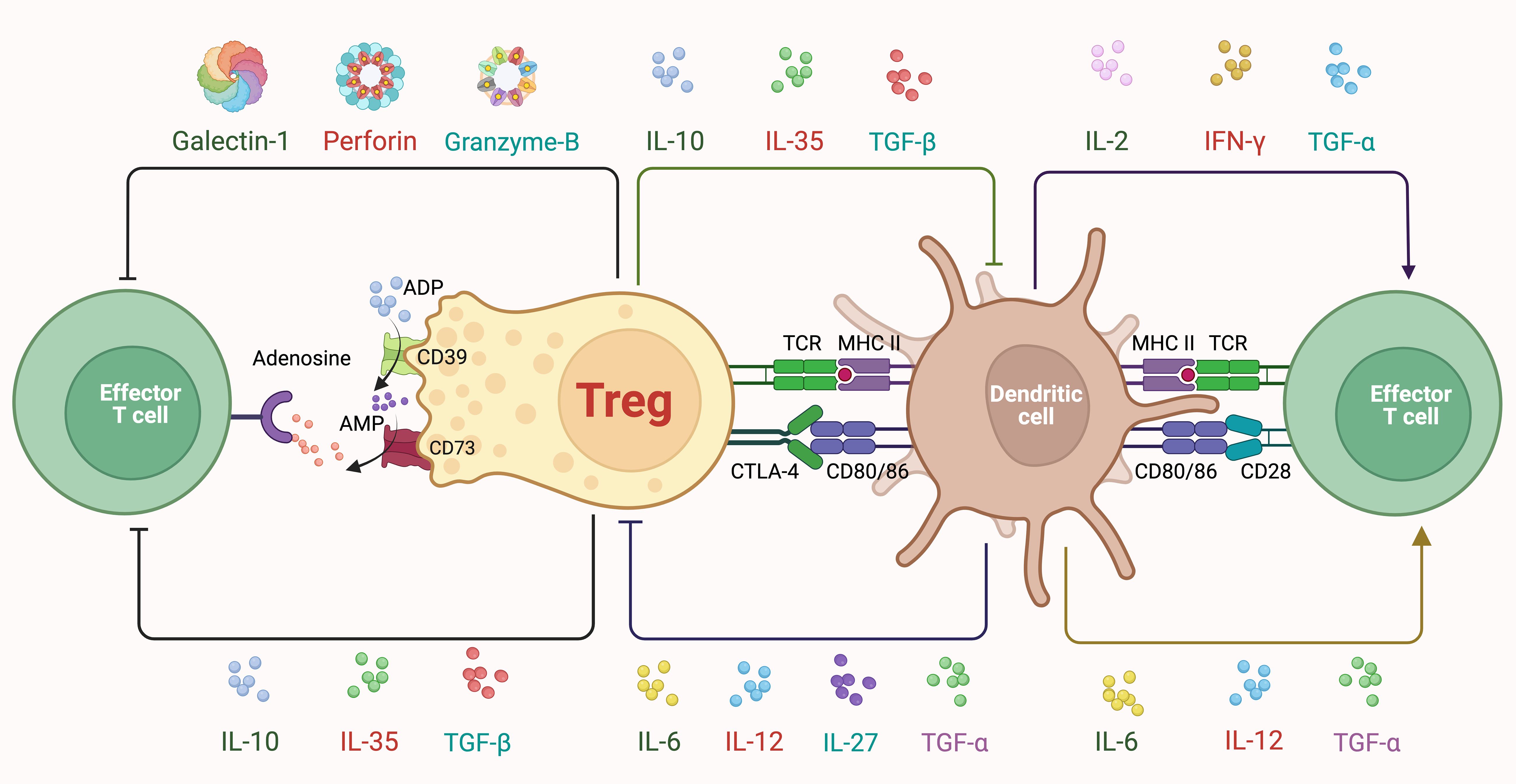
Figure 1. Classic mechanisms of treg suppression. Tregs express high levels of CD25, allowing them to efficiently capture and consume IL-2. This deprives nearby effector T cells of IL-2, limiting their proliferation and activity, and thereby contributing to immune suppression. Moreover, Tregs secrete several cytokines, including IL-10, IL-35, and TGF-β, which inhibit the activation and function of both DCs and effector T cells. IL-10 reduces the expression of co-stimulatory molecules and proinflammatory cytokines while IL-35 suppresses effector T cell proliferation, and TGF-β inhibits effector T cell activation while promoting the differentiation of other Tregs. Metabolically, Tregs express CD39 and CD73, converting ATP (Adenosine Triphosphate) and ADP (Adenosine Diphosphate) to adenosine, which then binds to adenosine receptors on effector T cells, leading to their suppression. Additionally, Tregs secrete Galectin-1, which induces apoptosis in effector T cells. Tregs also utilize cell-cell contact-dependent mechanisms such as CTLA-4, which competes with CD28 on effector T cells for binding to CD80/CD86 on DCs, thereby reducing co-stimulatory signals necessary for effector T cell activation. Tregs interact directly with DCs through TCR (T Cell Receptor) and MHC II (major histocompatibility complex class II) interactions, modulating DC function and indirectly suppressing effector T cell activation. Furthermore, Tregs can induce apoptosis in effector T cells via the release of cytotoxic molecules like perforin and granzyme B.
Classic mechanisms of Treg suppression
Tregs express high levels of CD25, allowing them to efficiently capture and consume IL-2. This deprives nearby effector T cells of IL-2, limiting their proliferation and activity, and thereby contributing to immune suppression (29, 30). Moreover, Tregs secrete several cytokines, including IL-10, IL-35, and TGF-β, which inhibit the activation and function of both DCs and effector T cells. IL-10 reduces the expression of co-stimulatory molecules and pro-inflammatory cytokines while IL-35 suppresses effector T cell proliferation, and TGF-β inhibits effector T cell activation while promoting the differentiation of other Tregs (31). Metabolically, Tregs express CD39 and CD73, converting ATP (Adenosine Triphosphate) and ADP (Adenosine Diphosphate) to adenosine, which then binds to adenosine receptors on effector T cells, leading to their suppression (2, 32). Additionally, Tregs secrete Galectin-1, which induces apoptosis in effector T cells (33). Tregs also utilize cell-cell contact-dependent mechanisms such as CTLA-4, which competes with CD28 on effector T cells for binding to CD80/CD86 on DCs, thereby reducing co-stimulatory signals necessary for effector T cell activation (34). Tregs interact directly with DCs through TCR (T Cell Receptor) and MHC II (major histocompatibility complex class II) interactions, modulating DC function and indirectly suppressing effector T cell activation. Furthermore, Tregs can induce apoptosis in effector T cells via the release of cytotoxic molecules like perforin and granzyme B (35).
Novel immune regulation mechanisms of Tregs
The immune regulatory mechanisms of Tregs exhibit high complexity, involving multi-layered interactions at the molecular, metabolic, and microenvironmental levels. These interactions are pivotal for maintaining immune homeostasis and for the modulation of immune responses within various contexts, including cancer. In tumor microenvironment, Tregs play a dual role by not only maintaining immune tolerance but also potentially facilitating tumor escape from immune surveillance (36–38). This paradoxical nature makes understanding the novel immune regulatory mechanisms of Tregs particularly significant, as it opens potential therapeutic avenues for tipping the balance in favor of tumor suppression without compromising systemic immune tolerance.
Firstly, metabolic adaptation plays a crucial role, as Tregs optimize their metabolism to survive in the nutrient-sparse tumor microenvironment by primarily using fatty acid oxidation (39). This enables them to maintain suppressive functionality under challenging conditions. Secondly, Tregs utilize exosome-mediated communication to extend their influence within the tumor microenvironment (40). These exosomes, loaded with microRNAs and other regulatory molecules, modulate the behavior of other immune cells and contribute to tumor immune escape (41).
Additionally, exploration of novel immune checkpoints such as TIGIT (T-cell Immunoreceptor with Ig and ITIM Domains) and LAG-3 (Lymphocyte-activation gene 3) extends beyond classic pathways like CTLA-4 and PD-1 (programmed cell death protein 1), providing new avenues for immunotherapeutic targets that influence Treg activity (42–44). Moreover, the plasticity of Tregs is highlighted by their potential conversion into ex-Tregs under specific environmental cues, which can then switch from suppressing to promoting immune responses. This plasticity underlines the dynamic nature of Tregs in response to the tumor microenvironment.
Furthermore, the role of hypoxia-inducible factors such as HIF1-α (Hypoxia-Inducible Factor 1-Alpha) is critical in adapting Treg functions to low-oxygen conditions typical in solid tumors (45, 46). HIF1-α enhances the stability and functionality of FoxP3, promoting Treg survival and suppressive activity under hypoxia (47). By elucidating these mechanisms, the manuscript can provide a detailed perspective on how Tregs crucially modulate the tumor microenvironment, reflecting the intricacy of their roles and the potential for targeted therapies that manipulate their regulatory functions. This comprehensive analysis will not only align with the latest research trends but also deepen our understanding of Treg dynamics within cancer biology, offering insights into potential therapeutic interventions.
The novel immune regulatory mechanisms of Tregs, especially within the context of the tumor microenvironment, underscore a critical area of oncological research that bridges immunology and tumor biology. The dynamic interplay between Tregs and the tumor microenvironment highlights the crucial role these cells play in modulating immune responses directly at the tumor site. Tregs not only influence the recruitment and activation of other immune cells through their secretory and cell-contact dependent mechanisms but also adapt to the harsh, often hypoxic and metabolically restricted conditions of the tumor microenvironment. This adaptation supports their survival and functional persistence, which in turn can either promote or inhibit tumor growth depending on the context.
Understanding these complex interactions is vital for developing targeted therapies that can modulate Treg activity to enhance anti-tumor immunity without inducing systemic autoimmunity. The insights gained from studying Treg interactions in the tumor microenvironment could lead to the development of biomarkers for better prognosis and tailored therapeutic strategies that specifically target the immune regulation in the tumor niche. This could potentially transform the approach to cancer therapy, moving towards more personalized and effective immunomodulatory treatments.
Recent advancements in Treg diversity
Recent studies have revealed that Tregs are not a homogenous population but rather comprise distinct subsets with specialized functions. These subsets, such as tissue-resident Tregs and follicular Tregs, have unique phenotypes and exhibit specific regulatory roles in different tissues and immune contexts (48–57). Traditionally, Tregs were considered stable and committed to their suppressive function. However, recent research has highlighted that Tregs can exhibit plasticity and acquire effector-like characteristics under certain conditions (58–62). This plasticity raises important questions about the stability of Treg function and their potential roles in autoimmune diseases and cancer. The tissue microenvironment plays a crucial role in shaping the functions and stability of Tregs (63, 64). Recent studies have focused on understanding the signals and cues within specific tissues that influence Treg function and stability (51, 65–67). This knowledge can provide insights into how Tregs adapt their regulatory properties based on the local tissue environment. In addition, Tregs have garnered significant attention as potential therapeutic agents for immune-mediated diseases (68, 69). Advances in the isolation, expansion, and genetic engineering of Tregs have opened up possibilities for their use in immunotherapy, including in the context of transplantation, autoimmune diseases, and graft-versus-host disease (70–72). Recent advances have deepened our understanding of Tregs and their roles in immune regulation. They hold promise for developing targeted strategies to modulate immune responses, restore immune balance, and potentially treat various immune-related disorders.
One fascinating area of progress is the development of antigen-specific regulatory T cells (73, 74). These cells have shown higher potency in immuno-suppression compared to polyclonal T cells in preclinical mouse models. Recent technical advances aim to generate antigen-specific Treg cells in sufficient quantities for therapeutic use, which includes redirecting Tregs using synthetic receptors and converting antigen-specific effector T cells into Tregs through FOXP3 overexpression. The use of CAR constructs is prevalent due to their ability to recognize a wide range of antigens, including non-protein targets. These advances have shown promise in treating hematological malignancies and offer a new strategy for autoimmune disease therapy and transplantation (75, 76).
The present research identified a distinct population of meningeal regulatory T cells (mTregs) residing in the meninges of the spinal cord and the leptomeninges surrounding dorsal root ganglia (77). These mTregs were enriched in the lumbosacral meninges and were found in proximity to IB4⁺ non-peptidergic sensory nerve fibers, indicating potential neuroimmune interaction (78). Using Foxp3-DTR mice, site-specific depletion of mTregs via intrathecal injection of PEGylated diphtheria toxin (pegDT) led to a sex-specific effect: only female mice exhibited reduced mechanical nociceptive thresholds, suggesting that mTregs exert a hormone-dependent analgesic function (77, 79). Conversely, intrathecal administration of low-dose IL-2 after nerve injury selectively expanded mTregs and alleviated mechanical hypersensitivity in females (77). These findings establish mTregs as key regulators of nociception in a sex-dependent manner and suggest that they may act independently of classical immune-suppressive pathways. The results provide novel insight into neuroimmune mechanisms of pain regulation and may help explain sex differences in pain sensitivity, offering potential for gender-specific pain therapies (77).
Tregs in cancer: a dual role
Tregs play a complex role in cancer, and their involvement can have both positive and negative effects on tumor progression (Figure 2): i. One of the primary functions of Tregs is to suppress immune responses, including those targeting tumor cells. Tregs can inhibit the activation and effector functions of cytotoxic T cells and others, thereby dampening anti-tumor immune responses. This immunosuppressive effect can create an environment that allows tumor cells to evade immune surveillance and promote tumor growth. ii. Tregs also play a crucial role in maintaining immune tolerance and preventing excessive immune reactions against self-antigens. In the context of cancer, this can result in Tregs suppressing immune responses against tumor-associated antigens, which are derived from normal self-proteins that are aberrantly expressed by tumor cells. iii. Tregs have been found to infiltrate tumors and are often enriched within the tumor microenvironment (80–82). The presence of Tregs within tumors has been associated with poor clinical outcomes in some cancers (83, 84). Tumor-infiltrating Tregs can actively suppress anti-tumor immune responses, promote immune evasion, and contribute to tumor immune evasion and progression. iv. Tregs can also contribute to resistance to certain cancer immunotherapies, such as immune checkpoint blockade (ICB) therapy. Tregs can upregulate immune checkpoint molecules, such as CTLA-4 and PD-1, which can dampen the activity of anti-tumor immune cells and limit the efficacy of ICB therapy (85). Targeting Tregs alongside ICB therapy is an active area of research to enhance treatment responses (86, 87). v. Some evidence suggests that Tregs can acquire tumor-specific antigen reactivity, leading to their differentiation into tumor-associated Tregs (TATs) (88–91). TATs exhibit an enhanced ability to suppress anti-tumor immune responses, further promoting tumor immune evasion (90, 92, 93). vi. Despite their suppressive role in anti-tumor immunity, Tregs are also involved in maintaining tissue homeostasis and preventing chronic inflammation, which can contribute to tumorigenesis (94, 95). Additionally, recent research suggests that not all Tregs exhibit the same suppressive properties, and subsets with pro-inflammatory properties or compromised suppressive function have been identified in certain cancer contexts (58–62). Targeting Tregs as a therapeutic strategy in cancer is an active area of investigation (96, 97). Several approaches are being explored, such as selectively depleting or inhibiting Tregs within tumors or modulating their function to enhance anti-tumor immune responses (62, 98). Combining Treg-targeted therapies with other immunotherapeutic approaches holds promise for improving outcomes in cancer treatment.
