- 1Department of Hematology, Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, China
- 2Clinical Research Ward of Cancer Center, Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, China
Purpose: CD19 Chimeric Antigen Receptor T-cell therapy (CART) represents a groundbreaking approach in the treatment of relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL). However, a subset of patients fails to achieve optimal outcomes with CD19-targeted CAR T-cells alone. To address these limitations, the development of multi-targeted CART therapies has become a focal point of innovative research. This study aims to compare the therapeutic efficacy and adverse events of dual-target versus single-target CART therapies in R/R DLBCL patients through a single-center retrospective analysis.
Methods: We included 70 patients with R/R DLBCL treated at Shanghai Tongji Hospital between January 1, 2019, and December 31, 2021. Among them, 20 patients received dual-target (CD19/20) CART, while 50 underwent CD19 CART.
Results: The CD19/20 CART group demonstrated significantly superior three-month efficacy to the CD19 CAR T-cell group, with a notably higher complete response (CR) rate. The median progression-free survival (PFS) and overall survival (OS) were 28.6 and 31.8 months longer in the Bi-CART group compared to the CD19 CAR T-cell group. However, the two groups had no significant differences in overall PFS, duration of response (DOR), or OS. The CD19/20 CART group exhibited a higher incidence of cytokine release syndrome (CRS), hematological toxicity, infections, and secondary primary tumors.
Conclusion: This study highlights the superior efficacy of dual-target CAR T-cell therapy in managing R/R DLBCL patients. The dual-target therapy significantly extended median survival compared to CD19 single-target CAR T-cell therapy. However, the enhanced therapeutic benefits were accompanied by a higher incidence of adverse effects.
1 Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common form of non-Hodgkin lymphoma (NHL), accounting for approximately 50% of newly diagnosed B-cell lymphomas globally. (1) The primary therapeutic modalities for DLBCL include chemotherapy, radiation, and stem cell transplantation, with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) being the established standard regimen, though 30-40% of patients still experience relapse or develop resistance to therapy. (2–4) Despite significant advancements, some patients experience treatment failure, leading to relapsed or refractory (R/R) DLBCL, which has a poor prognosis due to the absence of standardized, globally effective treatments.
Chimeric Antigen Receptor T-cell therapy (CART) marks a revolutionary breakthrough in the treatment of DLBCL, offering new hope for patients who have failed conventional therapies. The landmark ZUMA-1 trial reported a complete response rate of approximately 58%, a notable achievement given the poor prognosis with advanced DLBCL. (5, 6) Consistent with these findings, the JULIET and TRANSCEND trials have also demonstrated significant response rates, further underscoring the potential of this therapy. (7, 8) Despite these encouraging results, several challenges remain. Over 60% of patients experience relapse, often due to antigen escape—a phenomenon in which tumor cells lose or alter the expression of the target antigen (CD19)—thereby diminishing the efficacy of CAR T-cell therapy and contributing to disease recurrence (5, 9, 10). Additionally, resistance to subsequent CAR T-cell treatments can develop, underscoring the critical need for further innovations, such as multi-antigen targeting or strategies to enhance T-cell persistence, which are essential (11).
The development of dual-targeting strategies, particularly those addressing both CD19 and CD20 antigens, represents a promising advancement in overcoming antigen escape in CAR T-cell therapy. (12) This approach involves genetically engineering T-cells to either express two separate CAR molecules or a single bispecific CAR capable of recognizing both CD19 and CD20. (13) Early studies have indicated that this approach not only mitigates antigen heterogeneity but also enhances the durability of therapeutic responses, marking a critical step forward in addressing the limitations of single-target therapies. (14) A recent phase 1/2 trial conducted at our center demonstrated encouraging response rates and an acceptable safety profile, underscoring the potential of dual-target CAR (CD19/20) T-cell therapy in patients with refractory or relapsed DLBCL. These findings highlight the promise of this innovative approach in addressing treatment resistance and improving outcomes for this challenging patient population (15, 16).
To date, no rigorous Randomized Controlled Trials (RCT) have been conducted to directly compare the efficacy and safety of the two CART treatments. To address this gap, we conducted a single-center retrospective study to compare the therapeutic efficacy of dual-target versus single-target CART therapies in R/R DLBCL patients and to assess differences in the incidence and types of adverse events associated with each treatment.
2 Methods
2.1 Study population and design
Data from 70 patients diagnosed with R/R DLBCL, who received either CD19 CART or CD19/20 CART (Bi-CART), were retrospectively collected from Shanghai Tongji Hospital between January 1, 2019, and December 31, 2021. All patients exhibited stable disease (SD) or progressive disease (PD) status prior to undergoing cell therapy. This cohort included one case of primary mediastinal large B-cell lymphoma (PMBCL) and one case of follicular lymphoma that had histologically transformed into diffuse large B-cell lymphoma (tFL-DLBCL). The study protocols were approved by the Ethics Committee of Shanghai Tongji Hospital, Tongji University, and were conducted in compliance with the principles of the Declaration of Helsinki. All patients provided written informed consent.
2.2 Inclusion and exclusion
The CD19 CART group came from 4 different clinical trials, all of which used 4-1BB for costimulation (NCT02537977, NCT03154775, CTR20201986, CTR20200561). CD19/20 CART (Prizloncabtagene autoleucel, Prizlon-cel, C-CAR039) has been developed as a novel 2nd generation 4-1BB bi-specific CAR-T targeting both CD19 and CD20 antigens with an optimized bi-specific antigen binding domain, from clinical trial NCT04317885. In these clinical trials, patients aged 18–75 years who voluntarily participated and signed informed consent were included in the study. Inclusion of CD19 or CD20 positive DLBCL (including PMBCL and tFL) confirmed by cytology or histology according to 2016 WHO criteria. (17) For CD20-positive subjects, they should have received at least one regimen containing anti-CD20-targeted therapy (such as rituximab). And one follicular lymphoma patient and one primary central nervous system lymphoma patient were excluded.
2.3 Definitions of therapy and efficacy
The drug-eluting period between bridging therapy and initiating lymphodepletion for CAR-T therapy strictly adhered to the practice recommendations jointly issued by the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA) (18).
In accordance with the guidelines established by the U.S. Food and Drug Administration, the lymphodepletion regimen consisted of fludarabine at a dose of 25 mg/(m²*d) from day -5 to -3, and cyclophosphamide at 300 mg/(m²*d) from day -5 to -3, prior to the infusion of CD19 CAR/Bi-CAR T-cells. (7, 19, 20) The total dose of CAR-T cells ranged from 1-5^106/kg. The occurrence and severity of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity (ICANS) were documented and graded on the basis of the consensus guidelines provided by the American Society of Transplantation and Cellular Therapy (ASTCT) (21).
