- 1Department of General Surgery (Key Disciplines of Medicine in Quzhou City), Longyou County People’s Hospital, Longyou People’s Hospital Affiliated with Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Quzhou, China
- 2Department of Gastroenterology, Longyou County People’s Hospital, Quzhou, China
- 3Department of Hepatobiliary Surgery, Quzhou City People’s Hospital, Quzhou, China
Objective: To illuminate the pathological characteristics and treatment of synchronous pancreatic sarcomatoid carcinoma(SCP) and gastric cancer using a case report and comprehensive literature review.
Methods: Clinical presentation, pathological findings and immunohistochemical results of a patient with SCP and gastric cancer were reviewed. A surgical treatment strategy with best supportive care, including nutrition support, was adopted. A comprehensive literature review was conducted.
Results: A 69-year-old female patient presented to the general surgery clinic with ten days of abdominal pain. A Type IIc lesion measuring 30 mm in diameter was found in the gastric angle. Additionally,a thick-walled, solid mass with dimensions of 33 mm by 30 mm was detected in the pancreatic tail. HE staining revealed a moderately to poorly differentiated adenocarcinoma and partial mucinous adenocarcinoma in gastric angle and a sarcomatoid carcinoma with low-grade adenocarcinoma components in pancreatic tail. Immunohistochemical assay of the mass in pancreatic tail showed partial positivity for CK(pan), CK7, CD56, CD138, CEA, EMA, and Ki-67 (60%). The patient’s serum levels of CA199, CEA, CA125, and CA242 were sharply decreased postoperatively but remained above normal limits. However, these markers were predominantly increased in the presence of multiple liver metastases.Malnutrition and progressive disease occurred despite the adoption of EN plus PN and best supportive care, and the patient died on postoperative day 107.
Conclusions: Synchronous SCP and gastric cancer are first reported in this study. Surgical treatment remains effective despite an even poorer prognosis.
1 Introduction
In 2022, 968,350 patients were initially diagnosed with stomach cancer, and 659,853 died from it. Meanwhile, pancreatic cancer had 510,566 new cases and caused 467,005 deaths (1). The 5-year overall survival rate for pancreatic cancer was approximately 2-9%, due to limited treatment options (1). A total of 15 case reports on synchronous stomach cancer and pancreatic cancer have been discribed until now (2, 3) (Table 1). No reports on stomach cancer and pancreatic sarcomatoid carcinoma (SCP)have been found. Here is the case report and literature review.
The article is presented in line with the CARE reporting checklist (Supplementary Material).
2 Case presentation
2.1 General information
A 69-year-old female patient presented to the general surgery clinic with ten days of abdominal pain (+0 day). She experienced epigastric dull pain for 10 days, characterized by paroxysmal onset, more prominent at night and during the postprandial period, and relieved after sitting for 30 minutes. There was no radiation of pain to other areas, no acid regurgitation or belching, no nausea or vomiting, no hematemesis or melena. No risk factors for gastric cancer such as alcohol consumption, smoking, intake of salted and smoked foods, or gastroesophageal reflux disease were found. He had no history of psychosocial issues, oncological conditios, or genetic diseases, nor any relevant family history.
Upon physical examination, no signs of anemia, jaundice of the skin and sclera, enlargement of cervical or supraclavicular lymph nodes, liver palms, or spider nevi were detected. The abdomen was flat with no visible gastrointestinal or peristaltic waves, varicose veins, hepatomegaly, splenomegaly, shifting dullness, tenderness, rebound tenderness, or muscular tension.
Sonographically, a hypoechoic mass in the splenorenal space measured 33 mm by 30 mm. The lesion was poorly defined, with neither obvious motility nor significant blood flow signals (+0 day, Figure 1A). A CT scan demonstrated a low-density lesion in the pancreatic tail (+0 day, Figures 1B–D). Under gastroscopy, a Type IIc lesion measuring 30 mm in diameter was found on the posterior wall at the gastric angle.It showed an irregular microvascular pattern (IMVP), an irregular microsurface pattern (IMSP), and a distinct boundary line (DL)(+0 day, Figures 2A–D). Biopsies were taken with cold forceps for histology (+0 day).