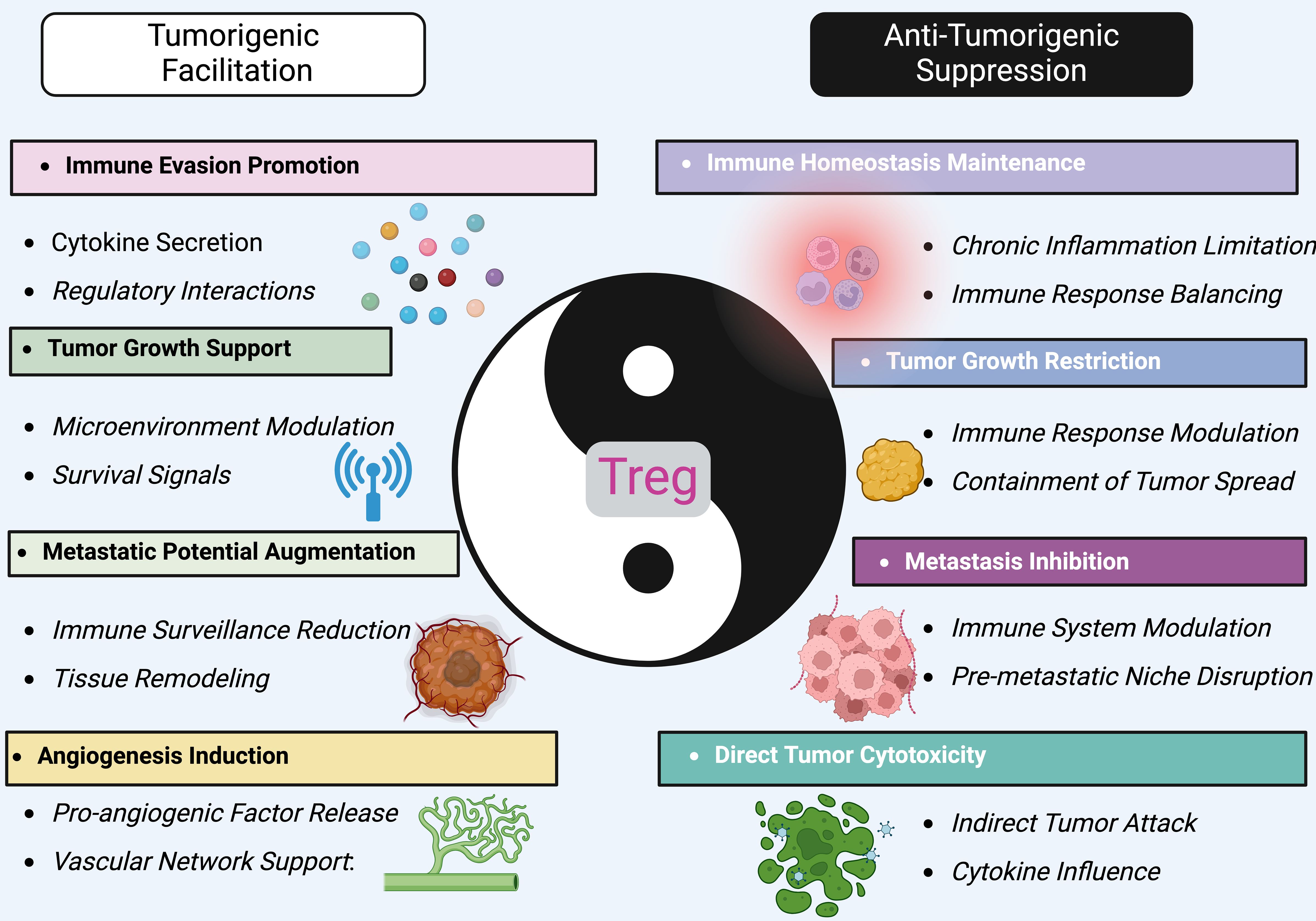
Figure 2. The dual role of treg in cancer. Tregs embody a paradigm of immunological dualism in the tumor microenvironment, oscillating between tumorigenesis facilitation and antitumor activity. On one side, Tregs promote tumorigenic processes, such as immune evasion, by secreting cytokines that modify the immune landscape to favor tumor growth and survival. They support the tumor by enhancing angiogenesis, which supplies necessary nutrients and oxygen, and by modulating the microenvironment to suppress anti-tumor immune surveillance. This leads to augmented metastatic potential and survival signaling within the tumor. Conversely, Tregs also exert anti-tumorigenic effects. They help maintain immune homeostasis, limiting chronic inflammation and immune responses that could otherwise support tumor progression. Tregs can restrict tumor growth by modulating immune responses that contain and potentially reduce tumor spread. Their role in inhibiting metastasis involves disrupting the formation of pre-metastatic niches, crucial for preventing the establishment of secondary tumors. Furthermore, Tregs can directly impact tumor cells through cytotoxic activities, though less commonly. They might indirectly attack tumor cells or influence other immune cells to target the tumor, mediated by their control over cytokine environments. By targeting specific pathways that influence Treg activity, it might be possible to shift their balance towards more consistent anti-tumorigenic actions, offering new avenues for therapeutic interventions in cancer management.
As research on Tregs evolves, the earlier notion that Tregs uniformly result in unfavorable cancer outcomes is increasingly recognized as oversimplified. Indeed, Tregs exhibit a dual role in the progression and onset of tumors. They mitigate systemic inflammation caused by overly active anti-tumor immune responses, crucial for maintaining immune equilibrium. Conversely, in advanced tumor stages, Tregs may attenuate these anti-tumor responses, promoting tumor cell survival. At earlier stages, their anti-inflammatory actions can inadvertently favor tumor growth. For instance, RORγT+ Helios− Tregs, activated by intestinal microbes, respond to IL-33 triggered by tissue damage, helping limit injury and forestall cancer development during colitis (99). In head and neck cancers, the accumulation of Foxp3+ Tregs correlates with improved prognoses, likely reflecting their diverse functions within different TMEs (tumor microenvironment) affecting their activity in various tumor areas (100, 101). In colorectal cancer, Tregs found in the tumor mesenchyme are associated with better outcomes, whereas those in tumor nests or edges correlate with worse prognoses (102, 103). Moreover, the impact of Tregs varies across cancer stages, with high Foxp3+ Treg presence in early gastric cancer stages linked to better survival rates, a trend that inverses in later stages. These discrepancies suggest changes in Treg function depending on the TME, influencing their effects on tumor prognosis and treatment strategies. Although it is speculative, the dual role of Tregs in tumor biology demands further investigation to elucidate their complex behaviors fully, advancing our understanding and application of Tregs in cancer therapeutics (Figure 2).
The dual role of treg in cancer
Tregs embody a paradigm of immunological dualism in the tumor microenvironment, oscillating between tumorigenesis facilitation and antitumor activity. On one side, Tregs promote tumorigenic processes, such as immune evasion, by secreting cytokines that modify the immune landscape to favor tumor growth and survival. They support the tumor by enhancing angiogenesis, which supplies necessary nutrients and oxygen, and by modulating the microenvironment to suppress anti-tumor immune surveillance. This leads to augmented metastatic potential and survival signaling within the tumor (104, 105). Conversely, Tregs also exert anti-tumorigenic effects. They help maintain immune homeostasis, limiting chronic inflammation and immune responses that could otherwise support tumor progression (106). Tregs can restrict tumor growth by modulating immune responses that contain and potentially reduce tumor spread (107–109). Their role in inhibiting metastasis involves disrupting the formation of pre-metastatic niches, crucial for preventing the establishment of secondary tumors (110, 111). Furthermore, Tregs can directly impact tumor cells through cytotoxic activities, though less commonly (112, 113). They might indirectly attack tumor cells or influence other immune cells to target the tumor, mediated by their control over cytokine environments. By targeting specific pathways that influence Treg activity, it might be possible to shift their balance towards more consistent anti-tumorigenic actions, offering new avenues for therapeutic interventions in cancer management (114).
Treg functions beyond immune suppression
In addition to their well-characterized roles in maintaining immune homeostasis and preventing autoimmunity, Tregs exhibit a range of other regulatory functions that are crucial for their versatility in the immune system. These expanded functions include modulation of tissue repair, influence on metabolic processes, and participation in the resolution of inflammation, highlighting the multifaceted roles Tregs play beyond traditional immune suppression (Figure 3).
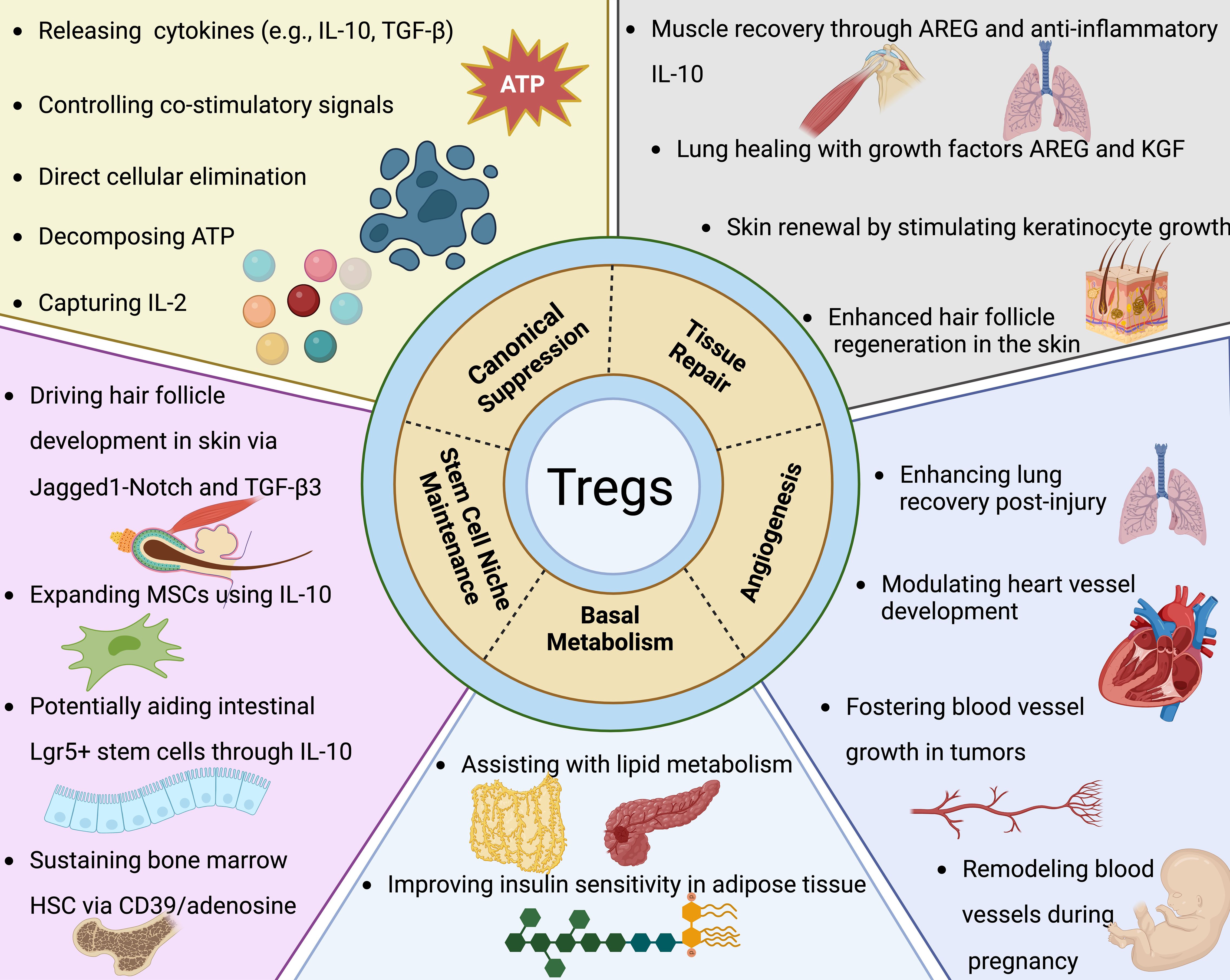
Figure 3. Tregs extend their functional repertoire well beyond mere immune suppression. As elaborated in the segment concerning canonical suppression, Tregs employ multiple strategies to curtail the proliferation and operational capacity of effector T cells alongside various other immune constituents. Moreover, Tregs exert extensive influence over processes such as tissue regeneration, angiogenesis, fundamental metabolism, and the preservation of stem cell microenvironments. Their contributions to tissue repair have been predominantly observed in muscle, lung, skin, and the central nervous system. Key to these processes are Treg-derived factors like Areg (amphiregulin) and Keratinocyte growth factor, which are essential for the proliferative responses of epithelial and satellite cells in lung, skin, and muscle tissues, respectively. Within the CNS (central nervous system), Tregs bolster the capacity of oligodendrocytes to restore myelin sheaths post-injury. The angiogenic role of Tregs is context-dependent, exhibiting both pro-angiogenic and anti-angiogenic activities based on the tissue type and experimental model. In the realm of oncology, Tregs distinctly support tumor angiogenesis, facilitating neoplastic growth. Their involvement in ischemic conditions reveals a complex picture: Tregs are necessary for revascularization in lung injury yet may counteract angiogenesis in cardiac ischemia. They also partake in the vascular adjustments occurring within the uterus throughout pregnancy. Specifically, visceral adipose tissue Tregs, which are among the most thoroughly characterized tissue-resident Tregs, play definitive roles in modulating insulin sensitivity and lipid metabolism. Additionally, Tregs underpin the specialized stem cell niches within the skin, bone marrow, and gastrointestinal tract. Through a variety of mechanisms, they directly uphold stem cell dormancy and indirectly modulate these cells by limiting the proliferation of the supporting mesenchymal stromal cells. While several of these observations have been corroborated in human studies, it's critical to acknowledge that a substantial portion of the insights is derived from murine research.
Tregs extend their functional repertoire well beyond mere immune suppression
As elaborated in the segment concerning canonical suppression, Tregs employ multiple strategies to curtail the proliferation and operational capacity of effector T cells alongside various other immune constituents. Moreover, Tregs exert extensive influence over processes such as tissue regeneration, angiogenesis, fundamental metabolism, and the preservation of stem cell microenvironments. Their contributions to tissue repair have been predominantly observed in muscle, lung, skin, and the central nervous system. Key to these processes are Treg-derived factors like Areg (amphiregulin) and Keratinocyte growth factor, which are essential for the proliferative responses of epithelial and satellite cells in lung, skin, and muscle tissues, respectively. Within the CNS (central nervous system), Tregs bolster the capacity of oligodendrocytes to restore myelin sheaths post-injury. The angiogenic role of Tregs is context-dependent, exhibiting both pro-angiogenic and anti-angiogenic activities based on the tissue type and experimental model. In the realm of oncology, Tregs distinctly support tumor angiogenesis, facilitating neoplastic growth. Their involvement in ischemic conditions reveals a complex picture: Tregs are necessary for revascularization in lung injury yet may counteract angiogenesis in cardiac ischemia. They also partake in the vascular adjustments occurring within the uterus throughout pregnancy. Specifically, visceral adipose tissue Tregs, which are among the most thoroughly characterized tissue-resident Tregs, play definitive roles in modulating insulin sensitivity and lipid metabolism. Additionally, Tregs underpin the specialized stem cell niches within the skin, bone marrow, and gastrointestinal tract. Through a variety of mechanisms, they directly uphold stem cell dormancy and indirectly modulate these cells by limiting the proliferation of the supporting mesenchymal stromal cells. While several of these observations have been corroborated in human studies, it’s critical to acknowledge that a substantial portion of the insights is derived from murine research.Tissue repair and regeneration
Recent advancements have expanded the understanding of Treg function, revealing significant contributions beyond traditional immunoregulation, particularly in the realms of tissue repair and regeneration. Tregs likely promote tissue repair and regeneration in a tissue-specific manner by modulating local immune responses and supporting tissue-specific progenitor cells. In the CNS, Tregs facilitate remyelination and oligodendrocyte differentiation via IL-33 and CCN3 signaling. In the lung, they promote alveolar epithelial cell regeneration and limit fibrosis. In bone, they suppress pro-inflammatory cells and enhance osteoblast differentiation. In skeletal muscle and cardiac tissue, Tregs are recruited through IL-33 and CCR5 (C-C chemokine receptor type 5) pathways, respectively, to modulate macrophage activity and support tissue regeneration. These findings highlight the context-dependent reparative roles of Tregs across organs (Figure 4). In tissue repair and regeneration, Tregs facilitate wound healing and reduce chronic inflammation that can interfere with the recovery of damaged tissues (115, 116). This function is evident across various tissue types, including skin, muscle, and pulmonary systems, where Tregs have been observed to enhance wound closure rates and improve structural restoration post-injury (116).
