- 1Department of Emergency, Sichuan Academy of Medical Sciences, Sichuan Provincial People’s Hospital, Chengdu, China
- 2Department of Pathology, Sichuan Academy of Medical Sciences, Sichuan Provincial People’s Hospital, Chengdu, China
Background: A secondary bacterial infection, which has a high incidence in patients with critical coronavirus disease 2019 (COVID-19), has been proven to have an association with increased mortality. Adaptive immune responses have been detected in almost all COVID-19 cases. This study aimed to determine whether the levels of immune-inflammatory factors are associated with coinfection in patients with critical COVID-19.
Methods: Patients with a confirmed critical severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection were enrolled in this single-center cohort study. Clinical data and venous blood samples were collected on the day of hospital admission. All patients were divided into two groups according to the presence of bacterial coinfection or absence of bacterial coinfection, which were then divided into two groups (survived group and deceased group) based on the outcome of the disease during hospitalization.
Results: Patients with coinfection had a higher mortality rate (83.3% VS 50.0%, P<0.001) and longer hospital stays (25.15 VS 13.80d, P<0.001). We observed that patients who developed coinfection tended to have a significantly lower number of CD4+ T cells (121.19 VS 207.83cells/µL, P=0.001) and CD8+ T cells (79 VS 158cells/µL, P=0.006) and a higher proportion of CD4+CD8+ double-positive T (DPT) cells (3.66% VS 1.91%, P=0.011) on the day of hospital admission. The tests for inflammatory cytokines showed a higher level of IL-4 (0.99 VS 0.42pg/mL, P<0.001) and IL-6 (109.60 VS 63.59pg/mL, P=0.009) in coinfection group. And the multivariant analyses also revealed that CD4+ cell counts < 199.5cells/µL, CD8+ cell counts < 124.5cells/µL, IL4 > 0.535pg/mL, IL6 > 388.9pg/mL could be independent risk factors for coinfection. Moreover, in the coinfection group, we observed that the deceased patients had a lower level of total lymphocytes, T cells, and albumin.
Conclusion: Our study found that lymphocyte subsets and cytokines play an important role in predicting bacterial coinfection in patients with critical COVID-19. Lower levels of CD4+ and CD8+ cells and higher level of IL4 and IL6 in patients on the day of admission were significantly correlated with the development of coinfection the following days in the hospital.
Introduction
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global public health challenge. According to the reports regarding patients from different districts in mainland China, while the signs and symptoms of most COVID-19 patients are usually mild to moderate, approximately 15–20% of individuals progress to severe interstitial pneumonia (1) with a 2–3% death rate (2). Secondary bacterial infection can be an important cause of mortality. A recent study reported that the prevalence of secondary bacterial infections was 18.4% (3). However, patients with critical COVID-19 are reported to have a 32.7% to 100% incidence of secondary bacterial infections (4). The presence of coinfection has been associated with increased mortality and prolonged hospital stay (5).
A previous study indicated that a higher white blood cell count, neutrophil count, and C-reactive protein (CRP) level were predictive of early bacterial coinfection in hospitalized patients with COVID-19 pneumonia (6). However, the role of cell-mediated immunity in association with COVID-19 coinfection has not been thoroughly elucidated. The adaptive immune system responds to pathogens in an antigen-specific manner, leading to protective immunity. T cell responses are detectable in nearly all patients infected with SARS-CoV-2 (7). Among the three major lymphocyte subsets of the adaptive immune system, the response of CD4+ T cells and B cells to SARS-CoV-2 is more prominent than that of CD8+ T cells (8) and are associated with the control of primary SARS-CoV-2 infection (9). Previous studies demonstrated that the level of CD8+ cells was an independent risk factor for the severity of COVID-19 (1). And it was reported that a specific phenotype of senescent effector CD8+ T cells exclusively present in critically ill patients with COVID-19 (10). Our study sought to examine whether levels of immune-inflammation factors and other clinical characteristics are associated with the development and prognosis of coinfection in critically ill patients with COVID-19.
Methods
A total of 98 patients with polymerase chain reaction (PCR) confirmed SARS-CoV-2 infection in Sichuan Provincial People’s Hospital for COVID-19 infection were enrolled between December 2022 and January 2023. The inclusion criteria were as follows: (1) age > 18 years; (2) ICU admission due to respiratory failure, shock, or other organ failure; and (3) SARS-CoV-2 infection confirmed by PCR testing.
