- 1Moderna, Inc., Cambridge, MA, United States
- 2Ragon Institute of Mass General Brigham, Massachusetts Institute of Technology, Harvard University, Cambridge, MA, United States
The global immune landscape of SARS-CoV-2 has progressively shifted from a naïve population several years ago to a population that possesses immunity to the virus through infection, vaccination, or a combination of both, known as hybrid immunity. Hybrid immunity offers a prolonged period of transmission-blocking activity, likely related to enhanced tissue-resident immunity, but also has been shown to be linked to broader humoral and cellular immune responses. Compared with vaccination or infection alone, the collective data have demonstrated that hybrid immunity offers enhanced protection against disease. Yet, despite the benefits of hybrid immunity, perpetual evolution of variants and the natural waning of immunity in vulnerable populations provides a strong rationale for revaccination. This article reviews the benefits of revaccination, including updating variant-specific immunity, bolstering humoral and cellular immune frequencies in those with hybrid immunity, and overcoming immune imprinting and enhancing effector mechanisms to raise surveillance and defense against the virus. As SARS-CoV-2 continues to evolve, updated booster vaccinations remain essential to enhance and sustain protection from disease by ensuring that the immune system is equipped to respond to contemporary strains, thereby reducing the impact of future outbreaks and mitigating the burden of COVID-19, especially among vulnerable populations.
1 Introduction
Several years after the first emergence of SARS-CoV-2 in 2019 (1), the global immune landscape has progressively evolved from a SARS-CoV-2–naïve population to one with hybrid immunity (2–5). Today, most of the world’s population has experienced a SARS-CoV-2 infection and/or received vaccination (2–4). The rapid and effective spread of SARS-CoV-2 is largely attributable to the unrelenting adaptation of the virus to population-level immunity, with an accumulation of escape mutations in the Spike antigen enabling successive surges of reinfections (6–8). While the majority of the world now possesses some level of immunity to the virus, these waves of reinfections continue to pose a real risk to vulnerable groups, including the aging population and individuals with compromised immunity, due to an inability of the immune system to adapt and block viruses with enhanced transmissibility or to respond with sufficient speed to contain the virus and prevent severe clinical outcomes (3, 6). In fact, older adults, those who are immunocompromised, and those with certain comorbid conditions continue to be hospitalized at the highest rates (9, 10), and COVID-19 continues to have a greater impact than influenza in terms of morbidity and mortality in the United States (11). Moreover, in the absence of robust immunity, these vulnerable populations are susceptible to post-acute sequelae, such as long COVID or post-COVID conditions, resulting in increased health burden and the risk of mortality (12, 13). Yet, the public health challenge is further complicated by the fact that the circulation of SARS-CoV-2 does not follow typical seasonality, but instead new variants emerge unpredictably throughout the year, rendering it difficult to time vaccine updates (14, 15).
Compared with vaccination or infection alone, emerging data have clearly demonstrated the superior level of protection conferred by the combination of vaccination and infection, termed hybrid immunity (5). Specifically, hybrid immunity offers a prolonged period of transmission-blocking activity (16, 17), likely related to enhanced tissue-resident immunity (18, 19), but also has been shown to be linked to broader humoral (20–22) and cellular immune responses (17, 23, 24). However, even in the setting of hybrid immunity, vulnerable populations continue to suffer from more severe disease and death, potentially related to reduced durability, compromised or senescent cellular immunity, or compromised capacity to elicit neutralizing antibodies in response to SARS-CoV-2 variants (25). SARS-CoV-2 variants are genetically mutated versions of the original virus that may have differing viral features, and are defined according to their lineages based on the genetic sequence of the Spike protein (26). Since the original SARS-CoV-2 was detected in 2019 (1), several variants have emerged, such as Alpha, Delta, and Omicron, each with varying degrees of transmissibility and disease severity (27). Development of first-generation COVID-19 vaccines targeting the original SARS-CoV-2 were proven to be effective, with subsequent development of variant-targeting vaccines as a response to manage emerging variants (28). Vaccination with the current COVID-19 seasonal vaccine provides an opportunity to not only bolster titers and effector cell frequencies, but also to reeducate and update the adaptive immune system to respond to the to the updated variant-focused vaccine (29–31). These more recently primed and educated immune responses create a higher barrier of immunity within these vulnerable populations and have the potential to reduce the risk of acute and post-acute COVID-19 events (32–35).
This review focuses on real-world evidence as well as the mechanisms of protection afforded by revaccination in individuals with hybrid immunity. We aim to explore and inform on the strategic use of vaccination to boost immunity and increase the window of protection, particularly among populations at higher risk for COVID-19. We also place this information in the context of subsequent protection against SARS-CoV-2 variants, particularly during multiple waves of omicron variants given the recent global recommendations for monovalent variant-updated vaccines (36).
2 Updating immunity to overcome imprinting
In October 2021, prior to the emergence of the highly transmissible omicron variant, global SARS-CoV-2 seroprevalence was estimated at 67% (37). SARS-CoV-2 seroprevalence in the United States has been largely attributed to vaccination (38). However, the steep increase in infections following the emergence of omicron in November 2021, due in part to the ineffectiveness of previous infection–induced immunity to protect against omicron, and expanded vaccine coverage led to increased vaccine- and infection-induced seroprevalence of >90%, with increases in hybrid immunity of 51%-60% by mid-2022 (37, 39–46). While it is difficult to determine the cause of seroprevalence at a population level, following repeated waves of global omicron sublineage evolution and transmission, it has been estimated that upwards of 90% of the globe has detectable SARS-CoV-2 antibodies from infection, vaccination, or hybrid immunity (46–49). The extraordinary speed of global transmission events coupled with the unprecedented speed of vaccine development, updating, and deployment has created remarkable heterogeneity in the frequency of vaccine and infection events across the population (40, 43, 47, 48, 50). However, emerging data suggest that the sequence and type of immune exposures substantially influence the quality of the immune response. For instance, the timing of vaccination following SARS-CoV-2 infection may influence post-vaccine IgG levels in hybrid immune individuals (51).
As SARS-CoV-2 continues to evolve, selective pressure through host immune responses can influence pathogen evolution to evade preexisting immunity (52). In the evolutionary arms race, the immune system rapidly adapts to respond to newly evolving variants, but often is heavily biased by preexisting memory B-cell specificities, also known as immune imprinting, that prevents the generation of truly de novo responses that find novel means to respond to evolving variants (53). This bias is due to the existence of a population of memory B cells that have high affinity and specificity to the initial strain, that compete aggressively for antigen in the germinal centers, preventing naïve B cells from competing for signals to proliferate and expand (53).
Despite the evolution of novel variants of concern and the inclusion of updated sequences in vaccines, both revaccination and reinfection result in the recall of the original virus-specific response to which an individual was first exposed (53). This imprinting manifests due to the rapid and preferential binding of preexisting SARS-CoV-2–specific B cells to shared epitopes on the circulating and original strain to which the individual was exposed (53). However, the non-shared, or escape, mutations found on the contemporaneous strain represent the critical immune targets for control, clearance, and future immunity against the current variant (54). Reinfection naturally stimulates evolution of the immune response to the circulating virus (55). However, for vulnerable populations that are susceptible to potential complications of infection, vaccination with updated strains represents a key approach to rapidly update and educate the immune response to adapt to respond to the evolving viral variants.
The importance of overcoming imprinting was clearly illustrated following the evolution of the delta and then omicron SARS-CoV-2 lineages (53). While vaccination with the original antigenic sequence conferred protection against delta variant infection, protection against omicron was reduced, and neutralizing antibody titers waned more quickly (56). This differential protection across the variants of concern was likely related to the higher conservation of delta to the original strain, resulting in more cross-reactive antibodies from the original Wuhan-based vaccine with the ability to neutralize delta compared with the more distant and less cross-reactive omicron (57–62). The critical importance of sequence degree of similarity between the variants became increasingly evident as reinfection rates were estimated to have increased from alpha-dominated periods (0.57, 95% confidence interval [CI]: 0.28–0.94]), through delta (1.25, 95% CI: 0.97–1.55), and through the initial wave of the highly divergent omicron (3.31, 95% CI: 1.15–6.53) (63). Of note, while comparing rates of re-infection across variants, the likelihood of exposure to the virus due to masking, social distancing, quarantining, etc. should be considered (64). The efficacy of infection-induced immunity alone against reinfection was estimated to be 65% (incidence rate ratio [IRR] = 0.35, 95% CI: 0.26–0.47), with the pooled IRRs for the alpha (IRR = 0.11), delta (IRR = 0.19), and omicron (IRR = 0.61) variants indicating progressively lower effectiveness (65). Despite widespread global immunity, the continued evolution of omicron subvariants JN.1, KP.2, and KP.3 subverted previous vaccine- and infection-induced immunity, necessitating updating the monovalent XBB.1.5-targeting vaccine.
Although genetic evolution occurs across the entire viral genome, continual changes in the receptor binding domain (RBD) of the Spike protein lead to evasion of population-level immunity to previous strains and are particularly relevant for vaccine-induced immunity (8, 36, 66). However, beyond their ability to evade preexisting humoral immune responses, several mutations, including those in the XBB variant and more recently in the KP sublineages of omicron, also exhibit increased transmissibility compared with SARS-CoV-2 variants that emerged during earlier phases of the pandemic (67, 68). This increased transmissibility has led to widespread waves of global infection (69). Yet, in the wake of these waves of infection and despite reduced neutralization of new variants, COVID-19 vaccination provided protection against severe disease and was associated with greater point estimates of protection against hospitalization among cases with XBB/XBB.1.5 versus non-XBB/XBB.1.5 cases (52). The XBB/XBB.1.5 lineage was more sensitive to immune responses triggered by vaccination than to those triggered by prior infection with pre-omicron or BA.4/BA.5 (52). However, updates to vaccine composition resulted in approximately 60% increased protection against infection caused by XBB.1.5, the variant targeted by the monovalent mRNA vaccine. The protection against infection provided by the monovalent XBB.1.5–targeting vaccine was only 49% against the emerging JN.1 variant (70), highlighting the critical importance of updating immunity to the more contemporaneously circulating variant. As such, the World Health Organization (WHO) recommended the inclusion of JN.1 antigen in vaccines to enhance protection against the JN.1 variant (71). Yet, JN.1 is now being replaced by JN.1 subvariants with mutations in the Spike protein, including FLiRT variants, KP.2, KP.3, and LB.1, which may have an even higher viral fitness (49). KP.2 has shown an almost 3-fold resistance to neutralization following XBB.1.5 vaccination and almost 2-fold resistance following previous infection (72). For the 2024–25 COVID-19 vaccine update, the US Food and Drug Administration (FDA) recommended including the KP.2 strain, intended to expose the immune system to the most recent dominant circulating variant to mount a variant-specific immune response (73). Particularly in vulnerable populations, updated vaccination may critically promote enhanced antibody titers to highly variable regions of the viral Spike glycoprotein, replenish effector T cell numbers, and update memory B cell clonal repertoires, enabling these cells to respond more effectively upon encounter with the next viral variant (31, 74).
Vaccination has been shown to provide protection from infection and enhance protection against illness in previously infected individuals (75). Conversely, in New Zealand, where vaccination rates were high with low levels of infection, the omicron wave early in 2022 led to nearly 24,000 daily cases and significant increases in hospitalization and intensive care unit admission, arguing that a combination of both infection and vaccination provided enhanced protection against severe disease (76). Omicron infected large swaths of the population globally, resulting primarily in upper respiratory disease (77, 78); however, the lack of concomitant increases in hospitalizations in vaccinated people (79) demonstrated the impact of vaccine-induced immunity against severe outcomes. In contrast, unvaccinated individuals in Hong Kong experienced high rates of hospitalization, severe disease, and death (80), highlighting the pathogenicity of omicron in non-immune populations. Moreover, additional revaccination with a fourth mRNA vaccine dose resulted in enhanced relative vaccine effectiveness against severe COVID-19 in adults aged ≥40 years, irrespective of infection history (81). The precise mechanism by which revaccination confers maximal protection against infection and disease in hybrid-immune individuals is likely via both quantitative and qualitative changes to the neutralizing/non-neutralizing antibody and adaptive cellular SARS-CoV-2–specific responses both peripherally and at mucosal barriers responsible for maximal immunity against this evolving pathogen. An overview of the features of SARS-CoV-2 infection-induced immunity, vaccination-induced immunity, and hybrid immunity is presented in Table 1.
