- 1Department of Hematology, Amsterdam University Medical Center, Amsterdam, Netherlands
- 2Red Cell Laboratory, Sanquin Research and Landsteiner Laboratory, Amsterdam, Netherlands
- 3Department of Pediatric Hematology, Emma Children’s Hospital, Amsterdam University Medical Center, Amsterdam, Netherlands
- 4Laboratory of Red Blood Cell Diagnostics and Iron, Sanquin Diagnostics, Amsterdam, Netherlands
- 5Department of Pediatric Immunology, Rheumatology and Infectious Diseases, Emma Children’s Hospital, Amsterdam University Medical Center, Amsterdam, Netherlands
Introduction: While sickle cell disease (SCD) is primarily acknowledged as an erythrocyte disorder, emerging evidence suggests a role for altered neutrophil phenotype and function in SCD pathophysiology and disease severity. Given the conflicting findings in previous studies, we performed a comprehensive exploration of neutrophil characteristics in SCD patients during steady state and vaso-occlusive crisis (VOC), as well as in response to therapeutic interventions.
Methods: Neutrophil phenotype was assessed by flow cytometry and functional properties were evaluated by measurement of neutrophil adhesion and reactive oxygen species (ROS) production.
Results: A total of 49 SCD patients (of whom 19 during both steady state and VOC) along with 16 healthy ethnicity-matched and 30 non-matched controls, were included in the study. Differences were observed between neutrophils from patients compared to controls and between control groups. Neutrophil phenotype was more activated in SCD patients compared to non-matched controls. Neutrophil adhesion was increased in steady-state SCD patients compared to both ethnicity-matched and non-matched controls.
Discussion: While neutrophil phenotype in SCD patients differed from non-matched controls, in contrast to earlier studies, the differences in neutrophil phenotype between SCD patients and ethnicity-matched controls were modest. In vitro neutrophil adhesion was higher in SCD patients than in ethnicity-matched and non-matched controls. Potential explanations for the discrepancies between earlier findings and our study are the large variation in neutrophil phenotypes between individuals, methodological variability between studies and differences in the time interval between blood sample collection and the measurements.
1 Introduction
SCD is a prevalent inherited hemoglobinopathy caused by a point mutation in the β-globin gene. The resulting hemoglobin S polymerizes upon deoxygenation, leading to the formation of the characteristic sickle erythrocytes (1). Clinically, these erythrocyte abnormalities result in hemolytic anemia and vaso-occlusion, causing periodic and painful episodes of VOC and ischemia-reperfusion injury in almost all organs.
Although primarily considered an erythrocyte disorder, there is an increasing interest in other cell types contributing to the pathophysiology of SCD, such as neutrophils (2–5). Circulating neutrophils are suggested to play a pivotal role in the disease process (2, 3, 6, 7). Increased neutrophil counts are associated with clinical complications such as cerebral infarction, hemorrhagic stroke, acute chest syndrome (ACS) and early death (8–13). Additionally, neutrophils have been suggested to play a key role in initiating VOCs (14). Neutrophils interact with sickle erythrocytes and platelets via the neutrophil integrin αmβ2 (CD11b/CD18) to form aggregates (15). Studies in sickle cell mice have shown that neutrophils adhere to vessel walls, decrease microvascular blood flow and interact with sickle erythrocytes, directly contributing to vaso-occlusion (16–19). Hemolysis and ischemia-reperfusion injury both lead to increased oxidative stress, resulting in chronic inflammation, increased neutrophil activation and adhesion (20). Neutrophil activation is characterized by increased expression of CD64 and decreased expression of CD62L (L-selectin) on neutrophils of SCD patients. In contrast, the neutrophil integrin αmβ2 and CXCR4 expression are associated with increased adhesion and aging of neutrophils in SCD patients (21–23). Neutrophil functions affected in SCD include migration, phagocytosis, production of reactive oxygen species (ROS) and formation of neutrophil extracellular traps (NET), which collectively might contribute to disease severity in SCD (Figure 1) (19, 24–27).
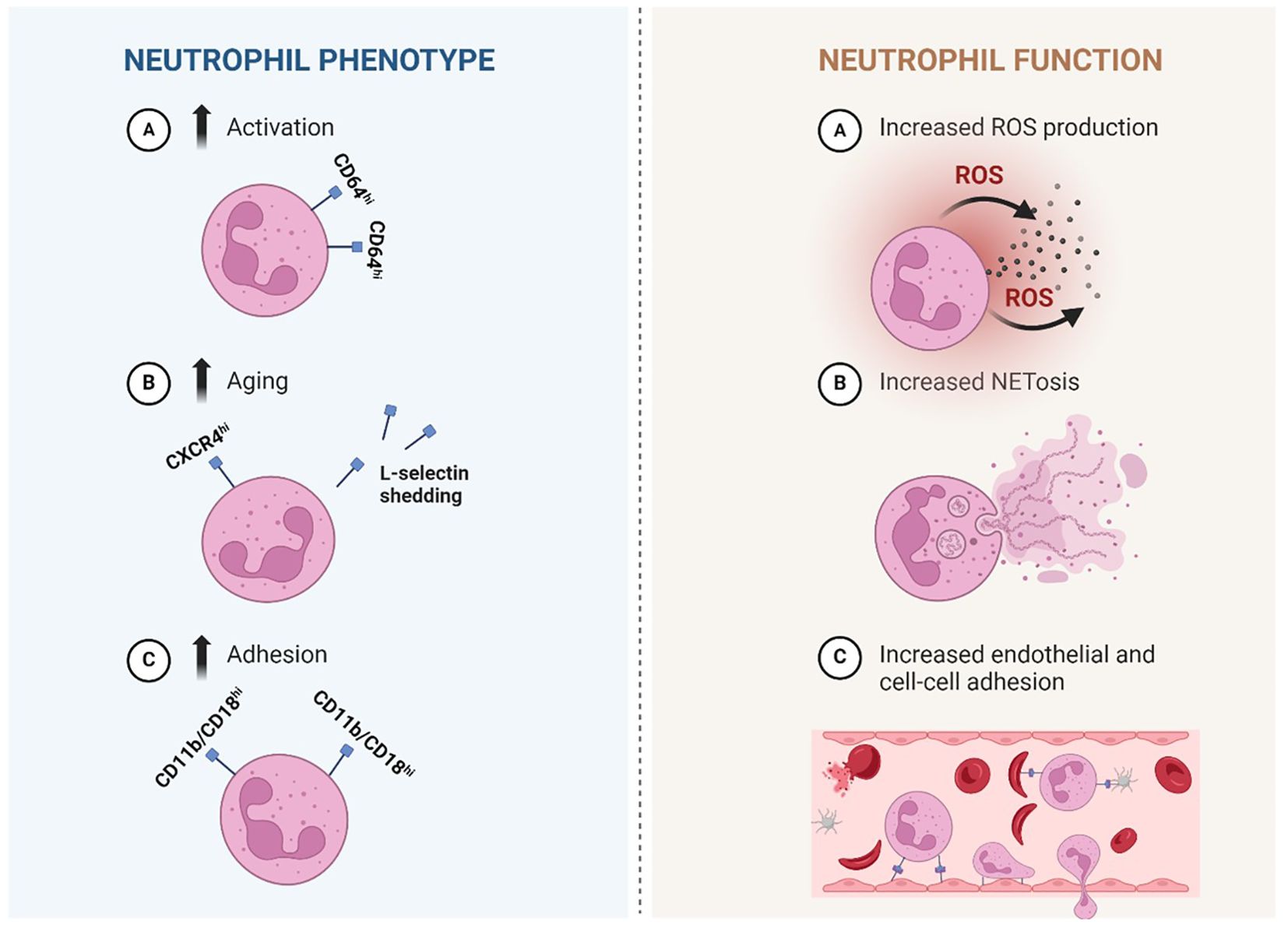
Figure 1. Simplified illustration of proposed neutrophil phenotypical and functional alterations in Sickle cell disease. Left: Neutrophil phenotype: Phenotypical changes that are described in sickle cell disease (SCD) are more activation, aging and adhesion. A: neutrophils that are activated express more CD64. B: increased aging is suggested by heightened expression of CXCR4 and reduced expression of L-selectin (CD62L) which is shed upon aging (and activation). C: increased expression of adhesion associated integrin CD11b/CD18 (αmβ2) is described, suggesting a more adhesive neutrophil. Right: Neutrophil function: several changes in neutrophil function are described in SCD. A: Increased production of ROS, which are highly reactive chemicals used in the body’s response against pathogens. B: Increased production of NET-like structures made of DNA-histone complexes and proteins and can capture micro-organisms. A+B: both processes have also been linked to inflammation. C: Increased adhesion of neutrophils to the endothelium (followed by crawling over- and migration through the endothelium) and other cell types such as (sickled) erythrocytes and platelets, which can actively contribute to vaso-occlusion. SCD, sickle cell disease; CD, cluster of differentiation; ROS, reactive oxygen species; NET, neutrophil extracellular trap. Created in Biorender.
