- 1Department of Pharmacy, The First People’s Hospital of Xiaoshan District, Hangzhou, China
- 2Department of Emergency, The First People’s Hospital of Xiaoshan District, Hangzhou, China
Bladder cancer is a prevalent malignancy, with muscle-invasive bladder cancer (MIBC) presenting a significant therapeutic challenge. Standard treatments, including radical cystectomy (RC) and neoadjuvant chemotherapy, pose substantial risks and impact quality of life, leading to increasing interest in bladder-preserving therapies (BPT). Immunotherapy has revolutionized bladder cancer management, with strategies ranging from intravesical Bacillus Calmette-Guérin (BCG) to immune checkpoint inhibitors targeting programmed cell death protein 1 (PD-1) and its ligand (PD-L1). In BCG-unresponsive non-muscle-invasive bladder cancer (NMIBC), PD-1 inhibitors such as pembrolizumab offer promising response rates. In MIBC, neoadjuvant immunotherapy with agents like atezolizumab and pembrolizumab improves pathological complete response (pCR) and facilitates bladder preservation. Combination regimens integrating radiotherapy, chemotherapy, and immunotherapy not only enhance treatment efficacy but also exploit mechanisms such as immunogenic cell death and antigen release that further augment antitumor immune responses. This review provides a comprehensive analysis of current immunotherapeutic strategies for invasive bladder cancer, highlighting their clinical applications and future potential.
1 Introduction
Bladder cancer ranks among the most prevalent malignancies of the urinary system and is the tenth most commonly diagnosed cancer globally (1, 2). It is categorized into non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC) based on the depth of tumor invasion, with MIBC accounting for approximately 25% of cases and associated with a poorer prognosis (3). The standard treatment for MIBC involves neoadjuvant chemotherapy followed by radical cystectomy (RC) and pelvic lymph node dissection (PLND) (3, 4). However, RC is high-risk, especially for elderly patients with comorbidities, and is linked to perioperative mortality and complications like infections (5). This has driven interest in bladder-preserving therapies (BPT) (6). Studies show BPT achieves tumor control comparable to RC, with no significant difference in overall survival (OS) (7, 8), and 80% of BPT patients maintain an intact bladder five years post-treatment, with improved quality of life (9–12).
In recent years, immunotherapy has emerged as a transformative approach in cancer management (13), particularly through its ability to reprogram the tumor microenvironment (TME). Immune checkpoint inhibitors (ICIs) (e.g., PD-1/PD-L1 inhibitors) can reduce T-cell exhaustion, reshape cytokine networks, and modulate tumor-associated macrophages (TAMs) in ways that enhance immune-mediated tumor clearance (14, 15). These advancements have expanded the therapeutic landscape, providing hope for improved outcomes and quality of life for bladder cancer patients. This review aims to synthesize current research on immunotherapy for invasive bladder cancer, focusing on its role in bladder preservation and its integration into multimodal treatment strategies. We will describe the mechanisms of action, clinical efficacy, and future potential of immunotherapeutic agents, including intravesical BCG, immune checkpoint inhibitors, and novel combination therapies. By examining the latest advancements in immunotherapy, this review seeks to highlight its growing importance as a cornerstone in the management of bladder cancer, offering new avenues for personalized and effective treatment.
2 Immunotherapy for NMIBC
2.1 Intravesical BCG treatment
2.1.1 Mechanism of action of BCG
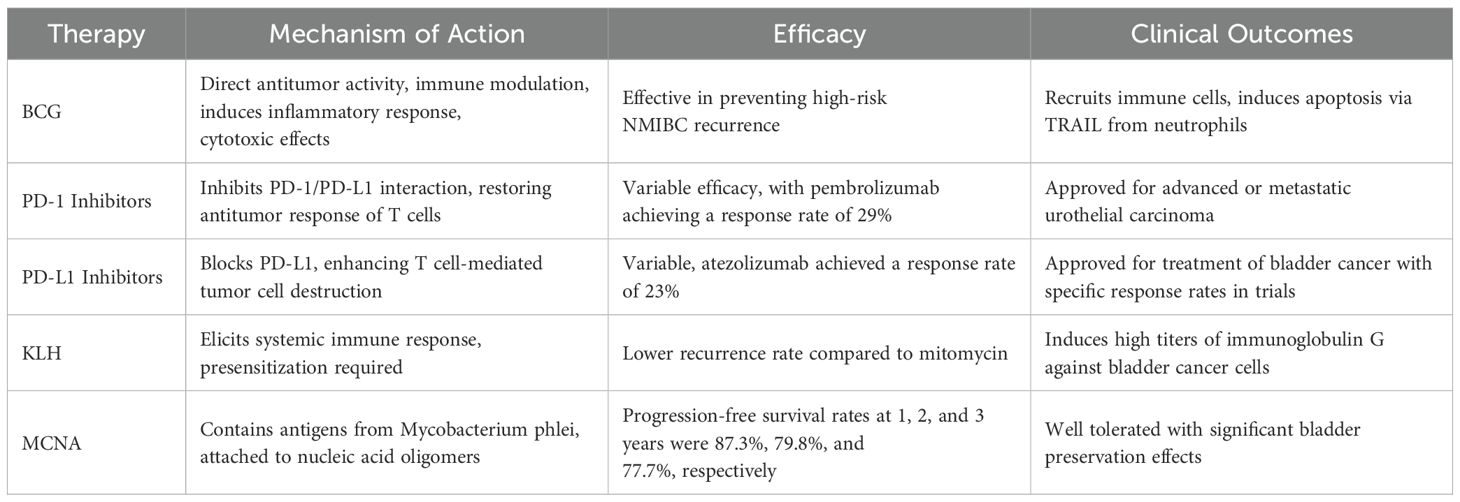
BCG, an attenuated live strain of Mycobacterium bovis, was initially used for tuberculosis treatment and approved in the U.S. in 1990 for stage I bladder cancer. Intravesical BCG is the preferred treatment for preventing high-risk NMIBC recurrence and is endorsed by the European Association of Urology and the National Comprehensive Cancer Network (16). BCG’s therapeutic mechanism involves direct antitumor activity and immunomodulation (17). It adheres to the bladder wall, exerting cytotoxic effects, and induces an inflammatory response, recruiting immune cells. BCG interacts with fibronectin on tumor cells, prompting neutrophil recruitment. Neutrophils release TRAIL, inducing apoptosis (18). This dual mechanism underscores BCG’s unique role in NMIBC management, combining direct and immune-mediated antitumor actions.