The Lugano classification (2014) was utilized to assess the response following the infusion of CD19 CAR T-cells and Bi-CAR T-cells. This classification relies on CT and PET-CT scans to evaluate the treatment response. (22) The response categories included complete response (CR), partial response (PR), stable disease, and progressive disease. The overall objective response (ORR) was evaluated based on the best response (CR+PR) within 3 months following CAR T-cells infusion. Progression-free survival (PFS) was defined as the interval from CAR T-cell infusion until disease progression, death from any cause, or the date of the last follow-up visit, whichever occurred first. Duration of response (DOR) referred to the time from the first assessment of CR or PR after CAR T-cell infusion to the first occurrence of disease progression or death from any cause, whichever came first. Overall survival (OS) was measured from the time of CAR T-cell infusion until death from any cause or the date of the last follow-up visit, whichever occurred first. And the follow-up period concluded on September 30, 2024.
According to the 2016 World Health Organization (WHO) classification for tumors of hematopoietic and lymphoid tissues, patients were diagnosed with DLBCL based on pathological evaluation. The classification of double-expressor lymphoma (characterized by overexpression of MYC and BCL-2 proteins) and double/triple-hit lymphoma (involving MYC and BCL2 and/or BCL6 rearrangements) followed standard diagnostic criteria. (17) The cell of origin (COO) classification, distinguishing between germinal center B-cell (GCB) and non-GCB subtypes, was determined using the Hans algorithm. (23) TP53 alterations were identified through mutations detected by next-generation sequencing (NGS) (24) or deletions observed via fluorescence in situ hybridization (FISH) analysis, based on the most recent pathological test conducted prior to CAR-T therapy (25).
2.4 Statistical analysis
The statistical analysis in this study was conducted primarily via R software (version 4.3.2, Boston, Massachusetts, USA)R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.12c, SPSS software (version 22.0, Chicago, Illinois, USA), and GraphPad Prism software (version 8.0.1). In this study, the normality test of continuous variables was performed via the Kolmogorov–Smirnov test. The Levene test was conducted for continuous variables with a normal distribution to assess homogeneity of variance. If there was no violation of the assumption of homogeneity of variance (P≥0.05), the data were reported as the mean standard deviation and analyzed via one-way ANOVA. Continuous variables with violated homogeneity of variances or nonnormal distributions are reported as medians edinterquartile ranges (IQR) and were analyzed via the Kruskal-Wallis test. The expected frequency of the cross-category in the categorical variable is not less than 5, the chi-square test is used for analysis. If the expected frequency was less than 1, then Fisher’s exact test was used for analysis. Given the exploratory nature of this retrospective study and the limited availability of CAR-T recipients during the study period, formal sample size calculation was not performed a priori. Post-hoc power analysis using the G*Power 3.1 (Z-test, two-tailed) demonstrated exceptional achieved power (98.8%) at α=0.05. (26) PFS, DOR and OS were visualized via Kaplan–Meier curves. The reported p values were two-sided, P<0.05 considered a statistically significant result.
3 Results
3.1 Baseline information
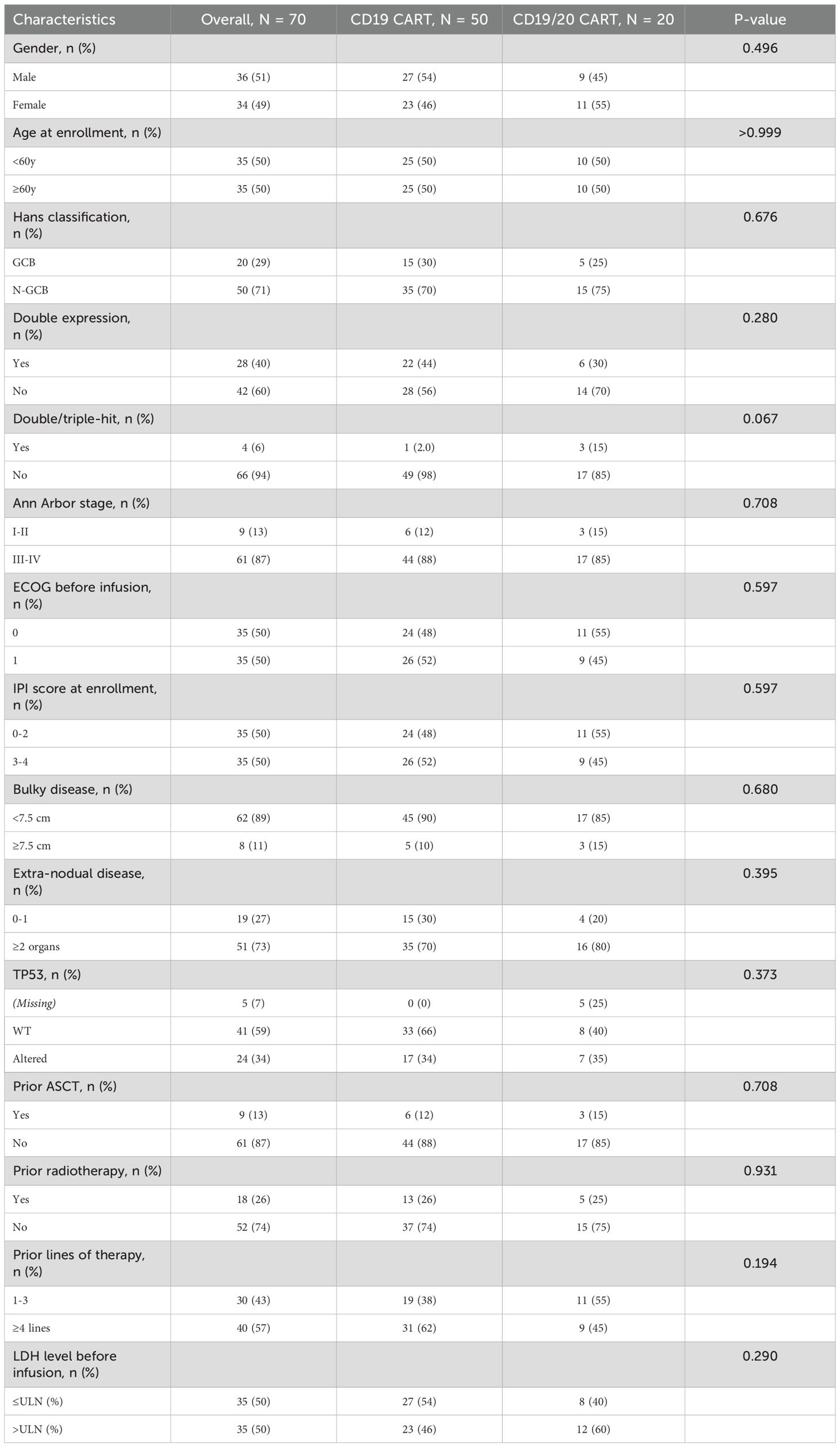
The median age of patients receiving CD19 CART therapy was 59.0 years (IQR: 49.3-67.0), the median follow-up date was 48.9 months (IQR: 40.13-58.6). While that of patients in the Bi-CART group was 58.5 years (IQR: 50.3-63.5), the median follow-up date was 52.5 months (IQR: 48.2-54.2), the median age between the two groups and the median follow-up date had no statistical difference. Baseline characteristics were well balanced between the two groups (Table 1), including general demographics [gender, age, Eastern Cooperative Oncology Group (ECOG) performance status prior to infusion], disease stage (Ann Arbor stage, proportion of extranodal disease), tumor burden [International Prognostic Index (IPI) score, percentage of bulky disease, lactate dehydrogenase (LDH) levels before infusion], tumor characteristics (Hans classification, double expression, double/triple-hit status, TP53 abnormalities), and prior treatments (percentage of prior ASCT, prior radiotherapy, and number of previous therapy lines). No significant differences were observed between the groups (P>0.05). It is worth noting that data on TP53 abnormalities were incomplete, as five patients in the Bi-CART group did not undergo NGS or FISH testing on their pathological biopsies.