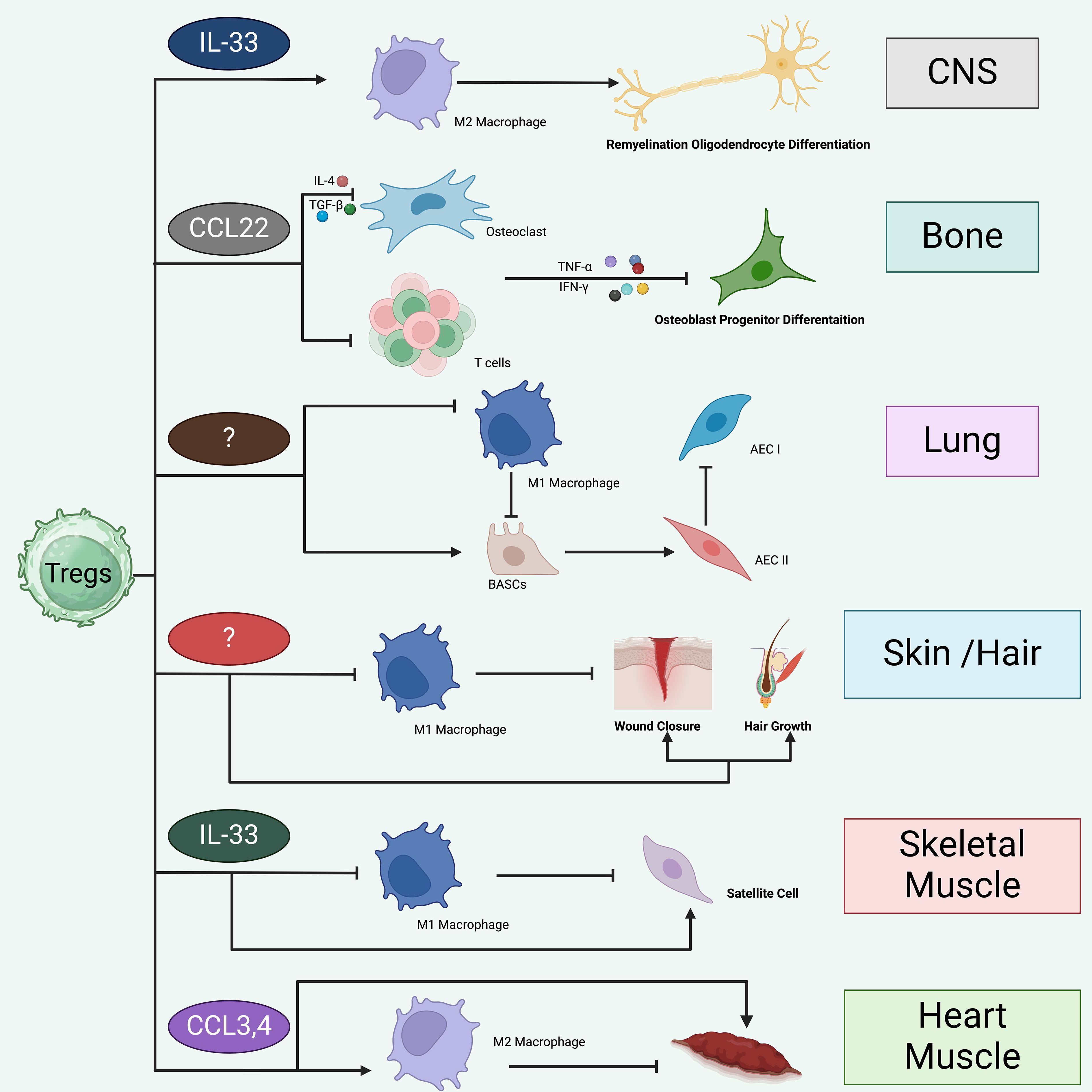
Figure 4. Treg likely promote tissue repair and regeneration in a tissue-specific manner. Tregs appear to facilitate tissue repair and regeneration in a manner specific to each tissue type. Tregs are crucial in the healing and regeneration processes of various tissues, including skeletal muscle, cardiac muscle, skin and hair, lungs, bones, and CNS. i. In the CNS, Tregs are drawn by IL-33 and contribute to repair by promoting M2 macrophage polarization, which aids in remyelination and the differentiation of oligodendrocytes. Additionally, Tregs may directly influence oligodendrocytes through mechanisms involving CCN3. ii. In bone tissue, Tregs are likely recruited through CCL22 signaling and function to inhibit Th1, CD8+ cells, and M1 macrophages, thereby promoting differentiation of osteoblast progenitors. iii. In the lung, Tregs suppress the inflammatory responses of M1 macrophages and support the proliferation and differentiation of damaged alveolar type 2 epithelial cells (AECII) into type 1 cells (AECIs). This activity may be mediated by Areg or the interaction of CD103 with E-cadherin. Tregs may also activate progenitor bronchioalveolar stem cells (BASCs) to differentiate into AECII cells while concurrently preventing fibrosis through the inhibition of fibrocyte recruitment and proliferation, mediated by CXCL12. iv. The mechanism by which Tregs are recruited to skin and hair remains unclear. However, once present, Tregs curb the inflammatory actions of M1 macrophages and enhance wound healing and hair regeneration through the Jag1-Notch signaling pathway. v. Within skeletal muscle, IL-33 aids in the recruitment of Tregs to injury sites. These cells suppress inflammation mediated by M1 macrophages, thereby fostering a shift towards the resolution phase of healing. Tregs also directly stimulate satellite cell proliferation and differentiation through Areg. vi. In cardiac tissue, Tregs are drawn to the site via CCR5 signaling, involving chemokines like CCL3 and CCL4, which leads to the suppression of Th1 cell activities and inhibition of M1 macrophages.
The molecular mechanisms underlying Treg involvement in tissue repair involve a sophisticated network of cytokines and growth factors. Tregs are known to secrete anti-inflammatory cytokines such as IL-10 and TGF-β (116). IL-10 contributes to the suppression of effector T cell activities and decreases the production of pro-inflammatory cytokines, thereby promoting an environment conducive to healing (117). TGF-β, on the other hand, supports fibrosis and angiogenesis, essential processes in tissue repair (118–120). Interactions between Tregs and other cell types within the damaged tissue are crucial. Tregs influence macrophages to adopt a reparative M2 phenotype, characterized by the secretion of growth factors and enzymes that aid in matrix remodeling and cellular proliferation (121, 122). A specific role of Tregs in regenerative processes includes their expression of Areg, a member of the epidermal growth factor family (123). Areg promotes the proliferation of epithelial cells, critical for restoring barrier functions in tissues such as the skin and gastrointestinal tract (123).
Tissue-resident ST2⁺ Tregs represent a specialized subset with pivotal roles in tissue repair and homeostasis (124). These cells are characterized by the expression of the IL-33 receptor ST2 and are enriched in non-lymphoid tissues such as the lung, muscle, and adipose tissue, particularly under conditions of injury or inflammation (124). Upon sensing IL-33, an alarmin released by damaged epithelial or stromal cells, ST2⁺ Tregs become activated and secrete Areg, which promotes epithelial proliferation and tissue regeneration (125).
However, the molecular pathways driving tissue repair, including IL-33/ST2 and Areg/EGFR signaling, are frequently co-opted by tumors to facilitate malignant progression (126). ST2⁺ Tregs have been reported to accumulate in various tumor microenvironments, where they may contribute not only to immune suppression but also to tumor-promoting inflammation and stromal remodeling via the same regenerative programs (127). The overlap between regenerative and tumorigenic signaling suggests that ST2⁺ Tregs play a dual role—beneficial in resolving inflammation and restoring tissue integrity, yet potentially deleterious in promoting tumor growth and metastasis (127). Therefore, a comprehensive understanding of their context-dependent functions is essential for designing targeted therapies that harness their reparative potential without exacerbating cancer progression.
Treg likely promote tissue repair and regeneration in a tissue-specific manner
Tregs appear to facilitate tissue repair and regeneration in a manner specific to each tissue type. Tregs are crucial in the healing and regeneration processes of various tissues, including skeletal muscle, cardiac muscle, skin and hair, lungs, bones, and CNS. i. In the CNS, Tregs are drawn by IL-33 and contribute to repair by promoting M2 macrophage polarization, which aids in remyelination and the differentiation of oligodendrocytes (128, 129). Additionally, Tregs may directly influence oligodendrocytes through mechanisms involving CCN3 (130). ii. In bone tissue, Tregs are likely recruited through CCL22 signaling and function to inhibit Th1, CD8+ cells, and M1 macrophages, thereby promoting differentiation of osteoblast progenitors (131). iii. In the lung, Tregs suppress the inflammatory responses of M1 macrophages and support the proliferation and differentiation of damaged alveolar type 2 epithelial cells (AECII) into type 1 cells (AECIs) (132). This activity may be mediated by Areg or the interaction of CD103 with E-cadherin (133). Tregs may also activate progenitor bronchioalveolar stem cells (BASCs) to differentiate into AECII cells while concurrently preventing fibrosis through the inhibition of fibrocyte recruitment and proliferation, mediated by CXCL12 (134, 135). iv. The mechanism by which Tregs are recruited to skin and hair remains unclear (65). However, once present, Tregs curb the inflammatory actions of M1 macrophages and enhance wound healing and hair regeneration through the Jag1-Notch signaling pathway (136). v. Within skeletal muscle, IL-33 aids in the recruitment of Tregs to injury sites. These cells suppress inflammation mediated by M1 macrophages, thereby fostering a shift towards the resolution phase of healing. Tregs also directly stimulate satellite cell proliferation and differentiation through Areg. vi. In cardiac tissue, Tregs are drawn to the site via CCR5 signaling, involving chemokines like CCL3 and CCL4, which leads to the suppression of Th1 cell activities and inhibition of M1 macrophages (137).
Metabolic regulation
The non-canonical function of Tregs in metabolic regulation highlights their importance in systemic homeostasis beyond mere immune modulation. Research has elucidated the involvement of Tregs in the modulation of lipid metabolism, insulin sensitivity, and overall energy balance (138, 139). These cells exert their effects in key metabolic sites, including adipose tissue, liver, and skeletal muscle, areas critical for metabolic homeostasis.
In adipose tissue, Tregs accumulate in visceral fat and contribute to the maintenance of insulin sensitivity and glucose homeostasis. This function is mediated by the secretion of anti-inflammatory cytokines such as IL-10 and TGF-β, which play roles in reducing inflammation — a known contributor to insulin resistance (140, 141). Moreover, adipose Tregs express the transcription factor PPAR-γ (peroxisome proliferator-activated receptor gamma), a master regulator of lipid metabolism, suggesting a direct role in lipid handling and storage (142, 143). Examination of the mechanisms underlying the function of Tregs in visceral adipose tissue (VAT) revealed that these cells, characterized by PPAR-γ expression, also express ST2 and depend on interleukin-33 (IL-33) for their accumulation in VAT (144–146). Administration of IL-33 to obese mice, which exhibit a reduced number of adipose Tregs, results in an expansion of these cells to levels seen in non-obese counterparts and leads to the restoration of insulin sensitivity (144). Although IL-33 signaling impacts other cell types such as innate lymphoid cells (ILCs), macrophages, and various additional cells, the critical role of ST2 signaling in sustaining visceral adipose tissue-Treg populations was confirmed through a Treg-specific knockout of the receptor (147, 148).
One present research identifies two unique Treg cell types, ST2+ and CXCR3+, which exhibit sex-specific distributions and functional differences influenced by hormonal variations (146). ST2+ Treg cells, found predominantly in males and driven by IL-33 and PPARγ, are essential for maintaining systemic glucose metabolism and controlling inflammation (149). Conversely, CXCR3+ Treg cells, more prevalent in females and influenced by IFN-γ and T-bet, play key roles in mitigating inflammation, especially under lean conditions (150). This study not only details the mechanisms through which these Treg cells regulate metabolic processes and inflammatory responses, including their interactions with other immune cells and response to environmental signals, but also emphasizes their potential as therapeutic targets in metabolic diseases like diabetes and obesity, highlighting the importance of understanding their regulatory mechanisms and developmental heterogeneity (146, 149).
Furthermore, Tregs influence liver metabolism, particularly in the context of inflammatory conditions such as non-alcoholic fatty liver disease (151, 152). By modulating the inflammatory milieu within the liver, Tregs can mitigate the progression of inflammation-driven hepatic damage, which is closely linked to metabolic dysregulation (153).
Support of the stem cell nich
Tregs exert profound effects on stem cell niches, impacting tissue regeneration and homeostasis through intricate mechanisms that are increasingly being elucidated in current research. The diverse roles of Tregs span several tissues, each characterized by unique interactions and signaling pathways that govern the fate and function of resident stem cells. Here, we try to explore the mechanisms by which Tregs influence stem cell niches in the bone marrow, skin, and intestines, highlighting the molecular underpinnings and potential therapeutic implications.
In the bone marrow, Tregs contribute to the maintenance of hematopoietic stem cell (HSC) quiescence and integrity through several pathways. Tregs express CXCR4, which is crucial for their homing to the bone marrow and positioning within the HSC niche (154). Once localized, Tregs modulate the HSCs via the secretion of adenosine, which is generated through the enzymatic activity of CD39 (2). This interaction is further exemplified by Treg-mediated inhibition of HSC proliferation and differentiation, a process reversed by antioxidant treatments that mitigate the oxidative milieu (155).
In skin, particularly around hair follicles, Tregs play a key role in modulating hair follicle stem cell (HFSC) cycles and regeneration (17). Tregs impact HFSC behavior through direct cell-cell contact and paracrine signaling mechanisms, notably involving the Notch pathway (156). Tregs increase the expression of the Notch ligand Jagged-1 (Jag-1), enhancing Notch signaling in HFSCs to promote hair growth and follicle regeneration (156). This process is critical during the telogen-to-anagen transition phase of the hair cycle, where Tregs have been shown to regulate the timing and extent of HFSC activation and subsequent hair follicle development (17).
In the intestinal mucosa, Tregs interact with intestinal stem cells through mechanisms involving MHC II (134, 157, 158). These interactions facilitate the maintenance of ISC integrity and function under inflammatory conditions, promoting tissue repair and barrier function. Tregs secrete IL-10, which not only dampens inflammatory responses but also supports the expansion and renewal of mesenchymal stromal cells that are crucial for ISC niche support (159). This regulatory axis is vital for preventing dysregulated immune responses from impairing ISC function, which is essential for maintaining intestinal homeostasis and responding to epithelial damage.
Regulation of angiogenesis
Tregs also exhibit capabilities in regulating angiogenesis, the process by which new blood vessels form from pre-existing vessels (160, 161). This non-canonical function of Tregs has significant implications for both tissue repair and tumor progression, highlighting their dual role in promoting or inhibiting angiogenesis under different pathological conditions.
Tregs could influence angiogenesis through several mechanisms, primarily involving the secretion of factors that can either promote or inhibit the angiogenic process. One of the key mediators in this context is vascular endothelial growth factor (VEGF), a potent angiogenic factor that Tregs can modulate indirectly through interactions with other immune cells or directly by cellular secretion (161, 162). By expressing or inducing the expression of VEGF, Tregs can enhance angiogenesis, facilitating tissue repair and regeneration (163). Conversely, Tregs can limit VEGF-induced angiogenesis through the secretion of anti-inflammatory cytokines such as TGF-β and IL-10, which are known to modulate inflammatory responses that influence angiogenesis (164, 165).
In addition, Tregs directly interact with endothelial cells, the primary cells involved in angiogenesis. Through direct cell-cell contact and the secretion of cytokines, Tregs can influence endothelial cell proliferation, migration, and tube formation (166, 167). These interactions are critical during the formation of new blood vessels, especially in contexts of wound healing and tissue regeneration.
Moreover, Tregs modulate the function of other immune cells in the angiogenic process. For example, Tregs can inhibit the angiogenic contributions of macrophages and other T cell subsets by suppressing their pro-inflammatory and pro-angiogenic activities (168, 169). This regulation ensures that angiogenesis does not proceed unchecked, which is crucial in preventing pathological conditions such as tumor growth and metastasis. Tregs play a pivotal role in promoting angiogenesis through multiple mechanisms (160). They enhance vascular growth by secreting VEGF, which activates endothelial cell proliferation, migration, and survival via Src, PI3K, and MAPK pathways (161). Tregs also release TGF-β and IL-10, modulating angiogenic gene expression through Smad and STAT3 signaling, respectively. Furthermore, direct interactions with endothelial cells via DLL4-Notch, TNFR1, and chemokine pathways such as CCL5/CCR5 influence endothelial cell behavior (170). Collectively, these mechanisms highlight Tregs as active participants in shaping the vascular microenvironment across various physiological and pathological contexts (Figure 5).