Hydroxyurea remains one of the main therapeutic options for SCD and has a significant impact on neutrophil counts and phenotype (26, 28–31). Induction of hemoglobin F (HbF) is hydroxyurea’s primary mechanism of action, but the clinical efficacy could at least partly be attributed to its effect on neutrophils (32, 33). Hydroxyurea treatment reduces neutrophil counts, but its effects on neutrophil activation, adhesion and phenotype are still largely unknown. Hydroxyurea is thought to interfere with the dysregulated L-selectin shedding, increased ROS production and increased myeloperoxidase levels in SCD neutrophils (28, 32). However, signs of increased neutrophil activity, such as spontaneous degranulation and enhanced production of NETs, have shown to persist in hydroxyurea-treated SCD patients (26).
This study aims to further investigate neutrophil phenotype and activity in SCD patients during steady state and VOC, as well as elucidate the effects of treatment, e.g. hydroxyurea or allogeneic hematopoietic stem cell transplantation (HSCT), on neutrophil characteristics.
2 Methods
2.1 Study population
In this prospective study, blood was collected from adult patients with a confirmed diagnosis of sickle cell anemia (HbSS) and heterozygous HbSβ0-thalassemia (HbSβ0-thal) during steady state and hospital admission for VOC. To assess the effects of treatment on neutrophil phenotype and functionality, we included a subgroup of patients before and during hydroxyurea treatment and before and after HSCT, which they received as standard of care. Healthy ethnicity-matched individuals were included as a control group. Controls were considered matched if at least one parent originated from an SCD-endemic region. Additionally, a healthy non-matched control group, primarily of Caucasian origin, was included as a quality control in all experiments. Results of SCD patients are compared with both ethnicity-matched healthy controls and non-ethnicity-matched healthy controls, hereafter referred to as ‘matched controls’ and ‘non-matched controls’.
Potential participants with a history of immune-related disorders or current use of medication influencing immune cells or inflammation at the moment of blood collection were excluded. Blood transfusion within 3 months were additional exclusion criteria. Participants who received a blood transfusion more than 3 months before inclusion, who still had an HbA1 of >10%, were also excluded from final analyses.
This study was approved by the Institutional Review Board of Amsterdam UMC and performed in accordance with the Declaration of Helsinki 2013. Informed consent was obtained from all participants before study inclusion.
2.2 Study procedures
Blood was obtained from SCD patients during a visit to the outpatient clinic or within 48 hours after hospital admission for VOC. In patients starting hydroxyurea, a second blood sample was collected after treatment for >90 days at a stable dose. In patients undergoing HSCT, a second blood sample was drawn after successful engraftment and at least mixed chimerism (≥6 months after transplantation). Patients that were included during VOC had a follow-up steady-state blood sample collected at least 4 weeks after the VOC.
Standard complete blood counts were performed in EDTA-anticoagulated blood and markers of hemolysis (levels of bilirubin and lactate dehydrogenase and reticulocyte counts) were measured in heparinized plasma with spectrophotometry according to local protocols. Erythrocyte assays were conducted within 24 hours after venous blood was collected in EDTA-anticoagulated tubes and stored at 4°C. Neutrophil assays were performed within 24 hours of collection in EDTA-anticoagulated blood samples, stored at room temperature.
2.3 Study assays
To assess neutrophil phenotype, neutrophil surface antigen expression was measured by flow cytometry. A set of directly conjugated antibodies was used. To facilitate readability, markers were roughly divided into subgroups of activation, adhesion and maturation (Supplementary Table S1). Flow cytometry data were quantified using a Canto II flow cytometer (BD Biosciences) and analyzed with FacsDiva software (Version 9). For FITC/AF488, the instance laser lines were 488 nm with filter set long-pass (LP) 502 nm and band-pass (BP) 530/30 nm. For APC/AF647, the instance laser lines were 633 nm with filter set no LP and BP 660/20 nm. Erythrocytes in whole blood were lysed twice with ice-cold lysis buffer (4.15 g NH4Cl, 0.5 g KHCO3 and 18 mg EDTA in 500ml H2O). Antibodies were added to the remaining cells. To distinguish neutrophils from other white blood cells, neutrophils were gated based on forward and side scatter. Results were depicted as Mean Fluorescent Intensity (MFI).
For in vitro neutrophil adhesion and neutrophil ROS production measurements, neutrophils were isolated from whole blood using a Percoll gradient and lysis buffer as previously described (34, 35). Neutrophils were kept at room temperature in a HEPES-buffered saline solution (20 mM HEPES, 132 mM NaCl, 6.0 mM KCl, 1.0 mM CaCl2, 1.0 mM MgSO4, 1.2 mM potassium phosphate, 5.5 mM glucose and 0.5% (w/v) human serum albumin, pH 7.4). For the adhesion assay, neutrophils (5 x 106/ml) were incubated with calcein-AM (1 μM; Molecular Probes) for 30 minutes at 37°C, washed twice, and resuspended in HEPES medium at a concentration of 2 x 106/ml. Adhesion was determined in an uncoated 96-well MaxiSorp plate (Nunc, Wiesbaden, Germany). Calcein-labeled cells (1.6 x 106/ml) were stimulated with 20 ng/ml granulocyte colony-stimulating factor (G-CSF), 10 mM dithiothreitol (DTT; Sigma Aldrich, St. Louis, MO, USA), 20 μg/ml Pam3Cys (EMC Microcollections, Tübingen, Germany), 20 ng/ml bacterial Toll Like Receptor-4 ligand lipopolysaccharide (LPS; isolated from E. coli strain 055:B5, Sigma Aldrich) in the presence of 50 ng/ml lipopolysaccharide-binding protein (LBP; R&D Systems, Minneapolis, MN, USA), 1 μM platelet-activation factor (PAF; Sigma Aldrich), 1 μM N-formyl-Met-Leu-Phe (fMLP), 10 ng/ml tumor necrosis factor-α (TNFα) or 100 ng/ml phorbol myristate acetate (PMA). Plates were incubated for 30 minutes at 37°C and washed with phosphate-buffered saline (PBS) twice. Adherent cells were lysed in 0.5% (w/v) Triton X-100 in PBS for 5 minutes at room temperature. Fluorescence was assessed using an Infinite F200-pro plate reader (Tecan, Mannedorf, Switzerland) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. Adhesion was determined as the percentage of the total input of calcein-labeled cells.