In the CD19 CART group, 13 patients (26.0%) received targeted drugs with chemotherapy as bridging therapy, and 2 patients (4.0%) received maintenance therapy with targeted drugs as part of their bridging protocol. In the Bi-CART group, 4 patients (20.0%) received targeted drugs with chemotherapy as bridging therapy.
3.2 Efficacy of CAR-T therapy
The cohort comprised 56 progressive disease and 14 stable disease patients at baseline (Supplementary Figure 1). While dual-target CAR-T recipients had numerically higher PD prevalence (95% vs. 74%, P=0.054), pretreatment status showed no association with ORR (P=0.903) or CR rates (CRR, P=0.719) in regression models. Subgroup analyses by treatment type confirmed consistent response patterns regardless of baseline disease status (Supplementary Table 1). Among patients who achieved ORR within 3 months of infusion (Figure 1A), 23 (46.0%) were from the CD19 CART group, and 18 (90.0%) were from the Bi-CART group, with the difference being statistically significant (P<0.001). CRR were 15 (30.0%) and 17 (85.0%) in the CD19 CART and Bi-CART groups (Figure 1B), respectively, also showing a statistically significant difference (P<0.001).
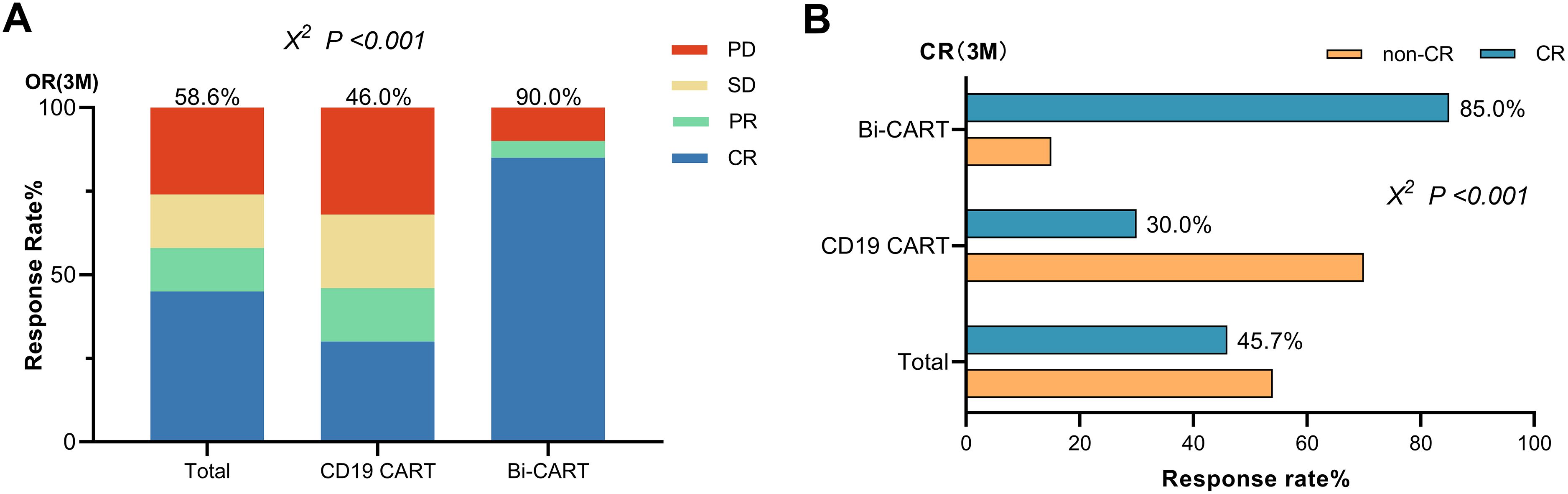
Figure 1. Efficacy of contrast in the CD19 CART and Bi-CART groups. (A) Best response (CR+PR) within 3 months of CARTs infusion (P<0.001). (B) The CR rate of the two groups within 3 months of CARTs infusion (P<0.001).
The proportion of patients suffering disease recurrence after treatment was 34 (68.0%) in the CD19 CART group and 10 (50.0%) in the Bi-CART group, with no statistically significant difference (P=0.159). Baseline characteristics of IPI score ≥3 (P<0.001) and TP53 abnormalities (P=0.007) as predictors of disease relapse (Supplementary Table 2), with no significant treatment-subgroup interactions detected (all P for interaction >0.1, Supplementary Table 3). The median PFS times were 4.0 months [95% Confidence Interval (CI): 2.4-42.9] and 32.6 months (95% CI: 11.0-Not Reach) for the CD19 CART and Bi-CART groups, respectively. The log-rank test for the PFS Kaplan-Meier curve yielded a statistic of 3.18, Hazard Ratio (HR) =0.54 (95% CI: 0.29-1.00), with a p-value of 0.074, indicating no statistically significant difference (Figure 2A). Within 3 months of achieving OR, there were 8 cases of recurrence (33.3%) in the CD19 CART group and 8 cases (44.4%) in the Bi-CART group, with no statistically significant difference (P=0.463). The median DOR in both groups was not reached (NR). The log-rank test for the DOR Kaplan-Meier curve produced a statistic of 0.42, HR = 1.38 (95% CI: 0.51-3.73), with a P-value of 0.516, indicating no statistically significant difference (Figure 2B). Deaths occurred in 28 patients (56.0%) in the CD19 CART group and 8 patients (40.0%) in the Bi-CART group, with no statistically significant difference (P=0.226). The median OS times were 22.1 months (95% CI: 11.8-NR) for CD19 CART and 53.9 months (95% CI: 51.8-NR) for Bi-CART. The log-rank test for the OS Kaplan-Meier curve yielded a statistic of 3.10, HR = 0.50 (95% CI: 0.25-0.99), with a P-value of 0.078, indicating no statistically significant difference (Figure 2C). While the extension of OR and median PFS within 3 months suggests an enhanced disease response, the depth of response remains uncertain due to recurrent events, relapses after achieving OR, and the lack of a difference in DOR.