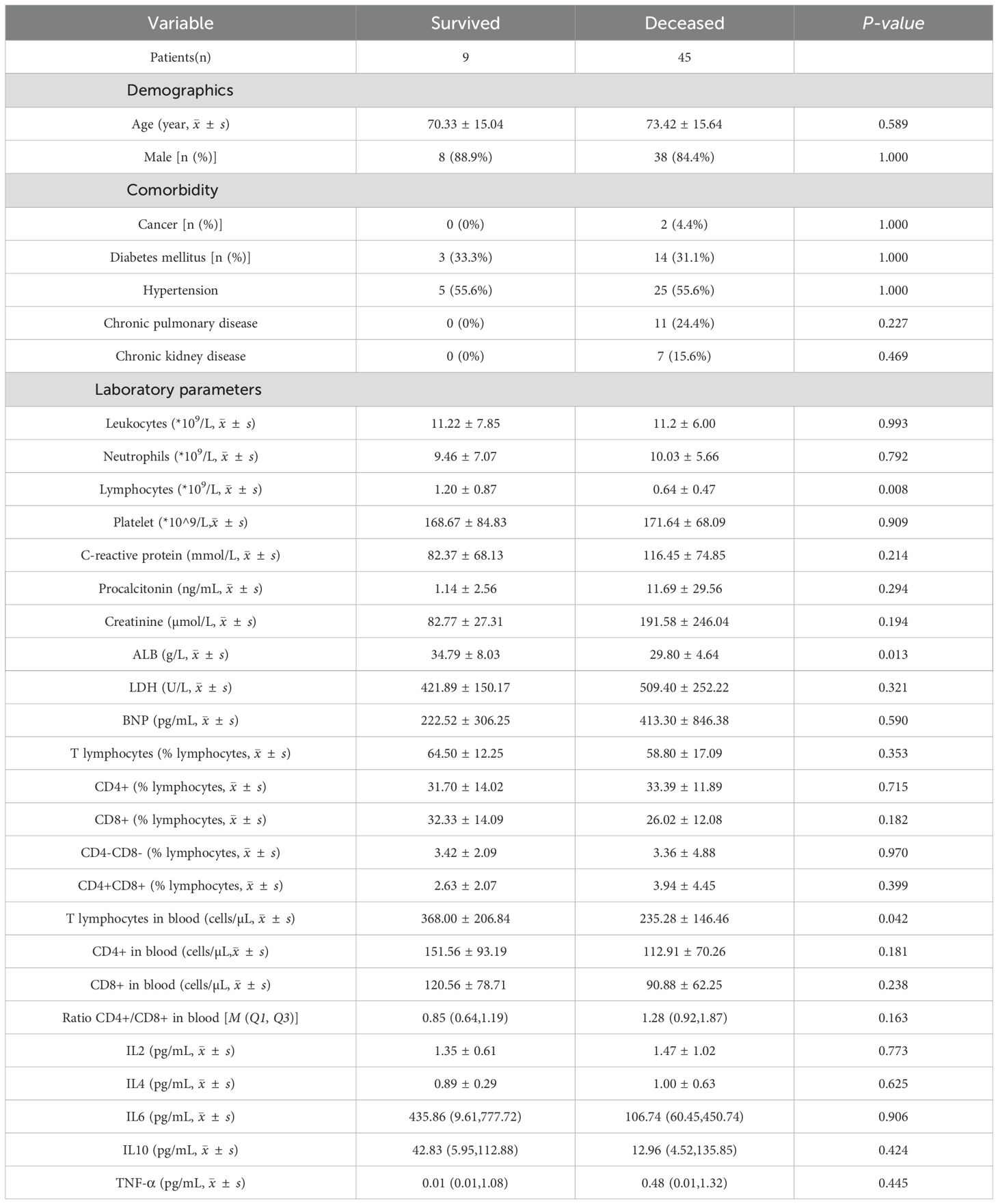
Patient clinical data, including age, sex, and medical history, were collected. Venous blood tests examining routine profiles, coagulation function, renal function, inflammatory cytokines, and lymphocyte subsets were performed on the day of hospital admission. Patients were divided into two groups according to the presence or absence of bacterial coinfection. Coinfection was defined by one or more positive microbiologicalevidence of bacterial coinfection (including positive hemoculture, sputum culture, urine culture, or other body fluid culture) obtained after confirmation of COVID-19 infection with the exclusion of the bacteria which considered to be colonization and contamination. Patients with bacterial coinfection were then divided into a survived group (n=9) and a deceased group (n=45) according to the outcome of the disease during hospitalization.
Statistical analyses were performed using the IBM SPSS Statistics version 25. The distribution normality of all continuous variables was assessed using the Shapiro–Wilk test. Variables with normal distribution were presented as means ± standard deviation and analyzed using the Student’s t-test, otherwise as medians (with interquartile ranges) and analyzed using the Mann–Whitney U test. Categorical variables are presented as percentages of the total and were analyzed using the chi-square test or Fisher’s exact test. A two-tailed P-value of < 0.05, was evaluated by receiver operating characteristic (ROC) curves and areas under the ROC curves (AUCs). For parameters that were significant based on univariate analyses, stepwise backward logistic regression was used to test the influence of the independent variables.