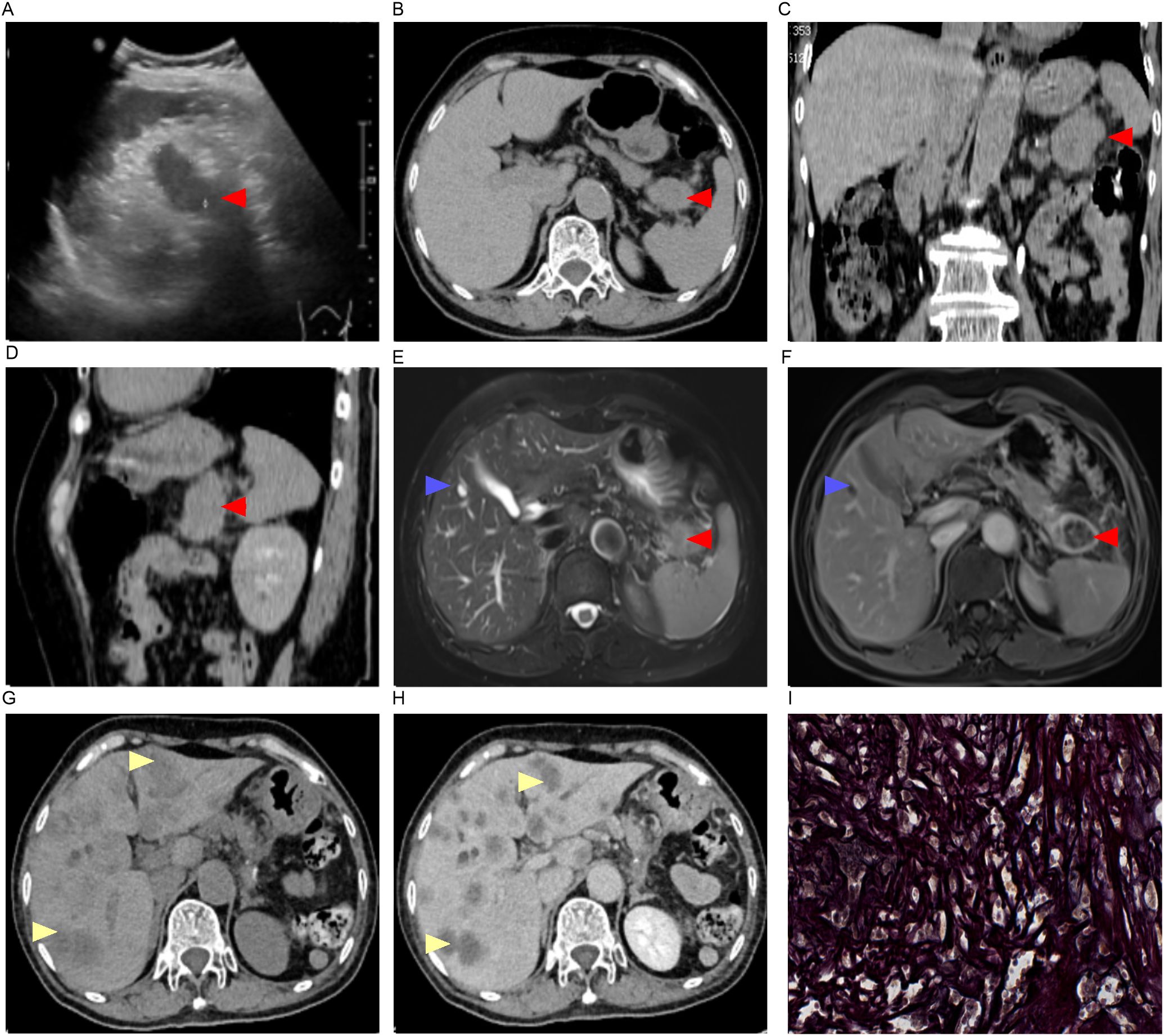
Figure 1. Imaging findings and pathological manifestations. (A) Ultrasound imaging (+0 day);(B–D), CT imaging [(C), coronal; (D), sagittal +0 day]; (E, F), MRI imaging (+1 day); (G, H), CT imaging (+56 day); (I) Reticular fiber staining of pancreatic tail tumor (–);Red arrow, pancreatic mass; Blue arrow, hepatic cyst; Yellow arrow, liver metastasis.