2.1.2 Factors influencing the efficacy of intravesical BCG therapy
Factors such as the strain of BCG, dosage, administration method, and the bladder microbiota significantly impact the efficacy of intravesical BCG therapy. Comparative studies reveal that, among various strains, the Russian and Connaught strains of BCG manifest a notably enhanced suppression of cell proliferation and cytokine responses in bladder cancer T24/J82 cells (19). Additionally, research indicates that patients with intermediate-risk NMIBC exhibit a more robust response to a full-dose BCG treatment over one year, whereas those with high-risk NMIBC benefit significantly from a three-year full-dose regimen (20). The local immune environment in cancer is also shaped by cytokine networks and immune cells (21–26), which can either promote or inhibit antitumor immunity (27–30). The bladder microbiota also plays a crucial role, potentially moderating mucosal inflammation by inhibiting pathways involving interleukins (IL-6, IL-8) and nuclear factor-kappa B (NF-kB), thereby influencing the BCG-dependent local inflammatory response essential for initiating immunotherapy. Preliminary findings suggest that pre-treatment with subcutaneous BCG injections prior to intravesical administration may enhance T-cell recruitment to the bladder and improve tissue responsiveness to BCG, correlating with increased recurrence-free survival rates and BCG-specific immunity (31).
2.1.3 Mechanisms of resistance in intravesical BCG therapy
Understanding resistance mechanisms to intravesical BCG therapy is critical, particularly in refractory, resistant, or recurrent cases, often leading to radical cystectomy (32). A key mechanism involves PD-L1 upregulation on tumor cells, which interacts with PD-1 on T-cells, suppressing immune responses (33–35), and enabling cancer evasion (36–39). Biomarkers such as PD-L1 expression and Th1/Th2 polarization can help predict which patients are more likely to develop BCG resistance (40, 41). This has made PD-1/PD-L1 inhibitors a focal point in bladder cancer immunotherapy. Recent studies highlight the role of ICIs, such as PD-1/PD-L1, in cancer immune escape, with ICIs reactivating cytotoxic T cells to target tumors (42). Additionally, BCG may inhibit TNF-α-mediated p53 expression, reducing apoptosis (43–45). Combining BCG therapy with PD-L1 inhibitors (intravesical or systemic) or TNF-α could overcome resistance, prolong BCG response, prevent recurrence, reduce BCG dosage, and minimize adverse effects.
2.1.4 BCG and IL-2 combined immunotherapy
Recent research has focused on enhancing the efficacy of BCG therapy and reducing the required dosage through combination treatments, which may improve prognosis and the safety of tumor immunotherapy, thereby reducing the incidence of adverse events leading to the discontinuation of BCG therapy. Steinberg (46) studied 52 high-risk NMIBC patients who had experienced at least one failure of previous BCG therapy and underwent a six-cycle regimen of quadruple immunotherapy (intravesical BCG + interferon + IL-2 + subcutaneous granulocyte-macrophage colony-stimulating factor). Among these patients, 55% remained recurrence-free at one year, and 53% at two years, thus avoiding cystectomy despite 6% being unable to tolerate the full induction.
2.2 Keyhole limpet hemocyanin and MCNA
KLH functions as an immunotherapeutic agent by eliciting a robust systemic immune response specifically targeting bladder cancer cells. This mechanism involves the induction of high titers of tumor-specific immunoglobulin G antibodies. Lammers et al. (47) conducted a pivotal prospective randomized Phase III clinical trial comparing the therapeutic efficacy and safety profile of KLH versus mitomycin in intermediate and high-risk non-muscle invasive bladder cancer (NMIBC) patients. The study demonstrated superior clinical outcomes in the KLH treatment arm, with a significantly lower tumor recurrence rate of 34% compared to 61% in the mitomycin group, indicating KLH’s potential as a more effective therapeutic option for this patient population. The Mycobacterium phlei cell wall-nucleic acid complex (MCNA) contains antigens derived from the cell wall of Mycobacterium phlei attached to a mixture of nucleic acid oligomers (48). Morales (49) performed a Phase II single-arm study to evaluate the efficacy and safety of MCNA in 129 patients with NMIBC who had failed BCG therapy. The study outcomes indicated that the one-year, two-year, and three-year progression-free survival rates were 87.3%, 79.8%, and 77.7% respectively, with a two-year overall disease-free survival rate of 19%. The patients tolerated MCNA well, making it an important option for bladder preservation.
2.3 PD-1 and PD-L1 inhibitors
Currently approved PD-1 inhibitors for the treatment of bladder cancer include pembrolizumab and nivolumab. Pembrolizumab exerts its antitumor immunotherapeutic effects by inhibiting the PD-1/PD-L1 interaction. A clinical trial involving 370 patients with advanced or metastatic urothelial carcinoma demonstrated that pembrolizumab achieved an objective response rate of 29% (50), with 7% of patients reaching complete remission. Nivolumab selectively blocks the interaction between PD-1 and PD-L1/Programmed Death-Ligand 2 expressed on tumor cells, disrupting PD-L1-mediated signaling and thereby restoring the antitumor response of effector T cells (51). Clinical trial data show that nivolumab treated patients with metastatic urothelial carcinoma had an overall objective response rate of 19.6% and a complete remission rate of 2% (52). Approved PD-L1 inhibitors in bladder cancer include atezolizumab, durvalumab, and avelumab. Atezolizumab demonstrated an objective response rate of 23% in metastatic urothelial carcinoma (53). Durvalumab, an immunoglobulin G monoclonal antibody, operates by obstructing the PD-1/PD-L1 interaction, thus enabling T cells to recognize and eliminate tumor cells. A clinical trial demonstrated that durvalumab treatment in patients with urothelial carcinoma achieved an overall objective response rate of 17.8%, and this increased to 27.6% in patients with high PD-L1 expression (54). Avelumab, an anti-PD-L1 immunoglobulin G1 monoclonal antibody, selectively blocks the PD-1/PD-L1 interaction. It reached an 18.2% overall response rate, with up to 53.8% in high PD-L1–expressing patients (55) (Table 1).
