- 1Department of Pharmacy, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, School of Clinical Medicine, Henan University, Zhengzhou, Henan, China
- 2Department of Pharmacy, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Oncology, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, People’s Hospital of Henan University, Zhengzhou, Henan, China
Objectives: We aimed to evaluate the effectiveness and safety of sintilimab versus camrelizumab, both plus targeted drugs, for advanced hepatocellular carcinoma (HCC) in the real world. Then the effectiveness was compared between sintilimab-lenvatinib and camrelizumab-apatinib.
Methods: Patients diagnosed as advanced HCC were included from January 2017 to December 2023. They were concurrently treated with targeted drugs and sintilimab or camrelizumab. Progression-free survival (PFS), overall survival (OS), and adverse events (AEs) were assessed. The subgroups analysis of PFS and OS based on baseline characteristics and univariate and multivariate COX analysis were done to test for heterogeneity of data and correct for confounding factors. Then subgroup analysis of sintilimab-lenvatinib versus camrelizumab-apatinib was conducted.
Results: A total of 300 eligible HCC patients (199 in sintilimab group and 101 in camrelizumab group) were included in our study. No significant difference in PFS (p=0.47) and OS (p=0.51) was observed between sintilimab and camrelizumab groups. The median PFS (mPFS) was 262 days in sintilimab group, and 220 days in camrelizumab group, and neither group has reached the median OS. There was no difference in AEs between two groups also. The effect of sintilimab and camrelizumab on PFS and OS based on baseline characteristics was consistent with primary outcomes, except for other metastatic sites and lenvatinib in terms of OS. Multivariable Cox analysis identified the number of metastatic sites ≥2 and AFP level ≥400ng/mL as independent predictors of shorter PFS and OS, but they had no effect on the primary outcomes. In subgroup analysis, the PFS and OS of sintilimab -lenvatinib and camrelizumab-apatinib in first-line treatment of advanced HCC were not clinically different, although sintilimab-lenvatinib had a longer mPFS (301 days in sintilimab-lenvatinib group vs. 194 days in camrelizumab-apatinib group).
Conclusions: Sintilimab and camrelizumab, both plus targeted agents, have equal clinical effectiveness and incidences of AEs. The effectiveness of sintilimab-lenvatinib and camrelizumab-apatinib are similar in first-line treatment of advanced HCC, despite a slight superiority in sintilimab-lenvatinib is observed.
1 Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in the world, with high morbidity and mortality. According to reports, 49.3% of new cases of liver cancer and 58.3% of deaths occurred in China (1). HCC has become the fourth malignant tumor with the fourth incidence rate and the second mortality rate in China (2). Due to the insidious onset of HCC, 70-80% of patients are initially diagnosed at a middle or advanced stage and miss opportunity for surgery (3). HCC with distant metastasis or progression has a dismal prognosis, with a 5-year survival rate of merely 3.1% for metastatic HCC (4).
Currently, systemic therapy is the mainstay of treatment for patients with unresectable or recurrent HCC, with the aim of controlling disease progression as much as possible and prolonging the survival time of patients (5). In 2007, the targeted antiangiogenic agent, sorafenib, was approved for the treatment of HCC (6), and then a variety of targeted agents appeared, such as bevacizumab (7), lenvatinib (8), apatinib (9), cabozantinib (10), et al. Given that targeted agents can no longer meet the clinical needs for lacking of alternative drugs after targeted therapy failure, researchers are exploring new therapeutic options to prolong the survival of patients with advanced HCC, such as immunotherapy combined with targeted therapy.
Two reviews revealed that immunotherapeutic drugs and antiangiogenic drugs theoretically have synergistic effects. Angiogenesis is an important step in the process of tumor growth and metastasis. Neoplastic tumor blood vessels, due to abnormal perfusion and increased permeability, cause tissue hypoxia and promote the release of tissue factor, which not only inhibits the function of immune cells in the tumor microenvironment, but also produces systemic immunosuppressive effects through blood circulation. Anti-angiogenic drugs can restore the balance in terms of angiogenesis in the tumor, remodel the tumour microenvironment (TME), promote T cell infiltration into the tumor, and then improve the effect of immunotherapy. Therefore, tumor vascular normalization and immune reprogramming have a synergistic effect on each other, and can enter a benign cycle of mutual enhancement by improving TME (11, 12).
Many clinical studies explored the role of immune checkpoint inhibitors (ICIs) combined with targeted anti-angiogenic drugs, based on molecular theory basis in advanced HCC, including atezolizumab plus bevacizumab (13, 14), pembrolizumab plus lenvatinib (15), sintilimab plus bevacizumab biosimilar (16), camrelizumab plus apatinib (9). These combinations further prolonged survival of patients compared to targeted drugs. Sintilimab-bevacizumab biosimilar and camrelizumab-apatinib are approved for first-line treatment of HCC in 2021 and 2023. Sintilimab and camrelizumab are cheaper and highly available compared to atezolizumab and pembrolizumab, because they are produced domestically and favored by health insurance policies in China. So sintilimab and camrelizumab are used widely in clinical practice. The ICIs are more commonly combined with oral targeted drugs than with bevacizumab and its biosimilars, because of their economics and convenience of drug administration. So sintilimab in combination with lenvatinib and other oral targeted drugs is widely used in real-world, and the same goes for camrelizumab (17, 18).
However, the head-to-head comparisons between sintilimab and camrelizumab are few (19). We aimed to evaluate the effectiveness and safety of sintilimab versus camrelizumab, both plus targeted drugs, for advanced HCC in the real world. Subsequently, the effectiveness was compared between sintilimab-lenvatinib and camrelizumab-apatinib in first-line treatment of advanced HCC.
2 Materials and methods
2.1 Study design and patients
In this retrospective study, the medical records and imaging data of HCC patients were obtained from the a large 3A hospital in China between January 2017 and December 2023. Our study was approved by the Ethics committee of Henan Provincial People’s Hospital and owing to the retrospective nature of the study, individual consent for this analysis was waived.