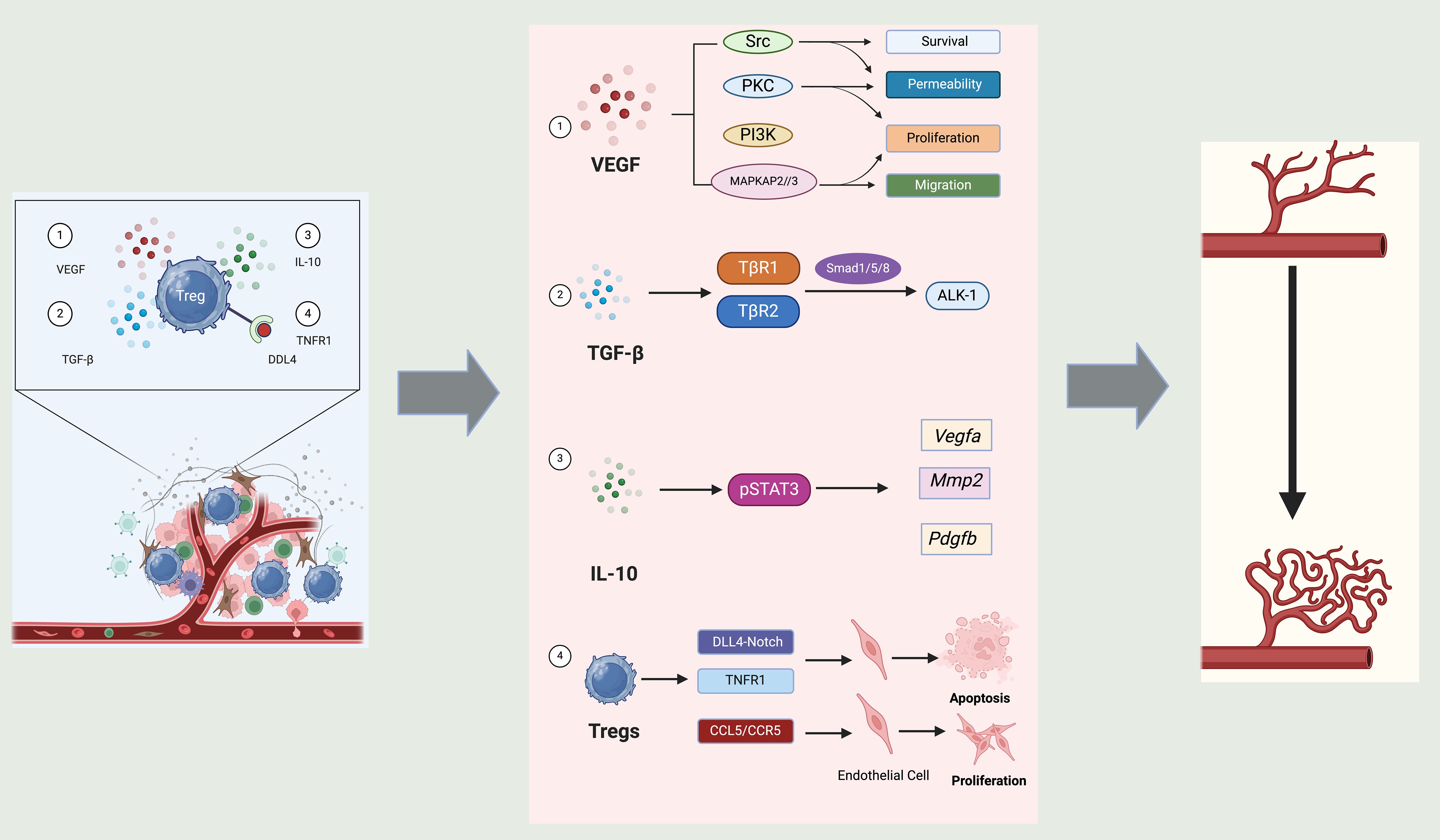
Figure 5. Role of Tregs in angiogenesis. Tregs influence angiogenesis through a variety of mechanisms mediated by distinct signals and interactions with endothelial cells. Firstly, Tregs contribute to the secretion of VEGF, a critical pro-angiogenic factor. This promotes several key functions in endothelial cells, including survival, permeability, proliferation, and migration, which are essential for new blood vessel formation. The signaling cascades involved, such as Src, PKC, PI3K, and MAPK pathways, underscore the multifaceted approach by which VEGF orchestrates the angiogenic process. Secondly, TGF-β secreted by Tregs interacts with its receptors, and through signaling intermediates like Smad1/5/8 and ALK-1, Tregs regulates the expression of angiogenic factors such as VEGF-A, MMP2, and PDGF-B. These molecules play crucial roles in modifying the extracellular matrix and promoting endothelial cell function, essential for angiogenesis. Thirdly, IL-10 produced by Tregs activates STAT3 signaling in target cells, which further influences the expression of angiogenic factors. This pathway underscores the anti-inflammatory and pro-angiogenic roles of IL-10, contributing to a conducive environment for vessel growth. Finally, Tregs directly interact with endothelial cells through mechanisms such as DLL4-Notch and TNFR1 signaling. These interactions can lead to endothelial cell apoptosis or proliferation, depending on the context, thereby influencing angiogenesis directly at the cellular level. Additionally, the interaction via CCL5/CCR5 pathways indicates a chemokine-mediated regulation of endothelial function by Tregs.
Role of Tregs in angiogenesis
Tregs influence angiogenesis through a variety of mechanisms mediated by distinct signals and interactions with endothelial cells. Firstly, Tregs contribute to the secretion of VEGF, a critical pro-angiogenic factor. This promotes several key functions in endothelial cells, including survival, permeability, proliferation, and migration, which are essential for new blood vessel formation. The signaling cascades involved, such as Src, PKC, PI3K, and MAPK pathways, underscore the multifaceted approach by which VEGF orchestrates the angiogenic process (160, 171). Secondly, TGF-β secreted by Tregs interacts with its receptors, and through signaling intermediates like Smad1/5/8 and ALK-1, Tregs regulates the expression of angiogenic factors such as VEGF-A, MMP2, and PDGF-B. These molecules play crucial roles in modifying the extracellular matrix and promoting endothelial cell function, essential for angiogenesis (172). Thirdly, IL-10 produced by Tregs activates STAT3 signaling in target cells, which further influences the expression of angiogenic factors. This pathway underscores the anti-inflammatory and pro-angiogenic roles of IL-10, contributing to a conducive environment for vessel growth (173). Last but not least, Tregs directly interact with endothelial cells through mechanisms such as DLL4-Notch and TNFR1 signaling (174, 175). These interactions can lead to endothelial cell apoptosis or proliferation, depending on the context, thereby influencing angiogenesis directly at the cellular level. Additionally, the interaction via CCL5/CCR5 pathways indicates a chemokine-mediated regulation of endothelial function by Tregs (176).
Further research is needed to elucidate the detailed mechanisms by which Tregs regulate angiogenesis and to determine how these mechanisms vary among different tissues and disease states. Developing targeted therapies that manipulate Treg functions in angiogenesis offers promising prospects for the treatment of a wide range of diseases, necessitating a collaborative effort between immunology and vascular biology fields to explore these therapeutic potentials fully (177).
Engineering antigen-specific Tregs
Tregs engineered to express CARs, known as CAR Tregs, are generated through genetic modification techniques that introduce CAR constructs into T cells (Figure 6) (178). This is typically achieved using viral vectors, such as lentiviral, retroviral, or adeno-associated viral systems, which facilitate stable integration of the CAR gene into the T cell genome. Alternatively, non-viral approaches like Sleeping Beauty or piggyBac transposon systems, and CRISPR-Cas9-based gene editing, offer site-specific integration with reduced risk of insertional mutagenesis (177, 179, 180). The starting cell population may include polyclonal Tregs, CD4⁺ T cells, or CD3⁺ T cells. In the case of polyclonal Tregs, cells are isolated and directly transduced with CAR constructs; however, their low abundance and potential phenotypic instability limit this approach. To overcome these limitations, CD4⁺ T cells can be co-transduced with CAR constructs and FOXP3 cDNA to induce a regulatory phenotype (181). This strategy enhances cell availability and helps maintain stable FOXP3 expression, which is essential for Treg function. These CAR Tregs are then expanded ex vivo under controlled conditions before being used for therapeutic applications, such as in autoimmunity, transplantation, or inflammation-related diseases. In 2022, a Phase 1/2 clinical trial (NCT04817774) investigated the safety and tolerability of autologous HLA-A2-specific CAR-Treg cells in kidney transplant recipients (182). A separate ongoing study (NCT05234190) is set to evaluate similar CAR-Treg therapy in liver transplant patients (182). These trials underscore the growing momentum and scientific interest in advancing CAR-Treg–based immunotherapies within the field of transplantation.
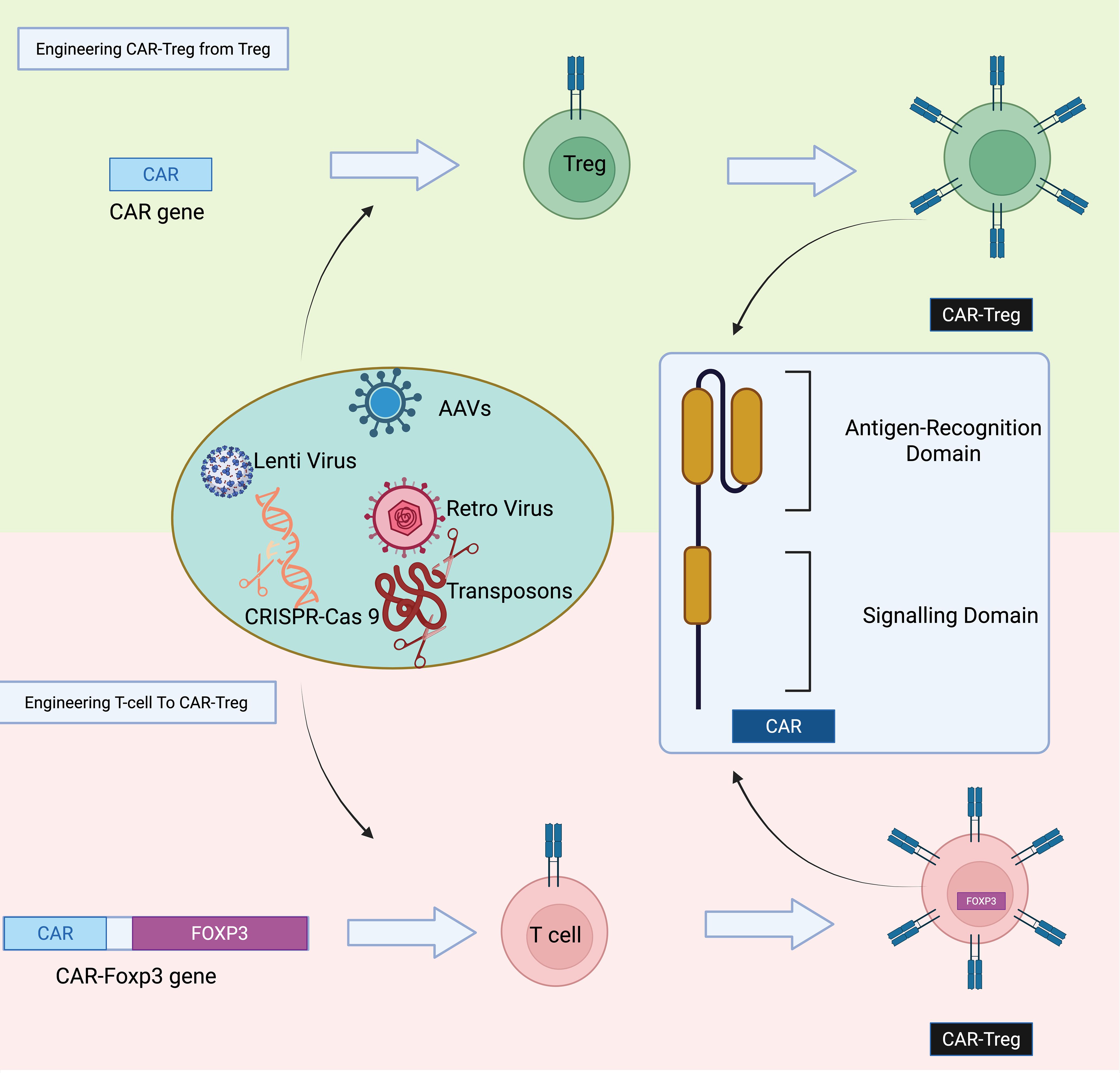
Figure 6. Strategies for generating CAR-Tregs. CAR-Tregs can be produced either by transducing polyclonal Tregs with a CAR construct or by co-transducing conventional T cells with both a CAR construct and the FoxP3 transcription factor gene to induce a regulatory phenotype.
Strategies for generating CAR-Tregs
CAR-Tregs can be produced either by transducing polyclonal Tregs with a CAR construct or by co-transducing conventional T cells with both a CAR construct and the FoxP3 transcription factor gene to induce a regulatory phenotype.
Introducing recombinant TCRs into Tregs enables antigen-specific activation by recognizing peptides presented by MHC molecules on antigen-presenting cells (182). Preclinical studies have shown that TCR-engineered Tregs specific for allo-MHC class II can prolong graft survival in transplant models, and autoreactive TCR-Tregs have shown therapeutic efficacy in autoimmune disease models such as EAE and type 1 diabetes (182–184). Future strategies may involve using TCRs naturally derived from tissue-resident Tregs to enhance tissue-specific immunoregulation, though knowledge about these TCRs and their target peptides remains limited.
To overcome the challenge of limited knowledge about relevant antigens and to enable MHC-independent activation, Treg cells have recently been engineered with artificial immune receptors (AIRs)—biosensors that detect inflammatory cues instead of antigens (185). These AIRs are designed to recognize membrane-bound inflammatory ligands such as TNF-α, TL1A (Tumor Necrosis Factor Superfamily Member 15), and LIGHT (Tumor Necrosis Factor Superfamily Member 14) (185). Structurally like CARs, AIRs include CD3ζ and CD28 intracellular signaling domains, enabling Tregs to respond to local inflammation. RNA sequencing has shown that AIR stimulation induces TCR-like signaling and promotes Treg proliferation. Importantly, AIRs respond only to membrane-bound ligands, enhancing tissue specificity. In vivo, AIR-Tregs expressing lymphotoxin-β receptor (LTBR) demonstrated superior protection against GvHD compared to polyclonal Tregs (185).
Though off-target effects are a potential concern, safety switches like inducible caspase 9 can be integrated to eliminate AIR-Tregs if needed. Comparative studies among TCR-Tregs, CAR-Tregs, and AIR-Tregs are needed to assess which approach offers better long-term persistence and therapeutic efficacy in chronic inflammatory settings or transplantation.
Tregs involved in the treatment of non-cancerous diseases
Tregs present unique opportunities for therapeutic innovation aimed at addressing the complex pathologies underlying many autoimmune and inflammatory diseases. Unlike other treatment modalities, Treg therapies have demonstrated the capacity to induce immune tolerance, allowing for short-term treatments that lead to prolonged periods of remission. Additionally, Tregs can function effectively in both lymphoid and non-lymphoid tissues, utilizing diverse mechanisms to modulate immune responses (186). In various preclinical studies, Treg-based adoptive immunotherapy has successfully reduced systemic inflammation and targeted organ damage across multiple autoimmune conditions such as inflammatory bowel disease (187), type 1 diabetes (188), experimental autoimmune encephalomyelitis (189), collagen-induced arthritis (190), and in models of organ transplant rejection and non-immune diseases like stroke (191) and amyotrophic lateral sclerosis (192), where inflammation contributes to the pathology. For example, researchers have recently developed a novel strategy by engineering Tregs to express a chimeric antigen receptor targeting the interleukin-23 receptor, thereby generating IL-23R CAR-Tregs as a potential therapy for Crohn’s disease (193). Given the critical role of IL-23R signaling and IL-23R-expressing mucosal cells in the development of inflammatory bowel diseases, targeting IL-23R using CAR-T cells represents a promising therapeutic strategy. The study showed that IL-23R-CAR-Treg cells displayed CAR-dependent suppressive effects on target cells in vitro and effectively protected mice from induced colitis (194–196). Following this, other research teams utilized autologous polyclonal Treg cells that were expanded ex vivo as a form of cellular therapy for treating inflammatory bowel diseases, as explored in clinical trials (NCT04691232; NCT03185000). A recent phase I, fast-track dose-escalation clinical trial in ulcerative colitis showed that a single infusion of Treg cells led to clinical improvement in certain subgroups of patients with active disease (68).
However, the ability of Tregs to act on nearby cells and the pivotal role of tissue-specific Tregs in managing local autoimmunity suggest that Tregs with single-antigen specificities may offer enhanced efficacy and safety. Studies in autoimmune and transplant settings have shown that Tregs expressing a singular T cell receptor can suppress autoreactive Teff cells and foster long-term tolerance. Early human trials indicate that alloantigen-specific Treg cells are promising in transplantation (197, 198). Recent advancements in synthetic biology have further enhanced Treg specificity through the introduction of specific TCRs and CARs, targeting inflamed tissues directly (199, 200). These innovations have not only proven effective in preclinical models but also demonstrated a significant increase in activity compared to polyclonal Treg populations. Therefore, with their versatile functionality across different diseases and the potential for genetic engineering, Treg therapies represent a groundbreaking approach to developing profound and enduring treatments.
Tregs involved in the treatment of cancer therapeutics
Recent advances in understanding and manipulating Tregs are transforming the landscape of cancer therapeutics. Tregs have a paradoxical role in cancer therapeutics by often enhancing tumor survival through immune suppression. This has led to a concentrated effort to either selectively deplete or functionally modulate Tregs in the tumor microenvironment to improve cancer immunotherapy outcomes without disrupting their protective role in healthy tissues (2, 201).
One significant area of development involves targeting Tregs that infiltrate tumors (TI-Tregs). These cells express high levels of immune checkpoint molecules such as CTLA-4 and PD-1, which are also targets of widely used cancer immunotherapies like ICB antibodies (147, 202). Research has shown that while blocking these pathways can reactivate effector T cells, it can also inadvertently enhance Treg suppression, underscoring the need for approaches that specifically target TI-Tregs without affecting those in non-tumoral tissues (203).
Recent studies have focused on exploiting the unique expression profiles of surface and intracellular molecules on TI-Tregs. For instance, differences in cytokine and metabolite dependency between TI-Tregs and conventional T cells provide opportunities for selective targeting (204–207). Molecules such as CD25 and CCR8 have emerged as potential targets to deplete TI-Tregs while sparing those necessary for immune homeostasis in healthy organs (208, 209). These approaches aim to evoke potent anti-tumor immunity while minimizing the risk of autoimmune responses.
