- 1State Key Laboratory of Experimental Hematology, National Clinical Research Center for Blood Diseases, Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, China
- 2Haihe Laboratory of Cell Ecosystem, Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, China
- 3Tianjin Institutes of Health Science, Tianjin, China
Introduction: The prognostic significance of body mass index (BMI) in elderly acute myeloid leukemia (AML) patients undergoing allogeneic hematopoietic cell transplantation (allo-HCT) remains controversial.
Methods: This retrospective study analyzed 142 AML patients aged ≥50 years receiving allo-HCT (2013-2022), stratified by Chinese BMI criteria: low BMI (<24 kg/m², n = 83) vs. high BMI (≥24 kg/m², n = 59).
Results: The median pre-transplant BMI was 23.63 (IQR, 22.07-25.78) kg/m². Multivariate analysis identified BMI <24 kg/m² as an independent risk factor for inferior OS (HR=1.80, p=0.037) and GRFS (HR=2.00, p = 0.003). Although BMI did not correlate with relapse, long-term non-relapse mortality (NRM), or the incidence of acute and chronic graft versus host disease (GVHD), the one-year NRM was significantly higher in the low BMI group compared to the high BMI group (p = 0.006). Subgroup analysis revealed that high-risk patients [not complete remission (NR) or CR but minimal residual disease (MRD)-positive) with low BMI had markedly reduced 3-year OS (20.87% vs. 57.69%, p=0.006), whereas no difference was observed in low-risk (CR/MRD-negative) patients.
Discussion: Pre-transplant BMI independently predicts inferior survival in older adults with AML undergoing allo-HCT. These findings highlight the need for BMI-guided nutritional interventions, especially for high-risk older patients.
1 Introduction
Acute myeloid leukemia (AML) is a prevalent malignant myeloid tumor, predominantly occurring in elderly patients. Allogeneic hematopoietic cell transplantation (allo-HCT) remains the only curative treatment for high-risk AML. In recent years, advancements in transplantation technology and increased graft availability have removed age as a contraindication for allo-HCT in elderly AML patients (1, 2).
Nutritional status significantly influences morbidity and mortality in cancer patients (3). Overweight and obesity are recognized health risk factors associated with cardiovascular and metabolic comorbidities (4). For patients undergoing HCT, a pre-transplant BMI of ≥35 kg/m² has been incorporated into the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) to predict post-transplant outcomes (5). However, the impact of BMI on prognosis after allo-HCT remains controversial. A study analyzing 310 adults with acute leukemia from a Chinese center found that a BMI ≥23 kg/m² improved overall survival (OS) following allo-HCT (6). Conversely, weight loss during chemotherapy was associated with inferior OS in AML patients after allo-HCT (3). Recently, a study reported that BMI at transplantation did not influence OS or non-relapse mortality (NRM) in myelofibrosis (MF) patients after allo-HCT, although its impact on relapse incidence was modestly significant (7). The role of BMI in predicting outcomes for elderly patients with AML after allo-HCT is not well established. In AML patients aged ≥60 years undergoing intensive induction chemotherapy, BMI ≥30 kg/m² emerged as an independent predictor of mortality, primarily due to obesity-related comorbidities (8). In contrast, in elderly (≥60 years) patients with myeloid malignancies undergoing allo-HCT, obesity did not impact clinical outcomes, including OS, progression-free survival (PFS), and graft-versus-host disease (GVHD) (9). Therefore, we conducted a retrospective analysis to assess the prognostic significance of BMI in elderly patients following allo-HCT.
2 Methods
2.1 Patients
This was a retrospective study including patients diagnosed with AML under the 2008 WHO guidelines who underwent their first allogeneic hematopoietic cell transplantation (allo-HCT) at age 50 years or older from January 2013 to December 2022 in our center. The stratification risk of AML was classified according to the 2022 European Leukemia Net (ELN) recommendations (10).
All patients in this study received modified myeloablative conditioning (MAC) regimens. The donors were limited to HLA-matched siblings (MSDs) or haploidentical related donors (HIDs). In total,142 patients were analyzed in this study. Patients were regularly followed up until December 2023. This research was approved by the Medical Ethics Committee of the Institute of Hematology & Blood Diseases Hospital and followed the Declaration of Helsinki.
2.2 Treatments
The MAC regimens consisted of intravenous busulfan (3.2 mg/kg/day for 3 days) combined with cyclophosphamide (40 mg/kg/day for 2 days). Anti-thymocyte globulin (ATG) was administered to patients for in vivo T-cell depletion. Calcineurin inhibitors (CNIs) with short-course methotrexate (MTX) were used for graft versus host disease (GVHD) prophylaxis. Comorbidities were assessed with the hematopoietic cell transplantation-specific comorbidity index (HCT-CI).
MRD was assessed by multiparametric flow cytometry (MFC) based on leukemia-associated immunophenotypes or by real-time quantitative PCR (qPCR) based on leukemia-associated fusion genes, including RUNX1/RUNX1T1, CBFβ-MYH11, and MLL.
2.3 Definitions and objectives
The primary endpoint was overall survival (OS). Relapse was defined as the presence of > 5% bone marrow blasts, blasts in peripheral blood, or extramedullary disease. Non-relapse mortality (NRM) was defined as death from any cause, except for relapse. Acute GVHD (aGVHD) was graded according to the Mount Sinai Acute GVHD International Consortium (MAGIC) criteria, and chronic GVHD (cGVHD) was classified as limited or extensive (11). GRFS was defined as survival without grade III–IV aGVHD, extensive cGVHD, or relapse. We defined MRD-positivity as an MRD ≥ 0.01% measured by MFC or MRD ≥ 0.001% measured by qPCR (12, 13).