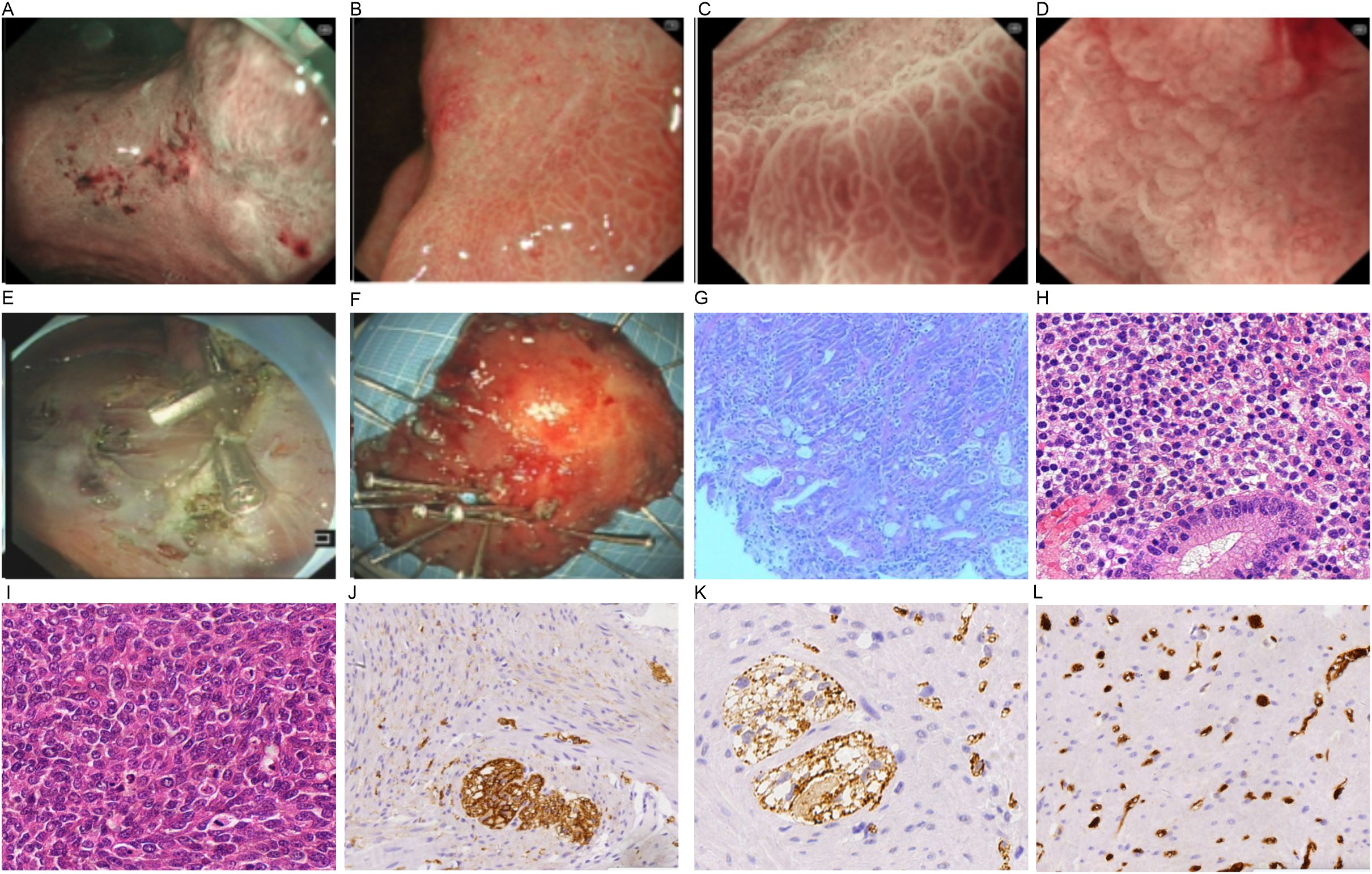
Figure 2. Endoscopic imaging (A–F) and pathological findings (G, H). (A) lesion in the gastric antrum; (B) DL (+); (C) IMVP(+); (D) IMSP(+); (E) endoscopic imaging after ESD; (F) specimen from ESD; (G–l) pathological manifestations [(G) gastric biopsy under endoscopy, (H) specimen from ESD, (I) tumor in pancreatic tail]; (J) CD68(+); (K) CK (pan) (+); (I) Ki67 (partial +).
The patient was admitted to the general surgical department with synchronous lesions: early gastric cancer at the gastric angle (T1N0M0, Stage I) and a pancreatic mass (+0 day).