The development of next-generation cell therapies, including engineered Tregs with CARs or modified TCRs, is also progressing (210–213). These engineered Tregs are designed to accurately target tumor cells or their microenvironment, enhancing specificity and minimizing off-target effects. Advanced gene-editing techniques, such as CRISPR/Cas9, are used to enhance the stability and functional properties of these cells under the harsh conditions of the tumor microenvironment (214, 215).
Furthermore, combination therapies that integrate Treg modulation with traditional cancer treatments are being tested. For example, combining Treg depletion strategies with immune checkpoint blockades or conventional chemotherapy has shown promise in preclinical models, suggesting that dual targeting of immune suppression and tumor cells can synergistically improve treatment outcomes (216–218).
Microbiota–Treg crosstalk in cancer and tissue repair
Unlike thymus-derived “natural” Tregs that form in a largely microbe-independent manner, RORγt⁺ Tregs in the colon are peripherally induced by commensal microbiota and carry a distinct microbial imprint (219–221). Gut bacteria produce metabolites—such as short-chain fatty acids (SCFAs) from dietary fiber and tryptophan catabolites—that drive the differentiation of these Foxp3⁺RORγt⁺ Tregs from naïve CD4⁺ T cells (222). SCFAs such as notably butyrate and propionate enhance Foxp3 expression through epigenetic modulation, thereby expanding the colonic Treg pool (223). Likewise, microbial tryptophan metabolites (e.g. indoles) serve as ligands for the aryl hydrocarbon receptor (AhR) on T cells, promoting the development of IL-10–producing RORγt⁺ Tregs. In parallel, commensal signals induce local dendritic cells and RORγt⁺ antigen-presenting cells to favor TGF-β–dependent Treg induction (224). The result is a specialized colonic Treg subset that co-expresses RORγt and is highly adapted to the intestinal environment – they often express gut-homing receptors (like CCR9) and secrete IL-10, which reinforces immune tolerance to microbiota (219, 225). By contrast, thymic Tregs recognize self-antigens and can function without microbial input, highlighting a fundamental difference in ontogeny and function between these subsets. Importantly, the influence of microbiota on RORγt⁺ Tregs extends beyond the gut, affecting systemic immunity, tissue repair, and cancer (13, 226–228). Colonic RORγt⁺ Tregs have been shown to migrate to distant sites of injury: for example, after muscle damage they transiently accumulate in the muscle to restrain IL-17A–mediated inflammation and thereby promote stem cell–driven regeneration (13). This “emissary” role suggests that a healthy microbiota can pre-condition regulatory T cells that aid in the resolution of inflammation and tissue healing throughout the body (13). In the context of cancer, microbiota-Treg interactions become a double-edged sword (229). On one hand, robust induction of RORγt⁺ Tregs by commensals helps keep chronic tissue inflammation in check – a factor known to reduce inflammation-driven tumorigenesis (229). On the other hand, an abundance of microbiota-educated Tregs in the tumor microenvironment may suppress anti-tumor effector responses, allowing tumors to evade immune surveillance (230). Thus, the balance of microbially tuned tolerance versus immunity can shape cancer outcomes. Understanding this crosstalk opens avenues for therapy: manipulating gut microbiota or their metabolites might modulate Treg activity to either enhance immune-mediated tumor clearance or, conversely, to reinforce regulatory pathways that limit tissue-damaging inflammation. In summary, RORγt⁺ Tregs represent a critical nexus between the microbiome and the immune system, influencing inflammatory diseases, cancer immunity, and the capacity for tissue regeneration.
Challenges and key features to be addressed in developing Treg therapeutics
Developing therapeutic strategies based on Tregs presents a promising frontier in cancer immunotherapy, yet it confronts numerous challenges and intricacies that necessitate detailed consideration. The key to advancing Treg-based therapies lies in overcoming these hurdles while harnessing the unique properties of Tregs to effectively target and modulate the tumor microenvironment.
i. Antigen-specificity: The antigen specificity of Tregs within the TME has garnered significant attention in recent years. While Tregs can suppress immune responses through antigen-independent mechanisms such as IL-2 consumption, secretion of inhibitory cytokines (e.g., IL-10, TGF-β), and CTLA-4-mediated modulation of dendritic cells, increasing evidence suggests that antigen recognition plays a crucial role in their tumor-suppressive function (231). Tumor-infiltrating Tregs often display a restricted TCR repertoire, indicating clonal expansion in response to tumor antigens. Several preclinical studies support this concept: in murine models, Tregs specific for tumor-associated antigens such as gp100 or carcino-embryonic antigen were shown to suppress effective antitumor T cell responses more potently than polyclonal Tregs (231–233). Furthermore, engineered Tregs expressing tumor-specific TCRs or CARs exhibit enhanced immunosuppressive activity within tumors (211). These findings imply that antigen specificity not only guides the accumulation of Tregs in tumors but may also enhance their suppressive capacity. However, the extent to which TCR engagement is required for Treg-mediated suppression remains debated, and further research is needed to dissect the balance between antigen-dependent and -independent mechanisms. Understanding this balance is critical for developing targeted strategies to modulate Tregs in cancer immunotherapy.
ii. Stability and Persistence in the Tumor Microenvironment: The suppressive and hypoxic conditions within tumors can affect the survival, function, and stability of infused or endogenously expanded Tregs. Modifying Tregs to enhance their resilience and functional stability in such hostile environments is critical (234, 235). This might involve genetic or pharmacological modulation of pathways involved in Treg survival and metabolic fitness, such as enhancing their oxidative phosphorylation capacity or their ability to respond to stress signals within the tumor microenvironment (236).
iii. Balancing Suppression: While the immunosuppressive functions of Tregs are beneficial in preventing autoimmunity and controlling inflammation, in the context of cancer, they can hinder effective anti-tumor immune responses. Innovatively designed strategies that modulate the suppressive functions of Tregs—potentially through transient and reversible approaches—are essential to maintain the balance between suppressing autoimmunity and enabling robust anti-tumor immunity.
iv. Off-Target Effects and Safety Concerns: The systemic effects of enhancing or inhibiting Tregs can lead to unintended consequences, such as increased susceptibility to infections or the development of autoimmune disorders. Developing localized delivery systems or ensuring that Treg activity is confined to the tumor site can mitigate these risks. Additionally, the potential for Tregs to convert into effector T cells under certain inflammatory conditions needs careful consideration to prevent adverse outcomes (11, 213).
v. Integration with Existing Therapies: Combining Treg-based approaches with existing cancer therapies such as chemotherapy, radiotherapy, and other immunotherapies could enhance overall treatment efficacy (237–239). However, the interactions between these therapies and Treg functions are complex and not fully understood. Research into how Tregs interact with other therapeutic agents and how they can be combined synergistically to improve patient outcomes is vital (11).
vi. Monitoring and Measuring Success: Effective biomarkers that can accurately monitor the function and impact of Treg therapies in real-time are lacking. Developing such biomarkers would enable better tracking of therapy efficacy and adjustment of treatment protocols in a personalized manner.
In conclusion, while the therapeutic potential of Tregs in cancer treatment is substantial, realizing this potential requires overcoming significant scientific and clinical challenges. Addressing these issues through innovative research and clinical trials will be crucial for the successful integration of Treg therapies into standard cancer treatment regimens. The journey from concept to clinic, therefore, involves not only understanding the fundamental biology of Tregs but also developing new technologies and therapeutic paradigms that can safely and effectively exploit these cells in the fight against cancer.
Concluding remarks
Tregs have emerged as central players not only in maintaining immune tolerance but also in orchestrating a wide range of physiological processes with significant therapeutic implications. The evolving understanding of Treg biology highlights their multifaceted roles across various disease contexts, including cancer, autoimmune disorders, metabolic diseases, and regenerative medicine.
In regenerative medicine, Tregs demonstrate significant potential through their ability to modulate inflammation and support tissue repair. Enhancing Treg function or mimicking their reparative actions offers promising strategies for improving outcomes in conditions characterized by chronic inflammation and impaired healing. Furthermore, Tregs contribute to angiogenesis and the maintenance of stem cell niches, suggesting a broader role in facilitating tissue regeneration. These insights emphasize the need to further dissect the molecular mechanisms by which Tregs interact with regenerative pathways, particularly in inflammatory and degenerative disease models.
Beyond tissue repair, Tregs are increasingly recognized for their regulatory influence over metabolic pathways. Their ability to modulate metabolic homeostasis positions them as attractive targets for treating complex metabolic disorders such as diabetes and metabolic syndrome. Therapeutically manipulating Tregs in this context could represent a novel immune-based approach to restoring metabolic balance and reducing chronic systemic inflammation.
In cancer, the dualistic role of Tregs presents both challenges and opportunities. While they are essential for maintaining immune tolerance, Tregs also suppress anti-tumor immunity within the tumor microenvironment, facilitating immune evasion and tumor progression. Therapeutic strategies that selectively disrupt the immunosuppressive functions of intratumoral Tregs—such as CAR-Tregs, monoclonal antibodies, and small-molecule inhibitors—have shown promise in preclinical and early clinical studies. These approaches aim to shift the immune balance toward effective tumor eradication while preserving systemic immune regulation.
Despite these advancements, several key challenges remain. Ensuring the stability and functional specificity of engineered or expanded Tregs in hostile environments, such as inflamed or hypoxic tumor tissues, is critical. Genetic modifications that enhance Treg resilience under such conditions are being explored. Equally important is the development of targeted delivery systems and reliable biomarkers to monitor Treg activity and therapeutic efficacy in real-time, minimizing off-target effects and improving patient stratification.
Taken together, the therapeutic manipulation of Tregs offers a compelling avenue for treating a spectrum of diseases. Their diverse functions—from immune suppression to tissue regeneration and metabolic regulation—underscore their potential as powerful tools in modern medicine. Future research should focus on refining Treg-based therapies to enhance their specificity, stability, and context-dependent functionality. By integrating advances in immunology, genetic engineering, and biomarker discovery, we can unlock the full therapeutic potential of Tregs and develop next-generation treatments that are both effective and safe across a wide range of clinical settings.
Author contributions
LZ: Investigation, Writing – original draft. DW: Methodology, Writing – original draft. HX: Validation, Visualization, Writing – original draft. HZ: Formal analysis, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was funded by the Shandong Province Natural Science Foundation grants ZR2022QH372.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hori S, Nomura T, and Sakaguchi S. Control of regulatory T cell development by the transcription factor foxp3. Science (80-). (2003) 299:1057–61. doi: 10.1126/science.1079490
2. Zhao H, Liao X, and Kang Y. Tregs: Where we are and what comes next? Front Immunol. (2017) 8:1578. doi: 10.3389/fimmu.2017.01578
3. Miyara M and Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. (2007) 13:108–16. doi: 10.1016/j.molmed.2007.01.003
4. Plitas G and Rudensky AY. Regulatory T cells in cancer. Annu Rev Cancer Biol. (2020) 4:459–77. doi: 10.1146/annurev-cancerbio-030419-033428
5. Shan F, Somasundaram A, Bruno TC, Workman CJ, and Vignali DAA. Therapeutic targeting of regulatory T cells in cancer. Trends Cancer. (2022) 8:944–61. doi: 10.1016/j.trecan.2022.06.008
6. Kang JH and Zappasodi R. Modulating Treg stability to improve cancer immunotherapy. Trends Cancer. (2023) 9:911–27. doi: 10.1016/j.trecan.2023.07.015
7. Iglesias-Escudero M, Arias-González N, and Martínez-Cáceres E. Regulatory cells and the effect of cancer immunotherapy. Mol Cancer. (2023) 22:26. doi: 10.1186/s12943-023-01714-0
8. Peng Y, Fu Y, Liu H, Zhao S, Deng H, Jiang X, et al. Non-IL-2-blocking anti-CD25 antibody inhibits tumor growth by depleting Tregs and has synergistic effects with anti-CTLA-4 therapy. Int J Cancer. (2024) 154:1285–97. doi: 10.1002/ijc.34823
9. Lax BM, Palmeri JR, Lutz EA, Sheen A, Stinson JA, Duhamel L, et al. Both intratumoral regulatory T cell depletion and CTLA-4 antagonism are required for maximum efficacy of anti-CTLA-4 antibodies. Proc Natl Acad Sci U.S.A. (2023) 120:e2300895120. doi: 10.1073/pnas.2300895120
10. Xu X, Dennett P, Zhang J, Sherrard A, Zhao Y, Masubuchi T, et al. CTLA4 depletes T cell endogenous and trogocytosed B7 ligands via cis-endocytosis. J Exp Med. (2023) 220:e20221391. doi: 10.1084/jem.20221391
11. Eskandari SK, Daccache A, and Azzi JR. Chimeric antigen receptor Treg therapy in transplantation. Trends Immunol. (2024) 45:48–61. doi: 10.1016/j.it.2023.11.005
12. MacDonald KN, Salim K, and Levings MK. Manufacturing next-generation regulatory T-cell therapies. Curr Opin Biotechnol. (2022) 78:102822. doi: 10.1016/j.copbio.2022.102822
13. Hanna BS, Wang G, Galván-Peña S, Mann AO, Ramirez RN, Muñoz-Rojas AR, et al. The gut microbiota promotes distal tissue regeneration via RORγ+ regulatory T cell emissaries. Immunity. (2023) 56:8249–846. doi: 10.1016/j.immuni.2023.01.033