Results
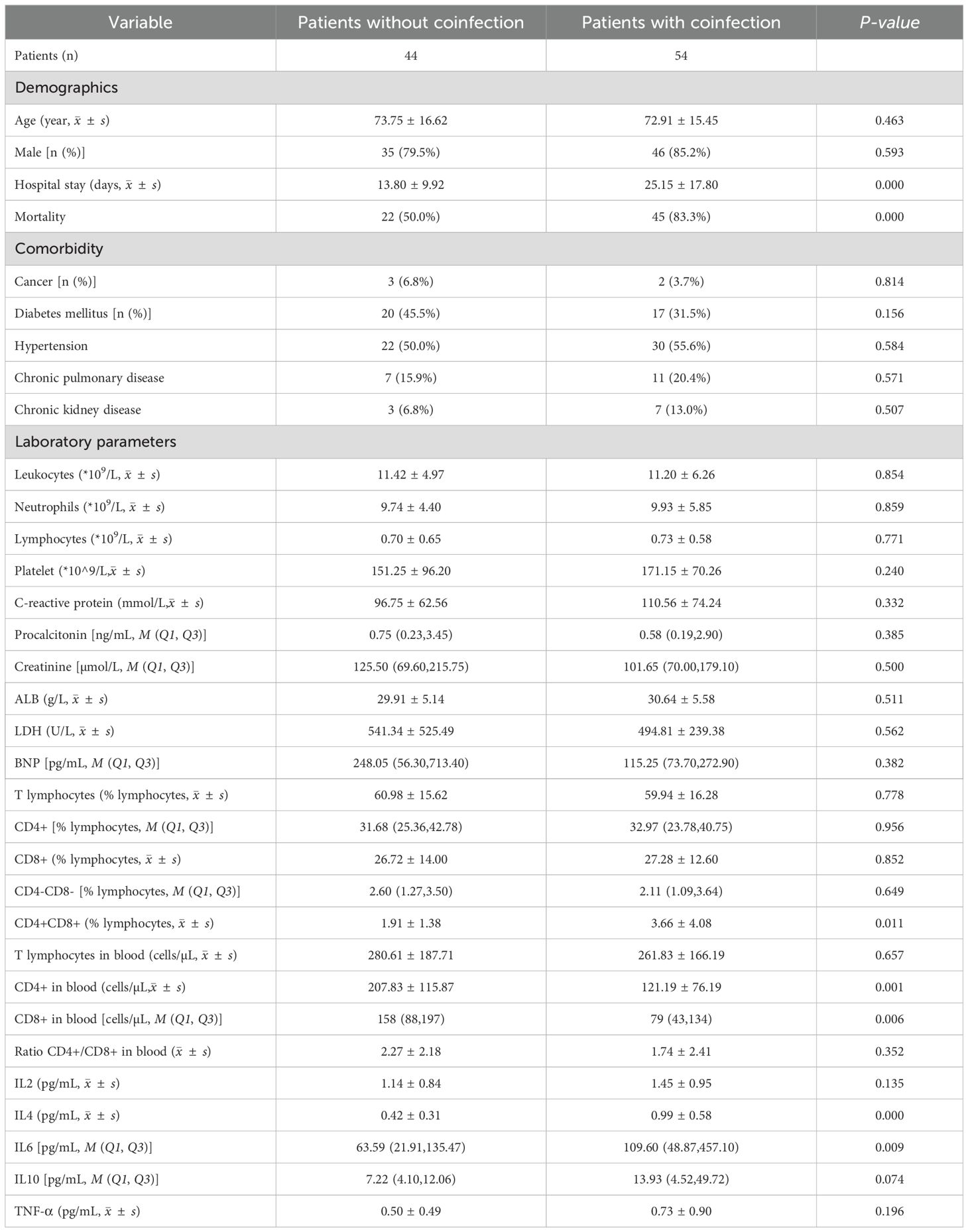
Among the 98 patients with COVID-19, 54 (55.1%) were diagnosed with confirmed bacterial infection, 30 of whom were infected by drug-resistant bacteria, including methicillin-resistant Staphylococcus aureus, carbapenem-resistant Acinetobacter baumannii, Klebsiella pneumoniae and pseudomonas aeruginosa. The identified seven bacteria were Acinetobacter baumannii, Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Enterobacter aerogenes, Staphylococcus aureus and Enterococcus faecium. Coinfections were diagnosed approximately 11 ± 7 days after hospital admission (Table 1). There were no significant differences in age, sex, BMI (body mass index) and the proportion of participants with comorbidities of cancer or hematologic malignancy, diabetes, hypertension, chronic cardiovascular disease, autoimmune disease, chronic kidney disease, or chronic pulmonary disease between patients with and without coinfection. Patients with co-infection had higher mortality (83.3% VS 50.0%, P<0.001) and longer hospital stays (25.15 VS 13.80, P<0.001) during hospital admission. Regarding laboratory parameters, we did not find significant differences in routine blood examination and CRP or procalcitonin (PCT) levels between the two groups. The lymphocyte subset showed that patients with co-infection had a lower amount of CD4+ T cells (121.19 VS 207.83cells/µL, P=0.001) and CD8+ T cells (79 VS 158cells/µL, P=0.006) on the day of hospital admission, with a higher proportion of CD4+CD8+ double-positive T (DPT) (3.66% VS 1.91%, P=0.011). And the data of inflammatory cytokines showed a higher level of IL-4 (0.99 VS 0.42pg/mL, P<0.001) and IL-6 (109.60 VS 63.59pg/mL, P=0.009) in patients who develop with coinfection than those without.