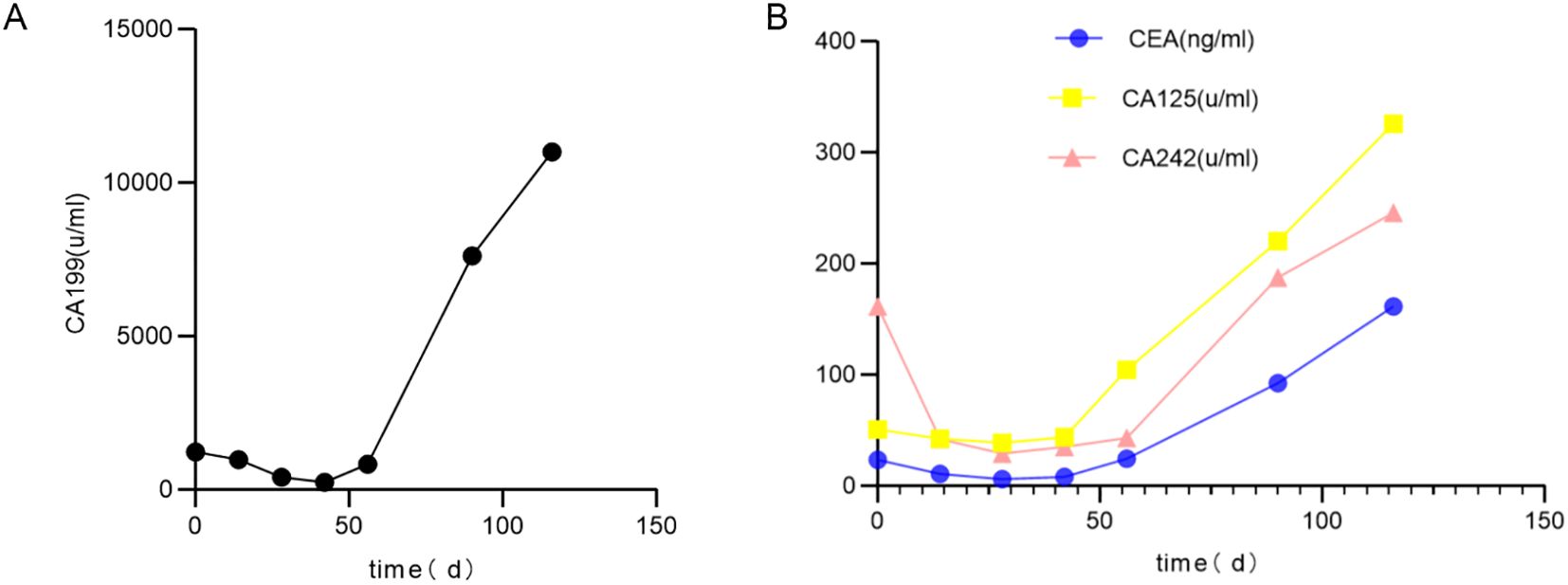
On Day 0, the serum levels of CA199, CEA, CA125, and CA242 were elevated (Figures 3A, B). The levels of CA125, AFP, CA724, CA50, and CA153 were found to be within normal ranges. The contrast -enhanced MRI identified a 21 mm intrahepatic cyst and a thick-walled, solid mass in the pancreatic tail. The mass measures 33 mm by 30 mm with mild to moderate heterogeneous enhancement. As such, a solid neoplasm in the pancreatic tail and a hepatic cyst were initially considered. (+1 day, Figures 1E, F). No enlarged lymph nodes around the stomach, pancreas, spleen or in postperiternium were delineated in ultrasonography, CT, and MRI (Figures 1A–F).
2.2 Treatment
According to the eighth edition of TNM classification system for gastric carcinoma (17), the patient was preoperatively staged as T1N0M0 (stomach cancer) and a solid neoplasm in pancreatic tail. The treatment strategy was confirmed to be gastric endoscopic submucosal dissection (ESD) combined with laparoscopic resection of the pancreatic tail and body, as determined by a multidisciplinary discussion. (+7 days, Figure 4).
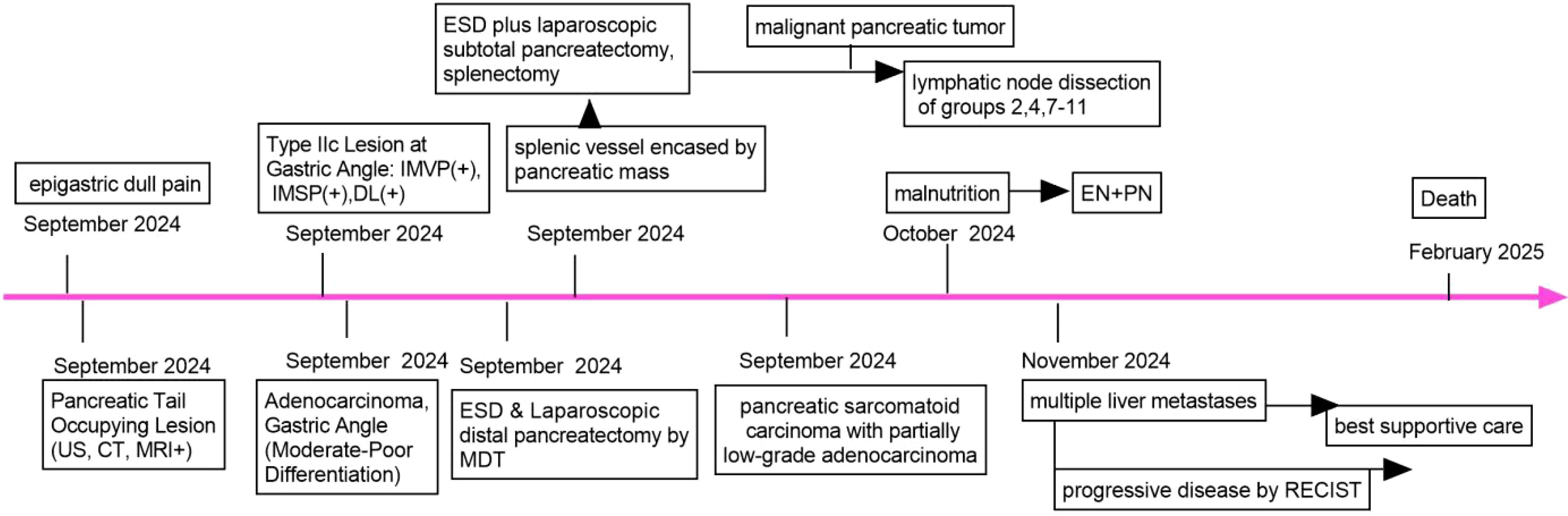
Figure 4. Timeline of the case report. US, ultrasound; CT, computer tomography; MRI, magnetic resonance imaging; EN, enteral nutrition; PN, parenteral nutrition; ESD, endoscopic submucosal dissection; IMVP, irregular microvascular pattern; IMSP,irregular microsurface pattern; DL, distinct boundary line; MDT, Multidisciplinary Discussion; RECIST, response evaluation criteria in solid tumors.
