- 1Department of Cardiology, State Key Laboratory of Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Hematology, China-Japan Friendship Hospital, Beijing, China
- 3Department of Rheumatology, Key Laboratory of Myositis, China-Japan Friendship Hospital, Beijing, China
Ruxolitinib is a selective JAK1/2 inhibitor, and its application in treating adult-onset dermatomyositis(DM) has rarely been described. Here, we report a case of an adult-onset DM patient with anti-TIF1γ antibody, who also suffered from essential thrombocytosis related to the JAK2 V617F mutation. Her clinical symptoms of DM markedly improved after ruxolitinib treatment, whereas her platelet count did not significantly change. In addition, we reviewed previous studies on the treatment of adult DM with ruxolitinib, and summarized reports of the JAK2 V617F mutation in adult idiopathic inflammatory myopathy.
Introduction
The Janus kinase(JAK)-signal transducer and activator of transcription (JAK-STAT) pathway plays a pivotal role in physiological functions, such as hematopoiesis and immune balance (1). JAK inhibitors are approved for treating some hematological and autoimmune diseases. For dermatomyositis(DM), increasing evidence suggests that JAK inhibitors can effectively manage interstitial lung disease(ILD) and refractory rashes (2–4). A recent Chinese retrospective study suggests the superiority of tofacitinib over calcineurin inhibitors in patients with anti-MDA5+DM (5). Therefore, JAK inhibitors are increasingly used to treat ILD or cutaneous symptoms despite being off-label in DM.
Ruxolitinib is a selective JAK1/2 inhibitor that has been approved for the treatment of primary and secondary myelofibrosis(MF). In addition, it is administered to treat essential thrombocytosis (ET) in clinical practice, although its efficacy remains controversial in this indication (6, 7). However, few reports focused on the simultaneous treatment of DM and ET with ruxolitinib. Here, we report a case of an adult DM with anti-TIF1γ antibody and ET with the JAK2 V617F mutation. In addition, we reviewed previously published cases of ruxolitinib treatment for adult-onset DM and JAK2 V617F mutation with adult idiopathic inflammatory myopathy(IIM) to broaden our understanding.
Case report
In May 2022, a 34-year-old woman developed heliotrope rash, Gottron’s sign, and V sign. These rashes were itchy. Her muscle strength was normal. DM was suspected by dermatologists. She received prednisone (30mg daily) and hydroxychloroquine (HCQ)(withdrawn in August 2022 due to adverse effects), a combination therapy of methotrexate and thalidomide(discontinued in March 2023 for the second relapse), and Chinese herbal medicine(stopped in December 2023 because of personal reason). This resulted in partial improvement of skin lesions with hyperpigmentation. Her symptoms relapsed twice as the dose of prednisone was decreased to 15mg daily in December 2022 and March 2023, respectively. She had to increase prednisone to 30 mg daily. She gradually reduced the dosage of prednisone and stopped all treatments in December 2023, while her symptoms did not completely alleviate. In February 2024, the patient developed skin lesions (in addition to the aforementioned rashes, erythema also appeared on the cheeks and trunk). Laboratory tests showed normal range of muscle enzyme profiles and elevated platelet count (PLT 690×109/L). Skin biopsy showed liquefaction degeneration of the basal layer and mucin deposition in the dermis. Bone marrow puncture confirmed thrombocythemia. In addition, a JAK2 V617F mutation was detected by quantitative real-time polymerase chain reaction, confirming the diagnosis of ET. The patient did not have family history of related hematological disorders or autoimmune diseases. She started taking aspirin 100mg daily to prevent thrombosis, and did not receive treatment for DM due to personal reason.