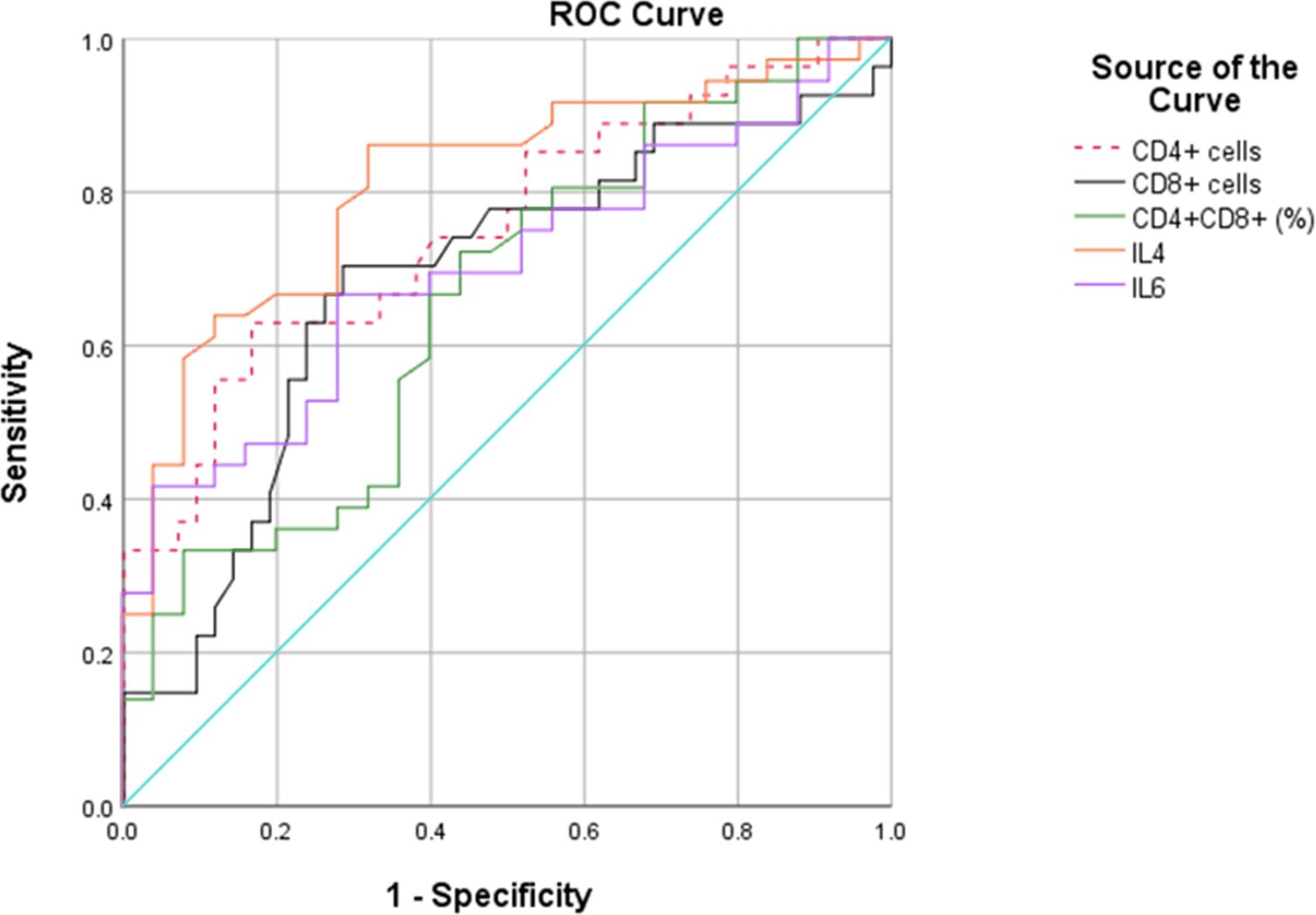
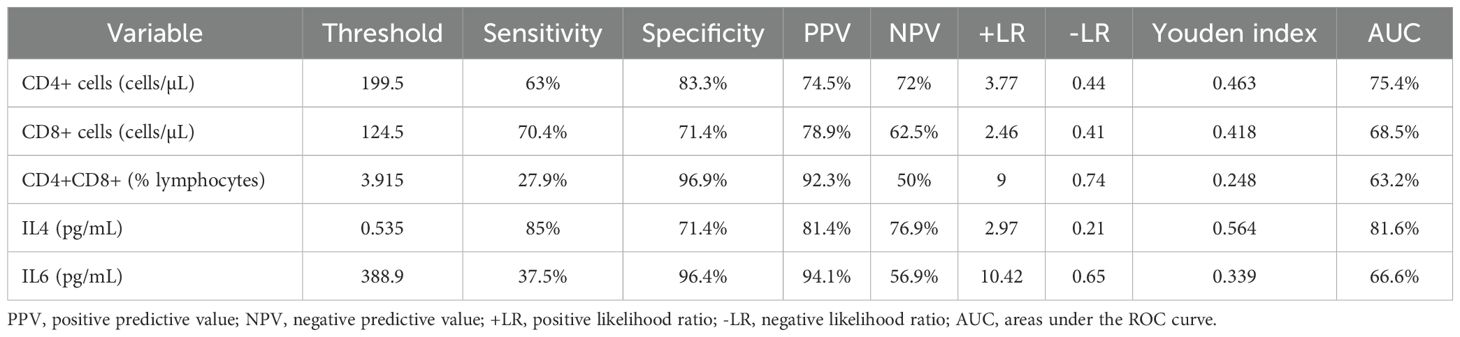
We then performed ROC curves to determine the best cut-off for all parameters that were statistically significant between the two groups (Figure 1, Table 2). The IL4 level of 0.535pg/mL and a CD4+ cell counts of 199.5cells/µL provided the best overall accuracy. The AUC of the IL4 level was 81.6%, with a positive predictive value (PPV) of 81.4% and a negative predictive value (NPV) of 76.9%. The AUC of the CD4+ cell count was 75.4%, with a PPV of 74.5% and an NPV of 72%. The other thresholds