In accordance with the recommendations of the Working Group on Obesity in China regarding weight classifications for the Chinese population, the suggested BMI categories are as follows: < 18.5 kg/m² (underweight); 18.5-23.9 kg/m² (normal-weight); 24.0-27.9 kg/m² (overweight); ≥ 28.0 kg/m² (obese) (14).Besides, we plotted the hazard ratio (HR) of OS against BMI and observed that the HR for OS approached 1 at a BMI of approximately 23.92 kg/m2 in this study. Therefore, we used a BMI of 24 kg/m2 as the cutoff to define high and low BMI groups, as illustrated in Supplementary Figure 1.
2.4 Statistical analysis
The rates of OS and GRFS were calculated using the Kaplan–Meier method. The probabilities of CIR, NRM, acute GVHD (aGVHD), and chronic GVHD (cGVHD) were calculated by competing risk analysis, accounting for competing risks. For GVHD-related NRM, relapse and NRM from other causes were defined as competing risks. Categorical variables were compared by the chi-squared test or Fisher’s exact test. The Mann–Whitney U test was used to compare continuous variables. Gray’s test and the log-rank test were used to compare variables between groups. Cox proportional hazard models were used for multivariate analysis of OS and GRFS. Fine-Gray methods were used for multivariate analysis of CIR and NRM. BMI at transplantation was included in the multivariate analysis. Besides, variables with P < 0.2 in the univariate analysis were included in the multivariate analysis. Statistical analyses were performed using R software, version 4.1.3, SPSS 20, and Graphpad Prism 10. All p values were two-tailed, and p < 0.05 was considered statistically significant.
3 Results
3.1 Patient characteristics
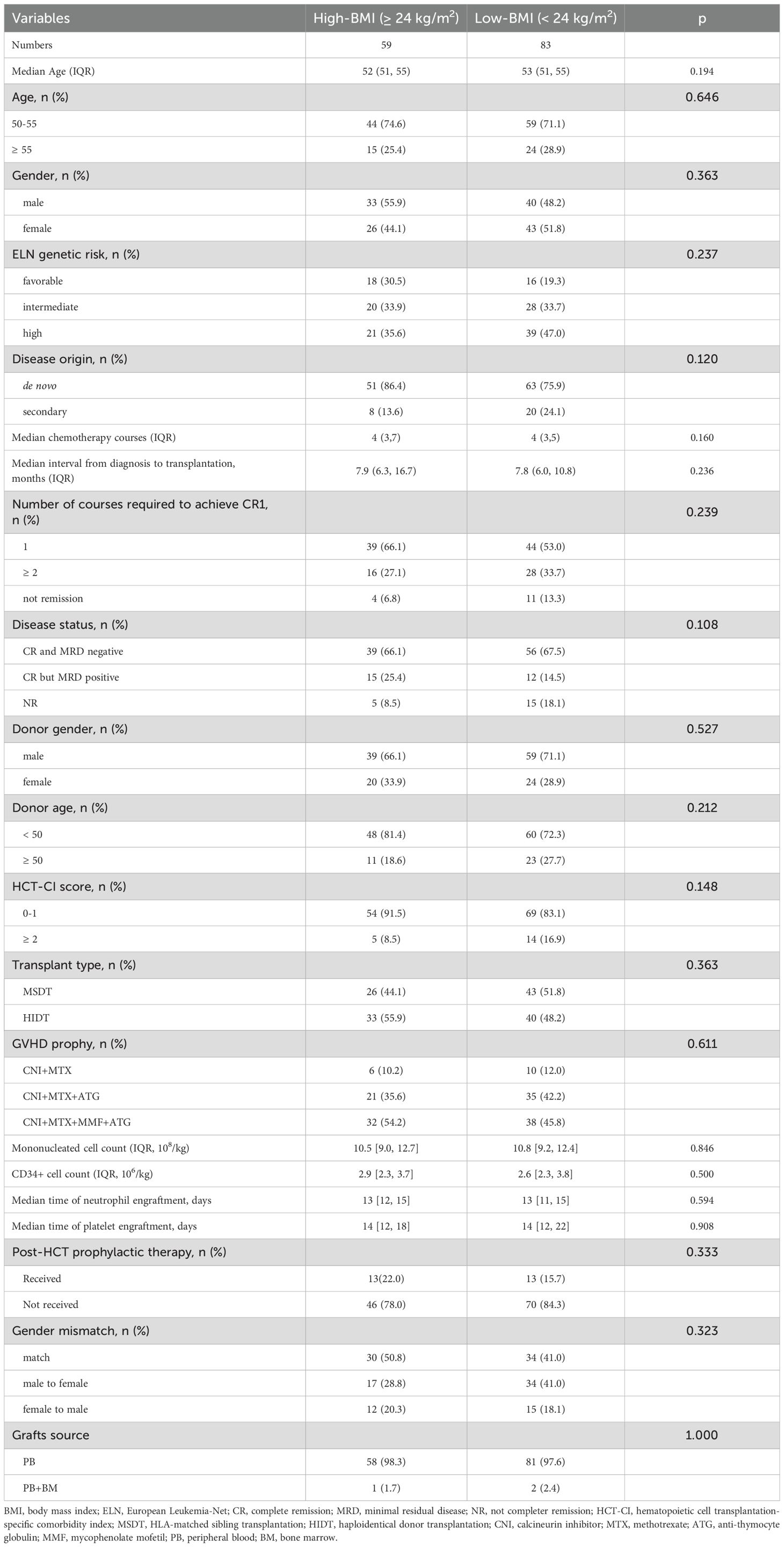
There were 142 AML patients aged 50 years or older who received MAC allo-HCT included in the analysis. The characteristics of the patients stratified by BMI groups are listed in Table 1. Among the 142 patients, 59 (41.5%) had a pre-transplant BMI of 24 kg/m² or higher. The remaining 83 patients (58.5%) had a BMI lower than 24 kg/m², of whom 3 (2.11%) had a BMI lower than 18.5 kg/m². The median pre-transplant BMI of patients was 23.63 (IQR, 22.07-25.78) kg/m². The frequency distribution of pre-transplant BMI values was illustrated in Supplementary Figure 2. The median follow-up time was 42.87 (95% CI, 32.82-52.92) months for patients in the low BMI group and 44.03 (95% CI, 37.73-50.33) months for those in the high BMI group (p = 0.864). A higher proportion of patients in the low BMI group did not achieve complete remission (CR) before transplantation, although the difference did not reach statistical significance (18.1% vs 8.5%; p = 0.108). Additionally, patients in the low BMI group exhibited a higher proportion of secondary AML (24.1% vs 13.6%; p = 0.120). There was no difference in other factors, including age, gender, HCT-CI scores, transplantation type, risk stratification, and GVHD prophylaxis.