The production of reactive oxygen species was measured with an Amplex Red kit (Molecular Probes, Eugene, OR, USA). In short, neutrophils (0.25 x 106/ml) were stimulated with 1 mg/ml unopsonized zymosan (MP Biomedicals, Solon, OH, USA), serum-treated zymosan (STZ), PMA (100 ng/ml; Sigma-Aldrich), fMLP (1 µM; Sigma-Aldrich) or PAF/fMLP (1/1 µM; Sigma-Aldrich) in the presence of Amplex Red (25 μM) and horseradish peroxidase (0.5 U/ml). Fluorescence was measured at 30-second intervals for 30 minutes with an Infinite F200-pro plate reader at an excitation wavelength of 535 nm and an emission wavelength of 595 nm. The activity of the NADPH oxidase of neutrophils was determined as nmol H2O2/minute x 106 cells. The maximal slope of hydrogen peroxide release was measured at a 2-minute interval.
2.4 Erythrocyte deformability
Erythrocyte deformability was assessed under shear stress and decreasing oxygen concentrations. Erythrocytes were counted using an ADVIA 2120 hematology cell counter (Siemens, Munich, Germany) and diluted according to standard procedure to a fixed cell number in oxy iso fluid before the cell suspension was introduced in a laser optical rotational red cell analyzer (Lorrca, RR Mechatronics, Zwaag, The Netherlands), with oxygenscan module, as described previously (36). The deformability of erythrocytes in relation to deoxygenation is interpreted as a maximal and minimal elongations index (EImax and EImin, defined as the elongation index at normal (47 mmHg) or low oxygen pressure (10 mmHg), respectively, and the point of sickling (PoS), defined as the oxygen tension at which 5% reduction of the EImax is observed.
2.5 Statistics
Data were analyzed using SPSS version 26 (IBM, Armonk, NY, USA) and GraphPad Prism version 9 (GraphPad Software, Boston, MA, USA). Data are presented as mean ± standard error of the mean (SE) or standard deviation (SD), or median and interquartile range (IQR) depending on the distribution of data. Patient characteristics were compared between the groups using standard descriptive statistics. Post-hoc analyses with Bonferroni correction or Dunns rule for multiple testing were performed when more than 2 groups were compared. Paired data were analyzed using the paired t-test, Wilcoxon signed-rank test or McNemar test as appropriate.
3 Results
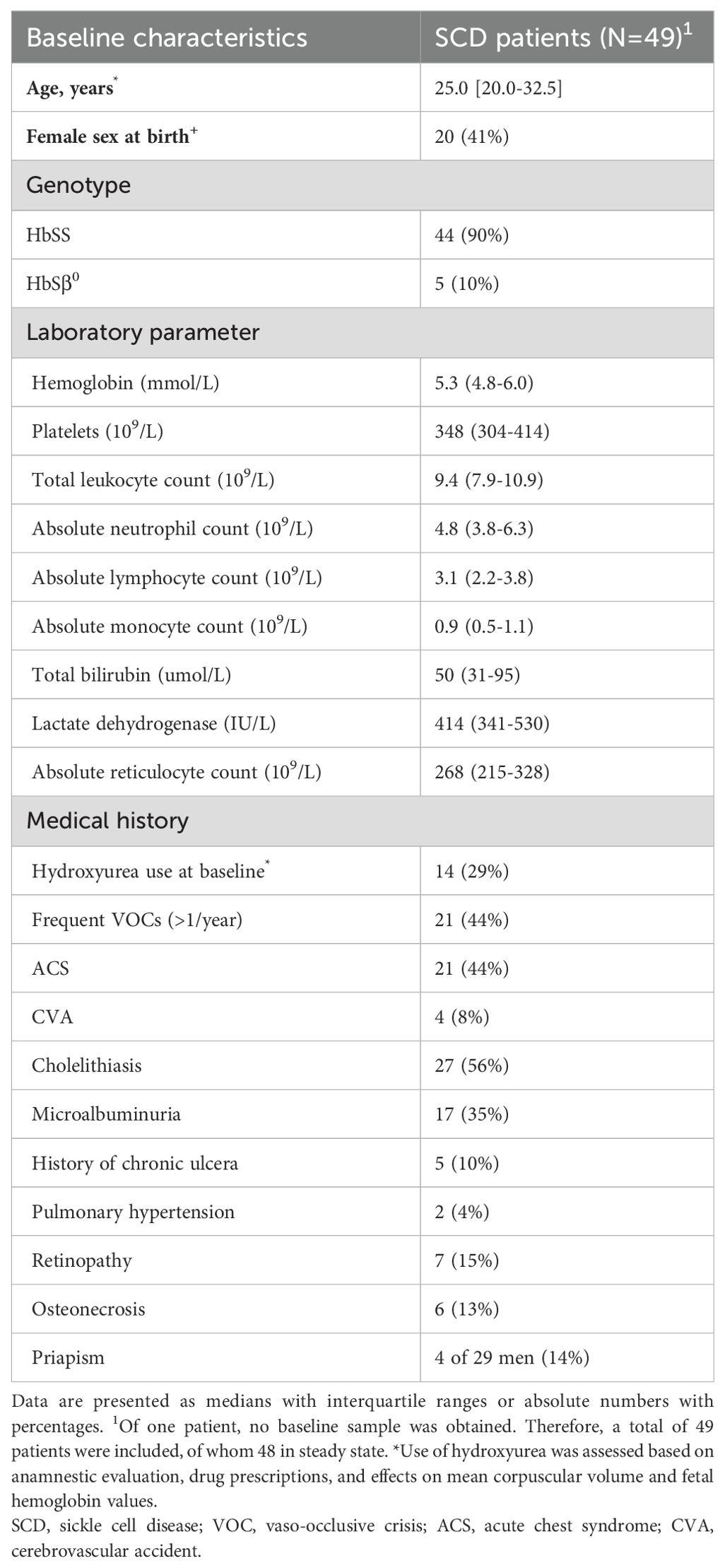
3.1 Baseline characteristics
A total of 49 SCD patients (median age 25.0 years [IQR 20.0-32.5], 41% female; Table 1), 16 matched controls (29.0 years [27.0-43.5], 50% female) and 30 non-matched controls (28.0 years [26.0-31.0]) were included in the final analyses. Nineteen patients were included during VOC and in steady state. Nine of the 48 steady-state patients were included before initiation of hydroxyurea with a follow-up sample after hydroxyurea treatment at a stable dose for >90 days. Seven of the 48 patients were included before HSCT with a follow-up sample ≥6 months after transplantation.