2.1 Revaccination as a mechanism to update neutralizing antibody responses
A significant number of studies have noted the critical immune synergy created by combined natural and vaccine-mediated protection in hybrid immunity, related to: broader proteomic coverage of humoral and cellular immune responses (74), improved breadth and magnitude of the neutralizing antibody response (51), enhanced B-cell affinity maturation (82, 83), shift in humoral immunodominance to more conserved regions of the Spike antigen (84, 85), and mucosal immune induction (86). As such, hybrid immunity allows for a broader, more diverse, and mucosally enriched humoral immune response compared with immunity from vaccination or infection alone (20, 24) (Figure 1). Specifically, vaccination alone induces highly potent but narrowly focused neutralizing antibody responses due to the stabilized presentation of the Spike antigen following COVID-19 vaccination (87). These responses are largely focused on the immunodominant RBD domain of the Spike antigen (88, 89), the primary region involved in attachment to the host angiotensin-converting enzyme 2 (ACE-2) receptor (90). However, infection alone or the combination of infection and vaccination results in an expansion of the humoral immune response to additional domains of the Spike antigen, including enhanced responses to the less mutable N-terminal (NTD) and S2 domains of the Spike antigen (85, 91). Despite their lower potency, expanded S2 responses have been shown to exhibit enhanced breadth of coverage across sarbecoviruses, and these findings may guide effective vaccine strategies for protection against evolving variants (92).
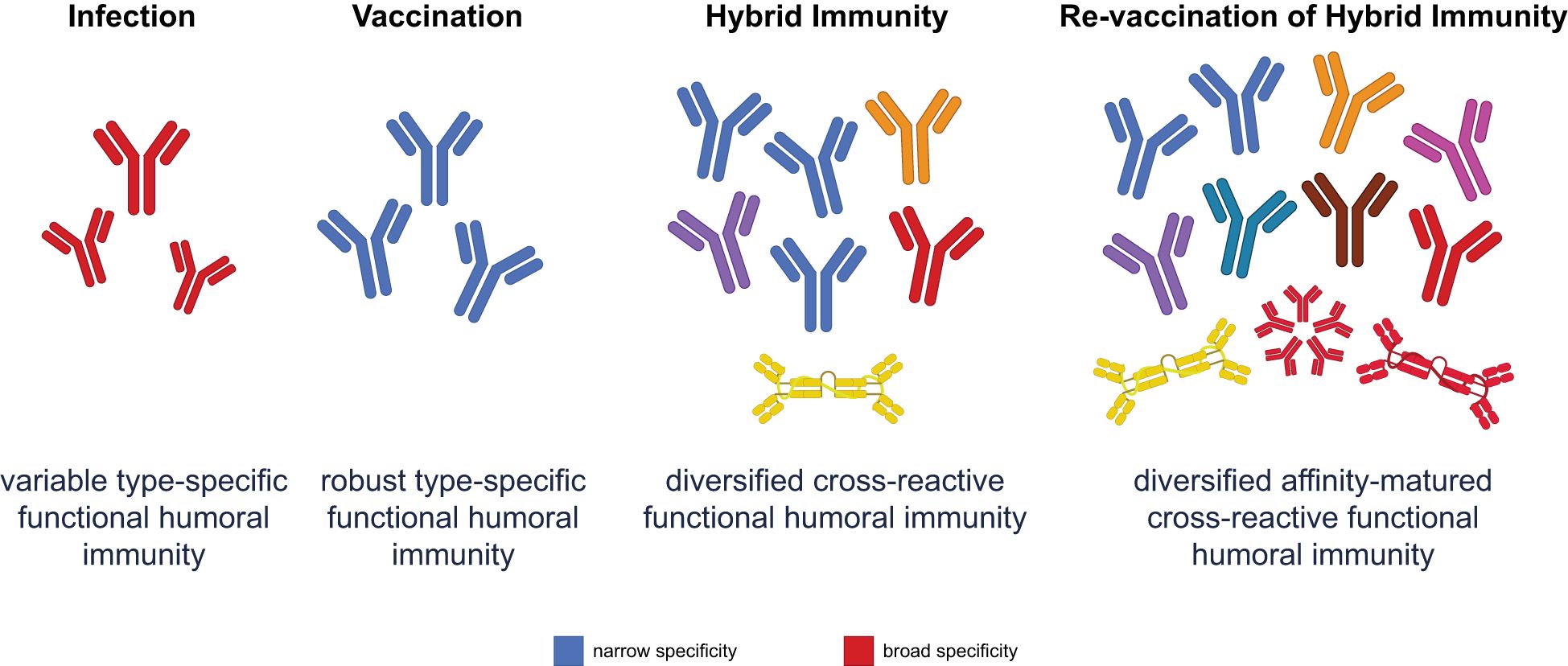
Figure 1. Functional humoral immunity varies across different immune scenarios. Infection produces a variable response with broad specificity, but limited affinity maturation. Vaccination elicits robust and specific antibodies, but narrowly focused protection. While hybrid immunity combines the strengths of both infection and vaccination, revaccination of hybrid-immune individuals further diversifies and matures the humoral response, leading to affinity-matured, highly cross-reactive antibodies. This approach optimally updates the immune repertoire to respond effectively to emerging variants, especially through improved mucosal and systemic functional immunity.
Critically, revaccination drives rapid and highly productive evolution of RBD-specific antibodies away from historical variants (93, 94) (Figure 1). Early revaccination data suggested that inclusion of the ancestral strain, in a combination vaccine, resulted in the continued selection and expansion of poorly cross-reactive, previously primed, B-cell responses (95, 96). Conversely, revaccination with updated variant vaccines only, ensured the evolution of the previously primed response towards the newer variant (97). However, the combination of infection and revaccination clearly illustrated the greatest increase in breadth of neutralization (98). The evolution of enhanced breadth of neutralization following breakthrough infection likely arose due to a combination of an updated Spike antigen, multiple structural presentations of the antigen on a virus or virally infected cell, the additional innate immune signals that are induced following infection (98), and potentially expanded T-cell helper signals. Together, these factors drive enhanced B-cell selection in the setting of hybrid immunity. However, upon revaccination, recall of these previously vaccine- and infection-primed B cells expands robustly, leveraging the rich preprogrammed memory B cells that can now adapt rapidly and robustly to potentially new viral variant sequences (98).
Mounting evidence suggests that hybrid immunity results in the presence of higher frequencies of memory B cells compared with infection or vaccination alone (22, 82); the B cells are primed for a more robust response upon revaccination (22) (Figure 2). Moreover, deeper B-cell clonal repertoire analyses illustrated that hybrid immunity resulted in the recruitment of broader, more affinity-matured, clonal repertoires that are resilient to evolving variants (99, 100). Specifically, RBD-specific neutralizing antibodies isolated from unvaccinated individuals following infection tend to show little or no somatic mutation, whereas antibodies following three doses of mRNA vaccine or breakthrough infection show high levels of somatic mutation, leading to greater cross-neutralization (101). These broader, richer, clonal repertoires still vary across hybrid populations, depending on the variant that caused infection. However, hybrid immunity likely provides a broader repertoire upon which novel variant boosting may help adapt immune responses more contemporaneously (93, 94, 99, 100).
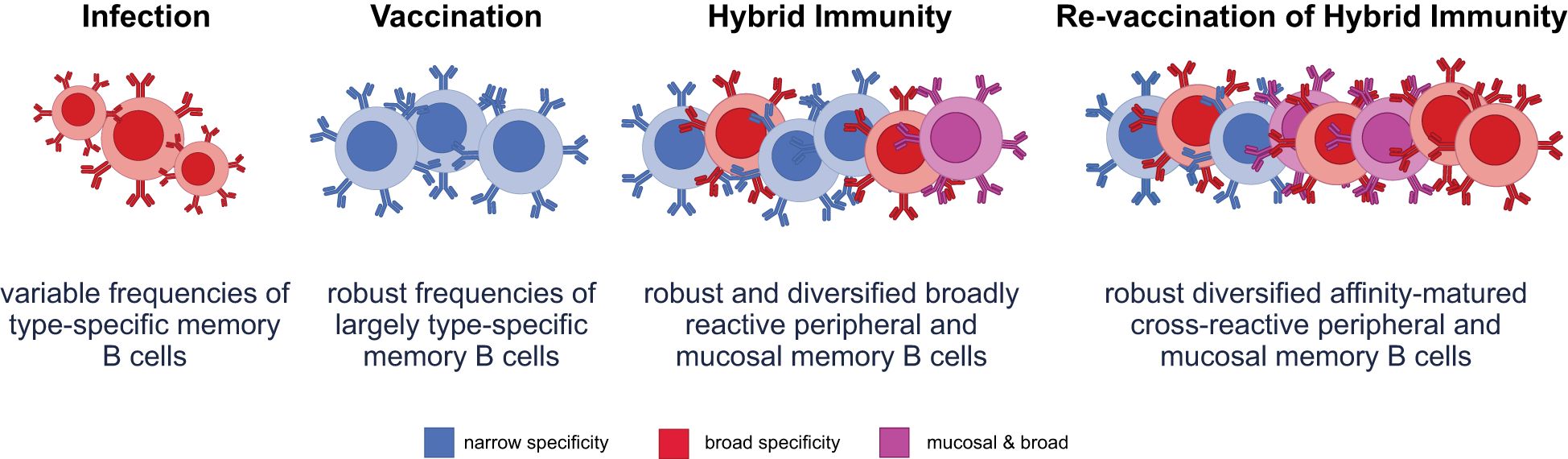
Figure 2. Varying immune exposures differentially characterize functional B-cell responses. Infection produces variable frequencies of memory B cells, with some broad reactivity due to exposure to diverse viral antigens, but typically limited by lower somatic hypermutation and affinity maturation. Vaccination induces robust frequencies of memory B cells that are largely type-specific and focused on the vaccine antigen, with limited breadth and minimal mucosal targeting. Hybrid immunity results in a robust and diversified memory B-cell pool, encompassing both peripheral and mucosal compartments. This broad reactivity enables improved protection across variants and sites of viral entry. Revaccination of hybrid-immune individuals enhances the diversity, affinity maturation, and mucosal targeting of memory B cells, producing a highly cross-reactive and protective memory B-cell repertoire optimized for variant recognition and clearance.
Revaccination in a hybrid-immune individual furthermore is likely to recall memory B-cell responses that were programmed at the mucosal barrier in the setting of previous infection (102). Specifically, while vaccination alone does not elicit high immunoglobulin A (IgA) titers that are critical for mucosal protection, infection elicits high levels of systemic and mucosal IgA responses (103). Notably, revaccination elicited higher titers of IgG and IgA in saliva compared with primary immunization (104), arguing that revaccination has the capacity to bolster mucosal humoral immunity. Furthermore, hybrid immunity was associated with an enrichment of mucosal B-cell responses (MBCs) and tissue-resident CD4 and CD8 T-cell responses in the bronchioalveolar lavage (BAL) not observed in individuals that had only received a vaccine (105).
Studies have shown the cross-recognition potential of memory B cells primed by vaccination alone, particularly those exposed initially to the ancestral Wuhan-Hu-1 strain. In vaccine-only individuals, memory B cells elicited by the original mRNA vaccines retain the ability to recognize and respond to diverse SARS-CoV-2 variants, though with reduced breadth and potency compared with those with hybrid immunity (83, 106). The effect of re-vaccination using either monovalent (Wuhan-Hu-1) or bivalent (Wuhan-Hu-1 + Omicron BA.4/5) mRNA boosters has been shown to expand memory B cell breadth (107). Importantly, individuals with hybrid immunity demonstrate superior somatic hypermutation and affinity maturation in memory B cells, with improved recognition of divergent Spike proteins (108, 109). This evolution of the B cell response is particularly relevant in immunocompromised populations, where both the magnitude and quality of vaccine-induced memory B cells are often impaired.
2.2 Revaccination as a mechanism to drive non-neutralizing antibodies
Beyond the ability of highly affinity-matured antibodies to neutralize the virus, emerging data suggest that additional properties of antibodies may contribute to attenuation of disease. Specifically, beyond their ability to block infection, once complexed with a virus or virally infected cell, antibodies are able to rapidly interact with Fc-receptors found on all innate immune cells and drive rapid clearance or destruction of the complex (110). Importantly, naturally produced non-neutralizing antibody functions during infection have been linked to protection against severe disease and death (111). Additionally, convalescent plasma with the ability to drive antibody-mediated cytotoxicity was associated with protection against disease (112), and therapeutic monoclonal antibodies targeting the Spike protein depended on the ability to recruit the innate immune system via Fc-receptors to provide protection against disease (110). Interestingly, while these non-neutralizing antibody functions are induced by vaccination and infection, hybrid immunity has been shown to bolster these responses (113).
