- 1Department of Experimental Therapy, Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wrocław, Poland
- 2Department of Immunology, Pathophysiology and Veterinary Preventive Medicine, Faculty of Veterinary Medicine, Wrocław University of Environmental and Life Sciences, Wrocław, Poland
- 3Endometriosis Institute of EuroMediCare Specialist Hospital, Wrocław, Poland
- 41st Department of Gynecology and Obstetrics, Wrocław Medical University, Wrocław, Poland
- 5Clinical Department of Oncological and Procreative Gynecology of the 4th Military Clinical Hospital with the Polyclinic, Wrocław, Poland
- 6Medical Faculty, Wrocław University of Science and Technology, Wrocław, Poland
- 7Department of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Wrocław University of Environmental and Life Sciences, Wrocław, Poland
Problem: Unbalanced production of pro- and anti-inflammatory cytokines by immune cells is a hallmark of endometriosis. IL-24, a member of the IL-10 family, is a pleiotropic cytokine produced by both non-immune cells like astrocytes, keratinocytes, pancreatic myofibroblasts, and endothelial cells and immune cells such as monocytes, macrophages, dendritic cells, NK cells, T cells (including Th2 and Th17), and B cells. However, its expression in regulatory T (Tregs) and B lymphocytes (Bregs) has not been explored. In this study, we determined the expression of IL-24 in Tregs and selected Breg subpopulations in women with endometriosis compared with healthy women.
Methods: Percentages of Tregs, B10 cells, immature B cells, and plasmablasts that produce IL-24 were measured in the peripheral blood of women with endometriosis (n=24) and healthy women (n=24) using flow cytometry.
Results: We observed an increased percentage of IL-24–producing Tregs in the total pool of women with endometriosis and in women with stages III and IV of endometriosis compared to controls. Within the Breg subpopulations, the percentages of IL-24–producing plasmablasts were higher in the overall endometriosis cohort as well as in women with stage IV endometriosis compared with healthy women. In contrast, the percentages of IL-24–producing immature B cells were lower in the endometriosis group than that in the control group.
Conclusions: We have shown, for the first time, that Tregs and Bregs secrete IL-24 and that their percentages are altered in endometriosis. The significance of this cytokine secretion by regulatory cells is unclear, but we speculated that IL-24 may enhance the improper immunosuppressive activity of Tregs and plasmablasts in endometriosis, which enables the implantation and growth of endometrial lesions outside the uterus.
1 Introduction
Endometriosis is a chronic disease characterized by the presence of uterine tissue outside the uterine cavity and persistent inflammation. Endometriosis is accompanied by chronic pelvic pain, dyspareunia, dysmenorrhea, and infertility (1–3). This condition affects up to 10% of the female population and as many as 50% of infertile women (4–9). The pathogenesis of endometriosis remains ambiguous; nevertheless, the most widely accepted hypothesis is retrograde menstruation, which implies that during menstruation, the endometrial lining migrates through the fallopian tubes into the pelvic space (10). These endometrial fragments may implant and survive at ectopic sites. However, retrograde menstrual blood is relatively common in women while endometriosis affects only a few women, suggesting that other factors such as genetic, hormonal, environmental, or immunologic are involved in the implantation and survival of endometrial lesions in the peritoneal cavity.
Abnormal immune responses, particularly the dysfunction of peripheral and peritoneal cavity immune cells that secrete various cytokines, chemokines, and growth factors, have been associated with the development of endometriosis (11–14). Therefore, women suffering from endometriosis exhibit chronic systemic inflammation, which is manifested by altered pro-inflammatory cytokines (IL-1β, IL-6, IL-8, and TNF-α), T cell cytokines (IL-12, IL-17, and IFN-γ), and anti-inflammatory cytokines like IL-4 and IL-10 (15–24). Among these, members of the IL-10 family have been implicated in suppressing immune response against endometriotic fragments, thereby contributing to the development of endometriosis (24). Regulatory T (Tregs) and B cells (Bregs), which secrete IL-10 and establish self-tolerance, play a key role in this process. The percentage of Tregs is significantly higher in the peritoneal fluid of women with endometriosis compared with healthy women (25, 26) and increases with disease progression (27). Moreover, elevated percentages of circulating Tregs have been found in patients with endometriosis than in controls (28). Recently Le et al., 2021 have shown no difference in peripheral natural Tregs and inducible Tregs between patients with endometriosis and controls (29). Furthermore, other findings indicate no significant variation in circulating Tregs during the menstrual cycle between women with and without endometriosis (30). The discrepancies between studies may be due to the low number of cases, the use of heterogeneous methodologies, and the fact that Tregs themselves constitute a heterogeneous group of T cells (31). In contrast, the percentages of Breg subpopulations at different developmental stages are lower in women with endometriosis than in healthy women (32).
Recently, altered levels of interleukin-24 (IL-24), a member of the IL-10 cytokine family also known as melanoma differentiation-associated gene-7 (mda-7), have been determined in the endometrium of women with endometriosis (33). IL-24 primarily functions as a tumor-suppressive cytokine via multiple mechanisms, including inhibition of invasion, migration, angiogenesis, and metastasis, induction of apoptosis, elimination of cancer stem cells, and sensitization of cancer cells to therapies (34–40). However, the physiological role of IL-24 is still being investigated. Various immune cells, such as monocytes, macrophages, dendritic cells, NK cells, T cells (including Th2 and Th17), and B cells, can express IL-24 upon stimulation with cytokines, concanavalin A, lipopolysaccharide, 12-myristate 13-acetate (PMA), and/or PMA/ionomycin (41–46). However, no data has focused on the ability of regulatory T and B lymphocytes to secrete IL-24. Therefore, we investigated whether Tregs and several subpopulations of Bregs at different developmental stages can produce IL-24 upon PMA/ionomycin stimulation and whether their percentages are altered in patients with endometriosis.
2 Methods
2.1 Patients
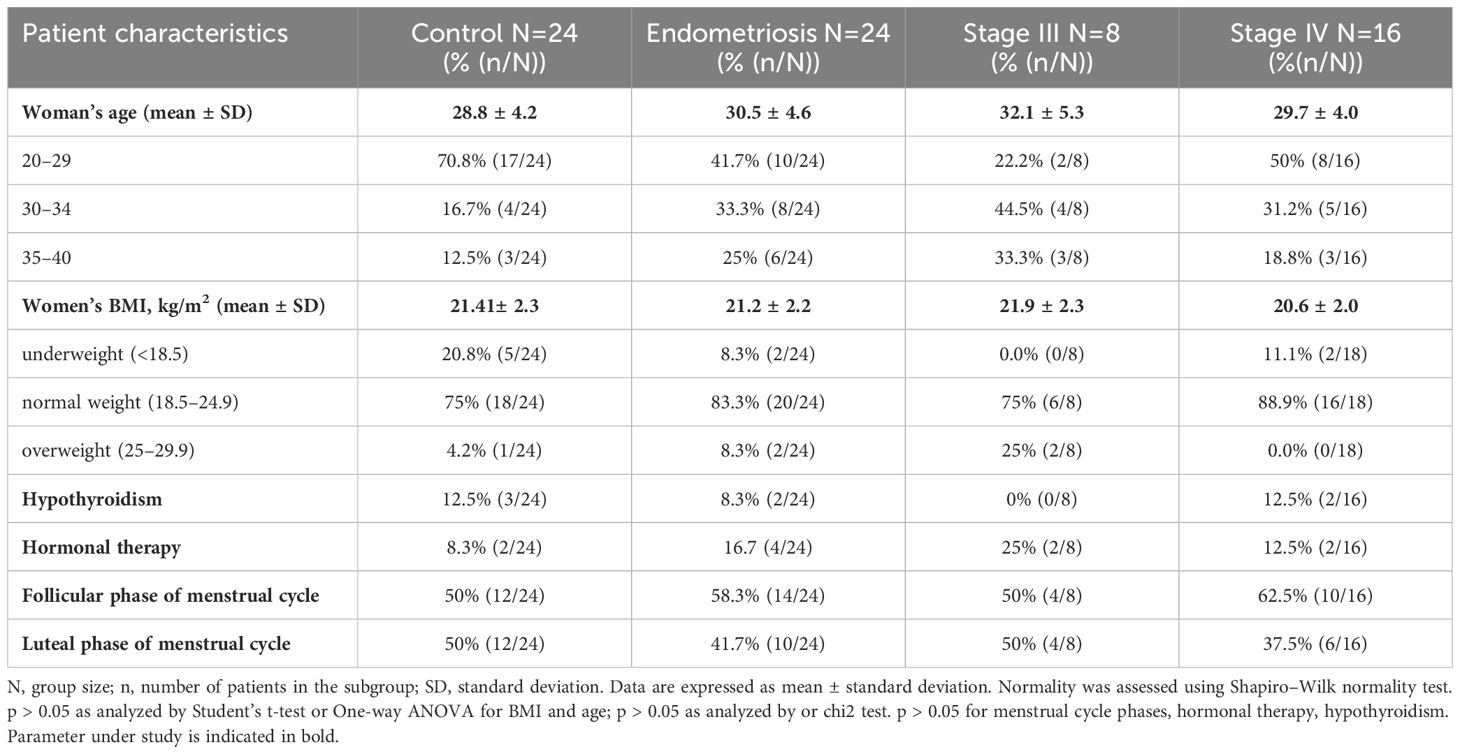
This study was approved by the Bioethical Commission of the Medical University of Wrocław (No. KB – 407/2018), and written informed consent was obtained from all participants to participate and process personal data for research purposes. Peripheral venous blood from healthy women (n=24) were obtained from the Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences. Blood samples from women with endometriosis (n=24) were collected from MultiMedica Clinics, Wrocław, and Clinical Department of Oncological and Procreative Gynecology, 4th Military Clinical Hospital in Wrocław. Women in the endometriosis group (aged 23–38 years old) were scheduled for laparoscopic surgery for suspected endometriosis based on specific pain symptoms and/or infertility evaluation. Women in the control group (aged 23–38 years old) had no history of endometriosis, pain symptoms, or pelvic abnormalities. Exclusion criteria included the presence of neoplastic disease, autoimmune disease, and pregnancy. A total of 48 women were enrolled in this study (Table 1). No differences were found in patients’ age, body mass index (BMI), menstrual cycle phases, or hormonal therapy for hypothyroidism (p > 0.05) between the examined groups. Endometriosis stage was assessed according to the ASRM classification (47). Samples were exclusively collected from women in advanced stages of endometriosis (stages III and IV), as collecting samples from patients in early stages of the disease (stages I and II) was challenging because of the typical diagnostic delay associated with the disease.