The patient underwent general anesthesia (+9 days). Upon ME-NBI observation, a 3.0 cm × 3.0 cm lesion within the resected area at the gastric angle was noted as IMSP+, IMVP+, and DL+ (Figures 2B–D). As depicted in Figures 2E, F, the gastric angle submucosa was resected during ESD, measuring 5.0 cm by 4.0 cm. During laparoscopic exploration, the splenic vessel was encased within the pancreatic mass, making it quite difficult to isolate. No intra-abdominal dissemination was found, and ascites cytology was negative. After obtaining informed consent from the patient’s family, laparoscopic subtotal pancreatectomy and splenectomy were performed (Figure 4).
Histologic examination of intraoperative frozen section indicated pancreatic malignancy (Figure 2I). The transection margin was free of tumor. Lymph nodes in groups 2, 4, 7–11 were laparoscopically cleared (Figure 4). No metastasis was reported in lymph nodes.
Enteral nutrition (EN) plus parenteral nutrition (PN) was administered when malnutrition occurred (+24 days, Figure 4). It is detrimental that the patient has rejected adjuvant therapy, including chemotherapy. Given that the disease had metastasized to the liver, making curative treatment unfeasible, best supportive care was provided (+56 days, Figure 4).
2.3 Outcome
Contrast-enhanced CT revealed multiple hepatic nodular hypodense lesions with a bull’s-eye appearance, which indicated a progressive disease stage according to the RECIST criteria (+56 days, Figures 1G, H). The patient passed away on February 1, 2025 (+116 days, Figure 4).
3 Result
3.1 Changes of tumor biomarkers
As shown in Figures 3A, B, the serum levels of CA199, CEA, CA125, and CA242 were elevated above normal range before the operation. These markers decreased sharply after the operation but remained abnormal. Subsequently, their levels increased predominantly in association with liver metastasis.
3.2 Pathological findings
Analysis of a biopsy sample from the gastric angle demonstrated chronic superficial antral gastritis, moderate intestinal metaplasia, partial disruption of the glandular structure, and the presence of atypical cells in focal stromal areas (Figure 2G).
As displayed in Figure 2H, the pathological result of the ESD specimen showed a moderately to poorly differentiated adenocarcinoma. The findings included partial mucinous adenocarcinoma, intestinal metaplasia, and an invasion depth of the submucosa, 0.22 cm away from the muscularis mucosae. The resection margins were negative, and there was no neural or vascular invasion (+16 days).
As illustrated in Figure 2I, the mass in the pancreatic tail was composed of spindle-shaped cells without a glandular component and was diagnosed as a sarcomatoid carcinoma with low-grade adenocarcinoma components. It exhibited neural invasion but neither lymphatic invasion nor vessel invasion.
The postoperative TNM classification is T1aN0M0,Stage I (stomach cancer) and T2N0M0,Stage IB(SCP) (17, 18).
3.3 Immunohistochemical results
The gastric lesion stained positively for CK(pan), CD68, and was partially positive for Ki-67 (Figures 2J–L).
The mass in the pancreatic tail was partially positive for CK(pan), CK7, CD56, CD138, CEA, EMA, and Ki-67 (60%), but negative for P40, P63, CgA, Syn, CD117, DOG1, S-100, HMB45, CD34, SMA, Desmin, Myogenin, TTF-1, and reticular fiber staining (+16 days, Figures 5A–T, Figure 1I).