All patients were diagnosed based on typical imaging features and/or biopsy or previous surgical resection (20). Patients, concurrently treated with targeted drugs and sintilimab or camrelizumab, were included in this study. The inclusion criteria are as follows: (i) age ≥18 years; (ii) Eastern Cooperative Oncology Group’s performance status (ECOG PS) score of 0-2; (iii) ChildPugh class A or B liver function; (iv) at least two usage records of ICIs and targeted drugs. The key exclusion criteria included: (i) prior diagnosis or treatment of other malignancies, severe dyspnea, cardiovascular or kidney disease, pregnant or lactating women; (ii) deficiency in imaging assessment; (iii) adjuvant or neoadjuvant therapy.
2.2 Treatment protocol
Sintilimab and camrelizumab were prescribed at a fixed dose of 200mg every 3 weeks. Bevacizumab biosimilar was prescribed 15mg/kg every 3 weeks by intravenous infusion (7), and six molecularly targeted drugs were administered orally. Sorafenib was administered at a dose of 400 mg twice daily, but it can be reduced to 400mg/day if the patient cannot tolerate (6). The dose of lenvatinib was dependent on weight of patients (≥60kg, 12 mg; <60kg, 8mg) (8). Regorafenib was prescribed 160mg/day during weeks 1–3 of each 4 week-cycle (21). Apatinib was prescribed 250mg/day (9). Anlotinib was administered 12mg/day during two weeks ago of each 3 week-cycle (22). Donafenib was prescribed 200mg twice daily (23).
2.3 Data collection and follow-up
The baseline data of patients, including patient age, sex, height, weight, accompanying disease, behavior of smoking and drinking, hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, tumor stage, laboratory data, surgical situation, imaging data, and medication information were collected and gathered from patients’ medical records. Patients were followed up and monitored via phone.
2.4 Outcome and safety assessment
All patients were evaluated according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (24). Progression-free survival (PFS) was defined as the time from treatment initiation of sintilimab or camrelizumab to disease progression or death from any cause. Overall survival (OS) was defined as the interval between the initial treatment and the time of death from any cause. The subgroups analysis of PFS and OS based on baseline characteristics was made to test for heterogeneity of data. Univariate and multivariate COX analysis were also done to correct for confounding factors. Then subgroup analysis of sintilimab-lenvatinib versus camrelizumab-apatinib for first-line treatment of advanced HCC was conducted.
Safety assessments and grading were recorded by the investigators from clinical examination, laboratory test results, medical records and follow-up information, using the Common Terminology Criteria for Adverse Events 5.0 (CTCAE 5.0) (25) and the management of immunotherapy-related toxicity (26) published by National Comprehensive Cancer Network (NCCN) as references.
2.5 Statistical analysis
Statistical analysis was performed using SPSS version 23.0 (IBM Corp., NY, USA) and the R software version 4.4.2 (http://www.r-project.org/). Differences in the clinical characteristics between the two groups were determined by chi-square test for categorical variables and independent samples t-test for continuous data. The PFS and OS were estimated using the Kaplan-Meier method, and the comparisons were analyzed using the log-rank test. Prognostic values were estimated using hazard ratios (HRs) with 95% confidence intervals (CIs). For univariate and multivariate analyses, a Cox proportional hazards regression model was constructed. The multivariate analysis included variables considered statistically significant by univariate analysis (p<0.1) and variables considered clinically closely related to the dependent variables. Mann-Whitney U test was used for ranked ordinal data, when AEs between the two groups were analyzed. Two-tailed p values < 0.05 were considered statistically significant.
3 Results
3.1 Demographics and clinical characteristics
A total of 467 patients with HCC were screened, and 167 patients were excluded based on inclusion and exclusion criteria (Figure 1). Then, a total of 300 eligible HCC patients who were treated with targeted drugs plus sintilimab (n=199) or camrelizumab (n=101) were included in our study.
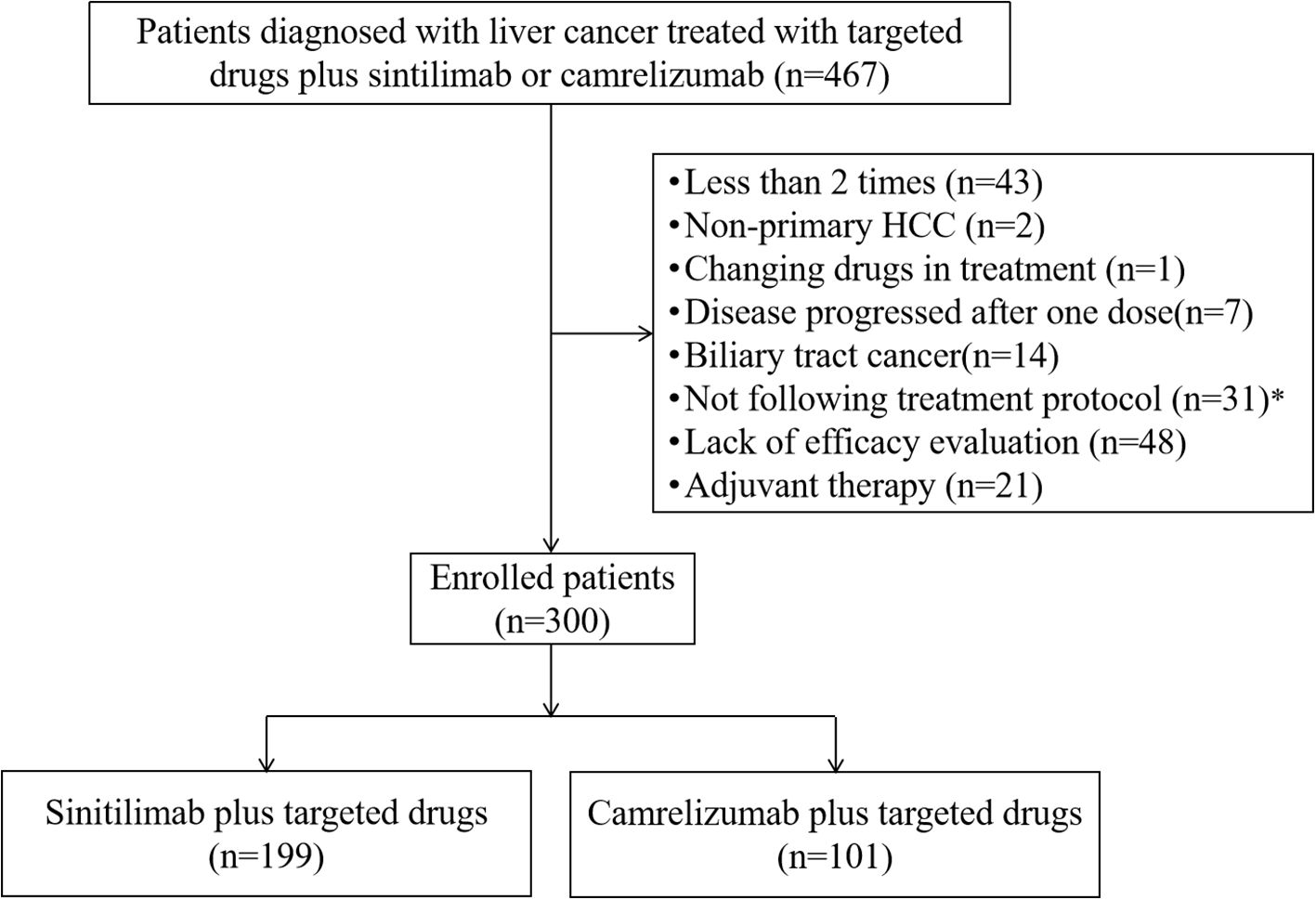
Figure 1. Patients’ selection flow. HCC, hepatocellular carcinoma.*Including patients have dose reductions not following the treatment protocol.