14. Guenin-Mace L, Konieczny P, and Naik S. Immune-epithelial cross talk in regeneration and repair. Annu Rev Immunol. (2023) 41:207–28. doi: 10.1146/annurev-immunol-101721-062818
15. Wu Y, Pu X, Wang X, and Xu M. Reprogramming of lipid metabolism in the tumor microenvironment: a strategy for tumor immunotherapy. Lipids Health Dis. (2024) 23:35. doi: 10.1186/s12944-024-02024-0
16. Oussaada SM, Kilicarslan M, de Weijer BA, Gilijamse PW, Şekercan A, Virtue S, et al. Tissue-specific inflammation and insulin sensitivity in subjects with obesity. Diabetes Res Clin Pract. (2024) 211:111663. doi: 10.1016/j.diabres.2024.111663
17. Cohen JN, Gouirand V, Macon CE, Lowe MM, Boothby IC, Moreau JM, et al. Regulatory T cells in skin mediate immune privilege of the hair follicle stem cell niche. Sci Immunol. (2024) 9:eadh0152. doi: 10.1126/sciimmunol.adh0152
18. Azari Z, Gorgani S, Hosseini SA, Wang AZ, Kim H-W, and Kargozar S. The role of immune cells in therapeutic angiogenesis: Concepts in tissue engineering. Curr Opin BioMed Eng. (2023) 28:100470. doi: 10.1016/j.cobme.2023.100470
19. Ramsdell F and Ziegler SF. FOXP3 and scurfy: how it all began. Nat Rev Immunol. (2014) 14:343–9. doi: 10.1038/nri3650
20. Rudensky AY. Regulatory T cells and foxp3. Immunol Rev. (2011) 241:260–8. doi: 10.1111/j.1600-065X.2011.01018.x
21. Lu L, Barbi J, and Pan F. The regulation of immune tolerance by FOXP3. Nat Rev Immunol. (2017) 17:703–17. doi: 10.1038/nri.2017.75
22. Sanidad KZ, Rager SL, Carrow HC, Ananthanarayanan A, Callaghan R, Hart LR, et al. Gut bacteria–derived serotonin promotes immune tolerance in early life. Sci Immunol. (2024) 9:eadj4775. doi: 10.1126/sciimmunol.adj4775
23. Su X, Wang X, Zhang X, Sun Y, and Jia Y. β-Indole-3-acetic acid attenuated collagen-induced arthritis through reducing the ubiquitination of Foxp3 via the AhR-TAZ-Tip60 pathway. Immunol Res. (2024) 72:1–13. doi: 10.1007/s12026-024-09480-x
24. Kanamori M, Nakatsukasa H, Okada M, Lu Q, and Yoshimura A. Induced regulatory T cells: their development, stability, and applications. Trends Immunol. (2016) 37:803–11. doi: 10.1016/j.it.2016.08.012
25. Trzonkowski P, Szmit E, Myśliwska J, Dobyszuk A, and Myśliwski A. CD4 +CD25 + T regulatory cells inhibit cytotoxic activity of T CD8 + and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol. (2004) 112:258–67. doi: 10.1016/j.clim.2004.04.003
26. Wang Z, He L, Li W, Xu C, Zhang J, Wang D, et al. GDF15 induces immunosuppression via CD48 on regulatory T cells in hepatocellular carcinoma. J Immunother Cancer. (2021) 9:e002787. doi: 10.1136/jitc-2021-002787
27. Sullivan JA, Tomita Y, Jankowska-Gan E, Lema DA, Arvedson MP, Nair A, et al. Treg-cell-derived IL-35-coated extracellular vesicles promote infectious tolerance. Cell Rep. (2020) 30:1039–1051.e5. doi: 10.1016/j.celrep.2019.12.081
28. Wang H, Franco F, and Ho PC. Metabolic regulation of tregs in cancer: opportunities for immunotherapy. Trends Cancer. (2017) 3:583–92. doi: 10.1016/j.trecan.2017.06.005
29. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, and Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. (1995) 155:1151–64. doi: 10.4049/jimmunol.155.3.1151
30. D’Cruz LM and Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. (2005) 6:1152–9. doi: 10.1038/ni1264
31. Josefowicz SZ, Lu LF, and Rudensky AY. Regulatory T cells: Mechanisms of differentiation and function. Annu Rev Immunol. (2012) 30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623
32. Zhao H, Bo C, Kang Y, and Li H. What else can CD39 tell us? Front Immunol. (2017) 8:727. doi: 10.3389/fimmu.2017.00727
33. Garín MI, Chu NC, Golshayan D, Cernuda-Morollón E, Wait R, and Lechler RI. Galectin-1: A key effector of regulation mediated by CD4 +CD25+ T cells. Blood. (2007) 109:2058–65. doi: 10.1182/blood-2006-04-016451
34. Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, et al. Basis of CTLA-4 function in regulatory and conventional CD4+ T cells. Blood J Am Soc Hematol. (2012) 119:5155–63. doi: 10.1182/blood-2011-11-388918
35. Gondek DC, Lu L-F, Quezada SA, Sakaguchi S, and Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ Regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. (2005) 174:1783–6. doi: 10.4049/jimmunol.174.4.1783
36. Zong Y, Deng K, and Chong WP. Regulation of Treg cells by cytokine signaling and co-stimulatory molecules. Front Immunol. (2024) 15:1387975. doi: 10.3389/fimmu.2024.1387975
37. Wang Y, Li J, Nakahata S, and Iha H. Complex role of regulatory T cells (Tregs) in the tumor microenvironment: their molecular mechanisms and bidirectional effects on cancer progression. Int J Mol Sci. (2024) 25:7346. doi: 10.3390/ijms25137346
38. Frydrychowicz M, Boruczkowski M, Kolecka-Bednarczyk A, and Dworacki G. The dual role of treg in cancer. Scand J Immunol. (2017) 86:436–43. doi: 10.1111/sji.12615
39. Zhang S, Lv K, Liu Z, Zhao R, and Li F. Fatty acid metabolism of immune cells: a new target of tumour immunotherapy. Cell Death Discov. (2024) 10:39. doi: 10.1038/s41420-024-01807-9
40. Wu T, Wang L, Jian C, Gao C, Liu Y, Fu Z, et al. Regulatory T cell-derived exosome mediated macrophages polarization for osteogenic differentiation in fracture repair. J Control Release. (2024) 369:266–82. doi: 10.1016/j.jconrel.2024.03.028
41. Hsu C-Y, Ahmed AT, Bansal P, Hjazi A, Al-Hetty HRAK, Qasim MT, et al. MicroRNA-enriched exosome as dazzling dancer between cancer and immune cells. J Physiol Biochem. (2024) 80:811–29. doi: 10.1007/s13105-024-01050-x
42. Zhang P, Liu X, Gu Z, Jiang Z, Zhao S, Song Y, et al. Targeting TIGIT for cancer immunotherapy: recent advances and future directions. biomark Res. (2024) 12:7. doi: 10.1186/s40364-023-00543-z
43. Chauvin J-M and Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. (2020) 8:e000957. doi: 10.1136/jitc-2020-000957
44. Huang C-T, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in regulatory T cells. Immunity. (2004) 21:503–13. doi: 10.1016/j.immuni.2004.08.010
45. Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med. (2015) 21:638–46. doi: 10.1038/nm.3868
46. Li Y, Zhang C, Jiang A, Lin A, Liu Z, Cheng X, et al. Potential anti-tumor effects of regulatory T cells in the tumor microenvironment: a review. J Transl Med. (2024) 22:293. doi: 10.1186/s12967-024-05104-y
47. Kim J, Li J, Wei J, and Lim SA. Regulatory T cell metabolism: a promising therapeutic target for cancer treatment? Immune Netw. (2025) 25:e13. doi: 10.4110/in.2025.25.e13
48. Zhou X, Tang J, Cao H, Fan H, and Li B. Tissue resident regulatory T cells: Novel therapeutic targets for human disease. Cell Mol Immunol. (2015) 12:543–52. doi: 10.1038/cmi.2015.23
49. Cipolletta D. Adipose tissue-resident regulatory T cells: Phenotypic specialization, functions and therapeutic potential. Immunology. (2014) 142:517–25. doi: 10.1111/imm.12262
50. Lee J, Kim D, and Min B. Tissue resident foxp3+ Regulatory T cells: sentinels and saboteurs in health and disease. Front Immunol. (2022) 13:865593. doi: 10.3389/fimmu.2022.865593
51. Liston A, Dooley J, and Yshii L. Brain-resident regulatory T cells and their role in health and disease. Immunol Lett. (2022) 248:26–30. doi: 10.1016/j.imlet.2022.06.005
52. Cheru N, Hafler DA, and Sumida TS. Regulatory T cells in peripheral tissue tolerance and diseases. Front Immunol. (2023) 14:1154575. doi: 10.3389/fimmu.2023.1154575
53. Cao Y, Hou Y, Zhao L, Huang Y, and Liu G. New insights into follicular regulatory T cells in the intestinal and tumor microenvironments. J Cell Physiol. (2023) 238:1465–77. doi: 10.1002/jcp.31039
54. Nguyen Q and Borrow P. Human T follicular regulatory cells with helper and regulatory lineage origins. Nat Rev Immunol. (2023) 23:204. doi: 10.1038/s41577-023-00850-4
55. Lin N, Yin W, Miller H, Byazrova MG, Herrada AA, Benlagha K, et al. The role of regulatory T cells and follicular T helper cells in HBV infection. Front Immunol. (2023) 14:1169601. doi: 10.3389/fimmu.2023.1169601
56. Graca L, Jacobsen J, and Kumar S. The expanding family of T follicular regulatory cells. Sci Immunol. (2023) 8:eadg7526. doi: 10.1126/sciimmunol.adg7526
57. Dikiy S and Rudensky AY. Principles of regulatory T cell function. Immunity. (2023) 56:240–55. doi: 10.1016/j.immuni.2023.01.004
58. Duhen T, Duhen R, Lanzavecchia A, Sallusto F, and Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. (2012) 119:4430–40. doi: 10.1182/blood-2011-11-392324
59. Mills KHG. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. (2023) 23(3):38–54. doi: 10.1038/s41577-022-00746-9
60. Malviya V, Yshii L, Junius S, Garg AD, Humblet-Baron S, and Schlenner SM. Regulatory T-cell stability and functional plasticity in health and disease. Immunol Cell Biol. (2023) 101:112–29. doi: 10.1111/imcb.12613
61. Zhu Y, Yan P, Wang R, Lai J, Tang H, Xiao X, et al. Opioid-induced fragile-like regulatory T cells contribute to withdrawal. Cell. (2023) 186:591–606. doi: 10.1016/j.cell.2022.12.030
62. Sugiyama D, Hinohara K, and Nishikawa H. Significance of regulatory T cells in cancer immunology and immunotherapy. Exp Dermatol. (2023) 32:256–63. doi: 10.1111/exd.14721
63. Chaudhary B and Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: Role and therapeutic targeting. Vaccines. (2016) 4:1–25. doi: 10.3390/vaccines4030028
64. Scott EN, Gocher AM, Workman CJ, and Vignali DAA. Regulatory T cells: barriers of immune infiltration into the tumor microenvironment. Front Immunol. (2021) 12:702726. doi: 10.3389/fimmu.2021.702726
65. Ali N and Rosenblum MD. Regulatory T cells in skin. Immunology. (2017) 152:372–81. doi: 10.1111/imm.12791
66. Burzyn D, Benoist C, and Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. (2013) 14:1007–13. doi: 10.1038/ni.2683
67. Wu J, Ren B, Wang D, and Lin H. Regulatory T cells in skeletal muscle repair and regeneration: recent insights. Cell Death Dis. (2022) 13:680. doi: 10.1038/s41419-022-05142-8
68. Voskens C, Stoica D, Rosenberg M, Vitali F, Zundler S, Ganslmayer M, et al. Autologous regulatory T-cell transfer in refractory ulcerative colitis with concomitant primary sclerosing cholangitis. Gut. (2023) 72:49–53. doi: 10.1136/gutjnl-2022-327075
69. Tough DF and Lombardi G. Therapeutic opportunities for regulatory T-cell enhancing approaches. Clin Exp Immunol. (2023) 211:93–5. doi: 10.1093/cei/uxad009
70. Wood KJ and Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. (2003) 3:199–210. doi: 10.1038/nri1027
71. Grant CR, Liberal R, Mieli-Vergani G, Vergani D, and Longhi MS. Regulatory T-cells in autoimmune diseases: Challenges, controversies and-yet-unanswered questions. Autoimmun Rev. (2015) 14:105–16. doi: 10.1016/j.autrev.2014.10.012
72. Hippen KL, Hefazi M, Larson JH, and Blazar BR. Emerging translational strategies and challenges for enhancing regulatory T cell therapy for graft-versus-host disease. Front Immunol. (2022) 13:926550. doi: 10.3389/fimmu.2022.926550
73. Mills KHG and McGuirk P. Antigen-specific regulatory T cells—their induction and role in infection. Semin Immunol. (2004) 16:107–17. doi: 10.1016/j.smim.2003.12.006
74. Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro–expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. (2004) 199:1455–65. doi: 10.1084/jem.20040139
75. Arjomandnejad M, Kopec AL, and Keeler AM. CAR-T regulatory (CAR-treg) cells: engineering and applications. Biomedicines. (2022) 10:287. doi: 10.3390/biomedicines10020287
76. Beheshti SA, Shamsasenjan K, Ahmadi M, and Abbasi B. CAR Treg: A new approach in the treatment of autoimmune diseases. Int Immunopharmacol. (2022) 102:108409. doi: 10.1016/j.intimp.2021.108409
77. Midavaine É, Moraes BC, Benitez J, Rodriguez SR, Braz JM, Kochhar NP, et al. Meningeal regulatory T cells inhibit nociception in female mice. Science (80-). (2025) 388:96–104. doi: 10.1126/science.adq6531
78. Othy S, Jairaman A, Dynes JL, Dong TX, Tune C, Yeromin AV, et al. Regulatory T cells suppress Th17 cell Ca2+ signaling in the spinal cord during murine autoimmune neuroinflammation. Proc Natl Acad Sci. (2020) 117:20088–99. doi: 10.1073/pnas.2006895117
79. Pereira MMA, Mahú I, Seixas E, Martinéz-Sánchez N, Kubasova N, Pirzgalska RM, et al. A brain-sparing diphtheria toxin for chemical genetic ablation of peripheral cell lineages. Nat Commun. (2017) 8:14967. doi: 10.1038/ncomms14967
80. Itahashi K, Irie T, and Nishikawa H. Regulatory T-cell development in the tumor microenvironment. Eur J Immunol. (2022) 52:1216–27. doi: 10.1002/eji.202149358
81. Nishikawa H and Koyama S. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer. (2021) 9:e002591. doi: 10.1136/jitc-2021-002591
82. Montauti E, Weinberg SE, Chu P, Chaudhuri S, Mani NL, Iyer R, et al. A deubiquitination module essential for Treg fitness in the tumor microenvironment. Sci Adv. (2023) 8:eabo4116. doi: 10.1126/sciadv.abo4116
83. Lee YY, Choi CH, Do IG, Song SY, Lee W, Park HS, et al. Prognostic value of the copper transporters, CTR1 and CTR2, in patients with ovarian carcinoma receiving platinum-based chemotherapy. Gynecol Oncol. (2011) 122:361–5. doi: 10.1016/j.ygyno.2011.04.025
84. Yu S, Wang Y, Hou J, Li W, Wang X, Xiang L, et al. Tumor-infiltrating immune cells in hepatocellular carcinoma: Tregs is correlated with poor overall survival. PloS One. (2020) 15:e0231003. doi: 10.1371/journal.pone.0231003
85. Pereira JA, Lanzar Z, Clark JT, Hart AP, Douglas BB, Shallberg L, et al. PD-1 and CTLA-4 exert additive control of effector regulatory T cells at homeostasis. Front Immunol. (2023) 14:997376. doi: 10.3389/fimmu.2023.997376
86. Tay C, Tanaka A, and Sakaguchi S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell. (2023) 41:450–65. doi: 10.1016/j.ccell.2023.02.014
87. Maharaj K, Uriepero A, Sahakian E, and Pinilla-Ibarz J. Regulatory T cells (Tregs) in lymphoid Malignancies and the impact of novel therapies. Front Immunol. (2022) 13:943354. doi: 10.3389/fimmu.2022.943354
88. Andersen MH. Tumor microenvironment antigens. Semin Immunopathol. (2023) 45:253–64. doi: 10.1007/s00281-022-00966-0
89. McRitchie BR and Akkaya B. Exhaust the exhausters: Targeting regulatory T cells in the tumor microenvironment. Front Immunol. (2022) 13:940052. doi: 10.3389/fimmu.2022.940052
90. Noyes D, Bag A, Oseni S, Semidey-Hurtado J, Cen L, Sarnaik AA, et al. Tumor-associated Tregs obstruct antitumor immunity by promoting T cell dysfunction and restricting clonal diversity in tumor-infiltrating CD8+ T cells. J Immunother Cancer. (2022) 10:e004605. doi: 10.1136/jitc-2022-004605
91. Huppert LA, Green MD, Kim L, Chow C, Leyfman Y, Daud AI, et al. Tissue-specific Tregs in cancer metastasis: opportunities for precision immunotherapy. Cell Mol Immunol. (2022) 19:33–45. doi: 10.1038/s41423-021-00742-4
92. Marangoni F, Zhakyp A, Corsini M, Geels SN, Carrizosa E, Thelen M, et al. Expansion of tumor-associated Treg cells upon disruption of a CTLA-4-dependent feedback loop. Cell. (2021) 184:3998–4015.e19. doi: 10.1016/j.cell.2021.05.027
93. Huang L, Guo Y, Liu S, Wang H, Zhu J, Ou L, et al. Targeting regulatory T cells for immunotherapy in melanoma. Mol BioMed. (2021) 2:11. doi: 10.1186/s43556-021-00038-z