were CD8+ cell counts < 124.5cells/µL, CD4+CD8+ (% lymphocytes) > 3.915 and IL6 > 388.9 pg/mL. The proportion of CD4+CD8+ cells had the highest specificity with a PPV of 92.3%; however, the NPV was only 50%.
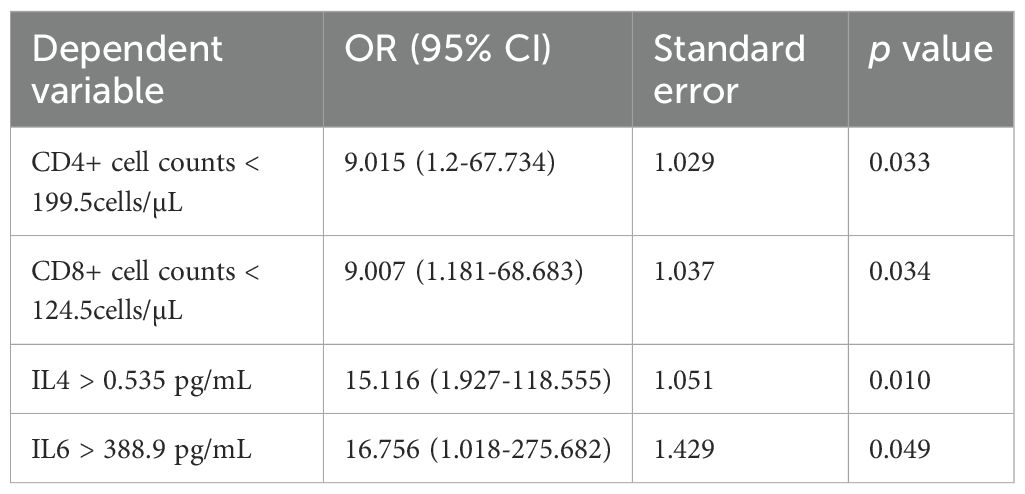
We then conducted a stepwise logistic regression analysis to identify independent associations between co-infection and the variables (Table 3). The results showed that CD4+ cell counts < 199.5cells/µL, CD8+ cell counts < 124.5cells/µL, IL4 > 0.535 pg/mL, IL6 > 388.9 pg/mL could be independent risk factors for coinfection.