3.2 Steady-state SCD patients versus healthy controls
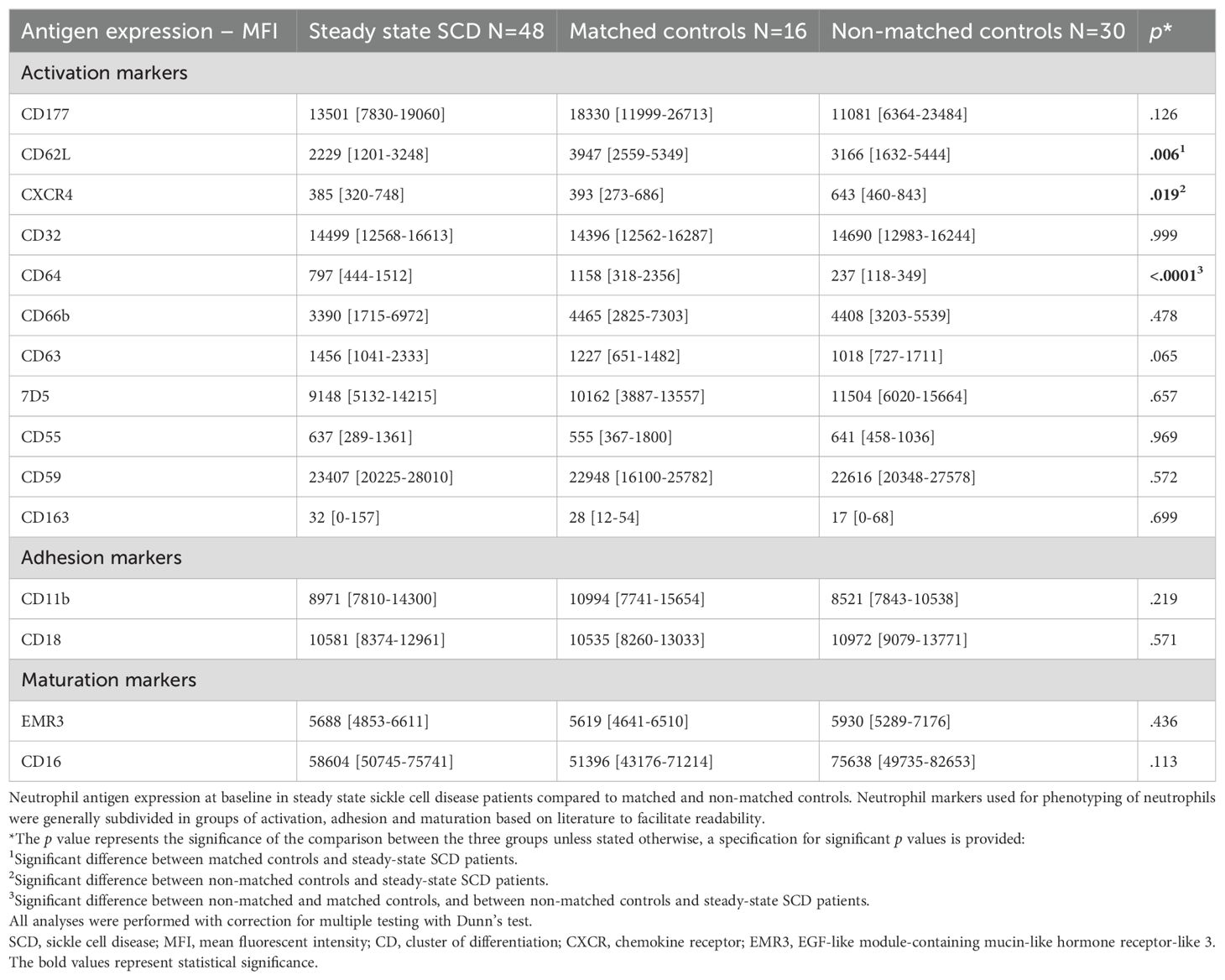
Results of neutrophil antigen expression in steady-state SCD patients and controls are depicted in Table 2. Expression of CD62L (L-selectin), which is shed upon activation, was significantly lower in steady-state SCD patients than in matched controls (median MFI: 2229 versus 3947, p=0.006). No other differences were observed between steady-state SCD patients and matched controls. CXCR4 expression was comparable between SCD patients and matched controls, but significantly higher in non-matched controls (p=0.019). CD64 expression was comparable between SCD patients and matched controls, but significantly lower in non-matched controls (p<0.0001). Neutrophil expression of the αmβ2 integrin CD11b/CD18 did not differ between SCD patients and the two control groups.
No significant correlations between markers of hemolysis (levels of bilirubin and lactate dehydrogenase and reticulocyte counts), a proposed driver of neutrophil activation, and expression of markers of neutrophil activation were found (data not shown). We hypothesized that hemolysis would be a driver of neutrophil activation due to the release of reactive oxygen species during hemolysis, however this was not supported by our data. We believe this could be due to the variation in neutrophil data and limited sample size.
Neutrophil adhesion was not different between SCD patients and matched controls. However, neutrophil adhesion was significantly lower in non-matched controls as compared to SCD patients and matched controls, both under unstimulated and stimulated conditions (Figure 2; Supplementary Table S2). ROS production of neutrophils stimulated by zymosan was lower in SCD patients compared to matched controls (median 0.40 [0.36-0.43] versus 0.44 nmol H2O2/minute x 106 cells [0.40-0.50], p=0.029). There were no differences in unstimulated ROS production between SCD patients and matched and non-matched controls (Supplementary Table S2).

Figure 2. Neutrophil adhesion in steady-state SCD patients compared to controls. Results of neutrophil adhesion assay in healthy matched and non-matched controls and steady-state sickle cell disease patients with, or without hydroxyurea treatment. Results are expressed as percentage (%) of total input of calcein-labeled cells. Grey, non-matched controls (n=30); green, matched controls (n=16); red, unpaired steady-state SCD without hydroxyurea (n=34); blue, unpaired steady-state SCD patients during hydroxyurea treatment (n=14). Unstimulated, non-matched controls significantly lower compared to all other groups; G-CSF, non-matched significantly lower than matched controls and hydroxyurea+; LBP/LPS, non-matched controls significantly lower than hydroxyurea-; Pam3Cys, non-matched controls significantly lower compared to all other groups; PAF, non-matched controls significantly lower than hydroxyurea-; TNF, significant difference between non-matched controls and matched controls. *Significant after correcting for multiple testing. #Not significant after correcting for multiple testing. Created in Biorender.
No differences in neutrophil antigen expression were seen between patients using and those not using hydroxyurea. Neutrophil adhesion under unstimulated conditions and upon G-CSF stimulation seemed higher in patients using hydroxyurea than in those not using hydroxyurea, although this did not reach statistical significance (Figure 2). No differences in ROS production were observed in SCD patients using and those not using hydroxyurea (data not shown).
3.3 Steady state versus vaso-occlusion
In the VOC subgroup (n=19), neutrophil expression of CXCR4 was significantly higher in steady state than during VOC with paired data analysis (median MFI: 500 versus 265, p=0.015), suggesting a younger subset of neutrophils during VOC. Likewise, expression of CD11b was significantly higher in steady state compared to VOC (median MFI: 9117 versus 6875, p=0.041). There was no difference in CD18 expression (Table 3).