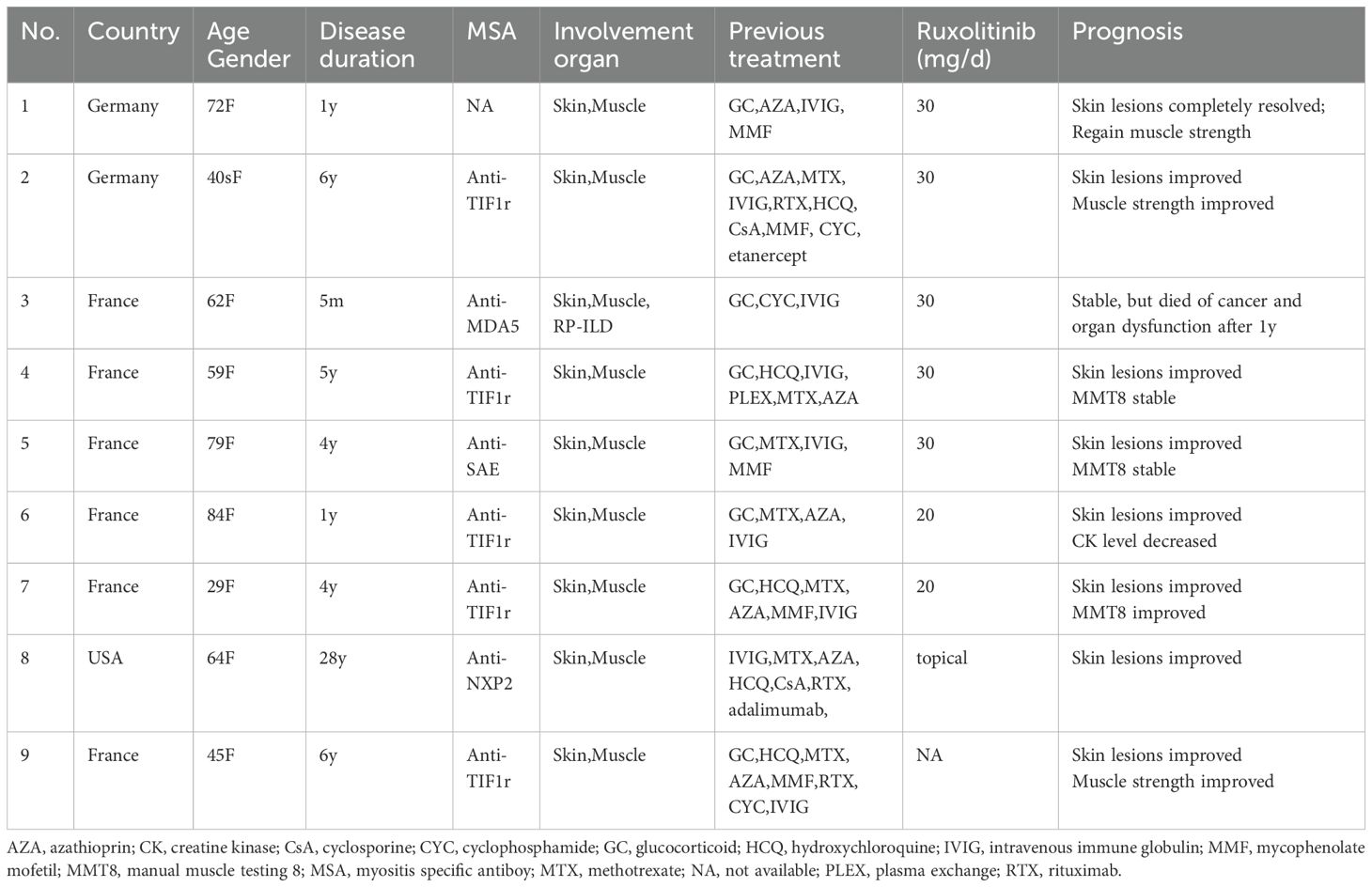
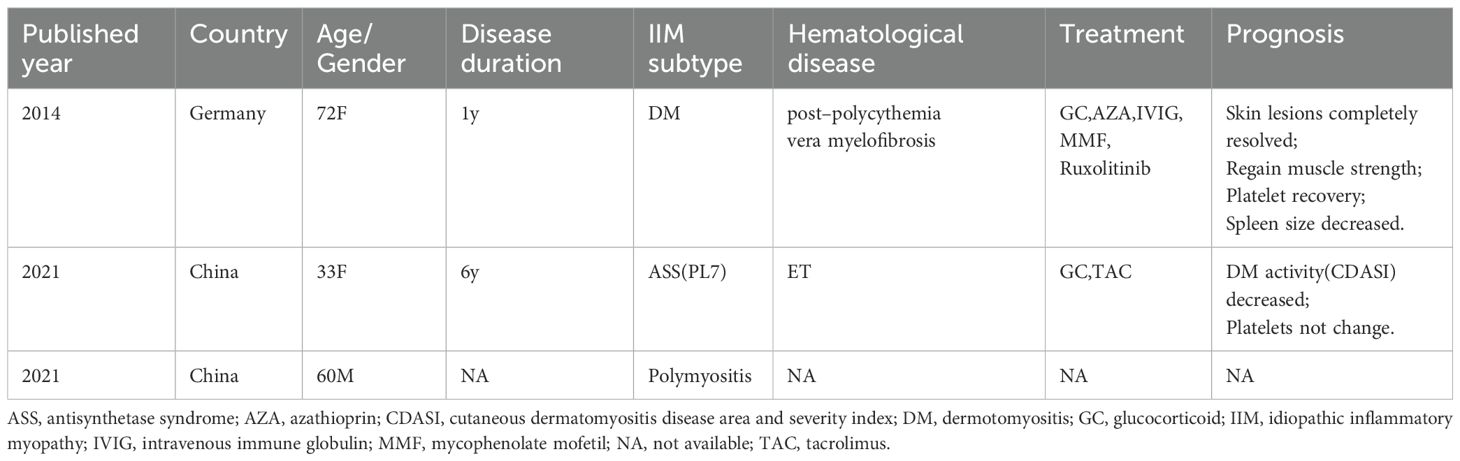
In November 2024, she came to our department due to mild muscle weakness and diffuse rashes, which lasted for four months. On physical examination, she had heliotrope rash, Gottron’s sign, V sign, diffuse erythema in cheeks, arms, and trunk (Figure 1a). She had muscle weakness with an MMT8 score of 76. Myositis-specific autoantibodies(MSA) test(immunoblotting test) showed positive result of anti-TIF1γ antibody. The level of creatine kinase (CK) was normal. Further whole-body muscle MRI showed muscle inflammation (Figure 1b). PET-CT imaging showed normal metabolic activity, and no significant abnormalities were observed during gastroscopy and colonoscopy. Therefore, TIF1γ+DM was diagnosed according to the 2018 ENMC classifications (8). Considering the patient with ET simultaneously, we consulted a hematologist for treatment advice. She took low dose ruxolitinib (5 mg twice a day), prednisone 30mg daily(equivalent to 0.5mg/kg) and aspirin 100mg daily. There was a significant improvement in skin lesions and a reduction in itching within 1 weeks. Her muscle strength gradually returned to normal, and her rash almost disappeared after taking ruxolitinib for 2 months. The prednisone dose was successfully reduced to 10mg daily at the last follow-up in April 2025, but there was no significant change in platelet count (Figures 1c, d).