Figure 5. IHC result of the mass in pancreatic tail. (A) Ki67 (60%+); (B) CD56 (partial +); (C) P40 (–): (D) TTF-1 (–); (E) Syn (–); (F) CgA (–); (G) CD117 (–); (H) S-100 (–); (I) DOG-1 (–); (J) CD34 (–); (K) HMB45 (–); (L) Myogenin (–); (M) SMA (–); (N) Desmin (–); (O) CEA(partial +); (P) EMA(partial+); (Q) CK7(partial +); (R) CD138 (partial +), (S) P63 (–) (T) CK (pan) (partial+).
Taken together, the IHC test supported a diagnosis of synchronous gastric adenocarcinoma and SCP.
4 Discussion
On the basis of the GLOBOCAN data, gastric cancer ranks 5th and pancreatic cancer ranks 12th in terms of incidence (1). Regarding mortality rate, gastric cancer is the 5th leading cause of cancer- related deaths, while pancreatic cancer ranks 6th (1). As summarized in Table 1, the average age of patients with synchronous gastric and pancreatic cancer is 70.06 ± 8.81 years. The female proportion is 23.5%. Nevertheless, it is widely accepted that there are no reports in the literature on synchronous gastric cancer and SCP to date. This study highlights a rare case involving a 69-year-old female patient with this unusual combination.
SCP constitutes a variant of undifferentiated pancreatic carcinoma and is associated with an extremely poor prognosis (19). Radical resection and adjuvant therapy, such as gemcitabine plus capecitabine, are mainly effective for this rare but deadly disease (20). In the context of sarcomatoid features, both mesenchymal and epithelial markers are typically co-expressed (19). Immunohisto- chemical markers are helpful in the differential diagnosis of pancreatic tumors. Gastrointestinal stromal tumor (GIST) was ruled out due to the negativity of CD117, DOG1, CD34, and SMA. The negativity of CgA and Syn enabled us to exclude pancreatic neuroendocrine tumors (pNETs). Negative staining for S-100 and HMB45 supported the exclusion of pancreatic malignant melanoma. Negative results for P40, P63, and TTF-1 aid in eliminating pancreatic metastasis of pulmonary squamous cell carcinoma and pulmonary adenocarcinoma. Negativity for Desmin and Myogenin staining typically precludes malignant tumors of skeletal muscle origin. A 60% positivity rate for Ki-67, partial positivity for CK(pan), CK7, CEA, EMA, CD56, and CD138, along with the pathological features, support the diagnosis of SCP with adenocarcinoma components.
Drawing on the histological findings and IHC results, the patient was diagnosed with synchronous gastric adenocarcinoma (T1aN0M0) and SCP (T2N0M0). At the same time, the following tumors should be differentiated:
1. Pancreatic invasion by gastric cancer. In this setting, the lesion is generally located in the greater curvature or posterior wall of the stomach. No clear space between these structures and enlarged lymph nodes surrounding the stomach was usually found on CT or MRI scans. The expressions of HER2, MLH1, MSH2, PMS2, MSH6, Ki67, and PD-L1 in the IHC test help us to identify this disease.
2. Pancreatic cancer metastasizing to stomach. In this scenario, encasements between the two organs were always noted on CT or MRI scans. No lesion was normally noticed in the gastric mucosa under gastroscopy. The positivity of CEA, CA19-9, MUC-1, CK-19, and Ki67 makes this diagnosis appropriate.
There is no consensus on treatment options for synchronous gastric and pancreatic cancer. As outlined in Table 1, both the surgically-treated strategy and Fluorouracil-based chemotherapy have been shown to be effective in treating simultaneous gastric and pancreatic cancer (2, 4–13, 15, 16). Ebihara M, et al. suggested that laparoscopic radical total gastrectomy and pancreatosplenectomy are preferred for the treatment of concurrent cancer of the stomach and pancreas (2). It was generally acknowleged that surgical resection should be conducted for pancreatic tail masses larger than 30 mm. In this study, to avoid the risk of SCP metastasizing to the stomach, endoscopic ultrasound-guided biopsy was not performed (16). Since Type IIc is a kind of early gastric cancer, ESD is considered appropriate. Considering the malignancy in the pancreatic tail, laparoscopic subtotal pancreatectomy, splenectomy, and lymph node resection were ultimately conducted.