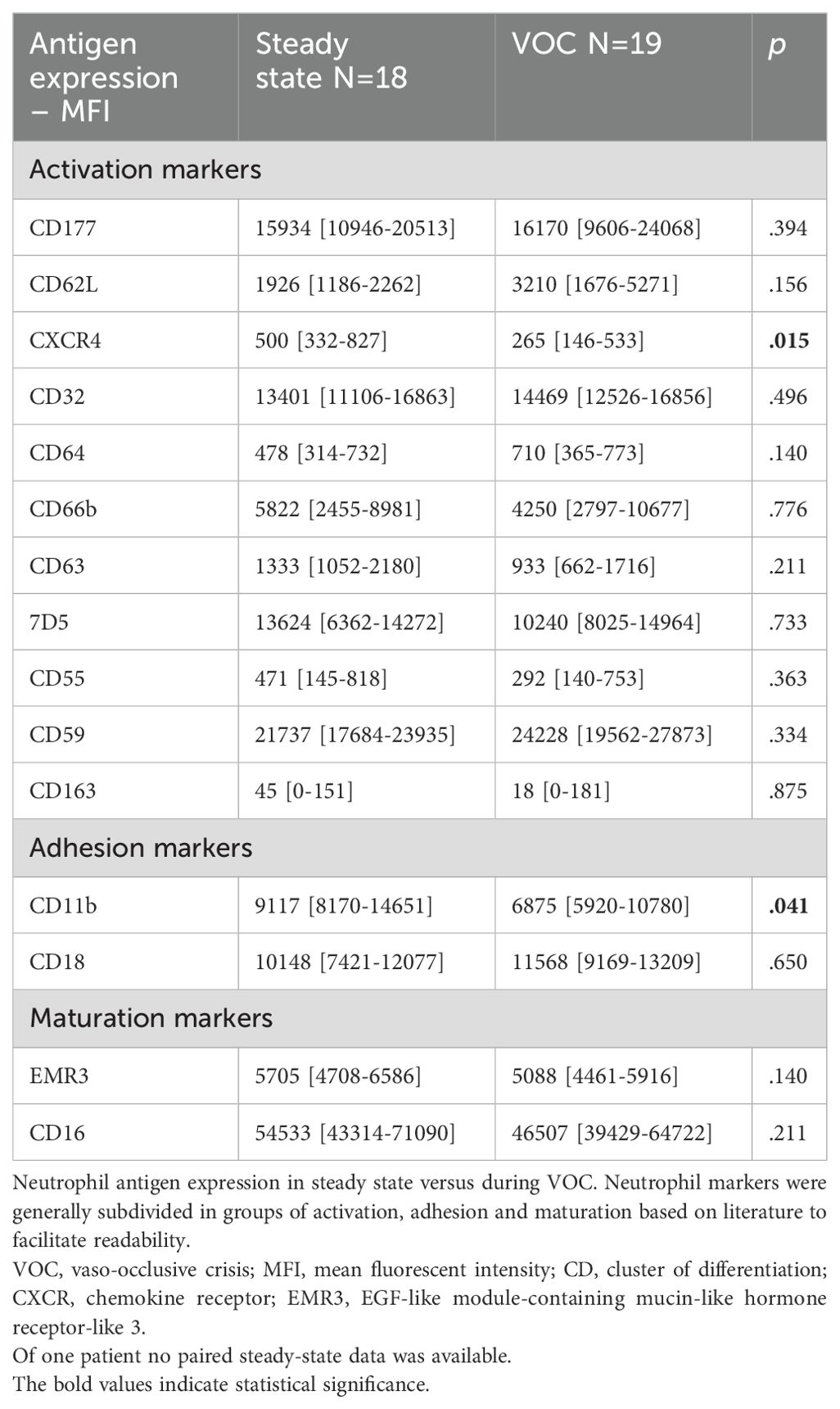
Table 3. Neutrophil antigen expression in SCD patients in steady state and during VOC – paired data.
Neutrophil adhesion upon stimulation with LBP/LPS was significantly lower in steady state than during VOC (median 29.5% [24.7-40.3] versus 34.1% [25.7-46.1], p=0.043). Neutrophil adhesion upon stimulation with other stimuli did not differ between VOC and steady state. There were no differences in neutrophil ROS production between steady state and VOC (Supplementary Table S3).
3.4 Hydroxyurea treatment and hematopoietic stem cell transplantation
Paired data of neutrophil phenotypical changes in a subgroup of patients starting hydroxyurea treatment or undergoing HSCT are depicted in Table 4. Upon hydroxyurea treatment (n=9), a decrease in the expression of the activation marker CD63 was observed (from median MFI 1939 to 1298, p=0.038). CD62L seemed to increase following the use of hydroxyurea, but this increase was not statistically significant (median MFI 2644 to 3654, p=0.441).
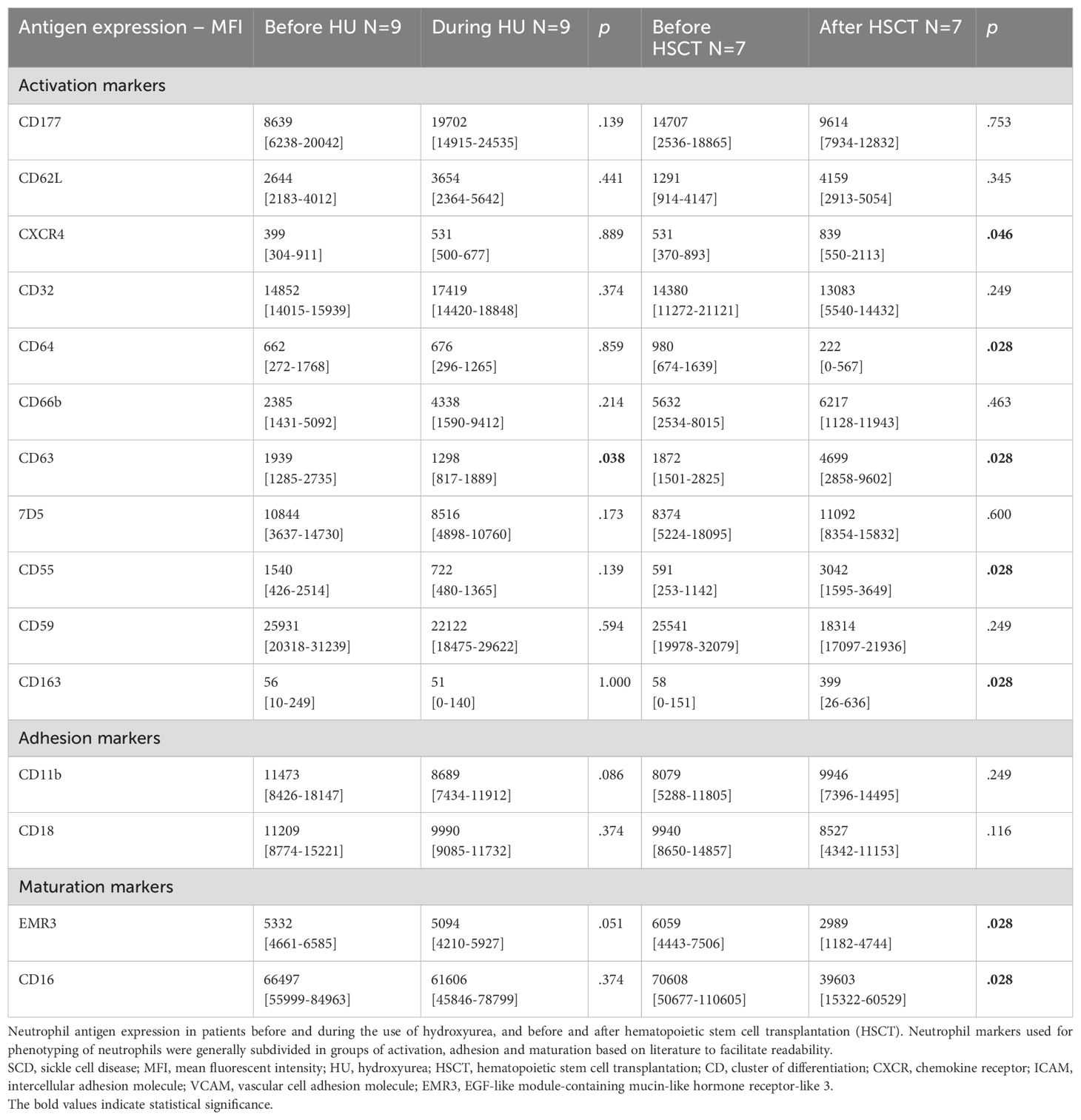
Table 4. Neutrophil antigen expression in SCD patients treated with hydroxyurea and hematopoietic stem cell transplantation – paired data.