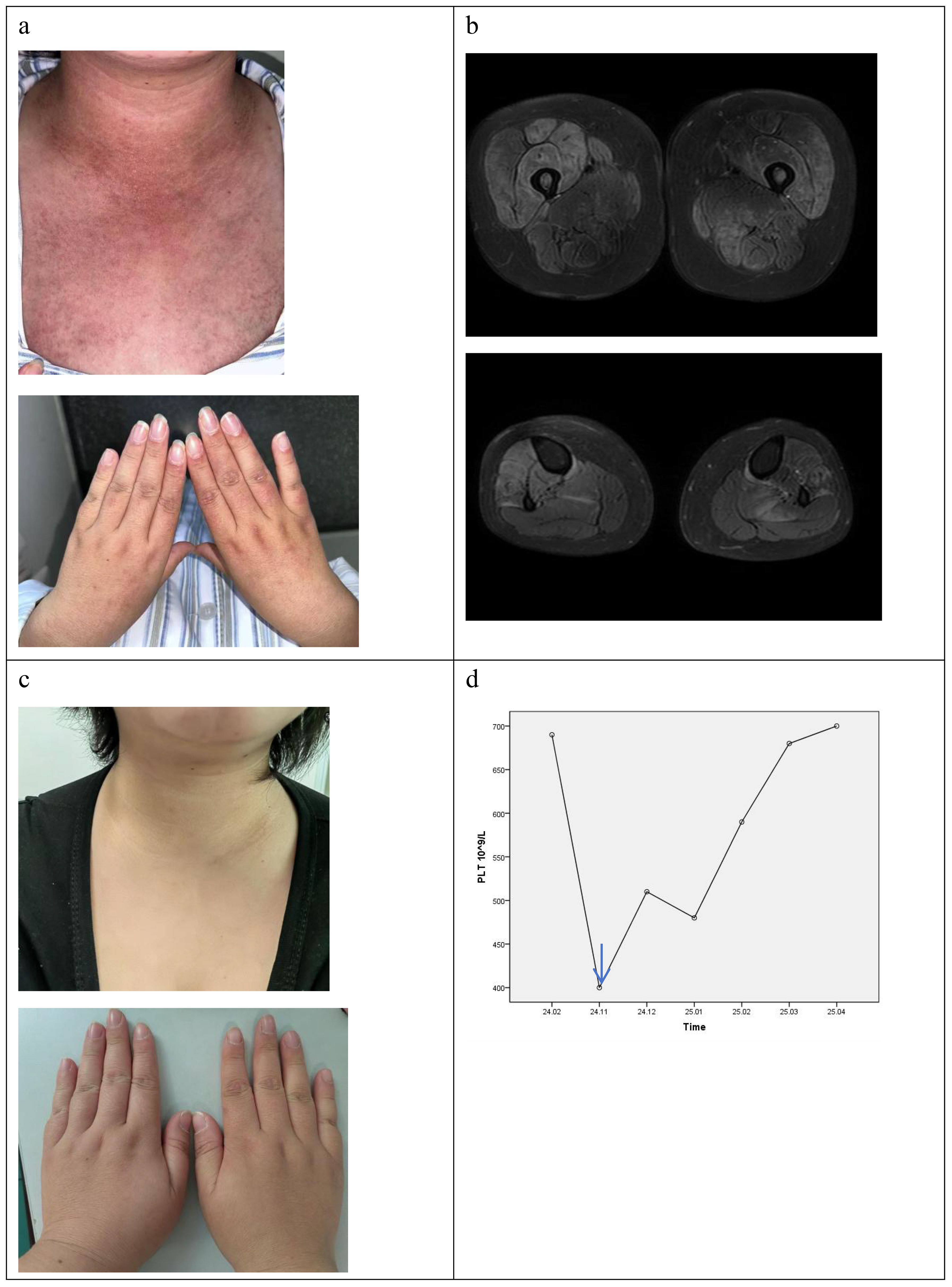
Figure 1. the clinical feature of patient with DM and ET. (a) the rash before ruxolitinib. (b) the T2 MRI of lower limb before ruxolitinib. (c) the rash after ruxolitinib at 5th month. (d) the change of platelet count (blue arrow: ruxolitinib start).
Literature review
We searched PubMed for studies on ruxolitinib in adult-onset DM patients using the keywords “ruxolitinib” and “dermatomyositis” in English from 2010 to 2024. We reviewed literature that clearly diagnosed DM and excluded juvenile DM, and identified 9 adult patients with DM (9–14). All patients were female with ages ranging from 29 to 79 years old. Among them, 5 cases were with anti-TIF1γ antibody, and 3 cases were with anti-NXP2 antibody, anti-MDA5 antibody and anti-SAE antibody, respectively. The earliest published case(no.1) did not specify the type of MSA. One of the patients was topically treated with ruxolitinib, whereas the remaining patients received oral treatment. Except for one case not described, the ruxolitinib dosage was usually between 20 to 30 mg daily. In addition, case no.1 suffered from MF secondary to polycythemia with the JAK2 V617F mutation. All patients’ skin lesions had improved. One patient died of cancer and organ dysfunction. The clinical data are shown in Table 1. In addition, a case of adult antisynthetase syndrome (ASS) with anti-PL12 antibody was treated with ruxolitinib, which improved cutaneous lesions, fever, synovitis, and maintaining stable ILD (15).
Next, we searched PubMed for studies on JAK2 V617F mutation with adult IIM using the keywords “JAK2 V617F” and “idiopathic inflammatory myopathy”(IIM) or “dermatomyositis” or “polymyositis(PM)” or “antisynthetase syndrome(ASS)” or “immune-mediated necrotizing myopathy” or “inclusion body myositis” in English from 2000 to 2024. Literature that clearly diagnosed the above disease and excluded juvenile DM was reviewed. Overall, only 3 cases of IIM combined with JAK2 V617F mutation were reported (9, 16). The first patient was diagnosed with post-polycythemia vera MF 1 year after the onset of DM. The second patient was diagnosed with ET 6 years after the onset of ASS (anti-PL7 positive antibody). The third patient was only described to have PM combined with the JAK2 V617F mutation (Table 2).