Unlike neutralizing antibodies that must target sites involved in host-cell binding or fusion mechanisms, non-neutralizing antibodies can target the whole surface of the Spike antigen (114). Both vaccination and infection have the potential to expand antibody function and responses across the Spike protein; however, infection-induced non-neutralizing antibodies may target a broader array of Spike antigen presentation states (pre- and post-fusion) as well as other virally encoded antigens (110, 115). Moreover, hybrid immunity has the potential to benefit from both vaccine- and infection-associated immune programming, inducing functional antibodies focused on an array of Spike antigen presentation states, programmed to elicit diverse antibody effector functions due to priming/boosting across inductive sites (22). Additionally, because non-neutralizing antibodies can target epitopes outside of the RBD, emerging data suggest that non-neutralizing functional antibodies are more resilient (85, 111, 113, 116), targeting less mutable segments of the Spike antigen. Thus, revaccination has the capacity to rapidly recall and boost variant-resilient, highly functional, humoral immune responses.
As mentioned above, revaccination in hybrid-immune populations is associated with the induction of broader antibody isotypes, including IgA, due to preexisting priming within mucosal inductive sites (117–122). Within mucosal tissues, IgA typically dimerizes, forming a quadrivalent molecule, able to bind incoming virus with higher avidity, which may enable IgA to continue to neutralize even in the setting of viral evolution (123, 124). Moreover, monomeric IgA may also interact with neutrophils that may be rapidly recruited to the mucosal barrier upon infection and drive rapid opsonophagocytosis of the virus (125–128). The importance of IgA responses to protection against COVID-19 was illustrated among healthcare workers in Barcelona, where higher IgA responses were observed among those with hybrid immunity compared with vaccination alone (129). Additionally, higher levels of IgG and IgA antibodies were shown to be a correlate of protection against breakthrough infection following revaccination (129, 130). While IgA responses in the nasal washes are higher in individuals following infection compared with vaccination, significantly higher levels of IgA and IgG are detectable in the nasal washes following boosting in individuals with hybrid immunity, with IgA antibodies contributing dominantly to neutralization in the mucosa (103).
Collectively, revaccination of hybrid-immune individuals leverages key humoral immune features that are likely key for protection against infection and disease: rapid affinity maturation and clonal diversification, enabling the rapid adaptation and updating of the humoral immune response for the most current viral variant (120, 131, 132), and expansion of functional humoral immunity to the whole surface of the Spike antigen, resulting in a highly resilient immune response able to rapidly capture and clear the infection (22). Thus, boosting of hybrid-immune B-cell repertoires is likely able to elicit more contemporaneously adapted immune responses at the mucosal barrier that may be essential to protect those most vulnerable in our population.
2.3 Revaccination as a mechanism to bolster T-cell immunity
As novel variants arose, protection against hospitalization and death in vaccinated populations also pointed to a potential role for T cells as key correlates of protective immunity against COVID-19 disease (133). Along the same lines, early in the pandemic, studies highlighted an association between preexisting cross-reactive human coronavirus T cells and reduced risk of severe COVID-19 disease (134–137), due to conserved T-cell targets across previously circulating coronaviruses and SARS-CoV-2 (138). Moreover, the fact that vaccination provided benefit from severe disease and death in individuals lacking B cells due to treatments for autoimmunity or malignancies (111, 139) and was demonstrated to be effective in individuals with inborn errors of B-cell development (e.g., X-linked agammaglobulinemia) (140), further highlights the importance of T-cell immunity in disease attenuation (141, 142). Unlike neutralizing antibodies, vaccine-induced T-cell responses have exhibited remarkable resilience across variants (143–146), demonstrating persistent recognition of variant sequences (138). In the setting of hybrid immunity, T-cell–mediated immunity was characterized by detectable viral proteome-wide T-cell responses (147–149), broader clonal composition (150, 151), enhanced cytotoxic CD8 T-cell responses (150), and presence of T cells at mucosal sites (18) (Figure 3). Revaccination resulted in increased expansions of both CD8 and CD4 T-cell receptor (TCR) clonal repertoires and large numbers of CD8 T-cell responses (117–121), arming the immune system with a diverse and rich repertoire of T cells primed for antiviral activity upon viral re-exposure.

Figure 3. The landscape of functional T-cell immunity is shaped by varying immune scenarios. T-cell responses (CD4 and CD8) differ in frequency and localization across immune scenarios, impacting protection at peripheral and mucosal sites. Infection generates variable frequencies of peripheral and mucosal CD4 and CD8 T cells, influenced by the severity and location of infection, with limited consistency in tissue-resident T-cell populations. Vaccination induces robust frequencies of largely peripheral CD4 T cells, with limited impact on CD8 or tissue-resident T cells, providing systemic protection but minimal mucosal immunity. Hybrid immunity combines features of infection and vaccination, resulting in robust frequencies of both peripheral and tissue-resident CD4 and CD8 T cells, enhancing protection at mucosal sites and against reinfection. Revaccination of hybrid-immune individuals further diversifies and amplifies frequencies of both peripheral and tissue-resident CD4 and CD8 T cells, driving optimal immune surveillance and response at systemic and mucosal sites.
Importantly, after priming or revaccination, T-cell immune responses reach maximal numbers of both memory and effector cells. However, over time, terminally differentiated effector cells contract, resulting in the persistence of largely central memory T cells (152). Thus, while T-cell responses appear to persist over time following vaccination, infection, as well as in the setting of hybrid immunity (153), effector cell responses contract in all populations, resulting in the circulation of only memory T cells, and not armed effector cells ready to respond immediately upon reinfection. Activation of memory T cells occurs in response to active viral replication or following vaccination. However, the speed of activation and conversion of memory, also known as anamnestic immunity, is a critical determinant of the ability of T cells to control and clear infection (154, 155). Due to age- or inflammation-associated senescence, anamnestic immunity is dampened in older and immunocompromised individuals (156, 157). Instead, revaccination is able to bolster T-cell numbers, and particularly effector cells, ready to act upon encounter with a virally infected cell, which may be of the utmost importance in vulnerable populations that exhibit delayed proliferative kinetics and/or require higher frequencies of T cells due to compromised humoral immune responses (158). Within the cellular arm of the immune response, cytotoxic T cells (CD8 T cells) play a role in viral control and clearance by directly interacting with SARS-CoV-2–infected cells to suppress viral replication or kill the infected cells (159). In contrast, CD4 T cells play diverse roles, as helpers for B- and T-cell programming (159), but can also differentiate into cytotoxic cells that are induced at higher levels with hybrid immunity and are linked to protection against symptomatic disease (22). Importantly, among the CD4 T-cell subpopulations, follicular helper (Tfh) subpopulations play a key role in promoting effective humoral immune responses (160, 161). While vaccination alone was shown to elicit robust SARS-CoV-2 Spike-specific CD4 T-helper responses and low-level CD8 T-cell immunity (162–164), infection induces enhanced CD8 T-cell immunity (150). This observation argues that viral infection may promote enhanced cytotoxic CD8 T-cell responses (150). However, vaccination following infection and revaccination of hybrid-immune individuals resulted in a robust expansion of both cytotoxic CD4 and CD8 T-cell responses (117–121) (Figure 3) as well as Tfh levels (160, 161) that are likely key to robust affinity maturation and evolution of neutralizing antibody responses.
Additionally, unlike vaccination alone, hybrid immunity results in the induction of tissue-resident T-cell populations that can respond rapidly to infection (18, 19), pointing to a critical opportunity for revaccination to increase the number of effector cells at the site of viral replication. A study evaluating phenotype, specificity, function, and persistence of nasal-resident SARS-CoV-2–specific T cells showed almost exclusive detection of SARS-CoV-2–specific CD4 and CD8 T cells in nasal mucosa among individuals with hybrid immunity versus those with only vaccine-induced immunity (18), with nasal-resident T-cell responses persisting for ≥140 days post infection (18). Differences observed in hybrid immunity compared with vaccine-only immunity may be related to critical signals delivered to T cells during SARS-CoV-2 infection within the mucosa, which may be key to retaining T cells at the site of infection. Along these lines, preclinical studies have suggested that, following infection, nasal-associated lymphoid aggregates remain active in the tissue, supporting the persistence of virus-specific T cells (18, 165), particularly in the lower respiratory tract (105). Because memory T cells perpetually survey tissues, revaccination is likely to bolster both systemic and tissue-resident immunity, amplifying the number of effector cells able to respond to infection.
Both infection- and vaccine-induced T cells exhibit broad cross-reactivity against newly emerging variants (86), including XBB.1, BA.2.86, and beyond (87). Interestingly, the order of vaccination/infection appears to affect the CD8 T-cell response (166). Additionally, the frequency and functional diversity of T cells expands with successive doses of mRNA vaccination in a hybrid-immune population (121). Importantly, these expanded CD4 and CD8 T-cell responses lead to increased viral control and clearance (159).
In addition to enhanced humoral responses, hybrid immunity is associated with robust and sustained cellular immune responses, particularly characterized by elevated interferon-gamma (IFN-γ) secretion by antigen-specific CD4+ and CD8+ T cells. These T-cell responses are often polyfunctional, co-expressing IFN-γ, IL-2, and TNF-α, and display greater breadth and magnitude than those elicited by vaccination or infection alone (135). Importantly, IFN-γ production remains detectable for several months following antigen exposure and appears to be less affected by Spike protein mutations, contributing to more durable and cross-variant protection in hybrid- immune individuals (167, 168). Moreover, evidence suggests that different cytokine profiles lead to various helper functions, with similarities across both vaccinated and hybrid individuals (169). This enhanced T cell–mediated immunity likely plays a critical role in limiting disease severity, especially in the context of emerging variants with partial escape from neutralizing antibodies.
Overall, revaccination significantly enhances T-cell responses following infection, particularly within the mucosal compartments, leading to increased numbers of both CD4 and CD8 T cells at the sites of viral replication. This augmentation is critical for vulnerable populations, as it improves the body’s ability to rapidly respond to and clear infections, including by highly mutated SARS-CoV-2 variants. Thus, boosting not only strengthens the overall T-cell–mediated immune response but also ensures a robust and versatile defense mechanism against emerging variants of concern, as T-cell epitopes are more conserved across viral variants (170).
2.4 Revaccination as a mechanism to improve immune durability
The benefits of revaccination in hybrid-immune populations over time was modeled in the setting of individuals living in prison settings (171). Specifically, the impact of infection alone, vaccination alone, hybrid immunity, and revaccination on reducing the risk of infection in their close contacts was assessed (171). While hybrid immunity provided the greatest and most durable level of indirect protection, additional vaccine doses, especially those targeting circulating variants, provided additive benefits in those with infection-acquired immunity (171). Whether this enhanced durability of protection was due to characteristics of the infecting variant, improved quality of the immune response, or simply higher levels of antibody titers or T-cell frequencies remains incompletely defined.
Vaccination-induced immunity, while initially highly effective in limiting COVID-19 (172), clearly wanes over time, both due to decreasing systemic antibody concentrations but also due to the emergence of viral variants (132, 173). Importantly, the same trend of waning immunity was observed with infection-induced immunity, which has been shown to initially provide a high degree of protection against reinfection, but over time provides incomplete protection against emerging SARS-CoV-2 variants (174). Specifically, titers following vaccine-induced immunity have been shown to be more durable than those following infection, particularly with mRNA-based COVID-19 vaccines, with a median duration of 29.6 months (175). In comparison, infection offered a substantial initial immune response, but with a shorter median durability of 21.5 months (175). Hybrid immunity, resulting from infection followed by vaccination, shows enhanced and more durable immune responses than either vaccination or infection alone, leading to higher antibody titers and better cross-reactivity against different variants (176, 177). However, strikingly, following a third vaccine dose, waning declined, and after a fourth dose antibody titers remained remarkably stable (173). Furthermore, boosting of individuals with hybrid immunity exhibited more durable protection from reinfection (39), both in the pre-omicron and post-omicron era, suggesting that COVID-19 mRNA vaccination induces long-lasting transmission-blocking activity (178).