3.2 Overall survival
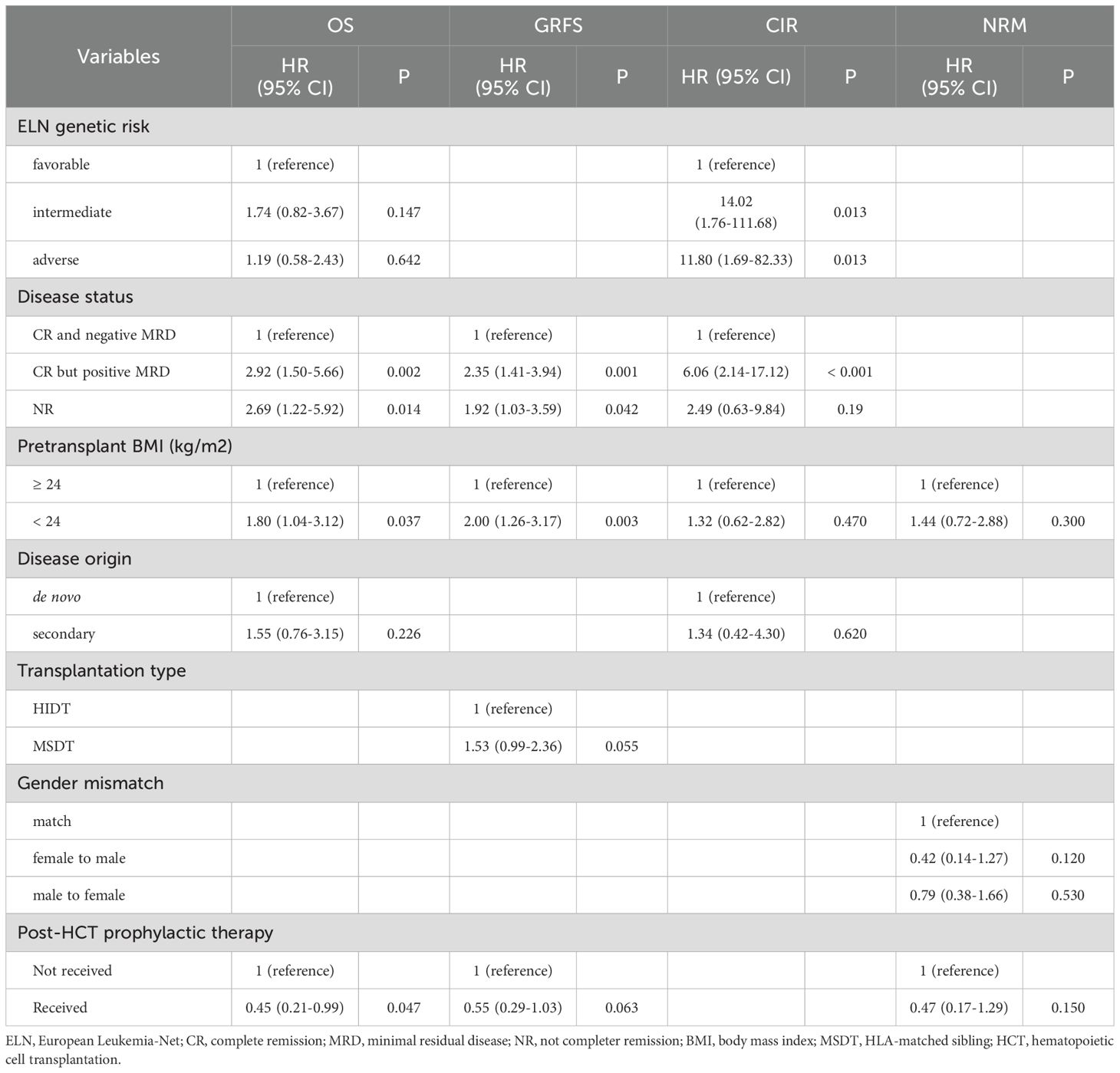
During the follow-up period, 21(21/59, 35.6%) patients in the high BMI group and 39 (47.0%, 39/83) in the low BMI group died. The causes of death are detailed in Supplementary Table 1. GVHD was the cause of death for 8/39 (20.5%) low-BMI patients and 2/21 (9.5%) high-BMI patients. The 3-year OS was 57.10% (95% CI, 49.12%-66.36%) in the entire cohort. The 3-year OS were 65.56% (95% CI, 54.00%-79.60%) for patients in the high BMI group and 51.02% (95% CI, 40.81%-63.79%) for those in the low BMI group (p = 0.067; Figure 1A). Besides, patients who achieved CR and were MRD-negative had a 3-year OS of 66.51% (95% CI, 57.28%-77.24%), compared to 39.32% (95% CI, 23.91%-64.66%) for those who were CR but MRD-positive, and 35.29% (95% CI, 18.86%-66.06%) for those who did not achieve CR (NR). (p = 0.013 for CR/MRD-positive patients vs. CR/MRD-negative patients; p = 0.002 for NR vs. CR/MRD-negative patients; Supplementary Figure 3).