2.2 Flow cytometry
Blood samples were collected into lithium heparin collection tubes and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Biowest, France) supplemented with 0.1 μg/ml phorbol 12-myristate 13-acetate (Cayman, USA), 1 μg/ml ionomycin (Cayman, USA), 10 μg/ml Brefeldin A (Biolegend, USA), and 2 μM Monensin A (Biolegend, USA) for 4 h at 37°C in 5% CO2. After stimulation, samples were stained with Zombie Red Viability Dye (Biolegend, USA) for 15 min at 4°C in the dark. Anti-human CD19 FITC (Biolegend, clone: HIB19), CD24 APC-Cy7 (Biolegend, clone: ML5), CD27 Pacific Blue (Biolegend, clone: M-T271), and CD38 Alexa Fluor 700 (Biolegend, clone: HB-7) antibodies were added for Bregs phenotyping, whereas anti-human CD4 Pacific Blue (Biolegend, clone: OKT4), CD25 APC (Invitrogen, clone: CD25-3G10), and CD127 Alexa Fluor 700 (Biolegend, clone: A019D5) antibodies were used for Tregs phenotyping. Samples were then washed with staining buffer (0.5 mM EDTA, 0.002% sodium azide, 1% fetal bovine serum) and fixed with a fixation buffer (eBioscience, USA) for 14 h at 4°C in the dark. After two washes with permeabilization buffer (eBioscience, USA), samples were blocked with Human True Stain FcX (Biolegend, USA) for 10 min at 4°C in the dark. Samples for Bregs phenotyping were stained with anti-IL-10 APC (Biolegend, clone: JES3-19F1) and anti-IL-24 Biotin (BAF1965; R&D, USA) antibodies and those for Tregs phenotyping were stained with anti-Foxp3 PE-Cy7 (Invitrogen, USA; clone PCH101) and anti-IL-24 Biotin (BAF1965; R&D, USA) antibodies or normal goat IgG biotinylated control antibody (BAF108, R&D, USA) for 1 h at 4°C in the dark. Samples were washed twice with permeabilization buffer and stained with Streptavidin PE (Biolegend, USA) for 30 min at 4°C in the dark. After washing again with permeabilization buffer, samples were analyzed using an LSR Fortessa Cell Analyzer (Becton Dickinson, USA). A total of 200,000 events were recorded at a rate of 600–800 events per second. Cytometer Setup and Tracking beads (CS&T Research Beads, Becton Dickinson, USA) were used for automated quality assurance and control of machine performance. Data were analyzed using FlowJo™ software version 10.8.1 (Becton Dickinson, USA). The relative levels of IL-24 in CD4+CD127-CD25hiFOXP3+ cells, CD19+CD27+CD24hi cells, CD19+CD38hi24hi cells, and CD19-CD38hi27int cells were calculated as follows: median fluorescence of cells stained with anti-IL-24 antibody minus median fluorescence of cells stained with isotype-matched control. FlowJo tSNE plugin software was used for the two-dimensional reduction of the manual gates. Classical gating and tSNE algorithms (48) were used to assess IL-24-positive subpopulations. The percentage of each analyzed subpopulation producing IL-24 was calculated as the total number of viable cells, CD4+ or CD19+ cells or the parent population during gating. For 2-dimensional reduction, data from each patient were evenly downsampled and concatenated into a new data file, and then the tSNE algorithm was applied with the following settings: perplexity - 200; learning rate - 32200; iterations - 1000; and theta - 0.5.
2.3 Statistical analysis
All statistical analyses were performed using the GraphPad Prism 7 software (GraphPad Software, USA). Normal distribution was assessed using the Shapiro–Wilk normality test, while homoscedasticity was examined using the Brown-Forsythe test. Normally distributed data were examined using Student’s t-test (control group vs. endometriosis group) or one-way ANOVA (control group vs. stage III vs. stage IV endometriosis) (parametric). Non-normally distributed data were analyzed using Mann-Whitney U or Kruskal–Wallis test (non-parametric). One-way ANOVA and Kruskal–Wallis tests were followed by Tukey’s and Dunnett’s multiple comparisons post-hoc tests, respectively. The distribution of women in the menstrual cycle phases, receiving hormonal therapy for hypothyroidism, or receiving hormonal contraception was tested using chi2 test. Differences were considered statistically significant at p < 0.05. The statistical power was calculated based on the mean values of IL-24-producing Tregs percentages and assuming a significance level of 0.05 using the online calculator available at https://clincalc.com/stats/Power.aspx. The calculated power was 78.3%.
3 Results
3.1 IL-24 is produced by different Breg subpopulations and women with endometriosis have higher percentages of IL-24+ plasmablasts
We investigated specific subpopulations of B lymphocytes, namely Bregs, to determine their ability to produce IL-24 and whether their percentages varied in women suffering from endometriosis. First, we visualized the population of IL-24–producing B cells in healthy controls (Figure 1A) and women with endometriosis (Figure 1B) using t-distributed stochastic neighbor embedding (tSNE) algorithms. Next, the FLOWSOM algorithm was used to identify distinct clusters of IL-24–producing cells (Figure 1C). Heatmap of IL-24 expression revealed that distinct Breg subpopulations were located in specific clusters: A1 (CD19+CD24hiCD38hiCD27-IL-24+), A2 (CD19-CD24-CD38hiCD27intIL-24+), and A3 (CD19+CD24hiCD38midCD27+IL-24+). tSNE analysis, presented as IL-24 heatmaps of different B cell subsets, revealed IL-24 expression in all analyzed clusters and similarity in complexity and distribution of IL-24 expression in healthy controls (Figure 1A) and women with endometriosis (Figure 1B).
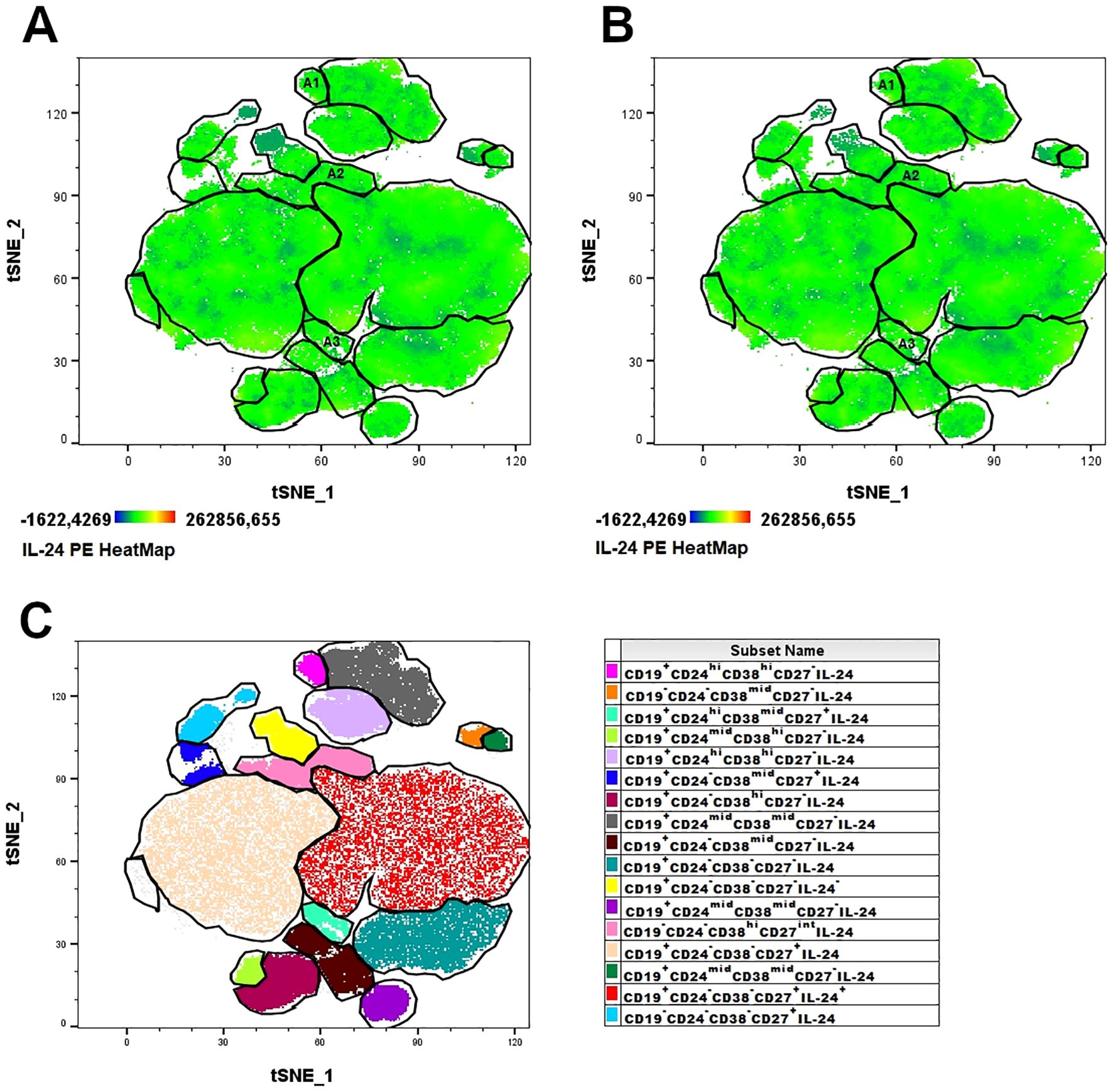
Figure 1. Combined t-SNE heatmap of IL-24–producing B cells. Combined t-SNE analysis was performed using 24 paired samples obtained from healthy women (A) and women with endometriosis (B). (C) t-SNE plot presenting 17 distinct clusters of IL-24–producing cells identified using FLOWSOM algorithm. Regulatory B lymphocytes (Bregs) clusters were identified in A1 (immature B cells, CD19+CD24hiCD38hiCD27-IL-24+), A2 (plasmablasts, CD19-CD38hiCD27intCD24-IL-24+) and A3 (B10 cells, CD19+CD38midCD24hiCD27+IL-24+) clusters.
Next, the number of CD19+CD27+CD24hiIL-24+ (B10 cells), CD19+CD38hiCD24hiIL-24+ (immature B cells), and CD19-CD38hiCD27intIL-24+ (plasmablasts) cells in whole peripheral blood were analyzed based on the gating strategy shown in Figure 2.