Beyond the effects of high-titer antibodies that provide a first line of defense against an incoming infection, the anamnestic immune response has been linked to long-term protection against COVID-19 (179). Higher frequencies of the circulating memory T and B cells that survey for infection increase the probability of a rapid and effective response to infection (152, 180, 181). Along these lines, the speed of the anamnestic antibody response was a critical predictor of viral control 1 year after mRNA vaccination in a non-human primate model (182). Importantly, the speed of the humoral, but not the T cell, response was a key determinant of viral load control in this preclinical model (182). Similarly, a rapid immune response upon reinfection with SARS-CoV-2 has been shown to be improved with hybrid immunity compared with vaccination alone in humans (22, 117), potentially via the rapid generation of germinal centers able to quickly adapt to the incoming variant resulting in accelerated generation of antibody-secreting cells producing up-to-date RBD-specific antibodies (22). Moreover, revaccination, as described above, increases both the frequency and quality of memory B cells and T cells, providing the potential for effective immune recall of diverse and high-quality memory cells in response to reinfection (16, 24, 82, 178). These data point to 2 potential mechanisms underlying durable immunity: 1) the persistence of a strong initial defense against the virus, characterized by sustained antibody titers and effector T cells, and 2) the maintenance of an expanded pool of memory B and T cells that can be quickly recalled. These memory cells are capable of rapidly recognizing and adapting to sequence changes, as well as proliferating in response to viral exposure with the capacity to traffic and respond rapidly at the site of infection. Routine revaccination is likely a key mechanism by which both of these lines of defense may be bolstered to provide both a first and second line of defense against SARS-CoV-2.
Among immunocompromised individuals, dampened antibody responses following SARS-CoV-2 infection and vaccination have been well established. Immunocompromised individuals often required multiple vaccine doses (up to 4 or 5) to achieve antibody titers comparable to those of immunocompetent individuals (183). Revaccination after a SARS-CoV-2 infection offers substantial benefits to these individuals by significantly enhancing both humoral and cellular immune responses (183). The combination of infection and vaccination has been shown to lead to a significant increase in neutralization capacity and cross-protection against emerging variants, including omicron variants, which have been more challenging due to their antigenic drift from the original virus strain (184). T-cell activation and memory T-cell formation were also stronger following revaccination in this group, providing a more robust and long-term immune defense against SARS-CoV-2 (158, 185). Moreover, this improved immune response translated into more favorable clinical outcomes for immunocompromised individuals with hybrid immunity compared with those who did not receive a vaccine post-infection, where individuals with hybrid immunity showed lower hospitalization rates and a reduced risk of severe COVID-19 complications (186). Yet, revaccination of immunocompromised populations with hybrid immunity was shown to update and replenish rapidly waning humoral immune responses (187), suggesting that such revaccination may be key to ensuring that these individuals experience mild or moderate symptoms when subsequently exposed to SARS-CoV-2.
2.5 Revaccination to prevent post-acute sequelae of COVID-19
In addition to acute phase outcomes, it is important to consider the post-acute sequelae of COVID-19. Long COVID, or post-acute sequelae of COVID-19, includes a myriad of complications affecting multiple organ systems, which, beyond 30 days after COVID-19 diagnosis, cause a substantial burden of health loss and increased risk of mortality (12). Findings from a cohort study utilizing electronic health record databases in the United States showed that risk of post-acute sequelae and death were substantially lower in individuals with hybrid immunity versus those with infection alone (188). Similarly, findings from a prospective study showed that unvaccinated individuals reported higher rates of post-acute sequelae at 6 months compared with those who were vaccinated (45.2% vs. 33.3%; p = 0.018) (189). However, additional doses of vaccine in those with hybrid immunity was shown to provide a protective effect in a large study of over 109,000 individuals in Germany, with a direct protective effect against post-COVID condition observed following a fourth vaccination (190).
Several hypotheses have been raised to explain the etiology of long COVID, including the potential persistence of viral reservoirs in specific organs/tissues that may lead to persistent inflammation and tissue damage (191, 192). Additionally, the association of long COVID with the emergence of pathological immune responses following resolution of acute infection remains a critical area of research in the field. However, the ability to improve immune surveillance, across all organs and tissues, to both prevent an initial acute tissue pathological insult or to contribute to persistent surveillance and elimination of reservoirs likely could play an important role in limiting long COVID. Vaccination after recovery from COVID-19 boosts the immune response (39), potentially helping to clear any residual virus and preventing its reactivation, which could contribute to prolonged symptoms. Thus, the expanded T- and B-cell responses observed with revaccination, in addition to hybrid immunity, may offer a critical means to not only provide protection against the acute inflammatory consequences of COVID-19, but also provide an additional defense against post-acute consequence of infection (121, 189).
3 Discussion
The global SARS-CoV-2–specific immune landscape has progressed to one that is largely hybrid immune (2–4). However, hybrid immunity is highly heterogenous due to exposure to different variants and differences in vaccination history, as well as differences in levels of natural antigen exposure. Conversely, annual revaccination robustly raises immunity across all populations, bolstering humoral and cellular immunity irrespective of infection and vaccination history and thereby providing protective immunity across vaccinees. Yet, revaccination with variant-updated vaccines also provides critical value, inducing an immune response to protect against novel SARS-CoV-2 variants, reinforcing the need for updated booster vaccines as new variants of concern emerge (5). While there is no distinct seasonality to COVID-19, similar to other respiratory viruses, peaks are typically seen in the winter (50). As such, preseason boosting can play a critical role in harmonizing the magnitude, breadth, and contemporaneousness of the immune response, equipping revaccinated individuals with more effective immunity when there is a higher probability of exposure to the virus. While immune imprinting provides a critical evolutionary mechanism to rapidly adapt and expand a new antibody-secreting response from memory B cells, the narrower range of potential B-cell responses may incompletely explore the potential landscape of more potent humoral immune responses that may provide the highest level of protection (53). Thus, while vaccine design is underway to define novel antigen design approaches to overcome imprinting, boosting with contemporaneous variants provides a means to rapidly update a memory B-cell population and create a broader repertoire of clones. Although this repertoire may not be perfectly matched to the next strain that may emerge, it provides a mechanism to shift the response to accumulating mutations in the variant evolutionary landscape. Additionally, revaccination may increase the population of tissue-resident effector T cells, which are crucial for a rapid and timely response to infection (18, 19). As such, updated variant–containing booster vaccines are needed to avoid perpetual recall of archived immune responses, and instead enable preexisting immunity to adapt and provide optimal protection against newly circulating strains. Accordingly, global recommending bodies have begun to harmonize COVID-19 vaccine composition to more closely match the predominantly circulating variants and simplify the vaccine schedules (8, 36). Monovalent variant–updated vaccines are currently recommended to continue to shift the humoral immune response forward and avoid back-boosting, to protect against the currently circulating sublineages (36).
Future strategies should consider the prospect of blocking transmission of SARS-CoV-2 by modulating immune responses in the respiratory mucosa to provide localized protection and recall responses at the sites of viral entry (193). However, given that a vast majority of the population has hybrid immunity with both systemic and mucosal immune responses to SARS-CoV-2, repeated intramuscular vaccination may be sufficient for continued protection from COVID-19. A more thorough understanding of the extent and duration of protection against reinfection through hybrid immunity is crucial for continued public health planning, as such information may guide recommendations for optimal COVID-19 vaccine timing following SARS-CoV-2 infection (66).
Although hybrid immunity confers broader and more durable immune protection than either modality alone, it is increasingly challenged by the rapid antigenic evolution of SARS-CoV-2. Variants such as XBB.1.5, EG.5, and JN.1 exhibit substantial immune escape from neutralizing antibodies generated by previous exposures, even in individuals with hybrid immunity (107, 194). While cellular responses (e.g., memory T cells) provide protection against severe disease, these responses alone do not prevent infection or transmission, and the growing frequency of reinfections illustrates this limitation, highlighting the need for continued vaccination against the current variants.
Epidemiological data show that reinfections are rising in frequency and occurring at shorter intervals, with some individuals experiencing three or more infections within a two-year span, particularly during periods of Omicron subvariant dominance (195, 196). Furthermore, while prior immunity continues to reduce the risk of hospitalization and death, susceptibility to symptomatic infection remains significant with each new wave of antigenically distinct variants (197). In hybrid-immune populations, susceptibility to symptomatic reinfection persists, particularly among older adults, immunocompromised individuals, and those with comorbidities (25). This highlights the critical limitation of relying solely on existing immunity shaped by outdated antigen exposures.
This ongoing pattern of reinfection underscores a critical problem: current vaccine-induced and infection-induced immunity is often based on outdated antigenic exposures. Immune imprinting—where immune memory preferentially targets epitopes from the original viral strain—may further limit the effectiveness of responses to new variants (198). Re-vaccination with updated formulations is therefore essential to realign the immune response to the circulating virus. In the context of hybrid immunity, re-vaccination can amplify and refocus immune memory, mitigating the risk of breakthrough infections and potentially curbing onward transmission. Revaccination in hybrid-immune individuals drives the maturation and expansion of neutralizing and non-neutralizing antibodies, and enhancing mucosal and tissue-resident immunity. It facilitates rapid adaptation of pre-existing memory B cells, promotes the development of broader and more variant-resilient antibody repertoires, and boosts mucosal IgA and effector T-cell responses at key sites of viral entry. Importantly, updated boosters have been shown to improve protection against highly immune-evasive strains such as XBB.1.5 and KP.2 (72, 199). Revaccination also improves the durability of immune protection, enhances immune responses in immunocompromised individuals, and reduces the risk of post-acute sequelae such as long COVID (12, 16, 81). Thus, in the context of an ever-shifting viral landscape, revaccination with updated formulations is not only a mechanism to restore and broaden protection but also a critical tool to reduce infection, transmission, severe disease, and long-term complications in both general and vulnerable populations.
Author contributions
MB-J: Conceptualization, data curation, formal analysis, Writing – original draft, Writing – review & editing. GA: Conceptualization, data curation, formal analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Moderna, Inc.
Acknowledgments
Medical writing and editorial assistance were provided by Kate Russin, PhD, of MEDiSTRAVA in accordance with Good Publication Practice (GPP 2022) guidelines, funded by Moderna, Inc., and under the direction of the authors.
Conflict of interest
MB-J and GA were employed by Moderna, Inc. at the time of development of the manuscript.
The authors declare that this study received funding from Moderna, Inc. The funder had the following involvement in the study: manuscript development and approval.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Our World in Data. Coronavirus (COVID-19) vaccinations (2024). Available online at: https://ourworldindata.org/covid-vaccinations. (Accessed May 1, 2024).
3. World Health Organization. Global Covid-19 vaccination strategy in a changing world (2022). Available online at: https://www.who.int/docs/default-source/coronaviruse/global-covid-19-vaccination-strategy-in-a-changing-world—july-2022-update.pdf. (Accessed May 1, 2024).
4. World Health Organization. WHO COVID-19 dashboard (2024). Available online at: https://data.who.int/dashboards/covid19/cases?n=c. (Accessed May 1, 2024).
5. The Lancet Infectious Diseases. Why hybrid immunity is so triggering Vol. 22. The Lancet Infectious Diseases (2022). p. 1649.
6. Fang L, Xu J, Zhao Y, Fan J, Shen J, Liu W, et al. The effects of amino acid substitution of spike protein and genomic recombination on the evolution of SARS-CoV-2. Front Microbiol. (2023) 14:1228128. doi: 10.3389/fmicb.2023.1228128
7. Kumar R, Srivastava Y, Muthuramalingam P, Singh SK, Verma G, Tiwari S, et al. Understanding mutations in human SARS-CoV-2 spike glycoprotein: a systematic review & meta-analysis. Viruses. (2023) 15:856. doi: 10.3390/v15040856
8. World Health Organization. Tracking SARS-CoV-2 variants (2024). Available online at: https://www.who.int/activities/tracking-SARS-CoV-2-variants. (Accessed May 1, 2024).