3 Immunotherapy for MIBC
3.1 Bladder-preserving treatment options and advantageous populations
Currently, a variety of BPTs are available, such as tri-modality therapy (TMT), maximal transurethral resection of bladder tumor (mTURBT) combined with chemotherapy and/or radiotherapy, partial cystectomy (PC) combined with chemotherapy and/or radiotherapy, and integrative immunotherapy-based approaches. The implementation of BPTs requires multidisciplinary consultation and rigorous evaluation. Patients considered to be particularly suited for BPTs meet the following criteria: (1) Patient factors: high compliance, good bladder function and capacity; and (2) Tumor factors: stage T2, solitary tumor, absence of concomitant carcinoma in situ, absence of hydronephrosis, and complete TURBT (56). For high-risk patients, such as those where TURBT cannot achieve complete tumor removal, widespread carcinoma in situ, presence of hydronephrosis, diffuse tumors, and stage cT3-4, TMT remains a viable option, although the likelihood of cure is significantly reduced (57). Studies have demonstrated that immunotherapy can markedly downstage tumors, potentially converting non-ideal candidates into suitable ones for bladder preservation (4).
3.2 The Role of immunotherapy in bladder preservation for patients with BCG-unresponsive MIBC
National guidelines strongly recommend one year of BCG bladder instillation therapy post-TURBT for high-risk MIBC. However, approximately 30% of patients experience tumor recurrence, 10% progress (58), and 50% do not respond to BCG (59). Adverse reactions like fever, cystitis, and hematuria further limit tolerability. For BCG-unresponsive cases, RC is the primary guideline-recommended treatment, though immunotherapy has emerged as a bladder-preserving alternative (60). Studies show Pembrolizumab monotherapy achieves a 41% complete response (CR) rate at three months in BCG-unresponsive high-risk MIBC patients refusing RC, with 80% maintaining CR for over six months and a median remission duration of 16.2 months (61). These results led to FDA approval of Pembrolizumab for such patients in January 2020. Similarly, Atezolizumab monotherapy reported CR rates of 41.1% at three months and 26.0% at six months in BCG-unresponsive carcinoma in situ patients, with an 83.6% adverse event rate (62).
Clinical trials have further validated the role of ICIs in NMIBC and MIBC settings. The KEYNOTE-057 trial updated results indicated durable CR rates in high-risk BCG-unresponsive NMIBC treated with pembrolizumab, reinforcing its approval status (61). Additionally, the CheckMate 274 trial in the adjuvant setting for MIBC demonstrated that nivolumab significantly improved disease-free survival compared with placebo in patients at high risk of recurrence post-RC (63). Inflammatory factors are pivotal in disease’s progression (64–67). Innovative immunotherapies like the IL-15 superagonist N-803 have shown promise, with a 92% bladder preservation rate at 12 months and 99.5% tumor-specific survival at 24 months, alongside a low grade 3+ adverse event rate of 3% (68). Additionally, the antibody-drug conjugate vicinium achieved a 40% CR rate at three months in carcinoma in situ patients, with a 96% two-year overall survival rate (69). These advancements highlight the potential of novel immunotherapies as effective alternatives for BCG-unresponsive high-risk NMIBC patients, offering viable bladder-preserving options as clinical trials progress.
3.3 Applications of immunotherapy in neoadjuvant therapy prior to bladder-sparing surgery
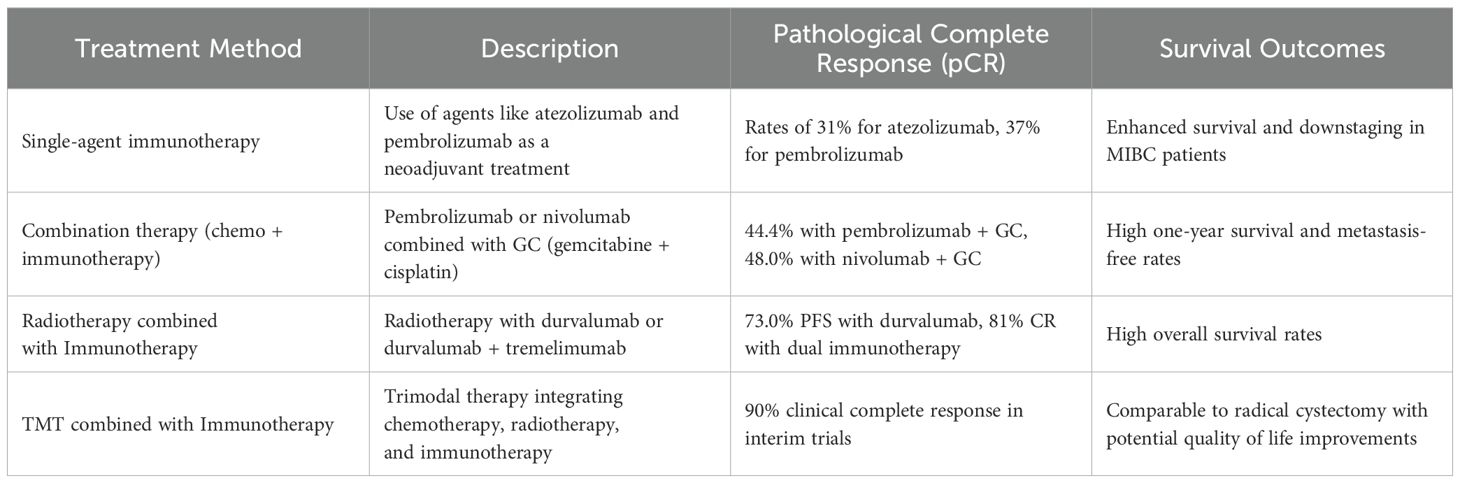
MIBC constitutes a lethal malignancy with a five-year survival rate of 50%guidelines universally recommend a neoadjuvant chemotherapy regimen based on cisplatin (70), followed by RC. However, given the significant decline in quality-of-life post-RC, a subset of patients strongly prefers bladder preservation. A critical concern with BPT is their ability to achieve survival outcomes comparable to those of current RC protocols. The pursuit of bladder preservation without compromising prognosis has emerged as a focal area of research. The application of single-agent or combination immunotherapeutic agents as neoadjuvant interventions has enhanced the rates of pathological complete response (pCR) and downstaging in MIBC patients (71). Compared to chemotherapy, the majority of adverse reactions associated with immunotherapy are mild (grades 1–2), offering a safer and more effective treatment modality for elderly and frail MIBC patients. Neoadjuvant immunotherapy has expanded the possibility of bladder preservation for a broader cohort of MIBC patients (71).
