- 1School of Public Health, Xinjiang Medical University, Urumqi, China
- 2Department of Maternal and Child Health, School of Public Health, Peking University, Beijing, China
- 3Department of Urology, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, China
- 4Xinjiang Clinical Medical Research Center for Urological and Male Reproductive System Diseases, Urumqi, China
Background: Prostate cancer (PCa) is an important cause of fatality in older men, with inflammation and metabolic disorders as risk factors for PCa. This study examined how systemic inflammation, measured by inflammatory indices, interacts with the cardiometabolic index (CMI), a marker of obesity and dyslipidemia, to influence the risk of developing PCa.
Methods: This study consisted of 1,591 male patients recruited from the Department of Urology at the First Affiliated Hospital of Xinjiang Medical University between 2022 and 2024. Propensity score matching was employed to adjust the sample size, resulting in a final cohort of 149 PCa patients and 296 matched controls. Logistic regression models and restricted cubic spline (RCS) analyses were employed to evaluate the associations between CMI and various inflammatory indices (e.g., PIV, SIRI, PLR, NLR, LMR) with prostate cancer. Interaction tests were conducted to investigate the impact of the interplay between inflammatory indices and CMI on the risk of prostate cancer.
Results: NLR, PLR, PIV, and SIRI were significantly positively associated with prostate cancer (PCa) risk, whereas LMR exhibited a significant negative association. The CMI was significantly associated with an elevated risk of prostate cancer (PCa) (OR = 1.97, 95% CI: 1.38~2.81). Restricted cubic spline (RCS) analysis revealed a nonlinear dose-response relationship between CMI and prostate cancer (PCa) risk, with the risk plateauing at CMI ≈ 0.65. Sensitivity analyses confirmed the robustness of these results. Significant interactions were observed between CMI and inflammatory indices, particularly NLR, PLR, and LMR, suggesting synergistic effects on prostate cancer (PCa) risk.
Conclusions: The present study demonstrated that inflammation indicators and CMI exhibited a strong association with the risk of PCa. Furthermore, a significant interaction was observed between CMI and inflammation indicators. These findings provide a novel perspective for PCa risk prediction and prevention, suggesting that inflammation and metabolic status should be considered together when assessing PCa risk.
1 Introduction
Prostate cancer (PCa) is one of the most common cancers worldwide and the fifth leading cause of cancer deaths in men (1). According to Global Cancer Statistics 2022, PCa accounts for 7.3% of all cancer cases, with over 1.46 million new cases and more than 390,000 deaths reported (1). Indeed, PCa is commonly diagnosed in men aged 50 years and older (2). With the aging of the global population and shifts in lifestyle patterns (3), the incidence of PCa is projected to rise annually, thereby posing a significant threat to the health of elderly men (4).
The etiology of PCa is complex (2). Some studies have shown that inflammation is a key factor that influences the progression of PCa (5). To illustrate, inflammation impacts every step of tumorigenesis, such as tumor initiation, promotion, and metastatic progression (6). The inflammatory tumor microenvironment drives PCa development. As inflammation progresses, tumor cells recruit additional leukocytes, promoting angiogenesis, proliferation, vascular and tissue growth, and remodeling, ultimately leading to the development of PCa (5). In the tumor microenvironment, the number of immuno-inflammatory cells such as lymphocytes, monocytes, neutrophils, and platelets is significantly altered, and these changes may reflect the extent of cancer progression (7–10), which can help in the diagnosis and prognostic assessment of the tumor (11, 12). Combined inflammatory indices provide a holistic assessment of systemic inflammation compared to individual markers (e.g., neutrophil count, lymphocyte count, etc.), significantly enhancing disease prediction (13, 14). For instance, indices such as the pan-immune-inflammation value (PIV), neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), systemic inflammation response index (SIRI), and lymphocyte-monocyte ratio (LMR) have demonstrated prognostic value across multiple cancers (15–17), and they have predictive value for the prognosis of PCa (18–23).
Numerous pro-inflammatory and anti-inflammatory molecules are recognized as secretory products of human adipocytes or are associated with adipose tissue (24). Wakabayashi et al. (2015) developed a novel marker known as the cardiometabolic index (CMI), which integrates key metabolic factors such as waist circumference, triglycerides, and high-density lipoprotein cholesterol (HDL-C). By combining lipid and obesity parameters into a simple and reproducible marker, CMI effectively reflects obesity and dyslipidemia (25). Subsequent research has validated CMI not only as a robust marker of obesity and metabolic syndrome but also as a superior diagnostic tool for visceral adiposity compared to conventional abdominal obesity indices (e.g., waist circumference, waist-to-hip ratio), demonstrating enhanced sensitivity in identifying early metabolic dysregulation (26, 27). While obesity is thought to be a chronic low-grade inflammatory state that is linked to PCa (28), CMI remains a relatively new area of research in the cancer field with many studies still in the exploratory stage. The association between CMI and prostate cancer risk has not yet been reported in the literature. Furthermore, existing studies have primarily focused on the correlation between inflammation and the cardiometabolic index (CMI), as well as the mediating role of inflammation in the relationship between CMI and chronic diseases among older adults (29–31). The exploration of the association between inflammation and CMI remains limited, and the impact of their interaction on prostate cancer risk has yet to be reported.
The present study investigates the effect of the interaction between the inflammatory index and CMI on the risk of PCa. This research aims to fill the gap in the field, enrich our understanding of the mechanisms underlying PCa development, and provide a foundation for more accurate risk prediction, screening, intervention, prevention, and treatment of PCa in the future.
2 Methods
2.1 Data source
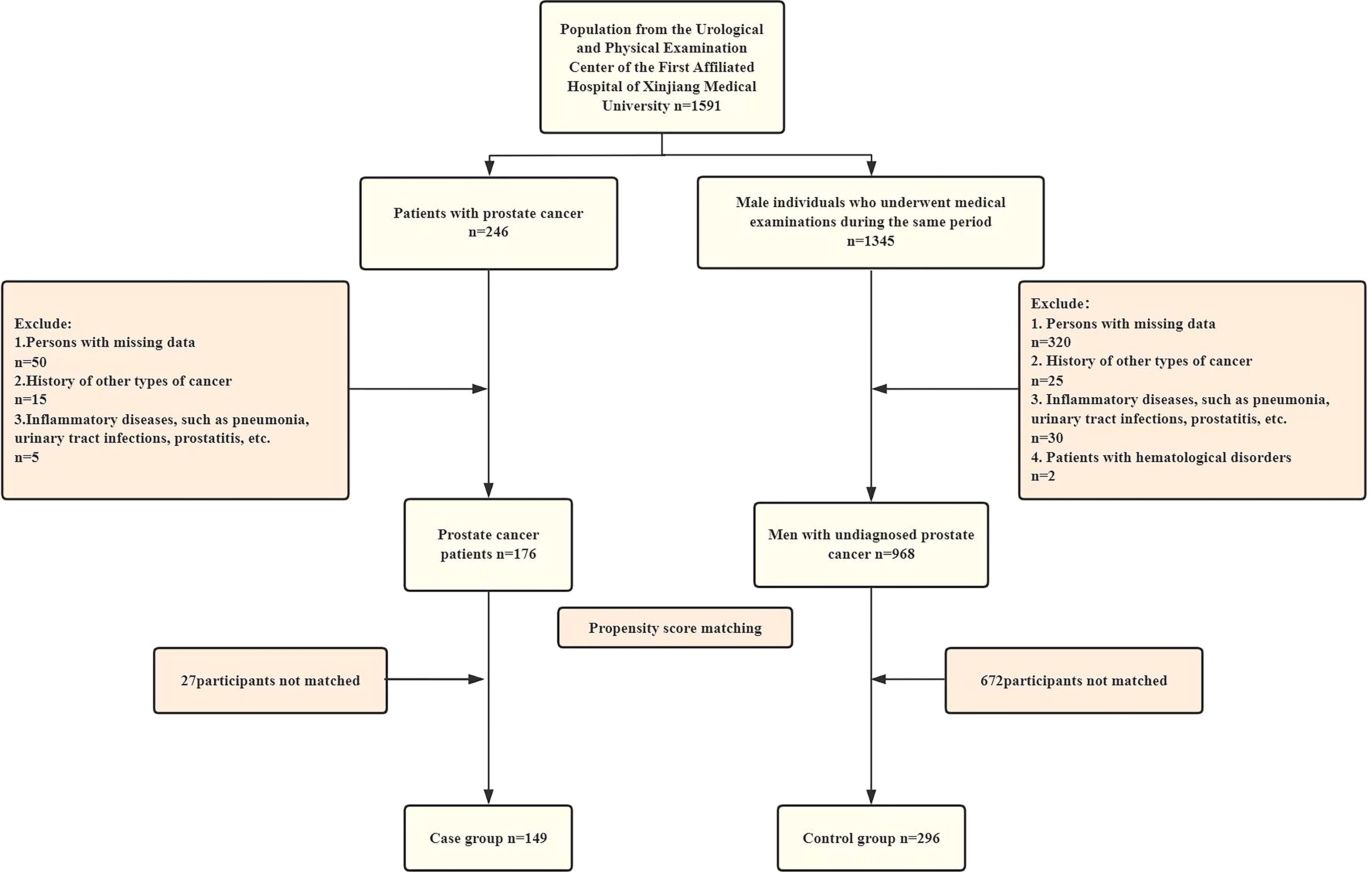
This study collected clinical data from 1591 male patients who visited the Department of Urology at the First Affiliated Hospital of Xinjiang Medical University between 2022 and 2024. After applying the exclusion criteria, the sample included 246 patients with PCa diagnosed through prostate biopsy and puncture, along with 1,345 individuals who underwent examinations at a medical examination center. Propensity score matching (PSM) was employed to mitigate the impact of confounding variables. Nearest neighbor matching was performed with a 1:2 case-to-control ratio. Post-matching analysis revealed standardized mean differences (SMD) <0.1 for all covariates, demonstrating effective control of intergroup differences. The final matched cohort comprised 149 cases and 296 controls (Figure 1).