Functional neutrophil assays of the hydroxyurea subgroup are shown in Supplementary Table S4. Unstimulated adhesion of neutrophils was significantly higher during hydroxyurea use than before starting hydroxyurea (from median 14.3% before [IQR 9.4-17.9] to 18.8% after [14.5-24.1], p=0.038). Trends were seen towards higher LBP/LPS, Pam3Cys and TNF-a stimulated neutrophil adhesion upon hydroxyurea treatment, although these differences were not statistically significant. Under hydroxyurea treatment, the neutrophil oxidative burst stimulated by PAF/fMLP significantly decreased (1.32 [1.19-1.49] to 1.14 nmol H2O2/minute x 106 cells [0.97-1.23], p=0.036).
After HSCT (n=7), neutrophil expression of CXCR4 increased (median MFI 531 to 839, p=0.046; Table 4). Expression of CD62L also seemed to increase after HSCT, though the difference was not statistically significant. Expression of CD64 decreased (from median MFI 980 to 222, p=0.028), while the complement related protein CD55 increased after HSCT (from median MFI 591 to 3042, p=0.028). The expression of EMR3 and CD16 decreased after HSCT (from median MFI 6059 to 2989, p=0.028, and 70608 to 39603, p=0.028, respectively). There were no differences in neutrophil adhesion or ROS production measurements (Supplementary Table S5).
3.5 Point of sickling
Point of sickling did not differ between steady state and VOC (20.9 mmHg [17.3-23.6] versus 21.8 mmHg [19.9-23.8], p=0.38). During treatment with hydroxyurea, the PoS was lower as compared to baseline in the patients measured both before and after starting hydroxyurea (n=9), though this did not reach statistical significance (from 24.5 before [14.7-25.5] to 15.2 mmHg after [15.0-19.9], p=0.091). The PoS was no longer detectable after transplant, comparable to healthy controls (data not shown). No significant changes in EImax and EImin were observed between steady state and VOC or after hydroxyurea (data not shown).
4 Discussion
While neutrophil involvement in SCD pathophysiology is widely described, the ex vivo assessment of SCD neutrophil phenotype and function has yielded conflicting results (37). Our study extensively evaluated these aspects in distinct SCD patient subgroups, comparing them with both (ethnicity-) matched and non-matched controls. Neutrophil characteristics were largely comparable between SCD patients and matched controls, with some differences observed when SCD patients were compared with non-matched controls. Treatment effects on neutrophil characteristics were observed, though most previously published findings could not be confirmed.
Neutrophils of SCD patients have previously been characterized as activated and aged, marked by reduced L-selectin and increased CD64 and CXCR4 expression (3, 21, 23, 28, 38). While reduced L-selectin expression in SCD patients suggested increased neutrophil activation in our study, other markers such as CXCR4 and CD64 did not. Additionally, while some previous studies have reported increased expression of adhesion-associated integrin CD11b/CD18 on SCD neutrophils, we and others did not observe increased CD11b/CD18 expression (3, 21, 22, 28, 39, 40).
Variations in experimental methods, including centrifugation, processing time, and exposure to activating compounds such as lysis buffer during neutrophil isolation, have been proposed as potential contributors to discrepancies in antigen expression across studies (22). Furthermore, sample handling practices vary. In some studies, neutrophils were fixed on ice immediately after collection, in other studies samples were stored at room temperature until isolation (22, 28). In the present study, experiments were performed within 24 hours after collection of blood and not directly due to practical constraints, which potentially influenced the results.
Notably, differences were observed between neutrophils of matched and non-matched controls, raising the question of whether disparities in neutrophil activity between SCD patients and healthy controls may be, in part, related to ethnicity. Total leukocyte, neutrophil, and platelet counts are lower in African Americans compared to Caucasians and increased platelet-erythrocyte and platelet-monocyte aggregates are described in African Americans compared to Caucasians (41–44). Additionally, neutrophils of African Americans showed an upregulation of CD11b/CD18 at a lower dose of IL-8 stimulation than neutrophils of Caucasians (22). Our CD11b/CD18-dependent functional adhesion assay revealed significant differences between SCD patients and non-matched controls, but not between SCD patients and matched controls. This might support the notion that both neutrophil numbers, as well as phenotypical and functional characteristics, may differ across ethnicities. Some studies into neutrophils in SCD patients lack an ethnicity-matched control group, which might contribute to data variation. Based on the differences observed between the non-matched and ethnicity-matched control groups, we recommend including ethnicity matched healthy controls for future studies on neutrophil function and phenotype.
In the present study, we did not observe any differences in ROS production at baseline (unstimulated) or after stimulation between SCD patients in steady state and during VOC and both control groups. Benkerrou et al. reported increased ROS production of SCD neutrophils upon stimulation, while Evans et al. observed an impaired (decreased) oxidative burst capacity under stimulated conditions (28, 45). As Benkerrou et al. included pediatric SCD patients, this could contribute to the differences in results between the studies. In addition, differences in the methods used to measure ROS production, as well as differences in handling of the samples could also account for the contradictory results in these studies (28).
Differences in neutrophil adhesion could be restricted to VOCs (40, 46). Although our study found no statistically significant differences during VOC compared to steady-state conditions or matched controls, the observed trends might suggest biologically meaningful changes. In addition, the LBP/LPS-stimulated condition revealed increased adhesion in SCD neutrophils during VOC. In contrast, CD11b expression was decreased during VOC compared to steady-state, possibly reflecting shifts in intravascular neutrophil subpopulations, where more activated and adhesive neutrophils may have adhered and transmigrated in vivo. Alternatively, stress-induced neutrophilia during VOC (indeed, the total leukocyte count and absolute neutrophil count increased during VOC in our study population) may result in the presence of a more immature, less activated, and less adhesive neutrophil population in the circulation, explaining the decreased expression of the aging marker CXCR4.
Hydroxyurea treatment is hypothesized to exert direct and indirect effects on neutrophils, potentially normalizing activation markers and ROS production, although conflicting results exist (3, 26, 28). In our study, a decrease in CD63 expression, indicative of azurophilic granule release, was found after hydroxyurea treatment, suggesting a deactivating effect. The trend towards increased L-selectin expression post-treatment also suggests de-activation. Other activation markers such as CD64 and CXCR4 as well as adhesion molecules CD11b and CD18 remained unchanged, although it is difficult to compare these markers due to different mechanisms that lead to their expression. Surprisingly, the baseline neutrophil adhesion (a CD11b/CD18 dependent assay) increased upon hydroxyurea treatment, despite unaltered expression of CD11b/CD18. Our study does not provide conclusive information on the cause of this observation and this might be influenced by the small number of patients in the hydroxyurea group and large assay variation. One hypothesis could be that changes in the composition of intravascular neutrophils occur, as hydroxyurea leads to reduced generation of neutrophils that might adhere less readily to the endothelial cells in vivo. In this study, we measured surface expression of CD11b/CD18 and not changes in its activity that can occur through functional upregulation by conformational changes (inside-out signaling) (38, 47). Integrin activation is regulated on many different levels, and it might be that hydroxyurea treatment influences integrin clustering or conformation by other means (48). ROS production upon stimulation with PMA and PAF/fMLP was reduced upon hydroxyurea treatment, falling below levels observed in healthy controls. This observation is in line with previous findings by Benkerrou et al. (28), although the mechanism(s) behind this lower activity remain unclear. When comparing all steady state SCD patients using hydroxyurea to those without hydroxyurea treatment, a trend towards increased expression of CD64 and increased unstimulated adhesion in patients using hydroxyurea was observed. This suggests an association between hydroxyurea use and increased neutrophil adhesion, possibly due to indication bias, as patients with more severe disease are more likely to be prescribed hydroxyurea.