The following delineates several immunotherapy-based neoadjuvant bladder preservation strategies: (I) Single-agent immunotherapy neoadjuvant bladder-preserving protocol: To benefit patient intolerant to chemotherapy, a multicenter phase II clinical trial on Atezolizumab (ABACUS study) demonstrated a pCR rate of 31% across the general population, with a one-year tumor recurrence-free survival (RFS) of 79%. In PD-L1 positive patients, the pCR rate reached 37%, with 54% experiencing downstaging to NMIBC, and a one-year RFS of 75% (72). Another phase II clinical trial using Pembrolizumab (PURE-01 study) reported a pCR rate of 37%, with 55% of patients achieving tumor downstaging (73). (II) Combination therapy and chemotherapy in neoadjuvant bladder-preserving protocols: The GC (gemcitabine + cisplatin) regimen remains the standard chemotherapy for bladder cancer and the conventional neoadjuvant therapy for MIBC. However, recent studies show improved efficacy with immunotherapeutic agents: Pembrolizumab combined with GC achieved a 44.4% pCR rate (74); Nivolumab with GC reached a 48.0% cCR rate, with cCR patients showing one-year survival and metastasis-free rates of 100.0% and 81.2%, respectively, and 78.0% maintaining bladder preservation (75). These combination regimens harness immunogenic cell death induced by chemotherapy, enhancing tumor antigen presentation and T-cell priming. The synergy between chemotherapeutic agents and ICIs potentially increases tumor immunogenicity, resulting in improved response rates (76). While single-agent immunotherapy may be safer in cisplatin-intolerant populations, combination strategies could yield more robust pCR and downstaging rates. Long-term follow-up data, ideally from randomized controlled trials, are needed to confirm survival benefits.
3.4 Application of immunotherapy in the adjuvant comprehensive treatment following bladder-sparing surgery
Current clinical guidelines universally endorse the implementation of rigorous adjuvant chemotherapy and/or radiotherapy for MIBC patients who have been meticulously selected and assessed following PC or mTURBT procedures (4, 77). Radiotherapy can release tumor-specific antigens, facilitate T-cell priming, and increase MHC-I expression on tumor cells, thereby amplifying the effects of ICIs. For cisplatin-ineligible MIBC patients, radiotherapy plus durvalumab yielded a one-year progression-free survival (PFS) rate of 73.0% and an OS rate of 83.8% (78). Another protocol combining radiotherapy with durvalumab and tremelimumab reported a CR rate of 81% (48), demonstrating the synergistic potential of multimodal regimens (79). TMT is a conventional bladder-preserving adjuvant treatment, which emphasizes the synchronous application of chemotherapy and radiotherapy. Ongoing clinical trials investigating TMT combined with immunotherapy have reported encouraging results. For instance, intermediate trial outcomes for MIBC patients undergoing mTURBT followed by Pembrolizumab combined with concurrent chemoradiotherapy revealed a cCR rate of 90%, with final study results highly anticipated (80–82). As immunotherapy continues to evolve, combining TMT with ICIs could increase cCR rates and further improve bladder preservation. These synergistic effects, coupled with a generally favorable safety profile, position immunotherapy as a key component of future bladder preservation strategies (Table 2).
4 Conclusion
Immunotherapy is transforming the management of invasive bladder cancer by offering viable alternatives to standard radical cystectomy, particularly for patients with comorbidities or those seeking bladder preservation. Intravesical BCG remains essential for high-risk NMIBC, but its limitations and resistance patterns highlight the growing role of ICIs such as pembrolizumab, nivolumab, atezolizumab, durvalumab, and avelumab. In MIBC, neoadjuvant and adjuvant immunotherapy, especially when combined with chemotherapy or radiotherapy, can enhance pathological complete response and long-term survival, facilitating bladder preservation without sacrificing oncologic outcomes. Looking forward, biomarker-driven clinical trials are urgently needed to personalize immunotherapy and identify patients most likely to benefit; overcoming primary and acquired resistance to ICIs remains a crucial challenge, spurring research into novel agents and combination approaches; and optimizing treatment sequencing, particularly how best to integrate immunotherapy with chemo- and radiotherapy, will be pivotal for advancing the standard of care. As research expands our understanding of the TME, immunotherapy is poised to become an even more integral component of bladder cancer management, ultimately improving patient outcomes and quality of life.