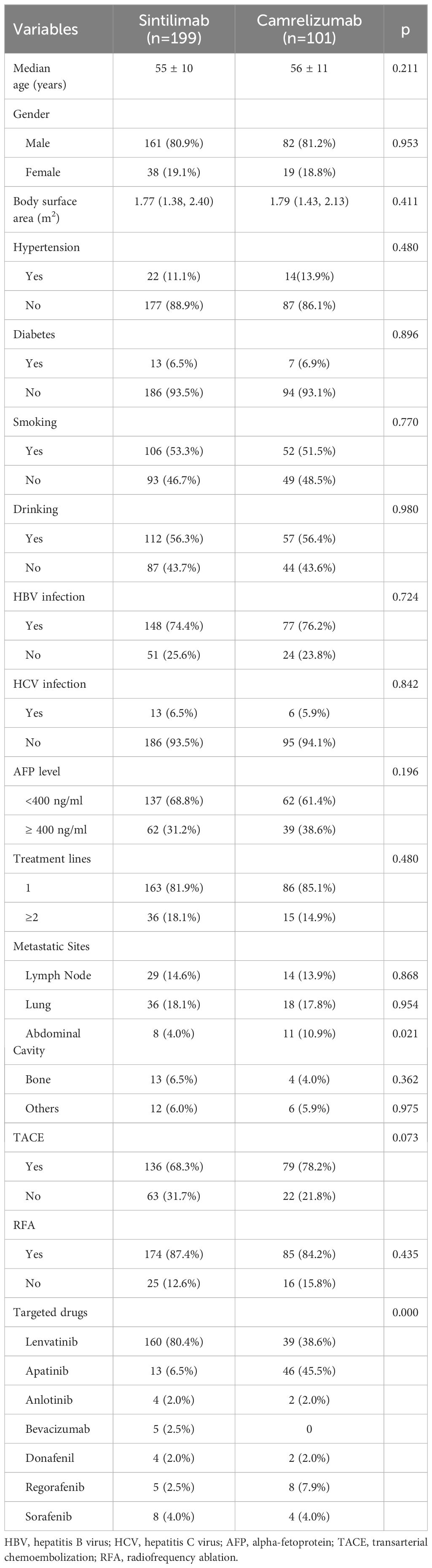
Characteristics including sex, age, body surface area, smoking and drinking history, diabetes, hypertension, HBV and HCV infection, alpha-fetoprotein (AFP) level, treatment lines, tumour metastasis, transarterial chemoembolization (TACE), radiofrequency ablation (RFA) and kinds of targeted drugs are shown in Table 1. There were no significant differences in the baseline characteristics between the two groups (p>0.05), excepting for the proportion of abdominal cavity metastasis (p=0.021) and targeted drugs (p=0.000).
3.2 Clinical outcomes
At the data cutoff for the primary analysis of PFS, the median follow-up was 178 days and 156 days in sintilimab and camrelizumab groups, respectively. 131 (66%) of 199 patients in the sintilimab group and 69 (68%) of 101 patients in camrelizumab group had disease progression as assessed by mRECIST or had died. The median PFS (mPFS) in the sintilimab and camrelizumab groups was 262 days and 220 days, respectively, with no significant difference (p=0.47, Figure 2A). The effect of sintilimab and camrelizumab on PFS was consistent across subgroups based on baseline characteristics (Figure 3A).