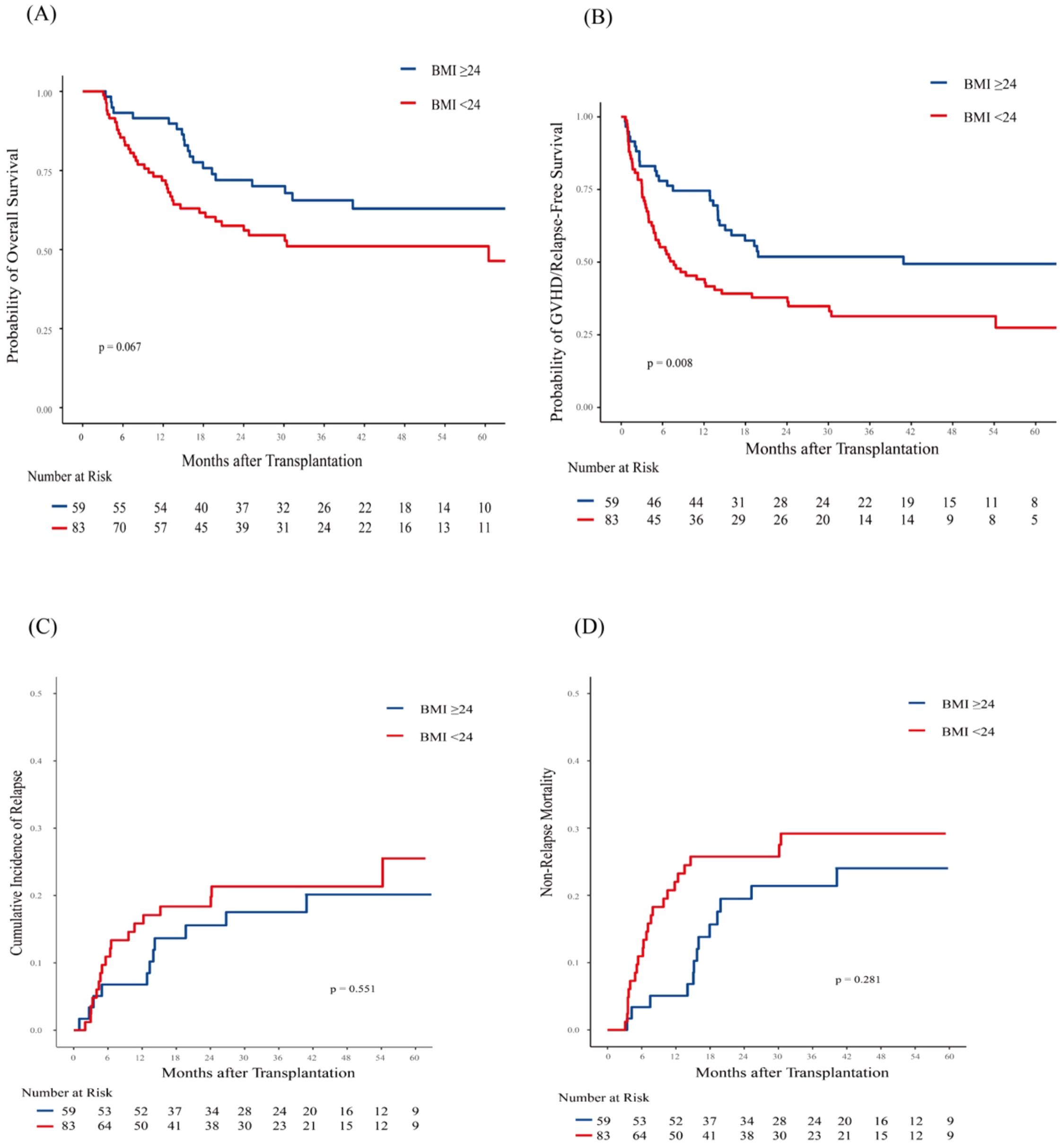
Figure 1. Association of body mass index (BMI) with Post-Transplantation Outcomes in 142 AML patients. Shown are (A) Overall survival (OS); (B) GVHD-free/relapse-free survival (GRFS); (C) Cumulative incidence of Relapse (CIR); (D) Non-relapse mortality (NRM). Patients with low BMI (< 24 kg/m²) exhibited significantly worse GRFS (p = 0.008). OS also showed a trend toward inferiority with low BMI, although this association did not reach statistical significance (p=0.067). No statistically significant associations were observed for CIR (p = 0.551) and NRM (p = 0.281).
In multivariate analysis, pre-transplant BMI was found to have a significant effect on OS (HR, 1.80, 95%CI, 1.04-3.12; p = 0.037). Besides, CR but MRD positive (HR,2.92; 95%CI, 1.50-5.66; p = 0.002) and NR (HR, 2.69, 95% CI, 1.22-5.92; p = 0.014) at allo-HCT were identified as risk factors for inferior OS as well (Table 2).
3.3 Graft versus host disease-free/relapse-free survival, relapse and nonrelapse mortality
Patients in the low BMI group had a significantly lower 3-year GRFS rate (31.38%, 95% CI, 22.46%-43.84%) compared to those in the high BMI group (51.86%, 95% CI, 40.43%-66.53%; p = 0.008; Figure 1B). In the multivariate analysis, pre-transplant BMI was an independent risk factor for inferior GRFS (HR,2.00, 95%CI, 1.26-3.17; p = 0.003).