Author contributions
YW: Writing – original draft. MH: Writing – original draft. JL: Writing – original draft. LL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Chen H, Shi D, Guo C, Zhang W, Guo Y, Yang F, et al. et al: Can uric acid affect the immune microenvironment in bladder cancer? A single-center multi-omics study. Mol Carcinog. (2024) 63:461–78. doi: 10.1002/mc.23664
3. Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. (2017) 71:447–61. doi: 10.1016/j.eururo.2016.05.041
4. Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. (2021) 79:82–104. doi: 10.1016/j.eururo.2020.03.055
5. Ramani VA, Maddineni SB, Grey BR, Clarke NW. Differential complication rates following radical cystectomy in the irradiated and nonirradiated pelvis. Eur Urol. (2010) 57:1058–63. doi: 10.1016/j.eururo.2009.12.002
6. Gray PJ, Fedewa SA, Shipley WU, Efstathiou JA, Lin CC, Zietman AL, et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: results from the National Cancer Data Base. Eur Urol. (2013) 63:823–9. doi: 10.1016/j.eururo.2012.11.015
7. Ding H, Fan N, Ning Z, Ma D. Trimodal therapy vs. Radical cystectomy for muscle-invasive bladder cancer: A meta-analysis. Front Oncol. (2020) 10:564779. doi: 10.3389/fonc.2020.564779
8. Fahmy O, Khairul-Asri MG, Schubert T, Renninger M, Malek R, Kübler H, et al. A systematic review and meta-analysis on the oncological long-term outcomes after trimodality therapy and radical cystectomy with or without neoadjuvant chemotherapy for muscle-invasive bladder cancer. Urol Oncol. (2018) 36:43–53. doi: 10.1016/j.urolonc.2017.10.002
9. Premo C, Apolo AB, Agarwal PK, Citrin DE. Trimodality therapy in bladder cancer: who, what, and when? Urol Clin North Am. (2015) 42:169–180, vii. doi: 10.1016/j.ucl.2015.02.002
10. Mak KS, Smith AB, Eidelman A, Clayman R, Niemierko A, Cheng JS, et al. Quality of life in long-term survivors of muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys. (2016) 96:1028–36. doi: 10.1016/j.ijrobp.2016.08.023
11. Guo Y, Zheng Z, Mao S, Yang F, Wang R, Wang H, et al. Metabolic-associated signature and hub genes associated with immune microenvironment and prognosis in bladder cancer. Mol Carcinog. (2023) 62:185–99. doi: 10.1002/mc.23475
12. Guo Y, Zheng Z, Zhang W, Mao S, Yang F, Li W, et al. Gender dimorphism in survival of patients with lymph node metastasis of bladder cancer. Ther Adv Med Oncol. (2022) 14:17588359221108690. doi: 10.1177/17588359221108690
13. Jin W, Yang Q, Chi H, Wei K, Zhang P, Zhao G, et al. Ensemble deep learning enhanced with self-attention for predicting immunotherapeutic responses to cancers. Front Immunol. (2022) 13:1025330. doi: 10.3389/fimmu.2022.1025330
14. Hoshi R, Gorospe KA, Labouta HI, Azad T, Lee WL, Thu KL. Alternative strategies for delivering immunotherapeutics targeting the PD-1/PD-L1 immune checkpoint in cancer. Pharmaceutics. (2024) 16:1181. doi: 10.3390/pharmaceutics16091181
15. Chamseddine AN, Assi T, Mir O, Chouaib S. Modulating tumor-associated macrophages to enhance the efficacy of immune checkpoint inhibitors: A TAM-pting approach. Pharmacol Ther. (2022) 231:107986. doi: 10.1016/j.pharmthera.2021.107986
16. Krajewski W, Zdrojowy R, Grzególka J, Krajewski P, Wróbel M, Luczak M, et al. Does mantoux test result predicts BCG immunotherapy efficiency and severe toxicity in non-muscle invasive bladder cancer. Urol J. (2019) 16:458–62. doi: 10.22037/uj.v0i0.4542
17. Shariat SF, Enikeev DV, Mostafaei H. Six essential conditions for bladder-sparing strategies in bacillus Calmette-Guérin unresponsive bladder cancer. Immunotherapy. (2019) 11:1083–6. doi: 10.2217/imt-2019-0083
18. Fulda S. Tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL). Adv Exp Med Biol. (2014) 818:167–80. doi: 10.1007/978-1-4471-6458-6_8
19. Witjes JA, Dalbagni G, Karnes RJ, Shariat S, Joniau S, Palou J, et al. The efficacy of BCG TICE and BCG Connaught in a cohort of 2,099 patients with T1G3 non-muscle-invasive bladder cancer. Urol Oncol. (2016) 34:484.e419–484.e425. doi: 10.1016/j.urolonc.2016.05.033
20. McNeel DG, Bander NH, Beer TM, Drake CG, Fong L, Harrelson S, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of prostate carcinoma. J Immunother Cancer. (2016) 4:92. doi: 10.1186/s40425-016-0198-x
21. Xie H, Xi X, Lei T, Liu H, Xia Z. CD8(+) T cell exhaustion in the tumor microenvironment of breast cancer. Front Immunol. (2024) 15:1507283. doi: 10.3389/fimmu.2024.1507283
22. Deng Y, Shi M, Yi L, Naveed Khan M, Xia Z, Li X. Eliminating a barrier: Aiming at VISTA, reversing MDSC-mediated T cell suppression in the tumor microenvironment. Heliyon. (2024) 10:e37060. doi: 10.1016/j.heliyon.2024.e37060
23. Xiong J, Chi H, Yang G, Zhao S, Zhang J, Tran LJ, et al. Revolutionizing anti-tumor therapy: unleashing the potential of B cell-derived exosomes. Front Immunol. (2023) 14:1188760. doi: 10.3389/fimmu.2023.1188760