Within the coinfection group, only nine (16.7%) patients survived, while the other 45 (83.3%) patients died during hospitalization (Table 4). Age, sex, BMI, and the proportion of participants with comorbidities were not significantly different between the deceased and survived groups. However, we observed that deceased patients had lower levels of total lymphocytes, T cells, and albumin.
Discussion
The presence of bacterial coinfection has been proved to be associated with increased mortality. A previous study revealed an excess of 50% deceased COVID-19 patients compared with patients without coinfection (11). Our study showed an incidence of 55.1% among patients with a confirmed bacterial infection during admission. Consistent with previous studies (5), more patients in the coinfection group died during hospital admission, which is most likely due to cytokine dysregulation, changes to immune cell activation and function, mucociliary dysfunction, and alterations to the respiratory tract epithelium (12). Procalcitonin (PCT) is used as a biomarker to predict bacterial co-infection. However, the results showed that it is not reliable (13). Patients with severe COVID-19 may have elevated PCT levels, but this does not seem to correlate with the presence of bacterial infection. Elevated C-reactive protein (CRP) and ferritin levels have been found in patients with coinfection; however, elevation did not occur before the diagnosis of infection (4). Likewise, neither PCT nor CRP levels were considered to have the ability to predict the onset of infection in our study.
Adaptive immune responses are important in controlling viral infections that cause diseases in humans. Lymphocytopenia is considered to be a prominent cause of severe COVID-19 (14). T lymphocyte subsets are key components of the adaptive immune system and are important factors in killing infected cells and supporting antibody-generating B cells (15). T cell dysfunction was considered to be highly connected and associated with COVID-19 severity (10). Previous studies (1, 16) have found that CD4+ and CD8+ T cells are significantly decreased in severe patients compared to non-severe patients, and are independently predictive of patient outcomes. We found that patients with co-infection had significantly lower levels of lymphocytes and T cells. However, no study has examined the relationship between T cell subsets and coinfection in patients with COVID-19. In our study, we revealed that patients with lower levels of CD4+ T cells and CD8+ T cells were more prone to co-infection with bacteria. Given that the immunosuppression could hamper bacterial clearance by inhibiting neutrophil and T-cell responses, we suggested that CD4+T cell counts lower than 199.5cells/µL, CD8+ T cells < 124.5cells/µL could be strong predisposing factors for the coinfection. Moreover, using two-color fluorescence analysis, peripheral CD4+CD8+ double-positive (DP) T cells were first found in humans in 1986 (17), and their levels vary depending on tissue distribution, health status, and age (18). However, the function of CD4+CD8+ DP T cells has not yet been clearly described and remains controversial in different studies. Cytotoxicity (19, 20) and suppressive roles (21, 22) are the two most common views. In our study, patients with co-infections tended to have a higher proportion of CD4+CD8+ DP T cells. We also found higher level of IL4 in patients with co-infections. This was consistent with a previous study that revealed that CD4+CD8+ DP T cells can produce elevated levels of IL-4, but not IFN-γ, IL-2, or IL-10, compared with CD4+ T cells or CD8+ T cells (23). Therefore, our results support a cytotoxic role for CD4+CD8+ DP T cells which expanded in response to SARS-CoV-2.
The levels of IL-1β, IL-2R, IL-6, and TNF-α were found to be significantly higher than the upper limits of normal in patients with confirmed secondary infections (24, 25). In another study, increased IL-6, IL-8, IL-10, and IFN-γ levels were found in severe patients compared to those in non-severe patients (1). We showed that patients with coinfection had higher level of IL4 and IL6 on the day of admission. IL-6 plays an important role in initiating antibacterial inflammation and can trigger a cascade of inflammatory mediators (26), is negatively correlated with NK cells and CD8+ T cells (27) and is considered to have the ability to predict the development of fatal SARS-CoV-2 pneumonia (28). Therefore, clinicians expected that interleukin-6 receptor blockade (Tocilizumab) could interrupt this inflammatory cascade at a early stage. However, even though several retrospective observational studies showed a positive effect on mortality, one randomised double-blind, placebo-controlled study suggested they found no significant effect on 28-day survival in severe COVID-19 cases while another two clinical trials were stopped early after an increased number of deaths in the tocilizumab group (29). Another prospective clinical trial in moderately ill patients hospitalized with COVID-19 also suggested that tocilizumab was not effective for preventing intubation or death (30). Meanwhile, IL-4 exerts both immunostimulatory and immunosuppressive activities. Previous studies have suggested that IL‐4 shown a protective role in acute lung injury, acute kidney injury and influenza/S. pneumoniae co‐infection (31). In our study, IL4 andIL6 were found to be potential biomarkers for predicting the development of coinfection in patients with COVID-19. And IL6 > 388.9 pg/mL and IL4>0.535pg/mL were independent risk factors for co-infection.