9. Taylor CA, Patel K, Pham H, Kirley PD, Kawasaki B, Meek J, et al. COVID-19-associated hospitalizations among U.S. adults aged ≥18 years - COVID-NET, 12 states, October 2023-April 2024. MMWR Morb Mortal Wkly Rep. (2024) 73:869–75. doi: 10.15585/mmwr.mm7339a2
10. Antinori A and Bausch-Jurken M. The burden of COVID-19 in the immunocompromised patient: implications for vaccination and needs for the future. J Infect Dis. (2023) 228:S4–S12. doi: 10.1093/infdis/jiad181
11. Xie Y, Choi T, and Al-Aly Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect Dis. (2024) 24:239–55. doi: 10.1016/S1473-3099(23)00684-9
12. Al-Aly Z, Xie Y, and Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. (2021) 594:259–64. doi: 10.1038/s41586-021-03553-9
13. Song Z and Giuriato M. Demographic and clinical factors associated with long COVID. Health Aff (Millwood). (2023) 42:433–42. doi: 10.1377/hlthaff.2022.00991
14. Callaway E. COVID’s future: mini-waves rather than seasonal surges. Nature. (2023) 617:229–30. doi: 10.1038/d41586-023-01437-8
15. Townsend JP, Hassler HB, Lamb AD, Sah P, Alvarez Nishio A, Nguyen C, et al. Seasonality of endemic COVID-19. mBio. (2023) 14:e0142623. doi: 10.1128/mbio.01426-23
16. Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman LS, Ash N, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med. (2022) 386:2201–12. doi: 10.1056/NEJMoa2118946
17. Andeweg SP, de Gier B, Eggink D, van den Ende C, van Maarseveen N, Ali L, et al. Protection of COVID-19 vaccination and previous infection against Omicron BA.1, BA.2 and Delta SARS-CoV-2 infections. Nat Commun. (2022) 13:4738. doi: 10.1038/s41467-022-31838-8
18. Lim JME, Tan AT, Le Bert N, Hang SK, Low JGH, and Bertoletti A. SARS-CoV-2 breakthrough infection in vaccinees induces virus-specific nasal-resident CD8+ and CD4+ T cells of broad specificity. J Exp Med. (2022) 219:e20220780. doi: 10.1084/jem.20220780
19. Nowill AE, Caruso M, and de Campos-Lima PO. T-cell immunity to SARS-CoV-2: what if the known best is not the optimal course for the long run? Adapting to evolving targets. Front Immunol. (2023) 14:1133225. doi: 10.3389/fimmu.2023.1133225
20. Lasrado N and Barouch DH. SARS-CoV-2 hybrid immunity: the best of both worlds. J Infect Diseases. (2023) 228:1311–3. doi: 10.1093/infdis/jiad353
21. Gancino M and Vispo NS. Hybrid immunity: the immune response of COVID-19 survivors to vaccination. Rev Bionatura. (2021) 6:1890–2. doi: 10.21931/RB
22. Rodda LB, Morawski PA, Pruner KB, Fahning ML, Howard CA, Franko N, et al. Imprinted SARS-CoV-2-specific memory lymphocytes define hybrid immunity. Cell. (2022) 185:1588–601 e14. doi: 10.1016/j.cell.2022.03.018
23. Buggert M, Cai C, Gao Y, Ballesteros OR, Hansson L, Österborg A, et al. Booster mRNA vaccination post-SARS-CoV-2 infection enhances functional qualities of T cell immunity. Res Square. (2023). doi: 10.21203/rs.3.rs-2587292/v1
24. Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. (2022) 386:1207–20. doi: 10.1056/NEJMoa2118691
25. Tsagkli P, Geropeppa M, Papadatou I, and Spoulou V. Hybrid immunity against SARS-CoV-2 variants: a narrative review of the literature. Vaccines (Basel). (2024) 12:1051. doi: 10.3390/vaccines12091051
26. Chen Y, Liu Q, Zhou L, Zhou Y, Yan H, and Lan K. Emerging SARS-CoV-2 variants: Why, how, and what’s next? Cell Insight. (2022) 1:100029. doi: 10.1016/j.cellin.2022.100029
27. Centers for Disease Control and Prevention. CDC museum COVID-19 timeline (2024). Available online at: https://www.cdc.gov/museum/timeline/covid19.html. (Accessed May 10, 2024).
28. Chakraborty C, Bhattacharya M, and Dhama K. SARS-CoV-2 Vaccines, vaccine development technologies, and significant efforts in vaccine development during the pandemic: the lessons learned might help to fight against the next pandemic. Vaccines (Basel). (2023) 11:682. doi: 10.3390/vaccines11030682
29. Cromer D, Reynaldi A, Mitchell A, Schlub TE, Juno JA, Wheatley AK, et al. Predicting COVID-19 booster immunogenicity against future SARS-CoV-2 variants and the benefits of vaccine updates. Nat Commun. (2024) 15:8395. doi: 10.1038/s41467-024-52194-9
30. Page L, Dennehy K, Mueller K, Girl P, Loell E, Buijze H, et al. Antigen-specific T helper cells and cytokine profiles predict intensity and longevity of cellular and humoral responses to SARS-CoV-2 booster vaccination. Front Immunol. (2024) 15:1423766. doi: 10.3389/fimmu.2024.1423766
31. Reinscheid M, Luxenburger H, Karl V, Graeser A, Giese S, Ciminski K, et al. COVID-19 mRNA booster vaccine induces transient CD8+ T effector cell responses while conserving the memory pool for subsequent reactivation. Nat Commun. (2022) 13:4631. doi: 10.1038/s41467-022-32324-x
32. Wee LE, Lim JT, Goel M, Malek MIA, Chiew CJ, Ong B, et al. Bivalent boosters and risk of post-acute sequelae following vaccine-breakthrough SARS-CoV-2 Omicron infection: a cohort study. Clin Infect Dis. (2025) 17;80:520–8. doi: 10.1093/cid/ciae598
33. Arbel R, Sergienko R, Friger M, Peretz A, Beckenstein T, Yaron S, et al. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med. (2022) 28:1486–90. doi: 10.1038/s41591-022-01832-0
34. Bello-Chavolla OY, Fermin-Martinez CA, Ramirez-Garcia D, Vargas-Vazquez A, Fernandez-Chirino L, Basile-Alvarez MR, et al. Prevalence and determinants of post-acute sequelae after SARS-CoV-2 infection (Long COVID) among adults in Mexico during 2022: a retrospective analysis of nationally representative data. Lancet Reg Health Am. (2024) 30:100688. doi: 10.1016/j.lana.2024.100688
35. Subramanian V. Susceptibility to SARS-CoV-2 infection and immune responses to COVID-19 vaccination among recipients of solid organ transplants. J Infect Dis. (2023) 228:S34–45. doi: 10.1093/infdis/jiad152
36. World Health Organization. Statement on the antigen composition of COVID-19 vaccines(2023). Available online at: https://www.who.int/news/item/13-12-2023-statement-on-the-antigen-composition-of-covid-19-vaccines. (Accessed June 10, 2024).
37. World Health Organization. Interim statement on hybrid immunity and increasing population seroprevalence rates (2022). Available online at: https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-immunity-and-increasing-population-seroprevalence-rates. (Accessed April 15, 2024).
38. Chervo TC, Elkin EP, Nugent JR, Valice E, Amsden LB, Ergas IJ, et al. Relative contribution of COVID-19 vaccination and SARS-CoV-2 infection to population-level seroprevalence of SARS-CoV-2 spike antibodies in a large integrated health system. PLoS One. (2024) 19:e0303303. doi: 10.1371/journal.pone.0303303
39. Bobrovitz N, Ware H, Ma X, Li Z, Hosseini R, Cao C, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. (2023) 23:556–67. doi: 10.1016/S1473-3099(22)00801-5
40. Busch MP, Stramer SL, Stone M, Yu EA, Grebe E, Notari E, et al. Population-weighted seroprevalence from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, vaccination, and hybrid immunity among US blood donations from January to December 2021. Clin Infect Dis. (2022) 75:S254–s63. doi: 10.1093/cid/ciac470
41. Frei A, Kaufmann M, Amati R, Butty Dettwiler A, von Wyl V, Annoni AM, et al. Development of hybrid immunity during a period of high incidence of Omicron infections. Int J Epidemiol. (2023) 52:1696–707. doi: 10.1093/ije/dyad098
42. Solastie A, Nieminen T, Ekström N, Nohynek H, Lehtonen L, Palmu AA, et al. Changes in SARS-CoV-2 seroprevalence and population immunity in Finland, 2020–2022. Emerg Microbes Infect. (2023) 12:2222849. doi: 10.1080/22221751.2023.2222849
43. Sulcebe G, Ylli A, Cenko F, Kurti-Prifti M, Shyti E, Dashi-Pasholli J, et al. Trends in SARS-CoV-2 seroprevalence in Albania during the 2021–2022 pandemic year. New Microbes New Infect. (2024) 56:101208. doi: 10.1016/j.nmni.2023.101208
44. Our World in Data. Coronavirus (COVID-19) cases (2024). Available online at: https://ourworldindata.org/covid-cases. (Accessed April 15, 2024).
45. Weill Cornell Medicine. Omicron: What we know so far (2021). Available online at: https://research.weill.cornell.edu/about-us/news-updates/omicron-what-we-know-so-far. (Accessed April 15, 2024).
46. Jones JM, Manrique IM, Stone MS, Grebe E, Saa P, Germanio CD, et al. Estimates of SARS-CoV-2 seroprevalence and incidence of primary SARS-CoV-2 infections among blood donors, by COVID-19 vaccination status - United States, April 2021-September 2022. MMWR Morb Mortal Wkly Rep. (2023) 72:601–5. doi: 10.15585/mmwr.mm7222a3
47. Clarke KEN, Jones JM, Deng Y, Nycz E, Lee A, Iachan R, et al. Seroprevalence of infection-induced SARS-CoV-2 antibodies - United States, September 2021-February 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:606–8. doi: 10.15585/mmwr.mm7117e3
48. Verheul MK, Kaczorowska J, Hofstee MI, Schepp RM, Smits GP, Wessels Beljaars D, et al. Protective mucosal SARS-CoV-2 antibodies in the majority of the general population in the Netherlands. Mucosal Immunol. (2024) 17:554–64. doi: 10.1016/j.mucimm.2024.03.008
49. IDSA and CDC. COVID-19 variant update . Available online at: https://www.idsociety.org/covid-19-real-time-learning-network/diagnostics/covid-19-variant-update//+/0/publishedDate_na_dt/desc/. (Accessed August 10, 2024).
50. Centers for Disease Control and Prevention. COVID-19 can surge throughout the year (2024). Available online at: https://www.cdc.gov/ncird/whats-new/covid-19-can-surge-throughout-the-year.html. (Accessed August 10, 2024).
51. Epsi NJ, Richard SA, Lindholm DA, Mende K, Ganesan A, Huprikar N, et al. Understanding ‘hybrid immunity’: comparison and predictors of humoral immune responses to SARS-CoV-2 infection and COVID-19 vaccines. Clin Infect Dis. (2023) 76:e439–e49. doi: 10.1093/cid/ciac392
52. Lewnard JA, Hong V, Kim JS, Shaw SF, Lewin B, Takhar H, et al. Increased vaccine sensitivity of an emerging SARS-CoV-2 variant. Nat Commun. (2023) 14:3854. doi: 10.1038/s41467-023-39567-2
53. Zhou Z, Barrett J, and He X. Immune imprinting and implications for COVID-19. Vaccines (Basel). (2023) 11:875. doi: 10.3390/vaccines11040875
54. Miyakawa K, Jeremiah SS, Yamaoka Y, Koyama T, Tokumasu R, Kudo M, et al. Molecular and epidemiological characterization of emerging immune-escape variants of SARS-CoV-2. Front Med (Lausanne). (2022) 9:811004. doi: 10.3389/fmed.2022.811004
55. Jiang XL, Song XD, Shi C, Yang GJ, Wang XJ, Zhang YW, et al. Variant-specific antibody response following repeated SARS-CoV-2 vaccination and infection. Cell Rep. (2024) 43:114387. doi: 10.1016/j.celrep.2024.114387
56. Paul P, El-Naas A, Hamad O, Salameh MA, Mhaimeed N, Laswi I, et al. Effectiveness of the pre-Omicron COVID-19 vaccines against Omicron in reducing infection, hospitalization, severity, and mortality compared to Delta and other variants: a systematic review. Hum Vaccin Immunother. (2023) 19:2167410. doi: 10.1080/21645515.2023.2167410
57. Nikolaidis M, Papakyriakou A, Chlichlia K, Markoulatos P, Oliver SG, and Amoutzias GD. Comparative analysis of SARS-CoV-2 variants of concern, including omicron, highlights their common and distinctive amino acid substitution patterns, especially at the spike ORF. Viruses. (2022) 14:707. doi: 10.3390/v14040707
58. Chavda VP, Bezbaruah R, Deka K, Nongrang L, and Kalita T. The delta and omicron variants of SARS-CoV-2: what we know so far. Vaccines (Basel). (2022) 10:1926. doi: 10.3390/vaccines10111926
59. Windsor IW, Tong P, Lavidor O, Moghaddam AS, McKay LGA, Gautam A, et al. Antibodies induced by an ancestral SARS-CoV-2 strain that cross-neutralize variants from Alpha to Omicron BA.1. Sci Immunol. (2022) 7:eabo3425. doi: 10.1126/sciimmunol.abo3425
60. Vogt AS, Augusto G, Martina B, Chang X, Nasrallah G, Speiser DE, et al. Increased receptor affinity and reduced recognition by specific antibodies contribute to immune escape of SARS-CoV-2 variant omicron. Vaccines (Basel). (2022) 10:743. doi: 10.3390/vaccines10050743
61. Moulana A, Dupic T, Phillips AM, Chang J, Roffler AA, Greaney AJ, et al. The landscape of antibody binding affinity in SARS-CoV-2 Omicron BA.1 evolution. Elife. (2023) 12:e83442. doi: 10.7554/eLife.83442
62. Bekliz M, Adea K, Vetter P, Eberhardt CS, Hosszu-Fellous K, Vu DL, et al. Neutralization capacity of antibodies elicited through homologous or heterologous infection or vaccination against SARS-CoV-2 VOCs. Nat Commun. (2022) 13:3840. doi: 10.1038/s41467-022-31556-1
63. Flacco ME, Acuti Martellucci C, Baccolini V, De Vito C, Renzi E, Villari P, et al. Risk of reinfection and disease after SARS-CoV-2 primary infection: meta-analysis. Eur J Clin Invest. (2022) 52:e13845. doi: 10.1111/eci.13845
64. Hadley E, Yoo YJ, Patel S, Zhou A, Laraway B, Wong R, et al. Insights from an N3C RECOVER EHR-based cohort study characterizing SARS-CoV-2 reinfections and Long COVID. Commun Med (Lond). (2024) 4:129. doi: 10.1038/s43856-024-00539-2
65. Hu WH, Cai HL, Yan HC, Wang H, Sun HM, Wei YY, et al. Protective effectiveness of previous infection against subsequent SARS-Cov-2 infection: systematic review and meta-analysis. Front Public Health. (2024) 12:1353415. doi: 10.3389/fpubh.2024.1353415
66. Wei J, Stoesser N, Matthews PC, Khera T, Gethings O, Diamond I, et al. Risk of SARS-CoV-2 reinfection during multiple Omicron variant waves in the UK general population. medRxiv. (2023). doi: 10.1101/2023.06.29.23292043
67. Yue C, Song W, Wang L, Jian F, Chen X, Gao F, et al. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect Dis. (2023) 23:278–80. doi: 10.1016/S1473-3099(23)00010-5
68. Qu P, Faraone JN, Evans JP, Zheng Y-M, Carlin C, Anghelina M, et al. Extraordinary evasion of neutralizing antibody response by omicron XBB.1.5, CH.1.1 and CA.3.1 variants. bioRxiv. (2023). doi: 10.1016/j.celrep.2023.112443
69. Thakur V, Bhola S, Thakur P, Patel SKS, Kulshrestha S, Ratho RK, et al. Waves and variants of SARS-CoV-2: understanding the causes and effect of the COVID-19 catastrophe. Infection. (2022) 50:309–25. doi: 10.1007/s15010-021-01734-2
70. Link-Gelles R, Ciesla AA, Mak J, Miller JD, Silk BJ, Lambrou AS, et al. Early estimates of updated 2023-2024 (monovalent XBB.1.5) COVID-19 vaccine effectiveness against symptomatic SARS-CoV-2 infection attributable to co-circulating omicron variants among immunocompetent adults - increasing community access to testing program, United States, September 2023-January 2024. MMWR Morb Mortal Wkly Rep. (2024) 73:77–83. doi: 10.15585/mmwr.mm7304a2
71. World Health Organization. Statement on the antigen composition of COVID-19 vaccines (2024). Available online at: https://www.who.int/news/item/23-12-2024-statement-on-the-antigen-composition-of-covid-19-vaccines. (Accessed January 10, 2025).