At 3 years, the CIR was similar between patients in the high BMI group (17.54%, 95% CI, 8.92%-28.55%) and those in the low BMI group (21.34%, 95% CI, 13.03%-31.02%; p = 0.551; Figure 1C). Similarly, NRM at 3 years was comparable between patients in the high BMI group (21.4%, 95% CI, 11.70%-33.03%) and those in the low BMI group (29.29%, 95% CI, 19.47%-39.80%; p = 0.281; Figure 1D). However, patients in the low BMI group had significantly higher 1-year NRM compared to those in the high BMI group ([22.01%, 95% CI,13.72%-31.55%] vs. [5.08%, 95% CI,1.32%-12.87%]; p = 0.006). Multivariate analysis showed that pre-transplant BMI did not significantly influence relapse and NRM (Table 2).
3.4 Subgroup analyses
To gain a deeper understanding of the relationship between pre-transplant BMI and patient outcomes, we conducted a subgroup analysis based on disease status. Given that both CR but MRD positivity and NR status at allo-HCT significantly impacted inferior OS, we categorized these patients into high-risk group. In high-risk group, 3-year OS was 57.69% (95% CI, 39.03%-85.27%) for patients with BMI ≥ 24 kg/m² and 20.87% (95% CI, 9.28%-46.92%) for patients with BMI < 24 kg/m² (p = 0.006; Figure 2A). In low-risk group (CR and MRD negative), 3-year OS was 69.54% (95% CI, 55.84%-86.61%) for patients with BMI ≥ 24 kg/m² and 64.37% (95% CI, 52.53%-78.87%) for patients with BMI < 24 kg/m² (p = 0.579; Figure 2B). Notably, in the low-risk group, the 1-year OS was 92.31% (95% CI, 84.31%-100%) for patients with BMI ≥ 24 kg/m² and 76.79% (95% CI, 66.49%-88.68%) for those with BMI < 24 kg/m² (p = 0.054). BMI showed no significant impact on 3-year CIR and NRM in patients with different disease status at allo-HCT (Supplementary Figure 4).
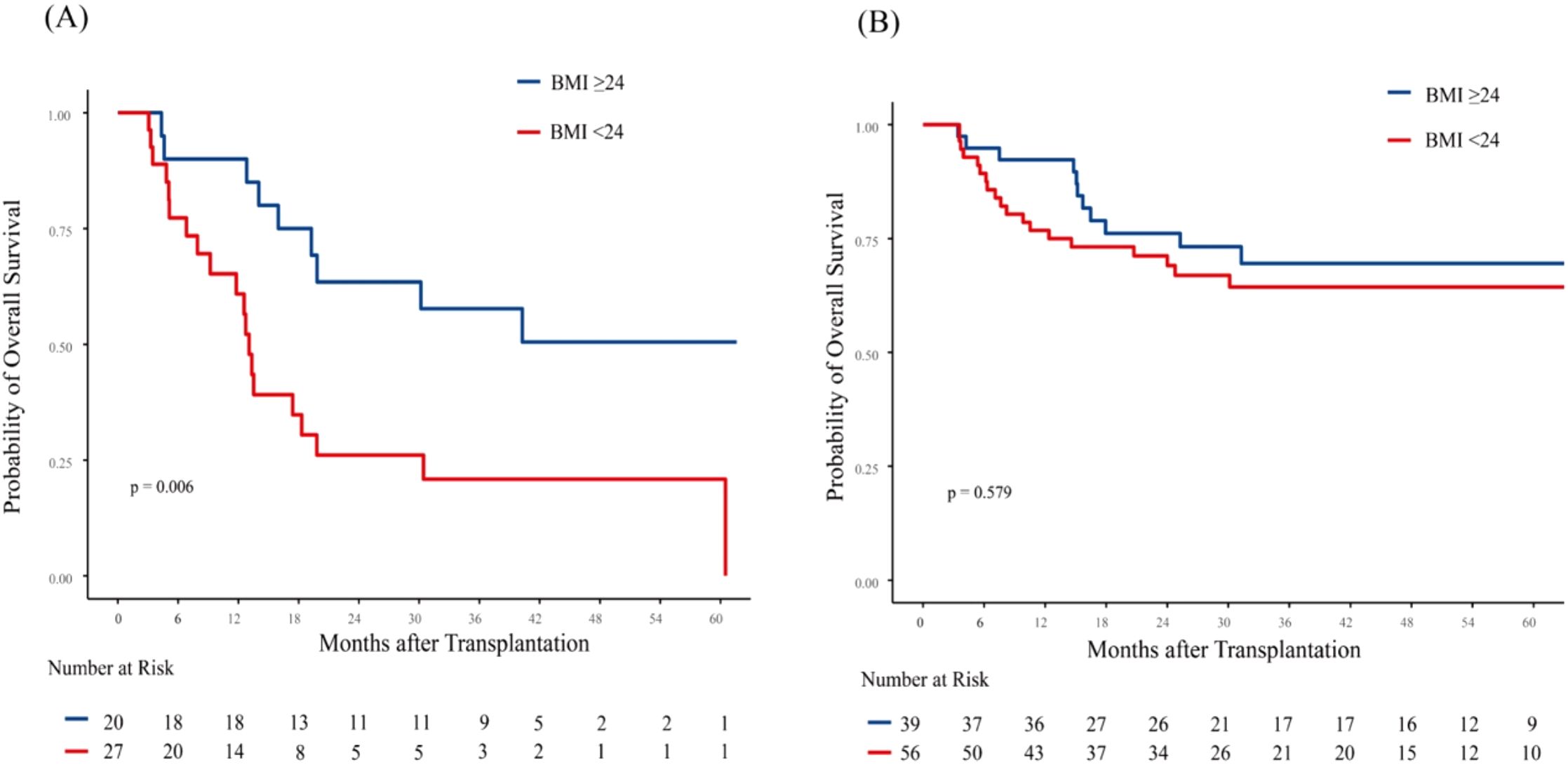
Figure 2. Association of body mass index (BMI) with overall survival (OS) based on disease status in AML patients. Kaplan-Meier curves for OS are shown for: (A) High-risk patients (NR or CR but MRD-positive), and (B) Low-risk patients (CR and MRD-negative). Among high-risk patients, low BMI was significantly associated with inferior OS (p = 0.006). In low-risk patients, no significant association between BMI and OS was observed (p=0.579) NR, not complete remission; CR, complete remission; MRD, minimal residual disease.