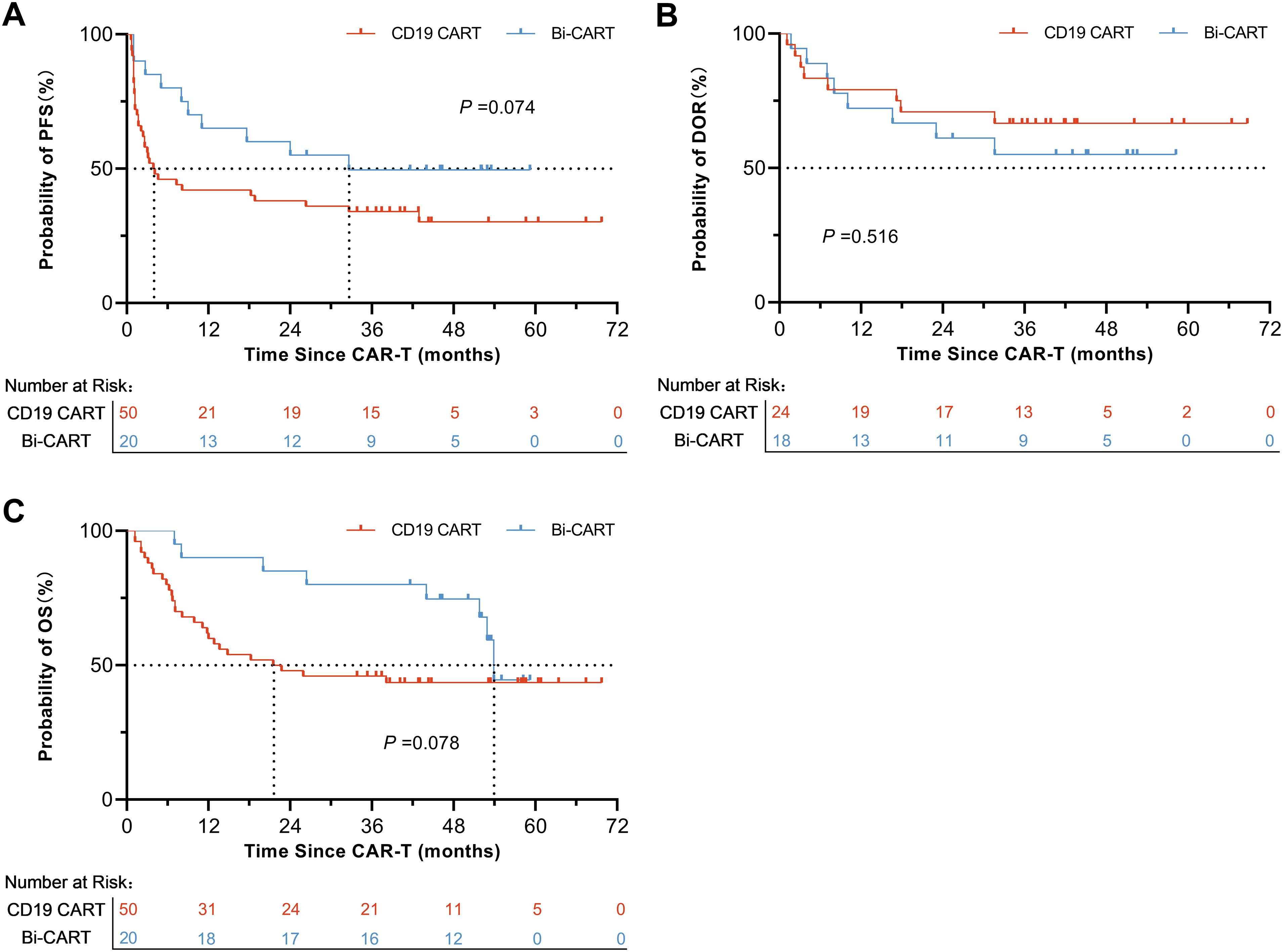
Figure 2. Prognosis of contrast in the CD19 CART and Bi-CART groups. Survival of patients with r/r DLBCL treated with CD19 CAR-T, (A) PFS (log-rank test P=0.074), (B) DOR (log-rank test P=0.516) and (C) OS (log-rank test P=0.076).
All deaths in the CD19 CART group were due to disease progression. In the Bi-CART group, two patients died from causes unrelated to primary disease progression: one from acute myeloid leukemia (AML) at 26.4 months and another from a cerebrovascular accident at 40.8 months.
3.3 Adverse reactions of CAR-T therapy
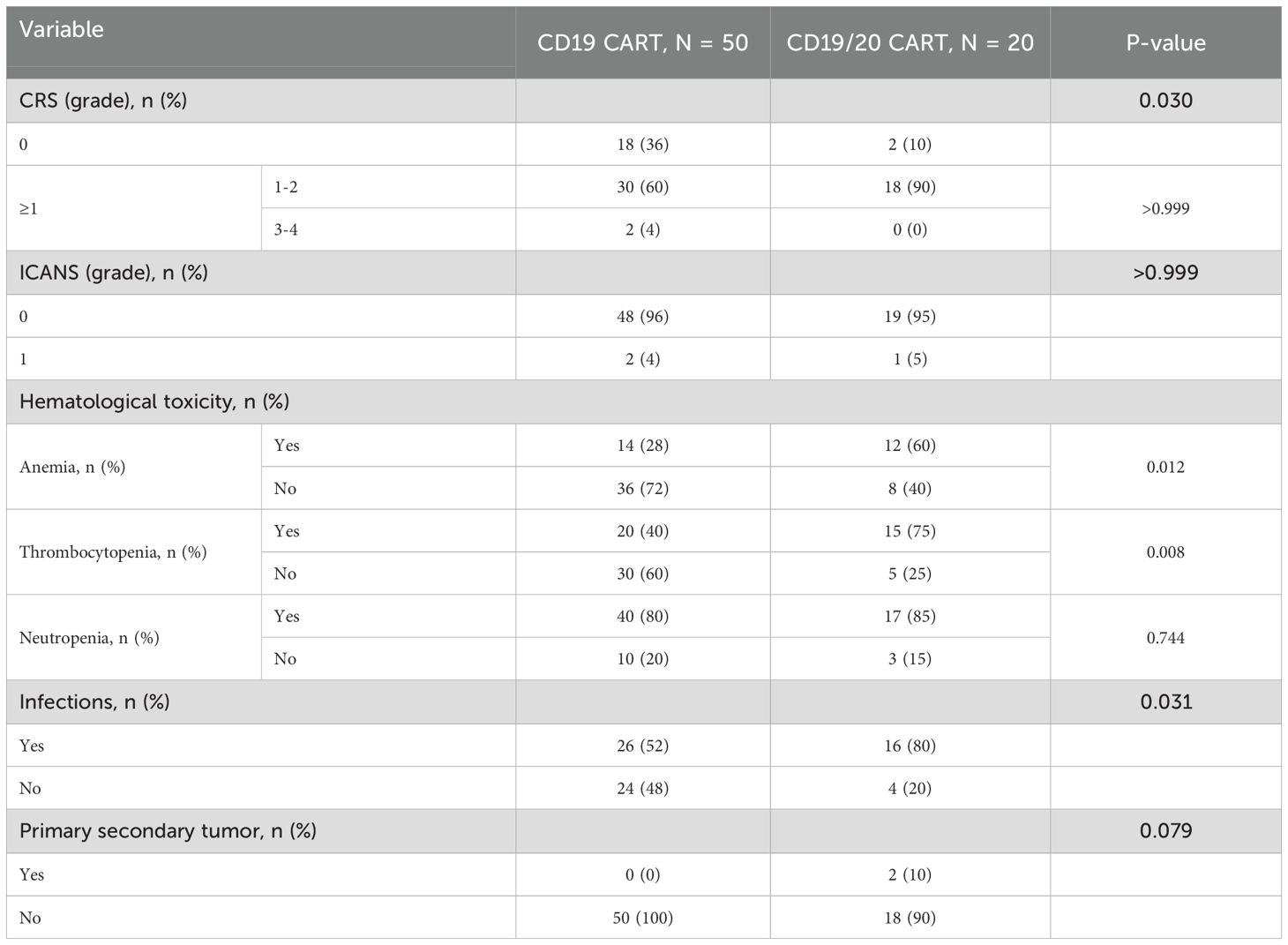
CRS occurred in 32 patients (64.0%) in the CD19 CART group and 18 patients (90.0%) in the Bi-CART group (Table 2), with a statistically significant difference (P=0.030). Grade 3–4 CRS was observed in 2 patients (4.0%) in the CD19 CART group, while no cases were reported in the Bi-CART group (P>0.999). The median time to CRS onset in the CD19 CART group was 5.5 days (IQR: 0.8-8.0 days), and the median duration was 5.5 days (IQR: 3.8-10.2 days). For the Bi-CART group, the median time (4.0 days, IQR: 2.0-7.8 days) to CRS onset was not significantly different (P=0.943), with a median duration of 6.5 days (IQR: 3.3-8.0 days, P= 0.887). Grade 1 ICANS was observed in 2 patients (4.0%) in the CD19 CART group and 1 patient (5.0%) in the Bi-CART group, with no statistically significant difference (P>0.999). In the CD19 CAR-T group, ICANS occurred on Day 5 (lasting 1 day) and Day 23 (lasting 4 days). In the Bi-CART group, ICANS occurred on Day 18 and lasted 2 days. All cases of CRS and ICANS were managed according to the ASTCT and 2022 Chinese consensus guidelines (27) using Nonsteroidal Anti-inflammatory Drugs (NSAIDs), Corticosteroids, and Tocilizumab, with no deaths occurring during these reactions. All events resolved completely under protocolized management; the occurrence, severity and management of CRS were not associated with clinical outcomes (Supplementary Table 4).