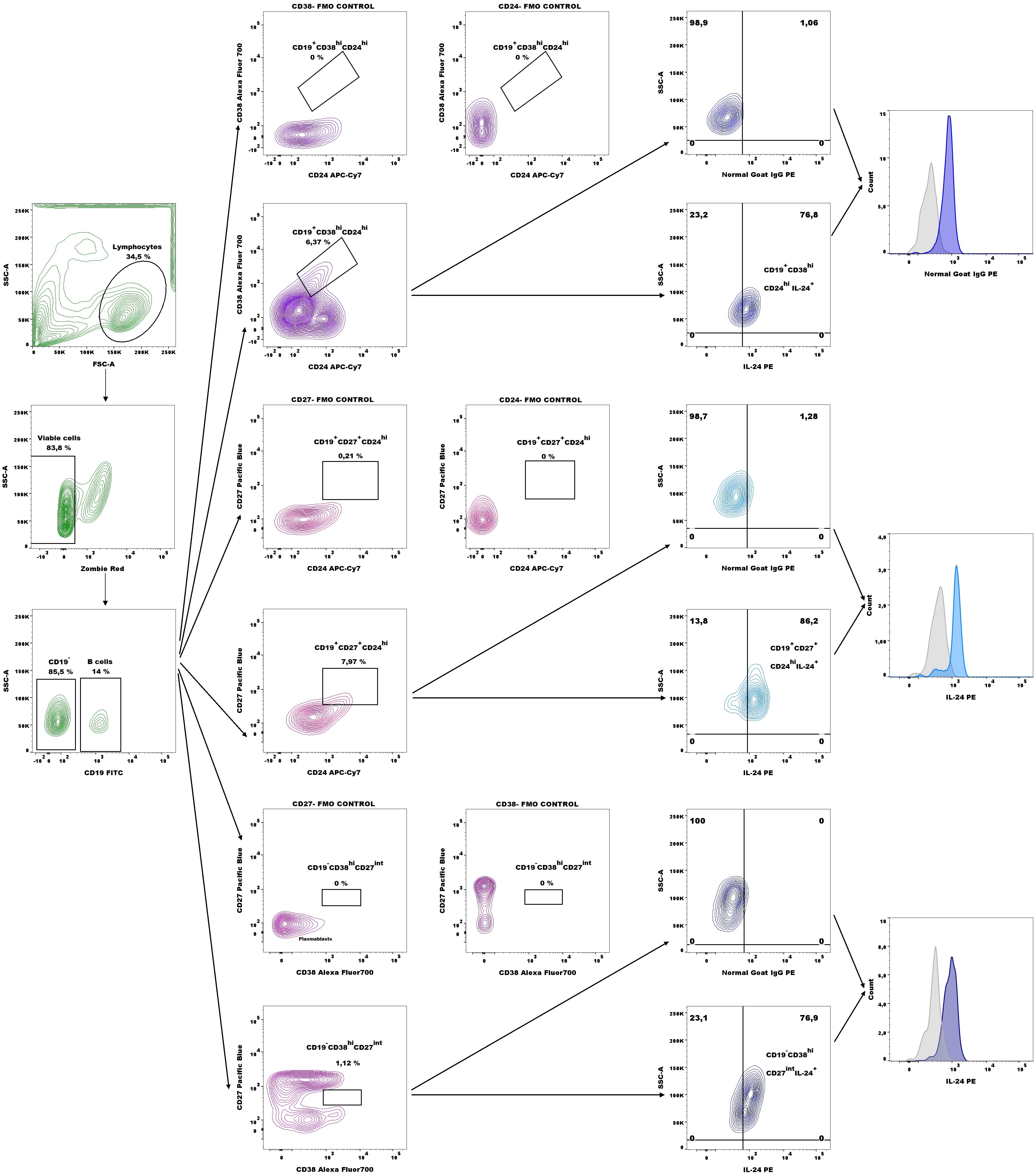
Figure 2. Representative dot plots for IL-24–expressing B10 cell (CD19+CD27+CD24hi), immature B cells (CD19+CD38hiCD24hi), and plasmablasts (CD19-CD38hiCD27int) gating and representative histograms of IL-24 expression (blue histograms) overlaid with those in the respective isotype-matched controls (grey histograms) derived from whole blood of patients with endometriosis.
Comparing women with endometriosis with healthy women revealed no differences in the percentages of IL-24–producing B10 cells (Figure 3A) and IL-24–producing B10 cells among CD19+CD27+CD24hi cells (Figure 3B), or median fluorescence intensity (MFI) of IL-24 in B10 cells (Figure 3C) (all p > 0.05).
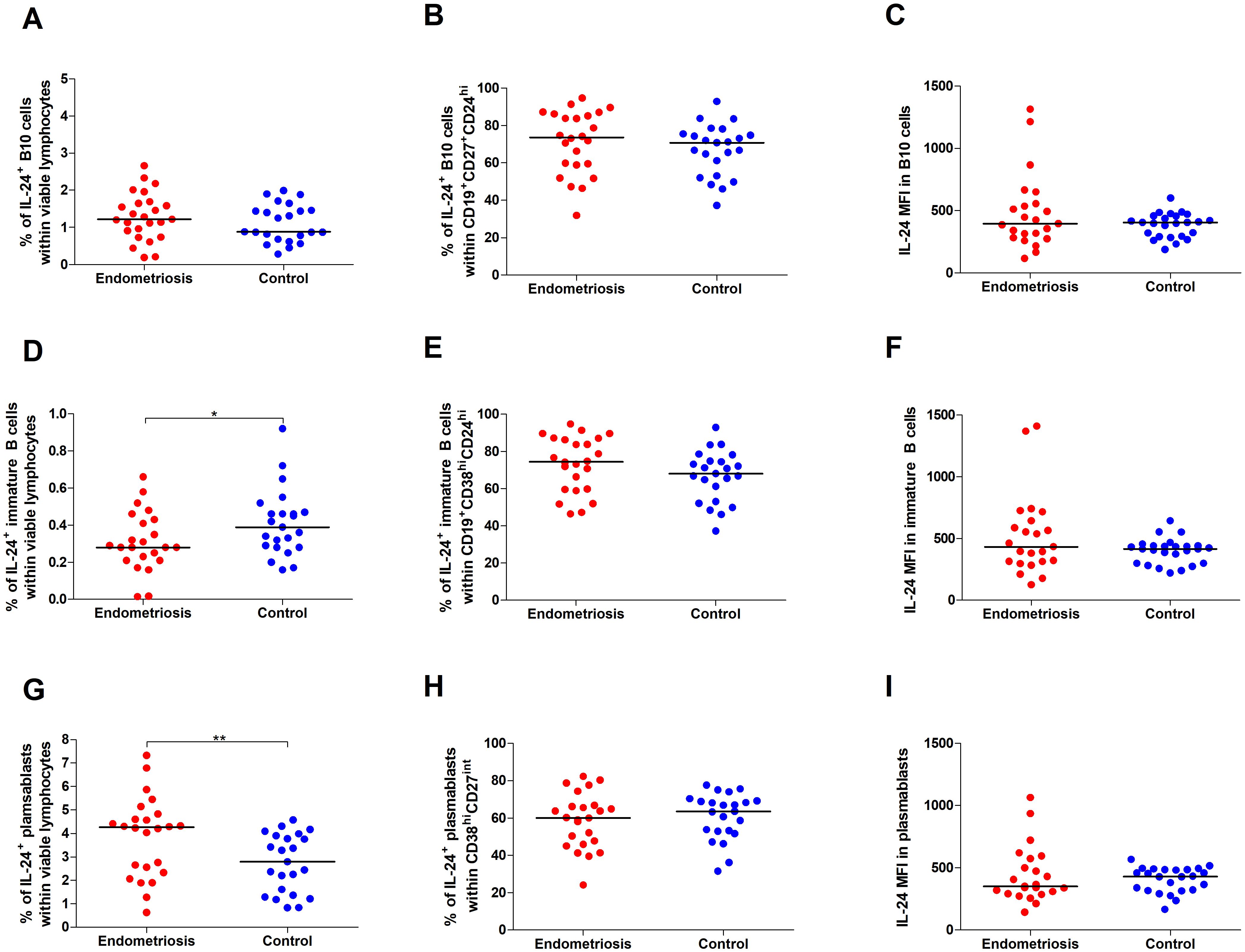
Figure 3. Percentage and IL-24 expression in selected subpopulations of Bregs in women with endometriosis compared with control. Whole peripheral blood obtained from women with endometriosis (red dots) and control group (blue dots) were stimulated with 12-myristate 13-acetate (PMA) and ionomycin in the presence of Brefeldin A and Monensin A. The following parameters were measured by flow cytometry: (A) Percentage of IL-24–producing B10 cells (CD19+CD27+CD24hi) within viable lymphocytes, (B) Percentage of IL-24-producing cells among B10 cells, (C) Median fluorescence intensity (MFI) of IL-24 within B10 cells, (D) frequency of IL-24–producing immature B cells (CD19+CD38hiCD24hi) within viable lymphocytes, (E) Percentage of IL-24-producing cells among immature B cells, (F) MFI of IL-24 within immature B cells, (G) Percentage of IL-24–producing plasmablasts (CD19-CD38hiCD27int) within viable lymphocytes, (H) Percentage of IL-24-producing cells among plasmablasts, and (I) MFI of IL-24 within plasmablasts. Data are presented as individual values with median. Data were analyzed using Student’s t-test (normal distribution) or Mann–Whitney U test (non-normal distribution). *p < 0.05 and **p < 0.01.
However, we observed decreased percentages of IL-24–producing immature B cells in women with endometriosis compared with those in the control group (p < 0.05; Figure 3D). The percentage of IL-24–producing immature B cells among CD19+CD38hiCD24hi cells (Figure 3E) and IL-24 (MFI) in these cells (Figure 3F) showed no significant differences between the two groups. Furthermore, the percentage of IL-24–producing plasmablasts was significantly higher in women with endometriosis (p < 0.01; Figure 3G), although the percentage of IL-24–producing plasmablasts among CD19-CD38hiCD27int cells (Figure 3H) or their IL-24 expression levels (MFI; Figure 3I) was similar in both groups. No significant differences were observed in the percentages of IL-24–producing B10 cells (Figure 4A) and IL-24 producing B10 cells among CD19+CD27+CD24hi cells (Figure 4B), as well as IL-24 expression in these cells (Figure 4C) between healthy and endometriosis-positive groups (all p > 0.05). Similarly, immature B cells among CD19+CD38hiCD24hi cells showed no significant difference) between control, stage III, and stage IV groups (Figures 4D–F; all p > 0.05). However, the percentages of IL-24–producing plasmablasts (Figure 4G) were significantly higher in women with stage IV endometriosis (p < 0.05) compared with those in the control group. No differences were observed in the percentage of IL-24–producing plasmablasts among CD19-CD38hiCD27int cells (Figure 4H) and IL-24 expression in these cells (Figure 4I; p > 0.05).