3.5 Graft versus host disease
The incidence of grades II-IV aGVHD was comparable between patients in the high BMI group (22.03%, 95% CI, 12.44%-33.36%) and those in the low BMI group (30.12%, 95% CI, 20.60%-40.20%; p = 0.131). For grades III-IV aGVHD, the observed rates were 15.25% (95% CI, 7.45%-25.63%) in the high BMI group and 20.48% (95% CI, 12.55%-29.78%) in the low BMI group, respectively (p = 0.194). The cumulative incidence of GVHD-related NRM at 3 years was 9.76% (95% CI, 4.53%-17.38%) in the low BMI group and 3.39% (95% CI, 0.62%-10.49%) in the high BMI group (p = 0.154). Additionally, there was no significant difference in the proportion of steroid refractory aGVHD between the low BMI group (38.2%, 13/34) and high BMI group (33.3%, 6/18).
The rate of cGVHD at 3 years was 30.77% (95% CI, 19.39%-42.89%) for patients in the high BMI group and 34.82% (95% CI, 24.48%-45.34%) for patients in the low BMI group (p = 0.933).
4 Discussion
Although the impact of BMI on transplant outcomes has been extensively studied, few investigations have specifically focused on older adults, particularly in the Chinese population. In this retrospective study, we evaluated the impact of pre-transplant BMI on clinical outcomes of Chinese patients aged ≥ 50 years undergoing MAC allo-HCT. Our analysis identified low pre-transplant BMI (< 24 kg/m²) was an independent predictor of inferior OS and GRFS. These findings highlight the critical role of nutritional optimization in this vulnerable population.
A BMI ≥ 35 kg/m² has been identified as a risk factor for an increased NRM and reduced OS post-transplantation (5). However, the prevalence of obesity among Asian patients undergoing allo-HCT remains low. A study of 267 Chinese patients aged ≥ 18 years post- allo-HCT reported that 22.1% (59/267) had a BMI ≥ 25 kg/m² and 9.7% (26/267) ≤ 18.5 kg/m² by Asian standards (6). Similarly, in a large Japanese cohort of 12,050 adults receiving allo-HCT, 83.3% had BMIs in the normal-to-overweight range (WHO criteria), with only 1.9% (224/12,050) classified as obese (BMI ≥ 30 kg/m²) (15). In our cohort of 142 patients, only 3 (2.11%) had a BMI below 18.5 kg/m², and 12 (8.45%) had a BMI above 28 kg/m². We speculate this distribution likely results from two key factors: (1) lower obesity prevalence in Asian populations, and (2) exclusion of patients with extreme BMIs from MAC due to frailty or comorbidities. Thus, our findings are most relevant to elderly patients with BMIs in the normal-to-overweight range.
The impact of BMI on transplant outcomes remains controversial. While some studies associate low BMI with inferior OS, leukemia-free survival (LFS), and increased NRM in allo-HCT recipients (16, 17), others suggest that pre-HCT BMI itself does not significantly influence relapse or NRM (3). Our study provides new insights into this debate by demonstrating that pre-transplant BMI significantly affects OS and GRFS in high-risk older AML patients (≥50 years) undergoing MAC allo-HCT. Obesity has been identified as an independent risk factor for acute and extensive chronic GVHD (15). Furthermore, obesity is associated with exacerbated gut aGVHD, potentially through increased pro-inflammatory cytokine production, as demonstrated in both mouse models and patient studies (18). However, in a study analyzing elderly patients undergoing allo-HCT, it was found that obesity was not significantly associated with the incidence of aGVHD or cGVHD (9). In this study, overweight status did not elevate the risk of post-transplant GVHD in elderly patients as well. We observed that the incidence of aGVHD was higher in the low BMI group, although this trend did not reach statistical significance. Notably, patients in the low BMI group exhibited higher 1-year NRM compared to those in the high BMI group (22.01% vs. 5.08%, p=0.006). However, the mortality directly attributable to aGVHD did not significantly differ between the two groups. This lack of significance may be related to the relatively small sample size. Beyond this, we speculate that patients in the low BMI group may not have a significantly increased mortality due to a single cause, but rather exhibit a mild upward trend in multiple risk factors, such as GVHD and infections. The cumulative effect of these multifactorial risks may ultimately contribute to the observed difference in the composite endpoint.
Pretransplant disease status and MRD levels significantly impact clinical outcomes in elderly patients after allo-HCT (19, 20). Li et al. found that positive pre-transplantation MRD and active disease were both risk factors for inferior survival in AML patients after allo-HCT (21). While CIR did not differ significantly between the two groups (p = 0.411), OS was significantly lower in patients with active disease (p = 0.011). Conversely, another study reported comparable OS rates in MRD-positive AML patients or those with active disease aged 65 years or older after allo-HCT (adjusted HR = 1.033, p = 0.76) (22). In this study, we also found pre-transplantation MRD positivity or NR status was a risk factor for survival, and no significant difference in OS between the two groups. Based on this finding, we categorized patients with either MRD positivity or NR status into a single high-risk group for subsequent subgroup analysis. Subgroup analyses showed that NR or MRD-positive CR patients with BMI < 24 kg/m² had poorer OS, whereas this association was absent in MRD-negative CR patients. We believe that adequate nutritional reserves may buffer against treatment-related stressors after allo-HCT.