72. Kaku Y, Uriu K, Kosugi Y, Okumura K, Yamasoba D, Uwamino Y, et al. Virological characteristics of the SARS-CoV-2 KP.2 variant. Lancet Infect Dis. (2024) 24:e416. doi: 10.1016/S1473-3099(24)00298-6
73. US Food and Drug Administration. Updated COVID-19 xaccines for use in the United States beginning in fall 2024–2024. Available online at: https://www.fda.gov/vaccines-blood-biologics/updated-covid-19-vaccines-use-united-states-beginning-fall-2024. (Accessed November 13, 2024).
74. Zhang Y, Fu Z, Zhang H, Lin K, Song J, Guo J, et al. Proteomic and cellular characterization of omicron breakthrough infections and a third homologous or heterologous boosting vaccination in a longitudinal cohort. Mol Cell Proteomics. (2024) 23:100769. doi: 10.1016/j.mcpro.2024.100769
75. Tartof SY, Xie F, Yadav R, Wernli KJ, Martin ET, Belongia EA, et al. Prior SARS-CoV-2 infection and COVID-19 vaccine effectiveness against outpatient illness during widespread circulation of SARS-CoV-2 Omicron variant, US Flu VE network. Influenza Other Respir Viruses. (2023) 17:e13143. doi: 10.1111/irv.13143
76. Baker MG, Kvalsvig A, Plank MJ, Geoghegan JL, Wall T, Tukuitonga C, et al. Continued mitigation needed to minimise the high health burden from COVID-19 in Aotearoa New Zealand. N Z Med J. (2023) 136:67–91. doi: 10.26635/6965.6247
77. Nori W and Ghani Zghair MA. Omicron targets upper airways in pediatrics, elderly and unvaccinated population. World J Clin Cases. (2022) 10:12062–5. doi: 10.12998/wjcc.v10.i32.12062
78. Hui KPY, Ng KC, Ho JCW, Yeung HW, Ching RHH, Gu H, et al. Replication of SARS-CoV-2 Omicron BA.2 variant in ex vivo cultures of the human upper and lower respiratory tract. EBioMedicine. (2022) 83:104232. doi: 10.1016/j.ebiom.2022.104232
79. Tseng HF, Ackerson BK, Bruxvoort KJ, Sy LS, Tubert JE, Lee GS, et al. Effectiveness of mRNA-1273 vaccination against SARS-CoV-2 omicron subvariants BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. Nat Commun. (2023) 14:189. doi: 10.1038/s41467-023-35815-7
80. Ma A and Parry J. When Hong Kong’s “dynamic zero” covid-19 strategy met omicron, low vaccination rates sent deaths soaring. BMJ. (2022) 377:o980. doi: 10.1136/bmj.o980
81. Lazar Neto F, Hitchings MDT, Amin AB, de Franca GVA, Lind ML, Scaramuzzini Torres MS, et al. Effectiveness of the fourth dose of COVID-19 vaccines against severe COVID-19 among adults 40 years or older in Brazil: a population-based cohort study. Lancet Reg Health Am. (2024) 34:100755. doi: 10.1016/j.lana.2024.100755
83. Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. (2021) 374:abm0829. doi: 10.1126/science.abm0829
84. Sokal A, Barba-Spaeth G, Hunault L, Fernández I, Broketa M, Meola A, et al. Omicron BA.1 breakthrough infection drives long-term remodeling of the memory B cell repertoire in vaccinated individuals. bioRxiv. (2023). doi: 10.1101/2023.01.27.525575
85. Kaplonek P, Deng Y, Shih-Lu Lee J, Zar HJ, Zavadska D, Johnson M, et al. Hybrid immunity expands the functional humoral footprint of both mRNA and vector-based SARS-CoV-2 vaccines. Cell Rep Med. (2023) 4:101048. doi: 10.1016/j.xcrm.2023.101048
86. He X, Jiang J, Li G, Liu J, Gan J, Zhou L, et al. Heterogeneous hybrid immunity against Omicron variant JN.1 at 11 months following breakthrough infection. Signal Transduct Target Ther. (2024) 9:180. doi: 10.1038/s41392-024-01898-x
87. Carnell GW, Ciazynska KA, Wells DA, Xiong X, Aguinam ET, McLaughlin SH, et al. SARS-CoV-2 spike protein stabilized in the closed state induces potent neutralizing responses. J Virol. (2021) 95:e0020321. doi: 10.1128/JVI.00203-21
88. Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. (2021) 19:409–24. doi: 10.1038/s41579-021-00573-0
89. Yassini P, Hutchens M, Paila YD, Schoch L, Aunins A, Siangphoe U, et al. Interim analysis of a phase 1 randomized clinical trial on the safety and immunogenicity of the mRNA-1283 SARS-CoV-2 vaccine in adults. Hum Vaccin Immunother. (2023) 19:2190690. doi: 10.1080/21645515.2023.2190690
90. Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, and Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. (2020) 176:104742. doi: 10.1016/j.antiviral.2020.104742
91. Andreano E, Paciello I, Pierleoni G, Maccari G, Antonelli G, Abbiento V, et al. mRNA vaccines and hybrid immunity use different B cell germlines against Omicron BA.4 and BA.5. Nat Commun. (2023) 14:1734. doi: 10.1038/s41467-023-37422-y
92. Guenthoer J, Garrett ME, Lilly M, Depierreux DM, Ruiz F, Chi M, et al. The S2 subunit of spike encodes diverse targets for functional antibody responses to SARS-CoV-2. PloS Pathog. (2024) 20:e1012383. doi: 10.1371/journal.ppat.1012383
93. Wratil PR, Stern M, Priller A, Willmann A, Almanzar G, Vogel E, et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med. (2022) 28:496–503. doi: 10.1038/s41591-022-01715-4
94. Paciello I, Pierleoni G, Pantano E, Antonelli G, Pileri P, Maccari G, et al. Antigenic sin and multiple breakthrough infections drive converging evolution of COVID-19 neutralizing responses. Cell Rep. (2024) 43:114645. doi: 10.1016/j.celrep.2024.114645
95. Wang Q, Guo Y, Tam AR, Valdez R, Gordon A, Liu L, et al. Deep immunological imprinting due to the ancestral spike in the current bivalent COVID-19 vaccine. Cell Rep Med. (2023) 4:101258. doi: 10.1016/j.xcrm.2023.101258
96. Cobey S. Vaccination against rapidly evolving pathogens and the entanglements of memory. Nat Immunol. (2024) 25:2015–23. doi: 10.1038/s41590-024-01970-2
97. Kurhade C, Zou J, Xia H, Liu M, Chang HC, Ren P, et al. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat Med. (2023) 29:344–7. doi: 10.1038/s41591-022-02162-x
98. Pradenas E, Marfil S, Urrea V, Trigueros M, Pidkova T, Pons-Grifols A, et al. Impact of hybrid immunity booster vaccination and Omicron breakthrough infection on SARS-CoV-2 VOCs cross-neutralization. iScience. (2023) 26:106457. doi: 10.1016/j.isci.2023.106457
99. Chernyshev M, Sakharkar M, Connor RI, Dugan HL, Sheward DJ, Rappazzo CG, et al. Vaccination of SARS-CoV-2-infected individuals expands a broad range of clonally diverse affinity-matured B cell lineages. Nat Commun. (2023) 14:2249. doi: 10.1038/s41467-023-37972-1
100. Andreano E, Paciello I, Piccini G, Manganaro N, Pileri P, Hyseni I, et al. Hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants. Nature. (2021) 600:530–5. doi: 10.1038/s41586-021-04117-7
101. Seydoux E, Homad LJ, MacCamy AJ, Parks KR, Hurlburt NK, Jennewein MF, et al. Analysis of a SARS-CoV-2-infected individual reveals development of potent neutralizing antibodies with limited somatic mutation. Immunity. (2020) 53:98–105.e5. doi: 10.1016/j.immuni.2020.06.001
102. Liu S, Tsun JGS, Fung GPG, Lui GCY, Chan KYY, Chan PKS, et al. Comparison of the mucosal and systemic antibody responses in Covid-19 recovered patients with one dose of mRNA vaccine and unexposed subjects with three doses of mRNA vaccines. Front Immunol. (2023) 14:1127401. doi: 10.3389/fimmu.2023.1127401
103. Puhach O, Bellon M, Adea K, Bekliz M, Hosszu-Fellous K, Sattonnet P, et al. SARS-CoV-2 convalescence and hybrid immunity elicits mucosal immune responses. eBioMedicine. (2023) 98:104893. doi: 10.1016/j.ebiom.2023.104893
104. Azzi L, Dalla Gasperina D, Veronesi G, Shallak M, Maurino V, Baj A, et al. Mucosal immune response after the booster dose of the BNT162b2 COVID-19 vaccine. EBioMedicine. (2023) 88:104435. doi: 10.1016/j.ebiom.2022.104435
105. Mitsi E, Diniz MO, Reiné J, Collins AM, Robinson RE, Hyder-Wright A, et al. Respiratory mucosal immune memory to SARS-CoV-2 after infection and vaccination. Nat Commun. (2023) 14:6815. doi: 10.1038/s41467-023-42433-w
106. Sokal A, Barba-Spaeth G, Fernandez I, Broketa M, Azzaoui I, de la Selle A, et al. mRNA vaccination of naive and COVID-19-recovered individuals elicits potent memory B cells that recognize SARS-CoV-2 variants. Immunity. (2021) 54:2893–907.e5. doi: 10.1016/j.immuni.2021.09.011
107. Wang Q, Bowen A, Valdez R, Gherasim C, Gordon A, Liu L, et al. Antibody response to omicron BA.4-BA.5 bivalent booster. N Engl J Med. (2023) 388:567–9. doi: 10.1056/NEJMc2213907
108. Kaku CI, Bergeron AJ, Ahlm C, Normark J, Sakharkar M, Forsell MNE, et al. Recall of preexisting cross-reactive B cell memory after Omicron BA.1 breakthrough infection. Sci Immunol. (2022) 7:eabq3511. doi: 10.1126/sciimmunol.abq3511
109. Samanovic MI, Cornelius AR, Gray-Gaillard SL, Allen JR, Karmacharya T, Wilson JP, et al. Robust immune responses are observed after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2-experienced individuals. Sci Transl Med. (2022) 14:eabi8961. doi: 10.1126/scitranslmed.abi8961
110. Zhang A, Stacey HD, D’Agostino MR, Tugg Y, Marzok A, and Miller MS. Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection. Nat Rev Immunol. (2023) 23:381–96. doi: 10.1038/s41577-022-00813-1
111. Beaudoin-Bussières G, Tauzin A, Dionne K, Gendron-Lepage G, Medjahed H, Perreault J, et al. A recent SARS-CoV-2 infection enhances antibody-dependent cellular cytotoxicity against several omicron subvariants following a fourth mRNA vaccine dose. Viruses. (2023) 15:1274. doi: 10.3390/v15061274
112. Tso FY, Lidenge SJ, Poppe LK, Pena PB, Privatt SR, Bennett SJ, et al. Presence of antibody-dependent cellular cytotoxicity (ADCC) against SARS-CoV-2 in COVID-19 plasma. PloS One. (2021) 16:e0247640. doi: 10.1371/journal.pone.0247640
113. Bowman KA, Stein D, Shin S, Ferbas KG, Tobin NH, Mann C, et al. Hybrid immunity shifts the Fc-effector quality of SARS-CoV-2 mRNA vaccine-induced immunity. bioRxiv. (2022). doi: 10.1101/2022.06.10.495727