Inclusion criteria for case group: (1) patients first diagnosed with PCa by prostate biopsy from 2022 to 2024; (2) complete test data; (3) can read, understand and sign the informed consent form.
Exclusion criteria for case group: (1) PCa patients with a history of other types of cancer;(2) patients with inflammatory diseases, such as pneumonia, urinary tract infections, or prostatitis.
Inclusion criteria for control group:(1) male individuals who underwent physical examinations at the Physical Examination Center of the First Affiliated Hospital of Xinjiang Medical University during the same period; (2) complete physical examination data; (3) can read, understand and sign the informed consent form.
Exclusion criteria for control group: (1) participants with any type of cancer or history of cancer;(2) patients with inflammatory diseases, such as pneumonia, urinary tract infections, or prostatitis 11.
The protocol was approved by the Ethics Committee of the First Hospital of Xinjiang Medical University (Approval No. 20220 308-166), and all study participants signed an informed consent form with a clear understanding of the purpose of the study protocol.
2.2 Data collection and measurements
Information regarding Participants’ general information (age), anthropometric measurements (including height, weight, and waist circumference), blood pressure, and blood samples were collected during standardized physical examinations. Participants were instructed to wear light clothing and remove their shoes for height and weight measurements. Waist circumference was measured by trained surveyors using a soft tape measure at the midpoint between the lower edge of the rib cage and the iliac crest. After a 10-minute rest period, blood pressure was measured using an upper-arm electronic sphygmomanometer. Following a 10–12 hour fasting period, blood samples were collected in the early morning via venipuncture from the antecubital vein. Fasting blood glucose(FBG), triglycerides(TG), HDL cholesterol(HDL-C), LDL cholesterol(LDL-C), serum creatinine(SCr), serum uric acid(SUA), monocyte count, neutrophil count, lymphocyte count, and platelet count were measured from the collected blood samples. All blood samples were analyzed enzymatically by trained laboratory technicians using an automated analyzer. Fasting blood glucose, triglycerides, and HDL cholesterol were measured using hexokinase, enzymatic, and clearance methods, respectively. Diabetes mellitus was defined as a fasting blood glucose level ≥126 mg/dL or current use of antidiabetic medication (32). Hypertension was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg, or current use of antihypertensive medication (33, 34). Information on comorbidities, including coronary heart disease, was obtained from participants’ medical history records.
2.3 Calculation of the inflammation index
NLR, PLR, PIV, SIRI, and LMR were calculated using the neutrophil count (NEU), lymphocyte count (LYM), monocyte count (MON), and platelet count (PLT) obtained from the patient’s routine blood tests at the initial visit. According to the following formulas:
2.4 Calculation of the CMI
The cardiometabolic index (CMI) was calculated as:
Where WHtR (waist-to-height ratio) is Waist circumference (cm) /Height (cm).
2.5 Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) for normally distributed data or median with interquartile range (IQR; P25, P75) for skewed distributions. Group comparisons for continuous variables were performed using Student’s t-test (parametric) or Mann-Whitney U test (non-parametric) based on normality assessed via the Shapiro-Wilk test. Categorical variables were analyzed using Pearson’s χ² or Fisher’s exact test. To investigate the association between inflammatory indices, CMI index, and prostate cancer risk, we constructed multivariate logistic regression models with adjustments for potential confounders. The stability of these associations was verified through sensitivity analyses employing alternative variable categorization strategies and the exclusion of potential outliers. Nonlinear relationships were explored using generalized additive models (GAM) with cubic spline smoothing functions (3 degrees of freedom). Interaction effects between inflammatory indices were formally tested through GAM-based interaction models. Three-dimensional surface plots were generated to visualize interaction effects, where elevated Z-axis values (log-odds ratios) indicated stronger synergistic effects on prostate cancer risk. All statistical analyses were performed using R software (version 4.2.1; R Foundation for Statistical Computing) with two-tailed α level set at 0.05 for statistical significance. Smoothing functions in GAM were implemented using the mgcv package, and interaction plots were created using the plotly visualization toolkit.
3 Results
3.1 Baseline characteristics
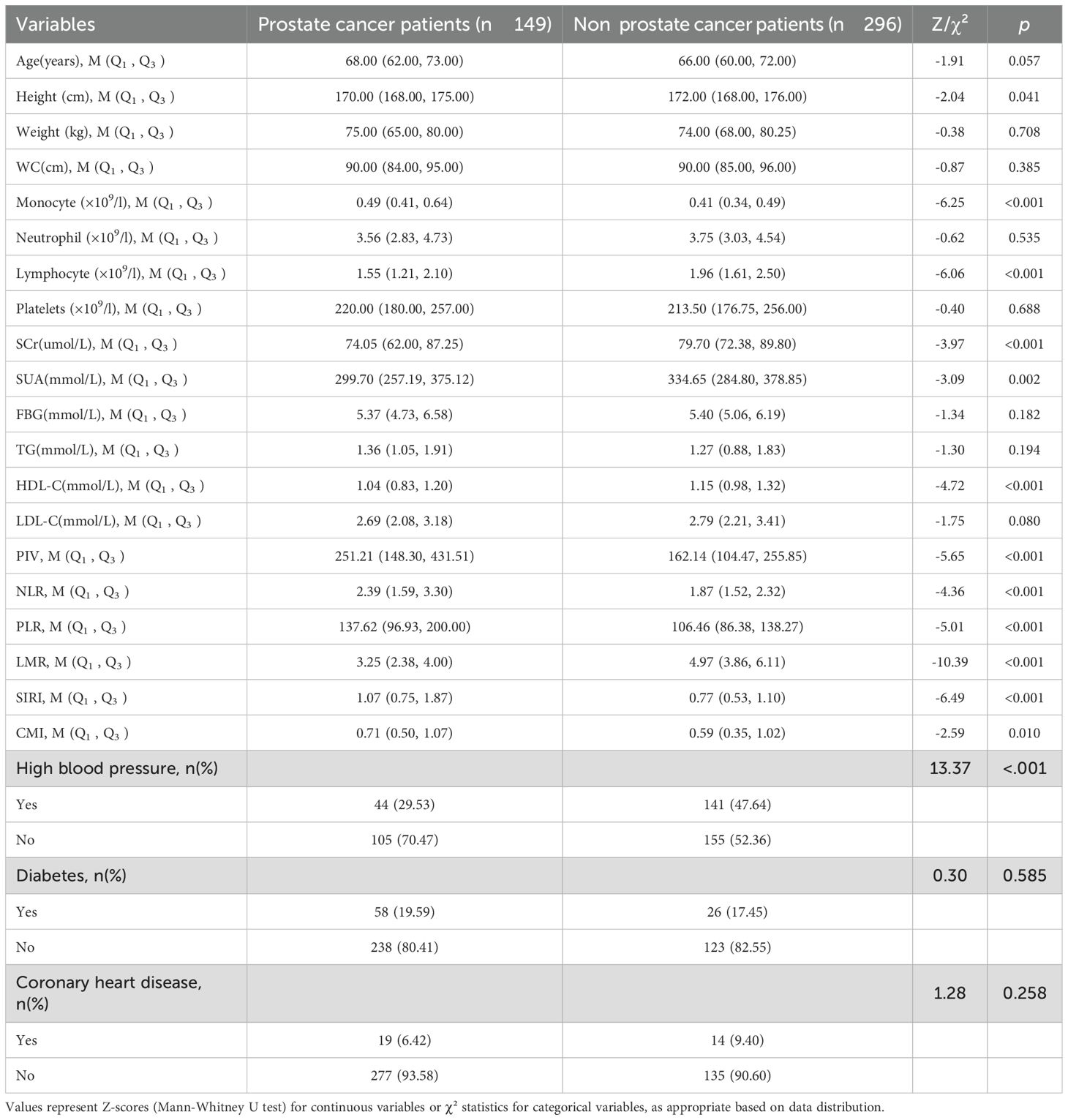
A total of 445 study subjects were included by propensity score matching (PSM), of which 149 prostate cancer patients were in the case group and 296 in the control group. Height, monocyte count, lymphocyte count, HDL cholesterol, NLR, PLR, PIV, SIRI, LMR and CMI were compared between the two groups and the differences were statistically significant (p < 0.05). See Table 1.