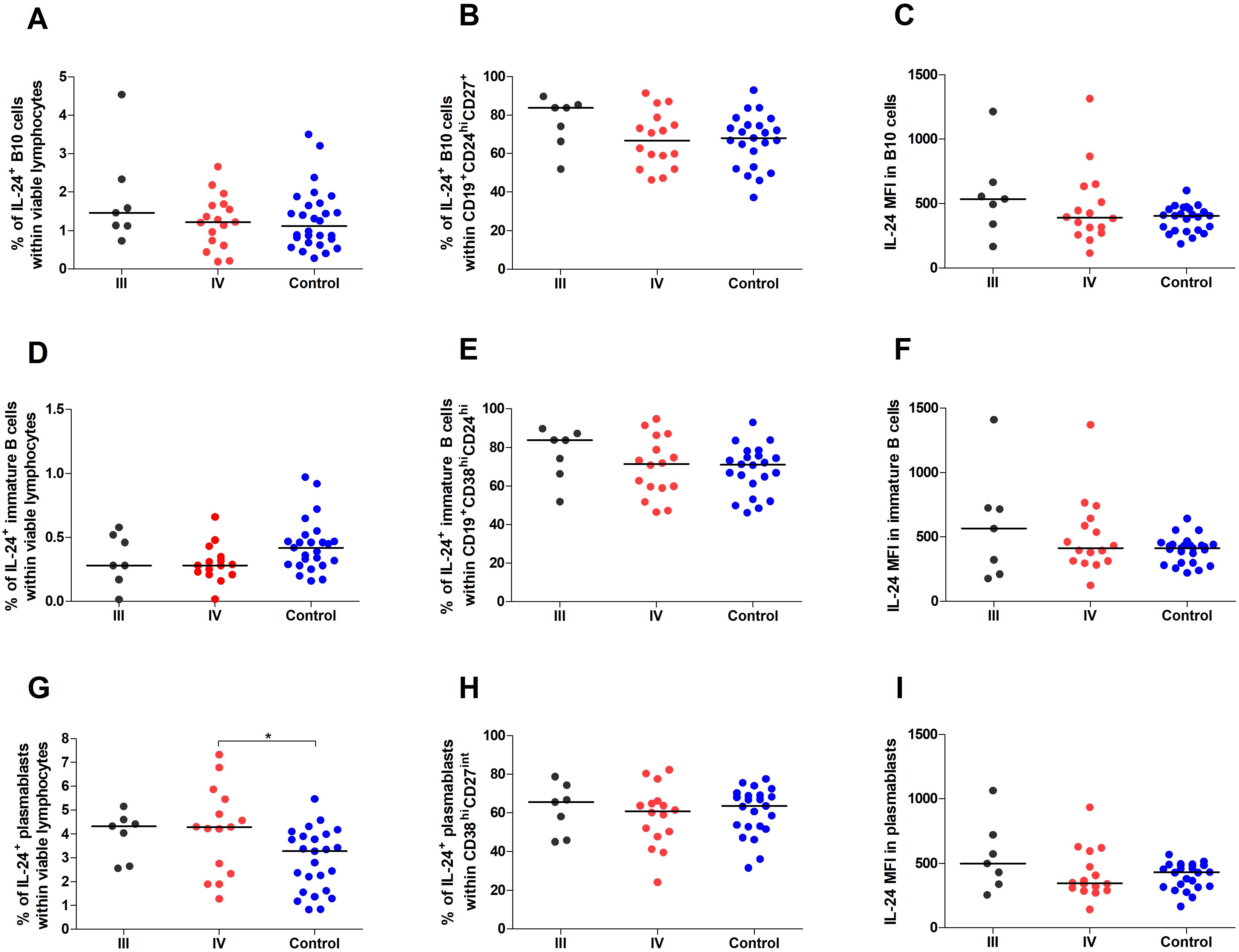
Figure 4. Percentage and IL-24 expression in Breg subpopulations stratified by endometriosis stage (III and IV) compared to control. Whole peripheral blood obtained from women with stages III (black dots) and IV (red dots) of endometriosis and control group (blue dots) were stimulated with PMA and ionomycin in the presence of Brefeldin A and Monensin A. The following parameters were measured by flow cytometry: (A) Percentage of IL-24–producing B10 cells (CD19+CD27+CD24hi) within viable lymphocytes, (B) Percentage of IL-24-producing cells among B10 cells, (C) Median fluorescence intensity (MFI) of IL-24 within B10 cells, (D) Percentage of IL-24–producing immature B cells (CD19+CD38hiCD24hi) within viable lymphocytes, (E) Percentage of IL-24-producing cells among immature B cells, (F) MFI of IL-24 within immature B cells, (G) Percentage of IL-24–producing plasmablasts (CD19-CD38hiCD27int) within viable lymphocytes, (H) Percentage of IL-24-producing cells among plasmablasts, and (I) MFI of IL-24 within plasmablasts. Data are presented as individual values with median. Statistical analysis was performed using one-way analysis of variance (ANOVA; normal distribution) or Kruskal–Wallis test (non-normal distribution) Dunnets’s multiple comparison post hoc test (p < 0.05). *p < 0.05.
3.2 Women with endometriosis exhibit higher percentage of IL-24–producing Tregs
We explored whether Tregs produce IL-24 and whether their percentages change in women with endometriosis. Using the tSNE algorithm, we visualized IL-24–producing T cells in healthy controls (Figure 5A) and women with endometriosis (Figure 5B). Next, the FLOWSOM algorithm was used to identify distinct clusters of IL-24–producing cells (Figure 5C). IL-24–producing T cell heatmap revealed the following Tregs clusters: B1 (CD4+CD127-CD25hiFOXP3+IL-24+) and B2 (CD4+CD127-CD25midFOXP3int IL-24+). tSNE analysis, presented as IL-24 heatmaps in T cell subsets, showed that all the analyzed clusters expressed IL-24 and that no significant differences were observed in the complexity of T cell clusters or distribution of IL-24 between women suffering from endometriosis (Figure 5A) and healthy controls (Figure 5B).
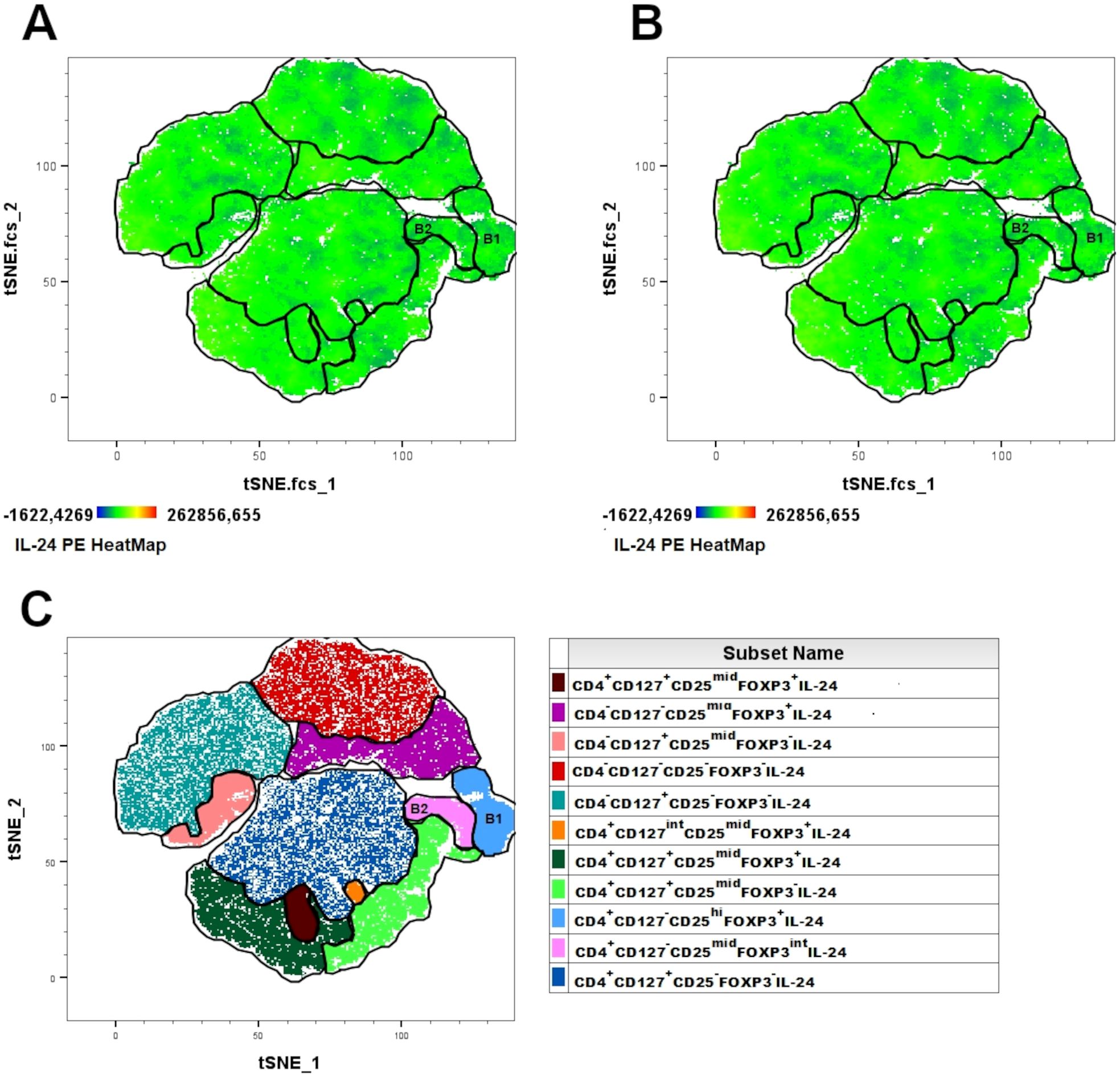
Figure 5. Combined t-SNE heatmap of IL-24–producing T cells. Combined t-SNE analysis was performed using 24 paired samples obtained from healthy women (A) and women with endometriosis (B). (C) t-SNE plot presenting 11 distinct clusters of IL-24–producing cells identified using FLOWSOM algorithm. Regulatory T lymphocytes were identified in B1 and B2 clusters.
Next, we analyzed the percentage of CD4+CD127-CD25hiFOXP3+IL-24+ (IL-24–producing Tregs) based on the gating strategy shown in Figure 6. Women with endometriosis showed a higher percentage of IL-24–producing Tregs (CD4+CD127-CD25hiFOXP3+IL-24+) (Figure 7A), IL-24–producing Tregs among CD4+ cells (Figure 7B), and among Tregs (Figure 7C) in comparison to controls (both p < 0.05). However, no differences were observed in IL-24 expression (IL-24 MFI) in Tregs among the examined groups (p > 0.05; Figure 7D). Similar results were noted when the data were stratified by the American Society for Reproductive Medicine (ASRM) classification of endometriosis (Figures 7E, G, H). The only exception was that the percentage of CD4+CD127-CD25hiFOXP3+ IL-24+ cells within CD4+ cells did not differ between women with stage III endometriosis compared with that in the control group (Figure 7F).