24. Lin L, Zou J, Pei S, Huang W, Zhang Y, Zhao Z, et al. Germinal center B-cell subgroups in the tumor microenvironment cannot be overlooked: Their involvement in prognosis, immunotherapy response, and treatment resistance in head and neck squamous carcinoma. Heliyon. (2024) 10:e37726. doi: 10.1016/j.heliyon.2024.e37726
25. Zhou W, Li S, Zhang X, Li C, Zhang J. miR-143-3p shuttled by M2 macrophage-derived extracellular vesicles induces progression of colorectal cancer through a ZC3H12A/C/EBPbeta axis-dependent mechanism. Int Immunopharmacol. (2023) 119:110137. doi: 10.1016/j.intimp.2023.110137
26. Liu T, Li C, Zhang J, Hu H, Li C. Unveiling efferocytosis-related signatures through the integration of single-cell analysis and machine learning: a predictive framework for prognosis and immunotherapy response in hepatocellular carcinoma. Front Immunol. (2023) 14:1237350. doi: 10.3389/fimmu.2023.1237350
27. Di Spirito A, Balkhi S, Vivona V, Mortara L. Key immune cells and their crosstalk in the tumor microenvironment of bladder cancer: insights for innovative therapies. Explor Target Antitumor Ther. (2025) 6:1002304. doi: 10.37349/etat
28. Zhao Y, Wei K, Chi H, Xia Z, Li X. IL-7: A promising adjuvant ensuring effective T cell responses and memory in combination with cancer vaccines? Front Immunol. (2022) 13:1022808. doi: 10.3389/fimmu.2022.1022808
29. Gong X, Chi H, Strohmer DF, Teichmann AT, Xia Z, Wang Q. Exosomes: A potential tool for immunotherapy of ovarian cancer. Front Immunol. (2022) 13:1089410. doi: 10.3389/fimmu.2022.1089410
30. Li X, Lin Z, Zhao F, Huang T, Fan W, Cen L, et al. Unveiling the cellular landscape: insights from single-cell RNA sequencing in multiple myeloma. Front Immunol. (2024) 15:1458638. doi: 10.3389/fimmu.2024.1458638
31. Niwa N, Kikuchi E, Matsumoto K, Kosaka T, Mizuno R, Oya M. Purified Protein Derivative Skin test prior to bacillus Calmette-Guérin Therapy May have Therapeutic Impact in Patients with Nonmuscle Invasive Bladder Cancer. J Urol. (2018) 199:1446–51. doi: 10.1016/j.juro.2017.12.057
32. Wang YH, Cao YW, Yang XC, Niu HT, Sun LJ, Wang XS, et al. Effect of TLR4 and B7-H1 on immune escape of urothelial bladder cancer and its clinical significance. Asian Pac J Cancer Prev. (2014) 15:1321–6. doi: 10.7314/APJCP.2014.15.3.1321
33. Sun L, Shao W, Lin Z, Lin J, Zhao F, Yu J. Single-cell RNA sequencing explored potential therapeutic targets by revealing the tumor microenvironment of neuroblastoma and its expression in cell death. Discovery Oncol. (2024) 15:409. doi: 10.1007/s12672-024-01286-5
34. Li H, Bian Y, Xiahou Z, Zhao Z, Zhao F, Zhang Q. The cellular signaling crosstalk between memory B cells and tumor cells in nasopharyngeal carcinoma cannot be overlooked: Their involvement in tumor progression and treatment strategy is significant. J Cancer. (2025) 16:288–314. doi: 10.7150/jca.101420
35. Zhou JG, Liang R, Wang HT, Jin SH, Hu W, Frey B, et al. Identification and characterization of circular RNAs as novel putative biomarkers to predict anti-PD-1 monotherapy response in metastatic melanoma patients - Knowledge from two independent international studies. Neoplasia. (2023) 37:100877. doi: 10.1016/j.neo.2023.100877
36. Xia Z, Chen S, He M, Li B, Deng Y, Yi L, et al. Editorial: Targeting metabolism to activate T cells and enhance the efficacy of checkpoint blockade immunotherapy in solid tumors. Front Immunol. (2023) 14:1247178. doi: 10.3389/fimmu.2023.1247178
37. Ren Q, Zhang P, Lin H, Feng Y, Chi H, Zhang X, et al. A novel signature predicts prognosis and immunotherapy in lung adenocarcinoma based on cancer-associated fibroblasts. Front Immunol. (2023) 14:1201573. doi: 10.3389/fimmu.2023.1201573
38. Lin Z, Wang F, Yin R, Li S, Bai Y, Zhang B, et al. Single-cell RNA sequencing and immune microenvironment analysis reveal PLOD2-driven Malignant transformation in cervical cancer. Front Immunol. (2024) 15:1522655. doi: 10.3389/fimmu.2024.1522655
39. Hou M, Zhao Z, Li S, Zhang Z, Li X, Zhang Y, et al. Single-cell analysis unveils cell subtypes of acral melanoma cells at the early and late differentiation stages. J Cancer. (2025) 16:898–916. doi: 10.7150/jca.102045
40. Thoma C. PD-L1 and BCG response prediction. Nat Rev Urol. (2020) 17:8. doi: 10.1038/s41585-019-0267-2
41. Martinez R, Tapia G, De Muga S, Hernandez A, Cao MG, Teixido C, et al. Combined assessment of peritumoral Th1/Th2 polarization and peripheral immunity as a new biomarker in the prediction of BCG response in patients with high-risk NMIBC. Oncoimmunology. (2019) 8:1602460. doi: 10.1080/2162402X.2019.1602460
42. Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. (2017) 8:561. doi: 10.3389/fphar.2017.00561
43. Han J, Gu X, Li Y, Wu Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. BioMed Pharmacother. (2020) 129:110393. doi: 10.1016/j.biopha.2020.110393
44. Soltani M, Zhao Y, Xia Z, Ganjalikhani Hakemi M, Bazhin AV. The importance of cellular metabolic pathways in pathogenesis and selective treatments of hematological Malignancies. Front Oncol. (2021) 11:767026. doi: 10.3389/fonc.2021.767026