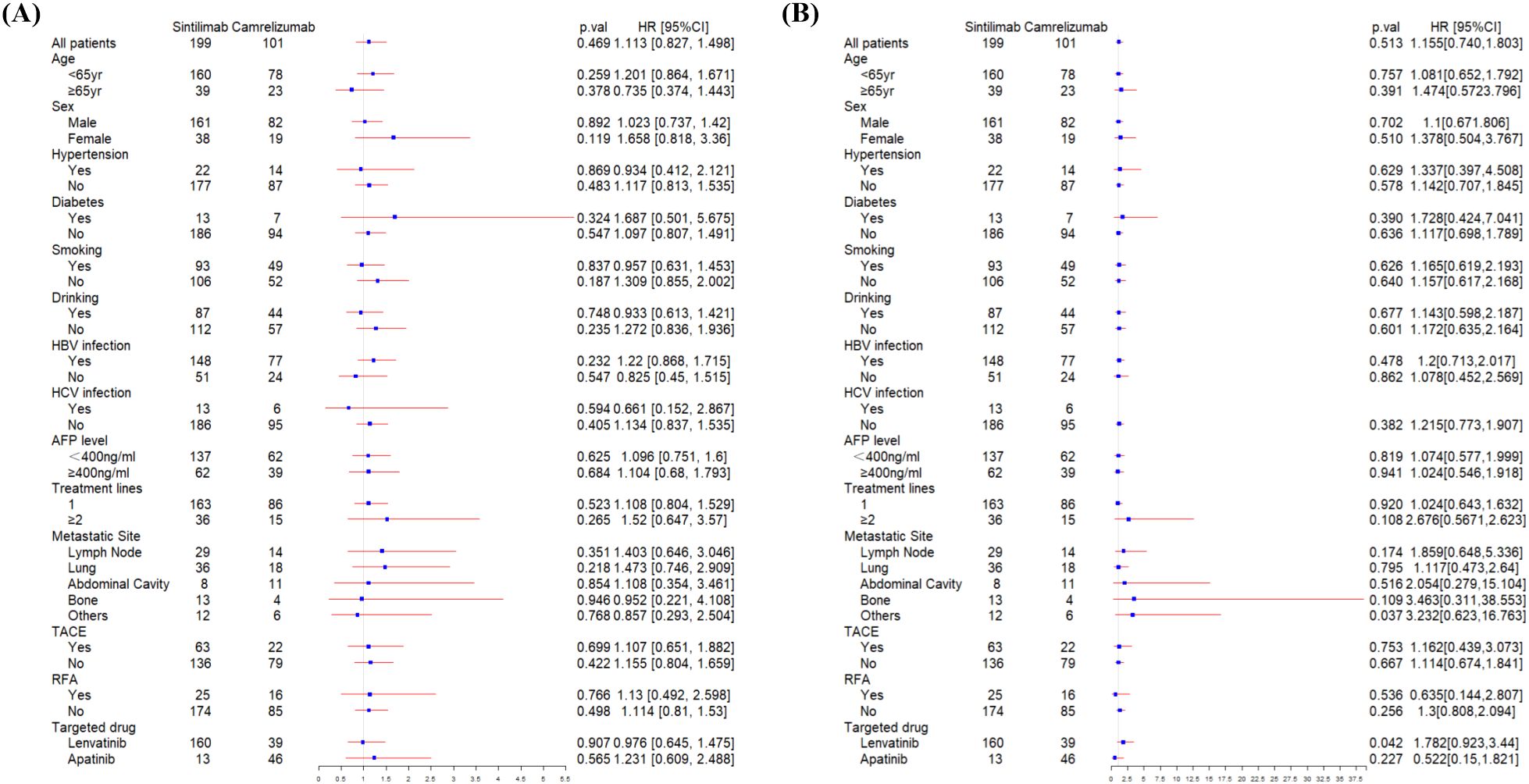
Figure 2. Kaplan-Meier plots of progression-free survival (A) and overall survival (B) in sintilimab and camrelizumab groups. ICIs, immune checkpoint inhibitor.
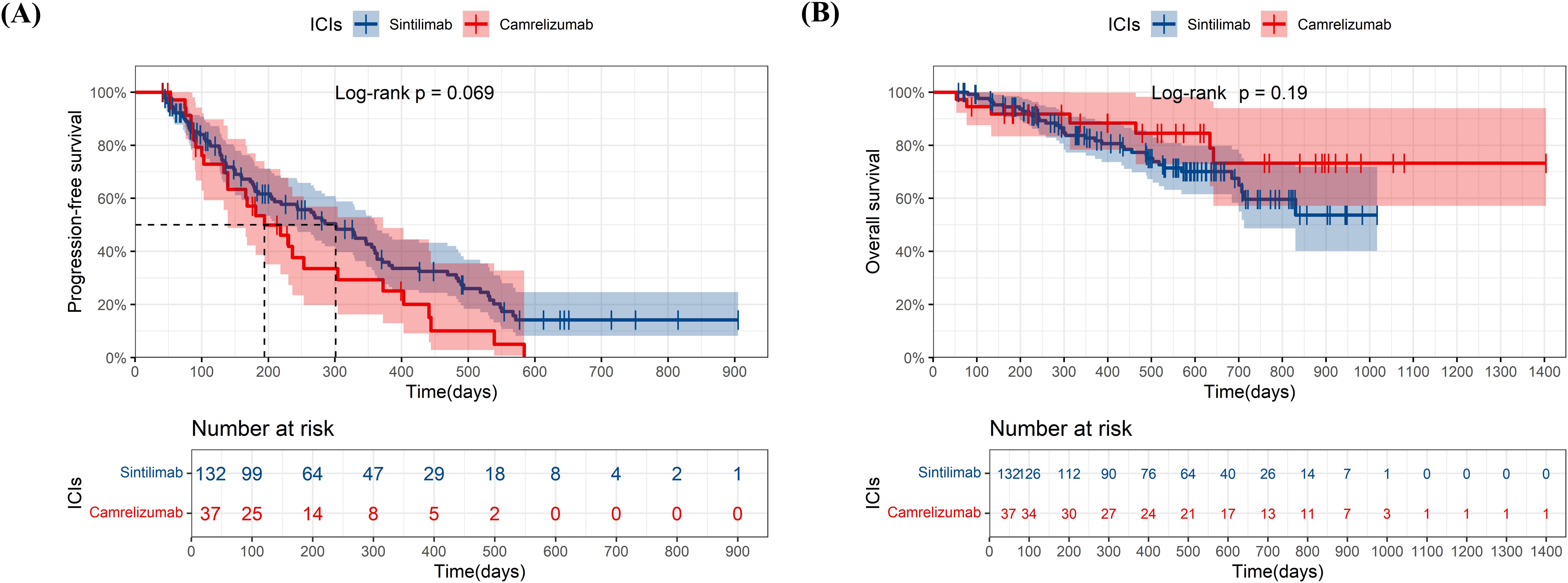
Figure 3. Subgroup analysis of progression-free survival (A) and overall survival (B) according to baseline characteristics. HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, alpha-fetoprotein; TACE, transarterial chemoembolization; RFA, radiofrequency ablation.