Discussion
This case highlights a rare and complex manifestation of DM with anti-TIF1γ antibody and ET with JAK2 V617F mutation. On the one hand, ruxolitinib significantly improved the clinical symptoms of DM, providing new evidence for the efficacy of ruxolitinib in treating DM; on the other hand, the special feature of this case was that the patient had a JAK2 V617F mutation, which may be one of the reasons for the excellent therapeutic effect of ruxolitinib.
JAK inhibitors reduce the production of various cytokines including type I interferons(IFN-I) and suppress inflammation, which is a novel treatment option for DM in recent years. Most studies have focused on the efficacy of tofacitinib, baricitinib, and upadacitinib in the treatment of DM (2–4). Here, we reported that another JAK inhibitor, ruxolitinib, had successfully treated DM. Although it has been studied in juveniles and adults with refractory DM, few studies focused on the latter, with only several case reports. A previous study confirmed that IFN-I reproduced the main DM pathological findings, including muscle atrophy and vasculopathy, and ruxolitinib inhibited the pathogenic effects of IFN-I in both muscle and endothelial cells in vitro (14). This provided evidence for the treatment of DM with ruxolitinib. In general, ruxolitinib will take effect within 3 months and can be sustained over a long-term follow-up (12). In terms of the therapeutic effect, previous literature indicated that ruxolitinib was more effective for refractory rashes than for myositis (14). In the literature review, all 9 patients demonstrated significant improvement in skin lesions after switching to ruxolitinib. However, 7 out of 9 patients showed stable or improved muscle strength, indicating that ruxolitinib may exhibit a good effect on muscle involvement.
Noteworthily, the coexistence of DM and ET with JAK2 V617F mutation indicated the possible complex interaction between hematologic and autoimmune diseases. The JAK2 V617F mutation drives the activation of the JAK-STAT pathway, which is crucial to hematopoiesis and immune regulation (17). According to reports, 50% of patients with ET have JAK2 V617F mutation (18). Case reports described patients harboring this gene mutation and various autoimmune diseases, such as rheumatoid arthritis, Sjögren syndrome and anti-neutrophil cytoplasmic antibody associated vasculitis (16, 19). In our patient, the JAK2 V617F mutation may have resulted in both hematologic and autoimmune manifestations through immune dysregulation and chronic inflammation, although the exact mechanism remains unclear. Therefore, JAK inhibitors may be a valuable option for patients who exhibit this gene mutation.
At present, there are over 10 registered studies on JAK inhibitors in the treatment of DM, among which the results of tofacitinib and baricitinib in DM had shown good effects in improving the disease (20, 21). These results further enhanced the confidence in using JAK inhibitors to treat DM. However, we should be alert to the adverse effects caused by the widespread administration of JAK inhibitors, especially when prescribed off-label in the treatment of DM. Tofacitinib has increased the risk of tumor and cardiovascular event in RA (22, 23). According to the published data in RA, surveillance demonstrated an HR of 1.48 (95% CI 1.04 to 2.09) for tofacitinib versus tumor necrosis factor inhibitors (23). However, the subsequent published meta-analysis showed that JAK inhibitors did not associate with a higher incidence of malignancy compared with placebo in RA or other autoimmune/inflammatory diseases (24). Baricitinib, another JAK1/2 inhibitor, has been more widely administered than ruxolitinib in the treatment of DM. Some adverse effects of baricitinib have been reported, such as cytomegalovirus infection and venous thrombophlebitis (3). Ruxolitinib has been approved for the treatment of some hematological malignancies; however, when administered for treating DM, the issue of adverse effects should also be addressed. For example, patient no.3 in the review section developed cancer in the second year. In addition, as anti-TIF1γ antibody is considered a tumor associated myositis antibody, the administration of ruxolitinib needs to be given extra attention (25).