3.2 Association between the inflammation index and CMI with PCa risk
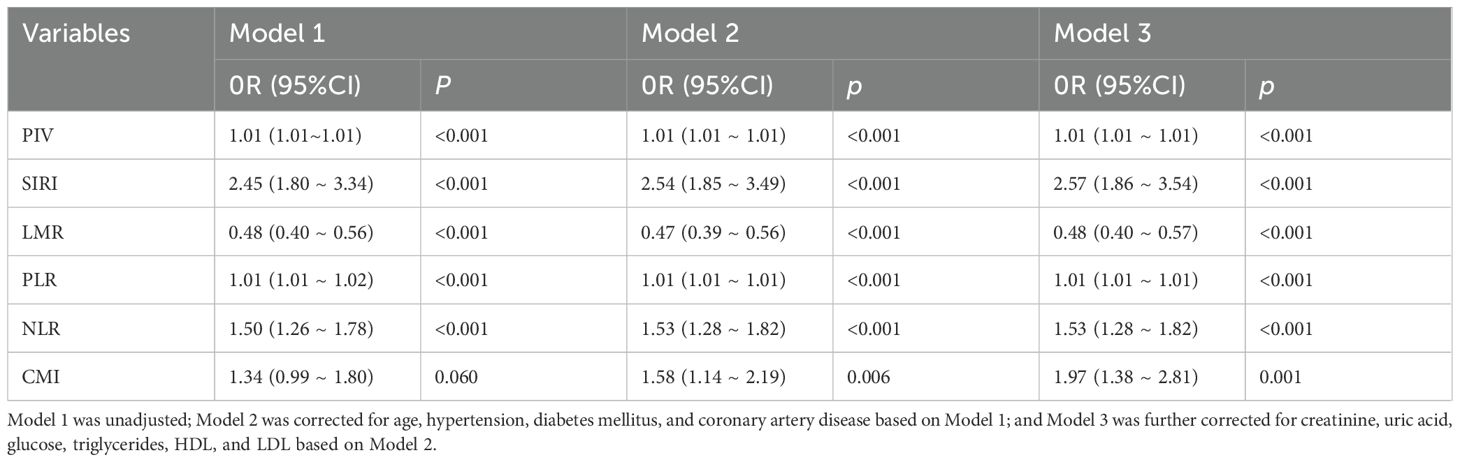
The differences between CMI and inflammation indices (PIV, SIRI, LMR, PLR, NLR) were statistically significant in all three models (p < 0.001). In models 1 and 2, PIV, SIRI, PLR, and NLR were positively associated with PCa, and LMR was negatively associated with PCa. After fully adjusting for covariates (model 3), the pattern remained the same (PIV: OR=1.01, 95%CI:[1.01 ~ 1.01]; SIRI: OR=2.57, 95%CI:[1.86 ~ 3.54]; PLR: OR=1.01, 95%CI:[1.86 ~ 3.54]; PLR: OR=1.01, 95%CI. 95%CI:[1.01 ~ 1.01];NLR: OR=1.53,95%CI:[1.28 ~ 1.82];LMR: OR=0.48,95%CI:[0.40 ~ 0.57]). In model 1 without adjustment, the correlation between CMI and PCa was not statistically significant (p > 0.05); however, after adjusting for the covariates (model 2, model 3), CMI was significantly associated with PCa (p < 0.05). In model 3, for every 1-unit standard deviation increase in CMI, there was a 97% increase in the risk of PCa (OR=1.97, 95%CI:[1.38~ 2.81]. See Table 2.
3.3 RCS curve assesses inflammatory index and CMI about PCa risk
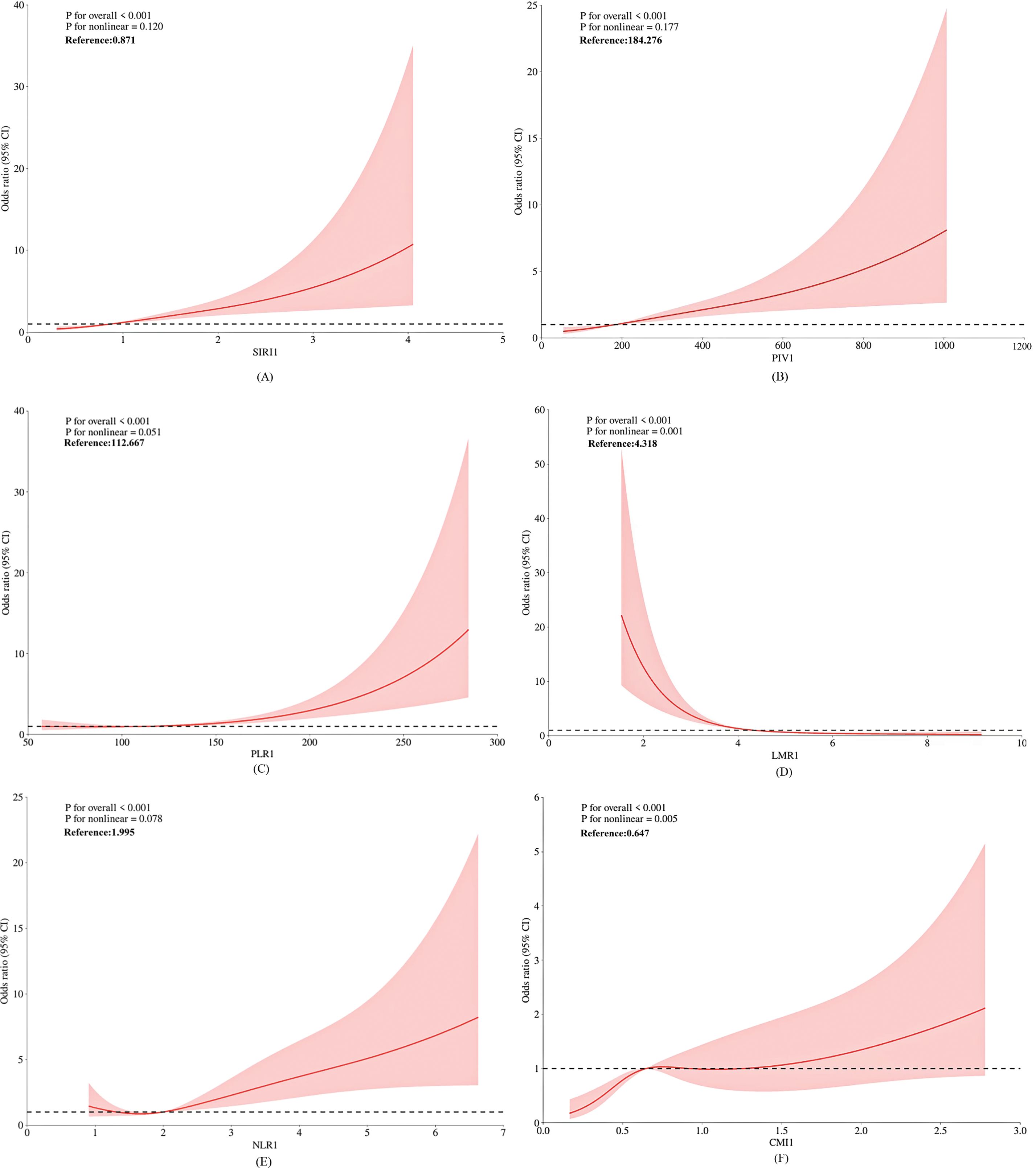
The restricted cubic spline (RCS) model was constructed to evaluate the relationship between the inflammatory index, the cardiometabolic index (CMI), and the risk of PCa (Figure 2). As demonstrated in Figures 2A-E, sIRI () and PIV() exhibited a linear dose-response relationship with PCa risk (p for overall association < 0.001; p for nonlinearity > 0.05). PLR(), LMR(), NLR(), and CMI() demonstrated a nonlinear dose-response relationship with PCa risk (p for overall association < 0.001; p for nonlinearity < 0.05). As shown in Figure 2F, there is an initial sharp increase in prostate cancer risk in association with increasing CMI, and this increase attenuates once CMI is around 0.65.