114. Bahnan W, Wrighton S, Sundwall M, Blackberg A, Larsson O, Hoglund U, et al. Spike-dependent opsonization indicates both dose-dependent inhibition of phagocytosis and that non-neutralizing antibodies can confer protection to SARS-CoV-2. Front Immunol. (2021) 12:808932. doi: 10.3389/fimmu.2021.808932
115. Amanat F, Thapa M, Lei T, Ahmed SMS, Adelsberg DC, Carreno JM, et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell. (2021) 184:3936–48.e10. doi: 10.1016/j.cell.2021.06.005
116. Richardson SI, Madzorera VS, Spencer H, Manamela NP, van der Mescht MA, Lambson BE, et al. SARS-CoV-2 Omicron triggers cross-reactive neutralization and Fc effector functions in previously vaccinated, but not unvaccinated, individuals. Cell Host Microbe. (2022) 30:880–6.e4. doi: 10.1016/j.chom.2022.03.029
117. Chen Y, Tong P, Whiteman N, Moghaddam AS, Zarghami M, Zuiani A, et al. Immune recall improves antibody durability and breadth to SARS-CoV-2 variants. Sci Immunol. (2022) 7:eabp8328. doi: 10.1126/sciimmunol.abp8328
118. Rossler A, Knabl L, von Laer D, and Kimpel J. Neutralization profile after recovery from SARS-CoV-2 omicron infection. N Engl J Med. (2022) 386:1764–6. doi: 10.1056/NEJMc2201607
119. Carreno JM, Alshammary H, Tcheou J, Singh G, Raskin AJ, Kawabata H, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. (2022) 602:682–8. doi: 10.1038/s41586-022-04399-5
120. Manali M, Bissett LA, Amat JAR, Logan N, Scott S, Hughes EC, et al. SARS-CoV-2 evolution and patient immunological history shape the breadth and potency of antibody-mediated immunity. J Infect Dis. (2022) 227:40–9. doi: 10.1093/infdis/jiac332
121. Ford ES, Mayer-Blackwell K, Jing L, Laing KJ, Sholukh AM, St. Germain R, et al. Repeated mRNA vaccination sequentially boosts SARS-CoV-2-specific CD8+ T cells in persons with previous COVID-19. Nat Immunol. (2023) 25:166–77. doi: 10.1038/s41590-023-01692-x
122. den Hartog G, Andeweg SP, Hoeve CE, Smits G, Voordouw B, Eggink D, et al. Assessment of hybrid population immunity to SARS-CoV-2 following breakthrough infections of distinct SARS-CoV-2 variants by the detection of antibodies to nucleoprotein. Sci Rep. (2023) 13:18394. doi: 10.1038/s41598-023-45718-8
123. Matsumoto ML. Molecular mechanisms of multimeric assembly of IgM and IgA. Annu Rev Immunol. (2022) 40:221–47. doi: 10.1146/annurev-immunol-101320-123742
124. Terauchi Y, Sano K, Ainai A, Saito S, Taga Y, Ogawa-Goto K, et al. IgA polymerization contributes to efficient virus neutralization on human upper respiratory mucosa after intranasal inactivated influenza vaccine administration. Hum Vaccin Immunother. (2018) 14:1351–61. doi: 10.1080/21645515.2018.1438791
125. Brandsma AM, Bondza S, Evers M, Koutstaal R, Nederend M, Jansen JHM, et al. Potent Fc receptor signaling by IgA leads to superior killing of cancer cells by neutrophils compared to IgG. Front Immunol. (2019) 10:704. doi: 10.3389/fimmu.2019.00704
126. Gimpel AK, Maccataio A, Unterweger H, Sokolova MV, Schett G, and Steffen U. IgA complexes induce neutrophil extracellular trap formation more potently than IgG complexes. Front Immunol. (2021) 12:761816. doi: 10.3389/fimmu.2021.761816
127. Steffen U, Koeleman CA, Sokolova MV, Bang H, Kleyer A, Rech J, et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat Commun. (2020) 11:120. doi: 10.1038/s41467-019-13992-8
128. Chan C, Stip M, Nederend M, Jansen M, Passchier E, van den Ham F, et al. Enhancing IgA-mediated neutrophil cytotoxicity against neuroblastoma by CD47 blockade. J Immunother Cancer. (2024) 12:e008478. doi: 10.1136/jitc-2023-008478
129. Martín Pérez C, Aguilar R, Jiménez A, Salmerón G, Canyelles M, Rubio R, et al. Correlates of protection and determinants of SARS-CoV-2 breakthrough infections 1 year after third dose vaccination. BMC Med. (2024) 22:103. doi: 10.1186/s12916-024-03304-3
130. Hertz T, Levy S, Ostrovsky D, Oppenheimer H, Zismanov S, Kuzmina A, et al. Correlates of protection for booster doses of the SARS-CoV-2 vaccine BNT162b2. Nat Commun. (2023) 14:4575. doi: 10.1038/s41467-023-39816-4
131. Bellusci L, Golding H, and Khurana S. Comparison of SARS-CoV-2 hyperimmune immunoglobulins following infection plus vaccination vs infection. JAMA Netw Open. (2023) 6:e2327307. doi: 10.1001/jamanetworkopen.2023.27307
132. Suryawanshi RK, Chen IP, Ma T, Syed AM, Brazer N, Saldhi P, et al. Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. Nature. (2022) 607:351–5. doi: 10.1038/s41586-022-04865-0
133. Wherry EJ and Barouch DH. T cell immunity to COVID-19 vaccines. Science. (2022) 377:821–2. doi: 10.1126/science.add2897
134. Swadling L, Diniz MO, Schmidt NM, Amin OE, Chandran A, Shaw E, et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature. (2022) 601:110–7. doi: 10.1038/s41586-021-04186-8
135. Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. (2022) 185:847–59 e11. doi: 10.1016/j.cell.2022.01.015
136. Ledford H. How ‘killer’ T cells could boost COVID immunity in face of new variants. Nature. (2021) 590:374–5. doi: 10.1038/d41586-021-00367-7
137. Noh JY, Jeong HW, Kim JH, and Shin E-C. T cell-oriented strategies for controlling the COVID-19 pandemic. Nat Rev Immunol. (2021) 21:687–8. doi: 10.1038/s41577-021-00625-9
138. Muik A, Lui BG, Quandt J, Diao H, Fu Y, Bacher M, et al. Progressive loss of conserved spike protein neutralizing antibody sites in Omicron sublineages is balanced by preserved T cell immunity. Cell Rep. (2023) 42:112888. doi: 10.1016/j.celrep.2023.112888
139. Hodl I, Sallegger C, Forstner P, Sareban N, Moritz M, Dreo B, et al. Altered cellular immune response to vaccination against SARS-CoV-2 in patients suffering from autoimmunity with B-cell depleting therapy. Microbes Infect. (2023) 25:105103. doi: 10.1016/j.micinf.2023.105103
140. Squire JD and Joshi AY. Response to mRNA COVID-19 vaccination in three XLA patients. Vaccine. (2022) 40:5299–301. doi: 10.1016/j.vaccine.2022.07.046
141. Bange EM, Han NA, Wileyto P, Kim JY, Gouma S, Robinson J, et al. CD8(+) T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. (2021) 27:1280–9. doi: 10.1038/s41591-021-01386-7
142. DeWolf S, Laracy JC, Perales MA, Kamboj M, van den Brink MRM, and Vardhana S. SARS-CoV-2 in immunocompromised individuals. Immunity. (2022) 55:1779–98. doi: 10.1016/j.immuni.2022.09.006
143. Barouch DH. Covid-19 vaccines - immunity, variants, boosters. N Engl J Med. (2022) 387:1011–20. doi: 10.1056/NEJMra2206573
144. Lasrado N, Collier AY, Miller J, Hachmann NP, Liu J, Anand T, et al. Waning immunity and IgG4 responses following bivalent mRNA boosting. Sci Adv. (2024) 10:eadj9945. doi: 10.1126/sciadv.adj9945
145. Lasrado N, Rowe M, McMahan K, Hachmann NP, Miller J, Jacob-Dolan C, et al. SARS-CoV-2 XBB.1.5 mRNA booster vaccination elicits limited mucosal immunity. Sci Transl Med. (2024) 16:eadp8920. doi: 10.1126/scitranslmed.adp8920
146. Sette A, Sidney J, and Grifoni A. Pre-existing SARS-2-specific T cells are predicted to cross-recognize BA.2.86. Cell Host Microbe. (2024) 32:19–24.e2. doi: 10.1016/j.chom.2023.11.010
147. Almendro-Vázquez P, Chivite-Lacaba M, Utrero-Rico A, González-Cuadrado C, Laguna-Goya R, Moreno-Batanero M, et al. Cellular and humoral immune responses and breakthrough infections after three SARS-CoV-2 mRNA vaccine doses. Front Immunol. (2022) 13:981350. doi: 10.3389/fimmu.2022.981350
148. Lozano-Ojalvo D, Camara C, Lopez-Granados E, Nozal P, Del Pino-Molina L, Bravo-Gallego LY, et al. Differential effects of the second SARS-CoV-2 mRNA vaccine dose on T cell immunity in naive and COVID-19 recovered individuals. Cell Rep. (2021) 36:109570. doi: 10.1016/j.celrep.2021.109570
149. Payne RP, Longet S, Austin JA, Skelly DT, Dejnirattisai W, Adele S, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. (2021) 184:5699–714.e11. doi: 10.1016/j.cell.2021.10.011
150. Phan JM, Layton ED, Yu KKQ, Aguilar MS, Golez I, Franko NM, et al. Cytotoxic T cells targeting spike glycoprotein are associated with hybrid immunity to SARS-CoV-2. J Immunol. (2023) 210:1236–46. doi: 10.4049/jimmunol.2200815
151. Dykema AG, Zhang B, Woldemeskel BA, Garliss CC, Rashid R, Westlake T, et al. SARS-CoV-2 vaccination diversifies the CD4+ spike-reactive T cell repertoire in patients with prior SARS-CoV-2 infection. EBioMedicine. (2022) 80:104048. doi: 10.1016/j.ebiom.2022.104048
152. Cohen KW, Linderman SL, Moodie Z, Czartoski J, Lai L, Mantus G, et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep Med. (2021) 2:100354. doi: 10.1016/j.xcrm.2021.100354
153. Ozbay Kurt FG, Lepper A, Gerhards C, Roemer M, Lasser S, Arkhypov I, et al. Booster dose of mRNA vaccine augments waning T cell and antibody responses against SARS-CoV-2. Front Immunol. (2022) 13:1012526. doi: 10.3389/fimmu.2022.1012526
154. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. (2022) 23:186–93. doi: 10.1038/s41590-021-01122-w
155. Mok CKP, Chen C, Zhao S, Sun Y, Yiu K, Chan TO, et al. Omicron BA.1-specific T-cell responses in adults vaccinated with CoronaVac or BNT162b2 in Hong Kong: an observational cohort study. Lancet Microbe. (2023) 4:e418–e30. doi: 10.1016/S2666-5247(23)00006-X
156. Debbag R, Rudin D, Ceddia F, and Watkins J. The impact of vaccination on COVID-19, influenza, and respiratory syncytial virus-related outcomes: a narrative review. Infect Dis Ther. (2025) 14:63–97. doi: 10.1007/s40121-024-01079-x
157. Gustafson CE, Kim C, Weyand CM, and Goronzy JJ. Influence of immune aging on vaccine responses. J Allergy Clin Immunol. (2020) 145:1309–21. doi: 10.1016/j.jaci.2020.03.017
158. Azzolini E, Pozzi C, Germagnoli L, Oresta B, Carriglio N, Calleri M, et al. mRNA COVID-19 vaccine booster fosters B- and T-cell responses in immunocompromised patients. Life Sci Alliance. (2022) 5:e202201381. doi: 10.26508/lsa.202201381
159. Lu X and Yamasaki S. Current understanding of T cell immunity against SARS-CoV-2. Inflammation Regen. (2022) 42:51. doi: 10.1186/s41232-022-00242-6