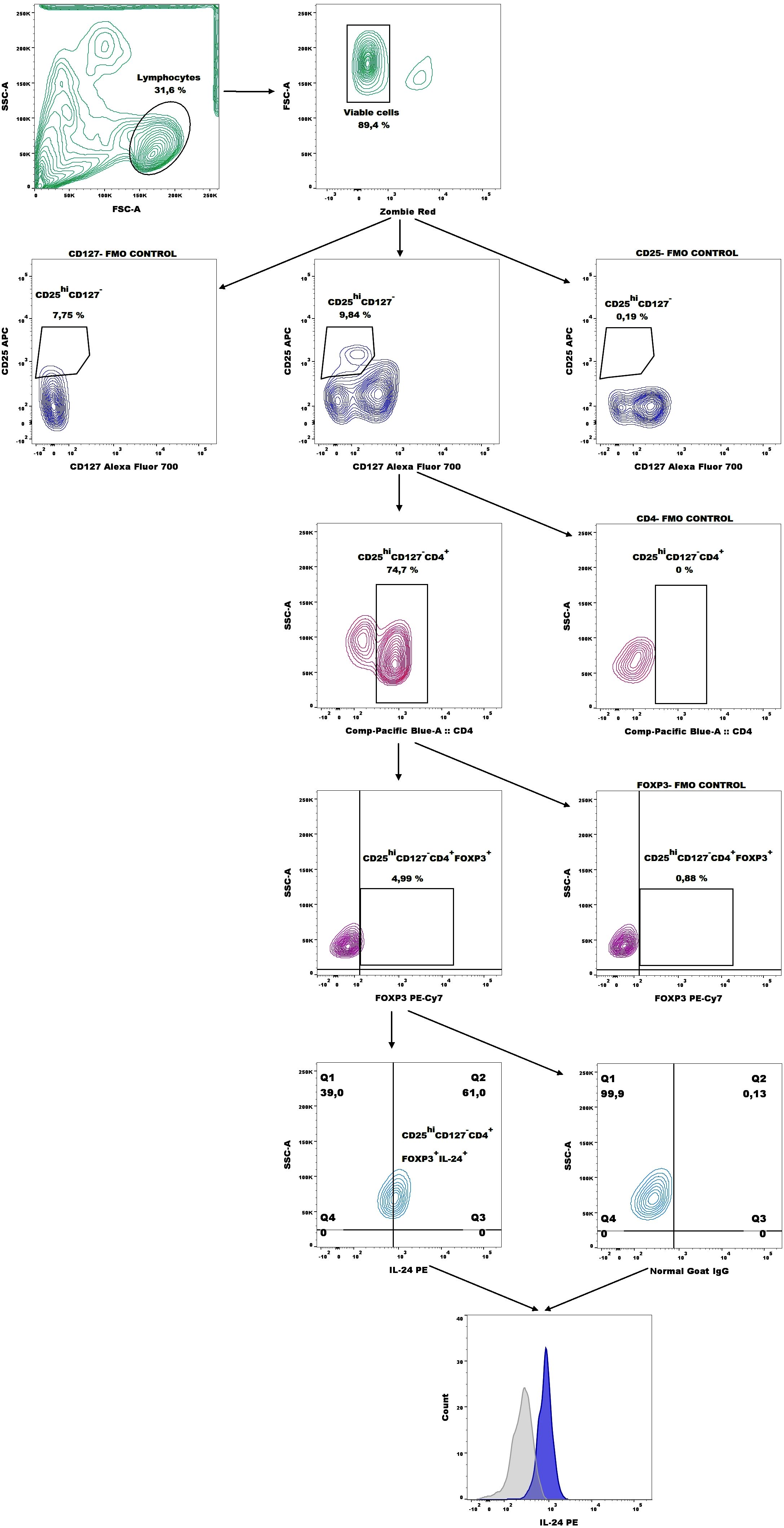
Figure 6. Representative dot plots showing the gating strategy for IL-24–producing Tregs (CD4+CD127-CD25hiFOXP3+ cells) and representative histograms of IL-24 expression (blue histogram) overlaid with the respective isotype-matched control (grey histogram) derived from whole blood of patients with endometriosis.
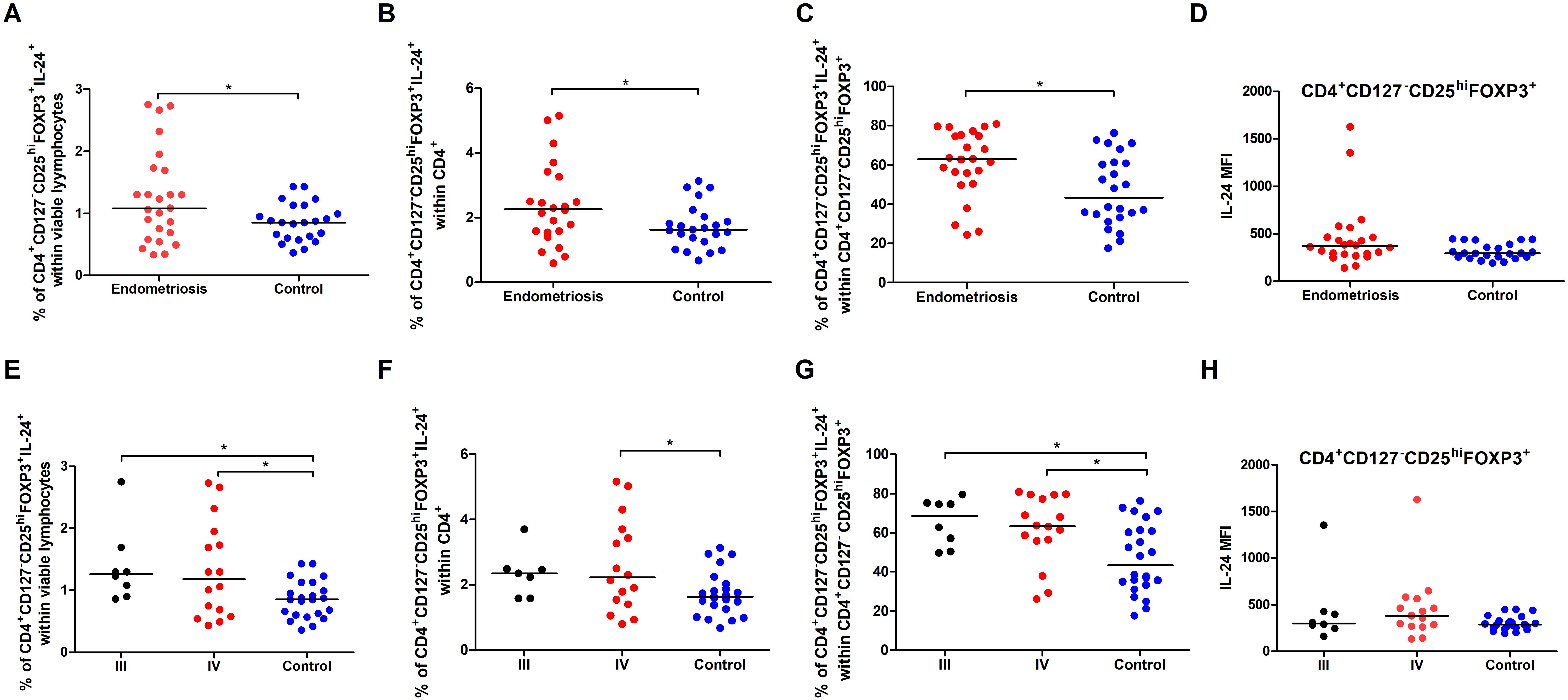
Figure 7. Percentage and IL-24 expression in Tregs in total pool of women with endometriosis (red dots in A–D) and women with stages III (black dots in E–H) and IV (red dots in E–H) endometriosis compared to control (blue dots). Whole peripheral blood were stimulated with PMA and ionomycin in the presence of Brefeldin A and Monensin A. The following parameters were measured by flow cytometry: (A, E) Percentage of IL-24–producing Tregs (CD4+CD127-CD25hiFOXP3+) within viable lymphocytes, (B, F) Percentage of IL-24–producing Tregs within CD4+ cells, (C, G) Percentage of IL-24–producing Tregs among total Tregs, and (D, H) Median fluorescence intensity (MFI) of IL-24 within Tregs. Data are presented as individual values with median. Data presented in graphs a-d were analyzed using Student’s t-test (normal distribution) or Mann–Whitney U test (non-normal distribution), whereas data in graphs e-f were analyzed using one-way ANOVA (normal distribution) or Kruskal–Wallis test (non-normal distribution) with Dunnets’s multiple comparison post-hoc test (p < 0.05). *p < 0.05.
4 Discussion
In endometriosis, the growth of endometrial tissue outside the uterine cavity disrupts peripheral and local tissue homeostasis. The dysfunctional response of peripheral and peritoneal cavity immune cells has been associated with the development of endometriosis (20, 21, 49, 50). Regulatory cells play a fundamental role in developing suppressive immune responses that allow endometriotic fragments to grow or spread outside the uterine cavity. Among these, Tregs and Bregs are key players in establishing self-tolerance. Their regulatory abilities are linked to the secretion of inhibitory cytokines, such as IL-35, TGF-beta, and IL-10, all of which are dysregulated in endometriosis. IL-24, a member of the IL-10 cytokine family, has also been implicated in the pathogenesis of endometriosis (33).
IL-24 plays a significant role in immune responses, particularly in cancer. As a tumor-suppressive cytokine, IL-24 induces apoptosis in cancer cells, while sparing normal cells, and inhibiting angiogenesis within the tumor, thereby restricting tumor growth and spread and enhancing the immune system’s ability to detect and destroy tumor cells (34, 35, 37–40, 51). IL-24 is produced by both non-immune cells like astrocytes, keratinocytes, pancreatic myofibroblasts, and endothelial cells (52–54) and immune cell populations such as monocytes, macrophages, dendritic cells, NK cells, T cells, and B cells (42–45). It is inducible in peripheral blood mononuclear cells (PBMCs) by pro-inflammatory cytokines, suggesting a systemic immunomodulatory role (45). Macrophages and NK cells produce IL-24 under the control of STAT3 and STAT4, respectively, reflecting their distinct roles in inflammation (42, 43). Furthermore, activated T helper (Th) cells stimulate macrophages to produce IL-24, leading to the suppression of mammary tumor growth (55). In B cells, IL-24 acts as a negative regulator of humoral immunity by inhibiting plasma cell differentiation (44). In non-immune contexts, IL-24 contributes to epithelial inflammation in the skin (52), modulates fibrogenic activity in pancreatic myofibroblasts (53), and exerts cytoprotective effects in endothelial cells under oxidative stress conditions (54). Taken together, the aforementioned studies indicate the multifunctional nature of IL-24 in regulating a diverse range of cellular responses across both immune and non-immune cell types. IL-24 exhibits dose-dependent and context-sensitive biological activity, which underlies its paradoxical roles in inflammation, immune regulation, and cancer. At low concentrations, IL-24 acts predominantly as an immunomodulatory cytokine, particularly in T cells (56). Furthermore, low-dose IL-24 has been shown to promote immune homeostasis by inducing IL-10 production in T helper 17 (Th17) cells, modulating mitochondrial signaling, and reducing signal transducer and activator of transcription 3 (STAT3) activity, thereby limiting the production of excessive pro-inflammatory cytokines (57, 58). This regulatory role is especially evident in autoimmune conditions, where IL-24 helps limit excessive immune activation. At moderate concentrations, IL-24 signals through IL-10 family receptors (IL-20RA/IL-20RB and IL-22RA1/IL-20RB) on immune and epithelial cells, promoting the production of cytokines and tissue repair mediators, which supports immune activation and maintains epithelial barrier integrity (59). At high concentrations, IL-24 activates the proteins PERK and eIF2, leading to reduced expression of survival proteins and simultaneously activating a ceramide-dependent pathway that induces apoptosis in tumor cells (60). Notably, IL-24’s cytotoxicity in cancer cells can also occur independently of receptor engagement, further highlighting its selective action toward malignant cells (61). Furthermore, IL-24’s function is heavily influenced by the tissue microenvironment (34). For example, in cancer models, its anti-tumor effects can be both direct through local overexpression or therapeutic delivery and indirect, mediated by immune cells (62–65).