3.4 Sensitivity analyses
Sensitivity analyses were performed to evaluate the robustness of the results. After excluding individuals with coronary heart disease, hypertension, and diabetes, the results consistently demonstrated significant correlations between PIV, SIRI, LMR, PLR, NLR, CMI, and prostate cancer. See Tables 3–5.
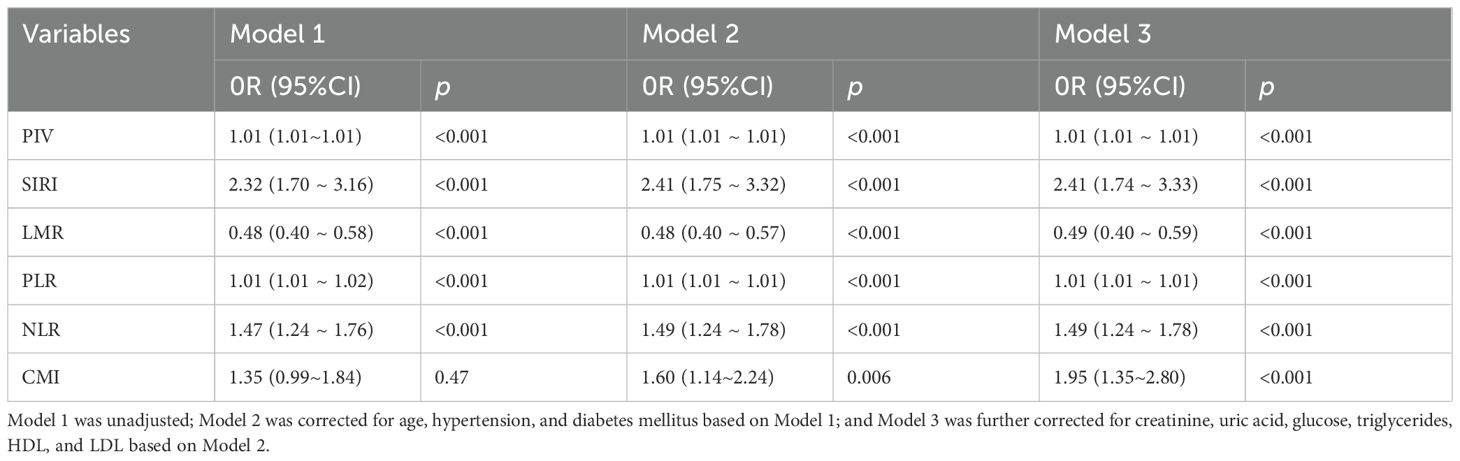
Table 3. Association between inflammatory indices and CMI with PCa risk after excluding coronary heart disease patients.
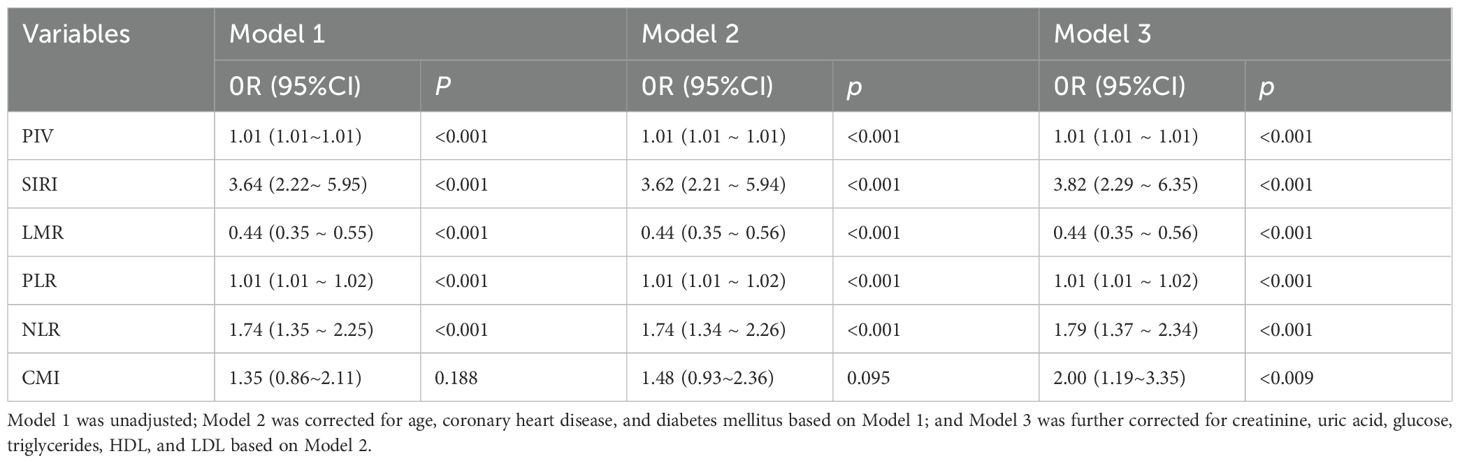
Table 4. Association between inflammatory indices and CMI with PCa risk after excluding hypertensive patients.
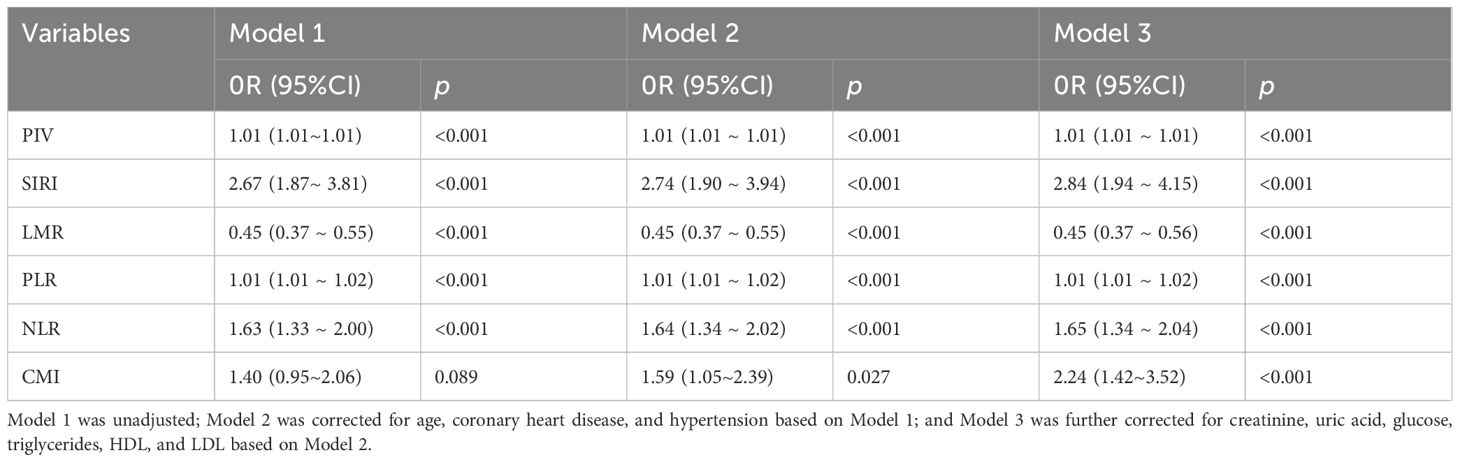
Table 5. Association between inflammatory indices and CMI with PCa risk after excluding diabetic patients.