160. Tincati C, Cannizzo ES, Giacomelli M, Badolato R, d’Arminio Monforte A, and Marchetti G. Heightened circulating interferon-inducible chemokines, and activated pro-cytolytic Th1-cell phenotype features Covid-19 aggravation in the second week of illness. Front Immunol. (2020) 11:580987. doi: 10.3389/fimmu.2020.580987
161. Cui D, Tang Y, Jiang Q, Jiang D, Zhang Y, Lv Y, et al. Follicular helper T cells in the immunopathogenesis of SARS-CoV-2 infection. Front Immunol. (2021) 12:731100. doi: 10.3389/fimmu.2021.731100
162. Naranbhai V, Garcia-Beltran WF, Chang CC, Berrios Mairena C, Thierauf JC, Kirkpatrick G, et al. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 vaccines. J Infect Dis. (2022) 225:1141–50. doi: 10.1093/infdis/jiab593
163. Painter MM, Mathew D, Goel RR, Apostolidis SA, Pattekar A, Kuthuru O, et al. Rapid induction of antigen-specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. (2021) 54:2133–42.e3. doi: 10.1016/j.immuni.2021.08.001
164. Gao F, Mallajosyula V, Arunachalam PS, van der Ploeg K, Manohar M, Röltgen K, et al. Spheromers reveal robust T cell responses to the Pfizer/BioNTech vaccine and attenuated peripheral CD8(+) T cell responses post SARS-CoV-2 infection. Immunity. (2023) 56:864–78.e4. doi: 10.1016/j.immuni.2023.03.005
165. Pizzolla A, Wang Z, Groom JR, Kedzierska K, Brooks AG, Reading PC, et al. Nasal-associated lymphoid tissues (NALTs) support the recall but not priming of influenza virus-specific cytotoxic T cells. Proc Natl Acad Sci U S A. (2017) 114:5225–30. doi: 10.1073/pnas.1620194114
166. Minervina AA, Pogorelyy MV, Kirk AM, Crawford JC, Allen EK, Chou C-H, et al. SARS-CoV-2 antigen exposure history shapes phenotypes and specificity of memory CD8+ T cells. Nat Immunol. (2022) 23:781–90. doi: 10.1038/s41590-022-01184-4
167. Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. (2022) 603:488–92. doi: 10.1038/s41586-022-04460-3
168. Qui M, Le Bert N, Chan WPW, Tan M, Hang SK, Hariharaputran S, et al. Favorable vaccine-induced SARS-CoV-2-specific T cell response profile in patients undergoing immune-modifying therapies. J Clin Invest. (2022) 132:e159500. doi: 10.1172/JCI159500
169. Benhamouda N, Besbes A, Bauer R, Mabrouk N, Gadouas G, Desaint C, et al. Cytokine profile of anti-spike CD4(+)T cells predicts humoral and CD8(+) T cell responses after anti-SARS-CoV-2 mRNA vaccination. iScience. (2024) 27:110441. doi: 10.1016/j.isci.2024.110441
170. Meyer S, Blaas I, Bollineni RC, Delic-Sarac M, Tran TT, Knetter C, et al. Prevalent and immunodominant CD8 T cell epitopes are conserved in SARS-CoV-2 variants. Cell Rep. (2023) 42:111995. doi: 10.1016/j.celrep.2023.111995
171. Tan ST, Rodríguez-Barraquer I, Kwan AT, Blumberg S, Park HJ, Hutchinson J, et al. Dynamics of indirect protection against SARS-CoV-2 infection through vaccine and infection-acquired immunity. medRxiv. (2024). doi: 10.1101/2024.07.23.24310889
172. Zaeck LM, GeurtsvanKessel CH, and de Vries RD. COVID-19 vaccine effectiveness and evolving variants: understanding the immunological footprint. Lancet Respir Med. (2023) 11:395–6. doi: 10.1016/S2213-2600(23)00140-6
173. Srivastava K, Carreno JM, Gleason C, Monahan B, Singh G, Abbad A, et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity. (2024) 57:587–99 e4. doi: 10.1016/j.immuni.2024.01.017
174. Mongin D, Burgisser N, Laurie G, Schimmel G, Vu DL, Cullati S, et al. Effect of SARS-CoV-2 prior infection and mRNA vaccination on contagiousness and susceptibility to infection. Nat Commun. (2023) 14:5452. doi: 10.1038/s41467-023-41109-9
175. Townsend JP, Hassler HB, Sah P, Galvani AP, and Dornburg A. The durability of natural infection and vaccine-induced immunity against future infection by SARS-CoV-2. Proc Natl Acad Sci U S A. (2022) 119:e2204336119. doi: 10.1073/pnas.2204336119
176. Xie X, Zou J, Kurhade C, Liu M, Ren P, and Shi PY. Neutralization of SARS-CoV-2 Omicron sublineages by 4 doses of mRNA vaccine. bioRxiv. (2022). doi: 10.1101/2022.07.29.502055
177. Di Chiara C, Cantarutti A, Raffaella Petrara M, Bonfante F, Benetti E, Boracchini R, et al. Stronger and durable SARS-CoV-2 immune response to mRNA vaccines in 5–11 years old children with prior COVID-19. Vaccine. (2024) 42:263–70. doi: 10.1016/j.vaccine.2023.12.006
178. Srivastava K, Carreño JM, Gleason C, Monahan B, Singh G, Abbad A, et al. Kinetics and durability of humoral responses to SARS-CoV-2 infection and vaccination. medRxiv. (2023).
179. Yorsaeng R, Atsawawaranunt K, and Riad A. Editorial: COVID-19 booster vaccination: increasing immunity against life-threatening infection. Front Public Health. (2023) 11:1342118. doi: 10.3389/fpubh.2023.1342118
180. Hartley GE, Edwards ESJ, Aui PM, Varese N, Stojanovic S, McMahon J, et al. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci Immunol. (2020) 5:eabf8891. doi: 10.1126/sciimmunol.abf8891
181. Neidleman J, Luo X, George AF, McGregor M, Yang J, Yun C, et al. Distinctive features of SARS-CoV-2-specific T cells predict recovery from severe COVID-19. Cell Rep. (2021) 36:109414. doi: 10.1016/j.celrep.2021.109414
182. Gagne M, Corbett KS, Flynn BJ, Foulds KE, Wagner DA, Andrew SF, et al. Protection from SARS-CoV-2 Delta one year after mRNA-1273 vaccination in rhesus macaques coincides with anamnestic antibody response in the lung. Cell. (2022) 185:113–30.e15. doi: 10.1016/j.cell.2021.12.002
183. Hauser D, Urda L, Lang C, Mittelholzer C, Otte F, Kipfer E, et al. Benefits of repeated SARS-CoV-2 vaccination and virus-induced cross-neutralization potential in immunocompromised transplant patients and healthy individuals. Open Forum Infect Dis. (2024) 11:ofae527. doi: 10.1093/ofid/ofae527
184. Quek AML, Wang S, Teng O, Shunmuganathan B, Er BGC, Mahmud NFB, et al. Hybrid immunity augments cross-variant protection against COVID-19 among immunocompromised individuals. J Infect. (2024) 89:106238. doi: 10.1016/j.jinf.2024.106238
185. Blixt L, Gao Y, Wullimann D, Muren Ingelman-Sundberg H, Muschiol S, Healy K, et al. Hybrid immunity in immunocompromised patients with CLL after SARS-CoV-2 infection followed by booster mRNA vaccination. Blood. (2022) 140:2403–7. doi: 10.1182/blood.2022016815
186. Orbo HS, Bjorlykke KH, Sexton J, Jyssum I, Tveter AT, Christensen IE, et al. Incidence and outcome of COVID-19 following vaccine and hybrid immunity in patients on immunosuppressive therapy: identification of protective post-immunisation anti-RBD antibody levels in a prospective cohort study. RMD Open. (2024) 10:e003545. doi: 10.1136/rmdopen-2023-003545
187. Johnston TS, Hage C, Abedon AT, Panda S, Alejo JL, Eby Y, et al. Rapid wane and recovery of XBB sublineage neutralization after sequential omicron-based vaccination in solid organ transplant recipients. Clin Infect Dis. (2024) 79:652–5. doi: 10.1093/cid/ciae279
188. Al-Aly Z, Bowe B, and Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. (2022) 28:1461–7. doi: 10.1038/s41591-022-01840-0
189. Peghin M, De Martino M, Palese A, Gerussi V, Bontempo G, Graziano E, et al. Post-COVID-19 syndrome and humoral response association after 1 year in vaccinated and unvaccinated patients. Clin Microbiol Infect. (2022) 28:1140–8. doi: 10.1016/j.cmi.2022.03.016
190. Mikolajczyk R, Diexer S, Klee B, Pfrommer L, Purschke O, Fricke J, et al. Likelihood of Post-COVID Condition in people with hybrid immunity; data from the German National Cohort (NAKO). J Infect. (2024) 89:106206. doi: 10.1016/j.jinf.2024.106206
191. de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. (2021) 13:eabf8396. doi: 10.1126/scitranslmed.abf8396
192. Zuo W, He D, Liang C, Du S, Hua Z, Nie Q, et al. The persistence of SARS-CoV-2 in tissues and its association with long COVID symptoms: a cross-sectional cohort study in China. Lancet Infect Dis. (2024) 24:845–55. doi: 10.1016/S1473-3099(24)00171-3
193. Pilapitiya D, Wheatley AK, and Tan H-X. Mucosal vaccines for SARS-CoV-2: triumph of hope over experience. eBioMedicine. (2023) 92:104585. doi: 10.1016/j.ebiom.2023.104585
194. Cao Y, Jian F, Wang J, Yu Y, Song W, Yisimayi A, et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature. (2023) 614:521–9. doi: 10.1038/s41586-022-05644-7
195. UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England: Technical briefing (2023). Available online at: https://www.gov.uk/government/publications/investigation-of-sars-cov-2-variants-technical-briefings. (Accessed August 20, 2024).
196. US Centers for Disease Control and Prevention. COVID data tracker: Rates of COVID-19 reinfection (2024). Available online at: https://covid.cdc.gov/covid-data-tracker. (Accessed August 20, 2024).
197. Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. (2022) 387:21–34. doi: 10.1056/NEJMoa2203965
198. Reynolds CJ, Pade C, Gibbons JM, Otter AD, Lin KM, Munoz Sandoval D, et al. Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science. (2022) 377:eabq1841. doi: 10.1126/science.abq1841
Keywords: infectious diseases, COVID-19, hybrid immunity, immunology, public health
Citation: Bausch-Jurken M and Alter G (2025) The immunological impact of revaccination in a hybrid-immune world. Front. Immunol. 16:1588259. doi: 10.3389/fimmu.2025.1588259
Received: 05 March 2025; Accepted: 20 May 2025;
Published: 09 June 2025.
Edited by:
Paul Licciardi, Royal Children’s Hospital, AustraliaReviewed by:
Emily S. J. Edwards, Monash University, AustraliaAnne M. Hahn, Peter Doherty Institute for Infection and Immunity, Australia
Copyright © 2025 Bausch-Jurken and Alter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mary Bausch-Jurken, bWFyeS5qdXJrZW5AZ21haWwuY29t
 Mary Bausch-Jurken
Mary Bausch-Jurken Galit Alter2
Galit Alter2