Conclusion
This patient simultaneously suffered from DM with anti-TIF1γ antibody and ET harboring JAK2 V617F mutation, and ruxolitinib improved the clinical symptoms of DM. The underlying association between DM and JAK2 V617F mutation warrants further attention and investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of China-Japan Friendship Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XJ: Resources, Writing – original draft. WM: Resources, Writing – review & editing. LX: Methodology, Writing – review & editing. LS: Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by National High Level Hospital Clinical Research Funding (2022-NHLHCRF-YS-02) and Beijing Municipal Science and Technology Commission(Z191100006619112).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer KT declared a shared parent affiliation with the author JX to the handling editor at the time of review.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lv Y, Qi J, Babon JJ, Cao L, Fan G, Lang J, Zhang J, et al. The JAK-STAT pathway: from structural biology to cytokine engineering. Signal Transduct Target Ther. (2024) 9:221. doi: 10.1038/s41392-024-01934-w
2. Beckett M, Tan J, Bonnardeaux E, Dutz J, Shojania K, To F, et al. Tofacitinib therapy in refractory inflammatory myositis: a retrospective cohort study of 41 patients. Rheumatol (Oxford). (2024) 63:1432–6. doi: 10.1093/rheumatology/kead404
3. Harada H, Shoda H, Tsuchiya H, Misaki M, Sawada T, and Fujio K. Baricitinib for anti-melanoma differentiation-associated protein 5 antibody-positive dermatomyositis-associated interstitial lung disease: a case series and literature review on Janus kinase inhibitors for the disease. Rheumatol Int. (2024) 44:961–71. doi: 10.1007/s00296-024-05551-2
4. Beckett M, Dutz J, and Huang K. Upadacitinib therapy in refractory inflammatory myositis: a case series of 10 patients. RMD Open. (2024) 10:e003837. doi: 10.1136/rmdopen-2023-003837
5. Wu W, Guo B, Sun W, Chen D, Xu W, Chen Z, et al. Effectiveness and safety of tofacitinib versus calcineurin inhibitor in interstitial lung disease secondary to anti-MDA5-positive dermatomyositis: a multi-center cohort study. Eur Respir J. (2025) 65:2401488. doi: 10.1183/13993003.01488-2024
6. Pieri L, Pancrazzi A, Pacilli A, Rabuzzi C, Rotunno G, Fanelli T, et al. JAK2V617F complete molecular remission in polycythemia vera/essential thrombocythemia patients treated with ruxolitinib. Blood. (2015) 125:3352–3. doi: 10.1182/blood-2015-01-624536
7. Harrison CN, Mead AJ, Panchal A, Fox S, Yap C, Gbandi E, et al. Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide. Blood. (2017) 130:1889–97. doi: 10.1182/blood-2017-05-785790
8. Mammen AL, Allenbach Y, Stenzel W, Benveniste O, and ENMC 239th Workshop Study Group. 239th ENMC international workshop: classification of dermatomyositis, amsterdam, the Netherlands, 14–16 december 2018. Neuromuscul Disord. (2020) 30:70–92. doi: 10.1016/j.nmd.2019.10.005
9. Hornung T, Janzen V, Heidgen FJ, Wolf D, Bieber T, and Wenzel J. Remission of recalcitrant dermatomyositis treated with ruxolitinib. N Engl J Med. (2014) 371:2537–8. doi: 10.1056/NEJMc1412997
10. Fetter T, Rios GC, Niebel D, Bieber T, and Wenzel J. Unexpected hair regrowth in a patient with longstanding alopecia universalis during treatment of recalcitrant dermatomyositis with the janus kinase inhibitor ruxolitinib. Acta Derm Venereol. (2020) 100:adv00144. doi: 10.2340/00015555-3481
11. Jalles C, Deroux A, Tardieu M, Lugosi M, Viel S, Benveniste O, et al. Severe MDA5 dermatomyositis associated with cancer and controlled by JAK inhibitor. Rev Med Interne. (2020) 41:421–4. doi: 10.1016/j.revmed.2020.02.015
12. Landon-Cardinal O, Guillaume-Jugnot P, Toquet S, Sbeih N, Rigolet A, Champtiaux N, et al. JAK inhibitors for the treatment of adult dermatomyositis: A pilot study. J Am Acad Dermatol. (2023) 88:924–6. doi: 10.1016/j.jaad.2022.10.055