3.5 Interaction of CMI with inflammation index
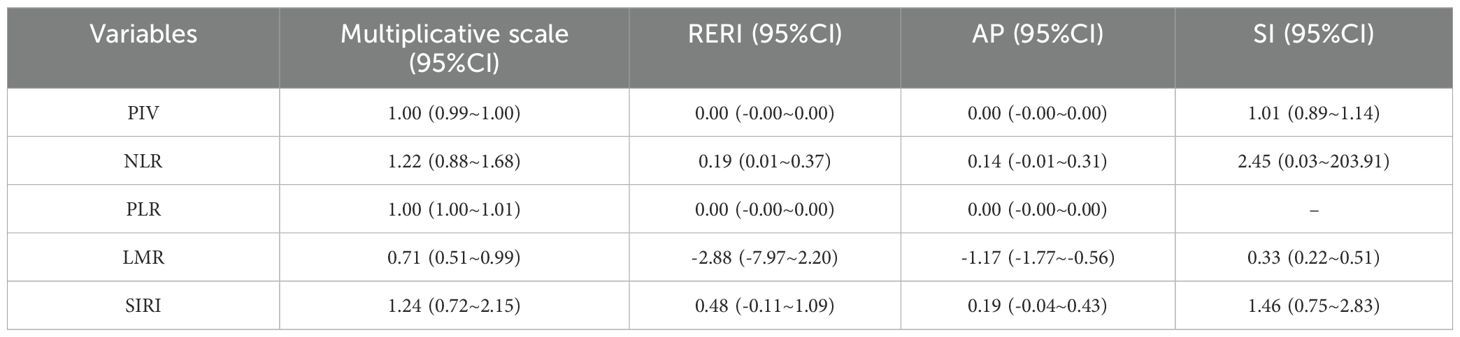
Concerning prostate cancer risk, the NLR exhibited a significant additive interaction with the CMI (RERI = 0.19, 95% CI: 0.01 to 0.37), indicating a potential synergistic effect. Nonetheless, further research is warranted to validate this additive interaction, given the non-significant results observed in the AP versus SI analysis (AP=0.14, 95%CI=-0.01~0.31; SI=2.45, 95%CI=0.03~203.91). See Table 6.
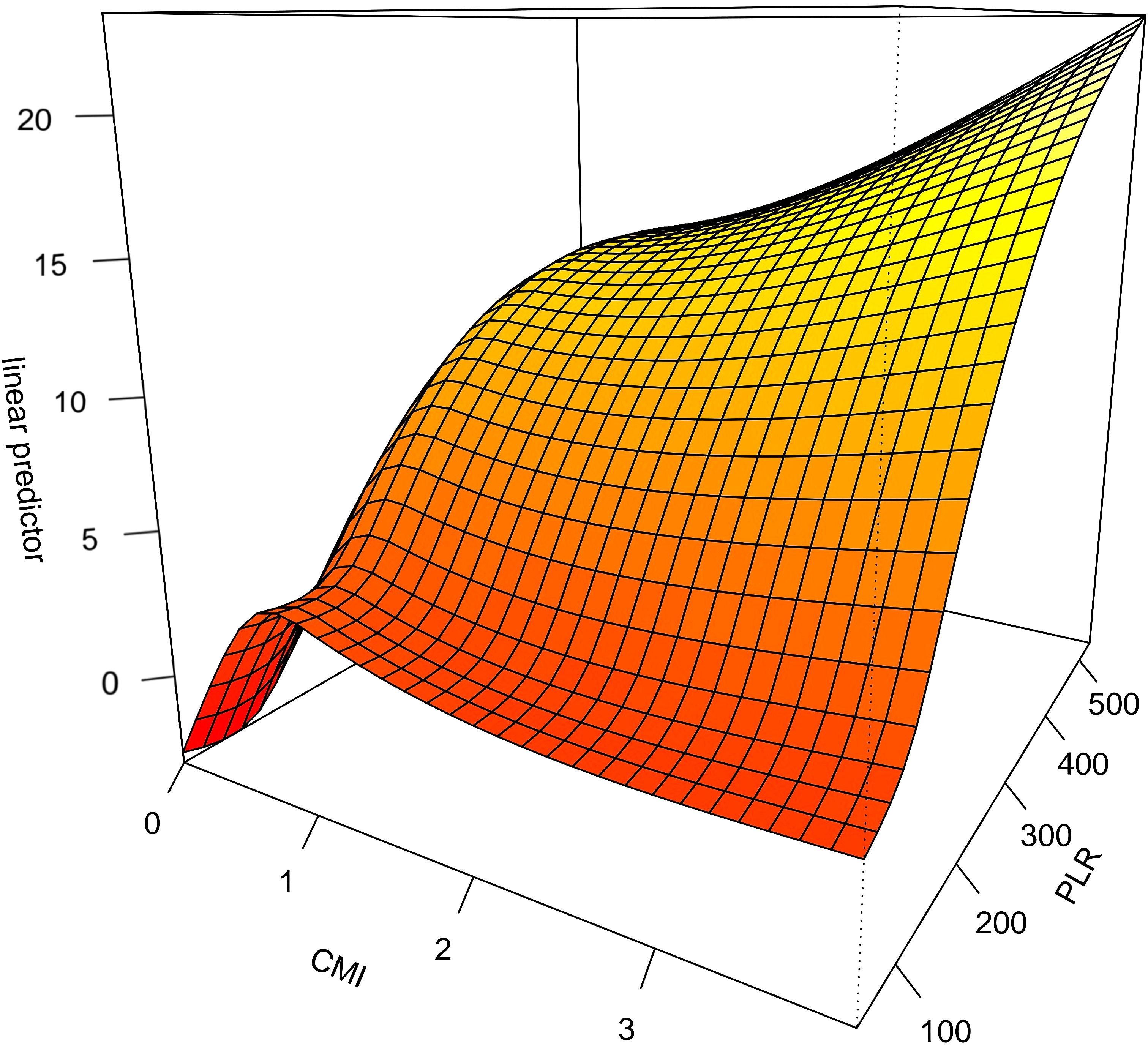
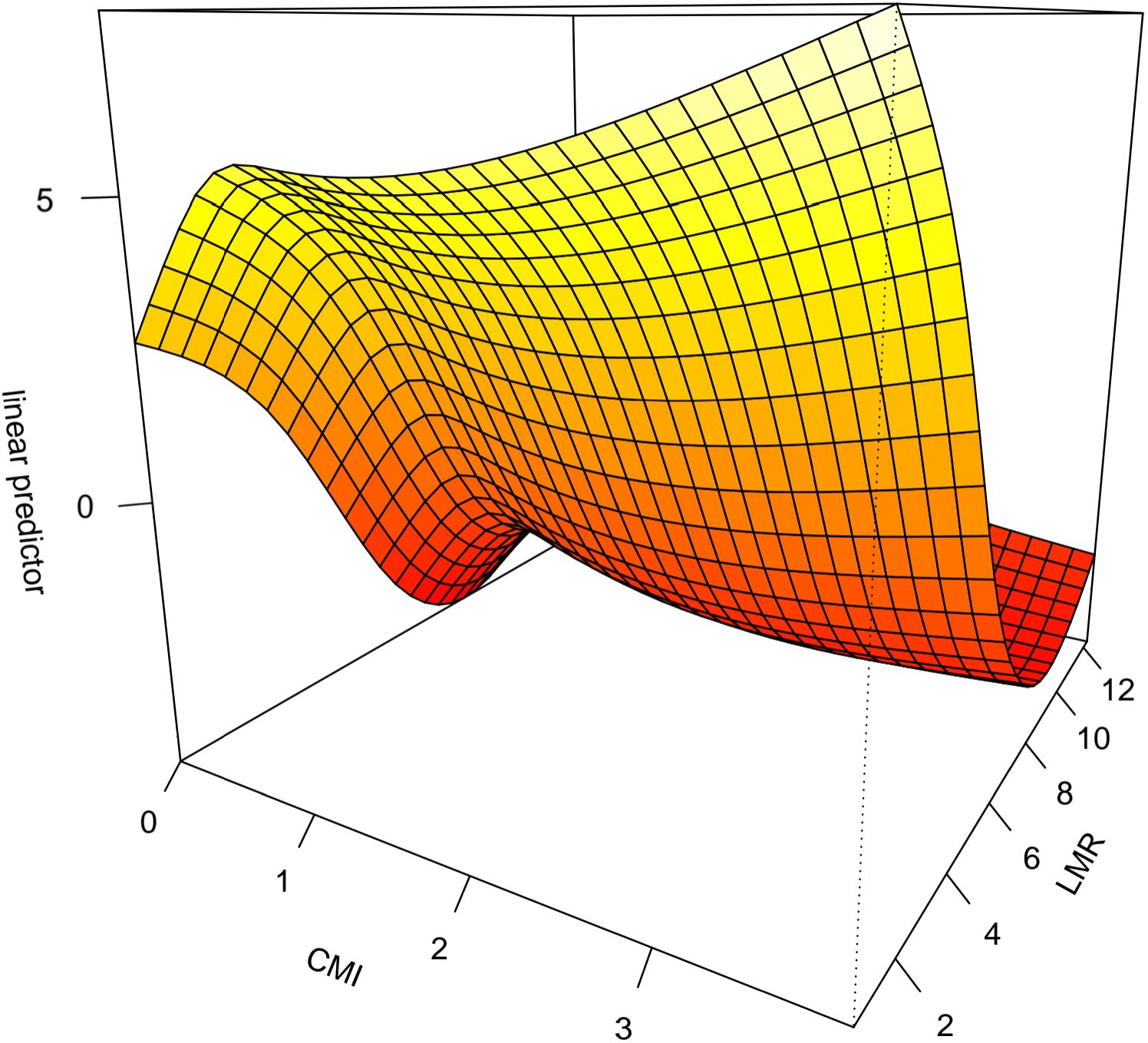
The interaction between CMI and different inflammatory factors in prostate cancer was assessed by constructing an interaction test and drawing a three-dimensional interaction model. There was a multiplicative interaction between PLR and CMI for prostate cancer (Multiplicative scale>1, p < 0.05).CMI > 3 and PLR > 400 were associated with the highest ratio (OR) for prostate cancer; the ratio (OR) for prostate cancer progressively decreased when CMI < 3 and PLR < 400. See Figure 3. There was a negative multiplicative interaction between LMR and CMI for prostate cancer (Multiplicative scale<1, p < 0.05). The highest ratio (OR) for prostate cancer was observed when CMI >3 and LMR <2. See Figure 4.