Our study had several limitations. First, it included a small number of patients. These results should be verified in larger cohorts. Second, our study lacks follow-up data. We did not follow the patient survival after discharge. Third, CD4+ and CD8+ lymphocyte subpopulations were not detected. Further mechanistic studies are needed to determine the exact role of the T cell subsets. Therefore, a larger patient cohort with more laboratory tests is warranted to validate our findings.
Conclusion
In conclusion, the levels of lymphocyte subsets and cytokines can be used as biomarkers for predicting bacterial coinfection in patients with critical COVID-19. Patients with lower levels of total lymphocytes and T cells tend to have poor prognosis. Therefore, monitoring the levels of immune inflammatory factors might be important for assessing the development and prognosis of coinfection in patients with critical COVID-19.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Sichuan Academy of Medical Science & Sichuan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Because the study design was retrospective.
Author contributions
XL: Data curation, Methodology, Resources, Validation, Writing – original draft. HP: Methodology, Resources, Writing – original draft. YW: Conceptualization, Data curation, Writing – original draft. SH: Data curation, Resources, Writing – original draft. XY: Writing – original draft, Data curation. JC: Conceptualization, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kang Y, Lu S, Zhong R, You J, Chen J, Li L, et al. The immune inflammation factors associated with disease severity and poor prognosis in patients with COVID-19: A retrospective cohort study. Heliyon. (2024) 10:e23583. doi: 10.1016/j.heliyon.2023.e23583
2. Dansana D, Kumar R, Bhattacharjee A, Hemanth DJ, Gupta D, Khanna A, et al. Early diagnosis of COVID-19-affected patients based on X-ray and computed tomography images using deep learning algorithm. Soft computing. (2023) 27:2635–43. doi: 10.1007/s00500-020-05275-y
3. Langford BJ, So M, Simeonova M, Leung V, Lo J, Kan T, et al. Antimicrobial resistance in patients with COVID-19: a systematic review and meta-analysis, The Lancet. Microbe. (2023) 4:e179–91. doi: 10.1016/S2666-5247(22)00355-X
4. Orsini EM, Sacha GL, Han X, Wang X, Duggal A, Rajendram P. Risk factors associated with development of coinfection in critically Ill patients with COVID-19. Acute Crit Care. (2022) 37:312–21. doi: 10.4266/acc.2022.00136
5. Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PloS One. (2021) 16:e0251170. doi: 10.1371/journal.pone.0251170
6. Satjawattanavimol S, Teerapuncharoen K, Kaewlai R, Disayabutr S. Prevalence of early bacterial co-infection in hospitalized patients with COVID-19 pneumonia: a retrospective study. J thoracic Dis. (2023) 15:3568–79. doi: 10.21037/jtd-22-1681
7. Sette A, Crotty S. Adaptive immunity to SARS-coV-2 and COVID-19. Cell. (2021) 184:861–80. doi: 10.1016/j.cell.2021.01.007
8. Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T cell responses to SARS-coV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. (2020) 181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015
9. Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-coV-2 in acute COVID-19 and associations with age and disease severity. Cell. (2020) 183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038
10. Li W, Qian R, Zhou Z, Wen L, Yin Q, Zhou X, et al. T cell senescence may contribute to immunothrombosis via Th17 immune transition in COVID-19. Sci Bull. (2024) 69:3501–6. doi: 10.1016/j.scib.2024.04.068
11. Zhu X, Tian F, Li Y, Lu Q, Long Q, Long X, et al. High prevalence of respiratory co-infections and risk factors in COVID-19 patients at hospital admission during an epidemic peak in China. Infection Drug resistance. (2023) 16:6781–93. doi: 10.2147/IDR.S435143
12. Mochan E, Sego TJ. Mathematical modeling of the lethal synergism of coinfecting pathogens in respiratory viral infections: A review. Microorganisms. (2023) 11(12):2974. doi: 10.3390/microorganisms11122974
13. Vanhomwegen C, Veliziotis I, Malinverni S, Konopnicki D, Dechamps P, Claus M, et al. Procalcitonin accurately predicts mortality but not bacterial infection in COVID-19 patients admitted to intensive care unit. Irish J Med Sci. (2021) 190:1649–52. doi: 10.1007/s11845-020-02485-z
14. Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Deng Y, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int J Infect diseases: IJID: Off Publ Int Soc Infect Dis. (2020) 96:131–5. doi: 10.1016/j.ijid.2020.04.086