After the infusion of CAR T-cells, anemia occurred in 14 patients (28.0%) in the CD19 CART group and 12 patients (60.0%) in the Bi-CART group, with a statistically significant difference (P=0.012). Thrombocytopenia occurred in 20 patients (40.0%) in the CD19 CART group and 15 patients (75.0%) in the Bi-CART group, with a significant difference (P=0.008); Neutropenia occurred in 40 patients (80.0%) in the CD19 CART group and 17 patients (85.0%) in the Bi-CART group, with no significant difference (P=0.744). Infections occurred in 26 patients (52.0%) in the CD19 CART group and 16 patients (80.0%) in the Bi-CART group, showing a statistically significant difference (P=0.031). Two patients (10.0%) in the Bi-CART group developed secondary primary malignancies. One patient developed AML in the 10th month after treatment and later died. The other developed Epstein-Barr virus-positive cytotoxic T-cell lymphoma at 8 months, with no CAR transgene detected via tumor biopsy (qPCR). No cases of secondary malignancies were observed in the CD19 CART group (P=0.079).
3.4 Treatment following CAR-T therapy
The treatment strategy for the entire study cohort is illustrated in the treatment thread diagram (Figure 3). All patients who experienced relapses within 3 months of CAR-T therapy, as well as those with subsequent relapses, received later-line therapy, except for 4 patients with ultra-rapid disease progression who only underwent life-sustaining treatment. As for later-line treatments, 25 patients received a new targeted immunotherapy combined with chemotherapy; 13 patients were treated with a novel CAR-T therapy, and 1 patient received a novel CAR-T therapy combined with autologous stem cell transplantation (ASCT); 6 patients received maintenance therapies, including immune checkpoint inhibitors (ICIs), Bruton’s tyrosine kinase (BTK) inhibitors, Lenalidomide, and Sidanidine. Follow-up information was missing for 3 patients who experienced disease progression after CAR-T therapy.
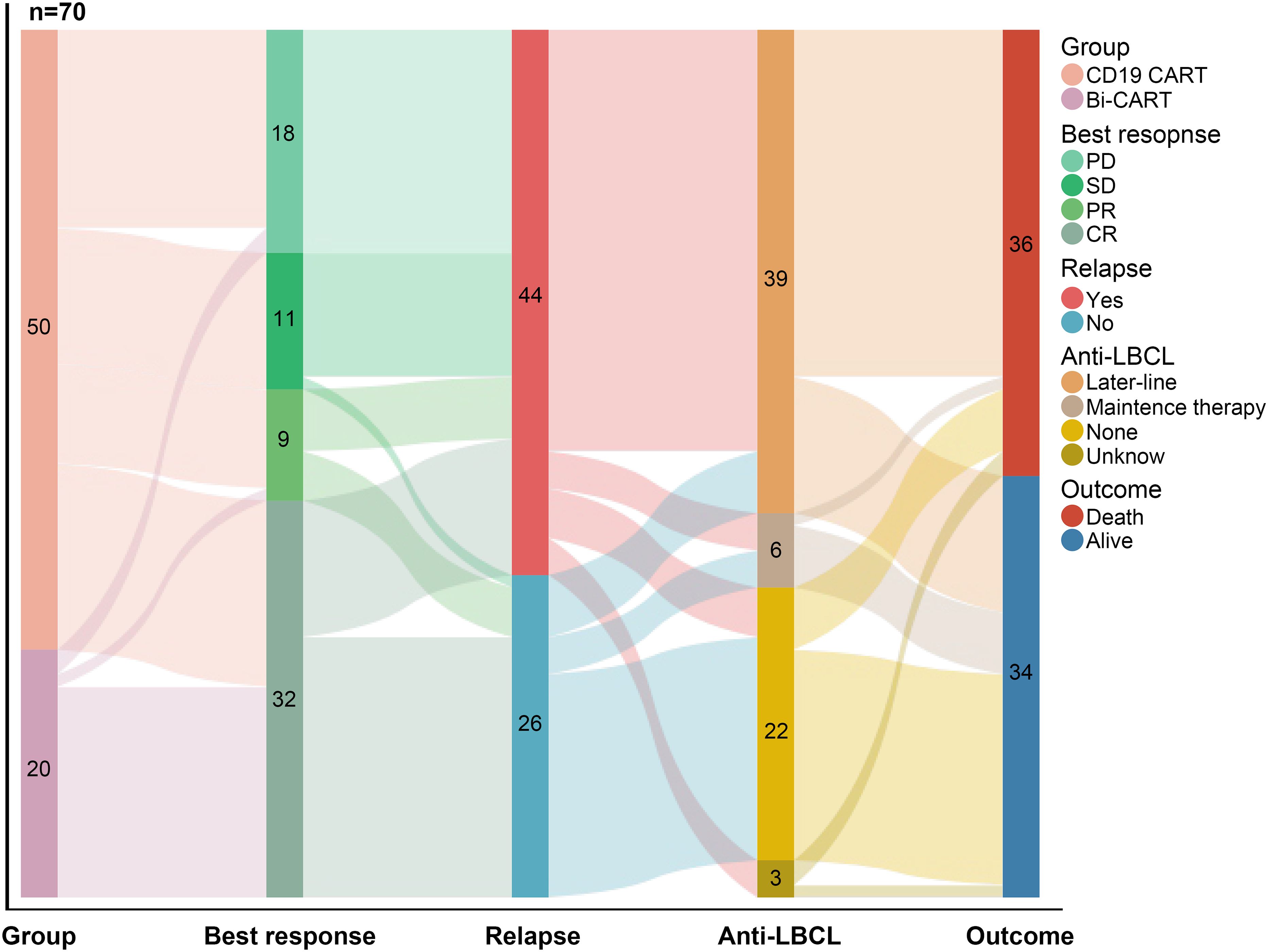
Figure 3. The treatment thread diagram for the entire study cohort. As of 2024/09/30, of the 70 patients, 41 achieved OR, 44 experienced disease recurrence, 45 received continued treatment, and 36 eventually died.