13. Lanis A, Kim H, Lu S, Tsai WL, Kaneshiro A, Ehrlich A, et al. Efficacy of topical ruxolitinib for cutaneous dermatomyositis. JAAD Case Rep. (2023) 45:24–6. doi: 10.1016/j.jdcr.2023.09.043
14. Ladislau L, Suárez-Calvet X, Toquet S, Landon-Cardinal O, Amelin D, Depp M, et al. JAK inhibitor improves type I interferon induced damage: proof of concept in dermatomyositis. Brain. (2018) 141:1609–21. doi: 10.1093/brain/awy105
15. Sengupta S, Law B, Sennett R, Jedrych JJ, Albayda J, Kang JK, et al. Ruxolitinib for refractory PL-12 antisynthetase syndrome-associated angioedema-like panniculitis with clonal T-cell receptor gene rearrangement. JAMA Dermatol. (2024) 160:363–6. doi: 10.1001/jamadermatol.2023.4940
16. Xu Q, Jin X, Jiang Y, Dang X, and Han Y. The relationship between JAK2(V617F) mutation and dermatomyositis-a case report and literature review. Clin Rheumatol. (2021) 40:1147–57. doi: 10.1007/s10067-020-05286-y
17. Samra S, Bergerson JRE, Freeman AF, and Turvey SE. JAK-STAT signaling pathway, immunodeficiency, inflammation, immune dysregulation, and inborn errors of immunity. J Allergy Clin Immunol. (2025) 155:357–67. doi: 10.1016/j.jaci.2024.09.020
18. Schieber M, Crispino JD, and Stein B. Myelofibrosis in 2019: moving beyond JAK2 inhibition. Blood Cancer J. (2019) 9:74. doi: 10.1038/s41408-019-0236-2
19. Álvarez-Reguera C, Prieto-Peña D, Herrero-Morant A, Sánchez-Bilbao L, Batlle-López A, Fernández-Luis S, et al. Features of immune mediated diseases in JAK2 (V617F)-positive myeloproliferative neoplasms and the potential therapeutic role of JAK inhibitors. Eur J Intern Med. (2024) 123:102–6. doi: 10.1016/j.ejim.2023.11.019
20. Paik JJ, Casciola-Rosen L, Shin JY, Albayda J, Tiniakou E, Leung DG, et al. Study of tofacitinib in refractory dermatomyosits: an open-label pilot study of ten patients. Arthritis Rheumatol. (2021) 73:858–65. doi: 10.1002/art.41602
21. Kim H, Dill S, O'Brien M, Vian L, Li X, Manukyan M, et al. Janus kinase (JAK) inhibition with baricitinib in refractory juvenile dermatomyositis. Ann Rheum Dis. (2021) 80:406–8. doi: 10.1136/annrheumdis-2020-218690
22. Charles-Schoeman C, Fleischmann R, Mysler E, Greenwald M, Ytterberg SR, Koch GG, et al. Risk of venous thromboembolism with tofacitinib versus tumor necrosis factor inhibitors in cardiovascular risk-enriched rheumatoid arthritis patients. Arthritis Rheumatol. (2024) 76:1218–29. doi: 10.1002/art.42846
23. Curtis JR, Yamaoka K, Chen YH, Bhatt DL, Gunay LM, Sugiyama N, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open-label, randomized controlled ORAL Surveillance trial. Ann Rheum Dis. (2023) 82:331–43. doi: 10.1136/ard-2022-222543
24. Russell MD, Stovin C, Alveyn E, Adeyemi O, Chan CKD, Patel V, et al. JAK inhibitors and the risk of Malignancy: a meta-analysis across disease indications. Ann Rheum Dis. (2023) 82:1059–67. doi: 10.1136/ard-2023-224049
25. Oldroyd AGS, Callen JP, Chinoy H, Chung L, Fiorentino D, Gordon P, et al. International guideline for idiopathic inflammatory myopathy-associated cancer screening: an international myositis assessment and clinical studies group (IMACS) initiative. Nat Rev Rheumatol. (2023) 19:805–17. doi: 10.1038/s41584-023-01045-w
Keywords: ruxolitinib, dermatomyositis, essential thrombocytosis, JAK2 V617F mutation, anti-TIF1γ antibody
Citation: Jing X, Min W, Xia L and Shanshan L (2025) Ruxolitinib in adult dermatomyositis with anti-TIF1γ antibody: a case report and literature review. Front. Immunol. 16:1591631. doi: 10.3389/fimmu.2025.1591631
Received: 11 March 2025; Accepted: 23 May 2025;
Published: 12 June 2025.
Edited by:
Dennis Niebel, University Medical Center Regensburg, GermanyReviewed by:
Keyun Tang, Peking Union Medical College Hospital (CAMS), ChinaLaurent Sailler, INSERM CIC1436 Centre d’Investigation Clinique de Toulouse, France
Copyright © 2025 Jing, Min, Xia and Shanshan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Shanshan, bGlzaGFuc2hhbmJqbXVAMTYzLmNvbQ==
 Xu Jing
Xu Jing Wang Min2
Wang Min2 Li Shanshan
Li Shanshan