4 Discussion
Inflammation and obesity are two important risk factors for PCa (2)., Indeed, recent studies have demonstrated a significant association between inflammation and the cardiometabolic index (CMI) (29, 31). Furthermore, the cardiometabolic index (CMI) is a valuable marker for assessing obesity and lipid metabolism disorders (25–27) and has been strongly associated with various obesity-related diseases (35), as confirmed by multiple studies. In terms of obesity, adipose tissue, particularly visceral fat, serves as a significant source of chronic low-grade inflammation in vivo. This inflammation can promote PCa progression through mechanisms such as shaping the tumor inflammatory microenvironment and modulating lipid metabolism (28, 36). This suggests a possible complex link between inflammation, CMI, and PCa.
A chronic inflammatory state is strongly associated with the development of PCa (5, 37, 38). In this study, peripheral blood inflammatory indices were utilized to assess the systemic inflammatory status. The results demonstrated that NLR, PLR, SIRI, and PIV were significantly positively correlated with prostate cancer (PCa), whereas LMR exhibited a significant negative correlation. This finding is consistent with previous studies (20, 21, 39) and further confirms the correlation between inflammation and PCa. The mechanism by which inflammation leads to cancer development may be the inflammatory microenvironment established by inflammatory cells as well as various cytokines and chemokines (37). Cancer cells recruit and activate large numbers of leukocytes and other types of immune cells to infiltrate into the developing tumor site to induce inflammation (40). These immune cells facilitate tumor angiogenesis, invasion, metastasis, and proliferation (6). In the tumor microenvironment, the monocyte lineage promotes PCa cell progression by upregulating monocyte chemotactic protein-1 (MCP-1) expression and activating the nuclear factor-κB (NF-κB) signaling pathway (7). Neutrophils, on the other hand, accelerate tumor growth by releasing inflammatory cytokines, constructing an immunosuppressive microenvironment, and producing reactive oxygen species (ROS) to induce DNA damage (8). Furthermore, lymphopenia impairs anti-tumor immune surveillance, which leads to tumor immune escape and ultimately promotes tumor progression (9). Platelets and their metabolites promote tumor metastasis by influencing the coagulation cascade, activating oncogenic mutations, maintaining proliferative signals, and inducing angiogenesis (e.g., through the release of vascular endothelial growth factor) (10).
Numerous studies have demonstrated that obesity and lipid metabolism are strongly associated with tumorigenesis and metastasis (41). In this study, CMI was significantly associated with PCa risk. The risk of PCa increased rapidly with higher CMI levels. However, this trend plateaued when CMI reached approximately 0.65(see Figure 2F). This suggests that interventions targeting CMI may have greater clinical utility when CMI values are below 0.65.Elevated triglyceride levels and lowered high-density lipoprotein cholesterol (HDL-C) levels can lead to increased CMI, reflecting abnormal fatty acid metabolism (41). PCa cells may exploit these abnormal fatty acid metabolic pathways to generate energy and facilitate cell membrane synthesis, thereby promoting tumor growth and metastasis (36). Indeed, numerous studies have demonstrated an association between hypertriglyceridemia and the severity of PCa (42). Additionally, hypertriglyceridemia may reduce sex hormone-binding globulin (SHBG) levels, leading to increased free estrogen concentrations and further promoting PCa progression (43). Furthermore, reduced HDL levels and elevated LDL levels are associated with a poor prognosis in PCa patients (44), as the protective effects of HDL are diminished, while the tumor-promoting effects of LDL are enhanced (41).
The association between CMI and inflammation has been increasingly supported by numerous studies (29, 31). In our study, we found that the interaction between inflammatory indices and CMI affected the risk of PCa. The interaction between NLR, PLR, LMR, and CMI may synergistically promote tumor development by exacerbating inflammation, promoting angiogenesis, and suppressing anti-tumor immunity, respectively (45–49). Numerous studies have shown that inflammation is a key driver of obesity-related cardiometabolic diseases (50, 51). In obesity, the body typically exhibits chronic low-grade inflammation, which not only impairs vascular endothelial function and contributes to organ dysfunction but also triggers adipose tissue inflammation. This further exacerbates peripheral insulin resistance and amplifies the systemic inflammatory response via the release of cytokines and adipokines (51, 52). As a comprehensive indicator of cardiometabolic disease risk, CMI reflects visceral adiposity and lipid levels, particularly indicating central obesity (25). Central obesity can decrease plasma lipocalin, a hormone that inhibits angiogenesis and inflammation, by elevating pro-inflammatory adipokines. This reduction in lipocalin contributes to vascular and systemic inflammation (45, 47, 53). Additionally, central obesity plays a significant role in promoting tumor cell proliferation (54). Metabolic disorders reflected by CMI (e.g., abdominal obesity) release pro-inflammatory factors, while the inflammatory state itself activates immune cells to produce more inflammatory cytokines. Both act together to exacerbate the chronic low-grade inflammatory state (46). This enhanced chronic inflammation exacerbates the pro-inflammatory state of the tumor microenvironment and promotes tumor cell proliferation, survival, metastasis, and immune escape (46, 48). Furthermore, metabolic disorders and inflammation may promote tumor development by altering gene expression patterns through epigenetic modifications, which influence the expression of tumor suppressor genes and oncogenes (49).
In contrast to previous studies, the present study reveals the correlation between CMI and PCa, as well as elucidates the effect of the interaction between CMI and inflammatory indices on the risk of PCa. The value of CMI as a readily available indicator of visceral obesity for PCa was found, as well as the fact that CMI and inflammatory indices can work together as an adjunct tool to help in the prediction of PCa. These findings provide new perspectives for understanding the etiology of PCa; and provide important references for risk prediction and development of prevention strategies for PCa. Furthermore, it may pave the way for more refined screening and diagnostic strategies in the future.
5 Research limitations
This study has several limitations that need to be acknowledged. First, as a cross-sectional study, it is inherently limited in establishing causal relationships between the investigated variables and prostate cancer risk. Longitudinal studies are required to confirm temporal associations and to further explore the prognostic implications of these findings. Second, the additive interaction between NLR and prostate cancer requires validation in future studies, potentially due to the small sample size, which led to nonsignificant results for the attributable proportion (AP) and the confidence interval (CI). Future studies involving larger and more diverse populations are necessary to validate these findings. Third, although several potential confounders were adjusted for, residual confounding from unmeasured variables, such as lifestyle factors (e.g., diet, physical activity) and genetic predisposition, could not be entirely ruled out. Fourth, the absence of detailed data on prostate cancer subtypes, tumor stages, and treatment histories limited our ability to assess the impact of these factors on the observed associations. Finally, the single-center design of this study may have introduced selection bias and limited the generalizability of the findings. Multicenter studies are recommended to enhance the robustness and generalizability of the findings.