4 Discussion
CD19 CAR-T therapy has demonstrated remarkable advancements over traditional chemotherapy, offering new hope to patients with R/R DLBCL patients. (7, 19, 28–31) Despite these successes, our previous research indicates that CD19 CAR-T therapy remains ineffective for certain patients. (32) To overcome resistance and antigen escape in R/R DLBCL, research is focusing on durable strategies, including alternative antigen targeting (CD20, CD22, CD70), combination therapies, and next-generation CAR-T or bispecific antibodies (CD19/20, CD19/22, CD19/70) to expand treatment options for CD19-negative relapses. (33–38) Dual-target CAR-T therapy may outperform single-target approaches by broadening tumor cell elimination, enhancing T-cell activation, extending CAR-T persistence for long-term surveillance, and improving immune penetration to strengthen anti-tumor efficacy (39, 40).
Notably, only one two-arm study has been reported so far, and dual-target studies showed no significant improvement in complete response or recurrence rates compared to single-target studies. (41) Other existing research and review articles primarily compare patients across different studies, which lack methodological rigor. (40) For the first time, we conducted a retrospective comparison of single-target CD19 CART and dual-target CART in both arms with long-term follow-up of more than 5 years. Results indicate that CD19/20 CART therapy achieves superior short-term efficacy compared to CD19 CART, as evidenced by metrics such as the best response at 3 months, median PFS, and median OS. However, dual-target CART does not show a significant advantage in terms of long-term survival. The dissociation between early CR superiority and comparable long-term outcomes in dual-target therapy suggests distinct biological mechanisms governing initial response versus sustained remission. While enhanced antigen coverage may improve tumor clearance efficiency, TP53-driven genomic instability and high tumor burden appear to ultimately determine relapse risk through CAR-T-resistant clonal evolution (39, 40, 42, 43). A comparison between the DOR curves of the two groups could also confirm this argument, with CAR-T responders achieving sustained remission regardless of target configuration.
Integrating dual-target CAR-T therapy into current treatment regimens demands robust evidence of its safety profile. Clinical trials and real-world studies are essential to assess potential side effects, particularly CRS and ICANS. Previous studies have indicated that CD19 CAR-T therapy is an independent factor contributing to the development of CRS, potentially resulting in more severe levels of CRS. (41) Our study showed that CD19/20 CART had a higher probability of CRS, but there was no significant difference in the severity or duration of CRS between the two groups, nor was there a significant difference in the incidence of ICANS. Further analysis of CRS grade and treatment intensity was not associated with CART response. The dissociation between CRS incidence and severity highlights that risk-adapted management (27) can effectively mitigate severe toxicity while preserving anti-tumor efficacy.
Another point of note is that patients who received CD19/20 CART had a higher incidence of primary secondary tumor (2 in 20, although no statistical difference). The big data analysis of the FDA Adverse Events Reporting System sheds light on the increased reporting of myeloid neoplasms, T-cell lymphomas, and certain types of solid tumor after commercial CART in 2024 (4.3%, 536 of 12,394). Considering the imbalance of analysis and the low incidence, secondary tumor cannot be considered directly related to CART. (44) Case reports describe CART-associated T-cell lymphomas potentially linked to viral vector integration mutagenesis, though comprehensive genomic analyses suggest different characteristics rather than direct CART causality. (45, 46) In our study, the two secondary malignancies observed in CD19/20 CART recipients were not considered to be related to CART, because no CAR transgene was detected via tumor biopsy. At present, there are few clinical retrospective statistics on secondary tumors after dual-target CART, but some studies suggest that the design of dual-target CART may increase the risk of insertional mutagenesis (47) and replicative stress (48). Secondary tumor occurrence of single-/dual-target CART will be reported in further follow-up.
Several limitations of this study should be acknowledged. First, the single-center cohort and relatively small sample size may restrict the generalizability of our findings to broader populations, and unmeasured confounding factors inherent to retrospective designs cannot be fully excluded. While post-hoc analyses suggested sufficient statistical power (98.8%) to detect the observed ORR difference, larger prospective cohorts are needed to validate subgroup findings and long-term outcomes. Second, the inability to control post-relapse therapeutic heterogeneity, such as secondary CAR-T reinfusion or conventional salvage chemotherapy, prevents definitive assessment of how subsequent interventions modulate long-term survival outcomes. Third, inter-group CAR-T platform disparities (multi-trial CD19 products vs. uniform CD19/20 bi-specific constructs) introduce confounding from divergent manufacturing protocols and pharmacokinetic behaviors.
To address these limitations, future prospective multicenter randomized controlled trials are warranted. Such studies should prioritize (a) standardized patient stratification based on tumor burden, prior treatment lines, and molecular biomarkers; (b) protocol-defined allocation of relapse interventions (e.g., randomized assignment to secondary CAR-T or chemotherapy) with rigorous adjustment for baseline prognostic variables; and (c) harmonized therapeutic protocols across institutions to minimize inter-center variability. This will help clinicians select more precise and less harmful CAR-T therapy options for patients with relapsed/refractory disease.