45. Zhang H, Zhai X, Liu Y, Xia Z, Xia T, Du G, et al. et al: NOP2-mediated m5C Modification of c-Myc in an EIF3A-Dependent Manner to Reprogram Glucose Metabolism and Promote Hepatocellular Carcinoma Progression. Res (Wash D C). (2023) 6:0184. doi: 10.34133/research.0184
46. Steinberg RL, Nepple KG, Velaer KN, Thomas LJ, O’Donnell MA. Quadruple immunotherapy of Bacillus Calmette-Guérin, interferon, interleukin-2, and granulocyte-macrophage colony-stimulating factor as salvage therapy for non-muscle-invasive bladder cancer. Urol Oncol. (2017) 35:670.e677–670.e614. doi: 10.1016/j.urolonc.2017.07.024
47. Lammers RJ, Witjes WP, Janzing-Pastors MH, Caris CT, Witjes JA. Intracutaneous and intravesical immunotherapy with keyhole limpet hemocyanin compared with intravesical mitomycin in patients with non-muscle-invasive bladder cancer: results from a prospective randomized phase III trial. J Clin Oncol. (2012) 30:2273–9. doi: 10.1200/JCO.2011.39.2936
48. Yuksel ZS, Buber E, Kocagoz T, Alp A, Saribas Z, Acan NL. Mycobacterial strains that stimulate the immune system most efficiently as candidates for the treatment of bladder cancer. J Mol Microbiol Biotechnol. (2011) 20:24–8. doi: 10.1159/000324331
49. Morales A, Herr H, Steinberg G, Given R, Cohen Z, Amrhein J, et al. Efficacy and safety of MCNA in patients with nonmuscle invasive bladder cancer at high risk for recurrence and progression after failed treatment with bacillus Calmette-Guérin. J Urol. (2015) 193:1135–43. doi: 10.1016/j.juro.2014.09.109
50. Plimack ER, Gupta S, Bellmunt J, Berger R, Montgomery B, Gonzalez EJ, et al. A phase 1B study of pembrolizumab (Pembro; mk-3475) in patients (Pts) with advanced urothelial tract cancer. Ann Oncol. (2014) 25:v1. doi: 10.1093/annonc/mdu438.24
51. Song D, Powles T, Shi L, Zhang L, Ingersoll MA, Lu YJ. Bladder cancer, a unique model to understand cancer immunity and develop immunotherapy approaches. J Pathol. (2019) 249:151–65. doi: 10.1002/path.v249.2
52. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicenter, single-arm, phase 2 trial. Lancet Oncol. (2017) 18:312–22. doi: 10.1016/S1470-2045(17)30065-7
53. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicenter, phase 2 trial. Lancet. (2017) 389:67–76. doi: 10.1016/S0140-6736(16)32455-2
54. Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. (2017) 3:e172411. doi: 10.1001/jamaoncol.2017.2411
55. Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase ib study. J Clin Oncol. (2017) 35:2117–24. doi: 10.1200/JCO.2016.71.6795
56. Ye DW. Expert consensus of multi-disciplinary collaboration on bladder-preserving treatment for bladder cancer in China (2024 edition). Zhonghua Zhong Liu Za Zhi. (2024) 46:1136–55. doi: 10.3760/cma.j.cn112152-20240602-00231
57. Tholomier C, Souhami L, Kassouf W. Bladder-sparing protocols in the treatment of muscle-invasive bladder cancer. Transl Androl Urol. (2020) 9:2920–37. doi: 10.21037/tau.2020.02.10
58. Jallad S, Thomas P, Newport MJ, Kern F. Baseline cytokine profiles of tuberculin-specific CD4(+) T cells in non-muscle-invasive bladder cancer may predict outcomes of BCG immunotherapy. Cancer Immunol Res. (2018) 6:1212–9. doi: 10.1158/2326-6066.CIR-18-0046
59. Compérat E, Amin MB, Cathomas R, Choudhury A, De Santis M, Kamat A, et al. Current best practice for bladder cancer: a narrative review of diagnostics and treatments. Lancet. (2022) 400:1712–21. doi: 10.1016/S0140-6736(22)01188-6
60. Donin NM, Lenis AT, Holden S, Drakaki A, Pantuck A, Belldegrun A, et al. Immunotherapy for the treatment of urothelial carcinoma. J Urol. (2017) 197:14–22. doi: 10.1016/j.juro.2016.02.3005
61. Balar AV, Kamat AM, Kulkarni GS, Uchio EM, Boormans JL, Roumiguie M, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicenter, phase 2 study. Lancet Oncol. (2021) 22:919–30. doi: 10.1016/S1470-2045(21)00147-9
62. Black PC, Tangen CM, Singh P, McConkey DJ, Lucia MS, Lowrance WT, et al. et al: phase 2 trial of atezolizumab in bacillus calmette-guérin-unresponsive high-risk non-muscle-invasive bladder cancer: SWOG S1605. Eur Urol. (2023) 84:536–44. doi: 10.1016/j.eururo.2023.08.004
63. Mercinelli C, Moschini M, Cigliola A, Mattorre B, Tateo V, Basile G, et al. First results of NURE-combo: A phase II study of neoadjuvant nivolumab and nab-paclitaxel, followed by postsurgical adjuvant nivolumab, for muscle-invasive bladder cancer. J Clin Oncol. (2024) 42:4196–205. doi: 10.1200/JCO.24.00576
64. Zhai X, Zhang H, Xia Z, Liu M, Du G, Jiang Z, et al. Oxytocin alleviates liver fibrosis via hepatic macrophages. JHEP Rep. (2024) 6:101032. doi: 10.1016/j.jhepr.2024.101032
65. Xiao J, Lin H, Liu B, Xia Z, Zhang J, Jin J. Decreased S1P and SPHK2 are involved in pancreatic acinar cell injury. biomark Med. (2019) 13:627–37. doi: 10.2217/bmm-2018-0404