6 Conclusions
The results of this study demonstrated that PIV, SIRI, PLR, NLR, and CMI were positively associated with prostate cancer risk, whereas LMR exhibited a negative association. Furthermore, the findings revealed that the interaction between NLR, LMR, PLR, and CMI significantly influenced prostate cancer risk. These interactions suggest that inflammatory and metabolic factors may jointly contribute to prostate cancer development through distinct pathways, underscoring the importance of considering both inflammatory and metabolic status in prostate cancer risk assessment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the First Hospital of Xinjiang Medical University (Approval No. 20220 308-166). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YX: Conceptualization, Writing – original draft, Formal Analysis, Investigation, Methodology, Validation, Visualization. BT: Data curation, Formal Analysis, Investigation, Writing – original draft. JW: Data curation, Formal Analysis, Software, Writing – original draft. ZC: Data curation, Formal Analysis, Software, Validation, Writing – original draft. HA: Data curation, Funding acquisition, Methodology, Resources, Software, Supervision, Writing – review & editing. NT: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the Excellence Youth Science Foundation of Xinjiang Uygur Autonomous Region (Grant No.2023D01E05), Autonomous Region Regional Collaborative Innovation Special Science and Technology Aid Program Projects in Xinjiang (Grant No. 2024E02054), National Natural Science Foundation of China (Grant No. 82360476), Key Projects of Xinjiang Uyghur Autonomous Region (Grant No. 2022D01D39), Xinjiang Uygur Autonomous Region “Tianshan Talents” youth science and technology top talent project (Grant No. 2022TSYCCX0026), Innovation and Entrepreneurship Training Program for College Students (Grant No. S202310760037).
Acknowledgments
The authors would like to express their gratitude to all patients and research staff who participated in this study. Special thanks are extended to the nurses at the medical examination center for their invaluable assistance during the data collection process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer NZ declared a shared parent affiliation with the author BT to the handling editor at the time of review.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CMI, Cardiometabolic Index; NLR, Neutrophil-to-Lymphocyte Ratio; PLR, Platelet-to-Lymphocyte Ratio; PIV, Pan-Immune-Inflammation Value; SIRI, Systemic Inflammation Response Index; LMR, Lymphocyte-to-Monocyte Ratio; WHtR, Waist-to-Height Ratio; HDL-C, High-Density Lipoprotein Cholesterol; TG, Triglycerides; LDL-C, Low-Density Lipoprotein Cholesterol; FBG, Fasting blood glucose; SCr, serum creatinine; SUA, serum uric acid; GAM, generalized additive models; NEU, the neutrophil count; LYM, lymphocyte count; MON, monocyte count; PLT, platelet count; MCP-1, monocyte chemotactic protein-1; NF-κB, the nuclear factor-κB; ROS, reactive oxygen species.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors-a Systematic Review. Eur Urol (2023) 84(2):191–206. doi: 10.1016/j.eururo.2023.04.021
3. Fuster V. Changing demographics: A new approach to global health care due to the aging population. J Am Coll Cardiol. (2017) 69:3002–5. doi: 10.1016/j.jacc.2017.05.013
4. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA: Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
5. Haitian L, Weiming O, and Chuanshu H. Inflammation, a key event in cancer development. Mol Cancer research: MCR. (2006) 4:221–33. doi: 10.1158/1541-7786.MCR-05-0261
6. Lin WW and Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. (2007) 117:1175–83. doi: 10.1172/JCI31537
7. Lindholm PF, Sivapurapu N, Jovanovic B, and Kajdacsy-Balla A. Monocyte-Induced Prostate Cancer Cell Invasion Is Mediated by Chemokine Ligand 2 and Nuclear Factor-Κb Activity. J Clin Cell Immunol (2015) 6(2). doi: 10.4172/2155-9899.1000308
8. Sounbuli K, Mironova N, and Alekseeva L. Diverse neutrophil functions in cancer and promising neutrophil-based cancer therapies. Int J Mol Sci. (2022) 23(24):20221213. doi: 10.3390/ijms232415827
9. Gutiérrez-Melo N and Baumjohann D. T follicular helper cells in cancer. Trends Cancer. (2023) 9:309–25. doi: 10.1016/j.trecan.2022.12.007
10. Franco AT, Corken A, and Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. (2015) 126:582–8. doi: 10.1182/blood-2014-08-531582
11. Rani A, Dasgupta P, and Murphy JJ. Prostate cancer. Am J Pathol. (2019) 189:2119–37. doi: 10.1016/j.ajpath.2019.07.007
12. Fei M, Chao Y, Weiyu L, Yijin W, Jie X, and Hengbing W. Peripheral blood lymphocyte subsets are associated with the clinical outcomes of prostate cancer patients. Int Immunopharmacol. (2022) 113:109287. doi: 10.1016/j.intimp.2022.109287
13. Gavriilidis P and Pawlik TM. Inflammatory indicators such as systemic immune inflammation index (Siii), systemic inflammatory response index (Siri), neutrophil-to-lymphocyte ratio (Nlr) and platelet-to-lymphocyte ratio (Plr) as prognostic factors of curative hepatic resections for hepatocellular carcinoma. Hepatobiliary Surg Nutr. (2024) 13:509–11. doi: 10.21037/hbsn-23-631
14. Kian HG, Vida S, Afshin H, Alireza FM, Sina N, and Sina R. The neutrophil-to-lymphocyte ratio as a new prognostic factor in cancers: A narrative review&13. Front Oncol. (2023) 13:1228076. doi: 10.3389/fonc.2023.1228076
15. Jifeng F, Liang W, Xun Y, Qixun C, and Xiangdong C. Clinical utility of preoperative pan-immune-inflammation value (Piv) for prognostication in patients with esophageal squamous cell carcinoma. Int Immunopharmacol. (2023) 123:110805–. doi: 10.1016/j.intimp.2023.110805
16. Misiewicz and Piekarska D. Fashionable, but what is their real clinical usefulness? Nlr, lmr, and plr as a promising indicator in colorectal cancer prognosis: A systematic review. J Inflammation Res. (2023) 2023:69–81. doi: 10.2147/JIR.S391932
17. Bei C, Xiaoli J, Lirong Z, Xin X, and Yan Z. A novel prognostic marker systemic inflammation response index (Siri) for operable cervical cancer patients. Front Oncol. (2020) 10:766. doi: 10.3389/fonc.2020.00766
18. Atike P, Ahmet O, Gokhan SZ, Ali SU, Ruchan U, and Burcak K. Prognostic role of neutrophil-to-lymphocyte ratio in castration-resistant prostate cancer patients treated with docetaxel. J Clin Oncol. (2015) 33:e16050–e. doi: 10.1200/jco.2015.33.15_suppl.e16050
19. Heshmat-Ghahdarijani K, Sarmadi V, Heidari A, Falahati Marvasti A, Neshat S, Raeisi S, et al. The Neutrophil-to-lymphocyte ratio as a new prognostic factor in cancers: a narrative review. Front Oncol. (2023) 13:1228076. doi: 10.3389/fonc.2023.1228076
20. Yazgan SC, Yekedüz E, Utkan G, and Ürün Y. Prognostic role of pan-immune-inflammation value in metastatic castration-resistant prostate cancer. Prostate (2022) 82(15):1456–61. doi: 10.1002/pros.24419
21. Chen P, Huang Z, and Wu X. Association between lymphocyte-to-monocyte ratio and prostate cancer in men: A population-based study. Medicine. (2024) 103:e38826. doi: 10.1097/MD.0000000000038826
22. Donate-Moreno MJ, Lorenzo-Sánchez MV, Díaz de Mera-Sánchez Migallón I, Herraiz-Raya L, Esper-Rueda JA, Legido-Gómez O, et al. Inflammatory markers as prognostic factors in metastatic castration-resistant prostate cancer. Actas Urológicas Españolas (English Edition). (2020) 44:692–700. doi: 10.1016/j.acuroe.2020.11.009
23. Maeve B and Stefan A. Systemic inflammation indices and association with prostate cancer survival in a diverse patient cohort. Cancers. (2023) 15:1869–. Z. MT, H. DT, J. SC, A. LC. doi: 10.3390/cancers15061869
24. Pourshahidi LK, Wallace JM, Mulhern MS, Horigan G, Strain JJ, McSorley EM, et al. Indices of adiposity as predictors of cardiometabolic risk and inflammation in young adults. J Hum Nutr dietetics: Off J Br Dietetic Assoc. (2016) 29:26–37. K PL, W WJM, S MM, G H, J SJ, M ME, et al. doi: 10.1111/jhn.2016.29.issue-1
25. Wakabayashi I and Daimon T. The “Cardiometabolic index” as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clinica Chimica Acta. (2015) 438:274–8. doi: 10.1016/j.cca.2014.08.042
26. Liu X, Wu Q, Yan G, Duan J, Chen Z, Yang P, et al. Cardiometabolic index: A new tool for screening the metabolically obese normal weight phenotype. J endocrinological Invest. (2020) 44:1–9. X L, Q W, G Y, J D, Z C, P Y, et al.