Dysregulation of IL-24 expression has been linked to several autoimmune diseases such as psoriasis (66), inflammatory bowel disease (67), and rheumatoid arthritis (19). The immune-related changes observed in endometriosis closely resemble those found in autoimmune diseases, suggesting a shared underlying pathogenesis between the two conditions. Individuals with endometriosis show signs of systemic immune dysregulation and face an increased risk, ranging from 30% to 80% of developing immune-related comorbidities, including classical autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, and coeliac disease; autoinflammatory conditions like osteoarthritis and mixed-pattern disorders such as psoriasis (68–70). Additionally, as reported by Shigesi et al. (2025), endometriosis shares a significant genetic correlation with both rheumatoid arthritis and multiple sclerosis. The presence of concurrent autoimmune diseases has also been associated with a more severe progression of endometriosis (71). As noted previously, interleukin-24 (IL-24) can activate various immune cells, including macrophages, dendritic cells, and T lymphocytes, leading to increased production of pro-inflammatory cytokines and reactive oxygen species. This immune activation contributes to the maintenance of chronic inflammation and progressive tissue damage. In the context of endometriosis, IL-24–driven inflammatory signaling may intensify local immune activation, contribute to the persistence of ectopic endometrial lesions, and disrupt the structural integrity of surrounding tissues, including the peritoneum and reproductive organs. The only study that addressed IL-24 expression in endometriosis was by Shao et al. (2016), which reported reduced IL-24 expression in the eutopic and ectopic endometrium of women with endometriosis compared with women without the condition. They demonstrated that monocyte-derived macrophages from healthy women and co-cultured with the eutopic endometrium of women with endometriosis downregulated IL-24 production and its receptor expression (calculated as MFI) compared to endometrial stromal cells cultured alone. Moreover, they revealed that macrophages significantly restricted the inhibitory effects of IL-24 on the viability, invasion, and proliferation markers of endometrial stromal cells. Although these findings were interesting, the experimental conditions and selection of control group were not in line with physiological conditions. Specifically, macrophages from healthy women were co-cultured with antigenically foreign endometria, in which created an artificial system conducive to immune responses against foreign endometrium. A more physiologically relevant approach would involve immune cells from women with endometriosis that would reflect the altered cytokine milieu characteristic of the disease (15–24).
In our study, we aimed to assess whether regulatory cells in their most “natural” state produce IL-24 and whether endometriosis is characterized by altered IL-24 expression. Unlike Shao et al., 2016 (33), who investigated local expression, we examined IL-24 in peripheral blood. These differences in scientific questions and experimental design likely account for observed discrepancies between our findings and their results. We revealed for the first time that circulating regulatory T and B lymphocytes secrete IL-24 and their percentages are altered in women with endometriosis compared with healthy women. Other studies have mainly investigated IL-24 expression at the mRNA level, but rarely at the protein level (western blotting, ELISA, and immunochemistry), and predominantly in non-immune or basic immune cells (activated T cells, NK cells, or B cells) (41–45, 53, 56, 72). Our results, based on flow cytometry, indicated that over 60% of Tregs and Bregs secrete IL-24. However, the relative IL-24 expression in these cells (as measured by MFI) was similar in both groups. Nonetheless, we observed an increase in IL-24+ Tregs percentage in women with endometriosis, regardless of endometriosis stages. This suggests that the functional capacity of individual IL-24+ Tregs cells remains relatively stable, and the observed changes are primarily due to variations in the number of IL-24+ cells rather than altered expression per cell. Previously, Tregs percentage in women with endometriosis was reported to be higher than in healthy women (25, 26, 28), with proportions increasing with the disease progresses (33). The influence of Tregs on endometriosis may be multifaceted, as they are responsible for shaping the immune environment by modulating the behavior of other immune cells. For example in ovarian ectopic lesions, Tregs contribute to macrophage polarization toward the anti-inflammatory M2 phenotype (73). Furthermore, Tregs can also influence the activity of T helper cells, cytotoxic CD8+ T cells, and NK cells, either by suppressing or promoting their inflammatory responses (74). This indicates that Tregs may, both directly and indirectly, contribute to the pathogenesis of endometriosis by promoting the adhesion and growth of endometrial implants. However, the exact role of IL-24+ Tregs in immunotolerance remains unclear. IL-24 may directly influence Tregs differentiation and function by modulating cytokine environments, or indirectly affect the balance between pro-inflammatory and anti-inflammatory responses, affecting Tregs activity in immune regulation (75). Zhang et al., 2019 (56) showed that low IL-24 concentration promotes FoxP3 mRNA expression in tumor-infiltrating T cells, whereas high concentrations decrease Tregs percentage, FoxP3 mRNA expression, and IL-10/IL-35 secretion in cells obtained from patients with colorectal adenocarcinoma. The only other study describing the influence of IL-24 on Tregs showed that this cytokine plays a crucial role in enhancing the suppressive function of mice CD4+CD25+ T cells/Peyer’s patch B cells co-cultures, named Treg-of-B (P) cells (76). In the absence of IL-24, the Treg-of-B (P) cells exhibited a decreased ability to inhibit responder T cell proliferation, whereas IL-24 blocking impaired the suppressive function of Treg-of-B (P) cells. They further suggested that IL-24 functions through autocrine signaling to stimulate IL-10 expression in Treg-of-B (P) cells. Therefore, it is tempting to speculate the induction in IL-24+ Tregs observed in our study might have enhanced adverse immune tolerance in endometriosis.
In the present study, we also determined the percentages of IL-24–producing Bregs. Bregs may help regulate the inflammatory milieu in endometriosis. They suppress autoreactive T cell activation, reduce pro-inflammatory cytokine production, and promote tissue repair (77–81). This function may be compromised in individuals with endometriosis, which could contribute to persistent inflammation and disease development. In our previous study, we demonstrated that B10 cell and plasmablast percentages were lower in women with endometriosis (32). Simultaneously, we revealed that the percentages of the same Breg subpopulation expressing IL-35 were higher in patients with endometriosis. Here, we also observed a similar trend of increased IL-24+ plasmablast percentages in women with endometriosis compared with the healthy controls. Plasmablasts are B cells that might secrete antibodies that contribute to tissue damage and inflammation in the pelvic region, which causes pain and fibrosis characteristic of endometriosis. Patients with endometriosis exhibit an increased prevalence of anti-endometrial (82) as well as IgG and IgM autoantibodies that target phospholipids, histones, or DNA (83). Another study demonstrated the significant presence of plasma cells and macrophages in endometriotic lesions (15). Normally, B cells mature into plasma cells that produce antibodies. However, in endometriosis, plasmablasts may be inappropriately activated, leading to an overproduction of antibodies that attack the body’s own tissues. The role of IL-24 in B cell function is still being actively studied, especially in cancer, with research suggesting that it may influence both B cell activation and regulation. IL-24 is expressed in naïve and memory B cells but is repressed in centroblasts (44). Moreover, the same authors reported that CD27+ memory B cells and CD5+ B cells exhibit elevated IL-24 expression upon BCR activation and CD40–CD40L ligation. In vitro experiments have demonstrated that IL-24 promotes CD40L-induced B cell proliferation, but inhibits plasma cell formation, immunoglobulin (Ig) G production, and IL-10 expression (44). Conversely, another study found that IL-24 induces B cell apoptosis by activating genes involved in the mitochondrial apoptotic pathway during the later stages of B cell differentiation, while inhibiting genes related to DNA replication and metabolism in the early stages (84). The aforementioned findings provide valuable insights, yet the precise impact of IL-24 on B cell differentiation and function, including plasmablasts, remains unclear. A deeper understanding of how this cytokine influences B cell activation, antibody production, and immune regulation in disease contexts could open up new potential treatment strategies.
Despite the novel findings on the role of IL-24 in endometriosis, our study has several limitations that should be considered when interpreting the results. Firstly, our study lacks patients with early stages (stage I/II) of endometriosis, which is due to diagnostic challenges as the disease is often asymptomatic or undiagnosed until it reaches more advanced stages. This limits the ability to assess the dynamics of interleukin-24 in the full spectrum of disease progression. Secondly, the study’s focus on peripheral blood samples excludes the analysis of peritoneal fluid or ectopic endometrial lesions, which could offer additional insights into the local immune microenvironment where IL-24 may play a role. Thirdly, stimulation of cells with PMA and ionomycin, may non-specifically induce IL-24 expression, potentially confounding the interpretation of cytokine levels in the context of physiological conditions. However, the PMA/Ionomycin stimulation is a well-established approach for inducing cytokine production. In our study, the stimulation conditions were consistent across both groups, thereby eliminating the potential confounding effect of the stimulation procedure. Finally, the inclusion of functional assays in upcoming research may help elucidate whether IL-24 secretion is associated with the immunosuppressive activity of regulatory cells. Addressing these limitations in future studies will be essential for a deeper understanding of IL-24’s role in the progression of endometriosis, and its potential clinical relevance.