66. Xiao J, Huang K, Lin H, Xia Z, Zhang J, Li D, et al. Mogroside II(E) inhibits digestive enzymes via suppression of interleukin 9/interleukin 9 receptor signaling in acute pancreatitis. Front Pharmacol. (2020) 11:859. doi: 10.3389/fphar.2020.00859
67. Zhang H, Xia T, Xia Z, Zhou H, Li Z, Wang W, et al. KIF18A inactivates hepatic stellate cells and alleviates liver fibrosis through the TTC3/Akt/mTOR pathway. Cell Mol Life Sci. (2024) 81:96. doi: 10.1007/s00018-024-05114-5
68. Chang SS, Chamie K, Gonzalgo ML, Kramolowsky EV, Sexton WJ, Reddy SK, et al. Positive efficacy and safety phase 3 results in both CIS and papillary cohorts BCG-unresponsive nonmuscle invasive bladder cancer (NMIBC) after IL-15RαFc superagonist N-803 (Anktiva) and BCG infusion. J Clin Oncol. (2022) 40:431–1. doi: 10.1200/JCO.2022.40.6_suppl.431
69. Shore N, O’Donnell M, Keane T, Jewett MAS, Kulkarni GS, Dickstein R, et al. pd03-02 phase 3 results of vicinium in bcg-unresponsive non-muscle invasive bladder cancer. J Urol. (2020) 203:e72. doi: 10.1097/JU.0000000000000823.02
70. Stein JP, Skinner DG. Radical cystectomy for invasive bladder cancer: long-term results of a standard procedure. World J Urol. (2006) 24:296–304. doi: 10.1007/s00345-006-0061-7
71. Kim IH, Lee HJ. Perioperative systemic treatment for muscle-invasive bladder cancer: current evidence and future perspectives. Int J Mol Sci. (2021) 22:7201. doi: 10.3390/ijms22137201
72. Powles T, Kockx M, Rodriguez-Vida A, Duran I, Crabb SJ, van der Heijden MS, et al. et al: Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. (2019) 25:1706–14. doi: 10.1038/s41591-019-0628-7
73. Necchi A, Raggi D, Gallina A, Madison R, Colecchia M, Lucianò R, et al. Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur Urol. (2020) 77:439–46. doi: 10.1016/j.eururo.2019.10.026
74. Hoimes CJ, Adra N, Fleming MT, Kaimakliotis HZ, Picus J, Smith ZL, et al. Phase Ib/II neoadjuvant (N-) pembrolizumab (P) and chemotherapy for locally advanced urothelial cancer (laUC): Final results from the cisplatin (C)-eligible cohort of HCRN GU14-188. Am Soc Clin Oncol. (2020) 38:15. doi: 10.1200/JCO.2020.38.15_suppl.5047
75. Galsky MD, Daneshmand S, Chan KG, Dorff TB, Cetnar JP, O Neil B, et al. Phase 2 trial of gemcitabine, cisplatin, plus nivolumab with selective bladder sparing in patients with muscle-invasive bladder cancer (MIBC): HCRN GU 16-257. Wolters Kluwer Health. (2021) 39:15. doi: 10.1200/JCO.2021.39.15_suppl.4503
76. Rouanne M, Bajorin DF, Hannan R, Galsky MD, Williams SB, Necchi A, et al. Rationale and outcomes for neoadjuvant immunotherapy in urothelial carcinoma of the bladder. Eur Urol Oncol. (2020) 3:728–38. doi: 10.1016/j.euo.2020.06.009
77. Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. Bladder cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2020) 18:329–54. doi: 10.6004/jnccn.2020.0011
78. Joshi M, Kaag M, Tuanquin L, Liao J, Kilari D, Emamekhoo H, et al. Phase II clinical study of concurrent durvalumab and radiation therapy (DUART) followed by adjuvant durvalumab in patients with localized urothelial cancer of bladder: Results for primary analyses and survival. BTCRC-GU15-023. Am Soc Clin Oncol. (2021) 39:6. doi: 10.1200/JCO.2021.39.6_suppl.398
79. Garcia del Muro X, Valderrama BP, Medina A, Cuellar MA, Etxaniz O, Gironés Sarrió R, et al. Phase II trial of durvalumab plus tremelimumab with concurrent radiotherapy (RT) in patients (pts) with localized muscle invasive bladder cancer (MIBC) treated with a selective bladder preservation approach: IMMUNOPRESERVE-SOGUG trial. Wolters Kluwer Health. (2021) 39:15. doi: 10.1200/JCO.2021.39.15_suppl.4505
80. Weickhardt AJ, Foroudi F, Lawrentschuk N, Galleta L, Seegum A, Herschtal A, et al. Pembrolizumab with chemoradiotherapy as treatment for muscle invasive bladder cancer: A planned interim analysis of safety and efficacy of the PCR-MIB phase II clinical trial (ANZUP 1502). Am Soc Clin Oncol. (2020) 38:6. doi: 10.1200/JCO.2020.38.6_suppl.485
81. Balar AV, Milowsky MI, O’Donnell PH, Alva AS, Kollmeier M, Rose TL, et al. Pembrolizumab (pembro) in combination with gemcitabine (Gem) and concurrent hypofractionated radiation therapy (RT) as bladder sparing treatment for muscle-invasive urothelial cancer of the bladder (MIBC): A multicenter phase 2 trial. Wolters Kluwer Health. (2021) 39:15. doi: 10.1200/JCO.2021.39.15_suppl.4504
Keywords: bladder cancer, immunotherapy, PD-1/PD-L1 inhibitors, bladder-preserving therapy, neoadjuvant immunotherapy
Citation: Wang Y, He M, Li J and Li L (2025) Immunotherapeutic strategies for invasive bladder cancer: a comprehensive review. Front. Immunol. 16:1591379. doi: 10.3389/fimmu.2025.1591379
Received: 11 March 2025; Accepted: 10 April 2025;
Published: 30 April 2025.
Edited by:
Zhiheng Lin, Shanghai University of Traditional Chinese Medicine, ChinaReviewed by:
Fang Yang, Charité University Medicine Berlin, GermanyCopyright © 2025 Wang, He, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Li, YWJjbGlseTE5ODJAMTYzLmNvbQ==
 Yingying Wang1
Yingying Wang1 Li Li
Li Li