All seven transplanted patients included in the study showed successful engraftment; their erythrocyte phenotypes were comparable to those of their donors with normalized PoS levels. A decrease in neutrophil CD64 expression, together with a non-significant rise in L-selectin expression after transplantation and decreased CD55 expression, suggests reduced neutrophil activation. However, conflicting results were observed for markers such as CXCR4, CD163, and CD63 after transplantation, indicating incomplete normalization of neutrophil phenotype despite a complete normalization of the PoS. This disparity may be explained by the presence of sickle cell trait in 57% of donors and post-transplant medication like sirolimus in all patients. Lastly, it is important to note that the comparison between pre- and post-HSCT antigen expression is not strictly paired, as pre-HSCT neutrophils are patient-derived, where post-HSCT neutrophils are donor-derived.
The strength of our study lies in providing an extensive evaluation of neutrophil characteristics in SCD patients, featuring paired data during VOC and from those undergoing treatment with hydroxyurea or HSCT. However, limitations include the relatively small sample sizes of the VOC, hydroxyurea, and HSCT subgroups, reducing statistical power. Considerable variability in antigen expression and functional assays further challenge robust conclusions within these groups. Differences in experimental methods complicate comparisons with other studies. Addressing limitations in experimental methods, such as delays in measurements after collecting blood samples, storing temperature and the use of lysis buffer, is essential.
In conclusion, our study highlights the significant influence of ethnicity on neutrophil phenotype, which may have biased previous observations. While confirming some findings from previous reports regarding neutrophil characteristics in SCD, our study emphasizes the substantial variation in study outcomes. Larger studies with enhanced statistical power and uniformed protocols are needed to comprehensively unravel the function and phenotype of neutrophils in SCD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Amsterdam University Medical Centers IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing, Project administration. LdL: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. BMB: Investigation, Methodology, Resources, Writing – review & editing. AT: Investigation, Methodology, Resources, Validation, Writing – review & editing. MV: Investigation, Writing – review & editing. TK: Writing – review & editing. RZ: Methodology, Resources, Writing – review & editing. BB: Writing – review & editing. RB: Conceptualization, Methodology, Resources, Supervision, Validation, Writing – review & editing. EN: Conceptualization, Methodology, Project administration, Formal Analysis, Supervision, Resources, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by a grant from the AMC Foundation. The SHOW Foundation (Stichting Hematologisch-Oncologisch Wetenschapsonderzoek).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1591283/full#supplementary-material
References
1. Buchanan GR, DeBaun MR, Quinn CT, Steinberg MH. Sickle cell disease. Hematology. (2004) 2004:35–47. doi: 10.1182/asheducation-2004.1.35
2. Allali S, Maciel TT, Hermine O, de Montalembert M. Innate immune cells, major protagonists of sickle cell disease pathophysiology. Haematologica. (2020) 105:273–83. doi: 10.3324/haematol.2019.229989
3. Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. (2016) 127:801–9. doi: 10.1182/blood-2015-09-618538
4. Conran N, Embury SH. Sickle cell vaso-occlusion: The dialectic between red cells and white cells. Exp Biol Med (Maywood). (2021) 246:1458–72. doi: 10.1177/15353702211005392
5. Dominical VM, Samsel L, Nichols JS, Costa FF, McCoy JP Jr., Conran N, et al. Prominent role of platelets in the formation of circulating neutrophil-red cell heterocellular aggregates in sickle cell anemia. Haematologica. (2014) 99:e214–7. doi: 10.3324/haematol.2014.108555
6. Okpala I. The intriguing contribution of white blood cells to sickle cell disease - a red cell disorder. Blood Rev. (2004) 18:65–73. doi: 10.1016/S0268-960X(03)00037-7
7. Okpala I. Leukocyte adhesion and the pathophysiology of sickle cell disease. Curr Opin Hematol. (2006) 13:40–4. doi: 10.1097/01.moh.0000190108.62414.06
8. West MS, Wethers D, Smith J, Steinberg M. Laboratory profile of sickle cell disease: a cross-sectional analysis. The Cooperative Study of Sickle Cell Disease. J Clin Epidemiol. (1992) 45:893–909. doi: 10.1016/0895-4356(92)90073-V
9. Anyaegbu CC, Okpala IE, Akren’Ova YA, Salimonu LS. Peripheral blood neutrophil count and candidacidal activity correlate with the clinical severity of sickle cell anaemia (SCA). Eur J Haematol. (1998) 60:267–8. doi: 10.1111/j.1600-0609.1998.tb01036.x
10. Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. (1994) 330:1639–44. doi: 10.1056/NEJM199406093302303
11. Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. (1998) 91:288–94.
12. Kinney TR, Sleeper LA, Wang WC, Zimmerman RA, Pegelow CH, Ohene-Frempong K, et al. Silent cerebral infarcts in sickle cell anemia: a risk factor analysis. The Cooperative Study of Sickle Cell Disease. Pediatrics. (1999) 103:640–5. doi: 10.1542/peds.103.3.640
13. Castro O, Brambilla DJ, Thorington B, Reindorf CA, Scott RB, Gillette P, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. (1994) 84:643–9. doi: 10.1182/blood.V84.2.643.643
14. Frenette PS. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr Opin Hematol. (2002) 9:101–6. doi: 10.1097/00062752-200203000-00003
15. Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. (2009) 15:384–91. doi: 10.1038/nm.1939
16. Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci U S A. (2002) 99:3047–51. doi: 10.1073/pnas.052522799
17. Finnegan EM, Turhan A, Golan DE, Barabino GA. Adherent leukocytes capture sickle erythrocytes in an in vitro flow model of vaso-occlusion. Am J Hematol. (2007) 82:266–75. doi: 10.1002/ajh.20819
18. Bennewitz MF, Jimenez MA, Vats R, Tutuncuoglu E, Jonassaint J, Kato GJ, et al. Lung vaso-occlusion in sickle cell disease mediated by arteriolar neutrophil-platelet microemboli. JCI Insight. (2017) 2:e89761. doi: 10.1172/jci.insight.89761
19. Vats R, Kaminski TW, Brzoska T, Leech JA, Tutuncuoglu E, Katoch O, et al. Liver-to-lung microembolic NETs promote gasdermin D-dependent inflammatory lung injury in sickle cell disease. Blood. (2022) 140:1020–37. doi: 10.1182/blood.2021014552
20. Nader E, Romana M, Connes P. The red blood cell-inflammation vicious circle in sickle cell disease. Front Immunol. (2020) 11:454. doi: 10.3389/fimmu.2020.00454
21. Lard LR, Mul FP, de Haas M, Roos D, Duits AJ. Neutrophil activation in sickle cell disease. J Leukoc Biol. (1999) 66:411–5. doi: 10.1002/jlb.66.3.411
22. Lum AF, Wun T, Staunton D, Simon SI. Inflammatory potential of neutrophils detected in sickle cell disease. Am J Hematol. (2004) 76:126–33. doi: 10.1002/ajh.20059
23. Garcia F, Mendonca R, Miguel LI, Dominical VM, Saad STO, Costa FF, et al. CXCR4(hi) effector neutrophils in sickle cell anemia: potential role for elevated circulating serotonin (5-HT) in CXCR4(hi) neutrophil polarization. Sci Rep. (2020) 10:14262. doi: 10.1038/s41598-020-71078-8
24. Qari MH, Zaki WA. Flow cytometric assessment of leukocyte function in sickle cell anemia. Hemoglobin. (2011) 35:367–81. doi: 10.3109/03630269.2011.571329
25. Amer J, Ghoti H, Rachmilewitz E, Koren A, Levin C, Fibach E. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br J Haematol. (2006) 132:108–13. doi: 10.1111/j.1365-2141.2005.05834.x
26. Barbu EA, Dominical VM, Mendelsohn L, Thein SL. Neutrophils remain detrimentally active in hydroxyurea-treated patients with sickle cell disease. PloS One. (2019) 14:e0226583. doi: 10.1371/journal.pone.0226583
27. Schimmel M, Nur E, Biemond BJ, van Mierlo GJ, Solati S, Brandjes DP, et al. Nucleosomes and neutrophil activation in sickle cell disease painful crisis. Haematologica. (2013) 98:1797–803. doi: 10.3324/haematol.2013.088021
28. Benkerrou M, Delarche C, Brahimi L, Fay M, Vilmer E, Elion J, et al. Hydroxyurea corrects the dysregulated L-selectin expression and increased H(2)O(2) production of polymorphonuclear neutrophils from patients with sickle cell anemia. Blood. (2002) 99:2297–303. doi: 10.1182/blood.V99.7.2297
29. Guarda CC, Silveira-Mattos PSM, Yahouédéhou S, Santiago RP, Aleluia MM, Figueiredo CVB, et al. Hydroxyurea alters circulating monocyte subsets and dampens its inflammatory potential in sickle cell anemia patients. Sci Rep. (2019) 9:14829. doi: 10.1038/s41598-019-51339-x
30. Haynes J Jr., Obiako B, Hester RB, Baliga BS, Stevens T. Hydroxyurea attenuates activated neutrophil-mediated sickle erythrocyte membrane phosphatidylserine exposure and adhesion to pulmonary vascular endothelium. Am J Physiol Heart Circ Physiol. (2008) 294:H379–85. doi: 10.1152/ajpheart.01068.2007
31. Pedrosa AM, Leal L, Lemes RPG. Effects of hydroxyurea on cytotoxicity, inflammation and oxidative stress markers in neutrophils of patients with sickle cell anemia: dose-effect relationship. Hematol Transfus Cell Ther. (2021) 43:468–75. doi: 10.1016/j.htct.2020.07.011
32. Saleh AW, Hillen HF, Duits AJ. Levels of endothelial, neutrophil and platelet-specific factors in sickle cell anemia patients during hydroxyurea therapy. Acta Haematol. (1999) 102:31–7. doi: 10.1159/000040964