In conclusion, we have shown for the first time that circulating regulatory T cells and selected B cell subpopulations (plasmablasts, immature B cells and B10 cells) secrete IL-24. Interestingly, their percentages are altered in women with endometriosis compared to that in healthy women. Based on our findings, we speculate that IL-24–producing cells with a regulatory phenotype are involved in the pathogenesis of endometriosis. IL-24 secretion appears to enhance the improper immunosuppressive activity of Tregs and plasmablasts in endometriosis, which enables the implantation and growth of endometrial lesions outside the uterus. Further research is required to elucidate the exact role of IL-24–producing regulatory lymphocytes in the development and progression of endometriosis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Bioethical Commission of the Medical University of Wrocław (No. KB – 407/2018). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AEK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft. DL: Investigation, Writing – review & editing. AS: Visualization, Writing – review & editing. MK: Investigation, Writing – review & editing. AK: Investigation, Writing – review & editing. JP: Investigation, Writing – review & editing. AC: Formal analysis, Writing – review & editing. KG: Funding acquisition, Writing – review & editing. AC-S: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wrocław, Poland, and National Science Center Poland grant number 2020/38/E/NZ6/00182.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bonavina G and Taylor HS. Endometriosis-associated infertility: From pathophysiology to tailored treatment. Front Endocrinol (Lausanne). (2022) 13:1020827. doi: 10.3389/fendo.2022.1020827
2. Bailleul A, Niro J, Du Cheyron J, Panel P, and Fauconnier A. Infertility management according to the Endometriosis Fertility Index in patients operated for endometriosis: What is the optimal time frame? PloS One. (2021) 16:1–11. doi: 10.1371/journal.pone.0251372
3. Endometriosis and infertility: a committee opinion. Fertil Steril. (2012) 98:591–8. doi: 10.1016/j.fertnstert.2012.05.031
4. Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, et al. ESHRE guideline: endometriosis. Hum Reprod Open. (2022) 2022):hoac009. doi: 10.1093/hropen/hoac009
5. Kohl Schwartz AS, Wölfler MM, Mitter V, Rauchfuss M, Haeberlin F, Eberhard M, et al. Endometriosis, especially mild disease: a risk factor for miscarriages. Fertil Steril. (2017) 108:806–814.e2. doi: 10.1016/j.fertnstert.2017.08.025
6. Koninckx PR, Ussia A, Adamyan L, Wattiez A, and Donnez J. Deep endometriosis: Definition, diagnosis, and treatment. Fertil Steril. (2012) 98:564–71. doi: 10.1016/j.fertnstert.2012.07.1061
7. Vannuccini S, Lazzeri L, Orlandini C, Morgante G, Bifulco G, Fagiolini A, et al. Mental health, pain symptoms and systemic comorbidities in women with endometriosis: a cross-sectional study. J Psychosom Obstet Gynaecol. (2018) 39:315–20. doi: 10.1080/0167482X.2017.1386171
8. Zondervan KT, Becker CM, and Missmer SA. Endometriosis. N Engl J Med. (2020) 382:1244–56. doi: 10.1056/NEJMra1810764
9. Darbà J and Marsà A. Economic implications of endometriosis: A review. Pharmacoeconomics. (2022) 40:1143–58. doi: 10.1007/s40273-022-01211-0
10. Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. (1927) 14:422–69. doi: 10.1016/S0002-9378(15)30003-X
11. Weisheng B, Nezhat CH, Huang GF, Mao Y-Q, Sidell N, and Huang R-P. Discovering endometriosis biomarkers with multiplex cytokine arrays. Clin Proteomics. (2019) 16:28. doi: 10.1186/s12014-019-9248-y
12. Rafi U, Ahmad S, Bokhari SS, Iqbal MA, Zia A, Khan MA, et al. Association of inflammatory markers/cytokines with cardiovascular risk manifestation in patients with endometriosis. Mediators Inflammation. (2021) 2021:3425560. doi: 10.1155/2021/3425560
13. O D, Waelkens E, Vanhie A, Peterse D, Fassbender A, and D’Hooghe T. The use of antibody arrays in the discovery of new plasma biomarkers for endometriosis. Reprod Sci. (2020) 27:751–62. doi: 10.1007/s43032-019-00081-w
14. Jørgensen H, Hill AS, Beste MT, Kumar MP, Chiswick E, Fedorcsak P, et al. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil Steril. (2017) 107:1191–1199.e2. doi: 10.1016/j.fertnstert.2017.03.013
15. Hever A, Roth RB, Hevezi P, Marin ME, Acosta JA, Acosta H, et al. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc Natl Acad Sci U.S.A. (2007) 104:12451–6. doi: 10.1073/pnas.0703451104
16. Hirata T, Osuga Y, Hamasaki K, Yoshino O, Ito M, Hasegawa A, et al. Interleukin (IL)-17A stimulates IL-8 secretion, cyclooxygensase-2 expression, and cell proliferation of endometriotic stromal cells. Endocrinology. (2008) 149:1260–7. doi: 10.1210/en.2007-0749
17. Ho HN, Wu MY, Chao KH, Chen CD, Chen SU, and Yang YS. Peritoneal interleukin-10 increases with decrease in activated CD4+ T lymphocytes in women with endometriosis. Hum Reprod. (1997) 12:2528–33. doi: 10.1093/humrep/12.11.2528
18. Hsu CC, Yang BC, Wu MH, and Huang KE. Enhanced interleukin-4 expression in patients with endometriosis. Fertil Steril. (1997) 67:1059–64. doi: 10.1016/s0015-0282(97)81439-2
19. Kragstrup TW, Otkjaer K, Holm C, Jørgensen A, Hokland M, Iversen L, et al. The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine. (2008) 41:16–23. doi: 10.1016/j.cyto.2007.10.004
20. Kubatova A, Erdem A, Erdem M, FiratMutlu M, and Korucuoglu U. Serum cytokine and growth factor levels in patients with endometriosis. Cent Eur J Immunol. (2013) 38:500–4. doi: 10.5114/ceji.2013.39768
21. Mihalyi A, Gevaert O, Kyama CM, Simsa P, Pochet N, De Smet F, et al. Non-invasive diagnosis of endometriosis based on a combined analysis of six plasma biomarkers. Hum Reprod. (2010) 25:654–64. doi: 10.1093/humrep/dep425
22. Milewski Ł, Barcz E, Dziunycz P, Radomski D, Kamiński P, Roszkowski PI, et al. Association of leptin with inflammatory cytokines and lymphocyte subpopulations in peritoneal fluid of patients with endometriosis. J Reprod Immunol. (2008) 79:111–7. doi: 10.1016/j.jri.2008.08.007
23. Punnonen J, Teisala K, Ranta H, Bennett B, and Punnonen R. Increased levels of interleukin-6 and interleukin-10 in the peritoneal fluid of patients with endometriosis. Am J Obstet Gynecol. (1996) 174:1522–6. doi: 10.1016/s0002-9378(96)70600-2
24. Suen J-L, Chang Y, Chiu P-R, Hsieh T-H, Hsi E, Chen Y-C, et al. Serum level of IL-10 is increased in patients with endometriosis, and IL-10 promotes the growth of lesions in a murine model. Am J Pathol. (2014) 184:464–71. doi: 10.1016/j.ajpath.2013.10.023
25. Hanada T, Tsuji S, Nakayama M, Wakinoue S, Kasahara K, Kimura F, et al. Suppressive regulatory T cells and latent transforming growth factor-β-expressing macrophages are altered in the peritoneal fluid of patients with endometriosis. Reprod Biol Endocrinol. (2018) 16:9. doi: 10.1186/s12958-018-0325-2
26. Olkowska-Truchanowicz J, Bocian K, Maksym RB, Białoszewska A, Włodarczyk D, Baranowski W, et al. CD4+ CD25+ FOXP3+ regulatory T cells in peripheral blood and peritoneal fluid of patients with endometriosis. Hum Reprod. (2013) 28:119–24. doi: 10.1093/humrep/des346
27. Wei C, Mei J, Tang L, Liu Y, Li D, Li M, et al. 1-Methyl-tryptophan attenuates regulatory T cells differentiation due to the inhibition of estrogen-IDO1-MRC2 axis in endometriosis. Cell Death Dis. (2016) 7:e2489. doi: 10.1038/cddis.2016.375
28. Delbandi A-A, Mahmoudi M, Shervin A, Moradi Z, Arablou T, and Zarnani A-H. Higher frequency of circulating, but not tissue regulatory T cells in patients with endometriosis. J Reprod Immunol. (2020) 139:103119. doi: 10.1016/j.jri.2020.103119
29. Le NXH, Loret de Mola JR, Bremer P, Groesch K, Wilson T, Diaz-Sylvester P, et al. Alteration of systemic and uterine endometrial immune populations in patients with endometriosis. Am J Reprod Immunol. (2021) 85:e13362. doi: 10.1111/aji.13362
30. Hey-Cunningham AJ, Riaz A, Fromm PD, Kupresanin F, Markham R, and McGuire HM. Circulating and endometrial regulatory T cell and related populations in endometriosis and infertility: endometriosis is associated with blunting of endometrial cyclical effects and reduced proportions in moderate-severe disease. Reprod Sci. (2022) 29:229–42. doi: 10.1007/s43032-021-00658-4
31. Knez J, Kovačič B, and Goropevšek A. The role of regulatory T-cells in the development of endometriosis. Hum Reprod. (2024). doi: 10.1093/humrep/deae103
32. Slawek A, Lorek D, Kedzierska AE, Kubik P, Pajak J, Chrobak A, et al. Peripheral blood subpopulations of Bregs producing IL-35 in women with endometriosis. Am J Reprod Immunol. (2023) 89:e13675. doi: 10.1111/aji.13675
33. Shao J, Zhang B, Yu J-J, Wei C-Y, Zhou W-J, Chang K-K, et al. Macrophages promote the growth and invasion of endometrial stromal cells by downregulating IL-24 in endometriosis. Reproduction. (2016) 152:673–82. doi: 10.1530/REP-16-0278
34. Emdad L, Bhoopathi P, Talukdar S, Pradhan AK, Sarkar D, Wang X-Y, et al. Recent insights into apoptosis and toxic autophagy: The roles of MDA-7/IL-24, a multidimensional anti-cancer therapeutic. Semin Cancer Biol. (2020) 66:140–54. doi: 10.1016/j.semcancer.2019.07.013
35. Gupta P, Su Z, Lebedeva IV, Sarkar D, Sauane M, Emdad L, et al. mda-7/IL-24: Multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther. (2006) 111:596–628. doi: 10.1016/j.pharmthera.2005.11.005
36. Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, and Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci U.S.A. (1996) 93:9160–5. doi: 10.1073/pnas.93.17.9160
37. Menezes ME, Bhoopathi P, Pradhan AK, Emdad L, Das SK, Guo C, et al. Role of MDA-7/IL-24 a multifunction protein in human diseases. Adv Cancer Res. (2018) 138:143–82. doi: 10.1016/bs.acr.2018.02.005
38. Sarkar D, Su Z, Lebedeva IV, Sauane M, Gopalkrishnan RV, Dent P, et al. mda-7 (IL-24): signaling and functional roles. Biotechniques. (2002) Suppl:30–9. doi: 10.2144/Oct0204
39. Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P, et al. Melanoma differentiation associated gene-7 (mda-7)/IL-24: a “magic bullet” for cancer therapy? Expert Opin Biol Ther. (2007) 7:577–86. doi: 10.1517/14712598.7.5.577
40. Whitaker EL, Filippov VA, and Duerksen-Hughes PJ. Interleukin 24: mechanisms and therapeutic potential of an anti-cancer gene. Cytokine Growth Factor Rev. (2012) 23:323–31. doi: 10.1016/j.cytogfr.2012.08.004