5 Conclusion
For the first time, our study demonstrates that dual-target CAR T-cell therapy (CD19/20) achieves a superior therapeutic compared to single-target CAR T-cell therapy (CD19) in the treatment of R/R DLBCL, significantly extending median survival. However, it is associated with a higher incidence of adverse effects, including CRS, hematological toxicity, infections, and secondary primary tumors. While the integration of dual-target CAR T-cell therapy into the DLBCL treatment landscape holds great promise, further optimization is essential. This will help enhance tumor remission rates while mitigating adverse effects, ultimately improving patient outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shanghai Tongji Hospital, Tongji University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
BX: Writing – original draft, Writing – review & editing. YFL: Writing – original draft, Writing – review & editing. BL: Writing – original draft, Writing – review & editing. YL: Data curation, Writing – original draft. LZ: Data curation, Writing – original draft. SY: Formal analysis, Writing – original draft. HL: Software, Writing – original draft. XL: Writing – review & editing. AL: Writing – review & editing. PL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by funds from the National Key R&D Program of China (2021YFA1100800), National Natural Science Foundation of China (Nos. U23A20418 and 82470225), and Foundation of Shanghai Shen Kang Hospital Development Center (SHDC 2023CRD021).
Acknowledgments
We thank all the patients who participated in this study, as well as clinical research unit colleagues who provided support for this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1582944/full#supplementary-material
References
1. Garg M, Takyar J, Dhawan A, Saggu G, Agrawal N, Hall A, et al. Diffuse large B-cell lymphoma (DLBCL): A structured literature review of the epidemiology, treatment guidelines, and real-world treatment patterns. Blood. (2022) 140:12106–7. doi: 10.1182/blood-2022-169045
2. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. (2002) 346:235–42. doi: 10.1056/NEJMoa011795
3. Fried S, Avigdor A, Bielorai B, Meir A, Besser MJ, Schachter J, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. (2019) 54:1643–50. doi: 10.1038/s41409-019-0487-3
4. Poletto S, Novo M, Paruzzo L, Frascione PMM, Vitolo U. Treatment strategies for patients with diffuse large B-cell lymphoma. Cancer Treat Rev. (2022) 110:102443. doi: 10.1016/j.ctrv.2022.102443
5. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. (2017) 377:2531–44. doi: 10.1056/NEJMoa1707447
6. Ayuk F, Gagelmann N, Von Tresckow B, Wulf G, Rejeski K, Stelljes M, et al. Real-world results of CAR T-cell therapy for large B-cell lymphoma with CNS involvement: a GLA/DRST study. Blood Adv. (2023) 7:5316–9. doi: 10.1182/bloodadvances.2023010336
7. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. (2020) 396:839–52. doi: 10.1016/S0140-6736(20)31366-0
8. Paillassa J, Di Blasi R, Chevret S, Bernard S, Darmon M, Meignin V, et al. CD19 CAR T-cell therapy in patients with relapse/refractory DLBCL: retrospective analysis of the eligibility criteria. Blood. (2019) 134:2887–7. doi: 10.1182/blood-2019-129532
9. Plaks V, Rossi JM, Chou J, Wang L, Poddar S, Han G, et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood. (2021) 138:1081–5. doi: 10.1182/blood.2021010930
10. Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. (2017) 377:2545–54. doi: 10.1056/NEJMoa1708566
11. Neelapu SS, Jacobson CA, Ghobadi A, Miklos DB, Lekakis LJ, Oluwole OO, et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood. (2023) 141(19):2307–15. doi: 10.1182/blood.2022018893
12. Jackson HJ, Brentjens RJ. Overcoming antigen escape with CAR T-cell therapy. Cancer Discovery. (2015) 5:1238–40. doi: 10.1158/2159-8290.CD-15-1275
13. Ma Y, Dai H, Cui Q, Liu S, Kang L, Lian X, et al. Decitabine in combination with fludarabine and cyclophosphamide as a lymphodepletion regimen followed by CD19/CD22 bispecific targeted CAR T-cell therapy significantly improves survival in relapsed/refractory B-ALL patients. Exp Hematol Oncol. (2023) 12:36. doi: 10.1186/s40164-023-00397-z
14. Yang J, Guo H, Han L, Song Y, Zhou K. Dual-targeted CAR T-cell immunotherapies for hematological Malignancies: latest updates from the 2023 ASH annual meeting. Exp Hematol Oncol. (2024) 13:25. doi: 10.1186/s40164-024-00485-8
15. Li P, Yu W-J, Zhou L, Yang M, Ye S, Zhu J, et al. C-CAR039, a novel anti-CD20/CD19 bi-specific CAR T-cell therapy shows deep and durable clinical benefits in patients with relapsed or refractory (r/r) B-cell non-Hodgkin lymphoma (B-NHL) in long term follow up. Blood. (2023) 142:1025–5. doi: 10.1182/blood-2023-182817
16. Yu W, Li P, Zhou L, Yang M, Ye S, Zhu D, et al. A phase 1 trial of prizloncabtagene autoleucel, a CD19/CD20 CAR T-cell therapy for relapsed/refractory B-cell non-Hodgkin lymphoma. Blood. (2025) 145(14):1526–1535. doi: 10.1182/blood.2024026401
17. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569
18. Hayden PJ, Roddie C, Bader P, Basak GW, Bonig H, Bonini C, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann Oncol. (2022) 33:259–75. doi: 10.1016/j.annonc.2021.12.003
19. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, Mcguirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. (2019) 380:45–56. doi: 10.1056/NEJMoa1804980
20. Ying Z, Xie Y, Zheng W, Liu W, Lin N, Tu M, et al. Efficacy and safety of relmacabtagene autoleucel, an anti-CD19 chimeric antigen receptor T cell, in relapsed/refractory B-cell non-Hodgkin’s lymphoma: 2-year results of a phase 1 trial. Bone Marrow Transplant. (2023) 58:288–94. doi: 10.1038/s41409-022-01888-z
21. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. (2019) 25:625–38. doi: 10.1016/j.bbmt.2018.12.758
22. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. (2014) 32:3059–68. doi: 10.1200/JCO.2013.54.8800
23. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. (2004) 103:275–82. doi: 10.1182/blood-2003-05-1545