The rate of postoperative pancreatic fistula (POPF) is about 23%, which is a severe complication during pancreatic surgery and a significant challenge for surgeons (21). A soft pancreatic texture and a main pancreatic duct (MPD) diameter of no more than 3 mm are considered objective pancreatic- specific factors associated with an increased risk of POPF (21). Intraoperative ultrasonography provides precise information about pancreatic texture and MPD size for surgeons, which offers a real-time, comprehensive evaluation of pancreatic anatomy when laparoscopic pancreatectomy is performed. It is intriguing that an innovative intraoperative Wirsung duct high-quality ultrasonography(IWU) has been utilized to assess the structural characteristics of the main pancreatic duct (22). IWU can also be used as a mitigation strategy by evaluating the MPD during laparoscopic distal pancreatectomy. Only laparoscopic exploration was performed during the operation in this study. No other pancreatic lesions were detected, which was postoperatively verified by contrast-enhanced CT, and no POPF developed.
Compared to pancreatic ductal adenocarcinoma, SCP has a worse prognosis, with a median overall survival of 6 months (23). Gemcitabine plus capecitabine chemotherapy was postoperatively administered as adjuvant therapy for SCP, and the patient was reported to be alive 3 months after the operation (20). Since the patient refused adjuvant therapy, including chemotherapy, EN plus PN was administered when malnutrition manifested. Best supportive care was adopted as progressive disease occurred. The patient died on the 107th postoperative day.
5 Conclusion
A rare case, with a comprehensive literature review, demonstrates compelling evidence that positivity for CK(pan), CK7, CD56, CD138, CEA, EMA, and Ki-67 (60%) in spindle-shaped cells without a glandular component contributes significantly to diagnosing SCP with low-grade adenocarcinoma components. Furthermore, this study reports the first case of synchronous SCP and gastric cancer. Minimally invasive surgical treatment, including ESD and laparoscopic radical pancreatosplenectomy, remains effective despite an even poorer prognosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by longyou county people’s hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GL: Writing – original draft, Writing – review & editing. JT: Writing – original draft, Writing – review & editing. RJ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research, authorship, and publication of this article was supported by Science and TechnologyPlan Project of Quzhou City (No.2023 K198), Guiding Science and Technology Project of Longyou County (No.2023079 and 2023088).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1584504/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Ebihara M, Fujisawa K, Haruta S, Uruga H, and Ueno M. Laparoscopic radical total gastrectomy and pancreatosplenectomy for synchronous cancer of the stomach and pancreas. Cureus. (2024) 16:e55927. doi: 10.7759/cureus.55927
3. Yonenaga Y, Kurosawa M, Mise M, Yamagishi M, and Higashide S. Pancreatic-type acinar cell carcinoma of the stomach included in multiple primary carcinomas. Anticancer Res. (2016) 36:2855–2864.
4. Eriguchi N, Aoyagi S, Hara M, Okuda K, Tamae T, Fukuda S, et al. A case of synchronous double cancers of the pancreas and stomach. Kurume Med J. (2000) 47:169–71. doi: 10.2739/kurumemedj.47.169
5. Fang T, Liang TT, Wang YZ, Wu HT, Liu SH, and Wang C. Synchronous concomitant pancreatic acinar cell carcin and gastric adenocarcinoma: A case report and review of literature. World J Clin cases. (2021) 9):8509–17. doi: 10.12998/wjcc.v9.i28.8509
6. Ghothim M, Havlík R, Skalický P, Klos D, Vrba R, Strážnická J, et al. Synchronous cancer duplicities of pancreas and stomach/kidney and their surgical treatment. Rozhl Chir. (2015) 94:251–5.
7. Inoue M, Hakoda K, Sawada H, Hotta R, Ohmori I, Miyamoto K, et al. Synchronous gastric cancer and carcinoma in situ of the pancreas. Clin Case Rep. (2021) 9:e04892. doi: 10.1002/ccr3.4892
8. Kourie HR, Markoutsaki N, Roussel H, Rahmi G, van der Stiegel M, Palazzo L, et al. Double pancreatic and gastric adenocarcinomas: a rare association. Clin Res Hepatol Gastroenterol. (2013) 37:e137–40. doi: 10.1016/j.clinre.2012.09.008
9. Kubota E, Kataoka H, Hayashi K, Kamiya T, Sasaki M, Ogasawara N, et al. Advanced stomach and pancreas cancer successfully treated with combination chemotherapy with S-1/paclitaxel/lentinan. Hepatogastroenterology. (2009) 56:106–10.
10. Ma YH, Yamaguchi T, Yasumura T, Kuno T, Kobayashi S, Yoshida T, et al. Pancreatic cancer secondary to intraductal papillary mucinous neoplasm with collision between gastric cancer and B-cell lymphoma: A case report. World J Clin Cases. (2021) 9:2400 –2408. doi: 10.12998/wjcc.v9.i10.2400
11. Muroni M, D’Angelo F, Pezzatini M, Sebastiani S, Noto S, Pilozzi E, et al. Synchronous gastric adenocarcinoma and pancreatic ductal adenocarcinoma. Hepatobiliary Pancreat Dis Int. (2010) 9:97–9.