41. Chong WP, Mattapallil MJ, Raychaudhuri K, Bing SJ, Wu S, Zhong Y, et al. The cytokine IL-17A limits Th17 pathogenicity via a negative feedback loop driven by autocrine induction of IL-24. Immunity. (2020) 53:384–397.e5. doi: 10.1016/j.immuni.2020.06.022
42. Dabitao D, Hedrich CM, Wang F, Vacharathit V, and Bream JH. Cell-specific requirements for STAT proteins and type I IFN receptor signaling discretely regulate IL-24 and IL-10 expression in NK cells and macrophages. J Immunol. (2018) 200:2154–64. doi: 10.4049/jimmunol.1701340
43. Garn H, Schmidt A, Grau V, Stumpf S, Kaufmann A, Becker M, et al. IL-24 is expressed by rat and human macrophages. Immunobiology. (2002) 205:321–34. doi: 10.1078/0171-2985-00135
44. Maarof G, Bouchet-Delbos L, Gary-Gouy H, Durand-Gasselin I, Krzysiek R, and Dalloul A. Interleukin-24 inhibits the plasma cell differentiation program in human germinal center B cells. Blood. (2010) 115:1718–26. doi: 10.1182/blood-2009-05-220251
45. Poindexter NJ, Walch ET, Chada S, and Grimm EA. Cytokine induction of interleukin-24 in human peripheral blood mononuclear cells. J Leukoc Biol. (2005) 78:745–52. doi: 10.1189/jlb.0205116
46. Schaefer G, Venkataraman C, and Schindler U. Cutting edge: FISP (IL-4-induced secreted protein), a novel cytokine-like molecule secreted by Th2 cells. J Immunol. (2001) 166:5859–63. doi: 10.4049/jimmunol.166.10.5859
47. Lee S-Y, Koo Y-J, and Lee D-H. Classification of endometriosis. Yeungnam Univ J Med. (2021) 38:10–8. doi: 10.12701/yujm.2020.00444
48. Belkina AC, Ciccolella CO, Anno R, Halpert R, Spidlen J, and Snyder-Cappione JE. Automated optimized parameters for T-distributed stochastic neighbor embedding improve visualization and analysis of large datasets. Nat Commun. (2019) 10:5415. doi: 10.1038/s41467-019-13055-y
50. Kwak J-Y, Park S-W, Kim K-H, Na Y-J, and Lee K-S. Modulation of neutrophil apoptosis by plasma and peritoneal fluid from patients with advanced endometriosis. Hum Reprod. (2002) 17:595–600. doi: 10.1093/humrep/17.3.595
51. Dash R, Bhutia SK, Azab B, Su Z, Quinn BA, Kegelmen TP, et al. mda-7/IL-24: a unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine Growth Factor Rev. (2010) 21:381–91. doi: 10.1016/j.cytogfr.2010.08.004
52. Van Belle AB, Cochez PM, de Heusch M, Pointner L, Opsomer R, Raynaud P, et al. IL-24 contributes to skin inflammation in Para-Phenylenediamine-induced contact hypersensitivity. Sci Rep. (2019) 9:1852. doi: 10.1038/s41598-018-38156-4
53. Imaeda H, Nishida A, Inatomi O, Fujiyama Y, and Andoh A. Expression of interleukin-24 and its receptor in human pancreatic myofibroblasts. Int J Mol Med. (2011) 28:993–9. doi: 10.3892/ijmm.2011.793
54. Wang Z, Wang Y, Chen Y, and Lv J. The IL-24 gene protects human umbilical vein endothelial cells against H2O2-induced injury and may be useful as a treatment for cardiovascular disease. Int J Mol Med. (2016) 37:581–92. doi: 10.3892/ijmm.2016.2466
55. Boieri M, Malishkevich A, Guennoun R, Marchese E, Kroon S, Trerice KE, et al. CD4+ T helper 2 cells suppress breast cancer by inducing terminal differentiation. J Exp Med. (2022) 219. doi: 10.1084/jem.20201963
56. Zhang Y, Liu Y, and Xu Y. Interleukin-24 regulates T cell activity in patients with colorectal adenocarcinoma. Front Oncol. (2019) 9:1401. doi: 10.3389/fonc.2019.01401
57. Feng K-N, Meng P, Zhang M, Zou X-L, Li S, Huang C-Q, et al. IL-24 contributes to neutrophilic asthma in an IL-17A-dependent manner and is suppressed by IL-37. Allergy Asthma Immunol Res. (2022) 14:505–27. doi: 10.4168/aair.2022.14.5.505
58. Sie C, Kant R, Peter C, Muschaweckh A, Pfaller M, Nirschl L, et al. IL-24 intrinsically regulates Th17 cell pathogenicity in mice. J Exp Med. (2022) 219. doi: 10.1084/jem.20212443
59. Dumoutier L, Leemans C, Lejeune D, Kotenko SV, and Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. (2001) 167:3545–9. doi: 10.4049/jimmunol.167.7.3545
60. Sauane M, Su Z-Z, Dash R, Liu X, Norris JS, Sarkar D, et al. Ceramide plays a prominent role in MDA-7/IL-24-induced cancer-specific apoptosis. J Cell Physiol. (2010) 222:546–55. doi: 10.1002/jcp.21969
61. Kreis S, Philippidou D, Margue C, Rolvering C, Haan C, Dumoutier L, et al. Recombinant interleukin-24 lacks apoptosis-inducing properties in melanoma cells. PloS One. (2007) 2:e1300. doi: 10.1371/journal.pone.0001300
62. Persaud L, De Jesus D, Brannigan O, Richiez-Paredes M, Huaman J, Alvarado G, et al. Mechanism of action and applications of interleukin 24 in immunotherapy. Int J Mol Sci. (2016) 17(6), 869. doi: 10.3390/ijms17060869
63. Panneerselvam J, Munshi A, and Ramesh R. Molecular targets and signaling pathways regulated by interleukin (IL)-24 in mediating its antitumor activities. J Mol Signal. (2013) 8:15. doi: 10.1186/1750-2187-8-15
64. Ma Y-F, Ren Y, Wu C-J, Zhao X-H, Xu H, Wu D-Z, et al. Interleukin (IL)-24 transforms the tumor microenvironment and induces anticancer immunity in a murine model of colon cancer. Mol Immunol. (2016) 75:11–20. doi: 10.1016/j.molimm.2016.05.010
65. Menezes ME, Bhatia S, Bhoopathi P, Das SK, Emdad L, Dasgupta S, et al. MDA-7/IL-24: multifunctional cancer killing cytokine. Adv Exp Med Biol. (2014) 818:127–53. doi: 10.1007/978-1-4471-6458-6_6
66. Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. (2007) 178:2229–40. doi: 10.4049/jimmunol.178.4.2229
67. Andoh A, Shioya M, Nishida A, Bamba S, Tsujikawa T, Kim-Mitsuyama S, et al. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J Immunol. (2009) 183:687–95. doi: 10.4049/jimmunol.0804169
68. Shigesi N, Kvaskoff M, Kirtley S, Feng Q, Fang H, Knight JC, et al. The association between endometriosis and autoimmune diseases: a systematic review and meta-analysis. Hum Reprod Update. (2019) 25:486–503. doi: 10.1093/humupd/dmz014
69. Blanco LP, Salmeri N, Temkin SM, Shanmugam VK, and Stratton P. Endometriosis and autoimmunity. Autoimmun Rev. (2025) 24:103752. doi: 10.1016/j.autrev.2025.103752
70. Shigesi N, Harris HR, Fang H, Ndungu A, Lincoln MR, Cotsapas C, et al. The phenotypic and genetic association between endometriosis and immunological diseases. Hum Reprod. (2025) 40(6):1195–209. doi: 10.1093/humrep/deaf062
71. Vanni VS, Villanacci R, Salmeri N, Papaleo E, Delprato D, Ottolina J, et al. Publisher Correction: Concomitant autoimmunity may be a predictor of more severe stages of endometriosis. Sci Rep. (2021) 11:17715. doi: 10.1038/s41598-021-97506-x
72. Liao S, Yang Y, Chen S, Bi Y, Huang Q, Wei Z, et al. IL-24 inhibits endometrial cancer cell proliferation by promoting apoptosis through the mitochondrial intrinsic signaling pathway. BioMed Pharmacother. (2020) 124:109831. doi: 10.1016/j.biopha.2020.109831
73. Hou X-X, Wang X-Q, Zhou W-J, and Li D-J. Regulatory T cells induce polarization of pro-repair macrophages by secreting sFGL2 into the endometriotic milieu. Commun Biol. (2021) 4:499. doi: 10.1038/s42003-021-02018-z
74. Wu X-G, Chen J-J, Zhou H-L, Wu Y, Lin F, Shi J, et al. Identification and validation of the signatures of infiltrating immune cells in the eutopic endometrium endometria of women with endometriosis. Front Immunol. (2021) 12:671201. doi: 10.3389/fimmu.2021.671201
75. Chu K-H and Chiang B-L. CD200R activation on naïve T cells by B cells induces suppressive activity of T cells via IL-24. Cell Mol Life Sci. (2024) 81:231. doi: 10.1007/s00018-024-05268-2
76. Subramaniam S, Anandha Rao JS, Ramdas P, Ng MH, Kannan Kutty M, Selvaduray KR, et al. Reduced infiltration of regulatory T cells in tumours from mice fed daily with gamma-tocotrienol supplementation. Clin Exp Immunol. (2021) 206:161–72. doi: 10.1111/cei.13650
77. Ahsan NF, Lourenço S, Psyllou D, Long A, Shankar S, and Bashford-Rogers R. The current understanding of the phenotypic and functional properties of human regulatory B cells (Bregs). Oxford Open Immunol. (2024) 5:iqae012. doi: 10.1093/oxfimm/iqae012
78. Mauri C and Menon M. Human regulatory B cells in health and disease: therapeutic potential. J Clin Invest. (2017) 127:772–9. doi: 10.1172/JCI85113
79. Guzman-Genuino RM and Diener KR. Regulatory B cells in pregnancy: lessons from autoimmunity, graft tolerance, and cancer. Front Immunol. (2017) 8:172. doi: 10.3389/fimmu.2017.00172
80. Catalán D, Mansilla MA, Ferrier A, Soto L, Oleinika K, Aguillón JC, et al. Immunosuppressive mechanisms of regulatory B cells. Front Immunol. (2021) 12:611795. doi: 10.3389/fimmu.2021.611795
81. Chekol Abebe E, Asmamaw Dejenie T, Mengie Ayele T, Dagnew Baye N, Agegnehu Teshome A, and Tilahun Muche Z. The role of regulatory B cells in health and diseases: A systemic review. J Inflammation Res. (2021) 14:75–84. doi: 10.2147/JIR.S286426
82. Hicks BR, Yudkin PL, Kennedy S, Barlow DH, and Starkey PM. Anti-endometrial and anti-endothelial auto-antibodies in women with endometriosis. Hum Reprod (1993) 8(2):310–5. doi: 10.1093/oxfordjournals.humrep.a138042
83. Gleicher N, el-Roeiy A, Confino E, and Friberg J. Is endometriosis an autoimmune disease? Obstet Gynecol. (1987) 70:115–22.
84. Hadife N, Nemos C, Frippiat J-P, Hamadé T, Perrot A, and Dalloul A. Interleukin-24 mediates apoptosis in human B-cells through early activation of cell cycle arrest followed by late induction of the mitochondrial apoptosis pathway. Leuk Lymphoma. (2013) 54:587–97. doi: 10.3109/10428194.2012.717079
Keywords: IL-24, Bregs, Tregs, endometriosis, plasmablasts, B10, immature B cells
Citation: Kedzierska AE, Lorek D, Slawek A, Karmowski M, Kalota A, Pajak J, Chrobak A, Grzymajlo K and Chelmonska-Soyta A (2025) IL-24 producing regulatory T and B lymphocytes in endometriosis. Front. Immunol. 16:1582762. doi: 10.3389/fimmu.2025.1582762
Received: 24 February 2025; Accepted: 29 May 2025;
Published: 18 June 2025.
Edited by:
Sander De Kivit, Leiden University Medical Center (LUMC), NetherlandsReviewed by:
Raj Raghupathy, Kuwait University, KuwaitIwona Wertel, Medical University of Lublin, Poland
Fengyi Xiao, Fudan University, China
Copyright © 2025 Kedzierska, Lorek, Slawek, Karmowski, Kalota, Pajak, Chrobak, Grzymajlo and Chelmonska-Soyta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Ewa Kedzierska, YW5uYS5rZWR6aWVyc2thQGhpcnN6ZmVsZC5wbA==
 Anna Ewa Kedzierska
Anna Ewa Kedzierska Daria Lorek2
Daria Lorek2 Anna Slawek
Anna Slawek Aleksandra Kalota
Aleksandra Kalota Krzysztof Grzymajlo
Krzysztof Grzymajlo Anna Chelmonska-Soyta
Anna Chelmonska-Soyta