24. Hayashi H, Takiguchi Y, Minami H, Akiyoshi K, Segawa Y, Ueda H, et al. Site-specific and targeted therapy based on molecular profiling by next-generation sequencing for cancer of unknown primary site: A nonrandomized phase 2 clinical trial. JAMA Oncol. (2020) 6:1931–8. doi: 10.1001/jamaoncol.2020.4643
25. Fioretos T, Strömbeck B, Sandberg T, Johansson B, Billström R, Borg A, et al. Isochromosome 17q in blast crisis of chronic myeloid leukemia and in other hematologic Malignancies is the result of clustered breakpoints in 17p11 and is not associated with coding TP53 mutations. Blood. (1999) 94:225–32. doi: 10.1182/blood.V94.1.225.413k24_225_232
26. Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/BF03193146
27. Li P, Liu Y, Liang Y, Bo J, Gao S, Hu Y, et al. 2022 Chinese expert consensus and guidelines on clinical management of toxicity in anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Cancer Biol Med. (2023) 20:129–46. doi: 10.20892/j.issn.2095-3941.2022.0585
28. Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. (2022) 399:2294–308. doi: 10.1016/S0140-6736(22)00662-6
29. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. (2022) 386:640–54. doi: 10.1056/NEJMoa2116133
30. Cappell KM, Sherry RM, Yang JC, Goff SL, Vanasse DA, Mcintyre L, et al. Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy. J Clin Oncol. (2020) 38:3805–15. doi: 10.1200/JCO.20.01467
31. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. (2019) 20:31–42. doi: 10.1016/S1470-2045(18)30864-7
32. Xue B, Liu Y, Zhou J, Zhou L, Ye S, Lu Y, et al. CD19 CAR-T treatment shows limited efficacy in r/r DLBCL with double expression and TP53 alterations. Cytotherapy. (2024) 26(12):1465–1471. doi: 10.1016/j.jcyt.2024.07.011
33. Xu X, Sun Q, Liang X, Chen Z, Zhang X, Zhou X, et al. Mechanisms of relapse after CD19 CAR T-cell therapy for acute lymphoblastic leukemia and its prevention and treatment strategies. Front Immunol. (2019) 10:2664. doi: 10.3389/fimmu.2019.02664
34. Dourthe ME, Rabian F, Yakouben K, Chevillon F, Cabannes-Hamy A, MéChinaud F, et al. Determinants of CD19-positive vs CD19-negative relapse after tisagenlecleucel for B-cell acute lymphoblastic leukemia. Leukemia. (2021) 35:3383–93. doi: 10.1038/s41375-021-01281-7
35. Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. (2021) 398:491–502. doi: 10.1016/S0140-6736(21)01222-8
36. Pan J, Tang K, Luo Y, Seery S, Tan Y, Deng B, et al. Sequential CD19 and CD22 chimeric antigen receptor T-cell therapy for childhood refractory or relapsed B-cell acute lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. (2023) 24:1229–41. doi: 10.1016/S1470-2045(23)00436-9
37. Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. (2021) 21:145–61. doi: 10.1038/s41568-020-00323-z
38. Zhang Y, Wang Y, Liu Y, Tong C, Wang C, Guo Y, et al. Long-term activity of tandem CD19/CD20 CAR therapy in refractory/relapsed B-cell lymphoma: a single-arm, phase 1–2 trial. Leukemia. (2022) 36:189–96. doi: 10.1038/s41375-021-01345-8
39. Tong C, Zhang Y, Liu Y, Ji X, Zhang W, Guo Y, et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma. Blood. (2020) 136:1632–44. doi: 10.1182/blood.2020005278
40. Xie B, Li Z, Zhou J, Wang W. Current status and perspectives of dual-targeting chimeric antigen receptor T-cell therapy for the treatment of hematological Malignancies. Cancers (Basel). (2022) 14(13):3230. doi: 10.3390/cancers14133230
41. Wang Y, Yang Y, Hong R, Zhao H, Wei G, Wu W, et al. A retrospective comparison of CD19 single and CD19/CD22 bispecific targeted chimeric antigen receptor T cell therapy in patients with relapsed/refractory acute lymphoblastic leukemia. Blood Cancer J. (2020) 10:105. doi: 10.1038/s41408-020-00371-6
42. Shouval R, Alarcon Tomas A, Fein JA, Flynn JR, Markovits E, Mayer S, et al. Impact of TP53 genomic alterations in large B-cell lymphoma treated with CD19-chimeric antigen receptor T-cell therapy. J Clin Oncol. (2022) 40:369–81. doi: 10.1200/JCO.21.02143
43. Locke FL, Rossi JM, Neelapu SS, Jacobson CA, Miklos DB, Ghobadi A, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. (2020) 4:4898–911. doi: 10.1182/bloodadvances.2020002394
44. Elsallab M, Ellithi M, Lunning MA, D’angelo C, Ma J, Perales M-A, et al. Second primary Malignancies after commercial CAR T-cell therapy: analysis of the FDA Adverse Events Reporting System. Blood. (2024) 143:2099–105. doi: 10.1182/blood.2024024166
45. Hamilton MP, Sugio T, Noordenbos T, Shi S, Bulterys PL, Liu CL, et al. Risk of second tumors and T-cell lymphoma after CAR T-cell therapy. N Engl J Med. (2024) 390:2047–60. doi: 10.1056/NEJMoa2401361
46. Ghilardi G, Fraietta JA, Gerson JN, Van Deerlin VM, Morrissette JJD, Caponetti GC, et al. T cell lymphoma and secondary primary Malignancy risk after commercial CAR T cell therapy. Nat Med. (2024) 30:984–9. doi: 10.1038/s41591-024-02826-w
47. Braun T, Rade M, Merz M, Klepzig H, Große F, Fandrei D, et al. Multiomic profiling of T cell lymphoma after therapy with anti-BCMA CAR T cells and GPRC5D-directed bispecific antibody. Nat Med. (2025) 31(4):1145–1153. doi: 10.1038/s41591-025-03499-9
Keywords: CAR-T, lymphoma, dual-target, CD19, CD19/20
Citation: Xue B, Liu Y, Li B, Lu Y, Zhou L, Ye S, Lu H, Luo X, Liang A and Li P (2025) Comparison of efficacy and adverse effects of CD19/20 CART versus CD19 single-target CART in R/R DLBCL: a single-center retrospective study. Front. Immunol. 16:1582944. doi: 10.3389/fimmu.2025.1582944
Received: 25 February 2025; Accepted: 14 April 2025;
Published: 06 May 2025.
Edited by:
Renata Stripecke, University Hospital of Cologne, GermanyReviewed by:
Ravi Kumar Sharma, All India Institute of Medical Sciences Bilaspur, IndiaChristian Augsberger, GSK, Germany
Copyright © 2025 Xue, Liu, Li, Lu, Zhou, Ye, Lu, Luo, Liang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiu Luo, bHVveWluZ2JpbmZlbjIwMDRAMTI2LmNvbQ==; Aibin Liang, bGFiNzE4MkB0b25namkuZWR1LmNu; Ping Li, bGlseWZvcmV2ZXI3NkAxMjYuY29t
†These authors have contributed equally to this work
 Bin Xue
Bin Xue Yifan Liu
Yifan Liu Bing Li
Bing Li Yan Lu1
Yan Lu1 Lili Zhou
Lili Zhou Shiguang Ye
Shiguang Ye Aibin Liang
Aibin Liang Ping Li
Ping Li