Global application of BMI standards shows considerable regional variability, with the World Health Organization (WHO) criteria being the most widely recognized (23). However, these universal benchmarks have been adapted by different regions to better match the specific anthropometric profiles and health data of their populations. In this study, we adopted the Chinese BMI classification instead of the Asian or WHO standard because our analysis of BMI data from 142 patients revealed that the threshold point of the HR for OS was around 24, which aligns more closely with the Chinese classification. BMI is influenced by age, and body fat percentage may vary between younger and older individuals with identical BMI (24). Besides, reliance solely on BMI for diagnosing obesity is insufficient; it is essential to incorporate additional anthropometric indicators and direct measures of body fat for a comprehensive assessment (25). Future investigations incorporating body composition analysis could provide more nuanced insights. Bioelectrical impedance analysis (BIA) can provide a comprehensive analysis of body composition and is currently the most commonly used method in clinical practice (26). The lack of additional nutritional status indicators is a limitation of this study. Future efforts will focus on integrating multiple nutritional assessment indicators to better tailor nutritional support for elderly patients after transplantation.
Our study has several limitations that should be acknowledged. First, the relatively small sample size and the single-center nature of our study limit the generalizability of our findings. Second, residual confounding cannot be ruled out despite multivariable adjustments. This includes the potential influence of trends in disease status distribution observed between BMI groups, even though these differences did not reach statistical significance. Third, our analysis focused solely on pre-transplantation BMI as the exposure variable, and did not incorporate longitudinal BMI measurements (from diagnosis to post-transplantation) to assess dynamic changes. This limits our understanding of how BMI fluctuations during the treatment course may influence outcomes. To overcome these limitations, future research should aim to include larger and more diverse patient cohorts. Multi-center studies would be particularly beneficial in providing more patients and reducing the impact of center-specific biases.
In conclusion, our study demonstrates pre-transplant BMI significantly impacts outcomes of MAC allo-HCT for older adults with AML. Greater emphasis should be placed on maintaining optimal nutritional status and providing nutritional support for these individuals, especially for high-risk patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of the Institute of Hematology & Blood Diseases Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the patient or patient's legal guardian/next of kin.
Author contributions
WG: Writing – original draft. HZ: Data curation, Writing – review & editing. HG: Data curation, Writing – review & editing. YZ: Data curation, Writing – review & editing. MW: Writing – review & editing. WC: Writing – review & editing. RZ: Data curation, Resources, Writing – review & editing. QM: Data curation, Resources, Writing – review & editing. YH: Data curation, Resources, Writing – review & editing. WZ: Data curation, Resources, Writing – review & editing. DY: Data curation, Resources, Writing – review & editing. AP: Data curation, Resources, Writing – review & editing. SF: Conceptualization, Writing – review & editing. MH: Conceptualization, Supervision, Writing – review & editing. YC: Methodology, Supervision, Writing – original draft. EJ: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by funds from Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0502400); the CAMS Innovation Fund for Medical Sciences, CIFMS (2023-I2M-2-007, 2023-I2M-C&T-B-108 to YZ); the National Natural Science Foundation of China (82300249 to YC); and Tianjin Natural Science Foundation (23JCZXJC00220); Fundamental Research Funds for the Central Universities Funds (No.3332023058 to HG, 2023-RW320-12 to YC), National Clinical Research Center for Hematological Disorders (No. 2023NCRCA0105 to YC).
Acknowledgments
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1586523/full#supplementary-material
References
1. Aoki J, Kanamori H, Tanaka M, Yamasaki S, Fukuda T, Ogawa H, et al. Impact of age on outcomes of allogeneic hematopoietic stem cell transplantation with reduced intensity conditioning in elderly patients with acute myeloid leukemia. Am J Hematol. (2016) 91:302. doi: 10.1002/ajh.24270
2. Duda K, Wieczorkiewicz-Kabut A, Koclęga A, Zielińska P, Woźniczka K, Krzemień H, et al. Allogeneic hematopoietic stem cell transplantation remains a feasible approach for elderly with acute myeloid leukemia: a 10-year experience. Ann Hematol. (2023) 102:1907. doi: 10.1007/s00277-023-05226-1
3. Brauer D, Backhaus D, Pointner R, Vucinic V, Niederwieser D, Platzbecker U, et al. Nutritional status at diagnosis and pre-transplant weight loss impact outcomes of acute myeloid leukemia patients following allogeneic stem cell transplantation. Hemasphere. (2021) 5:e532. doi: 10.1097/hs9.0000000000000532
4. Abdelaal M, le Roux CW, and Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. (2017) 5:161. doi: 10.21037/atm.2017.03.107
5. Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. (2005) 106:2912. doi: 10.1182/blood-2005-05-2004
6. Yang J, Xue SL, Zhang X, Zhou YN, Qin LQ, Shen YP, et al. Effect of body mass index on overall survival of patients with allogeneic hematopoietic stem cell transplantation. Eur J Clin Nutr. (2017) 71:750. doi: 10.1038/ejcn.2016.225
7. Polverelli N, Bonneville EF, de Wreede LC, Koster L, Kröger NM, Schroeder T, et al. Impact of comorbidities and body mass index on the outcomes of allogeneic hematopoietic cell transplantation in myelofibrosis: A study on behalf of the Chronic Malignancies Working Party of EBMT. Am J Hematol. (2024) 99:993. doi: 10.1002/ajh.27262
8. Enßle JC, Wolf S, Scheich S, Weber S, Kramer M, Ruhnke L, et al. Impact of BMI on patient outcome in acute myeloid leukaemia patients receiving intensive induction therapy: a real-world registry experience. Br J Cancer. (2023) 129:1126. doi: 10.1038/s41416-023-02362-3
9. Voshtina E, Szabo A, Hamadani M, Fenske TS, D’Souza A, Chhabra S, et al. Impact of obesity on clinical outcomes of elderly patients undergoing allogeneic hematopoietic cell transplantation for myeloid Malignancies. Biol Blood Marrow Transplant. (2019) 25:e33. doi: 10.1016/j.bbmt.2018.08.031
10. Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. (2022) 140:1345. doi: 10.1182/blood.2022016867
11. Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: A report from the mount sinai acute GVHD international consortium. Biol Blood Marrow Transplant. (2016) 22:4. doi: 10.1016/j.bbmt.2015.09.001
12. Guo W, Liu X, Wang M, Liu J, Cao Y, Zheng Y, et al. Application of prophylactic or pre-emptive therapy after allogeneic transplantation for high-risk patients with t(8;21) acute myeloid leukemia. Hematology. (2023) 28:2205739. doi: 10.1080/16078454.2023.2205739
13. Wang Y, Chang YJ, Chen J, Han M, Hu J, Hu J, et al. Consensus on the monitoring, treatment, and prevention of leukaemia relapse after allogeneic haematopoietic stem cell transplantation in China: 2024 update. Cancer Lett. (2024) 605:217264. doi: 10.1016/j.canlet.2024.217264
14. Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. BioMed Environ Sci. (2002) 15:83.