12. Ohtsubo K, Ishikawa D, Nanjo S, Takeuchi S, Yamada T, Mouri H, et al. Synchronous triple cancers of the pancreas, stomach, and cecum treated with S-1 followed by pancrelipase treatment of pancreatic exocrine insufficiency. JOP. (2013) 14:515–20. doi: 10.6092/1590-8577/1719
13. Read C, Hughes M, and Itani O. Presentation of gastrointestinal stromal tumor, pancreatic adenocarcinoma, and gastric adenocarcinoma in a woman with no identifiable genetic abnormalities. Am Surg. (2023) 89:6393–5. doi: 10.1177/00031348231201884
14. Santos-Fernández J, Arenal-Vera JJ, Cítores-Pascual MÁ, Fernández-Orcajo P, de-Benito-Sanz M, Benito-Fernández C, et al. Synchronic gastric and pancreatic ductal adenocarcinoma. A case report. Rev Esp Enferm Dig. (2015) 107:642–3. doi: 10.17235/reed.2015.3720/2014
15. Shupp B, Liaquat H, Rollins S, Stoll L, Singh G, Quiros RM, et al. A rare case of synchronous intraductal papillary mucinous neoplasm-associated pancreatic adenocarcinoma and signet ring cell gastric adenocarcinoma. Am J Case Rep. (2022) 23:e935242. doi: 10.12659/AJCR.935242
16. Yokoyama Y, Sakata H, Uekusa T, Tajima Y, and Ishimaru M. Solitary pancreatic metastasis of gastric cancer with synchronous pancreatic ductal carcinoma: A case report. Int J Surg Case Rep. (2020) 70:164–7. doi: 10.1016/j.ijscr.2020.04.006
17. Peyroteo M, Martins PC, Canotilho R, Correia AM, Baía C, Sousa A, et al. Impact of the 8th edition of the AJCC TNM classification on gastric cancer prognosis-study of a western cohort. Ecancermedicalscience. (2020) 14:1124. doi: 10.3332/ecancer.2020.1124
18. Kang H, Kim SS, Sung MJ, Jo JH, Lee HS, Chung MJ, et al. Evaluation of the 8th edition AJCC staging system for the clinical staging of pancreatic cancer. Cancers (Basel). (2022) 14:4672. doi: 10.3390/cancers14194672
19. Silvestris N, Argentiero A, Brunetti O, Sonnessa M, Colonna F, Delcuratolo S, et al. PD-L1 and notch as novel biomark ers in pancreatic sarcomatoid carcinoma: a pilot study. Expert Opin Ther Targets. (2021) 25:1007. doi: 10.1080/14728222
20. Blair AB, Burkhart RA, Griffin JF, Miller JA, Weiss MJ, Cameron JL, et al. Long-term survival after resection of sarcomatoid carcinoma of the pancreas: an updated experience. J Surg Res. (2017) 219:238–43. doi: 10.1016/j.jss.2017.05.065
21. Schuh F, Mihaljevic AL, Probst P, Trudeau MT, Müller PC, Marchegiani G, et al. A simple classification of pancreatic duct size and texture predicts postoperative pancreatic fistula: A classification of the international study group of pancreatic surgery. Ann Surg. (2023) 277:e597 –e608. doi: 10.1097/SLA.0000000000004855
22. Huscher CGS, Lazzarin G, Marchegiani G, and Boggi U. Intraoperative intraductal ultrasonography of the main pancreatic duct during pancreatoduodenectomy: technical description of a pilot series. Updates Surg. (2024) 76:2471–6. doi: 10.1007/s13304-024-01949-6
Keywords: pancreatic sarcomatoid carcinoma, gastric cancer, surgical treatment, enteral nutrition, parenteral nutrition
Citation: Lu G, Tu J and Jiang R (2025) Case Report: Synchronous pancreatic sarcomatoid carcinoma and gastric cancer: a case study with literature review. Front. Immunol. 16:1584504. doi: 10.3389/fimmu.2025.1584504
Received: 27 February 2025; Accepted: 08 May 2025;
Published: 27 May 2025.
Edited by:
Wei Wang, Jiangsu Institute of Parasitic Diseases (JIPD), ChinaReviewed by:
Alessio Vagliasindi, Oncological Center of Basilicata (IRCCS), ItalyGianni Lazzarin, Abano Terme Hospital, Italy
Tao Lu, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, China
Copyright © 2025 Lu, Tu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Genlin Lu, bHVnZW5saW4wMDdAMTYzLmNvbQ==
 Genlin Lu
Genlin Lu Jinming Tu
Jinming Tu Renya Jiang3
Renya Jiang3