15. Shepherd FR, McLaren JE. T cell immunity to bacterial pathogens: mechanisms of immune control and bacterial evasion. Int J Mol Sci. (2020) 21(17):6144. doi: 10.3390/ijms21176144
16. Huang W, Berube J, McNamara M, Saksena S, Hartman M, Arshad T, et al. Lymphocyte subset counts in COVID-19 patients: A meta-analysis. Cytometry Part A: J Int Soc Analytical Cytology. (2020) 97:772–6. doi: 10.1002/cyto.a.24172
17. Blue ML, Daley JF, Levine H, Craig KA, Schlossman SF. Biosynthesis and surface expression of T8 by peripheral blood T4+ cells in vitro. J Immunol (Baltimore Md: 1950). (1986) 137:1202–7. doi: 10.4049/jimmunol.137.4.1202
18. Overgaard NH, Jung JW, Steptoe RJ, Wells JW. CD4+/CD8+ double-positive T cells: more than just a developmental stage? J leukocyte Biol. (2015) 97:31–8. doi: 10.1189/jlb.1RU0814-382
19. Kitchen SG, Jones NR, LaForge S, Whitmire JK, Vu BA, Galic Z, et al. CD4 on CD8(+) T cells directly enhances effector function and is a target for HIV infection. Proc Natl Acad Sci United States America. (2004) 101:8727–32. doi: 10.1073/pnas.0401500101
20. Kitchen SG, Whitmire JK, Jones NR, Galic Z, Kitchen CM, Ahmed R, et al. The CD4 molecule on CD8+ T lymphocytes directly enhances the immune response to viral and cellular antigens. Proc Natl Acad Sci United States America. (2005) 102:3794–9. doi: 10.1073/pnas.0406603102
21. Parel Y, Aurrand-Lions M, Scheja A, Dayer JM, Roosnek E, Chizzolini C. Presence of CD4+CD8+ double-positive T cells with very high interleukin-4 production potential in lesional skin of patients with systemic sclerosis. Arthritis rheumatism. (2007) 56:3459–67. doi: 10.1002/art.v56:10
22. Das G, Augustine MM, Das J, Bottomly K, Ray P, Ray A. An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc Natl Acad Sci United States America. (2003) 100:5324–9. doi: 10.1073/pnas.0831037100
23. Zloza A, Sullivan YB, Connick E, Landay AL, Al-Harthi L. CD8+ T cells that express CD4 on their surface (CD4dimCD8bright T cells) recognize an antigen-specific target, are detected in vivo, and can be productively infected by T-tropic HIV. Blood. (2003) 102:2156–64. doi: 10.1182/blood-2002-07-1972
24. Wu G, Lu J, Liu D, He Y. Characteristics and risk factors of secondary bacterial infections in COVID-19 patients. Antimicrobial stewardship healthcare epidemiology: ASHE. (2023) 3:e156. doi: 10.1017/ash.2023.425
25. Lee JS, Park S, Jeong HW, Ahn JY, Choi SJ, Lee H, et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol. (2020) 5:eabd1554. doi: 10.1126/sciimmunol.abd1554
26. Ulhaq ZS, Soraya GV. Interleukin-6 as a potential biomarker of COVID-19 progression. Medecine maladies infectieuses. (2020) 50:382–3. doi: 10.1016/j.medmal.2020.04.002
27. Bordoni V, Sacchi A, Cimini E, Notari S, Grassi G, Tartaglia E, et al. An inflammatory profile correlates with decreased frequency of cytotoxic cells in coronavirus disease 2019. Clin Infect diseases: an Off Publ Infect Dis Soc America. (2020) 71:2272–5. doi: 10.1093/cid/ciaa577
28. Wang Y, Perlman S. COVID-19: inflammatory profile. Annu Rev Med. (2022) 73:65–80. doi: 10.1146/annurev-med-042220-012417
29. McElvaney OJ, Curley GF, Rose-John S, McElvaney NG. Interleukin-6: obstacles to targeting a complex cytokine in critical illness, The Lancet. Respiratory Med. (2021) 9:643–54. doi: 10.1016/S2213-2600(21)00103-X
30. Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with covid-19. New Engl J Med. (2020) 383:2333–44. doi: 10.1056/NEJMoa2028836
Keywords: COVID-19, critical pneumonia, bacterial coinfection, immunological characteristics, lymphocyte subsets
Citation: Li X, Peng H, Wang Y, He S, Yang X and Chen J (2025) The subsets of blood circulating T-cells associated with the development and prognosis of coinfection in patients with critical COVID-19. Front. Immunol. 16:1586302. doi: 10.3389/fimmu.2025.1586302
Received: 04 March 2025; Accepted: 22 April 2025;
Published: 09 May 2025.
Edited by:
Prof Shailendra Saxena, King George’s Medical University, IndiaReviewed by:
Wenxing Li, Columbia University, United StatesMohammad Arish, University of Virginia, United States
Copyright © 2025 Li, Peng, Wang, He, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiayue Chen, eGRjaGVuamlheXVlQDE2My5jb20=
†These authors have contributed equally to this work
 Xingming Li1†
Xingming Li1† Xueting Yang
Xueting Yang Jiayue Chen
Jiayue Chen