15. Fuji S, Takano K, Mori T, Eto T, Taniguchi S, Ohashi K, et al. Impact of pretransplant body mass index on the clinical outcome after allogeneic hematopoietic SCT. Bone Marrow Transplant. (2014) 49:1505. doi: 10.1038/bmt.2014.178
16. Le Blanc K, Ringdén O, and Remberger M. A low body mass index is correlated with poor survival after allogeneic stem cell transplantation. Haematologica. (2003) 88:1044.
17. Doney K, McMillen K, Buono L, Deeg HJ, and Gooley T. Impact of body mass index on outcomes of hematopoietic stem cell transplantation in adults. Biol Blood Marrow Transplant. (2019) 25:613. doi: 10.1016/j.bbmt.2018.10.006
18. Khuat LT, Le CT, Pai CS, Shields-Cutler RR, Holtan SG, Rashidi A, et al. Obesity induces gut microbiota alterations and augments acute graft-versus-host disease after allogeneic stem cell transplantation. Sci Transl Med. (2020) 12(571):eaay7713. doi: 10.1126/scitranslmed.aay7713
19. Ma R, Liao Y, Zhang XH, Xu LP, Wang Y, Mo XD, et al. Pre-transplant disease burden rather than age or donor type was associated with survival of hematopoietic cell transplantation for elderly patients with acute myeloid leukemia. Bone Marrow Transplant. (2025) 60:107. doi: 10.1038/s41409-024-02457-2
20. Gilleece MH, Labopin M, Yakoub-Agha I, Volin L, Socié G, Ljungman P, et al. Measurable residual disease, conditioning regimen intensity, and age predict outcome of allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first remission: A registry analysis of 2292 patients by the Acute Leukemia Working Party European Society of Blood and Marrow Transplantation. Am J Hematol. (2018) 93:1142. doi: 10.1002/ajh.25211
21. Li SQ, Yu CZ, Xu LP, Wang Y, Zhang XH, Chen H, et al. Pretransplantation risk factors for positive MRD after allogeneic stem cell transplantation in AML patients: a prospective study. Bone Marrow Transplant. (2025) 60:277. doi: 10.1038/s41409-024-02466-1
22. Veltri L, Rezvani K, Oran B, Mehta R, Rondon G, Kebriaei P, et al. Allotransplants for patients 65 years or older with high-risk acute myeloid leukemia. Biol Blood Marrow Transplant. (2019) 25:505. doi: 10.1016/j.bbmt.2018.09.032
23. Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. (2011) 377:557. doi: 10.1016/s0140-6736(10)62037-5
24. Müller MJ, Braun W, Enderle J, and Bosy-Westphal A. Beyond BMI: conceptual issues related to overweight and obese patients. Obes Facts. (2016) 9:193. doi: 10.1159/000445380
25. Rubino F, Cummings DE, Eckel RH, Cohen RV, Wilding JPH, Brown WA, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. (2025) 13(3):221–262. doi: 10.1016/s2213-8587(24)00316-4
Keywords: allogeneic hematopoietic cell transplantation, elderly, acute myeloid leukemia, body mass index, survival
Citation: Guo W, Zhang H, Gao H, Zheng Y, Wang M, Cao W, Zhang R, Ma Q, He Y, Zhai W, Yang D, Pang A, Feng S, Han M, Cao Y and Jiang E (2025) Impact of pre-transplant body mass index on outcomes in AML patients aged ≥ 50 years after allogeneic hematopoietic cell transplantation. Front. Immunol. 16:1586523. doi: 10.3389/fimmu.2025.1586523
Received: 03 March 2025; Accepted: 18 June 2025;
Published: 04 July 2025.
Edited by:
Irene Lidoriki, Harvard University, United StatesReviewed by:
Elisabetta Xue, National Cancer Institute Bethesda, United StatesOzlem Candan, İstanbul Kanuni Sultan Süleyman Eğitim ve Araştırma Hastanesi, Türkiye
Copyright © 2025 Guo, Zhang, Gao, Zheng, Wang, Cao, Zhang, Ma, He, Zhai, Yang, Pang, Feng, Han, Cao and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erlie Jiang, ZG9jdG9yX2VsamlhbmdAMTYzLmNvbQ==; Yigeng Cao, Y2FveWlnZW5nQGloY2Ftcy5hYy5jbg==
 Wenwen Guo
Wenwen Guo Haixiao Zhang
Haixiao Zhang Hongye Gao1,2,3
Hongye Gao1,2,3 Mingyang Wang
Mingyang Wang Yi He
Yi He Weihua Zhai
Weihua Zhai Donglin Yang
Donglin Yang Aiming Pang
Aiming Pang Sizhou Feng
Sizhou Feng Yigeng Cao
Yigeng Cao Erlie Jiang
Erlie Jiang