At the date of cutoff, the median follow-up of OS was 493 days and 423 days in sintilimab and camrelizumab groups, respectively. 55 (38%) of 199 patients in the sintilimab group and 32 (31%) of 101 patients in the camrelizumab group had died. There was no statistically significant difference in OS between the two groups (p=0.51, Figure 2B), and neither group has reached the median OS (mOS). The effect of sintilimab and camrelizumab on OS was consistent across subgroups except for other metastatic sites and lenvatinib (Figure 3B). Patients with other metastatic sites [HR (95% CI): 3.232 (0.623,16.763), p=0.037] or with lenvatinib administration [HR (95% CI): 1.782 (0.923,3.44), p=0.042] in camrelizumab group had higher risk of death, compared to sintilimab group.
The univariate and multivariate COX analysis results of the factors on PFS and OS are shown in Supplementary Material Tables S1, S2. Multivariable Cox analysis identified the number of metastatic sites (≥2 vs. 0-1; HR=1.823, 95% CI: 1.162-2.862, p=0.009) and AFP level ≥400ng/mL (HR=1.539, 95% CI: 1.113-2.127, p=0.009) as independent predictors of shorter PFS. Similar associations were observed for OS (metastatic sites: HR=2.272, 95% CI: 1.238-4.172, p=0.008; AFP: HR=2.775, 95% CI: 1.768-4.355, p=0.000). Diabetes was related to shorter PFS (HR=1.975, 95% CI: 1.12-3.485, p=0.019). HCV infection and RFA were separately independent predictors of longer PFS and OS (HCV infection for PFS: HR=0.409, 95% CI: 0.186-0.901, p=0.027; RFA for OS: HR=0.381, 95% CI: 0.171-0.85, p=0.018). But our primary outcomes were not affected by these factors, despite incomplete matching of baseline levels (PFS: HR=0.918, 95% CI: 0.653-1.291, p=0.623; OS: HR=1.106, 95% CI: 0.66-1.851, p=0.702).
Propensity score matching (PSM) was conducted to eliminate the effect of baseline characteristics (abdominal cavity metastasis and targeted drugs) with caliper for 0.1, and results were showed in Supplementary Material Figure S1. After adjusting for confounding factors by PSM, no statistically significant difference was still found between sintilimab and camrelizumab groups in the comparison of the PFS (mPFS: 280 vs. 247 days, p=0.95) and OS (p=0.94), suggesting that confounding factors (abdominal cavity metastasis and targeted drugs) have a small effect on the primary outcomes.
3.3 Subgroup analysis of sintilimab-lenvatinib versus camrelizumab-apatinib
We compare the effectiveness between sintilimab-lenvatinib and camrelizumab-apatinib for first-line treatment (Figures 4A, B). No significant differences in PFS (p=0.069) and OS (p=0.19) were observed between two combinations, although combination of the sintilimab-lenvatinib had a longer mPFS than the camrelizumab-apatinib (301 days versus 194 days). Neither group reached the mOS.
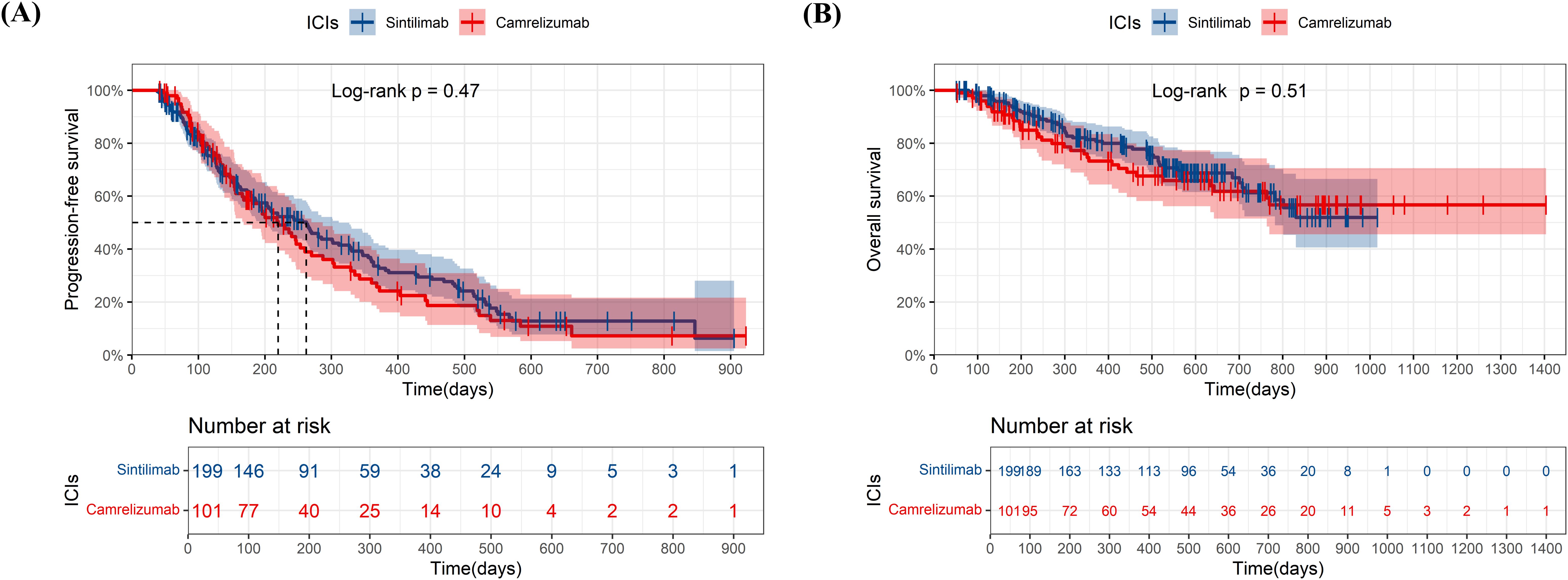
Figure 4. Kaplan-Meier plots of progression-free survival (A) and overall survival (B) in sintilimab plus lenvatinib group and camrelizumab plus apatinib group. ICIs, immune checkpoint inhibitor.