33. Charache S. Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults. Semin Hematol. (1997) 34:15–21.
34. Kuijpers TW, van Bruggen R, Kamerbeek N, Tool AT, Hicsonmez G, Gurgey A, et al. Natural history and early diagnosis of LAD-1/variant syndrome. Blood. (2007) 109:3529–37. doi: 10.1182/blood-2006-05-021402
35. Kuijpers TW, Tool ATJ, van der Bijl I, de Boer M, van Houdt M, de Cuyper IM, et al. Combined immunodeficiency with severe inflammation and allergy caused by ARPC1B deficiency. J Allergy Clin Immunol. (2017) 140:273–277 e10. doi: 10.1016/j.jaci.2016.09.061
36. Rab MAE, Kanne CK, Bos J, Boisson C, van Oirschot BA, Nader E, et al. Methodological aspects of the oxygenscan in sickle cell disease: A need for standardization. Am J Hematol. (2020) 95:E5–8. doi: 10.1002/ajh.25655
37. de Ligt LA, Gaartman AE, Biemond BJ, Fijnvandraat K, van Bruggen R, Nur E. Neutrophils in sickle cell disease: Exploring their potential role as a therapeutic target. Am J Hematol. (2024) 99(6):1119–28. doi: 10.1002/ajh.27224
38. Garcia NP, Junior ALS, Soares GAS, Costa TCC, Dos Santos APC, Costa AG, et al. Sickle cell anemia patients display an intricate cellular and serum biomarker network highlighted by TCD4+CD69+ Lymphocytes, IL-17/MIP-1beta, IL-12/VEGF, and IL-10/IP-10 axis. J Immunol Res. (2020) 2020:4585704. doi: 10.1155/2020/4585704
39. Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, et al. Neutrophil ageing is regulated by the microbiome. Nature. (2015) 525:528–32. doi: 10.1038/nature15367
40. Assis A, Conran N, Canalli AA, Lorand-Metze I, Saad ST, Costa FF. Effect of cytokines and chemokines on sickle neutrophil adhesion to fibronectin. Acta Haematol. (2005) 113:130–6. doi: 10.1159/000083451
41. Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol. (1996) 49:664–6. doi: 10.1136/jcp.49.8.664
42. Bain BJ, England JM. Normal haematological values: sex difference in neutrophil count. Br Med J. (1975) 1:306–9. doi: 10.1136/bmj.1.5953.306
43. Wun T, Cordoba M, Rangaswami A, Cheung AW, Paglieroni T. Activated monocytes and platelet-monocyte aggregates in patients with sickle cell disease. Clin Lab Haematol. (2002) 24:81–8. doi: 10.1046/j.1365-2257.2002.t01-1-00433.x
44. Wun T, Paglieroni T, Tablin F, Welborn J, Nelson K, Cheung A. Platelet activation and platelet-erythrocyte aggregates in patients with sickle cell anemia. J Lab Clin Med. (1997) 129:507–16. doi: 10.1016/S0022-2143(97)90005-6
45. Evans C, Orf K, Horvath E, Levin M, de la Fuente J, Chakravorty S, et al. Impairment of neutrophil oxidative burst in children with sickle cell disease is associated with heme oxygenase-1. Haematologica. (2015) 100:1508–16. doi: 10.3324/haematol.2015.128777
46. Fadlon E, Vordermeier S, Pearson TC, Mire-Sluis AR, Dumonde DC, Phillips J, et al. Blood polymorphonuclear leukocytes from the majority of sickle cell patients in the crisis phase of the disease show enhanced adhesion to vascular endothelium and increased expression of CD64. Blood. (1998) 91:266–74. doi: 10.1182/blood.V91.1.266
47. Klugewitz K, Ley K, Schuppan D, Nuck R, Gaehtgens P, Walzog B. Activation of the beta2 integrin Mac-1 (CD11b/CD18) by an endogenous lipid mediator of human neutrophils and HL-60 cells. J Cell Sci. (1997) 110:985–90. doi: 10.1242/jcs.110.8.985
Keywords: sickle cell disease, neutrophil activation, neutrophil adhesion, neutrohil aging, neutrophil phenotype, hemolysis, reactive oxygen species
Citation: Gaartman AE, de Ligt LA, Beuger BM, Tool ATJ, Veldthuis M, Kuijpers TW, van Zwieten R, Biemond BJ, van Bruggen R and Nur E (2025) Dynamics of neutrophil phenotype and function in sickle cell disease. Front. Immunol. 16:1591283. doi: 10.3389/fimmu.2025.1591283
Received: 10 March 2025; Accepted: 14 April 2025;
Published: 02 May 2025.
Edited by:
Nicola Conran, State University of Campinas, BrazilReviewed by:
John D. Belcher, University of Minnesota Twin Cities, United StatesThiago Trovati Maciel, Institut National de la Santé et de la Recherche Médicale (INSERM), France
Ravi Vats, Q32 Bio Inc., United States
Copyright © 2025 Gaartman, de Ligt, Beuger, Tool, Veldthuis, Kuijpers, van Zwieten, Biemond, van Bruggen and Nur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erfan Nur, ZS5udXJAYW1zdGVyZGFtdW1jLm5s
†These authors have contributed equally to this work
 Aafke E. Gaartman
Aafke E. Gaartman Lydian A. de Ligt1,2,3
Lydian A. de Ligt1,2,3 Taco W. Kuijpers
Taco W. Kuijpers Rob van Zwieten
Rob van Zwieten Bart J. Biemond
Bart J. Biemond Robin van Bruggen
Robin van Bruggen Erfan Nur
Erfan Nur