94. Rocamora-Reverte L, Melzer FL, Würzner R, and Weinberger B. The complex role of regulatory T cells in immunity and aging. Front Immunol. (2020) 11:616949. doi: 10.3389/fimmu.2020.616949
95. van der Veeken J, Gonzalez AJ, Cho H, Arvey A, Hemmers S, Leslie CS, et al. Memory of inflammation in regulatory T cells. Cell. (2016) 166:977–90. doi: 10.1016/j.cell.2016.07.006
96. Dees S, Ganesan R, Singh S, and Grewal IS. Regulatory T cell targeting in cancer: Emerging strategies in immunotherapy. Eur J Immunol. (2021) 51:280–91. doi: 10.1002/eji.202048992
97. Berasain C and Avila MA. Amphiregulin. Semin Cell Dev Biol. (2014) 28:31–41. doi: 10.1016/j.semcdb.2014.01.005
98. Seputra K, Kalim H, Susianti H, Purnomo B, Rasyid H, and Purnomo A. Modulation of T-cell regulators associated with advanced stage of prostate cancer. Acta Inform Med. (2021) 29:182. doi: 10.5455/aim.2021.29.182-186
99. Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, et al. Individual intestinal symbionts induce a distinct population of RORγ + regulatory T cells. Science (80-). (2015) 349:993–7. doi: 10.1126/science.aaa9420
100. Whiteside TL. Immunobiology of head and neck cancer. Cancer Metastasis Rev. (2005) 24:95–105. doi: 10.1007/s10555-005-5050-6
101. Seminerio I, Descamps G, Dupont S, de Marrez L, Laigle J-A, Lechien JR, et al. Infiltration of foxP3+ Regulatory T cells is a strong and independent prognostic factor in head and neck squamous cell carcinoma. Cancers (Basel). (2019) 11:227. doi: 10.3390/cancers11020227
102. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (80-). (2006) 313:1960–4. doi: 10.1126/science.1129139
103. Xu W, Liu H, Song J, Fu H-X, Qiu L, Zhang B-F, et al. The appearance of Tregs in cancer nest is a promising independent risk factor in colon cancer. J Cancer Res Clin Oncol. (2013) 139:1845–52. doi: 10.1007/s00432-013-1500-7
104. Togashi Y, Shitara K, and Nishikawa H. Regulatory T cells in cancer immunosuppression — implications for anticancer therapy. Nat Rev Clin Oncol. (2019) 16:356–71. doi: 10.1038/s41571-019-0175-7
105. Raffin C, Vo LT, and Bluestone JA. Treg cell-based therapies: challenges and perspectives. Nat Rev Immunol. (2020) 20:158–72. doi: 10.1038/s41577-019-0232-6
106. Santegoets SJ, Duurland CL, Jordanova ES, van Ham JJ, Ehsan I, van Egmond SL, et al. Tbet-positive regulatory T cells accumulate in oropharyngeal cancers with ongoing tumor-specific type 1 T cell responses. J Immunother Cancer. (2019) 7:14. doi: 10.1186/s40425-019-0497-0
107. Huang C, Zhang F, Li P, and Song C. Low-dose IL-2 attenuated depression-like behaviors and pathological changes through restoring the balances between IL-6 and TGF-β and between th17 and treg in a chronic stress-induced mouse model of depression. Int J Mol Sci. (2022) 23:13856. doi: 10.3390/ijms232213856
108. Kleinewietfeld M and Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol. (2013) 25:305–12. doi: 10.1016/j.smim.2013.10.009
109. Downs-Canner S, Berkey S, Delgoffe GM, Edwards RP, Curiel T, Odunsi K, et al. Suppressive IL-17A+ Foxp3+ and ex-Th17 IL-17AnegFoxp3+ Treg cells are a source of tumour-associated Treg cells. Nat Commun. (2017) 8:14649. doi: 10.1038/ncomms14649
110. Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, et al. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. (2009) 69:5490–7. doi: 10.1158/0008-5472.CAN-09-0304
111. Zítka O, Kukacka J, Krizkov S, Húska D, Adam V, Masarik M, et al. Matrix metalloproteinases. Curr Med Chem. (2010) 17:3751–68. doi: 10.2174/092986710793213724
112. Freuchet A, Roy P, Armstrong SS, Oliaeimotlagh M, Kumar S, Orecchioni M, et al. Identification of human exTreg cells as CD16+ CD56+ cytotoxic CD4+ T cells. Nat Immunol. (2023) 24:1748–61. doi: 10.1038/s41590-023-01589-9
113. West NR, Kost SE, Martin SD, Milne K, Deleeuw RJ, Nelson BH, et al. Tumour-infiltrating FOXP3+ lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer. (2013) 108:155–62. doi: 10.1038/bjc.2012.524
114. Li Y, Zhang C, Jiang A, Lin A, Liu Z, Cheng X, et al. Potential anti-tumor effects of regulatory T cells in the tumor microenvironment: a review. J Transl Med. (2024) 22:1–21. doi: 10.1186/s12967-024-05104-y
115. Knoedler S, Knoedler L, Kauke-Navarro M, Rinkevich Y, Hundeshagen G, Harhaus L, et al. Regulatory T cells in skin regeneration and wound healing. Mil Med Res. (2023) 10:49. doi: 10.1186/s40779-023-00484-6
116. Boothby IC, Cohen JN, and Rosenblum MD. Regulatory T cells in skin injury: At the crossroads of tolerance and tissue repair. Sci Immunol. (2020) 5:eaaz9631. doi: 10.1126/sciimmunol.aaz9631
117. Short WD, Steen E, Kaul A, Wang X, Olutoye OO, Vangapandu HV, et al. IL-10 promotes endothelial progenitor cell infiltration and wound healing via STAT3. FASEB J. (2022) 36:e22298. doi: 10.1096/fj.201901024RR
118. Xiaojie W, Banda J, Qi H, Chang AK, Bwalya C, Chao L, et al. Scarless wound healing: Current insights from the perspectives of TGF-β, KGF-1, and KGF-2. Cytokine Growth Factor Rev. (2022) 66:26–37. doi: 10.1016/j.cytogfr.2022.03.001
119. Xu X, Zheng L, Yuan Q, Zhen G, Crane JL, Zhou X, et al. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. (2018) 6:2. doi: 10.1038/s41413-017-0005-4
120. Pakyari M, Farrokhi A, Maharlooei MK, and Ghahary A. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care. (2013) 2:215–24. doi: 10.1089/wound.2012.0406
121. Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, and Saparov A. Immunology of acute and chronic wound healing. Biomolecules. (2021) 11:700. doi: 10.3390/biom11050700
122. Sim SL, Kumari S, Kaur S, and Khosrotehrani K. Macrophages in skin wounds: functions and therapeutic potential. Biomolecules. (2022) 12:1659. doi: 10.3390/biom12111659
123. Zaiss DMW, van Loosdregt J, Gorlani A, Bekker CPJ, Gröne A, Sibilia M, et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. (2013) 38:275–84. doi: 10.1016/j.immuni.2012.09.023
124. Zhang P, Wang J, Miao J, and Zhu P. The dual role of tissue regulatory T cells in tissue repair: return to homeostasis or fibrosis. Front Immunol. (2025) 16:1560578. doi: 10.3389/fimmu.2025.1560578
125. Popovic B, Golemac M, Podlech J, Zeleznjak J, Bilic-Zulle L, Lukic ML, et al. IL-33/ST2 pathway drives regulatory T cell dependent suppression of liver damage upon cytomegalovirus infection. PloS Pathog. (2017) 13:e1006345. doi: 10.1371/journal.ppat.1006345
126. Sun R, Zhao H, Gao DS, Ni A, Li H, Chen L, et al. Amphiregulin couples IL1RL1+ regulatory T cells and cancer-associated fibroblasts to impede antitumor immunity. Sci Adv. (2023) 9:eadd7399. doi: 10.1126/sciadv.add7399
127. Jiang W, Lian J, Yue Y, and Zhang Y. IL-33/ST2 as a potential target for tumor immunotherapy. Eur J Immunol. (2021) 51:1943–55. doi: 10.1002/eji.202149175
128. Fiyouzi T, Pelaez-Prestel HF, Reyes-Manzanas R, Lafuente EM, and Reche PA. Enhancing regulatory T cells to treat inflammatory and autoimmune diseases. Int J Mol Sci. (2023) 24:7797. doi: 10.3390/ijms24097797
129. Dombrowski Y, O’hagan T, Dittmer M, Penalva R, Mayoral SR, Bankhead P, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci. (2017) 20:674–80. doi: 10.1038/nn.4528
130. Peng L, Wei Y, Shao Y, Li Y, Liu N, and Duan L. The emerging roles of CCN3 protein in immune-related diseases. Mediators Inflammation. (2021) 2021:5576059. doi: 10.1155/2021/5576059
131. Tyler CJ, McCarthy NE, Lindsay JO, Stagg AJ, Moser B, and Eberl M. Antigen-presenting human γδ T cells promote intestinal CD4 + T cell expression of IL-22 and mucosal release of calprotectin. J Immunol. (2017) 198:3417–25. doi: 10.4049/jimmunol.1700003
132. Zhang D, Chen Z, Wang DC, and Wang X. Regulatory T cells and potential inmmunotherapeutic targets in lung cancer. Cancer Metastasis Rev. (2015) 34:277–90. doi: 10.1007/s10555-015-9566-0
133. Bernatchez E, Gold MJ, Langlois A, Lemay A-M, Brassard J, Flamand N, et al. Pulmonary CD103 expression regulates airway inflammation in asthma. . Am J Physiol Cell Mol Physiol. (2015) 308:L816–26. doi: 10.1152/ajplung.00319.2014
134. Cho I, Lui PP, and Ali N. Treg regulation of the epithelial stem cell lineage. J Immunol Regener Med. (2020) 8:100028. doi: 10.1016/j.regen.2020.100028
135. Wang F, Xia H, and Yao S. Regulatory T cells are a double-edged sword in pulmonary fibrosis. Int Immunopharmacol. (2020) 84:106443. doi: 10.1016/j.intimp.2020.106443
136. Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. (2010) 184:3964–77. doi: 10.4049/jimmunol.0903356
137. Fung THW, Yang KY, and Lui KO. An emerging role of regulatory T-cells in cardiovascular repair and regeneration. Theranostics. (2020) 10:8924–38. doi: 10.7150/thno.47118
138. Field CS, Baixauli F, Kyle RL, Puleston DJ, Cameron AM, Sanin DE, et al. Mitochondrial integrity regulated by lipid metabolism is a cell-intrinsic checkpoint for treg suppressive function. Cell Metab. (2020) 31:422–437.e5. doi: 10.1016/j.cmet.2019.11.021
139. Bapat SP, Myoung Suh J, Fang S, Liu S, Zhang Y, Cheng A, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. (2015) 528:137–41. doi: 10.1038/nature16151
140. Tao L, Liu H, and Gong Y. Role and mechanism of the Th17/Treg cell balance in the development and progression of insulin resistance. Mol Cell Biochem. (2019) 459:183–8. doi: 10.1007/s11010-019-03561-4
141. Deng T, Liu J, Deng Y, Minze L, Xiao X, Wright V, et al. Adipocyte adaptive immunity mediates diet-induced adipose inflammation and insulin resistance by decreasing adipose Treg cells. Nat Commun. (2017) 8:15725. doi: 10.1038/ncomms15725
142. Zhu F, Wang A, Li Y, Liang R, Li D, and Li B. Adipose tissue-resident regulatory T cells. Adv Exp Med Biol. (2017) 1011:153–62. doi: 10.1007/978-94-024-1170-6_4
143. Gao Z, Xu X, Li Y, Sun K, Yang M, Zhang Q, et al. Mechanistic insight into PPARγ and tregs in atherosclerotic immune inflammation. Front Pharmacol. (2021) 12:750078. doi: 10.3389/fphar.2021.750078
144. Han JM, Wu D, Denroche HC, Yao Y, Verchere CB, and Levings MK. IL-33 reverses an obesity-induced deficit in visceral adipose tissue ST2+ T regulatory cells and ameliorates adipose tissue inflammation and insulin resistance. J Immunol. (2015) 194:4777–83. doi: 10.4049/jimmunol.1500020
145. Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. (2015) 16:276–85. doi: 10.1038/ni.3085
146. Grusdat M and Brenner D. Adipose Treg cells in charge of metabolism. Nat Immunol. (2024) 25:392–3. doi: 10.1038/s41590-024-01762-8
147. Zhao H, Feng R, Peng A, Li G, and Zhou L. The expanding family of noncanonical regulatory cell subsets. J Leukoc Biol. (2019) 106:369–83. doi: 10.1002/JLB.6RU0918-353RRRR
148. Li C, DiSpirito JR, Zemmour D, Spallanzani RG, Kuswanto W, Benoist C, et al. TCR transgenic mice reveal stepwise, multi-site acquisition of the distinctive fat-treg phenotype. Cell. (2018) 174:285–99. doi: 10.1016/j.cell.2018.05.004
149. Papatriantafyllou M. A Treg cell duo for VAT control. Nat Rev Immunol. (2024) 1:232. doi: 10.1038/s41577-024-01018-4
150. Moreno Ayala MA, Campbell TF, Zhang C, Dahan N, Bockman A, Prakash V, et al. CXCR3 expression in regulatory T cells drives interactions with type I dendritic cells in tumors to restrict CD8+ T cell antitumor immunity. Immunity. (2023) 56:1613–1630.e5. doi: 10.1016/j.immuni.2023.06.003
151. Osei-Bordom D, Bozward AG, and Oo YH. The hepatic microenvironment and regulatory T cells. Cell Immunol. (2020) 357:104195. doi: 10.1016/j.cellimm.2020.104195
152. Zhang C and Yang M. Targeting T cell subtypes for NAFLD and NAFLD-related HCC treatment: an opinion. Front Med. (2021) 8:789859. doi: 10.3389/fmed.2021.789859
153. Wawman RE, Bartlett H, and Oo YH. Regulatory T cell metabolism in the hepatic microenvironment. Front Immunol. (2018) 8:1889. doi: 10.3389/fimmu.2017.01889
154. Camacho V, Matkins VR, Patel SB, Lever JM, Yang Z, Ying L, et al. Bone marrow Tregs mediate stromal cell function and support hematopoiesis via IL-10. JCI Insight. (2020) 5:e135681. doi: 10.1172/jci.insight.135681
155. Hirata Y, Furuhashi K, Ishii H, Li HW, Pinho S, Ding L, et al. CD150high bone marrow tregs maintain hematopoietic stem cell quiescence and immune privilege via adenosine. Cell Stem Cell. (2018) 22:445–453.e5. doi: 10.1016/j.stem.2018.01.017
156. Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong H, Lai K, et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell. (2017) 169:1119–1129.e11. doi: 10.1016/j.cell.2017.05.002
157. Schiering C, Krausgruber T, Chomka A, Fröhlich A, Adelmann K, Wohlfert EA, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. (2014) 513:564–8. doi: 10.1038/nature13577
158. Park SG, Mathur R, Long M, Hosh N, Hao L, Hayden MS, et al. T Regulatory cells maintain intestinal homeostasis by suppressing γδ T cells. Immunity. (2010) 33:791–803. doi: 10.1016/j.immuni.2010.10.014
159. Zhang D, Lin Y, Li Y, Zhao D, and Du M. Mesenchymal stem cells enhance Treg immunosuppressive function at the fetal-maternal interface. J Reprod Immunol. (2021) 148:103366. doi: 10.1016/j.jri.2021.103366
160. Lužnik Z, Anchouche S, Dana R, and Yin J. Regulatory T cells in angiogenesis. J Immunol. (2020) 205:2557–65. doi: 10.4049/jimmunol.2000574
161. Qiao S, Hou Y, Rong Q, Han B, and Liu P. Tregs are involved in VEGFA/VASH1-related angiogenesis pathway in ovarian cancer. Transl Oncol. (2023) 32:101665. doi: 10.1016/j.tranon.2023.101665
162. Li J, Li X-L, and Li C-Q. Immunoregulation mechanism of VEGF signaling pathway inhibitors and its efficacy on the kidney. Am J Med Sci. (2023) 366:404–12. doi: 10.1016/j.amjms.2023.09.005
163. Zhang Y and Brekken RA. Direct and indirect regulation of the tumor immune microenvironment by VEGF. J Leukoc Biol. (2022) 111:1269–86. doi: 10.1002/JLB.5RU0222-082R
164. Wang X-Q, Zhou W-J, Luo X-Z, Tao Y, and Li D-J. Synergistic effect of regulatory T cells and proinflammatory cytokines in angiogenesis in the endometriotic milieu. Hum Reprod. (2017) 32:1304–17. doi: 10.1093/humrep/dex067
165. Palomares O, Martín-Fontecha M, Lauener R, Traidl-Hoffmann C, Cavkaytar O, Akdis M, et al. Regulatory T cells and immune regulation of allergic diseases: Roles of IL-10 and TGF-β. Genes Immun. (2014) 15:511–20. doi: 10.1038/gene.2014.45
166. Maganto-García E, Bu D, Tarrio ML, Alcaide P, Newton G, Griffin GK, et al. Foxp3+-inducible regulatory T cells suppress endothelial activation and leukocyte recruitment. J Immunol. (2011) 187:3521–9. doi: 10.4049/jimmunol.1003947
167. Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, et al. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation. (2019) 139:206–21. doi: 10.1161/CIRCULATIONAHA.118.036065
168. D’Alessio FR, Zhong Q, Jenkins J, Moldobaeva A, and Wagner EM. Lung angiogenesis requires CD4(+) forkhead homeobox protein-3(+) regulatory T cells. Am J Respir Cell Mol Biol. (2015) 52:603–10. doi: 10.1165/rcmb.2014-0278OC
169. Facciabene A, Motz GT, and Coukos G. T-Regulatory cells: Key players in tumor immune escape and angiogenesis. Cancer Res. (2012) 72:2162–71. doi: 10.1158/0008-5472.CAN-11-3687