3.4 Safety
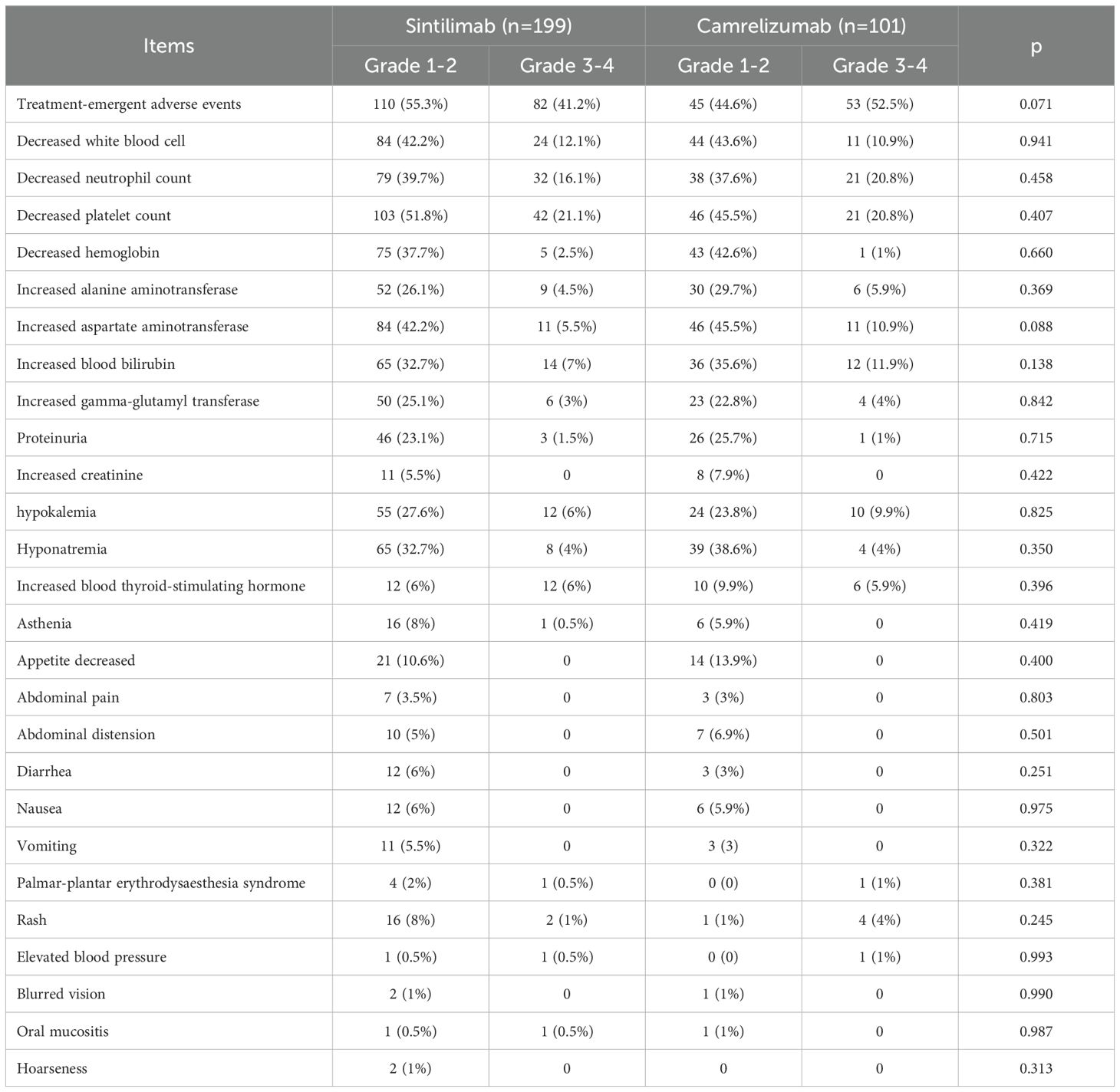
192 (96.5%) of 199 patients and 98 (97.0%) of 101 patients experienced at least one adverse event (AE) of any grade in sintilimab and camrelizumab groups (Table 2). The numbers of patients discontinued due to AEs in sintilimab and camrelizumab groups were 3 (1.5%) and 2 (2.0%), respectively. The number of patients with dose adjustments according to treatment protocol was 4 (2%) in sintilimab group and 1 (1%) in camrelizumab group. The incidences of serious adverse events (SAE), defined as AE of grade 3-4, were 41.2% and 52.5%, respectively. There were no significant differences in AEs whether it’s grade 1–2 or grade3–4 between two groups (p=0.071). The main AEs of any grade in two groups included decreased platelet count, decreased white blood cell, decreased neutrophil count, decreased hemoglobin, increased alanine aminotransferase, increased aspartate aminotransferase, increased blood bilirubin and hyponatremia, et al.
4 Discussion
Our study revealed a real-world comparison of the effectiveness and safety between sintilimab and camrelizumab for the treatment of advanced HCC. There was no statistically significant difference in PFS and OS between sintilimab and camrelizumab groups. And there was no significant difference in AEs between two groups. In subgroup analysis, no significant difference was observed in PFS and OS between sintilimab-lenvatinib and camrelizumab-apatinib for first-line treatment, although combination of the sintilimab-lenvatinib had a longer mPFS than the camrelizumab group.
Sintilimab and camrelizumab are both monoclonal antibodies that target the PD-1 receptor on T cells and have been approved for the treatment of advanced HCC in China. A retrospective study (19) on effectiveness and safety of toripalimab (n=23), camrelizumab (n=33), and sintilimab (n=14) in HCC patients with HBV showed that no significant difference in PFS and OS among them was observed. A network meta-analysis, about first-line systemic treatment for HCC, showed that camrelizumab plus apatinib showed a statistically significant superiority in all comparisons, such as sintilimab plus bevacizumab (27). Another network meta-analysis (28), about phase III trials of first-line systemic therapy for advanced HCC, revealed sintilimab plus bevacizumab biosimilars and camrelizumab plus apatinib reduced the risk of death with HRs of 0.57 and 0.62 compared with sorafenib indicating a slight advantage of sintilimab. Therefore, it’s ambiguous as to who is more effective between sintilimab and camrelizumab, and the evidence for direct comparisons is insufficient. Our study shows that sintilimab and camrelizumab have equal clinical effectiveness in all patients, but sintilimab-lenvatinib is possibly superior to camrelizumab-apatinib in first-line treatment with HCC.