27. Lazzer S, Isola M, De Martino M, Caroli D, Bondesan A, et al. Cardiometabolic index (Cmi) and visceral adiposity index (Vai) highlight a higher risk of metabolic syndrome in women with severe obesity. J Clin Med. (2023) 12.
28. Xiaoyi Z, Guiqin Z, Bo S, Guohua Z, Dezhong L, Jiage S, et al. Impact of obesity upon prostate cancer-associated mortality: A meta-analysis of 17 cohort studies. Oncol Lett. (2015) 9:1307–12. doi: 10.3892/ol.2014.2841
29. Carvalho RL, Brito TRP, Amaral JB, Monteiro FR, Lima DB, Pereira TAM, et al. Unraveling the interaction between inflammation and the cardiometabolic index in older men: A pilot study. Nutrients. (2024) 16:2529–. doi: 10.3390/nu16152529
30. Xu B, Wu Q, Yin G, Lu L, La R, Zhang Y, et al. Associations of cardiometabolic index with diabetic statuses and insulin resistance: the mediating role of inflammation-related indicators. BMC Public Health. (2024) 24:2736–. doi: 10.1186/s12889-024-20048-0
31. Xu B, Wu Q, La R, Lu L, Abdu FA, Yin G, et al. Is systemic inflammation a missing link between cardiometabolic index with mortality? Evidence from a large population-based study. Cardiovasc Diabetol. (2024) 23:212–. doi: 10.1186/s12933-024-02251-w
32. Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. (2019) 35(6):e3158. doi: 10.1002/dmrr.3158
33. Chen Y, Hu S, Wu L, Fang X, Xu W, and Shen G. Clinical practice guidelines for hypertension in China: a systematic review of the methodological quality. BMJ Open. (2015) 5(7):e008099. doi: 10.1136/bmjopen-2015-008099
34. 2018 Chinese guidelines for prevention and treatment of hypertension-a report of the revision committee of Chinese guidelines for prevention and treatment of hypertension. J Geriatr Cardiol. (2019) 16(3):182–241. doi: 10.11909/j.issn.1671-5411.2019.03.014
35. Tamini S, Bondesan A, Caroli D, and Sartorio A. The lipid accumulation product index (Lap) and the cardiometabolic index (Cmi) are useful for predicting the presence and severity of metabolic syndrome in adult patients with obesity. J Clin Med. (2024) 13(10):2843. doi: 10.3390/jcm13102843
36. Dłubek J, Rysz J, Jabłonowski Z, Gluba-Brzózka A, and Franczyk B. The Correlation between lipid metabolism disorders and prostate cancer. Curr Med Chem. (2021) 28(10):2048–61. doi: 10.2174/0929867327666200806103744
37. Coussens LM and Werb Z. Inflammation and cancer. Nature. (2002) 420:860–7. doi: 10.1038/nature01322
38. Ke-Qin Y, Yan L, Qing-Hua H, Ning M, Qing-Yun Z, Qing-Gui M, et al. Bone marrow-derived mesenchymal stem cells induced by inflammatory cytokines produce angiogenetic factors and promote prostate cancer growth. BMC Cancer. (2017) 17:878. doi: 10.1186/s12885-017-3879-z
39. Wang L, Li X, Liu M, Zhou H, and Shao J. Association between monocyte-to-lymphocyte ratio and prostate cancer in the U.S. Population: A population-based study. Front Cell Dev Biol. (2024) 12:1372731–. doi: 10.3389/fcell.2024.1372731
40. Jelena T, Laura A, and Michael K. Targeting inflammation in cancer prevention and therapy. Cancer Prev Res (Philadelphia Pa). (2016) 9:895–905. doi: 10.1158/1940-6207.CAPR-16-0209
41. Long J, Zhang CJ, Zhu N, Du K, Yin YF, Tan X, et al. Lipid metabolism and carcinogenesis, cancer development. Am J Cancer Res. (2018) 8:778–91.
42. Hong-Qun M, Lian-Hua C, Cheng-Cheng L, Zhuang Y, and Jin-Mei P. Effects of serum triglycerides on prostate cancer and breast cancer risk: A meta-analysis of prospective studies. Nutr Cancer. (2016) 68:1073–82. doi: 10.1080/01635581.2016.1206582
43. Cvetkovic Z, Cvetkovic B, Petrovic M, Ranic M, Debeljak-Martarcic J, Vucic V, et al Lipid profile as a prognostic factor in cancer patients. J Buon. (2009) 14(3):501–6.
44. Kotani K, Sekine Y, Ishikawa S, Ikpot IZ, Suzuki K, and Remaley AT. High-density lipoprotein and prostate cancer: an overview. J Epidemiol. (2013) 23:313–9. doi: 10.2188/jea.JE20130006
45. Stefan E, Mareike F, Kerstin G, Frauke H, Ute H, Jürgen J, et al. Association between adiponectin and mediators of inflammation in obese women. Diabetes. (2003) 52:942–7. doi: 10.2337/diabetes.52.4.942
46. Kershaw EE and Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. (2004) 89:2548–56. doi: 10.1210/jc.2004-0395
47. Xie L and Wang W. Weight control and cancer preventive mechanisms: role of insulin growth factor-1-mediated signaling pathways. Exp Biol Med. (2013) 238:127–32. doi: 10.1177/1535370213477602
48. Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: A review. Mediators Inflammation. (2010) 2010:1–20. doi: 10.1155/2010/513948
49. Lyssiotis CA and Nagrath D. Metabolic reprogramming and vulnerabilities in cancer. Cancers. (2019) 12:90–. doi: 10.3390/cancers12010090
50. Shaikh SR, Beck MA, Alwarawrah Y, and MacIver NJ. Emerging Mechanisms of Obesity-Associated Immune Dysfunction. Nat Rev Endocrinol (2024) 20(3):136–48. doi: 10.1038/s41574-023-00932-2
51. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25:1822–32. doi: 10.1038/s41591-019-0675-0
52. Soták M, Clark M, Suur BE, and Börgeson E. Inflammation and resolution in obesity. Nat Rev Endocrinol. (2025) 21(1):45–61. doi: 10.1038/s41574-024-01047-y
53. El-Wakkad A, Hassan NE-M, Sibaii H, and El-Zayat SR. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine. (2013) 61:682–7. doi: 10.1016/j.cyto.2012.11.010
Keywords: prostate cancer, cardiometabolic index, inflammation, neutrophillymphocyte ratio, platelet lymphocyte ratio, systemic inflammation response index, lymphocyte monocyte ratio
Citation: Xiao Y, Tang B, Wang J, Cai Z, An H and Tao N (2025) Study of the interaction between cardiometabolic index and inflammatory index on the risk of prostate cancer development. Front. Immunol. 16:1591879. doi: 10.3389/fimmu.2025.1591879
Received: 11 March 2025; Accepted: 23 June 2025;
Published: 17 July 2025.
Edited by:
Wenyi Jin, City University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Francesca Fanini, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyNing Zhang, Peking University, China
Copyright © 2025 Xiao, Tang, Wang, Cai, An and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hengqing An, OTI2OTczNUBxcS5jb20=; Ning Tao, Mzg1MTg0MTJAcXEuY29t
†These authors have contributed equally to this work
 Yunyun Xiao
Yunyun Xiao Bei Tang
Bei Tang Jueqi Wang
Jueqi Wang Zhiruo Cai
Zhiruo Cai Hengqing An
Hengqing An Ning Tao
Ning Tao