170. Aldinucci D, Borghese C, and Casagrande N. The CCL5/CCR5 axis in cancer progression. Cancers (Basel). (2020) 12:1765. doi: 10.3390/cancers12071765
171. Wang X, Bove AM, Simone G, and Ma B. Molecular bases of VEGFR-2-mediated physiological function and pathological role. Front Cell Dev Biol. (2020) 8:599281. doi: 10.3389/fcell.2020.599281
172. Goumans MJ, Liu Z, and Ten Dijke P. TGF-β signaling in vascular biology and dysfunction. Cell Res. (2009) 19:116–27. doi: 10.1038/cr.2008.326
173. Fan Y, Zhang W, Huang X, Fan M, Shi C, Zhao L, et al. Senescent-like macrophages mediate angiogenesis for endplate sclerosis via IL-10 secretion in male mice. Nat Commun. (2024) 15:2939. doi: 10.1038/s41467-024-47317-1
174. Tkachev V, Vanderbeck A, Perkey E, Furlan SN, McGuckin C, Gómez Atria D, et al. Notch signaling drives intestinal graft-versus-host disease in mice and nonhuman primates. Sci Transl Med. (2023) 15:eadd1175. doi: 10.1126/scitranslmed.add1175
175. Huysentruyt J, Steels W, Ruiz Perez M, Verstraeten B, Vadi M, Divert T, et al. RIPK1 protects naive and regulatory T cells from TNFR1-induced apoptosis. Cell Death Differ. (2024) 31:1–13. doi: 10.1038/s41418-024-01301-w
176. Zeng Z, Lan T, Wei Y, and Wei X. CCL5/CCR5 axis in human diseases and related treatments. Genes Dis. (2022) 9:12–27. doi: 10.1016/j.gendis.2021.08.004
177. Kebriaei P, Singh H, Huls MH, Figliola MJ, Bassett R, Olivares S, et al. Phase i trials using sleeping beauty to generate CD19-specific CAR T cells. J Clin Invest. (2016) 126:3363–76. doi: 10.1172/JCI86721
178. Holzinger A and Abken H. CAR T cells: A snapshot on the growing options to design a CAR. HemaSphere. (2019) 3:172. doi: 10.1097/HS9.0000000000000172
179. Maiti SN, Huls H, Singh H, Dawson M, Figliola M, Olivares S, et al. Sleeping beauty system to redirect T-cell specificity for human applications. J Immunother. (2013) 36:112–23. doi: 10.1097/CJI.0b013e3182811ce9
180. Monjezi R, Miskey C, Gogishvili T, Schleef M, Schmeer M, Einsele H, et al. Enhanced CAR T-cell engineering using non-viral Sleeping Beauty transposition from minicircle vectors. Leukemia. (2017) 31:186–94. doi: 10.1038/leu.2016.180
181. Henschel P, Landwehr-Kenzel S, Engels N, Schienke A, Kremer J, Riet T, et al. Supraphysiological FOXP3 expression in human CAR-Tregs results in improved stability, efficacy, and safety of CAR-Treg products for clinical application. J Autoimmun. (2023) 138:103057. doi: 10.1016/j.jaut.2023.103057
182. Cui Y, David M, Bouchareychas L, Rouquier S, Sajuthi S, Ayrault M, et al. IL23R-specific CAR Tregs for the treatment of Crohn’s disease. J Crohn’s Colitis. (2025) 19:jjae135. doi: 10.1093/ecco-jcc/jjae135
183. Gao Y, Tang J, Chen W, Li Q, Nie J, Lin F, et al. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc Natl Acad Sci U.S.A. (2015) 112:E3246–54. doi: 10.1073/pnas.1421463112
184. Hosseinalizadeh H, Rabiee F, Eghbalifard N, Rajabi H, Klionsky DJ, and Rezaee A. Regulating the regulatory T cells as cell therapies in autoimmunity and cancer. Front Med. (2023) 10:1244298. doi: 10.3389/fmed.2023.1244298
185. Bittner S, Ruhland B, Hofmann V, Schmidleithner L, Schambeck K, Pant A, et al. Biosensors for inflammation as a strategy to engineer regulatory T cells for cell therapy. Proc Natl Acad Sci U.S.A. (2022) 119:e2208436119. doi: 10.1073/pnas.2208436119
186. Akkaya B and Shevach EM. Regulatory T cells: Master thieves of the immune system. Cell Immunol. (2020) 355:104160. doi: 10.1016/j.cellimm.2020.104160
187. Laukova M and Glatman Zaretsky A. Regulatory T cells as a therapeutic approach for inflammatory bowel disease. Eur J Immunol. (2023) 53:e2250007. doi: 10.1002/eji.202250007
188. Spanier JA, Fung V, Wardell CM, Alkhatib MH, Chen Y, Swanson LA, et al. Tregs with an MHC class II peptide-specific chimeric antigen receptor prevent autoimmune diabetes in mice. J Clin Invest. (2023) 133:e168601. doi: 10.1172/JCI168601
189. Moorman CD, Yu S, Briseno CG, Phee H, Sahoo A, Ramrakhiani A, et al. CAR-T cells and CAR-Tregs targeting conventional type-1 dendritic cell suppress experimental autoimmune encephalomyelitis. Front Immunol. (2023) 14:1235222. doi: 10.3389/fimmu.2023.1235222
190. Wang L, Wang Y, Liu C, He J, He X, Zhang X, et al. Treg-targeted efficient-inducible platform for collagen-induced arthritis treatment. Mater Today Bio. (2023) 19:100557. doi: 10.1016/j.mtbio.2023.100557
191. Wang M, Thomson AW, Yu F, Hazra R, Junagade A, and Hu X. Regulatory T lymphocytes as a therapy for ischemic stroke. Semin Immunopathol. (2023) 45:329–46. doi: 10.1007/s00281-022-00975-z
192. Mandrioli J, D’Amico R, Zucchi E, De Biasi S, Banchelli F, Martinelli I, et al. Randomized, double-blind, placebo-controlled trial of rapamycin in amyotrophic lateral sclerosis. Nat Commun. (2023) 14:4970. doi: 10.1038/s41467-023-40734-8
193. Colombel J-F, Ungaro RC, Sands BE, Siegel CA, Wolf DC, Valentine JF, et al. Vedolizumab, adalimumab, and methotrexate combination therapy in crohn’s disease (EXPLORER). Clin Gastroenterol Hepatol. (2024) 22:1487–1496.e12. doi: 10.1016/j.cgh.2023.09.010
194. Feagan BG, Panés J, Ferrante M, Kaser A, D’Haens GR, Sandborn WJ, et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol. (2018) 3:671–80. doi: 10.1016/S2468-1253(18)30233-4
195. Cui Y, Boulakirba S, David M, Bouchareychas L, Rouquier S, Sajuthi S, et al. OP02 IL23R-CAR-Tregs: creating a therapeutic breakthrough for Crohn’s Disease. J Crohn’s Colitis. (2024) 18:i3–3. doi: 10.1093/ecco-jcc/jjad212.0002
196. Neurath MF. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. (2019) 45:1–8. doi: 10.1016/j.cytogfr.2018.12.002
197. Pilat N, Steiner R, and Sprent J. Treg therapy for the induction of immune tolerance in transplantation—Not lost in translation? Int J Mol Sci. (2023) 24:1752. doi: 10.3390/ijms24021752
198. Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. (2016) 64:632–43. doi: 10.1002/hep.28459
199. Elinav E, Waks T, and Eshhar Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology. (2008) 134:2014–24. doi: 10.1053/j.gastro.2008.02.060
200. Pierini A, Iliopoulou BP, Peiris H, Pérez-Cruz M, Baker J, Hsu K, et al. T cells expressing chimeric antigen receptor promote immune tolerance. JCI Insight. (2017) 2:e92865. doi: 10.1172/jci.insight.92865
201. Liu Z, Zhou J, Wu S, Chen Z, Wu S, Chen L, et al. Why Treg should be the focus of cancer immunotherapy: The latest thought. BioMed Pharmacother. (2023) 168:115142. doi: 10.1016/j.biopha.2023.115142
202. Tan CL, Kuchroo JR, Sage PT, Liang D, Francisco LM, Buck J, et al. PD-1 restraint of regulatory T cell suppressive activity is critical for immune tolerance. J Exp Med. (2021) 218:e20182232. doi: 10.1084/JEM.20182232
203. Ha D, Tanaka A, Kibayashi T, Tanemura A, Sugiyama D, Wing JB, et al. Differential control of human Treg and effector T cells in tumor immunity by Fc-engineered anti-CTLA-4 antibody. Proc Natl Acad Sci U.S.A. (2019) 116:609–18. doi: 10.1073/pnas.1812186116
204. Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. (2007) 450:566–9. doi: 10.1038/nature06306
205. Watson MLJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. (2021) 591:645–51. doi: 10.1038/s41586-020-03045-2
206. Kumagai S, Koyama S, Itahashi K, Tanegashima T, tzu LY, Togashi Y, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. (2022) 40:201–18. doi: 10.1016/j.ccell.2022.01.001
207. Setoguchi R, Hori S, Takahashi T, and Sakaguchi S. Homeostatic maintenance of natural Foxp3 + CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. (2005) 201:723–35. doi: 10.1084/jem.20041982
208. Van Damme H, Dombrecht B, Kiss M, Roose H, Allen E, Van Overmeire E, et al. Therapeutic depletion of CCR8 + tumor-infiltrating regulatory T cells elicits antitumor immunity and synergizes with anti-PD-1 therapy. J Immunother Cancer. (2021) 9:e001749. doi: 10.1136/jitc-2020-001749
209. Solomon I, Amann M, Goubier A, Arce Vargas F, Zervas D, Qing C, et al. CD25-Treg-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity. Nat Cancer. (2020) 1:1153–66. doi: 10.1038/s43018-020-00133-0
210. Requejo Cier CJ, Valentini N, and Lamarche C. Unlocking the potential of Tregs: innovations in CAR technology. Front Mol Biosci. (2023) 10:1267762. doi: 10.3389/fmolb.2023.1267762
211. Bittner S, Hehlgans T, and Feuerer M. Engineered Treg cells as putative therapeutics against inflammatory diseases and beyond. Trends Immunol. (2023) 44:468–83. doi: 10.1016/j.it.2023.04.005
212. Houston S. Remembering treg cells. Nat Immunol. (2023) 24:1966. doi: 10.1038/s41590-023-01703-x
213. Stucchi A, Maspes F, Montee-Rodrigues E, and Fousteri G. Engineered Treg cells: The heir to the throne of immunotherapy. J Autoimmun. (2024) 144:102986. doi: 10.1016/j.jaut.2022.102986
214. Chen X, Zhong S, Zhan Y, and Zhang X. CRISPR–Cas9 applications in T cells and adoptive T cell therapies. Cell Mol Biol Lett. (2024) 29:52. doi: 10.1186/s11658-024-00561-1
215. Arroyo-Olarte R, Mejía-Muñoz A, and León-Cabrera S. Expanded alternatives of CRISPR-cas9 applications in immunotherapy of colorectal cancer. Mol Diagn Ther. (2024) 28:69–86. doi: 10.1007/s40291-023-00680-z
216. Kotsias F, Janot-Sardet C, Caudana PP, Russo E, Dubois M, Zucchetti A, et al. EGL-001 is a novel immunocytokine designed to specifically target and disarm T regulatory cells in the tumor microenvironment. Cancer Res. (2024) 84:4079. doi: 10.1158/1538-7445.AM2024-4079
217. van Gulijk M, van Krimpen A, Schetters S, Eterman M, van Elsas M, Mankor J, et al. PD-L1 checkpoint blockade promotes regulatory T cell activity that underlies therapy resistance. Sci Immunol. (2023) 8:eabn6173. doi: 10.1126/sciimmunol.abn6173
218. Sun Q, Hong Z, Zhang C, Wang L, Han Z, and Ma D. Immune checkpoint therapy for solid tumours: clinical dilemmas and future trends. Signal Transduct Target Ther. (2023) 8:320. doi: 10.1038/s41392-023-01522-4
219. Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, et al. Expression of RORγt marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med. (2012) 4:164ra159–164ra159. doi: 10.1126/scitranslmed.3004566
220. Zhu Y, Wang Y, Zuo X, Liu S, Cao L, Wang J, et al. Inhibition SIRT1 to regulate FOXP3 or RORγt can restore the balance of Treg/Th17 axis in ulcerative colitis and enhance the anti-inflammatory effect of moxibustion. Front Immunol. (2025) 15:1525469. doi: 10.3389/fimmu.2024.1525469
221. Conrad ML, Barrientos G, Cai X, Mukherjee S, Das M, Stephen-Victor E, et al. Regulatory T cells and their role in allergic disease. Allergy. (2025) 80:77–93. doi: 10.1111/all.16326
222. Mann ER, Lam YK, and Uhlig HH. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol. (2024) 24:577–95. doi: 10.1038/s41577-024-01014-8
223. Yu Z, Xiaojia L, Wei Z, Jian Z, Aiting W, Jing W, et al. Baicalin circumvents anti-PD-1 resistance by regulating the gut microbiota metabolite short-chain fatty acids. Pharmacol Res. (2024) 199:107033. doi: 10.1016/j.phrs.2023.107033
224. Scott SA, Fu J, and Chang PV. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci. (2020) 117:19376–87. doi: 10.1073/pnas.2000047117
225. Pathak M, Padghan P, Halder N, Shilpi, Kulkarni N, Sonar SA, et al. CCR9 signaling in dendritic cells drives the differentiation of Foxp3+ Tregs and suppresses the allergic IgE response in the gut. Eur J Immunol. (2020) 50:404–17. doi: 10.1002/eji.201948327
226. Hegazy AN and Powrie F. Microbiota RORgulates intestinal suppressor T cells. Science (80-). (2015) 349:929–30. doi: 10.1126/science.aad0865
227. Rizzo A, Di Giovangiulio M, Stolfi C, Franzè E, Fehling H-J, Carsetti R, et al. RORγt-expressing tregs drive the growth of colitis-associated colorectal cancer by controlling IL6 in dendritic cells. Cancer Immunol Res. (2018) 6:1082–92. doi: 10.1158/2326-6066.CIR-17-0698
228. Garidou L, Pomié C, Klopp P, Waget A, Charpentier J, Aloulou M, et al. The gut microbiota regulates intestinal CD4 T cells expressing RORγt and controls metabolic disease. Cell Metab. (2015) 22:100–12. doi: 10.1016/j.cmet.2015.06.001
229. da Silva EM, Alves RW, Doretto-Silva L, and Andrade-Oliveira V. Cross-talk between gut microbiota and immune cells and its impact on inflammatory diseases. Biotechnol Appl to Inflammatory Diseases: Cell Mech Nanomedicine. (2023), 139–62.
230. Najar TA, Hao Y, Hao Y, Romero-Meza G, Dolynuk A, and Littman DR. Microbiota-induced plastic T cells enhance immune control of antigen-sharing tumors. bioRxiv. (2024) [Preprint]. 2024 Aug 14:2024.08.12.607605. doi: 10.1101/2024.08.12.607605
231. Cochrane RW, Allen E, and Ferreira LMR. Expanding the engineered Treg multiverse. Mol Ther. (2025) 33:833–6. doi: 10.1016/j.ymthe.2025.02.007
232. Shoushtari AN and Powell DJ. Tumor-infiltrating lymphocyte therapy for melanoma and other solid tumors: looking back, yet moving forward. Transplant Cell Ther. (2025) 31:S581–90. doi: 10.1016/j.jtct.2024.11.017
233. Marei HE, Bedair K, Hasan A, Al-Mansoori L, Caratelli S, Sconocchia G, et al. Current status and innovative developments of CAR-T-cell therapy for the treatment of breast cancer. Cancer Cell Int. (2025) 25:3. doi: 10.1186/s12935-024-03615-8
234. Sakaguchi S, Kawakami R, and Mikami N. Treg-based immunotherapy for antigen-specific immune suppression and stable tolerance induction: a perspective. Immunother Adv. (2023) 3:ltad007. doi: 10.1093/immadv/ltad007
235. Tuomela K, Salim K, and Levings MK. Eras of designer Tregs: Harnessing synthetic biology for immune suppression. Immunol Rev. (2023) 320:250–67. doi: 10.1111/imr.13254
236. Raud B, Roy DG, Divakaruni AS, Tarasenko TN, Franke R, Ma EH, et al. Etomoxir actions on regulatory and memory T cells are independent of cpt1a-mediated fatty acid oxidation. Cell Metab. (2018) 28:504–15. doi: 10.1016/j.cmet.2018.06.002
237. Cresta S, Tessari A, Aiello A, Paolini B, Martinetti A, Mariani L, et al. 114P Effects of chemotherapy on TREG lymphocytes in early breast cancer. ESMO Open. (2024) 9:102339. doi: 10.1016/j.esmoop.2024.102339
238. Frijlink E, Bosma DMT, Busselaar J, Battaglia TW, Staal MD, Verbrugge I, et al. PD-1 or CTLA-4 blockade promotes CD86-driven Treg responses upon radiotherapy of lymphocyte-depleted cancer in mice. J Clin Invest. (2024) 134:e171154. doi: 10.1172/JCI171154
Keywords: regulatory T cells, immune suppression, cancer immunotherapy, tissue repair and regeneration, metabolic regulation, angiogenesis
Citation: Zheng L, Wu D, Xie H and Zhao H (2025) Revisiting Tregs in cancer and beyond: immunological control and therapeutic potential. Front. Immunol. 16:1581093. doi: 10.3389/fimmu.2025.1581093
Received: 21 February 2025; Accepted: 03 June 2025;
Published: 01 September 2025.
Edited by:
Athanasia Mouzaki, University of Patras, GreeceReviewed by:
Kai Hildner, University Hospital Erlangen, GermanyZheng Liu, Virginia Commonwealth University, United States
Copyright © 2025 Zheng, Wu, Xie and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai Zhao, emhhbzI3MTQwNEBpY2xvdWQuY29t
 Lei Zheng1
Lei Zheng1 Hai Zhao
Hai Zhao