The randomized controlled trial (RCT) study (ORIENT-32) (16) showed that patients in the sintilimab-bevacizumab biosimilar group had a significantly longer mPFS (4.6 months) than did patients in the sorafenib group. A real-world analysis of sintilimab-bevacizumab biosimilar in patients with advanced HCC indicated that mPFS was 238 days (29). The mPFS of sintilimab plus anlotinib as first-line treatment for advanced HCC was 12.2 months in real-world study (30). So the survival time of sintilimab plus targeted drugs was different between RCT and real-world studies, and was inconsistent among real-world studies also. The same can be said for camrelizumab. The mPFS was 5.6 months in camrelizumab-apatinib group as first-line therapy for unresectable HCC in the RCT study (CARES-310) (9). The mPFS was 9.6 months in camrelizumab and apatinib group (31). The mPFS in second-line treatment of camrelizumab combined with apatinib was 10.5 months (32). The mPFS was 10.3 months in treatment group of camrelizumab and lenvatinib as first-line treatment of unresectable HCC (33). The mPFS of camrelizumab plus apatinib in first-line and second-line setting cohorts was 5.7 months and 5.5 months respectively in a phase 2 trial (34). In our study, the mPFS of sintilimab and camrelizumab is 262 days and 220 days in all patients, and 301 days and 194 days in patients receiving first-line treatment, respectively. The differences in survival time between researches are possibly due to the usage and dosage of ICIs and targeted drugs, patients included, follow-up time and so on.
The distribution of abdominal cavity metastasis is unequal between sintilimab and camrelizumab groups. But it doesn’t affect our primary outcomes. Because the subgroups analysis of PFS and OS based on baseline characteristics was consistent with primary outcomes, and results of the survival analysis after PSM were consistent with the primary outcomes, too. The distributional difference in type of targeted drugs was related to physicians’ drug habits, drug instructions, medical insurance policy and drug availability in hospital. So we performed survival analysis between two common combinations (sintilimab-lenvatinib and camrelizumab-apatinib) for first-line treatment of HCC. No significant difference was seen between the two groups in terms of PFS and OS, despite an advantage in the sintilimab group in terms of mPFS. However, this result is still questionable: (i) the sample size in the camrelizumab group is small (n=37); (ii) it was unknown whether the baseline data of the two groups were matched, as this was only a subgroup analysis. Therefore, future confirmation is needed in large-sample and head-to-head RCTs or real-world studies.
The difference in other metastatic sites, which was observed in subgroup analysis of OS, is not clinically significant in our opinion, because the sample size is small (sintilimab 12 vs camrelizumab 6). Sintilimab-lenvatinib showed longer OS then camrelizumab-lenvatinib showing the advantage of sintilimab than camrelizumab, which maybe fits with subgroup analysis of sintilimab-lenvatinib versus camrelizumab-apatinib.
Sintilimab and camrelizumab had an acceptable safety profile. No new AE was reported in the study. AEs in laboratory test results were collected easily from electronic medical record. But other AEs, such as reactive cutaneous capillary endothelial proliferation (RCCEP), nausea and vomiting, were difficult to obtained, because they were extracted from patient’s medical records or telephone follow-up. These AEs would be failed to be obtained if they were not recorded in medical records by doctors or patient was unclear or had forgotten. So they were possibly not captured in some patients, leading to low incidence in our study.
Comparisons between sintilimab and camrelizumab for the treatment of advanced HCC have some limitations. Firstly, residual confounding may persist due to imbalanced baseline characteristics, such as the higher proportion of abdominal cavity metastasis in the camrelizumab group and preferential use of lenvatinib/apatinib. These imbalances reflect real-world clinical practice, where treatment selection is often influenced by factors like medical insurance policy or drug availability. Future randomized trials with protocol-defined combinations are warranted to validate our findings. Secondly, the median duration of follow-up was not long enough and the mOS, as a major primary endpoint, was not met. Thirdly, the analyzable data were limited to those available in electronic medical record and telephone follow-up, so that certain AEs, such RCCEP, nausea and vomiting might be underreported. Fourthly, this is a single-center retrospective study. Therefore, we hope that multi-center, head-to-head clinical studies, including RCTs and real-world studies which can complement each other, will validate our results.
In conclusion, our study shows that sintilimab and camrelizumab, both plus targeted drugs, have equal clinical effectiveness and incidence of AEs. There is no difference between sintilimab-lenvatinib and camrelizumab-apatinib in first-line treatment for advanced HCC, since the former is seemed to be a slight superiority.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics committee of Henan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
CZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition. QZ: Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. XQ: Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. ZF: Data curation, Investigation, Methodology, Software, Writing – review & editing. WZ: Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing. YW: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1585956/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Center. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
3. Villanueva A. Hepatocellular carcinoma. N Engl J Med. (2019) 380:1450–62. doi: 10.1056/NEJMra1713263
4. Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, and Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. (2023) 79:516–37. doi: 10.1016/j.jhep.2023.03.017
5. Vogel A, Meyer T, Sapisochin G, Salem R, and Saborowski A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/S0140-6736(22)01200-4
6. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
7. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
8. Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
9. Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. (2023) 402:1133–46. doi: 10.1016/S0140-6736(23)00961-3
10. Chan SL, Ryoo B-Y, Mo F, Chan LL, Cheon J, Li L, et al. Multicentre phase II trial of cabozantinib in patients with hepatocellular carcinoma after immune checkpoint inhibitor treatment. J Hepatol. (2024) 81:258–64. doi: 10.1016/j.jhep.2024.03.033
11. Huang Y, Kim BYS, Chan CK, Hahn SM, Weissman IL, and Jiang W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat Rev Immunol. (2018) 18:195–203. doi: 10.1038/nri.2017.145
12. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, and Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. (2018) 15:325–40. doi: 10.1038/nrclinonc.2018.29
13. Casak SJ, Donoghue M, Fashoyin-Aje L, Jiang X, Rodriguez L, Shen Y-L, et al. FDA approval summary: atezolizumab plus bevacizumab for the treatment of patients with advanced unresectable or metastatic hepatocellular carcinoma. Clin Cancer Res. (2021) 27:1836–41. doi: 10.1158/1078-0432.CCR-20-3407
14. Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim T-Y, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. (2021) 22:991–1001. doi: 10.1016/S1470-2045(21)00151-0
15. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
16. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. (2021) 22:977–90. doi: 10.1016/S1470-2045(21)00252-7
17. Zhang W, Zhang K, Liu C, Gao W, Si T, Zou Q, et al. Hepatic arterial infusion chemotherapy combined with anti-PD-1/PD-L1 immunotherapy and molecularly targeted agents for advanced hepatocellular carcinoma: a real world study. Front Immunol. (2023) 14:1127349. doi: 10.3389/fimmu.2023.1127349
18. Xie D, Sun Q, Wang X, Zhou J, Fan J, Ren Z, et al. Immune checkpoint inhibitor plus tyrosine kinase inhibitor for unresectable hepatocellular carcinoma in the real world. Ann Transl Med. (2021) 9:652. doi: 10.21037/atm-20-7037
19. Chen J, Hu X, Li Q, Dai W, Cheng X, Huang W, et al. Effectiveness and safety of toripalimab, camrelizumab, and sintilimab in a real-world cohort of hepatitis B virus associated hepatocellular carcinoma patients. Ann Transl Med. (2020) 8:1187. doi: 10.21037/atm-20-6063
20. Bureau of Medical Administration and National Health Commission of the People’sRepublic of China. Guideline for diagnosis and treatment of hepatocellular carcinoma (2024 edition). Chin J Pract Surg. (2024) 44:361–86.
21. Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2017) 389:56–66. doi: 10.1016/S0140-6736(16)32453-9
22. Zhou J, Fan J, Jiao SC, Bai L, Luo J, Bai Y, et al. LBA40 Primary results from the phase III ALTN-AK105-III-02 study: Anlotinib plus penpulimab versus sorafenib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. (2024) 35:S1231. doi: 10.1016/j.annonc.2024.08.2280
23. Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: A randomized, open-label, parallel-controlled phase II-III trial. J Clin Oncol. (2021) 39:3002–11. doi: 10.1200/JCO.21.00163
24. Lencioni R and Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. (2010) 30:52–60. doi: 10.1055/s-0030-1247132
25. Freites-Martinez A, Santana N, Arias-Santiago S, and Viera A. Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed). (2021) 112:90–2. doi: 10.1016/j.ad.2019.05.009
26. Thompson JA, Schneider BJ, Brahmer J, Zaid MA, Achufusi A, Armand P, et al. NCCN guidelines® Insights: management of immunotherapy-related toxicities, version 2.2024. J Natl Compr Canc Netw. (2024) 22:582–92. doi: 10.6004/jnccn.2024.0057
27. Ciliberto D, Caridà G, Staropoli N, Romeo C, Arillotta GM, Napoli C, et al. First-line systemic treatment for hepatocellular carcinoma: A systematic review and network meta-analysis. Heliyon. (2023) 9:e18696. doi: 10.1016/j.heliyon.2023.e18696
28. Fulgenzi CAM, Scheiner B, Korolewicz J, Stikas C-V, Gennari A, Vincenzi B, et al. Efficacy and safety of frontline systemic therapy for advanced HCC: A network meta-analysis of landmark phase III trials. JHEP Rep. (2023) 5:100702. doi: 10.1016/j.jhepr.2023.100702
29. Zeng X, Jia Y, Chen H, Luo Q, Zhao H, Liang G, et al. A real-world analysis of survival and cost-effectiveness of sintilimab plus bevacizumab biosimilar regimen in patients with advanced hepatocellular carcinoma. J Cancer Res Clin Oncol. (2023) 149:9213–9. doi: 10.1007/s00432-023-04775-2
30. Chen X, Li W, Wu X, Zhao F, Wang D, Wu H, et al. Safety and efficacy of sintilimab and anlotinib as first line treatment for advanced hepatocellular carcinoma (KEEP-G04): A single-arm phase 2 study. Front Oncol. (2022) 12:909035. doi: 10.3389/fonc.2022.909035
31. Zuo M, Cao Y, Yang Y, Zheng G, Li D, Shao H, et al. Hepatic arterial infusion chemotherapy plus camrelizumab and apatinib for advanced hepatocellular carcinoma. Hepatol Int. (2024) 18:1486–98. doi: 10.1007/s12072-024-10690-6
32. Zeng Z, Jiang Y, Liu C, Zhu G, Ma F, Yang L, et al. Efficacy and biomarker exploration of camrelizumab combined with apatinib in the treatment of advanced primary liver cancer: a retrospective study. Anticancer Drugs. (2021) 32:1093–8. doi: 10.1097/CAD.0000000000001127
33. Li Q, Cao M, Yuan G, Cheng X, Zang M, Chen M, et al. Lenvatinib plus camrelizumab vs. Lenvatinib monotherapy as first-line treatment for unresectable hepatocellular carcinoma: A multicenter retrospective cohort study. Front Oncol. (2022) 12:809709. doi: 10.3389/fonc.2022.809709
Keywords: sintilimab, camrelizumab, hepatocellular carcinoma, survival analysis, real-world study
Citation: Zhao C, Zhu Q, Qian X, Fu Z, Zhang W and Wang Y (2025) The safety and effectiveness of sintilimab versus camrelizumab, both plus targeted drugs, in advanced hepatocellular carcinoma. Front. Immunol. 16:1585956. doi: 10.3389/fimmu.2025.1585956
Received: 01 March 2025; Accepted: 06 June 2025;
Published: 23 June 2025.
Edited by:
Lekshmi R. Nath, Amrita Vishwa Vidyapeetham, IndiaReviewed by:
Wan Zhuo, Air Force Medical University, ChinaMerin Babu, Amrita Vishwa Vidyapeetham University, India
Copyright © 2025 Zhao, Zhu, Qian, Fu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaqin Wang, eXF3YW5nXzA4MTNAenp1LmVkdS5jbg==
 Chenglong Zhao
Chenglong Zhao Qiongni Zhu
Qiongni Zhu Xiaoyan Qian
Xiaoyan Qian Zhonghua Fu
Zhonghua Fu Wei Zhang1
Wei Zhang1 Yaqin Wang
Yaqin Wang